Abstract
Sepsis‐induced myocardial dysfunction (SIMD) is the leading cause of death in patients with sepsis in the intensive care units. The main manifestations of SIMD are systolic and diastolic dysfunctions of the myocardium. Despite our initial understanding of the SIMD over the past three decades, the incidence and mortality of SIMD remain high. This may be attributed to the large degree of heterogeneity among the initiating factors, disease processes, and host states involved in SIMD. Previously, organ dysfunction caused by sepsis was thought to be an impairment brought about by an excessive inflammatory response. However, many recent studies have shown that SIMD is a consequence of a combination of factors shaped by the inflammatory responses between the pathogen and the host. In this article, we review the mechanisms of the inflammatory responses and potential novel therapeutic strategies in SIMD.
Keywords: host‐pathogen interactions, inflammation, myocardial dysfunction, sepsis, targeted therapy
The interaction of inflammatory environment promotes the occurrence and development of myocardial injury induced by sepsis.
1. INTRODUCTION
According to the latest international consensus, sepsis is defined as a life‐threatening organ dysfunction caused by a dysregulated host response to infection, and septic shock is classified as a subtype of sepsis. 1 Sepsis is shaped by “a combination of factors between the pathogen and the host,” and the guidelines for the management of sepsis and septic shock further highlight the role of organ dysfunction in sepsis. 2 Notaby, sepsos is a group of syndromes rather than a single desiase. Sepsis‐induced myocardial dysfunction (SIMD), one of the manifestations of organ dysfunction in sepsis, is the main cause of septic shock, and is characterized by myocardial systolic and diastolic dysfunctions. 3 It has been reported that the mortality rates of patients with septic shock can be as high as 38%. 4
However, the current knowledge about SIMD is still lacking in many aspects, as reflected in the controversies abounding its definition, identification, and therapeutic management. For example, it is still unclear whether myocardial dysfunction extends from the left to the right ventricle (RV) nd what role diastolic dysfunction plays in SIMD. 5 , 6 Additionally, compared to systolic dysfuntion, diastolic dysfuntion is often ignored. Moreover, even when the myocardial decreases, the ejection fraction (EF) of the myocardium remains preserved because the reduced ejection. In terms of treatment, there are no standardized or uniformly specified targeted measures. In the past 30 years, despite the large number of mechanistic studies on sepsis, there has been no satisfactory clinical transformation. The main reasons for these contradictions are the differences in the pathogenic infections and the host response mechanisms in patients. Effective treatment and management need to be adapted according to the evolving disease processes in the patients. 7
Persistent and excessive inflammation can trigger an unrecoverable inflammatory imbalance in the body, which ultimately leads to tissue and organ damage. 8 SIMD is unique considering the damage to the other tissues and organs during sepsis. In addition to the direct effects of the pathogen, the host's inflammatory response to the actions of the infectious agents (e.g., activation of the immune cells and the massive release of inflammatory mediators) can also damage the myocardium. Sometimes, these conditions may not be sequential but may act synergistically to amplify the damage to the heart and can be more likely to cause fatal septic shock. These conditions create a vicious cycle and ultimately exacerbate sepsis‐induced damage. This review focuses on the mechanisms of inflammatory response in SIMD.
2. MAIN BODY
2.1. Immune cell activation in sepsis
Activation of the immune cells is a prerequisite for inflammation. During the occurrence and development of SIMD, the local environment of the myocardium is closely connected with the state of the host, and the two affect each other. Inflammatory mechanisms include proinflammatory and anti‐inflammatory imbalance and immunosuppression. The activation of the host's immune cells after infection forms the basis of the inflammatory response in sepsis. In the interaction between the host and the pathogens, the immune response by the immune cells forms an important cause of SIMD. Therefore, an understanding of the pathogenesis, the exact mechanism behind the inflammatory reactions, and the resulting myocardial injury are the basic premise for developing effective interventions (Figure 1).
Figure 1.
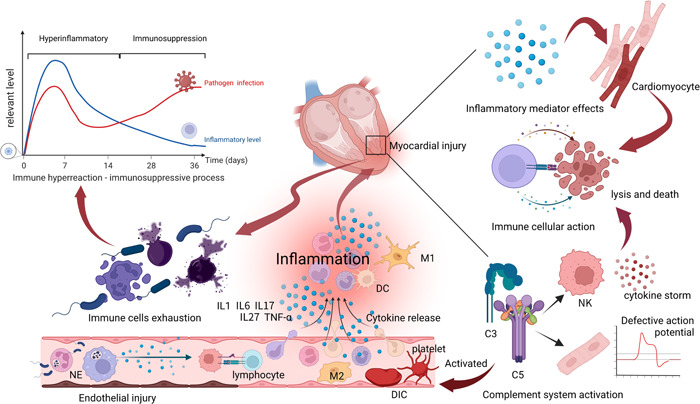
Immune response mediates sepsis‐induced myocardial dysfunction. Early microorganisms activate the immune cells to release immune mediators. Release of inflammatory mediators that target cardiomyocytes leads to cell death. Simultaneously, complement activation amplifies the inflammatory response and affects the myocardial action potential and coagulation system. After several days of infection, the body begins to enter the phase of immune suppression (immune cell failure), and the level of pathogen infection rises again. DC, dendritic cell; IL, interleukin; NE, neutrophil; NK, natural killer cell; M1/2, macrophage 1/2.
One of the most critical routes for activating immune cells is the recognition of pathogen‐associated molecular patterns (PAMPs). PAMPs are a group of highly conserved structures within the cell or in the cell walls of certain pathogens. These structures include lipids, glycoproteins, and nucleic acid components on microbial membranes. They activate immune cells through pattern recognition receptors (PRRs). 9 PRRs include NOD‐like receptors (NLRs), c‐type lectins (CLRs), and RIG‐I‐like receptors (RLRs); Toll‐like receptors (TLRs) and NLRs are the commonly activated receptors in sepsis. 10 Damage‐associated molecular patterns (DAMPs) are another group of molecules common to septic immune cells, and these include high‐mobility group box 1 (HMGB1), extracellular cold‐inducible RNA‐binding protein (eCIRP), adenosine triphosphate (ATP), and histones. 11 , 12 Chromatin‐associated molecular patterns and metabolism‐associated molecular patterns have also been proposed recently, in succession. The former mainly include DNA, cell‐free RNAs, microRNAs, extracellular traps (ETs), and RNA‐or DNA‐binding proteins. The latter mainly refer to metabolism‐related products: free fatty acids, glucose, advanced glycation end products, cholesterol, oxidized phospholipids, ceramides, and uric acid. These concepts broaden the previous understanding of inflammatory activation in sepsis and demonstrate the complexity of the inflammatory environment in which sepsis occurs. 13 , 14 The hosts may exhibit different immune characteristics to different pathogens. 15 , 16 Different types of immune cells are activated in different ways, at different times, and play different roles. Neutrophils are one of the most abundant leukocytes in the peripheral circulation, and play a key role in the early recognition of pathogens and initiation of host resistance to infection. 17 , 18 Bacterial lipopolysaccharides (LPS) and peptidoglycans, as well as the inflammatory factors (such as chemokines and interleukins) released after tissue injury are important signals for neutrophil activation. 19 The activated neutrophils are recruited at the site of infection to trap and kill the bacteria by forming neutrophil extracellular traps. They also secrete proinflammatory factors, such as chemokines, growth factors and interleukins, to activate other cells. 20 , 21 Monocytes/macrophages are another group of immune cells in an infection. Among them, macrophage polarization (M1/M2) plays a key role that involves a complex regulatory network. 22 , 23 , 24 Such polarization can be initiated not only by external infectious factors, but also by endogenous signaling molecules and pathways, thus maintaining a relative balance. 25
Similar to neutrophils, dendritic cells (DCs) are also important immune cells, activated in response to sepsis, and involved mainly in antigen presentation and in enhancing immune response lethality. 26 In the early stages of infection, the host mobilizes its defense system to clear the invading pathogens, a process accomplished by innate immunity. Antigen‐presenting cells (APCs) in the blood, mainly monocytes/macrophages, DCs, and B lymphocytes, are the most critical immune cells activated in the early stages of sepsis. After recognizing the pathogens, the APCs activate, phagocytose, and remove the pathogenic factors and then present the antigen information to T lymphocytes and natural killer cells (NK cells), thereby, inducing their differentiation into corresponding effector cells to enhance and amplify the immune clearance effect. In this process, the phenotype of the immune cells changes constantly according to the function, accompanied by the release of many inflammatory cytokines. 27 , 28
2.2. Activated immune cells and cytokines damage the heart
Normally, there are different immune cell subsets in the heart, including macrophages, monocytes, lymphocytes, and DCs. These immune cells, together with the cardiomyocytes, form the myocardial immune cluster that regulates the myocardial function. In sepsis, crosstalk between immune cells and cardiomyocytes is common. Neutrophil infiltration is one of the causes of organ damage, including the heart. 29 Emigrated neutrophils can affect the Na+ and K+ currents of cardiomyocytes, and then change the rhythm of the myocardium. 30 In SIMD, macrophage migration inhibitory factor (MIF) mediates interactions between cardiomyocytes and immune cells. It has been shown that inhibiting MIF reduces infiltration of myocardial macrophages in mice, decreases endoplasmic reticulum stress, and alleviates cardiomyocyte diastolic disorders and apoptosis. 31 , 32 Previous studies have shown that regulatory T lymphocytes play a reparative role in myocardial tissue after myocardial infarction, 33 , 34 but the activation of CD4+ T‐cell receptors in the presence of stress can promote cardiac dysfunction. 35 Whether these immune cells play similar roles in SIMD, remains unknown.
