Abstract
Objective
The objective of this study is to explore the clinicopathological characteristics of gastric cancer and precancerous conditions in patients with primary gastric lymphoma.
Methods
We analyzed 474 cases of primary gastric lymphoma, mainly DLBCL and MALT, from three clinical centres retrospectively, and compared the clinicopathological parameters of primary gastric lymphoma patients complicated with gastric cancer, precancerous conditions, or with no complications.
Results
A total of 5.1% of the patients with primary gastric lymphoma were diagnosed with gastric cancer, including metachronous gastric adenocarcinoma (3.2%) and synchronous gastric adenocarcinoma (1.9%). Of the patients with gastric lymphoma, 14.6% had precancerous conditions including atrophy (14.6%), intestinal metaplasia (8.9%), and low-grade intraepithelial neoplasia (1.9%). Primary gastric lymphoma patients with an ulcerative type (p = 0.009) and Lugano classification stage IIE + IV (p < 0.001) lymphoma had a higher risk of complicating with gastric cancers or precancerous conditions. The rate of infection of Helicobacter pylori (Hp) was 68.4% in patients with primary gastric lymphoma, which was higher in patients with MALT lymphoma (p < 0.001), Lugano classification stage I + II (p < 0.001), and patients complicated with precancerous conditions and gastric cancer (p < 0.001), especially gastric cancer of the intestinal type (p = 0.04). Gastric cancer (95.8%) and precancerous conditions (91.3%) occurred mostly in Hp-infected primary gastric lymphoma patients, with a minor subset of Hp-eradicated patients. Primary gastric lymphoma patients had a higher detection rate of early gastric cancer (25.0%) and a five-year survival rate (40.0%) than the general Chinese population.
Conclusions
Patients with primary gastric lymphoma have a high risk of developing gastric cancer and precancerous conditions, and this risk may be related to Helicobacter pylori infection. Follow-up of primary gastric lymphoma provides an opportunity for the detection of early gastric cancer.
Key messages
5.1% of the patients with primary gastric lymphoma were diagnosed with gastric cancer.
14.6% of the patients with gastric lymphoma had premalignant lesions including atrophy (14.6%), intestinal metaplasia (8.9%), and low-grade intraepithelial neoplasia (1.9%).
Primary gastric lymphoma patients complicating with gastric cancer had a higher infection rate of Helicobacter pylori (100.0%), a detection rate of early gastric cancer (25.0%) and a five-year survival rate (40.0%) than the general Chinese population.
Keywords: Primary gastric lymphoma, mucosa-vassociated lymphoid tissue lymphoma, diffuse large B-cell lymphoma, gastric cancer, precancerous conditions, Helicobacter pylori
1. Introduction
The incidence and mortality of gastric cancer rank first among malignant tumours of the stomach, with about 1.09 million new cases and 768,000 deaths each year globally [1]. Approximately 90% of gastric cancer is gastric adenocarcinoma [2], and the next primary gastric lymphoma is the second most common gastric malignant tumour [3]. In treating these cancers, clinicians need to differentiate between gastric cancer and primary gastric lymphoma if masses, ulcers, or other lesions are found in the stomach, and current identification methods include pathology biopsy and endoscopy, as well as other imaging examinations [4]. However, rare cases of primary gastric lymphoma complicated with synchronous gastric cancer and metachronous gastric cancer have attracted the attention of clinical researchers in particular. The occurrence of primary gastric lymphoma is closely related to Helicobacter pylori (H. pylori), especially mucosa-associated lymphoid tissue (MALT) lymphomas and some diffuse large B-cell lymphomas (DLBCL) [5]. H. pylori infection can lead to chronic inflammation, atrophy, intestinal metaplasia, low-grade intraepithelial neoplasia, and the intestinal type of gastric adenocarcinoma [6–8]. However, few studies have shown that patients with primary gastric lymphoma have a significantly increased risk of developing malignant solid tumours, such as gastric cancer [9,10], and the question of whether patients with primary gastric lymphoma have an increased risk for gastric cancer and precancerous conditions remains controversial [11,12].
This study retrospectively analyzes the clinicopathological characteristics of gastric cancer and precancerous conditions in patients with primary gastric lymphoma from three clinical cancer centres in China and aims to provide clinical evidence for the correlation between primary gastric lymphoma and gastric cancer.
2. Materials and methods
2.1. Study objects and groups
Our study sample consisted of 474 primary gastric lymphoma patients diagnosed at three Chinese cancer centers between January 2010 to August 2020, including 268 patients from the First Affiliated Hospital of Xi’an Jiao Tong University, 119 patients from Shaanxi Provincial People’ss Hospital, and 87 patients from Beijing Tsinghua Changgung Hospital. Our inclusion criteria were the presence of the following: clinical and pathological diagnosis of primary gastric lymphoma and regular endoscopic follow-up, and our exclusion criteria were the presence of complications with malignant tumours other than the stomach, previous upper digestive tract surgery, failure to cooperate with gastroscopy, serious cardiopulmonary disease, and serious liver, kidney, or mental disease. A diagnosis of primary gastric lymphoma was made based on the Dawson criteria [13]. We broke down the sample into two experimental groups and a control group. First, the gastric cancer group included cases diagnosed with synchronous gastric adenocarcinoma within 6 months or metachronous gastric adenocarcinoma beyond 6 months from primary gastric lymphoma diagnosis. Second, the gastric precancerous conditions group included cases with atrophy, intestinal metaplasia, or low-grade intraepithelial neoplasia at initial endoscopy and during follow-up, excluding patients who progressed to gastric cancer. The cases that didn’t develop gastric cancer or precancerous conditions were placed in the control group.
2.2. Gastroscopy
Gastroscopy follow-up of patients diagnosed with primary gastric lymphoma was carried out regularly in accordance with the guidelines presented in Matysiak-Budnik et al. [14]. A standard gastroscopy requires adequate preparation before the examination, where the endoscopist should carefully observe a patient’s H. pylori infection status, background mucosa, changes in the original lesions, and suspicious malignant lesions by white light endoscopy, chromoendoscopy, linked colour imaging, narrow band imaging, and magnifying endoscopy. After careful observation, we took a biopsy of suspicious lesions for histopathological examination as well.
2.3. Diagnosis of gastric cancer and precancerous conditions
Atrophy and intestinal metaplasia can be diagnosed by gastroscopy or pathology alone. Diagnosis of gastric cancer and low-grade intraepithelial neoplasia must be confirmed by histopathological examination of a biopsy or surgical specimens. Endoscopic manifestations of atrophic gastritis include the pale appearance of gastric mucosa, thinning of the gastric mucosa, increased visibility of vasculature, and loss of gastric folds [15,16]. The degree of atrophic gastritis is assessed using the Kimura-Takemoto classification system or Operative Link for Gastritis Assessment (OLGA) classification [15–17]. The pathological diagnosis of atrophic gastritis is based on the loss of normal glandular epithelium gastric glands [15,16]. Intestinal metaplasia is manifested as a gray-white flat bulge on white light endoscopy, and regular vessels with ridge/tubular or tubulovillous glands, particularly with a light blue crest on magnified narrow band imaging, mostly on an atrophic background [18]. The degree of intestinal metaplasia is assessed using the Operative Link on Gastric Intestinal Metaplasia Assessment (OLGIM) system [15,16]. The pathological manifestations of intestinal metaplasia are divided into complete intestinal metaplasia and incomplete intestinal metaplasia. Complete intestinal metaplasia resembles the small intestinal epithelium, while incomplete intestinal metaplasia resembles the colonic epithelium [19]. The classification of gastric cancer and intraepithelial neoplasia was defined by World Health Organization [20]. Low-grade intraepithelial neoplasia has limited architectural abnormalities and only mild to moderate cytological atypia, with hyperchromatic, elongated, pseudostratified nuclei [21].
