Abstract
The monkeypox virus (MPOX) is an uncommon zoonotic illness brought on by an orthopoxvirus (OPXV). MPOX can occur with symptoms similar to smallpox. Since April 25, 2023, 110 nations have reported 87,113 confirmed cases and 111 fatalities. Moreover, the outspread prevalence of MPOX in Africa and a current outbreak of MPOX in the U.S. have made it clear that naturally occurring zoonotic OPXV infections remain a public health concern. Existing vaccines, though they provide cross-protection to MPOX, are not specific for the causative virus, and their effectiveness in the light of the current multi-country outbreak is still to be verified. Furthermore, as a sequel of the eradication and cessation of smallpox vaccination for four decades, MPOX found a possibility to re-emerge, but with distinct characteristics. The World Health Organization (WHO) suggested that nations use affordable MPOX vaccines within a framework of coordinated clinical effectiveness and safety evaluations. Vaccines administered in the smallpox control program and conferred immunity against MPOX. Currently, vaccines approved by WHO for use against MPOX are replicating (ACAM2000), low replicating (LC16m8), and non-replicating (MVA-BN). Although vaccines are accessible, investigations have demonstrated that smallpox vaccination is approximately 85% efficient in inhibiting MPOX. In addition, developing new vaccine methods against MPOX can help prevent this infection. To recognize the most efficient vaccine, it is essential to assess effects, including reactogenicity, safety, cytotoxicity effect, and vaccine-associated side effects, especially for high-risk and vulnerable people. Recently, several orthopoxvirus vaccines have been produced and are being evaluated. Hence, this review aims to provide an overview of the efforts dedicated to several types of vaccine candidates with different strategies for MPOX, including inactivated, live-attenuated, virus-like particles (VLPs), recombinant protein, nucleic acid, and nanoparticle-based vaccines, which are being developed and launched.
Keywords: Monkeypox, Vaccine, Immunity, Nanovaccine, Nucleic acid vaccine
Graphical abstract
1. Introduction
The orthopoxviruses (OPXVs) are a genus of the Poxviridae's chordopoxvirus subfamily, including members that cause sickness in humans and other animals. The most well-known member of this genus, Variola virus (VARV), is a human-specific pathogen that caused smallpox, which was eliminated more than three decades ago after widespread immunization with the closely related vaccinia virus (VACV) [1,2]. The monkeypox virus (MPOX) is prevalent in several African nations. However, rodents are likely the primary reservoir, with monkeys and humans serving as accidental hosts. Human-to-human transmission of MPOX is expected to occur predominantly via close contact with symptomatic patients. It is assumed to occur through direct or indirect interaction with living or dead infected animals [3,4]. Phylogenetic analysis revealed that viral diversity is geographically structured into two major clades. These were named for the places where the virus is most prevalent: West Africa (WA) and the Congo Basin (CB). There have been five significant MPOX outbreaks documented, occurring in 1970, 1996–97, 2003, and 2018 [[5], [6], [7], [8], [9]]. A multi-country epidemic that is continuing in 2023, with 86,724 confirmed cases and 112 fatalities recorded from 110 countries (https://worldhealthorg.shinyapps.io/mpx_global/) [10]. As of 24 July 2022, the highest number of cases was in the countries of the U.S., Gibraltar, Portugal, the Netherlands, and Malta. The U.S. has the highest number of infected patients, with 29,980 cases [11,12]. However, the MPOX generates relatively subtle symptoms in immunocompetent persons. If the virus spreads to those with weakened immune systems, children, the elderly, pregnant women, and people with co-morbidities like HIV/AIDS and diabetes, severe sickness or death may follow. The precise cause of MPOX's reappearance has yet to be determined. However, one of the likely drivers of the MPOX worldwide expansion might be the 1980 discontinuation of smallpox vaccination, which resulted in a lack of waning of people's immunity [7,13,14]. MPOX infection was characterized by a distinctive rash of several hundred simultaneous lesions on the face, arms, legs, and, less often, the palms, soles, or genitalia. Prodromal signs such as fever, lymphadenopathy, and influenza-like symptoms usually preceded the rash. However, preliminary research on the virus suggests that cases in the current outbreak are atypical, with the rash appearing in more unusual areas of the body, particularly the genital and perianal areas, without spreading to other body regions and with a relative mildness or absence of prodromal symptoms [15,16]. The patient experiences a rash on the face that extends to other body parts after the onset of a fever. The oropharynx is where lesions originate and progress throughout the body. After exposure, serum Abs might be found around two weeks later. Depending on the genetic makeup of the MPOX strain causing the infection and the accessibility to contemporary treatment, the mortality rate ranges between 1 and 10% [17]. The severe skin problem raises worries about subsequent bacterial infections of the skin, which have been seen in 19% of unvaccinated MPOX patients [18].
As stated by the WHO, MPOX is treated with supportive care. Vaccines and remedial produced for smallpox and accepted in some countries can be utilized for MPOX in some situations [19]. MPOX is recently being treated with medications formerly administered for smallpox or other infections arising from the Orthopoxvirus, including tecovirimat, cidofovir, and brincidofovir. Furthermore, vaccinia immune globulin (VIG), which is created from composed human plasma of individuals who have been administered the smallpox vaccination, is one of the treatments for MPOX [20]. International health administrators have proposed many methods to combat MPOX transmission, including the introduction of vaccinations for those who have had close contact with MPOX patients and for populations at risk of occupational exposure to MPOX. Close contact with lesions, bodily fluids, respiratory droplets, and fomite may transfer MPOX from person to person. However, the current epidemiological environment raises questions regarding the viral transmission dynamics and epidemic scale [21]. Since the vaccine's intended effect is protection against MPOX, it makes sense to create it directly from the virus. A pure, attenuated, or already dead virus is used in one of the more conventional methods [22]. Although the smallpox vaccine preserves immunity against MPOX, it is doubtful to be of any use given the current incidence in the year 2022. This is shown by the fact that smallpox vaccination programs were abandoned 50 years ago and that there is now no vaccine available for nations with minimal resources. It is possible to use smallpox and MPOX vaccinations as pre-exposure prophylaxis (PrEP) and post-exposure preventive (PEP) measures. To protect contacts and vulnerable individuals at high risk, PrEP injection is best delivered in conjunction with second or third-generation vaccinations, such as ACAM2000, LC16m18, and JYNNEOS vaccines. Within 4–14 days of exposure, PEP injections can be given to treat the condition and lessen the severity of the infection [[23], [24], [25]]. ACAM2000 and JYNNEOS are two U.S. Food and Drug Administration-confirmed vaccines that can inhibit MPOX infection. However, ACAM2000 may cause significant adverse events, such as coronary artery disease, whereas JYNNEOS is related to fewer side effects. The current worldwide prevalence has once again focused on the requirement for continuous monitoring and the advance of new prevention and curative methods. In 2019, a new vaccine based on a modified attenuated Vaccinia virus (Ankara strain) was allowed for MPOX inhibition. While the ACAM2000 vaccine led to some adverse events in atopic dermatitis individuals and immunocompromised individuals, the modified vaccinia Ankara (MVA) vaccine is safe to utilize in those sick individuals [26]. Smallpox vaccine is believed to be cross-protective against MPOX, with some information from minor investigations appraising preservation as high as 85% [27]. Based on existing data, there is still a need to progress an efficient and safe novel generation of vaccines particular for MPOX that are developed into novel vaccine methods, such as virus-like particles (VLPs), recombinant protein, nucleic acid (mRNA or DNA), and nanoparticle-based vaccines, before MPOX is declared a pandemic [22,26,28]. To create better-defined vaccines, subunit vaccines targeting preservative antigens and delivered as purified protein or plasmid DNA or virally vectored vaccines targeting one or both of the two immunologically distinct infected types of poxviruses, the mature virion and the enveloped virion, have been developed [29]. Nucleic acid vaccines are safe and successfully mimic the immunization of inactivated vaccines. Furthermore, the industrial generation of such vaccines is affordable and straightforward [30,31]. To recognize the most efficient vaccine, it is essential to assess effects, including reactogenicity, safety, cytotoxicity effect, and vaccine-associated side effects, especially for high-risk and vulnerable people. The 2022 extra-African MPOX epidemic has highlighted the absence of vaccines with proven effects and low reactogenicity. It is considered that the utilization of this vaccine in the MPOX outbreak may play a function in the inhibition or attenuation of the infection as PrEP is in close contact with approved cases [32,33].
In this review, we will summarize the various characteristics of MPOX, such as structure, genome, and pathogenesis. In addition, we present an up-to-date discussion of the current state of knowledge regarding the MPOX virus, with a particular focus on innate and adaptive immune response and vaccination against the MPOX. The advantages and disadvantages of all the traditionally used MPOX vaccines, including LC16m8, ACAM2000, JYNNEOS, and Dryvax, were reviewed. Additionally, this review aims to give a general overview of the work done to develop and assess various novel vaccine candidates for MPOX, including inactivated VLPs, recombinant protein, nucleic acid, and nanoparticle-based vaccines. This review article may aid in the creation of an innovative and valuable MPOX preventive strategy.
1.1. Characteristics and structure of the monkeypox virus
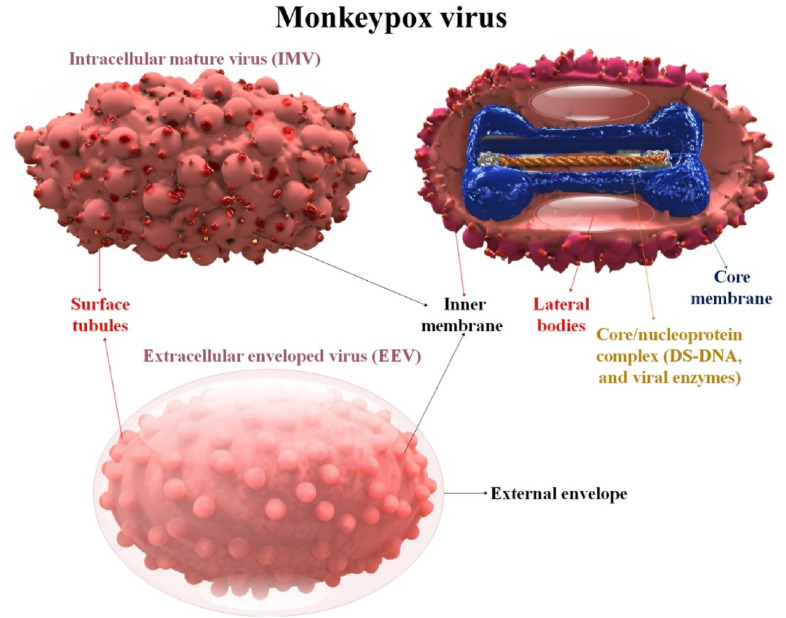
Despite having a sizable linear double-stranded DNA (ds-DNA) that contains all the enzymes required for replication and assembly, MPOX translates their genetic material in the cytoplasm of infected cells using the ribosomes of their host cells. Approximately 200 by 250 nm-sized lipoprotein outer membrane surrounds the ovoid or brick-shaped MPOX [34,35]. Intracellular mature virions (MVs) and extracellular enveloped virions (EVs) are the two types of virions (EVs). EVs have a separate envelope that MVs do not. A dumbbell-shaped nucleus and two lateral bodies are positioned between the concavities and the outer membrane within the viral particle. Scientists recently developed confident assumptions regarding the structure of MPOX variations' whole proteome, as well as the pockets that contain tiny molecules. MPOX has immunological cross-reactivity with three additional OPXVs that infect humans, soluble antigens, nucleoprotein antigens, and erythrocyte agglutinins [36,37]. Each MPOX contains more than 30 viral structural and membrane proteins, DNA-dependent RNA polymerase, and associated transcriptional enzymes [38]. The viral core and lateral body, which include some proteins, were coated by a lipoprotein envelope on the surface of the MV. When a cell is destroyed, MV is released, and it is generally stable in the outside environment. It is used chiefly for transmission. A lipid membrane is wrapped around MVs to create EV, which is then released by exocytosis. It came from the movement of endosomes or the Golgi apparatus [39] (Fig. 1 ).
Fig. 1.
The structural features of MPOX and its structure of the extracellular enveloped and intracellular mature orthopox virion. MPOX is an enveloped ds-DNA virus that belongs to the genus OPXVs in the family Poxviridae.
1.2. Monkeypox genome
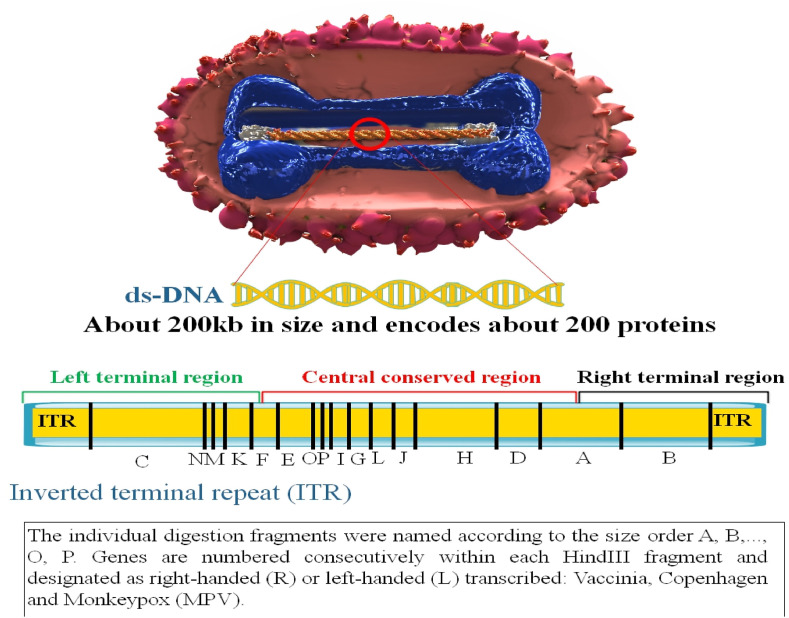
The inverted terminal repeats (ITRs) of the MPOX are made up of hairpin loops, tandem repeats, and a few open reading frames (ORFs), and the MPOX is a 197 kb linear ds-DNA covalently joined at its ends by palindromic hairpins [40]. Variable ends with inverted terminal repeat protect the genomes favorably conserved central coding site. At least 90 ORFs are required for poxvirus replication and morphogenesis. Many extra non-essential ORFs contribute to variances in poxvirus host tropism, immunomodulation, and pathogenicity, with many ORFs currently awaiting functional characterization [41]. The outer membrane protects membrane junctions and the densely packed core, which contains enzymes, a ds-DNA genome, and transcription factors. All proteins required for viral DNA replication, transcription, virion assembly, and egress are encoded by the MPOX genome. According to a comparison of the VARV and MPOX genomes, the core region of the MPOX genome contains essential enzymes and structural proteins and is 96.3% similar to that of the VARV. However, the terminal portions of the MPOX genome that encode virulence and host range factors vary dramatically [42]. These areas of MPOX and other OPXVs contain multiple known host ranges and immunomodulatory (IMM) genes. These genes have developed precisely to block many processes simultaneously, including pattern-recognition receptor signaling, apoptosis, chemokine and cytokine function, and lymphocyte and antibody (Ab) activity [43]. According to O'Toole and Rambaut, a cytidine deaminase termed apolipoprotein B editing complex (APOBEC3) may be driving the recent fast development of MPOX. Cytidine deaminases cause double-stranded breaks in DNA at switch sites [44] (Fig. 2 ).
Fig. 2.
MPOX genome structure. There are about 197 kilobase pairs (kbp) in the MPOX genome, with the core genomic region totaling 101,476 kbp. The coding area, NR1, and NR2 sections, and inverted terminal repeats (ITR) totaling 6379 base pairs are present in both ends of the molecule [45].
1.3. Monkeypox virus pathogenesis
During viral entry, fusion, replication, and release, known as pathogenesis, MPOX generates two infectious forms, EVs and MVs. EVs are MVs that have been changed to have three membranes, each of which is antigenically distinct (the double membrane is obtained via translocation to Golgi bodies), as opposed to MVs, which are restricted by a single membrane and are only released following host cell lysis. It has been shown that EV antigen-targeting vaccines and Abs provide less protection than those that do [29,46,47]. Enveloped virions (EVs) are those that leave the host cell, while cell-associated enveloped virions (CAEVs) are those that remain on the cell surface. There are differences between CEVs and EVs, but because of their resemblance (and for convenience), we shall use the word EV to designate both CEV and EV. Previously, MPOX was produced, collected from cell culture, sonicated, frozen, and thawed to release MV. So, the inoculum's EVs lose their protective outer membrane and are replaced by MV [48]. MPOX may enter the body intradermally, via the nasopharynx, or through the oropharynx. Before spreading to local lymph nodes, the virus multiplies at the injection site. Once the virus has established itself in the bloodstream (viremia), it travels to other body parts. MPOX has morphological similarities with other recognized OPXVs. MPOXs have an exterior membrane composed of lipoproteins and can be either oval or brick-shaped [49].