However, in contrast to the direct damaging effects of the immune cells, the cytokines (including those secreted by the immune cells and the tissues) are the cause of the myocardial inflammatory response. Interleukins (ILs) are one of the most common cytokines in the inflammatory response. 36 As a “strong responsive soldier” of inflammation, IL6 can exacerbate meningococcal septicemia‐induced myocardial depression by targeting P38 signaling in the mitogen‐activated protein kinase (MAPK) pathway. 37 , 38 However, a study has shown that moderate concentrations of IL6 may play a protective role in early LPs‐induced sepsis in mice by upregating the nuclear factor erythroid 2‐associated factor 2 (Nrf2) to reduce intracellular oxidative stress levels. 39 These complicate the role of ILs in regulating myocardial injury in sepsis. Recently, many rare cytokines and new regulatory mechanisms have been studied; these cytokines are not only secreted by immune cells, such as monocytes and macrophages, but also synthesized and secreted by damaged tissue cells, including cardiomyocytes, endothelial cells and fibroblasts 40 , 41
2.3. Activation of the complement system
In addition to the antibody and cellular responses, complement is another immune response pathway that recognizes common structures on the surface of pathogens, such as bacteria and fungi. The complement system may act through any of the three pathways: the classic, the bypass, and the lectin pathway, all leading to the cleavage of C3 convertase into C3a and C3b to initiate downstream terminal reactions. 42 In vivo experiments in mice have shown that the complement system of cardiomyocytes can be activated at an early stage. 43 C5a can synergistically activate NK and NKT cells to mediate the development of a cytokine storm in septic mice, drive the recruitment of NKT and NK cells to the site of infection, and promote the release of TNF‐γ from NK and DC cells. 44 C5a not only promotes the expression of the reactive oxygen species/NOD‐like receptor protein 3 (ROS/NLRP3) pathway and leads to pyroptosis, but also affects the ion channels and intracellular calcium flux in the cytosol, thereby inhibiting cellular contractility and diastolic functions. 45 , 46 Furthermore, C5a receptors on cardiomyocytes can directly affect the physiological function of the myocardium. A previous study in mice, using the patch clamp recording technique, revealed that C5a can affect Na+/K+‐ATPase, sarcoplasmic/endoplasmic reticulum calcium ATPase 2, and Na+/Ca2+ exchanger activities, resulting in impaired Ca2+ clearance in cardiomyocytes. This impairment leads to defective action potential in myocytes and ultimately affects myocardial contractility and diastolic capacity. 47
Another important mechanism by which complement affects septic shock is by affecting the function of the coagulation system, which in turn promotes the development of disseminated intravascular coagulation (DIC) and circulatory system disorders. 48 The presence of DIC can promote the development of multiple organ dysfunction syndrome and greatly increase the risk of patient death. 49 , 50 During sepsis, cardiomyocytes from mice with cecal‐ligation and puncture‐induced septicemia release complement‐dependent components, which are characterized by elevated expression of C5a and C5a receptors (C5aR and C5L2). 51 Activated C3a and C5a can induce platelet activation, and blocking C5a can prevent endothelial cell activation and inhibit platelet function to prevent coagulation. 52 , 53
2.4. Immunosuppression
Immunosuppression is another feature of immune imbalance in sepsis, which manifests as immune cell anergy (CD4+ T lymphocytes, CD8+ T lymphocytes, B lymphocytes, and DC exhaustion) and a decreased ability to fight primary bacterial infections; this condition usually develops a few days after infection due to a highly inflammatory response that may cause further secondary infections and worsen disease progression. 54 It is worth noting that the states of immunosuppression and immune overactivation are not strictly differentiated but show a dynamic range, depending on the type of pathogen, virulence, and defense capacity of the host. 55 In fact, immunosuppression, following an immune overreaction, may have more serious consequences than the immune response itself. 56 The exact mechanism of immunosuppression is still unclear, and immune cell apoptosis, endotoxin tolerance, central neuromodulation, reprogramming of the inflammatory response, and metabolic reprogramming are all important contributors to the development of immunosuppression. 57
One of the main causes of immunosuppression is the inability of immune cells to function, which makes the infection persistent and recurrent. In sepsis, different immune cells have different mechanisms associated with apoptosis and crosstalk, thus differing in their effects on host organs. 58 , 59 Chen et al. found that peripheral blood mononuclear cells from sepsis patients had defective energy metabolism, and impaired glycolysis, oxidative phosphorylation, and β‐oxidation, which caused energy failure in the mitochondria and contributed to the loss of immune function. 60 This conclusion was a significant point of view, and these experiments provided a rational connection between two important mechanisms (mitochondrial disorders and immunosuppression) in sepsis.
During immunosuppression, the inflammatory overreaction in the organ may be transiently relieved to some extent, but this may be followed by fatal exacerbation of the infection. It has been reported that the spleen and lungs can be immunosuppressed during sepsis (e.g., by upregulating the expression of inhibitory receptors on the surface of T cells that infiltrate the organs), thereby worsening the patient's condition. 55 Apoptotic immune cells can also promote tissue damage in the organs they infiltrate. Immunosuppression has started to gain attention in recent years, and therapeutic strategies have been developed to address this phenomenon. However, because of the organ‐specific nature of these infiltrating immune cells, the exact mechanism of immunosuppression in SIMD is unknown. 61
2.5. Mitochondrial energy metabolism disorders
The heart is one of the organs with a high energy requirement, thus it contains a lot of mitochondria, whose energy metabolism is susceptible to injury. These mitochondria can control myocardial organ damage through a variety of quality control mechanisms. (Figure 2). 62 , 63 Mitochondrial danger‐associated molecular patterns (mtDAMPs), released after mitochondrial damage and including mitochondrial DNA (mtDNA), mROS, cardiolipin, ATP, and N‐formyl peptide, can activate innate immune receptors to promote inflammatory responses. 64 mtDNA is recognized by various PRRs and interacts with NLRP3 inflammasomes to promote the maturation of IL1β and IL18. 65 In a previous study, rats injected with liver mitochondrial DAMPs developed systemic inflammation and acute lung damage, suggesting that circulating mtDAMPs can harm other organs. 66
Figure 2.
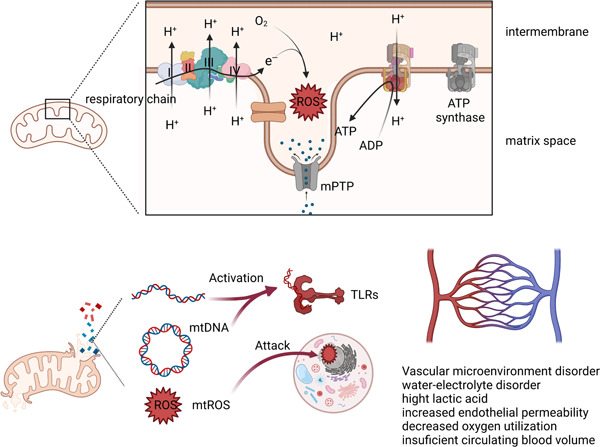
Mechanisms of mitochondrial dysfunction and local metabolic abnormalities. Under the action of inflammatory factors, electrons escape to form ROS, leading to a decrease in ATP production. ROS further attack nucleic acid structures and cause cell damage. The release of large amounts of mtDNA also activates inflammatory pathways. When aerobic respiration is impaired, the local glycolytic pathway gets switched to lactate metabolism, resulting in local metabolic and vasoconstriction disorders that affect the hemodynamic stability. mPTP, mitochondrial permeability transition pore; mtROS, mitochondrial reactive oxygen species; TLRs, toll‐like receptors.
Mitochondria are the main source of ROS in cardiomyocytes during sepsis, which is due to the disruption of the electron respiratory transport chain caused by abnormal mitochondrial membrane potential levels and uncoupling of energy transport. 67 , 68 Mitochondrial permeability transition is a typical characteristic of mitochondrial damage in SIMD, which is caused by the abnormal opening of the mitochondrial permeability transition pore (mPTP) in the mitochondrial membrane for a long time. 69 , 70 Calcium overload, changes in mitochondrial enzymatic activities, decreased ATP production, and mtDNA produced after ROS attack on mitochondria during sepsis can aggravate the energy metabolism disorder of the mitochondria. 71 , 72 , 73 Furthermore, ROS attack normal intracellular structures and disrupt intracellular metabolic homeostasis, and a decrease in ATP accelerates cellular dysfunction, including a decrease in the resistance to scavenging oxides. 74 Previous studies have shown that LPS‐induced excess ROS can exacerbate hyperglycemia and hypoxia/reoxygenation‐induced myocardial injury by mediating cardiomyocyte death through the NLRP3 inflammasome. 75 Excessive ROS can also induce HIFα nuclear translocation and downstream events by inhibiting prolyl hydroxylase‐mediated ubiquitination of HIFα, which mediates a shift in energy production from oxygen‐dependent oxidative phosphorylation to hypoxic glycolysis. 76 , 77 Under hypoxic conditions, the myocardium can induce HIF1α expression to modulate myocardial glucose uptake and alter myocardial contractility. 78
Notably though, the above‐mentioned processes should not be considered as independent events, rather as closely interacting elements. Organ damage control during sepsis may also be regulated by mitochondrial fission/fusion events and autophagy events. It has been demonstrated that in animals lacking DNA‐dependent protein kinase, sepsis results in less cardiac damage. 79 This is a serine/threonine protein kinase that induces mitochondrial fragmentation in kidney injury by interacting with the mitochondrial fission 1 protein. 80 Additionally, autophagy is essential for the mitochondrial control of SIMD. Activation of mitochondrial autophagy related genes can reduce myocardial damage during sepsis and maintain mitochondrial functional metabolism in mice. 81 Early inflammatory signals may affect the enzyme metabolism in mitochondria, leading to the production of free radicals. On the one hand, free radicals destroy the potential on the mitochondrial membrane, thereby amplifying the damage to the mitochondria, and on the other hand, they induce the generation of mtDNA, which in turn acts as an intracellular DAMP and aggravates the inflammatory response of cells, thus forming a vicious cycle. Oxidative phosphoric acid uncoupling of mitochondria is a very attractive research direction in these studies. In mammalian mitochondria, the respiratory chain pumps protons into the membrane space, where they produce ATP by pushing ATP synthase back into the matrix. However, when protons do not pass through ATP synthase, they flow into the mitochondrial matrix through some “shortcut paths” in the inner mitochondrial membrane to form “proton leakage.” Te energy utilization efficiency of the cell is reduced, and uncoupling protein (UCP) acts as the “shortcut path.” 82 UCP2 is one of the UCP family of proteins closely associated with metabolic diseases, such as diabetes, cardiovascular diseases, obesity, and cancer. 83 Previous studies have identified the upregulation of UCP2 protein in response to LPS‐induced myocardial injury. 84 , 85 It is noteworthy that most of the studies suggest that UCP2 plays a protective role in SIMD, which seems to contradict the conventional wisdom. The mechanism behind these phenomena may be explained by the presence of UCPs that make the electrons in the membrane space leak back into the matrix, thus reducing the excessive production of ROS and subsequently reduce the oxidative stress and apoptosis of cardiomyocytes. 86 , 87 , 88 , 89 , 90 However, it is unclear whether other members of the UCP play a similar role in SIMD.
