Abstract
Introduction
Spinal dural arterio-venous fistula (SDAVF) is a rare and underdiagnosed cause of myelopathy which can result in a devastating neurological outcome if not properly managed.
Case description
We report a case of SDAVF in a middle-aged man with gradual progressively deteriorating myelopathy and associated symptoms. This was first managed as demyelinating disease but was refractory to steroid therapy. Vigilant review of his spinal magnetic resonance imaging (MRI) scans showed dilated perimedullary veins, suspicious for SDAVF. The diagnosis was confirmed with catheter angiography. Neurological symptoms resolved after surgical treatment.
Discussion
SDAVF can closely mimic demyelinating conditions such as transverse myelitis or multiple sclerosis. MRI finding of dilated perimedullary veins can be subtle and masked in the late stage, posing a diagnostic challenge for physicians. It is potentially curable after timely treatment.
Conclusion
Clinicians should maintain a high level of suspicion for SDAVF and actively review all available radiological imaging for clues, particularly when there is a lack of response to treatment for other causes of myelopathy.
LEARNING POINTS
Spinal dural arterio-venous fistula (SDAVF) can have clinical and radiological features similar to those of demyelinating disease, often causing a diagnostic dilemma for physicians. Neurological sequalae can be devastating if left untreated.
Clinicians should be aware of this rare yet important differential diagnosis for myelopathy and its classic MRI findings (spinal cord oedema and dilated perimedullary veins).
The gold standard for diagnosis is catheter spinal angiography. Treatment options include endovascular embolization and surgical ligation of the fistula.
Keywords: Spinal dural arterio-venous fistula, myelopathy, spinal cord oedema, demyelinating disease, dilated perimedullary veins
CASE DESCRIPTION
A 54-year-old man in good health presented with insidious onset of lower back pain radiating to the left lower limb. Physical examination showed reduced power and touch sensation in the lower limbs. Radiographs and magnetic resonance imaging (MRI) of the spine revealed lumbar spondylosis with severe spinal stenosis at the L3/4 and L4/5 levels. Spinal surgery of L3/4 and L4/5 discectomy with posterior spinal fusion from L3 to L5 was performed by an orthopaedic surgeon.
Two years after surgery, the patient experienced worsening back pain, bilateral lower limb weakness and numbness. He became wheelchair-bound outdoors.
Radiographs showed no malalignment of the surgical implants. Repeat MRI of the spine revealed longitudinally extensive T2 hyperintense signals in the intramedullary region of the spinal cord, extending from the T5 level down to the conus medullaris with associated cord expansion (Fig. 1). No abnormal contrast enhancement was seen in the cord. MRI of the brain was unremarkable.
Figure 1.
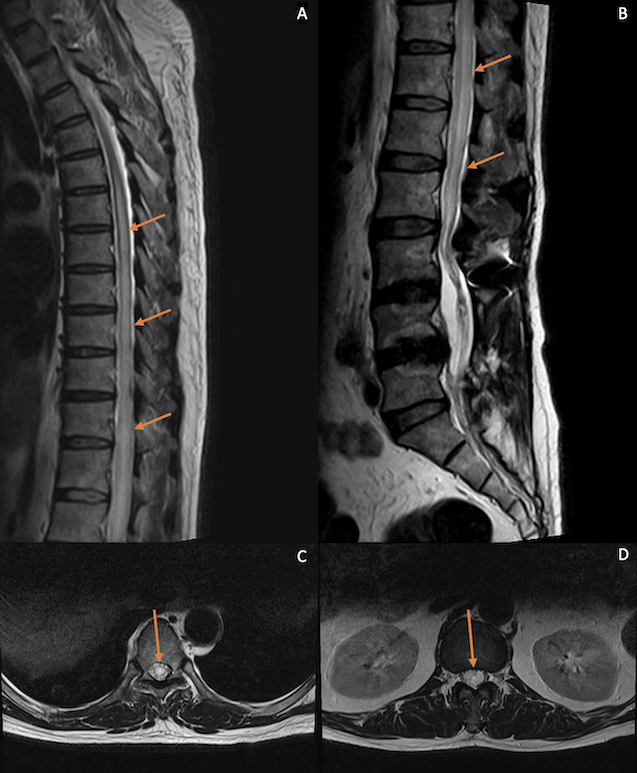