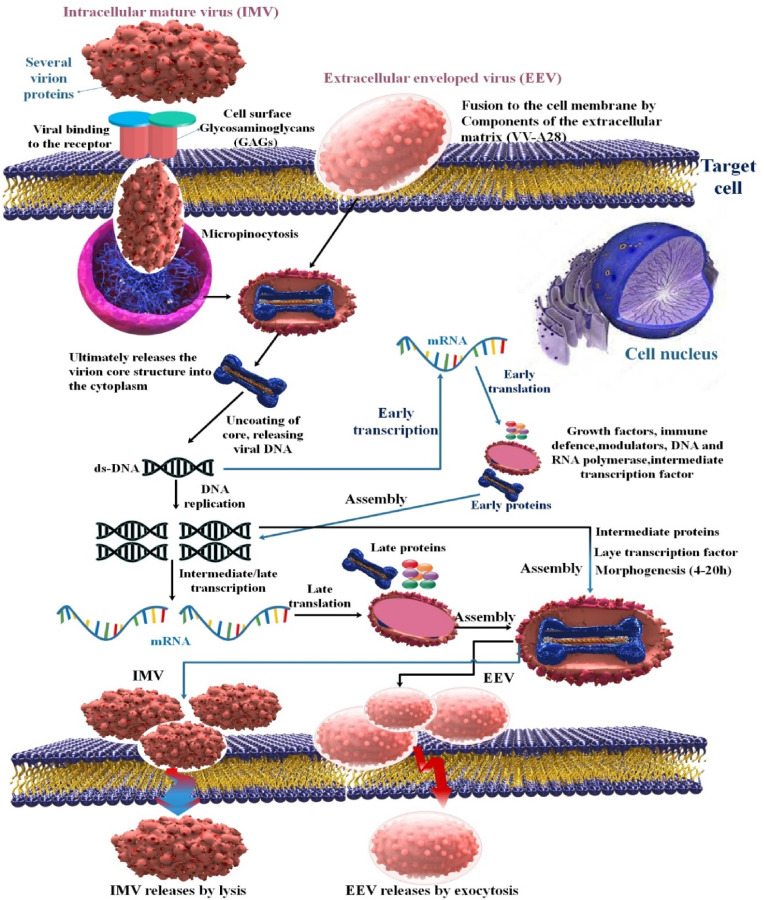
The expression of early genes is required for both the start of viral DNA synthesis and the production of transcriptional regulatory factors for intermediate genes. The middle transcription factor family is a subset of late transcription factors. Virions can be produced and assembled in the cytoplasm's electron-rich regions where mature protein molecules and enzymes are present. This is important for several reasons, including the attraction of early transcription factors intended to be packed into developing virions. In developing virions, emerging genomic DNA is organized into genome units and crammed into enormous concatemeric precursors. Some IMVs undergo a subsequent envelope whereby they acquire even more glycoproteins; this is facilitated by proteolytic activities that also encourage the formation of immature components in IMVs. Successful lysis of the host cell is necessary to release the most mature intracellular virions. After maturing within a cell, most viruses must develop extra membranes before escaping. They may exit as an enveloped virus that can infect other cells, or they may choose to stay on the cell surface. Mature intracellular virions can also be released when the cell's outer membrane is ruptured, either by mechanical stress or components of the host's complement system [16,50,51]. Recent studies have shown that several cellular proteins, including vacuolar protein sorting 52 (VPS52), conserved oligomeric Golgi 4 (COG4), COG7, and VPS54, are essential for the viral replication cycle. Identifying these proteins as host targets may provide viable intervention strategies for MPOX infection, even if it is unclear how their interactions together exacerbate MPOX-induced host disease [52] (Fig. 3 ).
Fig. 3.
Schematic representation of an MPOX life cycle. Enveloped Virion (EV) enters the host cell by fusion and the mature virion (MV) by micropinocytosis or fusion.
1.4. Monkeypox infection immunopathology (innate and adaptive immune response)
To spread, viruses must replicate inside host cells since they rely on the host and aim to disrupt cellular growth and immune control. MPOX delays or blocks apoptosis, impairs antiviral host defense, and hijacks host cell machinery. Although the virus was identified decades ago, the topic of human immunity to MPOX infection has not been explored extensively. Because of this, it is common practice to interact findings about MPOX interactions with the host immune system from studies of VACV and related OPXVs [53,54].
1.5. Monkeypox infection innate immune response
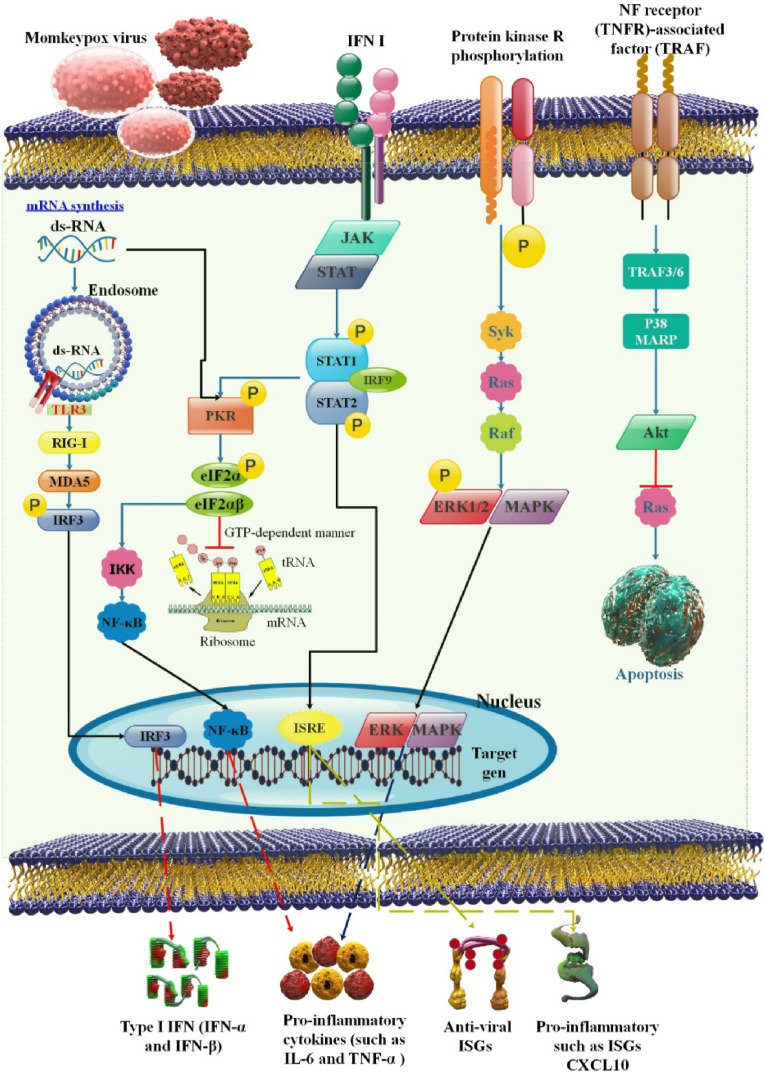
The innate immune response to viruses serves as the body's initial line of defense against infection and is necessary for developing adaptive immunity. In the first step of the innate immune response, DNA sensors such as cyclic GMP-AMP synthase (cGAS), DNA-dependent protein kinase (DNA-PK), and interferon-inducible protein 16 (IFN-IP16) recognize the viral DNA genome (IFI16). Next, adaptor proteins like stimulator of interferon genes (STING) are activated, initiating innate immune signal transduction and leading to the production of a wide range of host defense molecules like IFN and pro-inflammatory cytokines and chemokines. Infection with the poxvirus triggers a cytosolic DNA sensor, which then activates an adaptor, which triggers a cascade of downstream effectors to create interferons, cytokines, and interleukins [55].
Many genes related to immunity, such as those that regulate leukocyte chemotaxis and immune cell activation, were altered by the cowpox virus (CPXV) and MPOX. Most of these genes exhibited considerable upregulation after infection with CPXV and MPOX, which may suggest that the hosts' antiviral response is not sufficiently subverted. This is supported by the finding that immune-related genes, including IL6, were upregulated in response to modified VACV Ankara infection of HeLa cells that had been attenuated but not in response to non-attenuated VACV WR infection. Other studies have also connected increased levels of pro-inflammatory cytokines and chemokines to CPXV and MPOX infection. Alkhalil et al. discovered increased IL-8 gene expression after MPOX infection of MK2 cells. After MPOX or CPXV infection, significant secretion of IL-6, IL-8, and G-CSF or IL-6, IL-8, and CCL-2 was observed in vivo in cynomolgus macaques (Macaca fascicularis) [56]. Data from transcriptomic analysis of an in vitro infection model showed that MPOX might inhibit the expression of the MAPK-ERK pathway and other components of the innate immune signaling system. The phosphorylation of extracellular signal-regulated kinases 1/2 (ERK1/2) was also observed to be attenuated in an infected rhesus monkey tissue segment. However, MPOX may cause many cytokine productions in critically ill individuals, including granulocyte-mAbs yet colony-stimulating factor (GM-CSF), IL-10, and IL-2 receptors [54,57] (Fig. 4 ).
Fig. 4.
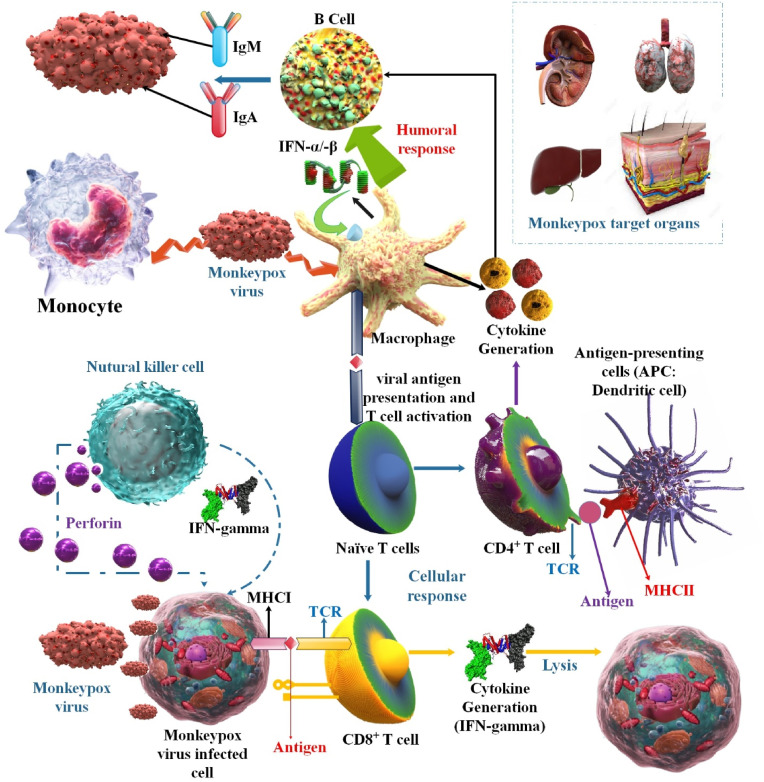
This diagram shows how MPOX infection may have an immunopathogenesis (innate immunity, adaptive immunity, and humoral immunity). The NK cell's ability to destroy virus-infected cells and release pro-inflammatory cytokines may be inhibited by MPOX. By preventing T cell receptor trans-signaling, MPOX may also obstruct the adaptive immune response [54].
To control MPOX, natural killer (NK) cells are crucial. Study results in infected rhesus macaques show that the MPOX infection significantly increases the number of all NK cell subsets in the blood and lymph nodes. The resistance loci remain unknown, although most wild strains of lab mice are unsusceptible to OPXVs. Wild mice may be very susceptible to MPOX. The CAST/EiJ mouse strain is one of the few laboratory mouse strains especially vulnerable to OPXVs because of low NK cell numbers and insufficient IFN response. These mice are protected from fatal MPOX infection by receiving either IFN- γ or IL-15, which promotes NK cell proliferation [[58], [59], [60], [61]]. The studies showed that MPOX affects NK-cell activities and suppresses IFN-α and TNF-secretion as well as C-C Motif Chemokine Ligand 5 (CCL5), C-X-C Motif Chemokine Receptor 3 (CXCR3), and C-C Motif Chemokine Receptor 6 (CCR6) expression. Changes in immunological mediators brought on by MPOX infection are associated with sickness severity. Regulating the complement system is one more method used by MPOX to evade the immune system. The D14L gene encodes the MPOX inhibitor of complement enzyme (MOPICE) in the Central African MPOX strain but not in the West African strain; MOPICE hinders the formation of a C3 convertase complex [52,62] (Fig. 5 ).
Fig. 5.
This illustration shows how MPOX infection may have an immunopathogenesis (innate immunity, adaptive immunity, and humoral immunity). The NK cell's ability to destroy virus-infected cells and release pro-inflammatory cytokines may be inhibited by MPOX. By preventing T cell receptor trans-signaling, MPOX may also obstruct the adaptive immune response.
1.6. Humoral immunity to monkeypox
Recent studies have emphasized the significance of cellular and humoral immunity in the defense against poxviruses. The vital importance that vaccine-induced anti-body reactions play in protecting against re-exposure to poxviruses is a critical finding from this study [63,64]. IgA, IgM, and total Abs can be found using serological AB assays specific to certain MPOX antigens [65,66]. These primates generated 23 different OPXV proteins identified by the anti-MPOX IgG. Vaccination results in a more limited serologic response to MPOX than natural infection since the Abs detected in smallpox vaccine recipients were specific to just 14 OPXV proteins. In the wake of the 2003 smallpox pandemic in the United States, scientists analyzed the prevalence of MPOX infections among patients who had and had not had a smallpox vaccination. Within the first week of infection, both groups showed signs of IgM and IgG Abs. The IgM levels in the unprotected group spiked more dramatically during the first two weeks of illness and were detectable for 126 days compared to 77 days in the vaccinated group. Although the unvaccinated group had a more extended period of IgG positive (139 days vs. 147 days), the vaccinated group had a more strong IgG response within the first 56 days [58] (Fig. 5).
1.7. Monkeypox infection adaptive immune response
T cells are essential for containing and getting rid of viral infections. The research also shows that WT MPOX-Z-infected animals produce a humoral and cellular immune response against the virus, as demonstrated by an increase in the number of memory B cells that are proliferating, the production of IgG that is specific for MPOX, the growth of IFN-γ/TNF-α—secreting T cells that are specific for OPXV antigens, and the proliferation of memory T cells. Beginning with the rise in viral loads found in infected animals, the adaptive immune response closely mirrored the management and healing of the MPOX infection [[67], [68], [69]]. T-cell-mediated cytokine responses after co-infection with VARV and MPOX were decreased by 95% when compared to responses after VARV infection alone and by 80% when a low dose of MPOX was administered (VARV: MPOX ratio of 10:1). Researchers demonstrated that MPOX suppressed T cells that had been stimulated by VARV and that MPOX encodes the IMM protein that VARV lacks. An early gene product was discovered after infecting peripheral blood mononuclear cells with VARV or MPOX in the presence or absence of cytosine arabinoside (AraC), which inhibits the production of late genes. This was attributed to AraC's ability to reduce the body's anti-CD3 responses. This suggests that an ancient gene was responsible for MPOX's immunosuppression of T cells (CD4+ and CD8+) [40].
