INTRODUCTION
Communication and swallowing are highly complex sensorimotor events that are tightly linked to respiration and vital to health and well-being. The tongue is a complex organ, often described as a muscular hydrostat, that is crucial for maintaining airway patency, preparing and safely transporting food/liquid, and rapidly changing position and shape for speech. As with any complex behavior, tongue function can be compromised with aging, diseases/conditions, trauma, or as a pharmacologic side effect. As such, modeling lingual function and dysfunction for basic and translational research is paramount; understanding how the nervous system controls tongue function for complex behavior is foundational to this work. Non-invasive access to tongue tissues and kinematics during awake behavior has been historically challenging, creating a critical need to measure tongue function in model systems. Germane to this field of study are the instruments and assays of licking/lapping and drinking, including tongue force and timing measures, many of which were designed or modified by Dr. Stephen C. Fowler. The focus of this paper is to review some of the important contributions of measuring tongue behaviors in awake rats and mice and how these have been modified by other researchers to advance translational science.
LICKING/LAPPING BEHAVIOR IN RATS AND MICE
Licking behavior in experimental rodent models, most commonly rats and mice, is used to determine fluid consumption in a variety of behavioral contexts (Stellar & Hill, 1952), such as orolingual motor function (Stanford et al., 2003), the impact of aging or disease conditions on tongue force and with tongue exercise (Ciucci et al., 2011; Cullins & Connor, 2019), and the assessment of dose-related behavioral effects of chronic or acute drug treatment (Fowler & Mortell, 1992; Fowler & Wang, 1998; Moss et al., 2001). Both licking and lapping are terms used to describe licking behavior interchangeably in the literature, though some researchers have defined them as distinct behavioral patterns. Licking behavior is driven by central pattern generators and involves repetitive tongue and jaw movements (Jüch et al., 1985; Marder & Calabrese, 1996; Brozek et al., 1996; Travers et al., 1997). Lapping is a form of drinking from an open source of water involving licking behavior but differing in tongue-movement patterns influenced by the position of the water source relative to the tongue (Halpern, 1977).
Different instruments have been used in research to measure licking and lapping behavior and to collect data based on tongue protrusion, timing, and force. Through modifications of these established basic systems, novel instruments have been introduced, discussed, and described by various researchers. Commercially available experimental instruments collect information via installed light beams (optical sensors) or electrical signals generated by either contact with the tongue or through application of tongue force on a lever (force sensors).
Many of Fowler’s assays and investigations targeted measurements of tongue force and licking outcomes in behavioral (Fowler et al., 2002) and pharmacological contexts (Fowler & Mortell, 1992; Fowler & Das, 1994; Das & Fowler, 1996, 1996b; Skitek et al., 1999; Wang & Fowler, 1999; Moss et al., 2001). Throughout his scientific work, he introduced, established, and modified rodent tongue force and licking instrument models of his own design, which inspired further modifications by various researchers in multiple scientific fields, described below (Ciucci & Connor, 2009; Connor et al., 2009; Lever et al., 2010; Cullins & Connor, 2019; Plowman et al., 2014).
THE LICKOMETER AS A BLUEPRINT FOR FOWLER’S LICKING INSTRUMENTS FOR RODENTS
The lickometer was first described by Wall, Walters, and England as an operant device for recording dry, unreinforced tube licking in rats (Wall et al., 1972). Prior to the development of this novel experimental device, an electronic drinkometer was commonly used for measuring rate of consummatory licking (Williams & Teitelbaum, 1956) and to track the amount of liquid intake in experimental settings (Hill & Stellar, 1951; Stricker & Miller, 1965). A drinkometer reinforces each lick with a liquid reward, which poses some limitations in studies that focus on licking as an operant, due to potential satiety.
The lickometer as described by Wall and colleagues consists of a solid, tube-like rod mimicking a standard water bottle drinking tube with an electrically sensitive tip (Wall et al., 1972). This facilitates the recording of licks, allowing for independently programmed reinforcement and direct comparison of licking with other operants under identical reinforcement conditions. In some models, each lick completes an electrical circuit, allowing for the measurement of licking frequency, lick duration, and/or tongue contact.
FOWLER’S EARLY WORK USING A MODIFIED LICKOMETER/DRINKOMETER MODEL
In a pharmacological study from 1984, Fowler described a contact-circuit model used to record licking behavior in rats. Licking behavior was quantified by recording lick duration and licking frequency to study the effects of pimozide on non-water-restricted rats (Gramling et al., 1984). A similar mechanism was used to study the effects of neuroleptics on rate and duration of operant versus reflexive licking in rats (Gramling & Fowler, 1985).
Fowler used a simple setup of a closed-circuit system. A circular opening permitted head entry of the experimental rat into a cylindrical recession that extended from the front panel of the chamber wall, with a circular opening parallel to the chamber floor, to permit tongue access to a lowered water/sucrose reservoir (Gramling et al., 1984). In a later model, the lick surface consisted of a dry horizontal disk and a solenoid valve to deliver water reinforcement into a cup. This was used for the investigation of operant licking condition, as opposed to the reflexive licking condition, using a tap water reservoir. Fluid levels were raised in the reservoir beneath the cylindrical recession prior to the experimental session, and levels were dropped slowly during the course of each session. Lick durations (amount of contact time between tongue and fluid/disk) and interlick intervals (ILI, amount of time between licks) were recorded. An electrical current (1.5 μA) passed through the experimental animal in the contact circuit to record licking measurements (Gramling et al., 1984; Gramling & Fowler, 1985).
In the following years, modifications of this apparatus were used to assess several outcome measures. These included licking rate, contact duration, inter contact intervals (ICI), and ILI measurements in a pharmacological context (Gramling & Fowler, 1986). Additionally, these advances shifted the focus toward tongue force and contact time measurements for a more detailed assessment of tongue dynamics in the context of various behavioral and drug studies (Fowler & Mortell, 1992; Fowler & Das, 1994). Further technical modifications allowed for measurement of peak tongue force strikes, number of separate tongue contacts, lapping rhythms, and duration of tongue contact (Das & Fowler, 1996, 1996b).
THE FORCE TRANSDUCER AND THE FORCE DISK
Key to these novel measurement approaches was Fowler’s implementation of a force transducer and force disk. A pressure transducer was used to measure the exerted force by the tongue on the water source or force disk (Fowler & Mortell, 1992). While electrical lick sensor models commonly use an electrical current in the experimental chamber floor and on the tip of the water bottle to create a closed circuit, force lick sensors do not pass an electrical current through the experimental animal (Weijnen, 1998). Force sensors detect the pressure of the experimental rodent’s tongue upon either a lick surface (force disk) or a drinking straw/tube to record tongue force. With his innovative modification to the lickometer, i.e. the single lick force recording chamber, Fowler was able to derive various new measurements studying muscle force timing and the underlying neurobiology in diverse behavioral (Fowler et al., 2002; Skitek et al., 1999; Stanford et al., 2003) and pharmacological contexts, such as the effects of haloperidol on rodent licking behavior (Fowler & Mortell, 1992; Fowler & Das, 1994; Das & Fowler, 1996; Fowler & Wang, 1998; Wang & Fowler, 1999), the effects of clozapine on licking in rats (Das & Fowler, 1995b, 1996b), and the effects of 3-Acetylpyridine on tongue protrusion and lick rhythm in rats (Moss et al., 2001).
