Abstract
These NCCN Guidelines Insights focus on recent updates to the NCCN Guidelines for Malignant Pleural Mesothelioma (MPM). These NCCN Guidelines Insights discuss systemic therapy regimens and surgical controversies for MPM. The NCCN panel recommends cisplatin/pemetrexed (category 1) for patients with MPM. The NCCN panel also now recommends bevacizumab/cisplatin/pemetrexed as a first-line therapy option for patients with unresectable MPM who are candidates for bevacizumab. The complete version of the NCCN Guidelines for MPM, available at NCCN.org, addresses all aspects of management for MPM including diagnosis, evaluation, staging, treatment, surveillance, and therapy for recurrence and metastasis; NCCN Guidelines are intended to assist with clinical decision-making.
Overview
Mesothelioma is a rare cancer that is estimated to occur in approximately 2,500 people in the United States every year.1,2 These NCCN Guidelines Insights focus on malignant pleural mesothelioma (MPM), which is the most common type; mesothelioma can also occur in the lining of other sites, such as the peritoneum, pericardium, and tunica vaginalis testis. Histologic subtypes of mesothelioma include epithelioid (most common), sarcomatoid, and biphasic (mixed) epithelioid and sarcomatoid (see MPM-2, above).2–4 Patients with epithelioid histology have better outcomes than those with either sarcomatoid or biphasic (mixed) histologies. MPM is difficult to treat, because most patients have pleural dissemination at presentation. Median overall survival for MPM is approximately 1 year; cure is rare.5–7 MPM occurs mainly in older men (median age at diagnosis, 72 years) who have been exposed to asbestos, although it occurs decades after exposure (20–40 years later).8–10 Reports of MPM have also been described following radiation therapy (RT) for other malignancies, including breast cancer and Hodgkin lymphoma.11–13 Patients with suspected MPM often have dyspnea and chest pain; they may also have pleural effusion, fatigue, insomnia, cough, chest wall mass, loss of appetite, and weight loss.14–16 Patients with MPM often have a high symptom burden; therefore, supportive care is important for patients, especially management of pleural effusions.14,17–21 A phase III randomized trial is currently assessing whether early palliative care will improve survival in patients with MPM.22 The NCCN panel recommends palliative RT for chest pain, bronchial or esophageal obstruction, or other symptomatic sites (see MPM-D, pages 830 and 831).14,23,24
The NCCN Guidelines recommend that patients with MPM be managed by a multidisciplinary team with experience in MPM. Treatment options for patients with MPM include surgery, RT, and/or chemotherapy2; select patients with clinical stages I–III disease who are medically operable and have good performance status (PS) are candidates for multimodality therapy.25–29 These NCCN Guidelines Insights focus on systemic therapy regimens and surgical controversies for MPM. Surgery for MPM is controversial, because sufficient data from randomized controlled trials are limited.14,30–33 Some surgical procedures, such as extrapleural pneumonectomy (EPP), are associated with greater morbidity than others, such as pleurectomy/decortication (P/D); therefore, EPP is are not recommended for patients with MPM who have sarcomatoid histology. When comparing EPP with P/D, it is not clear which surgical procedure will yield better oncologic outcomes.14
Systemic Therapy
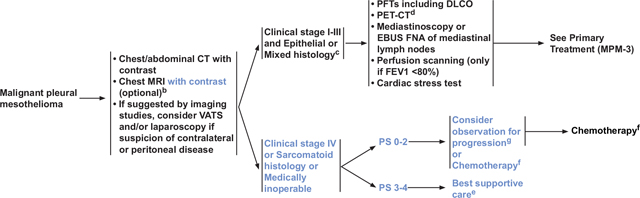
Many patients with MPM receive systemic therapy either alone or as part of multimodality therapy. Because most patients present with unresectable or medically inoperable MPM, they are not candidates for surgery, although a board-certified thoracic surgeon with experience in multimodality mesothelioma management should make the decision regarding resectability (see “Surgery,” page 831). The NCCN Guidelines currently recommend 4 combination systemic therapy options for patients with MPM, depending on clinical characteristics such as PS, histology, and whether patients are medically operable or inoperable. Three of the combination regimens are recommended as first-line therapy options for patients with unresectable clinical stage IV, sarcomatoid histology, or medically inoperable MPM or for those who refuse surgery (see MPM-B, page 828). The 3 combination regimens include (1) cisplatin/pemetrexed (category 1), (2) carboplatin/pemetrexed, and (3) cisplatin/gemcitabine.34–45 Pemetrexed-based regimens are typically used, with gemcitabine recommended only for patients who cannot receive pemetrexed. These 3 combination regimens can also be used as adjuvant therapy for patients as part of multimodality therapy.46 Several regimens can also be used as induction therapy as part of a modality regimen, including cisplatin/pemetrexed.46 The fourth combination regimen is bevacizumab/cisplatin/pemetrexed, which is only recommended for patients with unresectable disease and should only be considered for patients who are candidates to receive bevacizumab.47 For patients with clinical stage IV MPM, sarcomatoid histology, or medically inoperable MPM who are asymptomatic and have a minimal burden of disease, observation may be considered if chemotherapy is planned at the time of symptomatic or radiographic progression (see MPM-2, page 827).48,49 Best supportive care is recommended for patients with PS 3 to 4 who have clinical stage IV MPM, sarcomatoid histology, or medically inoperable MPM.
