Summary
Therapeutic drug monitoring (TDM) has emerged as a useful tool for optimizing biologics, and in particular anti-tumor necrosis factor (anti-TNF) therapy, in both inflammatory bowel disease (IBD) and other immune-mediated inflammatory diseases such as rheumatoid arthritis and psoriasis. However, there are still some challenges hindering the widespread implementation of TDM in clinical practice. These barriers include identification of the optimal drug concentration to target, the lag time between sampling and results, and the proper interpretation of anti-drug antibody titers among different assays. Solutions to overcome these barriers include the harmonization of TDM assays and the use of point-of-care testing. Other unmet needs include well-designed prospective studies and randomized controlled trials focusing on proactive TDM particularly during induction therapy. Future studies should also investigate the utility of TDM in biologics other than anti-TNFs in both IBD and other immune-mediated inflammatory diseases and the use of pharmacokinetic dashboards and pharmacogenetics towards individual personalized medicine.
Keywords: inflammatory bowel disease, Crohn’s disease, ulcerative colitis, therapeutic drug monitoring, infliximab, adalimumab, vedolizumab, ustekinumab, pharmacokinetic dashboards
Introduction
Therapeutic drug monitoring (TDM) has emerged as a useful tool for optimizing biologic therapy, specifically anti-tumor necrosis factor (anti-TNF) therapy, in inflammatory bowel disease (IBD) and other immune-mediated inflammatory diseases (IMID).1 Reactive TDM is defined as the evaluation of drug concentrations and anti-drug antibodies (ADA) in the setting of primary non-response (PNR) or secondary loss of response (SLR). Proactive TDM is utilizing the regular measurement of drug trough concentrations and ADA with dose adaptation to target appropriate drug concentration. Reactive TDM has rationalized the management of PNR and SLR and has proven more cost-effective than empiric dose optimization of infliximab.2-4 Moreover, preliminary data suggest that proactive TDM may be associated with better therapeutic outcomes than empiric dose optimization and/or reactive TDM.5-13 In addition numerous exposure-outcome relationship data both in IBD (Table 1)14-53 and other IMID including rheumatoid arthritis (RA) and psoriasis (Table 2) not only from retrospective but also prospective studies and post-hoc analyses of randomized controlled trials (RCTs), demonstrate that higher drug concentrations are associated with improved therapeutic outcomes.54-91 Conversely, lower drug concentrations, with or without ADA, are associated with treatment failure and drug discontinuation.2,3 Preliminary data also suggest that proactive TDM of infliximab can potentially safely guide treatment de-escalation92-94 and support the concept of optimized monotherapy in lieu of combination therapy with an immunomodulator (IMM).95,96
Table 1.
Biologic drug exposure-outcome relationship data in IBD from prospective studies and post-hoc analysis of RCTs.
| TDM time point |
Study type / acronym |
IBD type |
Drug concentration threshold (μg/mL) |
Therapeutic outcome (time point) |
Assay type |
Ref. |
|---|---|---|---|---|---|---|
| Infliximab | ||||||
| Week 2 | TAILORIX* | CD | >23.1 | Endoscopic remission (w12) | ELISA | 14 |
| Week 2 | JAPIC* | UC | >21.3 | Clinical remission (w14) | ELISA | 15 |
| Week 2 | ACT 1 & 2* | UC | ≥18.6 | MES<2 (w8) | ELISA | 16 |
| Week 2 | Prospective** | CD | ≥26.7 | Clinical response (w14) | ELISA | 17 |
| Week 2 | Prospective*** | CD | >20.4 | Clinical remission (w14) | ELISA | 18 |
| Week 2 | Prospective*** | UC | >15.3 | Clinical remission (w14) | ELISA | 18 |
| Week 2 | Prospective | CD/UC | >22.9 | Clinical response (w14) | ELISA | 19 |
| Week 6 | Prospective | CD/UC | >11.8 | Clinical response (w14) | ELISA | 19 |
| Week 6 | TAILORIX* | CD | >10 | Endoscopic remission (w12) | ELISA | 14 |
| Week 6 | Prospective** | CD | ≥15.9 | Clinical response (w14) | ELISA | 17 |
| Week 6 | Prospective | UC | >6.6 | Endoscopic response (w8) | ELISA | 20 |
| Week 6 | ACT 1 & 2* | UC | ≥10.6 | MES<2 (w8) | ELISA | 16 |
| Week 6 | ACT 1 & 2* | UC | >22 | Clinical response (w8) | ELISA | 21 |
| Week 8 | ACT 1 & 2* | UC | >41.1 | Clinical response (w8) | ELISA | 21 |
| Week 8 | ACT 1 & 2* | UC | ≥34.9 | MES<2 (w8) | ELISA | 16 |
| Week 10 | Prospective** | CD | ≥9.1 | Drug retention (w52) | ELISA | 22 |
| Week 14 | TAILORIX* | CD | >7.8 | Radiological remission (w54) | ELISA | 23 |
| Week 14 | ACCENT II* | CD | ≥7.2 | Complete fistula response & CRP normalization (w14) | ELISA | 24 |
| Week 14 | ACCENT I* | CD | >3.5 | Clinical response (w54) | ELISA | 25 |
| Week 14 | ACT 1 & 2* | UC | >5.1 | Clinical response (w30) | ELISA | 21 |
| Week 14 | ACT 1 & 2* | UC | ≥5.1 | MES<2 (w30) | ELISA | 16 |
| Week 14 | ACT 1 & 2* | UC | ≥6.7 | MES=0 (w30) | ELISA | 16 |
| Week 14 | Prospective | CD/UC | >4.8 | Clinical response (w14) | ELISA | 26 |
| Week 14 | Prospective*** | UC | >3.2 | Mucosal healing (w14) | ELISA | 27 |
| Week 14 | Prospectivea | CD | ≥7 | Clinical remission (w14/54) | ELISA | 28 |
| Week 14 | Prospective** | CD | >11.5 | FC<100μg/g (w14) | ELISA | 29 |
| Week 30 | SONIC* | CD | ≥3 | Mucosal healing (w26) | ELISA | 30 |
| Week 30 | ACT 1 & 2* | UC | >2.4 | Clinical response (w54) | ELISA | 21 |
| Week 30 | ACT 1 & 2* | UC | ≥2.3 | MES<2 (w30) | ELISA | 16 |
| Week 30 | ACT 1 & 2* | UC | ≥3.8 | MES=0 (w30) | ELISA | 16 |
| Adalimumab | ||||||
| Week 2 | Prospectiveb | CD | >6.7 | Clinical remission (w14) | ELISA | 31 |
| Week 4 | Prospective | CD | >12 | CRP≤5mg/L (w12) | ELISA | 32 |
| Week 4 | Prospective | CD/UC | >3.5 | Clinical response (w4) | ELISA | 26 |
| Week 4 | Prospective** | CD | >22.5 | PCDAI<10, CRP≤5mg/L & FC<250μg/g (w24) | ELISA | 33 |
| Week 8 | Prospective** | CD | >12.5 | PCDAI<10, CRP≤5mg/L & FC<250μg/g (w24) | ELISA | 33 |
| Week 12 | Prospective | CD | >7.3 | HBI<5 (w12) | ELISA | 34 |
| Week 14 | Prospectiveb | CD | >3.7 | CRP normalization (w14) | ELISA | 31 |
| Week 14 | Prospectivea | CD | ≥12 | Clinical remission (w14/54) | ELISA | 28 |
| Week 16 | Prospective** | CD | >8.8 | SES-CD=0 (w16) | ELISA | 35 |
| Week 26 | DIAMOND* | CD | >5 | Clinical remission (w52) | ELISA | 36 |
| Certolizumab pegol | ||||||
| Week 6 | 9 RCTs* | CD | >31.9 | CRP≤5mg/L (w6) | ELISA | 37 |
| Week 6 | 9 RCTs* | CD | >36.1 | FC<250μg/g & CDAI≤150 (w26) | ELISA | 37 |
| Week 8 | MUSIC* | CD | >23.3 | Endoscopic remission (w10) | ELISA | 38 |
| Week 12 | 9 RCTs* | CD | >14.8 | FC<250μg/g & CDAI≤150 (w26) | ELISA | 37 |
| Golimumab | ||||||
| Week 2 | PURSUIT* | UC | >8.9 | Clinical response (w6) | ECLIA | 39 |
| Week 4 | PURSUIT* | UC | >7.4 | Clinical response (w6) | ECLIA | 39 |
| Week 6 | PURSUIT* | UC | >2.5 | Clinical response (w6) | ECLIA | 39 |
| Week 6 | Prospective | UC | >10.7 | MES ≤1 (w52) | ELISA | 40 |
| Week 6 | Prospectivec | UC | >3.8 | SCCAI<3 & FC<250μg/g (w6) | ELISA | 41 |
| Week 28 | PURSUIT* | UC | >0.9 | Clinical remission (w30/54) | ECLIA | 39 |
| Week 44 | PURSUIT* | UC | >1.4 | Clinical remission (w30/54) | ECLIA | 39 |
| Vedolizumab | ||||||
| Week 2 | Prospective | CD/UC | ≥23.2 | Steroid-free endoscopic remission (w52) | HMSA | 42 |
| Week 6 | Prospective | CD/UC | ≥19.8 | Steroid-free endoscopic remission (w52) | HMSA | 42 |
| Week 6 | Prospective | CD/UC | >18 | Mucosal healing (w52) | ELISA | 44 |
| Week 6 | Prospective | CD/UC | >22 | Endoscopic & clinical remission (w46) | ELISA | 45 |
| Week 6 | Prospective | CD/UC | >29.9 | Clinical remission (w14) | ELISA | 46 |
| Week 6 | GEMINI 1* | UC | > 37.1 | Clinical response (w14) | ELISA | 43 |
| Week 14 | GEMINI 1* | UC | > 18.4 | Clinical response (w14) | ELISA | 43 |
| Week 14 | Prospective | CD/UC | >16.6 | Drug persistence (w52) | ELISA | 46 |
| Week 22 | Prospective | CD/UC | >8 | Endoscopic & clinical remission (w46) | ELISA | 45 |
| Week 22 | Prospectived | CD | >10 | Endoscopic remission (w26) | ELISA | 47 |
| Ustekinumab | ||||||
| Week 2 | Prospective | CD | >24.7 | FC<100μg/g (w8/16) | ELISA | 48 |
| Week 4 | Prospective | CD | >15.9 | 50% decrease in FC (w8) | ELISA | 49 |
| Week 4 | Prospective | CD | >13 | HBI<5, CRP<5mg/L, FC<250μg/g (w16) | ELISA | 50 |
| Week 4 | Prospective | CD | >23.7 | SES-CD<4 without ulceration (w24) | ELISA | 48 |
| Week 8 | Prospective | CD | >4.2 | 50% decrease in FC (w8) | ELISA | 49 |
| Week 8 | Prospective | CD | >7.2 | CRP≤5mg/L (w8) | ELISA | 49 |
| Week 8 | Prospective | CD | >2 | HBI<5, CRP<5mg/L, FC<250μg/g (w16) | ELISA | 50 |
| Week 8 | Prospective | CD | >11.1 | SES-CD<4 without ulceration (w24) | ELISA | 48 |
| Week 8 | UNITI 1&2* | CD | >3.3 | Clinical remission (w8) | ECLIA | 51 |
| Week 8 | UNIFI* | UC | >3.7 | Histologic improvement (w8) | ECLIA | 52 |
| Week 12 | Prospective | CD | >1.1 | Biological response (w26) | ELISA | 53 |
| Week 16 | Prospective | CD | >2.3 | Endoscopic response (w24) | ELISA | 49 |
| Week 16 | Prospective | CD | >1.4 | HBI<5, CRP<5mg/L, FC<250μg/g (w16) | ELISA | 50 |
| Week 24 | Prospective | CD | >1.9 | Endoscopic response (w24) | ELISA | 49 |
| Week 24e | UNITI 1&2* | CD | >0.8 | Clinical remission (w24) | ECLIA | 51 |
| Week 40f | UNITI 1&2* | CD | >1.4 | Clinical remission (w44) | ECLIA | 51 |
Post-hoc analysis of RCT
Pediatric
CT-P13
PANTS: The personalised anti-TNF therapy in Crohn's disease study
POETIC: Prospective Observational Evaluation of Time-Dependency of Adalimumab Immunogenicity and Drug Concentrations
GO-LEVEL: Study of the Golimumab Exposure-Response Relationship Using Serum Trough Levels
LOVE-CD: A Study to Evaluate Efficacy, of Early Versus Late Use of Vedolizumab in Crohn's Disease
Combined q8w and q12w
q8w only.