Fowler’s extensive collaboration with Shyamal Das investigating pharmacological effects on rats lapping and licking behavior accelerated the evolution of measurement derivatives, the standardization and specification of tongue force measurements, and development of testing device modifications (Fowler & Das, 1994, Das & Fowler, 1995, 1996, 1996b;). This important work laid the foundation for wide-ranging, innovative, and novel investigations. For example, researchers used modifications of the lickometer-based force disk and force transducer to study the onset, progression, and treatment of swallowing dysfunction-related conditions modeled in rats, such as functional swallowing changes post-stroke (Cullins & Connor, 2019), the effects of aging on tongue protrusion forces (Nagai et al., 2008), and tongue force and timing deficits in various models of Parkinson disease (Ciucci et al., 2011).
THE FORCE SENSING DISK AND THE ACTOMETER
The force-sensing disk was another method employed extensively by Fowler for studying force in forelimb (Fowler et al., 1990, 1994) and licking tasks (Fowler & Das, 1994, Fowler & Mortell, 1992). The apparatus includes a 23×20×19 cm chamber with stainless steel rods (6.5 mm in diameter) running parallel to the front of the chamber and a light mounted to the top. Chambers used for forelimb force have a cylindrical recession (5.7 cm in diameter) in the front panel with access to a solenoid-operated dipper and an opening to a manipulanda. This design allows rats to press an operandum with their forelimb and drink from the dipper simultaneously (see Figure 1) (Fowler et al., 2002b; Liao et al., 1997; Stanford et al., 2000; Stanford & Fowler, 1997, 1998, 2000, 2002). Force emissions from the operandum, as well as response durations, forelimb tremor, and time measurements are recorded and subsequently analyzed (Fowler et al., 1990, 1994, Fowler & Das, 1994). Similarly, chambers used for licking behavioral tasks include a 6×6×3 cm recession in the front panel with access to a circular (18 mm in diameter) metal disk, which is connected to a force transducer and is used as the lick contact surface (see Figure 2) (Das & Fowler, 1996; Fowler et al., 2002; Fowler & Mortell, 1992). Outcomes including the number of licks within a specific time frame, lick peak force (g), and lick rhythm (Hz) have been measured over the past several decades using these force transducers.
Figure 1.
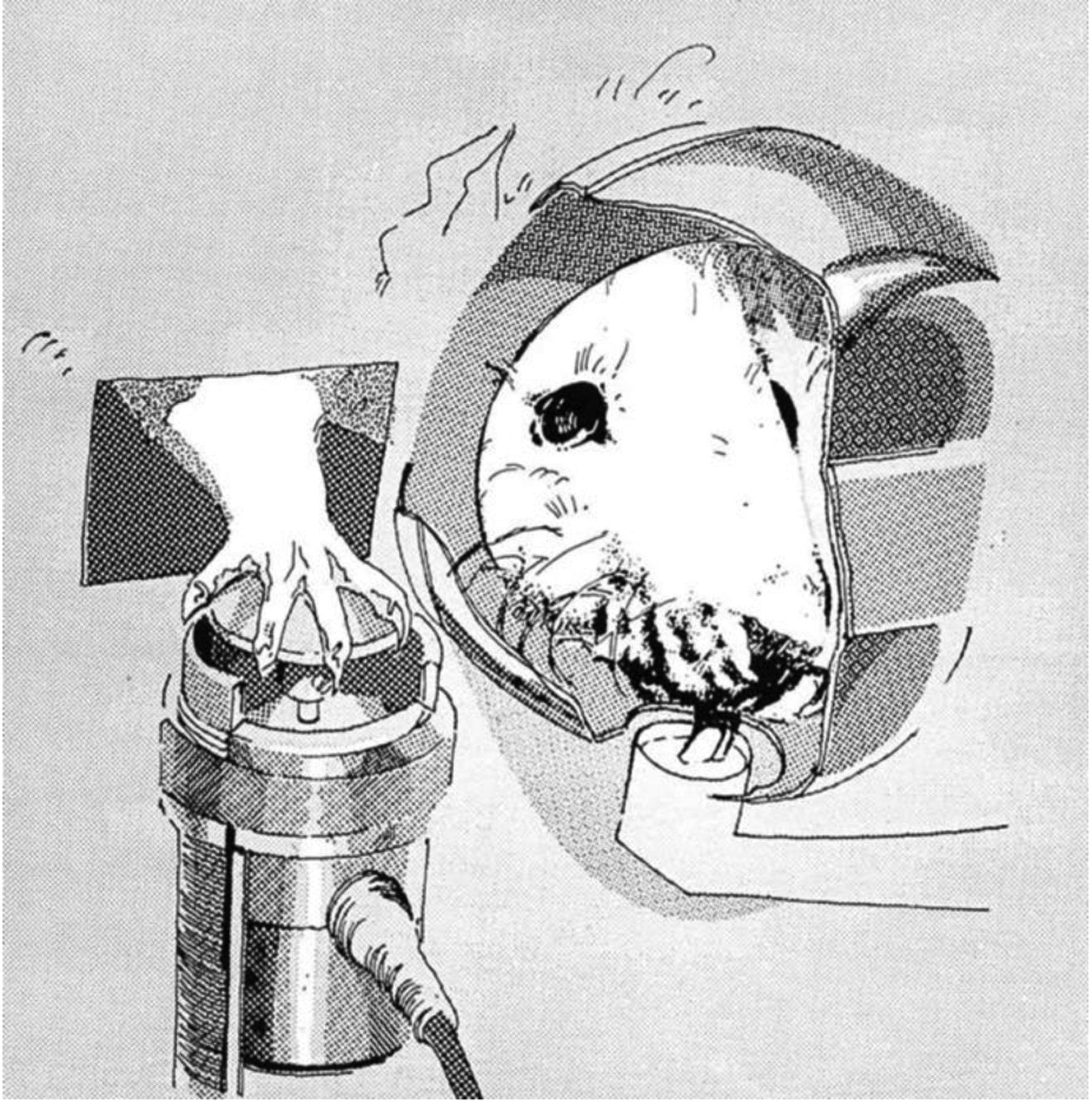
Outside-the-chamber view of a rat drinking from a dipper and exerting force on an isometric force transducer (Fowler et al., 1990).
Figure 2.
