Abstract
Purpose:
To investigate predictors associated with post-treatment biopsy outcomes after stereotactic body radiotherapy (SBRT) for localized prostate cancer.
Materials and methods:
257 patients treated with prostate SBRT to dose levels of 32.5 Gy to ≥40 Gy in 5–6 fractions underwent a post-treatment biopsy performed approximately two years after treatment to evaluate local control status. 73 had% intermediate-risk disease (n=187) and the remaining 17% (n=43) and 10% (n=27) had low-risk and high-risk disease, respectively.
Results:
The incidence of positive, negative, and treatment-effect post-treatment biopsies were 15.6%, 57.6%, and 26.8%, respectively. The incidence of a positive biopsy according to dose was 37.5% (n=9/24), 21.4% (n=6/28), 19.4% (n=6/31), and 10.9% (n=19/174) for 32.5 Gy, 35 Gy, 37.5 Gy, and ≥40 Gy, respectively. In a multivariable model, patients treated with SBRT doses of <40 Gy and those with unfavorable-intermediate-risk or high-risk disease had higher likelihood of a positive post-treatment biopsy. A positive post-SBRT biopsy was associated with a significantly higher likelihood of subsequent PSA relapse at five years (Positive biopsy: 57%, 95% CI: 29–77% compared to negative biopsy: 7%, 95% CI: 3–14%; p<0.001).
Conclusion:
Based on two-year post-SBRT biopsies, excellent tumor control was achieved when dose levels of 40 Gy or higher were used. Standard SBRT dose levels of 35–37.5 Gy were associated with a higher likelihood of a positive post-treatment biopsy. Two-year positive post-treatment biopsies pre-dated the development of PSA failure in the majority of patients.
Keywords: localized prostate cancer, stereotactic body radiotherapy, predictive factors, post-treatment biopsy
INTRODUCTION
Stereotactic body radiotherapy (SBRT) is a well-recognized treatment option for low- and intermediate-risk prostate cancer. Five-year prostate-specific antigen (PSA) relapse-free survival rates of approximately 90% have been reported for patients with low-risk and intermediate-risk disease when delivering SBRT prescription dose levels ranging from 34–50 Gy [1–7]. Recent reports of prospective randomized trials comparing SBRT to moderate hypo-fractionated radiotherapy have demonstrated comparable disease-related outcomes for patients treated for low-risk and intermediate-risk prostate cancer [8,9]. Yet, despite low rates of PSA-relapsing disease, positive post-treatment biopsies may be more often observed when using lower prescription dose levels. We previously reported results of a Phase 1 dose escalation SBRT trial that evaluated radiation doses ranging from 32.5 Gy to 40 Gy [10]. In that report, a significant increase in intraprostatic tumor control was achieved at the higher dose levels.
A positive post-treatment biopsy following definitive radiotherapy carries significant prognostic implications. Previous studies have demonstrated that a positive post-treatment biopsy is associated with inferior progression-free survival outcomes [11–13] and an increased risk of distant metastases and prostate cancer mortality [14–16]. In this study, we aimed to identify disease- and treatment-related variables associated with post-SBRT biopsy outcomes in a larger cohort of patients.
MATERIALS AND METHODS
Patient characteristics
Between April 2009 and August 2018, 529 patients were treated with SBRT monotherapy for clinically-staged T1-T3, prostate cancer based on the 2017 AJCC staging classification system. All patients had institutional confirmation of a histologic diagnosis of prostatic adenocarcinoma according to the Gleason grading system. Low-risk, favorable-intermediate-risk, unfavorable-intermediate-risk, and high-risk cohorts were stratified according to the NCCN prognostic risk group classification. Details of treatment planning and delivery were previously described [7, 10]. As per our institutional standard of practice, all patients were encouraged to undergo a post-treatment biopsy approximately two years following treatment completion. Two hundred and fifty-seven patients (of the 529 patients who underwent SBRT) underwent a post-treatment biopsy and are the subjects of this IRB-approved retrospective analysis. Included in this cohort are 96 patients enrolled in a Phase I dose escalation study who underwent a two-year post-treatment biopsy. In general, patients who did not undergo a biopsy avoided the procedure due to medical contra-indications, age ≥80 years, or patient refusal (n=270). No significant differences were found between the characteristics of patients who underwent a post-treatment biopsy and those who did not —based on androgen deprivation therapy (ADT) usage (26% vs. 34%, p=0.07), age at time of treatment (median 69 vs. 70 years, p=0.09), NCCN risk (unfavorable/high 51% vs. 56%, p=0.22), or pre-treatment PSA (median 6.4 vs. 6.7 ng/mL, p=0.30). The treatment characteristics of the 257 patients who underwent post-treatment biopsy are shown in Table 1.
