ABSTRACT
As a hallmark of COVID-19 progression, lymphopenia alongside its subtle immune disturbance has been widely reported, but yet to be thoroughly elucidated. Aiming at exploring clinical immune biomarkers with accessibility in the current and acute omicron epidemic abrupted in China post-control era, we design a real-world prospective observation cohort in Peking Union Medical College Hospital to describe immunological, haematological profiles inducing lymphocyte subsets related to SARS-CoV-2 infection. In this COVID-19 cohort, we enrolled 17 mild/moderate (M/M), 24 severe (S) and 25 critical (C) patients. The dynamics of lymphocytes of COVID-19 demonstrated that the sharp decline of NK, CD8+, and CD4+ T cell counts was the main contributor to lymphopenia in the S/C group, compared to the M/M group. Expressions of activation marker CD38 and proliferation marker Ki-67 both in CD8+ T and NK cells were significantly higher in all COVID-19 patients than that in healthy donors, independent of disease severity. The subsequent analysis showed in contrast to the M/M group, NK and CD8+ T cell counts remained low-level after therapy in the S/C group. CD38 and Ki-67 expressions in NK and CD8+ T cells still stay at a high level, despite active treatment. Targeting relatively elderly patients with SARS-CoV-2 infection, severe COVID-19 features the unreversible reduction of NK and CD8+ T cells with persistent activation and proliferation, which assist clinicians in early recognizing and saving severe or critical COVID-19 patients. Given that immunophenotype, the new immunotherapy improving NK and CD8+ T lymphocyte antiviral efficiency should be considered.
KEYWORDS: NK cell, CD8+ T cell, immunophenotype, COVID-19, CD38
Introduction
In China, the State Council’s joint prevention and control mechanism against Coronavirus disease 2019 (COVID-19) has been continuously regulating the measures to decrease the damage of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) since December 2019. On 7th December 2022, new 10 prevention and control measures were released to optimize the COVID-19 response, the surging number of infected population with omicron, mainly variant of concern (VOC) BF.7, in Beijing on December appeared in Chinese mainland [1]. During this COVID-19 wave in Beijing, the clinicians at the front lines suggested that patients with omicron are usually mild without organ dysfunction among younger people but severe even critical in an elderly population with an overwhelming inflammatory storm. The latest outbreak of COVID-19 in Beijing still claims more lives among the elderly population due to the shortage of specific treatments and efficient vaccination. The treatment based on patients’ symptoms usually lagged behind the underlying immune disorders induced by the rapidly evolving virus into emerging variants [2]. Facing such high contagious respiratory virus, it is imperative to explore more timely and accessible biomarkers related to immune disturbance in the COVID-19 pandemic.
Previous studies have shown that old age, co-morbidities, lymphocytopenia combined with significant elevation of neutrophils and high inflammatory factors (ESR, CRP, IL-6, ferritin, etc.) are highly related to COVID-19 progression [3–5]. A prominence of severe or critical COVID-19 is lymphopenia [6]. Furthermore, lymphocyte subset analysis has reported a general reduction in T cells, and natural killer (NK) cells among severe and critical patients [7]. Moreover, the total number of CD8+ and CD4+ T cells was demonstrated to decrease in patients with SARS-CoV-2 infection, particularly in elderly patients over 60 years old [8]. Based on our previous research and recent observation about the clinical features associated with SARS-CoV-2 infection [2], the unsuppressed viremia alongside lymphocytopenia and out-of-control inflammatory storm resulted in severe organ dysfunction, usually occurring at 7–14 days after onset. Based on that, it is essential for clinicians to distinguish critical COVID-19 patients according to underlying immune response changes.
Theoretically, the human body’s response to a virus enrols an innate and adaptive immune system, including monocyte, NK cells, B cells and T cells, and then ignites the inflammation [9]. The high elevation of the serum inflammatory factors may result in predicting the clinical symptoms and prognoses [10]. Based on what we have found in SARS, progressive lymphocytopenia has also been discovered and usually precedes the radiography abnormality [11]. Additionally, we have proposed rational and widely acceptable hypothetical pathogenesis of SARS-CoV-2 infection in 2020 [2], suggesting that lymphocyte subsets should be monitored during the COVID-19 course and optimal therapy could be given as early as possible when lymphocyte counts in peripheral blood show decreasing trend to avoid more severe outcomes caused by a subsequent inflammatory storm. Given that, the dynamic lymphocyte subset monitoring during the disease course would be conducive to early recognition of critical illness. The detailed immunophenotype parameters associated with lymphocytopenia in COVID-19 may assist clinicians to evaluate who is at high risk of severe or critical status. Moreover, to further elucidate the immunopathogenesis of COVID-19, it is vital to portray the lymphocyte subset profile for COVID-19 patients with different clinical manifestations, varying from mild or moderate to severe even critical illness. Thereafter, our patient may benefit from the early detection of the underlying immune disturbance related to COVID-19. In other words, no matter what therapy will be given, early alert for severe or critical illness and optimal intervention plays a pivotal role in blocking the impending inflammatory cascade related to COVID-19 progression.