The NCCN panel recommends cisplatin/pemetrexed (category 1) based on a phase III randomized trial and FDA approval.50 The phase III trial assessed cisplatin/pemetrexed versus cisplatin alone in patients who were not candidates for surgery; the combined regimen increased survival by 2.8 months compared with cisplatin alone (12.1 vs 9.3 months; P=.02). Patients receiving cisplatin/pemetrexed had less pain and dyspnea than those receiving cisplatin alone. Other recommended first-line combination chemotherapy options include (1) pemetrexed/carboplatin, which was assessed in 3 large phase II studies (median survival, 12.7, 14, and 14 months, respectively) and a large expanded access nonrandomized study34,51–53; or (2) gemcitabine/cisplatin, which was assessed in phase II studies (median survival, 9.6–14.7 months).35,36,40,54,55 The carboplatin/pemetrexed regimen is a better choice for patients with poor PS or comorbidities.51 Gemcitabine/cisplatin is only recommended for patients who cannot take pemetrexed. First-line single-agent options include pemetrexed or vinorelbine, which are recommended only for patients who cannot receive platinum-doublet therapy.48,56,57 New agents are being assessed in the frontline setting for MPM.43,58–61
A recent multicenter phase III randomized trial assessed the addition of bevacizumab to cisplatin/pemetrexed (with maintenance bevacizumab) compared with cisplatin/pemetrexed alone for patients 75 years of age or younger with unresectable MPM and PS 0 to 2 who did not have significant cardiovascular history, including history of stroke or transient ischemic attack.47 Most patients (97%) were PS 0 to 1. Overall survival was increased in the bevacizumab plus chemotherapy arm by 2.7 months when compared with chemotherapy alone: bevacizumab triplet arm (median, 18.8 months; 95% CI, 15.9–22.6) compared with cisplatin/pemetrexed (16.1 months; 95% CI, 14.0–17.9; hazard ratio, 0.77; 95% CI, 0.62–0.95; P=.0167). Grade 3 to 4 adverse events were reported in 71% of patients (158 of 222) receiving the bevacizumab regimen when compared with 62% (139 of 224) of those receiving cisplatin/pemetrexed alone. More grade 3 or higher hypertension (23% vs 0%), grade 3 proteinuria (3.1% vs 0%), and grade 3 to 4 thrombotic events (6% vs 1%) were observed in patients receiving the triplet arm. Based on this trial, the NCCN panel added a recommendation (category 2A) in 2015 (Version 2) for the bevacizumab/cisplatin/pemetrexed regimen.
Recommended second-line chemotherapy options include pemetrexed (if not administered first-line) (category 1), vinorelbine, or gemcitabine (see MPM-B, page 828).57,58,62–67 If patients experienced a good response to first-line pemetrexed, data suggest that repeating pemetrexed is effective, especially in those who achieved a treatment-free interval of at least 3 months.58,68–70 Several agents are in clinical trials.58,61,68,71–73 Preliminary data suggest that immune checkpoint inhibitors and agents targeting mesothelin may be useful in MPM.74–79
Surgery
For patients with MPM, the goals of surgery may differ depending on the needs of the patient. Surgery will be recommended in select patients with good PS and epithelioid or mixed histology if a complete gross cytoreduction can be achieved, with the goal to increase survival.46,80 However, palliative surgery and/or RT may be recommended to relieve pain, free a trapped lung, decrease pleural effusions, and/or improve respiration.14 As previously mentioned, most patients with MPM are not candidates for surgery because they present with unresectable or medically inoperable disease. Board-certified thoracic surgeons with expertise in managing MPM should decide whether a patient has unresectable or resectable MPM and should perform the surgical resection if indicated. Surgery is not usually recommended for patients with anticipated short-term survival and/or at high risk of morbidity and mortality, poor PS, or comorbidities, as well as unfavorable oncologic outcomes due to unfavorable histology such as sarcomatoid.5,81–83 The NCCN Guidelines do not recommend surgery for patients with clinical stage IV MPM who have locally advanced unresectable tumors (T4), N3 disease, and/or distant metastases (see Table 1 in the complete version of these guidelines, available at NCCN.org). In addition, patients with N2 disease, mixed histology, or sarcomatoid histology should not routinely be resected outside of a clinical trial and in a center with MPM experience (see MPM-C, page 829).
Surgical resection for patients with MPM can include either P/D (also known as total pleurectomy or lung-sparing surgery), which is complete removal of the involved pleura and all gross tumor, or EPP, which is en bloc resection of the involved pleura, lung, ipsilateral diaphragm, and often the pericardium.84 Extended P/D refers to the resection of the diaphragm and pericardium in addition to total pleurectomy.84 Mediastinal nodal dissection is recommended in patients with either P/D or EPP; at least 3 nodal stations should be obtained.