Table 1.
Patient Characteristics
| # Patients | 257 | |
|---|---|---|
| Age at SBRT, years | Median (Range) | 69 (47–89) |
| N = | 257 | |
| Clinical T-Stage | T1 | 176 (68.5) |
| T2 | 73 (28.4) | |
| T3 | 7 (2.7) | |
| T4 | 1 (0.4) | |
| NCCN Risk | Low | 43 (16.7) |
| Favorable-Intermediate | 84 (32.7) | |
| Unfavorable-Intermediate | 103 (40.1) | |
| High | 27 (10.5) | |
| Gleason Score | 6 | 50 (19.5) |
| 7 | 186 (72.4) | |
| 8 | 10 (3.9) | |
| 9 | 11 (4.3) | |
| RT Dose | 32.5 Gy | 24 (9.3) |
| 35.0 Gy | 28 (10.9) | |
| 37.5 Gy | 31 (12.1) | |
| 40.0 Gy | 174 (67.7) | |
| Pre-treatment PSA, ng/mL | Median (Range) | 6.4 (0.5–134.0) |
|
| ||
| Pre-treatment Prostate Volume | Median (Range) | 37.0 (9.0–121.0) |
| N = | 251 | |
| Extraprostatic capsule invasion | Negative-Possible | 192 (74.7) |
| Suspicious-Positive | 58 (22.6) | |
| Unknown | 7 (2.7) | |
| Use of ADT | Yes | 68 (26.5) |
| No | 189 (73.5) | |
|
| ||
| ADT Duration, months | Median (Range) | 4.6 (0.0–30.5) |
| N = | 67 | |
|
| ||
| IPSS Baseline Score | Median (Range) | 6.0 (0.0–24.0) |
| N = | 256 | |
|
| ||
| IIEF Baseline Score | Median (Range) | 9.0 (0.0–30.0) |
| N = | 250 | |
| Rectal Spacer Insertion | Yes | 90 (35) |
| No | 167 (65) | |
Treatment technique and schedules
All patients underwent fiducial marker placement — with or without hydrogel rectal spacer placement — prior to simulation (SpaceOAR, Boston Scientific), as previously described [7]. CT-based simulations were performed until 2016, after which MR-only simulation for prostate SBRT were routinely used instead at our institution. The details of CT- and MR-simulated protocols have been previously described [17–19].
The clinical target volume (CTV) included the entire prostate and bilateral seminal vesicles (SV). The planning target volume (PTV) was generated using a 5-mm circumferential margin around the CTV, except at the prostate-rectal interface where a 3-mm margin was used. The prescription dose for the PTV ranged from 32.5–42.5 Gy across 5–6 fractions delivered on alternating days. In cases where the lymph nodes were included within the target volume for high-risk disease, the prostate PTV was treated to 40 Gy in five fractions, while the lymph nodes were concomitantly treated with 25 Gy in five fractions, as previously described [20].
Treatment was delivered with 6–15 MV linear accelerators using multiple coplanar fields via intensity-modulated radiotherapy or multiple arcs of volumetric arc therapy. Image acquisition of 2D orthogonal KVs followed by cone beam CT was used to validate patient position based on implanted fiducial markers and anatomy. Patients were repositioned if the fiducial markers were mis-aligned by more than 0.2 cm. Intrafraction motion was corrected using the Calypso beacons tracking system or with the kV -MV technique, as previously described [19]. These treatment techniques were routinely employed for this SBRT delivery in this cohort of patients.
For selected low-risk and intermediate-risk patients, ADT was used for pre-treatment volume reduction for prostate sizes >75g. In general, high-risk patients received 18 months duration of neoadjuvant, concurrent, and adjuvant ADT.
Follow-up and outcomes assessment
Patients were followed at three months after SBRT completion and subsequently approximately every six months for the first five years and annually thereafter; serum PSA was drawn at these visits. Biochemical failure was established according to a PSA nadir plus 2 ng/mL definition. Follow-up time was calculated from the end date of SBRT until the last follow-up visit for survivors. The overall median follow-up time for survivors in this cohort was 44 months (range: 18–119 months).
Transrectal ultrasound-guided prostate template biopsies were performed with a minimum of 12 cores and two additional cores directed at the seminal vesicles bilaterally. The biopsies were performed at a median time of 2.2 years after SBRT completion. Pathological findings were classified as positive (prostatic adenocarcinoma with assigned Gleason score), negative (no evidence of carcinoma), and adenocarcinoma with severe treatment effect (STE); the latter categorization had similar clinical and biological outcomes as those with negative outcomes, as previously reported [14, 15]. Therefore, for formal statistical analyses, patients with post-treatment biopsies consistent with STE were grouped with patients whose biopsies were categorized as negative.
