ABSTRACT
Background
Real‐time quaking‐induced conversion (RT‐QuIC) and protein misfolding cyclic amplification (PMCA) have been developed to detect minute amounts of amyloidogenic proteins via amplification techniques and have been used to detect misfolded α‐synuclein (αSyn) aggregates in the cerebrospinal fluid (CSF) and other source materials of patients with Parkinson's Disease and other synucleinopathies.
Objectives
The aim of this systematic review and meta‐analysis was to evaluate the diagnostic accuracy of αSyn seed amplification assays (αSyn‐SAAs), including RT‐QuIC and PMCA, using CSF as source material to differentiate synucleinopathies from controls.
Methods
The electronic MEDLINE database PubMed was searched for relevant articles published until June 30, 2022. Study quality assessment was performed using the QUADAS‐2 toolbox. A random effects bivariate model was exploited for data synthesis.
Results
Our systematic review identified 27 eligible studies according to the predefined inclusion criteria, of which 22 were included in the final analysis. Overall, 1855 patients with synucleinopathies and 1378 non‐synucleinopathies as control subjects were included in the meta‐analysis. The pooled sensitivity and specificity to differentiate synucleinopathies from controls with αSyn‐SAA were 0.88 (95% CI, 0.82–0.93) and 0.95 (95% CI, 0.92–0.97), respectively. Evaluating the diagnostic performance of RT‐QuIC in a subgroup analysis for the detection of patients with multiple system atrophy the pooled sensitivity decreased to 0.30 (95% CI, 0.11–0.59).
Conclusions
While our study clearly demonstrated a high diagnostic performance of RT‐QuIC and PMCA for differentiating synucleinopathies with Lewy bodies from controls, results for the diagnosis of multiple system atrophy were less robust.
Keywords: protein misfolding cyclic amplification (PMCA), real‐time quaking‐induced conversion (RT‐QuIC), alpha‐synuclein, synucleinopathy, biomarker
Synucleinopathies is used as an umbrella term for a class of neurodegenerative diseases characterized by the misfolding and aberrant accumulation of α‐synuclein leading to the formation of Lewy bodies in Parkinson's disease (PD) and dementia with Lewy bodies (DLB) or to the appearance of oligodendroglial cytoplasmic inclusion in multiple system atrophy (MSA). 1 Pure autonomic failure (PAF) or idiopathic rapid‐eye movement sleep behavior disorder (iRBD) may precede synucleinopathies and are increasingly recognized as the prodromal stage of these neurodegenerative diseases. 2 , 3 The differential diagnosis of PD from atypical Parkinsonian disorders (APDs), including MSA and progressive supranuclear palsy (PSP), which represents a tauopathy, remains a clinical challenge. Particularly in the early stages patients are frequently misdiagnosed, even by movement disorder experts. 4 Therefore, validated tools and biomarkers are urgently needed to correctly classify these patients early in the disease enabling adequate care, counseling and whenever available initiate disease‐modifying therapies.
In recent years, a prion‐like propagation of α‐synuclein, spreading from neuron to neuron in synucleinopathies, has been demonstrated experimentally in vitro and in vivo. 5 , 6 Importantly, there is no evidence of transmissibility in humans which is a prerequisite for a prion disease. 7 Nevertheless, the similarities opened an avenue for the development of novel diagnostic and therapeutic approaches in the field of neurodegenerative diseases. With regards to diagnostic biomarkers, the real‐time quaking‐induced conversion assay (RT‐QuIC), a well‐established tool in the diagnosis of Creutzfeld‐Jakob disease (CJD), 8 appeared to be of particular interest. Green and his group 9 were the first to use their expertise in the RT‐QuIC assay to adopt it for the detection of α‐synuclein aggregates in the cerebrospinal fluid (CSF) of PD patients and patients with RBD. Shortly afterwards Groveman et al. 10 reported about optimized reaction conditions for completing the α‐synuclein RT‐QuIC assay in a significantly shorter amount of time. Both of these two initial studies 9 , 10 yielded an excellent sensitivity and specificity for discriminating synucleinopathies from controls.
Already small amounts of misfolded α‐synuclein can be detected by RT‐QuIC via incubation of a pathogenic seed derived from biological fluids or tissues with a reaction buffer containing recombinant α‐synuclein, which acts as a substrate. 11 , 12 Protein misfolding cyclic amplification (PMCA) is another concept of seeding assays working in a similar way to RT‐QuIC with some methodical differences and was first introduced for the detection of α‐synuclein aggregates in CSF by Soto et al. 13
So far, α‐synuclein seeding activity has mainly been tested with CSF as a seed for discriminating patients with synucleinopathies from controls via RT‐QuIC or PMCA. Therefore, the aim of this systematic review and meta‐analysis was to evaluate the diagnostic accuracy of αSyn‐SAAs using CSF as source material for the diagnosis of synucleinopathies.
Methods
Literature Search Strategy
This meta‐analysis followed the PRISMA statement. Two reviewers (AG, GH) systematically searched the electronic MEDLINE database PubMed by the following search strategy:
((“real‐time”[All Fields] AND “quaking‐induced”[All Fields] AND (“conversion”[All Fields] OR “conversions”[All Fields])) OR “RT‐QuIC”[All Fields]) AND (“lewy body disease”[MeSH Terms] OR (“Lewy”[All Fields] AND “body”[All Fields] AND “disease”[All Fields]) OR “lewy body disease”[All Fields] OR (“Lewy”[All Fields] AND “body‐associated”[All Fields] AND (“synucleinopathies”[MeSH Terms] OR “synucleinopathies”[All Fields] OR “synucleinopathy”[All Fields])) OR (“parkinson disease”[MeSH Terms] OR (“parkinson”[All Fields] AND “disease”[All Fields]) OR “parkinson disease”[All Fields]) OR (“pharmacology”[MeSH Subheading] OR “pharmacology”[All Fields] OR “pd”[All Fields]) OR ((“dementia”[MeSH Terms] OR “dementia”[All Fields] OR “dementias”[All Fields] OR “dementia s”[All Fields]) AND (“lewy bodies”[MeSH Terms] OR (“Lewy”[All Fields] AND “bodies”[All Fields]) OR “lewy bodies”[All Fields] OR (“Lewy”[All Fields] AND “body”[All Fields]) OR “lewy body”[All Fields])) OR “DLB”[All Fields] OR (“lewy bodies”[MeSH Terms] OR (“Lewy”[All Fields] AND “bodies”[All Fields]) OR “lewy bodies”[All Fields] OR (“Lewy”[All Fields] AND “body”[All Fields]) OR “lewy body”[All Fields]) OR (“synucleinopathies”[MeSH Terms] OR “synucleinopathies”[All Fields] OR “synucleinopathy”[All Fields]) OR (“synucleinopathies”[MeSH Terms] OR “synucleinopathies”[All Fields] OR “synucleinopathy”[All Fields]) OR (“synucleinopathies”[MeSH Terms] OR “synucleinopathies”[All Fields] OR “a synucleinopathies”[All Fields]) OR (“synucleinopathies”[MeSH Terms] OR “synucleinopathies”[All Fields] OR “a synucleinopathy”[All Fields]) OR (“synucleinopathies”[MeSH Terms] OR “synucleinopathies”[All Fields] OR (“alpha”[All Fields] AND “synuclein”[All Fields] AND “pathology”[All Fields]) OR “alpha synuclein pathology”[All Fields]) OR (“synucleinopathies”[MeSH Terms] OR “synucleinopathies”[All Fields] OR (“alpha”[All Fields] AND “synuclein”[All Fields] AND “pathologies”[All Fields]) OR “alpha synuclein pathologies”[All Fields]) OR (“synucleinopathies”[MeSH Terms] OR “synucleinopathies”[All Fields] OR (“alpha”[All Fields] AND “synucleinopathies”[All Fields]) OR “alpha synucleinopathies”[All Fields]) OR ((“alpha”[All Fields] OR “alpha s”[All Fields] OR “alphas”[All Fields]) AND “Synucleinopathie”[All Fields]) OR (“synucleinopathies”[MeSH Terms] OR “synucleinopathies”[All Fields] OR (“alpha”[All Fields] AND “synucleinopathy”[All Fields]) OR “alpha synucleinopathy”[All Fields])).
