Abstract
Although host responses to the ancestral SARS-CoV-2 strain are well described, those to the new Omicron variants are less resolved. We profiled the clinical phenomes, transcriptomes, proteomes, metabolomes, and immune repertoires of >1,000 blood cell or plasma specimens from SARS-CoV-2 Omicron patients. Using in-depth integrated multi-omics, we dissected the host response dynamics during multiple disease phases to reveal the molecular and cellular landscapes in the blood. Specifically, we detected enhanced interferon-mediated antiviral signatures of platelets in Omicron-infected patients, and platelets preferentially formed widespread aggregates with leukocytes to modulate immune cell functions. In addition, patients who were re-tested positive for viral RNA showed marked reductions in B cell receptor clones, antibody generation, and neutralizing capacity against Omicron. Finally, we developed a machine learning model that accurately predicted the probability of re-positivity in Omicron patients. Our study may inspire a paradigm shift in studying systemic diseases and emerging public health concerns.
Keywords: COVID-19; Omicron, platelet; blood; plasma; platelet-leukocyte aggregate; re-positive; proteome; metabolome; single-cell RNA-seq
The blood parameters altered by SARS-CoV-2 Omicron infection have not been sufficiently resolved. Wang et al. profile more than 1,000 human blood or plasma samples to generate a multi-omics atlas, revealing unique features of the systemic host response and facilitating the development of screening and therapeutic strategies for Omicron breakthrough infection.
Introduction
The concept of an ecosystem that describes all components and interactions in a defined environment or space has been well-established in earth and ocean ecosystems and is now adapted for use in health sciences, especially in the context of tumors and gut microbes.1 , 2 , 3 , 4 , 5 Circulating blood is the most accessible source of samples that can be used to examine the health condition of a given organism. The blood ecosystem consists of complex and highly orchestrated interactions between distinct blood cells and diverse types of molecules in the plasma. These interactions are coordinated to maintain physiological homeostasis that is often destroyed after infections or other systematic diseases. Multi-omics investigations of the blood ecosystem enable us to capture a more complete picture of these pathological landscapes.6 , 7 , 8 , 9
Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 has resulted in infections in over 600 million people and the cumulative death of over 6 million by October 2022, making it one of the worst pandemics in human history.10 As the most widespread variant of concern (VOC) to date, Omicron has diminished toxicity compared with the ancestral SARS-CoV-2 strain, although its transmissibility is dramatically enhanced.11 , 12 As such, Omicron remains a life-threatening infection for individuals with immune deficiencies or underlying medical conditions that require immune suppressor therapy.13 , 14 However, the dynamics of the molecular and cellular changes across the different disease phases and severities associated with the new variant Omicron have not been clarified.
High percentages of SARS-CoV-2 Omicron patients have been reported to retest positive for viral RNA.15 , 16 This “re-positivity” is a special clinical phenomenon involving the recurrence of detectable viral RNA that is not caused by reinfection or false-positive/false-negative testing.17 , 18 Although re-positivity is an emerging public health concern, the underlying molecular mechanisms and their potential effects on Omicron’s enhanced transmissibility remain largely unknown.15 , 17 There are also no effective strategies to treat re-positive patients or predict those at risk of re-positivity at present.
In this study, we systematically dissected the blood ecosystem of patients with Omicron breakthrough infection across multiple disease phases using multi-omics factor analysis (MOFA) of both the cellular and fluid compartments of peripheral blood. We revealed three categories of time-resolved host responses to provide diverse mechanistic and clinical insights into Omicron infection. The blood ecosystem concept enabled us to unveil the essential roles of platelets as immune modulatory components, suggesting an uncanonical immune landscape during Omicron breakthrough infection. We developed a machine learning model that accurately predicted Omicron patients at risk of having re-positivity. We further showed the potential of antibody or plasma therapy for preventing and treating Omicron re-positive patients. Together, these findings can facilitate the development of both screening and treatment strategies for this emerging public health concern.
Results
Multi-dimensional depiction of the blood ecosystem in Omicron-infected patients
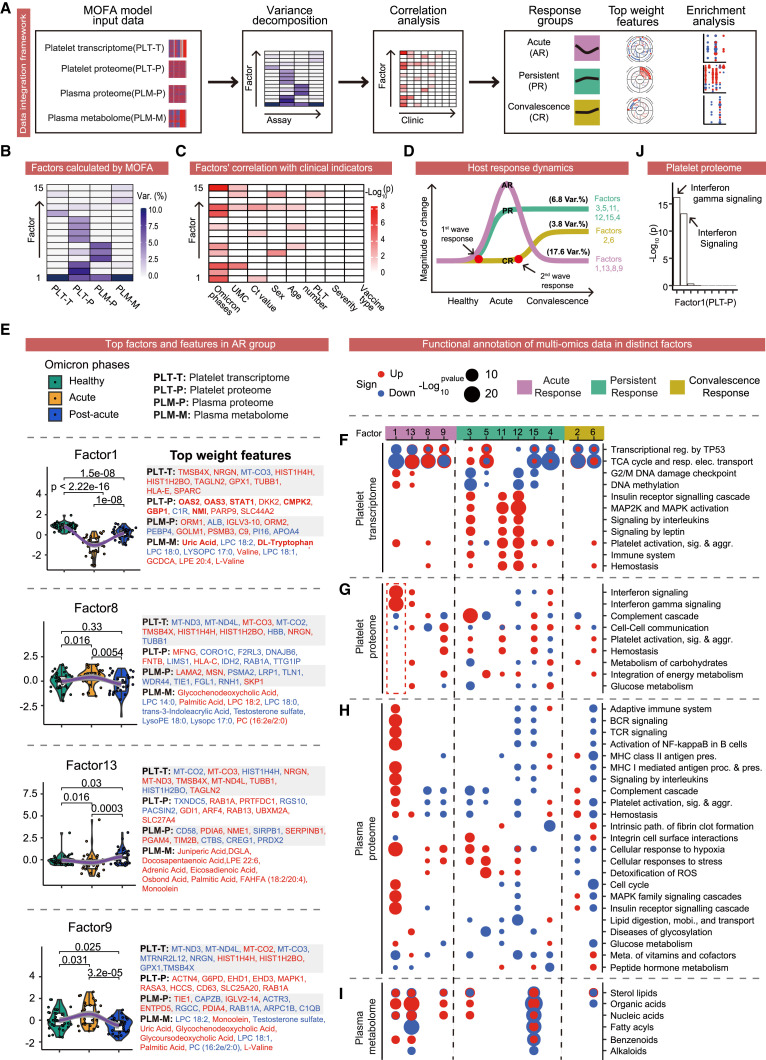
To systematically characterize the molecular and cellular features of patient peripheral blood after SARS-CoV-2 Omicron breakthrough infection, we analyzed the molecular signatures of distinct blood components across different disease phases using multi-omics including (1) clinical phenomes covering laboratory biochemistry, interferon, immune repertoire, and viral RNA PCR tests; (2) bulk omics, including plasma proteome and metabolome and platelet transcriptome and proteome; and (3) single-cell omics, including RNA-seq along with B cell receptor sequencing (BCR-seq) and T cell receptor sequencing (TCR-seq) of peripheral blood mononuclear cells (PBMCs) (Figures 1 A and 1B). Collectively, we have analyzed more than 1,000 specimens and generated a comprehensive molecular and cellular landscape in the blood of SARS-CoV-2 Omicron-infected patients (Figure 1A; Table S1).
Figure 1.
Study design for multi-dimensional dissection of the blood ecosystem in SARS-CoV-2 Omicron patients
(A) Overview of assay modalities and Omicron disease phases analyzed.
(B) Summary of omics measurements, and clinical parameters for patients enrolled in the omics study. The y axis displays patient IDs from XGO_001 to XGO_110, while the x axis shows days since disease onset.
See also Table S1.
In the omics studies, 110 of the 430 first-wave Omicron patients in China were recruited. Most of these patients displayed mild (n = 42) to moderate (n = 67) symptoms, apart from one severe case (n = 1) (Figure 1B). We evaluated the associations among basic clinical factors, including patient age, sex, duration of hospitalization, viral RNA PCR threshold cycle (Ct) value, vaccine type, clinical spectrum, underlying medical conditions, and the incidence of viral RNA PCR test re-positivity. No noticeable confounding factors were detected that may have hindered the subsequent omics analyses (Table S1). Like previous SARS-CoV-2 variant infections, older patients with underlying medical conditions were more likely to have more severe symptoms compared with younger patients (Table S1).
We first assayed the clinical phenomes of the Omicron patients at different disease phases with a focus on clinical laboratory biomarkers that were dysregulated in infections with the ancestral SARS-CoV-2 strain (Figure S1; Table S1).19 , 20 , 21 The serum liver and heart damage biomarkers aspartate aminotransferase (AST), AST/alanine transaminase (ALT) ratio, and lactate dehydrogenase (LDH) were elevated in Omicron patients, whereas ALT and ferritin, which are reported to be highly increased in ancestral strain infections,19 , 20 remained within normal ranges across multiple disease phases (Figures S1A and S1B). Other essential organ markers, including CK and Hs-Tnl (heart) and urea (kidney),19 , 20 were within clinically normal ranges (Figure S1B). Vitamin deficiencies and high serum cortisol levels are associated with severe disease and high mortality in patients infected with the ancestral SARS-CoV-2 strain.22 , 23 , 24 , 25 We observed no changes in serum vitamin B12, folate, and cortisol levels among the phases of disease caused by Omicron infection (Figures S1C and S1D). Although cortisol levels stayed within the clinically normal range, they were elevated across all measured disease phases compared with those of healthy controls, suggesting a minor but enduring suppression of inflammation in the peripheral blood (Figure S1D). High levels of interferons and interferon-stimulated genes (ISGs) were reported in the nasal swabs and bronchoalveolar lavage fluids, but not in the blood, of COVID-19 patients.26 , 27 Consistently, Omicron breakthrough infection patients displayed low serum interferon α and γ levels (Figure S1E). Despite mild patients displaying evidence of less severe respiratory symptoms compared with those with moderate disease, there were no differences in most of the above-mentioned clinical molecular parameters between mild and moderate patients (Figure S1F). Together, the clinical phenome profiles demonstrated that Omicron breakthrough infections are associated with limited systemic inflammation.
Plasma proteome and metabolome analyses reveal platelet-associated dysregulation after Omicron breakthrough infection
We also performed plasma proteomic and metabolomic profiling of Omicron patients and healthy donors and obtained high-quality quantitative data for 546 metabolites and 803 proteins using the TMTpro 16-plex platform (Figure S2A; Tables S1 and S2). In accordance with the results of clinical laboratory tests, there were no significant differences in the metabolic and proteomic data between mild and moderate patients (Figure S2B). However, comparison of the corresponding data for the healthy, acute, and post-acute samples revealed 447 differentially expressed metabolites (DEMs) and 476 differentially expressed proteins (DEPs) within the plasma metabolomes and proteomes (Benjamin-Hochberg [B-H]-adjusted p value < 0.05) (Figures 2 A–2C; Table S2). These DEMs and DEPs were grouped into five and four clusters, respectively. Uniform Manifold Approximation and Projection (UMAP) visualization of the DEMs and DEPs showed high accuracy for classifying and clustering different disease phases (Figures S2C–S2F). Many platelet-associated responses were detected in the different Omicron disease phases, including platelet degranulation, activation, signaling and aggregation, hemostasis, and complement and coagulation cascades (Figures 2C–2E and S2G–S2I; Table S3). Joint analyses of DEMs and DEPs again identified significant associations with pathways including platelet activation, complement and coagulation cascades, and biosynthesis of unsaturated fatty acids (Figures S3A and S3B). Together, plasma proteomic and metabolomic analyses suggest that platelets play critical roles in Omicron breakthrough infections.
Figure 2.
Plasma proteome and metabolome analyses unveil evident platelet-associated dysregulation in Omicron patients
(A) Heatmap of 447 differentially expressed metabolites (DEMs) (ANOVA B-H-adjusted p < 0.05) clustered using mFuzz into five discrete significant clusters.
(B) Super classes enrichment based on the metabolites in (A) using MetaboAnalyst.
(C) Heatmap of 449 differentially expressed proteins (DEPs) (ANOVA B-H adjusted p < 0.05) clustered using mFuzz into four discrete significant clusters.
(D and E) Networks generated by String database analysis using dysregulated proteins in clusters 2 and 3 for (D) and (E), respectively.
See also Figures S2 and S3 and Table S2.
In-depth platelet proteome and transcriptome analyses reveal enhanced immune signatures and reduced thrombosis in Omicron breakthrough compared with ancestral strain infections
Blood clotting is a common complication arising in 20%–30% of critically ill COVID-19 patients and is a frequent cause of COVID-19 death.28 Our plasma proteome and metabolome profiles of Omicron patients exhibited platelet-relevant dysregulations, underpinning a role for platelets in COVID-19 pathogenesis. Nevertheless, there is a paucity of proteomic studies of platelets in COVID-19 patients.29
To dissect the molecular mechanisms underlying the function(s) of platelets in Omicron breakthrough infection, we isolated platelets from COVID-19 patients and matched healthy donors for parallel proteomic and RNA-seq-based transcriptomic profiling (Table S1), resulting in a quantitative analysis of 4,804 platelet proteins using the TMTpro 16-plex platform. To analyze the dynamic expression patterns during various phases of the infection, the 415 DEPs identified were classified into four patterns (clusters 1–4) using Mfuzz (Figure 3 A; Table S3), independent of other factors such as age, sex, disease typing, and re-positivity (Figure 3A). Proteins in cluster 1 were enriched in ATP metabolic process, oxidative phosphorylation, and aerobic respiration (Figure 3B). Cluster 2 proteins were associated with hemostasis (Figures 3A and 3B), and proteins in cluster 3 were mainly involved in epidermal and keratinocyte differentiation (Figures 3A and 3B).
