ABSTRACT
Chlamydia muridarum has been used to study chlamydial pathogenesis because it induces mice to develop hydrosalpinx, a pathology observed in C. trachomatis-infected women. We identified a C. muridarum mutant that is no longer able to induce hydrosalpinx. In the current study, we evaluated the mutant as an attenuated vaccine. Following an intravaginal immunization with the mutant, mice were protected from hydrosalpinx induced by wild-type C. muridarum. However, the mutant itself productively colonized the mouse genital tract and produced infectious organisms in vaginal swabs. Nevertheless, the mutant failed to produce infectious shedding in the rectal swabs following an oral inoculation. Importantly, mice orally inoculated with the mutant mounted transmucosal immunity against challenge infection of wild-type C. muridarum in the genital tract. The protection was detected as early as day 3 following the genital challenge infection and the orally immunized mice were protected from any significant pathology in the upper genital tract. However, the same orally immunized mice failed to prevent the colonization of wild-type C. muridarum in the gastrointestinal tract. The transmucosal immunity induced by the oral mutant was further validated in the airway. The orally vaccinated mice were protected from both lung infection and systemic toxicity caused by intranasally inoculated wild-type C. muridarum although the same mice still permitted the gastrointestinal colonization by the wild-type C. muridarum. These observations suggest that the mutant C. muridarum may be developed into an intracellular oral vaccine vector (or IntrOv) for selectively inducing transmucosal immunity in extra-gut tissues.
KEYWORDS: Chlamydia muridarum, CMG28.51.1, TC0237, TC0668, attenuated oral vaccine, transmucosal immunity, attenuated, hydrosalpinx, airway infection, genital tract infection, oral vaccines
INTRODUCTION
Sexually transmitted infection with the obligate intracellular bacterium species Chlamydia trachomatis can lead to inflammatory pathologies in the upper genital tract, potentially resulting in pelvic inflammatory diseases and infertility (1, 2). An effective vaccine against C. trachomatis is necessary for preventing the sequelae because C. trachomatis infection is asymptomatic or lacks specific symptoms. However, despite extensive research and development efforts, there is still no licensed Chlamydia vaccine for humans (3). When chlamydial organisms were successfully isolated from human samples by Tang et al. more than 70 years ago (4, 5), intramuscular injection of formalin-inactivated whole chlamydial organisms was tested for preventing trachoma caused by C. trachomatis in school children (6–8). Unfortunately, the inactivated whole organism vaccines not only failed to induce lasting protection, but also exacerbated conjunctivitis in some cases. The failed trachoma vaccine trials motivated chlamydial researchers to search for subunit vaccines. Over the years, many chlamydial components have been evaluated as vaccine candidates in various preclinical models (9, 10). A recent study has demonstrated that the inactivated chlamydial organisms may be modified to induce protective immunity with no detrimental effects in preclinical models (11). However, none of the above has ever been advanced to efficacy evaluations in humans, although a phase I safety evaluation has been carried out for one of the candidates (12).
Mouse infection with Chlamydia muridarum is one of the most extensively used preclinical models for investigating chlamydial pathogenesis and evaluating chlamydial vaccines (9, 13–17). C. muridarum is known to cause hydrosalpinx and infertility in mice following intravaginal inoculation (18–20), which mimics the tubal adhesion/infertility observed in women under laparoscopy (2, 21, 22). The fact that genital C. muridarum infection is self-limited and often ends up with oviduct scaring suggests that there may be a partial adaptation of C. muridarum to the female mouse genital tract mucosal tissue. In contrast, intranasal inoculation with C. muridarum leads to severe acute pneumonia or death (23, 24), suggesting that there is a lack of adaptation of C. muridarum to the mouse airway mucosal tissue. Thus, C. muridarum can be used for evaluating immunity against chlamydial infection under different infection conditions (25, 26).
Besides infecting the mucosal tissues in the mouse genital tract and airway, C. muridarum is also known to colonize the gastrointestinal (GI) tract for a long period of time but without causing significant pathology (27, 28). Interestingly, various chlamydial species have been detected in their corresponding host GI tracts (29–33). Although the significance of chlamydial species in the GI tract remains unknown, recent studies based on the models of C. muridarum interaction with mouse mucosal tissues have revealed that the order of chlamydial exposure to mouse tissue sites may significantly influence the consequence of the gut C. muridarum (34, 35). When a naive mouse is exposed to C. muridarum in the GI tract first, C. muridarum may function as an oral vaccine. It has been reported that oral inoculation with C. muridarum can induce robust transmucosal immunity against subsequent chlamydial infections in both the genital tract (25) and airway (26), suggesting that C. muridarum may be developed into an oral vaccine for inducing transmucosal immunity. To further improve the safety of oral Chlamydia vaccines, efforts have been made to evaluate various genital tract pathogenicity-attenuated C. muridarum mutants (36, 37).
We recently identified a mutant C. muridarum that is highly attenuated in inducing pathogenicity in the female mouse genital tract following an intravaginal inoculation (38, 39). In the current study, we compared the genital pathogenicity-attenuated mutant for its ability to induce protection in the genital tract following intravaginal immunization versus oral immunization. Intravaginal immunization with the mutant protected mice from developing hydrosalpinx induced by wild-type C. muridarum. However, the protection was accompanied with productive colonization of the mutant itself in the mouse genital tract, which led to the release of infectious organisms in vaginal swabs. In contrast, oral inoculation with the mutant failed to produce infectious shedding in the rectal swabs. More importantly, orally immunized mice developed robust transmucosal immunity against challenge infection by wild-type C. muridarum in the genital tract, although these mice permitted the colonization of wild-type C. muridarum in the GI tract. The oral mutant-induced protection in the extra-gut tissues, but not the gastrointestinal tract, was further validated in a mouse airway model in which mice were intranasally challenged with wild-type C. muridarum. Thus, the mutant C. muridarum may be developed into an intracellular oral vaccine vector (or IntrOv) for selectively inducing transmucosal immunity.
RESULTS
A genital pathogenicity-attenuated C. muridarum mutant induces protection against subsequent infection by wild-type C. muridarum.
