Abstract
Background:
The impact of margin status on resection of primary pancreatic neuroendocrine tumors has been poorly defined. The objectives of the present study were to determine the impact of margin status on long-term survival of patients with pancreatic neuroendocrine tumors after curative resection and evaluate the impact of reresection to obtain a microscopically negative margin.
Methods:
Patients who underwent curative-intent resection for pancreatic neuroendocrine tumors between 2000 and 2016 were identified at 8 hepatobiliary centers. Overall and recurrence-free survival were analyzed relative to surgical margin status using univariable and multivariable analyses.
Results:
Among 1,020 patients, 866 (84.9%) had an R0 (>1 mm margin) resection, whereas 154 (15.1%) had an R1 (≤1 mm margin) resection. R1 resection was associated with a worse recurrence-free survival (10-year recurrence-free survival, R1 47.3% vs R0 62.8%, hazard ratio 1.8, 95% confidence interval 1.2–2.7, P = .002); residual tumor at either the transection margin (R1t) or the mobilization margin (R1m) was associated with increased recurrence versus R0 (R1t versus R0: hazard ratio 1.8, 95% confidence interval 1.0–3.0, P = .033; R1m versus R0: hazard ratio 1.3, 95% confidence interval 1.0–1.7, P = .060). In contrast, margin status was not associated with overall survival (10-year overall survival, R1 71.1% vs R0 71.8%, P = .392). Intraoperatively, 539 (53.6%) patients had frozen section evaluation of the surgical margin; 49 (9.1%) patients had a positive margin on frozen section analysis; 38 of the 49 patients (77.6%) had reresection, and a final R0 (secondary R0) margin was achieved in 30 patients (78.9%). Extending resection to achieve an R0 status remained associated with worse overall survival (hazard ratio 3.1, 95% confidence interval 1.6–6.2, P = .001) and recurrence-free survival (hazard ratio 2.6, 95% confidence interval 1.4–5.0, P = .004) compared with primary R0 resection. On multivariable analyses, tumor-specific factors, such as cellular differentiation, perineural invasion, Ki-67 index, and major vascular invasion, rather than surgical margin, were associated with long-term outcomes.
Conclusion:
Margin status was not associated with long-term survival. The reresection of an initially positive surgical margin to achieve a negative margin did not improve the outcome of patients with pancreatic neuroendocrine tumors. Parenchymal-sparing pancreatic procedures for pancreatic neuroendocrine tumors may be appropriate when feasible.
Introduction
Gastroenteropancreatic neuroendocrine tumors are a heterogeneous group of neoplasms that can present with variable biologic behavior ranging from benign or indolent to frankly malignant or aggressive.1,2 Among the various anatomic locations from which gastroenteropancreatic neuroendocrine tumors can arise, pancreatic neuroendocrine tumors (pNETs) generally have the worst outcome.3 The incidence of pNETs has been reported to be 0.43 per 100,000 people and appears to be increasing worldwide.4,5 Operative treatment plays a central role in the treatment of patients with pNET, because resection represents the best chance at potentially curative therapy for locoregional pNETs. Depending on the location of the lesion, complete oncologic resection may require pancreatoduodenectomy, distal pancreatectomy, central pancreatectomy, or—very rarely—total pancreatectomy. Goals of resection are aimed typically at complete removal of the tumor and adequate lympadenectomy.5,6
Traditionally a guiding principle for any curative-intent resection of pancreatic cancer is to achieve free or negative surgical margins.7,8 The precise definition of a “positive” margin is not always clear, however.8 In turn, previous studies that investigated the association of margin status and long-term prognosis after curative-intent resection have been inconsistent.8–12 In addition, whether residual tumor at the surgical margin affects survival remains somewhat controversial.13 Whether a final negative margin affects outcome after re-resection of an initially positive margin on intraoperative frozen section analysis has also been debated among patients with pancreatic ductal cancer9,13–15 but does not appear to have been investigated among patients with pNETs. As such, the objective of the present study was to determine the impact of margin status on long-term survival of patients with pNETs after curative resection. In addition, we sought to evaluate potential differences in outcomes relative to tumor presence at the mobilization versus transection margin after an apparent curative resection of pNET. The impact of reresection to obtain a microscopically negative margin after an initially positive intraoperative frozen section margin was also examined.
Materials and methods
Study cohort and data collection
Patients who underwent curative-intent resection for pNETs between 2000 and 2016 were identified from the US Neuroendocrine Tumor Study Group. The study group included the Ohio State University Wexner Medical Center and James Comprehensive Cancer Center, Columbus, OH; the Winship Cancer Institute, Emory University, Atlanta, GA; Stanford University, Palo Alto, CA; the Virginia Mason Medical Center, Seattle, WA; University of Wisconsin, School of Medicine and Public Health, Madison, WI; Washington University School of Medicine, St. Louis, MO; Vanderbilt University, Nashville, TN; and University of Michigan, Ann Arbor, MI. All patients were diagnosed with pNET and the diagnosis confirmed by histologic examination.
Standard patient demographic, clinicopathologic, and perioperative data were collected based on a prospectively maintained database. Disease recurrence was defined as identification of suspicious imaging findings on postoperative surveillance or biopsy-proven, recurrent pNET. Overall survival (OS) was calculated from the date of operation to the date of death or date of last follow-up, and recurrence-free survival (RFS) was defined as the time interval between the date of operation and the date of recurrence. The Institutional Review Board of each participating institution approved the study.
Pathologic assessment
Frozen section examination was performed depending on the intraoperative judgment of the surgeon. In general, for pancreatoduodenectomy specimens the investigated sites included the pancreatic transection margin, including the uncinate or superior mesenteric artery margins, the common bile duct margin, and the anterior and posterior surface margins. When the margin was positive, additional resection was performed to obtain a clear margin when deemed technically feasible at the surgeon’s discretion. All resected specimens were submitted for permanent section histopathologic analysis. R status was determined by the pathologists based on examination of all specimen margin sites on permanent sections. An R0 resection was defined as a minimum margin length of >1 mm; the microscopic presence of tumor at the margin or a minimum margin length of ≤1 mm was designated as an R1 resection. The inability to resect all gross residual disease was defined as an R2 resection.16 For purposes of the present analyses, a positive margin at the common bile duct, pancreatic transection surface, uncinate, or superior mesenteric artery (SMA) margins was categorized as R1 at the transection margin (R1t); a positive margin at the anterior, posterior, or other peripancreatic soft tissues was categorized as R1 at the mobilization margin (R1m). Patients with synchronous involvement of the mobilization and transection margins were allocated to the R1t group. Tumor-related characteristics, including maximal tumor diameter, number, location, tumor morphology, histologic grade, lymph-vascular/perineural invasion, Ki-67, mitotic rate, and lymph node status were recorded based on final pathologic examination.
Statistical analysis
Continuous variables were expressed as medians with interquartile ranges (IQR) and categorical variables were expressed as totals and percentages. Statistical analyses were performed with the Mann-Whitney U test, χ2 test, or Fisher exact test, as appropriate. Survival probabilities were estimated by Kaplan-Meier methodology and compared by log-rank analysis. Factors statistically associated with OS and RFS on univariable analysis were included in the multivariable analyses; results were reported as hazard ratios (HRs) and 95% confidence intervals (95% CI). Statistical analysis was performed using SPSS Version 22.0 (IBM Corporation, Armonk, NY).