The final search was conducted on June 30, 2022 and resulted in a total of 134 articles.
Inclusion and Exclusion Criteria
For this systematic review, studies had to fulfill the following predefined criteria: (1) manuscripts were required to be published in English language; (2) patients with synucleinopathies were included; (3) the source material for the aggregation assays was CSF; (4) the detection method for α‐synuclein aggregates was RT‐QuIC or PMCA; (5) studies had to report either true positive (TP), true negative (TN), false positive (FP), and false negative (FN) rates, or overall sample size and sensitivity and specificity values. The criteria for exclusion were as follows: (1) Non‐diagnostic test studies; (2) When subjects of two studies overlapped, the study with the smaller sample size was excluded from further analysis. Sample sizes informing the decisions on study inclusion were determined individually for each subgroup analysis resulting in a difference in the composition of studies in different subgroup analyses.
Data Extraction
Two investigators (AG, GH) independently extracted data on the (1) first author; (2) year of publication; (3) overall sample size; (4) number of participants in each group; (5) diagnostic criteria for the clinical diagnosis of PD, DLB, MSA, RBD, PAF, progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and Alzheimer's disease (AD); (6) αSyn‐SAA used (RT‐QuIC or PMCA); (7) cut‐off values to be considered as positive samples; (8) TP, TN, FP, and FN rates, or alternatively, sensitivity and specificity.
Quality Assessment
The Quality Assessment Tool for Diagnostic Accuracy Studies 2 (QUADAS‐2), 14 which was rated and documented using Review Manager 5.3 (Nordic Cochrane Centre, Copenhagen, Denmark), was used to assess each study's methodological quality concerning risk of bias and applicability.
Data Analysis
Statistical analyses were performed for the following subgroups: (1) synucleinopathies versus non‐synucleinopathies; (2) synucleinopathies with Lewy bodies (LBs) versus non‐synucleinopathies; (3) synucleinopathies with LBs versus healthy controls (HC); (4) synucleinopathies with LBs versus PSP/CBD; (5) established PD/DLB versus non‐synucleinopathies; (6) MSA versus non‐synucleinopathies; (7) MSA versus PSP/CBD; (8) prodromal stages of synucleinopathies versus non‐synucleinopathies; (9) RBD versus non‐synucleinopathies.
Synucleinopathies comprised patients with PD, DLB, MSA, prodromal disease stages of synucleinopathies, and patients with neuropathologically confirmed existence of Lewy bodies mixed with other pathologies. Prodromal stages of synucleinopathies included patients with RBD, PAF or mild cognitive impairment with Lewy bodies (MCI‐LB) as well as non‐manifesting carriers (NMC) of genetic mutations known to cause PD.
Synucleinopathies with LBs included patients with PD, genetic forms of PD, DLB, and neuropathologically confirmed existence of LBs mixed with other pathologies. Prodromal disease stages were not included in the patient group for these analyses as they could also proceed to MSA.
Non‐synucleinopathies included patients with other neurodegenerative and neurological disorders as well as HC.
For data synthesis, MetaDTA, an online toolbox applying a random effects bivariate model, was used. 15 , 16 Forest plots were created in Stata 16.1. Hierarchical summary receiver operating characteristics curves (HSROC) with 95% confidence intervals (95% CI) were mapped to summarize sensitivities and specificities of each study. 17 Furthermore, to assess heterogeneity of sensitivities and specificities, Chi‐square tests were applied, the null hypothesis being in both cases that all studies are equal.
Results
Study Selection
A total of 134 articles were identified in the final search. In total, 27 articles were eligible according to our predefined inclusion criteria and 22 articles, comprising 1855 patients with synucleinopathies and 1378 non‐synucleinopathies as controls (566 HC), were included in the main analysis. A detailed overview about the study selection process is given in Figure 1.
Figure 1.
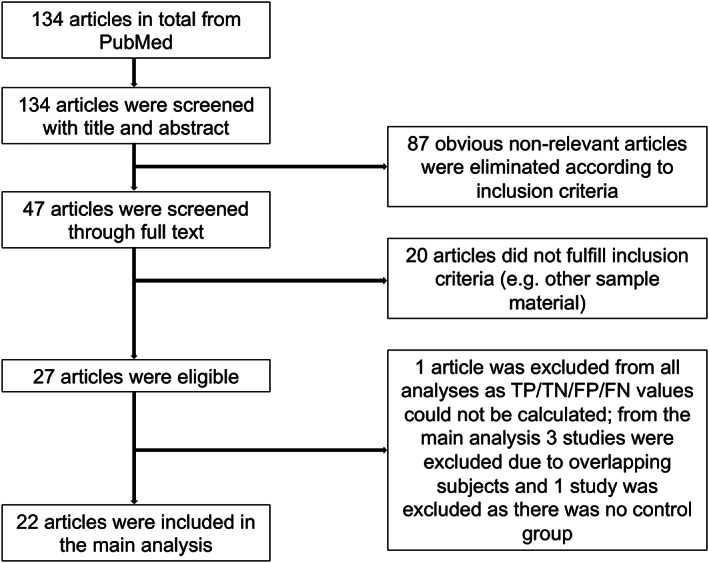
Flowchart for the identification of included studies.
Two studies 18 , 19 were excluded from all analyses. In one of these studies 18 sensitivity and specificity values were only given according to ROC analysis and no TP/FN/FP/TN values were reported. In the other study 19 there was no control group for non‐synucleinopathies.
For the main analysis of the diagnostic value of αSyn‐SAAs in the differential diagnosis of synucleinopathies versus non‐synucleinopathies two studies 20 , 21 were excluded as the subjects overlapped with other studies 22 , 23 and the sample size was smaller. An additional study 9 was excluded as CSF samples were obtained from the same longitudinal cohort used in another study 24 and an overlap of subjects could not be excluded.