Therefore, during the current and acute SARS-CoV-2 omicron infection wave in Beijing, China on December 2022, we design a prospectively real-world observation study to further disclose which lymphocyte subgroup is the main contributor to lymphopenia and evaluate the extent of innate and/or adaptive immune reaction upon COVID-19 severity. Furthermore, it is urgent to determine the immunophenotype biomarkers with clinical accessibility to early distinguish and treat severe or critical illness.
Materials and methods
Patients and study design
We designed a prospective real-world observational study in Peking Union Medical College Hospital (PUMCH), which consecutively enrolled COVID-19 patients treated from 28th December 2022 to 10th January 2023. The enrolled COVID-19 patients were confirmed to be infected with SARS-CoV-2 by RT–PCR assays and/or antigen tests. All patients were categorized into mild or moderate patients, severe patients and critical patients, according to the guidelines on the diagnosis and treatment of new coronavirus pneumonia (version 9) issued by the National Health Commission of China issued on 15th March 2022. Based on that, mild COVID-19 was defined as mild clinical symptoms without radiological findings of pneumonia; moderate COVID-19 was identified as fever and respiratory symptoms with mild radiological abnormal but not requiring supplemental oxygen; severe COVID-19 was considered if satisfying any of the following criteria: respiration rate ≥30 breaths/min, oxygen saturation ≤93% at rest, oxygenation index ≤300 mmHg, or progressive severe clinical symptoms and chest imaging progressed >50% within 24–48 h; and critical COVID-19 was considered as respiratory failure requiring mechanical ventilation, shock, and/or other organs failures requiring ICU care. All baseline medical record information, including demographic data and clinical characteristics, was obtained within the first day after recruitment. Blood samples were first collected at enrolment and then 7 days after enrolment (supplement figure 1). For comparison, data from age-matched healthy volunteers, collected before the pandemic, were included as controls. Patients’ consent was required before enrolment. This study was approved by the Ethical Committee of Peking Union Medical College Hospital (Ethical Committee Number: I-23PJ463).
Peripheral blood mononuclear cell isolation and cytometric analysis
The fresh whole blood was collected in an EDTA anticoagulant tube at the time points described above. Immunophenotyping of peripheral blood lymphocytes was analysed by 18-colour flow cytometry (LSRFortessa & trade; BD Biosciences, USA). Isolated PBMC from whole blood was incubated and tested with a panel of monoclonal antibodies against CD3/CD8/CD4, CD3/CD16 plus CD56, HLA-DR/CD38/PD-1/Ki-67/CD56 plus CD16, HLA-DR/CD38/PD-1/Ki-67/CD8, CD38/PD-1/Ki-67/CD19, CD28/CD8/CD4, CD62L/CD45RA/ CD4, CD25/CD127/CD4, and isotype controls (Immunotech, France). The proportion of subsets was calculated using FACSDiva software (Becton Dickinson, NJ, USA). Cell counts of lymphocyte subsets were calculated with the white blood cell counts and lymphocyte differentials obtained from blood routine tests of the same specimen.
Statistics
All data parameters were subject to normality testing using the Shapiro–Wilk normality test. The continuous variables were presented as median with interquartile range and compared by Kruskal–Wallis Rank Sum Test or Mann–Whitney U test when data did not conform to a normal distribution. Categorical variables were compared using Chi-square testing. The probability value was obtained from two-sided tests, and p < 0.05 was considered statistically significant. Given the potential confounding effects of gender, vaccination status, and pre-blood-sampling treatment on the lymphocyte subgroups, we used a linear model to test the robustness of our main findings (details in supplement table 2). To illustrate the changes in lymphocyte subgroups at different stages of COVID-19, we used the data of all patients at the enrolment timepoint with different median onset-sampling delays ranging from 12 days for the C group to 16 days for the M/M group. Based on what we obtained at baseline, we split the observational onset-sampling period into 4-time points as 7th, 12th, 17th, and 22nd day after onset. We showed the median of lymphocyte subgroups at 2 days before and after each time point. Based on the observation of the line trend, we illustrated a hypothesis depicting an immunopathology model among mild/moderate, and severe/critical illness. All statistical analysis and figure plot was performed using the R programming language (R, version 4.1.2, 2021-11-01) [12].
