Abstract
Ticks are obligatory hematophagous ectoparasites that transmit pathogens among various vertebrates, including humans. The composition of the microbial and viral communities in addition to the pathogenic microorganisms is highly diverse in ticks, but the factors driving the diversity are not well understood. The tropical horse tick, Dermacentor nitens, is distributed throughout the Americas and it is recognized as a natural vector of Babesia caballi and Theileria equi, the causal agents of equine piroplasmosis. We characterized the bacterial and viral communities associated with partially-fed D. nitens females collected by a passive survey on horses from field sites representing three distinct geographical areas in Colombia (Bolivar, Antioquia, and Cordoba). RNA-seq and sequencing of the V3 and V4 hypervariable regions of the 16S rRNA gene were performed using the Illumina-Miseq platform. A total of 356 operational taxonomic units (OTUs) were identified, in which the presumed endosymbiotic Francisellaceae/Francisella spp. was predominantly found. Nine contigs corresponding to six different viruses were identified in three viral families: Chuviridae, Rhabdoviridae, and Flaviviridae. Differences in the relative abundance of the microbial composition among the geographical regions were found to be independent of the presence of Francisella-Like Endosymbiont (FLE). The most prevalent bacteria found on each region were Corynebacterium in Bolivar, Staphylococcus in Antioquia, and Pseudomonas in Cordoba. Rickettsia-like endosymbionts, mainly recognized as the etiological agent of rickettsioses in Colombia were detected in the Cordoba samples. Metatranscriptomics revealed 13 contigs containing FLE genes, suggesting a trend of regional differences. These findings suggest regional distinctions among the ticks and their bacterial compositions.
Keywords: next-generation sequencing, metatranscriptomics, 16s rRNA, RNA-seq, Francisella-like endosymbiont
1. Introduction
Ticks are important vectors of pathogens that cause livestock and human diseases, such as ehrlichiosis, borreliosis, Lyme disease, human and cattle babesiosis, and theileriosis. Tick-borne encephalitis virus, Powassan virus, and Crimean-Congo hemorrhagic fever virus are one of the most prevalent tick-borne viral infections.(1,2). The risks of emerging and re-emerging tick-borne diseases remain a continuing threat since prevention and management are hampered by suboptimal diagnostics, lack of treatment options for emerging pathogens, and scarcity of vaccines (3,4). Habitat changes of the ticks by human activities and globalization have been described as direct factors driving migration and colonization of hosts, vectors, and pathogens (5). In addition, global climate change caused by human activities has increased the incidence and diversity of circulating pathogens in new habitats (6).
Ticks harbor diverse microorganisms, including symbionts, in addition to pathogenic organisms, which may have direct positive/negative effects on the tick or other members of the microbial communities (1,7,8). Interactions among the microorganisms in the bacterial communities in the ticks are considered an important factor in the transmission of human/animal pathogenic organisms. (9,10). Among non-pathogenic communities, common bacterial endosymbionts found in ticks are mainly related to Rickettsia, Coxiella, and Francisella genera (1,11,12). These microorganisms act as primary endosymbionts providing essential nutrients involved in survival, development, and tick-fitness, such as biosynthesis of B vitamins and cofactors like riboflavin, folic acid, and biotin (13). Tick-endosymbionts are generally tissue-specific with microbial guilds well established in salivary glands, gut, ovaries, among other tissues (14). Some of these microorganisms, including pathogenic and non-pathogenic bacteria, can be transovarially transmitted to tick offspring (15). Given the importance of ticks as vectors of many important pathogens, understanding ticks and their symbiont compositions in different ecological systems has arisen as an important area of study (2).
The tick microbiome includes communities of viruses, bacteria, protozoa, and fungi (8,14). Recent experimental approaches to characterize the bacterial diversity in various species of ticks used next-generation sequencing (NGS) of the 16S rRNA gene sequence amplicons (16–18). Those studies revealed tick bacterial communities, including mammalian pathogens, that are dependent on the tick species, type of host, and geographic location (4,11,19). Characterizing the microbial tick populations may give us a better understanding of the different potential roles in intra- and interspecific microbial interactions and their involvement in vector competence (4,7,20).
Viruses are present in all domains of life, particularly rich in the phylum Arthropoda, which includes ticks (21). Metatranscriptomics is a widely used tool to investigate RNA viruses in ticks. Despite considerable insights into bacterial diversity, our understanding of tick-associated viruses is still limited, and largely unexplored compared with bacterial diversity (22). Virome studies of ticks collected in Asia, Europe, and North America have revealed the emergence of novel pathogenic tick-borne viruses as well as the dearth of data on tick viromes which suggest a need for viral surveillance and discovery in this group of arthropods (23–25). Progress in sequencing technology and metagenomics data have provided an approximation to the viral community composition present in a few tick species (22,24,26–30). In addition, more information from different species may be an efficient strategy to mitigate potential threats of tick-borne disease to public health (2,3,25,30).
The tropical horse tick, Dermacentor nitens, is distributed throughout the Americas and it is recognized as a natural vector of Babesia caballi and Theileria equi, the causal agents of equine piroplasmosis (31,32). Dermacentor nitens is a one-host tick, with three to four generations per year (33). Severe infestation in vertebrate animals can cause severe lesions, especially in the ears, and predispose the host to secondary bacterial infections (34). Although equines are the primary host, natural infestations have been reported in other domestic, and companion animals, as well as wild animals (35–37). Dermacentor nitens is considered a sporadic ectoparasite of humans, where tick infestations are probably a consequence of humans entering infested livestock environments, resulting in a transference of ticks from infested animals to persons (38). Accidental infestations by D. nitens in humans related to agricultural activities may represent a potential danger to human health, although the vectorial capacity of D. nitens for pathogens related to public health remains unknown. Occurrence of human pathogenic agents in this tick species have been previously reported (39,40).
To gain an in-depth understanding of the microbial communities of D. nitens, we used 16S rRNA gene sequences combined with metatranscriptomic analysis to identify the main bacterial and viral communities present in the ticks collected in different geographical populations. These results provide large numbers of sequences annotated as tick viruses and operons of Francisella-like endosymbionts (FLE) and revealed a trend of differences among the three geographical populations.
2. Materials and methods
2.1. Sample collection and nucleic acid extraction
Tick collection was carried out by passive survey at “La Rinconada” slaughterhouse (06°11’26.0”N; 75°22’43.4”W) in the municipality of Rionegro, Antioquia, Colombia in July, and September 2019. A total of 45 blood-fed D. nitens adults were obtained from three horses native to each region, Bolivar, Antioquia, and Cordoba (Supplementary Figure 1). The three departments are located in the northwest of Colombia and share borders with the department of Antioquia. Live ticks were transported to the Universidad de Antioquia facilities, where taxonomical identification was made following morphological keys (41), and specimens subsequently stored at −20 or −80°C until shipment to Kansas State University facilities. Blood-fed female D. nitens collected from horses were pooled and processed based on host (individual animal) and region (Bolivar, Antioquia, and Cordoba). From a total of three horses per region and one pool of five ticks per horse were chosen by using the random selection method, thus sampling a total of 45 ticks (nine pools). Genomic DNA and RNA were extracted independently following manufacturer instructions using Zymo™ DNA and RNA extraction kits (Irvine, California, US) from the pools previously separated from the tick-exoskeleton.
2.2. NGS library preparations and data processing
Genomic DNA of the pools of ticks was sent to the Genome Sequencing Core at the University of Kansas. Amplicon libraries were prepared by Illumina Miseq targeting the V3-V4 region with the primers 16S-F (5’-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3’) and 16S-R (5’- GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3’) of the 16S rRNA, with an expected length of ~465 base-pair (bp) for the DNA analysis (16).