2.4. H. pylori detection and eradication
H. pylori infection status was determined by H. pylori detection and patients’ histories of eradication therapy. For detection, we used a 13C-urea breathing test, a 14C-urea breath test, or a rapid urease test to detect H. pylori. A positive result for any of the above tests confirmed the presence of an infection with H. pylori, which we denote as ‘Hp-infected’, and negative results without previous H. pylori eradication therapy were judged to be ‘Hp-uninfected’. Negative results after H. pylori eradication therapy was denoted as ‘Hp-eradicated’. In order to avoid false negative test results, we required an interval of at least 4 weeks from antibiotics and at least 2 weeks from proton pump inhibitor and bismuth before H. pylori detection could take place. Patients were also required to fast for at least 2 h before the examination.
2.5. Clinicopathological data collection
Clinicopathological and demographic data of all the study subjects were also collected and analyzed. Demographic data included age and sex, and the clinicopathological characteristics of primary gastric lymphoma included diagnosis time, pathological type, lesion location, endoscopy manifestation, Lugano Classification, B symptoms, H. pylori infection status, treatment and prognosis. The clinicopathological characteristics of gastric cancer included symptoms, diagnosis time, lesion location, largest tumour size, TNM staging, pathological type, Lauren classification, H. pylori infection status, treatment and prognosis. Finally, the clinicopathological characteristics of gastric precancerous lesions included diagnosis time, lesion location, pathological type, H. pylori infection status, treatment and prognosis as well. We successfully contacted 391 patients by telephone on 22 May 2022 to 124 months (median: 49 months) after their initial diagnosis of primary gastric lymphoma. The remaining patients were lost.
2.6. Statistical analysis
We used SPSS 24.0 (SPSS Inc., Chicago, IL, USA) for the statistical analysis of all of our data. Categorical variables were expressed as the number of cases (percentage) and analyzed by Pearson χ2 tests or Fisher’s exact test between groups, and continuous variables that conformed to normal distribution were represented by mean ± standard deviation and analyzed by Student’s t-tests and ANOVA tests. Continuous variables that didn’t conform to normal distribution were presented as median (interquartile range) and analyzed by Mann–Whitney U-tests. The overall survival rate and its risk factors were evaluated by the Kaplan–Meier survival analysis. Our threshold for a statistically significant test result was p < 0.05 for all tests.
3. Results
3.1. Demographic and clinicopathological characteristics of patients with primary gastric lymphoma
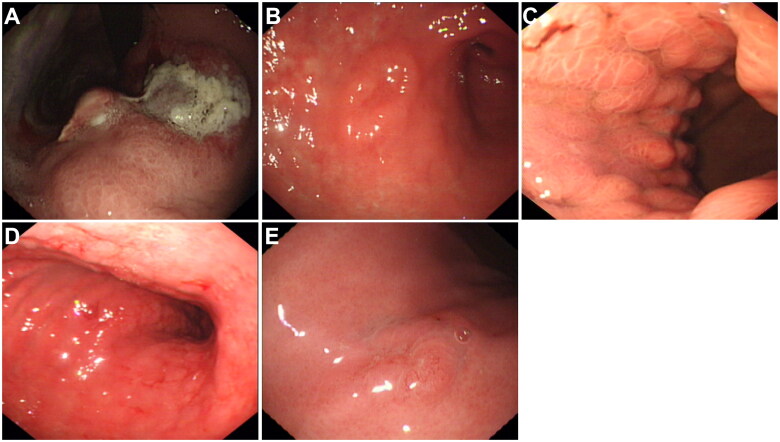
In total 474 patients with primary gastric lymphoma were enrolled in our study. As shown in Table 1, the patients’ average age was 52.6 ± 5.5 years (27 to 73 years), and 63.5% of the patients were male. The most common involved locations were the gastric body (55.9%) and gastric antrum (45.1%), and ulcerative type (32.5%) was the most common endoscopic manifestation of primary gastric lymphoma, and the pathological types of primary gastric lymphoma were mainly DLBCL (47.5%) and MALT lymphoma (45.6%). Other pathological types of primary gastric lymphoma included follicular lymphoma (1.9%), mantle cell lymphoma (0.8%), Burkitt lymphoma (0.8%), and T cell lymphoma (3.4%). Most primary gastric lymphomas were assessed as Lugano Classification stage I (43.5%) and stage II (33.1%) without B symptoms (70.9%). Only 9.1% of the patients were Lugano Classification stage IV with bone marrow invasion (5.5%) or supradiaphragmatic nodal involvement (3.6%). Figure 1 shows endoscopic manifestations of primary gastric lymphomas.
Table 1.
Demographic and clinicopathological characteristics of patients with primary gastric lymphoma, mean ± SD or N(%).
| Characteristic | Primary gastric lymphoma (n = 474) |
|---|---|
| Sex | |
| Male | 301 (63.5%) |
| Female | 173 (36.5%) |
| Age, y | 52.6 ± 5.5 |
| Lesion location | |
| Gastric antrum | 214 (45.1%) |
| Gastric body | 265 (55.9%) |
| Gastric fundus | 115 (24.3%) |
| Pylorus | 103 (21.7%) |
| Cardia | 42 (8.9%) |
| Lesion distribution | |
| Multi-site | 78 (16.5%) |
| Single-site | 396 (83.5%) |
| Endoscopic manifestation | |
| Ulcerative type | 154 (32.5%) |
| Mass or polypoid type | 121 (25.5%) |
| Fold change type | 103 (21.7%) |
| Infiltrative type | 48 (10.1%) |
| Superficial type | 48 (10.1%) |
| Pathological type | |
| DLBCL | 225 (47.5%) |
| MALT lymphoma | 216 (45.6%) |
| Others | 33 (7.0%) |
| Lugano classification | |
| I | 206 (43.5%) |
| II | 157 (33.1%) |
| IIE | 68 (14.3%) |
| IV | 43 (9.1%) |
| B symptoms | |
| Yes | 138 (29.1%) |
| No | 336 (70.9%) |
DLBCL: diffuse large B-cell lymphoma; MALT: mucosa-associated lymphoid tissue.
Figure 1.
Endoscopic manifestation of primary gastric lymphoma. (A) ulcerative type; (B) mass or polypoid type; (C) fold change type; (D) infiltrative type; (E) superficial type.
3.2. The risk of gastric cancer in patients with primary gastric lymphoma
A total of 5.1% (24/474) of primary gastric lymphoma patients had been diagnosed with gastric cancer, all of which were gastric adenocarcinomas. As shown in Table 2, MALT lymphoma (50%, 12/24) and DLBCL (50%, 12/24) were the main pathological types. Primary gastric lymphoma patients with an ulcerative type (with gastric cancer, 14/24, 58.3% vs. without gastric cancer, 146/450, 32.4%; p = 0.009) and Lugano classification stage IIE + IV (with gastric cancer, 14/24, 58.3% vs. without gastric cancer, 113/450, 25.1%; p < 0.001) had a higher risk of gastric cancer. The odds ratios for ulcerative type and Lugano classification stage IIE + IV were 2.748 (95% CI 1.248–6.047) and 3.825 (95% CI 1.744–8.392), respectively.
Table 2.
Demographic and clinicopathological characteristics of primary gastric lymphoma patients with or without gastric cancer, mean ± SD or N(%).
| Characteristic | With gastric cancer (n = 24) | Without gastric cancer (n = 450) | p Value |
|---|---|---|---|
| Sex | 0.741 | ||
| Male | 16 (66.7%) | 285 (63.3%) | |
| Female | 8 (33.3%) | 165 (36.7%) | |
| Diagnosis age of lymphoma, y | 54.0 ± 8.4 | 52.2 ± 5.2 | 0.053 |
| Lesion location of lymphoma | |||
| Gastric antrum | 10 (41.6%) | 218 (48.4%) | 0.517 |
| Gastric body | 14 (58.3%) | 261 (58.0%) | 0.974 |
| Others | 4 (16.7%) | 54 (12.0%) | 0.497 |
| Lesion distribution of lymphoma | |||
| Multi-site | 3 (12.5%) | 62 (13.8%) | 0.859 |
| Single-site | 21 (87.5%) | 388 (86.2%) | |
| Endoscopic manifestation of lymphoma | 0.009 | ||
| Ulcerative type | 14 (58.3%) | 146 (32.4%) | |
| Other types | 10 (41.7%) | 304 (67.6%) | |
| Pathological type of lymphoma | 0.485 | ||
| DLBCL | 12 (50.0%) | 221 (49.1%) | |
| MALT lymphoma | 12 (50.0%) | 204 (45.3%) | |
| Others | 0 (0.0%) | 25 (5.6%) | |
| Lugano classification of lymphoma | < 0.001 | ||
| I + II | 10 (41.6%) | 337 (74.9%) | |
| IIE + IV | 14 (58.3%) | 113 (25.1%) | |
| B symptoms of lymphoma | 0.807 | ||
| Yes | 7 (29.2%) | 121 (26.9%) | |
| No | 17 (70.8%) | 329 (73.1%) |
DLBCL: diffuse large B-cell lymphoma; MALT: mucosa-associated lymphoid tissue.