Hammarlund et al. postulated that MPOX has similar immune evasion mechanisms to CPXV because MPOX encodes a close homolog of CPXV203, which maintains a major histocompatibility complex (MHC) class I in the endoplasmic reticulum. However, the MPOX evasion mechanism prevented CD4+ and CD8+ T cell activation following cognate contacts with MPOX-infected cells, protecting the viral reservoir from immune surveillance. Even though MPOX can infect primary human monocytes, antiviral CD4+ and CD8+ T cells were not able to identify infected monocytes. This demonstrated that virus-specific T lymphocytes were not stimulated to produce inflammatory cytokines (such as IFN-γ or TNF-α) in response to MPOX [70]. Even though CD8+ T cells are well known to be essential for recovery from primary infection, recent research called into question their role in recovery from secondary poxvirus infections. B cell-deficient mice infected with Ectromelia virus, a member of the same genus as the human smallpox pathogen VARV, cannot recover from primary infection despite mounting a solid CD8+ T cell response, suggesting that Abs are required for clearance of a poxvirus. In mice, protective Ab responses to the poxvirus appear T cell-dependent and necessitate MHC-II molecules, CD40, and B cells during a secondary infection [[71], [72], [73]] (Fig. 5).
1.8. Different types of vaccines for monkeypox infection
It has been shown that smallpox vaccination provides 85% pre-immunization against MPOX infection. Approximately 50 years afterward the stop of immunization, with the enhancement in the number of individuals infected with MPOX, re-emergence of this disease was detected. Therefore, MPOX was categorized as an emergent disease via the WHO and Development (WHO R&D) Blueprint in 2018 [74]. There have been hints since the 1960s that immunological responses unique to the vaccine may protect MPOX. Vaccination with Dryvax (Wyeth Laboratories, PA, USA) or another first-generation smallpox vaccine offered complete protection against illness in almost all vaccinated animals in three experiments, including chimpanzees, rhesus macaques, and cynomolgus macaques. The only exception was an unusual animal that failed to produce a take (a characteristic blister at the injection site) following immunization [75]. There are two confirmed accessible to prevent smallpox in the USA, such as ACAM2000 and JYNNEOS [68]. Smallpox vaccinations show an effective defense that may be used to reduce the prevalence of monkeypox. The replication-competent, second-generation vaccinations, however, are not allowed since smallpox vaccines have side effects [75].
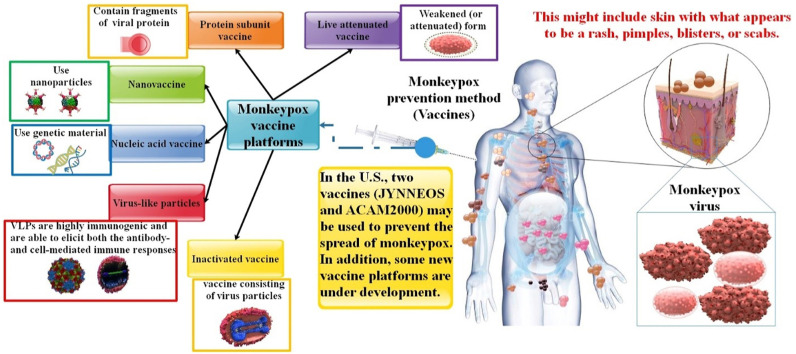
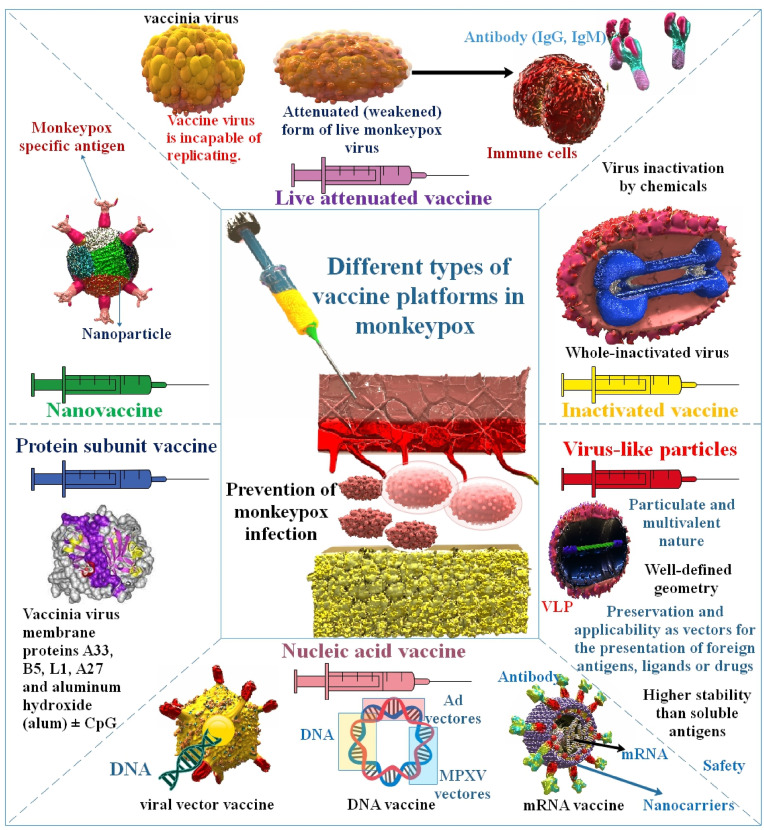
The choice of antigens, vaccine platforms, vaccination regimens, and routes are all aspects of vaccine design. The relative immunogenic potency of vaccine-derived viral antigens, whether an immune adjuvant is necessary, and the type of protective immunity all depend on the vaccine platform selected. These characteristics also affect if a vaccine is appropriate for a particular immunization method and whether a prime-boost vaccination schedule is necessary to promote vaccine-mediated protective immunity and its longevity. Additionally, choosing a respiratory mucosal vaccination route or a live attenuated (LA) viral vaccine will require more rigorous safety testing [76]. However, the vaccinia vaccine (replication-competent vaccine, authorized for smallpox) or the newer JYNNEOS ring vaccination approach may be helpful for early-identified close contacts (replication-deficient vaccine). Due to the extended incubation period of MPOX, early immunization may decrease symptoms or potentially prevent the illness [77,78]. Although current vaccinations give cross-protection against MPOX, they are not specific for the Pathogenic virus. Their effectiveness in light of the recent multi-country epidemic remains to be determined. Furthermore, due to the elimination and halt of smallpox immunizations for four decades, MPOX found a way to re-emerge, albeit with new features [22] (Table 1 ). In this part, we will look at some examples of how application types of vaccination platforms have been used to successfully induce distinct immune responses in preventive regimens against MPOX infection. It is possible to classify the vaccine development platforms as either “traditional” (inactivated or live-virus vaccines), “recently licensed” (recombinant protein vaccines and vectored vaccines), or “not yet licensed” (vectored and recombinant protein vaccines) (RNA and DNA vaccines) (Fig. 6 ).
Table 1.
The production method of first-, second-, and third-generation smallpox vaccines.
| Vaccine generation | Name of vaccines | The production method of vaccine | Ref |
|---|---|---|---|
| First-generation | Dryvax | A live VACV preparation made from calf lymph is called Dryvax®. Lyophilization is used to purify, concentrate, and dry the calf lymph. In government vaccine stockpiles, a more recent vaccine made in labs is replacing the antigen extracted by scraping viruses from the skin of diseased calves. | [79,80] |
| Aventis Pasteur Smallpox Vaccine (APSV) | Another replication-competent VACV vaccine with a projected similar safety profile to ACAM2000® is APSV, held in the Strategic National Stockpile. Currently, research is being done on vaccination. If ACAM2000® becomes unavailable, is challenging to procure, or is contraindicated for a specific person, APSV would be made accessible under an IND or EUA for use in the case of a smallpox emergency. | [81] | |
| Second-generation | ACAM2000 | A clonally purified master seed stock of the vaccinia virus, derived from the strain of vaccinia used by the New York City Board of Health, is used to create it in cell culture. Without the intrinsic mutations associated with serial replication, the clonally purified master seed ensures a more uniform vaccine, and the cell culture restricts accidental and bacterial contamination in vaccine manufacture. | [82,83] |
| Lister strain isolate of vaccinia in rabbit kidney cells (RIVM) | Thus, using rabbit kidney cells, the first second-generation Lister-based vaccine, RIVM, was created in 1960. No additional passages were carried out during the creation of this vaccine; the virus was transferred directly from the calf lymph vaccine to cells. Similar take rates and neutralizing Abs were seen with the freeze-dried vaccine and the calf lymph-derived vaccine. In clinical trials, this vaccine was used in the Netherlands and Indonesia without leading to serious side effects. | [84] | |
| Elstree-BN | Bavarian Nordic's (BN) Lister strain-based Elstree-BN vaccine demonstrated safety and immunogenicity in monkey and human clinical studies beginning in 2004. The embryonic cells of chickens were used in the experiment. Similarly, a vaccine was developed in Japan utilizing chicken embryo fibroblast (CEF) cells before smallpox was eliminated; although its safety profile was adequate, its effectiveness was not. | [[85], [86], [87]] | |
| VACV Lister/CEP | Sanofi Pasteur created a second-generation VACV vaccination by undergoing three passages in chicken embryonic primary cells using a batch of the first-generation Lister vaccine (CEP). Regarding immunogenicity and safety, Lister/CEP was comparable to the original first-generation Lister vaccination. | [84,88] | |
| Third-generation | IMVAMUNE | The dermal vaccinia strain Ankara (chorioallantois vaccinia virus Ankara (CVA)) is used to create modified vaccinia Ankara; it was first isolated from a lesion on a horse and is now housed at the Vaccination Institution Ankara in Ankara, Turkey. Although initially developed to protect cattle against OPXVs, MVA has currently been researched as a main vaccination booster for at-risk human populations using the first generation of smallpox vaccine. Over 570 consecutive passages in primary chick embryo fibroblast cells suppressed MVA. | [89] |
| LC16m8 | On chorioallantois membranes, a clone of LC16 that produced small pocks, a trait associated with reduced growth capacity in mammalian tissues, was chosen to minimize the possibility of autoinoculation complications brought on by a prolonged pock reaction (CAM). In the primary rabbit kidney, the virus was passed through six more times at a low temperature before growing on CAM. Small (2–3 mm) pocks were isolated and designated LC16mO. (Lister Clone 16 medium pock size on CAM original clone). The LC16mO clones were grown on CAM and passed through three additional PRK cell passages before being chosen for small (0.5–1 mm) pocks. The last attenuated clone, designated as LC16m8 (clone 8). | [90] |
Fig. 6.
Infection with MPOX and several vaccination platforms (e.g., live attenuated vaccines, inactivated vaccines, protein subunit vaccines, VLP, nucleic acid vaccines, nanovaccine).
1.9. Traditional vaccine methods for monkeypox
Conventional vaccines contain live vaccinia strains, live attenuated strains defective in one or more viral genes, and inactivated vaccines. The LV vaccines utilized in the extirpation attempts were not without adverse events and problems. They are among the most reactogenic of all FDA-accepted vaccines. From a biodefense point of view, these vaccine harmlessness worries would be of secondary importance to the need to rapidly and effectively break the chain of transmission and include the prevalence [63,91]. Three smallpox vaccines are currently available in the National Pharmaceutical Stockpile (NPS) of the United States: JYNNEOSTM (also known as IMVANEX, IMVAMUNE, and MVA-BN), ACAM2000®, and the Aventis Pasteur Smallpox Vaccine (APSV), which could be used for smallpox under an IND protocol [92].
1.10. Inactivated vaccines
Since more than a century ago, inactivated vaccines have created immunity against viral infections. Historically, purified inactivated viruses have been used to develop vaccinations. These vaccines have been proven to be both safe and effective at preventing illnesses brought on by viruses like the poliovirus and the influenza virus [93,94]. Compared to their live counterparts, this well-established method of vaccine manufacture is relatively easy to do and has a higher safety profile. To create significant amounts of antigen, the pathogen must first be grown on a substrate, a typical manufacturing process for all inactivated viral vaccines. These regrettable occurrences serve as a caution to all vaccine developers: viral epitopes required for induction of protective immunity should be conserved after inactivation since pathogen inactivation does not always translate into a vaccine that, by default, induces protective immunity [95]. Vaccines against inactivated viruses comprise whole viruses that have undergone chemical or thermal treatment. The surface proteins are denaturized by heat and chemicals like phenol and formaldehyde, rendering them inactive and non-infectious. Some epitopes remain unaffected by the treatment of the entire virus, maintaining some of their integrity and triggering the production of Abs to elicit an adaptive immune response. When a virus is injected, phagocytic immature dendritic cells break it into smaller antigenic fragments. These antigenic fragments are then presented as antigens on the surface of MHC cells, activating B cells and T helper cells. Inactivated vaccines are risk-free and do not cause any side effects. Since they cannot reproduce, they are heat-stable, non-infectious, and disease-transmissible. To induce a robust enough immune response, booster doses may sometimes be required since they are inadequate as a single dose and typically do not confer immunity for the same period as live vaccinations [22].
1.11. Live attenuated vaccines
Live attenuated (LA) vaccines have prevented more viral infections than other vaccines in history, and most current human vaccines for viral infections are safe LA vaccines. LA vaccines can inhibit completely viral diseases and outbreaks of related viruses and their variants. This vaccine method suppresses the appearance of vaccine‐escape and virulence‐increasing variants and preserves immunologically abnormal persons better in general [96,97]. LA vaccines against human viral infections have been among the most effective and economical therapies in medical history. In most parts of the globe, measles is under control, poliomyelitis is on the verge of extinction, and smallpox was declared eradicated in 1980 [98]. Using LA viruses (LAVs), produced by infecting and replicating inside cultured cells, is an efficient means of stopping the spread of several viral illnesses. However, empirical attenuation may be iffy, and LAVs raise safety issues. To some extent, inactivated viruses and subunit vaccines address these issues, although they are still less effective than LAVs [99]. Successful usage of live vaccinia vaccines (such as Dryvax® and ACAM2000) suggests that they may be utilized without further Phase III testing. While the hazards have been studied and may be acceptable in the case of an epidemic, the formulation of the presently marketed items differs from the original product. It may need further stability and safety testing. Large safety databases exist for the LA LC16m8 and replication-defective vaccinia (MVA, NYVAC), which have been shown to protect nonhuman primates (NHP) from the MPOX challenge [33,91,100].
1.12. LC16m8
A live, attenuated, cell-cultured smallpox vaccine called LC16m8 was developed in Japan in the 1970s to replace first-generation vaccines like the Lister and Dryvax strains, which have rare but severe side effects. There were no adverse severe responses in a clinical study involving 10,578 of the almost 50,000 kids who got the LC16m8 immunization. Both immunogenicity and efficacy were on par with first-generation vaccinations. Because of its decreased virulence and replication competence, LC16m8 is a promising vaccine. An essential EV antigen, B5R, undergoes a frameshift mutation that causes the production of a shortened protein, which reduces the strain's pathogenicity [101]. However, serious side effects such as encephalitis, encephalopathy, widespread and generalized vaccinia, ocular VACV infections, and cardiac dysfunction have been recorded in recipients and are very worrying. As a result, LC16m8 is seen as a possible replacement for the VACV vaccinations that are already available [102,103]. A single LC16m8 vaccine protects NHPs against MPOX infection. Post-exposure vaccination with LC16m8 for MPOX-infected NHPs improved clinical manifestation, but all naïve individuals were practically deadly. LC16m8 vaccination 7 days before the MPOX challenge protected NHPs against MPOX [104]. This vaccine has been utilized mainly in Japan, and 2 current investigations in Japan and the USA showed no safety concerns [105]. However, present appropriation policies have concentrated on inactivated-virus vaccines; other countermeasures, including antiviral drugs and live-virus vaccines, also need proportionated appropriation. Significantly, LC16m8 is stocked in Japan, where it has been approved and effectively utilized for vaccinating non-immunocompromised persons, such as children [106].