16S rRNA sequences were analyzed with Mothur v.1.45, according to the MiSeq Standard Operating Procedure (42). Operational Taxonomic Units (OTUs) with 97% of identity were clustered and classified using the database SILVA v.138. Raw reads were filtered to a maximum length of 465 base-pair without ambiguous bases (43). Another filtering step was done in Excel to remove low-count OTUs with a prevalence in samples of less than 0.005% (44). Bacterial relative abundance was analyzed in R studio (vegan and ggpubr packages), and GraphPad Prism 9.2.0 software (45–47). We also compared the differences in the proportion of the bacterial composition of the regions through a Non-Metric Multidimensional Scaling (NMDS) ordination plot. It is important to note that there is the potential for low-frequency background noises in this dataset due to the absence of blank extraction control during the nucleic acid extraction and bioinformatics workflows (44).
RNA-seq library preparation was done with the NEB Next Stranded RNA library kit without PolyA selection of the mRNA, the nine pooled RNAs were sent to the Genome Sequencing Core at the University of Kansas. For the metatranscriptomics analysis, the RNA-seq reads were processed for removal of Illumina adaptor sequences, trimmed, and quality-based filtered using Fastp software v.0.20.0 (48). The high-quality reads (Phred-score >30) were removed by mapping onto the reference genome of D. silvarum (assembly ASM1333974v1) and Equus caballus (assembly EquCab3.0) using STAR v.2.7 (49). The unmapped reads (Supplementary Table 1) were used to perform the assembly and annotation of the transcriptome by using Trinity and Blast2GO suite in OmicsBox v.2.0.36 software (50–52). Contigs annotated in Blast2GO were reexamined manually by BLASTn and BLASTx (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to confirm the results and eliminate potential false positives. Empirical Bayes estimation and Fisher’s exact tests (α = 0.05) by pairwise comparison based on the negative binomial distribution analysis were done with edgeR by using the Galaxy platform to test statistically significant differences in abundance between the bacterial and viral sequences annotated with the geographic location for the blood-fed D. nitens.
2.3. Phylogenetic analyses of viral and Francisella spp. contigs
Phylogenetic analyses by comparison of Bayesian inference, Maximum-Likelihood, Minimum-Evolution, and Neighbor-Joining methods were performed as an initial assessment with the bacterial protein sequences and the OTUs detected in this study compared to the reference sequences pulled out from the NCBI GenBank database by doing homology-based search using Blast search. Bacterial protein sequences, partial 16s rRNA nucleotide sequences of FLE, and viral protein sequences were retrieved from the GenBank database as indicated with the GenBank accession numbers in Figures 2 to 4. Sequences were aligned by using Muscle in MEGA-X software (53). Bayesian inference analysis was done using BEAST v1.10.4 software (54). Phylogenetic trees for the analysis of the 16s rRNA nucleotide sequences were constructed based on the Neighbor-Joining method with a pairwise deletion. The tree for the V3-V4 regions sequenced in this study were constructed with 500 bootstrap replicates (55–57) unless otherwise specified. For metatranscriptomic analyses of the FLE and viral proteins sequences, the cladograms were constructed using annotated and concatenated genes for each contig by using the Maximum Likelihood method with Tamura-Nei model and 500 bootstrap replicates (58).
Figure 2.
Phylogenetic analyses for the Francisella-Like endosymbionts (FLE, A) and Rickettsia-like endosymbionts (RLE, B) identified in this study for Dermacentor nitens samples. (A) Neighbor-joining cladogram rooted to Francisella tularensis strains representing the phylogenetic relationship of 16S rDNA sequences OTUs classified as Francisella spp. in D. nitens. The tree was built using the pairwise deletion method. The blue branches represent the FLE clade, the green branches represent opportunistic pathogenic Francisella species, and the red branches represent the pathogenic Francisella tularensis strains as an outgroup. (B) Neighbor-joining cladogram rooted to pathogenic Rickettsia strains to represent the phylogenetic relationship of rickettsial 16S rDNA sequences with the OTU184 classified as Rickettsia spp. in the D. nitens sample. The red branches represent pathogenic Rickettsia spp., blue branches represent the sequences of RLE, and dark branches represent candidate-human pathogenic Rickettsia. The OTUs were determined by a 97% identity threshold. Bootstrapping percentages in 500 replications are shown on the nodes with a 60% cut-off. The GenBank accession numbers for each sequence are shown at the beginning of names of taxa.
Figure 4.
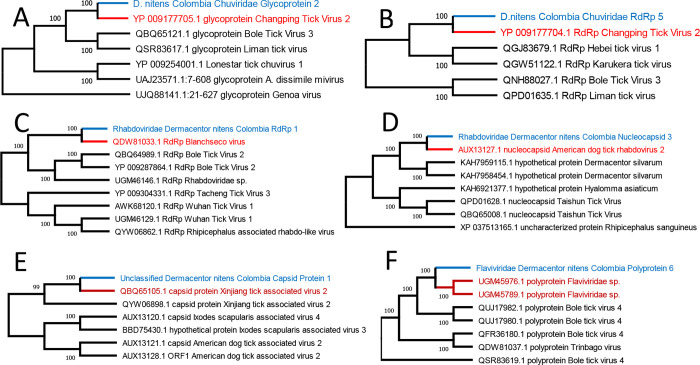
Phylogenetic relationship of the contigs for to RNA viruses captured in the D. nitens samples in this study. The maximum likelihood cladograms were constructed with complete deletion of assembly gaps. Bootstrapping percentages in 500 replications are shown at the nodes. The contig D. nitens Colombia Chuviridae Glycoprotein 2 encodes a Glycoprotein gene with a length of 668 bp (A), D.nitens_Colombia_Chuviridae_Polymerase_5 encodes an RNA-dependent RNA polymerase with a length of 2156 (B), Rhabdoviridae_Dermacentor_nitens_Colombia_Polymerase_1 encodes an RNA-dependent RNA polymerase with a length of 7061 bp (C), Rhabdoviridae_Dermacentor_nitens_Colombia_Nucleocapsid_3 encodes a nucleocapsid with a length of 524 bp (D), Unclassified_Dermacentor_nitens_Capsid_Protein_1 encodes a capsid protein with a length of 168 bp (E), Flaviviridae_Dermacentor_nitens_Colombia_Polyprotein_6 encodes a polyprotein with a length of 5140 bp (F). Names in blue correspond to the viral contigs found in this study, and red names correspond to the closest viral protein sequence in the GenBank database. The GenBank accession numbers are shown at the beginning of the names of taxa.
2.4. Ethical approval
This study was approved by the Bioethics Committee of the Universidad de Antioquia (Approval record No. 15–32-436 of June 2015). It was also granted an environmental license issued by the Colombian government through the National Environmental Licensing Authority (Autoridad Nacional de Licencias Ambientales-ANLA, Resolution ANLA 00908 of May 27, 2017).
3. Results
3.1. Bacterial diversity investigated using V3-V4 regions of the 16S rRNA sequences.
A total of 372,493 sequences after filtering 392,819 raw reads were assembled into 6,686 contigs and assigned to 356 OTUs with a threshold of 97% of sequence identity (Table 1). Notably, the sequences consisted of three main OTUs, all identified as FLE (>80%) in all nine samples (Figure 1A). Among the remaining <20% OTUs, the most prevalent bacteria in different regions were Corynebacterium in Bolivar, Staphylococcus in Antioquia, and Pseudomonas in Cordoba (Figure 1B). We also compared the differences in bacterial compositions of the regions through Non-Metric Multidimensional Scaling (NMDS) in the data sets before and after excluding FLE (Figures 1C and 1D). Our NMDS plots suggest that regional bacterial composition is unique and independent of the presence of FLE and can be useful to differentiate the bacterial composition from different geographical regions (Figure 1).