Using a Pasteur passage selection scheme (38), we previously identified a C. muridarum mutant (CMmut, clone CMG28.51.1) that was no longer able to induce hydrosalpinx following an intravaginal inoculation (39). Intravaginal inoculation with CMmut was evaluated for inducing protective immunity against subsequent challenge infection by wild-type C. muridarum (CMwt). As shown in Fig. 1, in the sucrose-phosphate-glutamate buffer (SPG)-treated mice (mock immunization control), intravaginal challenge infection with CMwt produced a typical 1-month live chlamydial organism shedding course from the genital tract. All mice cleared infection by day 35. However, the CMwt challenge infection-induced shedding course was significantly shortened in the CMmut-immunized mice. All mice cleared infection by day 21. The shedding levels in terms of inclusion forming units (IFUs) per swab were significantly reduced on days 3, 7, and 14. Clearly, prior genital tract exposure to CMmut was sufficient for inducing anti-chlamydial immunity in the female genital tract. Further, the CMmut-induced protective immunity significantly protected mice from developing hydrosalpinx induced by CMwt. Only one of the eight mice in the immunized group developed hydrosalpinx, while six of eight control mice did so. The immunized group only had a mean hydrosalpinx score of 0.25 while the control group had a score of 4.5. These results have demonstrated that although intravaginal inoculation with the CMmut clone itself can no longer induce hydrosalpinx (39), CMmut can still induce protective immunity, suggesting that it can be developed into an attenuated vaccine. However, the CMmut-induced protection was accompanied with its own colonization, resulting in the release of live CMmut organisms from the genital tract, which raises a safety concern on the potential spreading of the vaccine strain in the vaccinated host population.
FIG 1.
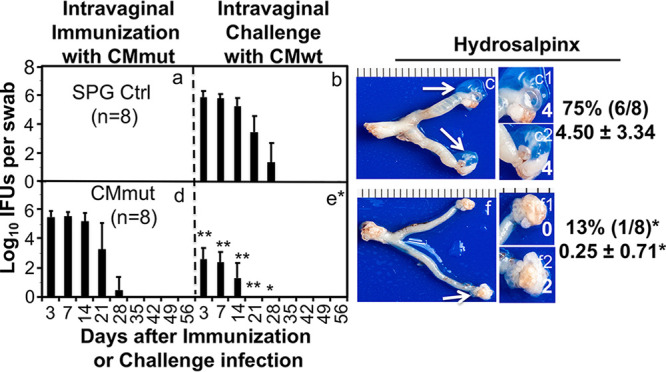
Effect of intravaginal immunization with an attenuated Chlamydia on subsequent challenge infection by wild-type Chlamydia. Groups of female C57BL/6J mice were intravaginally inoculated with SPG buffer (Ctrl, n = 8) (a); or a mutant C. muridarum (CMmut at an inoculum dose of 2 × 105 inclusion forming units or IFUs, n = 8) (d) as immunization for 56 days. Subsequently, both groups of mice were intravaginally challenged with wild-type C. muridarum (CMwt at an inoculum dose of 2 × 105 IFUs) (b and e). All mice were monitored for live Chlamydia burdens in the genital tract by taking vaginal swabs on days 3, 7, and weekly thereafter (X-axis) following the immunization (Panels a and d) and challenge infection (b and e), respectively. The number of live organisms recovered from each vaginal swab was expressed as log10IFUs (Y-axis). Then, 56 days after the challenge infection, all mice were sacrificed for observing hydrosalpinx (c and f). Only one representative image of the entire genital tract was shown for each group. Oviducts positive for hydrosalpinx were marked with white arrows. The magnified images of oviduct/ovary regions (with hydrosalpinx scores indicated in white numbers) were shown on the right of the overall genital tract image. Both the hydrosalpinx incidence (along with group sample size) and severity score from each group were listed next to the corresponding group images. *, P < 0.05; **, P < 0.01 (Fisher’s exact for comparing incidences while Wilcoxon for scores or IFUs). Data were from two or three independent experiments. Note that intravaginal immunization with CMmut induced significant protection against both infection and pathogenicity of CMwt.
Mice orally inoculated with CMmut fail to release live chlamydial organisms.
We previously reported that CMmut failed to spread from the genital tract into the gastrointestinal (GI) tract (40) although CMwt readily did so (41), suggesting that CMmut might not produce infectious shedding in the GI tract. As shown in Fig. 2, following a direct oral inoculation with 1 × 107 IFUs of CMmut, mice failed to release any live chlamydial organisms from either the rectal or vaginal swabs, while mice orally inoculated with CMwt continuously shed live chlamydial organisms from the GI tract. Further, following an intracolon inoculation, only a minimal level of CMmut live chlamydial organisms was detected in the rectal swabs and all CMmut-inoculated mice cleared infection by day 14. However, the intracolon-inoculated CMwt maintained a steady level of live organism shedding. These observations indicate that CMmut can be rapidly cleared from the mouse colon, which may partially explain why oral CMmut failed to shed live chlamydial organisms in the rectal swabs. Thus, CMmut may not cause spreading when it is delivered orally.
FIG 2.
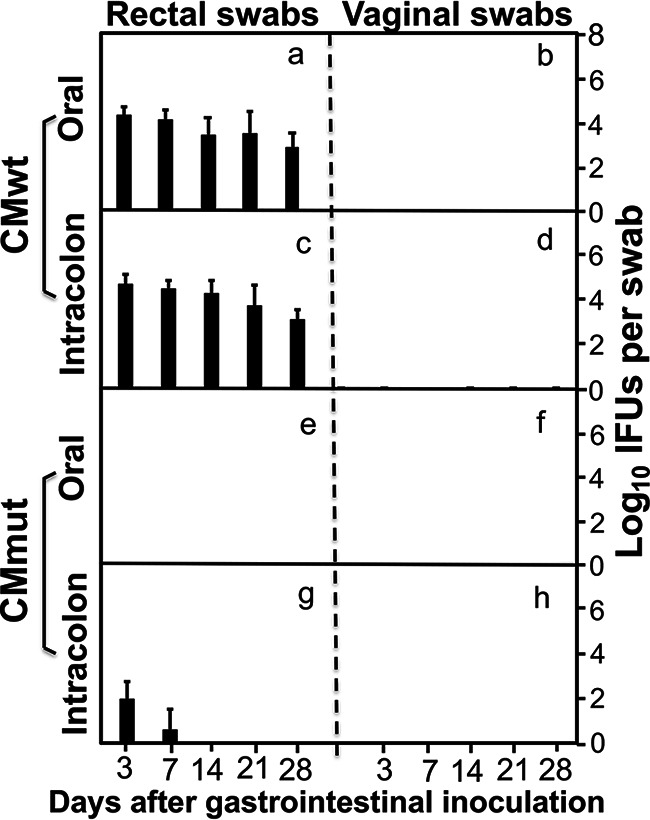
Comparison of live chlamydial organism shedding courses between mice orally or intrarectally inoculated with wild or mutant Chlamydia. Groups of female C57BL/6J mice (n = 5) were either orally (panels a, b, e, and f) or intracolonally (c, d, g, and h) inoculated with wild-type Chlamydia (CMwt at an inoculum dose of 2 × 105 IFUs) (a to d); or mutant Chlamydia (CMmut at an inoculum dose of 1 × 107 IFUs) (e to h). All mice were monitored for live Chlamydia burdens in the gastrointestinal tract and genital tract by taking rectal and vaginal swabs on days 3, 7, and weekly thereafter (X-axis). The number of live organisms recovered from each swab was expressed as log10IFUs (Y-axis). Data were from two experiments. Note that orally inoculated CMmut failed to shed live chlamydial organisms from either gastrointestinal tract or genital tract although minimal shedding was detected following intrarectal inoculation.