1.13. ACAM2000™
ACAM2000, a second-generation vaccination with a higher level of safety than first-generation vaccines, is likewise based on live VARV. For use against MPOX, this vaccine is presently accessible in the US thanks to an extended access investigational new drug (IND) application. The smallpox vaccine ACAM2000™ (Emergent Biosolutions) is produced utilizing cutting-edge cell culture technology and is derived from Dryvax® (Wyeth). ACAM2000 has been associated with severe adverse reactions, just like the original vaccination. When given within three days of contact, smallpox vaccination post-exposure prevention is thought to be 80–93% effective in preventing VARV infection. However, its efficiency quickly dwindles following the onset of smallpox clinical signs [107,108]. Each VACV-based vaccine has been shown to produce potent T-cell responses and significant levels of neutralizing Abs while having different safety and replication characteristics [108]. The finding that vaccinia immune globulin may reduce vaccine side effects and can guard against smallpox infection emphasizes the significance of Abs in immunity. Additionally, whereas vaccine-induced Abs alone are adequate for MPOX challenge protection, Abs are necessary for the rhesus macaque MPOX infection model. In one Phase I clinical study (vaccinia-naive), one of the two Phase II trials (vaccinia-experienced), and both Phase III trials (vaccinia-nave and vaccinia-experienced), the ACAM2000TM vaccine produced a poorer Ab response than Dryvax®. Although the titer of neutralizing Abs increased by a factor of four after ACAM2000TM vaccination, it was still roughly 40% lower than in those who had received the Dryvax® vaccine [109]. Post-exposure administration of tecovirimat alone or in combination with ACAM2000 protects against a fatal MPOX challenge, even if treatment initiation is delayed to the point where vaccination with ACAM2000 alone does not protect from severe MPOX disease and mortality. That immunity acquired from surviving the initial MPOX exposure due to treatment with tecovirimat is long-lasting and effective [110,111]. ACAM2000 adverse events comprise fundamental symptoms, including fever, malaise, headache, muscle pain, and adenopathy. Acute adverse events are infrequent but may happen, including skin infection, vaccinia gangrenosa, generalized vaccinia, Stevens-Johnson syndrome, inflammatory cardiomyopathy, idiopathic pericarditis, post-vaccination encephalitis, acute brain failure, and acute disseminated encephalomyelitis have been reported [112,113].
1.14. JYNNEOS™
JYNNEOS, a more recent vaccination that also goes by the names Imvamune and Imvanex, gives protection against OPXV and may be administered to immunocompromised people without risk. The JYNNEOS™ (Imvamune or Imvanex) smallpox/MPOX vaccine is created using attenuated live VACV and cannot cause smallpox, MPOX, or any other infectious illness [114]. In 2020–2021, Advisory Committee on Immunization Practices (ACIP) examined the evidence supporting the use of JYNNEOS, a VACV vaccine with replication defects, in place of ACAM2000. The ACIP voted overwhelmingly in support of JYNNEOS as a replacement for ACAM2000 for the first immunization and booster shots in November 2021. Two vaccines (ACAM2000 and JYNNEOS) are now available and advised for preexposure prophylaxis against OPXV infection in persons at risk for such exposures due to these recommendations for using JYNNEOS [115]. Third-generation smallpox vaccine IMVAMUNE has undergone testing in HIV-positive and atopic dermatitis-prone individuals. Furthermore, various research using animal models have shown that protection against MPOX is also possible. The risk/benefit ratio has been considerably shifted by the discovery of third-generation vaccinations with superior safety profiles, making them currently practical for MPOX prevention. However, since they were created after eradicating smallpox, their capacity to stop natural human OPXV infections has never been shown [116]. Investigations revealed that a standard dosage of IMVAMUNE (0.5 mL of 1108 TCID/mL) injected subcutaneously was safe and well tolerated. more significant Ab responses and the highest number of responders were produced by a double dosage of IMVAMUNE on day 28 instead of day 7. By day 14, after the double dosage, IFN-y ELISPOT responses for Groups 0 + 28 and 0 + 7 were similar [117]. Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN) is given subcutaneously via injection and is often well tolerated. It may induce injection site responses (such as discomfort, redness, and swelling) and minor systemic adverse effects similar to other vaccinations (e.g., nausea, headache, and chills) [82]. The FDA has given the JYNNEOS vaccine nonreplicating vaccine protection against smallpox and MPOX. The JYNNEOS and ACAM2000 vaccines, however, pose different difficulties. The ACAM2000 vaccine is a single vaccination that has can potentially cause severe adverse effects. In contrast, the JYNNEOS vaccine is administered as a 2-dose regimen with a mild side effect profile. However, vaccination with ACAM2000 may be an option in areas that urgently require MPOX vaccinations due to ongoing JYNNEOS shortages that are impeding pre-exposure and post-exposure prophylaxis efforts. It is still unknown whether either vaccine will be effective in the current outbreak [118]. In a novel investigation, researchers evaluated and compared the effectiveness of both vaccines versus each other and other vaccines on the market. They observed that using JYNNEOS vaccines led to a better humoral immune response than ACAM2000. Moreover, although the information was not statistically considerable, researchers showed that ACAM2000 led to more side effects, including myocarditis, than MVA. The non-replicating character of JYNNEOS inhibits the incidence of the side effects detected with other vaccines [119].
1.15. Aventis Pasteur smallpox vaccine (APSV)
The Aventis Pasteur smallpox vaccine (APSV) is a second-generation smallpox vaccine that is replication-competent. It is predicted that APSV will have a comparable effectiveness and safety profile as ACAM2000. A VACV seed from the New York City Board of Health (NYCBOH) strain creates the APSV. To make the vaccine, live VACV is combined with 50% glycerol, 0.4% phenol, and 0.00017% Brilliant Green. In those who have never had poxvirus, the APSV vaccination is more than 95% effective. It is anticipated that the safety profiles of APSV and ACAM2000 would be comparable. It is less likely that young people who got the first dose of the smallpox vaccination from the NYCBOH strain of VACV may have serious side outcomes, but it is still possible. The three most frequent severe side effects from vaccination are encephalitis, increasing vaccinia, and vaccinatum eczema. Similar to ACAM2000, it was anticipated that APSV would increase the risk of myopericarditis. When ACAM2000 is unavailable or inappropriate, the US FDA has granted APSV an IND or emergency use authorization (EUA) for use in specific cases [120]. APSV could be utilized with an IND use when neither of the licensed vaccines are accessible. However, it is not recognized how efficient the APSV is versus MPOX. Previous investigations with other orthopoxviruses have demonstrated that smallpox drugs, including Brincidofovir and Cidofovir, could be utilized to treat MPOX. However like the APSV, there is a science gap about their efficacy against MPOX [121].
1.16. Dryvax
One of the interferences for blocking and regulating MPOX is VACV, first developed against smallpox. The three primary VACV-based vaccination types are as follows. First-generation vaccinations include live VACV, such as Dryvax, used to eradicate smallpox in the previous century. Dryvax, a live VACV smallpox vaccine, protects against both smallpox and MPOX but should not be administered to those with impaired immune systems [108,122]. A safe subunit protein-based vaccination that can elicit a protective Ab response might be used instead of a live virus vaccine since Abs to VACV mediate protection. Smallpox vaccinations traditionally made from calf lymph and used to eradicate the disease, like Dryvax, are based on replicating VACV. Despite their excellent efficacy, they may have uncommon but serious adverse effects, especially in those with impaired immune systems. The adverse reactions are Stevens-Johnson syndrome, myo/pericarditis, fetal vaccinia, encephalitis, progressive vaccinia, eczema vaccinatum, and occasionally death [123]. This vaccine has several side effects, such as generalized vaccinia, ocular vaccinia, and eczema vaccinatum, happened rarely, and deadly developing vaccinia related to immunodeficiency, postvaccinal encephalitis (PVE), and embryonic vaccinia during early pregnancy occurred infrequently. Inflammatory cardiomyopathy, coronary heart disease, and idiopathic cardiomyopathy were newly identified side effects of smallpox vaccination. Clone 3 was particularly harmful in animal studies and was generated from Dryvax, the vaccine most widely used in the United States during the smallpox extirpation effort. Researchers found that their differential in pathogenicity may be attributed to the full-length IFN-α/β decoy receptor in Clone 3 and a truncated receptor in the clone used for the second-generation smallpox vaccine ACAM2000. When given to mice through intranasal, intraperitoneal, or intracranial injection, viruses expressing the full-length decoy receptor were more lethal than ACAM2000, and infection was made worse by T cell depletion. Therefore, smallpox vaccinations with high rates of adverse effects and a Dryvax clone obtained from a lesion in a patient with advanced vaccinia both have the full-length decoy receptor [124,125].
1.17. Novel vaccine method
Every vaccination works on the fundamental tenet that the vaccine may trigger an immune response faster than the pathogen. Even though traditional immunizations gave animals the ability to create powerful neutralizing and preservative Abs, these vaccines are allergenic, expensive, and time-consuming. They suggest the in vitro growth of harmful viruses, raising serious safety concerns. Therefore, a safe and efficient vaccine should be produced to inhibit MPOX. Unlike conventional vaccinations, novel vaccine generation is highly secure and affordable. Moreover, licensed vaccines and several other vaccine candidates are at different levels of progression [92,126]. However, it will need accurate assessments to certify that they stimulate immunity equivalent to traditional MPOX vaccines. To solve this problem, further tests require to be done to refine animal models for the evaluation of novel vaccines, as animal investigations utilizing several poxviruses can generate inconsistent outcomes, and non-human primate models do not entirely recapitulate human exposure to MPOX. New vaccine methods against MPOX include virus-like particles (VLPs), recombinant protein, nucleic acid, and nanoparticle-based vaccines [127,128].
1.18. Protein subunit vaccine for monkeypox infection
These difficulties may be solved by subunit vaccinations built on microbe fragments. Only the pathogen antigenic components necessary to elicit efficient immune responses are included in subunit vaccinations. An antigen may be a polysaccharide, a nucleic acid, or a protein [129]. The antigenicity of the vaccine's ingredients is a significant barrier to developing safe, effective vaccines. Adjuvants are now essential parts of many vaccines and help to maximize the vaccine's protective effect by lengthening and intensifying immune responses. Recent research has concentrated on a novel class of adjuvants targeting innate immune cells' pattern recognition receptors (PRRs). Targeting specific innate pathways with adjuvants has improved Ab and T cell responses. Innate immune cells are important immune system modulators because they orchestrate adaptive immunity responses [130]. Since recombinant protein or peptide vaccines often produce suboptimal immune responses, there is an urgent need for safe and effective vaccine adjuvants that boost immunization efficiency. Examples of approved vaccine adjuvants include inorganic aluminium salts (alum), the oil-in-water emulsion MF59, monophosphoryl lipid A (MPL) absorbed on aluminium salts (AS04), and the toll-like receptor 9 (TLR9) agonist CpG. TLR agonists have also been studied as vaccine adjuvants, along with cytosolic pattern recognition receptor activators such as stimulators of interferon genes (STING) agonists. According to a notion, vaccination adjuvants that imitate natural diseases, such as live attenuated vaccines, may activate innate immune-sensing pathways to create potent and long-lasting immune responses [131].
Subunit vaccines containing the membrane proteins A33, B5, L1, A27, and alum CpG were tested on primates for the etiology of smallpox. Then, a lethal dosage of MPOX was administered intravenously to the primates. Whereas adjuvanted alum vaccinations only exhibited little protection, those with adjuvant CpG added provided complete protection and a more uniform Ab response and more significant IgG1 responses. These results provide encouraging evidence for developing a highly effective subunit vaccine against OPXV infections as a safer alternative to live VACV immunization. In a study, two doses of an adjuvanted protein-based subunit vaccination protected NHP from a fatal MPOX challenge [132]. In an investigation to identify typical (B and T cell) 9-mer antigenic epitopes, the MPOX genome-encoded proteins were examined. The epitopes having the highest antigenic score among these were then selected for use in vaccine construction. The potential vaccine candidate was also characterized and validated using several in silico approaches, and research was done to get a better understanding of the molecular interaction with the human TLR4/MD2 complex. In conclusion, researchers applied a machine learning technique to assess the immunological simulation profile of this new peptide-based vaccination in the mammalian immune system. With no reported adverse effects, this novel vaccine candidate has shown protection against MPOX and may be employed shortly [133].
Reverse vaccinology, along with other bioinformatics and immunoinformatic tools, have been used in recent research to create a vaccine against MPOX that specifically targets numerous viral proteins. The cupin domain-containing protein, the ABC transporter ATP-binding protein, and the DUF192 domain-containing protein were all examined for their antigenic potential as extracellular proteins. B and T-cell epitopes were predicted from selected proteins to induce cellular and humoral immunity. The simulation findings showed a higher positive response from B and T cells after the immunization phase. In-depth computational modeling demonstrated that the suggested vaccine would stimulate an effective immune response against MPOX infection, giving it a promising prospect for future clinical trials [92]. Researchers showed that the appropriate MHC-I, MHC-II, and B-cell epitopes were chosen to develop multi-epitope vaccination constructions connected with acceptable linkers and adjuvants to increase immune responses to vaccine designs. The most promising epitopes discovered bound to MHC-I and MHC-II alleles were also found to have high binding affinities and low binding energies between −7.0 and −8.6 kcal/mol. Study results from immunological simulations indicate that immunization induced a protective immune response against MPOX. Finally, the suggested vaccination was stable against the MHC-I allele in a dynamic molecular study, exhibiting low Root Mean Square Fluctuations (RMSF) values. According to the researchers of this study, an essential protein in MPOX pathogenesis is the cell surface-binding protein [134]. Due to its potential safety and effectiveness, vaccination using replication-deficient recombinant adenovirus (rAd) vaccines may be preferred to live VACV vaccination. Six distinct poxvirus glycoproteins (A33R, A34R, A36R, B5R, A27L, or L1R), all of which are present on the surface of infectious VACV, were delivered as rAd vaccines to mice. A deadly intranasal challenge with VACV was avoided four weeks after mice received a single intramuscular injection of rAd encoding A27L. Remarkable reductions in post-challenge morbidity were seen by week 10 post-vaccination, and these improvements were linked to the development of neutralizing solid Ab responses and the induction of individual polyfunctional T cell responses. rAd-A27L vaccination was highly immunogenic and protective for at least 35 weeks post-vaccination. Therefore, the mortality and morbidity caused by poxvirus infection are likely preventable with a single-dose subunit vaccine expressing a poxvirus protein [135]. In a study, researchers developed mRNA and multi-epitopes vaccines (MVC) vs. MPOX using structural vaccinology and proteomics methodologies. To create MVC and mRNA-based vaccines, 9 cytotoxic T lymphocytes, 6 B cells, and 5 helper T lymphocyte epitopes were linked using the suitable linkers. The results of immune induction showed that the antigen titer after administration peaked on day five and that a sharp decline in antigen titer was observed upon the production of IgM, IgG, IgM + IgG, dendritic cells, IFN-γ, and IL, demonstrating the ability of this vaccine to elicit an immune response against MPOX [136].
1.19. Virus-like particles for monkeypox infection
VLPs are particles that self-assemble because of the expression of proteins encoding viral capsids, cores, or envelopes, or even preparations of monolayered particles obtained from a multilayered virus. VLPs have a unique mix of high immunogenicity and outstanding safety characteristics, making them suitable platforms for vaccine development. Since the first description of VLP-based hepatitis E virus (HBV) vaccines, the field has made significant progress, and VLPs are now the foundation of many marketed vaccines, including those against human papillomavirus (HPV), human immunodeficiency virus (HIV), and hepatitis E virus (HEV) [137,138]. Foreign epitopes, such as those from nonstructural viral proteins or nonviral proteins, will be attached to or shown on the VLP in the future. The antigen delivery and the VLP's capacity to induce immune solid responses make for a powerful treatment strategy. Almost any platform may be used to make VLPs, and many different antigens can be attached to them, so the results frequently seem promising at first [139,140].
Naïve and immune individuals have had severe side effects after receiving the traditional VACV-based smallpox vaccination. Despite the absolute risk of the illness and the known risks of the vaccination, there have been conversations about restoring live-vaccine injection because of the concern that it may be used as a biological weapon. During the vaccination drive in the United States before the Iraq War, serious safety issues about the live VACV vaccine were uncovered. Weak immunogenicity or using bacterial toxins as adjuvants in current killed-virus vaccine formulations for mucosal delivery leads to inflammation and autoimmune reactions. Smallpox vaccination of the mucosa was studied using a nanoscale (400 nm) oil-in-water emulsion as a formulation for the killed virus. This study analyzed the immunogenicity and effectiveness of a nanoemulsions (NE) adjuvant mixed with VACV purified from tissue culture (VACV/NE) as a possible smallpox vaccine. Their findings showed that NE inactivates VACV and that when administered to the nares of mice, this combination produces protective mucosal and systemic immunity. In conclusion, the study's authors established that a modified version of NE-inactivated VACV is an efficient mucosal vaccination that stimulates the development of Ab in the mucosa, as well as systemic Ab and TH-1 cellular immunity. By mixing NE with the purified virus, this prototype vaccine is easy to produce and has the potential to be safer than live-viral immunization [85,141].