Table 1.
Nine sequencing libraries for the pools for D. nitens, targeting V3-V4 regions of the 16 rRNA gene.
| Library (Paired Reads) | Region | Raw reads | Mapped Reads | Contigs |
|---|---|---|---|---|
|
| ||||
| DNA_Pool_1 | Bolivar | 48852 | 46109 | 706 |
| DNA_Pool_2 | Bolivar | 41430 | 39512 | 508 |
| DNA_Pool_3 | Bolivar | 37846 | 36438 | 503 |
| DNA_Pool_4 | Antioquia | 45141 | 42948 | 842 |
| DNA_Pool_5 | Antioquia | 39380 | 37847 | 1044 |
| DNA_Pool_6 | Antioquia | 43778 | 41116 | 886 |
| DNA_Pool_7 | Cordoba | 47878 | 45604 | 665 |
| DNA_Pool_8 | Cordoba | 41244 | 38268 | 583 |
| DNA_Pool_9 | Cordoba | 47270 | 44651 | 949 |
|
| ||||
| Total | 392819 | 372493 | 6686 | |
Figure 1.
Bacterial diversity shown by the genera in 16S rDNA sequences from Dermacentor nitens samples collected from three different regions of Colombia. (A) Relative abundance is shown by bacterial genera. (B) The relative abundance after excluding the sequences of endosymbionts Francisellaceae/Francisella spp. (C) Non-metric multidimensional scaling plot (NMDS) plot showing the differences among tick samples from different regions. (D) NMDS plot showing the differences among tick samples after excluding the endosymbionts.
The FLEs categorized by a 97% identity threshold were three different OTUs (OTU001, 002, and 010 in Figure 2A and Supplementary Table 2). These sequences are significantly different from each other with 20 nucleotides (nt) mismatches between OTU001 and OTU002, 21 nt mismatches between OTU002 and OTU010, and 8 nt mismatches between OTU001 and OTU010. High frequencies of the reads for each FLE OTUs, which are in independent libraries, suggest that the three different FLE OTUs are not sequencing artifacts. The cladogram of the FLE sequences showed these three OTU clustered in a branch with the bootstrapping value of 100 (Figure 2A). A single OTU, OTU184, was categorized into Rickettsia-like endosymbiont (RLE) in one pool of the Cordoba region. Phylogenetic analysis supports the position of this sequence in the tree clustered with RLE of Amblyomma latepunctatum and a clear separation from the pathogenic Rickettsia although the bootstrapping value was 68 (Figure 2B).
3.2. Metatranscriptome containing viral and Francisella spp. RNA
A total of 152.2 million raw reads were obtained from the nine pools representing the three different regions. After quality trimming and filtering out against E. caballus and D. silvarum sequences, 92.18 million reads were used for downstream analysis (Supplementary Table 1). De novo assembly was conducted using the TRINITY pipeline built in OmicsBox software. After cleaning and filtering, 16.8 million reads were assembled into 81 contigs. Homology-based taxonomic assignment and gene function for each contig was made in Blast2Go and using manual BLAST searches.
Thirteen contigs were categorized as FLE, containing presumed independent operons with an average length of 4,794 bp. Table 2 represents the length and coverage information, the sequence name, the gene encoded, and the putative gene size for each contig (Supplementary Figure 2). The highest coverage of the FLE contigs was Contig_ORF_FLE_of_D. nitens_13, which partially encodes the Mechanosensitive ion channel protein MscS with a length of 596 and 1,892.14 TPM (transcripts per million reads) (Supplementary Figure 3 and Supplementary Table 3). FLE putative operon sequences were submitted to GenBank with the accession numbers contained in the BioProject PRJNA953638.
Table 2.
Annotations of bacterial contigs captured in the metatranscriptome of Dermacentor nitens.
| Sequence ID | Gene name | Open reading frame (bp) |
|---|---|---|
| Contig_FLE_D.nitens_1, length = 9969bp, Coverage = 1628 | ||
| TRINITY_DN179725_c0_g1_Gene1 | 3-Oxoacyl-ACP synthase CDS | 972 |
| TRINITY_DN179725_c0_g1_Gene2 | Phosphate acyltransferase CDS | 1047 |
| TRINITY_DN179725_c0_g1_Gene3 | rpmF CDS | 183 |
| TRINITY_DN179725_c0_g1_Gene4 | Hypothetical protein CDS | 504 |
| TRINITY_DN179725_c0_g1_Gene5 | Transketolase CDS | 1992 |
| TRINITY_DN179725_c0_g1_Gene6 | Glyceraldehyde-3-phospate dehydrogenase CDS | 1002 |
| TRINITY_DN179725_c0_g1_Gene7 | Phosphoglycerate kinase CDS | 1179 |
| TRINITY_DN179725_c0_g1_Gene8 | Pyruvate kinase CDS | 1437 |
| TRINITY_DN179725_c0_g1_Gene9 | Fructose-1,6-bisphosphate aldolase CDS | 1065 |
| Contig_FLE_D.nitens_2, length = 5250bp, Coverage = 696 | ||
| TRINITY_DN15830_c0_g2_Gene1 | Nucleotide exchange factor GrpE CDS | 588 |
| TRINITY_DN15830_c0_g2_Gene2 | Molecular chaperone DnaK CDS | 1929 |
| TRINITY_DN15830_c0_g2_Gene3 | Molecular chaperone DnaJ CDS | 1122 |
| TRINITY_DN15830_c0_g2_Gene4 | LysR family transcriptional regulator CDS | 906 |
| TRINITY_DN15830_c0_g2_Gene5 | Hypothetical protein CDS | 705 |
| Contig_FLE_D.nitens_3, length = 8089bp, Coverage = 675 | ||
| TRINITY_DN25174_c0_g1_Gene1 | Hypothetical protein CDS | 1444 |
| TRINITY_DN25174_c0_g1_Gene2 | Hypothetical protein CDS | 620 |
| TRINITY_DN25174_c0_g1_Gene3 | Hypothetical protein CDS | 1006 |
| TRINITY_DN25174_c0_g1_Gene4 | Hypothetical protein CDS | 1003 |
| TRINITY_DN25174_c0_g1_Gene5 | Membrane protein CDS | 478 |
| TRINITY_DN25174_c0_g1_Gene6 | Hypothetical protein CDS | 934 |
| TRINITY_DN25174_c0_g1_Gene7 | moxR CDS | 962 |
| TRINITY_DN25174_c0_g1_Gene8 | Hypothetical protein CDS | 444 |
| TRINITY_DN25174_c0_g1_Gene9 | pdcY CDS | 853 |
| TRINITY_DN25174_c0_g1_Gene10 | Hypothetical protein CDS | 345 |
| Contig_FLE_D.nitens_4, length = 5373bp, Coverage = 660 | ||
| TRINITY_DN3539_c0_g1_Gene1 | Carbamoyl phosphate synthase small subunit CDS | 1167 |
| TRINITY_DN3539_c0_g1_Gene2 | Carbamoyl phosphate synthase large subunit CDS | 3285 |
| TRINITY_DN3539_c0_g1_Gene3 | Aspartate carbamoyltransferase CDS | 921 |
| Contig_FLE_D.nitens_5, length = 5215bp, Coverage = 617 | ||
| TRINITY_DN112697_c0_g1_Gene1 | Coproporphyrinogen III oxidase CDS | 1143 |
| TRINITY_DN112697_c0_g1_Gene2 | Polysacccharide biosynthesis protein GtrA CDS | 378 |