Results
In total, 1,176 patients diagnosed with a pNET were identified; 156 patients were excluded (liver metastasis, n = 101; R2 resection, n = 47; multiple primary tumors, n = 8). Among the 1,020 patients included in the final analytic cohort, median patient age was 58 (47–66) years, and the cohort was balanced with regards to sex (male versus female, 51.4% vs 48.6%; Table 1). The overwhelming majority of patients had sporadic pNETs with no designated syndrome (n = 895, 87.7%); a small subset of individuals had multiple endocrine neoplasia type 1 (n = 85, 8.3%) or Von Hippel–Lindau syndrome (n = 10, 1.0%). Most patients (n = 778, 76.3%) underwent an open resection, although 239 patients (23.4%) had laparoscopic or robotic resection. Resections included distal pancreatectomy (56.5%), pylorus-preserving pancreatoduodenectomy (15.6%), classic pancreatoduodenectomy (12.6%), enucleation (10.5%), central pancreatectomy (n = 3.1%), and total pancreatectomy (1.7%). Intraoperative frozen section examination was performed in 539 patients (52.8%) (Table 2). Median tumor size on final pathologic examination was 2.1 cm (IQR 1.4–3.5); the majority of patients (89.1%) had a single lesion. Among the 858 patients (84.1%) who underwent lymphadenectomy, 235 patients (27.4%) had at least 1 lymph node metastasis. In the postoperative setting, approximately half of patients developed one or more postoperative complications (Table 2). Only 21 patients (2.1%) received neoadjuvant chemotherapy, 8 (0.8%) patients received neoadjuvant somatostatin analogs; 25 (2.5%) patients were administered adjuvant chemotherapy, 49 (4.8%) patients had adjuvant somatostatin analog treatment, and 11 patients (1.1%) received postoperative radiotherapy. There was no discernible pattern or variation among the centers with regard to perioperative treatment regimens.
Table 1.
Clinicopathologic characteristics and surgical procedures of patients undergoing operative resection of pancreatic neuroendocrine tumors (pNETS) stratified by final surgical margins.
| Overall (n = 1020) | R0 (n = 866) | R1 (n = 154) | P value | |
|---|---|---|---|---|
| Age (y) | 58 (47–66) | 58 (47–65) | 58 (48–67) | .195 |
| Sex | .168 | |||
| Male | 524 (51.4%) | 437 (49.5%) | 87 (56.5%) | |
| Female | 496 (48.6%) | 429 (50.5%) | 67 (43.5%) | |
| BMI (kg/m2 ) | 27.9 (24.5–32.8) | 28.0 (24.5–32.8) | 27.6 (24.5–32.7) | .443 |
| Functional status | .113 | |||
| Nonfunctional | 838 (82.2%) | 720 (83.1%) | 118 (76.6%) | |
| Functional | 164 (16.1%) | 133 (15.4%) | 31 (20.1%) | |
| NA | 18 (1.8%) | 13 (1.5%) | 5 (3.2%) | |
| Genetic syndrome | .664 | |||
| None | 895 (87.7%) | 763 (88.1%) | 132 (85.7%) | |
| MEN 1 | 85 (8.3%) | 72 (8.3%) | 13 (8.4%) | |
| VHL | 10 (1.0%) | 7 (0.8%) | 3 (1.9%) | |
| Neurofibromatosis | 1 (0.1%) | 1 (0.1%) | 0 | |
| Tuberous sclerosis | 2 (0.2%) | 2 (0.2%) | 0 | |
| NA | 27 (2.5%) | 21 (2.4%) | 6 (3.9%) | |
| Symptomatic | .087 | |||
| No | 442 (43.3%) | 387 (44.7%) | 55 (35.7%) | |
| Yes | 558 (54.7%) | 466 (53.8%) | 92 (59.7%) | |
| Unknown | 20 (2.0%) | 13 (1.5%) | 7 (4.5%) | |
| Primary location | .206 | |||
| Head | 284 (27.8%) | 231 (26.7%) | 53 (34.4%) | |
| Uncinated | 44 (4.3%) | 38 (4.4%) | 6 (3.9%) | |
| Neck/body | 234 (22.9%) | 195 (22.5%) | 39 (25.3%) | |
| Tail | 401 (39.3%) | 351 (40.5%) | 50 (32.5%) | |
| Multiple | 51 (5.0%) | 45 (5.2%) | 6 (3.9%) | |
| Operative technique | <.001 | |||
| Open | 778 (76.3%) | 643 (74.2%) | 135 (87.7%) | |
| Laparoscopic/robotic | 239 (23.4%) | 220 (25.4%) | 19 (12.3%) | |
| Type of resection | .220 | |||
| Enucleation | 107 (10.5%) | 83 (9.6%) | 24 (15.6%) | |
| Classic PD | 129 (12.6%) | 111 (12.8%) | 18 (11.7%) | |
| Pylorus-preserving PD | 159 (15.6%) | 133 (15.4%) | 26 (16.9%) | |
| Central pancreatectomy | 32 (3.1%) | 28 (3.2%) | 4 (2.6%) | |
| Distal pancreatectomy | 576 (56.5%) | 498 (57.5%) | 78 (50.6%) | |
| Total pancreatectomy | 17 (1.7%) | 13 (1.5%) | 4 (2.6%) | |
| Major vascular resection | 49 (4.8%) | 29 (3.3%) | 20 (13.0%) | <.001 |
| Frozen section | .426 | |||
| No | 467 (45.8%) | 392 (45.3%) | 75 (48.7%) | |
| Yes | 539 (52.8%) | 463 (53.5%) | 76 (49.4%) | |
| Operation time (min) | 235 (185–315) | 231 (185–310) | 248 (188–331) | .020 |
| Blood loss (mL) | 200 (100–400) | 200 (100–400) | 300 (100–500) | .017 |
| Largest tumor size (cm) | 2.1 (1.4–3.5) | 2.0 (1.3–3.4) | 3.0 (1.8–5.0) | <.001 |
| Tumor number | .392 | |||
| Single | 909 (89.1%) | 774 (89.4%) | 135 (87.7%) | |
| Multiple | 106 (10.4%) | 87 (10.0%) | 19 (12.3%) | |
| Tumor differentiation | .033 | |||
| Well differentiated | 781 (76.6%) | 670 (77.4%) | 111 (72.1%) | |
| Moderately differentiated | 89 (8.7%) | 68 (7.9%) | 21 (13.6%) | |
| Poorly differentiated | 20 (2.0%) | 15 (1.7%) | 5 (3.2%) | |
| NA | 130 (12.7%) | 113 (13.0%) | 17 (11.0%) | |
| Lymphadenectomy | 858 (84.1%) | 731 (84.4%) | 127 (82.5%) | .394 |
| Lymph nodes status | <.001 | |||
| Negative | 623 (61.1%) | 555 (64.1%) | 68 (44.2%) | |
| Positive | 235 (23.0%) | 176 (20.3%) | 59 (38.3%) | |
| Ki-67 | .517 | |||
| <3% | 412 (40.4%) | 351 (40.5%) | 61 (39.6%) | |
| 3%–20% | 249 (24.4%) | 205 (23.7%) | 44 (28.6%) | |
| >20% | 26 (2.5%) | 23 (2.7%) | 3 (1.9%) | |
| Mitotic rate | .383 | |||
| <2 | 507 (49.7%) | 433 (50.0%) | 74 (48.1%) | |
| 2–20 | 100 (9.8%) | 80 (9.2%) | 20 (13.0%) | |
| >20 | 8 (0.8%) | 7 (0.8%) | 1 (0.6%) | |
| Lymph-vascular invasion | <.001 | |||
| Absent | 587 (57.5%) | 513 (59.2%) | 74 (48.1%) | |
| Present | 252 (24.7%) | 186 (21.5%) | 66 (42.9%) | |
| Perineural invasion | <.001 | |||
| Absent | 601 (58.9%) | 516 (59.6%) | 85 (55.2%) | |
| Present | 169 (16.6%) | 119 (13.7%) | 50 (32.5%) | |
| Postoperative morbidity | 544 (53.3%) | 461 (53.2%) | 83 (53.9%) | .860 |
| Severe complication (III-V) | 225 (22.1%) | 195 (22.5%) | 30 (19.5%) | .333 |
BMI, body mass index; MEN 1, multiple endocrine neoplasia type 1; NA, not available; PD, pancreaticoduodenectomy; VHL, Von Hippel-Lindau.