TAILORIX: A Study investigating Tailored Treatment With Infliximab for Active Crohn’s Disease; JAPIC: Clinical study to assess the efficacy and safety of TA-650 in patients with active ulcerative colitis; ACT: Active Ulcerative Colitis Trials; ACCENT-I: A Randomized, Double-blind, Placebo-controlled Trial of Anti-TNFa Chimeric Monoclonal Antibody (Infliximab, Remicade) in the Long-term Treatment of Patients With Moderately to Severely Active Crohn’s Disease; ACCENT-II: A Randomized, Double-blind, Placebo-controlled Trial of Anti-TNFa Chimeric Monoclonal Antibody (Infliximab; REMICADE Janssen Biotech, Inc, Malvern, PA) in the Long-term Treatment of Patients with Fistulizing Crohn’s Disease; SONIC: The Study of Biologic and Immunomodulator Naïve Patients in Crohn’s Disease; DIAMOND: Comparison of Adalimumab Monotherapy and a Combination With Azathioprine for Patients With Crohn’s Disease: A Prospective, Multicenter, Open-Labeled Clinical Trial; MUSIC: Endoscopic Mucosal Improvement in Patients with Active Crohn’s Disease Treated with Certolizumab Pegol; PURSUIT: A study to Evaluate the Safety and Efficacy of Golimumab Maintenance Therapy, Administered Subcutaneously, in Subjects With Moderately to Severely Active Ulcerative Colitis; GEMINI: A Study of Vedolizumab (MLN0002) in Patients With Moderate to Severe Ulcerative Colitis; UNITI: A Study to Evaluate the Safety and Efficacy of Ustekinumab Induction Therapy in Subjects With Moderately to Severely Active Crohn’s Disease; UNIFI: A Study to Evaluate the Safety and Efficacy of Ustekinumab Induction Therapy in Subjects With Moderately to Severely Active Ulcerative Colitis; IBD: inflammatory bowel disease; TDM: therapeutic drug monitoring; RCT: randomized controlled trial; CD: Crohn’s disease; UC: ulcerative colitis; MES: Mayo endoscopic score; CRP: C-reactive protein, FC: fecal calprotectin; CDAI: Crohn’s disease activity index; HBI: Harvey Bradshaw index; PCDAI: Pediatric Crohn’s disease activity index; SES-CD: Simple Endoscopic Score-CD; SCCAI: simple clinical colitis activity index. ECLIA: electrochemiluminescent immunoassay; ELISA: enzyme-linked immunosorbent assay; HMSA: homogenous mobility shift assay; w: week; ref.: reference.
Table 2.
Association of biologic drug concentrations and clinical outcomes in other IMID.
| IMID type |
Study type | Threshold, μg/mL (time point) |
Clinical outcome (time point) | Ref. |
|---|---|---|---|---|
| Infliximab | ||||
| RA | Prospective | >2.5 (week 6) | Good EULAR response (week 26) | 54 |
| RA | Retrospective | <4.4 (week 6) | Drug discontinuation (week 52) | 55 |
| RA | Retrospective | >1 | DAS28≤3.2 (week 42) | 56 |
| RA | Post-hoc analysis of RCTa | Higher drug concentrations were associated with higher rates of clinical response and a greater reduction of CRP | 57 | |
| RA | Prospective | Patients who did not respond after 14 weeks of treatment had significantly lower drug concentrations compared with responders and CRP levels were negatively correlated with drug concentrations | 58 | |
| Psoriasis | Prospective | >1 (week 48) | PASI75 (week 48) | 59 |
| Psoriasis | Prospective | PASI score and PASI 90/100 response were significantly associated with trough drug concentrations | 60 | |
| AS | Retrospective | Higher drug concentrations were associated with lower ASDAS-ESR/CRP scores | 61 | |
| AS | RCT | No association of drug concentrations with treatment failure | 62 | |
| Adalimumab | ||||
| RA | Prospective | >1.3 | Good EULAR response (week 26) | 63 |
| RA | Prospective | >6.4 | Persistent remission after dose-halving (week 24) | 64 |
| RA | Prospective | >5 | EULAR response (week 28) | 65 |
| RA | Prospective | <5 (week 12) | No EULAR response (week 52) | 66 |
| AS | Retrospective | >6.4 >7.7 >4.6 |
BASDAI<4 ASDAS-ESR< 2.1 ASDAS-CRP< 2.1 |
67 |
| AS | Retrospective | <3.4 (week 2) <4.3 (week 4) |
Primary non-response | 68 |
| AS | Prospective | Association of drug concentrations with ASDAS | 69 | |
| Psoriasis | Prospective | >7.8 (week 48) | PASI75 (week 48) | 59 |
| Psoriasis | Prospective | >3.2 | PASI75 | 70 |
| Psoriasis | Prospective | >3.51 | ΔPASI75-100 | 71 |
| Psoriasis | Retrospective | Drug concentrations at weeks 4, 12 and 24 were higher in responders than non-responders who failed to t achieve PASI50 | 72 | |
| Psoriasis | Prospective | There was a correlation between drug serum levels and PASI scores | 73 | |
| PsA | Prospective | Patients with detectable ADA compared with patients without had lower drug concentrations and a poorer clinical outcome | 74 | |
| PsA | Prospective | ADA were associated with lower drug concentrations and reduced clinical response | 75 | |
| Peripheral SpA | RCT | No clear association between drug concentrations or ADA with clinical response or with relapse upon treatment discontinuation | 76 | |
| Certolizumab pegol | ||||
| RA | Prospective | Higher drug concentrations were associated with better EULAR response (12 months) | 77 | |
| RA, Axial SpA, PsA | RCTb | ≥20 | Treatment response (3 and 6 months) | 78 |
| Golimumab | ||||
| RA | Prospective | EULAR responders compared with non-responders were found to have higher golimumab concentrations at week 52. | 79 | |
| Ustekinumab | ||||
| Psoriasis | Retrospective | >3.6 (w4) | PASI≤2 (w4) | 80 |
| Psoriasis | Prospective | Inverse correlation between drug concentrations at week 6 and absolute PASI score | 81 | |
| Psoriasis | Prospective | Early drug concentration (1-12 weeks after starting treatment) were associated with PASI75 response (6 months) | 82 | |
| Etanercept | ||||
| RA | Prospective | >1.2 | Good EULAR response (week 26) | 63 |
| RA | Prospective | EULAR good responders compared with EULAR moderate and non-responders had higher drug concentrations | 83 | |
| AS | Prospective | Drug concentrations were higher in patients with ASDAS<2.1 compared to those with ASDAS≥ 2.1 | 84 | |
| Psoriasis | Retrospective | Positive correlation between drug concentration and decrease in the PASI scale with respect to the baseline value | 85 | |
| Psoriasis | Prospective | Inverse correlation between drug concentration and PASI in patients <50 years old | 86 | |
| Secukinumab | ||||
| PsA | RCT | >33.2 | PASI≤2 | 87 |
| PsA | RCT | Patients with low drug concentrations were at higher risk of radiographic progression | 88 | |
| Ixekizumab | ||||
| Psoriasis | RCT | Steady-state drug trough concentrations were associated with high clinical responses at week 12 | 89 | |
| Psoriasis | Post-hoc analysis of 3 RCTs | Higher drug concentrations were associated with higher response levels based on static physician global assessment and PASI | 90 | |
| Tocilizumab | ||||
| RA | Prospective | Association between drug concentrations and ΔDAS28 | 91 | |
ATTRACT: Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy
NOR-DMARD: Norwegian-biologic disease-modifying antirheumatic drug.
IMID: immune mediated inflammatory diseases; RA: rheumatoid arthritis; EULAR: European league against rheumatism; PsA: psoriatic arthritis; AS: Ankylosing Spondylitis; RCT: randomized controlled trial; Δ: delta; PASI: Psoriasis area and severity index; DAS: disease activity score; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; ASDAS: Ankylosing Spondylitis Disease Activity Score- ESR Erythrocyte Sedimentation Rate; CRP: C-reactive protein; SpA: spondyloarthritis; ADA: anti-drug antibodies; Ref.: reference.
However, there are still several issues the use of TDM in patients with IBD. These include the identification of the optimal drug concentrations to target taking into account also inter-individual variability, the lag time between testing and TDM results and interpretation of ADA titers among different assays. These issues could efficiently be addressed by the harmonization of assays and the use of rapid, point-of-care, testing. Further unmet needs include well-designed prospective studies and RCTs focused on proactive TDM particularly during induction therapy when the inflammatory burden and drug clearance is greatest. Research should also emphasize the role of TDM of non anti-TNF biologics. Future perspectives towards a more personalized application of TDM ought to embrace pharmacokinetic (PK) modeling and dashboards as well as pharmacogenetics. This would also allow selection of those patients at high risk of accelerated drug clearance who would benefit more from proactive TDM.
This collaborative state-of-the-art review from members of the intErnational Consortium Therapeutic dRUg Monitoring (spECTRUM) aims to present the most recent data from RCTs, prospective studies and post-hoc analyses of RCTs examining the role of TDM for optimizing biologics in IBD. Moreover, we provide up-to-date information regarding the role of TDM in other IMID. Finally, emphasis is given to the unmet needs and future perspectives for TDM. The spECTRUM consortium is a group that consists of IBD, rheumatology, and dermatology TDM specialists from thirteen countries on five different continents. It is a consortium with global perspectives launched to determine the unmet need, design research to address the issues and expand the utility of TDM with the ultimate aim of improving patient care.
Search strategy and selection criteria
References for this Review were identified through searches of PubMed with the search terms ‘inflammatory bowel disease’; ‘Crohn’s disease’; ‘ulcerative colitis’; ‘psoriasis’; ‘rheumatoid arthritis’; ‘ankylosing spondylitis’; ‘anti-drug antibodies’; ‘immunogenicity’; ‘therapeutic drug monitoring’; ‘point of care assays’; ‘pharmacokinetics’; ‘pharmacogenetics’; ‘infliximab’; ‘adalimumab’; ‘certolizumab pegol’; ‘golimumab’; ‘vedolizumab’; ‘ustekinumab’; ‘etanercept’; ‘secukinumab’; ‘ixekizumab’; ‘tocilizumab’ from 2000 until March, 2021. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this review focusing mainly on the more recent publications.
What is already known regarding the role of TDM of biologics in IBD
Two main types of studies have defined the role of TDM of biologics in IBD; exposure-outcome relationship studies and studies assessing the utility of TDM for optimizing anti-TNF therapy. The latter have compared (1) reactive TDM to empiric treatment optimization and (2) proactive TDM to reactive TDM and/or empiric treatment optimization. Although the majority of these investigations are retrospective that are characterized by inherited limitations and biases there are now several prospective studies, RCTs and post-hoc analyses of RCTs that are presented in relevant sections of this review.
Randomized controlled trials
There are currently five RCTs9,10,97-99 that have investigated the role of TDM for anti-TNF therapy in IBD patients with inconsistent results probably also due to differences in study design and population, primary end-points and TDM-based algorithms (Table 3).100 Four studies examined infliximab. Steenholdt et al.97examined the cost-effectiveness and clinical efficacy of reactive TDM compared to empiric treatment optimization in patients with CD and SLR to infliximab, whereas TAXIT (Trough Concentration Adapted Infliximab Treatment)9 and TAILORIX (A Study investigating Tailored Treatment With Infliximab for Active Crohn’s Disease)98 RCTs examined the role of proactive TDM. The fourth study, the PRECISION (Precision Dosing of Infliximab Versus Conventional Dosing of Infliximab) RCT99 was designed to investigate the efficacy of dashboard-driven infliximab dosing compared to standard dosing. For Adalimumab, the PAILOT (Pediatric Crohn’s Disease Adalimumab Level-based Optimization Treatment) RCT10 specifically investigated the role of proactive TDM in pediatric CD.
Table 3.