| TRINITY_DN112697_c0_g1_Gene3 | Peroxidase CDS | 882 |
| TRINITY_DN112697_c0_g1_Gene4 | Aconitate hydratase CDS | 2812 |
| Contig_FLE_D.nitens_6, length = 1350bp, Coverage = 787 | ||
| TRINITY_DN1678_c0_g1_Gene1 | Glutamate dehydrogenase CDS | 1350 |
| Contig_FLE_D.nitens_7, length = 2846bp, Coverage = 942 | ||
| TRINITY_DN396500_c0_g1_Gene1 | Glycine dehydrogenase CDS | 1381 |
| TRINITY_DN396500_c0_g1_Gene2 | Glycine dehydrogenase CDS | 1465 |
| Contig_FLE_D.nitens_8, length = 4254bp, Coverage = 880 | ||
| TRINITY_DN1569_c0_g1_Gene1 | ATP synthase subunit alpha CDS | 1542 |
| TRINITY_DN1569_c0_g1_Gene2 | ATP F0F1 synthase subunit gamma CDS | 897 |
| TRINITY_DN1569_c0_g1_Gene3 | ATP synthase subunit beta CDS | 1377 |
| TRINITY_DN1569_c0_g1_Gene4 | atpC CDS | 438 |
| Contig_FLE_D.nitens_9, length = 7945bp, Coverage = 1393 | ||
| TRINITY_DN253568_c0_g1_Gene1 | Leucyl aminopeptidase CDS | 1440 |
| TRINITY_DN253568_c0_g1_Gene2 | lptF CDS | 1087 |
| TRINITY_DN253568_c0_g1_Gene3 | lptG CDS | 1063 |
| TRINITY_DN253568_c0_g1_Gene4 | Insulinase family protein CDS | 1254 |
| TRINITY_DN253568_c0_g1_Gene5 | Insulinase family protein CDS | 1254 |
| TRINITY_DN253568_c0_g1_Gene6 | rsmD CDS | 579 |
| TRINITY_DN253568_c0_g1_Gene7 | Trimeric intracellular cation channel family protein CDS | 654 |
| TRINITY_DN253568_c0_g1_Gene8 | tRNA-(ms[2]io[6]A)-hydrolase CDS | 614 |
| Contig_FLE_D.nitens_10, length = 3170bp, Coverage = 221 | ||
| TRINITY_DN182378_c0_g1_Gene1 | Amino acid transporter CDS | 705 |
| TRINITY_DN182378_c0_g1_Gene2 | Oxidoreductase, short chain dehydrogenase/reductase family CDS | 827 |
| TRINITY_DN182378_c0_g1_Gene3 | Hypothetical protein CDS | 471 |
| TRINITY_DN182378_c0_g1_Gene4 | NAD(FAD)-utilizing dehydrogenase CDS | 1167 |
| Contig_FLE_D.nitens_11, length = 4745bp, Coverage = 306 | ||
| TRINITY_DN15837_c0_g1_Gene1 | Hypothetical protein CDS | 653 |
| TRINITY_DN15837_c0_g1_Gene2 | Hypothetical protein CDS | 417 |
| TRINITY_DN15837_c0_g1_Gene3 | Alanine--tRNA ligase CDS | 2598 |
| TRINITY_DN15837_c0_g1_Gene4 | Transporter CDS | 1077 |
| Contig_FLE_D.nitens_12, length = 3517bp, Coverage = 491 | ||
| TRINITY_DN182530_c0_g1_Gene1 | Hypothetical protein CDS | 537 |
| TRINITY_DN182530_c0_g1_Gene2 | rpIT CDS | 357 |
| TRINITY_DN182530_c0_g1_Gene3 | 50S ribosomal protein L35 CDS | 199 |
| TRINITY_DN182530_c0_g1_Gene4 | Translation initiation factor IF-3 CDS | 519 |
| TRINITY_DN182530_c0_g1_Gene5 | Threonine--tRNA ligase CDS | 1905 |
| Contig_FLE_D.nitens_13 length = 596bp, Coverage = 3219 | ||
| TRINITY_DN15777_c0_g1_Gene1 | Mechanosensitive ion channel protein MscS-Partial | 596 |
| Total coverage | 12515 | |
Six different putative viruses covered by nine viral contigs with an average length of 1,749 bp were identified in BLAST searches for the non-redundant protein database of NCBI and the Viral Genomes database. The sequences were manually inspected and annotated for the coding regions. Table 3 shows the viral contigs with the length and coverage information. The highest coverage for the viral contigs was the D. nitens_Colombia_Flaviviridae_Polyprotein_6 contig with a total of 2,346.25 TPM with the coverage predominantly higher in the region of Cordoba (Supplementary Figure 4 and Supplementary Table 4). The D. nitens virus contig sequences were submitted to GenBank with the accession numbers contained in the BioProject PRJNA953638.
Table 3.
Viral contigs captured in the metatranscriptome of D. nitens, shown for the lengths, coverages, and Blast results.
| Contig ID | Length | Coverage | Sequence name | Blast result | ||
|---|---|---|---|---|---|---|
| GenBank ID | e-value | Name of Virus | ||||
|
| ||||||
| Unclassified_Capsid_Protein_1 | 198 | 1 | TRINITY_DN36539_c0_g1 | QBQ65105.1 | 4.00E-140 | Xinjiang Tick associated virus 2 |
| Chuviridae_Glycoprotein_2 | 668 | 168 | TRINITY_DN179920_c0_g1 | YP_009177705.1 | 0 | Changping Tick Virus 2 |
| Chuviridae_Polymerase_5 | 2156 | 355 | TRINITY DN180002 c0 g1 | YP_009177704.1 | 0 | Changping Tick Virus 2 |
| Rhabdoviridae_Nucleocapsid_3 | 524 | 4 | TRINITY_DN327528_c0_g1 | AUX13127.1 | 0 | American dog tick rhabdovirus 2 |
| Rhabdoviridae_Polymerase_1 | 7061 | 218 | TRINITY_DN16706_c0_g1 | QDW81034.1 | 0 | Blanchseco virus |
| TRINITY_DN399801_c0_g1 | QDW81033.1 | 0 | Blanchseco virus | |||
| TRINITY_DN405583_c0_g1 | QDW81033.1 | 0 | Blanchseco virus | |||
| TRINITY_DN31349_c0_g1 | QDW81033.1 | 0 | Blanchseco virus | |||
| Flaviviridae_Polyprotein_6 | 5140 | 3374 | TRINITY_DN544_c0_g1 | UGM45976.1 | 0 | Flaviviridae sp. |
|
| ||||||
| Total coverage | 4120 | |||||
3.3. Phylogenetic analyses of viral and Francisella spp contigs
Thirteen FLE groups and nine viral contigs identified by metatranscriptomics were further analyzed for their phylogenetic positions. All 13 FLE contigs clustered with other FLE identified in tick species when rooted in the pathogenic and opportunistic Francisella groups. The sequences had a 100% bootstrapping value for the tick endosymbiont clade represented by Amblyomma maculatum and Ornithodoros moubata (59) Figure 3 showing the phylogeny of concatenated sequences of 13 contigs. The overall similarity was 90% with the FLE of the Ixodidae family represented by A. maculatum. The green branched clade, containing F. persica, F. opportunistica, and F. hispaniensis represents the opportunistic pathogens that have been linked as potential causative agents of illness episodes in humans (12,59,60). The red branched cluster, shown as the outgroup, are the pathogenic strains of Francisella tularensis sl. To show the relationship of the contigs identified with the FLE clade, the sequence named Contig_ORF_FLE_of_D.nitens_1 was used as a representative sequence for the phylogenetic analysis, mainly because all 13 contigs grouped with the tick endosymbiont clade. The total coverage found for the 13 contigs classified as FLE was 12,515, with contigs 13 and 1 being the most predominant among all pools of samples (Supplementary Table 3).