Results
PUMCH cohort for SARS-CoV-2
As of writing this report, we had enrolled 66 COVID-19 admission patients including 17 in the M/M group, 24 in the S group, and 25 in the C group. The median disease duration before admission was 11 days [10,13] in the M/M group, 10 days [7,14] in the S group and 9 days [7,10] in the C group. The median age of patients in the three COVID-19 groups was 70 years [57, 83], 77 years [68, 82], and 74 years [67, 80], respectively. The proportion of males was significantly higher in the C group (88.0%), compared to the S (54.2%) and M/M group (47.1%). The boosted-vaccinated rates were 28% in the C group, 33.3% in the S group and 29.4% in the M/M group without statistical significance. Around half of the patients in the three groups received oral antiviral therapy. The patients in the S/C group received glucocorticoid therapy (91.7% for the S group and 96% for the C group), anticoagulant therapy (75% for the S group and 56% for the C group), intravenous immunoglobulin (62.5% for the S group and 32% for the C group) and biologics (20.8% for the S group and 44% for the C group), while the M/M COVID-19 patients had a lower frequency of using glucocorticoid therapy (58.8%), intravenous immunoglobulin (35.3%), anticoagulant therapy (17.6%) (details shown in Table 1). In Table 1, the most frequent comorbidities observed in our cohort were systemic arterial hypertension, diabetes mellitus, chronic kidney disease and cardiovascular disorders, which occurred similarly between M/M and S/C clinical conditions.
Table 1.
Clinical characteristics of COVID-19 admission patients.
| Mild/Moderate | Severe | Critical | pa | |
|---|---|---|---|---|
| N | 17 | 24 | 25 | |
| Age (years, median [IQR]) | 70 [57, 83] | 77 [68, 82] | 74 [67, 80] | 0.545 |
| Positive PCR result (n, %) | 10 (58.8) | 9 (37.5) | 15 (60.0) | 0.226 |
| Male (n, %) | 8 (47.1) | 13 (54.2) | 22 (88.0) | 0.009 |
| Onset-admission delays (days, median [IQR]) | 11 [10, 15] | 10 [7, 13] | 9 [7, 10] | 0.150 |
| Onset-sampling interval (days, median [IQR]) | 16 [12, 17] | 14 [13, 18] | 12 [11, 17] | 0.399 |
| Comorbidities | ||||
| HTN (n, %) | 4 (23.5) | 13 (54.2) | 14 (56.0) | 0.079 |
| CAD (n, %) | 5 (29.4) | 12 (50.0) | 8 (32.0) | 0.304 |
| CVD (n, %) | 3 (17.6) | 2 (8.3) | 2 (8.0) | 0.549 |
| COPD (n, %) | 0 (0.0) | 3 (12.5) | 2 (8.0) | 0.328 |
| DM (n, %) | 3 (17.6) | 8 (33.3) | 12 (48.0) | 0.126 |
| CLD (n, %) | 0 (0.0) | 0 (0.0) | 2 (8.0) | 0.184 |
| CKD (n, %) | 2 (11.8) | 4 (16.7) | 8 (32.0) | 0.229 |
| Tumour (n, %) | 2 (11.8) | 4 (16.7) | 8 (32.0) | 0.229 |
| IDD (n, %) | 3 (17.6) | 7 (29.2) | 5 (20.0) | 0.631 |
| CTD (n, %) | 3 (17.6) | 7 (29.2) | 5 (20.0) | 0.631 |
| Vaccination number (n, %) | 0.538 | |||
| ≥ 2 | 5 (29.4) | 8 (33.3) | 7 (28.0) | |
| 1 | 1 (5.9) | 0 (0.0) | 3 (12.0) | |
| 0 | 11 (64.7) | 16 (66.7) | 15 (60.0) | |
| The interval of vaccine (days, median [IQR]) | 264 [220, 301] | 394 [279, 439] | 446 [235, 536] | 0.445 |
| Respiratory support (n, %) | <0.001 | |||
| None | 5 (29.4) | 0 (0.0) | 0 (0.0) | |
| Nasal catheter | 11 (64.7) | 13 (54.2) | 1 (4.0) | |
| Mask | 0 (0.0) | 9 (37.5) | 2 (8.0) | |
| HFNC | 1 (5.9) | 2 (8.3) | 3 (12.0) | |
| MV | 0 (0.0) | 0 (0.0) | 19 (76.0) | |
| Antiviral therapy (n, %) | 0.649 | |||
| None | 10 (58.8) | 12 (50.0) | 15 (60.0) | |
| Nirmatrelvir/ritonavir | 5 (29.4) | 8 (33.3) | 7 (28.0) | |
| Molnupinavir | 1 (5.9) | 0 (0.0) | 0 (0.0) | |
| Azvudine | 1 (5.9) | 4 (16.7) | 3 (12.0) | |
| Glucocorticoid (n, %) | 10 (58.8) | 22 (91.7) | 24 (96.0) | 0.002 |