Study Characteristics
The criteria used for the clinical diagnosis of PD, DLB, PSP, and AD were not consistent between the studies: For PD diagnosis, the UK Brain Bank diagnostic criteria 25 were used in ten studies, 9 , 13 , 18 , 20 , 22 , 24 , 26 , 27 , 28 , 29 the MDS clinical diagnostic criteria 30 were used in eight studies, 10 , 19 , 21 , 23 , 31 , 32 , 33 , 34 the diagnostic criteria of the National Institute of Neurological Disorders and Stroke (NINDS) 35 were used in one study 36 and three studies 37 , 38 , 39 did not explicitly state which criteria for PD diagnosis were used. One study 26 used the CSF from PD patients enrolled in the NINDS Parkinson's Disease Biomarker Program, in which diagnostic criteria were only explicitly mentioned for one site.
For DLB diagnosis, two studies 10 , 36 used the third report of the DLB consortium 40 and ten studies 19 , 21 , 23 , 28 , 32 , 33 , 38 , 39 , 41 , 42 used the fourth report of the DLB consortium. 43 Two studies 13 , 31 did not explicitly state which diagnostic criteria for DLB were used.
For the clinical diagnosis of PSP, five studies 21 , 23 , 31 , 34 , 42 used the MDS criteria, 44 two studies 36 , 39 used the criteria proposed by the NINDS and Society for PSP (SPSP) international workshop, 45 and two studies 10 , 26 did not explicitly state diagnostic criteria for PSP.
For the clinical diagnosis of AD, three studies 10 , 41 , 42 used the National Institute on Aging and Alzheimer's Association (NIA‐AA) 2011 criteria, 46 one study 23 used the International Working Group (IWG)‐2 criteria, 47 one study 39 used the CSF “Alzheimer profile” proposed by Duits et al. 48 and two studies 13 , 32 did not explicitly state the diagnostic criteria used.
For the clinical diagnosis of MSA, all studies 13 , 19 , 21 , 23 , 24 , 29 , 34 , 36 , 38 , 39 , 41 except one 31 used the second consensus statement. 49 The latter 31 did not explicitly report which diagnostic criteria were used.
For the clinical diagnosis of CBD, all studies 21 , 23 , 31 , 34 , 42 used the Armstrong et al. criteria. 50
One study 51 included patients with MCI in their cohort and classified them into three groups: MCI‐LB, MCI‐AD, and unspecified MCI. MCI‐LB was diagnosed when the current criteria for probable MCI‐LB 52 were fulfilled and MCI‐AD was diagnosed when in vivo evidence of AD pathology was present. 47 , 48 , 53
The clinical diagnosis of RBD was confirmed by polysomnography in two studies 9 , 32 and explicitly established according to the International classification of sleep disorders‐third edition (ICSD3) 54 in three studies. 21 , 23 , 24
Seven studies 9 , 10 , 23 , 33 , 41 , 55 , 56 included a cohort with neuropathologically confirmed diagnoses as well, in which pathologies with incidental LBs could also be detected.
RT‐QuIC was used as Syn‐SAA in 22 studies 9 , 10 , 20 , 21 , 22 , 23 , 24 , 26 , 27 , 28 , 31 , 33 , 34 , 36 , 37 , 39 , 41 , 42 , 51 , 55 , 57 and PMCA was used in five studies. 13 , 18 , 19 , 29 , 38 One study 56 used a quantitative RT‐QuIC approach.
Details about TP/TN/FP/FN values, sensitivity, specificity and cut‐off values to be considered as positive samples in each included study are given in Table 1. Table S1 gives a detailed overview about patient and control groups in each of the eligible studies.
TABLE 1.
Diagnostic results and methodological aspects for all included studies in the analysis of synucleinopathies versus non‐synucleinopathies
| Author | TP | FN | FP | TN | Sens | Spec | Assay | Cut‐off value |
|---|---|---|---|---|---|---|---|---|
| Groveman et al. 2018 | 27 | 2 | 0 | 31 | 0.93 | 1.00 | RT‐QuIC | Mean of all samples +3SD |
| Manne et al. 2019 | 15 | 0 | 2 | 14 | 1.00 | 0.88 | RT‐QuIC | Mean of all samples +10SD |
| Garrido et al. 2019 | 18 | 23 | 2 | 8 | 0.44 | 0.80 | RT‐QuIC | Mean of negative controls +2SD |
| Van Rumund et al. 2019 | 62 | 21 | 5 | 72 | 0.75 | 0.94 | RT‐QuIC | Mean of negative controls +2SD |
| Bongianni et al. 2019 | 43 | 11 | 2 | 57 | 0.80 | 0.97 | RT‐QuIC | Mean of all samples +3SD |
| Rossi et al. 2020 | 166 | 39 | 10 | 224 | 0.81 | 0.96 | RT‐QuIC | Mean of neuropathological controls +30SD |
| Orru et al. 2020 | 105 | 3 | 11 | 74 | 0.97 | 0.87 | RT‐QuIC | 10% of maximum value |
| Donadio et al. 2021 | 6 | 2 | 0 | 26 | 0.75 | 1.00 | RT‐QuIC | Mean of negative controls +3SD |
| Iranzo et al. 2021 | 47 | 5 | 4 | 47 | 0.90 | 0.92 | RT‐QuIC | Mean of negative controls +2SD |
| Bargar et al. 2021 | 143 | 3 | 0 | 68 | 0.98 | 1.00 | RT‐QuIC | Mean background fluorescence +5SD |
| Rossi et al. 2021 | 77 | 4 | 20 | 188 | 0.95 | 0.90 | RT‐QuIC | Mean of negative controls +30SD |
| Russo et al. 2021 | 24 | 4 | 1 | 29 | 0.86 | 0.97 | RT‐QuIC | 10% of maximum value |
| Brockmann et al. 2021 | 244 | 54 | 2 | 24 | 0.82 | 0.92 | RT‐QuIC | Mean of negative controls +30SD |
| Poggiolini et al. 2021 | 113 | 30 | 2 | 53 | 0.79 | 0.96 | RT‐QuIC | Mean of initial fluorescence at 120 h + 5SD |
| Mammana et al. 2021 | 22 | 1 | 0 | 57 | 0.96 | 1.00 | RT‐QuIC | 15% of maximum value |
| Perra et al. 2021 | 16 | 0 | 3 | 29 | 1.00 | 0.91 | RT‐QuIC | Mean fluorescence during initial 10 h + 10SD |
| Sokratian et al. 2021 | 27 | 4 | 0 | 14 | 0.87 | 1.00 | qRT‐QuIC | >200 FFUs/ml |
| Compta et al. 2022 | 19 | 38 | 4 | 51 | 0.33 | 0.93 | RT‐QuIC | Mean of negative controls +2SD |
| Hall et al. 2022 | 68 | 13 | 13 | 49 | 0.84 | 0.79 | RT‐QuIC | 10% of maximum value |
| Shahnawaz et al. 2017 | 85 | 11 | 12 | 85 | 0.89 | 0.88 | PMCA | ≥50 FU |
| Singer et al. 2020 | 87 | 4 | 0 | 29 | 0.96 | 1.00 | PMCA | ≥150 AU |
| Shahnawaz et al. 2020 | 153 | 16 | 0 | 56 | 0.91 | 1.00 | PMCA | ≥50 FU |
Abbreviations: SD, standard deviation; WT, wild type; FFUs, fibril forming units; FU, fluorescence units; AU, arbitrary units.