Oral immunization with CMmut protects against infection and pathogenicity of CMwt in the genital tract.
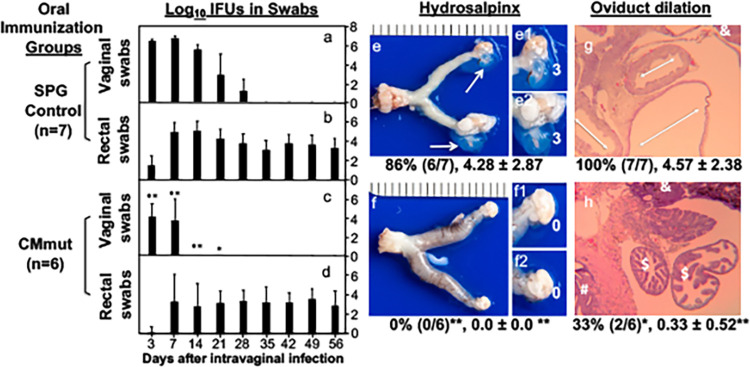
Because oral CMmut failed to shed live organisms, oral CMmut was further evaluated for the induction of protective immunity in the genital tract. As shown in Fig. 3, mice orally immunized with CMmut significantly reduced the shedding levels of CMwt live organisms in the genital tract. The SPG mock-immunized control group developed a typical 1-month long shedding course caused by intravaginal challenge infection with CMwt while the CMmut-immunized group significantly shortened the shedding course of CMwt. Live CMwt organisms were only detected on days 3 and 7 with significantly reduced levels at both time points in the oral CMmut-immunized mice. These observations have demonstrated that oral CMmut is able to induce transmucosal immunity in the female genital tract. It is worth noting that a steady level of live organism shedding was detected in the rectal swabs of the CMmut-immunized mice following intravaginal challenge infection with CMwt. The rectal live organisms might be caused by the intravaginally challenged CMwt since CMwt is known to spread from the genital tract into the GI tract (41). More importantly, the oral CMmut-induced transmucosal immunity was sufficient for preventing pathology in the upper genital tract. None of the oral CMmut-immunized mice developed hydrosalpinx following intravaginal challenge infection with CMwt. The lack of hydrosalpinx in the CMmut-immunized mice was validated by examining oviduct dilation under microscopy. Only two of the six immunized mice developed oviduct dilation with a mean dilation score of 0.33 while all control mice developed significant oviduct dilation with a mean score of 4.57. The above observations have demonstrated that oral immunization with CMmut is an efficient approach for inducing transmucosal immunity in the female genital tract.
FIG 3.
Effect of oral immunization with attenuated Chlamydia on subsequent challenge infection by wild-type Chlamydia in the genital tract. Female C57BL/6J mice orally inoculated with SPG (controls a and b) (n = 7) or mutant C. muridarum (CMmut at an inoculum dose of 1 × 107 IFUs) (c and d, n = 6) as immunization for 28 days. Both groups of mice were then intravaginally challenged with 2 × 105 IFUs of wild-type C. muridarum (CMwt). All mice were monitored for live chlamydial organisms from both the genital tract (vaginal swabs) (a and c) and gastrointestinal tract (rectal swabs) (b and d) on days 3, 7, and weekly thereafter following the challenge infection. The results were expressed as Log10 IFUs per swab (Y-axis). On day 56, all mice were sacrificed for observing genital tract pathology. Hydrosalpinx was visually evaluated (e and f). Only one representative image of the entire genital tract was shown for each group. Oviducts positive for hydrosalpinx were marked with white arrows. The magnified images of oviduct/ovary regions (with hydrosalpinx scores indicated in white numbers) were shown on the right of the overall image (e1, e2, f1, and f2). Both the hydrosalpinx incidence (along with group sample size) and severity score from each group were listed under the corresponding group images. The same excised genital tract tissues were further processed for monitoring oviduct dilation under microscopy (g and h). After H&E staining, tissue sections of the genital tissues were first examined for the overall appearance of the oviduct tissues under a 4× objective lens. Representative normal oviduct cross-section was labelled with a “$” sign, ovary with “&” and uterine horn tissue with “#” while dilated oviducts were indicated with the white double arrowhead arrows. Both hydrosalpinx (e and f) and oviduct dilation (g and h) were semiquantitatively measured as described the materials and method section. Data were from two independent experiments. Note that mice orally immunized with CMmut were protected from genital tract infection (*, P < 0.05; **, P < 0.01, Wilcoxon for comparing IFUs at different time points; a versus c) and pathology (Wilcoxon for comparing scores while Fisher’s Exact for comparing incidence rates).
Oral CMmut induces transmucosal protection against infection and pathogenicity of CMwt in the airway.