| Intravenous Immunoglobulin (n, %) | 6 (35.3) | 15 (62.5) | 8 (32.0) | 0.070 |
| Anticoagulant therapy (n, %) | 3 (17.6) | 18 (75.0) | 14 (56.0) | 0.001 |
| Biologics (n, %) | 0 (0.0) | 5 (20.8) | 11 (44.0) | 0.004 |
| SpO2 at room air (%, median [IQR]) | 96 [96, 99] | 90 [85, 92] | 80 [75, 85] | <0.001 |
| Clinical parameters | ||||
| PLT (109/ml, median [IQR]) | 194 [171, 226] | 193 [160, 262] | 214 [148, 262] | 0.946 |
| NEUT (109/ml, median [IQR]) | 5.03 [3.86, 5.69] | 6.92 [5.86, 8.89] | 8.63 [6.8 4, 9.99] | <0.001 |
| HGB (g/L, median [IQR]) | 119 [112, 124] | 118 [94, 125] | 115 [101, 126] | 0.614 |
| ALT (U/L, median [IQR]) | 29 [21, 36] | 38 [16, 57] | 25 [18, 47] | 0.940 |
| Urea (mmol/L, median [IQR]) | 6.8 [5.7, 7.8] | 7.7 [6.5, 11.0] | 9.8 [7.5, 18.3] | 0.010 |
| hsCRP (mg/L, median [IQR]) | 7.6 [2.5, 17.4] | 26.6 [6.5, 51.4] | 52.3 [26.3, 93.2] | 0.005 |
| D-Dimer (mg/L FEU, median [IQR]) | 1.21 [1.00, 1.53] | 1.83 [1.29, 3.00] | 4.79 [1.77, 7.99] | 0.013 |
| IL-6 (pg/ml, median [IQR]) | 6.5 [2.8, 43.4] | 7.6 [3.4, 28.7] | 94.6 [23.4, 1000] | 0.052 |
| Ferritin (μg/L, median [IQR]) | 316 [138, 380] | 354 [244, 648] | 359 [249, 702] | 0.540 |
HTN, hypertension; CAD, coronary artery disease; CVD, cerebrovascular disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; CLD, chronic liver disease; CKD, chronic kidney disease; IDD, immunologic deficiency disease; CTD, connective tissue disease; HFNC, high flows through the nasal cannula; MV, mechanical ventilation; PLT, platelet; NEUT, neutrophil count; HGB, haemoglobin; ALT, alanine aminotransferase; Urea, urea nitrogen; hsCRP, hypersensitive C-reactive protein; IL-6, interleukin-6.
The Wilcoxon rank-sum test was used for all continuous variables and the χ2 test was used for all categorical variables.
Circulating lymphocyte subsets varied in different COVID-19 groups
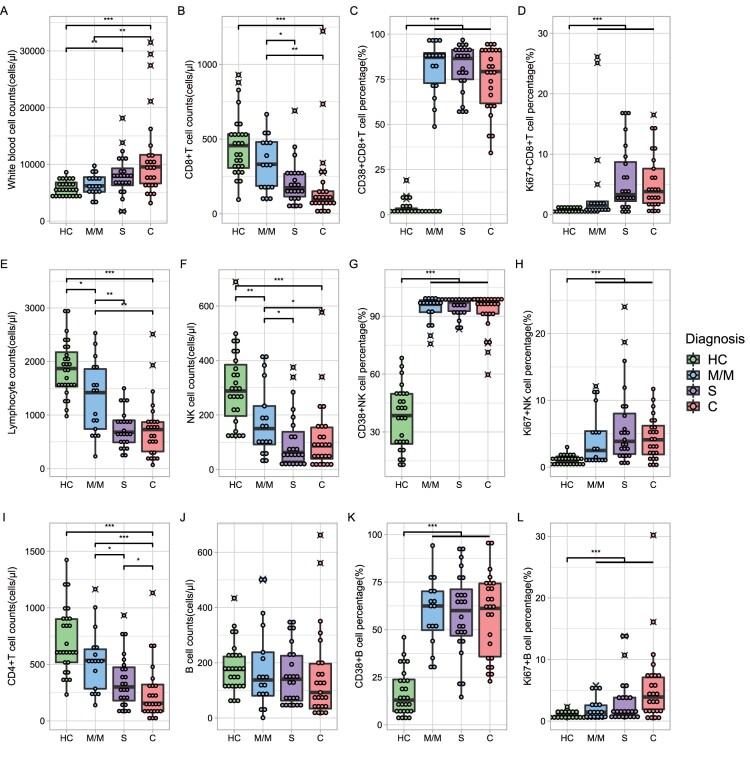
Flow cytometry analysis of lymphocyte subsets was performed in all patients at the enrolment (Figure 1, supplement table 1 and supplement figure 2). Data for a total of 27 healthy donors aged more than 60 years were collected as a control. Lymphopenia was a key feature of COVID-19 regardless of the severity (Figure 1E), as reported in many previous studies [8,14]. The reduction of CD8+ T cells (CD3+ CD8+), CD4+ T cells (CD3+ CD4+) and NK cells (CD56+ CD16+) contributed to lymphopenia. The median of CD8+ T cells was 99 cells/μl [72, 151] in the C group, 173 cells/μl [115, 268] in the S group, and 332 cells/μl [186, 481] in the M/M group, respectively, significantly lower than that in the HC group (456 cells/μl [307, 533]) (Figure 1B, p < 0.001). A similar trend was observed for CD4+ T cell counts (HC 610 cells/μl [518, 901], MM 534 cells/μl [285, 634], S 300 cells/μl [179, 475], C 156 [84, 321]) (Figure 1I, HC vs COVID-19 group p < 0.001) and NK cells (HC 288 cells/μl [196, 385], MM 150 cells/μl [92, 233], S 62 cells/μl [30, 138], C 86 cells/μl [39, 154]) (Figure 1F, HC vs COVID-19 group p < 0.001). Furthermore, a statistically significant difference in CD8+T cells, CD4+ T cells, and NK cells were seen between the M/M group and the C group (Figure 1I,B,F). The decreasing trend of CD8+ T cell counts is more obvious than that of CD4+ T cell counts at the enrolment timepoint (Figure 1I,B). However, compared to HC, COVID-19 patients had stable CD19+ B cell counts (HC 176 cells/μl [117, 223], MM 138 cells/μl [81, 238], S 140 cells/μl [63, 225], C 92 cells/μl [34, 197], Figure 1J).