Figure 3.
Phylogenetic relationship of the Francisella-Like Endosymbiont in the D. nitens samples in this study. The sequence is the translated sequence for the concatenated open reading frames. The selected contig contains nine genes (Table 2) annotated with a total length for the concatenated contig of 3323 amino acids (9969 bp). and 1892 transcript per million (TPM) in the pooled metatranscriptome. The tree is for maximum likelihood cladogram built using the complete deletion method. Bootstrapping percentage values are based on 500 replications and are shown at the nodes. The outgroup is for the sequences of pathogenic F. tularensis strains. The blue lines correspond to tick FLE, the green lines correspond to opportunistic pathogens, and the red lines correspond to pathogenic strains of F. tularensis. The GenBank accession numbers are shown at the beginning of each label.
Phylogenetic analysis of nine viral contigs found three different families for all different viral species. The genes were capsid protein, glycoprotein, nucleocapsid, polyprotein, and RNA-dependent RNA polymerase (RdRp) (Table 3). Most of the putative viruses were found by identifying genes encoding RdRp with five annotated sequences and classified into two viral families, Chuviridae and Rhabdoviridae. Two different contigs, D. nitens_Colombia_Chuviridae_Glycoprotein_2, and D. nitens_Colombia_Chuviridae_RdRp_5 were grouped into the same family Chuviridae. Based on the sequence similarities and the tree pattern (Figures 4A and 4B), these contigs are likely presenting two different viruses although the name of the closely related virus is the same as Changping Tick Virus 2, a virus that has been reported in China and Turkey infecting Dermacentor spp. and Hyalomma asiaticum ticks (23,24). These two viruses were found to be more abundant in the region of Antioquia (Supplementary Table 4). The Family Rhabdoviridae is represented by five sequences clustered into two putative viruses (Figures 4C and 4D). Four of them targeting RdRp were grouped in a clade with Blanchseco virus. The remaining sequence was found encoding a nucleocapsid protein and clustered with the American dog tick Rhabdovirus-2. The contig D. nitens_Colombia_Unclassified_Capsid_Protein_1 showed a close relationship with the capsid protein of Xinjiang tick-associated virus-2, a virus sequence that was presumably reported for the first time in the province of Xinjiang in China. This virus remains as unclassified for the family, and it is grouped with other tick viruses found in Ixodes scapularis and D. variabilis (Figure 4E). The family Flaviviridae was found to be represented by one contig named D. nitens_Colombia_Flaviviridae_Polyprotein_6 (Figure 4F). This name was assigned due to the high similarity found with a portion of a Flaviviridae polyprotein from Haemaphysalis longicornis and Rhipicephalus microplus infesting goats (30).
4. Discussion
Hard ticks harbor a considerable diversity of bacteria and viruses, of which there are significant pathogens to humans or domestic animals (2,4,6,8,61–63). A comprehensive survey of tick microorganisms may allow us to uncover the spectrum of the vectorial capacity of ticks for known pathogens and yield novel potential pathogenic microorganisms. In addition, it may provide a better understanding of the interactions among microorganisms under different environmental conditions. Thus, identifying symbiotic microorganisms and their effects on the vectorial capacity is critical for predicting future outbreaks caused of febrile diseases of unknown etiology (3). In this study, metatranscriptome and bacterial 16S rRNA sequencing enriched the sequence database with newly uncovered Francisella-like Endosymbionts (FLE) and virus genes in the blood- fed D. nitens originating from three different geographical areas in Colombia.
Differences in the bacterial compositions of ticks collected from animals coming from Bolivar, Antioquia, and Cordoba populations were found in either inclusion or exclusion of the FLE sequences. (Figures 1C and 1D). The NMDS plot for 16S sequences revealed clusters for tick geographical origin with a unique bacterial assortment. Geographically separated populations of ticks have previously been shown to have distinctive microbial compositions in a number of tick species (17,39,40,64,65). Microbial compositions could be influenced by other factors, such as the degree of tick engorgement, which has been reported previously (66–68). The capacity of ticks to acquire and spread pathogens may be significantly impacted by these variations in the microbial composition.
We found that the most abundant bacterium was FLE (80% of classified reads), which is phylogenetically related to the pathogenic bacteria F. tularensis, and causes tularemia in humans (9). While Dermacentor variabilis and Dermacentor andersoni, are known to carry this pathogen and are common in the northern hemisphere, the effect of FLE interaction with pathogens and their role in disease transmission remain unknown (1,11,17,69,70). Previous results have shown a positive association of vertically-transmitted FLE against pathogenic Francisella novicida artificial infection in D. andersoni, however F.novicida is not considered a tick-borne pathogen, which means this interaction is unlikely to happen in natural conditions (7).
Our result shows that the microbial composition of D. nitens appears to vary depending on the geographic location of the species’ population. We observed overall higher proportion of FLE compared to those previously reported in D. variabilis (62%), and D. occidentalis (41%) in the Americas (17,71). This highly abundant FLE was in accordance with previous 16S rRNA sequencing studies on whole-body samples obtained from partially or fully-engorged adult Dermacentor spp., females as D. variabilis, D. marginatus, D. reticulatus, D. silvarum, and D. albipictus (71–74). Metatranscriptomic analysis suggested high levels of FLE coverage (i.e., transcript per million reads TPM) for Cordoba samples, but without statistical significance in all pairwise comparisons by Student t-test. 16S rRNA analysis, showing the relative abundance, also suggested that the Cordoba population is richer in FLE. The department of Cordoba, an agricultural stronghold in northern Colombia, has a constant flow and exchange of animals. Thus, the associated ticks may be exposed to a more diverse bacterial environment, which may explain the increased detection frequency of main endosymbiont and transient bacteria, through mechanisms such as horizontal transfer (1,64). These tendencies of small differences in the communities of endosymbionts related to the geographical origin of the ticks have also been reported for D. occidentalis (17). In other tick species, such as Ixodes scapularis, the endosymbiont population has been shown to impact pathogen infection processes. An unaltered intestinal microbiota favored colonization of Borrelia burgdorferi s.l., while an induced microbial dysbiosis environment showed a negative effect by blocking colonization of Anaplasma phagocytophilum (1,19). In D. nitens, the transmission of human pathogens is yet unknown; however, D. nitens ticks collected from equines in Brazil were found positive for B. burgdorferi s.l., the complex known as the causal agent of Lyme disease in the Americas (75). While D. nitens’ potential as a Lyme disease vector, and the roles of FLE population have not been documented, the initial characterization of FLE population, may provide insights into their involvement in tick vector competence.