Trimodality therapy—chemotherapy, EPP, and hemithoracic RT—has been shown to benefit select patients with epithelioid histology, good PS, and low-volume disease on the basis of single-arm phase II studies at centers with experience.14,25–28,46 Median survival of up to 20 to 29 months has been reported for patients who complete trimodality therapy.26,46 Lung-sparing options, such as P/D, decrease the risk for perioperative mortality and yield either equal or better long-term survival than nonsurgical therapy in patients with more advanced disease.85,86 However, the choice of surgery for MPM is controversial, because data from randomized controlled trials are not available.14,30–33
A retrospective analysis (n=663) suggested that survival was greater after P/D than EPP, but this may have been confounded by patient selection.2,80 A recent meta-analysis suggested a trend in favor of overall survival for extended PD when compared with EPP.30 The Mesothelioma and Radical Surgery (MARS) trial assessed whether patients treated with induction chemotherapy would accept randomization either to EPP with hemithoracic radiation or to no further treatment; 112 were patients enrolled in the trial, and 50 patients were randomized.87 In this trial, overall 30-day mortality was 18.7% (3 of 16 patients). Median survival was 14.4 months in the EPP arm and 19.5 months in the no-EPP arm. The authors concluded that EPP was not beneficial because of the high rate of surgical mortality when compared with chemotherapy alone treatment. However, these results were controversial because survival was not the primary outcome of the study, the sample size was small, and the surgical mortality was higher than expected.88
Neither P/D nor EPP will achieve an R0 resection2,85,89; it is not clear which surgical procedure will yield better oncologic outcomes.14 When compared with P/D, EPP is associated with more morbidity and more short-term mortality.30,90–92 Some surgeons prefer to use P/D, because they feel it is a safer procedure.33,80,90,93–97 Some surgeons mainly use P/D for palliation.14
The surgical goal for MPM is cytoreductive surgery to achieve macroscopic complete resection by removing all visible or palpable tumors.84,98,99 If macroscopic complete resection is not possible, such as in patients with multiple sites of chest wall invasion, then surgery should be aborted. However, to help with postoperative management, surgery should be continued if most of the gross disease can be removed and if there will be a minimal impact on morbidity (see MPM-C, page 829). The NCCN panel feels that P/D and EPP are both reasonable surgical options that should be considered in select patients to achieve complete gross cytoreduction.30,80,87,91,100 For patients having surgery, either preoperative chemotherapy or postoperative chemotherapy (with or without adjuvant hemithoracic RT, depending on which surgical procedure is used) is recommended in the NCCN Guidelines.14,46 Surgical procedures can also be done to obtain diagnostic samples and to provide palliative benefit.14 Palliative surgical procedures include pleurodesis to decrease pleural effusions and P/D to debulk the tumor with the goals of relieving pain and decreasing pleural effusions.14,20,101 Video-assisted thoracic surgery (VATS) has a diagnostic role and a palliative role (eg, pleurodesis) in patients with MPM, but it is not an accepted technique for P/D.84
Summary
These NCCN Guidelines Insights discuss surgical controversies and systemic therapy regimens for MPM. The NCCN Guidelines recommend that patients with MPM be managed by a multidisciplinary team with experience in MPM. Patients with suspected MPM often have dyspnea and chest pain; they may also have pleural effusion, fatigue, insomnia, cough, chest wall mass, loss of appetite, and weight loss. Patients with MPM often have a high symptom burden; therefore, supportive care is important for patients, especially management of pleural effusions. The NCCN panel recommends palliative RT for chest pain, bronchial or esophageal obstruction, or other symptomatic sites. Treatment options for patients with MPM include surgery, RT, and/or chemotherapy; select patients with clinical stages I to III disease who are medically operable and have good PS are candidates for multimodality therapy. Board-certified thoracic surgeons with expertise in managing MPM should decide whether a patient has resectable MPM and should perform the surgical resection if indicated. Surgery is not usually recommended for patients with anticipated short-term survival and/or at high risk of morbidity and mortality, poor PS, or comorbidities, as well as unfavorable oncologic outcomes because of unfavorable histology such as sarcomatoid. The choice of surgery for MPM is controversial, because data from randomized controlled trials are not available. Neither P/D nor EPP will achieve an R0 resection; it is not clear which surgical procedure will yield better oncologic outcomes. The NCCN panel feels that P/D and EPP are both reasonable surgical options that should be considered in select patients to achieve complete gross cytoreduction.
The NCCN Guidelines currently recommend 4 combination systemic therapy options for patients with MPM. Three of the combination regimens are recommended as first-line therapy for patients with unresectable, metastatic, sarcomatoid histology, or medically inoperable MPM or those who refuse surgery. The 3 regimens include (1) cisplatin/pemetrexed (category 1), (2) carboplatin/pemetrexed, and (3) cisplatin/gemcitabine. The NCCN panel now also recommends bevacizumab/cisplatin/pemetrexed as a first-line therapy option for patients with unresectable MPM who are candidates for bevacizumab. Observation may be considered if chemotherapy is planned at the time of symptomatic or radiographic progression for select patients with clinical stage IV, sarcomatoid histology, or medically inoperable MPM who are asymptomatic and have a minimal burden of disease.
NCCN Categories of Evidence and Consensus.
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B: Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate.
Category 3: Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
All recommendations are category 2A unless otherwise noted.
Clinical trials: NCCN believes that the best management for any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.