Figure 3.
Deep platelet proteome and transcriptome analyses reveal enhanced immune signatures and reduced thrombosis in Omicron breakthrough compared with ancestral strain patients
(A) Heatmap showing the four dynamic expression patterns of 415 DEPs (B-H-adjusted p < 0.05), clustered using mFuzz, during different phases of Omicron breakthrough infection.
(B) Dot plot showing representative gene ontology (GO) terms enriched in the four clusters.
(C) Boxplot displaying expression levels of proteins related to “response to virus” (GO:0009615) in distinct phases of infection. The y axis shows log2 normalized protein expression. Wilcoxon test: ∗ p ≤ 0.05; ∗∗ p ≤ 0.01; ∗∗∗ p ≤ 0.001.
(D) Boxplots showing expression of IFIT1, IFIT5, and PARP14 in different phases of Omicron breakthrough infection.
(E) Heatmap of differentially expressed genes (DEGs) in platelets from healthy participants and COVID-19 patients with Omicron breakthrough and ancestral SARS-CoV-2 infections (Wilcoxon test B-H-adjusted p < 0.05 and fold change > 2).
(F) Bar plots showing GO terms for platelet DEGs enriched in patients with Omicron breakthrough or ancestral SARS-CoV-2 infections.
(G) Boxplots showing expression of representative DEGs in (E).
(H) Schematic diagram showing the differences in platelets between patients with ancestral COVID-19 and Omicron breakthrough infection.
Cluster 4 proteins, which were rapidly increased before returning to normal levels, displayed characteristic features of antiviral immunity (Figure 3B). This was further supported by the augmented antivirus response score (Figure 3C). This suggests that platelets may play a strong immune-support role during the rapid host responses to Omicron breakthrough infection. Proteins in cluster 4 included: the interferon-induced proteins IFIT1 and IFIT5 (Figures 3D and S3C), which inhibit viral infections by blocking the replication and propagation of viruses,30 and PARP14 (Figures 3D and S3C), an ADP-ribosyltransferase that controls interferon responses and balances proinflammatory cytokines in COVID-19.31 Our proteomic results revealed that platelets might have antiviral ability during acute Omicron breakthrough infections.
We compared the transcriptomic profiles of platelets from healthy donors and patients infected with the ancestral32 or Omicron strains. In accordance with the attenuated clinical phenome, 943 of the 1,083 differentially expressed platelet genes were decreased in Omicron breakthrough infection samples compared with ancestral strain samples (Figure 3E; Table S4). Genes related to the procoagulant features of platelets characterized by hemostasis, platelet activation, and wound healing, such as THBS1, MYL6, and MYL9, were lower during Omicron breakthrough compared with ancestral SARS-CoV-2 infections (Figures 3F and 3G), perhaps providing some explanation for why Omicron breakthrough infections rarely cause severe thrombotic symptoms.
We observed the strongest enrichment in immune system processes with 12% of DEGs higher in Omicron breakthrough compared with ancestral strain patients (p value = 1.83E−08) (Figure 3F), including antigen presentation regulators CD74 33 and HLA-E 34 and the neutrophil-activating chemokine CXCL3 35 (Figures 3F, 3G, and S3D). The interferon-inducible transmembrane (IFITM) genes (including IFITM1/2/3), which were significantly elevated in platelets from patients with ancestral SARS-CoV-2 infections,32 were only slightly increased in platelets during Omicron breakthrough infection (Figures 3G and S3D). Endogenous IFTIMs can be hijacked as cell entry cofactors by SARS-CoV-2 and allow efficient viral replication and transmission to various organs.36 , 37 Thus, the reduction of IFITMs in Omicron compared with ancestral strain patients (Figures 3G and S3D) might represent an enhanced antiviral activity of platelets in Omicron patients. Despite globally attenuated molecular responses (i.e., 943 of the 1,082 DEGs were lower in Omicron vs. the ancestral strain), platelets in Omicron breakthrough infection patients had markedly enhanced immune features compared those with the ancestral strain (Figure 3E).
Taken together, platelets in ancestral COVID-19 strain patients showed enhanced pro-thrombotic characteristics contributing to severe microthrombosis and multiple organ failure. In contrast, platelets in Omicron breakthrough patients exhibited much stronger antiviral immune signatures, implying possible platelet involvement in immune responses during the acute phase of infection (Figure 3H).
Multi-omics integration reveals the landscape of host response dynamics
In a cohort of 342 patients, lymphocyte levels inversely correlated with symptom severity even 1 month after hospital discharge (Figure S1G), suggesting that the physiological impacts of viral infection may persist even after symptoms have cleared.38 Moreover, clinical parameters, such as age and underlying medical conditions, can often distort omics profiles; therefore, unbiased deconstruction of the massive variances in multi-layer omics and extraction of the disease-phase-relevant changes are vital for the accurate depiction of host response dynamics. To accomplish this, we integrated the four omics datasets from 102 specimens via MOFA39 and further dissected the clinical relevance to obtain biological insights (Figure 4 A). Each individual omics dataset explained a substantial percentage of the variances within the 15 top-ranking factors. Factor 1 was functionally explained by all four omics layers, whereas the functions of other factors were explained by more specific omics (Figure 4B). Each individual omics output provided additional and complementary information (Table S5).
Figure 4.
Multi-omics integration reveals the landscape of time-resolved host responses
(A) Workflow of MOFA integrating the four omics datasets.
(B) MOFA deconstructed vast variances into 15 factors in PLT-T, PLT-P, PLM-T, and PLM-M.
(C) Correlation analysis of the top 15 MOFA factors with clinical parameters.
(D) Top 15 MOFA factors were classified into three groups and two waves of host responses. AR, acute response; PR, persistent response; CR, convalescence response; and Var.%, percentage of variances explained.
(E) Differences of factors among distinct Omicron phases (left) along with top-weighted features from the four omics datasets (right) in the AR group. Red font indicates positive weighting, whereas blue font shows negative weighting.
(F–I) Pathway enrichment analyses utilizing the four omics datasets for each factor from the three response groups. Red dots indicate enrichment in positive changes, whereas blue dots show enrichment in negative changes in Omicron patients compared with healthy controls.
(J) Pathway enrichment of the platelet proteome in factor 1. The x axis shows enriched pathways, whereas the y axis shows the −log (10) p value.
We next evaluated the clinical relevance of these factors by correlation with major clinical parameters. Omicron phases were most associated with the top 15 factors, confirming that disease phase was a primary cause of variances in the blood ecosystem (Figure 4C). By comparing the factor values of samples from different Omicron phases, we identified three groups that we classified as the acute response (AR), persistent response (PR), and convalescence response (CR) (Figure 4D). The AR group was dysregulated during the acute phase and quickly recovered in the convalescence phase, explaining 17.6% of the total variances (Figures 4D and 4E). The PR group represents enduring responses that emerged in the acute phase and persisted into the convalescence phase, explaining 6.8% of the total variances (Figures 4D and S4A). The CR group exhibits changes that emerged in the convalescence phase and explained 3.8% of the total variances (Figures 4D and S4A).
We further explored the biological functions of the three groups of disease phase-associated factors in the distinct omics datasets via top-weighted transcript, protein, metabolite (Figures 4E and S4B–S4E), and pathway analysis (Figures 4F–4I). In the AR group, top-weight dysregulated platelet transcripts were related to pathways including the tricarboxylic acid (TCA) cycle and respiratory electron transport, transcriptional regulation by TP53, and DNA methylation, indicating that platelets are involved in preparing the host response to stress early in Omicron breakthrough infection (Figure 4F). In the AR platelet proteome, as expected, gene expression was altered in multiple classic platelet pathways, including platelet activation, signaling and aggregation, and hemostasis (Figure 4G). In contrast to the low serum interferon levels, interferon signaling signatures were highest in factor 1 in the platelet proteome (Figures 4G–4J). Furthermore, six of the top 10 weighted platelet proteins in factor 1 were classic ISGs (i.e., OAS2, OAS3, STAT1, CMPK2, GBP1, and NMI) (Figures 4E and S4C). Interferons are important antiviral cytokines, and early ISG induction in platelets might promote the swift inhibition of viral replication following infection.40 In the AR plasma proteome, we observed broad-scale signatures of adaptive immunity, including TCR signaling, BCR signaling, and complement cascade (Figure 4H), which highlighted the rapid activation of adaptive immunity essential for the efficient inhibition of SARS-CoV-2 viral replication.11 In the AR plasma metabolome, fatty acids were markedly decreased, whereas organic acids, the breakdown products of fatty acids, were elevated. Fatty acid oxidation reduces inflammation.41 (Figure 4I). Additionally, uric acid, the highest weighted metabolite in factor 1, is a reported COVID-19 severity marker, with low levels associated with increased disease severity and death42 (Figures 4E and S4E). The acute elevation of uric acid and other organic acids suggests possible associations among enhanced fatty acid oxidation, reduced inflammation, and better clinical outcomes during acute infection. Similarly, low tryptophan and increased levels of its intermediate metabolites, which have immunosuppressive properties, are associated with more severe COVID-19.43 , 44 Thus, tryptophan, the third highest weight metabolite in factor 1 (Figures 4E and S4E), is likely a good indicator of milder clinical symptoms. Taken together, the AR group had features of reduced inflammation and rapid adaptive immune activation. Furthermore, the boosted interferon-stimulated antiviral signatures in platelets provide a potential molecular mechanism of quickly inhibiting viral replication following infection.
Many of the adaptive immune signatures elevated in the platelet and plasma proteomes in the AR group were lower in the PR group. This is consistent with the widespread metabolic reprogramming (Figures 4G and 4H) and broad metabolite changes observed in the plasma metabolome profiles in factor 15 (Figure 4I). Moreover, many of the altered metabolites were inflammation- or immune-related. For instance, carnitine, which has anti-inflammatory effects,45 was consistently the highest weight-ranked metabolite in factor 15 (Figure S4E). Higher carnitine levels are also associated with lower COVID-19 susceptibility and severity.46 In addition, signatures of cellular responses to stress and hypoxia, and detoxification of reactive oxygen species was evident in the plasma proteome of the PR group (Figure 4H), indicating enduring stress in the host blood ecosystem during convalescence.
Finally, many of the molecular signatures that were dysregulated in the AR or PR group were reversed in the CR group, including organic acids, immune signatures, and platelet signatures (Figures 4G–4I). For instance, peptidylprolyl isomerase A (PPIA), the second highest-ranked plasma protein in factor 6 (Figure S4D), is hijacked by SARS-CoV-2 to support its replication in host cells, and pharmacological inhibition of PPIA restricts viral replication.47 Another top-weight protein in factor 6, TREML1, is known to protect against inflammation-associated hemorrhage.48 Together, our data revealed many characteristic signatures of recovery in the CR group that may serve as markers for monitoring the recovery progress.
In summary, through an advanced multi-layer-omics integration approach, we dissected the vast range of molecular changes during the phases of Omicron infection into three groups and two waves of host responses, providing important insights into the mechanisms underlying the rapid inhibition of viral replication, diminished systematic damage to various organs, and globally attenuated viral toxicity.
The single-cell transcriptome landscape reveals that platelets form aggregates with leukocytes to modulate immune cell function
To dissect the immunological dysregulation among the different severities and phases of Omicron infection, we obtained single-cell transcriptomic profiles of PBMCs derived from patients in the acute, post-acute, and 1-month follow-up stages as well as healthy controls using the 10× Genomics platform. Single-cell BCR and TCR sequencing were also performed (Figure 5 A; Tables S1 and S6). To accurately annotate the types of PBMCs, single-cell data were projected onto the Atlas of Blood Cells (ABC).49 Addition of UMAP visualization of canonical signature genes verified the identities of the PBMCs. We detected 318,359 NK and T cells (characterized by CD3D/IL7R and KLRF1), 44,997 B cells (characterized by MS4A1), 89,456 monocytes (characterized by FCN1), 10,125 platelets (characterized by PF4), and 7,425 neutrophils (characterized by MNDA, CXCR2, and FCGR3B). For subsequent higher resolution analysis, we further divided each cell population into sub-clusters: twelve for NK/T cells, nine for B cells, eight for monocytes, and six for neutrophils, as well as seven clusters of platelets including the platelet-leukocyte aggregates (platelet-naive T, platelet-effector T (Teff), platelet-NK, platelet-B cells, and platelet-monocytes)50 , 51 , 52 , 53 , 54 , 55 (Figures 5B and S5A–S5C; Table S7).
Figure 5.
Single-cell transcriptome landscape of peripheral blood cells in Omicron patients
(A) Schematic diagram showing sample information for single-cell RNA-seq coupled with scBCR-seq and scTCR-seq analyses of frozen PBMCs and fresh neutrophil extraction.
(B) UMAP plots displaying the clusters of the total of 462,937 frozen PBMCs (left) and PBMCs from healthy donors and mild and moderate patients (right). Colors indicate cell determined by unsupervised Leiden clustering.
(C) Variation in cellular composition measured by log2 (fold-change) for each cluster of cells comparing Omicron patients to healthy donors across the acute, post-acute, and follow-up phases.
(D) Variation in cellular composition for each cluster of neutrophils from mild- and moderate-severity infectious patients.
