Abstract
Purpose:
Retinopathy of Prematurity (ROP) has been suggested to be increasing in Africa. However, it was only previously documented as a cause of blindness in 8 of 48 (16.7%) sub-Saharan African countries. The purpose of this study was to better understand the magnitude and breadth of blindness from ROP in sub-Saharan Africa.
Methods:
A questionnaire was sent to 455 ophthalmologists practicing in sub-Saharan Africa; the questionnaire was available in English, French and Portuguese.
Results:
Responses were received from 132 of 455 (29%) ophthalmologists to whom the survey was sent. Eighty-three respondents were identified as ROP-involved ophthalmologists and were from 26 of 48 (54%) sub-Saharan African countries. Ophthalmologists in 23 countries reported that they examined at least one child who was blind from ROP during the last 5 years. Sixteen of these countries had not previously reported cases of blindness from ROP in the literature. The perceived occurrence of Type 1 or more severe ROP was reported to be increasing by 31 of 77 (40%) ROP-involved ophthalmologists. ROP-involved pediatric ophthalmologists and retinal surgeons reported the number of infants they examined annually with Type 1 or more severe ROP increased from a median of 1 (range: 0–15) to a median of 4 (range: 0–40) from 2015 to 2019. ROP was estimated to be the cause of blindness for 10% of all blind children examined by ROP-involved pediatric ophthalmologists and retinal surgeons during 2019.
Conclusions:
ROP is becoming a more important and widespread cause of childhood blindness in sub-Saharan Africa.
Keywords: Retinopathy of Prematurity, Childhood Blindness, sub-Saharan Africa, Blindness, Children
Introduction
Advances in the screening and treatment of preterm infants have reduced the incidence of blindness from retinopathy of prematurity (ROP) in high-income countries.1,2 Similar advances in the management of ROP in middle-income countries have reduced the prevalence of severe ROP. For example, a concerted effort to improve neonatal care in Argentina reduced blindness from ROP in a school for the blind from 115 (64%) students in 1998 to 9 (20%) students in 2012.3 In low-income countries, the leading cause of childhood blindness was thought to be corneal opacification resulting from vitamin A deficiency, measles and harmful traditional medical practices.4,5 Whereas in the past ROP was not recognised as an important cause of blindness in low-income countries,6–8 the expansion of neonatal care to preterm infants in these countries has increasingly put them at risk of becoming blind from ROP.8 In 2014, it was estimated that there were 4.2 million pre-term births in sub-Saharan Africa.9 Many of these infants who are being cared for in neonatal care units10 are receiving unregulated supplemental oxygen,11 putting them at increased risk of developing ROP.12 Blindness from ROP has only been reported in a few countries in sub-Saharan Africa in the last 10 years.13–24 In the absence of data on blindness from ROP in many sub-Saharan African countries, the goal of our study was to better understand the current burden of blindness from ROP by surveying a large number of ophthalmologists throughout sub-Saharan Africa.
Subjects and methods
The International Pediatric Ophthalmology and Strabismus Council (IPOSC) recently established an ROP task force entitled Stop Infant Blindness in Africa (SIBA) comprised of more than 30 physicians from many countries around the world. SIBA’s mission is to reduce preventable blindness from ROP in preterm infants in sub-Saharan Africa. The task force was asked to begin by better understanding how much of a problem ROP is in sub-Saharan Africa. To ascertain this information, a questionnaire was created to query African ophthalmologists on screening criteria and treatment methods for ROP, the occurrence of Type 1 ROP in their clinic or hospital unit, and the number of children they have recently examined who are blind from ROP. The study was approved by the Stanford University School of Medicine Institutional Review Board (eProtocol number: 57247) as exempt from review and the study was conducted in accordance with the tenets of the Declaration of Helsinki.
The contact information of ophthalmologists was collected between April 27-July 30, 2020. The contact information of these individuals was gathered through IPOSC’s 2018 Cape Town, South Africa symposium attendee list, corresponding authors of ophthalmological papers in sub-Saharan Africa, secondary contacts of these authors, ophthalmological societies, online resources, and personal contacts of colleagues. In total, 455 individuals were sent the survey via email, WhatsApp Messenger or both.
The questionnaire was created in Qualtrics Survey Software (Qualtrics Provo, USA) and accessed through a personal hyperlink that could be completed once. There were a total of 11 questions in the survey, created by SIBA committee members. The questionnaire was field tested in Nigeria prior to submission to the Institutional Review Board. Available languages included English, French, and Portuguese.