Among the patients with gastric adenocarcinoma, 1.9% (9/474) and 3.2% (15/474) had synchronous gastric cancer and metachronous gastric cancer, respectively. Only one patient who had synchronous gastric cancer was diagnosed at the same time with gastric lymphoma. The median diagnosis interval between metachronous gastric cancer and primary gastric lymphoma was about 3 years (–5 to 11 years). Only one patient who had metachronous gastric cancer was diagnosed before gastric lymphoma. We found no significant differences in sex, age, lesion location, largest tumour size, pathological type, Lauren classification, or TNM stage between the synchronous gastric adenocarcinoma group and the metachronous gastric adenocarcinoma group (Table 3).
Table 3.
Demographic and clinicopathological characteristics of gastric cancer patients with primary gastric lymphoma, mean ± SD, median (interquartile range) or N(%).
| Characteristic | Gastric cancer (n = 24) |
||
|---|---|---|---|
| Synchronous gastric adenocarcinoma (n = 9) | Metachronous gastric adenocarcinoma (n = 15) | p Value | |
| Sex | >0.999 | ||
| Male | 6 (66.7%) | 9 (60.0%) | |
| Female | 3 (33.3%) | 6 (40.0%) | |
| Age, ya | 54 (43.5) | 55 (54) | 0.857 |
| Diagnosis interval, yb | 0 (0) | 3 (2) | <0.001 |
| Lesion location of the cancer | >0.999 | ||
| Gastric antrum | 5 (55.6%) | 7 (46.7%) | |
| Gastric body | 2 (22.2%) | 4 (26.7%) | |
| Others | 2 (22.2%) | 4 (26.7%) | |
| Largest tumor size, cm | 4 (3.25) | 4 (3) | 0.627 |
| Pathological type of cancer | >0.999 | ||
| Differentiated adenocarcinoma | 7 (77.8%) | 12 (80.0%) | |
| Undifferentiated adenocarcinoma | 2 (22.2%) | 3 (20.0%) | |
| Lauren classification of cancer | 0.829 | ||
| Intestinal type | 6 (66.7%) | 8 (53.3%) | |
| Diffuse type | 3 (33.3%) | 5 (33.3%) | |
| Mixed type | 0 (0.0%) | 2 (13.3%) | |
| T stage | >0.999 | ||
| T1 | 3 (33.3%) | 6 (40.0%) | |
| T2 + T3 + T4 | 6 (66.7%) | 9 (60.0%) | |
| N stage | >0.999 | ||
| N0 | 5 (55.6%) | 8 (53.3%) | |
| N1 + N2 + N3 | 4 (44.4%) | 7 (46.7%) | |
| M stage | 0.326 | ||
| M0 | 6 (66.7%) | 13 (86.7%) | |
| M1 | 3 (33.3%) | 2 (13.3%) | |
| TNM stage | >0.999 | ||
| Advanced gastric cancer | 7 (77.8%) | 11 (73.3%) | |
| Early gastric cancer | 2 (22.2%) | 4 (26.7%) | |
aAge of patients when diagnosed with gastric cancer.
bDiagnosis interval between gastric cancer and primary gastric lymphoma of each case is presented as an estimated whole year. The diagnosis of gastric cancer earlier than gastric lymphoma is indicated by a negative number, and the diagnosis of gastric cancer later than gastric lymphoma is indicated by a positive number.
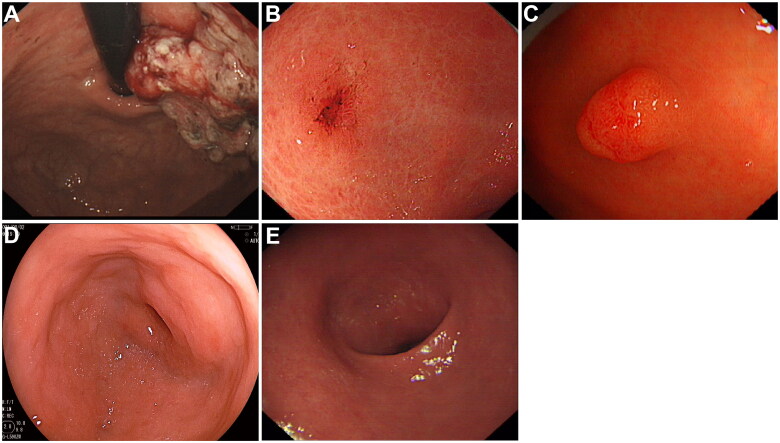
Most primary gastric lymphoma patients who had gastric cancer had advanced gastric cancer (18/24, 75.0%), accounting for 77.8% (7/9) of synchronous gastric adenocarcinoma and 86.7% (11/15) of metachronous gastric adenocarcinoma. Figure 2(A,B) show advanced gastric adenocarcinoma and early gastric adenocarcinoma developed in primary gastric lymphoma patients. The patients with advanced gastric cancer had larger tumour sizes (advanced gastric cancer, 4.8 cm (1.3 cm) vs. early gastric cancer, 3.0 cm (0.9 cm); p = 0.001), higher T stage (advanced gastric cancer, T1, 3/18, 16.7% vs. early gastric cancer, T1, 6/6, 100.0%; p<0.001), and higher N stage (advanced gastric cancer, N0, 11/18, 61.1% vs. early gastric cancer, N0, 0/6, 0.0%; p = 0.009) than those with early gastric cancer.
Figure 2.
Endoscopic manifestation of gastric cancer and precancerous conditions in primary gastric lymphoma patients. (A) advanced gastric adenocarcinoma; (B) early gastric adenocarcinoma; (C) low-grade intraepithelial neoplasia; (D) intestinal metaplasia; (E) atrophy.
3.3. The risk of gastric precancerous conditions in patients with primary gastric lymphoma
A total of 14.6% (69/474) of the patients with primary gastric lymphoma had gastric precancerous conditions, including atrophy (14.6%, 69/474), intestinal metaplasia (8.9%, 42/474), and low-grade intraepithelial neoplasia (1.9%, 12/474) (Figure 2(C–E)). Endoscopic manifestation (p < 0.001) and Lugano classification (p < 0.001) of primary gastric lymphoma differed among the groups of patients with gastric cancer, precancerous conditions or not (Table 4). However, more ulcerative-type primary gastric lymphoma was seen in patients with gastric precancerous conditions (gastric precancerous conditions, 38/69, 55.1% vs. control, 108/381, 28.3%; p < 0.001) as well as gastric cancer (gastric cancer, 14/24, 58.3% vs. control, 108/381, 28.3%; p = 0.004). Primary gastric lymphoma patients with Lugano classification stage IIE + IV developed more gastric precancerous conditions (gastric precancerous conditions, 38/69, 55.1% vs. control, 75/381, 19.7%; p < 0.001) and gastric cancer as well (gastric cancer, 14/24, 58.3% vs. control, 75/381, 19.7%; p < 0.001). The median diagnosis interval between gastric precancerous conditions and primary gastric lymphoma was about 3 years (3 to 11 years).
Table 4.