Sagittal T2-weighted MRI of the thoracic (A) and lumbosacral spine (B) after orthopaedic surgery. Axial T2-weighted images at the T7 (C) and T12 levels (D). A longitudinally extensive hyperintense signal in the intramedullary region of the spinal cord from the T5 level down to the conus medullaris was seen, associated with cord expansion. Axial images show involvement of both sides of the cord
Blood tests including auto-antibodies (e.g., anti-aquaporin-4, anti-myelin oligodendrocyte glycoprotein (MOG), anti-ganglioside and other anti-neuronal antibodies), markers of vasculitis (e.g., anti-nuclear and anti-extractable nuclear antibodies, rheumatoid factor) and markers for infection (e.g., human immunodeficiency virus and syphilis serology) were all negative. Lumbar puncture results were unremarkable. The serum vitamin B12 level was normal. Nerve conduction study of the lower limbs showed no signs of peripheral neuropathy. There was no sign of optic neuropathy.
The patient was managed as a case of longitudinally extensive transverse myelitis. Whole body computed tomography was performed to exclude a paraneoplastic syndrome as the cause of this diagnosis; the results were unremarkable. The patient was started on high-dose steroids, plasma exchange and later immunosuppressants such as mycophenolate mofetil. Unfortunately, his neurological symptoms continued to deteriorate despite frequent medication adjustment. The patient became bedbound with episodes of urinary incontinence. Follow-up MRI of the spine showed progression of myelopathy from the T3/4 level down to the conus medullaris.
A clinical-radiological conference involving neurologists, neurosurgeons and radiologists was conducted to review the patient’s condition. On scrutiny of the initial pre-operative MRI of the lumbosacral spine, there was evidence of serpentine and tortuous hypointense signals around the lower spinal cord and cauda equina on a T2-weighted sagittal sequence (Fig. 2). These signal intensities represented abnormal dilated vasculature. No cord oedema signal was observed. These dilated vessels were completely masked in subsequent MRI studies due to mass effect from significant congestion of the spinal cord. Spinal dural arterio-venous fistula (SDAVF) was considered to be the most likely differential diagnosis.
Figure 2.
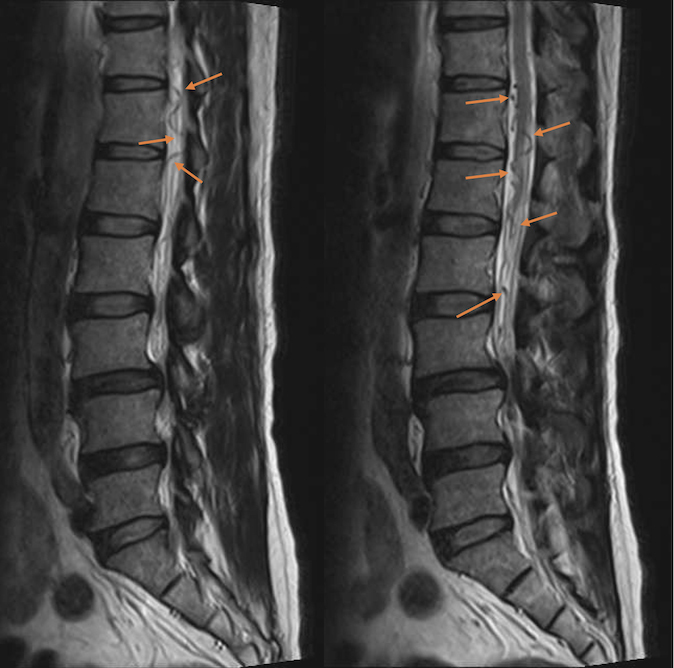
Sagittal T2-weighted MRI of the lumbosacral spine before orthopaedic surgery. Note the prominent, serpentine, tortuous, hypointense lesions around the lower spinal cord and cauda equina, representing dilated perimedullary veins
MRI angiography of the spine showed a prominent intradural early enhancing vein at the L1–L4 levels, but no arterial feeder was identified (Fig. 3). Catheter digital subtraction angiography of the spinal arteries was performed. An arterio-venous fistula (AVF) between a sacral branch of the posterior division of the left internal iliac artery and the anterior spinal vein was found (Fig. 4). The diagnosis of SDAVF was finally established.