Table 2.
Association of final surgical margins with clinicopathologic characteristics among 1,006 eligible patients with operatively resected pNETs∗.
| Primary R0 (n = 825) | Secondary R0 (n = 30) | R1 (n = 151) | P value | |
|---|---|---|---|---|
| Age (y) | 58 (47–66) | 51 (45–62) | 59 (48–67) | .187 |
| Sex | ||||
| Male | 420 (50.9%) | 12 (40.0%) | 84 (55.6%) | .257 |
| Female | 405 (49.1%) | 18 (60.0%) | 67 (44.4%) | |
| BMI (kg/m2 ) | 28.1 (24.7–33.0) | 24.4 (21.2–29.6) | 27.6 (24.5–32.7) | .025 |
| Functional status | .428 | |||
| Nonfunctional | 687 (83.3%) | 23 (76.7%) | 126 (83.4%) | |
| Functional | 22 (2.7%) | 0 | 8 (5.3%) | |
| NA | 116 (14.0%) | 7 (23.3%) | 17 (11.3%) | |
| Genetic syndrome | .594 | |||
| None | 729 (88.4%) | 26 (86.7%) | 129 (85.4%) | |
| MEN 1 | 68 (8.2%) | 3 (10.0%) | 13 (8.6%) | |
| VHL | 7 (0.8%) | 0 | 3 (2.0%) | |
| Neurofibromatosis | 1 (0.1%) | 0 | 0 | |
| Tuberous sclerosis | 2 (0.2%) | 0 | 0 | |
| NA | 18 (2.2%) | 1 (3.3%) | 6 (4.0%) | |
| Symptomatic | 442 (53.6%) | 18 (60.0%) | 90 (59.6%) | .163 |
| Primary location | .221 | |||
| Head | 220 (26.75) | 8 (26.7%) | 53 (35.1%) | |
| Uncinated | 35 (4.2%) | 2 (6.7%) | 6 (4.0%) | |
| Neck/body | 184 (22.3%) | 11 (36.7%) | 37 (24.5%) | |
| Tail | 338 (41.0%) | 8 (26.7%) | 49 (32.5%) | |
| Multiple | 44 (5.3%) | 1 (3.3%) | 6 (4.0%) | |
| Largest tumor size (cm) | 2.0 (1.3–3.4) | 2.8 (1.2–4.8) | 3.0 (1.8–5.0) | <.001 |
| Multiple tumor nodules | 82 (9.9%) | 4 (13.3%) | 19 (12.6%) | .550 |
| Operative technique | <.001 | |||
| Open | 606 (73.5%) | 27 (90.0%) | 132 (87.4%) | |
| Laparoscopic/robotic | 217 (26.3%) | 3 (10.0%) | 19 (12.6%) | |
| Type of resection | .410 | |||
| Enucleation | 78 (9.5%) | 1 (3.3%) | 23 (15.2%) | |
| Classic PD | 103 (12.5%) | 5 (16.7%) | 18 (11.9%) | |
| Pylorus-preserving PD | 129 (15.6%) | 4 (13.3%) | 26 (17.2%) | |
| Central pancreatectomy | 26 (3.2%) | 2 (6.7%) | 4 (2.6%) | |
| Distal pancreatectomy | 476 (57.7%) | 18 (60.0%) | 76 (50.3%) | |
| Total pancreatectomy | 13 (1.6%) | 0 | 4 (2.6%) | |
| Major vascular resection | 25 (3.0%) | 3 (10.0%) | 19 (12.6%) | <.001 |
| Operation time (min) | 230 (185–307) | 291 (216–351) | 248 (188–331) | .008 |
| Blood loss (mL) | 200 (100–400) | 400 (200–500) | 300 (100–500) | .056 |
| Lymphadenectomy | 539 (65.3%) | 22 (73.3%) | 114 (75.5%) | .054 |
| Lymph nodes metastasis | 163 (19.8%) | 8 (26.7%) | 58 (38.4%) | <.001 |
| Postoperative morbidity | 438 (53.1%) | 18 (60.0%) | 81 (53.6%) | .767 |
| Severe complication (III–V) | 183 (22.2%) | 9 (30.0%) | 28 (18.5%) | .336 |
BMI, body mass index; MEN 1, multiple endocrine neoplasia type 1; NA, not available; PD, pancreaticoduodenectomy; VHL, Von Hippel-Lindau.
Fourteen patients were excluded who had no information available on intraoperative margin status.
Relationship between survival and R status
Among the 1,020 patients, 866 (84.9%) underwent an R0 resection, whereas 154 (15.1%) had an R1 resection. The distribution of main clinicopathologic and operative parameters according to R0/R1 margin status are summarized in Table 1. There was no difference in patient age, sex, body mass index, history of a genetic syndrome, tumor location, or functional status among patients relative to R0 versus R1 surgical margin status (all P > .05). Perhaps not unexpectedly, there was a greater incidence of R1 resection among patients with a larger tumor and patients who had an extended resection, combined major vascular invasion, a prolonged operative time, and increased intraoperative blood loss (all P < .05).
Median and 10-year OS among the entire cohort were 176.4 months (IQR 134.3–218.6) and 72%, respectively. Of note, patients undergoing an R1 resection had comparable OS compared with patients who had an R0 resection (10-year OS, 71.1% vs 71.8%, P = .392) (Fig. 1, A). In contrast, R1 resection was correlated with a worse RFS compared with patients who had an R0 resection (10-year RFS, R1 47.3% vs R0 62.8%, HR 1.8, 95% CI 1.2–2.7, P = .002; Fig. 1, B).
Fig. 1.