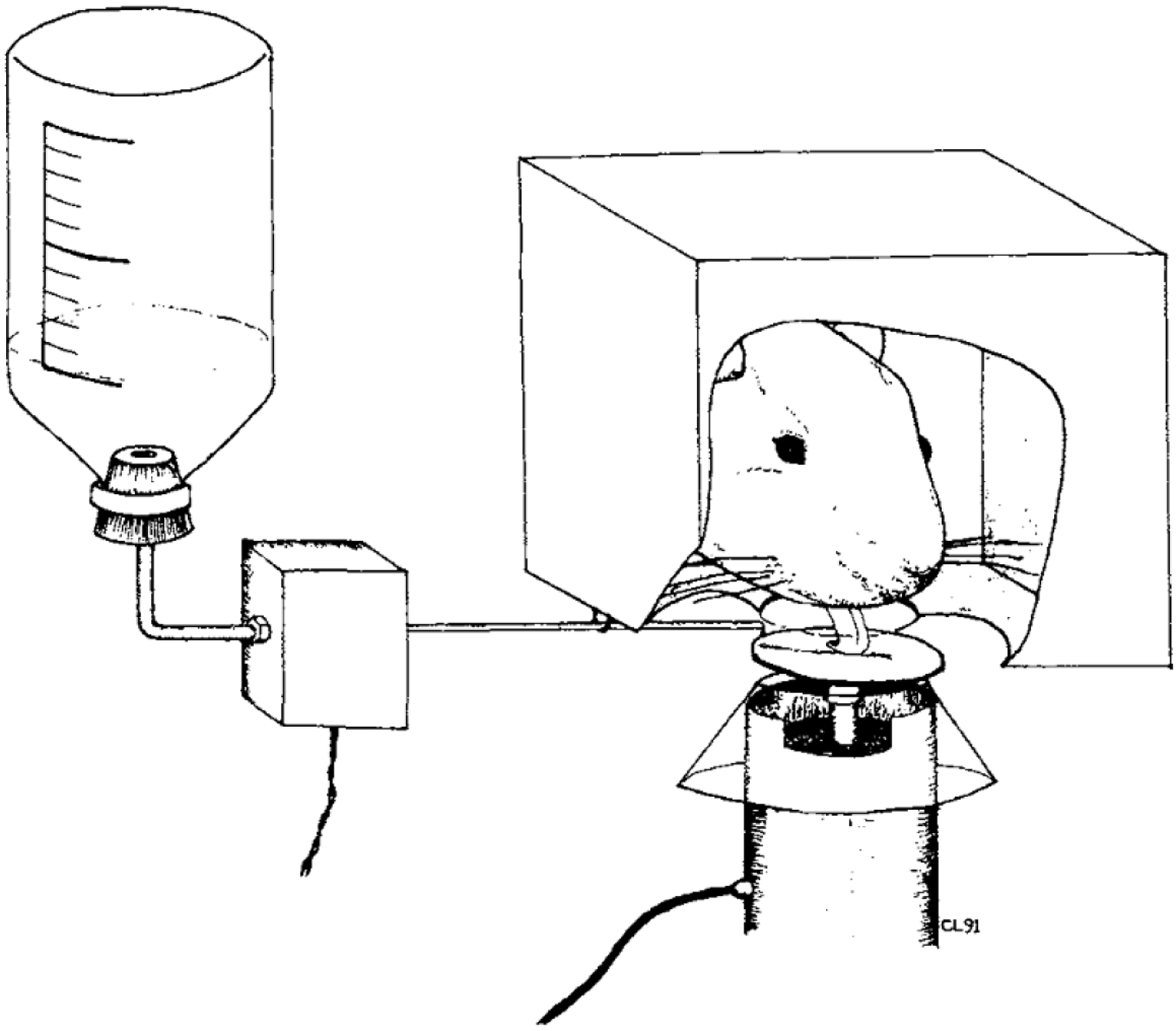
Line drawing of a rat licking the force-sensing lick surface (Fowler & Mortell, 1992).
In 2001, Fowler and colleagues published another method for measuring rodent motor behavior: the force-plate actometer (Fowler et al., 2001). The computer-based instrument uses an immobile, low-mass horizontal plate and four supporting force transducers to monitor and score animal movements, including locomotion, rotation around the center, whole-body tremor, rearing, and amphetamine-induced stereotypies (Fowler et al., 2001). The force-plate actometer combines mechanical, electronic, and computational properties, resulting in an apparatus that allows for the objective collection of behavioral data. Throughout the literature, the actometer is often coupled with the force-sensing disk, as both inform motor function. For example, both methods have been used to assess behavioral deficits in genetically modified animals (Fowler et al., 2002, 2002b). Depending on the research question and the context of the study, tongue function can also inform body function or vice versa. For example, the tongue can influence the neuromotor control of a lower limb (Bordoni et al., 2018). Specifically, tongue positioning (extended up to the palatine spot) significantly increased knee flexion peak torque (Di Vico et al., 2013). This demonstrates that tongue function may have implications for lower limb movement, beyond just the classically considered oromotor behaviors like chewing and swallowing. As such, we will review the actometer briefly, as future work may benefit from the combined use of the force-sensing disk and the actometer, to measure cranial and appendicular movements.
The force-plates were created to be 280×280 mm, a size deemed suitable for both rats and mice, eliminating the need to make changes for either species. Force plates were made from 0.125 mm thick aluminum foil and aluminum honeycomb, achieving low mass and high stiffness. Wide (15 mm) strips of aluminum tape were used to cover sharp edges of the plates. Four of the plates were attached to the transducer shafts. A stainless-steel reference plate was used to fix the position of the transducers. An approximately 20 lb square piece of granite (349×349×28.6 mm) was used as a ballast plate to ensure no environmental interference (e.g. vibrations) was picked up by the sensors.
The animal chambers are constructed 2 mm above the force plates using plexiglass, aluminum, and brass, all non-toxic materials likely to withstand animal urine and feces. Transparency of the plexiglass allows for direct visualization of animals. Holes at the top of the chamber provide ventilation. All interior surfaces are smooth and level as to prevent the biased use of space in the chamber (e.g. some animals are prone to climbing). The chamber also includes unique rotometer inserts that, unlike other commonly used rotometers, do not require that the animal be harnessed or tethered. Furthermore, compared to traditional video systems or photobeams, the force-plate actometer provides high spatial and temporal resolution, significantly improving the quality of locomotor research.
The force-plate actometer and its associated specialized computer algorithms have revolutionized behavioral data collection. Since its inception, the force-plate actometer has been adapted and used to measure a number of motor behaviors in various animal models of disease, including Huntington’s disease (Fowler et al., 2009), Parkinson disease (Meredith & Kang, 2006), Alzheimer’s disease (Winkler et al., 2015), and Tourette syndrome (Fowler et al., 2017).
MODIFICATIONS OF FOWLER’S INSTRUMENTS IN RESEARCH
Fowler’s work has been adapted for numerous lines of research in recent years. For example, modified techniques using the force transducer have been established by Connor and Russell for aging research, and this approach has extended to Connor trainees for examining complex tongue behavior in models of aging (Cullins et al., 2018, 2019; Cullins & Connor, 2017; Glass et al., 2021; Kletzien et al., 2012, 2020; Nagai et al., 2008; Schaser et al., 2016, Connor et al., 2009), Parkinson disease (PD) (Ciucci et al., 2011, 2013; Glass et al., 2020), stroke (Cullins & Connor, 2019), and head and neck cancer (Russell & Connor, 2014). Further, this paradigm has been used to measure tongue muscle function and subsequent correlation with histopathological assays in various models of aging and disease (Cullins et al., 2019; Cullins & Connor, 2017, 2019; Glass et al., 2020; Ota et al., 2005; Connor et al., 2009).