Our FLE sequence analysis revealed three different D. nitens FLE variants, OTU001, 002, and 010, with relatively large variations (8 to 21 bp or 1.7 to 4.5% difference) in the V3-V4 region. The source of these variants are likely from different strains that occurs in all three geographical locations. While the genus Francisella contains three 16S rRNA copies, we exclude the possibility of intra-genomic variations from these copies based on a study that described 99.65% minimum similarity average in 1374 Proteobacteria genomic sequences of 16S rRNA (76). These results are comparable to our previously reported study in Amblyomma americaun, where at least two different strains of Coxiella-like endosymbionts were found, at the individual tick level (44). Three D. nitens FLE OTUs were monophyletic and clustered while this cluster is also grouped with the FLE of other Dermacentor FLEs (Figure 2). However, FLEs of R. microplus and I. scapularis were also grouped in this clade (77), indicating, first that endosymbionts are more diverse than previously thought, and second that relatively recent independent invasions or transfers of FLEs frequently occurred, as it has been shown that the FLE initially evolved from the pathogenic Francisella species (1,12,13,59,60,62,71,77,78).
Metatranscriptomics revealed several contigs highly similar to viral families. Rhabdoviridae family was found as the most abundant and common in the pools of all sequences. This group of Rhabdoviridae viruses (Figure 4D) were also reported for different Ixodidae species such as Rhipicephalus annulatus, R. sanguineus, Hyalomma marginatum, H. asiaticum, and D. variabilis in the United States (23,24,26). Blanchseco virus (Rhabdoviridae family) was found in one pool of Amblyomma ovale ticks infesting cattle and dogs in Trinidad and Tobago (27). Similarly, we have identified Chuviridae- related sequences in the D. nitens RNA pools as the second predominant viral family (Figure 4A). Chuviridae is a newly-proposed viral family, that constitutes a large monophyletic group, clustering in an intermediate phylogenetic branch between segmented and unsegmented negative-sense RNA viruses identified in ticks, true flies, mosquitoes, cockroaches, and crabs (23). The most closely related to the D. nitens virus found in this study was previously identified in China (Figure 4A) with 90.2% (11,275 out of 12,500 bp) nucleotide sequence identity. The similar viruses in different continents may originate from historical commerce of animals.
We found geographical differences in the Rhabdoviridae family according to the contig Rhabdoviridae_RdRp that showed differences between Antioquia and Cordoba regions (p = 0.02), and the sequence coverage for Rhabdoviridae_Nucleocapsid is predominant in Bolivar when compared with those in other two regions (p = 0.03). The frequency data support unique viral compositions in different region (Supplementary Table 4). The coverage of the viral gene composition among the ticks in three different populations showed statistical differences in transcripts classified into the Rhabdoviridae family (Supplementary Table 5). A previous study with R. microplus, D. nitens, and R. sanguineus s.l. in the Magdalena Valley and Magdalena/Urabá ecoregions in Colombia reported the presence of Flaviviridae, Rhabdoviridae, Chuviridae, and Unclassified viruses (29). We conclude that the core RNA virome composition appears to be poor compared with the bacterial endosymbiotic communities. However, identifying viruses by using preexisting viral sequences in the GenBank may be limited for the discovery of novel viruses. This sequence-based survey needs further investigation to understand whether those are transiently acquired with the mammalian blood or established and vertically transmitted.
Overall, this study offers a description of the diversity of bacterial and viral communities of partially-fed D. nitens female ticks collected in animals originating from three Colombian regions based on our 16S rRNA sequences and transcriptomic analysis. In addition to the differentiated geographical populations in the bacterial and viral composition, we also found multiple co-existing strains of FLE and six different viruses in D. nitens, which offers the foundation for future studies. A deeper understanding of the microbial and viral communities hosted by ticks can be utilized to develop future measures to mitigate tick pathogen transmission.
10. Acknowledgments
The authors thank the employees of “La Rinconada” slaughterhouse, the collaborators from Universidad de Antioquia, the Kansas State Department of Entomology, and the College of Agriculture.
7. Funding
This study was partially funded by the Armed Forces Health Surveillance Division (AFHSD), Global Emerging Infections Surveillance (GEIS) Branch, PROMIS ID 2019 P0143_19_N6_04, 2020 P0144_20_N6_04 to MLF and GMV, USDA-multistate fund KS17MS1443-NE1443 to BL-R, NIH-NIAID R21 AI163423 and USDA-NIFA GRANT13066347 to YP.
Footnotes
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Disclaimer
The views expressed in this article reflect the results of research conducted by the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
Copyright statement
Some authors of this manuscript are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government”. Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
11 References
- 1.Bonnet SI, Binetruy F, Hernández-Jarguín AM, Duron O. The Tick Microbiome: Why Non-pathogenic Microorganisms Matter in Tick Biology and Pathogen Transmission. Frontiers in Cellular and Infection Microbiology [Internet]. 2017. [cited 2022 Apr 8];7. Available from: 10.3389/fcimb.2017.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madison-Antenucci S, Kramer LD, Gebhardt LL, Kauffman E. Emerging Tick-Borne Diseases. Clinical Microbiology Reviews. 2020;33(2):e00083–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad N, Murdoch DR, Reyburn H, Crump JA. Etiology of Severe Febrile Illness in Low- and Middle-Income Countries: A Systematic Review. PLOS ONE. 2015. Jun 30;10(6):e0127962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabezas-Cruz A, Vayssier-Taussat M, Greub G. Tick-borne pathogen detection: what’s new? Microbes and Infection. 2018. Aug 1;20(7):441–4. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard C, Dibernardo A, Koffi J, Wood H, Leighton P, Lindsay L. N Increased risk of tick-borne diseases with climate and environmental changes. Can Commun Dis Rep. 2019. Apr 4;45(4):83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a One Health perspective. Trends in Parasitology. 2012. Oct 1;28(10):437–46. [DOI] [PubMed] [Google Scholar]
- 7.Gall CA, Reif KE, Scoles GA, Mason KL, Mousel M, Noh SM, et al. The bacterial microbiome of Dermacentor andersoni ticks influences pathogen susceptibility. ISME J. 2016. Aug;10(8):1846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narasimhan S, Swei A, Abouneameh S, Pal U, Pedra JHF, Fikrig E. Grappling with the tick microbiome. Trends in Parasitology. 2021. Aug;37(8):722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JM, Oliva Chávez AS, Shaw DK. Ticks: More Than Just a Pathogen Delivery Service. Frontiers in Cellular and Infection Microbiology [Internet]. 2021. [cited 2022 Apr 25];11. Available from: 10.3389/fcimb.2021.739419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu-Chuang A, Obregon D, Mateos-Hernández L, Cabezas-Cruz A. Anti-tick microbiota vaccines: how can this actually work? Biologia. 2022. Jun 1;77(6):1555–62. [Google Scholar]