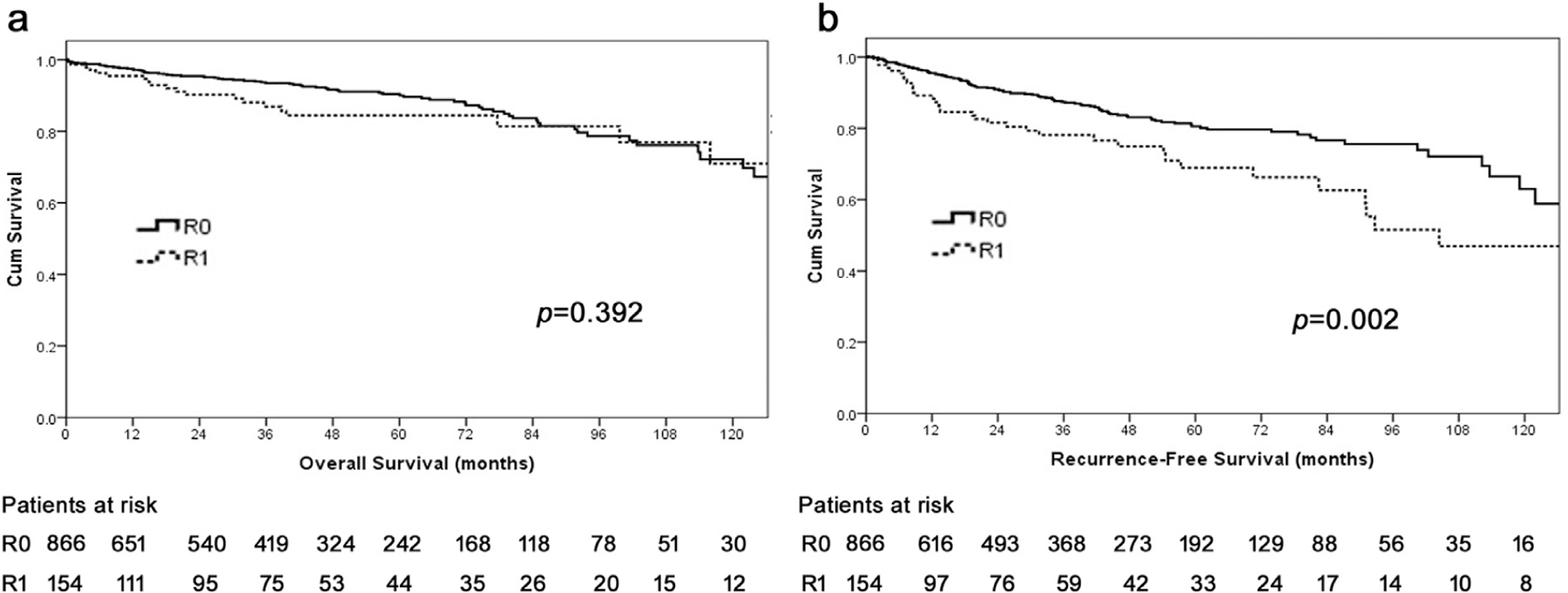
Overall (A) and recurrence-free (B) survival of patients undergoing curative-intent resection for pancreatic neuroendocrine tumor (pNET).
Impact of different positive margin sites on survival
Among the 154 patients who had R1 margin status, 141 patients had detailed information on the site of the positive surgical margin. Among these 141 patients, 76 patients had a R1 transection margin (R1t) and 65 had only a positive mobilization margin (R1m). Compared with patients who had an R0 resection, OS was similar among patients who had R0, R1t, and R1m margin status (10-year OS, R0 72.2%, R1t 74.3% vs R1m 69.1%, P = .672; Fig. 2, A). Both R1t and R1m were associated with increased tumor recurrence after resection compared with patients who had an R0 margin (R1t versus R0: HR 1.8, 95% CI 1.0–3.0, P = .033; R1m versus R0: HR 1.3, 95% CI 1.0–1.7, P = .060); there was, however, no difference in RFS among patients who had an R1t and R1m (HR 0.9, 95% CI 0.4–1.8, P = .739; Fig. 2, B).
Fig. 2.
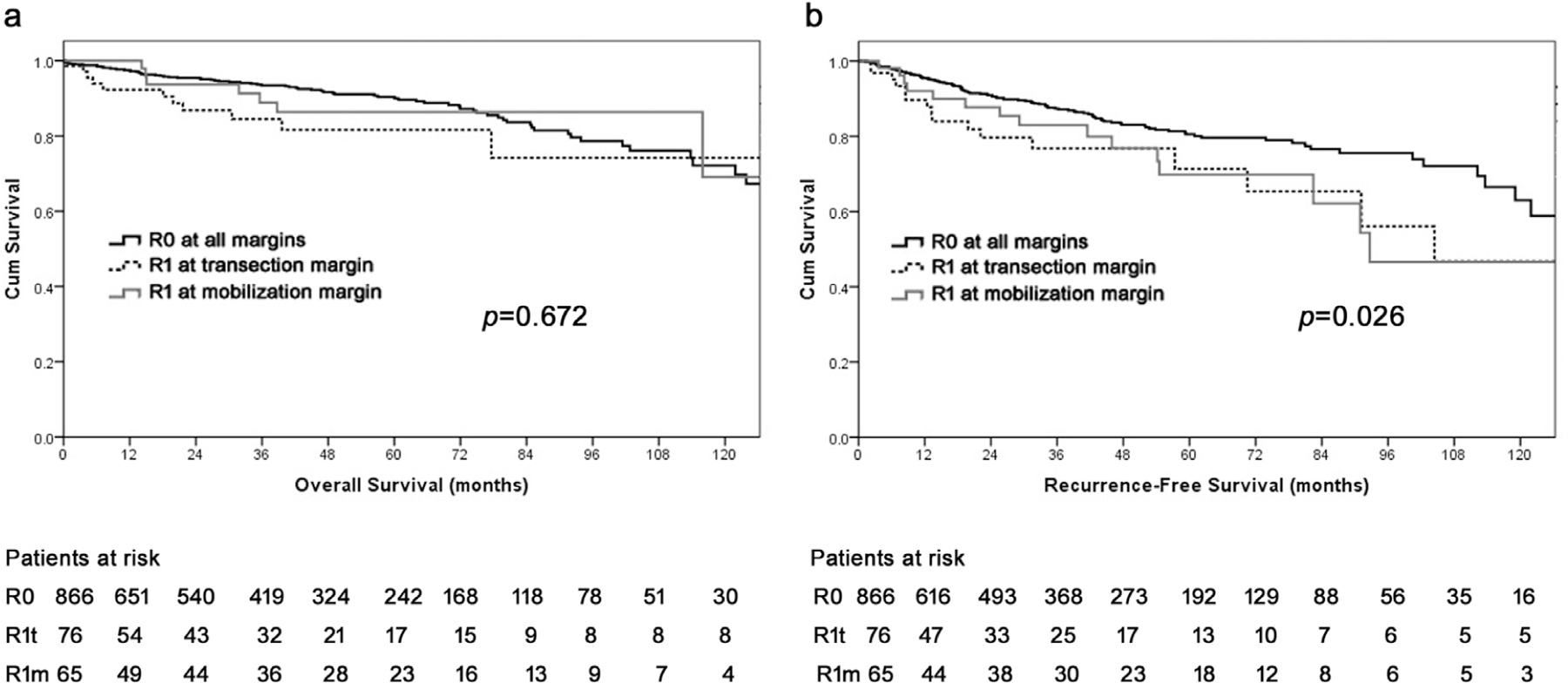
Flow chart of patients undergoing operative resection of pancreatic neuroendocrine tumors stratified by frozen section examination, frozen section status, and final surgical margin.
Impact of intraoperative reresection of positive margin on survival
Among the 1,006 patients who had information of intraoperative frozen section examination, 539 patients (53.6%) had frozen section evaluation of the surgical margin at the time of operation. Among the 539 patients, 49 patients (9.1%) had a positive surgical margin on frozen section analysis; 38 of the 49 patients (77.6%) had reresection, and a final R0 (secondary R0) margin was achieved in 30 patients (78.9%). As such, 825 patients had an initial negative surgical margin with no reresection (primary R0), 30 patients had a negative margin after reresection (secondary R0), and 151 patients had a microscopically positive margin (R1; Fig. 3). Of note, patients who had a secondary R0 resection and patients who had a final R1 resection had larger tumors, a greater incidence of lymph node metastasis, and were more likely to have combined major vascular resection and greater operative times versus patients who had a primary R0 resection (all P < .01; Table 2). The incidence of postoperative morbidity or severe complications was, however, comparable among patients who had a primary, secondary, and R1 resection (both P > .1; Table 2).