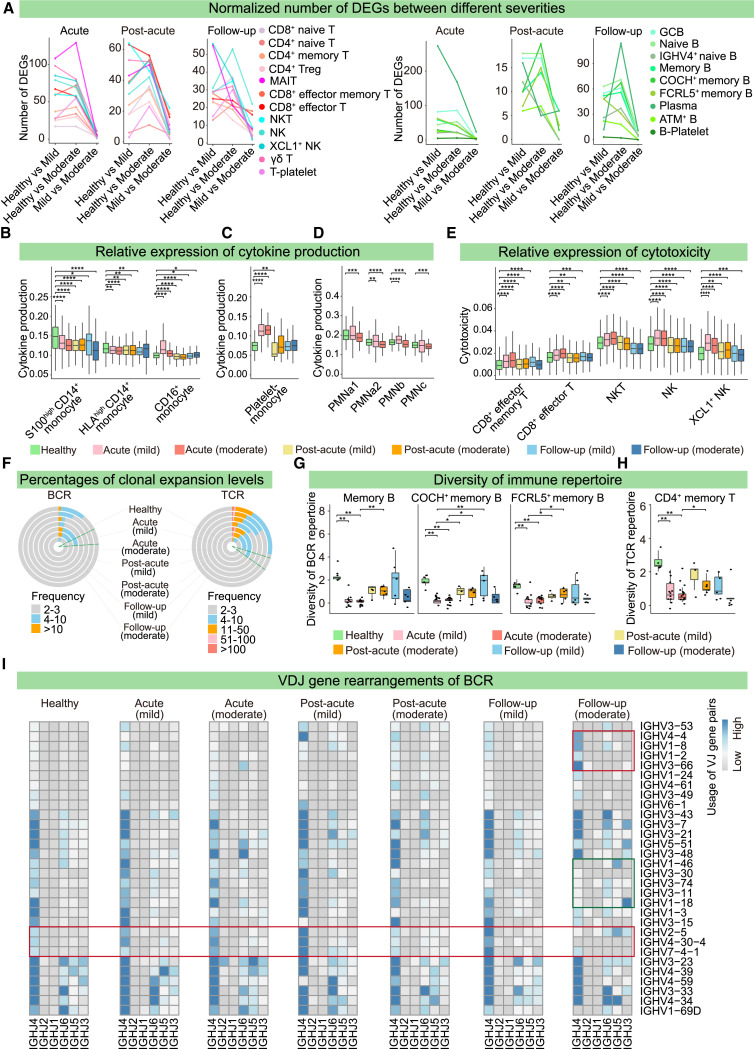
(E) Normalized number of DEGs between the two phases for NK and T cells, B cells, monocytes, and platelets in mild (top) and moderate (bottom) patients. For each cell cluster, the number of DEGs was scaled by (X-min, max-min) + 1.
Compared with healthy donors, the cellular composition of PBMC from Omicron patients fluctuated dramatically (Figures 5C and5D). Specifically, the decreased number of naive T cells and increased abundance of monocytes in the acute phase were consistent with previously reported phenomena in COVID-19 cases.56 CD8+ Teff, MAIT, and γδ T cells maintained at normal levels after infection, which might contribute to the attenuated clinical severity in Omicron patients.57 , 58 Only a proportion of cell clusters, such as naive CD4+ T and XCL1 + NK cells, recovered in the post-acute and follow-up phases. In contrast, naive B and memory B cells recovered in mild patients, but not in moderate patients. In addition, we found that platelets preferentially formed aggregates with other blood cells, displaying both platelet- and other blood cell-related gene signatures (Figure S5C and S5D). The number of platelet-leukocyte aggregates increased in the acute phase, which is consistent with the described activation of platelets in COVID-19 pathophysiology.32 , 59 The decreased platelet-leukocyte aggregates in the post-acute phase re-accumulated in the follow-up phase (Figure 5C). Our data suggest that PBMCs, especially memory B cells and platelet-leukocyte aggregates, had not returned to normal levels by the 1-month follow-up. Next, we found that the transcriptomes of NK and T cells and monocytes gradually recovered from the post-acute phase to the follow-up phase (Figure 5E). Although the transcriptomes of B cells and platelets approached a healthy state in the post-acute phase, transcriptomic fluctuation recurred in B cells in the follow-up phase (Figure 5E). In summary, these data suggested that both the cellular quantity and the molecular function in B cells, along with the abundance of platelet-leukocyte aggregates, were still abnormal in the follow-up phase.
The aggregates that formed between platelets and various immune cells suggest that platelets may regulate immune responses against Omicron in the form of platelet-leukocyte aggregates. To verify this hypothesis, we first measured the interactions between platelets and immune cells ex vivo (Figure S5E). We confirmed an increase in platelet-leukocyte aggregates in Omicron patients with dynamic changes observed among the disease stages, which was consistent with the single-cell analysis results (Figures 5C and S5F). Furthermore, the dynamics of platelet-leukocyte aggregation were not associated with leukocyte counts (Figure S5G) and thus likely not driven by the changes in the quantity of immune cells. To further define the functional relevance of these interactions, we also co-cultured leukocytes with platelets isolated from healthy donors and Omicron samples (Figure S5H). Since platelet-neutrophil, platelet-monocyte, and platelet-NK aggregates have been investigated previously in COVID-19,60 , 61 , 62 we selected T cells as representative leukocytes for the platelet co-culture assay. Instead of enhancing the immune properties, platelets from Omicron patients at different disease stages, but not those from healthy donors, inhibited T cell activation, as supported by the decrease in expression of the activation marker CD69 in CD3+, CD4+, and CD8+ T cells (Figure S5I). We further compared the molecular profiles of Teff cells with or without platelet interactions in the single-cell sequencing data. Similarly, platelet interaction inhibited T cell cytotoxicity gene expression signatures (Figure S5J). Thus, our findings suggest that platelets modulate T cell function by aggregate formation. Overall, we reveal that platelets preferentially form aggregates with leukocytes to modulate immune cell functions, suggesting an uncanonical immune landscape during Omicron breakthrough infection.
Immune responses to Omicron variant at single-cell resolution
The number of DEGs between patients with mild and moderate disease severity revealed reduced transcriptomic differences in NK and T cells, B cells, monocytes, and platelets from each phase, which contrasted the differences between healthy donors and patients (Figures 6 A and S6A). This suggested that unlike the distinct effects seen in mild and moderate cases of ancestral SARS-CoV-2,63 responses to the Omicron variant were comparable between mild and moderate patients. We also observed a greater transcriptomic difference between the neutrophils of healthy donors and mild patients (Figure S6B), indicative of the sensitive response of neutrophils in mild patients.
Figure 6.
Immune responses to Omicron variant at single-cell resolution
(A) Number of DEGs in NK and T cells (left) and B cells (right) from mild- and moderate-severity infection patients in the acute, post-acute, and follow-up phases.
(B–E) Relative expression gene of cytokines in CD14+ and CD16+ monocytes (B), platelet-monocytes (C), and neutrophils (D), and cytotoxicity-related genes in CD8+ Teff and NK cells (E) from healthy donors and mild and moderate patients during the acute, post-acute, and follow-up phases. Wilcoxon test: ∗ p ≤ 0.05; ∗∗ p ≤ 0.01; ∗∗∗ p ≤ 0.001; ∗∗∗∗ p ≤ 0.0001.
(F) Percentages of different clonal expansion levels for BCR (left) and TCR (right) across seven severity and stage conditions.
(G and H) Diversities of BCR repertoires in memory B cells (G) and TCR repertoires in memory T cells (H) across the seven conditions. Diversity was evaluated as Shannon entropy = ΣP∗log2(1/P), in which P represents the frequency of a given BCR and TCR clone among that of all BCR and TCR clones. Wilcoxon test: ∗ p ≤ 0.05; ∗∗ p ≤ 0.01; ∗∗∗ p ≤ 0.001; ∗∗∗∗ p ≤ 0.0001.
(I) Heatmap showing the VDJ gene rearrangements of BCRs across the seven conditions. Red and green rectangles indicate biased and declined usage, respectively.
In accordance with known COVID-19 phenomena,51 , 64 neutrophils, monocytes, and platelets showed high cytokine, inflammation, and interferon gene signature expressions in Omicron patients (Figure S6C). Unlike the increased inflammatory signatures in both CD14+ and CD16+ monocytes of ancestral SARS-CoV-2-infected patients,50 , 65 only CD16+ monocytes displayed a high expression of cytokine/interferon signatures in the Omicron acute phase, suggesting the transcriptomic response to the Omicron variant was attenuated (Figures 6B and S6D). The cytokine, inflammation, and interferon signature expressions were elevated in platelet-monocytes in the acute phase and were subsequently reduced in the post-acute and follow-up phases (Figures 6C and S6E). We also observed that the unique molecular identifier (UMI) levels of cytokine and interferon signatures were enhanced in neutrophils of mild patients but were lower in moderate patients, further confirming the unique sensitivity of the neutrophil response in mild patients (Figures 6D and S6F). Furthermore, effector CD8+ T cells and NK cells exhibited higher cytotoxicity signatures in acute infectious patients before gradually decreasing in the post-acute and follow-up phases (Figure 6E).
Single-cell BCR-seq (scBCR-seq) and single-cell TCR-seq (scTCR-seq) were also conducted to dissect the variation in the immune repertoires of Omicron patients. First, we observed that the complementarity-determining region 3 (CDR3) lengths of BCR heavy chain and TCR β chain were increased in acute phase patients compared with those of healthy donors (Figure S6G), and such increases might give rise to the polyreactivity and autoimmunity of antibodies.66 Moreover, the percentages of BCR and TCR detected in different subtypes of B cells and T cells in the acute phase were higher than those in the post-acute phase (Figure S6H). Thus, we analyzed the clonal expansion across different disease statuses. Clones expanded in the acute phase were subsequently diminished in the post-acute phase but re-emerged in the follow-up phase, especially in moderately symptomatic patients (Figure 6F). To verify this, BCR and TCR diversities were evaluated by Shannon entropy. We observed that the diminished BCR and TCR diversities of memory B and T cells in the acute phase recovered in the post-acute phase but were reduced again during the follow-up phase for moderate patients (Figures 6G, 6H, S6I, and S6J). Finally, the VDJ gene rearrangements for BCRs indicated that mild patient B cells preferentially expressed IGHV2-5, 4-30-4, and 7-4-1. In contrast, moderate patients in the follow-up phase exhibited biased usage of IGHV4-4, 1-8, 1-2, and 3-66, with less frequent usage of IGHV1-46, 3-30, 3-74, 3-11, and 1-18 (Figure 6I). These findings provide preliminary clues for the optimum design of vaccines against Omicron.
Together, the immune responses of B and T cells are strongly activated in the acute phase, but diminish rapidly, which differs from the high-immunity reactions reported during acute and convalescent phases of ancestral SARS-CoV-2-infected patients.50 Immune responses were re-activated in moderate patients after discharge from the hospital (Figures 6F–6H, S6I, and S6J), implying patient-to-patient variability in Omicron immunity.
Re-positive patients exhibit diminished BCR clones, lower antibody levels, and reduced Omicron-neutralizing capacity
Considering the high percentage of re-positivity in Omicron patients, we attempted to explain the mechanism of this phenomenon in terms of cellular composition, biological processes, and immune repertoires. Although the abundance of most cell clusters remained stable, effector memory CD8+ T cells and XCL1 + NK cells were greatly depleted, accompanied by increased naive B cells (Figure 7 A). These observations were supported by decreased cytotoxic and suppressor T cell counts in 342 routine blood samples (Figure S7A), which may have resulted in impaired immune responses in re-positive patients.
Figure 7.
Machine-learning models accurately predict re-positive patients with decreased immunity
(A) Variation in cellular composition in each cluster of cells from re-positive patients (RPs) and non-re-positive patients (NRPs).
(B–G) Relative expression of cytokine production in CD14+ and CD16+ monocytes (B), platelet-monocytes (C), and neutrophils (D), cytotoxicity-related genes in CD8+ memory Teff, CD8+ Teff, and NK cells (E), exhaustion-related genes in CD8+ Teff and NK cells (F), and apoptosis signaling pathway-related genes in CD8+ memory Teff and NK cells (G) from healthy donors, RP and NRP. Wilcoxon test: ∗ p ≤ 0.05; ∗∗ p ≤ 0.01; ∗∗∗ p ≤ 0.001; ∗∗∗∗ p ≤ 0.0001.
(H) Percentages of different clonal expansion levels for BCR (top) and TCR (bottom) in healthy donors, RP and NRP.
(I and J) Boxplot showing levels of antibodies against Omicron spike (S) protein (I) and the inhibition effects of neutralization antibodies (J) in RP and NRP, as detected by ELISA assay.
(K) Workflow of the machine-learning models, and performance of the model in a test cohort of 26 Omicron patients.
(L–N) Top four clinical indicators (L), top four metabolites (M), and top 10 proteins (N) prioritized by random forest analysis and ranked by the Gini index.
(O) Importance of three types of features, as determined by combined coefficient of the model.
(P and Q) Boxplot displaying the abundance of four metabolites (P) and four representative proteins (Q) in the RP and NRP groups in the training cohort.
See also Figure S7.
We also observed wider transcriptomic variation in the peripheral blood cells of re-positive patients (Figure S7B). To ascertain the factors leading to this dysregulation, we investigated the expression of inflammation, cytokine, and interferon signatures. In re-positive patients, we found that CD16+ monocytes, platelet-monocytes, and neutrophils exhibited higher inflammation, cytokine, and interferon signature expressions (Figures 7B–7D and S7C–S7E). However, CD8+ Teff cells showed reduced cytotoxic signatures and increased exhaustion signals (Figures 7E and 7F). NK cells from re-positive patients displayed a higher expression of cytotoxicity signatures and low levels of apoptosis signatures (Figures 7E–7G), suggesting that these cells may compensate for dysregulated CD8+ Teff cells in re-positive patients.