Demographic and clinicopathological characteristics of primary gastric lymphoma patients with gastric cancer and gastric precancerous conditions, mean ± SD, median (interquartile range), or N(%).
| Characteristic | Gastric cancer (n = 24) | Gastric precancerous conditions (n = 69) | Control (n = 381) | p Value |
|---|---|---|---|---|
| Sex | 0.804 | |||
| Male | 16 (66.7%) | 46 (66.7%) | 240 (63.0%) | |
| Female | 8 (33.3%) | 23 (33.3%) | 141 (37.0%) | |
| Diagnosis age of lymphoma, y | 52 (5.5) | 51 (12) | 53 (9) | 0.502 |
| Diagnosis age of gastric cancer or precancerous conditions, y | 55 (5) | 55 (9) | / | 0.795 |
| Diagnosis intervala, y | 2 (5) | 3 (3) | / | 0.102 |
| Lesion location of lymphoma | ||||
| Gastric antrum | 10 (41.6%) | 34 (49.3%) | 184 (48.3%) | 0.802 |
| Gastric body | 14 (58.3%) | 32 (46.4%) | 229 (60.1%) | 0.104 |
| Others | 4 (16.7%) | 7 (10.1%) | 47 (12.3%) | 0.697 |
| Endoscopic manifestation of lymphoma | ||||
| Ulcerative type | 14 (58.3%) | 38 (55.1%) | 108 (28.3%) | < 0.001 |
| Mass or polypoid type | 5 (20.8%) | 15 (21.7%) | 77 (20.2%) | |
| Other type | 5 (20.8%) | 16 (23.2%) | 196 (51.4%) | |
| Pathological type of lymphoma | 0.816 | |||
| DLBCL | 12 (50.0%) | 35 (50.7%) | 186 (48.8%) | |
| MALT lymphoma | 12 (50.0%) | 30 (43.5%) | 174 (45.7%) | |
| Others | 0 (0.0%) | 4 (5.8%) | 21 (5.5%) | |
| Lugano classification of lymphoma | < 0.001 | |||
| I + II | 10 (41.6%) | 31 (44.9%) | 306 (80.3%) | |
| IIE + IV | 14 (58.3%) | 38 (55.1%) | 75 (19.7%) | |
| B symptoms of lymphoma | 0.268 | |||
| Yes | 7 (29.2%) | 24 (34.8%) | 97 (25.5%) | |
| No | 17 (70.8%) | 45 (65.2%) | 284 (74.5%) |
DLBCL: diffuse large B-cell lymphoma; MALT: mucosa-associated lymphoid tissue.
aDiagnosis interval between gastric cancer or gastric precancerous conditions and primary gastric lymphoma of each case is presented as an estimated whole year. The diagnosis of gastric cancer or gastric precancerous conditions earlier than gastric lymphoma is indicated by a negative number, and the diagnosis of gastric cancer or gastric precancerous conditions later than gastric lymphoma is indicated by a positive number.
3.4. The risk of H. pylori infection in patients with primary gastric lymphoma, gastric cancer, or precancerous conditions
The H. pylori infection rate of primary gastric lymphoma patients was 68.4% (324/474), and as defined above the remaining patients were Hp-uninfected (20.0%, 95/474), and Hp-eradicated (11.6%, 55/474) (Table 5). Gastric antrum (p < 0.001) and pylorus (p = 0.014) involvement were more common in primary gastric lymphoma patients who were HP-infected, and patients with MALT lymphoma accounted for 56.2% of Hp-infected primary gastric lymphoma patients. MALT lymphoma was more common in Hp-infected primary gastric lymphoma patients compared to Hp-uninfected and Hp-eradicated patients (p < 0.001). The H. pylori infection rate of MALT lymphoma patients was significantly higher than that of DLBCL patients as well (MALT lymphoma, 182/216, 84.3% vs. DLBCL 123/225, 54.7%; p < 0.001). In addition, primary gastric lymphoma patients with Lugano classification stage I + II also had a higher H. pylori infection rate than patients with Lugano classification stage IIE + IV (p < 0.001). The H. pylori infection rate of primary gastric lymphoma with Lugano Classification stage I + II was as high as 89.5% (Table 5).
Table 5.
Helicobacter pylori infection status of patients with primary gastric lymphoma, mean ± SD or N(%).
| Characteristic | Primary gastric lymphoma (n = 474) |
|||
|---|---|---|---|---|
| Hp-infected (n = 324) | Hp-eradicated (n = 55) | Hp-uninfected (n = 95) | p Value | |
| Sex | 0.808 | |||
| Male | 203 (62.7%) | 35 (63.6%) | 63 (66.3%) | |
| Female | 121 (37.3%) | 20 (36.4%) | 32 (33.7%) | |
| Age, y | 52.4 ± 5.9 | 54.3 ± 8.3 | 48.2 ± 6.8 | 0.327 |
| Lesion location of lymphoma | ||||
| Gastric antrum | 171 (52.8%) | 23 (41.8%) | 20 (21.1%) | < 0.001 |
| Gastric body | 181 (55.9%) | 26 (47.3%) | 58 (61.1%) | 0.261 |
| Gastric fundus | 81 (25.0%) | 11 (20.0%) | 23 (24.2%) | 0.726 |
| Pylorus | 82 (25.3%) | 10 (30.9%) | 11 (11.6%) | 0.014 |
| Cardia | 26 (8.0%) | 6 (10.9%) | 10 (10.5%) | 0.640 |
| Lesion distribution of lymphoma | 0.587 | |||
| Multi-site | 57(17.6%) | 7 (12.7%) | 14 (14.7%) | |
| Single-site | 267(82.4%) | 48 (87.3%) | 81 (85.3%) | |
| Endoscopic manifestation of lymphoma | 0.373 | |||
| Ulcerative type | 95 (28.4%) | 19 (34.5%) | 40 (42.1%) | |
| Mass or polypoid type | 81 (25.0%) | 14 (25.5%) | 26 (27.4%) | |
| Fold change type | 75 (23.1%) | 13 (23.6%) | 15 (15.8%) | |
| Infiltrative type | 36 (11.1%) | 5 (9.1%) | 7 (7.8%) | |
| Superficial type | 37 (13.1%) | 4 (7.3%) | 7 (7.8%) | |
| Pathological type of lymphoma | < 0.001 | |||
| DLBCL | 123 (38.0%) | 31 (56.4%) | 71 (74.7%) | |
| MALT lymphoma | 182 (56.2%) | 17 (30.9%) | 17 (17.9%) | |
| Others | 19 (5.9%) | 7 (12.7%) | 7 (7.4%) | |
| Lugano classification of lymphoma | < 0.001 | |||
| I + II | 290 (89.5%) | 29 (52.7%) | 44 (46.3%) | |
| IIE + IV | 34 (33.1%) | 26 (47.3%) | 51 (53.7%) | |
| B symptoms of lymphoma | 0.050 | |||
| Yes | 105 (32.4%) | 10 (18.2%) | 23 (24.2%) | |
| No | 219 (67.6%) | 45 (81.8%) | 72 (75.8%) | |
| Complicated with gastric cancer | 0.011 | |||
| Yes | 23 (7.1%) | 1 (1.8%) | 0 (0.0%) | |
| No | 301 (92.9%) | 54 (98.2%) | 95 (100.0%) | |
| Complicated with gastric cancer of the intestinal type | 0.040 | |||
| Yes | 14 (4.3%) | 0 (0.0%) | 0 (0.0%) | |
| No | 310 (95.7%) | 55 (100.0%) | 95 (100.0%) | |
| Complicated with gastric cancer or precancerous conditions | < 0.001 | |||
| Gastric cancer | 23 (7.1%) | 1 (2.8%) | 0 (0.0%) | |
| Gastric precancerous conditions | 63 (19.4%) | 4 (7.3%) | 2 (2.1%) | |
| None | 238 (73.5%) | 50 (90.9%) | 93 (97.9%) | |
DLBCL: diffuse large B-cell lymphoma; MALT; mucosa-associated lymphoid tissue; Hp: Helicobacter pylori.