Blue Water Vaccines Inc. (“BWV” or “Blue Water Vaccines” or “the Company”), a biopharmaceutical company working on transformational vaccines to solve major global health problems, announced plans to look into the possibility of making a new MPX vaccine using its norovirus shell and protrusion (S&P) VLP platform. For example, BWV-101 for influenza, BWV-301 for gastroenteritis brought on by norovirus or rotavirus infection, and BWV-302 for malaria are all being developed using the company's S&P platform. In this new initiative, BWV will try to offer MPOX antigens inside the S&P platform to create a vaccine candidate that might shield people against MPOX [142,143].
1.20. Nucleic acid vaccines
It is anticipated that vaccination-induced immunity would focus on the native antigens that diseases express. Therefore, it is crucial to produce vaccine antigens that are identical to natural antigens immunologically. DNA, mRNA, or recombinant viral vector vaccines are examples of nucleic acid vaccines that inject the genetic material encoding the antigenic protein for the host to express. Due to the host posttranslational alterations these proteins will experience, host glycosylation may change the antigen's structure and immunological effectiveness [144].
1.21. DNA-based vaccine for monkeypox infection
Due to their lack of replication, DNA-based vaccinations have a good safety profile. In comparison to alternative methods, such as inactivated viral vaccinations, DNA vaccines offer the following advantages: They can be generated fast at a large scale, are simple and quick to make using synthetic or polymerase chain reaction (PCR) techniques, are safer than other methods, and are more thermostable than other forms of vaccines [145,146]. Additionally, DNA-based vaccinations do not need to be kept in a cold chain, which makes them a fantastic substitute for the lack of vital, life-saving vaccines in places with limited resources. Complementary DNAs encoding desired immunogens are cloned into eukaryotic expression plasmids using recombinant DNA technology. After being purified and amplified in bacteria, vaccine plasmids are then administered directly to the host being immunized. DNA-based vaccines offer both benefits and drawbacks compared to classic immunization techniques. The immunogen's capacity to be presented by both MHC-I and II molecules is its primary immunological benefit. Highly active expression vectors based on those created for synthesizing recombinant proteins are used to get the most extraordinary immune responses. DNA vaccines have been explored against various viral, bacterial, and parasitic infections due to their simplicity in development and testing [[147], [148], [149]].
Rhesus macaques were immunized by intradermal and intramuscular injection with the MPOX orthologs of the VACV L1R, A27L, A33R, and B5R proteins, either alone or in combination with the corresponding recombinant proteins produced in Escherichia coli. They found that animals given just DNA had trouble producing significant titers. Skin lesions appeared after the challenge and disappeared similarly to placebo controls. The protein-vaccinated animals, however, suffered from mild to severe sickness (20–155 skin lesions), yet they lived. DNA-vaccinated and protein-boost recipients had mild diseases with no lesions or fewer lesions that cleared up within days. DNA/protein vaccination resulted in Th responses as well as binding Ab titers to all four proteins, and these responses were inversely related to the total number of lesions. Only a few numbers of linear B cell epitopes that are highly conserved across OPXVs were identified by the sera of the inoculated macaques. Their finding might direct future work on creating MPOX and smallpox vaccinations that are easier, safer, and more efficient [150].
In a separate study, scientists created the DNA vaccine 4pox, which shielded NHP from the severe illness caused by MPOX. The protective efficacy of the 4pox DNA vaccine was tested in rabbits by challenging them with aerosolized rabbitpox virus (RPXV). This model mimics the respiratory route of exposure and low dose associated with natural smallpox exposure in humans. Researchers showed that after receiving the 4pox vaccination, rabbits did not exhibit any clinical symptoms and produced immunogen-specific Abs, including neutralizing Abs, demonstrating protection against RPXV aerosol. On the other hand, unvaccinated animals showed significant disease symptoms, such as lesions, and were put down. These findings indicate the protective potential of an unformulated, non-adjuvanted DNA vaccine given intramuscularly [29].
Researchers found that a synthetic, multivalent, highly concentrated DNA vaccine may induce polyvalent immunity in macaques and provide defense against a highly pathogenic MPOX challenge. The vaccine is given via a unique, less invasive skin EP microarray. It has never been documented how such a varied, high-titer Ab response may be elicited against several DNA-encoded antigens administered concurrently in micro volumes. Researchers vaccinated cynomolgus macaques with multivalent smallpox DNA vaccine through the intradermal or intramuscular route to evaluate the effectiveness of these methods. They kept an eye on the scope, character, and effectiveness of the vaccine-induced response to ensure that it offered protection during a fatal MPOX challenge. Furthermore, they noted that the vaccination could stimulate an Ab response that was broadly and powerfully binding and neutralizing, comparable to the reaction elicited by Dryvax. Additionally, solid cellular immunity was seen. A deadly poxvirus challenge in macaques was significantly impacted by the mix of immunological responses [151].
1.22. mRNA-based vaccine for monkeypox infection
Research has shown that the mRNA therapy's prototype, direct mRNA injection, expresses specific proteins that cause an immune response. With their high potency, possibility for safe delivery, cheap production costs, and capacity for rapid development, RNA-based vaccines offer several benefits over conventional vaccination techniques. According to reports, the mRNA-based vaccination induces a more significant CD4+ or CD8+ T cell response than protein immunization [152]. Clinical trials have shown that the two most used mRNA vaccines are safe and effective against SARS-CoV-2 and its various mutations [153]. To prevent another possibly catastrophic viral outbreak and all the severe health and economic impacts that would follow, it would be more accessible and purely based on the information gathered by the COVID-19 mRNA vaccines to build mRNA vaccinations for MPOX. The only immunization authorized by the US Food and Drug Administration (FDA) is modified Vaccinia Ankara-Bavarian Nordic. However, in the case of an MPOX epidemic, the ACAM2000® smallpox vaccination and the experimental Aventis Pasteur smallpox vaccine will be accessible [51].
In a study, scientists used the mRNA sequences of chimeric monoclonal Abs (mAbs) against the MV, c7D11 (anti-L1), and the EV, c8A (anti-B5) and c6C (anti-A33) types of poxviruses, as well as those made by chimpanzees and humans. The safety and efficacy of using unmodified mRNA that encodes the mAbs c8A, c6C, and c7D11 in preventing smallpox infection were tested in a relatively large (>3 kg) laboratory animal. Investigators used a mouse VACV model to choose monoclonal Ab target levels that showed therapeutic efficacy, in vitro translation, secretion, and biological activity of mRNA constructs. Only one day after giving rabbits an injection of a lipid nanoparticle (LNP) encasing mRNA, they found c7D11, c8A, and c6C in their blood. Combining three LNP-created mRNA constructs that encode distinct Abs led to serum levels almost identical to those attained when each construct was given alone. Their results provide some of the first evidence that numerous Abs may be generated via mRNA constructions in a big, nonrodent species. Considering the experimentally set target serum level and the observed decay rate, the Ab levels attained were, therefore, unlikely to provide protection [154]. Two MPOX quadrivalent lipid-based nanoparticle mRNA vaccines (mRNA-A-LNP and mRNA-B-LNP) were created by researchers as part of a study. These vaccinations were created using the specific MPOX antigens A29L, A35R, M1R, and B6R. Mice are stimulated to produce MPOX-specific IgG and VACV-specific neutralizing Abs by administering mRNA-A-LNP and mRNA-B-LNP twice intramuscularly. In addition, these vaccines induce effective MPOX, particularly Th-1 biased cellular immune response, also persisting effector memory T and germinal center B cell reactions in mice. A double injection of mRNA-A-LNP and mRNA-B-LNP protects animals against the VACV challenge. Additionally, the passive transfer of sera from mice immunized with mRNA-A-LNP and mRNA-B-LNP protects naked animals against the VACV challenge. These mRNA-based vaccinations seem to be effective and safe against MPOX epidemics as well as against outbreaks brought on by other orthopoxviruses, such as the smallpox virus [155]. In the USA, researchers created the polyvalent mRNA vaccine MPXVac-097 against MPOX and tested the immunogenicity of the vaccine in mice. Using 2A peptides and codon optimization, five MPOX viral antigens—A29L, E8L, M1R, A35R, and B6R—were connected in tandem. This vaccine induces widespread neutralizing Abs, MPOX-particular T cell reaction, and preservation versus vaccinia virus challenge. Administration of MPXVac-097 did not produce considerable pathological alterations in mice [156].
1.23. Viral vector vaccines for monkeypox infection
Recombinant viral vectors have been employed to convey specific disease antigens for more than 40 years. The first viral vector carrying a foreign gene was built on the SV40 virus in 1972. Viral vectors have some benefits over conventional subunit vaccines, one of which is that they trigger not only robust Ab responses but also cellular reactions that are essential for destroying pathogen-infected cells. Viral vectors may also produce long-lasting immune responses, in some situations, after only one dose, as well as solid immunogenicity without needing an adjuvant. Moreover, it is possible to build viral vectors to deliver vaccination antigens to particular cells or tissues [157]. There is still room to increase immunogenicity despite the licensed viral vector vaccines' good immunogenicity and effectiveness. Genetic or molecular adjuvants may be a helpful strategy, particularly with vaccination vectors that only trigger mild or short transgene expression. In addition, it has been shown that heterologous prime-boost regimens, which combine different viral vectors or technologies, such as mRNA vaccines, enhance the immunogenicity of homologous regimens, and they are likely to play a significant role in viral vector vaccine regimens in the future [158] (Table 2 ).
Table 2.
Novel MPOX vaccines in the development phase.
| Vaccine platform | Description of the vaccine composition | Type of the study and Vaccine groups | Results | Ref. |
|---|---|---|---|---|
| Protein subunit vaccine | Combining the adjuvants Alhydrogel and CpG with the purified protein ectodomains of A33 and B5 derived from EV and L1 and A27 derived from MV. | Animal study: Cynomolgus macaques | An adjuvanted protein-based subunit vaccination administered twice defended NHP against a fatal MPOX challenge. Additionally, it might be utilized to immunize those who reject VACV and to establish baseline protection against the poxvirus safely. | [132] |
| Protein subunit vaccine | 10 epitopes (9-mer) containing 147 amino acid residues, the PADRE sequence, the CTxB adjuvant, and the necessary peptide linker. The adjuvant and the EAAAK peptide linker are incorporated into the vaccine's N-terminal end. The PADRE sequence, which serves as a helper T cell epitope for triggering the CTL response in response to various antigens, was also connected to the EAAAK linker. | In silico study | The MPOX epitopic vaccine design has shown excellently defined features regarding antigenicity, non-allergenicity, and physicochemical properties. The vaccine was produced using whole-genome-encoded proteins. Researchers might thus conclude that the vaccine architecture they created is not only optimal but also efficient and secure to administer against MPOX. | [133] |
| Protein subunit vaccine | In a multi-epitope vaccination, the epitopes were linked by “GPGPG” linkers and to the adjuvant for cholera toxin B by another EAAAK linker. | Immunoinformatic and molecular docking studies | The modeling of the immune system revealed that the vaccination component stimulated more robust responses from both B and T cells. To induce an immune response against MPOX, a novel risk-free and nearly symptom-free multi-epitope vaccination has been developed that target explicitly three potentially antigenic extracellular proteins. | [92] |
| Protein subunit vaccine | MHC-I, MHC-II, and B-cell epitopes | In silico study | A specific immune response to the MPOX was discovered to be elicited by the vaccination, according to immunological simulation studies. The dynamic molecular investigation, which concludes, demonstrates that the vaccine is stable with a minimum RMSF against the MHC-I allele. Studies concluded that one of the main proteins implicated in the pathogenesis of MPOX is the cell surface-binding protein. | [134] |
| virus-like particle | Norovirus shell and protrusion (S&P) VLP platform. | Developing | In this new endeavor, BWV will try to present MPOX antigens within the S&P platform to potentially develop a vaccine candidate that can shield people from MPOX infection. | [142,143] |
| DNA vaccine | Plasmid DNA encoding the MPOX orthologs of the VACV L1R, A27L, A33R, and B5R proteins | Animal study: Rhesus macaques | In one study, individuals immunized with DNA and boosted with proteins had minor illnesses with no lesions or fewer lesions that went away in a matter of days. Th responses and binding Ab titers to all four proteins were induced by DNA/protein vaccination, and these responses were adversely linked with the overall number of lesions. Only a few numbers of linear B cell epitopes that are highly conserved across OPXVs were identified by the sera of the inoculated macaques. | [150] |
| DNA vaccine | A27, F9, H3, and L1 are the MV-neutralizing Ab targets in the plasmid cocktail. Researchers also incorporated the EV antigens A33, A56, and B5. In an MPOX challenge model, the core antigen A4 was also used to increase the impact of cytotoxic T lymphocytes. | Animal study: Cynomolgus macaques | These findings show a considerable increase in the DNA vaccine platform's effectiveness, producing immune responses that resemble those of live viral infections. They are considered relevant for developing vaccines against difficult-to-treat human and animal illnesses. | [151] |
| mRNA vaccine | Unmodified mRNA that encodes three mAbs, c8A, c6C, and c7D11. | Animal study: rabbits | When the three LNP-formulated mRNA constructs encoding the three different Abs were injected together, the resulting blood levels were almost equal to those obtained when each construct was treated separately. Based on the experimentally established target serum level and the observed decay rate, the Ab levels achieved were not expected to offer protection. | [154] |
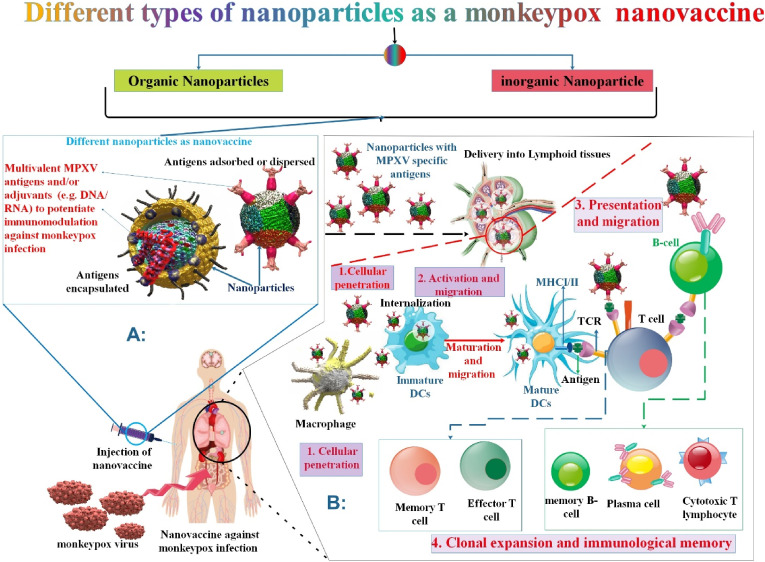
1.24. Nanovaccine
In recent years, proteins have been found in multiple copies on various nanoparticle types, including metals, polymers, lipids, and others, to extend half-life, promote a deposit effect, or primarily activate specific immune cells. The usage of protein-only nanovaccines produced by recombinant protein self-assembly, on the other hand, has been around for a while and offers a technique to meet the specifications of the ideal vaccine formulation. Additionally, it avoids employing non-protein nanoparticles as carriers, which might be problematic if using xenobiotic, resistant, or poorly biocompatible materials because of potential adverse effects [159]. An appropriate nanovaccine design aims to induce an efficient adaptive immune response that results in immunological memory. This describes a very long-lived surveillance system of specialized memory B cells that may accumulate many plasma cells via rapid clonal proliferation before maturing into cells able to produce particular Ab in response to pathogen exposure. These specific Abs ought to neutralize the virus and ultimately lead to viral clearance by preventing it from interacting with the host cell. The activation of cytotoxic CD8+ T cells (CD, cluster of differentiation) is necessary for the elimination of virus-infected host cells in addition to the creation of neutralizing Abs [160]. The viral vaccines that are live-attenuated and inactivated may be seen as nanoparticles in and of themselves. A viral or non-viral nanoparticle may be a nanocarrier to encapsulate or convey the antigen payload or nucleic acid encoding the antigen rather than acting as the vaccine itself. Nanocarriers may impart intrinsic adjuvant behavior and offer stability when delivering these payloads to antigen-presenting cells (APCs). Target immune cells get both the antigen and the adjuvant simultaneously, thanks to nanocarriers [161] (Fig. 7 ). Furthermore, not utilizing the nanoparticle antiviral vaccines frequently needs the help of adjuvants to trigger the immune systems successfully. Virosome- and lipid-based nanoparticle (LNP) vaccines were evaluated in the face of viral infection and demonstrated potential effectiveness in the human body. Several studies have supported the promising efficacy of nanovaccines versus viral infections. Such a study can be utilized to produce a nanovaccine that could inhibit the outbreak of the MPOX while enhancing the immune reaction, especially with nanoparticles improving the delivery yield, delivering adjuvants, and slowly releasing packed factors [[162], [163], [164], [165], [166], [167]]. For instance, researchers demonstrated in one study that an mRNA-lipid nanoparticle vaccine encoding a collection of four highly preserved MPOX surface proteins involved in virus binding, fusion, and transfer can stimulate MPOX-specific immunity and heterologous preservation versus a lethal VACV challenge. To the four MPOX antigens and the four VACV homologs, mRNA vaccination induced more significant Fc-effector Th1-biased humoral immune responses than MVA. It also produced superior neutralizing and cellular spread-inhibitory activities than MPOX and VACV. During combinations of two, three, or four MPOX antigen-expressing mRNAs maintained against infection-related weight loss and death, single MPOX antigen mRNA vaccines showed relative preservation vs. VACV challenge. Due to a combination of neutralizing and non-neutralizing Ab activities, cross-conservation through multivalent MPOX mRNAs was much better than homologous preservation by MVA. This material demonstrates essential VACV protection using an mRNA-based vaccination that targets four highly protected viral surface antigens together with the induction of highly functional Abs to reduce viral infection swiftly [168]. In a different study, scientists created mRNA-ALAB-LNP, a novel poxvirus vaccine that encodes the four vaccinia viral antigens A27, L1, A33, and B5. After a single injection, animals developed potent anti-L1-particular Abs and controlled anti-A33, anti-A27, and anti-B5-particular Ab responses. Following the second injection, the Ab responses to all four antigens were noticeably enhanced, with all IgG titers above 5 logs and the highest being anti-A33 IgG. The high rate of binding Abs revealed a significant ability to neutralize the vaccinia virus. Exclusive IFN-γ reactions were shown to all four antigens, with the stimulation of the same antigen, A33, eliciting the most robust cellular response. This mRNA-LNP vaccine, which encodes four vaccinia antigens, promoted immunity to MPOX and the vaccinia virus, suggesting that the mRNA-ALAB may be a contender for future manufacture of a vaccine against infection with MPOX, smallpox, and likely additional orthopoxviruses [169].