Specifically, custom instruments have been created by Connor and Russell to acquire maximal tongue force and timing measures in an awake rat to assess tongue strength and timing. Such modifications include an operandum within a single animal enclosure that measures lick rate, number of licks, lick interval, and tongue force (see Figure 3) (Connor et al., 2009). An 18 mm aluminum disk is fitted with a force transducer on the shaft; when pressed with a designated amount of force, a 0.10 mL water reward is dispensed onto the disk via a variable ratio schedule. The water reward is delivered as more force is generated by the experimental rat. The operandum is connected to computer software that allows for real-time capture of relevant measures and can be programmed to reinforce at force-specific targets. Measurements obtained provide functional behavioral data that are correlated to other physiologic findings, discussed below.
Figure 3.
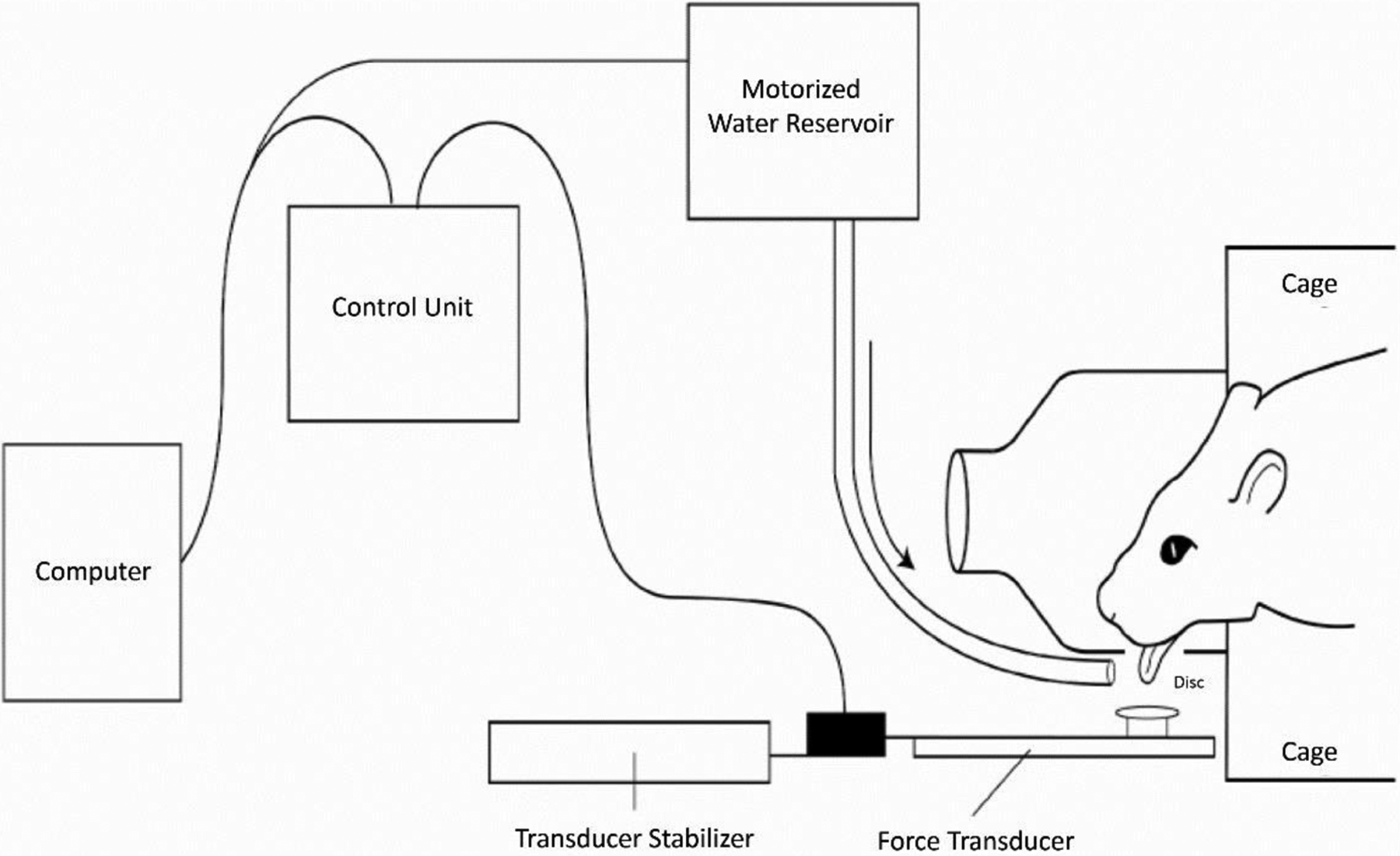
Schematic of the tongue force operandum (Connor et al., 2009).
Examples of these physiologic correlates include studies focused on neuromuscular electrical stimulation (NMES). Muscle contractile properties have been elicited and subsequently evaluated using bilateral hypoglossal nerve stimulation, specifically maximum forces and fatigability of the tongue in an aging model (Ota et al., 2005). Examination of protrusive tongue force and timing characteristics (e.g., via bilateral hypoglossal nerve stimulation (Ciucci et al., 2013) in the rat model have key clinical implications related to oromotor and swallow function. Correlating behavioral findings with physiologic measures such as stimulated forces allows more nuanced analysis of muscle function as it relates to swallow function (Nagai et al., 2008; Russell & Connor, 2014).
The ability to assess tongue force and timing using the modified operandum has also been described by the Connor and Ciucci research groups in several models of PD, including the Pink 1−/− rat model (Glass et al., 2020; Grant et al., 2015) and 6-hydroxydopamine (6-OHDA) neurotoxin model of PD (Ciucci et al., 2011, 2013). The tongue press paradigm allows for measurement of function in extrinsic tongue muscles, including force and timing measures (Ciucci et al., 2013; Glass et al., 2020). Subsequent correlations to histopathological findings allow for comparisons between behavioral and neurologic-based outcomes, such as striatal dopamine depletion, neuronal loss in cranial brainstem regions, and pathology of peripheral nerves and muscles (Ciucci et al., 2013; Glass et al., 2020). Conclusions provide crucial insight into the mechanism of tongue motor function (central vs. peripheral), as well as critical information regarding early disease signs that manifest in functional licking.
Tongue force has also been measured by Plowman in the 6-OHDA rat model of PD and in autologous muscle-derived stem cell (MdSC) therapy in denervated tongue muscle of ewes (Plowman et al., 2014, 2014b). In 2014, Plowman and colleagues piloted a study on denervated tongue muscle of ewes using hypoglossal nerve stimulation coupled with a tensometer and high-resolution manometry to gather data on tongue strength, allowing for functional and histological comparisons pre- and post-mortem. Specifically, maximal contractile force and tongue pressure were measured to assess the utility of MdSC therapy in the presence of denervation; preliminary findings demonstrated increased tongue strength post-therapy. Measurement of tongue strength is crucial in assessing key mechanistic function related to swallowing, as well as the potential utility and validity of intervention methodologies such as MdSC therapy.