2.6. Microcirculatory dysfunction
Microcirculatory dysfunction is an important core in the progression of sepsis. Coagulation dysfunction and DIC are the pathophysiological manifestations of sepsis, which may be caused by an imbalance between the coagulant and the anticoagulant systems after activation of inflammatory factors. 91 The mechanisms by which inflammatory factors contribute to sepsis‐associated coagulopathy have been previously described in detail, including microbial diffusion, neutrophil activation, and DAMP interaction with coagulation factors. 92 , 93 , 94 However, the mechanism underlying the interaction between coagulation and myocardial dysfunction is not well understood. Abnormal activation of the coagulation system may lead to local blood hypoperfusion and abnormal distribution of the peripheral circulation, especially capillary microcirculation disorders. 95 , 96
In the early stages, tissue hypoxia caused by hypoperfusion hypoxia may not be obvious because the heart is in a hyperdynamic state and can compensate for the circulation. However, with the peripheral vasodilatation disorders, increased endothelial vascular permeability results in tissue edema and hypovolemia. The circulatory hemodynamics, then, become unstable, perfusion changes from “High Power” to “High Resistance” and further aggravates the dysfunction of the local coagulation system. On the one hand, the formation of microthrombi causes abnormal distribution of peripheral circulation, and on the other hand, it causes local hypoxia in peripheral tissues and accelerates the inflammatory reaction. 97 , 98 , 99 Abnormal blood flow in the local tissues, in turn, promotes local metabolic abnormalities, such as the accumulation of harmful metabolites. Vascular endothelial cells, macrophages, neutrophils, and histiocytes promote the synthesis of excessive nitric oxide (NO) by NO synthase (NOS) under the microenvironmental changes, such as an increase in the inflammatory factors and hemodynamic changes, thus leading to the dysfunction of immune cells, such as the induction of T‐cell apoptosis. 100 Furthermore, excessive NO can also affect vascular reactivity through downstream effector molecules, such as guanylate cyclase and potassium ion channels, leading to vascular paralysis and further aggravating abnormal blood perfusion in tissues. 101 , 102
2.7. Autonomic nerve activation
During sepsis, the autonomic nervous system, including the sympathetic and parasympathetic nerves, is abnormally activated, releasing large amounts of catecholamines (mainly epinephrine and norepinephrine) and acetylcholine, which constrict blood vessels and increase peripheral resistance, while enhancing tissue energy metabolism. In addition to the high levels of inflammatory factors, this activation also comes from the abnormal distribution of peripheral blood volume, leading to hypoperfusion and reduced peripheral vascular tension. 103 , 104 , 105 Excessive catecholamine hormones can increase the energy consumption of cardiomyocytes, increase the metabolic burden of myocardial microcirculation, enhance the oxidative stress levels of cardiomyocytes, and affect the myocardial rhythm. The compensatory work of the heart and inadequate coronary blood perfusion accelerate the imbalance in myocardial oxygen supply and demand. 105 , 106 , 107 , 108
2.8. Pathogenic microorganisms mediate damage
In addition to mediating myocardial injury by inducing host immune responses, pathogenic microorganisms can also act directly on cardiomyocytes. 109 , 110 , 111 , 112 Most microorganisms that can cause sepsis, such as bacteria, fungi, parasites, and viruses (Table 1), can infect the heart. The most common pathogens that cause sepsis are gram‐negative bacteria, including Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Streptococcus pneumoniae, and these pathogens usually spread from peritonitis or pneumonia. 126
Table 1.
Common pathogens and mechanism of myocardial injury in sepsis.
| Pathogens | Common mechanisms | References |
|---|---|---|
| Bacteria | ||
|
|
[113, 114, 115, 116, 117, 118] |
| Fungi | ||
|
|
[119, 120, 121] |
| Virus | ||
|
|
[122, 123, 124, 125] |
Endotoxin, a characteristic cell wall component of gram‐negative bacteria, was previously used to study sepsis. Activation of toll‐like receptor 4 (TLR‐4) is an important mechanism of endotoxin. TLR‐4 promotes the activation of interferon (IFN) regulatory factors, nuclear factor‐κB and MAPK signaling through early myeloid differentiation factor 88 (MyD88)‐dependent and MyD88‐independent pathways and promotes the production of inflammatory factors such as TNF‐α, IL‐6, IL‐8, TNF‐α and granulocyte colony‐stimulating factor. 127
Similarly, lipoteichoic acid, another conserved bacterial structure (gram‐negative) that mediates septic injury, triggers immune inflammatory damage to the myocardium by activating TLR‐2, TLR‐1, and TLR‐6, which are considered receptor sites for this response. 128 The most well‐represented gram‐positive bacteria are Staphylococcus aureus, which carry toxins with potent pathogenic effects, and some of these bacteria secrete coagulases that predispose tissues to the formation of infectious foci (myocardial abscesses). 129 , 130 The misuse of antibiotics in recent years has increased potent virulence factors and made therapeutic interventions more difficult. 131 The remaining antigenic structures on bacteria can exert cytopathogenic effects, for example, bacterial flagella can mediate innate immune inflammation through TLR5 receptors, leading to acute myocardial contractile dysfunction in rat. 132
Another common cause of sepsis is viral infection. The mechanism of viral injury in SIMD is very similar to that in viral myocarditis, which sometimes makes it difficult to distinguish between the two; the completion of infection—entry—replication leads to an immune response and cell death. 133 , 134 , 135 Most viruses invade cardiomyocytes by specifically recognizing receptors on the cell membrane. Human immunodeficiency virus, hepatitis C virus, influenza A or B viruses, the coronavirus family, Middle East respiratory syndrome coronavirus, severe acute respiratory syndrome coronavirus (SARS‐CoV), and severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), can recognize angiotensin‐converting enzyme receptor 2 (ACE2) to infect the myocardium and promote cellular damage. 136 , 137 , 138 Pathogens that invade cardiomyocytes can release inflammatory cytokines, such as NF‐α, IL‐1β, and IL‐6 by affecting inflammatory pathways, such as the NFκB, MAPK, and the complement system. These viruses may also change the characteristic phenotypes of cells by affecting intracellular calcium concentrations, mitochondrial conversion, and membrane permeability. 45 , 127 , 139 , 140 , 141 , 142 , 143
3. TREATMENT STRATEGIES
The fundamental principles of sepsis treatment and management include early recognition, early control of infection, early restoration of organ tissue perfusion, close monitoring of patient vital signs and markers of organ damage, aggressive fluid resuscitation, active prevention of complications, and optimization of nursing management. The inflammatory reaction to sepsis is systemic, and the prevention and treatment of SIMD should not be limited to the treatment of the heart alone but should be considered from the overall perspective of the host‐pathogen interaction (Figure 3).
Figure 3.
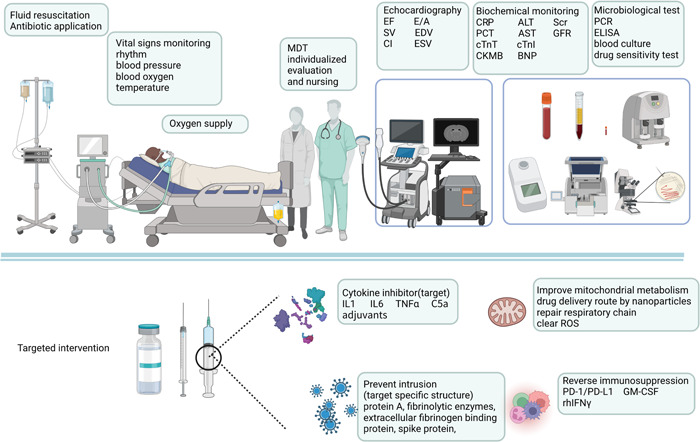
Treatment strategies for septic shock. Based on traditional strategies, a new generation of monitoring and treatment approaches are being developed. Highly sensitive PCR and sequencing technologies allow for faster identification of pathogens. A variety of small molecule targeted drug development is expected to be the future solution. ALT, alanine aminotransferase; AST, aspartate aminotransferase; ELISA, enzyme‐linked immunosorbent assay; GFR, glomerular filtration rate; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; IL, interleukin; MDT, multidisciplinary treatment; PCR, polymerase chain reaction; rhIFNγ, recombinant human interferon; ROS, reactive oxygen species; Scr, serum creatinine.
3.1. Early identification and monitoring
Currently, there are no uniform standards for diagnosing SIMD. The diagnosis and treatment of SIMD depends on a combination of the patient's clinical symptoms and the relevant indicators of cardiac function assessment. However, the early symptoms in patients may not be obvious, especially in severe patients (such as comatose and speech‐impaired), and it is difficult to determine the occurrence of SIMD in a timely manner. Electrocardiography may be a quick and convenient method to evaluate SIMD, however, due to specificity limitations, it cannot be used for the timely determination of SIMD in many cases. Echocardiography (ECHO), especially two‐dimensional ultrasound imaging, is an important means of SIMD diagnosis and monitoring. Routine measures should include left ventricular ejection fraction (LVEF), stroke volume and cardiac index, RV systolic dysfunction, and diastolic dysfunction (E/e’). 144 However, due to the limitation of consistent dependence, LVEF and other traditional indicators may not fully reflect the degree of myocardial injury. More and more studies support the superiority of speckle tracking ECHO over conventional ECHO in evaluating myocardial function; therefore, this may be the new major monitoring method for SIMD in the future. 145 , 146 , 147
Although they are controversial, biomarkers for SIMD monitoring are still based on traditional common indicators of myocardial damage, such as cardiac troponin T (cTnT) and cardiac troponin I (cTnI), brain natriuretic peptide, and creatine kinase isoenzyme‐MB. Dynamic monitoring of C‐reactive protein, procalcitonin and other inflammation‐related biomarkers are also effective measures to prevent further development of sepsis. 148 , 149 , 150 , 151 , 152 In fact, in addition to heart‐related biomarkers, other biomarkers of organ dysfunction should also be included in the category for comprehensive evaluation because damage to other organs will also result in heart damage. 95
Several novel biomarkers have been developed to identify and track SIMD. Copper (Cu) and zinc (Zn) levels in the blood, Cu/Zn ratio, myeloperoxidase (MPO), pregnancy‐associated plasma protein A (PAPP‐A), and noncoding RNA (Micro) are good performance predictors for SIMD, which can improve the recognition efficiency for SIMD. 153 , 154 , 155 , 156 , 157 However, owing to the complex monitoring process and equipment, clinical applications may be limited, and biomarkers should not be used alone.