Fig. 7.
In addition to facilitating effective vaccine delivery to tissues and cells, MPOX nanovaccines also allow for the simultaneous delivery of adjuvants and antigens. Furthermore, nanovaccines enable the potentiation of immunomodulation by multivalent antigens and, or adjuvants. When compared to conventional formulations, nanotechnology has the potential to increase therapeutic efficacy and change the viral vaccine market significantly.
1.25. Current status of monkeypox vaccines
According to the WHO's proclamation that MPOX infection is a public health emergency of global concern, governments must fund early and timely efficacy/effectiveness research and ensure that they are prepared to introduce vaccination as soon as necessary. The WHO released interim guidelines on June 24, 2022, noting that mass immunization is neither essential nor advised for MPOX and encourages the prudent use of presently available vaccinations due to supply constraints [170]. Within collaborative vaccine effectiveness studies using standardized protocols and data collection tools, the WHO emphasizes the importance of supporting vaccination programmed with surveillance and contact tracing, as well as vital public health messaging and robust pharmacovigilance [171]. The recommended postexposure vaccination for MPOX prophylaxis is JYNNEOS. The use of vaccinia immune globulin in postexposure prevention for severely immunocompromised individuals who may not establish an adequate immunologic response to JYNNEOS and for whom ACAM2000 is contraindicated is currently under consideration. If general case prevalence becomes a problem, ensuring prompt and protocolized access to JYNNEOS or vaccinia immune globulin should be a top priority for managing infection in immunocompromised individuals [172]. JYNNEOS, a replication-deficient MVA, was suggested as an alternative to ACAM2000 for primary immunization in the newly released recommendations from the Advisory ACIP in 2022. Due to the lack of a significant cutaneous response (or “take”), the vaccine does not represent a danger for autoinoculation or unintentional inoculation. The vaccination is given in two doses, separated by 28 days. For those who have come into contact with more virulent OPXVs, a booster is advised every two years and every ten years for those who have come into contact with less virulent OPXVs. The ACIP also suggested using JYNNEOS as a booster for anyone who has received ACAM2000 as their primary vaccine. However, the scientists showed in a sero-study that although the illness severity is decreased, childhood immunization against MPOX does not entirely protect against it [46,115] (Table 3 and Table 4 ). MVA has shown a good safety profile in phase III clinical assessments, large-scale vaccination investigations are still required, and MVA has not been accepted for utilization in the public crowd to inhibit smallpox and MPOX infections. Furthermore, its cost is also one of the agents to be considered. Therefore, ACAM2000 and MVA vaccines are still the more significant elections for postexposure inhibition of MPOX and smallpox [173]. However, the availability also the possible safety worries of the ACAM2000 and MVA vaccines are far from the global medical request. Significantly, a novel investigation showed that immunization with JYNNEOS stimulated partly low rates of neutralizing Abs versus MPOX. Therefore, a safe, efficient, and available MPOX-particular vaccine is immediately required to face the ongoing MPOX epidemic [26]. Nucleic acid vaccines can safely and efficiently mimic the immunization of inactivated vaccines. Furthermore, the large-scale generation of such vaccines is affordable and straightforward. mRNA vaccine has been well shown as an efficient method to inhibit viral infections with benefits such as fast and scalable generation, excellent safety profile without nuclear entrance, and the capability to successfully stimulate humoral and cellular immune reactions [174,175]. Several investigations offered the advantage of utilizing LNPs as a delivery system for the mRNA vaccines since excellent progress was made in MPOX inhibition. This is because these nanoparticles are considered a constant delivery method that can protect mRNA integrity, preserve their cellular absorption, and improve the yield of their transfer and nucleic acid discharge in the host cells [176]. Additionally, issues that may be encountered when developing a nanovaccine for MPOX and need to be addressed are vaccine affordability, capability to scale up, and durability in various environments because many MPOX patients are in several counties that cannot afford strict storage and transportation requirements. Moreover, it is crucial to thoroughly study and evaluate the toxicity, immune responses, and efficacy of nanoparticles on the transferring factor each time a nanovaccine is prepared for the MPOX [162].
Table 3.
MPOX vaccines in the advanced clinical development phase (https://clinicaltrials.gov/).
| Vaccine platform | Vaccine Name | Vaccine information | Recruitment Status | participants | Clinical Trials.gov Identifier | Ref. |
|---|---|---|---|---|---|---|
| Live attenuated, non-replicating vaccine | IMVAMUNE | While simultaneously assessing the immunogenicity and safety of the vaccine, IMVAMUNE® (also known as MVA-BN, JYNNEOS, and IMVANEX), among healthcare workers in the Democratic Republic of the Congo, the trial will record participant exposure to and infection with MPOX. | Active, not recruiting | 1600 | NCT02977715 | [116] |
| Live attenuated vaccines | Vaccination with MVA vaccine (IMVANEX® and JYNNEOS®) | Even while they feel that two doses of the MVA vaccine used in post-exposure immunization may not prevent the disease, the Centres for Disease Control and Prevention (CDC) believe that one rapid vaccination of high-risk contacts may minimize the severity of symptoms. | Recruiting | 226 | NCT05438953 | [177] |
| Live attenuated vaccines | MVA-BN | This research will compare two intradermal MVA-BN vaccination regimens to the typical subcutaneous regimen in Phase 2 randomized, open-label, non-placebo controlled, multi-site clinical trial. One-fifth (2 x 10^7) and one-tenth (1 x 10^7) of the usual dosage of MVA-BN is delivered intradermally on Days 1 and 29, respectively, as part of the two dose-sparing methods (Arm 1 and 2, respectively). The 2-dose conventional (1 x 10^8) MVA-BN subcutaneous regimen will be the comparative arm (Arm 3). | Recruiting | 210 | NCT05512949 | [178] |
| Live attenuated vaccines | Imvanex® | Imvanex® was first used as a public health intervention for an MPOX outbreak during the UK outbreak, and this study will give researchers a chance to measure and characterize Ab responses to Imvanex® given in a non-trial setting. | Completed | 120 | NCT03745131 | [179] |
Table 4.
| Monkeypox vaccine | Advantages | Disadvantages |
|---|---|---|
| ACAM2000 | Replication‐competent Recombinant vaccinia viruses make VACV vaccinations typically safe and effective. Since the vaccine does not include the variola virus, it cannot cause smallpox. It is provided as a lyophilized powder reconstituted with a packed diluent. | Possible adverse effects include itchiness, swelling (lymphadenopathy), pain (administration site), fever, headaches, myalgia, weakness (fatigue), and infection (bacteria). Other severe adverse reactions to vaccinations, in addition to myopericarditis and pericarditis, include encephalitis, progressive vaccinia, erythema multiforme major, eczema, vaccinatum, generalized vaccinia, post-vaccinal encephalitis or encephalomyelitis, blindness from autoinoculation, and fetal death in expectant mothers. |
| LC16m18 | Since it was successfully given to more than 50,000 children in the 1970s, LC16m8, an attenuated, replicating smallpox vaccine developed from the Lister strain of vaccinia, has been licensed in Japan. In nonclinical studies, it was found to have significantly less neurotoxicity than vaccines that had not been attenuated. Recent research in two different animal models has shown that LC16m8 is immunogenic after a single dose and has protective efficacy comparable to the only smallpox vaccine approved by the FDA. | Common adverse effects include weariness, pruritus, lymphadenopathy, pain at the injection site, fever, headache, myalgia, and rash. |
| JYNNEOS | People with immune weaknesses may be vaccinated with a live, attenuated, replication-deficient vaccine since it has fewer side effects than some others and no serious adverse events. | Side effects that are often experienced include myalgia, headaches, tiredness, nausea, and chills. Clinical studies did not have a sufficient number of participants 65 years of age and over, which cannot be established by adequate human studies. The human data on expectant mothers that are currently available are insufficient to determine the risks of vaccination during pregnancy, and it is unknown if the vaccine is excreted in human milk. Individuals under 18 have not been studied for safety and efficacy. |
In addition to DNA, VLPs, replication-competent and replication-defective vectors, and novel protein or gene-based subunit methods, some have shown effectiveness against MPOX in NHP. The results of the animal studies demonstrate that smallpox vaccinations induce immunological responses capable of providing significant and often total protection against MPOX infection. All of these products will need to undergo a lengthy clinical development procedure that includes determining the protection's level of durability and a thorough safety assessment and formulation considerations to maximize stability [91] (Table 2).
2. Conclusion
Resources must be gathered to stop the worldwide spread of MPOX, a disease that is now relevant and concerning globally. According to data, smallpox vaccination before infection may prevent MPOX and alleviate clinical symptoms of illness. Infants and young children (under 8), pregnant women, and those with impaired immune systems are probably at the most significant risk. The Strategic National Stockpile of the United States has two antivirals that might be employed, as well as smallpox and MPOX vaccinations. Only a few other nations have made similar preparations. JYNNEOS, LC16m8, and ACAM2000 are approved mainly for groups at high risk for exposure to MPOX. However, the availability also the possible safety worries of the ACAM2000 and MVA vaccines are far from the global medical request. Continuing assessment of available vaccines for safety and effectiveness in preventing MPOX is essential. Therefore, developing a novel vaccine with a wide range of use potential for MPOX and its emerging variants could present a good preventive method to deal with the evolving MPOX infection. According to various studies, mRNA-based LNP induces more neutralizing Abs, more effective MPOX-specific T-cell responses, and protection against MPOX than traditional vaccine methods. Considering that the safety profiles of LNPs relate to dose and combination and that long-time side effects, particularly following numerous dose use, cannot be seriously forecasted as data on long-time health results is clearly not accessible yet. More advanced studies should re-assess the disadvantages and advantages ratio before a continuing comprehensive utilization of mRNA vaccines in low-risk individuals considered for life-threatening periods. In addition, to recognize the most efficient vaccine between traditional and novel vaccines, it is crucial to assess efficiency, including reactogenicity, cytotoxicity test, safety, and adverse effects, especially for high-risk and vulnerable patients. It is necessary to control and contain the MPOX using the lessons learned from the COVID-19 pandemic. It is essential to immediately create, produce, and license safe and effective countermeasures as the COVID-19 pandemic spreads throughout the world. Platforms based on nucleic acids can be ideal for such unanticipated situations. Success will be determined over time, but the quick creation and testing of the now FDA-approved mRNA vaccines are unique. The next stage might be to test and approve preventive or therapeutic effector molecules, like Abs, using nucleic acid technology. As shown by SARS-CoV-2, a responsive platform is probably necessary to keep up with an agent that is continually and quickly changing. The threshold toward such a therapy may be approaching fast, given how swiftly technology is developing in delivery and stability. Furthermore, the groundwork for the rapid development of novel vaccine technologies to have an effect during the MPOX infection has been laid by breakthroughs in bio/nanotechnology and enhanced nano/manufacturing with open reporting and data sharing. Based on the available information, it is still necessary to create a new generation of MPOX-specific vaccinations that are safe and efficient and either eradicate the virus or create innovative vaccine platforms before MPOX is declared a pandemic.
Ethics approval statement
Not applicable.
Clinical trial registration
Not applicable.
Human and animal rights and informed consent
This article does not contain any studies with animal or human subjects performed by any of the authors.
Consent for publication
The authors guarantee that, once their material has been accepted for publication by the journal, they will not submit the same material or portions thereof to another journal.
Permission to reproduce material from other sources
Not applicable.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Mohamed J. Saadh: Conceptualization, Investigation, Writing – review & editing. Tahmineh Ghadimkhani: Data curation, Investigation, Writing – review & editing. Narges Soltani: Investigation, Software, Writing – review & editing. Arian Abbassioun: Investigation, Visualization, Writing – review & editing. Renzon Daniel Cosme Pecho: Conceptualization, Investigation, Writing – review & editing. Ali taha: Conceptualization, Investigation, Writing – review & editing. Tareq Jwad Kazem: Conceptualization, Visualization, Writing – review & editing. Saman Yasamineh: Writing – review & editing, Writing – original draft, Software, Investigation, Conceptualization. Omid Gholizadeh: Writing – review & editing, Writing – original draft, Visualization, Supervision, Software, Project administration, Investigation, Conceptualization.
Declaration of competing interest
The authors declared no conflicts of interest regarding the research, authorship, and publication of this article.
Acknowledgment
The authors would like to thank the Department of Medical Biotechnology and Department of Bacteriology and Department of Virology, Tabriz University of Medical Sciences for supporting this project.