Recommended Doses for Conventionally Fractionated Radiation Therapy
| Treatment type | Total dose | Fraction size | Treatment duration |
|---|---|---|---|
|
| |||
| Postoperative after EPP | |||
| Negative margins | 50–54 Gy | 1.8–2 Gy | 4–5 weeks |
| Microscopic-macroscopic positive margins | 54–60 Gy | 1.8–2 Gy | 5–6 weeks |
|
| |||
| Palliative Chest wall pain from recurrent nodules Multiple brain or bone metastasis |
20–40 Gy or 30 Gy 30 Gy |
≥4 Gy 3 Gy 3 Gy |
1–2 weeks 2 weeks 2 weeks |
|
| |||
| Prophylactic radiation to prevent surgical tract recurrence | 21 Gy | 7 Gy | 1 week |
After EPP, RT should only be considered for patients who meet the following criteria: ECOG PS ≤1; good functional pulmonary status; good function of contralateral kidney confirmed by renal scan; and absence of disease in abdomen, contralateral chest, or elsewhere. Patients who are on supplemental oxygen should not be treated with adjuvant RT.
Radiation Techniques
• Use of conformal radiation technology is the preferred choice based on comprehensive consideration of target coverage and clinically relevant normal tissue tolerance.
• CT simulation-guided planning using either intensity-modulated radiation therapy (IMRT) or conventional photon/electron RT is acceptable.7 IMRT is a promising treatment technique that allows for a more conformal high-dose RT and improved coverage to the hemithorax. IMRT or other modern technology (such as tomotherapy or protons) should only be used in experienced centers or on protocol. When IMRT is applied, the NCI and ASTRO/ACR IMRT guidelines should be strictly followed.13,14 Special attention should be paid to minimize radiation to the contralateral lung,15 as the risk of fatal pneumonitis with IMRT is excessively high when strict limits are not applied.16 The mean lung dose should be kept as low as possible, preferably <8.5 Gy. The low-dose volume should be minimized.17
• The gross tumor volume (GTV) should include any grossly visible tumor. Surgical clips (indicative of gross residual tumor) should be included for postoperative adjuvant RT.
• The clinical target volume (CTV) for adjuvant RT after EPP should encompass the entire pleural surface (for partial resection cases), surgical clips, and any potential sites with residual disease.
• Extensive elective nodal irradiation (entire mediastinum and bilateral supraclavicular nodal regions) is not recommended.
• The planning target volume (PTV) should consider the target motion and daily setup errors. The PTV margin should be based on the individual patien’s motion, simulation techniques used (with and without inclusion motion), and reproducibility of each clinic’s daily setup.
See General Principles and Radiation Dose and Volume (MPM-D 1 of 3)
See References MPM-D (3 of 3)
Acknowledgments
This activity is supported by educational grants from AstraZeneca, Bayer Healthcare Pharmaceuticals Inc., Bristol-Myers Squibb, Clovis Oncology, Foundation Medicine, Genentech, Novartis Oncology, Otsuka America Pharmaceutical, Inc., Seattle Genetics, Inc., and Takeda Oncology; support provided by Actelion Pharmaceuticals US, Inc.; and by an independent educational grant from Astellas and Medivation, Inc.
Footnotes
Provided content development and/or authorship assistance.
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) are a statement of consensus of the authors regarding their views of currently accepted approaches to treatment. The NCCN Guidelines® Insights highlight important changes to the NCCN Guidelines® recommendations from previous versions. Colored markings in the algorithm show changes and the - discussion aims to further the understanding of these changes by summarizing salient portions of the NCCN Guideline Panel discussion, including the literature reviewed.
These NCCN Guidelines Insights do not represent the full NCCN Guidelines; further, the National Comprehensive Cancer Network® (NCCN®) makes no representation or warranties of any kind regarding the content, use, or application of the NCCN Guidelines and NCCN Guidelines Insights and disclaims any responsibility for their applications or use in any way.
The full and most current version of these NCCN Guidelines are available at NCCN.org.
© National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines and the illustrations herein may not be reproduced in any form without the express written permission of NCCN.
Disclosure of Relevant Financial Relationships
Editor:
Kerrin M. Green, MA, Assistant Managing Editor, JNCCN—Journal of the National Comprehensive Cancer Network, has disclosed that she has no relevant financial relationships.
CE Authors:
Deborah J. Moonan, RN, BSN, Director, Continuing Education, NCCN, has disclosed that she has no relevant financial relationships.
Kristina M. Gregory, RN, MSN, OCN, Vice President, Clinical Information Operations, NCCN, has disclosed that she has no relevant financial relationships.
Rashmi Kumar, PhD, Senior Manager, Clinical Content, NCCN, has disclosed that she has no relevant financial relationships.
Individuals Who Provided Content Development and/or Authorship Assistance:
David S. Ettinger, MD, Panel Chair, has disclosed that he is a scientific advisor for ARIAD Pharmaceuticals, Inc.; Boehringer Ingelheim GmbH; Eli Lilly and Company; EMD Serono; Genentech, Inc.; and Helsinn Pharmaceutical. He receives consulting fees/honoraria from Bristol-Myers Squibb Company, and receives grant/research support from Golden Biotechnology Corporation.
Douglas E. Wood, MD, Panel Vice Chair, has disclosed that he receives grant/research support from and is a scientific advisor for Spiration, Inc.
James Stevenson, MD, Panel Member, has disclosed that he receives grant/research support from Merck & Co., Inc.