Figure 1.
Immunophenotype of COVID-19 admission patients. Green dots and boxes, healthy control; blue dots and boxes, mild or moderate COVID-19 admission patients; purple dots and boxes, severe COVID-19 admission patients; red dots and boxes, critical COVID-19 admission patients. The number of samples in the M/M, S, and C groups was 27, 17, and 24, respectively (details in supplement table 1). Significance was determined by the Wilcoxon rank-sum test: ***, < 0.001; **, < 0.01; *, < 0.05. M/M, mild or moderate illness; S, severe illness; C, critical illness.
To identify the kinetics of the functional lymphocytes upon severity of COVID-19, we showed the percentage of activation marker CD38 and proliferation marker Ki67 on T cells, NK cells, and B cells. Compared with the HC group, the percentage of CD38+ CD8+ T cells in all COVID-19 patients expressed higher than that in HC with statistical significance (HC 1.9% [1.3, 3.5], MM 87.2% [72.9, 89.6], S 86.6% [75.0, 91.4], C 79.3% [61.7, 90.7]) (Figure 1C, p < 0.001), so did the percentage of CD38 in NK cells (HC 38.5% [23.8, 49.8], MM 96.2% [92.1, 98.3], S 97.4% [92.7, 97.9], C 97.2% [91.3, 98.3]) (Figure 1G, p < 0.001), and in B cells (Figure 1K, p < 0.001). But the differences in CD38 expression in CD8+ T, NK and B cells among three COVID-19 groups were not significant (Figure 1C,G,K). We also found a higher proportion of Ki67 expression in CD8+ T cells, B cells and NK cells compared with the HC group (Figure 1D,H,L). We utilized a linear regression model that corrected for confounding factors such as gender, vaccine status, and treatment to validate the robustness of our conclusions regarding CD8 and NK cells (details in supplementary table 2).
Immunophenotype changes of COVID-19 patients following 7 days after enrolment
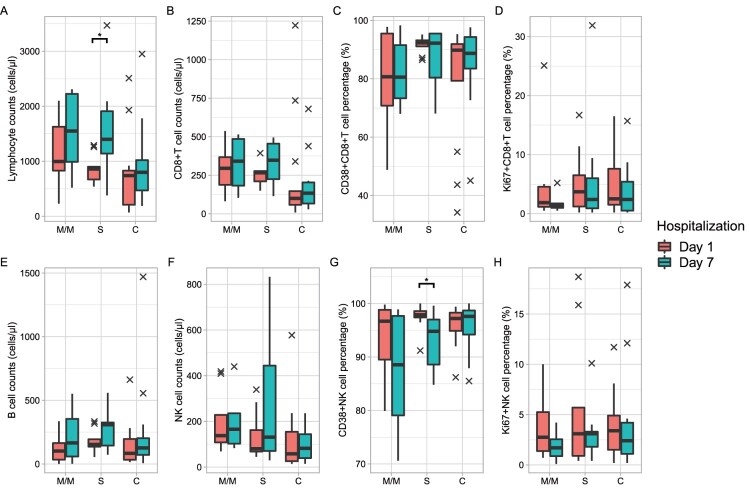
All patients in this cohort were treated with active therapy after enrolment. Therefore, we further examined the lymphocyte subsets following 7 days of treatment. As of writing this report, a total of 8 in the M/M group, 9 in the S group, and 13 in the C group completed blood collection one week after enrolment. Lymphocyte subsets did not change dramatically in just a few 7 days of intervention although most of the patients received glucocorticoid and anti-coagulation therapy. The number of lymphocytes increased slightly in the M/M and C groups, and increased significantly from 265 cells/μl to 347 cells/μl in the S group (Figure 2A). There was also mild recovery in CD8+ T cell counts (Figure 2B), CD4+ T cell counts (supplement Figure 3), and NK cell counts (Figure 2F). The proportion of CD38+ CD8+ T cells showed downregulations in all three groups. A greater downregulation of CD38+NK cells was observed in M/M and S groups compared with the C group (Figure 2G). The expression of Ki67 in NK cells remains high. CD8+ T cells showed a similar change for absolute count, activation, and proliferation proportion in all the three groups.
Figure 2.