Statistical analysis
Differences between patient and baseline characteristics were compared across post-treatment biopsy groups using the Wilcoxon rank-sum test for continuous characteristics and Fisher’s exact test for categorical characteristics. We also compared the characteristics of patients who received a biopsy to a cohort of patients who did not receive a biopsy and had at least two years of follow-up (N=270) using the same testing methods.
Multivariable logistic regression was conducted to assess associations with post-treatment biopsy outcomes (positive vs negative/STE) and clinically-relevant covariates, including radiation dose, risk group (low/intermediate-favorable vs. intermediate-unfavorable/high), and ADT use. All three variables were incorporated into the multivariable analyses and interactions between covariates were assessed.
PSA relapse-free survival was calculated from the time of biopsy until biochemical relapse (BCR) for overall estimates. Patients alive at the last follow-up were censored, and death without BCR was treated as a competing risk. For comparison of BCR rates by biopsy outcomes, BCR was measured from the time of biopsy; patients who had BCR prior to biopsy were excluded. BCR was estimated with competing risks using Fine & Gray’s methods, and differences in BCR by biopsy finding were assessed using Gray’s test. Two-sided p<0.05 was considered statistically significant. All analyses were conducted using SAS 9.4 TS1M6 software (The SAS Institute, Cary, NC).
RESULTS
Forty patients were found to have a positive post-treatment biopsy after SBRT. The rates of a positive biopsy stratified by the radiation dose level were 37.5% (95% CI: 18.8–59.4%; 9/24) for 32.5 Gy, 21.4% (95% CI: 8.3–41.0%; 6/28) for 35 Gy, 19.4% (95% CI: 7.5–37.5%; 6/31) for 37.5 Gy, and 10.9% (95% CI: 6.7–16.5%; 19/174) for ≥40 Gy. The incidence of positive, negative, and treatment-effect post-treatment biopsies were 15.6%, 57.6%, and 26.8%, respectively.
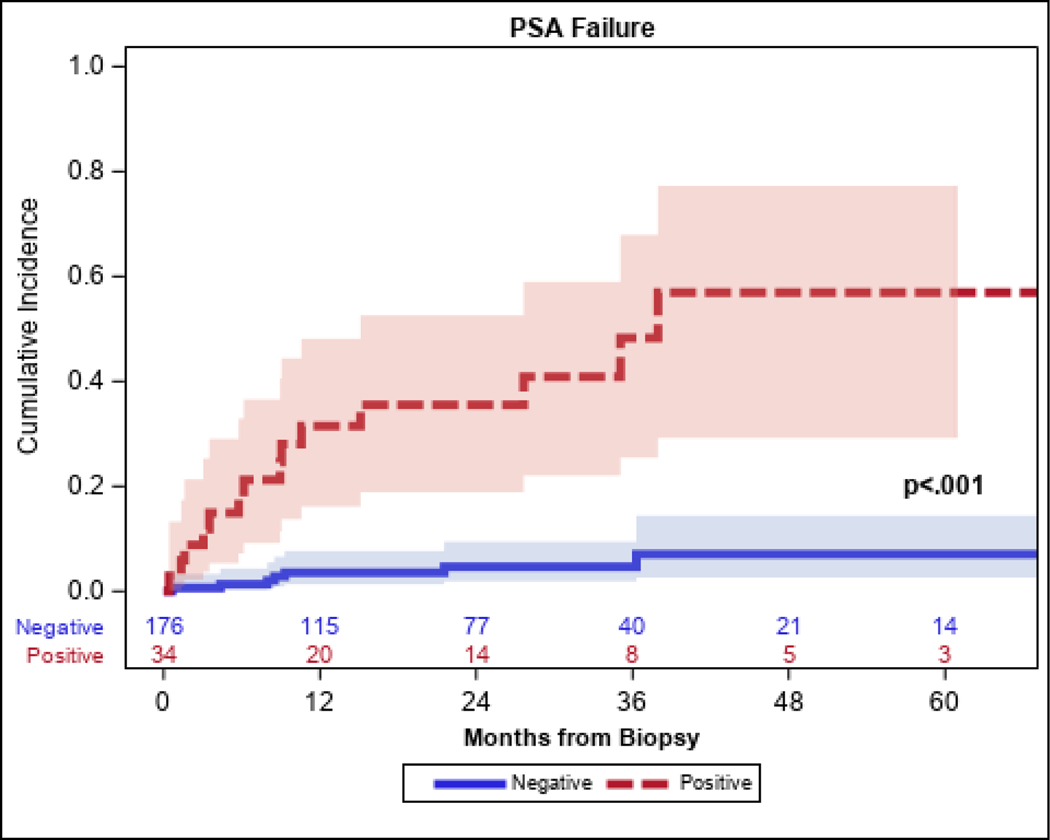
Table 3 shows the results of the univariate and multivariable analysis. In the multivariable model, a radiation dose of <40 Gy compared to higher doses was associated with 2.75-fold higher odds of a positive biopsy (OR: 2.34, 95% CI: 1.30–5.84; p=0.008). In addition, patients with unfavorable-intermediate-risk or high-risk disease had significantly higher likelihood of a positive biopsy outcome (OR: 2.34, 95% CI: 1.11–4.96; p=0.026). The absence of ADT did not reach statistical significance as a variable associated with a positive post-treatment biopsy outcome (OR: 0.37, 95% CI: 0.13–1.07; p=0.07). A significant interaction was found where the association between dose and positive biopsy was stronger for those with unfavorable-intermediate-risk or high-risk