References
- 1.Wang J., et al. An overview of antivirals against monkeypox virus and other orthopoxviruses. J. Med. Chem. 2023 doi: 10.1021/acs.jmedchem.3c00069. [DOI] [PubMed] [Google Scholar]
- 2.Meseko C., et al. Orthopoxvirus infections in rodents, Nigeria. Emerg. Infect. Dis. 2023;29(2):433. doi: 10.3201/eid2902.221411. 2018–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Baetselier I., et al. Retrospective detection of asymptomatic monkeypox virus infections among male sexual health clinic attendees in Belgium. Nat. Med. 2022:1–5. doi: 10.1038/s41591-022-02004-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dashraath P., et al. Guidelines for pregnant individuals with monkeypox virus exposure. Lancet. 2022;400(10345):21–22. doi: 10.1016/S0140-6736(22)01063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunge E.M., et al. The changing epidemiology of human monkeypox—a potential threat? A systematic review. PLoS Neglected Trop. Dis. 2022;16(2) doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luna N., et al. Phylogenomic analysis of the monkeypox virus (MPXV) 2022 outbreak: emergence of a novel viral lineage? Trav. Med. Infect. Dis. 2022;49 doi: 10.1016/j.tmaid.2022.102402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alakunle E.F., Okeke M.I. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat. Rev. Microbiol. 2022:1–2. doi: 10.1038/s41579-022-00776-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forni D., et al. Geographic structuring and divergence time frame of monkeypox virus in the endemic region. J. Infect. Dis. 2022 doi: 10.1093/infdis/jiac298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dogra S., et al. Monkeypox: a new global health emergency with predominant dermatological manifestations. Int. J. Dermatol. 2023;62(1):3–11. doi: 10.1111/ijd.16504. [DOI] [PubMed] [Google Scholar]
- 10.Ulaeto D., et al. New nomenclature for mpox (monkeypox) and monkeypox virus clades. Lancet Infect. Dis. 2023;23(3):273–275. doi: 10.1016/S1473-3099(23)00055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiwari A., et al. Monkeypox outbreak: wastewater and environmental surveillance perspective. Sci. Total Environ. 2023;856 doi: 10.1016/j.scitotenv.2022.159166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karagoz A., et al. Monkeypox (mpox) virus: classification, origin, transmission, genome organization, antiviral drugs, and molecular diagnosis. Journal of Infection and Public Health. 2023 doi: 10.1016/j.jiph.2023.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahase E. British Medical Journal Publishing Group; 2022. Monkeypox: What Do We Know about the Outbreaks in Europe And North America? [DOI] [PubMed] [Google Scholar]
- 14.Philpott D. Morbidity and Mortality Weekly Report; 2022. Epidemiologic And Clinical Characteristics of Monkeypox cases—United States, May 17–July 22, 2022. MMWR; p. 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarín-Vicente E.J., et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022;400(10353):661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler H., et al. The Lancet Infectious Diseases; 2022. Clinical Features and Management of Human Monkeypox: a Retrospective Observational Study in the UK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar N., et al. The 2022 outbreak and the pathobiology of the monkeypox virus. J. Autoimmun. 2022 doi: 10.1016/j.jaut.2022.102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huangfu L., et al. Novel prognostic marker LINC00205 promotes tumorigenesis and metastasis by competitively suppressing miRNA-26a in gastric cancer. Cell death discovery. 2022;8(1):1–10. doi: 10.1038/s41420-021-00802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maruotti A., et al. Estimating the undetected infections in the Monkeypox outbreak. J. Med. Virol. 2023;95(1) doi: 10.1002/jmv.28099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabaan A.A., et al. Monkeypox outbreak 2022: what we know so far and its potential drug targets and management strategies. J. Med. Virol. 2023;95(1) doi: 10.1002/jmv.28306. [DOI] [PubMed] [Google Scholar]
- 21.Isidro J., et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 2022;28(8):1569–1572. doi: 10.1038/s41591-022-01907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdelaal A., et al. Preventing the next pandemic: is live vaccine efficacious against monkeypox, or is there a need for killed virus and mRNA vaccines? Vaccines. 2022;10(9):1419. doi: 10.3390/vaccines10091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Najeeb A., Eltalkhawy Y.M., Reda O. The current Monkeypox outbreak: updates and concerns. Journal of Taibah University Medical Sciences. 2023;18(1):137–139. doi: 10.1016/j.jtumed.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferdous J., et al. A review on monkeypox virus outbreak: new challenge for world. Health Science Reports. 2023;6(1):e1007. doi: 10.1002/hsr2.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrar J.L. MMWR. Morbidity and Mortality Weekly Report; 2022. Demographic and Clinical Characteristics of Mpox in Persons Who Had Previously Received 1 Dose of JYNNEOS Vaccine and in Unvaccinated Persons—29 US Jurisdictions; p. 71. May 22–September 3, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaeck L.M., et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med. 2023;29(1):270–278. doi: 10.1038/s41591-022-02090-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obeid M.A., et al. Monkeypox: emerging virus of concern; antivirals and vaccines therapeutic options. Microb. Pathog. 2022 doi: 10.1016/j.micpath.2022.105799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng X., et al. Immunomodulatory nanosystems. Adv. Sci. 2019;6(17) doi: 10.1002/advs.201900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mucker E.M., et al. A nucleic acid-based orthopoxvirus vaccine targeting the vaccinia virus L1, A27, B5, and A33 proteins protects rabbits against lethal rabbitpox virus aerosol challenge. J. Virol. 2022;96(3) doi: 10.1128/JVI.01504-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baghban R., Ghasemian A., Mahmoodi S. Nucleic acid-based vaccine platforms against the coronavirus disease 19 (COVID-19) Arch. Microbiol. 2023;205(4):150. doi: 10.1007/s00203-023-03480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerji I., et al. Nucleic Acid Biology and its Application in Human Diseases. Springer; 2023. RNA vaccines: the evolution, applications, and the challenges ahead; pp. 349–364. [Google Scholar]
- 32.Reina J., Iglesias C. English Edition); 2023. Vaccines Against Monkeypox. Medicina Clínica. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roper R.L., et al. Vaccine; 2023. Monkeypox (Mpox) Requires Continued Surveillance, Vaccines, Therapeutics and Mitigating Strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sepehrinezhad A., Ashayeri Ahmadabad R., Sahab-Negah S. Monkeypox virus from neurological complications to neuroinvasive properties: current status and future perspectives. J. Neurol. 2022:1–8. doi: 10.1007/s00415-022-11339-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Q., et al. Highly accurate protein structure prediction and drug screen of monkeypox virus proteome. J. Infect. 2022 doi: 10.1016/j.jinf.2022.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu M., et al. Unusual global outbreak of monkeypox: what should we do? Front. Med. 2022:1–11. doi: 10.1007/s11684-022-0952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng L., et al. Acta Materia Medica; 2022. Structure Prediction of the Entire Proteome of Monkeypox Variants. [Google Scholar]
- 38.Manes N.P., et al. Comparative proteomics of human monkeypox and vaccinia intracellular mature and extracellular enveloped virions. J. Proteome Res. 2008;7(3):960–968. doi: 10.1021/pr070432+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong Q., et al. Monkeypox virus: a re-emergent threat to humans. Virol. Sin. 2022 doi: 10.1016/j.virs.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alakunle E., et al. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12(11):1257. doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kmiec D., Kirchhoff F. Monkeypox: a new threat? Int. J. Mol. Sci. 2022;23(14):7866. doi: 10.3390/ijms23147866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quarleri J., Delpino M., Galvan V. Monkeypox: considerations for the understanding and containment of the current outbreak in non-endemic countries. GeroScience. 2022:1–9. doi: 10.1007/s11357-022-00611-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopera J.G., et al. Attenuation of monkeypox virus by deletion of genomic regions. Virology. 2015;475:129–138. doi: 10.1016/j.virol.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marty A.M. Why is there expanding community transmission of monkeypox in 2022? The Lancet Microbe. 2022 doi: 10.1016/S2666-5247(22)00200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saxena, S.K., et al., Re‐emerging human monkeypox: a major public‐health debacle. J. Med. Virol.. [DOI] [PubMed]
- 46.Lansiaux E., et al. The Virology of human monkeypox virus (hMPXV): a brief overview. Virus Res. 2022 doi: 10.1016/j.virusres.2022.198932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Realegeno S., et al. Monkeypox virus host factor screen using haploid cells identifies essential role of GARP complex in extracellular virus formation. J. Virol. 2017;91(11) doi: 10.1128/JVI.00011-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mucker E.M., et al. Evaluation of virulence in cynomolgus macaques using a virus preparation enriched for the extracellular form of monkeypox virus. Viruses. 2022;14(9):1993. doi: 10.3390/v14091993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramalingam P., et al. Recent advances in nanomaterials-based drug delivery system for cancer treatment. Emerging Nanomaterials for Advanced Technologies. 2022:83–116. [Google Scholar]
- 50.Antinori A., et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200421. May 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aljabali A.A., et al. Microbial Pathogenesis; 2022. Monkeypox Virus: an Emerging Epidemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H., et al. Cytokine & Growth Factor Reviews; 2022. The Evolving Epidemiology of Monkeypox Virus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lum F.-M., et al. Monkeypox: disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022;22(10):597–613. doi: 10.1038/s41577-022-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ejaz H., et al. Emergence and dissemination of monkeypox, an intimidating global public health problem. Journal of Infection and Public Health. 2022 doi: 10.1016/j.jiph.2022.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu Y., Zhang L. DNA-sensing antiviral innate immunity in poxvirus infection. Front. Immunol. 2020;11:1637. doi: 10.3389/fimmu.2020.01637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bourquain D., Dabrowski P.W., Nitsche A. Comparison of host cell gene expression in cowpox, monkeypox or vaccinia virus-infected cells reveals virus-specific regulation of immune response genes. Virol. J. 2013;10(1):1–13. doi: 10.1186/1743-422X-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sood A., et al. Comparison of multiplexed immunofluorescence imaging to chromogenic immunohistochemistry of skin biomarkers in response to monkeypox virus infection. Viruses. 2020;12(8):787. doi: 10.3390/v12080787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Musa A., Chou J., LaBere B. The resurgence of a neglected orthopoxvirus: immunologic and clinical aspects of monkeypox virus infections over the past six decades. Clin. Immunol. 2022 doi: 10.1016/j.clim.2022.109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Earl P.L., Americo J.L., Moss B. Genetic studies of the susceptibility of classical and wild-derived inbred mouse strains to monkeypox virus. Virology. 2015;481:161–165. doi: 10.1016/j.virol.2015.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song H., et al. Monkeypox virus infection of rhesus macaques induces massive expansion of natural killer cells but suppresses natural killer cell functions. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0077804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Earl P.L., Americo J.L., Moss B. Lethal monkeypox virus infection of CAST/EiJ mice is associated with a deficient gamma interferon response. J. Virol. 2012;86(17):9105–9112. doi: 10.1128/JVI.00162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saghazadeh A., Rezaei N. Insights on Mpox virus infection immunopathogenesis. Rev. Med. Virol. 2023;33(2):e2426. doi: 10.1002/rmv.2426. [DOI] [PubMed] [Google Scholar]
- 63.Lozano J.M., Muller S. Monkeypox: potential vaccine development strategies. Trends Pharmacol. Sci. 2022 doi: 10.1016/j.tips.2022.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shafaati M., Zandi M. Human monkeypox (hMPXV) re‐emergence: host immunity status and current vaccines landscape. J. Med. Virol. 2023;95(1) doi: 10.1002/jmv.28251. [DOI] [PubMed] [Google Scholar]
- 65.Townsend M., et al. Humoral immunity to smallpox vaccines and monkeypox virus challenge: proteomic assessment and clinical correlations. J. Virol. 2013;87(2):900–911. doi: 10.1128/JVI.02089-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pittman P.R., et al. MedRxiv; 2022. Clinical Characterization of Human Monkeypox Infections in the Democratic Republic of the Congo; p. 2022. 05. 26.22273379. [Google Scholar]
- 67.Estep R.D., et al. Deletion of the monkeypox virus inhibitor of complement enzymes locus impacts the adaptive immune response to monkeypox virus in a nonhuman primate model of infection. J. Virol. 2011;85(18):9527–9542. doi: 10.1128/JVI.00199-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li H., et al. The land-scape of immune response to monkeypox virus. EBioMedicine. 2023;87 doi: 10.1016/j.ebiom.2022.104424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agrati C., et al. Immunological signature in human cases of monkeypox infection in 2022 outbreak: an observational study. Lancet Infect. Dis. 2023;23(3):320–330. doi: 10.1016/S1473-3099(22)00662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hammarlund E., et al. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc. Natl. Acad. Sci. USA. 2008;105(38):14567–14572. doi: 10.1073/pnas.0800589105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walsh S.R., et al. Diverse recognition of conserved orthopoxvirus CD8+ T cell epitopes in vaccinated rhesus macaques. Vaccine. 2009;27(36):4990–5000. doi: 10.1016/j.vaccine.2009.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Calvo-Calle J.M., et al. Human CD4+ T cell epitopes from vaccinia virus induced by vaccination or infection. PLoS Pathog. 2007;3(10):e144. doi: 10.1371/journal.ppat.0030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Panchanathan V., Chaudhri G., Karupiah G. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J. Virol. 2006;80(13):6333–6338. doi: 10.1128/JVI.00115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hemati S., et al. Travel Medicine and Infectious Disease; 2022. A Review on Insights and Lessons from COVID-19 to the Prevent of Monkeypox Pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poland G.A., Kennedy R.B., Tosh P.K. The Lancet Infectious Diseases; 2022. Prevention of Monkeypox with Vaccines: a Rapid Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jeyanathan M., et al. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bryer J.S., Freeman E.E., Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatological primer. J. Am. Acad. Dermatol. 2022 doi: 10.1016/j.jaad.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khalil A., et al. Monkeypox vaccines in pregnancy: lessons must be learned from COVID-19. Lancet Global Health. 2022;10(9):e1230–e1231. doi: 10.1016/S2214-109X(22)00284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Handley L., et al. The new ACAM2000™ vaccine and other therapies to control orthopoxvirus outbreaks and bioterror attacks. Expet Rev. Vaccine. 2009;8(7):841–850. doi: 10.1586/erv.09.55. [DOI] [PubMed] [Google Scholar]
- 80.Weltzin R., et al. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nat. Med. 2003;9(9):1125–1130. doi: 10.1038/nm916. [DOI] [PubMed] [Google Scholar]
- 81.Petersen B.W., et al. Clinical guidance for smallpox vaccine use in a postevent vaccination program. MMWR Recomm. Rep. (Morb. Mortal. Wkly. Rep.) 2015;64(2):1–26. [PubMed] [Google Scholar]
- 82.Chouchana L., et al. Facial nerve palsy as A possible adverse drug reaction of the modified vaccinia ankara-bavarian nordic (MVA-BN) smallpox vaccine: a pharmacovigilance analysis. J. Infect. 2023 doi: 10.1016/j.jinf.2023.01.005. [DOI] [PubMed] [Google Scholar]
- 83.Farahat R.A., Khan S.H., Memish Z.A. Travel Medicine and Infectious Disease; 2022. Availability of Monkeypox Vaccinations for Low and Middle-Income Countries: Challenges and Recommendations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sánchez-Sampedro L., et al. The evolution of poxvirus vaccines. Viruses. 2015;7(4):1726–1803. doi: 10.3390/v7041726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stittelaar K.J., et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J. Virol. 2005;79(12):7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wiser I., Balicer R.D., Cohen D. An update on smallpox vaccine candidates and their role in bioterrorism related vaccination strategies. Vaccine. 2007;25(6):976–984. doi: 10.1016/j.vaccine.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 87.Kennedy R.B., Ovsyannikova I., Poland G.A. Smallpox vaccines for biodefense. Vaccine. 2009;27:D73–D79. doi: 10.1016/j.vaccine.2009.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferrier-Rembert A., et al. Safety, immunogenicity and protective efficacy in mice of a new cell-cultured Lister smallpox vaccine candidate. Vaccine. 2007;25(49):8290–8297. doi: 10.1016/j.vaccine.2007.09.050. [DOI] [PubMed] [Google Scholar]
- 89.Kennedy J.S., Greenberg R.N. IMVAMUNE®: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expet Rev. Vaccine. 2009;8(1):13–24. doi: 10.1586/14760584.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kenner J., et al. LC16m8: an attenuated smallpox vaccine. Vaccine. 2006;24(47–48):7009–7022. doi: 10.1016/j.vaccine.2006.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rimoin A.W., Graham B.S. Whither monkeypox vaccination. Vaccine. 2011;29:D60–D64. doi: 10.1016/j.vaccine.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ullah A., et al. An integrative reverse vaccinology, immunoinformatic, docking and simulation approaches towards designing of multi-epitopes based vaccine against monkeypox virus. J. Biomol. Struct. Dyn. 2022:1–14. doi: 10.1080/07391102.2022.2125441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao Q., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pato T.P., et al. Purification of yellow fever virus produced in Vero cells for inactivated vaccine manufacture. Vaccine. 2019;37(24):3214–3220. doi: 10.1016/j.vaccine.2019.04.077. [DOI] [PubMed] [Google Scholar]