- 11.Cabezas-Cruz A, Pollet T, Estrada-Peña A, Allain E, Bonnet S I., Moutailler S. Handling the Microbial Complexity Associated to Ticks. In: Abubakar M K., Perera P, editors. Ticks and Tick-Borne Pathogens [Internet]. IntechOpen; 2019. [cited 2022 Apr 23]. Available from: https://www.intechopen.com/books/ticks-and-tick-borne-pathogens/handling-the-microbial-complexity-associated-to-ticks [Google Scholar]
- 12.Díaz-Sánchez S, Estrada-Peña A, Cabezas-Cruz A, de la Fuente J. Evolutionary Insights into the Tick Hologenome. Trends in Parasitology. 2019. Sep 1;35(9):725–37. [DOI] [PubMed] [Google Scholar]
- 13.Duron O, Morel O, Noël V, Buysse M, Binetruy F, Lancelot R, et al. Tick-Bacteria Mutualism Depends on B Vitamin Synthesis Pathways. Current Biology. 2018. Jun 18;28(12):1896–1902.e5. [DOI] [PubMed] [Google Scholar]
- 14.Narasimhan S, Fikrig E. Tick microbiome: the force within. Trends Parasitol. 2015. Jul;31(7):315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumrandee C, Hirunkanokpun S, Grubhoffer L, Baimai V, Trinachartvanit W, Ahantarig A. Phylogenetic relationships of Francisella-like endosymbionts detected in two species of Amblyomma from snakes in Thailand. Ticks and Tick-borne Diseases. 2014. Feb;5(1):29–32. [DOI] [PubMed] [Google Scholar]
- 16.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Research. 2013. Jan 1;41(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurfield N, Grewal S, Cua LS, Torres PJ, Kelley ST. Endosymbiont interference and microbial diversity of the Pacific coast tick, Dermacentor occidentalis, in San Diego County, California. PeerJ. 2017. Apr 13;5:e3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang L, Poźniak B, Cheng TY. Bacteriological analysis of saliva from partially or fully engorged female adult Rhipicephalus microplus by next-generation sequencing. Antonie van Leeuwenhoek. 2017. Jan 1;110(1):105–13. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet SI, Pollet T. Update on the intricate tango between tick microbiomes and tick-borne pathogens. Parasite Immunology. 2021;43(5):e12813. [DOI] [PubMed] [Google Scholar]
- 20.Wu-Chuang A, Hodžić A, Mateos-Hernández L, Estrada-Peña A, Obregon D, Cabezas-Cruz A. Current debates and advances in tick microbiome research. Current Research in Parasitology & Vector-Borne Diseases. 2021. Jan 1;1:100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pettersson JHO, Shi M, Bohlin J, Eldholm V, Brynildsrud OB, Paulsen KM, et al. Characterizing the virome of Ixodes ricinus ticks from northern Europe. Sci Rep. 2017. Sep 7;7(1):10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokarz R, Williams SH, Sameroff S, Sanchez Leon M, Jain K, Lipkin WI. Virome Analysis of Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis Ticks Reveals Novel Highly Divergent Vertebrate and Invertebrate Viruses. Journal of Virology. 2014. Oct;88(19):11480–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li CX, Shi M, Tian JH, Lin XD, Kang YJ, Chen LJ, et al. Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. Goff SP, editor. eLife. 2015. Jan 29;4:e05378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brinkmann A, Dinçer E, Polat C, Hekimoğlu O, Hacıoğlu S, Földes K, et al. A metagenomic survey identifies Tamdy orthonairovirus as well as divergent phlebo-, rhabdo-, chu- and flavi-like viruses in Anatolia, Turkey. Ticks and Tick-borne Diseases. 2018. Jul 1;9(5):1173–83. [DOI] [PubMed] [Google Scholar]
- 25.Shi J, Hu Z, Deng F, Shen S. Tick-Borne Viruses. Virol Sin. 2018. Feb 1;33(1):21–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tokarz R, Sameroff S, Tagliafierro T, Jain K, Williams SH, Cucura DM, et al. Identification of Novel Viruses in Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis Ticks. mSphere. 2018. Mar 7;3(2):e00614–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sameroff S, Tokarz R, Charles RA, Jain K, Oleynik A, Che X, et al. Viral Diversity of Tick Species Parasitizing Cattle and Dogs in Trinidad and Tobago. Sci Rep. 2019. Jul 18;9(1):10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gómez GF, Isaza JP, Segura JA, Alzate JF, Gutiérrez LA. Metatranscriptomic virome assessment of Rhipicephalus microplus from Colombia. Ticks and Tick-borne Diseases. 2020. Sep 1;11(5):101426. [DOI] [PubMed] [Google Scholar]
- 29.Orozco Orozco M, Gómez GF, Alzate JF, Isaza JP, Gutiérrez LA. Virome analysis of three Ixodidae ticks species from Colombia: A potential strategy for discovering and surveying tick-borne viruses. Infection, Genetics and Evolution. 2021. Dec 1;96:105103. [DOI] [PubMed] [Google Scholar]
- 30.Xu L, Guo M, Hu B, Zhou H, Yang W, Hui L, et al. Tick virome diversity in Hubei Province, China, and the influence of host ecology. Virus Evolution. 2021. Dec 1;7(2):veab089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwint ON, Knowles DP, Ueti MW, Kappmeyer LS, Scoles GA. Transmission of Babesia caballi by Dermacentor nitens (Acari: Ixodidae) Is Restricted to One Generation in the Absence of Alimentary Reinfection on a Susceptible Equine Host. Journal of Medical Entomology. 2008. Nov 1;45(6):1152–5. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues V da S, Garcia MV, Cruz BC, Maciel WG, Zimmermann NP, Koller WW, et al. Life cycle and parasitic competence of Dermacentor nitens Neumann, 1897 (Acari: Ixodidae) on different animal species. Ticks and Tick-borne Diseases. 2017. Mar 1;8(3):379–84. [DOI] [PubMed] [Google Scholar]
- 33.Labruna MB, Kasai N, Ferreira F, Faccini JLH, Gennari SM . Seasonal dynamics of ticks (Acari: Ixodidae) on horses in the state of São Paulo, Brazil. Veterinary Parasitology. 2002. Apr 19;105(1):65–77. [DOI] [PubMed] [Google Scholar]
- 34.Borges LMF, Oliveira PR, Ribeiro MFB. Seasonal dynamics of Anocentor nitens on horses in Brazil. Veterinary Parasitology. 2000. Apr 28;89(3):165–71. [DOI] [PubMed] [Google Scholar]
- 35.Borges LMF, Silva CRF da. IXODÍDEOS PARASITOS DE BOVINOS E EQUINOS DA MICRORREGIÁO DE GOIÂNIA, GOIÁS. Revista de Patologia Tropical / Journal of Tropical Pathology [Internet]. 1994. [cited 2022 Aug 4];23(1). Available from: https://revistas.ufg.br/iptsp/article/view/20035 [Google Scholar]
- 36.Martins TF, Teixeira RHF, Labruna MB. Ocorrência de carrapatos em animais silvestres recebidos e atendidos pelo Parque Zoológico Municipal Quinzinho de Barros, Sorocaba, São Paulo, Brasil. Brazilian Journal of Veterinary Research and Animal Science. 2015. Dec 10;52(4):319–24. [Google Scholar]
- 37.Nelson SL, Durden LA, Reuter JD. Rhipicephalus microplus and Dermacentor nitens (Acari: Ixodidae) Coparasitize White-Tailed Deer on St. John, U.S. Virgin Islands. Journal of Medical Entomology. 2017. Sep 1;54(5):1440–3. [DOI] [PubMed] [Google Scholar]
- 38.Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak IG. The Hard Ticks of the World [Internet]. Dordrecht: Springer Netherlands; 2014. [cited 2022 Jul 29]. Available from: 10.1007/978-94-007-7497-1 [DOI] [Google Scholar]
- 39.Santodomingo A, Sierra-Orozco K, Cotes-Perdomo A, Castro LR. Molecular detection of Rickettsia spp., Anaplasma platys and Theileria equi in ticks collected from horses in Tayrona National Park, Colombia. Exp Appl Acarol. 2019. Mar 1;77(3):411–23. [DOI] [PubMed] [Google Scholar]