Randomized controlled trials regarding the role of therapeutic drug monitoring in IBD.
| RCT acronym |
IBD type |
Study arms | Primary end point | Major limitations |
|---|---|---|---|---|
| Infliximab | ||||
| N/A97 | CD | Reactive TDM vs. empiric dose optimization | Cost-effectiveness and CDAI response after 12 weeks | - IFX TC to target of 0.5 μg/mL |
| TAXIT9 | CD / UC | Proactive TDM vs. clinically based dose optimization | Clinical and biochemical remission at one year after optimization phase | - All patients were optimized based on IFX TC, prior to randomization that may eliminated the opportunity for proactive TDM to prove its benefit - IFX TC to target 3-7 μg/mL - Only one year follow up |
| TAILORIX98 | CD | Dose optimization based on clinical symptoms and biomarkers and/or proactive TDM vs. dose optimization based on clinical symptoms alone | Sustained CS-free clinical remission from weeks 22 to 54 with no ulcers at week 54 | - IFX TC to target of 3 μg/mL - IFX dose in the ‘TDM’ arms could be escalated based also on symptoms and biomarkers - IFX dose in the ‘control’ group could be escalated based only on clinical symptoms and as a result a high number of dose optimizations were driven by nonspecific symptoms as demonstrated by normal drug and biomarker levels in these patients - IFX concentrations were similar in all 3 groups which likely accounted for the similar efficacy outcomes - Sustained IFX TC of >3μg/mL in <50% in the ‘TDM’ arms |
| PRECISION99 | CD / UC | Proactive TDM based on PK dashboard driven dosing vs. standard dosing | Sustained clinical remission after 1 year |
- IFX TC to target ≥ 3 μg/mL - No dosing adaptations were allowed in the control group - Lack of endoscopic outcomes |
| Adalimumab | ||||
| PAILOT10 | CD* | Proactive vs. reactive TDM | Sustained CS-free clinical remission from weeks 8 to 72 | - ADM TC to target ≥ 5 μg/mL - Lack of endoscopic endpoints - Rather small sample size |
Pediatric.
TAXIT: Trough Concentration Adapted Infliximab Treatment; TAILORIX: A Study investigating Tailored Treatment With Infliximab for Active Crohn’s Disease; PRECISION: Precision Dosing of Infliximab Versus Conventional Dosing of Infliximab; PAILOT: Pediatric Crohn’s Disease Adalimumab Level-based Optimization Treatment; RCT: randomized controlled trial; IBD: inflammatory bowel disease; N/A: not applicable; CD: Crohn’s disease; UC: ulcerative colitis; CS: corticosteroids; TDM: therapeutic drug monitoring; TC: trough concentration; PK: pharmacokinetic; CDAI: Crohn’s disease activity index; IFX: infliximab; ADM: adalimumab.
In the RCT by Steenholdt et al.97, although reactive TDM proved to be more cost-effective than routine dose intensification in patients with SLR, it did not improve clinical efficacy. The TAXIT9 and the TAILORIX98 RCTs did not also meet their primary endpoint. However, the TAXIT RCT showed that during the optimization phase in patients with CD with low drug concentrations, proactive TDM-based dose optimization led to higher rates of clinical remission (88% vs. 65%; p=0.020) and improvement in C-reactive protein (CRP) (3.2 vs. 4.3 mg/L; p<0.001) compared to before dose escalation. Moreover, some of the secondary endpoints of TAXIT favored the proactive TDM over the clinical-based dosing arm including disease relapse over time and the rate of undetectable drug trough concentrations. The PAILOT RCT10 assessed a pediatric population with CD naïve to biological therapy who had responded to adalimumab induction therapy and showed that the rate of sustained corticosteroid-free clinical remission was significantly higher in the proactive arm compared to the reactive TDM arm (82% vs. 48%; p=0.002) achieving its primary endpoint. Several secondary outcomes also favored proactive over reactive TDM, the most important being the composite outcome of sustained corticosteroid-free remission, normal CRP and normal FC (42% vs. 12%; p=0.003). The PRECISION99 was the first RCT to investigate the efficacy of PK-dashboard-driven infliximab dosing compared to standard dosing in patients with IBD in clinical remission. Meeting its primary endpoint the study showed that more patients in the PK model arm were in sustained clinical remission compared to the control group (88% vs. 64%; p=0.017). Furthermore, a composite outcome of combined clinical remission and normal fecal calprotectin levels was higher in the PK model arm compared to the clinical arm (95% vs. 68.2%; p=0.027).
The major limitations of these RCTs are described in Table 3. We would like to point out that there are two main issues common to all of them. The first is the use of a rather low targeted drug concentration. Numerous recent reports suggest that higher drug concentrations are associated with more stringent therapeutic outcomes such as endoscopic and histologic remission101 The other concern is that patients had to wait until the next dose before treatment changes were implemented. Future RCTs should address these issues with the hope of better defining the role of TDM.
Prospective studies and post-hoc analyses of RCTs
Multiple prospective exposure-outcome relationship studies in both adult and pediatric IBD and post-hoc analyses of RCTs have demonstrated a positive correlation between biologic drug concentrations and favorable therapeutic outcomes (Table 1).102,103 Studies demonstrated similar results during induction and maintenance therapy. In the largest prospective study, PANTS (The personalised anti-TNF therapy in Crohn's disease study), infliximab concentrations of > 7 μg/mL and adalimumab concentration of > 12 μg/ml at week 14 were associated with clinical remission at both week 14 and 54. Low drug concentrations at week 14 were independently associated with immunogenicity, PNR and non-remission at week 54.28 A recent post-hoc analysis of the ACCENT-II [A Randomized, Double-blind, Placebo-controlled Trial of Anti-TNFa Chimeric Monoclonal Antibody (Infliximab; REMICADE Janssen Biotech, Inc, Malvern, PA) in the Long-term Treatment of Patients with Fistulizing Crohn’s Disease] RCT showed that higher infliximab concentrations at week 14 were independently associated with composite remission at week 14 [odds ratio (OR): 2.32; 95% confidence interval (CI): 1.55–3.49; p<0.001) and week 54 (OR: 2.05; 95% CI: 1.10–3.82; p=0.023). Based on receiver operating characteristic curve analysis, infliximab concentration thresholds of ≥20.2 μg/mL at week 2, ≥15 μg/mL at week 6, and ≥7.2 μg/mL at week 14 were associated with composite remission at week 14.24 Preliminary data from prospective studies and post-hoc analyses of RCTs have also examined anti-TNF drug concentrations in relation to perioperative complications104 and post-operative recurrence in patients with CD undergoing ileocolic resection (Table 4).105-108
Table 4.
Association of anti-TNF drug concentrations with post-operative recurrence in patients undergoing an ileocolonic resection for CD.
| Biologic drug |
Study type |
Association of anti-TNF drug concentrations with POR in patients undergoing an ileocolonic resection for CD |
Ref. |
|---|---|---|---|
| IFX / ADM | Prospective | - Drug TC > 1μg/mL and > 3 μg/mL were not associated to increased rates of early (30-day) postoperative complications. | 104 |
| IFX | RCTa | - Inverse correlation between IFX concentrations at week 72 and POR rates at week 76. | 105 |
| ADM | Prospective | - Lower ADM concentration in patients with normal mucosa (Rutgeerts’ score ≤i1) compared to those with endoscopic POR (Rutgeerts’ score ≥i2) (7.95 μg/mL vs. 3.25μg/mL; p=0.048). - ADM concentration was inversely correlated to the Rutgeerts’ score. - Patients with ADM concentrations <4.2 μg/mL compared to those with concentrations ≥4.2 μg/mL had higher POR (86% vs. 15%; p=0.025). |
106 |
| ADM | Post-hoc analysis of RCTb | - ADM concentration did not statistically significant differ between patients in endoscopic remission vs. endoscopic POR defined as a Rutgeert’s score ≥i2. | 107 |
| ADM | Post-hoc analysis of RCT | - In patients with clinical or endoscopic POR ADM TC were lower than in those who maintained remission both at baseline (9.5 vs. 14.4 μg/mL; p<0.01) and during follow-up (7.5 vs. 13.9 μg/mL; p<0.01). | 108 |
PREVENT: Prospective, Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial Comparing REMICADE® [infliximab] and Placebo in the Prevention of Recurrence in Crohn’s Disease Patients Undergoing Surgical Resection Who Are at an Increased Risk of Recurrence
POCER: Postoperative Crohn's Endoscopic Recurrence.
IBD: inflammatory bowel disease; Ref.: reference; CD: Crohn’s disease; UC: ulcerative colitis; CRP: C-reactive protein, FC: fecal calprotectin; HBI: Harvey Bradshaw index; TC: trough concentrations; IFX: infliximab; ADM: adalimumab; POR: post-operative recurrence; RCT: randomized controlled trial.
A major limitation of prospective exposure-outcome relationship studies and post-hoc analyses of RCTs is that these studies only show an association and not causation as higher serum drug concentrations may just reflect lower disease activity related to lower drug clearance. Furthermore, the association of drug concentrations with outcomes typically is less clear for maintenance than induction therapy. This finding is probably because a significant portion of patients withdraws during the course of the study. This dropout eventually decreases the power to detect differences in drug concentrations of patients achieving the investigated outcome at latter time points. Another limitation is that the great majority of these studies investigate infliximab and adalimumab. Consequently, drug concentrations threshold to target for other biologics are not clearly defined. This is important as these studies often provide a starting point to define values when designing prospective studies of TDM.
What is already known regarding the role of TDM of biologics in other IMID
Evidence is growing regarding the role of TDM in other IMID. Similar to IBD, numerous exposure-outcome relationship studies demonstrate that higher biologic drug concentrations are associated with higher rates of favorable clinical outcomes, especially in RA and psoriasis (Table 2). 54-91 Studies have shown that TDM can help to identify possible causes of poor outcomes such as mechanistic failure or insufficient drug exposure due to PK issues or non-adherence.109-120 TDM has been demonstrated to efficiently guide treatment de-escalation for adalimumab121,122 and etanercept123 in patients with RA. Although, TDM-based therapeutic algorithms have already been described, such as in psoriasis,124 a recent survey showed that most dermatologists still perform dose adaptations empirically.125 Routine use of TDM in clinical practice is not widely applied outside of IBD.126,127 The NOR-DRUM (NORwegian DRUg Monitoring study) RCT included 398 adults with spondyloarthritis (n=117), rheumatoid arthritis (n=80), psoriatic arthritis (n=42), psoriasis (n=22) or IBD (n=137, 80 with UC and 57 with CD) who received their randomized intervention, either proactive TDM based on a predefined algorithm or standard infliximab therapy with treatment adjustments based on clinical assessment. The primary outcome of clinical remission at week 30 was comparable between the two groups (100/198, 50.5% for the standard therapy vs. 106/200, 53% for the TDM group; p=0.78).128 However, it is difficult to draw firm conclusions for IBD or other IMID as the trial did not have the statistical power to test hypotheses within each disease subgroup. Moreover, the therapeutic range for defining adequate infliximab concentrations for the TDM group was rather low based on recent data.1,101,129
Unmet needs regarding the role of TDM of biologics in IBD
There are several unmet needs regarding the role of TDM of biologics in IBD that are described in detail below.
Optimal drug concentrations to target
One of the most important unmet need is the identification of the optimal drug concentrations to target as these can be therapeutic outcome-, assay-, time- and IBD phenotype-dependent.129 Exposure-outcome relationship studies show that typically higher drug concentrations are needed to achieve more stringent outcomes such as endoscopic and histologic remission or fistula healing.101 Moreover, recent data show that there may be quantitative and qualitative discrepancies among assays concerning both drug concentrations and ADA titers.130-133 Regarding IBD phenotypes, preliminary data suggest that patients with perianal fistulising CD may require higher infliximab concentrations to achieve fistula closure.134,135
Proper interpretation of anti-drug antibody titers
Another difficulty is the correct interpretation of ADA titers across different assays, such as the commonly used enzyme-linked immunosorbent assay (ELISA), the homogeneous mobility shift assay (HMSA) and the electrochemiluminescence immunoassay (ECLIA). ADA titers are often expressed in arbitrary units and cannot be compared directly between different assays.133,136 This is important as physicians may inadequately stop a biologic due to hypothetical high titer ADAs. For example, Imbrechts et al.133 showed that a cut-off of 8 μg/ml measured with the first generation ELISA had a similar impact as the cut-off of 374 ng/ml with the second generation ELISA and a cut-off of 119 ng/ml in the ready-to-use ELISA kit. This is quite significant as different ADA titer thresholds may be associated with diverse clinical outcomes and guide management. A recent 3-year study of patients receiving infliximab who developed ADA >8 μg/ml evaluated by a drug-tolerant ELISA showed that ADA cut-off values of 16, 19, 37 and 45 μg/ml were associated with treatment failure, steroid use, development of infusion reactions and switch to another biologic, respectively.137
Other unmet needs regarding the role of TDM of biologics in IBD
A very important point to consider is that the current techniques used to measure drug concentrations and ADA, require significant incubation times and may have long turnaround times. Furthermore, dose-escalation may require a pre-authorization from payers that can add even more time to the process of dose optimization. These factors do not allow physicians to dose adjust promptly at the time of infusion or injection.136 Further investigation is also needed to determine the role of peak48 or intermediate concentrations22,95 as well as total drug exposure138 and the role of drug concentrations measured in tissue139,140 or stool samples.141 Beyond these issues, there is the need for high quality data demonstrating that strategic use of TDM changes clinically meaningful outcomes in IBD. As mentioned above, two of the large RCTs examining the role of proactive TDM-dose adjustment likely missed their primary endpoint due to methodological issues. Alternative trial designs are needed to address this deficit.