- 95.Sanders B., Koldijk M., Schuitemaker H. Vaccine Analysis: Strategies, Principles, and Control. Springer; 2015. Inactivated viral vaccines; pp. 45–80. [Google Scholar]
- 96.Chen J.M. Should the world collaborate imminently to develop neglected live‐attenuated vaccines for COVID‐19? J. Med. Virol. 2022;94(1):82–87. doi: 10.1002/jmv.27335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee A., et al. A molecular atlas of innate immunity to adjuvanted and live attenuated vaccines, in mice. Nat. Commun. 2022;13(1):549. doi: 10.1038/s41467-022-28197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Minor P.D. Live attenuated vaccines: historical successes and current challenges. Virology. 2015;479:379–392. doi: 10.1016/j.virol.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 99.Lauring A.S., Jones J.O., Andino R. Rationalizing the development of live attenuated virus vaccines. Nat. Biotechnol. 2010;28(6):573–579. doi: 10.1038/nbt.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang B., et al. Perceptions, precautions, and vaccine acceptance related to monkeypox in the public in China: a cross-sectional survey. Journal of Infection and Public Health. 2023;16(2):163–170. doi: 10.1016/j.jiph.2022.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eto A., et al. Recent advances in the study of live attenuated cell-cultured smallpox vaccine LC16m8. Vaccine. 2015;33(45):6106–6111. doi: 10.1016/j.vaccine.2015.07.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saijo M., et al. LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R, protects monkeys from monkeypox. J. Virol. 2006;80(11):5179–5188. doi: 10.1128/JVI.02642-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kennedy J.S., et al. Safety and immunogenicity of LC16m8, an attenuated smallpox vaccine in vaccinia-naive adults. JID (J. Infect. Dis.) 2011;204(9):1395–1402. doi: 10.1093/infdis/jir527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saijo M., et al. Post-exposure vaccination with a highly attenuated vaccinia vaccine, LC16m8, for protection of nonhuman primates from monkeypox. Int. J. Infect. Dis. 2008;12:e53. [Google Scholar]
- 105.mondiale de la Santé O., Organization W.H. Report of the second joint meeting (hybrid) of the WHO Global Advisory Committee on Vaccine Safety and the WHO Advisory Committee on Safety of Medicinal Products, 14–16 December 2022–Rapport de la deuxième réunion conjointe (hybride) du Comité consultatif mondial de l’OMS pour la sécurité des vaccins et du Comité consultatif de l’OMS sur la sécurité des produits médicaux, 14-16 décembre 2022. Weekly Epidemiological Record= Relevé épidémiologique hebdomadaire. 2023;98(9):83–92. [Google Scholar]
- 106.Persad G., et al. Fair domestic allocation of monkeypox virus countermeasures. Lancet Public Health. 2023;8(5):e378–e382. doi: 10.1016/S2468-2667(23)00061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Russo A.T., et al. Co-administration of tecovirimat and ACAM2000™ in non-human primates: effect of tecovirimat treatment on ACAM2000 immunogenicity and efficacy versus lethal monkeypox virus challenge. Vaccine. 2020;38(3):644–654. doi: 10.1016/j.vaccine.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ahmed S.F., et al. Vaccinia-virus-based vaccines are expected to elicit highly cross-reactive immunity to the 2022 monkeypox virus. Viruses. 2022;14(9):1960. doi: 10.3390/v14091960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nalca A., Zumbrun E.E. ACAM2000™: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des. Dev. Ther. 2010;4:71. doi: 10.2147/dddt.s3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chakraborty S., et al. Clinical management, antiviral drugs and immunotherapeutics for treating monkeypox. An update on current knowledge and futuristic prospects. Int. J. Surg. 2022;105 doi: 10.1016/j.ijsu.2022.106847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Berhanu A., et al. Treatment with the smallpox antiviral tecovirimat (ST-246) alone or in combination with ACAM2000 vaccination is effective as a postsymptomatic therapy for monkeypox virus infection. Antimicrob. Agents Chemother. 2015;59(7):4296–4300. doi: 10.1128/AAC.00208-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Katamesh B.E., et al. Monkeypox pandemic containment: does the ACAM2000 vaccine play a role in the current outbreaks? Expet Rev. Vaccine. 2023 doi: 10.1080/14760584.2023.2198600. (just-accepted)) [DOI] [PubMed] [Google Scholar]
- 113.Yeo K., et al. SG-APSIC1035: prospective safety surveillance study of ACAM2000 smallpox vaccine in deployed military personnel. Antimicrobial Stewardship & Healthcare Epidemiology. 2023;3(S1) s10-s10. [Google Scholar]
- 114.Petersen B.W. 2021. Clinical Guidance for the Use of JYNNEOS. [Google Scholar]
- 115.Rao A.K., et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the advisory committee on immunization practices—United States. MMWR (Morb. Mortal. Wkly. Rep.) 2022;71(22):734. doi: 10.15585/mmwr.mm7122e1. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Petersen B.W., et al. Vaccinating against monkeypox in the democratic republic of the Congo. Antivir. Res. 2019;162:171–177. doi: 10.1016/j.antiviral.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Frey S.E., et al. Safety and immunogenicity of IMVAMUNE® smallpox vaccine using different strategies for a post event scenario. Vaccine. 2013;31(29):3025–3033. doi: 10.1016/j.vaccine.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Frey S.E., Goll J.B., Beigel J.H. Erythema and induration after Mpox (JYNNEOS) vaccination revisited. N. Engl. J. Med. 2023;388(15):1432–1435. doi: 10.1056/NEJMc2215846. [DOI] [PubMed] [Google Scholar]
- 119.Kandeel M., et al. Efficacy of the modified vaccinia Ankara virus vaccine and the replication-competent vaccine ACAM2000 in monkeypox prevention. Int. Immunopharm. 2023;119 doi: 10.1016/j.intimp.2023.110206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Islam M.R., et al. Repositioning potentials of smallpox vaccines and antiviral agents in monkeypox outbreak: a rapid review on comparative benefits and risks. Health Science Reports. 2022;5(5):e798. doi: 10.1002/hsr2.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vega‐Rodriguez W., Ly H. Getting ahead of monkeypox: learning from the COVID‐19 pandemic experience to prevent the potentially new monkeypox pandemic. J. Med. Virol. 2023;95(1) doi: 10.1002/jmv.28146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sudarmaji N., et al. Prevention and treatment of monkeypox: a systematic review of preclinical studies. Viruses. 2022;14(11):2496. doi: 10.3390/v14112496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hatch G.J., et al. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J. Virol. 2013;87(14):7805–7815. doi: 10.1128/JVI.03481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu R., et al. Interferon α/β decoy receptor encoded by a variant in the Dryvax smallpox vaccine contributes to virulence and correlates with severe vaccine side effects. mBio. 2022;13(1) doi: 10.1128/mbio.00102-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cruz N.V.G., et al. Genomic characterization of the historical smallpox vaccine strain wyeth isolated from a 1971 seed vial. Viruses. 2023;15(1):83. doi: 10.3390/v15010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sarkar I., et al. Detection of the peptidyl epitope for vaccine development against MPV. J. King Saud Univ. Sci. 2023;35(1) doi: 10.1016/j.jksus.2022.102458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jiang F., et al. Developing a multiepitope vaccine for the prevention of SARS-CoV-2 and monkeypox virus co-infection: a reverse vaccinology analysis. Int. Immunopharm. 2023 doi: 10.1016/j.intimp.2023.109728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zaib S., et al. Designing multi-epitope monkeypox virus-specific vaccine using immunoinformatics approach. Journal of Infection and Public Health. 2023;16(1):107–116. doi: 10.1016/j.jiph.2022.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Heidary M., et al. A comprehensive review of the protein subunit vaccines against COVID-19. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.927306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee A., et al. A molecular atlas of innate immunity to adjuvanted and live attenuated vaccines, in mice. Nat. Commun. 2022;13(1):1–13. doi: 10.1038/s41467-022-28197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang N., et al. Heat-inactivated modified vaccinia virus Ankara boosts Th1 cellular and humoral immunity as a vaccine adjuvant. npj Vaccines. 2022;7(1):1–15. doi: 10.1038/s41541-022-00542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Buchman G.W., et al. A protein-based smallpox vaccine protects non-human primates from a lethal monkeypox virus challenge. Vaccine. 2010;28(40):6627–6636. doi: 10.1016/j.vaccine.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bhattacharya M., et al. Travel Medicine and Infectious Disease; 2022. Designing, Characterization, and Immune Stimulation of a Novel Multi-Epitopic Peptide-Based Potential Vaccine Candidate against Monkeypox Virus through Screening its Whole Genome Encoded Proteins: an Immunoinformatics Approach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shantier S.W., et al. Novel multi epitope-based vaccine against monkeypox virus: vaccinomic approach. Sci. Rep. 2022;12(1):1–17. doi: 10.1038/s41598-022-20397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rudraraju R., Ramsay A.J. Single-shot immunization with recombinant adenovirus encoding vaccinia virus glycoprotein A27L is protective against a virulent respiratory poxvirus infection. Vaccine. 2010;28(31):4997–5004. doi: 10.1016/j.vaccine.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jin Y., et al. Proteomics-based vaccine targets annotation and design of subunit and mRNA-based vaccines for Monkeypox virus (MPXV) against the recent outbreak. Comput. Biol. Med. 2023 doi: 10.1016/j.compbiomed.2023.106893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mohsen M.O., Bachmann M.F. Virus-like particle vaccinology, from bench to bedside. Cell. Mol. Immunol. 2022;19(9):993–1011. doi: 10.1038/s41423-022-00897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.García-Arriaza J., et al. HIV/AIDS vaccine candidates based on replication-competent recombinant poxvirus NYVAC-C-KC expressing trimeric gp140 and gag-derived virus-like particles or lacking the viral molecule B19 that inhibits type I interferon activate relevant HIV-1-specific B and T cell immune functions in nonhuman primates. J. Virol. 2017;91(9) doi: 10.1128/JVI.02182-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Plummer E.M., Manchester M. Viral nanoparticles and virus‐like particles: platforms for contemporary vaccine design. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2011;3(2):174–196. doi: 10.1002/wnan.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chu K.-B., Quan F.-S. Respiratory viruses and virus-like particle vaccine development: how far have we advanced? Viruses. 2023;15(2):392. doi: 10.3390/v15020392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bielinska A.U., et al. A novel, killed-virus nasal vaccinia virus vaccine. Clin. Vaccine Immunol. 2008;15(2):348–358. doi: 10.1128/CVI.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ghosh U., et al. The emerging roles of silver nanoparticles to target viral life cycle and detect viral pathogens. Chem.--Asian J. 2022;17(5) doi: 10.1002/asia.202101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gomez M., Vehring R. Spray drying and particle engineering in dosage form design for global vaccines. J. Aerosol Med. Pulm. Drug Deliv. 2022;35(3):121–138. doi: 10.1089/jamp.2021.0056. [DOI] [PubMed] [Google Scholar]
- 144.Ozdilek A., Avci F.Y. Glycosylation as a key parameter in the design of nucleic acid vaccines. Curr. Opin. Struct. Biol. 2022;73 doi: 10.1016/j.sbi.2022.102348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hawman D.W., et al. A DNA-based vaccine protects against Crimean-Congo haemorrhagic fever virus disease in a Cynomolgus macaque model. Nature microbiology. 2021;6(2):187–195. doi: 10.1038/s41564-020-00815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hayashi H., et al. Preclinical study of a DNA vaccine targeting SARS-CoV-2. Current research in translational medicine. 2022;70(4) doi: 10.1016/j.retram.2022.103348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lundstrom K. Plasmid DNA-based alphavirus vaccines. Vaccines. 2019;7(1):29. doi: 10.3390/vaccines7010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Seo Y.B., et al. Soluble spike DNA vaccine provides long-term protective immunity against SARS-CoV-2 in mice and nonhuman primates. Vaccines. 2021;9(4):307. doi: 10.3390/vaccines9040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kayyal M., et al. Silico design and immunological studies of two novel multiepitope DNA-based vaccine candidates against high-risk human papillomaviruses. Mol. Biotechnol. 2021;63(12):1192–1222. doi: 10.1007/s12033-021-00374-z. [DOI] [PubMed] [Google Scholar]
- 150.Heraud J.-M., et al. Subunit recombinant vaccine protects against monkeypox. J. Immunol. 2006;177(4):2552–2564. doi: 10.4049/jimmunol.177.4.2552. [DOI] [PubMed] [Google Scholar]
- 151.Hirao L.A., et al. Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. JID (J. Infect. Dis.) 2011;203(1):95–102. doi: 10.1093/infdis/jiq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang M., et al. mRNA-based modalities for infectious disease management. Nano Res. 2022:1–20. doi: 10.1007/s12274-022-4627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hussain A., et al. mRNA vaccines for COVID-19 and diverse diseases. J. Contr. Release. 2022 doi: 10.1016/j.jconrel.2022.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mucker E.M., et al. Lipid nanoparticle delivery of unmodified mRNAs encoding multiple monoclonal antibodies targeting poxviruses in rabbits. Mol. Ther. Nucleic Acids. 2022;28:847–858. doi: 10.1016/j.omtn.2022.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sang Y., et al. Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus. Signal Transduct. Targeted Ther. 2023;8(1):172. doi: 10.1038/s41392-023-01432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fang Z., et al. Cell Research; 2023. Polyvalent mRNA Vaccination Elicited Potent Immune Response to Monkeypox Virus Surface Antigens; pp. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Travieso T., et al. The use of viral vectors in vaccine development. npj Vaccines. 2022;7(1):1–10. doi: 10.1038/s41541-022-00503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McCann N., et al. Viral vector vaccines. Curr. Opin. Immunol. 2022;77 doi: 10.1016/j.coi.2022.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.de Pinho Favaro M.T., et al. Recombinant vaccines in 2022: a perspective from the cell factory. Microb. Cell Factories. 2022;21(1):1–17. doi: 10.1186/s12934-022-01929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Guerrini G., et al. Characterization of nanoparticles-based vaccines for COVID-19. Nat. Nanotechnol. 2022;17(6):570–576. doi: 10.1038/s41565-022-01129-w. [DOI] [PubMed] [Google Scholar]
- 161.Shin M.D., et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020;15(8):646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 162.Mohamed N.A., et al. Nanomedicine as a Potential Tool against Monkeypox. Vaccines. 2023;11(2):428. doi: 10.3390/vaccines11020428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yasamineh S., et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm. 2022 doi: 10.1016/j.ijpharm.2022.121878. [DOI] [PubMed] [Google Scholar]
- 164.Yasamineh S., et al. An overview on nanoparticle-based strategies to fight viral infections with a focus on COVID-19. J. Nanobiotechnol. 2022;20(1):440. doi: 10.1186/s12951-022-01625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Oveili E., et al. The potential use of mesenchymal stem cells-derived exosomes as microRNAs delivery systems in different diseases. Cell Commun. Signal. 2023;21(1):1–26. doi: 10.1186/s12964-022-01017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yasamineh S., et al. Natural Polymeric Materials Based Drug Delivery Systems in Lung Diseases. Springer; 2023. Future prospects of natural polymer-based drug delivery systems in combating lung diseases; pp. 465–482. [Google Scholar]
- 167.Gholizadeh O., et al. Therapeutic and diagnostic applications of nanoparticles in the management of COVID-19: a comprehensive overview. Virol. J. 2022;19(1):1–22. doi: 10.1186/s12985-022-01935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Freyn A.W., et al. bioRxiv; 2022. A Monkeypox mRNA-Lipid Nanoparticle Vaccine Targeting Virus Binding, Entry, and Transmission Drives Protection against Lethal Orthopoxviral Challenge. [Google Scholar]
- 169.Su C., et al. A Quadrivalent mRNA immunization elicits potent immune responses against vaccinia and monkeypox viral antigens–a step closer to a broad orthopoxvirus vaccine. bioRxiv. 2023:2023. 04. 23.537951. [Google Scholar]
- 170.Organization W.H. vol. 24. World Health Organization; August 2022. 2022. (Vaccines and Immunization for Monkeypox: Interim Guidance). [Google Scholar]
- 171.Gruber M.F. Current status of monkeypox vaccines. npj Vaccines. 2022;7(1):1–3. doi: 10.1038/s41541-022-00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ranganath N., et al. Mayo Clinic Proceedings. Elsevier; 2022. Monkeypox 2022: gearing up for another potential public health crisis. [DOI] [PubMed] [Google Scholar]
- 173.Dou Y.-M., Yuan H., Tian H.-W. Monkeypox virus: past and present. World Journal of Pediatrics. 2023;19(3):224–230. doi: 10.1007/s12519-022-00618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pardi N., et al. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang R.-R., et al. Emerging Microbes & Infections; 2023. Rational Development of Multicomponent mRNA Vaccine Candidates against Mpox. (just-accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mohamed N.A., et al. Think like a virus: toward improving nanovaccine development against SARS-CoV-2. Viruses. 2022;14(7):1553. doi: 10.3390/v14071553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.da Silva-Júnior E.F. The 2022 Monkeypox outbreak: how the medicinal chemistry could help us? Bioorg. Med. Chem. 2022;73 doi: 10.1016/j.bmc.2022.117036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chakraborty C., et al. Monkeypox virus vaccine evolution and global preparedness for vaccination. Int. Immunopharm. 2022;113 doi: 10.1016/j.intimp.2022.109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vaughan A., et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23(38) doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kidokoro M., Shida H. Vaccinia virus LC16m8Δ as a vaccine vector for clinical applications. Vaccines (Basel) 2014;2(4):755–771. doi: 10.3390/vaccines2040755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.