Responses were anonymous and collected between July 16–August 11, 2020. Each contact received an introductory message describing SIBA, the purpose of the questionnaire and that their responses would remain anonymous. Participants received up to two reminder messages if no response was received.
Four responses were excluded from analysis and not included in the number of surveys distributed due to a significant amount of missing data. If a response to a question was blank or seemed to be affected by a data entry error, it was excluded from analysis since the respondents were anonymous and could not be contacted for clarification. If terms such as “more than,” “less than,” “about,” “approx.” etc. were used in conjunction with a number reported then only the number indicated was used for analysis. The mean was used if a range of values was given. “Other:” responses to questions were grouped into like categories.
If a respondent only indicated the weight or gestational age in response to the question on screening criteria, then it was assumed they were indicating “less than” the value provided. Most of the analysis was conducted with the responses of those who indicated they are involved with the management of ROP or they had seen at least one infant/child with ROP during last 5 years. These respondents were termed “ROP-involved” ophthalmologists. The survey was terminated early through survey logic if a respondent indicated they were not “ROP-involved.” The perceived occurrence of blindness from ROP was calculated for each ROP-involved ophthalmologist by dividing the number of children blind from ROP examined last year by the total number of blind children examined by these ophthalmologists in the last year. To estimate the perceived occurrence of blindness from ROP in each country, the median occurrence seen by ROP-involved ophthalmologists within the country was used. If no blind children were seen, then the occurrence of blindness from ROP could not be calculated and the response was excluded from analysis.
Microsoft Excel was used for analysis and constructing tables and ArcGIS Pro geospatial software (Ersi Redlands, USA) was used to construct a map of sub-Saharan Africa.
Results
Responses were received from 132 of 455 (29%) ophthalmologists who were sent the survey from 35 of 48 (73%) sub-Saharan African countries, 20 of 23 (87%) English-speaking nations, 13 of 20 (65%) French-speaking nations, and 2 of 5 (40%) Portuguese-speaking nations in sub-Saharan Africa. Eighty-three of 132 respondents (63%) indicated they were ROP-involved; 36 (43%) general ophthalmologists, 26 (31%) paediatric ophthalmologists, 10 (12%) retinal surgeons, 7 (8%) who did not indicate a sub-specialty, and 4 (5%) anterior segment surgeons. These ophthalmologists were from 26 of 48 (54%) sub-Saharan African countries (Table 1), 19 of 23 (83%) English-speaking nations, 6 of 20 (30%) French-speaking nations, and 1 of 5 (20%) Portuguese-speaking nations.
Table 1.
Cases of severe ROP seen by ROP-involved ophthalmologists in 2019.
| Country | Responding ROP-Involved ophthalmologists | No. of Type 1 ROP per ophthalmologist | No. of blind from ROP per ophthalmologist | Perceived occurence of blindness from ROPa |
|---|---|---|---|---|
| No. | Median b | Median b | Median b (%) | |
| Benin | 1 | 10 | 1 | 20 |
| Botswana | 2 | 4 | 4 | 26 |
| Burundi | 1 | 0 | 1 | 0 |
| Cameroon | 2 | 0.5 | 0 | 0 |
| DR Congo | 2 | 0 | 1 | 10 |
| Ethiopia | 6 | 0.5 | 1 c | 17c |
| Gambia | 2 | 0 | 0 | 0 |
| Ghana | 2 | 1.5 | 2.5 | 33c |
| Kenya | 6 | 3.5 | 4 | 8c |
| Madagascar | 6 | 0 | 0 | 0c |
| Malawi | 1 | 0 | 1 | 7 |
| Mauritius | 1 | 0 | 1 | 100 |
| Mozambique | 9 | 0c | 0 | 0c |
| Namibia | 1 | 5 | 1 | 14 |
| Nigeria | 12 | 1 | 0.5 | 5d |
| Rwanda | 6 | 8c | 3.5 | 55 |
| Sierra Leone | 1 | 1 | 2 | 13 |
| Somalia | 1 | 15 | 45 | 23 |
| South Africa | 3 | 4 | 1 | 20 |
| South Sudan | 1 | 0 | 1 | 20 |
| Sudan | 1 | 10 | 5 | 17 |
| Swaziland | 1 | 0 | 0 | 0 |
| Tanzania | 2 | 3.5 | 2 | 11 |
| Uganda | 3 | 0 | 3 | 9c |
| Zambia | 3 | 0 | 0 | 0 |
| Zimbabwe | 7 | 0 | 2 | 3 |
Calculated by dividing the number of children blind from ROP examined last year by the total number of children blind from any condition examined last year.
In countries with only one respondent, the value reported by that respondent was used.