Quality Assessment
Quality assessment results based on the QUADAS‐2 tool 14 are represented in Figure 2. The overall risk of bias concerning the reference standard and index test was low, whereas for flow and timing the risk of bias was rated as high or unclear in most of the eligible studies. Overall, concerns regarding applicability were low in the majority of eligible studies.
Figure 2.
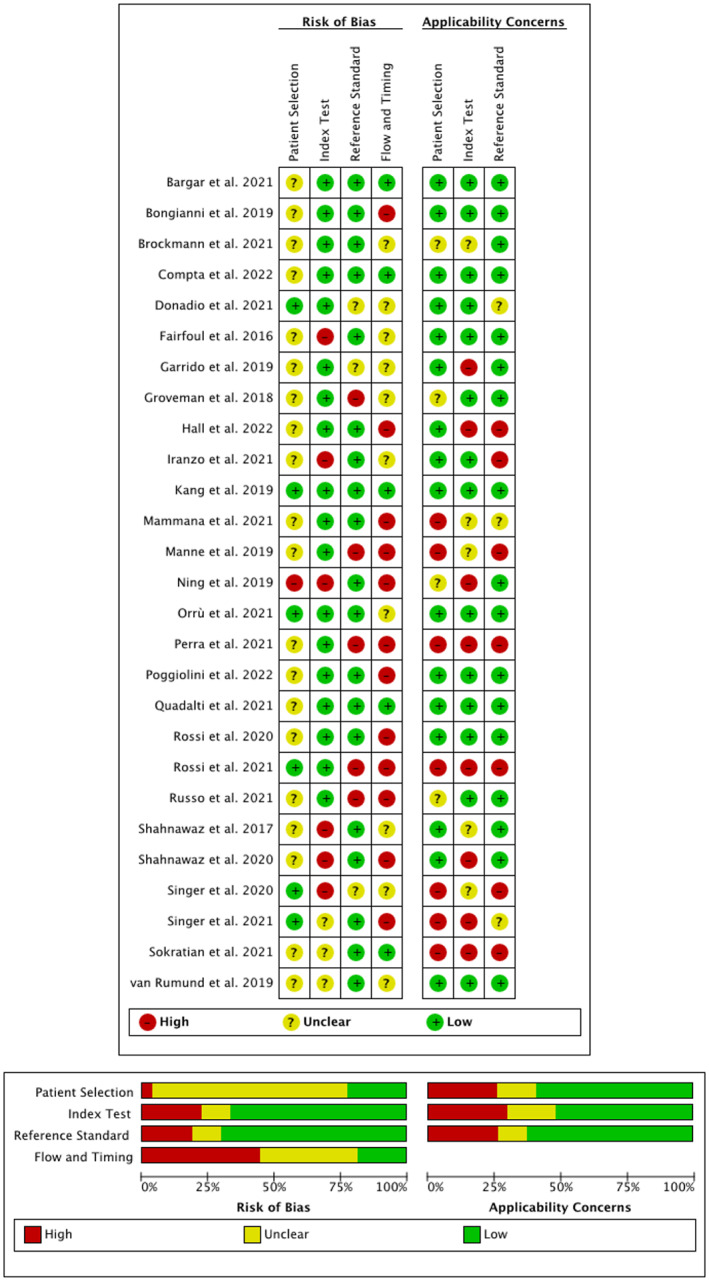
Quality assessment of all eligible studies based on QUADAS‐2.
Meta‐Analysis
The analysis of this systematic review showed a pooled sensitivity and specificity of αSyn‐SAAs, including RT‐QuIC and PMCA, for differentiating synucleinopathies from non‐synucleinopathies of 0.88 (95% CI, 0.82–0.93) and 0.95 (95% CI, 0.92–0.97), respectively (Fig. 3). Figure 4 represents the HSROC for this analysis.
Figure 3.
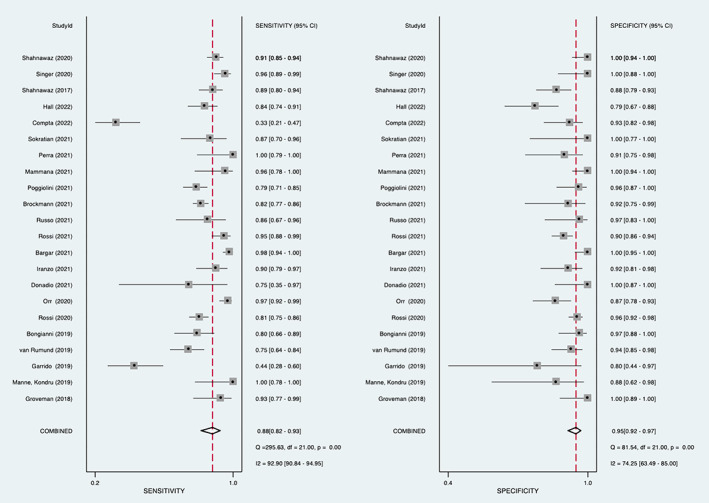
Forest plot of sensitivity and specificity of αSyn‐SAAs for the diagnosis of synucleinopathies versus non‐synucleinopathies.
Figure 4.
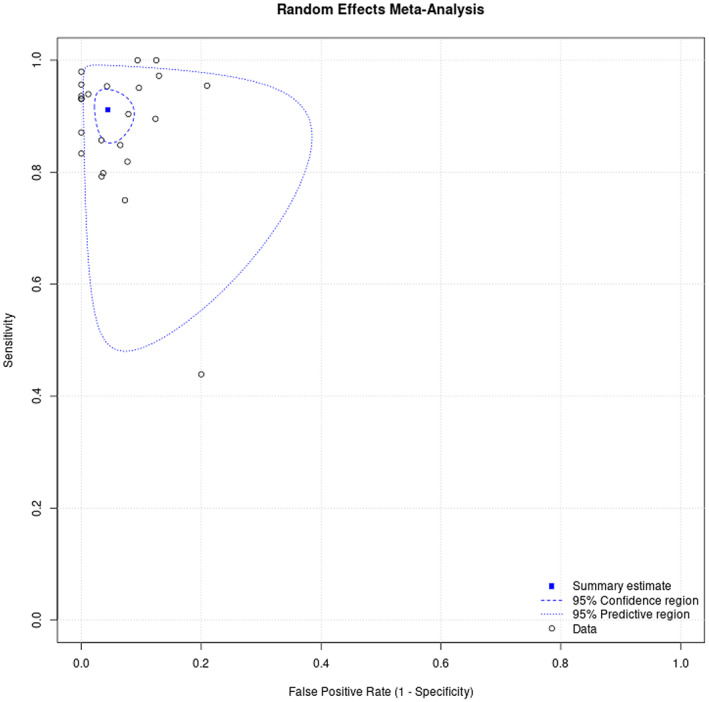
Summary receiver operating characteristic curve of αSyn‐SAAs for the diagnosis of synucleinopathies versus non‐synucleinopathies.