Having demonstrated the robust transmucosal protection in the female genital tract, we next tested whether the oral CMmut-induced transmucosal immunity can also be detected in the airway. Intranasal inoculation with CMwt is known to cause mouse pneumonia or death (24), which was used for evaluating transmucosal immunity induced by oral CMmut in the current study. As shown in Fig. 4, in the control group, intranasal challenge infection with CMwt significantly decreased mouse body weight, which is consistent with previous studies (23, 24, 26, 42). However, the oral CMmut-immunized mice gained body weight in the same period following the intranasal challenge infection with CMwt. Thus, oral CMmut is sufficient for protecting mice from the systemic toxicity caused by airway CMwt. When the live CMwt burden was monitored in the mouse lung tissue on day 9 after intranasal inoculation, the CMmut-immunized mice were completely protected from any live chlamydial organisms while the SPG control mice harbored >10 million IFUs of live chlamydial organisms per lung. The difference in CMwt burden in the lung may partially explain the lack of toxicity in the oral CMmut-immunized mice. It is worth noting again that despite the full protection of the lung, low levels of live chlamydial organisms were detected in the stomach and colon. These live chlamydial organisms may come from the airway CMwt because CMwt is known to establish lasting colonization in the gut following an inoculation elsewhere (43, 44).
FIG 4.
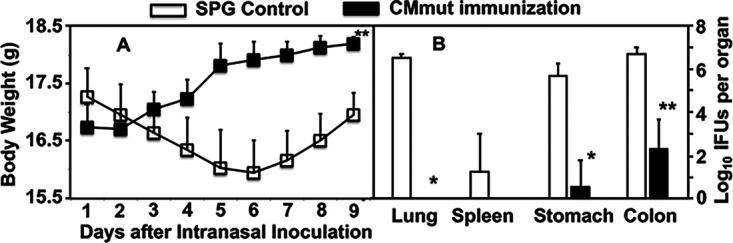
Effect of oral immunization with attenuated Chlamydia on subsequent infection by wild-type Chlamydia in the airway. Female C57BL/6J mice orally inoculated with SPG (control, open square/bar, n = 5) or mutant C. muridarum (CMmut at an inoculum dose of 1 × 107 IFUs, solid square/bar, n = 5) as immunization for 28 days. Both groups of mice were then intranasally challenged with 5,000 IFUs of wild-type C. muridarum (CMwt). All mice were monitored for body weight daily (A) and the mouse body weight were measured in grams (Y-axis). All mice were sacrificed for measuring live chlamydial organism burdens in lungs, spleen, stomach, and colon tissues on day 9 after intranasal infection (B). The results were expressed as Log10 IFUs per organ (Y-axis). Data were from two different experiments. *, P < 0.05; **, P < 0.01, Wilcoxon for comparing body weight (area under the curve) or IFUs between SPG group and CMmut immunization group. Note that mice orally immunized with CMmut were protected from airway infection by CMwt.
DISCUSSION
There is an urgent need to develop an effective vaccine against C. trachomatis in humans. It is obvious that an ideal Chlamydia vaccine would be a subunit vaccine. However, years of extensive efforts in evaluating hundreds of chlamydial components as vaccine candidates have yielded no licensable vaccines. Perhaps, it is time to explore or re-explore non-subunit vaccine approaches. A recent study has revealed that inactivated whole chlamydial organisms can be modified to induce protective immunity without exacerbating pathology (11), which is similar to the immunity induced by live chlamydial infection. Using the C. muridarum model, it has been shown that inoculation with live C. muridarum induces the strongest protection against subsequent challenge infection (45–47). However, inoculating live chlamydial organisms into either the genital tract or the airway causes pathologies. Instead, we have recently shown that oral inoculation with C. muridarum is not only nonpathological but also induces strong transmucosal protection in extra-gut tissues (25, 26). These observations suggest that it is possible to develop a safe and effective oral Chlamydia vaccine. To further improve the safety of oral Chlamydia vaccines, various genital tract pathogenicity-attenuated C. muridarum mutants have been evaluated as oral vaccines (36, 37).
In the current study, we compared the genital tract pathogenicity-attenuated C. muridarum mutant or CMmut (clone G28.51.1) for its ability to induce protection against infection with wild-type C. muridarum (CMwt) following either an intravaginal immunization or oral immunization. The results have led us to conclude that oral immunization is a more favorable approach. First, although either intravaginal or oral immunization route was sufficient for CMmut to induce protection against genital infection and pathogenicity caused by CMwt, intravaginal CMmut itself produced significant shedding of infectious organisms from the immunized mice, while oral CMmut failed to do so. Thus, oral immunization may minimize the spreading of the vaccine strain in the vaccinee population. Second, intravaginal inoculation is obviously more difficult to administer and may be hard to be accepted. Thus, the compliance rate will be a major concern. On the contrary, oral vaccination is easy to administer and has been successfully used for other vaccines. These benefits have motivated us to propose to develop the CMmut clone G28.51.1 into an intracellular oral vaccine or IntrOv for short. Finally, the genital tract protection induced by oral CMmut is based on transmucosal immunity. The robust transmucosal immunity was also validated in the airway. Thus, the clone G28.51.1 may also be developed into an intracellular oral vector for delivering prevention or intervention reagents against nonchlamydial diseases in extra-gut tissues.
Although oral CMmut- or IntrOv-induced immunity efficiently protected both the mouse genital and airway mucosal tissues from infection and pathogenicity caused by CMwt, the immunity was insufficient for protecting the same mouse’s GI tract against colonization by CMwt. First, the IFUs detected in the GI tract were not caused by the IntrOv organisms because oral inoculation with IntrOv did not produce any IFUs in either the rectal or vaginal swabs. Second, the GI IFUs detected in mice challenged with CMwt might be due to the spreading of genital or airway CMwt organisms into the GI tract. This is because it has been demonstrated that any mucosal inoculation with C. muridarum can always lead to systemic spreading (43) and the systemic C. muridarum can only establish long-lasting colonization in the GI tract (44). By following the spreading of genital C. muridarum into the GI tract (41), two distinct and complementary pathways have been identified for systemic C. muridarum to home to the GI tract (48). The primary pathway is the spleen-stomach pathway, while the secondary pathway is the liver-intestinal route. Nevertheless, the precise molecular and cellular basis of C. muridarum trafficking into the GI tract remains unclear although dendritic cells (DCs) have been proposed to play an essential role (49).