Fig. 3.
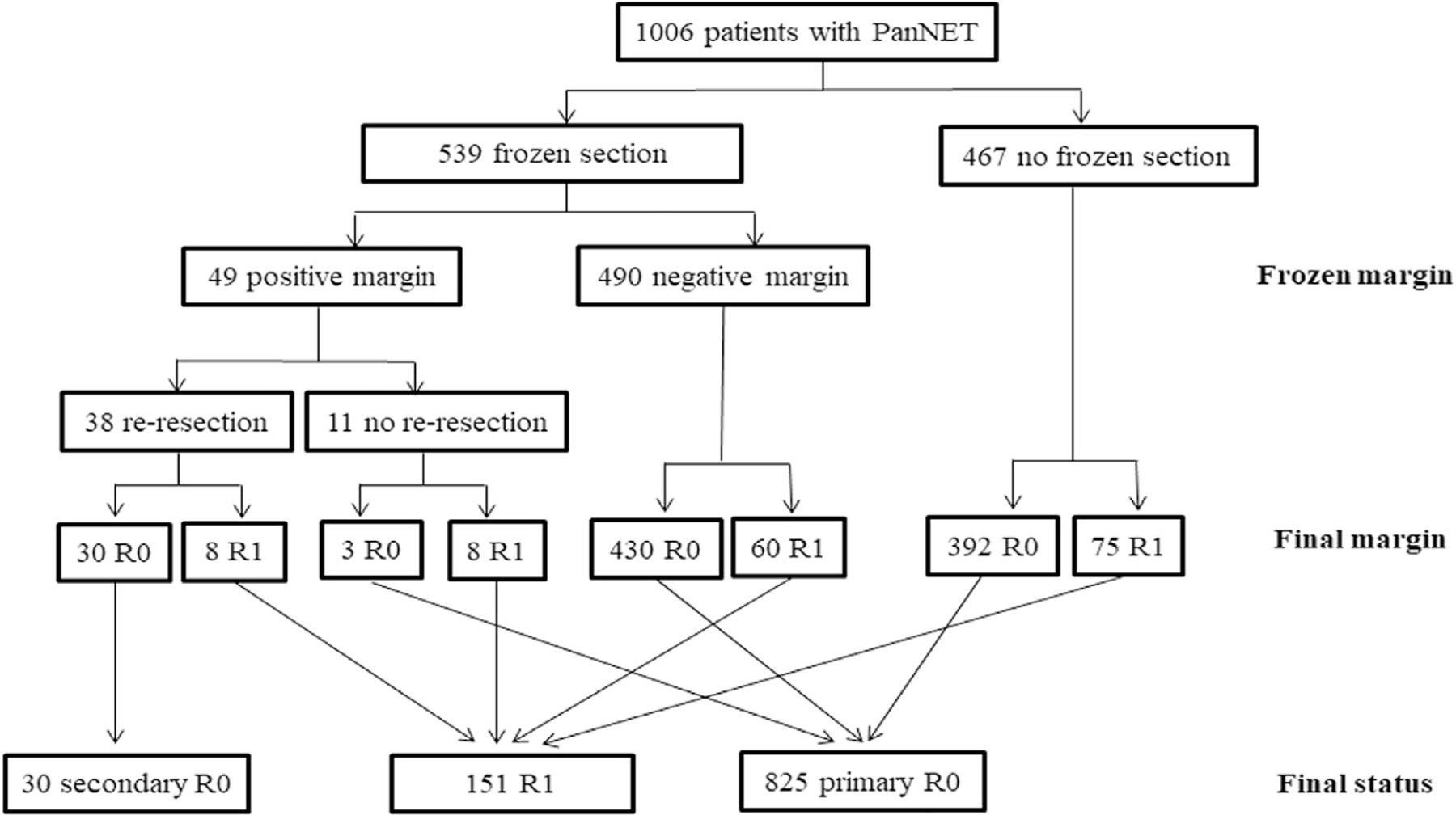
Overall (A) and recurrence-free (B) survival of patients with primary R0, secondary R0, and R1 status after curative-intent resection for pancreatic neuroendocrine tumors. PanNET, pancreatic neuroendocrine tumor.
Median and 10-year OS among patients who had a secondary R0 resection were 85.4 months and 32.9%, respectively; in comparison, median and 10-year OS among patients who had a primary R0 resection was 150.0 months and 74.5%, respectively (secondary versus primary R0, HR 3.1, 95% CI 1.6–6.2, P = .001), whereas patients who had a R1 resection had a median OS that was not attained and a 10-year OS of 71.4% (R1 versus primary R0, HR 1.3, 95% CI 0.8–2.1, P = .346; Fig. 4, A). The risk of tumor recurrence was greater among patients who had a secondary versus primary R0 margin (HR 2.6, 95% CI 1.4–5.1, P = .004) but similar among patients who had a secondary negative versus final R1 margin (HR 0.8, 95% CI 0.4–1.6, P = .471; Fig. 4, B).
Fig. 4.
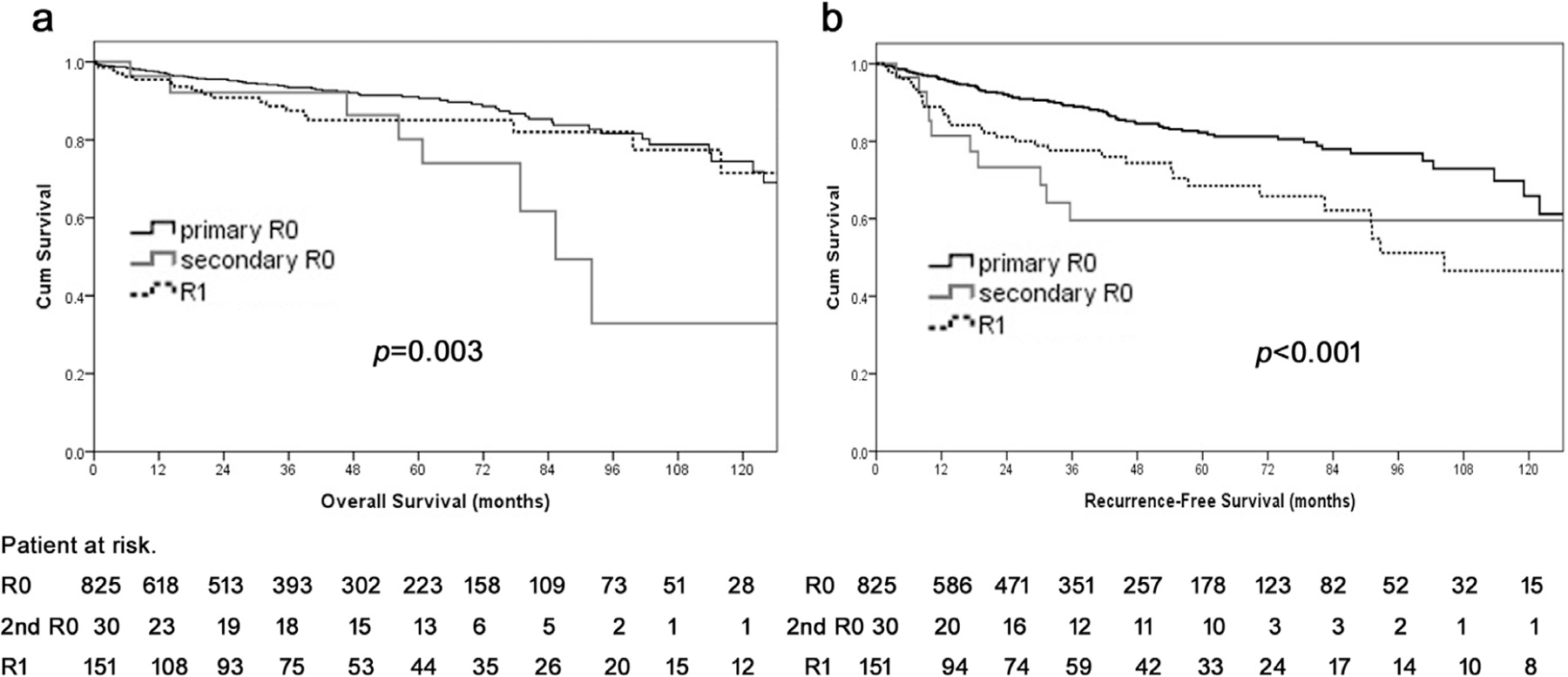
Overall (A) and recurrence-free (B) survival of patients with R0 status, R1 at transection margin, and R1 at mobilization margin after curative-intent resection for pancreatic neuroendocrine tumors.