disease (OR: 7.25, 95% CI: 2.69–19.55) compared to those with low-risk or favorable-intermediate-risk disease (OR: 1.45, 95%CI: 0.52–4.05) with p=0.027. The overall five-year PSA relapse-free survival outcome was 15.2% (95% CI: 14.2–33.1%) from the time of SBRT. Among the 40 positive post-treatment biopsies, the PSA level at the time of biopsy was 0–0.5, 0.5–1 ng/ml, 1–2 ng/ml, and >2 ng/ml in 32.5% (13/40), 20% (8/40), 25% (10/40), and 22.5% (9/40) of patients, respectively. Patients with a positive biopsy had a significantly higher cumulative incidence of BCR at five years (57%; 95% CI: 29–77%) compared to patients who had a negative post-RT biopsy (7% 95% CI: 3–14%; p<0.001), as shown in Figure 1. Of the 40 patients with a positive biopsy, 35% elected to be treated with salvage therapy which consisted of salvage low dose rate brachytherapy, salvage prostatectomy or androgen deprivation therapy. The remaining patients elected to be monitored with active surveillance.
Table 3.
Logistic regression analysis of associations with positive biopsy
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| OR | [95% CI] | p-value | OR | [95% CI] | p-value | ||
| Use of ADT | Yes | 0.35 | [0.13 – 0.93] | 0.036 | 0.37 | [0.13 – 1.07] | 0.07 |
| No | REF | --- | |||||
| RT Dose | <40 | 2.76 | [1.39 – 5.49] | 0.004 | 2.75 | [1.30 – 5.84] | 0.008 |
| 40+ | REF | --- | |||||
| NCCN Risk | Unfavorable-High | 1.39 | [0.70 – 2.75] | 0.34 | 2.34 | [1.11 – 4.96] | 0.026 |
| Low-Favorable | REF | --- | |||||
Figure 1.
Biochemical failure stratified by post-treatment biopsy outcome
DISCUSSION
Our findings highlight the importance of using higher SBRT dose levels, which are associated with improved local control as demonstrated on routine post-treatment biopsies. Patients who received ≥40 Gy had a significantly lower rate of a positive biopsy compared to those who received lower radiation dose levels. The prognostic significance of a positive post-treatment biopsy after definitive prostate radiotherapy is well-recognized. Post-treatment biopsy data from several cohorts of patients treated with external beam radiotherapy indicate that residual prostate cancer cells are seen at 2–3 years after completion of therapy in 20–40% of men clinically without evidence of biochemical or clinical recurrence [11, 12, 14]. We reported our long-term experience of patients treated with IMRT conventionally-fractionated external beam radiotherapy, and noted a strong association of local tumor control based on post-treatment biopsies with long-term distant metastases-free survival and cause-specific survival outcomes. We also showed two-year positivity was associated with eventual biochemical failure, disease-free survival, distant metastasis, and cause-specific survival outcomes [14,15].