The next question is why the oral IntrOv-induced immunity is effective against chlamydial infections in the mucosal tissues of both the genital tract and airway but not the GI tract. Using a combination of knockout mice and mutant C. muridarum (the IntrOv clone), we have recently shown that the long-lasting colonization of C. muridarum in mouse colon is dependent on its ability to evade colonic IFNγ that is produced by the group 3 innate lymphoid cells (ILC3s) or ex-ILC3s, but not other ILCs or conventional lymphocytes (50, 51). Although IFNγ can be produced by many different types of cells, it is likely that C. muridarum-infected cells in the GI tract may only be accessible by ex-ILC3s, but not other lymphocytes while the C. muridarum-infected cells in extra-gut tissues may be accessed by different lymphocytes (34). Oral C. muridarum is known to induce transmucosal immunity that is dependent on conventional lymphocytes (25). Thus, we hypothesize that oral IntrOv may also induce conventional lymphocyte-dependent immunity that can only transmucosally prevent C. muridarum infection in the female genital tract and airway but not the GI tract. Testing of this hypothesis is under way.
It is worth emphasizing that the attenuation phenotype of intrOv is mapped to the loss of function mutations in hypothetical genes tc0237 and tc0668. In contrast, the attenuation mutations for a previously reported chromosomal gene mutant that is also attenuated in genital pathogenicity have not been defined. Both TC0237 and TC0668 are hypothetical proteins and their functions are unknown. TC0237 is predicted to contain a domain of unknown function 720 (DUF720) motif. Loss of function mutation in TC0237 has been corelated with enhanced chlamydial infectivity in vitro, suggesting that the loss of function mutation in tc0237 gene may be a direct result of chlamydial adaptation to cell culture conditions applied during in vitro passaging. In contrast, the tc0668 mutation does not significantly alter chlamydial infectivity in cultured cells. Its weak homology with integrins may suggest its role in facilitating chlamydial interactions with host mucosal tissues. Clearly more studies are required for further characterizing TC0237 and TC0668.
The final but important question is whether the C. muridarum model-based findings described in the current study can be used to develop a C. trachomatis vaccine for humans. A direct approach is to make the same attenuation mutations in the genome of C. trachomatis and develop the mutant C. trachomatis clone into an oral vaccine. The C. trachomatis homologs of tc0237 and tc0668 are ct849 and ct389, respectively (38, 39). Genetic approaches for interrupting a gene in the C. trachomatis genome are now available (52). Because the mutation is restricted to two genes unlike other attenuated C. muridarum clones with multiple mutations (36), making the corresponding C. trachomatis mutant based on intrOv described in the current study should not be a problem. Although plasmid-free or plasmid gene deletion C. muridarum mutants have also been evaluated as oral vaccine candidates (37), plasmid gene-based mutants may be less stable than chromosomal gene mutants. Production of a stable C. trachomatis mutant clone with loss of function mutations in ct849 and ct398 may allow us to test the hypothesis that oral delivery of an attenuated C. trachomatis mutant may be sufficient for inducing transmucosal immunity in the genital tract.
MATERIALS AND METHODS
Chlamydial organism growth.
All Chlamydia muridarum (CM) clones used in the current study were derived from strain Nigg3 (GenBank accession# CP009760.1), including a wild-type clone CMG13.32.1 (CMwt) and a genital pathogenicity-attenuated clone CMG28.51.1 (CMmut or IntrOv) (38, 39). All chlamydial organisms were propagated in HeLa cells and purified as elementary bodies (EBs) as reported previously (41, 53). Aliquots of the purified EBs were stored at −80°C until use.
Mouse immunization and challenge infection.
Mouse experiments were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Laboratory Animal Experiments of the University of Texas Health Science Center at San Antonio.
Purified CMmut/intrOv or CMwt EBs were used to inoculate 6- to 7-week-old female C57BL/6J mice (000664, Jackson Laboratories, Inc., Bar Harbor, ME) intragastrically (for CMmut/IntrOv as oral immunization route), intravaginally (as both immunization and challenge infection), intracolonally or intranasally as described previously (20, 24, 26, 41, 54, 55). Five days before intravaginal inoculation, each mouse was injected with 2.5 mg subcutaneous medroxyprogesterone (Depo-Provera; Pharmacia Upjohn, Kalamazoo, MI) suspended in sterile phosphate-buffered saline (PBS). The inoculation dose for oral or intracolon intrOv was 1 × 107 IFUs (50, 56) while for intranasal, CMwt was 5,000 IFUs (based on pretitration in a pilot experiment). The dose of the remaining inoculation was kept at 2 × 105 IFUs regardless of the routes and C. muridarum clones. Following each inoculation, vaginal and/or rectal swabs as well as organ/tissues were taken at the designated time points for monitoring viable C. muridarum colonization as described previously (41, 44, 54).
Titrating live chlamydial organisms recovered from swabs and tissues.
To quantitate live chlamydial organisms in vaginal or rectal swabs, each swab was soaked in 0.5 mL of SPG, vortexed with glass beads, and the chlamydial organisms released into the supernatants were titrated on HeLa cell monolayers in duplicate. For tissue samples, each organ was transferred to a tube containing 2 mL SPG for homogenization using an automatic homogenizer (Omni Tissue Homogenizer, TH115, Kennesaw, GA). The homogenates were briefly sonicated and spun at 3000 rpm for 5 min to pellet remaining large debris. The supernatants were titrated for live C. muridarum organisms on HeLa cells. The infected cultures were processed for immunofluorescence assay as described previously (39, 57) and below. Inclusions were counted in five random fields per well under a fluorescence microscope. For wells with less than one IFU per field, entire wells were counted. Wells showing obvious cytotoxicity of HeLa cells were excluded. The total number of IFUs per swab was calculated based on the mean IFUs per view, the ratio of the view area to that of the well, dilution factor, and inoculation volumes. Where possible, a mean IFU/swab was derived from the serially diluted and duplicate samples for any given swab. The total number of IFUs/swab was converted into log10, which was used to calculate the mean and standard deviation across mice of the same group at each time point.
Immunofluorescence assay.
For immunofluorescence labelling of C. muridarum in HeLa cells, a rabbit antibody (designated as R1604, raised with purified C. muridarum EBs) was used as a primary antibody to label C. muridarum, which was visualized with a goat anti-rabbit IgG conjugated with FITC (green, cat#111-225-144, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). The DNA dye Hoechst 3328 (blue, Sigma-Aldrich, St. Louis, MO) was used to visualize nuclei. The dually labelled samples were used for counting for C. muridarum under a fluorescence microscope (IX80, Olympus) equipped with a CCD camera (Hamamatsu).