Kristina Gregory, RN, MSN, OCN, Vice President, Clinical Information Operations, NCCN, has disclosed that she has no relevant financial relationships.
Miranda Hughes, PhD, Oncology Scientist/Senior Medical Writer, NCCN, has disclosed that she has no relevant financial relationships.
References
- 1.Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol 2009;39:576–588. [DOI] [PubMed] [Google Scholar]
- 2.Tsao AS, Wistuba I, Roth JA, Kindler HL. Malignant pleural mesothelioma. J Clin Oncol 2009;27:2081–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galateau-Salle F, Churg A, Roggli V, et al. The 2015 World Health Organization classification of tumors of the pleura: advances since the 2004 Classification. J Thorac Oncol 2016;11:142–154. [DOI] [PubMed] [Google Scholar]
- 4.Henderson DW, Reid G, Kao SC, et al. Challenges and controversies in the diagnosis of malignant mesothelioma: part 2. Malignant mesothelioma subtypes, pleural synovial sarcoma, molecular and prognostic aspects of mesothelioma, BAP1, aquaporin-1 and microRNA. J Clin Pathol 2013;66:854–861. [DOI] [PubMed] [Google Scholar]
- 5.Meyerhoff RR, Yang CF, Speicher PJ, et al. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J Surg Res 2015;196:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musk AW, Olsen N, Alfonso H, et al. Predicting survival in malignant mesothelioma. Eur Respir J 2011;38:1420–1424. [DOI] [PubMed] [Google Scholar]
- 7.Linton A, Pavlakis N, O’Connell R, et al. Factors associated with survival in a large series of patients with malignant pleural mesothelioma in New South Wales. Br J Cancer 2014;111:1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taioli E, Wolf AS, Camacho-Rivera M, et al. Determinants of survival in malignant pleural mesothelioma: a Surveillance, Epidemiology, and End Results (SEER) study of 14,228 patients. PLoS One 2015;10:e0145039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanphear BP, Buncher CR. Latent period for malignant mesothelioma of occupational origin. J Occup Med 1992;34:718–721. [PubMed] [Google Scholar]
- 10.Selikoff IJ, Hammond EC, Seidman H. Latency of asbestos disease among insulation workers in the United States and Canada. Cancer 1980;46:2736–2740. [DOI] [PubMed] [Google Scholar]
- 11.Antman KH, Corson JM, Li FP, et al. Malignant mesothelioma following radiation exposure. J Clin Oncol 1983;1:695–700. [DOI] [PubMed] [Google Scholar]
- 12.Weissmann LB, Corson JM, Neugut AI, Antman KH. Malignant mesothelioma following treatment for Hodgkin’s disease. J Clin Oncol 1996;14:2098–2100. [DOI] [PubMed] [Google Scholar]
- 13.Pappo AS, Santana VM, Furman WL, et al. Post-irradiation malignant mesothelioma. Cancer 1997;79:192–193. [DOI] [PubMed] [Google Scholar]
- 14.van Zandwijk N, Clarke C, Henderson D, et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis 2013;5:E254–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyer DS, Mohammed TL, Kirsch J, et al. ACR appropriateness criteria chronic dyspnea: suspected pulmonary origin. J Thorac Imaging 2013;28:W64–66. [DOI] [PubMed] [Google Scholar]
- 16.Gadgeel S, Pass H. Malignant mesothelioma. Commun Oncol 2006;3:215–224. [Google Scholar]
- 17.Rintoul RC, Ritchie AJ, Edwards JG, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014;384:1118–1127. [DOI] [PubMed] [Google Scholar]
- 18.Tan C, Sedrakyan A, Browne J, et al. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg 2006;29:829–838. [DOI] [PubMed] [Google Scholar]
- 19.Rahman NM, Pepperell J, Rehal S, et al. Effect of opioids vs NSAIDs and larger vs smaller chest tube size on pain control and pleurodesis efficacy among patients with malignant pleural effusion: the TIME1 randomized clinical trial. JAMA 2015;314:2641–2653. [DOI] [PubMed] [Google Scholar]
- 20.Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383–2389. [DOI] [PubMed] [Google Scholar]
- 21.Srour N, Amjadi K, Forster A, Aaron S. Management of malignant pleural effusions with indwelling pleural catheters or talc pleurodesis. Can Respir J 2013;20:106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunatilake S, Brims FJ, Fogg C, et al. A multicentre non-blinded randomised controlled trial to assess the impact of regular early specialist symptom control treatment on quality of life in malignant mesothelioma (RESPECT-MESO): study protocol for a randomised controlled trial. Trials 2014;15:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price A What is the role of radiotherapy in malignant pleural mesothelioma? Oncologist 2011;16:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins P, Milliner R, Salmon C. Re-evaluating the role of palliative radiotherapy in malignant pleural mesothelioma. Eur J Cancer 2011;47:2143–2149. [DOI] [PubMed] [Google Scholar]
- 25.de Perrot M, Feld R, Cho BC, et al. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:1413–1418. [DOI] [PubMed] [Google Scholar]