- 40.Cotes-Perdomo AP, Oviedo Á, Castro LR. Molecular detection of pathogens in ticks associated with domestic animals from the Colombian Caribbean region. Exp Appl Acarol. 2020. Sep 1;82(1):137–50. [DOI] [PubMed] [Google Scholar]
- 41.Barros-Battesti DM, Arzua M, Bechara GH. Carrapatos de importância médico-veterinária da região neotropical: um guia ilustrado para identificação de espécies [Internet]. ICTTD-3;Instituto Butantan; 2006. [cited 2022 Aug 4]. Available from: https://repositorio.butantan.gov.br/handle/butantan/3153 [Google Scholar]
- 42.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Applied and Environmental Microbiology. 2009. Dec;75(23):7537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013. Sep;79(17):5112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maldonado-Ruiz LP, Neupane S, Park Y, Zurek L. The bacterial community of the lone star tick (Amblyomma americanum). Parasites Vectors. 2021. Jan 14;14(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dixon P. VEGAN, a package of R functions for community ecology. Journal of Vegetation Science. 2003;14(6):927–30. [Google Scholar]
- 46.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Research. 2013. Jan 1;41(D1):D590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Team RStudio. RStudio: Integrated Development for R. [Internet]. Boston, MA: RStudio, PBC; 2020. (RStudio: Integrated Development for R). Available from: http://www.rstudio.com/ [Google Scholar]
- 48.Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018. Sep 1;34(17):i884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013. Jan 1;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008. Jun;36(10):3420–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011. Jul;29(7):644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012. Apr;9(4):357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol Biol Evol. 2018. Jun;35(6):1547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ, Rambaut A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evolution. 2018. Jan 1;4(1):vey016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Felsenstein J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution. 1985;39(4):783–91. [DOI] [PubMed] [Google Scholar]
- 56.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987. Jul 1;4(4):406–25. [DOI] [PubMed] [Google Scholar]
- 57.Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences. 2004. Jul 27;101(30):11030–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution. 1993. May 1;10(3):512–26. [DOI] [PubMed] [Google Scholar]
- 59.Gerhart JG, Moses AS, Raghavan R. A Francisella-like endosymbiont in the Gulf Coast tick evolved from a mammalian pathogen. Sci Rep. 2016. Sep 20;6(1):33670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerhart JG, Auguste Dutcher H, Brenner AE, Moses AS, Grubhoffer L, Raghavan R. Multiple Acquisitions of Pathogen-Derived Francisella Endosymbionts in Soft Ticks. Genome Biology and Evolution. 2018. Feb 1;10(2):607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004. Oct;129(S1):S3–14. [DOI] [PubMed] [Google Scholar]
- 62.Duron O, Binetruy F, Noël V, Cremaschi J, McCoy KD, Arnathau C, et al. Evolutionary changes in symbiont community structure in ticks. Mol Ecol. 2017. Jun;26(11):2905–21. [DOI] [PubMed] [Google Scholar]
- 63.CDC. Tickborne Diseases of the United States [Internet]. CDC; 2022. [cited 2022 Oct 27]. Available from: https://www.cdc.gov/ticks/tickbornediseases/index.html [Google Scholar]
- 64.Van Treuren W, Ponnusamy L, Brinkerhoff RJ, Gonzalez A, Parobek CM, Juliano JJ, et al. Variation in the Microbiota of Ixodes Ticks with Regard to Geography, Species, and Sex. Goodrich-Blair H, editor. Appl Environ Microbiol. 2015. Sep 15;81(18):6200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar D, Downs LP, Adegoke A, Machtinger E, Oggenfuss K, Ostfeld RS, et al. An Exploratory Study on the Microbiome of Northern and Southern Populations of Ixodes scapularis Ticks Predicts Changes and Unique Bacterial Interactions. Pathogens. 2022. Jan 21;11(2):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moreno CX, Moy F, Daniels TJ, Godfrey HP, Cabello FC. Molecular analysis of microbial communities identified in different developmental stages of Ixodes scapularis ticks from Westchester and Dutchess Counties, New York. Environmental Microbiology. 2006;8(5):761–72. [DOI] [PubMed] [Google Scholar]
- 67.Clay K, Klyachko O, Grindle N, Civi℡lo D, Oleske D, Fuqua C. Microbial communities and interactions in the lone star tick, Amblyomma americanum. Molecular Ecology. 2008;17(19):4371–81. [DOI] [PubMed] [Google Scholar]
- 68.Clow KM, Weese JS, Rousseau J, Jardine CM. Microbiota of field-collected Ixodes scapularis and Dermacentor variabilis from eastern and southern Ontario, Canada. Ticks and Tick-borne Diseases. 2018. Feb 1;9(2):235–44. [DOI] [PubMed] [Google Scholar]
- 69.Ahantarig A, Trinachartvanit W, Baimai V, Grubhoffer L. Hard ticks and their bacterial endosymbionts (or would be pathogens). Folia Microbiol. 2013. Sep 1;58(5):419–28. [DOI] [PubMed] [Google Scholar]
- 70.Yeni DK, Büyük F, Ashraf A, Shah MS ud D. Tularemia: a re-emerging tick-borne infectious disease. Folia Microbiol (Praha). 2021;66(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Travanty NV, Ponnusamy L, Kakumanu ML, Nicholson WL, Apperson CS. Diversity and structure of the bacterial microbiome of the American dog tick, Dermacentor variabilis, is dominated by the endosymbiont Francisella. 2019;12. [Google Scholar]
- 72.Zhang YK, Yu ZJ, Wang D, Bronislava V, Branislav P, Liu JZ. The bacterial microbiome of field-collected Dermacentor marginatus and Dermacentor reticulatus from Slovakia. Parasites Vectors. 2019. Jun 27;12(1):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duan DY, Liu GH, Cheng TY. Microbiome analysis of the saliva and midgut from partially or fully engorged female adult Dermacentor silvarum ticks in China. Exp Appl Acarol. 2020. Apr 1;80(4):543–58. [DOI] [PubMed] [Google Scholar]
- 74.Sperling J, MacDonald Z, Normandeau J, Merrill E, Sperling F, Magor K. Within-population diversity of bacterial microbiomes in winter ticks (Dermacentor albipictus). Ticks and Tick-borne Diseases. 2020. Nov 1;11(6):101535. [DOI] [PubMed] [Google Scholar]
- 75.Gonçalves DD, Carreira T, Nunes M, Benitez A, Lopes-Mori FMR, Vidotto O, et al. First record of Borrelia burgdorferi B31 strain in Dermacentor nitens ticks in the northern region of Parana (Brazil). Braz J Microbiol. 2013. Sep;44:883–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ibal JC, Pham HQ, Park CE, Shin JH. Information about variations in multiple copies of bacterial 16S rRNA genes may aid in species identification. PLOS ONE. 2019. Feb 15;14(2):e0212090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scoles GA. Phylogenetic Analysis of the Francisella-like Endosymbionts of Dermacentor Ticks. Journal of Medical Entomology. 2004. May 1;41(3):277–86. [DOI] [PubMed] [Google Scholar]
- 78.Kumar D, Sharma SR, Adegoke A, Kennedy A, Tuten HC, Li AY, et al. Recently Evolved Francisella-Like Endosymbiont Outcompetes an Ancient and Evolutionarily Associated Coxiella-Like Endosymbiont in the Lone Star Tick (Amblyomma americanum) Linked to the Alpha-Gal Syndrome. Frontiers in Cellular and Infection Microbiology [Internet]. 2022. [cited 2022 Apr 23];12. Available from: 10.3389/fcimb.2022.787209 [DOI] [PMC free article] [PubMed] [Google Scholar]