On multivariable analyses, after controlling for other competing risk factors associated with prognosis, reresection of an initially positive surgical margin was not associated with OS (referent primary R0 margin: secondary R0, HR 0.8, 95% CI 0.1–6.3; final R1, HR 0.9, 95% CI 0.4–2.9; both P > .1; Table 3) or RFS (referent primary R0 margin: secondary R0, HR 1.4, 95% CI 0.4–5.5; final R1, HR 1.3, 95% CI 0.7–2.6; both P > .1; Table 4). In contrast, tumor-specific factors, such as poor cellular differentiation (HR 3.0, 95% CI 1.1–8.3) and perineural invasion (HR 2.6, 95% CI 1.1–6.0) were associated with worse OS (Table 4); pNETs associated with a genetic syndrome (HR 2.9, 95% CI 1.4–6.0), major vascular resection (HR 2.3, 95% CI 1.1–5.1), and Ki-67 index were each strongly correlated with lesser RFS (Table 4).
Table 3.
Factors associated with overall survival after curative resection of pNETs.
|
Univariate analysis
|
Multivariable analysis
|
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P value | |
| Functional status | .076 | |||
| Nonfunctional | Ref. | |||
| Functional | 2.2 (0.9–5.3) | |||
| Genetic syndrome | .782 | |||
| Not associated | Ref. | |||
| Associated | 0.9 (0.5–1.7) | |||
| Symptomatic | .571 | |||
| No | Ref. | |||
| Yes | 1.1 (0.8–1.7) | |||
| Operative technique | .192 | |||
| Open | Ref. | |||
| Laparoscopic/robotic | 0.7 (0.4–1.2) | |||
| Major vascular resection | 1.8 (1.0–3.5) | .035 | 1.1 (0.5–2.9) | .786 |
| Tumor size (cm) | <.001 | .081 | ||
| <3 cm | Ref. | Ref. | ||
| ≥3 cm | 2.0 (1.4–3.0) | 1.8 (0.9–3.6) | ||
| Multiple lesions | 1.2 (0.8–2.1) | .389 | ||
| Final margin | .392 | |||
| R0 | Ref. | |||
| R1 | 1.2 (0.8–2.0) | |||
| Surgical margin | ||||
| Primary R0 | Ref. | Ref. | ||
| Secondary R0 | 3.1 (1.6–6.2) | .001 | 0.8 (0.1–6.3) | .848 |
| R1 | 1.3 (0.8–2.1) | .346 | 0.9 (0.4–2.9) | .724 |
| Tumor differentiation | ||||
| Well differentiated | Ref. | Ref. | ||
| Moderately differentiated | 2.1 (1.1–3.8) | .018 | 1.1 (0.4–2.7) | .879 |
| Poorly differentiated | 8.1 (4.0–16.5) | <.001 | 3.0 (1.1–8.3) | .036 |
| Lymph nodes positive | 2.2 (1.4–3.3) | <.001 | 1.3 (0.6–2.9) | .477 |
| Ki-67 | ||||
| <3% | Ref. | |||
| 3%–20% | 1.2 (0.7–2.1) | .577 | ||
| >20% | 2.4 (0.8–6.9) | .106 | ||
| Lymph-vascular invasion | 1.7 (1.1–2.6) | .027 | 0.9 (0.4–2.4) | .866 |
| Perineural invasion | 2.7 (1.7–4.4) | <.001 | 2.6 (1.1–6.0) | .030 |
Table 4.
Factors associated with recurrence-free survival after curative resection of pNETs.
|
Univariate analysis
|
Multivariable analysis
|
|||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P value | |||
| Functional status | .660 | |||||
| Nonfunctional | Ref. | |||||
| Functional | 0.8 (0.3–2.4) | |||||
| Genetic syndrome | .017 | .004 | ||||
| Not associated | Ref. | Ref. | ||||
| Associated | 1.7 (1.1–2.5) | 2.9 (1.4–6.0) | ||||
| Symptomatic | <.001 | .528 | ||||
| No | Ref. | Ref. | ||||
| Yes | 1.9 (1.4–2.7) | 1.2 (0.7–2.2) | ||||
| Operative technique | <.001 | .083 | ||||
| Open | Ref. | Ref. | ||||
| Laparoscopic/robotic | 0.2 (0.1–0.5) | 0.5 (0.3–1.2) | ||||
| Major vascular resection | 4.1 (2.6–6.4) | <.001 | 2.3 (1.1–5.1) | .035 | ||
| Tumor size (cm) | <.001 | .210 | ||||
| <3 cm | Ref. | Ref. | ||||
| ≥3 cm | 4.4 (3.1–6.2) | 1.5 (0.8–2.9) | ||||
| Multiple lesions | 0.8 (0.5–1.4) | .451 | ||||
| Surgical margin | ||||||
| Primary R0 | Ref. | Ref. | ||||
| Secondary R0 | 2.6 (1.4–5.1) | .004 | 1.4 (0.4–5.5) | .585 | ||
| R1 | 2.0 (1.4–3.0) | <.001 | 1.3 (0.7–2.6) | .393 | ||
| Tumor differentiation | ||||||
| Well differentiated | Ref. | Ref. | ||||
| Moderately differentiated | 1.8 (1.1–3.1) | .024 | 0.9 (0.4–2.2) | .900 | ||
| Poorly differentiated | 7.3 (3.8–14.0) | <.001 | 1.4 (0.7–2.8) | .220 | ||
| Lymph nodes positive | 2.8 (2.0–3.8) | <.001 | 1.4 (0.8–2.5) | .260 | ||
| Ki-67 | ||||||
| <3% | Ref. | Ref. | ||||
| 3%–20% | 3.2 (2.0–5.3) | <.001 | 2.2 (1.2–4.2) | .016 | ||
| >20% | 14.0 (7.3–26.7) | <.001 | 8.1 (2.4–27.5) | .001 | ||
| Lymph-vascular invasion | 4.8 (3.3–7.0) | <.001 | 1.6 (0.8–3.2) | .216 | ||
| Perineural invasion | 2.6 (1.7–3.9) | <.001 | 1.4 (0.7–2.5) | .315 | ||
Discussion
Traditionally, obtaining a negative surgical margin has been the primary objective in curative-intent resection of a solid tumor because a micro- or macroscopically positive margin is generally accepted as a poor prognostic factor. Because there has been no universal definition of an R0 and R1 margin for pancreatic cancers, many studies that have examined the association of margin status and prognosis have yield conflicting results.8–12 In addition, the issue of tumor residue at different margin sites and whether reresection of an initially positive margin to achieve a negative margin has not been well investigated among patients with the different types of pancreatic cancer.9,13–15 Most previous studies have also focused on pancreatic ductal adenocarcinoma, with very few studies evaluating the impact of margin status on the long-term prognosis of pNETs after curative-intent resection. We believe the present study to be important because we used a large, multi-institutional cohort of patients to determine that an R1 (≤1 mm) versus R0 margin status was not associated with OS, despite R1 patients having a greater risk of tumor recurrence. Perhaps not surprisingly, patients who had an R1 margin status were more likely to have a high-risk tumor (eg, larger tumor size, lymph node metastasis, etc) and various operative characteristics (eg, vascular resection, greater operative times, etc). Data from the present study also suggested that additional reresection of a positive margin to achieve an R0 margin did not affect long-term outcomes compared with patients who had an initial R0 margin. Collectively, margin status was not an important factor relative to prognosis among patients with pNETs; rather, other tumor-specific factors, such as differentiation, tumor burden, and major vascular invasion affected long-term outcomes.2,17,18