- 26.Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolukbas S, Manegold C, Eberlein M, et al. Survival after trimodality therapy for malignant pleural mesothelioma: radical pleurectomy, chemotherapy with cisplatin/pemetrexed and radiotherapy. Lung Cancer 2011;71:75–81. [DOI] [PubMed] [Google Scholar]
- 28.Weder W, Stahel RA, Bernhard J, et al. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol 2007;18:1196–1202. [DOI] [PubMed] [Google Scholar]
- 29.Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54–63. [DOI] [PubMed] [Google Scholar]
- 30.Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240–245. [DOI] [PubMed] [Google Scholar]
- 31.Teh E, Fiorentino F, Tan C, Treasure T. A systematic review of lung-sparing extirpative surgery for pleural mesothelioma. J R Soc Med 2011;104:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479–495. [DOI] [PubMed] [Google Scholar]
- 33.Zahid I, Sharif S, Routledge T, Scarci M. Is pleurectomy and decortication superior to palliative care in the treatment of malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg 2011;12:812–817. [DOI] [PubMed] [Google Scholar]
- 34.Ceresoli GL, Zucali PA, Favaretto AG, et al. Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J Clin Oncol 2006;24:1443–1448. [DOI] [PubMed] [Google Scholar]
- 35.van Haarst JMW, Baas P, Manegold C, et al. Multicentre phase II study of gemcitabine and cisplatin in malignant pleural mesothelioma. Br J Cancer 2002;86:342–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nowak AK, Byrne MJ, Williamson R, et al. A multicentre phase II study of cisplatin and gemcitabine for malignant mesothelioma. Br J Cancer 2002;87:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blomberg C, Nilsson J, Holgersson G, et al. Randomized trials of systemic medically-treated malignant mesothelioma: a systematic review. Anticancer Res 2015;35:2493–2501. [PubMed] [Google Scholar]
- 38.Kondola S, Manners D, Nowak AK. Malignant pleural mesothelioma: an update on diagnosis and treatment options. Ther Adv Respir Dis 2016;10:275–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raynaud C, Greillier L, Mazieres J, et al. Management of malignant pleural mesothelioma: a French multicenter retrospective study (GFPC 0802 study). BMC Cancer 2015;15:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kindler HL, Karrison TG, Gandara DR, et al. Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol 2012;30:2509–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellis P, Davies AM, Evans WK, et al. The use of chemotherapy in patients with advanced malignant pleural mesothelioma: a systematic review and practice guideline. J Thorac Oncol 2006;1:591–601. [PubMed] [Google Scholar]
- 42.Krug LM. An overview of chemotherapy for mesothelioma. Hematol Oncol Clin North Am 2005;19:1117–1136. [DOI] [PubMed] [Google Scholar]
- 43.Kelly RJ, Sharon E, Hassan R. Chemotherapy and targeted therapies for unresectable malignant mesothelioma. Lung Cancer 2011;73:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell K, Brosseau S, Reviron-Rabec L, et al. [Malignant pleural mesothelioma: 2013 state of the art]. Bull Cancer 2013;100:1283–1293. [DOI] [PubMed] [Google Scholar]
- 45.Mansfield AS, Symanowski JT, Peikert T. Systematic review of response rates of sarcomatoid malignant pleural mesotheliomas in clinical trials. Lung Cancer 2014;86:133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thieke C, Nicolay NH, Sterzing F, et al. Long-term results in malignant pleural mesothelioma treated with neoadjuvant chemotherapy, extrapleural pneumonectomy and intensity-modulated radiotherapy. Radiat Oncol 2015;10:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405–1414. [DOI] [PubMed] [Google Scholar]
- 48.Muers MF, Stephens RJ, Fisher P, et al. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet 2008;371:1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Brien ME, Watkins D, Ryan C, et al. A randomised trial in malignant mesothelioma (M) of early (E) versus delayed (D) chemotherapy in symptomatically stable patients: the MED trial. Ann Oncol 2006;17:270–275. [DOI] [PubMed] [Google Scholar]
- 50.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636–2644. [DOI] [PubMed] [Google Scholar]
- 51.Santoro A, O’Brien ME, Stahel RA, et al. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaive patients with malignant pleural mesothelioma: results of the International Expanded Access Program. J Thorac Oncol 2008;3:756–763. [DOI] [PubMed] [Google Scholar]
- 52.Katirtzoglou N, Gkiozos I, Makrilia N, et al. Carboplatin plus pemetrexed as first-line treatment of patients with malignant pleural mesothelioma: a phase II study. Clin Lung Cancer 2010;11:30–35. [DOI] [PubMed] [Google Scholar]
- 53.Castagneto B, Botta M, Aitini E, et al. Phase II study of pemetrexed in combination with carboplatin in patients with malignant pleural mesothelioma (MPM). Ann Oncol 2008;19:370–373. [DOI] [PubMed] [Google Scholar]