Immunophenotype changes of COVID-19 admission patients following 7 days of hospitalization. Orange boxes, baseline immunophenotype; cyan boxes, after 7 days of therapy. The black farks represent outliers. The number of samples in the M/M, S, and C groups was 8, 9, and 13, respectively. Paired Wilcoxon’s rank-sum test was used to assess for a difference before and after 7 days of hospitalization. No significant differences were found except lymphocyte cell counts and CD38+ NK cell percentage in the S group. Significance: ***, <0.001; **, <0.01; *, <0.05. M/M, mild or moderate illness; S, severe illness; C, critical illness.
Longitudinal trajectory of the peripheral lymphocyte subpopulations in different COVID-19 groups
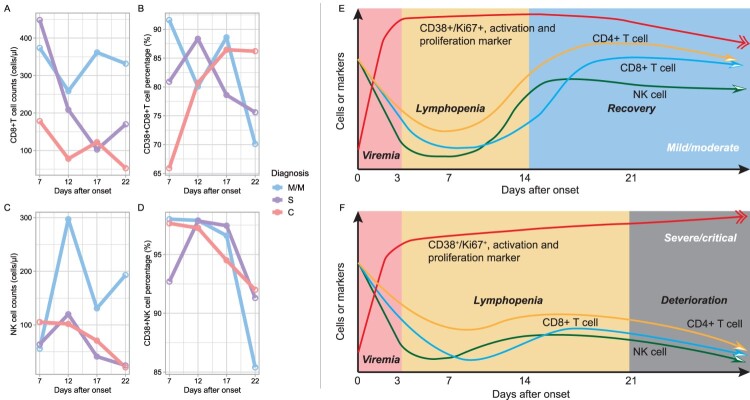
To understand how the lymphocyte subsets and their activation, and proliferation indicators varied in different onset time point upon COVID-19 severity, we investigated longitudinal dynamic pattern for the lymphocyte immunophenotypes in M/M and S/C COVID-19, based on data collected at enrolment. The lymphocyte subsets at different time points were calculated using a median value of 2 days before and after each time point. We found that CD8+ T cell counts recovered post about 3 weeks of disease course in the M/M group but showed a trend towards decreased numbers in the C group. The proportion of CD38+ CD8+ T cells decreased in the M/M group but remained high in the C group later in the disease course. The excessive activation and proliferation of CD8+ T cells did not compensate for the loss of the number of CD8+ T cells in critical illness. In the M/M group, NK cells rebound earlier than CD8+ T cells during the disease course (Figure 3A,C, details in supplement table 3), and the activation and proliferation of NK cells and CD8+ T also decreased smoothly (Figure 3B,D) in the M/M group, which is a contrast to that in the S/C group. Therefore, we depicted a schematic diagram to explain how the lymphocyte subsets fluctuated in COVID-19 (Figure 3E for M/M COVID-19, 3F for S/C COVID-19, all detailed data in supplemental figure 4). More robust NK and CD8+ T cell response at the early stage of disease, and more timely downregulation of activation and proliferation at the later stage of disease, were signatures of recovery. The unreversible reduction of NK and CD8+T cell with persistent activation and proliferation cause clinical deterioration.
Figure 3.
Dynamics of immunophenotype at early stages of COVID-19. Figures A–D refer to the dynamics of counts and activations of CD8+ T cell and natural killer cells based on 66 individual lymphocyte subset data. Each time point was a median of data of 2 days before and after. Figures E-F were hypothesis models describing distinct immunopathology among mild, moderate, severe, and critical illnesses. M/M, mild or moderate illness; S, severe illness; C, critical illness.
Discussion
In this report, we first describe the dynamics of the immune response profile upon clinical course in COVID-19 patients treated in PUMCH during the latest SARS-CoV-2 omicron prevailing period. The kinetic model of the peripheral lymphocyte subset has been portrayed among different COVID-19 illnesses varying from M/M to S/C. Based on what we have learned, lymphopenia is still an iconic feature of severe and critical COVID-19 from an immunological view. The comprehensive lymphocyte subset analysis demonstrates that the sharp reduction of NK, CD8+, and CD4+ T-lymphocyte counts mainly accounts for lymphopenia embedded in the COVID-19 progression, which is hard to restore in S/C illness despite active therapies. The persistent activation and proliferation of NK and CD8+ T-lymphocytes hardly compensate for the loss of those lymphocytes, which play a central role to eliminate pathogens in S/C ill. Our report displays an all-round evaluation of immune reaction against SARS-COV-2 at an early stage of illness, which may be helpful for clinicians to be vigilant to patients with critical dispositions and adopt optimal therapy timely.
In this cohort, the peripheral neutrophilia along with lymphopenia was more pronounced in patients with an S/C condition, which had been indicated as a predictor of the severity of COVID-19 due to the infiltration of neutrophils in the histopathological lung lesion [15]. Based on our observation, we captured the rapid restoration of peripheral lymphocyte counts in the M/M group rather than the S/C group (Figure 3), as previous data indicated a slow restoration capacity of lymphocytes in most severe cases [13]. Although the detailed mechanism of the rapid reduction and slow restoration of lymphocyte counts is still unclear, it reminded clinicians to alert to S/C COVID-19 with potentially worse outcomes, as did in SARS [11].