With regard to the 2014 6-OHDA study, Plowman and colleagues used targeted motor training to examine the potential impacts of said rehabilitation methodology on limb motor and cranial motor (licking) function, as well as corresponding corticobulbar and corticospinal circuits (Plowman, 2014b). They used a lick/force recording apparatus to study licking dynamics and to employ a targeted training paradigm (2 g lingual force requirement for 14 days) (Plowman, 2014b). Understanding orolingual motor deficits in models of PD allows for refined investigation and subsequent preclinical intervention methodology, aiding future clinical research in populations affected by dysphagia (Nuckolls et al., 2012).
Measurement of tongue function provides critical information regarding swallow function and degree of impairment for both speech and swallowing throughout amyotrophic lateral sclerosis (ALS) disease progression (Perry et al., 2018, 2021). Fowler’s instruments have been adapted for ALS research by scientists such as Stanford, Plowman, and Lever. Smittkamp and Stanford were the first to measure tongue function in mice and rats with ALS using the force transducer. Tongue force and lick rhythm were measured in pre-symptomatic, symptomatic, and end-stage points of disease using a familial model of ALS, specifically the SOD1-G93A model, allowing for characterization of bulbar motor deficits (Smittkamp et al., 2008, 2010). Additional research by Ma and colleagues used a modified operant chamber with a force transducer to assess both tongue motor function and employ a strength-training paradigm. This allowed for exploration into orolingual deficits and potential impacts of exercise related to bulbar function in an SOD1-G93A female rat model of ALS (Ma et al., 2016). In Lever’s research, the force lickometer allows for investigation of the potential benefit in implementing an individualized lingual exercise-based program for patients with neurodegenerative diseases, coupled with assessment of oral function using videofluoroscopy (Lever et al., 2008, 2010; Osman et al., 2020).
Beyond functional measurement, this modification has been used in the context of an exercise-based treatment paradigm (Behan et al., 2012; Cullins et al., 2018; Glass et al., 2021; Kletzien et al., 2012, 2020; Connor et al., 2009; Schaser et al., 2016, Ciucci et al., 2011, 2013; Plowman et al., 2014). The progressive resistance training paradigm employed by the Connor and Ciucci research groups is conducted 5x/week over the course of several weeks (e.g., 6–8 weeks). The paradigm involves training rats to press the disk with an increasing amount of force to obtain a water reward. Force requirements are individualized and determined through baseline and mid-point testing. The maximum force produced during a 5-minute session in the chamber is recorded, and a percentage of that maximum force is calculated to define the force requirement. Percent required is then incrementally increased by a specific amount (e.g. 20% for the first 2 weeks of training, 40% for the next 2, and so on). Midpoint recordings are conducted to update the maximum force produced, and percentages are re-calculated before continuing the second half of the paradigm. Final testing is done to collect relevant measures and allows for pre- to post-training comparisons. Tongue exercise treatment in the rat model aligns with clinical dysphagia intervention methods and enables exploration into how treatments may impact swallow function in both aging (Kletzien et al., 2012; Krekeler et al., 2018, 2020; Krekeler & Connor, 2017) and disease models, such as head and neck cancer (Russell & Connor, 2014).
Tongue exercise therapy using the force transducer has also been explored beyond the context of orolingual deficits. Such research includes upper airway stability, with implications for obstructive sleep apnea (OSA) (Huang et al., 2021; Rueda et al., 2020). Huang and colleagues described the use of an 8-week training paradigm, in which rats that underwent a progressive resistance tongue exercise training were found to have increased corticomotor excitability and EMG activity within the genioglossus muscle of the tongue, thus providing data for a potential framework using tongue exercise in patients diagnosed with OSA (Huang et al., 2021).
The modification and use of these instruments in research focused on aging and disease models has provided crucial information related to quantifying key aspects of swallow function. Measurements of lick rate, number of licks, tongue force, and variability in force and timing correlate with implications in the oral phases of swallowing, which has subsequent impacts on airway safety and swallowing efficiency. Further, this data allows for translation to therapy-based paradigms in which researchers assess the utility of tongue exercise on swallow function. In doing so, gaps in knowledge present in a wide variety of clinical populations can be addressed, allowing for improvements in clinical research and practice.
CONCLUSION AND FUTURE DIRECTIONS
The expansive contributions of Fowler continue to be used to measure tongue dynamics. His innovation has left a lasting impact on various fields of research. Even today, many of his innovations continue to be updated and adapted to address critical gaps in knowledge. Functional measurements of tongue force remain an unparalleled method for studying the onset, progression, and treatment of swallowing dysfunction related to ALS (Lever et al., 2008, 2010; Osman et al., 2020; Perry et al., 2018, 2021), Parkinson disease (Ciucci et al., 2011, 2013; Glass et al., 2020; Plowman et al., 2014b), aging (Cullins et al., 2018; Kletzien et al., 2012; Krekeler et al., 2018, 2020; Krekeler & Connor, 2017; Nagai et al., 2008; Ota et al., 2005; Connor et al., 2009), stroke (Cullins & Connor, 2019), and head and neck cancer (Russell & Connor, 2014). These advances are continuing to elucidate not only the mechanisms of diseases, but also pathways of treatment and care for a wide range of patient populations. As a scientific community, we have robust tools to study tongue dysfunction and swallowing impairment, made possible through the innovation of Fowler.