Evaluating genital tract gross pathology hydrosalpinx microscopically.
On day 56 after intravaginal infection, mice were euthanized for evaluating hydrosalpinx in the upper genital tract. Before the tissues were removed from mice, an in situ gross examination was performed for evidence of hydrosalpinx or any other related abnormalities. The severity of hydrosalpinx was scored based on the following criteria: no hydrosalpinx (0), hydrosalpinx detectable only under microscopic examination (1), hydrosalpinx clearly visible with naked eyes but the size was smaller than the ovary on the same side (2), equal to the ovary on the same side (3), or larger than the ovary on the same side (4). Bilateral hydrosalpinx severity was calculated for each mouse as the summed scores of the left and right oviducts. Hydrosalpinx incidence was calculated as the number of mice with a score of 1 or higher divided by the total number of mice in the group.
Evaluating oviduct dilation microscopically.
After microscopic evaluation of hydrosalpinx and photographing for documenting hydrosalpinx, the same mouse genital tissues were fixed in 10% neutral formalin, embedded in paraffin, and serially sectioned longitudinally (with 5 μm/each section). Efforts were made to include the cervix, both uterine horns and oviducts, as well as lumenal structures of each tissue in the same section. The sections were stained with hematoxylin and eosin (H&E). Three representative sections separated by 15 μM or more from each other were used for evaluating oviduct dilation. The oviduct dilation was assessed under a 4× subjective lens. The severity of oviduct dilation was semiquantitatively scored using the following criteria: 0, no significant oviduct lumenal dilatation; 1, mild dilation of a single cross section; 2, one to three dilated cross sections; 3, more than three dilated cross sections; and 4, confluent pronounced dilation. The median of the three scores served as a single value for each oviduct unilateral dilation score. Both unilateral scores for each mouse were combined to form a bilateral dilation score. Oviduct dilation incidence was calculated as the number of mice with a score of 1 or higher divided by the total number of mice in the group.
Statistics analyses.
The time courses of live organism shedding (IFUs) were compared using “area under the curve or AUC” between two groups using Wilcoxon rank sum test. The individual data point IFUs and pathology scores were also analyzed with Wilcoxon rank sum test. The category data, including the percentage of mice positive for live organism shedding or pathology, were analyzed using Fisher's Exact test. Prior to any two-group comparison, an ANOVA was used to determine whether there was an overall significant difference among all groups in the same experiment (https://goodcalculators.com/one-way-anova-calculator/).
ACKNOWLEDGMENT
This study is supported in part by US NIH grants (R01AI047997 and R56AI168479 to G.Z.).
Contributor Information
Cheng He, Email: chenghe@cau.edu.cn.
Guangming Zhong, Email: Zhongg@UTHSCSA.EDU.
Craig R. Roy, Yale University School of Medicine
REFERENCES
- 1.CDC. 2017. Sexually transmitted disease surveillance, 2016. Services USDoHaH, Atlanta, GA. https://www.cdc.gov/std/stats16/default.htm. [Google Scholar]
- 2.Budrys NM, Gong S, Rodgers AK, Wang J, Louden C, Shain R, Schenken RS, Zhong G. 2012. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstet Gynecol 119:1009–1016. doi: 10.1097/AOG.0b013e3182519326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong G, Brunham RC, de la Maza LM, Darville T, Deal C. 2019. National Institute of Allergy and Infectious Diseases workshop report: “Chlamydia vaccines: the way forward.” Vaccine 37:9. doi: 10.1016/j.vaccine.2017.10.075. [DOI] [PubMed] [Google Scholar]
- 4.Tang FF, Chang HL, Huang YT, Wang KC. 1957. Studies on the etiology of trachoma with special reference to isolation of the virus in chick embryo. Chin Med J 75:429–447. [PubMed] [Google Scholar]
- 5.Tang FF, Huang YT, Chang HL, Wong KC. 1957. Isolation of trachoma virus in chick embryo. J Hyg Epidemiol Microbiol Immunol 1:109–120. [PubMed] [Google Scholar]
- 6.Grayston JT, Wang SP, Yang YF, Woolridge RL. 1962. The effect of trachoma virus vaccine on the course of experimental trachoma infection in blind human volunteers. J Exp Med 115:1009–1022. doi: 10.1084/jem.115.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolridge RL, Grayston JT, Chang IH, Cheng KH, Yang CY, Neave C. 1967. Field trial of a monovalent and of a bivalent mineral oil adjuvant trachoma vaccine in Taiwan school children. Am J Ophthalmol 63:Suppl:1645–50. doi: 10.1016/0002-9394(67)94158-x. [DOI] [PubMed] [Google Scholar]
- 8.Woolridge RL, Grayston JT, Chang IH, Yang CY, Cheng KH. 1967. Long-term follow-up of the initial (1959–1960) trachoma vaccine field trial on Taiwan. Am J Ophthalmol 63:Suppl:1650–5. doi: 10.1016/0002-9394(67)94159-1. [DOI] [PubMed] [Google Scholar]
- 9.Rockey DD, Wang J, Lei L, Zhong G. 2009. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev Vaccines 8:1365–1377. doi: 10.1586/erv.09.98. [DOI] [PubMed] [Google Scholar]
- 10.de la Maza LM, Darville TL, Pal S. 2021. Chlamydia trachomatis vaccines for genital infections: where are we and how far is there to go? Expert Rev Vaccines 20:421–435. doi: 10.1080/14760584.2021.1899817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, Perro M, Vrbanac VD, Tager AM, Shi J, Yethon JA, Farokhzad OC, Langer R, Starnbach MN, von Andrian UH. 2015. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 348:aaa8205. doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abraham S, Juel HB, Bang P, Cheeseman HM, Dohn RB, Cole T, Kristiansen M, Korsholm KS, Lewis D, Olsen AW, McFarlane LR, Day S, Knudsen S, Moen K, Ruhwald M, Kromann I, Andersen P, Shattock RJ, Follmann F. 2019. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: a first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infectious Diseases 19:10. doi: 10.1016/S1473-3099(19)30279-8. [DOI] [PubMed] [Google Scholar]