3.2. Targeting the host inflammatory response
The host immune response imbalance has been a major concern in the treatment of sepsis. It should be noted that traditional anti‐inflammatory therapy should be based on the patient's clinical presentation. Precision immunotherapy for sepsis is an advocated research direction, but owing to the complex response mechanisms, current therapeutic advances in this area are still very limited. There are not many directly targeted therapies for inflammation in clinical practice. Most treatments are symptomatic, such as lowering the body temperature, full volume perfusion, and timely removal of pathogens, to reduce the level of inflammation in the patient's body. Clinical practice currently relies heavily on timely infection control, fluid resuscitation, and oxygen administration to achieve this goal. In the Sepsis Save Campaign, patients with sepsis are not recommended to use blood purification techniques such as dialysis to identify potential inflammatory agents unless absolutely necessary. This is because such methods appear to be of little benefit to patients. 2
Many studies have shown that blocking inflammation‐related pathways can effectively mitigate sepsis‐induced cardiomyocyte pyroptosis, including inhibition of TNF, IL1, IL6, IL7, IL15, coagulation factors, and complement C5a adjuvants. 158 , 159 , 160 , 161 However, most of these novel molecularly targeted drugs are still in the research and development stage and have not yet entered clinical application. Currently, a number of immunotherapy clinical trials for the treatment of sepsis are underway, such as the application of anakinra (IL‐1 inhibitor), granulocyte‐macrophage stimulating factor, recombinant human INF γ (rhIFNγ), allocetra‐OTS (off‐the‐shelf apoptotic cells) treatments, and Nangibotide (myeloid triggering receptor 1 receptor competitive inhibitor). 162 , 163 , 164 , 165 , 166 However, many problems still remain associated with immunotherapy. The inflammatory response is highly heterogeneous across individuals and during different stages. Pure immunotherapeutic interventions may lead to serious consequences. Besides, most current animal models of immunosuppression in sepsis are not very well developed and differ from the actual in vivo environment. 167 , 168 Therefore, more in‐depth studies are required to elucidate these mechanisms.
3.3. Improving perfusion and microcirculation disturbance
A timely fluid resuscitation can improve the hypoxic condition of local tissues, especially the microcirculation. Currently, norepinephrine, epinephrine, dopamine, and dobutamine are the most commonly used drugs in the clinical treatment of septic shock. These drugs can increase blood pressure and cardiac output in shock patients, and maintain tissue blood flow. The Surviving Sepsis Campaign‐2021 suggests that patients with septic shock should be resuscitated immediately, and recommends the use of norepinephrine instead of dopamine or epinephrine as vasoactive first‐line drugs. 2 Appropriate use of vasoactive drugs such as amrinone, milrinone, enoxidone and levosimendan on the basis of maintaining blood pressure can help improve the vascular resistance and blood flow status of patients. These have been clinically proven to be effective in the treatment of SIMD. However, the use of these drugs is governed strictly by the patient's organ function status, underlying disease, and progression, which may influence the selection of drugs. As much as possible, the clinician should make a comprehensive assessment of the patient's hemodynamics. Inappropriate vasoactive drug administration and oxygen supplementation may exacerbate the damage. In the absence of appropriate monitoring and care, overly aggressive resuscitation can increase the risk of patient death. 169 , 170 , 171 , 172 Resurrection fluids should use crystals rather than colloids, as the latter seem to increase the risk of organ damage and death. 173
In addition to improving local metabolism, interventions aimed at improving myocardial function are currently under study. Previous research showed that the β‐blocker esmolol improved 28‐day mortality, controlled ventricular rate, reduced myocardial energy loss, and improved hemodynamics in patients with sepsis after fluid resuscitation. 174 However, β‐blockers should still be used very cautiously, and their safety should be evaluated periodically on an individual basis, considering that sepsis itself is a complex environment, and that the application of β‐blockers in the case of hemodynamic instability would increase the risk to patients. 175 , 176 Other substances that can improve myocardial oxidation levels, such as melatonin, ferrostatin‐1, luteolin, dexmedetomidine, and other novel drugs, improve myocardial injury in vitro, but their efficacy and safety in vivo have not been verified. 177 , 178 , 179 , 180
Metabolic resuscitation therapy of mitochondria has recently been proposed to reduce oxidative stress, maintain stable mitochondrial function and improve local tissue metabolism by adjusting mitochondrial metabolism and maintaining normal electron transport chain function. 181 This strategy includes the use of hormonal drugs, reduction in tissue caloric requirements, promotion of the tricarboxylic acid cycle and ATP production, and the administration of antioxidants. 182 , 183 Several mitochondria‐targeted delivery systems based on lipophilic cations and nano pathways have been developed. In a previous study, the surface of the targeted peptide Szeto Schiller 31 (ss31) was modified to carry cyclosporine A (CsA) to inhibit mPTP opening. By using the specificity of the interaction between ss31 and cardiolipin, the researchers carried out targeted drug delivery to mitochondria, and compared to using CsA alone, targeted mitochondrial delivery significantly alleviated the myocardial injury induced by hypoxia/reoxygenation. 184 In addition to delivery systems based on lipophilic cations, temperature‐dependent mitochondrial drug delivery systems, near‐infrared light‐triggered drug delivery vehicles based on chemical photothermal therapy, and amphiphilic cell‐penetrating motifs have also been developed as promising mitochondria‐targeted drug delivery systems. Research on mitochondria‐targeted interventions is being performed for clinical translation, and several clinical trials targeting mitochondria to improve atherosclerosis, cardiomyopathy, diastolic dysfunction, aging, and peripheral vascular disease are currently enrolling patients. 185 , 186 , 187 , 188 , 189 It is expected that mitochondria‐targeted therapy will have broad applications in the prevention and treatment of SIMD.
3.4. Targeting pathogen invasion
Antibiotics are still the most effective measures for rapid infection control. The Surviving Sepsis Campaign 2021 recommends that adults with septic shock or a high risk of sepsis be administered antibiotics immediately, and unless there are clear contraindications to antibiotic use, antibiotics should be used as early as possible. To ensure coverage of multiple potential pathogens, use broad‐spectrum antibiotics in combination early before the pathogen is identified, such as carbapenems like meropenem and imipenem/cilastatin, or broad‐spectrum penicillin/beta‐lactamase inhibitors. 2 A retrospective study of 35,000 sepsis patients in an emergency department showed that hourly delays in antibiotic administration were associated with increased in‐hospital mortality. 190 A large multicenter, open‐label, randomized trial showed that pre‐hospital antibiotic use in ambulances appeared to improve prognostic outcomes for patients with sepsis. 191 However, it is still worth mentioning that timely and accurate dynamic assessment of sepsis patients is the primary position of rescue, especially to grasp the indication and time of antibiotic use. 192 , 193 It is suggested that in any patient with sepsis, the patient's medical history (such as pneumonia, trauma, local infection, and organ injury) should be combined with the biochemical indicators to make a rapid comprehensive assessment and identify the pathogen as soon as possible, if conditions permit. In addition, attending physicians and caregivers should also take into account local bacterial resistance, such as assessing the risk of methicillin‐resistant S. aureus (MRSA), candida, and fungal infections, and adjusting antibiotic regimens to cover the appropriate pathogens.
There have been reports of new therapies that have been developed to stop or slow the extent of pathogenic damage, such as targeted nanoparticles with multifunctional antimicrobial effects, monoclonal antibodies targeting pathogen‐specific structures, surface protein A (SasA), fibrinolytic enzymes, extracellular fibrinogen binding protein (a complement inhibitory protein), teichoic acid, and specific spike proteins. 194 , 195 , 196 , 197 , 198 However, these new target therapies are based on the identification of the pathogen, so the rapid identification of the pathogen is still the key to preventing the further spread of infection. Microbial monitoring, though, has shown more promising progress, especially through blood cultures and rapid detection polymerase chain reaction (PCR). Early identification of microorganisms is of great significance for clinical medication, as it can quickly control infection, and also prevent further organ failure in sepsis. As the technology improves, a combination of PCR and blood culture can be used to identify infectious pathogens more quickly. Compared to the traditional blood culture followed by nucleic acid extraction for PCR amplification, the specific sequence amplification of clinical samples can be more rapid and convenient. 199 , 200 , 201 , 202 Molecular diagnostics for pathogens (nucleic acid sequences, and specific protein fragments) can greatly improve the rapid identification of infectious agents and guide antibiotic dosage. 203 , 204 However, molecular diagnosis and prediction in sepsis still face many problems and challenges, and the transformaiional application of the study is not very satisfactory. 205 , 206 , 207 , 208 These novel technologies are still in the early stages of research, and sensitivity and specificity must be further examined in clinical studies with larger samples. The cost of the assay further hinders the use of these technologies in the clinical setting.
4. CONCLUSIONS
Despite many important insights into sepsis and septic shock over the past three decades, SIMD still remains a significant cause of death in sepsis patients. Early monitoring and prevention of organ dysfunction are more important than treatment. In the past, the anti‐inflammatory response was the key to treating sepsis, but new perspectives are increasingly focused on the role of immunosuppression in sepsis. SIMD is not just an organ disorder associated with sepsis but a manifestation of systemic damage, and the prevention and treatment of SIMD should include a holistic view of the host. SIMD remains an important challenge for cardiologists, intensivists, researchers, and patients
AUTHOR CONTRIBUTIONS
Yuxin Nong and Danqing Yu conceived, designed, wrote, and revised the manuscript together. Xuebiao Wei made referential comments on the content of the manuscript. All authors approved the manuscript for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by Provincial Key Laboratory of Coronary Heart Disease Prevention (No. Y0120220151), National Natural Science Foundation of China (No. 82002014), Natural Science Foundation of Guangdong Province (No. 2021A1515010107), Medical Research Foundation of Guangdong Province (No. A2019409), Science and Technology Planning Project of Guangzhou (No. 201704020124). The funders had no role in the study design, analysis, decision to publish, nor preparation of the manuscript.