Footnotes
Declarations of Interest:
None
REFERENCES
- Behan M, Moeser AE, Thomas CF, Russell JA, Wang H, Leverson GE, & Connor NP (2012). The effect of tongue exercise on serotonergic input to the hypoglossal nucleus in young and old rats. Journal of Speech, Language, and Hearing Research : JSLHR, 55(3), 919–929. 10.1044/1092-4388(2011/11-0091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordoni B, Morabito B, Mitrano R, Simonelli M, & Toccafondi A (2018). The Anatomical Relationships of the Tongue with the Body System. Cureus, 10(12). 10.7759/CUREUS.3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozek G, Zhuravint IA, Megiriant D, & Bures J (1996). Localization of the central rhythm generator involved in spontaneous consummatory licking in rats: Functional ablation and electrical brain stimulation studies. Psychology, 93, 3325–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, & Connor NP (2009). Dopaminergic influence on rat tongue function and limb movement initiation. Experimental Brain Research, 194(4), 587–596. 10.1007/S00221-0091736-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Russell JA, Schaser AJ, Doll EJ, Vinney LM, & Connor NP (2011). Tongue force and timing deficits in a rat model of Parkinson disease. Behavioural Brain Research, 222(2), 315–320. https://doi.org/ 10.1016/j.bbr.2011.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Schaser AJ, & Russell JA (2013). Exercise-induced rescue of tongue function without striatal dopamine sparing in a rat neurotoxin model of Parkinson disease. Behavioural Brain Research, 252, 239–245. 10.1016/J.BBR.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor NP, Russell JA, Wang H, Jackson MA, Mann L, & Kluender K (2009). Effect of Tongue Exercise on Protrusive Force and Muscle Fiber Area in Aging Rats. Journal of Speech, Language, and Hearing Research, 52(3), 732–744. 10.1044/1092-4388(2008/08-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullins MJ, & Connor NP (2017). Alterations of intrinsic tongue muscle properties with aging. Muscle & Nerve, 56(6), E119–E125. 10.1002/MUS.25605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullins MJ, & Connor NP (2019). Reduced tongue force and functional swallowing changes in a rat model of post stroke dysphagia. Brain Research, 1717, 160–166. 10.1016/j.brainres.2019.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullins MJ, Krekeler BN, & Connor NP (2018). Differential impact of tongue exercise on intrinsic lingual muscles. The Laryngoscope, 128(10), 2245–2251. 10.1002/LARY.27044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullins MJ, Wenninger JM, Cullen JS, Russell JA, Kleim JA, & Connor NP (2019). Tongue Force Training Induces Plasticity of the Lingual Motor Cortex in Young Adult and Aged Rats. In Frontiers in Neuroscience (Vol. 13, p. 1355). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, & Fowler SC (1995). Acute and subchronic effects of clozapine on licking in rats: tolerance to disruptive effects on number of licks, but no tolerance to rhythm slowing. Psychopharmacology, 120(3), 249–255. 10.1007/BF02311171 [DOI] [PubMed] [Google Scholar]
- Das S, & Fowler SC (1996). An update of Fowler and Das: Anticholinergic reversal of haloperidol-induced, within-session decrements in rats’ lapping behavior. Pharmacology Biochemistry and Behavior, 53(4), 853–855. 10.1016/0091-3057(95)02094-2 [DOI] [PubMed] [Google Scholar]
- Das S, & Fowler SC (1996b). Similarity of clozapine’s and olanzapine’s acute effects on rats’ lapping behavior. Psychopharmacology, 123(4), 374–378. 10.1007/BF02246648 [DOI] [PubMed] [Google Scholar]
- Di Vico R, Paolo Ardigò L, Salernitano G, Chamari K, & Padulo J (2013). The acute effect of the tongue position in the mouth on knee isokinetic test performance: a highly surprising pilot study. Ligaments and Tendons Journal, 3(4), 318–323. [PMC free article] [PubMed] [Google Scholar]
- Fowler SC, Birkestrand BR, Chen R, Moss SJ, Vorontsova E, Wang G, & Zarcone TJ (2001). A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. Journal of Neuroscience Methods, 107(1–2), 107–124. 10.1016/S0165-0270(01)00359-4 [DOI] [PubMed] [Google Scholar]
- Fowler SC, & Das S (1994). Haloperidol-induced decrements in force and duration of rats’ tongue movements during licking are attenuated by concomitant anticholinergic treatment. Pharmacology, Biochemistry and Behavior, 49(4), 813–817. 10.1016/0091-3057(94)90228-3 [DOI] [PubMed] [Google Scholar]
- Fowler SC, Davison KH, & Stanford JA (1994). Unlike haloperidol, clozapine slows and dampens rats’ forelimb force oscillations and decreases force output in a press-while-licking behavioral task. Psychopharmacology, 116(1), 19–25. 10.1007/BF02244866 [DOI] [PubMed] [Google Scholar]
- Fowler SC, Liao RM, & Skjoldager P (1990). A New Rodent Model for Neuroleptic-Induced Pseudo-Parkinsonism: Low Doses of Haloperidol Increase Forelimb Tremor in the Rat. Behavioral Neuroscience, 104(3), 449–456. 10.1037/0735-7044.104.3.449 [DOI] [PubMed] [Google Scholar]
- Fowler SC, Miller BR, Gaither TW, Johnson MA, & Rebec GV (2009). Force-plate quantification of progressive behavioral deficits in the R6/2 mouse model of Huntington’s disease. Behavioural Brain Research, 202(1), 130–137. 10.1016/J.BBR.2009.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SC, & Mortell C (1992). Low Doses of Haloperidol Interfere With Rat Tongue Extensions During Licking: A Quantitative Analysis. Behavioral Neuroscience, 106(2), 386–395. 10.1037/0735-7044.106.2.386 [DOI] [PubMed] [Google Scholar]
- Fowler SC, Mosher LJ, Godar SC, & Bortolato M (2017). Assessment of gait and sensorimotor deficits in the D1CT-7 mouse model of Tourette syndrome. Journal of Neuroscience Methods, 292, 37–44. 10.1016/j.jneumeth.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SC, & Wang G (1998). Chronic haloperidol produces a time- and dose-related slowing of lick rhythm in rats: Implications for rodent models of tardive dyskinesia and neuroleptic-induced parkinsonism. Psychopharmacology, 137(1), 50–60. 10.1007/s002130050592 [DOI] [PubMed] [Google Scholar]
- Fowler SC, Zarcone TJ, Vorontsova E, & Chen R (2002). Motor and associative deficits in D2 dopamine receptor knockout mice. International Journal of Developmental Neuroscience, 20(3–5), 309–321. 10.1016/S0736-5748(02)00009-6 [DOI] [PubMed] [Google Scholar]
- Fowler SC, Zarcone TJ, Chen R, Taylor MD, & Wright DE (2002b). Low grip strength, impaired tongue force and hyperactivity induced by overexpression of neurotrophin-3 in mouse skeletal muscle. International Journal of Developmental Neuroscience, 20(3–5), 303–308. 10.1016/S0736-5748(02)00010-2 [DOI] [PubMed] [Google Scholar]