For primary gastric lymphoma patients split into groups with gastric cancer, precancerous conditions, or neither, the H. pylori infection rate was significantly higher in the patients with gastric cancer and precancerous conditions (p < 0.001). Actually, gastric cancer (95.8%, 23/24) occurred mostly in Hp-infected primary gastric lymphoma patients and rarely occurred in patients after H. pylori eradication (4.2%, 1/24). None of our study subjects without H. pylori infection developed gastric cancer. Primary gastric lymphoma patients with a history of H. pylori infection (Hp-infected and Hp-eradicated) (with gastric cancer, 14/24, 58.3% vs. without gastric cancer, 146/450, 32.4%; p = 0.012) and present H. pylori infection (Hp-infected) (with gastric cancer, 23/24, 100.0% vs. without gastric cancer, 301/450, 66.9%; p = 0.003) had a higher risk of gastric cancer. The odds ratios for a history of H. pylori infection and present H. pylori infection were 1.068 (95% CI 1.040–1.096) and 1.069 (95% CI 1.035–1.105), respectively.
Similarly, gastric precancerous conditions also mostly occurred in Hp-infected primary gastric lymphoma patients (91.3%, 63/69), a few patients after H. pylori eradication (5.8%, 4/69), and rare Hp-uninfected patients (3.4%, 2/69). Additionally, gastric cancer of the intestinal type was more common in Hp-infected primary gastric lymphoma patients compared to Hp-uninfected and Hp-eradicated patients (p = 0.04) (Table 5). Gastric cancer of the intestinal type was only found in Hp-infected primary gastric lymphoma patients.
3.5. Treatment and prognosis of patients with primary gastric lymphoma, gastric cancer, or precancerous conditions
All Hp-infected primary gastric lymphoma patients received H. pylori eradication therapy, and the overall success rate of H. pylori eradication was 87.1%, with no difference in MALT lymphoma (89.9%), DLBCL (88.3%), and other pathological types (84.6%). H. pylori eradication therapy was effective in 40.9% of primary gastric lymphoma patients, 55.8% of MALT lymphomas patients, and 28.6% of DLBCL patients. Among them,17.7% of primary gastric lymphoma patients, 28.1% of MALT lymphoma patients, and 7.1% of DLBCL patients achieved complete remission. Only 2.9% of patients relapsed after complete remission with H. pylori eradication therapy. Finally, 7.4% of all primary gastric lymphoma patients achieved sustained complete remission after H. pylori eradication and didn’t need other treatments during follow-up, Most of which were MALT lymphoma patients (92.3%).
Primary gastric lymphoma patients with Lugano classification stage IIE + IV, and those who were Hp-uninfected, failed to eradicate H. pylori, or didn’t respond to H. pylori eradication therapy received chemotherapy (75.9%), targeted therapy (53.2%), surgery (12.4%), radiotherapy (6.8%), and immunotherapy (5.9%). Most patients (67.1%) received a combination of therapies. The prognosis of primary gastric lymphoma was generally well. The five-year overall survival rate and three-year overall survival rate of primary gastric lymphoma patients were 62.6% and 73.1%, respectively.
Among patients with primary gastric lymphoma and gastric precancerous conditions, 90.9% (10/11) of patients with low-grade intraepithelial neoplasia had atrophy before progressing, and 63.6% (7/11) had intestinal metaplasia during follow-up. All patients with intestinal metaplasia had atrophy either before or at the same time. While among patients with primary gastric lymphoma and gastric cancer, 91.7% (22/24) had atrophy, 66.7% (16/24) had intestinal metaplasia, and 16.7% (4/24) had low-grade intraepithelial neoplasia, either before or at the time of gastric cancer diagnosis. We found no statistical difference in the risk of developing metachronous gastric cancer between patients with primary gastric lymphoma who received and those who did not receive chemotherapy, as well as immunotherapy, radiotherapy, and targeted therapy.
Patients with primary gastric lymphoma complicated with synchronous gastric cancer and metachronous gastric cancer received surgery (70.8%), chemotherapy (37.5%), targeted therapy (25.0%), immunotherapy (16.7%), radiotherapy (8.3%), endoscopic submucosal dissection (16.7%), H. pylori eradication (75.0%), etc. The five-year overall survival rate and three-year overall survival rate of gastric adenocarcinoma in primary gastric lymphoma patients were 40.0% (6/15) and 57.9% (11/19), respectively, which were similar in patients with synchronous gastric cancer and metachronous gastric cancer. We found no significant difference in five-year overall survival rate (with gastric cancer, 6/15, 40% vs. without gastric cancer, 98/167, 58.7%; p = 0.161) and three-year overall survival (with gastric cancer, 12/19, 63.2% vs. without gastric cancer, 249/338, 73.7%; p = 0.315) between primary gastric lymphoma patients complicated with gastric cancer or not. The three-year overall survival rate (early gastric cancer, 5/5, 100.0% vs. advanced gastric cancer, 6/14, 42.9%; p = 0.045) and the five-year survival rate (early gastric cancer, 3/3, 100.0% vs. advanced gastric cancer, 3/12, 25.0%; p = 0.044) of early gastric cancer was significantly higher than that of the advanced gastric cancer in primary gastric lymphoma patients.
4. Discussion
We found a high risk of gastric cancer and precancerous conditions for primary gastric lymphoma patients from our multi-centre retrospective cohort study. A total of 5.1% and 14.6% of primary gastric lymphoma patients had gastric cancer and precancerous conditions, respectively. This is of particular concern in China, where the number of new cases and deaths of gastric cancer each year is close to 50% of all the world’s cases [22,23]. Chronic inflammation and the progressing cascade of precancerous conditions caused by H. pylori are high-risk factors for gastric cancer [24], and primary gastric lymphoma is a gastric malignant tumour second only to gastric adenocarcinoma in prevalence [3]. Pathological examination of gastroscopic biopsy is the gold standard for the diagnosis of primary gastric lymphoma. The majority of primary gastric lymphoma are of B-cell lineage pathologically, of which DLBCL accounts for 45% to 59%, and MALT lymphoma accounts for 38% to 48% of primary gastric lymphoma cases worldwide [25,26].
Cases of primary gastric lymphoma that are complicated with gastric adenocarcinoma are relatively rare in clinical practice, and therefore there are few studies on this topic, most of which are case reports and small-sample or single-centre studies. However, these previous studies and our research have shown that the presence of gastric malignant lymphoma increases the incidence of gastric cancer. Previous studies have shown that patients with primary gastric lymphoma face a significantly higher risk of developing other malignant tumours, including gastric cancer [27,28]. Amiot et al. [29] showed that patients with gastric MALT lymphoma had a 16-fold increased risk of gastric cancer compared with the French general population [29]. Our research also found that 5.1% of primary gastric lymphoma patients had gastric cancer, much higher than for the general Chinese population with a 5-year prevalence of 0.0276%. Inaba et al. [9] found that 7.2% (10/139) of Japanese patients with primary gastric lymphoma treated with radiotherapy developed metachronous gastric adenocarcinoma, which was higher than the prevalence of gastric adenocarcinoma in the general Japanese population. Old age, H. pylori infection, gastric mucosal change of chronic gastritis, and intestinal metaplasia were also found to be possible risk factors for metachronous gastric adenocarcinoma [9]. Capelle et al. [10] also showed that Dutch patients with gastric MALT lymphoma were six times (2.4%, 34/1419) more likely to have metachronous gastric adenocarcinoma than the general Dutch population, and Ishihama et al. [28] reported that 3.3% (4/121) of primary gastric malignant lymphoma patients develop synchronous gastric adenocarcinoma in Japan, a much higher figure than for the Japanese population in general (about 0.05%). The diagnosis interval between metachronous gastric cancer and primary gastric lymphoma varied widely. As previously reported, 91.2% to 100% of gastric cancer in primary gastric lymphoma patients were metachronous gastric adenocarcinoma developed after lymphoma [9,10]. Inaba et al. [9] reported that the mean latent period between primary gastric lymphoma and metachronous gastric adenocarcinoma was 43.1 months (range: 7.9 to 90.8 months). Our research reported 1.9% and 3.2% of primary gastric lymphoma patients had synchronous gastric cancer and metachronous gastric cancer, respectively, which were similar to those reported in previous literature, and higher than that in the general Chinese population.