Furthermore, the role of TDM in biologics other than anti-TNFs such as vedolizumab and ustekinumab, needs greater clarification, especially as data from exposure-outcome relationship studies are only available and as these drugs exhibit low immunogenicity.142 A post-hoc analysis of the UNITI (A Study to Evaluate the Safety and Efficacy of Ustekinumab Induction Therapy in Subjects With Moderately to Severely Active Crohn’s Disease) RCT identified a ustekinumab concentration of 0.8 μg/ml at week 24 and 1.4 μg/ml at week 40 for clinical remission at weeks 24 and 44, respectively.51 Another prospective study identified a ustekinumab concentration cut-off of 2.3 μg/ml at week 16 and 1.9 μg/ml at week 24 to be associated with endoscopic response at week 24.49 Löwenberg et al. demonstrated that drug concentrations >10 mg/L at week 22 were associated with endoscopic remission at week 26 in patients with IBD.47 A recent multi-center observational study assessed the outcome of vedolizumab dose-increase and pre-escalation drug concentrations. It demonstrated that pre-intensification vedolizumab trough concentrations were comparable or higher among patients who subsequently attained post-optimization clinical, biomarker and endoscopic remission, compared with non-remitting patients. This was true during induction and maintenance therapy. Moreover, the same study demonstrated that integrin-receptors on M1- and M2-macrophages were saturated by low concentrations of vedolizumab. Based on these data PK issues may not be the main mechanism for loss of response to vedolizumab and higher pre-escalation drug concentrations may indicate lower clearance due to a less severe disease and a higher likelihood of subsequent re-gaining of response regardless of therapy escalation.143 It is fortunate that the pharmaceutical industry has realized the importance of incorporating TDM into more recent drug trials. Some ongoing registration trials currently incorporate TDM arms, such as risankizumab for UC (https://www.clinicaltrials.gov/ct2/show/NCT03398135). If shown to be effective, this should allow for early adoption of TDM into clinical practice for these novel medications.
Future perspectives regarding the role of TDM of biologics in IBD
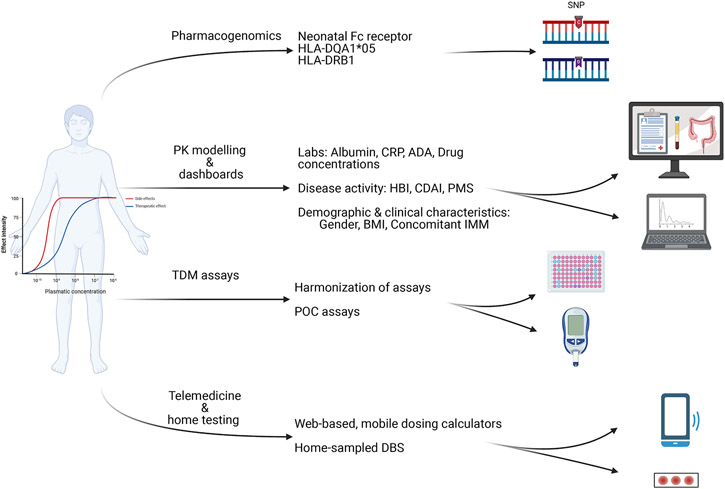
Potential investigations, addressing some of the unmet needs described above, should examine the harmonization of assays, the use of rapid point-of-care assays, the incorporation of PK modelling dashboards and pharmacogenomics as well as the application of telemedicine and home TDM testing (Figure 1).
Figure 1: Future perspectives of therapeutic drug monitoring of biologics in IBD.
PK: pharmacokinetic; TDM: therapeutic drug monitoring; HLA: human leukocyte antigen; SNP: Single nucleotide polymorphisms; CRP: C-reactive protein; ADA: anti-drug antibodies; HBI: Harvey-Bradshaw index; CDAI: clinical disease activity index; PMS: partial Mayo score; BMI: body mass index; IMM: immunomodulator; POC: point of care; DBS: dried blood spots.
Harmonization of TDM assays
Many academic groups and diagnostics companies have developed assays for the quantification of biologic drug concentration. Although drug concentrations correlate well between different assays, their agreement may not always be good; if possible, it may be best to use the same assay over time for individual patient follow-up.130-132,136 Efforts are ongoing to standardize assays for measuring drug concentrations by producing reference standards. Implementation of universal calibrators for quantifying ADA has also been proposed to facilitate inter-laboratory harmonization of ADA measurements.144,145 Since assays for determining ADA are still not harmonized worldwide, it is almost impossible to set ADA cut-off levels that would preclude successful dose escalation and overcoming of ADA. Consequently, generation of robust immunogenicity data for inclusion in product labels and TDM-based therapeutic algorithms to support clinical practice continues to be a challenge.
Point-of-care assays
Point-of-care assays are medical diagnostic tests applied at the time and place of patient care. When referring to TDM of biologics, this could be done either in the infusion unit, the clinic or even at home. Such assays allow clinicians to get results within minutes and therefore more timely adjust drug dosage, although the timely availability of the assay result may still not be able to be immediately implemented due to restrictions by payers and insurers on increased drug dosing.146 Point-of-care assays have been clinically validated for quantifying both infliximab and adalimumab in serum. 32,146 However, as the number of such tests increases, it is important that their quality is carefully assessed and their accuracy is compared with the commonly used laboratory measurements of drug concentrations and ADA as there may be discrepancies.147-149
Pharmacokinetic modelling and dashboards
Performing TDM is a smart way to evaluate variability in drug PK between patients and within a patient over time. Population PK modelling has been used to create algorithms which also include a variety of parameters that may affect drug concentrations. For biologics, common factors that have been shown to impact drug clearance include gender, body weight, serum albumin, inflammatory burden, immunogenicity, concomitant immunomodulation and polymorphisms in the neonatal Fc receptor.150,151 By applying population PK models, one can utilize previous and concurrent drug concentration measurements to predict the timing and/or magnitude of future doses required to reach a pre-specified drug concentration. Such methods are referred to as ‘dashboards’. Dashboards integrate individual clinical and PK data to generate dosing recommendations to achieve pre-specified target trough concentrations using adaptive Bayesian forecasting towards precision medicine.152-154 Clinical intuition alone does not accurately predict the need for dose escalation highlighting the importance of a more robust method of selecting the right dose at the right time for the individual patient.155 As detailed above the clinical efficacy of a PK-dashboard was recently demonstrated by the PRECISION RCT showing that PK-dashboard-driven infliximab dosing was superior than standard dosing in patients with IBD in terms of clinical remission.99 However, as an absolute value may be less informative at an individual level, TDM could be combined with pharmacodynamic measures, such as clinical disease activity, biomarkers and/or imaging for better guiding treatment optimization.
There is also the potential for a population PK model to be used prior to initiation of therapy, to calculate drug clearance at baseline in an individual. Drug clearance has been shown to correlate with endoscopic remission in patients with moderately to severely active UC starting infliximab. More specifically there was a linear relationship between baseline infliximab clearance and Mayo endoscopic scores (MES) at week 8 and a threshold of <0.397 L/d was associated with week 8 MES ≤1.156 Furthermore, it was found that in patients with acute severe UC, higher values of baseline infliximab clearance were associated with higher rates of treatment failure and colectomy.157,158 Using the data from the ACT (Active Ulcerative Colitis Trials)-1/2 phase 3 clinical trials of infliximab in patients with UC, a decision support tool was developed and validated to calculate at baseline the likelihood that a subject would achieve endoscopic remission at week 8 or week 30 following initiation of infliximab.159 Such a model could now be tested to stratify patients and identify those patients who are at risk of accelerated drug clearance and who may benefit from proactive TDM and optimized dosing. Model-informed precision dosing guided by real-world PK may also be available at the bedside in real-time as these decision-support PK dashboards can be embedded within the electronic health record allowing individual personalised TDM.160
Pharmacogenomics
Preliminary data suggest that patients with IBD carrying specific gene alleles are at high risk of low infliximab or adalimumab concentrations or developing immunogenicity to either of these drugs.161-166 A genome-wide analysis of the PANTS prospective study identified the HLA-DQA1*05 allele to increase the risk of immunogenicity to both infliximab and adalimumab in patients with CD.161 Another study showed that the same allele was associated with a high risk of antibodies to infliximab in addition to loss of response and infliximab discontinuation in patients with IBD.162 These high-risk patients could be treated with combination therapy with either thiopurines or methotrexate to help prevent immunogenicity and/or to undergo proactive TDM starting early during induction therapy.6,167 Pharmacogenomics is one more step towards personalized medicine and identifying patients that would benefit more from proactive TDM and combination therapy.
Telemedicine and home testing related to TDM
Even before COVID-19, there was growing evidence to support virtual healthcare and a clear desire from patients.168 However, the COVID-19 pandemic acted as a key driver for widespread adoption of virtual health and telemedicine.169 Additionally, electronic health smartphone applications are an adjunct to virtual healthcare, but in many cases have become synonymous with the delivery of telemedicine.170 Virtual healthcare and use of electronic health applications have been associated with reduced outpatient visits171,172 and hospital admissions172 as well as reduced costs to healthcare providers173 and patients.174 New easier sampling methods, such as home-sampled dried blood spots, may allow for “home TDM.”175,176 A prospective observational cohort study showed that the use of a web-based, mobile infliximab dosing calculator for therapy optimization is feasible and potentially effective, facilitating both standardization and individualization of therapy in clinical care.177
Conclusion
Reactive TDM is emerging as the new standard of care for optimizing biologic therapy in IBD. There is still a debate regarding the role of proactive TDM in clinical practice and there are still limited data from well-designed prospective studies and RCTs for both IBD and other IMID. Several areas of TDM that still need to be defined include the potential use of point-of-care assays, PK dashboard models and pharmacogenetics. Prospective studies of proactive TDM starting from the induction phase which is characterised by increased disease activity and consequently higher drug clearance are needed to better define its role in the management of IBD and other IMID.
Acknowledgment:
We would like to thank Nasim Sadat Seyed Tabib for her excellent contribution to drafting Figure 1.
Funding:
K.P. was supported by the Ruth L. Kirschstein NRSA Institutional Research Training Grant (T32 DK007760) from NIH. N.V.C. was supported by a Research Scholar Award from the American Gastroenterological Association (AGA). N.V.C. and W.J.S. are supported in part by the NIDDK-funded San Diego Digestive Diseases Research Center (P30 DK120515). M. F. and S. V. are senior clinical investigators of the Research Foundation Flanders (FWO).