One response excluded or left blank.
Three responses were excluded or left blank.
Ophthalmologists (n = 54) from 23 of 48 (48%) sub-Saharan African countries reported examining at least one child blind from ROP between 2015 and 2019 (Figure 1). During 2019, ROP-involved paediatric ophthalmologists and retinal surgeons (n = 36) diagnosed a median of 2 (n = 35; range: 0–40) infants/children with Type 1 ROP during 2019 and examined a median of 2 (n = 35; range: 0–20) infants/children who were blind secondary to ROP. They estimated that a median of 10% (n = 32; range: 0–100%) of all blind children they had examined in 2019 were blind secondary to ROP. ROP-involved general ophthalmologists who do not screen in a neonatal care unit (n = 18) examined a median of 1 (n = 18; range: 0–45) infants/children who were blind due to ROP during 2019 and reported a median of 13% (n = 17; range: 0–29%) of all the blind children they had examined during 2019 were blind secondary to ROP.
Figure 1.
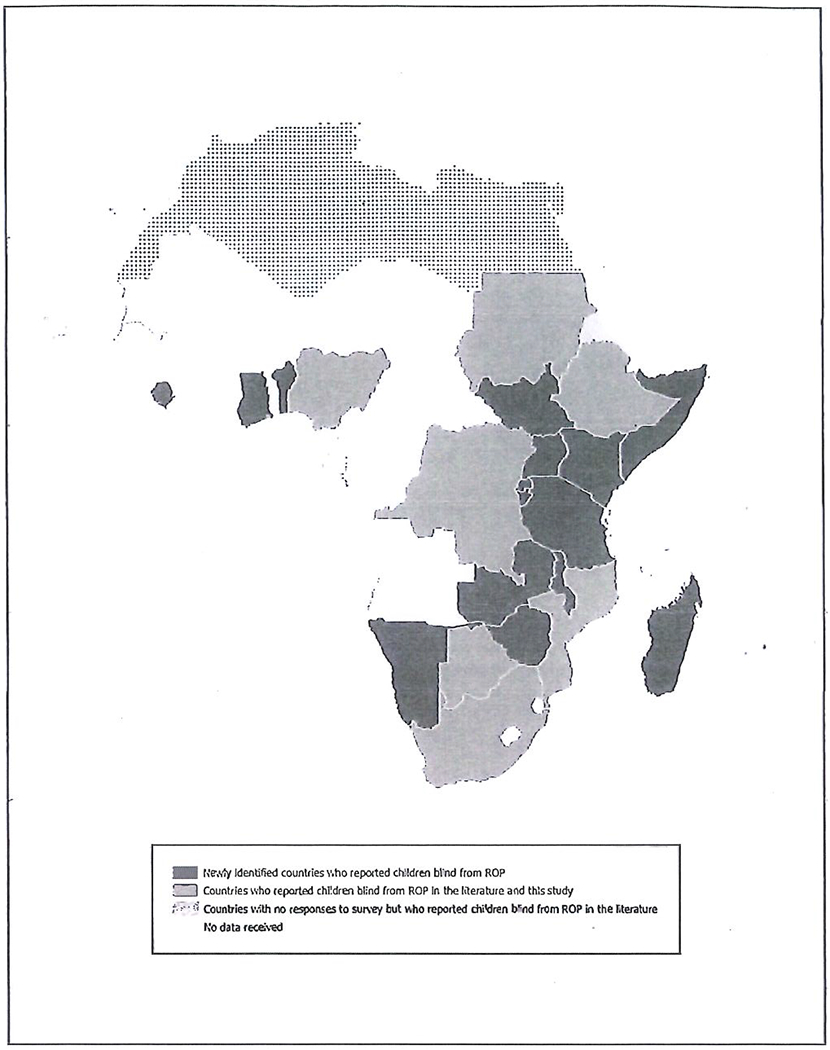
Sub-Saharan African countries reporting children blind from ROP during the last 10 years. No responses to the survey were obtained from ophthalmologists in Eritrea. This was the only country that has previously reported blindness from ROP for which we did not receive data.
The proportion of ROP-related blindness in children examined varied from country to country (Table 1). Excluding Mauritius, the highest perceived occurrence of childhood blindness from ROP was Rwanda, followed by Ghana and Botswana. In Rwanda, ROP caused the majority of childhood blindness seen by the 6 ROP-involved ophthalmologists, and all of them reported that Type 1 or more severe ROP had increased over the last 5 years. We did not find a significant correlation between the neonatal mortality rate25 and the perceived occurrence of blindness from ROP in these countries.