- 54.Kalmadi SR, Rankin C, Kraut MJ, et al. Gemcitabine and cisplatin in unresectable malignant mesothelioma of the pleura: a phase II study of the Southwest Oncology Group (SWOG 9810). Lung Cancer 2008;60:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arrieta O, Lopez-Macias D, Mendoza-Garcia VO, et al. A phase II trial of prolonged, continuous infusion of low-dose gemcitabine plus cisplatin in patients with advanced malignant pleural mesothelioma. Cancer Chemother Pharmacol 2014;73:975–982. [DOI] [PubMed] [Google Scholar]
- 56.Scagliotti GV, Shin DM, Kindler HL, et al. Phase II study of pemetrexed with and without folic acid and vitamin B12 as front-line therapy in malignant pleural mesothelioma. J Clin Oncol 2003;21:1556–1561. [DOI] [PubMed] [Google Scholar]
- 57.Taylor P, Castagneto B, Dark G, et al. Single-agent pemetrexed for chemonaive and pretreated patients with malignant pleural mesothelioma: results of an International Expanded Access Program. J Thorac Oncol 2008;3:764–771. [DOI] [PubMed] [Google Scholar]
- 58.Abdel-Rahman O, Kelany M. Systemic therapy options for malignant pleural mesothelioma beyond first-line therapy: a systematic review. Expert Rev Respir Med 2015;9:533–549. [DOI] [PubMed] [Google Scholar]
- 59.Christoph DC, Eberhardt WE. Systemic treatment of malignant pleural mesothelioma: new agents in clinical trials raise hope of relevant improvements. Curr Opin Oncol 2014;26:171–181. [DOI] [PubMed] [Google Scholar]
- 60.Ai J, Stevenson JP. Current issues in malignant pleural mesothelioma evaluation and management. Oncologist 2014;19:975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stahel RA, Weder W, Felley-Bosco E, et al. Searching for targets for the systemic therapy of mesothelioma. Ann Oncol 2015;26:1649–1660. [DOI] [PubMed] [Google Scholar]
- 62.Zauderer MG, Kass SL, Woo K, et al. Vinorelbine and gemcitabine as second- or third-line therapy for malignant pleural mesothelioma. Lung Cancer 2014;84:271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janne PA, Wozniak AJ, Belani CP, et al. Pemetrexed alone or in combination with cisplatin in previously treated malignant pleural mesothelioma: outcomes from a phase IIIB expanded access program. J Thorac Oncol 2006;1:506–512. [PubMed] [Google Scholar]
- 64.van Meerbeeck JP, Baas P, Debruyne C, et al. A Phase II study of gemcitabine in patients with malignant pleural mesothelioma. European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. Cancer 1999;85:2577–2582. [DOI] [PubMed] [Google Scholar]
- 65.Jassem J, Ramlau R, Santoro A, et al. Phase III trial of pemetrexed plus best supportive care compared with best supportive care in previously treated patients with advanced malignant pleural mesothelioma. J Clin Oncol 2008;26:1698–1704. [DOI] [PubMed] [Google Scholar]
- 66.Stebbing J, Powles T, McPherson K, et al. The efficacy and safety of weekly vinorelbine in relapsed malignant pleural mesothelioma. Lung Cancer 2009;63:94–97. [DOI] [PubMed] [Google Scholar]
- 67.Manegold C, Symanowski J, Gatzemeier U, et al. Second-line (post-study) chemotherapy received by patients treated in the phase III trial of pemetrexed plus cisplatin versus cisplatin alone in malignant pleural mesothelioma. Ann Oncol 2005;16:923–927. [DOI] [PubMed] [Google Scholar]
- 68.Ceresoli GL, Zucali PA, Gianoncelli L, et al. Second-line treatment for malignant pleural mesothelioma. Cancer Treat Rev 2010;36:24–32. [DOI] [PubMed] [Google Scholar]
- 69.Zucali PA, Simonelli M, Michetti G, et al. Second-line chemotherapy in malignant pleural mesothelioma: results of a retrospective multicenter survey. Lung Cancer 2012;75:360–367. [DOI] [PubMed] [Google Scholar]
- 70.Ceresoli GL, Zucali PA, De Vincenzo F, et al. Retreatment with pemetrexed-based chemotherapy in patients with malignant pleural mesothelioma. Lung Cancer 2011;72:73–77. [DOI] [PubMed] [Google Scholar]
- 71.Kotova S, Wong RM, Cameron RB. New and emerging therapeutic options for malignant pleural mesothelioma: review of early clinical trials. Cancer Manag Res 2015;7:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zauderer MG, Krug LM. Novel therapies in phase II and III trials for malignant pleural mesothelioma. J Natl Compr Canc Netw 2012;10:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomas A, Hassan R. Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol 2012;13:e301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alley EW, Molife LR, Santoro A, et al. Clinical safety and efficacy of pembrolizumab (MK-3475) in patients with malignant pleural mesothelioma: preliminary results from KEYNOTE-028 [abstract]. Cancer Res 2015;75:Abstract CT103. [Google Scholar]
- 75.Alley EW, Schellens JH, Santoro A, et al. Single-agent pembrolizumab for patients with malignant pleural mesothelioma (MPM) [abstract]. Presented at the 16th World Conference on Lung Cancer; September 6–9, 2016; Denver, Colorado. Abstract 3011. [Google Scholar]
- 76.Marcq E, Pauwels P, van Meerbeeck JP, Smits EL. Targeting immune checkpoints: new opportunity for mesothelioma treatment? Cancer Treat Rev 2015;41:914–924. [DOI] [PubMed] [Google Scholar]