- Glass TJ, Figueroa JE, Russell JA, Krekeler BN, & Connor NP (2021). Progressive Protrusive Tongue Exercise Does Not Alter Aging Effects in Retrusive Tongue Muscles. Frontiers in Physiology, 12, 740876. 10.3389/fphys.2021.740876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass TJ, Kelm-Nelson CA, Szot JC, Lake JM, Connor NP, & Ciucci MR (2020). Functional characterization of extrinsic tongue muscles in the Pink1−/− rat model of Parkinson disease. PLOS ONE, 15(10), e0240366. 10.1371/JOURNAL.PONE.0240366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramling SE, & Fowler SC (1985). Effects of neuroleptics on rate and duration of operant versus reflexive licking in rats. Pharmacology, Biochemistry and Behavior, 22(4), 541–545. 10.1016/0091-3057(85)90272-2 [DOI] [PubMed] [Google Scholar]
- Gramling SE, & Fowler SC (1986). Some effects of pimozide and of shifts in sucrose concentration on lick rate, duration, and interlick interval. Pharmacology, Biochemistry and Behavior, 25(1), 219–222. 10.1016/0091-3057(86)90256-X [DOI] [PubMed] [Google Scholar]
- Gramling SE, Fowler SC, & Collins KR (1984). Some effects of pimozide on nondeprived rats licking sucrose solutions in an anhedonia paradigm. Pharmacology Biochemistry and Behavior, 21(4), 617–624. 10.1016/S0091-3057(84)80047-7 [DOI] [PubMed] [Google Scholar]
- Grant LM, Kelm-Nelson CA, Hilby BL, Blue KV, Paul Rajamanickam ES, Pultorak JD, Fleming SM, & Ciucci MR (2015). Evidence for early and progressive ultrasonic vocalization and oromotor deficits in a PINK1 gene knockout rat model of Parkinson’s disease. Journal of Neuroscience Research, 93(11), 1713–1727. 10.1002/jnr.23625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern BP (1977). Functional Anatomy of the Tongue and Mouth of Mammals. Drinking Behavior, 1–92. 10.1007/978-1-4684-2319-8_1 [DOI] [Google Scholar]
- Hill JH, & Stellar E (1951). An electronic drinkometer. Science, 114(2950), 43–44. 10.1126/SCIENCE.114.2950.43 [DOI] [PubMed] [Google Scholar]
- Naga Hiromi, Russell John A, Jackson Michelle A, & Connor Nadine P. (2008). Effect of Aging on Tongue Protrusion Forces in Rats. Dysphagia, 23, 116–121. 10.1007/s00455-007-9103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Li W, Jin H, Zhang L, Wei Z, & Wang W (2021). Tongue Strength Training Increases Daytime Upper Airway Stability in Rats. Nature and Science of Sleep, 13, 1653–1661. 10.2147/NSS.S328214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Kusaka G, Takashima K, Kamochi H, & Shinoda S (2010). Intraoperative monitoring during surgery for hypoglossal schwannoma. Journal of Clinical Neuroscience, 17(8), 1053–1056. 10.1016/j.jocn.2009.09.040 [DOI] [PubMed] [Google Scholar]
- Jüch PJW, van Willigen JD, Broekhuijsen ML, & Ballintijn CM (1985). Peripheral influences on the central pattern-rhythm generator for tongue movements in the rat. Archives of Oral Biology, 30(5), 415–421. 10.1016/0003-9969(85)90069-X [DOI] [PubMed] [Google Scholar]
- Kim AM, Keenan BT, Jackson N, Chan EL, Staley B, Torigian DA, Alavi A, & Schwab RJ (2014). Metabolic activity of the tongue in obstructive sleep apnea: A novel application of FDG positron emission tomography imaging. American Journal of Respiratory and Critical Care Medicine, 189(11), 1416–1425. 10.1164/rccm.201310-1753OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien H, Kelm-Nelson CA, Wang S, Suzuki M, & Connor NP (2020). Myogenic marker expression as a function of age and exercise-based therapy in the tongue. Experimental Gerontology, 142, 111104. 10.1016/j.exger.2020.111104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien H, Russell JA, Leverson GE, & Connor NP (2012). Differential effects of targeted tongue exercise and treadmill running on aging tongue muscle structure and contractile properties. Journal of Applied Physiology, 114(4), 472–481. 10.1152/japplphysiol.01370.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krekeler BN, & Connor NP (2017). Age-related changes in mastication are not improved by tongue exercise in a rat model. The Laryngoscope, 127(1), E29–E34. 10.1002/lary.26045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krekeler BN, Leverson G, & Connor NP (2018). Tongue exercise and ageing effects on morphological and biochemical properties of the posterior digastric and temporalis muscles in a Fischer 344 Brown Norway rat model. Archives of Oral Biology, 89, 37–43. 10.1016/j.archoralbio.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krekeler BN, Weycker JM, & Connor NP (2020). Effects of Tongue Exercise Frequency on Tongue Muscle Biology and Swallowing Physiology in a Rat Model. Dysphagia, 35(6), 918–934. 10.1007/s00455-020-10105-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever TE, Gorsek A, Cox KT, O’Brien KF, Capra NF, Hough MS, & Murashov AK (2008). An Animal Model of Oral Dysphagia in Amyotrophic Lateral Sclerosis. Dysphagia, 24(2), 180. 10.1007/s00455-008-9190-z [DOI] [PubMed] [Google Scholar]
- Lever TE, Simon E, Cox KT, Capra NF, O’Brien KF, Hough MS, & Murashov AK (2010). A Mouse Model of Pharyngeal Dysphagia in Amyotrophic Lateral Sclerosis. Dysphagia, 25(2), 112–126. 10.1007/s00455-009-9232-1 [DOI] [PubMed] [Google Scholar]
- Liao RM, Fowler SC, & Kallman MJ (1997). Quantifying Operant Behavior Deficits in Rats with Bilateral 6-Hydroxydopamine Lesions of the Ventrolateral Striatum. Chinese Journal of Physiology, 40(2), 71–78. [PubMed] [Google Scholar]
- Ma D, Shuler JM, Kumar A, Stanford QR, Tungtur S, Nishimune H, & Stanford JA (2016). Effects of Tongue Force Training on Bulbar Motor Function in the Female SOD1-G93A Rat Model of Amyotrophic Lateral Sclerosis. Neurorehabilitation and Neural Repair, 31(2), 147–156. 10.1177/1545968316666956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, & Calabrese RL (1996). Principles of rhythmic motor pattern generation. Physiological Reviews, 76(3), 687–717. 10.1152/PHYSREV.1996.76.3.687 [DOI] [PubMed] [Google Scholar]
- Meredith GE, & Kang UJ (2006). Behavioral models of Parkinsons disease in rodents: A new look at an old problem. Movement Disorders, 21(10), 1595–1606. 10.1002/mds.21010 [DOI] [PubMed] [Google Scholar]
- Moss SJ, Wang G, Chen R, Pal R, & Fowler SC (2001). 3-Acetylpyridine reduces tongue protrusion force but does not abolish lick rhythm in the rat. Brain Research, 920(1–2), 1–9. 10.1016/S0006-8993(01)02790-1 [DOI] [PubMed] [Google Scholar]
- Nagai H, Russell JA, Jackson MA, & Connor NP (2008). Effect of Aging on Tongue Protrusion Forces in Rats. Dysphagia, 23(2), 116–121. 10.1007/s00455-007-9103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuckolls AL, Worley C, Leto C, Zhang H, Morris JK, & Stanford JA (2012). Tongue force and tongue motility are differently affected by unilateral vs bilateral nigrostriatal dopamine depletion in rats. Behavioural Brain Research, 234(2), 343–348. 10.1016/j.bbr.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman KL, Kohlberg S, Mok A, Brooks R, Lind LA, McCormack K, Ferreira A, Kadosh M, Fagan MK, Bearce E, Nichols NL, Coates JR, & Lever TE (2020). Optimizing the Translational Value of Mouse Models of ALS for Dysphagia Therapeutic Discovery. Dysphagia, 35(2), 343–359. 10.1007/s00455-019-10034-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota F, Connor NP, & Konopacki R (2005). Alterations in contractile properties of tongue muscles in old rats. The Annals of Otology, Rhinology, and Laryngology, 114(10), 799–803. 10.1177/000348940511401010 [DOI] [PubMed] [Google Scholar]