Margin status has been reported to be associated with OS after curative resection of pancreatic ductal cancer in some studies,8,9,19 yet not others.11,12 The variable definitions of what constitutes an R1 margin may have contributed to the discrepant results. Most studies, including the British Royal College of Pathologists and the International Study Group of Pancreatic Surgery have defined a minimum margin width >1 mm as R0 status for pancreatic ductal cancer.7,16,20–22 In the present study addressing pNETs, a margin >1 mm was consistently defined as R0, whereas a width ≤1 mm was categorized as R1 among patients with complete macroscopic resection of pNET. Of note, although an R1 margin status was associated with an increased incidence of recurrence versus R0 resection, OS among patients who underwent an R1 versus R0 resection was comparable. The greater incidence of recurrence among R1 patients was likely multifactorial and in part may be related to the findings that these patients generally had more aggressive tumor characteristics (eg, larger tumors, lymph node metastasis, etc). Of note, even though recurrence was more common among R1 patients, OS was similar, thereby confirming the general indolent nature of pNETs even among patients with recurrent disease. As such, operative resection for pNETs should be recommended even if a complete R0 resection may not be feasible, because resection can provide symptom control, can facilitate loco-control, and can be associated with long-term survival.23
A precise histopathologic evaluation of the pancreatic resection margin has been recommended.7,16,24 Specifically, the International Study Group of Pancreatic Surgery recommends the evaluation of 7 margins on the resected specimens: anterior, posterior, medial or SMA margin, pancreatic transection margin, bile duct, and enteric margin.7 The exact prognostic impact of residual disease relative to the specific site of R1 disease remains poorly defined. Jamieson et al13 evaluated the influence of positive mobilization margins versus positive transection margins on survival after pancreatoduodenectomy for pancreatic ductal cancer and reported that a positive mobilization margin was not an adverse prognostic factor. The authors proposed that a positive pancreatic parenchymal transection margin and the medial meso-pancreas adjacent to major vessels, such as the portal vein, SMA, and superior mesenteric vein or hepatoduodenal ligaments, involves contiguous adventitia and lymphovascular outflow that is likely the major route of dissemination. In contrast, the positive mobilization margin includes the posterior or anterior surface or lateral duodenal margins where 2 adjacent organ surfaces have been simply separated; these margins contain no vascular or lymphatic planes.13 In the present study, among patients who underwent resection of a pNET, tumor residue at either the transection or mobilization margin was associated with a greater risk of recurrence compared with patients who had an R0 margin. Local extension or residual tumor foci left in situ, as well as perineural invasion, were likely the most common reasons for recurrence, because each of these factors has been associated with relapse after resection after both ductal cancer and pNETs.25,26 A microscopically positive margin at either the transection or mobilization surface after pNET resection may therefore warrant closer surveillance for recurrence postoperatively.27
Frozen section is used intraoperatively to assess the resection margin because most surgeons believe empirically achieving a final R0 margin may benefit patients with pancreatic cancer.13,15 In the present study, frozen section analysis of the intraoperative surgical margin was used in roughly half of the cases (53.6%), suggesting that the importance of the use of frozen section varied considerably among surgeons. When an initial positive margin was noted on frozen section, most patients had reresection of the margin with a final negative margin achieved in a subset of patients. Of note, among patients undergoing curative-intent resection of the pNET, achieving a tumor-free margin with reresection of an initially positive margin offered no survival advantage on multivariable analysis. These findings were consistent with other reports that failed to identify an improved survival benefit of re-resection of an initial positive margin to achieve a final negative margin among patients with pancreatic ductaladenocarcinoma.9,14 As such, the data suggest that for pNETs, a positive margin per se did not in any meaningful way affect the OS and was more likely to reflect more biologically aggressiveneoplasms.2,9,28 In turn, although a negative margin should remain the goal of surgical resection, our study and others suggest that the biology of the pNET, and not surgical millimeters, ultimately drives prognosis. In fact, histologic grade and perineural invasion were strongly associated with the OS of these patients. Therefore enucleation or parenchyma-sparing resections of pNETs with minimal margins may be appropriate in select patients. In fact, parenchyma-sparing resection has been found to offer similar long-term outcomes compared with more aggressive oncologic resections among patients with small pNETs.29–32 In contrast, parenchymal-sparing resection can be demanding from a technical standpoint and can also be associated with complications, such as pancreatic fistula, depending on the location of the enucleated lesion. As such, clinical judgment should be exercised when selecting patients for enucleation versus a more formal pancreatic resection with lymphadenectomy. Decisions to perform a traditional pancreatic resection versus a parenchymal-sparing operation should depend on factors such as the size of the pNET, its location, and the surgeon’s experience.
The present study had several limitations. Although including multiple tertiary referral centers allowed for a larger sample size, the multicentric nature of the study did not allow for a dedicated rereview of the pathologic specimens. Nevertheless, the centers included in the present collaborative were all high-volume centers with experienced pathologists who used standardize protocols to interpret the specimens. Furthermore, the diagnosis and management of pNETs has evolved over the 2 decades, including selection of patients and use of various therapies (eg, small molecular targeted agents, everolimus and sunitinib, peptide receptor radionuclide therapy, somatostatin analogues, and systematic chemotherapy). Despite this, operative resection with curative intent or debulking has remained the treatment of choice for patients who are fit to undergo resection. The use of intraoperative frozen section analysis also varied. Variation may not have been dependent on intraoperative judgment but rather a more systematic bias of some pancreatic surgeons never to employ frozen section analysis; however, even among surgeons who used frozen section analysis, the data strongly suggested that reresection was not advantageous.