Regarding lymphopenia, further evaluation of peripheral lymphocyte subsets disclosed that clear signs and the number of NK cells and CD8+ T-lymphocyte declined sharply in the S/C condition, while CD19+ B lymphocyte remained stable at the early acute stage of illness. In M/M COVID-19, within early days after SARS-CoV-2 infection, NK cells as innate lymphocytes, first activated, recruited to the lung and then adaptive lymphocytes were enrolled to play an essential role to achieve immune protection without over-activation. In contrast, a dysfunction of NK cells may account for tissue damage related to virus-triggered immune disturbance in S/C conditions [16,17]. Interestingly, we observed the rapid restoration of CD8+ T-lymphocyte and NK cell counts in the M/M group rather than the S/C group alongside the disease progressing (Figure 3), which further elucidated “different immunophenotype pattern has a different prognosis.” Based on previous studies, whether apoptosis leading to CD8+ T lymphocyte subset shrinkage in S/C conditions at the very early clinical phase is still controversial [18,19]. Additionally, our knowledge about the loss of peripheral NK cells among S/C illnesses at the acute phase is limited. Therefore, we evaluated the exhausting marker PD-1 in CD8+ T-lymphocyte and NK cells (supplemental figure 2), which remained stable between the M/M group and S/C group during the first three weeks of illness. Moreover, the proliferation capacity (Ki67 expression) of CD8+ T-lymphocyte and NK cells is more energetic in the S/C group in comparison with the M/M group as the clinical course went forward. Based on that, we boldly speculated that apoptosis or an exhausting mechanism may not explain lymphopenia thoroughly in the early three weeks of COVID-19. The inflammatory factors related to innate and adaptive immune response against SARS-CoV-2 in this cohort will be further evaluated in future to identify critical illness along with lymphocyte immunophenotype.
In addition, we observed that in the second week of illness, the viral clearance in M/M patients was easier, compared with that in S/C patients. It had been reported that the viral shedding time varied among different SARS-CoV-2 VOC infections, and so did among diverse severity of COVID-19 [20]. Commonly, severe or critical illness bore lagged viral shedding period [20]. Although the human host’s immune system has delicate antiviral defence programmes, viruses could escape the immune attack and disturb the host’s immune response. NK cell as the first-line fighter recognizes and fights against the invader, and then T cells of the adaptive immune system play an important role in the elimination of viruses to maintain long-term protection from infections [21]. However, the battle between the pathogen and the human immune system may exhibit a dysfunctional immune response resulting in targeted tissue damage. Especially, patients in the C group exhibited inappropriate CD4 T cell exhaustion following treatment (supplement figure 3F), indicating possible difficulty in reducing cytotoxic cell activation in these patients due to early anti-inflammation cell damage. Based on that, we hypothesize that M/M COVID-19 patients could remove the virus with their delicate and optimal immune response which was supposed to recover quickly without over-activating immunity resulting in an overwhelming inflammatory storm, while the over-activated NK and/or cytotoxic T-lymphocytes elicited by persistently existing SARS-COV-2 in S/C illness may infiltrate lung tissue, leading to a worse prognosis. It still needs further research to confirm this speculation.
Recently, loads of clinical treatment strategies have been developed to impede the progression of COVID-19, such as new neutralizing antibodies, antiviral drugs, anticoagulation treatment, intravenous immunoglobulins, etc. In this cohort, at the recruitment time point (second week of illness), most patients with COVID-19 had been treated with steroids, high-dose IVIG, anticoagulant therapy, and other immunomodulators. Therefore, the elevation of inflammatory biomarkers (CRP or IL-6) and D-dimer seemed to be more moderate than that reported in other studies [5]. Despite adopting the current therapies above, in our cohort, there were still lots of S/C conditions occurring during this period. Based on our results (Figure 3), SARS-CoV-2 subtly interrupts the balance of immune responses, disrupting the cytolytic antiviral effects of NK cells and CD8+ T-lymphocytes, while seriously activating virus-infected macrophages and other immune cells to induce an uncontrollable cytokine storm, causing multiple organ failures in patients. Given this, approaches that improve the antiviral effect of the NK cell and CD8+ T-lymphocytes should be considered for severe or critical COVID-19 patients, especially in cases suffering from cytokine storms. From the view of new immunotherapy targeting saving severe and critical illness [22,23], it was valuable to monitor the dynamics of peripheral lymphocytes, especially NK and T-lymphocytes, to identify the onward progression of COVID-19 and implement optimal therapy promptly.