Furthermore, we performed several subgroup‐analyses to assess the diagnostic value of αSyn‐SAAs for synucleinopathies with LB pathology. The pooled sensitivity and specificity were 0.91 (95% CI, 0.87–0.95) and 0.96 (95% CI, 0.93–0.98), respectively, for differentiating synucleinopathies with LBs from non‐synucleinopathies. Considering only patients with established PD or DLB according to diagnostic criteria, the pooled sensitivity showed a slight increase to 0.92 (95% CI, 0.88–0.95). The pooled specificity for differentiating synucleinopathies with LBs from HC and from PSP/CBD was 0.97 (95% CI, 0.92–0.99) and 0.94 (95% CI, 0.79–0.98), respectively.
Additionally, the pooled sensitivity and specificity of αSyn‐SAAs was evaluated for the differential diagnosis of MSA versus non‐synucleinopathies. The pooled sensitivity and specificity were 0.57 (95% CI, 0.26–0.83) and 0.96 (95% CI, 0.91–0.99), respectively. The pooled sensitivity decreased to 0.30 (95% CI, 0.11–0.59) when only studies using RT‐QuIC as seeding method to differentiate MSA from non‐synucleinopathies were included. For the differentiation of MSA from PSP/CBD αSyn‐SAAs yielded a pooled sensitivity and specificity of 0.18 (95% CI, 0.08–0.37) and 0.90 (95% CI, 0.70–0.97), respectively.
For the differentiation of patients with prodromal signs of synulceinopathies from non‐synucleinopathies αSyn‐SAAs yielded a pooled sensitivity and specificity of 0.74 (95% CI, 0.36–0.93) and 0.93 (95% CI, 0.89–0.96), respectively. The diagnostic performance of αSyn‐SAAs for patients with RBD showed sensitivity rates of 0.64 (95% CI, 0.50–0.77), 24 0.80 (95% CI, 0.58–0.92) 32 and 1.00 (95% CI, 0.82–1.00). 23 Pooled sensitivity and specificity rates could not be computed for this analysis due to the small number of included studies.
Forest plots for all analyses can be found in the supplemental material (Data S1).
Discussion
In this systematic review and meta‐analysis, our aim was to systematically evaluate the diagnostic accuracy of αSyn‐SAAs, including RT‐QuIC and PMCA, for the differential diagnosis of synucleinopathies versus non‐synucleinopathies. Furthermore, we performed several subgroup analyses to address pertinent research questions.
Overall, our meta‐analysis showed a pooled sensitivity and specificity of 0.88 (95% CI, 0.82–0.93) and 0.95 (95% CI, 0.92–0.97), respectively, to distinguish synucleinopathies from non‐synucleinopathies. There is one previous meta‐analysis 58 assessing the performance of RT‐QuIC in the diagnosis of LBDs reporting a pooled sensitivity and specificity of 0.91 and 0.95, respectively, which is similar to the results of our analysis. Extending the previous meta‐analysis, 58 we have also included studies using PMCA methodology and our study also differs in further methodological aspects: (1) MSA patients were included in the patient group in our main analysis, as α‐synuclein aggregates in the form of oligodendroglial cytoplasmic inclusions represent the histopathological hallmark of the disease 1 ; (2) Non‐manifesting carriers of genetic mutations associated with synuclein pathology known to cause PD and patients with MCI‐LB were also included as they represent prodromal disease stages, in which diagnostic utility of αSyn‐SAAs may be of particular relevance for future clinical trials and clinical practice; (3) For the main analysis of the diagnostic accuracy of αSyn‐SAAs in the differential diagnosis of synucleinopathies versus non‐synucleinopathies, we included four more studies 34 , 36 , 51 , 56 using RT‐QuIC, and three more studies 13 , 29 , 38 using PMCA, while we excluded one study 9 of the former meta‐analysis because subjects were from the same longitudinal cohort reported in another study 24 ; (4) We performed several subgroup‐analyses focusing on different patient cohorts to generate more profound data on the diagnostic utility of RT‐QuIC and PMCA in specific clinical settings.
Regarding the quality of included studies the overall risk of bias was adequate, only for flow and timing the risk was rated as high in a substantial number of eligible studies (see Fig. 2). Concerns regarding applicability were particularly high or unclear in seven eligible studies. 19 , 26 , 33 , 38 , 42 , 51 , 56 Regarding the risk of bias in this tool the categories “reference standard” and “index test” refer to the conduction and interpretation of the tests. Knowledge of the results of one out of the index or reference test may influence interpretation of the other test, hence, a blinded rating is preferred. “Patient selection” refers to the recruitment of included patients, for example whether they were enrolled consecutively or whether a random sample was chosen. “Flow and timing” can introduce a risk of bias when for example the interval between the index test and reference standard is inappropriate or when patients are excluded. This is particularly important since improvement or deterioration of the condition (eg, disease progression or symptom alleviation through symptomatic therapies) may cause misclassification and thereby impacts the diagnostic accuracy. Concerns regarding applicability address the question, whether the patient selection, index test and reference standard match the specific research question. Moreover, diagnostic criteria used as a reference standard for the clinical diagnosis of PD, DLB, PSP, and AD were not consistent between the studies, which yields the risk of misdiagnosis in some cases. This aspect needs be taken into consideration when interpreting the obtained values for the overall sensitivity and specificity in each analysis. Particularly, post‐mortem verification of the clinical diagnosis of a synucleinopathy was only obtained in a minority of patients in a few studies. 9 , 10 , 23 , 33 , 41 , 55 , 56 Cut‐off values for samples to be considered as positive were also not the same across included studies, which should be consistently defined in future harmonized protocols. Indeed, standardization of diagnostic and pre‐analytical procedures are vital for the establishment of uniform cut‐off values.
The results of the present and of the previous meta‐analysis 58 demonstrate that αSyn‐SAAs represent a highly sensitive method for the diagnosis of synucleinopathies and support their usefulness as a diagnostic biomarker in clinical routine work‐up. The pooled specificity for differentiating synucleinopathies with LBs from PSP/CBD was high as well with 0.94 (95% CI, 0.79–0.98), although sample sizes for PSP/CBD were rather small in most of the included studies.
In the analysis of synucleinopathies versus non‐synucleinopathies, two studies 27 , 34 showed markedly lower sensitivity rates with 0.44 (95% CI, 0.28–0.60) and 0.33 (95% CI 0.21–0.47), respectively. In one study, 27 non‐manifesting carriers of the p.G2019S mutation in the LRRK2 gene were negative for α‐synuclein aggregates by RT‐QuIC and only 40% of manifest LRRK2‐PD patients presented with a positive RT‐QuIC result. This is consistent with the pleiotropic pathology associated with the p.G2019S mutation, 59 , 60 which may lack α‐synuclein pathology. 61 , 62 , 63
One study in MSA patients, 34 also reported a high proportion of patients presenting with negative CSF α‐synuclein RT‐QuIC results. Overall, CSF αSyn‐SAAs have shown inconsistent results in MSA so far 21 , 31 , 34 , 36 with detection rates ranging from <10% to 35%, except for one study 24 demonstrating a sensitivity of 75% for MSA. Two studies 39 , 41 only included a very small number of MSA patients, hence results are difficult to interpret.