Footnotes
Conflict of interests: K.P. received a lecture fee from Mitsubishi Tanabe Pharma. N.V.C. received research grants and personal fees from R-Biopharm, Takeda and UCB; and personal fees from Alimentiv, Inc. (formerly Robarts Clinical Trials, Inc.), Celltrion and Prometheus. A.S.C: received consultancy fees from AbbVie, Janssen, Takeda, Bacainn, Arena pharmaceuticals, Grifols, Prometheus, Samsung, Bristol Myers Squibb, and Pfizer, and research support from Inform Diagnostic. S.V. received grants from AbbVie, J&J, Pfizer, and Takeda; and has received consulting and/or speaking fees from AbbVie, Arena Pharmaceuticals, Avaxia, Boehringer Ingelheim, Celgene, Dr Falk Pharma, Ferring, Galapagos, Genentech-Roche, Gilead, Hospira, Janssen, Mundipharma, MSD, Pfizer, Prodigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Takeda, Theravance, and Tillots Pharma AG. N.A. received grant from Pfizer and lecture fees from Janssen, Pfizer and Takeda. T.K. reports personal fees from Alfresa Pharma, Covidien, Eli Lilly, Ferring Pharmaceuticals, Janssen, Kyorin Pharmaceutical, Mochida Pharmaceutical, Nippon Kyaku, Pfizer, Takeda Pharmaceutical, Thermo Scientific, Abbvie GK, Astellas, Celltrion, EA Pharma, Nippon Kyaku, Mochida Pharmaceutical, Mitsubishi Tanabe Pharma, ZERIA, Gilead Sciences, Janssen, JIMRO, grants from Abbvie GK, EA Pharma, Otsuka Holdings, ZERIA, Kyorin Pharmaceutical, Mochida Pharmaceutical, Thermo Fisher Scientific, Alfresa Pharma, Nippon Kyaku. W. J. S. reports: research grants from Abbvie, Abivax, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Genentech, Gilead Sciences, Glaxo Smith Kline, Janssen, Lilly, Pfizer, Prometheus Biosciences, Seres Therapeutics, Shire, Takeda, Theravance Biopharma; consulting fees from Abbvie, Abivax, Admirx, Alfasigma, Alimentiv (previously Robarts Clinical Trials, owned by Alimentiv Health Trust), Alivio Therapeutics, Allakos, Amgen, Applied Molecular Transport, Arena Pharmaceuticals, Bausch Health (Salix), Beigene, Bellatrix Pharmaceuticals, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Meyers Squibb, Celgene, Celltrion, Cellularity, Cosmo Pharmaceuticals, Escalier Biosciences, Equillium, Forbion, Genentech/Roche, Gilead Sciences, Glenmark Pharmaceuticals, Gossamer Bio, Immunic (Vital Therapies), Index Pharmaceuticals, Intact Therapeutics, Janssen, Kyverna Therapeutics, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pandion Therapeutics, Pfizer, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonists Therapeutics, Provention Bio, Reistone Biopharma, Seres Therapeutics, Shanghai Pharma Biotherapeutics, Shire, Shoreline Biosciences, Sublimity Therapeutics, Surrozen, Takeda, Theravance Biopharma, Thetis Pharmaceuticals, Tillotts Pharma, UCB, Vendata Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals, Vivreon Biosciences, Zealand Pharma; and stock or stock options from Allakos, BeiGene, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences, Prometheus Laboratories Progenity, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivreon Biosciences. Spouse: Iveric Bio - consultant, stock options; Progenity - stock; Oppilan Pharma - consultant, stock options; Prometheus Biosciences - employee, stock, stock options; Prometheus Laboratories – stock, stock options, consultant; Ventyx Biosciences – stock, stock options; Vimalan Biosciences – stock, stock options. P.M.I. reports lecture fees from AbbVie, BMS, Celgene, Falk Pharma, Ferring, Gilead, MSD, Janssen, Pfizer, Takeda, Tillotts, Sapphire Medical, Sandoz, Shire, Warner Chilcott, financial support for research from MSD, Pfizer, Takeda and advisory fees from AbbVie, Arena, Genentech, Gilead, Hospira, Janssen, Lilly, MSD, Pfizer, Pharmacosmos, Procise, Prometheus, Roche, Sandoz, Samsung Bioepis, Takeda, Topivert, VH2, Vifor Pharma, Warner Chilcott. M.F. reports financial support for research from Abbvie, Amgen, Biogen, Janssen, Pfizer, and Takeda, consultancy fees from Abbvie, Boehringer-Ingelheim, Celltrion, Janssen, Lilly, Medtronic, MSD, Pfizer, Sandoz, Takeda and Thermo Fisher and speakers fees from Abbvie, Amgen, Biogen, Boehringer-Ingelheim, Falk, Ferring, Janssen, Lamepro, MSD, Mylan, Pfizer, Sandoz, Takeda and Truvion Healthcare. P.G.K. received consultancy and speaking fees from Abvie, Janssen, Pfizer, Takeda, Ferring, Novartis and scientific grants from Takeda and Pfizer. D. D. has served as a speaker, a consultant and an advisory board member for MSD, Abbvie, Takeda, Pfizer, Janssen, Amgen, Biogen and Krka. J.L received unrestricted grants from AbbVie, Almirall, Celgene, Eli Lilly, Janssen-Cilag, LEO Pharma, Novartis, UCB, speaker fees for AbbVie, Almirall, BMS, Janssen-Cilag, Pfizer, UCB and consultant fees for AbbVie, BMS, Celgene, Eli Lilly, Janssen-Cilag, LEO Pharma, Novartis and UCB. N.K. has received speaker fees from Janssen. W.A. received consultancy fees from Abbvie, Amgen, Arena Pharmaceuticals, Dynacare, Janssen, Merck, Novartis, Pfizer, Sandoz, Takeda. A.J.Y. has received Consultant/Advisory board fees from Prometheus Labs, Takeda, Arena pharmaceuticals and Bristol Myers Squibb. S.D. has served as a speaker, consultant and advisory board member for Schering- Plough, AbbVie, MSD, UCB Pharma, Ferring, Cellerix, Millenium Takeda, Nycomed, Pharmacosmos, Actelion, Alphawasserman, Genentech, Grunenthal, Pfizer, Astra Zeneca, Novo Nordisk, Cosmo Pharmaceuticals, Vifor and Johnson & Johnson, Nikkiso Europe GMBH, Theravance. M.C.D. received consultant fees from Janssen, Abbvie, Pfizer, Takeda, UCB, Celgene, BMS, Prometheus biosciences, Arena, Lilly and grant support from Abbvie, Pfizer, Janssen. L.P-B: grant support from AbbVie, MSD, Takeda, consulting fees from AbbVie, Janssen, Genentech, Ferring, Tillots, Pharmacosmos, Celltrion, Takeda, Boerhinger Ingelheim, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Alma, Sterna, Nestle, Enterome, Allergan, MSD, Roche, Arena, Gilead, Hikma, Amgen, BMS, Vifor, Norgine; Mylan, Lilly, Fresenius, Oppilan Pharma, Sublimity Therapeutics, Applied Molecular Transport, OSE Immunotherapeutics, Enthera, and stock or stock options from CTMA. The remaining authors have nothing to disclose.
References
- 1.Papamichael K, Vogelzang EH, Lambert J, Wolbink G, Cheifetz AS. Therapeutic drug monitoring with biologic agents in immune mediated inflammatory diseases. Expert Rev Clin Immunol 2019;15:837–48. [DOI] [PubMed] [Google Scholar]
- 2.Sparrow MP, Papamichael K, Ward MG, et al. Therapeutic drug monitoring of biologics during induction to prevent primary non-response. J Crohns Colitis 2020;14:542–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fine S, Papamichael K, Cheifetz AS. Etiology and management of lack or loss of response to anti-tumor necrosis factor therapy in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2019;15:656–65. [PMC free article] [PubMed] [Google Scholar]
- 4.McNeill RP, Barclay ML Cost-effectiveness of therapeutic drug monitoring in inflammatory bowel disease. Curr Opin Pharmacol 2020;55:41–6. [DOI] [PubMed] [Google Scholar]
- 5.Syed N, Tolaymat M, Brown SA, Sivasailam B, Cross RK. Proactive drug monitoring is associated with higher persistence to infliximab and adalimumab treatment and lower healthcare utilization compared with reactive and clinical monitoring. Crohns Colitis 360 2020;2:otaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017;15:1580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaughn BP, Martinez-Vazquez M, Patwardhan VR, Moss AC, Sandborn WJ, Cheifetz AS. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis 2014;20:1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyles JL, Mulgund AA, Bauman LE, et al. Effect of a practice-wide anti-TNF proactive therapeutic drug monitoring program on outcomes in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2021;27:482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9. [DOI] [PubMed] [Google Scholar]
- 10.Assa A, Matar M, Turner D, et al. Proactive monitoring of adalimumab trough concentration associated with increased clinical remission in children with Crohn's disease compared with reactive monitoring. Gastroenterology 2019;157:985–96. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Hernández JG, Rebollo N, Martin-Suarez A, Calvo MV, Muñoz F. A 3-year prospective study of a multidisciplinary early proactive therapeutic drug monitoring programme of infliximab treatments in inflammatory bowel disease. Br J Clin Pharmacol 2020;86:1165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papamichael K, Juncadella A, Wong D, et al. Proactive therapeutic drug monitoring of adalimumab is associated with better long-term outcomes compared to standard of care in patients with inflammatory bowel disease. J Crohns Colitis 2020;14:976–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papamichael K, Vajravelu RK, Vaughn BP, MT, Cheifetz AS. Proactive infliximab monitoring following reactive testing is associated with better clinical outcomes than reactive testing alone in patients with inflammatory bowel disease. J Crohns Colitis 2018;12:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreesen E, Baert F, Laharie D, et al. , Monitoring a combination of calprotectin and infliximab identifies patients with mucosal healing of Crohn's disease. Clin Gastroenterol Hepatol 2020;18:637–46. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi T, Suzuki Y, Motoya S, et al. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis-results from a multicenter prospective randomized controlled trial and its post hoc analysis. J Gastroenterol 2016;51:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vande Casteele N, Jeyarajah J, Jairath V, Feagan BG, Sandborn WJ. Infliximab exposure-response relationship and thresholds associated with endoscopic healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2019;17:1814–21. [DOI] [PubMed] [Google Scholar]
- 17.Clarkston K, Tsai YT, Jackson K, Rosen MJ, Denson LA, Minar P. Development of infliximab target concentrations during induction in pediatric Crohn disease patients. J Pediatr Gastroenterol Nutr 2019;69:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonczi L, Gecse KB, Vegh Z, et al. Long-term efficacy, safety, and immunogenicity of biosimilar infliximab after one year in a prospective nationwide cohort. Inflamm Bowel Dis 2017;23:1908–15. [DOI] [PubMed] [Google Scholar]
- 19.Buhl S, Dorn-Rasmussen M, Brynskov J, et al. Therapeutic thresholds and mechanisms for primary non-response to infliximab in inflammatory bowel disease. Scand J Gastroenterol 2020;55:884–890. [DOI] [PubMed] [Google Scholar]
- 20.Brandse JF, Mathot RA, van der Kleij D, et al. Pharmacokinetic features and presence of anti-drug antibodies associate with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol 2016;14:251–8. [DOI] [PubMed] [Google Scholar]
- 21.Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014;147:1296–1307. [DOI] [PubMed] [Google Scholar]
- 22.Stein R, Lee D, Leonard MB, et al. Serum infliximab, antidrug antibodies, and tumor necrosis factor predict sustained response in pediatric Crohn's disease. Inflamm Bowel Dis 2016;22:1370–7. [DOI] [PubMed] [Google Scholar]
- 23.Bossuyt P, Dreesen E, Rimola J, et al. Infliximab exposure associates with radiologic evidence of healing in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2021;19:947–54. [DOI] [PubMed] [Google Scholar]
- 24.Papamichael K, Vande Casteele N, Jeyarajah J, Jairath V, Osterman MT, Cheifetz AS. Higher postinduction infliximab concentrations are associated with improved clinical outcomes in fistulizing Crohn’s disease: An ACCENT-II post-hoc analysis. Am J Gastroenterol 2021;116:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut 2014;63:1721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tighe D, Smith S, O'Connor A, Breslin N, Ryan B, McNamara D. Positive relationship between infliximab and adalimumab trough levels at completion of induction therapy with clinical response rates, at a tertiary referral center. JGH Open 2017;1:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farkas K, Rutka M, Golovics PA, et al. Efficacy of infliximab biosimilar CT-P13 induction therapy on mucosal healing in ulcerative colitis. J Crohns Colitis 2016;10:1273–8. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol 2019;4:341–53. [DOI] [PubMed] [Google Scholar]