Information on the perception of whether Type 1 or more severe ROP increased or decreased between 2015 and 2019 was provided by 77 of 83 (93%) ROP-involved ophthalmologists. Of these, 31 (40%) responded Type 1 or more severe ROP increased, 36 (47%) remained unchanged and 10 (13%) decreased between 2015 and 2019. However, when asked to estimate the number of children examined who were blind from ROP by year, ROP-involved paediatric ophthalmologists and retinal surgeons (n = 36) estimated an increase from a median of 1 (n = 25; range: 0–15) to a median of 4 (n = 31; range: 0–40) infants/children developing Type 1 or more severe ROP annually from 2015 to 2019. They also observed an increasing number of children blind from ROP, from a median of 0 (n= 25; range: 0–10) in 2015 to a median of 1 (n = 29; range: 0–30) in 2019.
A total of 51 (39%) of all respondents provided information on their ROP screening criteria: gestational age = 48 (94%), birthweight = 47 (92%), oxygen therapy = 12 (24%), prevalence of comorbidities = 8 (16%), and unstable clinical course 4 (8%). The birthweight and gestational age requirements for screening were also reported (Table 2).
Table 2.
Reported birthweight and gestational age for ROP screening.
| Criteria | ||
|---|---|---|
|
| ||
| Birthweight (g) | Gestational age (weeks) | |
| n | 35 | 35 |
| Median | 1500 | 34 |
| Max | 2500 | 37 |
| Min | 1250 | 28 |
| IQRa | 300 | 2.5 |
Interquartile range.
Forty-four of 83 (53%) ROP-involved respondents indicated that their facility does not treat ROP. Of respondents who treat ROP (n = 39), 35 (90%) indicated they use anti-VEGF medication, 26 (67%) laser therapy, 4 (10%) cryotherapy, 5 (13%) vitreoretinal surgery.
Discussion
Although Africa has been called the “new frontier” of ROP blindness,26 it was previously only documented to be a cause of childhood blindness in 8 of 48 (16.7%) sub-Saharan African countries within the past 10 years13–24 (Table 3). The prevalence of blindness due to ROP in these studies ranged from 0.1%22 to 15.4%.20 They likely underestimated the magnitude of this problem due to incomplete medical records,15,17,19,20 inadequate screening occurring within the facilities,16,21 high rates of patients lost to follow-up,22,24 a selection bias because many of these studies were conducted in schools for the blind,6 and an improved neonatal survival rate since these studies were conducted.27 A key finding in our study was the identification of 16 additional sub-Saharan African countries, beyond the 8 countries with previously published reports, where children are becoming blind from ROP during the last 5 years. These findings suggest that ROP is becoming a more widespread cause of childhood blindness in sub-Saharan Africa.
Table 3.
Reported cases of ROP blindness in sub-Saharan Africa 2013–2019.
| Country | Year | Participantsa | ROP blind | Prevalence (%) | Study |
|---|---|---|---|---|---|
| Botswana | |||||
| Neonatal Unit | Jun 2018–May 2019 | 200 | 7 | 3.5 | Gezmu13 |
| Democratic Republic of the Congo | |||||
| Eye Hospital | Jan 2014–Jun 2016 | 67 | 1 | 1.5 | Kabesha14 |
| Eritrea | |||||
| Eye Hospital | Jan 2011-Dec 2015 | 249 | 5b | 2.0 | Gyawali15 |
| Ethiopia | |||||
| Eye Hospital | Jun 2016-Dec 2019 | 93 | 12 | 12.9 | Melesse16 |
| Mozambique | |||||
| School for Visually Impaired | Sep 2015 | 99 | 1b | 1.0 | Almeida17 |
| Nigeria | |||||
| School for Visually Impaired | Jun-Jul 2017 | 114 | 1b | 0.9 | Olowoyeye18 |
| South Africa | |||||
| Neonatal Unit | Jan 2009-Dec 2014 | 919 | 7 | 0.8 | Jacoby19 |
| Referral Hospital | 2009-2015 | 13 | 2 c | 15.4 | Botha20 |
| Neonatal Unit | Jan 2013-Dec 2013 | 132 | 1 | 0.8 | Dadoo21 |
| Eye Hospital | 2013-2015 | 1,911 | 2d | 0.1 | Kana22 |
| Sudan | |||||
| Neonatal Unit | Nov 2011-Sep 2012 | NA | NA | 5.0 | Saleem23 |
Includes infants who were treated or met screening criteria, children with visual impairment seen in eye hospital, or students in schools for the blind.
Blind and/or visually impaired.