Studies using PMCA as seed amplification assay for α‐synuclein in MSA patients showed better diagnostic performance with sensitivities of 80% to 97%. 13 , 19 , 29 , 38 The pooled overall sensitivity for RT‐QuIC in the diagnosis of MSA was estimated to be 0.30 (95% CI, 0.11–0.59) in our analysis. When studies using PMCA were included, the pooled sensitivity increased to 0.57 (95% CI, 0.26–0.83). A possible explanation for this observed discrepancy could be the use of different reaction buffers and structural differences in α‐synuclein strains of MSA patients as described in one study. 64 Overall, MSA patients showed markedly lower maximum fluorescence values compared to PD patients in the PMCA assay. 13 , 19 , 29 , 38 Notably, differences in Thioflavin T (ThT) fluorescence do not seem to simply reflect different amounts of misfolded aggregates at the end of the reaction, but may rather be due to structural differences of α‐synuclein aggregates (‘strains’) in PD and MSA patients, leading to differences in the accessibility of the mode of interaction of ThT with aggregates. 29 By investigating different thiophene‐based ligands, it was shown that different ligands prefer binding to either PD or MSA α‐synuclein aggregates. 29 These findings illustrate a limitation of the current clinical applicability of RT‐QuIC and further research is required to better understand the discrepancies between RT‐QuIC and PMCA as well as to improve the differential diagnostic yield of these tests.
Intriguingly, one study 65 using olfactory mucosa as seeding material for RT‐QuIC reported a totally different behavior of MSA from the parkinsonian subtype (MSA‐P) and MSA from the cerebellar subtype (MSA‐C) with the latter being almost unresponsive to RT‐QuIC, while 90% of MSA‐P samples showed a positive seeding activity. The authors of this study 65 suggest that this again may be due to differences in α‐synuclein strains possessing different tropism for peripheral tissues.
Moreover, two studies 10 , 55 included in our meta‐analysis suggest a distinct pattern of RT‐QuIC fluorescence kinetics between CSF samples from PD and DLB patients, with PD samples showing a slower seeding reactivity with a lower maximum ThT fluorescence value. Again, variants in strains of α‐synuclein between PD and DLB patients were mentioned as a possible explanation for the observed differences in RT‐QuIC kinetics. 10
As MSA and PSP/CBD can be difficult to discriminate in the early disease stages, we assessed the diagnostic performance of αSyn‐SAAs to differentiate between these two types of APD as well. Only studies using RT‐QuIC could be included in this analysis and showed a pooled sensitivity of 0.18 (95% CI, 0.08–0.37). Therefore, our results do not support the use of this assay to differentiate MSA from PSP/CBD.
The time required for PMCA is significantly longer with 13–15 days 13 , 19 instead of 1–5 days for RT‐QuIC, depending on the protocol used, 9 , 10 which is a factor to be considered in future clinical routine use.
More recently, α‐synuclein RT‐QuIC has also been tested in sample materials other than CSF, including skin homogenates, 33 , 39 , 66 , 67 olfactory mucosa, 42 , 65 , 68 , 69 submandibular glands, 70 and most recently also neuronally derived exososomes in peripheral blood. 71 Generally, results have been encouraging and less invasive sampling techniques for α‐Syn‐SAAs like skin biopsies, nasal swabs or, ultimately blood may be preferable over CSF sampling in future clinical routine.
The present results also demonstrate that αSyn‐SAAs may be able to detect prodromal stages of synucleinopthies and differentiate this patient group from non‐synucleinopathies with a pooled sensitivity and specificity of 0.74 (95% CI, 0.36–0.93) and 0.93 (95% CI, 0.89–0.96). respectively. In patients with RBD sensitivity rates ranged from 0.64–1.00. 23 , 24 , 32 The use of αSyn‐SAAs might have significant implications in the clinical diagnosis of prodromal synucleinopathies, including prodromal PD, and in designing future clinical trials with disease‐modifiying therapies, which should target patients at pre‐clinical stages of the disease. However, with seven studies 23 , 24 , 27 , 28 , 32 , 39 , 51 the number included in our analysis is rather small. Therefore, more studies with larger numbers of this patient group and a longitudinal follow‐up to assess conversion into a synucleinopathy are needed.
Conclusion
Our systematic review and meta‐analysis clearly demonstrated a high diagnostic performance of CSF α‐synuclein RT‐QuIC and PMCA for differentiating synucleinopathies with LBs from non‐synucleinopathies, which should be considered when framing supporting criteria for the clinical diagnosis of PD or DLB. Results in MSA have so far been inconsistent and it is unclear, whether differences exist between performance in MSA‐P versus MSA‐C, which clearly needs further investigation. Furthermore, our analysis provides evidence for the potential usefulness of CSF αSyn‐SAAs in identifying prodromal stages of synucleinopathies. Importantly, alternative and less invasive sampling approaches for these assays have provided encouraging results that may suggest non‐inferiority to CSF tests. Ultimately, α‐synuclein SAAs on peripheral blood may become the future diagnostic tool of choice for the identification of synucleinopathies.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution. (2) Statistical analysis: A. Design, B. Execution, C. Review and Critique. (3) Manuscript preparation: A. Writing of the first draft, B. Review and Critique.
A.G.: 1A, 1B, 1C, 2A, 3A
G.H.: 1C, 3B
F.K.: 2A, 2B, 3B
M.P.: 1C, 2C, 3B
A.D.: 1A, 2C, 3B
W.P.: 1A, 2C, 3B
K.S.: 1A, 1B, 2A, 2C, 3B
B.H.: 1A, 1B, 1C, 2A, 2B, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. No specific approval by an ethics committee or informed patient consent was necessary for this work.
Funding Sources and Conflicts of Interest: No specific funding was received for this work. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months: Anna Grossauer received travel grants from Boston Scientific and Novartis. Greta Hemicker and Marina Peball declare that there are no additional disclosures to report. Florian Krismer received personal fees from Institut de Recherches Internationales Servier, Clarion Healthcare, Takeda Pharmaceuticals, Sanofi‐Aventis and the Austrian Society of Neurology; grant support from the National Institutes of Health outside of the submitted work. Atbin Djamshidian reveived honoraria from Novo Nordisk, Bial, Roche, Biogen und Abbvie. Werner Poewe reports personal fees from: AC Immune, Alterity, AbbVie, BIAL, Britannia, Lundbeck, Neuroderm, Roche, Sunovion, Stada, Takeda, UCB and Zambon (consultancy and lecture fees in relation to clinical drug development programmes for PD). Klaus Seppi reports personal fees from Ono Pharma UK Ltd, Teva, UCB, Lundbeck, AOP Orphan Pharmaceuticals AG, Roche, Grünenthal, Stada, Licher Pharma, Biogen, BIAL, EverPharma and Abbvie, honoraria from the International Parkinson and Movement Disorders Society, research grants from FWF Austrian Science Fund, Michael J. Fox Foundation, and AOP Orphan Pharmaceuticals AG, outside the submitted work. Beatrice Heim reports honoraria from AOP orphan Pharmaceuticals AG and research grants from FWF Austrian Science Fund, outside the submitted work.