Expanded BCR clones were diminished in re-positive patients, along with slightly increased BCR and TCR repertoire diversities (Figures 7H, S7F, and S7G). Re-positive patients exhibit a diminished generation of antiviral antibodies,17 but the underlying molecular mechanisms remain unknown. Our observation of diminished BCR clones provides a potential mechanistic explanation for the compromised humoral immunity in re-positive patients. To test this hypothesis, we measured the levels and neutralizing capacity of serum antibodies in individuals with or without re-positivity and confirmed that patients with re-positivity had lower antibody levels and neutralizing capacity against Omicron compared with non-re-positive patients (Figures 7I and 7J)
Re-positivity of patients has raised serious issues impairing strategies to fight the highly transmissible Omicron strain. Therefore, methods that can predict patients at high risk of re-positivity are urgently needed. We developed a machine learning model integrating clinical indicators, plasma proteomes, and metabolome data that predict the probability of re-positivity. Machine learning models were developed using random forests (Figure 7K). Based on the training sets, feature selection was conducted, leaving four clinical characteristics (information gain > 0), four metabolites (analysis of variance [ANOVA] p value < 0.001), and 10 proteins (top 10 most important proteins selected by random forest) (Figures 7L–7N). We then built three single-class random forest models, and their performances in analyzing the test set are summarized in Figure 7K. The clinical, metabolomic, and proteomic models achieved area under the receiver operating characteristic curve (AUC) scores of 0.674, 0.659, and 0.812, respectively (Figure 7K). Next, we set the threshold as the ratio of re-positive cases in the overlapped training set of 35 samples, and the three single-class models were integrated to form our ensemble logistic model, which achieved better performance (AUC = 0.855) (Figures 7K–7O). The proteomic model was further validated using another independent cohort of 11 Omicron patients (Test set 2) and achieved an AUC of 0.857 (Figure 7K).
Four clinical characteristics (nucleic_acid_result_CT35, sex, comorbidities, and disease severity), four plasma metabolites (Lysopc 18:3, (±)19(20)-DiHDPA, deoxycholic acid, and 5-ketogluconic acid), and 10 plasma proteins (PLTP, immunoglobulin kappa variable 1D-39 [IGKV1D-39], CPB1, LCP1, EIF5A, CLIC1, collectin-10, lambda variable 3-16 [IGLV3-16], and lipopolysaccharide-binding protein [LBP], and endoplasmic reticulum aminopeptidase 1 [ERAP1]) were selected for our machine learning models (Figures 7P, 7Q, and S7H). A network built via ingenuity pathway analysis showed that these selected molecular biomarkers were highly interconnected (Figure S7I). Based on our proteomic and ensemble models, only one re-positive patient from Test cohort 1 (XGO_73) was mispredicted. XGO_73 was a 60-year-old mild-severity patient, and the results were probably affected by the drugs the patient received to treat hypertension. In addition, one 63-year-old moderate patient in Test set 1 (XGO_78) was incorrectly predicted to be re-positive, possibly due to the long-term comorbidities of grade 2 hypertension, type 2 diabetes,67 abnormal liver function, and hyperlipidemia. In Test cohort 2, all non-re-positive patients were predicted accurately, whereas four re-positive patients were incorrectly predicted as non-re-positive. Possible reasons for this inaccuracy might be that two of these patients were among the youngest and had the lowest-severity clinical spectra (i.e., asymptomatic and mild), whereas the other two patients had underlying medical conditions and were on drug therapies for diabetes and hepatitis B. Despite these mispredictions, our prediction model accurately predicted individuals with re-positivity in two independent cohorts.
In summary, these results indicated that re-positivity was likely caused by an attenuated immune response featuring: fewer effector memory T cells; enhanced CD16+ inflammatory signatures in monocytes, platelet-monocyte aggregates, and neutrophils; a decline in cytotoxicity; and increased effector CD8+ T cell exhaustion. The diminished BCR clones, compromised virus-specific antibody generation, and lower antibody-neutralizing capacity in Omicron re-positive patients imply that antibody or plasma therapy could be used to prevent and treat re-positivity.68 , 69 We further developed a machine-learning model that accurately predicts re-positivity in Omicron patients.
Discussion
SARS-CoV-2 Omicron, which exhibits dramatically enhanced transmissibility, is a life-threatening global health issue for individuals with immune deficiencies.13 , 14 The high percentage of re-positivity in Omicron patients is an emerging problem, particularly as we have a limited mechanistic understanding of the phenomenon. Although previous multi-omics studies of COVID-19 patients have investigated factors such as disease severity, specificity, and therapeutic targets, omics studies of host response dynamics across different disease phases are still lacking. To fill this knowledge gap, we adapted the ecosystem concept from health and gut microbe science and dissected the blood ecosystems of Omicron patients across multiple disease phases utilizing a powerful and integrative multi-omics strategy.
We revealed that interferon-mediated antiviral signatures were dramatically boosted in platelets in Omicron-infected patients, whereas serum interferon levels remained low. It is possible that these platelets are recruited to the sites of virus-host interactions via the bloodstream, thus aiding rapid virus clearance. Moreover, we discovered that platelets preferentially formed aggregates with numerous immune cells and verified that platelet interactions inhibited T cell activation. We speculated that this platelet-driven T cell inhibition serves as a “brake” to avoid overreaction by immune cells, thereby attenuating the clinical symptoms of Omicron patients. Platelets that inhibit T cell activation were present in the bloodstream as platelet-leukocyte aggregates that are distinct from the platelets with enhanced interferon-mediated antiviral signatures detected by platelet proteomic analysis because platelet-leukocyte aggregates were removed during proteomic sample preparation. These results highlight the possible functional heterogeneity of platelets during Omicron host responses. Together, these intriguing findings demonstrate that platelets play more essential immunomodulatory roles than originally thought in the blood ecosystem during host responses to Omicron.
Beyond the numerous mechanistic insights, we also gained many valuable clinical insights into potential therapeutic targets and biomarkers as well as disease management and neutralizing antibody development strategies for COVID-19. For instance, evidence suggests that IFTIM family proteins, which are required for efficient viral replication and transmission to various organs, may serve as cofactors for the entry of SARS-CoV-2 into cells, thereby escalating the clinical symptoms of COVID-19.36 , 37 Silencing endogenous IFITM proteins (especially IFITM2) prevented productive infection of human lung cells by several SARS-CoV-2 VOCs.37 Similarly, our data revealed PPIA as a top-weighted protein associated with Omicron. Reportedly, PPIA in host cells can be hijacked by SARS-CoV-2 to support viral replication, which is restricted by the pharmacological inhibition of PPIA.47 Therefore, functional assays targeting these proteins might provide clues to potential new methods of inhibiting SARS-CoV-2 infections. Moreover, we identified a list of proteins and metabolites that can be used to identify patients in the acute and post-acute disease phases; thus, these DEMs and DEPs could potentially be employed in monitoring Omicron disease processes. Additionally, we extracted multiple top-weight molecules that are closely associated with different aspects of SARS-CoV-2 via MOFA-based multi-omics integration. These top-weight molecules (e.g., uric acid, tryptophan, carnitine, and TREML1) may serve as biomarkers for assessing the types of host responses and the progress of disease recovery or predicting disease outcomes.
In a recent study, no broad-spectrum antibodies against new Omicron variants were isolated from patients in the post-acute phase.70 However, the rapid diminution of BCR clones in the convalescent phase strongly suggests that it might be more suitable to identify neutralizing antibodies for COVID-19 in acute infectious than convalescent patients. Moreover, although the responses to Omicron decreased in convalescent patients, the immune repertoires had not completely returned to normal by the 1-month follow-up. These data suggest that attention needs to be paid to populations with weak immunity after discharge from the hospital. Moreover, deciphering the nature of immunopathological dysregulation after discharge could have crucial implications in the detection and treatment of post-COVID-19 sequelae.71
Finally, a high incidence of patients with SARS-CoV-2 Omicron RNA re-positivity has been widely reported.16 , 18 , 72 , 73 , 74 Although one study suggested that the viability and transmissibility of re-positive viral RNA may be diminished,16 it was also reported that re-positive patients can have active viral replication, possibly resulting in active viral shedding.17 The molecular mechanisms underlying the phenomenon of re-positivity remain unknown, and their potential effect on the markedly enhanced transmissibility of Omicron is still open to debate. We found that re-positivity is probably caused by an attenuated immune response. The drastically diminished BCR clone counts explain the significantly compromised virus-specific antibody generation reported in re-positive patients,17 , 75 implying that antibody or plasma therapy is a potential solution to the problem of preventing and treating re-positivity.68 , 69 Multiple studies have attempted to predict the likelihood of patients becoming re-positive using clinical parameters, but without success.16 , 74 In this study, we discovered a panel of plasma molecular biomarkers using machine-learning models that accurately predict re-positivity. These biomarkers are closely linked to host-immune responses. For instance, LBP, collectin-10, and ERAP1 are associated with innate immune responses, especially the acute phase immunologic response.76 IGKV1D-39, IGLV3-16, and ERAP1 participate in antibody secretion and humoral immunity. Network analysis further showed that these biomarkers are highly interconnected and interact with other features through major signaling hubs and master regulators, including AKT, NF-κB (complex), ESR1, and TNF, during host-immune responses. These highly interconnected features provide important insights into the potential mechanisms of re-positivity. Together, these findings will be valuable for developing screening and treatment strategies for this emerging public health concern.
Limitations of the study
The observed changes between Omicron breakthrough and ancestral strain infections may have been inevitably affected by other factors, such as vaccination, as most Omicron patients have been vaccinated. Therefore, these changes may reflect the combinatorial consequences of other factors besides the evolution of SARS-CoV2 virus, including vaccination and re-infection. Test data 2 used to validate the re-positivity prediction model were obtained from a retrospective cohort, and plasma metabolite quality was probably compromised by long-term storage (i.e., >6 months) and multiple freeze-thaw cycles, particularly as metabolites are generally less stable than proteins. These limitations may explain the non-optimal performance of the metabolomic panel for Test data 2. Hence, independent cohorts of high-quality specimens are required to further confirm the validity of the prediction model. Despite our systematic investigation of the roles of platelets in the bloodstream in the present study, further association analyses with the molecular and cellular features of swab samples were not conducted because of the lack of specimen availability. It would be interesting to assess, for instance, if and how interactions between platelets and immune cells in the bloodstream affect local host responses in the airways in future studies.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-human-CD41 BV421 (clone HIP8) | Biolegend | Cat#303730 RRID: AB_2629627 |
| Anti-human CD42b PE (clone HIP1) | BD Bioscience | Cat#555473 RRID: AB_395865 |
| Anti-human-CD3 FITC (clone UCHT1 ) | Biolegend | Cat#300440 RRID: AB_2562046 |
| Anti-human-CD4 PE/Cyanine7 (clone RPA-T4 ) | Biolegend | Cat#300512 RRID: AB_314080 |
| Anti-human-CD8 APC (clone RPA-T8) | BD Bioscience | Cat#555369 RRID: AB_398595 |
| Anti-human-CD69 PE (clone FN50) | BD Bioscience | Cat#555531 RRID: AB_395916 |
| Anti-human-CD11b PE/Cyanine7 (clone ICRF44) | Biolegend | Cat#301322 RRID: AB_830644 |
| Anti-human-CD56 APC (clone 5.1H11) | Biolegend | Cat#362504 RRID: AB_2563913 |
| Anti-human-CD19 APC (clone 4G7) | Biolegend | Cat#392504 RRID: AB_2728416 |
| Anti-human-CD14 APC/Cyanine7 (clone HCD14) | Biolegend | Cat#325620 RRID: AB_830693 |
| Biological samples | ||
| Multi-layers specimens from1188 patients and 47 healthy individuals | This paper | This paper (Table S1) |
| Fetal Bovine Serum (FBS) | Gibco | Cat#F8318 |
| Chemicals, peptides, and recombinant proteins | ||
| Prostaglandin E1 | Sigma-Aldrich | Cat#P7527 |
| Tyrode's solution | Solarbio | Cat#T1420 |
| Phosphate Buffer Saline (PBS) | Beyotime | Cat#C0221A |
| RNase-free water | Beyotime | Cat#ST876 |
| TRIzol | Invitrogen | Cat#15596026 |
| Red blood cell lysis buffer | Beyotime | Cat#C3702 |
| RPMI-1640 medium | Sigma-Aldrich | Cat#R7388 |
| Ficoll–Paque solution | TBDsciences | Cat#LTS1077 |
| Acetonitrile (ACN) | Thermo Fisher Scientific | Cat#A955-4 |