- 13.Vlcek KR, Li W, Manam S, Zanotti B, Nicholson BJ, Ramsey KH, Murthy AK. 2016. The contribution of Chlamydia-specific CD8(+) T cells to upper genital tract pathology. Immunol Cell Biol 94:208–212. doi: 10.1038/icb.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Maza LM, Peterson EM. 2002. Vaccines for Chlamydia trachomatis infections. Curr Opin Invest Drugs 3:980–986. [PubMed] [Google Scholar]
- 15.Morrison RP, Caldwell HD. 2002. Immunity to murine chlamydial genital infection. Infect Immun 70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu C, Peng B, Li Z, Lei L, Li Z, Chen L, He Q, Zhong G, Wu Y. 2013. Induction of protective immunity against Chlamydia muridarum intravaginal infection with the chlamydial immunodominant antigen macrophage infectivity potentiator. Microbes Infect 15:329–338. doi: 10.1016/j.micinf.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson RM, Kerr MS, Slaven JE. 2014. An atypical CD8 T-cell response to Chlamydia muridarum genital tract infections includes T cells that produce interleukin-13. Immunology 142:248–257. doi: 10.1111/imm.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, Inouye S, Ramsey KH. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis 32:49–56. doi: 10.1097/01.olq.0000148299.14513.11. [DOI] [PubMed] [Google Scholar]
- 19.de la Maza LM, Pal S, Khamesipour A, Peterson EM. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun 62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, Zhong E, Arulanandam B, Baseman J, Zhong G. 2014. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PLoS One 9:e95076. doi: 10.1371/journal.pone.0095076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodgers AK, Budrys NM, Gong S, Wang J, Holden A, Schenken RS, Zhong G. 2011. Genome-wide identification of Chlamydia trachomatis antigens associated with tubal factor infertility. Fertil Steril 96:715–721. doi: 10.1016/j.fertnstert.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodgers AK, Wang J, Zhang Y, Holden A, Berryhill B, Budrys NM, Schenken RS, Zhong G. 2010. Association of tubal factor infertility with elevated antibodies to Chlamydia trachomatis caseinolytic protease P Am J Obstet Gynecol 203:494.e7–494.e14. doi: 10.1016/j.ajog.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X, Brunham RC. 1998. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol 161:1439–1446. doi: 10.4049/jimmunol.161.3.1439. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Gao L, Lei L, Zhong Y, Dube P, Berton MT, Arulanandam B, Zhang J, Zhong G. 2009. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J Immunol 183:1291–1300. doi: 10.4049/jimmunol.0803075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Zhu C, Zhang T, Tian Q, Zhang N, Morrison S, Morrison R, Xue M, Zhong G. 2018. Nonpathogenic colonization with chlamydia in the gastrointestinal tract as oral vaccination for inducing transmucosal protection. Infect Immun 86:e00630-17. doi: 10.1128/IAI.00630-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu C, Lin H, Tang L, Chen J, Wu Y, Zhong G. 2018. Oral Chlamydia vaccination induces transmucosal protection in the airway. Vaccine 36:2061–2068. doi: 10.1016/j.vaccine.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Yeruva L, Spencer N, Bowlin AK, Wang Y, Rank RG. 2013. Chlamydial infection of the gastrointestinal tract: a reservoir for persistent infection. Pathog Dis 68:88–95. doi: 10.1111/2049-632X.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong G. 2021. Chlamydia overcomes multiple gastrointestinal barriers to achieve long-lasting colonization. Trends Microbiol 29:1004–1012. doi: 10.1016/j.tim.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campos-Hernandez E, Vazquez-Chagoyan JC, Salem AZ, Saltijeral-Oaxaca JA, Escalante-Ochoa C, Lopez-Heydeck SM, de Oca-Jimenez RM. 2014. Prevalence and molecular identification of Chlamydia abortus in commercial dairy goat farms in a hot region in Mexico. Trop Anim Health Prod 46:919–924. doi: 10.1007/s11250-014-0585-6. [DOI] [PubMed] [Google Scholar]
- 30.Peters RP, Dubbink JH, van der Eem L, Verweij SP, Bos ML, Ouburg S, Lewis DA, Struthers H, McIntyre JA, Morre SA. 2014. Cross-sectional study of genital, rectal, and pharyngeal Chlamydia and gonorrhea in women in rural South Africa. Sex Transm Dis 41:564–569. doi: 10.1097/OLQ.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 31.Gratrix J, Singh AE, Bergman J, Egan C, McGinnis J, Drews SJ, Read R. 2014. Prevalence and characteristics of rectal chlamydia and gonorrhea cases among men who have sex with men after the introduction of nucleic acid amplification test screening at 2 Canadian sexually transmitted infection clinics. Sex Transm Dis 41:589–591. doi: 10.1097/OLQ.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 32.Gratrix J, Singh AE, Bergman J, Egan C, Plitt SS, McGinnis J, Bell CA, Drews SJ, Read R. 2015. Evidence for increased Chlamydia case finding after the introduction of rectal screening among women attending 2 Canadian sexually transmitted infection clinics. Clin Infect Dis 60:398–404. doi: 10.1093/cid/ciu831. [DOI] [PubMed] [Google Scholar]
- 33.Musil K, Currie M, Sherley M, Martin S. 2016. Rectal chlamydia infection in women at high risk of chlamydia attending Canberra Sexual Health Centre. Int J STD AIDS 27:526–530. doi: 10.1177/0956462415586317. [DOI] [PubMed] [Google Scholar]