In contrast to PSA post-treatment assessments, which could reflect the status of regional or micro-metastatic disease, the efficacy of a local treatment intervention may be better evaluated with pathologic confirmation from a post-treatment biopsy. The post-treatment PSA nadir levels or biochemical relapse-free survival outcomes may not reflect the local tumor status after treatment. The PSA nadir plus 2 definition for this reason could be misconstrued to suggest that the rising PSA profile prior to reaching the defined threshold of a biochemical relapse is not concerning for residual local disease. In our reported experience [7] among patients with positive biopsies, only 2/21 or 9.5 % had a documented nadir plus 2 PSA failure at the time of biopsy, while the remaining patients manifested a positive biopsy prior to biochemical failure. In the current report, the majority of patients had PSA levels < 1 ng/ml at the time of the post-treatment biopsy, and only 12.5% of these patients had manifested at the time of the biopsy a biochemical nadir+2 relapse. We also noted that despite pre-biopsy PSA levels of ≤1ng/ml, the incidence of a positive post-treatment biopsy was as high as 25%. While Kass-Illya and colleagues have reported a lower incidence of a positive biopsy in the setting of low PSA levels, that study was very limited by the fact that the post-treatment biopsy only obtained 2–4 cores from the irradiated prostate which likely under-sampled the gland (13). In contrast to that report, our post-treatment biopsies routinely obtained 14–16 cores representing a more comprehensive sampling of the treated gland.
Our results also demonstrated a higher incidence of post-treatment biopsies among patients with unfavorable-intermediate-risk prostate cancer compared to those with low- and favorable-intermediate risk disease. In addition, we observed that the association between dose and positive biopsy was stronger for unfavorable-intermediate-risk and possibly high-risk disease (although limited number of the latter such patients were included in this study), suggesting that patients with more aggressive histologies may require higher dose levels to achieve local disease eradication. Excellent biochemical tumor control has been observed with dose levels of 45 Gy or higher, but a higher grade 2 rectal toxicity risk is possible and has been reported [21]. Yet, decreased toxicities have been observed even among patients treated at these higher dose levels with strict adherence dose volume constraints for surrounding normal tissues, tight margins around the CTV, the use of a rectal hydrogel spacer, and attention to reduced urethral and bladder neck exposure [7, 21].
Focal dose intensification to the dominant intraprostatic lesion (DIL) may be an attractive option to further improve local control rates, especially for patients with unfavorable-intermediate-risk disease. Several reports have demonstrated that the most common location for local relapse of disease after definitive radiotherapy is within the region of the initial dominant lesion as noted on multi-parametric MRI based imaging [24–26]. Focal dose boosting provides an opportunity to reduce normal tissue from potentially unnecessary exposure to the higher prescription radiation doses [25–27]. In a Phase III multicenter study (Focal Lesion Ablative Microboost in Prostate Cancer, or FLAME), 571 men with intermediate-risk and high-risk prostate cancer were randomized to receive an IMRT dose of 77 Gy in 35 fractions to the entire prostate or a similar IMRT regimen with an integrated boost up to 95 Gy to dominant intra-prostatic lesions (DIL) defined by multi-parametric MRI [28]. With a median follow-up of 72 months, there was no differences in the incidence of late genitourinary (GU) and gastrointestinal (GI) toxicity across focal dose escalated and the standard dose arm (GU: 28% vs 23%; GI: 13% vs 12%). These results indicate that an escalated dose delivered to the DIL was safe and feasible. In addition, at 5- years there was superior PSA relapse-free survival outcomes noted for the focal boost arm compared to the standard dose arm (92% vs 85%; p< 0.001). Draulans et al [29] recently reported on tolerance outcomes of a Phase II trial (hypo-FLAME trial) (NCT02853110), which again boosted dose delivered in five weekly fractions for MRI-visible tumor to 50 Gy, while the remainder of the gland received 35 Gy. With a minimum of six months follow-up, they observed a 34% rate of grade 2 urinary toxicity, a 5% rate of grade 2 rectal GI toxicity, and no grade 3 toxicities.
There are limitations to the current report including its retrospective cohort, the potential for selection biases, the variable follow-up between the dose levels, and the fact that not all 529 patients underwent the two-year biopsy. Nevertheless, all patients regardless of dose delivered were strongly encouraged to get the two-year biopsy, unless as noted, they were elderly with active comorbidities. In addition, when the cohort of patients who underwent a post-treatment biopsy were compared to patients treated during the same time period who did not undergo a biopsy, no significant differences in the baseline treatment characteristics were observed.
In summary, SBRT dose was associated with the post-treatment positive biopsy outcome after controlling for NCCN risk classification, and higher doses of SBRT were associated with lower rates of positive biopsy. While the 2-year biopsy is not necessarily a standard of care, it provides valuable prognostic information especially for patients who are potential candidates for local salvage therapies to be administered at earlier time points. In addition, the post-treatment biopsy could be helpful in evaluating new radiotherapy interventions and may serve as a valuable early surrogate endpoint to assess efficacy. Dose intensification strategies, such as the utilization of a focal SBRT or brachytherapy boost, are being prospectively studied to determine the impact of these strategies on local tumor control based on post-treatment biopsy outcomes.