Supporting information
Table S1. Detailed overview about patient and control groups in each of the eligible studies
Data S1. Forest plots for all subgroup analyses
Contributor Information
Klaus Seppi, Email: klaus.seppi@i-med.ac.at.
Beatrice Heim, Email: beatrice.heim@i-med.ac.atl.
References
- 1. Wong YC, Krainc D. α‐Synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med 2017;23(2):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahlknecht P, Marini K, Werkmann M, Poewe W, Seppi K. Prodromal Parkinson's disease: hype or hope for disease‐modification trials? Transl Neurodegener 2022;11(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coon EA, Mandrekar JN, Berini SE, Benarroch EE, Sandroni P, Low PA, Singer W. Predicting phenoconversion in pure autonomic failure. Neurology 2020;95(7):e889–e897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta‐analysis. Neurology 2016;86(6):566–576. [DOI] [PubMed] [Google Scholar]
- 5. Ma J, Gao J, Wang J, Xie A. Prion‐like mechanisms in Parkinson's disease. Front Neurosci 2019;13:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernis ME, Babila JT, Breid S, Wüsten KA, Wüllner U, Tamgüney G. Prion‐like propagation of human brain‐derived alpha‐synuclein in transgenic mice expressing human wild‐type alpha‐synuclein. Acta Neuropathol Commun 2015;3:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wenning G, Trojanowski JQ, Kaufmann H, Wisniewski T, Rocca WA, Low PA. Is multiple system atrophy an infectious disease? Ann Neurol 2018;83(1):10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zerr I, Parchi P. Sporadic Creutzfeldt‐Jakob disease. Handb Clin Neurol 2018;153:155–174. [DOI] [PubMed] [Google Scholar]
- 9. Fairfoul G, McGuire LI, Pal S, et al. Alpha‐synuclein RT‐QuIC in the CSF of patients with alpha‐synucleinopathies. Ann Clin Transl Neurol 2016;3(10):812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Groveman BR, Orrù CD, Hughson AG, et al. Rapid and ultra‐sensitive quantitation of disease‐associated α‐synuclein seeds in brain and cerebrospinal fluid by αSyn RT‐QuIC. Acta Neuropathol Commun 2018;6(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakagaki T, Nishida N, Satoh K. Development of α‐Synuclein real‐time quaking‐induced conversion as a diagnostic method for α‐Synucleinopathies. Front Aging Neurosci 2021;13:703984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okuzumi A, Hatano T, Fukuhara T, et al. α‐Synuclein seeding assay using RT‐QuIC. Methods Mol Biol 2021;2322:3–16. [DOI] [PubMed] [Google Scholar]
- 13. Shahnawaz M, Tokuda T, Waragai M, et al. Development of a biochemical diagnosis of Parkinson disease by detection of α‐Synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol 2017;74(2):163–172. [DOI] [PubMed] [Google Scholar]
- 14. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155(8):529–536. [DOI] [PubMed] [Google Scholar]
- 15. Patel A, Cooper N, Freeman S, Sutton A. Graphical enhancements to summary receiver operating characteristic plots to facilitate the analysis and reporting of meta‐analysis of diagnostic test accuracy data. Res Synth Methods 2021;12(1):34–44. [DOI] [PubMed] [Google Scholar]
- 16. Freeman SC, Kerby CR, Patel A, Cooper NJ, Quinn T, Sutton AJ. Development of an interactive web‐based tool to conduct and interrogate meta‐analysis of diagnostic test accuracy studies: MetaDTA. BMC Med Res Methodol 2019;19(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rutter CM, Gatsonis CA. A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Stat Med 2001;20(19):2865–2884. [DOI] [PubMed] [Google Scholar]
- 18. Ning H, Wu Q, Han D, et al. Baseline concentration of misfolded α‐synuclein aggregates in cerebrospinal fluid predicts risk of cognitive decline in Parkinson's disease. Neuropathol Appl Neurobiol 2019;45(4):398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singer W, Schmeichel AM, Shahnawaz M, et al. Alpha‐Synuclein oligomers and Neurofilament light chain predict Phenoconversion of pure autonomic failure. Ann Neurol 2021;89(6):1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kang UJ, Boehme AK, Fairfoul G, et al. Comparative study of cerebrospinal fluid α‐synuclein seeding aggregation assays for diagnosis of Parkinson's disease. Mov Disord 2019;34(4):536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quadalti C, Calandra‐Buonaura G, Baiardi S, et al. Neurofilament light chain and α‐synuclein RT‐QuIC as differential diagnostic biomarkers in parkinsonisms and related syndromes. NPJ Parkinsons Dis 2021;7(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Orrù CD, Ma TC, Hughson AG, et al. A rapid α‐synuclein seed assay of Parkinson's disease CSF panel shows high diagnostic accuracy. Ann Clin Transl Neurol 2021;8(2):374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rossi M, Candelise N, Baiardi S, et al. Ultrasensitive RT‐QuIC assay with high sensitivity and specificity for Lewy body‐associated synucleinopathies. Acta Neuropathol 2020;140(1):49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poggiolini I, Gupta V, Lawton M, et al. Diagnostic value of cerebrospinal fluid alpha‐synuclein seed quantification in synucleinopathies. Brain 2022;145(2):584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55(3):181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manne S, Kondru N, Hepker M, et al. Ultrasensitive detection of aggregated α‐Synuclein in glial cells, human cerebrospinal fluid, and brain tissue using the RT‐QuIC assay: new high‐throughput Neuroimmune biomarker assay for parkinsonian disorders. J Neuroimmune Pharmacol 2019;14(3):423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garrido A, Fairfoul G, Tolosa ES, Martí MJ, Green A. α‐Synuclein RT‐QuIC in cerebrospinal fluid of LRRK2‐linked Parkinson's disease. Ann Clin Transl Neurol 2019;6(6):1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brockmann K, Quadalti C, Lerche S, et al. Association between CSF alpha‐synuclein seeding activity and genetic status in Parkinson's disease and dementia with Lewy bodies. Acta Neuropathol Commun 2021;9(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shahnawaz M, Mukherjee A, Pritzkow S, et al. Discriminating α‐synuclein strains in Parkinson's disease and multiple system atrophy. Nature 2020;578(7794):273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30(12):1591–1601. [DOI] [PubMed] [Google Scholar]
- 31. van Rumund A, Green AJE, Fairfoul G, Esselink RAJ, Bloem BR, Verbeek MM. α‐Synuclein real‐time quaking‐induced conversion in the cerebrospinal fluid of uncertain cases of parkinsonism. Ann Neurol 2019;85(5):777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iranzo A, Fairfoul G, Ayudhaya ACN, et al. Detection of α‐synuclein in CSF by RT‐QuIC in patients with isolated rapid‐eye‐movement sleep behaviour disorder: a longitudinal observational study. Lancet Neurol 2021;20(3):203–212. [DOI] [PubMed] [Google Scholar]