At least two cases.
Unilateral cases.
Although the proportion of ROP-related blindness in children examined by ROP-involved paediatric ophthalmologists and retinal surgeons in this study cannot be directly compared to other studies due to differences in study design, it is informative to note that the proportion found in this study (10%) is higher than 9 of the previous reports in sub-Saharan Africa.13–15,17–19,21–23 A recent prospective hospital-based study in rural Kenya found a lower prevalence of ROP compared to other previous studies; however, the lower prevalence was likely due to a high mortality rates (12.6%) and a high proportion of infants lost to follow-up (25.2%). 28 As rural regions receive expanded neonatal services, it is likely that more infants will survive and develop severe ROP.8
General ophthalmologists who do not screen for ROP reported they have seen children blind from ROP in their clinics. This suggests a deficit in screening, treatment and follow-up care of infants with ROP. The high perceived proportion of blindness from ROP in sub-Saharan Africa is multifactorial and likely includes babies receiving unregulated oxygen as neonates,11 limited screening guidelines for ROP,29 inadequate resources for treating Type 1 ROP, and many of these infants being lost to follow-up after discharge.22,24 Continued efforts to improve ROP screening and follow-up care are needed.
ROP-involved physicians from four countries reported more than 25% of the blind children they examined in 2019 were blind secondary to ROP (Table 1). ROP-involved ophthalmologists from Rwanda reported that the majority of the blind children they examined during 2019 were blind due to ROP. One respondent examined 20 children in 2019 who were blind from ROP. This is important since the only previous ROP study conducted in Rwanda did not find any infants who progressed to stage 4 or 5 ROP.30 Respondents from Ghana also reported a high proportion of ROP-related blindness in children examined. This is important since two previous reports in Ghana indicated infants were at risk of developing ROP,31 but none were reported to become blind. 32 Benin, Sierra Leone, Somalia, and South Sudan all have neonatal mortality rates above 30 deaths in 1000 live births,25 but still reported that children are becoming blind from ROP, suggesting that despite high neonatal mortality rates, children in these countries are still becoming blind from ROP (Table 1). We did not receive any responses to the survey from ophthalmologists in Eritrea, but they have previously reported cases of blindness from ROP.15
Neonatal mortality rates in sub-Saharan Africa have decreased by 40% since 1990,27 which is likely why some respondents observed an increase in the number of Type 1 or more severe cases of ROP within the past 5 years. Lower gestational age of infants is associated with higher incidence of ROP.33 Since sub-Saharan Africa accounts for 28% of preterm births globally,9 this means that as neonatal care expands and neonatal mortality rates decline, more of these preterm infants will be at risk for developing severe ROP. It is likely that additional sub-Saharan African countries will experience a high proportion of blindness from ROP.3
ROP screening is important for early diagnosis of the disease,34 however, South Africa and Kenya are the only countries in sub-Saharan Africa to have published national screening guidelines,29 while guidelines for Nigeria are currently being drafted. National screening guidelines are needed in more sub-Saharan African countries. Of those who reported on the composition of screening criteria, most indicated birthweight and gestational age. The median birthweight for screening in this study is the same as recommended in the national screening guidelines of South Africa35 and Kenya,36 and the United States,37 but the median gestational age was higher than the recommended screening criteria in all of these countries.35–37 Since most infants in sub-Saharan neonatal care units are administered unregulated oxygen,11 larger and more mature babies may develop severe ROP.12 Thus, larger birthweight and higher gestational age criterion for screening may be warranted in some countries in sub-Saharan Africa.
Nearly all respondents who treat ROP indicated they use anti-VEGF agents for treating ROP. Although anti-VEGF treatment does not affect later visual acuity in most infants,38 there are still questions as to its longterm systemic effects.39 Anti-VEGF treatment has a high rate of recurrence,39 which poses a problem in sub-Saharan African countries given the high rate of patients lost to follow-up.22,24 A majority of those indicating they treat ROP reported they also use laser therapy. This is higher than was previously reported.11 Laser treatment has a lower recurrence rate, but comes with its own associated risks.39
Limitations to this study include a low response rate (29%). Many contacts had multiple email addresses and we may not have sent the survey to the contact’s most frequently checked email address. Some contacts may not have been involved in the management of ROP and felt no need to respond. In addition, the findings from this study are focused on ophthalmologists who are ROP-involved, and thus are more likely to see ROP cases. This could have inflated the perceived occurrence of blindness from ROP reported. Respondents may also have had recall bias when reporting numbers of infants developing ROP. Additionally, it is possible that some ophthalmologists throughout sub-Saharan Africa may not know how to recognise Type 1 ROP or ROP blindness correctly. There was also some internal inconsistency in some of the responses such as indicating a larger number of children blind from ROP examined last year compared to a subsequent question asking them to estimate the number of children blind from ROP examined in 2019. Some countries also only had one respondent who may not be representative of the country as a whole. Lastly, the proportion of ROP-related blindness in children examined was estimated as seen by ophthalmologists in their hospitals and clinics. It is likely that some children blind from ROP may not present to these facilities, thus the actual occurrence of blindness may be higher than what is documented in this study.