| Dimethylsulfoxide (DMSO) | Thermo Fisher Scientific | Cat#85190 |
| Urea | Sigma-Aldrich | Cat#U6504 |
| Triethylammonium bicarbonate buffer (TEAB) | Sigma-Aldrich | Cat#T7408 |
| Tris (2-carboxyethyl) phosphine (TCEP) | Adamas-beta | Cat#61820E |
| Bovine Serum Albumin (BSA) | Solarbio | Cat#9048-46-8 |
| Dithiothreitol | Sigma-Aldrich | Cat#D5545 |
| Iodoacetamide (IAA) | Sigma-Aldrich | Cat#16125 |
| Trypsin | Hualishi Tech | Cat#TRY001C |
| Lys C | Hualishi Tech | Cat#LYS001C |
| Trifluoroacetic acid (TFA) | Thermo Fisher Scientific | Cat#85183 |
| Formic acid (FA) | Thermo Fisher Scientific | Cat#A117-50 |
| Ammonium hydroxide solution | Sigma-Aldrich | Cat#221228 |
| Methanol | Sigma-Aldrich | Cat#348601 |
| Buffer EB | Qiagen | Cat#19086 |
| Low TE buffer | Thermo Fisher Scientific | Cat#12090-015 |
| Recombinant B.1.1.529 (Omicron) Spike S1+S2 protein | Sino Biological | Cat#40589-V08B33 |
| Recombinant SARS-CoV-2 Spike Neutralizing Antibody | Sino Biological | Cat#40591-MM48-100 |
| Critical commercial assays | ||
| VAHTS mRNA-seq V3 Library Prep Kit | Vazyme | Cat#NR611 |
| Human CD3 MicroBeads | Miltenyi Biotec | Cat#130-050-101 |
| SPRI select beads | Beckman Coulter | Cat#B23317 |
| High Sensitivity DNA Kit | Agilent | Cat#5067-4626 |
| Dead cell removal kit | Miltenyi Biotec | Cat#130-090-101 |
| Multitest 6-Color TBNK CD3/ CD16+CD56/CD45/CD4/CD19/CD8 reagent |
BD Bioscience | Cat#644611 |
| TMTpro 16plex reagents | Thermo Fisher Scientific | Cat#A44520 |
| Human affinity depletion resin | Thermo Fisher Scientific | Cat#A36372 |
| 3K MWCO filtering unit | Thermo Fisher Scientific | Cat#88512 |
| Chromium single-cell 5′ library kit | 10xGenomics | Cat#1000263 |
| Chromium Single Cell V(D)J Enrichment Kit | 10×Genomics | Cat#1000252 Cat#1000253 |
| SARS-CoV-2 (2019-nCoV) Spike Detection ELISA Kit | Sino Biological | Cat#KIT40591 |
| New Coronavirus Nucleic Acid Detection kit | Biogerm | Cat#20220516M |
| New Coronavirus Nucleic Acid Detection kit | Zybio | Cat#2210113 |
| New Coronavirus Nucleic Acid Detection kit | Liferiver | Cat#P20220149 |
| Deposited data | ||
| Platelet bulk RNA-seq data | This paper | National Omics Data Encyclopedia:OEP003718 and OEP003719 |
| All single-cell sequencing data | This paper | National Genomics Data Center: HRA003738 |
| Mass spectrometry data | This paper | iProX:IPX0004421000 |
| Data analysis codes | This paper | Zenodo:1188465 |
| Platelet RNA-seq reference data | Manne et al.32 | NCBI short-read archives:PRJNA634489 |
| Human reference genome NCBI build 38, GRCh38v22 | Genome Reference Consortium | http://www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/human/ |
| Kyoto Encyclopedia of Genes and Genomes (KEGG) | Kanehisa et al.77 | http://www.genome.jp/kegg/ |
| Human Metabolome Database (HMDB) | Wishart et al.78 | https://hmdb.ca/downloads |
| LIPID MAPS® Structure Database (LIPIDMaps) | Sud et al.79 | https://www.lipidmaps.org/ |
| Software and algorithms | ||
| Cellranger v6.1.2 | 10x genomics | https://support.10xgenomics.com/ |
| STAR v2.6.1d | Dobin et al.80 | https://github.com/star7th/showdoc/releases/tag/v2.6.1 |
| MetaboAnalyst v5.0 | Pang et al.81 | https://www.metaboanalyst.ca/ |
| Seurat v4.0.3 | Hao et al.82 | https://satijalab.org/seurat/ |
| Scanpy v1.5.1 | Wolf et al.83 | https://pypi.org/project/scanpy |
| Scrublet v0.2.1 | Wolock et al.84 | https://github.com/swolock/scrublet |
| Mfuzz v2.46.0/v2.54.0 | Bioconductor | http://mfuzz.sysbiolab.eu/ |
| Gene Ontology | GO Consortium | http://www.geneontology.org/ |
| Reactome | The Reactome project | http://www.reactome.org/ |
| Ingenuity Pathway Analysis | QIAGEN Digital Insights | https://www.qiagen.com/cn/ |
| Compound Discoverer v3.1 | ThermoFisher | https://www.thermofisher.cn/order/catalog/product/en/zh/OPTON-31055 |
| Proteome Discoverer v2.4.0.305 | Thermo Fisher Scientific | https://www.thermofisher.cn/order/catalog/product/cn/zh/OPTON-31101 |
| mzCloud | Thermo Fisher Scientific | https://www.mzcloud.org/ |
| Xcalibur | Thermo Fisher Scientific | Cat#OPTON-30965 |
| MOFA model | Argelaguet et al.39 | https://github.com/jcparkgithub/multiomics_for_mtpad_mipad |
| R v3.6.3/v3.4.3 | Comprehensive R Archive Network | https://cran.r-project.org/ |
| Python v2.7 | Python Software Foundation | https://www.python.org |
| FlowJo v10 | TreeStar | https://www.flowjo.com |
| GraphPad Prism v8.0 | GraphPad Software | https://www.graphpad.com |
| Other | ||
| Vanquish UHPLC system | Waters Corporation | Cat#186015001 |
| Orbitrap Q Exactive HF-X | Thermo Fisher Scientific | Cat#IQLAAEGAAPFALGMAZR |
| U3000 liquid chromatograph | Thermo Fisher Scientific | Cat#IQLAAAGABHFAPBMBFC |
| Orbitrap Exploris 480 | Thermo Fisher Scientific | Cat#BRE725539 |
| Hypersil GOLD column | Thermo Fisher Scientific | Cat#25003-032130 |
| ACQUITY BEH C18 column, 2.1 × 100 mm, 1.7 μm | Waters Corporation | Cat#186008316 |
| ACQUITY BEH Amide column, 2.1 × 100 mm, 1.7 μm | Waters Corporation | Cat#186008315 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Tao Cheng (chengtao@ihcams.ac.cn).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
Omicron patients in the acute and post-acute phases were recruited from Tianjin Haihe Hospital and Tianjin First Central Hospital respectively, and healthy donors were recruited from the Institute of Hematology and Blood Disease Hospital (Table S1). Sample collection, pre-processing, and laboratory operations were approved by the Ethics Committee for the Haihe Laboratory of Cell Ecosystem (ethical approval number: HHL2022005-EC-1), and written informed consent was obtained from all participants before enrolment.
Method details
Sample collection and processing
Peripheral blood samples (5 mL and 20 mL) were collected from healthy donors and Omicron patients who gave informed consent. The 5-mL peripheral blood samples were centrifuged at 300 x g for 10 min with a rise-and-fall speed of grade 5. The plasma supernatant was collected in a new tube, and the centrifugation step was repeated to completely remove other blood cells. The platelet-enriched plasma was then transferred to a new tube for centrifugation at 800 x g for 10 min. The plasma supernatant was transferred to new tubes for metabolome and protein extraction; 850 μl of cold ACN was added to 200 μl of the above-mentioned supernatant to precipitate plasma proteins for proteome analysis, while the remaining supernatant was used for metabolomic profiling. The platelet sediment was resuspended in Tyrode's solution containing 1 mM prostaglandin E1 and spun again (800 x g for 10 min), followed by RNA extraction and protein digestion. All Omicron sample-processing steps were conducted in a biosafety level 2+ laboratory.
To process the 20-mL peripheral blood samples, 1 mL fresh blood was mixed with red blood cell lysis buffer at a ratio of 1:9 for 10 min, followed by centrifugation at 300 × g for 5 min without braking. After removing the supernatant, the lysis and centrifugation steps were repeated to completely remove red blood cells. Then, the cells were resuspended in PBS containing 0.04% BSA and submitted to the 10x Genomics barcoding process. The remaining 19 mL of blood were frozen for later PBMC isolation. Frozen PBMCs were isolated using a Ficoll–Paque solution according to standard density gradient centrifugation methods. Cells were harvested and resuspended in freezing media (90% FBS, 10% DMSO) and frozen in a –80°C freezer for fewer than 20 days before use. Cells were thawed in a water bath at 37°C with agitation and removed from the water bath when a tiny ice crystal remained. After thawing, the cells were gently transferred to a 15-mL conical tube containing PBS + 2% FBS, and centrifuged at 300 × g for 5 min at 4°C. After removing the supernatant, the washing and centrifugation steps were repeated, and the cells were resuspended in PBS containing 0.04% BSA. The number and viability of the cells were measured using an automated cell counter (TC20, Bio-Rad). The optimal cell concentration range was 700-1200 cells/μl to maximize the likelihood of achieving the desired cell-recovery target.
Clinical laboratory assays
Clinical laboratory tests were conducted in the clinical laboratory and pathology center at the blood diseases hospital of the Chinese Academy of Medical Sciences, a China National Accreditation Service for Conformity Assessment (CNAS) ISO15189 and College of American Pathologists (CAP)-certified laboratory. Plasma AST, ALT, AST/ALT, LDH, CK, and urea levels were analyzed on a Beckman AU5800 (Beckman Coulter). Plasma ferritin, Hs-Tnl, vitamin B12, folate, FT4, and cortisol levels were analyzed on a Beckman Dxl800 (Beckman Coulter). All biochemistry analyses were done with reagents provided by the manufacturer. Plasma interferon α and γ were analyzed using the CBA kit from Cellgene on a flow cytometer (Navios, Beckman Coulter). Immune cells were analyzed using Multitest 6-Color TBNK reagent on a flow cytometer (FACSCanto ii, BD Biosciences). All clinical laboratory assays were performed by certified technicians following the manufacturers’ protocols.
Viral RNA PCR tests and definition of re-positivity
Omicron patients were diagnosed and treated according to the eighth version of the Protocol for Novel Coronavirus Pneumonia published by the Chinese National Health Commission. Patients with two consecutive positive throat swab PCR tests with sampling time intervals of over 24 h were diagnosed with COVID-19 infection. COVID-19 inpatients at Haihe Hospital were monitored with throat swab tests every 2 days. Patients with at least two consecutive negative throat swab PCR tests at a time interval of over 24 h, normal body temperature over 3 days, and significantly improved respiratory symptoms and chest computed tomography images were discharged from Haihe Hospital and were advised to spend another 14 days as inpatients in the First Central Hospital for health status monitoring. Patients in the First Central Hospital were again monitored with throat swab PCR tests every 2 days; during this time, if patients had at least two consecutive positive swab PCR tests, they were identified as re-positive patients. To avoid false-positive or false-negative results, throat swabs were collected by well-trained medical staff, and throat swabs were simultaneously tested for re-positivity by nucleic acid detection kits from three different venders (i.e., Biogerm, Zybio, and Liferiver) that were recommended by the Chinese Center for Disease Control and Prevention. Both internal and negative controls were included in each batch of tests, and all tests were conducted under stringent biosafety conditions.
Plasma and platelet sample preparation for proteomics
Plasma sample proteome processing was performed similarly to previously described.11 , 12 Briefly, samples were first inactivated and sterilized at 56°C for 30 min. For each sample, 10 μL of plasma were depleted for 14 high-abundance proteins using human affinity depletion resin and then concentrated into 50 μL through a 3K MWCO filtering unit (Thermo Fisher Scientific). Next, they were denatured in 8 M urea at 32°C for 30 min, reduced with 10 mM tris (2-carboxyethyl) phosphine (TCEP), and alkylated with 40 mM iodoacetamide (IAA). Finally, protein extracts were diluted and digested using double-step trypsinization for 16 h in total.
Platelet proteome samples were prepared as previously described.11 , 12 Briefly, 30 μL of lysis buffer in 100 mM TEAB with 20 mM TCEP and 40 mM IAA were added to PCT-microtubes for 60 min. The proteins were digested using a mixture of trypsin and Lys-C for 100 min. Digestion was then arrested by adding 10% trifluoroacetic acid. Digested peptides were cleaned-up and labeled using TMTpro 16plex label reagents as different batches. For each batch, peptides were separated into 60 fractions, which were later combined into 30 fractions. Subsequently, the fractions were dried and redissolved in 2% ACN/0.1% formic acid.
Plasma and platelet liquid chromatography tandem mass spectrometry analysis for proteome
All the samples were separated on a reverse-phase C18 column on an U3000 liquid chromatograph under a flow rate of 300 nL/min with 98% H2O, 2% ACN, 0.1% FA (buffer A) and 98% ACN, 2% H2O, 0.1% FA (buffer B). Then analyzed by Orbitrap Exploris 480 (Thermo Fisher Scientific) with a data-dependent acquisition method. During each acquisition, peptides were separated using a 45-minute-long liquid chromatography (LC) gradient (from 7% to 30% buffer B). The m/z range of MS1 was 375-1800, with a resolution of 60,000, normalized automatic gain control (AGC) target of 300%, maximum ion injection time (max IT) of 50 ms, and compensation voltages of −48 V and −68 V for FAIMS Pro. Tandem mass spectrometry (MS/MS) scans were performed at a resolution of 30,000, normalized AGC target of 200%, and 86 ms max IT. Turbo-TMT and advanced peak determination were enabled.
The mass spectrometric data were analyzed using Proteome Discoverer (v2.4.0.305) and the Homo sapiens protein database downloaded from UniProtKB on April 18, 2022 (Fasta file containing 20,307 reviewed protein sequences). The database search was performed as previously described.6 Other parameters followed the default setup. Precursor ion mass tolerance was set to 10 ppm, and product ion was 0.02 Da. The peptide-spectrum-match allowed a 1% target false discovery rate.
The quality of the proteomics data was ensured at multiple levels. First, two samples with a nucleic acid detection time of > 24 h were excluded. A pool of samples labeled by TMTpro-126 was used as the control for aligning the data from different batches. We also assessed the reproducibility of the data using technical replicates and used buffer A as a blank every four injections to avoid carry-over. After removing proteins of low quality, 803 proteins from plasma and 4,804 proteins from platelets underwent quality control. We then assessed the coefficient of variation in the pooled samples.