There are even fewer studies on primary gastric lymphoma complicated with gastric precancerous conditions. However, the studies that have been conducted have found that atrophy and intestinal metaplasia appear in 54% to 91% and 51% to 70% of the surrounding tissue of gastric MALT, respectively [12,30]. In addition, Capelle et al. [10] found that 31% (440/1419) of Dutch gastric MALT patients had gastric precancerous conditions, with 4.6% having atrophic gastritis, 21.3% having intestinal metaplasia, and 5.1% having dysplasia. Later, they also found that precancerous conditions existed in 67.5% (27/40) of gastric MALT patients simultaneously and that the prevalence of atrophy, intestinal metaplasia, and dysplasia was 20%, 35%, and 12.5%, respectively [11]. No investigations of primary gastric lymphoma patients complicated with gastric cancer and precancerous conditions in China have previously been conducted.
Despite this, our observations for the prevalence of gastric cancer in primary gastric lymphoma patients are consistent with most earlier studies. However, we must pay attention to the endoscopic diagnosis of gastric adenocarcinoma both for the initial endoscopy and the endoscopic review during the follow-up of patients with primary gastric lymphoma. The prevalence of gastric precancerous conditions in primary gastric lymphoma is lower in our study than in earlier ones, and possible reasons for this include a lack of high-definition endoscopy, and insufficient knowledge and limited diagnostic ability of endoscopists on gastric precancerous conditions in the earlier years. Moreover, the sample size and time span of the study subjects are also factors that cannot be ignored. Insufficient amounts taken for biopsy might lead to insufficient bases for pathological diagnosis. In addition, the follow-up of gastric precancerous conditions in our study was consistent with Correa’s cascade [24].
This study also analyzed the clinicopathological characteristics of primary gastric lymphoma, gastric cancer, and precancerous conditions, including H. pylori infection. We found that most primary gastric lymphomas were ulcerative type and Lugano Classification stage I + II, and were mostly confirmed to be DLBCL and MALT lymphomas, and we also found that primary gastric lymphoma patients with ulcerative type and Lugano classification stage IIE + IV had a higher risk of developing gastric cancer and precancerous conditions as well. Furthermore, the H. pylori infection rate was higher in patients with MALT lymphoma, Lugano classification stage I + II, and patients with precancerous conditions and gastric cancer, especially gastric cancer of the intestinal type. Our findings on clinicopathological features of primary gastric lymphoma are consistent with those of other studies, which we discuss below.
One earlier study found that about two-thirds of primary gastric MALT lymphoma patients were diagnosed at stage I, and that stage IV accounted for only 4.2% [31]. Primary gastric lymphoma with stage IV had disseminated extranodal involvement or concomitant supradiaphragmatic nodal involvement according to the modified Lugano staging of primary gastrointestinal lymphoma [32]. We also had less than 10% of stage IV patients in our study. The ulcerative type was the most frequent presentation at endoscopy [31]. The appearance of an ulcer generally indicates invasion of the muscularis mucosae and deeper layers, which is related to severe Lugano classification stage and high-grade lymphoma [31]. It has also been shown that high-grade lymphomas presented more commonly as ulcerative type, being more frequently diagnosed in stage > I when compared with low-grade lymphomas [33]. We also found that the ulcerative type was an important risk factor for developing gastric cancer and precancerous conditions in primary gastric lymphoma, as was having a severe Lugano classification stage. A possible reason for this is that patients with ulcerative types and severe stages may have had a longer course of the underlying disease. For patients under follow-up care for primary gastric lymphoma, we need to distinguish metachronous gastric cancer and precancerous conditions from the recurrence of gastric lymphoma. We noticed that the median largest tumour size of 4 cm in metachronous gastric cancer was too large considering the median diagnosis interval of 3 years between primary gastric lymphoma and metachronous gastric cancer. A study of minute gastric cancer also showed that the mean growth rate was 0.0071 mm/day, and it took an average of 3.42 years to grow to 5 mm, with 95% of cases being well-differentiated tubular adenocarcinoma [34]. One possible reason for the larger tumour size of the metachronous gastric cancers in our study is that some gastric cancers may have been present before or at the time of diagnosis of primary gastric lymphoma but were missed. The small sample size of 15 metachronous gastric cancer patients is also a very important factor. In addition, 20% of metachronous gastric cancers were undifferentiated adenocarcinomas, which grow fast. All of these factors may exist, but no matter what the cause, gastric cancer should not be missed. Therefore, careful endoscopic and pathological evaluation is required to make the diagnosis.
Rentien et al. [35] found that precancerous conditions were more frequent in gastric MALT lymphoma than DLBCL and H. pylori-associated gastritis, and more frequent in the MALT lymphoma area than other areas in the stomach. However, our study didn’t find differences in the prevalence of precancerous conditions in gastric MALT lymphoma and DLBCL. Therefore, the prevalences of precancerous conditions among different pathological types of gastric lymphoma need to be further studied with large samples.
For our observed high H. pylori infection rate of primary gastric lymphoma, studies have even shown that H. pylori infection is related to adenocarcinoma of the distal stomach and gastric lymphoma [5,6,8]. Some studies have even shown that H. pylori infection was present in nearly 90% of primary gastric lymphoma patients [12,31]. H. pylori can cause chronic inflammation, gastric precancerous conditions, and intestinal-type gastric adenocarcinoma [6,8]. Therefore, we indeed observed that the H. pylori infection rate was higher in patients with primary gastric lymphoma complicated with gastric cancer and with precancerous conditions, and the underlying biological relationship between H. pylori, primary gastric lymphoma and gastric cancer, and precancerous conditions warrants further study. We also found that H. pylori eradication was effective in 40.9% of patients with primary gastric lymphoma, including 55% in MALT lymphoma and 28% in DLBCL, suggesting the necessity of eradicating H. pylori therapy in patients with primary gastric lymphoma. H. pylori eradication is the first choice for the treatment of gastric MALT lymphoma, and it has been reported so far that eradication of H. pylori can achieve a complete pathological response in 47% to 100% of H. pylori-positive gastric MALT lymphoma [5]. However, the role of H. pylori eradication in gastric DLBCL is still controversial. Gastric DLBCL with evidence of MALT is classified as DLBCL (MALT), which is not high-grade transformed MALT lymphoma or de novo DLBCL [5]. Several studies have found that some gastric DLBCL patients respond to H. pylori eradication therapy. A multicenter prospective study has reported that 80% of patients with early-stage gastric DLBCL (MALT) achieve complete remission at a median of 4.0 months after H. pylori eradication [36]. Several other studies also showed that some H. pylori-positive gastric DLBCL(MALT) patients respond to H. pylori eradication [37–39]. There were also cases of gastric de novo DLBCL patients who regressed after H. pylori eradication [40,41]. A study in Japan showed 27% of gastric de novo DLBCL patients achieved complete remission with eradication [42]. The efficacy of H. pylori eradication in DLBCL patients might be related to histological type and depth of invasion [42]. BCL10, NF-κB (p65), and CagA expression were also found to help predict the efficacy of H. pylori eradication in patients with early-stage gastric DLBCL(MALT) [36]. The results of our study and previous studies suggest that prospective studies on the efficacy of H. pylori eradication in gastric DLBCL patients are promising.
In addition, a previous study suggested an increased risk of metachronous gastric cancer in gastric DLBCL patients treated with chemotherapy, which can be explained by the fact that patients with advanced stages needed chemotherapy [9]. Amiot et al. [29] also found an increased risk of metachronous gastric cancer in gastric MALT Lymphoma patients who received immunotherapy. Our study didn’t find the negative effects of chemotherapy, radiotherapy, immunotherapy, and targeted therapy on the development of metachronous gastric cancer in primary gastric lymphoma patients. The association and mechanism of various therapies for lymphoma and the risk of solid tumours, including gastric cancer, remain to be further investigated. Furthermore, whether gastric cancer or gastric malignant lymphoma was treated first depended on the time of diagnosis. For patients with synchronous gastric cancer, the pathological types, stages and expected prognosis of gastric cancer and lymphoma should be taken into consideration to determine the treatment plan. Extending survival and improving quality of life is the primary goal at all times.