Despite these limitations, our methodology is similar to other studies determining the occurrence of different etiologies of blindness.40 In the United Kingdom, Rahi and Cable41 determined the causes of childhood blindness by having doctors report patients who were diagnosed as severely visually impaired or blind. In the United States, Steinkuller et al.4 determined the causes of blindness through mailing surveys to schools for the blind. In New Zealand, Chong et al.42 estimated the prevalence and etiologies contributing to childhood blindness through a retrospective review of the medical records of students in a national blind and low vision network. Our study estimated the occurrence of blindness from ROP throughout sub-Saharan Africa by surveying ophthalmologists. Through this method, we estimated the burden of blindness secondary to ROP throughout sub-Saharan Africa and identified many additional sub-Saharan African countries, beyond the literature, where ROP is a contributor to childhood blindness.
Conclusions
Blindness secondary to ROP is becoming a more important contributor and more widespread cause of childhood blindness in sub-Saharan Africa. Our study reports 16 additional countries where children have been noted to become blind from ROP between 2015 and 2019. Some countries reported a high proportion of children blind from ROP in 2019. Most respondents indicated that birthweight and gestational age were part of their ROP screening criteria, but a minority indicated other risk factors were used as screening criteria. Anti-VEGF medication was the most common modality used to treat ROP. Efforts are needed to raise awareness and improve the management of ROP in order to mitigate an impending epidemic of blindness from ROP in sub-Saharan Africa.
Literature search.
The literature was searched between April 27-June 1, 2020. Google Scholar and PubMed were the primary databases used. The search was conducted systematically for each of the 48 sub-Saharan African countries. Search items included a combination of terms such as “retinopathy of prematurity,” “ROP,” “childhood blindness,” and “pediatric blindness” in conjunction with a specific country in sub-Saharan Africa. Where relevant, search terms were translated into French or Portuguese. Reports in French or Portuguese were translated using Google Translate in order to extract relevant information. Additional studies were located through the snowball method. A subsequent search was conducted in Embase on September 16, 2020. Search terms included “retinopathy of prematurity,” “ROP,” and “Africa.” Only studies reporting at least one child blind from ROP and conducted within the last 10 years were included. Studies were considered to have participants blind due to ROP if indicated in the text, or if stage 4 and 5 ROP was reported.
Acknowledgments
The survey was translated into French by Charlotte Tibi and into Portuguese by Ruth Baptista.
Funding
Supported by grant P30 EY026877 from the National Institutes of Health (Bethesda, MD), Research to Prevent Blindness, and time support from Brigham Young University College of Life Sciences; Brigham Young University.
Footnotes
Disclosure of Interest
None of the authors have any proprietary interests or conflicts of interest related to this submission.