Nong Y, Wei X, Yu D. Inflammatory mechanisms and intervention strategies for sepsis‐induced myocardial dysfunction. Immun Inflamm Dis. 2023;11:e860. 10.1002/iid3.860
REFERENCES
- 1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315:801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181‐1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walley KR. Sepsis‐induced myocardial dysfunction. Curr Opin Crit Care. 2018;24:292‐299. [DOI] [PubMed] [Google Scholar]
- 4. Vincent JL, Jones G, David S, Olariu E, Cadwell KK. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta‐analysis. Crit Care. 2019;23:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hollenberg SM, Singer M. Pathophysiology of sepsis‐induced cardiomyopathy. Nat Rev Cardiol. 2021;18:424‐434. [DOI] [PubMed] [Google Scholar]
- 6. Pal N, Lim A, Karamchandani K. Sepsis‐Induced myocardial dysfunction. Chest. 2021;159:1684‐1685. [DOI] [PubMed] [Google Scholar]
- 7. Kakihana Y, Ito T, Nakahara M, Yamaguchi K, Yasuda T. Sepsis‐induced myocardial dysfunction: pathophysiology and management. J Intensive Care. 2016;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407‐420. [DOI] [PubMed] [Google Scholar]
- 9. Moriyama K, Nishida O. Targeting cytokines, Pathogen‐Associated molecular patterns, and damage‐associated molecular patterns in sepsis via blood purification. Int J Mol Sci. 2021;22:8882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wiersinga WJ, Leopold SJ, Cranendonk DR, van der Poll T. Host innate immune responses to sepsis. Virulence. 2014;5:36‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou M, Aziz M, Wang P. Damage‐Associated molecular patterns as double‐edged swords in sepsis. Antioxid Redox Signaling. 2021;35:1308‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Denning NL, Aziz M, Gurien SD, Wang P. DAMPs and NETs in sepsis. Front Immunol. 2019;10:2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu X, Zhang W, Wu C, et al. The novel role of metabolism‐associated molecular patterns in sepsis. Front Cell Infect Microbiol. 2022;12:915099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nofi CP, Wang P, Aziz M. Chromatin‐Associated molecular patterns (CAMPs) in sepsis. Cell Death Dis. 2022;13:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reyes M, Filbin MR, Bhattacharyya RP, et al. An immune‐cell signature of bacterial sepsis. Nature Med. 2020;26:333‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kokkinopoulos I, Jordan WJ, Ritter MA. Toll‐like receptor mRNA expression patterns in human dendritic cells and monocytes. Mol Immunol. 2005;42:957‐968. [DOI] [PubMed] [Google Scholar]
- 17. Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459‐489. [DOI] [PubMed] [Google Scholar]
- 18. Alves‐Filho JC, de Freitas A, Spiller F, Souto FO, Cunha FQ. The role of neutrophils in severe sepsis. Shock. 2008;30(suppl 1):3‐9. [DOI] [PubMed] [Google Scholar]
- 19. Futosi K, Fodor S, Mócsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013;17:638‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen X, Cao K, Zhao Y, Du J. Targeting neutrophils in sepsis: from mechanism to translation. Front Pharmacol. 2021;12:644270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tamassia N, Bianchetto‐Aguilera F, Arruda‐Silva F, et al. Cytokine production by human neutrophils: revisiting the “dark side of the moon”. Eur J Clin Invest. 2018;48(suppl 2):e12952. [DOI] [PubMed] [Google Scholar]
- 22. Chen X, Liu Y, Gao Y, Shou S, Chai Y. The roles of macrophage polarization in the host immune response to sepsis. Int Immunopharmacol. 2021;96:107791. [DOI] [PubMed] [Google Scholar]
- 23. Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72:4111‐4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumar V. Targeting macrophage immunometabolism: dawn in the darkness of sepsis. Int Immunopharmacol. 2018;58:173‐185. [DOI] [PubMed] [Google Scholar]
- 25. Zhou D, Huang C, Lin Z, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cellular Signalling. 2014;26:192‐197. [DOI] [PubMed] [Google Scholar]
- 26. Wu DD, Li T, Ji XY. Dendritic cells in sepsis: pathological alterations and therapeutic implications. J Immunol Res. 2017;2017:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rimmelé T, Payen D, Cantaluppi V, et al. Immune cell phenotype and function in sepsis. Shock. 2016;45:282‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luan YY, Dong N, Xie M, Xiao XZ, Yao YM. The significance and regulatory mechanisms of innate immune cells in the development of sepsis. J Interferon Cytokine Res. 2014;34:2‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grabie N, Hsieh DT, Buono C, et al. Neutrophils sustain pathogenic CD8+ T cell responses in the heart. Am J Pathol. 2003;163:2413‐2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ward C, Bazzazi H, Clark R, Nygren A, Giles W. Actions of emigrated neutrophils on Na(+) and K(+) currents in rat ventricular myocytes. Prog Biophys Mol Biol. 2006;90:249‐269. [DOI] [PubMed] [Google Scholar]
- 31. Zhang J, Zhang X, Cui Y, Cui L, Zhao P. Macrophage migration inhibitory factor knockout attenuates endotoxin‐induced cardiac dysfunction in mice. Kardiol Pol. 2018;76:871‐880. [DOI] [PubMed] [Google Scholar]
- 32. Dhanantwari P, Nadaraj S, Kenessey A, et al. Macrophage migration inhibitory factor induces cardiomyocyte apoptosis. Biochem Biophys Res Commun. 2008;371:298‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lim GB. Mobilizing regulatory T cells to promote myocardial repair. Nat Rev Cardiol. 2019;16:200. [DOI] [PubMed] [Google Scholar]
- 34. Wang Y, Dembowsky K, Chevalier E, et al. C‐X‐C motif chemokine receptor 4 blockade promotes tissue repair after myocardial infarction by enhancing regulatory T cell mobilization and Immune‐Regulatory function. Circulation. 2019;139:1798‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ngwenyama N, Kirabo A, Aronovitz M, et al. Isolevuglandin‐Modified cardiac proteins drive CD4+ T‐Cell activation in the heart and promote cardiac dysfunction. Circulation. 2021;143:1242‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bartekova M, Radosinska J, Jelemensky M, Dhalla NS. Role of cytokines and inflammation in heart function during health and disease. Heart Fail Rev. 2018;23:733‐758. [DOI] [PubMed] [Google Scholar]
- 37. Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL‐6 blockade for cytokine storm. Immunotherapy. 2016;8:959‐970. [DOI] [PubMed] [Google Scholar]
- 38. Pathan N, Franklin JL, Eleftherohorinou H, et al. Myocardial depressant effects of interleukin 6 in meningococcal sepsis are regulated by p38 mitogen‐activated protein kinase. Crit Care Med. 2011;39:1692‐1711. [DOI] [PubMed] [Google Scholar]
- 39. Peng Y, Yang Q, Gao S, et al. IL‐6 protects cardiomyocytes from oxidative stress at the early stage of LPS‐induced sepsis. Biochem Biophys Res Commun. 2022;603:144‐152. [DOI] [PubMed] [Google Scholar]
- 40. Liang W, Li J, Bai C, et al. Interleukin‐5 deletion promotes sepsis‐inducedM1macrophage differentiation, deteriorates cardiac dysfunction, and exacerbates cardiac injury via theNF‐κBp65 pathway in mice. Biofactors. 2020;46:1006‐1017. [DOI] [PubMed] [Google Scholar]
- 41. Wang Z, Liu M, Ye D, et al. Il12a deletion aggravates Sepsis‐Induced cardiac dysfunction by regulating macrophage polarization. Front Pharmacol. 2021;12:632912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garred P, Tenner AJ, Mollnes TE. Therapeutic targeting of the complement system: from rare diseases to pandemics. Pharmacol Rev. 2021;73:792‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Riedemann NC, Guo RF, Neff TA, et al. Increased C5a receptor expression in sepsis. J Clin Invest. 2002;110:101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fusakio ME, Mohammed JP, Laumonnier Y, Hoebe K, Köhl J, Mattner J. C5a regulates NKT and NK cell functions in sepsis. J Immunol. 2011;187:5805‐5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fattahi F, Ward PA. Complement and sepsis‐induced heart dysfunction. Mol Immunol. 2017;84:57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kalbitz M, Fattahi F, Grailer JJ, et al. Complement‐induced activation of the cardiac NLRP3 inflammasome in sepsis. FASEB J. 2016;30:3997‐4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kalbitz M, Fattahi F, Herron TJ, et al. Complement destabilizes cardiomyocyte function in vivo after polymicrobial sepsis and in vitro. The Journal of Immunology. 2016;197:2353‐2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Abe T, Kubo K, Izumoto S, et al. Complement activation in human sepsis is related to Sepsis‐Induced disseminated intravascular coagulation. Shock. 2020;54:198‐204. [DOI] [PubMed] [Google Scholar]
- 49. Gando S, Shiraishi A, Yamakawa K, et al. Role of disseminated intravascular coagulation in severe sepsis. Thromb Res. 2019;178:182‐188. [DOI] [PubMed] [Google Scholar]
- 50. Umemura Y, Yamakawa K, Hayakawa M, Hamasaki T, Fujimi S, Japan Septic Disseminated Intravascular Coagulation study g . Screening itself for disseminated intravascular coagulation May reduce mortality in sepsis: a nationwide multicenter registry in Japan. Thromb Res. 2018;161:60‐66. [DOI] [PubMed] [Google Scholar]
- 51. Atefi G, Zetoune FS, Herron TJ, et al. Complement dependency of cardiomyocyte release of mediators during sepsis. FASEB J. 2011;25:2500‐2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grossklaus C, Damerau B, Lemgo E, Vogt W. Induction of platelet aggregation by the complement‐derived peptides C3a and C5a. Naunyn‐Schmiedeberg's Arch Pharmacol. 1976;295:71‐76. [DOI] [PubMed] [Google Scholar]
- 53. Rataj D, Werwitzke S, Haarmeijer B, et al. Inhibition of complement component C5 prevents clotting in an ex vivo model of xenogeneic activation of coagulation. Xenotransplantation. 2016;23:117‐127. [DOI] [PubMed] [Google Scholar]