Plasma LC-MS/MS analysis for metabolome
The pipeline for the metabolomics analysis, including sample preparation and quality control, was as previously described.85 UHPLC-MS/MS analyses were operated in positive or negative polarity mode and performed on a Vanquish UHPLC system (Thermo Fisher) coupled with an Orbitrap Q Exactive HF-X mass spectrometer (Thermo Fisher) in Novogene Co., Ltd. (Beijing, China). Samples were injected onto a Hypesil Gold column (100 × 2.1 mm, 1.9 μm) using a 17-min linear gradient at a flow rate of 0.2 mL/min. The eluents for the positive polarity mode were eluent A (0.1% FA in water) and eluent B (methanol). The eluents for the negative polarity mode were eluent A (5 mM ammonium acetate, pH 9.0) and eluent B (methanol). The raw data files generated by UHPLC-MS/MS were processed using Compound Discoverer 3.1 to perform peak alignment, peak picking, and quantitation for each metabolite. Peaks were matched with the mzCloud (https://www.mzcloud.org/), mzVault, and MassList databases to obtain the accurate qualitative and relative quantitative results. These metabolites were annotated using the KEGG database,77 HMDB database,78 and LIPIDMaps database.79 Three samples with nucleic acid detection times of > 24 h were then excluded. Finally, two outlier samples from healthy donors were removed. After data preprocessing, 546 metabolites in plasma underwent quality control, and log2-scaled data were used. We then assessed the coefficient of variation in the QC samples.
Platelet proteomic data analysis
The DEPs of platelets among healthy donors and Omicron patients were classified into four clusters using the R package “Mfuzz” (v2.46.0), and each cluster was functionally categorized using the biological processes of Gene Ontology (http://www.geneontology.org/). The gene set “response to virus (GO:0009615)” was download from Reactome (http://www.reactome.org/). Heatmaps were drawn up with the “pheatmap” package (v1.0.12), while bubble plots, box plots, bar plots, etc., were implemented in the “ggplot2” (v3.3.6) package in R language.
Plasma proteomic and metabolomic data analyses
One-way analysis of variance (ANOVA) was performed on data from the healthy, acute Omicron, and post-acute Omicron patients. Then, the adjusted p-values were corrected using the Benjamin–Hochberg method. DEMs and DEPs were selected by imposing a cutoff value of less than 0.05 for the B-H-adjusted p-values. Next, soft clustering of the DEPs selected by ANOVA was performed using MFuzz (v2.54.0) (Figures 2A and 2C). For comparing protein expression between groups, log2 (fold change) was calculated using the mean values of each group. A two-sided unpaired Welch's t-test was performed for each group pair. Totals of 258 DEMs and 31 DEPs were evaluated between acute Omicron and post-acute Omicron patients.
Library construction and sequencing of platelets
Purified platelets were lysed with Trizol reagent for RNA extraction. Platelet RNA-seq library preparation was performed using VAHTS mRNA-seq V3 Library Prep Kit according to the manual. The purified cDNA was sequenced on a NovaSeq 6000 platform (BerryGenomics).
Platelet transcriptome data analysis
To process the RNA-seq data, adapters were trimmed using Trimmomatic (v0.36),86 and trimmed reads were aligned to the reference genome (GRCh38v22) using STAR (v2.6.1d)80 to generate the aligned BAM files. GTF files (gencode.v22.annotation.gtf) were applied to convert the read counts into fragments per kilobase of exon per million reads mapped (FPKM). The R package biomaRt (org.Hs.eg.db v3.6.0) was used to retrieve gene annotations from Ensembl release 98. Heatmaps were generated using the “pheatmap” package v1.0.12 to show the up-regulated genes in each group. GO enrichment analysis of DEGs was performed and significant genes and the functional enrichment categories were displayed as box plots and bar plots, respectively, using “ggplot2”. Previously published RNA-seq data for platelets isolated from COVID-19 patients with the ancestral SARS-CoV-2 infection were downloaded from NCBI short-read archives under PRJNA634489. The data were processed following the same steps and compared with platelet transcriptomes of healthy donors and Omicron patients in this study.
Multi-omics integration by MOFA
Platelet transcriptomes from 85 cases, platelet proteomes from 95 cases, plasma proteomes from 94 cases, and plasma metabolomes from 95 cases were used for MOFA integration, resulting in 102 overlapping cases consisting of 65 Omicron patients and 37 non-Omicron healthy donors. Differential expression analysis with LIMMA R package was first implemented for each omics dataset, resulting in 961 platelet DEGs, 658 platelet DEPs, 777 Plasma DEPs, and 546 plasma DEMs. Individual datasets were then Z-score-standardized before inputting into the MOFA2 R package to build the MOFA model.39 The number of factors were set to 15, random seeds were set to 42, and default settings were applied for other parameters. In total, the platelet transcriptome explained 33.53% of total variances, the platelet proteome explained 39.66% of variances, the plasma proteome explained 34.74% of variances, and the plasma metabolome explained 20.72% of variances. Correlations between clinical parameters and the 15 MOFA factors were then performed using Spearman correlation analysis. The 15 MOFA factors were further classified to three groups according to the samples’ factor value differences for healthy donors, acute phase Omicron, and post-acute Omicron patients, and this differential analysis was done by Wilcoxon test. Feature weights in the platelet transcriptomes, platelet proteomes, and plasma proteomes of each factor in the different response groups were applied for GSEA enrichment analysis using the reactomeGS database,87 while feature weights in the plasma metabolome were applied for enrichment analysis using MetaboAnalyst.81
Library construction and sequencing of PBMCs
According to the results of the cell counts and the recommended cell concentration, each sample was immediately loaded onto a 10x Chromium Next GEM Chip K according to the manufacturer’s user guide (CG000331). In detail, libraries for samples without cryopreservation were constructed using the Chromium single-cell 5′ library, while libraries for samples with cryopreservation were constructed using the Chromium single-cell 5′ library and V(D)J enrichment kits. cDNA purification and size selection were achieved by SPRI select beads. The quality of the cDNA post-amplification, cDNA post-target enrichment, and the final libraries were assessed using a Qubit 4.0 and Agilent Bioanalyzer 2100 with high-sensitivity chips. The libraries were sequenced using a Novaseq6000 platform with 150-bp pair-end sequencing ( NovoGene).
Processing of single-cell RNA-seq data
Gene-barcode matrices for all samples (47 frozen samples and 16 fresh samples) were generated by the Cellranger (v6.1.2) “count” function plotted against the GRCh38 human genome. Firstly, Seurat (v4.0.3)82 was applied to calculate the total gene number, total UMI count, and percentages of mitochondrial UMIs for each sample, whose UMI matrices were simultaneously projected onto the ABC by “TransferData” from Seurat (v4.0.3) to resolve the blood cell types of PBMCs. Next, UMI matrices for 47 frozen samples and 16 fresh samples were integrated by Scanpy (v1.5.1).83 For frozen PBMCs, (1) > 500 genes; (2) > 1000 UMIs; (3) < 20% mitochondrial UMIs were excluded, and doublets identified by Scrublet (v0.2.1)84 with default parameters were removed. After the removal of doublets, fresh PBMCs with > 200 genes, > 500 UMIs, and < 10% mitochondrial UMIs were retained to extract neutrophils. The integrated and high-quality UMI matrices were submitted for normalization, logarithmization, and regression of cell cycle and scale, followed by PCA dimension reduction based on the highly variable genes (HVGs) generated by the “highly_variable_genes” function of Scanpy (v1.5.1). Batch effects among different samples were removed by the BBKNN algorithm, followed by UMAP dimension reduction, Leiden clustering, and UMAP visualization by Scanpy (v1.5.1). Finally, combining the annotated cell types projected onto ABC with canonical signature genes, 318,359 NK and T cells, 44,997 B cells, 89,456 monocytes, 10,125 platelets, and 7,425 neutrophils were precisely defined.
To further dissect the heterogeneity, UMI matrices of NK and T cells, B cells, monocytes, platelets, and neutrophils were separated from PBMCs and re-clustered. Considering the large numbers of NK and T cells and monocytes, several procedures, including normalization, HVG detection, scale, batch effect correction, dimension reduction, and clustering, were performed by Scanpy (v1.5.1) as aforementioned. With regards to B cells, platelets, and neutrophils, nearly identical processing, i.e., normalization by “NormalizeData”, HVG detection by “NormalizeData”, integration by “IntegrateData”, scaling by “ScaleData”, dimension reduction by “RunUMAP”, and clustering by “FindClusters”, was achieved using Seurat (v4.0.3). Eventually, 12 cell clusters for NK and T cells, 9 cell clusters for B cells, 8 cell clusters for monocytes, 7 cell clusters for platelets, and 6 cell clusters for neutrophils were identified for subsequent analysis.
Analysis of single-cell BCRs and TCRs
Assembled and annotated contigs of BCRs and TCRs were generated by the Cellranger (v6.1.2) “vdj” function by comparing to the reference genome (GRCh38). Only efficient contigs characterized by high-confidence and productive UMIs ≥ 2 were reserved for clonotype constitution. Only cells with at least one heavy chain (IGH for BCR and TRB for TCR) and one light chain (IGL/IGK for BCR and TRA for TCR) were retained for further analysis. In terms of B cells or T cells with two or more assembled contigs, the heavy or light chain with the highest UMI level was regarded as the dominant chain in each cell. Each unique IGH-IGL/IGK or TRB-TRA pair was defined as a clonotype whose expansion degree was indicated by the frequency of B cells or T cells harboring this clonotype. Clonotypes with identical VDJ sequences and rearranged VDJ genes were integrated for different samples belonging to one category. For a given B and T cell type, BCR and TCR diversity was evaluated as Shannon entropy, in which P represented the frequency of a given BCR and TCR clone among that of all BCR and TCR clones.
Identification of signature genes and DEGs for single-cell RNA-seq
Based on the cell cluster information acquired above, “FindAllMarkers” from Seurat (v4.0.3) was used to identify cell-cluster-specific signature genes for NK and T cells, B cells, monocytes, platelets, and neutrophils. While “FindMarkers” was applied to detect DEGs between any two given groups. The filtered criteria for signature genes and DEGs were fold-change ≥ 1.5 and adjusted p-value ≤ 0.05.
Calculation of the fraction of UMIs for single-cell RNA-seq
UMI fraction of cytokine production, inflammatory responses, and interferon-α and β responses (downloaded from GSEA) in neutrophils, platelets, and monocytes were compared between different groups.50 Additionally, 12 well-defined cytotoxicity genes (PRF1, IFNG, GNLY, NKG7, GZMB, GZMA, GZMH, KLRK1, KLRB1, KLRD1, CTSW, and CST7) and 6 well-defined exhaustion genes (LAG3, TIGIT, PDCD1, CTLA4, HAVCR2, and TOX)88 were used to evaluate the cytotoxicity and exhaustion state of NK and T cells. The UMI fraction was calculated as the total number of UMIs in each gene set divided by the total number of UMIs in each single cell.89
Functional analyses
Several pathway analysis tools were used to perform functional analysis of the significant DEPs. Enrichment analyses based on Gene Ontology processes, KEGG pathways, reactome gene sets, and Wiki pathways were performed using the web-based platform Metascape.76 With ingenuine pathway analysis90 of the regulated metabolites and proteins, we identified the most significantly regulated pathways. We then used MetaboAnalyst v5.0 for the metabolomics enrichment analysis and joint-pathways analysis between metabolome and proteome data based on the DEMs and DEPs we previously identified. The significantly enriched pathways had p-values < 0.05 and contained at least three proteins or metabolites from our dataset.
Machine learning
To predict an individual’s probability of having re-positive viral RNA, we used the samples from a training set (clinical [73 x 7], metabolic [36 x 546], and proteomic data [72 x 803]) for model training, and 26 (about 30% of the average number of samples) randomly selected test samples in the evaluation of model performance. Machine learning analysis was conducted using the “mlr3” R package.91 Different feature-selection strategies were used for clinical, metabolic, and proteomic datasets. In detail, based on the training set, we chose clinical characteristics with positive information gain, metabolites whose ANOVA p-values were less than 0.001, and the top 10 most important proteins selected by random forest for our clinical, metabolic, and proteomic models, respectively. These single-class models were built using a random forest algorithm and trained with their corresponding training sets. In particular, we grew 500 trees, with set node size 1, and measured the feature importance by Gini index, while setting other hyperparameters as default. We next developed a model that was an ensemble of the three previous ones. It was a logistic regression model that was trained on the intersection of three single-class training sets and took the output scores (ClinicalScore, MetaScore, and PlasmaScore) from single-class models as input. The proteomic model was further validated by another independent cohort using the same data processing pipeline as in the training and Test dataset 1.
Platelet-leukocyte aggregate detection
About 30 μL of peripheral blood from healthy donors (n = 8) or COVID-19 patients (Acute, n = 4; Post-acute, n = 7; Follow-up, n = 6) were mixed with 70 μL PBS buffer containing 2% FBS. Antibodies for T cells (CD3), B cells (CD19), NK cells (CD56), monocytes (CD14), neutrophils (CD11b), and platelets (CD42b) (1:100) were added into each sample for 30 min at room temperature in the dark, followed by treatment with red blood cell lysis buffer for 5 min. Then, cells were collected via a centrifugation step (400 × g, 5 min) and resuspended in 200 μL PBS buffer containing 2% FBS for flow cytometry (FACSCanto ii, BD Biosciences) analysis.
Platelet-T cell co-culture
Peripheral blood (∼10 mL) was obtained from healthy donors (n = 5) or COVID-19 patients (Acute, n = 3; Post-acute, n = 3; Follow-up, n = 5) and centrifugated at 300 × g for 10 min. The platelet-rich plasma supernatant was collected for platelet isolation (800 × g, 10 min). PBMCs in the sediment were isolated using Ficoll–Paque solution according to standard density gradient centrifugation methods. Then, the mononuclear selection fraction was subjected to immunomagnetic bead separation using a MiniMACS CD3+ isolation kit. For each sample, enriched CD3+ T cells (cell density, 3 × 105/mL) were cultured in RPMI-1640 medium containing 10% FBS with or without platelet addition (T cell:platelet ratio = 1:100). After 24 h of incubation, cells were collected and labeled with CD3, CD4, CD8 and CD69 (T cell activation antigen) antibodies (1:100) for 30 min at room temperature in the dark. The frequency of CD69 expression in CD3+, CD4+, and CD8+ T cells were then measured by flow cytometry (FACSCanto ii, BD Biosciences).