References
- 1.Chow LC, Wright KW, Sola A. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in veiy low birth weight infants? Pediatrics. 2003;111(2):339–345. doi: 10.1542/peds.111.2.339. [DOI] [PubMed] [Google Scholar]
- 2.Bullard SR, Donahue SP, Feman SS, Sinatra RB, Walsh WF. The decreasing incidence and severity of retinopathy of prematurity. J AAPOS. 1999;3(1):46–52. doi: 10.1016/S1091-8531(99)70094-7. [DOI] [PubMed] [Google Scholar]
- 3.Hariharan L, Gilbert CE, Quinn GE, et al. Reducing blindness from retinopathy of prematurity (ROP) in Argentina through collaboration, advocacy and policy implementation. Health Policy Plan. 2018;33:654–665. doi: 10.1093/heapol/czy004. [DOI] [PubMed] [Google Scholar]
- 4.Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK. Childhood blindness. J AAPOS. 1999;3:26–32. doi: 10.1016/S1091-8531(99)70091-1. [DOI] [PubMed] [Google Scholar]
- 5.Sommer A, Hussaini G, Tarwotjo I, Susanto D. Increased mortality in children with mild vitamin a deficiency. Lancet. 1983;322:585–588. doi: 10.1016/S0140-6736(83)90677-3. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert C, Rahi J, Eckstein M, O’Sullivan J, Foster A. Retinopathy of prematurity in middle-income countries. Lancet. 1997;350:12–14. doi: 10.1016/S0140-6736(97)01107-0. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert C, Foster A. Childhood blindness in the context of VISION 2020: the right to sight. Bull World Health Organ. 2001;79:227–232. doi: 10.1590/S0042-96862001000300011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert C Changing challenges in the control of blindness in children. Eye. 2007;21:1338–1343. doi: 10.1038/sj.eye.6702841. [DOI] [PubMed] [Google Scholar]
- 9.Chawanpaiboon S, Vogel JP, Moller A-B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7:e37–e46. doi: 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74(S1):35–49. doi: 10.1038/pr.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lloyd T, Isenberg S, Lambert SR. Current management of retinopathy of prematurity in sub-Saharan Africa. J AAPOS. 2020;24(3):151.e1–151.e6. Published online June 4. doi: 10.1016/j.jaapos.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah PK, Narendran V, Kalpana N. Aggressive posterior retinopathy of prematurity in large preterm babies in South India. Arch Dis Child - Fetal Neonatal Ed. 2012;97(5):F371–F375. doi: 10.1136/fetalneonatal-2011-301121. [DOI] [PubMed] [Google Scholar]
- 13.Gezmu AM, Shifa JZ, Quinn GE, et al. Incidence of retinopathy of prematurity in Botswana: a prospective observational study. Clin Ophthalmol. 2020;14:2417–2425. doi: 10.2147/OPTH.S265664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabesha TB, Kabesha D, Rehema G, et al. [Result of intravitreal injection of Bevacizumab (Avastin) in the treatment of retinopathy of prematurity (About 11 cases followed up at Bukavu from 01.01 2014 to 06.30 2016)]. Lubumbashi Med J Fac Med. December 2018; Published online. doi: 10.13140/RG.2.2.18193.28004. [DOI] [Google Scholar]
- 15.Gyawali R, Bhayal BK, Adhikary R, Shrestha A, Sah RP. Retrospective data on causes of childhood vision impairment in Eritrea. BMC Ophthalmol. 2017;17:1–8. doi: 10.1186/sl2886-017-0609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melesse MA. Retinopathy of prematurity - an emerging cause of childhood blindness in Ethiopia. Ethiop Med J. 2020;58:167–172. [Google Scholar]
- 17.Almeida AA Causes of blindness and severe visual impairment among students attending school for the blind in Beira, Mozambique [Thesis], Nairobi, KY; 2016. [Google Scholar]
- 18.Olowoyeye AO, Musa KO, Aribaba OT, Onakoya AO, Akinsola FB. Pattern of childhood visual impairment and blindness among students in schools for the visually impaired in Lagos State: an update. Niger Postgrad Med J. 2018;25:105–111. doi: 10.4103/npmj.npmj_27_18. [DOI] [PubMed] [Google Scholar]
- 19.Jacoby MR, Toit LD. Screening for retinopathy of prematurity in a provincial hospital in Port Elizabeth, South Africa. S Afr Med J. 2016;106(6):598–601. doi: 10.7196/SAMJ.2016.v106i6.10663. [DOI] [PubMed] [Google Scholar]
- 20.Botha TC Retinopathy of prematurity: a case series of treated infants [Thesis]. Bloemfontein, SA: University of the Free State; 2017 [Google Scholar]
- 21.Dadoo Z, Ballot DE. An evaluation of the screening for retinopathy of prematurity in very-low-birth-weight babies at a tertiary hospital in Johannesburg, South Africa. South Afr J Child Health. 2016;10(1):79–82. doi: 10.7196/SAJCH.2016.v10il.1099. [DOI] [Google Scholar]
- 22.Kana H, Mayet I, Soma D, Alli HD, Biddulph S. The efficacy of intravitreal antivascular endothelial growth factor as primary treatment of retinopathy of prematurity: experience from a tertiary hospital. S Afr Med J. 2017;107 (3):215–218. doi: 10.7196/SAMJ.2017.vl07i3.11080. [DOI] [PubMed] [Google Scholar]