Taking together, we have explored the trajectory of the peripheral innate and adaptive immune response. Based on our knowledge, it is pivotal to monitor the dynamic of virus-stimulating NK cell and CD8+ T-lymphocyte counts and their functional biomarkers, which account for lymphopenia predicting malicious outcomes. when we capture the kinetics of lymphocyte subsets which obey the severe or critical immunophenotype model (Figure 3F) among COVID-19 patients, optimal management should be implemented to block the onward tissue damage related to the inflammatory storm. Further studies are underway to support our findings, but time is of the essence in the current acute pandemic situation. This current study and findings will be proved to be valuable to save lives especially relatively elderly COVID-19 patients.
Limitations
There are several limitations to this study. First, the sample size is relatively small, which makes it hard to do further subgroup analysis about the relationship between the change of lymphocyte subsets and clinical features. Second, the functional studies related to inflammatory factors released by NK and T lymphocytes are not enrolled in this study yet. Finally, this study focuses on relatively elderly people and it should be cautious to extrapolate to the whole COVID-19 patients. Notwithstanding those problems, our prospective real-world study provides timely and critical guidelines for clinicians to better understand the immunopathogenesis of COVID-19 and mostly, to change clinical practice immediately to save lives now in China.
Supplementary Material
Acknowledgements
We thank Dr. Li Zhang, Dr. Di Wu, Dr. Shuang Zhou, Dr. Zhiyu Zeng, Dr. Yuhao Jiao, and Dr. Shengjun Liu for their assistance in data collection.
Funding Statement
This work was funded by the Key Clinical Specialties Program of Beijing, China, the Scientific and Technical Innovation in Medical and Health Project of the Chinese Academy of Medical Science [grant number: 2021-I2M-1-037], and Special Research Fund for the Central High-level Hospitals of Peking Union Medical College Hospital [grant number: 2022-PUMCH-D-008&2022-PUMCH-B-117].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Leung K, Lau EHY, Wong CKH, et al. Estimating the transmission dynamics of SARS-CoV-2 omicron BF.7 in Beijing after the adjustment of zero-COVID policy in November–December 2022. Nat Med. 2023 Jan 13;29:570–582. [DOI] [PubMed] [Google Scholar]
- 2.Lin L, Lu L, Cao W, et al. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020 Dec;9(1):727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ying-Hao P, Yuan-Yuan G, Hai-Dong Z, et al. Clinical characteristics and analysis of risk factors for disease progression of patients with SARS-CoV-2 omicron variant infection: a retrospective study of 25207 cases in a Fangcang hospital. Front Cell Infect Microbiol. 2022;12:1009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020 Jul 28;71(15):762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bivona G, Agnello L, Ciaccio M.. Biomarkers for prognosis and treatment response in COVID-19 patients. Ann Lab Med. 2021 Nov 1;41(6):540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng HY, Zhang M, Yang CX, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020 May;17(5):541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaech SM, Wherry EJ.. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007 Sep;27(3):393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020 Jun 25;181(7):1489–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T, Qiu Z, Zhang L, et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis. 2004 Feb 15;189(4):648–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. Available from: https://www.R-project.org/.
- 13.Gozzi-Silva SC, Oliveira LM, Alberca RW, et al. Generation of cytotoxic T cells and dysfunctional CD8 T cells in severe COVID-19 patients. Cells. 2022 Oct 25;11(21):3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Liu Z, Liu H, et al. Decreased T cell levels in critically ill coronavirus patients: single-center, prospective and observational study. J Inflamm Res. 2021;14:1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narasaraju T, Tang BM, Herrmann M, et al. Neutrophilia and NETopathy as key pathologic drivers of progressive lung impairment in patients With COVID-19. Front Pharmacol. 2020;11:870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Q, Xu Y, Wang T, et al. Innate and adaptive immune response in SARS-CoV-2 infection-current perspectives. Front Immunol. 2022;13:1053437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergantini L, d'Alessandro M, Cameli P, et al. NK and T cell immunological signatures in hospitalized patients with COVID-19. Cells. 2021 Nov 15;10(11):3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andre S, Picard M, Cezar R, et al. T cell apoptosis characterizes severe COVID-19 disease. Cell Death Differ. 2022 Aug;29(8):1486–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rha MS, Shin EC.. Activation or exhaustion of CD8(+) T cells in patients with COVID-19. Cell Mol Immunol. 2021 Oct;18(10):2325–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puhach O, Meyer B, Eckerle I.. SARS-CoV-2 viral load and shedding kinetics. Nat Rev Microbiol. 2023 Mar;21(3):147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva MJA, Ribeiro LR, Lima KVB, et al. Adaptive immunity to SARS-CoV-2 infection: a systematic review. Front Immunol. 2022;13:1001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghasemzadeh M, Ghasemzadeh A, Hosseini E.. Exhausted NK cells and cytokine storms in COVID-19: whether NK cell therapy could be a therapeutic choice. Hum Immunol. 2022 Jan;83(1):86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Garcia I, Guerra-Garcia P, Ferreras C, et al. A phase I/II dose-escalation multi-center study to evaluate the safety of infusion of natural killer cells or memory T cells as adoptive therapy in coronavirus pneumonia and/or lymphopenia: RELEASE study protocol. Trials. 2021 Oct 2;22(1):674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.