It’s also worth noting that we found no significant difference in prognosis between primary gastric lymphoma patients and complicated with gastric cancer. The main possible reason that gastric cancer didn’t significantly affect survival in primary gastric lymphoma patients was that the sample size was too small to represent the population. A previous study reported that the 5-year overall survival rates for gastric DLBCL and MALT lymphomas were 89.6% and 97.7%, respectively, while the 5-year survival rates for patients complicated with gastric cancer were 75.0% [10]. Differences in survival were not statistically analyzed in the study because only 139 patients with primary gastric lymphoma were included, 10 of whom had gastric cancer [10]. A few other studies had similar limitations. In addition, early gastric cancer accounted for 25% of patients with gastric cancer in our study, with a high survival rate and little impact on the prognosis. Regular follow-up of gastric lymphomas provided opportunities for the detection of early gastric cancer, which is also related to the detection and monitoring of precancerous conditions. One earlier study showed that up to 76% of gastric cancer in primary gastric lymphoma patients were of early type [28]. Other studies have shown increased prevalences and progression of intestinal metaplasia and dysplasia during follow-up for gastric lymphoma, which associated with the occurrence of carcinoma [32,43]. We also found a higher detection rate of early gastric cancer (25.0%) in primary gastric lymphoma patients than in the general Chinese population (11%) [44], which may explain the better prognosis and higher 5-year survival rate of gastric cancer complicated in primary gastric lymphoma patients (40.0%) than the gastric cancer patients (27.4%) in China [23]. Our study also found that the development of gastric cancer and precancerous conditions complicated in primary gastric lymphoma conformed to Correa’s cascade [24]. Therefore, the follow-up of patients with primary gastric lymphoma may provide an opportunity for early detection of gastric cancer, thereby improving the prognosis of gastric cancer.
The primary advantage of our study is that it is the first Chinese study of primary gastric lymphoma complicated with gastric cancer and precancerous conditions. Moreover, our study is a multi-centre study and has the largest sample size among the current studies on gastric lymphoma complicated with gastric cancer and precancerous conditions, followed by the Dutch study [10]. There are also some limitations to the study. The three centres included in this study are located in urban areas in China, two of which are located in the same city. Therefore, there may be bias among the study subjects. In addition, endoscopists could diagnose atrophic gastritis and intestinal metaplasia based on endoscopic findings, and biopsy pathology was not performed on all these patients. Although pathology is the ‘gold standard’, pathological evaluation of gastric atrophy and intestinal metaplasia is also based on endoscopic observation by endoscopists and is related to the sampling location and number. Endoscopic evaluation of gastric atrophy and intestinal metaplasia also relies on the endoscopic equipment and the experience of the endoscopists. Conventional white-light endoscopy showed a poor correlation between histological and endoscopic findings in the diagnosis of gastric atrophy and intestinal metaplasia [16]. High-definition white-light endoscopy can identify atrophy and intestinal metaplasia better than conventional white-light endoscopy [45]. Narrow-band imaging and white-light endoscopy nontargeted biopsies may present more promising results for the diagnosis of atrophy and intestinal metaplasia [46,47]. Narrow-band imaging-guided biopsies further improved the diagnostic yield of atrophy and intestinal metaplasia [48]. We included patients with atrophy and intestinal metaplasia both endoscopically and pathologically diagnosed in the gastric precancerous conditions group. While high definition white-light endoscopy and/or narrow-band imaging were used in most cases, except in some early cases. Furthermore, because of our lack of knowledge about the grading of atrophy and intestinal metaplasia, many patients were not graded, especially before 5 years ago. We cannot accurately judge the severity of atrophy or intestinal metaplasia according to the endoscopic pictures at present. However, if we discard cases that are not graded for precancerous conditions, we will lose a lot of cases and information, and we will underestimate the probability of precancerous conditions in patients with primary gastric lymphoma. Therefore, we included these patients with precancerous conditions that were not graded for severity in the precancerous conditions group. It does cause bias.
5. Conclusion
In summary, we find that patients with primary gastric lymphoma have a high risk of developing gastric cancer and precancerous conditions and that this risk may be related to H. pylori infection. Follow-up of primary gastric lymphoma may provide an opportunity for early detection of gastric cancer. Endoscopists should pay attention to the endoscopic findings of metachronous gastric cancer, synchronous gastric adenocarcinoma and precancerous conditions in patients with primary gastric lymphoma.
Acknowledgements
The authors thank the support of the science and technology department of the hospital.
Funding Statement
This work was supported by the Key Research and Development Program of Shaanxi [grant numbers 2019SF023, 2021ZDLSF02-06]; the Institutional Foundation of The First Affiliated Hospital of Xi’an Jiaotong University [grant number 2021QN-10].
Ethics approval
This work was approved by Ethics Committee of The First Affiliated Hospital of Xi’an Jiao Tong University (KYLLSL-2021-112) and complied with the Declaration of Helsinki.
Author contributions
Yun Feng and Hong-Xia Li were responsible for the study conception and design. Yun Feng, Tian-Jiao Duan, Qing Huang, and Zhi-Yi Li participated in subject enrollment and data collection. Ya-ping Liu, Miaosha Luo, Guifang Lu, Wen Shi, Zhi-Yong Zhang performed gastroscopy, data statistics and analysis. Yun Feng was responsible for drafting the manuscript. Hong-Xia Li supervised the whole process of the research and revised the manuscript. All the authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):1–14. [DOI] [PubMed] [Google Scholar]
- 2.Karimi P, Islami F, Anandasabapathy S, et al. . Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Akwaa AM, Siddiqui N, Al-Mofleh IA.. Primary gastric lymphoma. World J Gastroenterol. 2004;10(1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Z, Fang M, Huang Y, et al. . CT-based radiomics signature for differentiating borrmann type IV gastric cancer from primary gastric lymphoma. Eur J Radiol. 2017;91:142–147. [DOI] [PubMed] [Google Scholar]
- 5.Paydas S. Helicobacter pylori eradication in gastric diffuse large B cell lymphoma. World J Gastroenterol. 2015;21(13):3773–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SS, Ruiz VE, Carroll JD, et al. . Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Lett. 2011;305(2):228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Negovan A, Iancu M, Fulop E, et al. . Helicobacter pylori and cytokine gene variants as predictors of premalignant gastric lesions. World J Gastroenterol. 2019;25(30):4105–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki H, Matsuzaki J.. Gastric cancer: evidence boosts Helicobacter pylori eradication. Nat Rev Gastroenterol Hepatol. 2018;15(8):458–460. [DOI] [PubMed] [Google Scholar]
- 9.Inaba K, Kushima R, Murakami N, et al. . Increased risk of gastric adenocarcinoma after treatment of primary gastric diffuse large B-cell lymphoma. BMC Cancer. 2013;13:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capelle LG, de Vries AC, Looman CW, et al. . Gastric MALT lymphoma: epidemiology and high adenocarcinoma risk in a nation-wide study. Eur J Cancer. 2008;44(16):2470–2476. [DOI] [PubMed] [Google Scholar]