Table 2.
Dose-volume constraints for target and normal tissues for 40 Gy SBRT
| Structure | Parameter | Dose-volume constraint |
|---|---|---|
| Prescription dose (Gy) | PTV D95% | ≥90% of prescription dose |
| Rectum | Max point dose | 103% of prescription dose |
| D1cc | 38.5 Gy | |
| Mean | 16.4 Gy | |
| V24Gy | <25% | |
| V30.15Gy | ≤8 cc | |
| Bladder | Max point dose | 105% of prescription dose |
| D50 | 20 Gy | |
| Femoral heads | Max point dose | 31 Gy |
| Large bowel | Max point dose | 29 Gy |
| Small bowel | Max point dose | 25 Gy |
| Bladder trigone | Max point dose | 41.2 Gy |
| Urethra | Max point dose | 105% of prescription dose |
| D1cc | 40 Gy |
Highlights:
SBRT dose levels of 35–37.5 Gy of SBRT were associated with a higher likelihood of a positive post-treatment biopsy at 2 years.
Two-year positive post-treatment biopsies pre-dated the development of PSA failure in the majority of these patients.
Among patients with positive post-treatment biopsies, the PSA level at the time of 2-year biopsy was ≤ 1.00 in 52.5% of patients.
Patients with a positive biopsy had a significantly higher cumulative incidence of BCR at five years compared to patients who had a negative post-RT biopsy (57% versus 7%; p<0.001).
Funding
This research was partially supported by the NIH/NCI Cancer Center Support Grant/Core Grant (P30 CA008748).
Disclosures
Dr. Zelefsky serves as a consultant for Boston Scientific. Dr. McBride was given an honorarium from Bristol Meyers in 2016 and receives research funding from Janssen. Dr. Ehdaie serves as a consultant for Myriad Genetics and was given an honorarium by KOELIS. Dr. Tyagi reports grants from Philips healthcare and grants from Elekta healthcare, outside the submitted work. The remaining authors have no relevant disclosures.
Footnotes
Data Sharing Statement
Individual participant data that underlie the results reported in this article will be shared after deidentification. The statistical analysis plan and analytic code will also be shared. This data will be available — beginning three months and ending five years following publication of the article — upon request on a case-by-case basis to researchers who provide a methodologically sound proposal. Requests should be made to the corresponding author, which will then be evaluated by the participating study institutions.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Meier RM, Bloch DA, Cotrutz C, Beckman AC, Henning GT, Woodhouse SA, Williamson SK, Mohideen N, Dombrowski JJ, Hong RL, Brachman DG, Linson PW, Kaplan ID. Multicenter Trial of Stereotactic Body Radiation Therapy for Low- and Intermediate-Risk Prostate Cancer: Survival and Toxicity Endpoints. Int J Radiat Oncol Biol Phys. 2018; 102(2): 296–303. doi: 10.1016/j.ijrobp.2018.05.040 [DOI] [PubMed] [Google Scholar]
- 2.Fuller DB, Falchook AD, Crabtree T, Kane BL, Medbery CA, Underhill K, Gray JR, Peddada A, Chen RC. Phase 2 Multicenter Trial of Heterogeneous-dosing Stereotactic Body Radiotherapy for Low- and Intermediate-risk Prostate Cancer: 5-year Outcomes. Eur Urol Oncol. 2018; 1(6): 540–547. doi: 10.1016/j.euo.2018.06.013 [DOI] [PubMed] [Google Scholar]
- 3.Boike TP, Lotan Y, Cho LC, Brindle J, DeRose P, Xie XJ, Yan J, Foster R, Pistenmaa D, Perkins A, Cooley S, Timmerman R.J. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. Clin Oncol. 2011; 29(15): 2020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kishan AU, Dang A, Katz AJ, Mantz CA, Collins SP, Aghdam N, Chu FI, Kaplan ID, Appelbaum L, Fuller DB, Meier RM, Loblaw DA, Cheung P, Pham HT, Shaverdian N, Jiang N, Yuan Y, Bagshaw H, Prionas N, Buyyounouski MK, Spratt DE, Linson PW, Hong RL, Nickols NG, Steinberg ML, Kupelian PA, King CR. Long-term Outcomes of Stereotactic Body Radiotherapy for Low-Risk and Intermediate-Risk Prostate Cancer. JAMA Netw Open. 2019; 2(2): e188006. doi: 10.1001/jamanetworkopen.2018.8006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson WC, Silva J, Hartman HE, Dess RT, Kishan AU, Beeler WH, Gharzai LA, Jaworski EM, Mehra R, Hearn JWD, Morgan TM, Salami SS, Cooperberg MR, Mahal BA, Soni PD, Kaffenberger S, Nguyen PL, Desai N, Feng FY, Zumsteg ZS, Spratt DE. Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Systematic Review and Meta-Analysis of Over 6,000 Patients Treated On Prospective Studies. Int J Radiat Oncol Biol Phys. 2019; n104(4): 778–789. doi: 10.1016/j.ijrobp.2019.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alayed Y, Cheung P, Pang G, Mamedov A, D’Alimonte L, Deabreu A, et al. Dose escalation for prostate stereotactic ablative radiotherapy (SABR): Late outcomes from two prospective clinical trials. Radiother Oncol. 2018; 127: 213–8. [DOI] [PubMed] [Google Scholar]
- 7.Zelefsky MJ, Pinitpatcharalert A, Kollmeier M, Goldman DA, McBride S, Gorovets D, Zhang Z, Varghese M, Happersett L, Tyagi N, Hunt M. Early Tolerance and Tumor Control Outcomes with High-dose Ultrahypofractionated Radiation Therapy for Prostate Cancer. Eur Urol Oncol. 2019; S2588–9311(19)30147–6. doi: 10.1016/j.euo.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widmark A, Gunnlaugsson A, Beckman L, Thellenberg-Karlsson C, Hoyer M, Lagerlund M, Kindblom J, Ginman C, Johansson B, Björnlinger K, Seke M, Agrup M, Fransson P, Tavelin B, Norman D, Zackrisson B, Anderson H, Kjellén E, Franzén L, Nilsson P. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019; 394(10196): 385–395. [DOI] [PubMed] [Google Scholar]
- 9.Brand DH, Tree AC, Ostler P, van der Voet H, Loblaw A, Chu W, Ford D, Tolan S, Jain S, Martin A, Staffurth J, Camilleri P, Kancherla K, Frew J, Chan A, Dayes IS, Henderson D, Brown S, Cruickshank C, Burnett S, Duffton A, Griffin C, Hinder V, Morrison K, Naismith O, Hall E, van As N (PACE Trial Investigators). Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomized, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019; 20(11): 1531–1543. doi: 10.1016/j.euo.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zelefsky MJ, Kollmeier M, McBride S, Varghese M, Mychalczak B, Gewanter R, Garg MK, Happersett L, Goldman DA, Pei I, Lin M, Zhang Z, Cox BW. Five-Year Outcomes of a Phase 1 Dose-Escalation Study Using Stereotactic Body Radiosurgery for Patients With Low-Risk and Intermediate-Risk Prostate Cancer. Int J Radiat Oncol Biol Phys. 2019. May 1;104(1):42–49. doi: 10.1016/j.ijrobp.2018.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crook JM, Malone S, Perry G, et al. Twenty-four-month postradiation prostate biopsies are strongly predictive of 7-year disease-survival: results from a Canadian randomized trial. Cancer. 2009; 115: 673–9. [PubMed: 19117039] [DOI] [PubMed] [Google Scholar]
- 12.Vance W, Tucker SL, De Crevoisier R, Kuban DA, Cheung MR. The predictive value of 2-year posttreatment biopsy after prostate cancer radiotherapy for eventual biochemical outcome. Int J Radiat Oncol Biol Phys. 2007; 67: 828–33. [PubMed: 17161554] [DOI] [PubMed] [Google Scholar]
- 13.Kass- Illyya A, Jovic G, Murphy C et al. Two-years postradiotherapy biopsies: Lessons from the MRC RT01 trial Eur Urol 2018;73:968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zelefsky MJ, Reuter VE, Fuks Z, Scardino P, Shippy A. Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J Urol. 2008; 179: 1368–73. 10.1016/j.juro.2007.11.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zelefsky MJ, Goldman DA, Reuter V, Kollmeier M, McBride S, Zhang Z, Varghese M, Pei X, Fuks Z. Long-Term Implications of a Positive Posttreatment Biopsy in Patients Treated with External Beam Radiotherapy for Clinically Localized Prostate Cancer. J Urol. 2019; n201(6): 1127–1133. doi: 10.1097/JU.0000000000000110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krauss DJ, Hu C, Bhary JP et al. The importance of local control inearly stage prostate cancer : outcomes of patients with a positive post-radiotherapy biopsy treated on RTOG 94–08. Int J Radiat Oncol Biol Phys. 2015; 92:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tyagi N, Fontenla S, Zhang J, Cloutier M, Kadbi M, Mechalakos J, et al. Dosimetric and workflow evaluation of first commercial synthetic CT software for clinical use in pelvis. Phys Med Biol. 2017; 62: 2961–75. 