ELISA assay
The ELISA kits for detecting the levels of an-SARS-CoV-2 (B.1.1.529) spike S1+S2 ECD antibodies in serum and of neutralization antibodies were purchased from Sino Biological. For spike antibody detection, serum samples were diluted 2000 times and added to precoated microwells, followed by incubation for 1 h at room temperature. After washing, an HRP-goat anti-human IgG was added and incubated for 1 h at room temperature. Next, the mixed substrate solution was added and incubated for 15 min before the stop solution was added. The plate was read with a BIO-TEK (Synergy H4) at 450 nm wavelength. For neutralization antibody detection, serum samples were diluted 30 times and preincubated with HRP-RBD solution with a volume ratio of 1:1 at 37°C for 30 min. Then, the mixture was added into microwells and co-incubated for 30 min at 37°C. After washing, the mixed substrate solution was added and incubated for 15 min, followed by addition of the stop solution. The plate was read with a BIO-TEK (Synergy H4) at 450 nm wavelength.
Quantification and statistical analysis
During proteomics data analysis, first, missing values were imputed using SeqKNN by R.92 Then, the effect of platelet enrichment in some plasma samples due to experimental centrifuging speed was removed using the linear model, and the batch effects were removed using the R package combat (https://lifeinfor.shinyapps.io/batchserver/). No other significant batch effects were highlighted by UMAP analysis. We used fisher’s exact test to determine whether there is a significant association between qualitative clinical characteristics and label “re-positive” or “not re-positive”. In single-cell analysis, a nonparametric Wilcoxon test was conducted in R to compare differences between the two groups. Reported p-values were from two-sided tests, and a p-value < 0.05 was deemed significant. The construction of heatmaps was achieved in the “pheatmap” package, while bubble plots, box plots, bar plots, and so on, were implemented in the “ggplot2” package in R. For biological experiments, the number of biological replicates is indicated by the n value and all graphs depict mean ± SEM. The statistical procedures were performed using a two-tailed unpaired Student’s t test and all results were considered statistically significant at p-value < 0.05. The graphs and statistical evaluation were performed using GraphPad Prism 8.0 software.
Acknowledgments
This work was supported by Haihe Laboratory of Cell Ecosystem Innovation Fund (22HHXBSS00001, 22HHXBSS00049, 22HHXBSS00032, 22HHXBSS00034, 22HHXBSS00031, and 22HHXBSS00018), the National Key Research and Development Program of China (2021YFA1100900, 2022YFA1103500, 2021YFA1102800, 2020YFE0202200, 2021YFA1301602, and 2021YFA1103000), the National Natural Science Foundation of China (82270236, 81890990, 82022002, 82125003, 82200141, 32000803, and 32200763), the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2021-I2M-1-040, 2021-I2M-1-073, and 2022-I2M-JB-015), the Non-CAMS Fundamental Research Funds for Central Research Institutes (3332021093), Distinguished Young Scholars of Tianjin (22JCJQJC00090 and 21JCJQJC00070), Tianjin Applied Basic Research Project (22JCQNJC01270), and the Special Research Peking Union Medical College Fund for Central Universities, Peking Union Medical College (3332022060). We gratefully acknowledge Qing Ji, Jiajia Gao, Yi Yue, and Jiale Wang for coordinating the research and clinical teams, Dan Li for data analysis, and Lili Ren, Junmin Peng, and Timothy I. Shaw for participating in insightful discussions. We also acknowledge Novogene for conducting the metabolomic analysis.
Author contributions
T.C., Hong Wang, P.Z., J. Zhou, T.G., Z.S., and X.W. conceived the project. T.C., Hong Wang, P.Z., C.L., X.X., M.N., Yingrui Wang, and R.S. wrote the manuscript. All authors discussed the results and commented on the manuscript. Hong Wang, X.X., M.N., C.L., Yingrui Wang, D.Z., M.L., Y.M., Hongtao Wang, W.G., and J. Zhau performed data analysis. C.L., M.N., Yingrui Wang., X. Chen, B.Z., S.M., Huijun Wang, G.Z., Y.L., R.S., B.H., P.S., X. Cheng, He Wang, L.S., L.F., Q. Huang, Y.Y., C.W., C.C., Q. Huo, L.G., Ying Wang, Q.W., H.C., Yan Xie, Yi Xie, L.L., J.Q., J.W., E.J., and W.J. performed the experiments and provided the samples.
Declaration of interests
A patent related to this work has been filed.
Published: May 16, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.immuni.2023.05.007.
Supplemental information
Data and code availability
The bulk RNA-seq data of platelets generated during this study have been deposited at National Omics Data Encyclopedia with accession codes OEP003718 and OEP003719. All single-cell sequencing data have been deposited at the National Genomics Data Center with accession code HRA003738. All mass-spectrometry-based proteomic and metabolomic data are available in the platform iProX with ID IPX0004421000, password: coGQ. (https://www.iprox.cn/page/PSV023.html;?url=1683181621450EX28). All the data will be publicly released upon publication.
The data analyses codes are deposited at Zenodo: https://sandbox.zenodo.org/ [DOI: https://doi.org/10.5281/zenodo.7880998].
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Tirosh I., Izar B., Prakadan S.M., Wadsworth M.H., Treacy D., Trombetta J.J., Rotem A., Rodman C., Lian C., Murphy G., et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Price J., Arze C., Ananthakrishnan A.N., Schirmer M., Avila-Pacheco J., Poon T.W., Andrews E., Ajami N.J., Bonham K.S., Brislawn C.J., et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner J., Rapsomaniki M.A., Chevrier S., Anzeneder T., Langwieder C., Dykgers A., Rees M., Ramaswamy A., Muenst S., Soysal S.D., et al. A single-cell atlas of the tumor and immune ecosystem of human breast cancer. Cell. 2019;177:1330–1345.e18. doi: 10.1016/j.cell.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Y., Wu L., Zhong Y., Zhou K., Hou Y., Wang Z., Zhang Z., Xie J., Wang C., Chen D., et al. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell. 2021;184:404–421.e16. doi: 10.1016/j.cell.2020.11.041. [DOI] [PubMed] [Google Scholar]
- 5.López-Otín C., Kroemer G. Hallmarks of health. Cell. 2021;184:1929–1939. doi: 10.1016/j.cell.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Shen B., Yi X., Sun Y., Bi X., Du J., Zhang C., Quan S., Zhang F., Sun R., Qian L., et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell. 2020;182:59–72.e15. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shu T., Ning W., Wu D., Xu J., Han Q., Huang M., Zou X., Yang Q., Yuan Y., Bie Y., et al. Plasma proteomics identify biomarkers and pathogenesis of COVID-19. Immunity. 2020;53:1108–1122.e5. doi: 10.1016/j.immuni.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demichev V., Tober-Lau P., Lemke O., Nazarenko T., Thibeault C., Whitwell H., Röhl A., Freiwald A., Szyrwiel L., Ludwig D., et al. A time-resolved proteomic and prognostic map of COVID-19. Cell Syst. 2021;12 doi: 10.1016/j.cels.2021.05.005. 780.e7–794.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao J., Sun R., Ai J., Qian L., Liu F., Wang H., Tan L., Cai X., Shi Y., Liang X., et al. Proteomic characterization of Omicron SARS-CoV-2 host response. Cell Discov. 2022;8:46. doi: 10.1038/s41421-022-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 11.Shuai H., Chan J.F., Hu B., Chai Y., Yuen T.T., Yin F., Huang X., Yoon C., Hu J.C., Liu H., et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature. 2022;603:693–699. doi: 10.1038/s41586-022-04442-5. [DOI] [PubMed] [Google Scholar]
- 12.Tang X., Sun R., Ge W., Mao T., Qian L., Huang C., Kang Z., Xiao Q., Luo M., Zhang Q., et al. Enhanced inflammation and suppressed adaptive immunity in COVID-19 with prolonged RNA shedding. Cell Discov. 2022;8:70. doi: 10.1038/s41421-022-00441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., Pearson C.A.B., Russell T.W., Tully D.C., Washburne A.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372 doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mlcochova P., Kemp S.A., Dhar M.S., Papa G., Meng B., Ferreira I.A.T.M., Datir R., Collier D.A., Albecka A., Singh S., et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599:114–119. doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng H., Cao Y., Chen X., Wang F., Hu Y., Song W., Chai Y., Gu Q., Shi Y., Feng Y., et al. Disease profile and plasma neutralizing activity of post-vaccination Omicron BA.1 infection in Tianjin, China: a retrospective study. Cell Res. 2022;32:781–784. doi: 10.1038/s41422-022-00674-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L., Tang J., Xie Z., Gan Q., Tang G., Hu Z., Zeng H., Shi J., Li J., Li Y., et al. Characteristics of SARS-CoV-2 Delta variant-infected individuals with intermittently positive retest viral RNA after discharge. Natl. Sci. Rev. 2022;9:nwac141. doi: 10.1093/nsr/nwac141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu F., Chen F., Ou Z., Fan Q., Tan X., Wang Y., Pan Y., Ke B., Li L., Guan Y., et al. A compromised specific humoral immune response against the SARS-CoV-2 receptor-binding domain is related to viral persistence and periodic shedding in the gastrointestinal tract. Cell. Mol. Immunol. 2020;17:1119–1125. doi: 10.1038/s41423-020-00550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y., Xu H. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323:1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutledge A.C., Choi Y.H., Karp I., Bhayana V., Stevic I. Biochemistry tests in hospitalized COVID-19 patients: experience from a Canadian tertiary care centre. Clin. Biochem. 2021;95:41–48. doi: 10.1016/j.clinbiochem.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z., Xu W., Ma W., Shi X., Li S., Hao M., Fang Y., Zhang L. Clinical laboratory evaluation of COVID-19. Clin. Chim. Acta. 2021;519:172–182. doi: 10.1016/j.cca.2021.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jimenez-Guardeño J.M., Ortega-Prieto A.M., Moreno B.M., Maguire T.J.A., Richardson A., Diaz-Hernandez J.I., Diez Perez J., Zuckerman M., Playa A.M., Deline C.C., et al. 2021. Drug repurposing based on a quantum-inspired method versus classical fingerprinting uncovers potential antivirals against SARS-CoV-2 including vitamin B12. Preprint at bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batista K.S., Cintra V.M., Lucena P.A.F., Manhães-de-Castro R., Toscano A.E., Costa L.P., Queiroz M.E.B.S., de Andrade S.M., Guzman-Quevedo O., Aquino J.S. The role of vitamin B12 in viral infections: a comprehensive review of its relationship with the muscle-gut-brain axis and implications for SARS-CoV-2 infection. Nutr. Rev. 2022;80:561–578. doi: 10.1093/nutrit/nuab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Guo R., Kim S.H., Shah H., Zhang S., Liang J.H., Fang Y., Gentili M., Leary C.N.O., Elledge S.J., et al. SARS-CoV-2 hijacks folate and one-carbon metabolism for viral replication. Nat. Commun. 2021;12:1676. doi: 10.1038/s41467-021-21903-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal R., Banerjee M., Bhadada S.K. Cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020;8:809. doi: 10.1016/S2213-8587(20)30304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181:1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sposito B., Broggi A., Pandolfi L., Crotta S., Clementi N., Ferrarese R., Sisti S., Criscuolo E., Spreafico R., Long J.M., et al. The interferon landscape along the respiratory tract impacts the severity of COVID-19. Cell. 2021;184 doi: 10.1016/j.cell.2021.08.016. 4953–4968.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willyard C. Coronavirus blood-clot mystery intensifies. Nature. 2020;581:250. doi: 10.1038/d41586-020-01403-8. [DOI] [PubMed] [Google Scholar]
- 29.Trugilho M.R.O., Azevedo-Quintanilha I.G., Gesto J.S.M., Moraes E.C.S., Mandacaru S.C., Campos M.M., Oliveira D.M., Dias S.S.G., Bastos V.A., Santos M.D.M., et al. Platelet proteome reveals features of cell death, antiviral response and viral replication in Covid-19. Cell Death Discov. 2022;8:324. doi: 10.1038/s41420-022-01122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou X., Michal J.J., Zhang L., Ding B., Lunney J.K., Liu B., Jiang Z. Interferon induced IFIT family genes in host antiviral defense. Int. J. Biol. Sci. 2013;9:200–208. doi: 10.7150/ijbs.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tauber A.L., Schweiker S.S., Levonis S.M. The potential association between PARP14 and SARS-CoV-2 infection (COVID-19) Future Med. Chem. 2021;13:587–592. doi: 10.4155/fmc-2020-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manne B.K., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C., Petrey A.C., Tolley N.D., Guo L., Cody M., et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136:1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Rhijn I., Van den Berg L.H., Bosboom W.M., Otten H.G., Logtenberg T. Expression of accessory molecules for T-cell activation in peripheral nerve of patients with CIDP and vasculitic neuropathy. Brain. 2000;123:2020–2029. doi: 10.1093/brain/123.10.2020. [DOI] [PubMed] [Google Scholar]