- 54. Hotchkiss RS, Monneret G, Payen D. Sepsis‐induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Remy KE, Mazer M, Striker DA, et al. Severe immunosuppression and not a cytokine storm characterizes COVID‐19 infections. JCI Insight . 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Venet F, Monneret G. Advances in the understanding and treatment of sepsis‐induced immunosuppression. Nature Reviews Nephrology. 2018;14:121‐137. [DOI] [PubMed] [Google Scholar]
- 58. Delano MJ, Ward PA. The immune system's role in sepsis progression, resolution, and long‐term outcome. Immunol Rev. 2016;274:330‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bhan C, Dipankar P, Chakraborty P, Sarangi PP. Role of cellular events in the pathophysiology of sepsis. Inflamm Res. 2016;65:853‐868. [DOI] [PubMed] [Google Scholar]
- 60. Cheng SC, Scicluna BP, Arts RJW, et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nature Immunol. 2016;17:406‐413. [DOI] [PubMed] [Google Scholar]
- 61. Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594‐2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang J, Zhou H. Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia‐reperfusion injury. Acta Pharm Sin B. 2020;10:1866‐1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Klopstock T, Priglinger C, Yilmaz A, Kornblum C, Distelmaier F, Prokisch H. Mitochondrial disorders. Dtsch Arztebl Int. 2021;118:741‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. West AP. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology. 2017;391:54‐63. [DOI] [PubMed] [Google Scholar]
- 65. Zhong F, Liang S, Zhong Z. Emerging role of mitochondrial DNA as a major driver of inflammation and disease progression. Trends Immunol. 2019;40:1120‐1133. [DOI] [PubMed] [Google Scholar]
- 66. Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS‐induced ROS release. Physiol Rev. 2014;94:909‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Bioch et Biophy Acta. 2018;1859:940‐950. [DOI] [PubMed] [Google Scholar]
- 69. Larche J, Lancel S, Hassoun SM, et al. Inhibition of mitochondrial permeability transition prevents sepsis‐induced myocardial dysfunction and mortality. JACC. 2006;48:377‐385. [DOI] [PubMed] [Google Scholar]
- 70. Bonora M, Giorgi C, Pinton P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat Rev Mol Cell Biol. 2022;23:266‐285. [DOI] [PubMed] [Google Scholar]
- 71. Yang J, Zhang R, Jiang X, et al. Toll‐like receptor 4‐induced ryanodine receptor 2 oxidation and sarcoplasmic reticulum Ca(2+) leakage promote cardiac contractile dysfunction in sepsis. J Biol Chem. 2018;293:794‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fernandes CJ Jr., Campos AH. Sepsis‐induced myocardial depression and calcium mishandling: an acceptable unifying theory? Crit Care Med. 2008;36:2695‐2696. [DOI] [PubMed] [Google Scholar]
- 73. Bartz RR, Suliman HB, Piantadosi CA. Redox mechanisms of cardiomyocyte mitochondrial protection. Front Physiol. 2015;6:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bar‐Or D, Carrick MM, Mains CW, Rael LT, Slone D, Brody EN. Sepsis, oxidative stress, and hypoxia: are there clues to better treatment? Redox Rep. 2015;20:193‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Qiu Z, He Y, Ming H, Lei S, Leng Y, Xia Z. Lipopolysaccharide (LPS) aggravates high glucose‐ and hypoxia/reoxygenation‐induced injury through activating ROS‐dependent NLRP3 Inflammasome‐mediated pyroptosis in H9C2 cardiomyocytes. J Diabetes Res. 2019;2019:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shalova IN, Lim JY, Chittezhath M, et al. Human monocytes undergo functional re‐programming during sepsis mediated by Hypoxia‐Inducible Factor‐1α. Immunity. 2015;42:484‐498. [DOI] [PubMed] [Google Scholar]
- 77. Downes NL, Laham‐Karam N, Kaikkonen MU, Ylä‐Herttuala S. Differential but complementary HIF1α and HIF2α transcriptional regulation. Mol Ther. 2018;26:1735‐1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bateman RM, Tokunaga C, Kareco T, Dorscheid DR, Walley KR. Myocardial hypoxia‐inducible HIF‐1α, VEGF, and GLUT1 gene expression is associated with microvascular and ICAM‐1 heterogeneity during endotoxemia. Am J Physiol Heart Circul Physiol. 2007;293:H448‐H456. [DOI] [PubMed] [Google Scholar]
- 79. Zou R, Tao J, Qiu J, et al. DNA‐PKcs promotes sepsis‐induced multiple organ failure by triggering mitochondrial dysfunction. J Adv Res. 2022;41:39‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang S, Zhu H, Li R, et al. DNA‐PKcs interacts with and phosphorylates Fis1 to induce mitochondrial fragmentation in tubular cells during acute kidney injury. Sci Signaling. 2022;15:eabh1121. [DOI] [PubMed] [Google Scholar]
- 81. Wang Y, Jasper H, Toan S, Muid D, Chang X, Zhou H. Mitophagy coordinates the mitochondrial unfolded protein response to attenuate inflammation‐mediated myocardial injury. Redox Biol. 2021;45:102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhao RZ, Jiang S, Zhang L, Yu ZB. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med. 2019;44:3‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sreedhar A, Zhao Y. Uncoupling protein 2 and metabolic diseases. Mitochondrion. 2017;34:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tang R, Qi P, Liu Y, et al. Uncoupling protein 2 drives myocardial dysfunction in murine models of septic shock. BioMed Res Int. 2019;2019:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nicholls DG. Mitochondrial proton leaks and uncoupling proteins. Biochim Biophys Acta Bioenerg. 2021;1862:148428. [DOI] [PubMed] [Google Scholar]
- 86. Huang J, Peng W, Zheng Y, et al. Upregulation of UCP2 expression protects against LPS‐Induced oxidative stress and apoptosis in cardiomyocytes. Oxid Med Cell Longevity. 2019;2019:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pan P, Zhang H, Su L, Wang X, Liu D. Melatonin balance the autophagy and apoptosis by regulating UCP2 in the LPS‐Induced cardiomyopathy. Molecules. 2018;23:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chen W, Luo S, Xie P, Hou T, Yu T, Fu X. Overexpressed UCP2 regulates mitochondrial flashes and reverses lipopolysaccharide‐induced cardiomyocytes injury. Am J Transl Res. 2018;10:1347‐1356. [PMC free article] [PubMed] [Google Scholar]
- 89. Zheng G, Lyu J, Liu S, et al. Silencing of uncoupling protein 2 by small interfering RNA aggravates mitochondrial dysfunction in cardiomyocytes under septic conditions. Int J Mol Med. 2015;35:1525‐1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cheng J, Nanayakkara G, Shao Y, et al. Mitochondrial proton leak plays a critical role in pathogenesis of cardiovascular diseases. Adv Exp Med Biol. 2017;982:359‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Iba T, Levy JH, Warkentin TE, et al. Diagnosis and management of sepsis‐induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemostasis. 2019;17:1989‐1994. [DOI] [PubMed] [Google Scholar]
- 92. Iba T, Gando S, Thachil J. Anticoagulant therapy for sepsis‐associated disseminated intravascular coagulation: the view from Japan. J Thromb Haemostasis. 2014;12:1010‐1019. [DOI] [PubMed] [Google Scholar]
- 93. Chen Z, Zhang H, Qu M, et al. Review: the emerging role of neutrophil extracellular traps in sepsis and Sepsis‐Associated thrombosis. Front Cell Infect Microbiol. 2021;11:653228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Adelborg K, Larsen JB, Hvas AM. Disseminated intravascular coagulation: epidemiology, biomarkers, and management. Br J Haematol. 2021;192:803‐818. [DOI] [PubMed] [Google Scholar]
- 95. Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nature Reviews Nephrol. 2018;14:417‐427. [DOI] [PubMed] [Google Scholar]
- 96. Ince C. The microcirculation is the motor of sepsis. Crit Care. 2005;9(suppl 4):S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chang JC. Sepsis and septic shock: endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb J. 2019;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83:536‐545. [DOI] [PubMed] [Google Scholar]
- 99. Aird WC. Sepsis and coagulation. Crit Care Clin. 2005;21:417‐431. [DOI] [PubMed] [Google Scholar]
- 100. Fortin CF, McDonald PP, Fülöp T, Lesur O. Sepsis, leukocytes, and nitric oxide (NO): an intricate affair. Shock. 2010;33:344‐352. [DOI] [PubMed] [Google Scholar]
- 101. Fernandes D, Assreuy J. Nitric oxide and vascular reactivity in sepsis. Shock. 2008;30(suppl 1):10‐13. [DOI] [PubMed] [Google Scholar]
- 102. Burgdorff AM, Bucher M, Schumann J. Vasoplegia in patients with sepsis and septic shock: pathways and mechanisms. J Int Med Res. 2018;46:1303‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Carrara M, Herpain A, Baselli G, Ferrario M. Vascular decoupling in septic shock: the combined role of autonomic nervous system, arterial stiffness, and peripheral vascular tone. Front Physiol. 2020;11:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Badke CM, Marsillio LE, Weese‐Mayer DE, Sanchez‐Pinto LN. Autonomic nervous system dysfunction in pediatric sepsis. Front Pediatr. 2018;6:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Carrara M, Ferrario M, Bollen Pinto B, Herpain A. The autonomic nervous system in septic shock and its role as a future therapeutic target: a narrative review. Ann Intensive Care. 2021;11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Drosatos K, Lymperopoulos A, Kennel PJ, Pollak N, Schulze PC, Goldberg IJ. Pathophysiology of sepsis‐related cardiac dysfunction: driven by inflammation, energy mismanagement, or both? Curr Heart Fail Rep. 2015;12:130‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chang YT, Huang WC, Cheng CC, et al. Effects of epinephrine on heart rate variability and cytokines in a rat sepsis model. Bosn J Basic Med Sci. 2020;20:88‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Schmittinger CA, Wurzinger B, Deutinger M, et al. How to protect the heart in septic shock: a hypothesis on the pathophysiology and treatment of septic heart failure. Med Hypotheses. 2010;74:460‐465. [DOI] [PubMed] [Google Scholar]