- 29.Colman RJ, Tsai Y-T, Jackson K, et al. Achieving target infliximab drug concentrations improves blood and fecal neutrophil biomarkers in Crohn’s disease. Inflamm Bowel Dis 2020. Sep 18;izaa241. doi: 10.1093/ibd/izaa241. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reinisch W, Colombel JF, Sandborn WJ, et al. Factors associated with short- and long-term outcomes of therapy for Crohn's disease. Clin Gastroenterol Hepatol 2015;13:539–47. [DOI] [PubMed] [Google Scholar]
- 31.Ungar B, Engel T, Yablecovitch D, et al. Prospective observational evaluation of time-dependency of adalimumab immunogenicity and drug concentrations: The Poetic Study. Am J Gastroenterol 2018;113:890–8. [DOI] [PubMed] [Google Scholar]
- 32.Verstockt B, Moors G, Bian S, et al. Influence of early adalimumab serum levels on immunogenicity and long-term outcome of anti-TNF naive Crohn's disease patients: the usefulness of rapid testing. Aliment Pharmacol Ther 2018;48:731–9. [DOI] [PubMed] [Google Scholar]
- 33.Rinawi F, Ricciuto A, Church PC, et al. Association of early postinduction adalimumab exposure with subsequent clinical and biomarker remission in children with Crohn's disease. Inflamm Bowel Dis. 2020. Sep 26;izaa247. doi: 10.1093/ibd/izaa247. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Vande Casteele N, Baert F, Bian S, et al. Subcutaneous absorption contributes to observed interindividual variability in adalimumab serum concentrations in Crohn’s disease: A prospective multicentre study. J Crohn’s Colitis 2019;13:1248–56. [DOI] [PubMed] [Google Scholar]
- 35.Choi SY, Choi YO, Choe YH, et al. Potential utility of therapeutic drug monitoring of adalimumab in predicting short-term mucosal healing and histologic remission in pediatric Crohn's disease patients. J Korean Med Sci 2020;35:e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakase H, Motoya S, Matsumoto T, et al. Significance of measurement of serum trough level and anti-drug antibody of adalimumab as personalised pharmacokinetics in patients with Crohn's disease: a subanalysis of the DIAMOND trial. Aliment Pharmacol Ther 2017;46:873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vande Casteele N, Feagan BG, Vermeire S, et al. Exposure–response relationship of certolizumab pegol induction and maintenance therapy in patients with Crohn’s disease. Aliment Pharmacol Ther 2018;47:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colombel JF, Sandborn WJ, Allez M, et al. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:423–31. [DOI] [PubMed] [Google Scholar]
- 39.Adedokun OJ, Xu Z, Marano CW, et al. Pharmacokinetics and exposure-response relationship of golimumab in patients with moderately-to-severely active ulcerative colitis: results from phase 2/3 PURSUIT induction and maintenance studies. J Crohns Colitis 2017;11:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stefanovic S, Detrez I, Compernolle G, et al. Endoscopic remission can be predicted by golimumab concentrations in patients with ulcerative colitis treated with the changed label. Eur J Gastroenterol Hepatol 2021;33:54–61. [DOI] [PubMed] [Google Scholar]
- 41.Samaan MA, Cunningham G, Tamilarasan AG, et al. Therapeutic thresholds for golimumab serum concentrations during induction and maintenance therapy in ulcerative colitis: results from the GO-LEVEL study. Aliment Pharmacol Ther 2020;52:292–302. [DOI] [PubMed] [Google Scholar]
- 42.Yarur AJ, Bruss A, Naik S. et al. Vedolizumab concentrations are associated with long-term endoscopic remission in patients with inflammatory bowel diseases. Dig Dis Sci 2019;64:1651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osterman MT, Rosario M, Lasch K, et al. Vedolizumab exposure levels and clinical outcomes in ulcerative colitis: determining the potential for dose optimisation. Aliment Pharmacol Ther 2019;49:408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yacoub W, Williet N, Pouillon L, et al. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther 2018;47:906–12. [DOI] [PubMed] [Google Scholar]
- 45.Hanzel J, Sever N, Ferkolj I, et al. Early vedolizumab trough levels predict combined endoscopic and clinical remission in inflammatory bowel disease. United Eur Gastroenterol J 2019;7:741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guidi L, Pugliese D, Tonucci TP, et al. Early vedolizumab trough levels predict treatment persistence over the first year in inflammatory bowel disease. United European Gastroenterol J 2019;7:1189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Löwenberg M, Vermeire S, Mostafavi N, et al. Vedolizumab induces endoscopic and histologic remission in patients with Crohn's disease. Gastroenterology 2019;157:997–1006. [DOI] [PubMed] [Google Scholar]
- 48.Hanzel J, Zdovc J, Kurent T, et al. Peak concentrations of ustekinumab after intravenous induction therapy identify patients with Crohn's disease likely to achieve endoscopic and biochemical remission. Clin Gastroenterol Hepatol 2021;19:111–8. [DOI] [PubMed] [Google Scholar]
- 49.Verstockt B, Dreesen E, Noman M, et al. Ustekinumab exposure-outcome analysis in Crohn's disease only in part explains limited endoscopic remission rates. J Crohns Colitis 2019;13:864–72. [DOI] [PubMed] [Google Scholar]
- 50.Soufflet N, Boschetti G, Roblin X, et al. Concentrations of ustekinumab during induction therapy associate with remission in patients with Crohn's disease. Clin Gastroenterol Hepatol 2019;17:2610–2 [DOI] [PubMed] [Google Scholar]
- 51.Adedokun OJ, Xu Z, Gasink C, et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn’s disease. Gastroenterology 2018;154:1660–71. [DOI] [PubMed] [Google Scholar]
- 52.Adedokun OJ, Xu Z, Marano C, et al. Ustekinumab pharmacokinetics and exposure response in a phase 3 randomized trial of patients with ulcerative colitis: ustekinumab PK and exposure-response in UC. Clin Gastroenterol Hepatol 2020;18:2244–55. [DOI] [PubMed] [Google Scholar]
- 53.Painchart C, Brabant S, Duveau N, et al. Ustekinumab serum trough levels may identify suboptimal responders to ustekinumab in Crohn's disease. Dig Dis Sci 2020;65:1445–52. [DOI] [PubMed] [Google Scholar]
- 54.Van den Bemt BJ, Den Broeder AA, Wolbink GJ, et al. The combined use of disease activity and infliximab serum trough concentrations for early prediction of (non-)response to infliximab in rheumatoid arthritis. Br J Clin Pharmacol 2013;76:939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teresa J, Chamaida PR, Ana MF, et al. Predictive value of serum infliximab levels at induction phase in rheumatoid arthritis patients. Open Rheumatol J 2017;11:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mulleman D, Chu Miow Lin D, Ducourau E, et al. Trough infliximab concentrations predict efficacy and sustained control of disease activity in rheumatoid arthritis. Ther Drug Monit 2010;32:232–6. [DOI] [PubMed] [Google Scholar]
- 57.St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2002;46:1451–9. [DOI] [PubMed] [Google Scholar]
- 58.Wolbink GJ, Voskuyl AE, Lems WF, et al. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis 2005;64:704–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takahashi H, Tsuji H, Ishida-Yamamoto A, Iizuka H. Plasma trough levels of adalimumab and infliximab in terms of clinical efficacy during the treatment of psoriasis. J Dermatol 2013;40:39–42. [DOI] [PubMed] [Google Scholar]
- 60.Colls-Gonzalez M, Notario-Rosa J, Bas-Minguet J. et al. Association between infliximab concentrations and clinical response in psoriasis: a prospective cohort study. J Dermatolog Treat 2019;20:1–8. [DOI] [PubMed] [Google Scholar]
- 61.Patil A, Upadhyaya S, Dawar R, et al. Anti-drug antibodies and low serum trough infliximab levels correlate with disease activity measures in spondyloarthritis patients on an as-needed infliximab treatment. Int J Rheum Dis 2019;22:1638–43. [DOI] [PubMed] [Google Scholar]
- 62.Krzysiek R, Breban M, Ravaud P, et al. Circulating concentration of infliximab and response to treatment in ankylosing spondylitis: results from a randomized control study. Arthritis Rheum 2009;61:569–76. [DOI] [PubMed] [Google Scholar]
- 63.Chen D-Y, Chen Y-M, Tsai W-C, et al. Significant associations of antidrug antibody levels with serum drug trough levels and therapeutic response of adalimumab and etanercept treatment in rheumatoid arthritis. Ann Rheum Dis 2015;74:e16. [DOI] [PubMed] [Google Scholar]
- 64.Chen D-Y, Chen Y-M, Hsieh T-Y, et al. Drug trough levels predict therapeutic responses to dose reduction of adalimumab for rheumatoid arthritis patients during 24 weeks of follow-up. Rheumatology (Oxford) 2016;55:143–8. [DOI] [PubMed] [Google Scholar]
- 65.Pouw MF, Krieckaert CL, Nurmohamed MT, et al. Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann Rheum Dis 2015;74:513–8. [DOI] [PubMed] [Google Scholar]
- 66.Jani M, Chinoy H, Warren RB, et al. Clinical utility of random anti-tumor necrosis factor drug-level testing and measurement of antidrug antibodies on the long-term treatment response in rheumatoid arthritis. Arthritis Rheumatol 2015;678:2011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Senabre Gallego JM, Rosas J, Marco-Mingot M, et al. Clinical relevance of monitoring serum adalimumab levels in axial spondyloarthritis. Rheumatol Int 2019;39:841–9. [DOI] [PubMed] [Google Scholar]
- 68.Ding X, Zhu R, Wu J, Xue L, Gu M, Miao L. Early adalimumab and anti-adalimumab antibody levels for prediction of primary nonresponse in ankylosing spondylitis patients. Clin Transl Sci 2020;13:547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kneepkens EL, Wei JC, Nurmohamed MT, et al. Immunogenicity, adalimumab levels and clinical response in ankylosing spondylitis patients during 24 weeks of follow-up. Ann Rheum Dis 2015;74:396–401. [DOI] [PubMed] [Google Scholar]
- 70.Wilkinson N, Tsakok T, Dand N, et al. Defining the therapeutic range for adalimumab and predicting response in psoriasis: a multicenter prospective observational cohort study. J Invest Dermatol 2019;139:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Menting SP, Coussens E, Pouw MF, et al. Developing a therapeutic range of adalimumab serum concentrations in management of psoriasis: a step toward personalized treatment. JAMA Dermatol 2015;151:616–22. [DOI] [PubMed] [Google Scholar]
- 72.Mahil SK, Arkir Z, Richards G, Lewis CM, Barker JN, Smith CH. Predicting treatment response in psoriasis using serum levels of adalimumab and etanercept: a single-centre, cohort study. Br J Dermatol 2013;169:306–13. [DOI] [PubMed] [Google Scholar]
- 73.Carrascosa JM, Toro Montecinos M, Ballescá F, Teniente Serra A, Martínez Cáceres E, Ferrándiz C. Correlation between trough serum levels of adalimumab and absolute PASI score in a series of patients with psoriasis. J Dermatolog Treat 2018;29:140–4. [DOI] [PubMed] [Google Scholar]
- 74.Vogelzang EH, Kneepkens EL, Nurmohamed MT, et al. Anti-adalimumab antibodies and adalimumab concentrations in psoriatic arthritis; an association with disease activity at 28 and 52 weeks of follow-up. Ann Rheum Dis 2014;73:2178–82. [DOI] [PubMed] [Google Scholar]
- 75.van Kuijk AWR, de Groot M, Stapel SO, Dijkmans BAC, Wolbink GJ, Tak PP. Relationship between the clinical response to adalimumab treatment and serum levels of adalimumab and anti-adalimumab antibodies in patients with psoriatic arthritis. Ann Rheum Dis 2010;69:624–5. [DOI] [PubMed] [Google Scholar]
- 76.Paramarta JE, Baeten DL. Adalimumab serum levels and antidrug antibodies towards adalimumab in peripheral spondyloarthritis: no association with clinical response to treatment or with disease relapse upon treatment discontinuation. Arthritis Res Ther 2014;16:R160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jani M, Isaacs JD, Morgan AW, et al. High frequency of antidrug antibodies and association of random drug levels with efficacy in certolizumab pegol-treated patients with rheumatoid arthritis: results from the BRAGGSS cohort. Ann Rheum Dis 2017;76:208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gehin JE, Goll GL, Warren DJ, et al. Associations between certolizumab pegol serum levels, anti-drug antibodies and treatment response in patients with inflammatory joint diseases: data from the NOR-DMARD study. Arthritis Res Ther 2019;21:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kneepkens EL, Plasencia C, Krieckaert CL, et al. Golimumab trough levels, antidrug antibodies and clinical response in patients with rheumatoid arthritis treated in daily clinical practice. Ann Rheum Dis 2014;73:2217–9. [DOI] [PubMed] [Google Scholar]