- 34.Sharpe H.R., Bowyer G., Brackenridge S., Lambe T. HLA-E: exploiting pathogen-host interactions for vaccine development. Clin Exp Immunol. 2019;196:167–177. doi: 10.1111/cei.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulati K., Gangele K., Agarwal N., Jamsandekar M., Kumar D., Poluri K.M. Molecular cloning and biophysical characterization of CXCL3 chemokine. Int. J. Biol. Macromol. 2018;107:575–584. doi: 10.1016/j.ijbiomac.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 36.Prelli Bozzo C., Nchioua R., Volcic M., Koepke L., Krüger J., Schütz D., Heller S., Stürzel C.M., Kmiec D., Conzelmann C., et al. IFITM proteins promote SARS-CoV-2 infection and are targets for virus inhibition in vitro. Nat. Commun. 2021;12:4584. doi: 10.1038/s41467-021-24817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nchioua R., Schundner A., Kmiec D., Prelli Bozzo C., Zech F., Koepke L., Graf A., Krebs S., Blum H., Frick M., et al. SARS-CoV-2 variants of concern hijack IFITM2 for efficient replication in human lung cells. J. Virol. 2022;96:e0059422. doi: 10.1128/jvi.00594-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu D., Shu T., Yang X., Song J.X., Zhang M., Yao C., Liu W., Huang M., Yu Y., Yang Q., et al. Plasma metabolomic and lipidomic alterations associated with COVID-19. Natl. Sci. Rev. 2020;7:1157–1168. doi: 10.1093/nsr/nwaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Argelaguet R., Velten B., Arnol D., Dietrich S., Zenz T., Marioni J.C., Buettner F., Huber W., Stegle O. Multi-omics factor analysis—a framework for unsupervised integration of multi-omics data sets. Mol. Syst. Biol. 2018;14:e8124. doi: 10.15252/msb.20178124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.García-Sastre A. Ten strategies of interferon evasion by viruses. Cell Host Microbe. 2017;22:176–184. doi: 10.1016/j.chom.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Namgaladze D., Brüne B. Macrophage fatty acid oxidation and its roles in macrophage polarization and fatty acid-induced inflammation. Biochim. Biophys. Acta. 2016;1861:1796–1807. doi: 10.1016/j.bbalip.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Dufour I., Werion A., Belkhir L., Wisniewska A., Perrot M., De Greef J., Schmit G., Yombi J.C., Wittebole X., Laterre P.F., et al. Serum uric acid, disease severity and outcomes in COVID-19. Crit. Care. 2021;25:212. doi: 10.1186/s13054-021-03616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas T., Stefanoni D., Reisz J.A., Nemkov T., Bertolone L., Francis R.O., Hudson K.E., Zimring J.C., Hansen K.C., Hod E.A., et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020;5 doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mezrich J.D., Fechner J.H., Zhang X., Johnson B.P., Burlingham W.J., Bradfield C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moretti S., Alesse E., Di Marzio L., Zazzeroni F., Ruggeri B., Marcellini S., Famularo G., Steinberg S.M., Boschini A., Cifone M.G., et al. Effect of L-carnitine on human immunodeficiency virus-1 infection-associated apoptosis: a pilot study. Blood. 1998;91:3817–3824. [PubMed] [Google Scholar]
- 46.Li C., Ou R., Wei Q., Shang H. Carnitine and COVID-19 susceptibility and severity: A Mendelian randomization study. Front. Nutr. 2021;8:780205. doi: 10.3389/fnut.2021.780205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt N., Lareau C.A., Keshishian H., Ganskih S., Schneider C., Hennig T., Melanson R., Werner S., Wei Y., Zimmer M., et al. The SARS-CoV-2 RNA-protein interactome in infected human cells. Nat. Microbiol. 2021;6:339–353. doi: 10.1038/s41564-020-00846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Washington A.V., Gibot S., Acevedo I., Gattis J., Quigley L., Feltz R., De La Mota A., Schubert R.L., Gomez-Rodriguez J., Cheng J., et al. TREM-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. J. Clin. Invest. 2009;119:1489–1501. doi: 10.1172/JCI36175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie X., Liu M., Zhang Y., Wang B., Zhu C., Wang C., Li Q., Huo Y., Guo J., Xu C., et al. Single-cell transcriptomic landscape of human blood cells. Natl. Sci. Rev. 2021;8:nwaa180. doi: 10.1093/nsr/nwaa180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J.Y., Wang X.M., Xing X., Xu Z., Zhang C., Song J.W., Fan X., Xia P., Fu J.L., Wang S.Y., et al. Single-cell landscape of immunological responses in patients with COVID-19. Nat. Immunol. 2020;21:1107–1118. doi: 10.1038/s41590-020-0762-x. [DOI] [PubMed] [Google Scholar]
- 51.Ren X., Wen W., Fan X., Hou W., Su B., Cai P., Li J., Liu Y., Tang F., Zhang F., et al. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell. 2021;184:5838. doi: 10.1016/j.cell.2021.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X., Chen Y., Li Z., Huang B., Xu L., Lai J., Lu Y., Zha X., Liu B., Lan Y., et al. Single-cell RNA-seq of T cells in B-ALL patients reveals an exhausted subset with remarkable heterogeneity. Adv. Sci. (Weinh) 2021;8:e2101447. doi: 10.1002/advs.202101447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogega C.O., Skinner N.E., Blair P.W., Park H.S., Littlefield K., Ganesan A., Dhakal S., Ladiwala P., Antar A.A., Ray S.C., et al. Durable SARS-CoV-2 B cell immunity after mild or severe disease. J. Clin. Invest. 2021;131 doi: 10.1172/JCI145516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moeini Shad T., Yousefi B., Amirifar P., Delavari S., Rae W., Kokhaei P., Abolhassani H., Aghamohammadi A., Yazdani R. Variable abnormalities in T and B cell subsets in ataxia telangiectasia. J. Clin. Immunol. 2021;41:76–88. doi: 10.1007/s10875-020-00881-9. [DOI] [PubMed] [Google Scholar]
- 55.Descatoire M., Weller S., Irtan S., Sarnacki S., Feuillard J., Storck S., Guiochon-Mantel A., Bouligand J., Morali A., Cohen J., et al. Identification of a human splenic marginal zone B cell precursor with NOTCH2-dependent differentiation properties. J. Exp. Med. 2014;211:987–1000. doi: 10.1084/jem.20132203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.COvid-19 Multi-omics Blood ATlas (COMBAT) Consortium A blood atlas of COVID-19 defines hallmarks of disease severity and specificity. Cell. 2022;185 doi: 10.1016/j.cell.2022.01.012. 916–938.e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keeton R., Tincho M.B., Ngomti A., Baguma R., Benede N., Suzuki A., Khan K., Cele S., Bernstein M., Karim F., et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603:488–492. doi: 10.1038/s41586-022-04460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moss P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022;23:186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 59.Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pão C.R.R., Righy C., Franco S., Souza T.M.L., Kurtz P., et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136:1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malengier-Devlies B., Filtjens J., Ahmadzadeh K., Boeckx B., Vandenhaute J., De Visscher A., Bernaerts E., Mitera T., Jacobs C., Vanderbeke L., et al. Severe COVID-19 patients display hyper-activated NK cells and NK cell-platelet aggregates. Front. Immunol. 2022;13:861251. doi: 10.3389/fimmu.2022.861251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicolai L., Leunig A., Brambs S., Kaiser R., Weinberger T., Weigand M., Muenchhoff M., Hellmuth J.C., Ledderose S., Schulz H., et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su Y., Chen D., Yuan D., Lausted C., Choi J., Dai C.L., Voillet V., Duvvuri V.R., Scherler K., Troisch P., et al. Multi-omics resolves a sharp disease-state shift between mild and moderate COVID-19. Cell. 2020;183:1479–1495.e20. doi: 10.1016/j.cell.2020.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schulte-Schrepping J., Reusch N., Paclik D., Baßler K., Schlickeiser S., Zhang B., Krämer B., Krammer T., Brumhard S., Bonaguro L., et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182 doi: 10.1016/j.cell.2020.08.001. 1419–1440.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilk A.J., Rustagi A., Zhao N.Q., Roque J., Martínez-Colón G.J., McKechnie J.L., Ivison G.T., Ranganath T., Vergara R., Hollis T., et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020;26:1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meffre E., Milili M., Blanco-Betancourt C., Antunes H., Nussenzweig M.C., Schiff C. Immunoglobulin heavy chain expression shapes the B cell receptor repertoire in human B cell development. J. Clin. Invest. 2001;108:879–886. doi: 10.1172/JCI13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berbudi A., Rahmadika N., Tjahjadi A.I., Ruslami R. Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 2020;16:442–449. doi: 10.2174/1573399815666191024085838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raman R., Patel K.J., Ranjan K. COVID-19: unmasking emerging SARS-CoV-2 variants, vaccines and therapeutic strategies. Biomolecules. 2021;11 doi: 10.3390/biom11070993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Majumder J., Minko T. Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J. 2021;23:14. doi: 10.1208/s12248-020-00532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao Y., Yisimayi A., Jian F., Song W., Xiao T., Wang L., Du S., Wang J., Li Q., Chen X., et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608:593–602. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anka A.U., Tahir M.I., Abubakar S.D., Alsabbagh M., Zian Z., Hamedifar H., Sabzevari A., Azizi G. Coronavirus disease 2019 (COVID-19): an overview of the immunopathology, serological diagnosis and management. Scand. J. Immunol. 2021;93:e12998. doi: 10.1111/sji.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao H., Ruan L., Liu J., Liao W. The clinical characteristic of eight patients of COVID-19 with positive RT-PCR test after discharge. J. Med. Virol. 2020;92:2159–2164. doi: 10.1002/jmv.26017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tao W.Y., Wang X., Zhang G., Guo M., Ma H., Zhao D., Sun Y., He J., Liu L., Zhang K., et al. Re-detectable positive SARS-CoV-2 RNA tests in patients who recovered from COVID-19 with intestinal infection. Protein Cell. 2021;12:230–235. doi: 10.1007/s13238-020-00778-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Z., Xie W., Ge Z., Wang Y., Zhao H., Wang J., Xu Y., Zhang W., Song M., Cui S., et al. Reactivation of SARS-CoV-2 infection following recovery from COVID-19. J. Infect. Public Health. 2021;14:620–627. doi: 10.1016/j.jiph.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dao T.L., Hoang V.T., Gautret P. Recurrence of SARS-CoV-2 viral RNA in recovered COVID-19 patients: a narrative review. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:13–25. doi: 10.1007/s10096-020-04088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou Y., Zhou B., Pache L., Chang M., Khodabakhshi A.H., Tanaseichuk O., Benner C., Chanda S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019;10:1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wishart D.S., Guo A., Oler E., Wang F., Anjum A., Peters H., Dizon R., Sayeeda Z., Tian S., Lee B.L., et al. HMDB 5.0: the human metabolome database for 2022. Nucleic Acids Res. 2022;50:D622–D631. doi: 10.1093/nar/gkab1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sud M., Fahy E., Cotter D., Brown A., Dennis E.A., Glass C.K., Merrill A.H., Murphy R.C., Raetz C.R., Russell D.W., et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007;35:D527–D532. doi: 10.1093/nar/gkl838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pang Z., Zhou G., Ewald J., Chang L., Hacariz O., Basu N., Xia J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022;17:1735–1761. doi: 10.1038/s41596-022-00710-w. [DOI] [PubMed] [Google Scholar]
- 82.Hao Y., Hao S., Andersen-Nissen E., Mauck W.M., Zheng S., Butler A., Lee M.J., Wilk A.J., Darby C., Zager M., et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184 doi: 10.1016/j.cell.2021.04.048. 3573–3587.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolf F.A., Angerer P., Theis F.J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 2018;19:15. doi: 10.1186/s13059-017-1382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolock S.L., Lopez R., Klein A.M. Scrublet: computational identification of cell doublets in single-cell transcriptomic data. Cell Syst. 2019;8 doi: 10.1016/j.cels.2018.11.005. 281–291.e9-e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barri T., Dragsted L.O. UPLC-ESI-QTOF/MS and multivariate data analysis for blood plasma and serum metabolomics: effect of experimental artefacts and anticoagulant. Anal. Chim. Acta. 2013;768:118–128. doi: 10.1016/j.aca.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 86.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frost H.R., Li Z., Moore J.H. Principal component gene set enrichment (PCGSE) BioData Min. 2015;8:25. doi: 10.1186/s13040-015-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guo X., Zhang Y., Zheng L., Zheng C., Song J., Zhang Q., Kang B., Liu Z., Jin L., Xing R., et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat. Med. 2018;24:978–985. doi: 10.1038/s41591-018-0045-3. [DOI] [PubMed] [Google Scholar]
- 89.Li H., van der Leun A.M., Yofe I., Lubling Y., Gelbard-Solodkin D., van Akkooi A.C.J., van den Braber M., Rozeman E.A., Haanen J.B.A.G., Blank C.U., et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell. 2019;176 doi: 10.1016/j.cell.2018.11.043. 775–789.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krämer A., Green J., Pollard J., Jr., Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lang M., Binder M., Richter J., Schratz P., Pfisterer F., Coors S., Au Q., Casalicchio G., Kotthoff L., Bischl B. mlr3: A modern object-oriented machine learning framework in R. J. Open Source Software. 2019;4:1903. doi: 10.21105/joss.01903. [DOI] [Google Scholar]
- 92.Wang S., Li W., Hu L., Cheng J., Yang H., Liu Y. NAguideR: performing and prioritizing missing value imputations for consistent bottom-up proteomic analyses. Nucleic Acids Res. 2020;48:e83. doi: 10.1093/nar/gkaa498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The bulk RNA-seq data of platelets generated during this study have been deposited at National Omics Data Encyclopedia with accession codes OEP003718 and OEP003719. All single-cell sequencing data have been deposited at the National Genomics Data Center with accession code HRA003738. All mass-spectrometry-based proteomic and metabolomic data are available in the platform iProX with ID IPX0004421000, password: coGQ. (https://www.iprox.cn/page/PSV023.html;?url=1683181621450EX28). All the data will be publicly released upon publication.
The data analyses codes are deposited at Zenodo: https://sandbox.zenodo.org/ [DOI: https://doi.org/10.5281/zenodo.7880998].
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.