- 77.Karim S, Leighl N. Pembrolizumab for the treatment of thoracic malignancies: current landscape and future directions. Future Oncol 2016;12:9–23. [DOI] [PubMed] [Google Scholar]
- 78.Le DT, Brockstedt DG, Nir-Paz R, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res 2012;18:858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Golfier S, Kopitz C, Kahnert A, et al. Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther 2014;13:1537–1548. [DOI] [PubMed] [Google Scholar]
- 80.Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620–626. [DOI] [PubMed] [Google Scholar]
- 81.Baud M, Bobbio A, Lococo F, et al. Should we continue to offer extrapleural pneumonectomy to selected mesothelioma patients? A single center experience comparing surgical and non-surgical management. Jpn J Clin Oncol 2014;44:1127–1129. [DOI] [PubMed] [Google Scholar]
- 82.Zauderer MG, Krug LM. The evolution of multimodality therapy for malignant pleural mesothelioma. Curr Treat Options Oncol 2011;12:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaufman AJ, Flores RM. Surgical treatment of malignant pleural mesothelioma. Curr Treat Options Oncol 2011;12:201–216. [DOI] [PubMed] [Google Scholar]
- 84.Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J Thorac Oncol 2011;6:1304–1312. [DOI] [PubMed] [Google Scholar]
- 85.Friedberg JS. The state of the art in the technical performance of lung-sparing operations for malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 2013;25:125–143. [DOI] [PubMed] [Google Scholar]
- 86.Halstead JC, Lim E, Venkateswaran RM, et al. Improved survival with VATS pleurectomy-decortication in advanced malignant mesothelioma. Eur J Surg Oncol 2005;31:314–320. [DOI] [PubMed] [Google Scholar]
- 87.Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weder W, Stahel RA, Baas P, et al. The MARS feasibility trial: conclusions not supported by data. Lancet Oncol 2011;12:1093–1094; author reply 1094–1095. [DOI] [PubMed] [Google Scholar]
- 89.Hasani A, Alvarez JM, Wyatt JM, et al. Outcome for patients with malignant pleural mesothelioma referred for trimodality therapy in Western Australia. J Thorac Oncol 2009;4:1010–1016. [DOI] [PubMed] [Google Scholar]
- 90.Schipper PH, Nichols FC, Thomse KM, et al. Malignant pleural mesothelioma: surgical management in 285 patients. Ann Thorac Surg 2008;85:257–264; discussion 264. [DOI] [PubMed] [Google Scholar]
- 91.Spaggiari L, Marulli G, Bovolato P, et al. Extrapleural pneumonectomy for malignant mesothelioma: an Italian multicenter retrospective study. Ann Thorac Surg 2014;97:1859–1865. [DOI] [PubMed] [Google Scholar]
- 92.Taioli E, Wolf AS, Flores RM. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg 2015;99:472–480. [DOI] [PubMed] [Google Scholar]
- 93.Nakas A, von Meyenfeldt E, Lau K, et al. Long-term survival after lung-sparing total pleurectomy for locally advanced (International Mesothelioma Interest Group Stage T3-T4) non-sarcomatoid malignant pleural mesothelioma. Eur J Cardiothorac Surg 2012;41:1031–1036. [DOI] [PubMed] [Google Scholar]
- 94.Bille A, Belcher E, Raubenheimer H, et al. Induction chemotherapy, extrapleural pneumonectomy, and adjuvant radiotherapy for malignant pleural mesothelioma: experience of Guy’s and St Thomas’ hospitals. Gen Thorac Cardiovasc Surg 2012;60:289–296. [DOI] [PubMed] [Google Scholar]
- 95.Shahin Y, Wellham J, Jappie R, et al. How successful is lung-preserving radical surgery in the mesothelioma and radical surgery-trial environment? A case-controlled analysis. Eur J Cardiothorac Surg 2011;39:360–363. [DOI] [PubMed] [Google Scholar]
- 96.Sugarbaker DJ, Jaklitsch MT, Bueno R, et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg 2004;128:138–146. [DOI] [PubMed] [Google Scholar]
- 97.Yan TD, Boyer M, Tin MM, et al. Extrapleural pneumonectomy for malignant pleural mesothelioma: outcomes of treatment and prognostic factors. J Thorac Cardiovasc Surg 2009;138:619–624. [DOI] [PubMed] [Google Scholar]
- 98.Bolukbas S, Eberlein M, Fisseler-Eckhoff A, Schirren J. Radical pleurectomy and chemoradiation for malignant pleural mesothelioma: the outcome of incomplete resections. Lung Cancer 2013;81:241–246. [DOI] [PubMed] [Google Scholar]
- 99.Sugarbaker DJ, Wolf AS, Chirieac LR, et al. Clinical and pathological features of three-year survivors of malignant pleural mesothelioma following extrapleural pneumonectomy. Eur J Cardiothorac Surg 2011;40:298–303. [DOI] [PubMed] [Google Scholar]
- 100.Flores RM, Riedel E, Donington JS, et al. Frequency of use and predictors of cancer-directed surgery in the management of malignant pleural mesothelioma in a community-based (Surveillance, Epidemiology, and End Results [SEER]) population. J Thorac Oncol 2010;5:1649–1654. [DOI] [PubMed] [Google Scholar]
- 101.Zahid I, Routledge T, Bille A, Scarci M. What is the best treatment for malignant pleural effusions? Interact Cardiovasc Thorac Surg 2011;12:818–823. [DOI] [PubMed] [Google Scholar]