Figure 3.
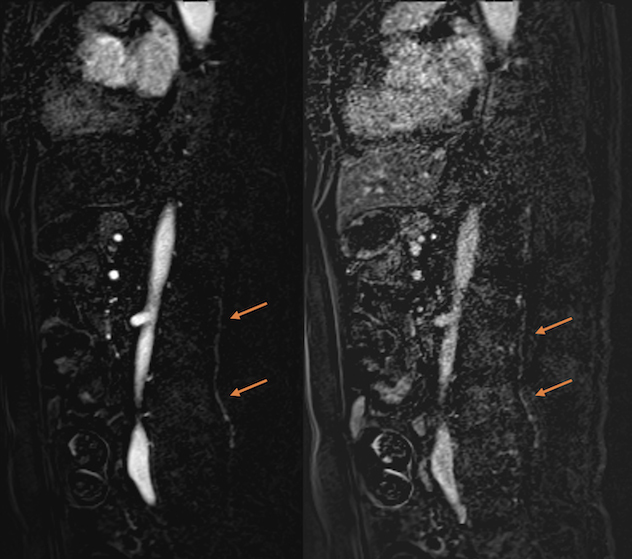
MR angiography (sagittal images) of the lumbosacral spine. Note the abnormal, prominent, intradural, early enhancing vein around the L1 to L4 levels
Figure 4.
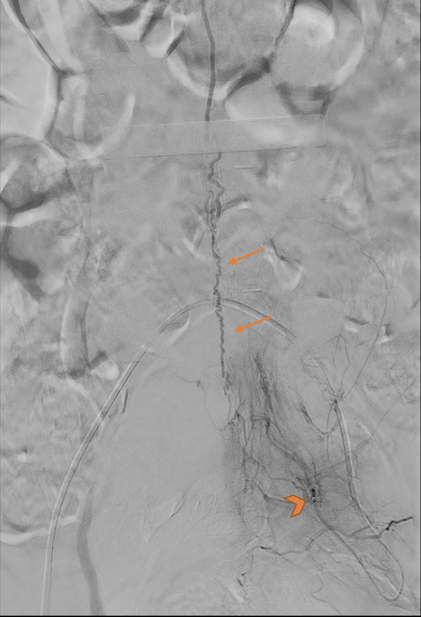
Catheter digital subtraction angiography. The tip of the catheter was within the posterior division of the left internal iliac artery (arrowhead). SDAVF with a supplying artery from a sacral branch of the posterior division of the left internal iliac artery gave rise to a dilated anterior spinal vein at the lumbar level (arrows)
Steroids and immunosuppressants were withheld. Neurosurgeons performed laminectomy to look for the fistula. The SDAVF was identified at the L5/S1 level and was completely disconnected.
The patient continued regular physiotherapy after the operation for SDAVF with gradual improvement of lower limb power on follow-up. Occasional incontinence was still present.
DISCUSSION
There are several types of spinal dural arterio-venous malformation with SDAVF being the most common (60–80%)[1]. The aetiology of this condition remains unclear. The majority of patients become symptomatic in middle age[1,2]. Men are more affected than women[1].
SDAVF results from abnormal direct communication between radicular arteries and veins[2]. The feeding arteries raise the pressure of the draining veins. The perimedullary venous plexus become tortuous and dilated. Chronic venous hypertension leads to congestion and oedema of the spinal cord[2,3]. The thoracolumbar cord is more affected than the cervical cord[2].