- 33. Mammana A, Baiardi S, Quadalti C, et al. RT‐QuIC detection of pathological α‐Synuclein in skin punches of patients with Lewy body disease. Mov Disord 2021;36(9):2173–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Compta Y, Painous C, Soto M, et al. Combined CSF α‐SYN RT‐QuIC, CSF NFL and midbrain‐pons planimetry in degenerative parkinsonisms: from bedside to bench, and back again. Parkinsonism Relat Disord 2022;99:33–41. [DOI] [PubMed] [Google Scholar]
- 35. Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56(1):33–39. [DOI] [PubMed] [Google Scholar]
- 36. Hall S, Orrù CD, Serrano GE, et al. Performance of αSynuclein RT‐QuIC in relation to neuropathological staging of Lewy body disease. Acta Neuropathol Commun 2022;10(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Russo MJ, Orru CD, Concha‐Marambio L, et al. High diagnostic performance of independent alpha‐synuclein seed amplification assays for detection of early Parkinson's disease. Acta Neuropathol Commun 2021;9(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Singer W, Schmeichel AM, Shahnawaz M, et al. Alpha‐Synuclein oligomers and Neurofilament light chain in spinal fluid differentiate multiple system atrophy from Lewy body Synucleinopathies. Ann Neurol 2020;88(3):503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Donadio V, Wang Z, Incensi A, et al. In vivo diagnosis of Synucleinopathies: a comparative study of skin biopsy and RT‐QuIC. Neurology 2021;96(20):e2513–e2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 2005;65(12):1863–1872. [DOI] [PubMed] [Google Scholar]
- 41. Bongianni M, Ladogana A, Capaldi S, et al. α‐Synuclein RT‐QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann Clin Transl Neurol 2019;6(10):2120–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perra D, Bongianni M, Novi G, et al. Alpha‐synuclein seeds in olfactory mucosa and cerebrospinal fluid of patients with dementia with Lewy bodies. Brain Commun 2021;3(2):fcab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology 2017;89(1):88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 2017;32(6):853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele‐Richardson‐Olszewski syndrome): report of the NINDS‐SPSP international workshop. Neurology 1996;47(1):1–9. [DOI] [PubMed] [Google Scholar]
- 46. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG‐2 criteria. Lancet Neurol 2014;13(6):614–629. [DOI] [PubMed] [Google Scholar]
- 48. Duits FH, Teunissen CE, Bouwman FH, et al. The cerebrospinal fluid "Alzheimer profile": easily said, but what does it mean? Alzheimers Dement 2014;10(6):713–723.e712. [DOI] [PubMed] [Google Scholar]
- 49. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71(9):670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80(5):496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rossi M, Baiardi S, Teunissen CE, et al. Diagnostic value of the CSF α‐Synuclein real‐time quaking‐induced conversion assay at the prodromal MCI stage of dementia with Lewy bodies. Neurology 2021;97(9):e930–e940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McKeith IG, Ferman TJ, Thomas AJ, et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 2020;94(17):743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Frisoni GB, Boccardi M, Barkhof F, et al. Strategic roadmap for an early diagnosis of Alzheimer's disease based on biomarkers. Lancet Neurol 2017;16(8):661–676. [DOI] [PubMed] [Google Scholar]
- 54. Sateia MJ. International classification of sleep disorders‐third edition: highlights and modifications. Chest 2014;146(5):1387–1394. [DOI] [PubMed] [Google Scholar]
- 55. Bargar C, Wang W, Gunzler SA, et al. Streamlined alpha‐synuclein RT‐QuIC assay for various biospecimens in Parkinson's disease and dementia with Lewy bodies. Acta Neuropathol Commun 2021;9(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sokratian A, Ziaee J, Kelly K, et al. Heterogeneity in α‐synuclein fibril activity correlates to disease phenotypes in Lewy body dementia. Acta Neuropathol 2021;141(4):547–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Iranzo A. Parasomnias and sleep‐related movement disorders in older adults. Sleep Med Clin 2018;13(1):51–61. [DOI] [PubMed] [Google Scholar]
- 58. Wang Y, Hu J, Chen X, et al. Real‐time quaking‐induced conversion assay is accurate for Lewy body diseases: a meta‐analysis. Neurol Sci 2022;43(7):4125–4132. [DOI] [PubMed] [Google Scholar]
- 59. Kalia LV, Lang AE, Hazrati LN, et al. Clinical correlations with Lewy body pathology in LRRK2‐related Parkinson disease. JAMA Neurol 2015;72(1):100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Poulopoulos M, Levy OA, Alcalay RN. The neuropathology of genetic Parkinson's disease. Mov Disord 2012;27(7):831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schneider SA, Alcalay RN. Neuropathology of genetic synucleinopathies with parkinsonism: review of the literature. Mov Disord 2017;32(11):1504–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cookson MR, Hardy J, Lewis PA. Genetic neuropathology of Parkinson's disease. Int J Clin Exp Pathol 2008;1(3):217–231. [PMC free article] [PubMed] [Google Scholar]
- 63. Gaig C, Martí MJ, Ezquerra M, Cardozo A, Rey MJ, Tolosa E. G2019S LRRK2 mutation causing Parkinson's disease without Lewy bodies. BMJ Case Rep 2009;2009:bcr0820080632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Martinez‐Valbuena I, Visanji NP, Kim A, et al. Alpha‐synuclein seeding shows a wide heterogeneity in multiple system atrophy. Transl Neurodegener 2022;11(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bargar C, De Luca CMG, Devigili G, et al. Discrimination of MSA‐P and MSA‐C by RT‐QuIC analysis of olfactory mucosa: the first assessment of assay reproducibility between two specialized laboratories. Mol Neurodegener 2021;16(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kuzkina A, Bargar C, Schmitt D, et al. Diagnostic value of skin RT‐QuIC in Parkinson's disease: a two‐laboratory study. NPJ Parkinsons Dis 2021;7(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Manne S, Kondru N, Jin H, et al. Blinded RT‐QuIC analysis of α‐Synuclein biomarker in skin tissue from Parkinson's disease patients. Mov Disord 2020;35(12):2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. De Luca CMG, Elia AE, Portaleone SM, et al. Efficient RT‐QuIC seeding activity for α‐synuclein in olfactory mucosa samples of patients with Parkinson's disease and multiple system atrophy. Transl Neurodegener 2019;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stefani A, Iranzo A, Holzknecht E, et al. Alpha‐synuclein seeds in olfactory mucosa of patients with isolated REM sleep behaviour disorder. Brain 2021;144(4):1118–1126. [DOI] [PubMed] [Google Scholar]
- 70. Manne S, Kondru N, Jin H, Anantharam V, Huang X, Kanthasamy A, Kanthasamy AG. α‐Synuclein real‐time quaking‐induced conversion in the submandibular glands of Parkinson's disease patients. Mov Disord 2020;35(2):268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kluge A, Bunk J, Schaeffer E, et al. Detection of neuron‐derived pathological α‐synuclein in blood. Brain 2022;145(9):3058–3071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Detailed overview about patient and control groups in each of the eligible studies
Data S1. Forest plots for all subgroup analyses