- 109. Lv X, Wang H. Pathophysiology of sepsis‐induced myocardial dysfunction. Mil Med Res. 2016;3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chang C, Hu L, Sun S, et al. Regulatory role of the TLR4/JNK signaling pathway in sepsisinduced myocardial dysfunction. Mol Med Rep. 2021;23:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yang C, Xia W, Liu X, Lin J, Wu A. Role of TXNIP/NLRP3 in sepsis‐induced myocardial dysfunction. Int J Mol Med. 2019;44:417‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Xu M, Liu PP, Li H. Innate immune signaling and its role in metabolic and cardiovascular diseases. Physiol Rev. 2019;99:893‐948. [DOI] [PubMed] [Google Scholar]
- 113. Knuefermann P, Sakata Y, Baker JS, et al. Toll‐like receptor 2 mediates Staphylococcus aureus‐induced myocardial dysfunction and cytokine production in the heart. Circulation. 2004;110:3693‐3698. [DOI] [PubMed] [Google Scholar]
- 114. Svennerholm K, Park KS, Wikström J, et al. Escherichia coli outer membrane vesicles can contribute to sepsis induced cardiac dysfunction. Sci Rep. 2017;7:17434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mutig N, Geers‐Knoerr C, Piep B, et al. Lipoteichoic acid from Staphylococcus aureus directly affects cardiomyocyte contractility and calcium transients. Mol Immunol. 2013;56:720‐728. [DOI] [PubMed] [Google Scholar]
- 116. Yao X, Carlson D, Sun Y, et al. Mitochondrial ROS induces cardiac inflammation via a pathway through mtDNA damage in a pneumonia‐related sepsis model. PLoS One. 2015;10:e0139416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Horton JW. A model of myocardial inflammation and dysfunction in burn complicated by sepsis. Shock. 2007;28:326‐333. [DOI] [PubMed] [Google Scholar]
- 118. Ranjani J, Pushpanathan M, Mahesh A, Niraimathi M, Gunasekaran P, Rajendhran J. Pseudomonas aeruginosa PAO1 induces distinct cell death mechanisms in H9C2 cells and its differentiated form. J Basic Microbiol. 2015;55:1191‐1202. [DOI] [PubMed] [Google Scholar]
- 119. Duggan S, Leonhardt I, Hünniger K, Kurzai O. Host response to Candida albicans bloodstream infection and sepsis. Virulence. 2015;6:316‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Buluc M, Ataoğlu H, Doğan D, et al. Effect of Candida albicans septicemia on the cardiovascular function of rabbits. Int Immunopharmacol. 2005;5:893‐901. [DOI] [PubMed] [Google Scholar]
- 121. Torres‐Tirado D, Knabb M, Castaño I, Patrón‐Soberano A, De Las Peñas A, Rubio R. Candida glabrata binds to glycosylated and lectinic receptors on the coronary endothelial luminal membrane and inhibits flow sense and cardiac responses to agonists. American J Physiol Regulat Integ Compar Physiol. 2016;310:R24‐R32. [DOI] [PubMed] [Google Scholar]
- 122. Lin GL, McGinley JP, Drysdale SB, Pollard AJ. Epidemiology and immune pathogenesis of viral sepsis. Front Immunol. 2018;9:2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhu P, Chen S, Zhang W, Duan G, Jin Y. Essential role of Non‐Coding RNAs in enterovirus infection: from basic mechanisms to clinical prospects. Int J Mol Sci. 2021;22:2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bratincsák A, El‐Said HG, Bradley JS, Shayan K, Grossfeld PD, Cannavino CR. Fulminant myocarditis associated with pandemic H1N1 influenza A virus in children. JACC. 2010;55:928‐929. [DOI] [PubMed] [Google Scholar]
- 125. Daba TM, Zhao Y, Pan Z. Advancement of mechanisms of coxsackie virus B3‐Induced myocarditis pathogenesis and the potential therapeutic targets. Curr Drug Targets. 2019;20:1461‐1473. [DOI] [PubMed] [Google Scholar]
- 126. Holmes CL, Anderson MT, Mobley HLT, Bachman MA. Pathogenesis of Gram‐Negative bacteremia. Clin Microbiol Rev. 2021;34:e00234‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Avlas O, Fallach R, Shainberg A, Porat E, Hochhauser E. Toll‐like receptor 4 stimulation initiates an inflammatory response that decreases cardiomyocyte contractility. Antioxid Redox Signal. 2011;15:1895‐1909. [DOI] [PubMed] [Google Scholar]
- 128. Salomao R, Brunialti MKC, Rapozo MM, Baggio‐Zappia GL, Galanos C, Freudenberg M. Bacterial sensing, cell signaling, and modulation of the immune response during sepsis. Shock. 2012;38:227‐242. [DOI] [PubMed] [Google Scholar]
- 129. Chikwe J. Myocardial abscess. Heart. 2004;90:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Thomer L, Schneewind O, Missiakas D. Pathogenesis of Staphylococcus aureus bloodstream infections. Annu Rev Pathol: Mech Dis. 2016;11:343‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Turner NA, Sharma‐Kuinkel BK, Maskarinec SA, et al. Methicillin‐resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17:203‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Rolli J, Rosenblatt‐Velin N, Li J, et al. Bacterial flagellin triggers cardiac innate immune responses and acute contractile dysfunction. PLoS One. 2010;5:e12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Pollack A, Kontorovich AR, Fuster V, Dec GW. Viral myocarditis‐‐diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12:670‐680. [DOI] [PubMed] [Google Scholar]
- 134. Miteva K, Pappritz K, Sosnowski M, et al. Mesenchymal stromal cells inhibit NLRP3 inflammasome activation in a model of coxsackievirus B3‐induced inflammatory cardiomyopathy. Sci Rep. 2018;8:2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Cooper LT. Jr. Myocarditis. N Engl J Med. 2009;360:1526‐1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bojkova D, Wagner JUG, Shumliakivska M, et al. SARS‐CoV‐2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc Res. 2020;116:2207‐2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Rezkalla SH, Kloner RA. Viral myocarditis: 1917‐2020: from the influenza A to the COVID‐19 pandemics. Trends Cardiovascul Med. 2021;31:163‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Tschöpe C, Ammirati E, Bozkurt B, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Knuefermann P, Schwederski M, Velten M, et al. Bacterial DNA induces myocardial inflammation and reduces cardiomyocyte contractility: role of toll‐like receptor 9. Cardiovasc Res. 2008;78:26‐35. [DOI] [PubMed] [Google Scholar]
- 140. Liu ZJ, Liu H, Wu C, Xue K. Effect of sepsis on the action potential and cardiac serotonin response in rats. Exp Ther Med. 2019;18:2207‐2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ock S, Ahn J, Lee SH, et al. Receptor activator of nuclear factor‐κB ligand is a novel inducer of myocardial inflammation. Cardiovasc Res. 2012;94:105‐114. [DOI] [PubMed] [Google Scholar]
- 142. You W, Min X, Zhang X, et al. Cardiac‐specific expression of heat shock protein 27 attenuated endotoxin‐induced cardiac dysfunction and mortality in mice through a PI3K/Akt‐dependent mechanism. Shock. 2009;32:108‐117. [DOI] [PubMed] [Google Scholar]
- 143. Li XQ, Cao W, Li T, et al. Amlodipine inhibits TNF‐α production and attenuates cardiac dysfunction induced by lipopolysaccharide involving PI3K/Akt pathway. Int Immunopharmacol. 2009;9:1032‐1041. [DOI] [PubMed] [Google Scholar]
- 144. L'Heureux M, Sternberg M, Brath L, Turlington J, Kashiouris MG. Sepsis‐Induced cardiomyopathy: a comprehensive review. Curr Cardiol Rep. 2020;22:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Beesley SJ, Weber G, Sarge T, et al. Septic cardiomyopathy. Crit Care Med. 2018;46:625‐634. [DOI] [PubMed] [Google Scholar]
- 146. Ng PY, Sin WC, Ng AKY, Chan WM. Speckle tracking echocardiography in patients with septic shock: a case control study (SPECKSS). Crit Care. 2016;20:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Orde S, Huang SJ, McLean AS. Speckle tracking echocardiography in the critically ill: enticing research with minimal clinical practicality or the answer to non‐invasive cardiac assessment? Anaesth Intensive Care. 2016;44:542‐551. [DOI] [PubMed] [Google Scholar]
- 148. Tan M, Lu Y, Jiang H, Zhang L. The diagnostic accuracy of procalcitonin and c‐reactive protein for sepsis: a systematic review and meta‐analysis. JCB. 2019;120:5852‐5859. [DOI] [PubMed] [Google Scholar]
- 149. Catenacci V, Sheikh F, Patel K, Fox‐Robichaud AE. The prognostic utility of protein C as a biomarker for adult sepsis: a systematic review and meta‐analysis. Crit Care. 2022;26:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kim J, Kim M, Kim YJ, et al. Troponin testing for assessing Sepsis‐Induced myocardial dysfunction in patients with septic shock. J Clin Med. 2019;8:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Post F, Weilemann LS, Messow CM, Sinning C, Münzel T. B‐type natriuretic peptide as a marker for sepsis‐induced myocardial depression in intensive care patients. Crit Care Med. 2008;36:3030‐3037. [DOI] [PubMed] [Google Scholar]
- 152. Innocenti F, Palmieri V, Stefanone VT, et al. Comparison of troponin I levels versus myocardial dysfunction on prognosis in sepsis. Intern Emerg Med. 2022;17:223‐231. [DOI] [PubMed] [Google Scholar]
- 153. Chen F, Xu Y, Zhang Z. Multi‐biomarker strategy for prediction of myocardial dysfunction and mortality in sepsis. J Zhejiang Univer. 2020;21:537‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Meng JB, Hu MH, Zhang M, Hu GP, Zhang W, Hu SJ. The correlation between whole blood copper (Cu), zinc (Zn) levels and Cu/Zn ratio and Sepsis‐Induced left ventricular systolic dysfunction (SILVSD) in patients with septic shock: a Single‐Center prospective observational study. Int J Gen Med. 2021;14:7219‐7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wang L, Xie W, Li G, et al. Lipocalin 10 as a new prognostic biomarker in Sepsis‐Induced myocardial dysfunction and mortality: a pilot study. Mediators Inflamm. 2021;2021:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Yang C, Wen K. Predictive value and regulatory mechanism of serum miR‐499a‐5p on myocardial dysfunction in sepsis. J Cardiothorac Surg. 2021;16:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sun B, Luan C, Guo L, Zhang B, Liu Y. Low expression of microRNA‐328 can predict sepsis and alleviate sepsis‐induced cardiac dysfunction and inflammatory response. Braz J Med Biol Res. 2020;53:e9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Cohen J, Vincent JL, Adhikari NKJ, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15:581‐614. [DOI] [PubMed] [Google Scholar]