The clinical course of SDAVF is usually slowly progressive, although acute presentations may occur. Symptoms are non-specific and may include pain, paraesthesia, weakness, gait disturbance and autonomic dysfunction such as urinary incontinence[2]. Intraspinal haemorrhage is rare but possible[1,2]. The diagnostic delay may extend for 10–15 months[3]. Initial misdiagnosis causing treatment delay is common and many patients are often primarily diagnosed as having demyelinating diseases[4]. Final diagnosis and management often require multi-disciplinary team input, involving physicians, neurosurgeons and radiologists. A shorter symptom duration before intervention is associated with a better clinical outcome[4]. Alternatively, if left untreated, irreversible para- or tetraplegia can result[3].
MRI plays a pivotal role in the diagnosis of SDAVF. There are two key MRI findings: spinal cord oedema and dilated perimedullary veins.
Spinal cord oedema is seen as hyperintense signals in the intramedullary region of the spinal cord on T2-weighted sequences. The aetiologies of myelopathy include demyelinating disease, spinal stenosis, vasculitis, tumours, infection, and metabolic as well as vascular causes including ischaemia and SDAVF. Although differentiating the cause on MRI is difficult and clinical correlation is often required, there are several useful clues. For instance, myelopathy caused by SDAVF tends to involve a longer segment than some other causes such as multiple sclerosis. An average of five to seven spinal levels are involved, with conus medullaris involvement seen in over 80% of cases[2]. Cord expansion is more typical for causes such as SDAVF, tumours, transverse myelitis and neuromyelitis optica. Contrast enhancement within the spinal cord is not characteristic for SDAVF, while causes such as cord tumours typically enhance.
The dilated perimedullary veins in SDAVF are seen as tortuous, serpentine, intradural, extramedullary, hypointense flow voids on T2-weighted sequences, commonly extending over an average of eight spinal levels[2]. These veins enhance after contrast administration. Perimedullary veins related to SDAVF are much more prominent than the vessels that may occasionally be seen in cases of spinal stenosis. As proven in our case, if the dilated perimedullary veins are not identified at an early stage of disease, they can be difficult to see on MRI in late stages of the disease due to compression by the swollen cord.
The gold standard for the diagnosis of SDAVF is catheter spinal angiography. Findings include stasis of contrast in feeding arteries, early filling of radicular veins and a dilated perimedullary venous plexus[2].
Treatment of SDAVF includes endovascular embolization and surgical ligation of the fistula[1–3]. After treatment, patients are more likely to experience improvement in motor function than in urinary function[4].
In summary, SDAVF is a rare and under-recognized cause of myelopathy, but is a vital differential diagnosis to consider. Clinicians should maintain a high level of suspicion for this diagnosis, particularly when there is a lack of response to treatment for other causes of myelopathy.
Footnotes
Conflicts of Interests: The Authors declare that there are no competing interests.
Patient Consent: Patient consent was obtained.
REFERENCES
- 1.Maimon S, Luckman Y, Strauss I. Spinal dural arteriovenous fistula: a review. Adv Tech Stand Neurosurg. 2016;43:111–137. doi: 10.1007/978-3-319-21359-0_5. [DOI] [PubMed] [Google Scholar]
- 2.Jeng Y, Chen DYT, Hsu HL, Huang YL, Chen CJ, Tseng YC. Spinal dural arteriovenous fistula: imaging features and its mimics. Korean J Radiol. 2015;16:1119–1131. doi: 10.3348/kjr.2015.16.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenck S, Bernat AL, Bresson D, Labeyrie MA, Saint-Maurice JP, Froelich S, et al. Spinal dural arteriovenous fistula. J Spine. 2016;5:287. [Google Scholar]
- 4.Ronald AA, Yao B, Winkelman RD, Piraino D, Masaryk TJ, Krishnaney AA. Spinal dural arteriovenous fistula: diagnosis, outcomes, and prognostic factors. World Neurosurg. 2020;144:e306–315. doi: 10.1016/j.wneu.2020.08.126. [DOI] [PubMed] [Google Scholar]