In conclusion, about 1 in 6 patients had a positive margin (≤1 mm) after curative-intent resection for pNETs. Margin status was associated with RFS, with residual tumor at either the transection or mobilization margin being associated with increased risk of recurrence. Margin status was not associated, however, with long-term survival. In addition, the reresection of an initially positive surgical margin to achieve a negative margin did not improve the outcome of patients with pNETs. Taken together, margin status was not a driving factor in determining survival of patients with pNETs and therefore parenchymal-sparing pancreatic resections may be appropriate when feasible.
Acknowledgments
Xu-Feng Zhang was supported by the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University of China (No. XJTU1AF-CRF-2017-004).
References
- 1.Kim SJ, Kim JW, Oh DY, Han SW, Lee SH, Kim DW, et al. Clinical course of neuroendocrine tumors with different origins (the pancreas, gastrointestinal tract, and lung). Am J Clin Oncol 2012;35:549–556. [DOI] [PubMed] [Google Scholar]
- 2.Merath K, Bagante F, Beal EW, Lopez-Aguiar AG, Poultsides G, Makris E, et al. Nomogram predicting the risk of recurrence after curative-intent resection of primary non-metastatic gastrointestinal neuroendocrine tumors: an analysis of the U.S. Neuroendocrine Tumor Study Group. J Surg Oncol 2018;117:868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi W, Warner RRP, Chan DL, Singh S, Segelov E, Strosberg J, et al. Long-term outcomes of gastroenteropancreatic neuroendocrine tumors. Pancreas 2018;47:321–325. [DOI] [PubMed] [Google Scholar]
- 4.Kuo JH, Lee JA, Chabot JA. Nonfunctional pancreatic neuroendocrine tumors. Surg Clin North Am 2014;94:689–708. [DOI] [PubMed] [Google Scholar]
- 5.Kasumova GG, Tabatabaie O, Eskander MF, Tadikonda A, Ng SC, Tseng JF. National Rise of primary pancreatic carcinoid tumors: comparison to functional and nonfunctional pancreatic neuroendocrine tumors. J Am Coll Surg 2017;224:1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 2016;103:153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977–988. [DOI] [PubMed] [Google Scholar]
- 8.Strobel O, Hank T, Hinz U, Bergmann F, Schneider L, Springfeld C, et al. Pancreatic cancer surgery: the new R-status counts. Ann Surg 2017;265:565–573. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez J, Mullinax J, Clark W, Toomey P, Villadolid D, Morton C, et al. Survival after pancreaticoduodenectomy is not improved by extending resections to achieve negative margins. Ann Surg 2009;250:76–80. [DOI] [PubMed] [Google Scholar]
- 10.Demir IE, Jager C, Schlitter AM, Konukiewitz B, Stecher L, Schorn S, et al. R0 versus R1 resection matters after pancreaticoduodenectomy, and less after distal or total pancreatectomy for pancreatic cancer. Ann Surg 2017. doi: 10.1097/SLA.0000000000002345. [DOI] [PubMed]
- 11.Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg 2007;246:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ethun CG, Kooby DA. The importance of surgical margins in pancreatic cancer. J Surg Oncol 2016;113:283–288. [DOI] [PubMed] [Google Scholar]
- 13.Jamieson NB, Chan NI, Foulis AK, Dickson EJ, McKay CJ, Carter CR. The prognostic influence of resection margin clearance following pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Gastrointest Surg 2013;17:511–521. [DOI] [PubMed] [Google Scholar]
- 14.Kooby DA, Lad NL, Squires MH, MaithelSK 3rd, Sarmiento JM, Staley CA, et al. Value of intraoperative neck margin analysis during Whipple for pancreatic adenocarcinoma: a multicenter analysis of 1399 patients. Ann Surg 2014;260:494–501 discussion 3. [DOI] [PubMed] [Google Scholar]
- 15.Nitschke P, Volk A, Welsch T, Hackl J, Reissfelder C, Rahbari M, et al. Impact of intraoperative re-resection to achieve R0 status on survival in patients with pancreatic cancer: a single-center experience with 483 patients. Ann Surg 2017;265:1219–1225. [DOI] [PubMed] [Google Scholar]
- 16.The Royal College of Pathologists. Standards and minimum datasets for reporting cancers. Minimum dataset for the histopathological reporting of pancreatic, ampulla of vater and bile duct carcinoma London: The Royal College of Pathologists; 2002. [Google Scholar]
- 17.Fang C, Wang W, Feng X, Sun J, Zhang Y, Zeng Y, et al. Nomogram individually predicts the overall survival of patients with gastroenteropancreatic neuroendocrine neoplasms. Br J Cancer 2017;117:1544–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang C, Wang W, Zhang Y, Feng X, Sun J, Zeng Y, et al. Clinicopathologic characteristics and prognosis of gastroenteropancreatic neuroendocrine neoplasms: a multicenter study in South China. Chin J Cancer 2017;36:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paniccia A, Hosokawa P, Henderson W, Schulick RD, Edil BH, McCarter MD, et al. Characteristics of 10-year survivors of pancreatic ductal adenocarcinoma. JAMA Surg 2015;150:701–710. [DOI] [PubMed] [Google Scholar]
- 20.Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg 2011;254:311–319. [DOI] [PubMed] [Google Scholar]
- 21.Verbeke CS. Resection margins in pancreatic cancer. Surg Clin North Am 2013;93:647–662. [DOI] [PubMed] [Google Scholar]
- 22.Esposito I, Kleeff J, Bergmann F, Reiser C, Herpel E, Friess H, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol 2008;15:1651–1660. [DOI] [PubMed] [Google Scholar]
- 23.Liu JB, Baker MS. Surgical management of pancreatic neuroendocrine tumors. Surg Clin North Am 2016;96:1447–1468. [DOI] [PubMed] [Google Scholar]
- 24.Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg 2006;93:1232–1237. [DOI] [PubMed] [Google Scholar]
- 25.Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer 2011;11:695–707. [DOI] [PubMed] [Google Scholar]
- 26.Genc CG, Jilesen AP, Partelli S, Falconi M, Muffatti F, van Kemenade FJ, et al. A new scoring system to predict recurrent disease in grade 1 and 2 nonfunctional pancreatic neuroendocrine tumors. Ann Surg 2018;267:1148–1154. [DOI] [PubMed] [Google Scholar]
- 27.Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas 2013;42:557–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neoptolemos JP, Stocken DD, Dunn JA, Almond J, Beger HG, Pederzoli P, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg 2001;234:758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitt SC, Pitt HA, Baker MS, Christians K, Touzios JG, Kiely JM, et al. Small pancreatic and periampullary neuroendocrine tumors: resect or enucleate? J Gastrointest Surg 2009;13:1692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casadei R, Ricci C, Rega D, D’Ambra M, Pezzilli R, Tomassetti P, et al. Pancreatic endocrine tumors less than 4 cm in diameter: resect or enucleate? A single–center experience. Pancreas 2010;39:825–828. [DOI] [PubMed] [Google Scholar]
- 31.Hackert T, Hinz U, Fritz S, Strobel O, Schneider L, Hartwig W, et al. Enucleation in pancreatic surgery: indications, technique, and outcome compared to standard pancreatic resections. Langenbecks Arch Surg 2011;396:1197–1203. [DOI] [PubMed] [Google Scholar]
- 32.Falconi M, Zerbi A, Crippa S, Balzano G, Boninsegna L, Capitanio V, et al. Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol 2010;17:1621–1627. [DOI] [PubMed] [Google Scholar]