10.1088/1361-6560/aa5452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyagi N, Fontenla S, Zelefsky M, Chong-Ton M, Ostergren K, Shah N, et al. Clinical workflow for MR-only simulation and planning in prostate. Radiat Oncol. 2017; 12: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorovets D, Burleson S, Jacobs L, Ravindranath B, Tierney K, Kollmeier M, McBride S, Happersett L, Hunt M, Zelefsky M. Prostate SBRT With Intrafraction Motion Management Using a Novel Linear Accelerator-Based MV-kV Imaging Method. Pract Radiat Oncol. 2020; S1879–8500(20)30112–0. [DOI] [PubMed] [Google Scholar]
- 20.Pinitpatcharalert A, Happersett L, Kollmeier M, McBride S, Gorovets D, Tyagi N, Varghese M, Zelefsky MJ. Early Tolerance Outcomes of Stereotactic Hypofractionated Accelerated Radiation Therapy Concomitant with Pelvic Node Irradiation in High-risk Prostate Cancer. Adv Radiat Oncol. 2019; 4(2): 337–344. doi: 10.1016/j.adro.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folkert MR, Zelefsky MJ, Hannan R, et al. Multi-Institutional Phase 2 Trial of High-Dose Stereotactic Body Radiation Therapy with Temporary Hydrogel Spacer for Low- and Intermediate-Risk Prostate Cancer. Int J Radiat Oncol Biol Phys. 2017; 99(5): 1319–1320. doi: 10.1016/j.ijrobp.2017.09.020 [DOI] [Google Scholar]
- 22.Pucar D, Hricak H, Shukla-Dave A, Kuroiwa K, Drobnjak M, Eastham J, et al. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys. 2007; 69: 62–9. 10.1016/j.ijrobp.2007.03.065 [DOI] [PubMed] [Google Scholar]
- 23.N, Morganti AG, Mattiucci GC, Valentini V, Leone M, Luzi S, et al. Analysis of intraprostatic failures in patients treated with hormonal therapy and radiotherapy: Implications for conformal therapy planning. Int J Radiat Oncol Biol Phys. 2002; 53: 595–9. PMID12062602 [DOI] [PubMed] [Google Scholar]
- 24.Arrayeh E, Westphalen AC, Kurhanewicz J, Roach M 3rd, Jung AJ, Carroll PR, et al. Does local recurrence of prostate cancer after radiation therapy occur at the site of primary tumor? Results of a longitudinal MRI and MRSI study. Int J Radiat Oncol Biol Phys. 2012; 82(5): e787–93. 10.1016/j.ijrobp.2011.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monninkhof EM, van Loon JWL, van Vulpen M, Kerkmeijer LGW, Pos FJ, Haustermans K, et al. Standard whole prostate gland radiotherapy with and without lesion boost in prostate cancer: Toxicity in the FLAME randomized controlled trial. Radiother Oncol. 2018; 127: 74–80. 10.1016/j.radonc.2017.12.022 [DOI] [PubMed] [Google Scholar]
- 26.Sundahl N, De Meerleer G, Villeirs G, Ost P, De Neve W, Lumen N, et al. Combining high dose external beam radiotherapy with a simultaneous integrated boost to the dominant intraprostatic lesion: Analysis of genito-urinary and rectal toxicity. Radiother Oncol. 2016; 119: 398–404. 10.1016/j.radonc.2016.04.031 [DOI] [PubMed] [Google Scholar]
- 27.Miralbell R, Mollà M, Rouzaud M, Hidalgo A, Toscas JI, Lozano J, et al. Hypofractionated boost to the dominant tumor region with intensity modulated stereotactic radiotherapy for prostate cancer: A sequential dose escalation pilot study. Int J Radiat Oncol Biol Phys. 2010; 78: 50–7. 10.1016/j.ijrobp.2009.07.1689 [DOI] [PubMed] [Google Scholar]
- 28.Kerkmeijer LGW, Groen VH, Pos FJ, et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J Clin Oncol. Published online January 20, 2021:JCO.20.02873. doi: 10.1200/JCO.20.02873 [DOI] [PubMed] [Google Scholar]
- 29.Draulans C, van der Heide UA, Haustermans K, Pos FJ, van der Voort van Zyp J, De Boer H, et al. Primary endpoint analysis of the multicentre phase II hypo-FLAME trial for intermediate and high risk prostate cancer. Radiother Oncol. 2020; 147: 92–8. [DOI] [PubMed] [Google Scholar]