- 159. Phares TW, Kotraiah V, Chung CS, et al. A Peptide‐Based checkpoint immunomodulator alleviates immune dysfunction in murine polymicrobial sepsis. Shock. 2021;55:806‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Li N, Zhou H, Wu H, et al. STING‐IRF3 contributes to lipopolysaccharide‐induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019;24:101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Liu JJ, Li Y, Yang MS, Chen R, Cen CQ. SP1‐induced ZFAS1 aggravates sepsis‐induced cardiac dysfunction via miR‐590‐3p/NLRP3‐mediated autophagy and pyroptosis. Arch Biochem Biophys. 2020;695:108611. [DOI] [PubMed] [Google Scholar]
- 162.Anonymous. Targeted reversal of inflammation in pediatric sepsis‐induced MODS. https://ClinicalTrials.gov/show/NCT05267821
- 163.Anonymous. GM‐CSF for reversal of immunoparalysis in pediatric sepsis‐induced MODS. https://ClinicalTrials.gov/show/NCT05266001
- 164.Anonymous. Personalized immunotherapy in sepsis. https://ClinicalTrials.gov/show/NCT04990232
- 165.Anonymous. A Phase 2 study evaluating efficacy, safety and tolerability of different doses and regimens of allocetra‐OTS for the treatment of organ failure in adult sepsis patients. https://ClinicalTrials.gov/show/NCT04612413
- 166.Anonymous. Efficacy, safety and tolerability of nangibotide in patients with septic shock. https://ClinicalTrials.gov/show/NCT04055909
- 167. Peters van Ton AM, Kox M, Abdo WF, Pickkers P. Precision immunotherapy for sepsis. Front Immunol. 2018;9:1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kingsley SMK, Bhat BV. Differential paradigms in animal models of sepsis. Curr Infect Dis Rep. 2016;18:26. [DOI] [PubMed] [Google Scholar]
- 169. Scheeren TWL, Bakker J, Kaufmann T, et al. Current use of inotropes in circulatory shock. Ann Intensive Care. 2021;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Damiani E, Donati A, Girardis M. Oxygen in the critically ill: friend or foe? Curr Opin Anaesthesiol. 2018;31:129‐135. [DOI] [PubMed] [Google Scholar]
- 171. Andrews B, Semler MW, Muchemwa L, et al. Effect of an early resuscitation protocol on in‐hospital mortality among adults with sepsis and hypotension: a randomized clinical trial. JAMA. 2017;318:1233‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Sakr Y, Rubatto Birri PN, Kotfis K, et al. Intensive Care Over Nations I . Higher fluid balance increases the risk of death from sepsis: results from a large international audit. Crit Care Med. 2017;45:386‐394. [DOI] [PubMed] [Google Scholar]
- 173. Brown RM, Semler MW. Fluid management in sepsis. J Intensiv Care Med. 2019;34:364‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Li J, Sun W, Guo Y, Ren Y, Li Y, Yang Z. Prognosis of β‐adrenergic blockade therapy on septic shock and sepsis: a systematic review and meta‐analysis of randomized controlled studies. Cytokine. 2020;126:154916. [DOI] [PubMed] [Google Scholar]
- 175. Pinsky MR. Is there a role for β‐blockade in septic shock? JAMA. 2013;310:1677‐1678. [DOI] [PubMed] [Google Scholar]
- 176. Lira A, Pinsky MR. Should β‐blockers be used in septic shock? Crit Care. 2014;18:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Zhang W, He B, Wu Y, Qiao J, Peng Z. Melatonin protects against sepsis‐induced cardiac dysfunction by regulating apoptosis and autophagy via activation of SIRT1 in mice. Life Sci. 2019;217:8‐15. [DOI] [PubMed] [Google Scholar]
- 178. Xiao Z, Kong B, Fang J, et al. Ferrostatin‐1 alleviates lipopolysaccharide‐induced cardiac dysfunction. Bioengineered. 2021;12:9367‐9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Wu B, Song H, Fan M, et al. Luteolin attenuates sepsisinduced myocardial injury by enhancing autophagy in mice. Int J Mol Med. 2020;45:1477‐1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wang C, Yuan W, Hu A, et al. Dexmedetomidine alleviated sepsisinduced myocardial ferroptosis and septic heart injury. Mol Med Rep. 2020;22:175‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Zhang H, Feng Y, Yao Y. Potential therapy strategy: targeting mitochondrial dysfunction in sepsis. Mil Med Res. 2018;5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Lin Y, Xu Y, Zhang Z. Sepsis‐Induced myocardial dysfunction (SIMD): the pathophysiological mechanisms and therapeutic strategies targeting mitochondria. Inflammation. 2020;43:1184‐1200. [DOI] [PubMed] [Google Scholar]
- 183. Reitsema VA, Star BS, de Jager VD, van Meurs M, Henning RH, Bouma HR. Metabolic resuscitation strategies to prevent organ dysfunction in sepsis. Antioxid Redox Signaling. 2019;31:134‐152. [DOI] [PubMed] [Google Scholar]
- 184. Zhang C, Cheng Y, Liu D, et al. Mitochondria‐targeted cyclosporin A delivery system to treat myocardial ischemia reperfusion injury of rats. J Nanobiotechnol. 2019;17:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Anonymous. Targeting mitochondrial fusion and fission to prevent atherosclerosis: getting the balance right. https://ClinicalTrials.gov/show/NCT03980548
- 186.Anonymous. Mitochondrial complex I dysfunction in PWS. https://ClinicalTrials.gov/show/NCT03831425
- 187.Anonymous. MitoQ supplementation and cardiovascular function in healthy men and women. https://ClinicalTrials.gov/show/NCT03586414
- 188.Anonymous. Effects of mitochondrial‐targeted antioxidant on mild cognitive impairment (MCI) Patients. https://ClinicalTrials.gov/show/NCT03514875
- 189.Anonymous. Impacts of Mitochondrial‐targeted antioxidant on peripheral artery disease patients. https://ClinicalTrials.gov/show/NCT03506633
- 190. Liu VX, Fielding‐Singh V, Greene JD, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196:856‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Alam N, Oskam E, Stassen PM, et al. Prehospital antibiotics in the ambulance for sepsis: a multicentre, open label, randomised trial. Lancet Respirat Med. 2018;6:40‐50. [DOI] [PubMed] [Google Scholar]
- 192. Weinberger J, Rhee C, Klompas M. A critical analysis of the literature on time‐to‐antibiotics in suspected sepsis. J Infect Dis. 2020;222:S110‐S118. [DOI] [PubMed] [Google Scholar]
- 193. Rhee C, Kadri SS, Dekker JP, et al. Program CDCPE Prevalence of Antibiotic‐Resistant pathogens in culture‐proven sepsis and outcomes associated with inadequate and broad‐spectrum empiric antibiotic use. JAMA Network Open. 2020;3:e202899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Yin C, Wang Z, Ding X, et al. Crystalline ruthenium polypyridine nanoparticles: a targeted treatment of bacterial infection with multifunctional antibacterial, adhesion and surface‐anchoring photosensitizer properties. Journal of Materials Chemistry B. 2021;9:3808‐3825. [DOI] [PubMed] [Google Scholar]
- 195. Yang Y, Qian M, Yi S, et al. Monoclonal antibody targeting Staphylococcus aureus surface protein A (SasA) protect against Staphylococcus aureus sepsis and peritonitis in mice. PLoS One. 2016;11:e0149460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Ohsawa H, Baba T, Enami J, Hiramatsu K. Protective activity of anti‐lipoteichoic acid monoclonal antibody in single or combination therapies in methicillin‐resistant Staphylococcus aureus‐induced murine sepsis models. J Infect Chemother. 2020;26:520‐522. [DOI] [PubMed] [Google Scholar]
- 197. Georgoutsou‐Spyridonos M, Ricklin D, Pratsinis H, et al. Attenuation of Staphylococcus aureus‐Induced bacteremia by human mini‐antibodies targeting the complement inhibitory protein efb. J Immunol. 2015;195:3946‐3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Biering SB, Akey DL, Wong MP, et al. Structural basis for antibody inhibition of flavivirus NS1‐triggered endothelial dysfunction. Science. 2021;371:194‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Opota O, Croxatto A, Prod'hom G, Greub G. Blood culture‐based diagnosis of bacteraemia: state of the art. Clin Microbiol Infect. 2015;21:313‐322. [DOI] [PubMed] [Google Scholar]
- 200. Ginn AN, Halliday CL, Douglas AP, Chen SCA. PCR‐based tests for the early diagnosis of sepsis. Where do we stand? Curr Opin Infect Dis. 2017;30:565‐572. [DOI] [PubMed] [Google Scholar]
- 201. Mishra D, Satpathy G, Wig N, Fazal F, Ahmed NH, Panda SK. Evaluation of 16S rRNA broad range PCR assay for microbial detection in serum specimens in sepsis patients. J Inf Pub Health. 2020;13:998‐1002. [DOI] [PubMed] [Google Scholar]
- 202. Jordana‐Lluch E, Giménez M, Quesada D, et al. Evaluation of the broad‐range PCR/ESI‐MS technology in blood specimens for the molecular diagnosis of bloodstream infections. PLoS One. 2015;10:e0140865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Riedel S, Carroll KC. Early identification and treatment of pathogens in sepsis. Clin Chest Med. 2016;37:191‐207. [DOI] [PubMed] [Google Scholar]
- 204. Grumaz S, Stevens P, Grumaz C, et al. Next‐generation sequencing diagnostics of bacteremia in septic patients. Genome Med. 2016;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Rocca C, De Bartolo A, Grande F, et al. Cateslytin abrogates lipopolysaccharide‐induced cardiomyocyte injury by reducing inflammation and oxidative stress through toll like receptor 4 interaction. Int Immunopharmacol. 2021;94:107487. [DOI] [PubMed] [Google Scholar]
- 206. Sun Y, Yao X, Zhang QJ, et al. Beclin‐1‐dependent autophagy protects the heart during sepsis. Circulation. 2018;138:2247‐2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Walley KR. Sepsis‐induced myocardial dysfunction and mammalian target of rapamycin signalling pathways. Can J Cardiol. 2019;35:809‐812. [DOI] [PubMed] [Google Scholar]
- 208. Cendejas‐Bueno E, Romero‐Gómez MP, Mingorance J. The challenge of molecular diagnosis of bloodstream infections. World J Microbiol Biotechnol. 2019;35:65. [DOI] [PubMed] [Google Scholar]