- Perry BJ, Martino R, Yunusova Y, Plowman EK, & Green JR (2018). Lingual and Jaw Kinematic Abnormalities Precede Speech and Swallowing Impairments in ALS. Dysphagia, 33(6), 840–847. 10.1007/s00455-018-9909-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry BJ, Stipancic KL, Martino R, Plowman EK, & Green JR (2021). Biomechanical Biomarkers of Tongue Impairment During Swallowing in Persons Diagnosed with Amyotrophic Lateral Sclerosis. Dysphagia, 36(1), 147–156. 10.1007/s00455-020-10116-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman EK, Bijangi-Vishehsaraei K, Halum S, Cates D, Hanenberg H, Domer AS, Nolta JA, & Belafsky PC (2014). Autologous myoblasts attenuate atrophy and improve tongue force in a denervated tongue model: A pilot study. The Laryngoscope, 124(2), E20–E26. 10.1002/lary.24352 [DOI] [PubMed] [Google Scholar]
- Plowman EK, Maling N, Thomas NJ, Fowler SC, & Kleim JA (2014b). Targeted motor rehabilitation dissociates corticobulbar versus corticospinal dysfunction in an animal model of parkinson’s disease. Neurorehabilitation and Neural Repair, 28(1), 85–95. 10.1177/1545968313498648 [DOI] [PubMed] [Google Scholar]
- Rueda J-R, Mugueta-Aguinaga I, Vilaró J, & Rueda-Etxebarria M (2020). Myofunctional therapy (oropharyngeal exercises) for obstructive sleep apnoea. The Cochrane Database of Systematic Reviews, 11(11), CD013449–CD013449. 10.1002/14651858.CD013449.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, & Connor NP (2014). Effects of age and radiation treatment on function of extrinsic tongue muscles. Radiation Oncology (London, England), 9, 254. 10.1186/s13014-014-0254-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaser AJ, Ciucci MR, & Connor NP (2016). Cross-activation and detraining effects of tongue exercise in aged rats. Behavioural Brain Research, 297, 285–296. 10.1016/J.BBR.2015.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skitek EB, Fowler SC, & Tessel RE (1999). Effects of unilateral striatal dopamine depletion on tongue force and rhythm during licking in rats. Behavioral Neuroscience, 113(3), 567–573. 10.1037/0735-7044.113.3.567 [DOI] [PubMed] [Google Scholar]
- Smittkamp SE, Brown JW, & Stanford JA (2008). Time-course and characterization of orolingual motor deficits in B6SJL-Tg(SOD1-G93A)1Gur/J mice. Neuroscience, 151(2), 613–621. 10.1016/j.neuroscience.2007.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smittkamp SE, Spalding HN, Brown JW, Gupte AA, Chen J, Nishimune H, Geiger PC, & Stanford JA (2010). Measures of bulbar and spinal motor function, muscle innervation, and mitochondrial function in ALS rats. Behavioural Brain Research, 211(1), 48–57. 10.1016/j.bbr.2010.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford JA, & Fowler SC (1997). Subchronic effects of clozapine and haloperidol on rats’ forelimb force and duration during a press-while-licking task. Psychopharmacology, 130(3), 249–253. 10.1007/s002130050236 [DOI] [PubMed] [Google Scholar]
- Stanford JA, & Fowler SC (1998). At low doses, harmaline increases forelimb tremor in the rat. Neuroscience Letters, 241(1), 41–44. 10.1016/S0304-3940(97)00974-9 [DOI] [PubMed] [Google Scholar]
- Stanford JA, & Fowler SC (2000). Clozapine-like motor effects of the atypical antipsychotic risperidone in rats. Neuroscience Letters, 285(3), 189–192. 10.1016/S0304-3940(00)01062-4 [DOI] [PubMed] [Google Scholar]
- Stanford JA, & Fowler SC (2002). Dantrolene diminishes forelimb force-related tremor at doses that do not decrease operant behavior in the rat. Experimental and Clinical Psychopharmacology, 10(4), 385–391. 10.1037/1064-1297.10.4.385 [DOI] [PubMed] [Google Scholar]
- Stanford JA, Vorontsova E, & Fowler SC (2000). The relationship between isometric force requirement and forelimb tremor in the rat. Physiology and Behavior, 69(3), 285–293. 10.1016/S0031-9384(99)00248-6 [DOI] [PubMed] [Google Scholar]
- Stanford JA, Vorontsova E, Surgener SP, Gerhardt GA, & Fowler SC (2003). Aged Fischer 344 rats exhibit altered orolingual motor function: Relationships with nigrostriatal neurochemical measures. Neurobiology of Aging, 24(2), 259–266. 10.1016/S0197-4580(02)00083-0 [DOI] [PubMed] [Google Scholar]
- Stellar E, & Hill JH (1952). The rat’s rate of drinking as a function of water deprivation. Journal of Comparative and Physiological Psychology, 45(1), 96–102. 10.1037/H0062150 [DOI] [PubMed] [Google Scholar]
- Stricker EM, & Miller NE (1965). THIRST MEASURED BY LICKING REINFORCED ON INTERVAL SCHEDULES: EFFECTS OF PREWATERING AND OF BACTERIAL ENDOTOXIN. Journal of Comparative and Physiological Psychology, 59(1), 112–115. 10.1037/H0021629 [DOI] [PubMed] [Google Scholar]
- Teruya PY, Farfán FD, Pizá ÁG, Soletta JH, Lucianna FA, & Albarracín AL (2021). Quantifying muscle alterations in a Parkinson’s disease animal model using electromyographic biomarkers. Medical and Biological Engineering and Computing, 59(9), 1735–1749. 10.1007/s11517-021-02400-3 [DOI] [PubMed] [Google Scholar]
- Travers JB, Dinardo LA, & Karimnamazi H (1997). Motor and premotor mechanisms of licking. Neuroscience and Biobehavioral Reviews, 21(5), 631–647. 10.1016/S0149-7634(96)00045-0 [DOI] [PubMed] [Google Scholar]
- Wall AM, Walters GC, & England RS (1972). The lickometer: A simple device for the analysis of licking as an operant. Behavior Research Methods & Instrumentation, 4(6), 320–322. 10.3758/BF03207315 [DOI] [Google Scholar]
- Wang G, & Fowler SC (1999). Effects of haloperidol and clozapine on tongue dynamics during licking in CD-1, BALB/c and C57BL/6 mice. Psychopharmacology, 147(1), 38–45. 10.1007/s002130051140 [DOI] [PubMed] [Google Scholar]
- Weijnen JAWM (1998). Licking Behavior in the Rat: Measurement and Situational Control of Licking Frequency. Neuroscience & Biobehavioral Reviews, 22(6), 751–760. 10.1016/S0149-7634(98)00003-7 [DOI] [PubMed] [Google Scholar]
- Williams DR, & Teitelbaum P (1956). Control of drinking behavior by means of an operant-conditioning technique. Science (New York, N.Y.), 124(3235), 1294–1296. 10.1126/SCIENCE.124.3235.1294 [DOI] [PubMed] [Google Scholar]
- Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, Sengillo JD, Hillman S, Kong P, Nelson AR, Sullivan JS, Zhao Z, Meiselman HJ, Wenby RB, Soto J, Abel ED, Makshanoff J, Zuniga E, De Vivo DC, & Zlokovic BV (2015). GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nature Neuroscience, 18(4), 521–530. 10.1038/nn.3966 [DOI] [PMC free article] [PubMed] [Google Scholar]