- 80.Van Den Berghe N, De Keyser E, Soenen R, et al. Clinical response correlates with 4-week post injection ustekinumab concentrations in moderate-to-severe psoriasis patients. Br J Dermatol 2020;182:390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toro-Montecinos M, Ballesca F, Ferrandiz C, Teniente-Serra A, Martinez-Caceres E, Carrascosa JM. Usefulness and correlation with clinical response of serum ustekinumab levels measured at 6 weeks versus 12 weeks. J Dermatolog Treat 2019;30:35–9. [DOI] [PubMed] [Google Scholar]
- 82.Tsakok T, Wilson N, Dand N, et al. Association of serum ustekinumab levels with clinical response in psoriasis. JAMA Dermatol 2019;155:1235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jamnitski A, Krieckaert CL, Nurmohamed MT, et al. Patients non-responding to etanercept obtain lower etanercept concentrations compared with responding patients. Ann Rheum Dis 2012;71:88–91. [DOI] [PubMed] [Google Scholar]
- 84.Kneepkens EL, Krieckaert CLM, van der Kleij D, et al. Lower etanercept levels are associated with high disease activity in ankylosing spondylitis patients at 24 weeks of follow-up. Ann Reum Dis 2015;74:1825–9. [DOI] [PubMed] [Google Scholar]
- 85.Elberdin L, Outeda M, Salvador P, et al. Positive correlation between etanercept concentration and the decrease in Psoriasis Area and Severity Index scale value. Int J Clin Pharm 2016;38:1142–8. [DOI] [PubMed] [Google Scholar]
- 86.Detrez I, Van Steen K, Segaert S, Gils A. The association between etanercept serum concentration and psoriasis severity is highly age-dependent. Clin Sci (Lond) 2017;131:1179–89. [DOI] [PubMed] [Google Scholar]
- 87.Soenen R, Meulewaeter E, Grine L, et al. Defining a minimal effective serum trough concentration of secukinumab in psoriasis: a step towards personalized therapy. J Invest Dermatol 2019;139:2232–5. [DOI] [PubMed] [Google Scholar]
- 88.van der Heijde D, Landewé RB, Mease PJ, et al. Brief report: secukinumab provides significant and sustained inhibition of joint structural damage in a phase III study of active psoriatic arthritis. Arthritis Rheumatol 2016;68:1914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reich K, Jackson K, Ball S, et al. Ixekizumab pharmacokinetics, anti-drug antibodies, and efficacy through 60 weeks of treatment of moderate to severe plaque psoriasis. J Invest Dermatol 2018;138:2168–73. [DOI] [PubMed] [Google Scholar]
- 90.Chigutsa E, de Mendizabal NV, Chua L, et al. Exposure-response modeling to characterize the relationship between ixekizumab serum drug concentrations and efficacy responses at week 12 in patients with moderate to severe plaque psoriasis. J Clin Pharmacol 2018;58:1489–1500. [DOI] [PubMed] [Google Scholar]
- 91.Kneepkens EL, van den Oever I, Plasencia CH, et al. Serum tocilizumab trough concentration can be used to monitor systemic IL-6 receptor blockade in patients with rheumatoid arthritis: a prospective observational cohort study. Scand J Rheumatol 2017;46:87–94. [DOI] [PubMed] [Google Scholar]
- 92.Lucidarme C, Petitcollin A, Brochard C, et al. Predictors of relapse following infliximab de-escalation in patients with inflammatory bowel disease: the value of a strategy based on therapeutic drug monitoring. Aliment Pharmacol Ther 2019;49:147–54. [DOI] [PubMed] [Google Scholar]
- 93.Petitcollin A, Brochard C, Siproudhis L, et al. Pharmacokinetic parameters of infliximab influence the rate of relapse after de-escalation in adults with inflammatory bowel diseases. Clin Pharmacol Ther 2019;106:605–15. [DOI] [PubMed] [Google Scholar]
- 94.Aguas Peris M, Bosó V, Navarro B, et al. , Serum adalimumab levels predict successful remission and safe deintensification in inflammatory bowel disease patients in clinical practice. Inflamm Bowel Dis 2017;23:1454–60. [DOI] [PubMed] [Google Scholar]
- 95.Lega S, Phan BL, Rosenthal CJ, et al. Proactively optimized infliximab monotherapy is as effective as combination therapy in IBD. Inflamm Bowel Dis 2019;25:134–41. [DOI] [PubMed] [Google Scholar]
- 96.Drobne D, Kurent T, Golob S, et al. Optimised infliximab monotherapy is as effective as optimised combination therapy, but is associated with higher drug consumption in inflammatory bowel disease. Aliment Pharmacol Ther 2019;49:880–9. [DOI] [PubMed] [Google Scholar]
- 97.Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn's disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27. [DOI] [PubMed] [Google Scholar]
- 98.D'Haens G, Vermeire S, Lambrecht G, et al. Increasing infliximab dose based on symptoms, biomarkers, and serum drug concentrations does not increase clinical, endoscopic, and corticosteroid-free remission in patients with active luminal Crohn's disease. Gastroenterology 2018;154:1343–51. [DOI] [PubMed] [Google Scholar]
- 99.Strik AS, Löwenberg M, Mould DR, et al. Efficacy of dashboard driven dosing of infliximab in inflammatory bowel disease patients; a randomized controlled trial. Scand J Gastroenterol 2021;569:145–54. [DOI] [PubMed] [Google Scholar]
- 100.Wang Z, Dreesen E. Therapeutic drug monitoring of anti-tumor necrosis factor agents: lessons learned and remaining issues. Curr Opin Pharmacol 2020;55:53–9. [DOI] [PubMed] [Google Scholar]
- 101.Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2019;17:1655–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kolho KL. Therapeutic drug monitoring and outcome of infliximab therapy in pediatric onset inflammatory bowel disease. Front Pediatr 2021;8:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Papamichael K, Cheifetz AS. Therapeutic drug monitoring in patients on biologics: lessons from gastroenterology. Curr Opin Rheumatol 2020;32:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fumery M, Seksik P, Auzolle C, et al. Postoperative complications after ileocecal resection in Crohn’s disease: A prospective study from the REMIND Group. Am J Gastroenterol 2017;112:337–45. [DOI] [PubMed] [Google Scholar]
- 105.Regueiro M, Feagan BG, Zou B, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn's disease after ileocolonic resection. Gastroenterology 2016;150:1568–78. [DOI] [PubMed] [Google Scholar]
- 106.Boivineau L, Guillon F, Altweg R. Serum adalimumab concentration after surgery is correlated with postoperative endoscopic recurrence in Crohn’s disease patients: one step before proactive therapeutic drug monitoring. J Crohn’s Colitis 2020;14:1500–1. [DOI] [PubMed] [Google Scholar]
- 107.Wright EK, Kamm MA, De Cruz P, et al. Anti-TNF therapeutic drug monitoring in postoperative Crohn’s disease. J Crohns Colitis 2018;12:653–61. [DOI] [PubMed] [Google Scholar]
- 108.Bodini G, Savarino V, Peyrin-Biroulet L, et al. Low serum trough levels are associated with post-surgical recurrence in Crohn’s disease patients undergoing prophylaxis with adalimumab. Dig Liver Dis 2014;46:1043–6. [DOI] [PubMed] [Google Scholar]
- 109.Plasencia C, Pascual-Salcedo D, Nuño L, et al. Influence of immunogenicity on the efficacy of long term treatment of spondyloarthritis with infliximab. Ann Rheum Dis 2012;71:1955–60. [DOI] [PubMed] [Google Scholar]
- 110.Plasencia C, Pascual-Salcedo D, García-Carazo S, et al. The immunogenicity to the first anti-TNF therapy determines the outcome of switching to a second anti-TNF therapy in spondyloarthritis patients. Arthritis Res Ther 2013;26:R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mazilu D, Opris D, Gainaru C, et al. Monitoring drug and antidrug levels: a rational approach in rheumatoid arthritis patients treated with biologic agents who experience inadequate response while being on a stable biologic treatment. Biomed Res Int 2014;2014:702701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bodio C, Grossi C, Pregnolato F, et al. Personalized medicine in rheumatoid arthritis: How immunogenicity impacts use of TNF inhibitors. Autoimmun Rev 2020;19:102509. [DOI] [PubMed] [Google Scholar]
- 113.Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. Antiinfliximab andantiadalimumab antibodies in relation to response to adalimumab in infliximax switchers and antitumour necrosis factor naive patients: a cohort study. Ann Rheum Dis 2010;69:817–21. [DOI] [PubMed] [Google Scholar]
- 114.Bartelds GM, Krieckaert CL, Nurmohamed MT, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 2011;305:1460–8. [DOI] [PubMed] [Google Scholar]
- 115.Ulijn E, den Broeder N, Wientjes M, et al. Therapeutic drug monitoring of adalimumab in RA: no predictive value of adalimumab serum levels and anti-adalimumab antibodies for prediction of response to the next bDMARD. Ann Rheum Dis 2020;79:867–73. [DOI] [PubMed] [Google Scholar]
- 116.Balsa A, Sanmarti R, Rosas J, et al. Drug immunogenicity in patients with inflammatory arthritis and secondary failure to tumour necrosis factor therapies: the REASON study. Rheumatology (Oxford) 2018;57:688–93. [DOI] [PubMed] [Google Scholar]
- 117.Lambert J, Nast A, Nestle FO, Prinz JC. Practical guidance on immunogenicity to biologic agents used in the treatment of psoriasis: What can be learnt from other diseases? J Dermatol Treat 2015;26:520–7. [DOI] [PubMed] [Google Scholar]
- 118.Vogelzang EH, Hebing RCF, Nurmohamed MT, et al. Adherence to etanercept therapy in rheumatoid arthritis patients during 3 years of follow-up. PLoS One 2018;13:e0205125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Benucci M, Grossi V, Manfredi V, et al. Laboratory monitoring of biological therapies in rheumatology: the role of immunogenicity. Ann Lab Med 2020;40:101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ducourau E, Mulleman D, Paintaud G, et al. Antibodies toward infliximab are associated with low infliximab concentration at treatment initiation and poor infliximab maintenance in rheumatic diseases. Arthritis Res Ther 2011;13:R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.l'Ami MJ, Krieckaert CL, Nurmohamed MT, et al. Successful reduction of overexposure in patients with rheumatoid arthritis with high serum adalimumab concentrations: an open-label, non-inferiority, randomised clinical trial. Ann Rheum Dis 2018;77:484–7. [DOI] [PubMed] [Google Scholar]
- 122.Chen DY, Chen YM, Hsieh TY, et al. Drug trough levels predict therapeutic responses to dose reduction of adalimumab for rheumatoid arthritis patients during 24 weeks of follow-up. Rheumatology (Oxford) 2016;55:143–8. [DOI] [PubMed] [Google Scholar]
- 123.Bouman C, van Herwaarden N, van den Hoogen F, van der Maas A, van den Bemt Bjf, den Broeder AA. Prediction of successful dose reduction or discontinuation of adalimumab, etanercept, or infliximab in rheumatoid arthritis patients using serum drug levels and antidrug antibody measurement. Expert Opin Drug Metab Toxicol 2017;13:597–604. [DOI] [PubMed] [Google Scholar]
- 124.Liau MM, Oon HH. Therapeutic drug monitoring of biologics in psoriasis. Biologics 2019;13:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schots L, Grine L, Soenen R, Lambert J. Dermatologists on the medical need for therapeutic drug monitoring of biologics in psoriasis: results of a structured survey. J Dermatolog Treat 2020. Oct 15:1–9. doi: 10.1080/09546634.2020.1832649. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 126.Perry M, Abdullah A, Frleta M, MacDonald J, McGucken A. The potential value of blood monitoring of biologic drugs used in the treatment of rheumatoid arthritis. Ther Adv Musculoskelet Dis 2020;12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abdalla T, Lowes MA, Kaur N, Micheletti RG, A Steinhart H, Alavi A. Is there a role for therapeutic drug monitoring in patients with hidradenitis suppurativa on tumor necrosis factor-alpha inhibitors? Am J Clin Dermatol 2021;22:139–47. [DOI] [PubMed] [Google Scholar]