- 23.Saleem M, Abdalla N. Screening of retinopathy of prematurity in Khartoum city, Sudan, 2011-2012. Merit Res J Microbiol Biol Sci. 2014;2:25–30. [Google Scholar]
- 24.Du Bruyn A, Visser L. Eight years of treatable retinopathy of prematurity in KwaZulu-Natal : are we winning the battle? South Afr Ophthalmol J. 2017;12:8–10. [Google Scholar]
- 25.The World Bank. Mortality rate, neonatal (per 1,000 live births). data.worldbank.org. Published 2018. Accessed August 15, 2020. https://data.worldbank.org/indicator/SH.DYN.NMRT?end=2018&name_desc=true&start=2016
- 26.Gilbert C, Malik ANJ, Nahar N, et al. Epidemiology of ROP update - Africa is the new frontier. Semin Perinatol. 2019;43:317–322. doi: 10.1053/j.semperi.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Hug L, Alexander M, You D, Alkema L. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. Lancet Glob Health. 2019;7:e710–e720. doi: 10.1016/S2214-109X(19)30163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sitati SM, Ojuma SM, Alba C de. Retinopathy of prematurity: prevalence and risk factors among infants in rural Kenya. J Ophthalmol East Cent South Afr. 2018;22:63–67. [Google Scholar]
- 29.Wang D, Duke R, Chan RP, Campbell JP. Retinopathy of prematurity in Africa: a systematic review. Ophthalmic Epidemiol. 2019;26:223–230. doi: 10.1080/09286586.2019.1585885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutangana F, Muhizi C, Mudereva G, et al. Retinopathy of prematurity in Rwanda: a prospective multi-centre study following introduction of screening and treatment services. Eye. 2019;34(5):847–856.doi: 10.1038/S41433-019-0529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nti AA, Essuman VA, Enweronu-Laryea C, Ofori-Darko A, Quinn GE. Babies at risk for retinopathy of prematurity in Ghana. J AAPOS. 2015;19:e55. doi: 10.1016/j.jaapos.2015.07.174. [DOI] [Google Scholar]
- 32.Braimah IZ, Enweronu-Laryea C, Sackey AH, et al. Incidence and risk factors of retinopathy of prematurity in Korle-Bu Teaching Hospital: a baseline prospective study. BMJ Open. 2020;10:e035341. doi: 10.1136/bmjopen-2019-035341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SJ, Port AD, Swan R, Campbell JP, Chan RVP, Chiang MF. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol. 2018;63:618–637. doi: 10.1016/j.survophthal.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulkarni S, Gilbert C, Zuurmond M, Agashe S, Deshpande M. Blinding retinopathy of prematurity in Western India: characteristics of children, reasons for late presentation and impact on families. Indian Pediatr. 2018;55:665–670. doi: 10.1007/sl3312-018-1355-8. [DOI] [PubMed] [Google Scholar]
- 35.Visser L, Singh R, Young M, Lewis H, McKerrow N. Guideline for the prevention, screening and treatment of retinopathy of prematurity (ROP). S Afr Med J. 2013;103:116–125. doi: 10.7196/SAMJ.6305. [DOI] [PubMed] [Google Scholar]
- 36.Sitati S, Mwangi N, Njambi L, et al. National guidelines for screening and management of retinopathy of prematurity in Kenya: an overview of the recommendations. JOECSA. July 2019; 23:3–5. Published online. [Google Scholar]
- 37.Fierson WM. Ophthalmology AAOPS on, Ophthalmology AAO, Strabismus AA for POA, Orthoptists AA of C. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018;142:e20183061. doi: 10.1542/peds.2018-3061. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez SH, Schechet SA, Shapiro MJ, Blair MP. Late visual outcomes in infants treated with primary bevacizumab for type 1 retinopathy of prematurity. J AAPOS. 2020;24(3):149.e1–149.e5. Published online May 24. doi: 10.1016/j.jaapos.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 39.VanderVeen DK, Melia M, Yang MB, Hutchinson AK, Wilson LB, Lambert SR. Anti-vascular endothelial growth factor therapy for primary treatment of type 1 retinopathy of prematurity: a report by the American academy of ophthalmology. Ophthalmology. 2017;124:619–633. doi: 10.1016/j.ophtha.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 40.Solebo AL, Teoh L, Rahi J. Epidemiology of blindness in children. Arch Dis Child. 2017;102:853–857. doi: 10.1136/archdischild-2016-310532. [DOI] [PubMed] [Google Scholar]
- 41.Rahi JS, Cable N. Severe visual impairment and blindness in children in the UK. Lancet. 2003;362:1359–1365. doi: 10.1016/S0140-6736(03)14631-4. [DOI] [PubMed] [Google Scholar]
- 42.Chong C, McGhee CNJ, Dai SH. Causes of childhood low vision and blindness in New Zealand. Clin Exp Ophthalmol. 2019;47(2):165–170. doi: 10.1111/ceo.13443. [DOI] [PubMed] [Google Scholar]