- 34.Winner H, Friesenhahn A, Wang Y, Stanbury N, Wang J, He C, Zhong G. 2022. Regulation of chlamydial colonization by IFNgamma delivered via distinct cells. Trends Microbiol 31:270–279. doi: 10.1016/j.tim.2022.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong G. 2018. Chlamydia spreading from the genital tract to the gastrointestinal tract - A two-hit hypothesis. Trends Microbiol 26:611–623. doi: 10.1016/j.tim.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison SG, Giebel AM, Toh E, Banerjee A, Nelson DE, Morrison RP. 2020. A genital infection-attenuated Chlamydia muridarum mutant infects the gastrointestinal tract and protects against genital tract challenge. mBio 11. doi: 10.1128/mBio.02770-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Z, Tian Q, Wang L, Zhong G. 2022. Chlamydia deficient in plasmid-encoded glycoprotein 3 (pGP3) as an attenuated live oral vaccine. Infect Immun 90:e0047221. doi: 10.1128/IAI.00472-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C, Zhou Z, Conrad T, Yang Z, Dai J, Li Z, Wu Y, Zhong G. 2015. In vitro passage selects for Chlamydia muridarum with enhanced infectivity in cultured cells but attenuated pathogenicity in mouse upper genital tract. Infect Immun 83:1881–1892. doi: 10.1128/IAI.03158-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conrad TA, Gong S, Yang Z, Matulich P, Keck J, Beltrami N, Chen C, Zhou Z, Dai J, Zhong G. 2016. The chromosome-encoded hypothetical protein TC0668 Is an upper genital tract pathogenicity factor of Chlamydia muridarum. Infect Immun 84:467–479. doi: 10.1128/IAI.01171-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao L, Zhang T, Liu Q, Wang J, Zhong G. 2017. Chlamydia muridarum with mutations in chromosomal genes tc0237 and/or tc0668 is deficient in colonizing the mouse gastrointestinal tract. Infect Immun 85:e00321-17. doi: 10.1128/IAI.00321-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Q, Huang Y, Gong S, Yang Z, Sun X, Schenken R, Zhong G. 2015. In vivo and ex vivo imaging reveals a long-lasting chlamydial infection in the mouse gastrointestinal tract following genital tract inoculation. Infect Immun 83:3568–3577. doi: 10.1128/IAI.00673-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Gartner J, Zhu L, Wang S, Brunham RC. 1999. IL-10 gene knockout mice show enhanced Th1-like protective immunity and absent granuloma formation following Chlamydia trachomatis lung infection. J Immunol 162:1010–1017. doi: 10.4049/jimmunol.162.2.1010. [DOI] [PubMed] [Google Scholar]
- 43.Perry LL, Hughes S. 1999. Chlamydial colonization of multiple mucosae following infection by any mucosal route. Infect Immun 67:3686–3689. doi: 10.1128/IAI.67.7.3686-3689.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai J, Zhang T, Wang L, Shao L, Zhu C, Zhang Y, Failor C, Schenken R, Baseman J, He C, Zhong G. 2016. Intravenous inoculation with Chlamydia muridarum leads to a long-lasting infection restricted to the gastrointestinal tract. Infect Immun 84:2382–2388. doi: 10.1128/IAI.00432-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu C, Zeng H, Li Z, Lei L, Yeh IT, Wu Y, Zhong G. 2012. Protective immunity against mouse upper genital tract pathology correlates with high IFNgamma but low IL-17 T cell and anti-secretion protein antibody responses induced by replicating chlamydial organisms in the airway. Vaccine 30:475–485. doi: 10.1016/j.vaccine.2011.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu H, Karunakaran KP, Kelly I, Shen C, Jiang X, Foster LJ, Brunham RC. 2011. Immunization with live and dead Chlamydia muridarum induces different levels of protective immunity in a murine genital tract model: correlation with MHC class II peptide presentation and multifunctional Th1 cells. J Immunol 186:3615–3621. doi: 10.4049/jimmunol.1002952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaharik ML, Nayar T, White R, Ma C, Vallance BA, Straka N, Jiang X, Rey-Ladino J, Shen C, Brunham RC. 2007. Genetic profiling of dendritic cells exposed to live- or ultraviolet-irradiated Chlamydia muridarum reveals marked differences in CXC chemokine profiles. Immunology 120:160–172. doi: 10.1111/j.1365-2567.2006.02488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Z, Tian Q, Wang L, Xue M, Xu D, Zhong G. 2021. Chlamydia spreads to the large intestine lumen via multiple pathways. Infect Immun 89:e0025421. doi: 10.1128/IAI.00254-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howe SE, Shillova N, Konjufca V. 2019. Dissemination of Chlamydia from the reproductive tract to the gastro-intestinal tract occurs in stages and relies on Chlamydia transport by host cells. PLoS Pathog 15:e1008207. doi: 10.1371/journal.ppat.1008207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koprivsek JJ, He Y, Song C, Zhang N, Tumanov A, Zhong G. 2020. Evasion of innate lymphoid cell-regulated gamma interferon responses by Chlamydia muridarum to achieve long-lasting colonization in mouse colon. Infect Immun 88:e00798-19. doi: 10.1128/IAI.00798-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He Y, Xu H, Song C, Koprivsek JJ, Arulanandam B, Yang H, Tao L, Zhong G. 2021. Adoptive transfer of group 3-like innate lymphoid cells restores mouse colon resistance to colonization of a gamma interferon-susceptible Chlamydia muridarum mutant. Infect Immun 89:e00533-20. doi: 10.1128/IAI.00533-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brothwell JA, Muramatsu MK, Zhong G, Nelson DE. 2018. Advances and obstacles in the genetic dissection of chlamydial virulence. Current Topics in Microbiology 412:133–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, Greenberg AH, Zhong G. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Zhang Q, Zhang T, Zhang Y, Zhu C, Sun X, Zhang N, Xue M, Zhong G. 2016. The Chlamydia muridarum organisms fail to auto-inoculate the mouse genital tract after colonization in the gastrointestinal tract for 70 days. PLoS One 11:e0155880. doi: 10.1371/journal.pone.0155880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shao L, Melero J, Zhang N, Arulanandam B, Baseman J, Liu Q, Zhong G. 2017. The cryptic plasmid is more important for Chlamydia muridarum to colonize the mouse gastrointestinal tract than to infect the genital tract. PLoS One 12:e0177691. doi: 10.1371/journal.pone.0177691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koprivsek JJ, Zhang T, Tian Q, He Y, Xu H, Xu Z, Zhong G. 2019. Distinct roles of chromosome- versus plasmid-encoded genital tract virulence factors in promoting chlamydia muridarum colonization in the gastrointestinal tract. Infect Immun 87:e00265-19. doi: 10.1128/IAI.00265-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang L, Zhang H, Lei L, Gong S, Zhou Z, Baseman J, Zhong G. 2013. Oviduct infection and hydrosalpinx in DBA1/j Mice is induced by intracervical but not intravaginal inoculation with Chlamydia muridarum. PLoS One 8:e71649. doi: 10.1371/journal.pone.0071649. [DOI] [PMC free article] [PubMed] [Google Scholar]