- 128.Syversen SW, Goll GL, Jørgensen KK, et al. Effect of therapeutic drug monitoring vs standard therapy during infliximab induction on disease remission in patients with chronic immune-mediated inflammatory diseases: A randomized clinical trial. JAMA 2021;325:1744–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vermeire S, Dreesen E, Papamichael K, Dubinsky MC. How, when, and for whom should we perform therapeutic drug monitoring? Clin Gastroenterol Hepatol 2020;18:1291–9. [DOI] [PubMed] [Google Scholar]
- 130.Berger AE, Duru G, de Vries A, et al. Comparison of immunoassays for measuring serum levels of golimumab and antibodies against golimumab in ulcerative colitis: a retrospective observational Study. Ther Drug Monit 2019;41:459–66. [DOI] [PubMed] [Google Scholar]
- 131.Papamichael K, Clarke WT, Vande Casteele N et al. Comparison of assays for therapeutic monitoring of infliximab and adalimumab in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2021;19:839–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Verdon C, Vande Casteele N, Heron V, Germain P, Afif W. Comparison of serum concentrations of ustekinumab obtained by three commercial assays in patients with Crohn’s disease. J Can Assoc Gastroenterol 2020;4:73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Imbrechts M, Van Stappen T, Compernolle G, Tops S, Gils A. Anti-infliximab antibodies: How to compare old and new data? J Pharm Biomed Anal 2020;177:112842. [DOI] [PubMed] [Google Scholar]
- 134.El-Matary W, Walters TD, Huynh HQ, et al. Higher postinduction infliximab serum trough levels are associated with healing of fistulizing perianal Crohn's disease in children. Inflamm Bowel Dis 2019;25:150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yarur AJ, Kanagala V, Stein DJ, et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn's disease. Aliment Pharmacol Ther 2017;45:933–40. [DOI] [PubMed] [Google Scholar]
- 136.Vande Casteele N. Assays for measurement of TNF antagonists in practice. Frontline Gastroenterol 2017;8:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mc Gettigan N, Afridi AS, Harkin G, et al. The optimal management of anti-drug antibodies to infliximab and identification of anti-drug antibody values for clinical outcomes in patients with inflammatory bowel disease. Int J Colorectal Dis 2021;36:1231–41. [DOI] [PubMed] [Google Scholar]
- 138.Landemaine A, Petitcollin A, Brochard C, et al. Cumulative exposure to infliximab, but not trough concentrations, correlates with rate of infection. Clin Gastroenterol Hepatol 2021;19:288–95. [DOI] [PubMed] [Google Scholar]
- 139.Yarur AJ, Jain A, Sussman DA, Barkin JS, et al. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: the ATLAS study. Gut 2016;65:249–55. [DOI] [PubMed] [Google Scholar]
- 140.Van den Berghe N, Verstockt B, Gils A, et al. Tissue exposure does not explain non-response in ulcerative colitis patients with adequate serum vedolizumab concentrations. J Crohns Colitis 2020. jjaa239. doi: 10.1093/ecco-jcc/jjaa239. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 141.Szántó KJ, Madácsy T, Kata D, et al. Advances in the optimisation of therapeutic drug monitoring using serum, tissue and faecal anti-tumour necrosis factor concentration in patients with inflammatory bowel disease treated with TNF-α antagonists. Expert Opin Biol Ther 2021;21:539–48. [DOI] [PubMed] [Google Scholar]
- 142.Vermeire S, Gils A, Accossato P, Lula S, Marren A. Immunogenicity of biologics in inflammatory bowel disease. Ther Adv Gastroenterol 2018;11:1756283X1775035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ungar B, Malickova K, Hanžel J, et al. Dose-optimization for loss-of-response to vedolizumab - pharmacokinetics and immune mechanisms. J Crohns Colitis 2021. Apr 10;jjab067. doi: 10.1093/ecco-jcc/jjab067. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 144.Gils A, Van Stappen T, Dreesen E, Storme R, Vermeire S, Declerck PJ. Harmonization of infliximab and anti-infliximab assays facilitates the comparison between originators and biosimilars in clinical samples. Inflamm Bowel Dis 2016;22:969–75. [DOI] [PubMed] [Google Scholar]
- 145.Van Stappen T, Billiet T, Vande Casteele N, et al. An optimized anti-infliximab bridging enzyme-linked immunosorbent assay for harmonization of anti-infliximab antibody titers in patients with inflammatory bowel diseases. Inflamm Bowel Dis 2015;21:2172–7. [DOI] [PubMed] [Google Scholar]
- 146.Van Stappen T, Bollen L, Vande Casteele N, et al. Rapid test for infliximab drug concentration allows immediate dose adaptation. Clin Transl Gastroenterol 2016;7:e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rocha C, Lago P, Fernandes S, et al. Rapid test detection of anti-infliximab antibodies: performance comparison with three different immunoassays. Therap Adv Gastroenterol 2020;13:1756284820965790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nasser Y, Labetoulle R, Harzallah I, Berger A-E, Roblin X, Paul S. Comparison of point-of-care and classical immunoassays for the monitoring infliximab and antibodies against infliximab in IBD. Dig Dis Sci 2018;63:2714–21. [DOI] [PubMed] [Google Scholar]
- 149.Dutzer D, Nasser Y, Berger AE, Roblin X, Paul S. Letter: new thresholds need to be defined when using point of care assays to monitor infliximab trough levels in IBD patients. Aliment Pharmacol Ther 2018;47:1571–3. [DOI] [PubMed] [Google Scholar]
- 150.Dreesen E, Berends S, Laharie D, et al. Modelling of the relationship between infliximab exposure, faecal calprotectin, and endoscopic remission in patients with Crohn’s disease. Br J Clin Pharmacol 2021;87:106–118. [DOI] [PubMed] [Google Scholar]
- 151.Lefevre PLC, Shackelton LM, Vande Casteele N. Factors influencing drug disposition of monoclonal antibodies in inflammatory bowel disease: Implications for personalized medicine. BioDrugs 2019;33:453–68. [DOI] [PubMed] [Google Scholar]
- 152.Eser A, Primas C, Reinisch S, et al. Prediction of individual serum infliximab concentrations in inflammatory bowel disease by a Bayesian dashboard system. J Clin Pharmacol 2018;58:790–802. [DOI] [PubMed] [Google Scholar]
- 153.Dubinsky MC, Phan BL, Singh N, Rabizadeh S, Mould DR. Pharmacokinetic dashboard-recommended dosing is different than standard of care dosing in infliximab-treated pediatric IBD patients. AAPS J 2017;19:215–22. [DOI] [PubMed] [Google Scholar]
- 154.Dave MB, Dherai AJ, Desai DC, Mould DR, Ashavaid TF. Optimization of infliximab therapy in inflammatory bowel disease using a dashboard approach-an Indian experience. Eur J Clin Pharmacol 2021;77:55–62. [DOI] [PubMed] [Google Scholar]
- 155.Papamichael K, Cheifetz AS. Letter: infliximab concentrations during induction therapy-one size does not fit all. Aliment Pharmacol Ther 2018;47:1334–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vande Casteele N, Jeyarajah J, Jairath V, Feagan BG, Sandborn WJ. Infliximab exposure-response relationship and thresholds associated with endoscopic healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2019;17:1814–21. [DOI] [PubMed] [Google Scholar]
- 157.Battat R, Hemperly A, Truong S, et al. Baseline clearance of infliximab is associated with requirement for colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2021;19:511–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kevans D, Murthy S, Mould DR, Silverberg MS. Accelerated clearance of infliximab is associated with treatment failure in patients with corticosteroid-refractory acute ulcerative colitis. J Crohns Colitis 2018;12:662–9. [DOI] [PubMed] [Google Scholar]
- 159.Vande Casteele N, Jairath V, Jeyarajah J, et al. Development and validation of a clinical decision support tool that incorporates pharmacokinetic data to predict endoscopic healing in patients treated with infliximab. Clin Gastroenterol Hepatol 2021;19:1209–17. [DOI] [PubMed] [Google Scholar]
- 160.Xiong Y, Mizuno T, Colman R, et al. Real-world infliximab pharmacokinetic study informs an electronic health record-embedded dashboard to guide precision dosing in children with Crohn's disease Clin Pharmacol Ther 2021;109:1639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sazonovs A, Kennedy NA, Moutsianas L, et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn's disease. Gastroenterology 2020;158:189–99. [DOI] [PubMed] [Google Scholar]
- 162.Wilson A, Peel C, Wang Q, Pananos AD, Kim RB. HLADQA1*05 genotype predicts anti-drug antibody formation and loss of response during infliximab therapy for inflammatory bowel disease. Aliment Pharmacol Ther 2020;51:356–63. [DOI] [PubMed] [Google Scholar]
- 163.Romero-Cara P, Torres-Moreno D, Pedregosa J, et al. A FCGR3A polymorphism predicts anti-drug antibodies in chronic inflammatory bowel disease patients treated with anti-TNF. Int J Med Sci 2018;15:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Billiet T, Vande Casteele N, Van Stappen T, et al. Immunogenicity to infliximab is associated with HLA-DRB1. Gut 2015;64:1344–5. [DOI] [PubMed] [Google Scholar]
- 165.Billiet T, Dreesen E, Cleynen I, et al. A genetic variation in the neonatal Fc-receptor affects anti-TNF drug concentrations in inflammatory bowel disease. Am J Gastroenterol 2016;111:1438–45. [DOI] [PubMed] [Google Scholar]
- 166.Salvador-Martín S, Pujol-Muncunill G, Bossacoma F, et al. Pharmacogenetics of trough serum anti-TNF levels in paediatric inflammatory bowel disease. Br J Clin Pharmacol 2021;87:447–57. [DOI] [PubMed] [Google Scholar]
- 167.Roblin X, Williet N, Boschetti G, et al. Addition of azathioprine to the switch of anti-TNF in patients with IBD in clinical relapse with undetectable anti-TNF trough levels and antidrug antibodies: a prospective randomised trial. Gut 2020;69:1206–12. [DOI] [PubMed] [Google Scholar]
- 168.Con D, Jackson B, Gray K, De Cruz P. eHealth for inflammatory bowel disease self-management–the patient perspective. Scand J Gastroenterol 2017;52:973–80. [DOI] [PubMed] [Google Scholar]
- 169.Lees CW, Regueiro M, Mahadevan U, International Organization for the Study of Inflammatory Bowel Disease. Innovation in IBD care during the COVID-19 pandemic: results of a global telemedicine survey by the International Organization for the Study of Inflammatory Bowel Disease. Gastroenterology 2020;159:805–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Walsh A, Travis S. What’s app? Electronic health technology in inflammatory bowel disease. Intest Res 2018;16:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.De Jong MJ, van der Meulen-de Jong AE, Romberg-Camps MJ, et al. Telemedicine for management of inflammatory bowel disease (myIBDcoach): a pragmatic, multicentre, randomised controlled trial. Lancet 2017;390(10098):959–68. [DOI] [PubMed] [Google Scholar]
- 172.Heida A, Dijkstra A, Kobold AM, et al. Efficacy of home telemonitoring versus conventional follow-up: A randomized controlled trial among teenagers with inflammatory bowel disease. J Crohns Colitis 2018;12:432–41. [DOI] [PubMed] [Google Scholar]
- 173.Hunter J, Claridge A, James S, et al. Improving outpatient services: the Southampton IBD virtual clinic. Frontline Gastroenterol 2012;3:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Habashi P, Bouchard S, Nguyen GC. Transforming access to specialist care for inflammatory bowel disease: The PACE telemedicine program. J Can Assoc Gastroenterol 2019;2:186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Berends SE, D'Haens G, Schaap T, et al. Dried blood samples can support monitoring of infliximab concentrations in patients with inflammatory bowel disease: A clinical validation. Br J Clin Pharmacol 2019;85:1544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Detrez I, Schops G, Lefrère J, et al. Golimumab dried blood spot analysis (GOUDA): a prospective trial showing excellent correlation with venepuncture samples and more detailed pharmacokinetic information. AAPS J 2018;21:10. [DOI] [PubMed] [Google Scholar]
- 177.Piester T, Frymoyer A, Christofferson M, Yu H, Bass D, Park KT. A mobile infliximab dosing calculator for therapy optimization in inflammatory bowel disease Inflamm Bowel Dis 2018;24:227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]