- 11.Capelle LG, den Hoed CM, de Vries AC, et al. . Premalignant gastric lesions in patients with gastric mucosa-associated lymphoid tissue lymphoma and metachronous gastric adenocarcinoma: a case-control study. Eur J Gastroenterol Hepatol. 2012;24(1):42–47. [DOI] [PubMed] [Google Scholar]
- 12.Lamarque D, Levy M, Chaumette MT, et al. . Frequent and rapid progression of atrophy and intestinal metaplasia in gastric mucosa of patients with MALT lymphoma. Am J Gastroenterol. 2006;101(8):1886–1893. [DOI] [PubMed] [Google Scholar]
- 13.Matysiak-Budnik T, Fabiani B, Hennequin C, et al. . Gastrointestinal lymphomas: french intergroup clinical practice recommendations for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFH). Dig Liver Dis. 2018;50(2):124–131. [DOI] [PubMed] [Google Scholar]
- 14.Dawson IM, Cornes JS, Morson BC.. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961;49:80–89. [DOI] [PubMed] [Google Scholar]
- 15.Shah SC, Piazuelo MB, Kuipers EJ, et al. . AGA clinical practice update on the diagnosis and management of atrophic gastritis: expert review. Gastroenterology. 2021;161(4):1325–1332.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pimentel-Nunes P, Libânio D, Marcos-Pinto R, et al. . Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): european society of gastrointestinal endoscopy (ESGE), european helicobacter and microbiota study group (EHMSG), european society of pathology (ESP), and sociedade Portuguesa de endoscopia digestiva (SPED) guideline update 2019. Endoscopy. 2019;51(4):365–388. [DOI] [PubMed] [Google Scholar]
- 17.Kimura K, Takemoto T.. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;3:87–97. [Google Scholar]
- 18.Huang RJ, Choi AY, Truong CD, et al. . Diagnosis and management of gastric intestinal metaplasia: current status and future directions. Gut Liver. 2019;13(6):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giroux V, Rustgi AK.. Metaplasia: tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat Rev Cancer. 2017;17(10):594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosman FT. WHO classification of tumours of the digestive system. 5th edn. Lyon: IARC: World Health Organization; 2010. p. 71–101. [Google Scholar]
- 21.Yakirevich E, Resnick MB.. Pathology of gastric cancer and its precursor lesions. Gastroenterol Clin North Am. 2013;42(2):261–284. [DOI] [PubMed] [Google Scholar]
- 22.GBD 2017 Stomach Cancer Collaborators The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Zheng R, Baade PD, et al. . Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 24.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process - First American cancer society award lecture on cancer epidemiology and prevention. Cancer Re. 1992;52(24):6735–6740. [PubMed] [Google Scholar]
- 25.Ferrucci PF, Zucca E.. Primary gastric lymphoma pathogenesis and treatment: what has changed over the past 10 years? Br J Haematol. 2007;136(4):521–538. [DOI] [PubMed] [Google Scholar]
- 26.Juarez-Salcedo LM, Sokol L, Chavez JC, et al. . Primary gastric lymphoma, epidemiology, clinical diagnosis, and treatment. Cancer Control. 2018;25(1):1073274818778256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carson HJ. Unexpected synchronous non-Hodgkin’s lymphoma encountered during the treatment of a previously-diagnosed carcinoma: report of three cases. Leuk Lymphoma. 1996;23(5-6):625–629. [DOI] [PubMed] [Google Scholar]
- 28.Ishihama T, Kondo H, Saito D, et al. . Clinicopathological studies on coexisting gastric malignant lymphoma and gastric adenocarcinoma: report of four cases and review of the japanese literature. Jpn J Clin Oncol. 1997;27(2):101–106 [DOI] [PubMed] [Google Scholar]
- 29.Amiot A, Jooste V, Gagniere C, et al. . Second primary malignancies in patients treated for gastric mucosa-associated lymphoid tissue lymphoma. Leuk Lymphoma. 2017;58(9):1–11. [DOI] [PubMed] [Google Scholar]
- 30.Herrera-Goepfert R, Arista-Nasr J, Alba-Campomanes A.. Pathologic features of the gastric mucosa adjacent to primary MALT-lymphomas. J Clin Gastroenterol. 1999;29(3):266–269. [DOI] [PubMed] [Google Scholar]
- 31.Zullo A, Hassan C, Andriani A, et al. . Primary low-grade and high-grade gastric MALT-lymphoma presentation. J Clin Gastroenterol. 2010;44(5):340–344. [DOI] [PubMed] [Google Scholar]
- 32.Rohatiner A, D’Amore F, Coiffier B, et al. . Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994;5(5):397–400. [DOI] [PubMed] [Google Scholar]
- 33.Andriani A, Zullo A, Di Raimondo F, et al. . Clinical and endoscopic presentation of primary gastric lymphoma: a multicentre study. Aliment Pharmacol Ther. 2006;23(6):721–726. [DOI] [PubMed] [Google Scholar]
- 34.Liu ZH, Lu SY, Li XB, et al. . Endoscopic, clinicopathological, and growth characteristics of minute gastric cancer. J Dig Dis. 2022;23(11):628–635. [DOI] [PubMed] [Google Scholar]
- 35.Rentien AL, Lévy M, Copie-Bergman C, et al. . Long-term course of precancerous lesions arising in patients with gastric MALT lymphoma. Dig Liver Dis. 2018;50(2):181–188. [DOI] [PubMed] [Google Scholar]
- 36.Tsai HJ, Tai JJ, Chen LT, Taiwan Cooperative Oncology Group, et al.. A multicenter prospective study of first-line antibiotic therapy for early-stage gastric mucosa-associated lymphoid tissue lymphoma and diffuse large B-cell lymphoma with histological evidence of mucosa-associated lymphoid tissue. Haematologica. 2020;105(7):e349-54–e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferreri AJ, Govi S, Raderer M, et al. . Helicobacter pylori eradication as exclusive treatment for limited-stage gastric diffuse large B-cell lymphoma: results of a multicenter phase 2 trial. Blood. 2012;120(18):3858–3860. [DOI] [PubMed] [Google Scholar]
- 38.Cavanna L, Pagani R, Seghini P, et al. . High grade B-cell gastric lymphoma with complete pathologic remission after eradication of Helicobacter pylori infection: report of a case and review of the literature. World J Surg Oncol. 2008;6(6):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuo SH, Yeh KH, Wu MS, et al. . Helicobacter pylori eradication therapy is effective in the treatment of early-stage H pylori-positive gastric diffuse large B-cell lymphomas. Blood. 2012;119(21):4838–4844; quiz 5057. [DOI] [PubMed] [Google Scholar]
- 40.Sugimoto M, Kajimura M, Sato Y, et al. . Regression of primary gastric diffuse large B-cell lymphoma after eradication of Helicobacter pylori. Gastrointest Endosc. 2001;54(5):643–645. [DOI] [PubMed] [Google Scholar]
- 41.Alsolaiman MM, Bakis G, Nazeer T, et al. . Five years of complete remission of gastric diffuse large B cell lymphoma after eradication of Helicobacter pylori infection. Gut. 2003;52(4):507–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tari A, Asaoku H, Kashiwado K, et al. . Predictive value of endoscopy and endoscopic ultrasonography for regression of gastric diffuse large B-cell lymphomas after Helicobacter pylori eradication. Dig Endosc. 2009;21(4):219–227. [DOI] [PubMed] [Google Scholar]
- 43.Zullo A, Rago A, Felici S, et al. . Onset and progression of precancerous lesions on gastric mucosa of patients treated for gastric lymphoma. J Gastrointestin Liver Dis. 2020;29(1):27–31. [DOI] [PubMed] [Google Scholar]
- 44.Strong VE, Wu AW, Selby LV, et al. . Differences in gastric cancer survival between the U.S. and China. J Surg Oncol. 2015;112(1):31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kikuste I, Marques-Pereira R, Monteiro-Soares M, et al. . Systematic review of the diagnosis of gastric premalignant conditions and neoplasia with high-resolution endoscopic technologies. Scand J Gastroenterol. 2013;48(10):1108–1117. [DOI] [PubMed] [Google Scholar]
- 46.Pimentel-Nunes P, Libanio D, Lage J, et al. . A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy. 2016;48(8):723–730. [DOI] [PubMed] [Google Scholar]
- 47.Xirouchakis E, Laoudi F, Tsartsali L, et al. . Screening for gastric premalignant lesions with narrow band imaging, white light and updated Sydney protocol or both? Dig Dis Sci. 2013;58(4):1084–1090. [DOI] [PubMed] [Google Scholar]
- 48.Buxbaum JL, Hormozdi D, Dinis-Ribeiro M, et al. . Narrow-band imaging versus white light versus mapping biopsy for gastric intestinal metaplasia: a prospective blinded trial. Gastrointest Endosc. 2017;86(5):857–865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.