Background:
Atrial functional mitral regurgitation (AFMR) is associated with increased morbidity and mortality. Left atrial (LA) size and function in AFMR are poorly characterized. We aimed to assess LA function by reservoir strain (LASr) and estimated reservoir work (LAWr) and their impact on outcome in AFMR.
Methods:
Consecutive patients at our institution between 2001 and 2019 and with significant (moderate or greater) AFMR were examined. LAWr was estimated as LASr×LA reservoir volume, and patients were grouped by median LASr and LAWr. Outcomes were all-cause death or heart failure hospitalizations.
Results:
Five hundred fifteen AFMR patients were followed up for 5 (1–17) years. Patients had previously documented atrial fibrillation (AF; 37%), heart failure with preserved ejection fraction (HFpEF) without AF (24%), or both (HFpEF+AF, 39%). LA volume was largest in AF, while LA function parameters were most impaired in the combined HFpEF+AF group. During follow-up, patients with low LASr or LAWr had higher risk of death (P<0.001) and heart failure hospitalization (P<0.05). In Cox regression analyses, low LASr and LAWr, but not LA volume or left ventricular function, were associated with a higher risk of death (LASr: hazard ratio, 2.3 [95% CI, 1.6–3.5]; LAWr: hazard ratio, 3.4 [95% CI, 2.4–4.9]; both P<0.001) after adjustment for clinical and echocardiographic confounders. Low LASr and LAWr were strongest associated with death in HFpEF and HFpEF+AF.
Conclusions:
LA reservoir function but not LA size is a robust predictor of outcome in significant AFMR. This provides mechanistic insights into the interplay of functional versus geometric LA changes in AFMR.
Keywords: atrial function, left; echocardiography; mitral valve; mitral valve insufficiency; mortality
CLINICAL PERSPECTIVE.
In a large cohort of patients with moderate and greater atrial functional mitral regurgitation (AFMR), we found that AFMR is commonly associated with heart failure with preserved ejection fraction or atrial fibrillation. Depending on the linked pathology, we identified distinct patterns of left atrial remodeling in AFMR, with more severely dilated atria with higher storage capacity in patients with atrial fibrillation and smaller, noncompliant atria in heart failure with preserved ejection fraction. Patients with significant AFMR experienced a high number of adverse events, including a 38% mortality and 30% first-time heart failure hospitalizations during a 5-year follow-up. Impaired left atrial function assessed by either the reservoir strain or the product of reservoir strain×left atrial reservoir volume (a simplified index of left atrial reservoir work) was a strong predictor of death in AFMR, particularly in the setting of heart failure with preserved ejection fraction. The maximum left atrial volume—a guideline-recommended criterion for grading and considering intervention in chronic mitral regurgitation—was not related to outcome in significant AFMR. The current findings support future investigations to evaluate different treatment strategies addressing both the mitral regurgitation and the left atrial dysfunction in AFMR.
See Editorial by Vannan et al
Functional mitral regurgitation (MR) is caused by geometric changes of the mitral apparatus without concomitant structural leaflet pathology. A common type of secondary MR is associated with left ventricular (LV) remodeling in the context of ischemic heart disease or dilated cardiomyopathy. More recently, atrial functional MR (AFMR) has been recognized as a type of secondary MR with distinct mechanisms and clinical features than that due to ischemic or myopathic LV remodeling. AFMR is mostly described in patients with normal LV size and preserved ejection fraction (EF), mitral leaflets with normal echocardiographic appearance, and dilated left atrium (LA).1
AFMR is common in elderly individuals with atrial arrhythmias but is also associated with heart failure (HF) with preserved EF (HFpEF).2 The clinical impact of this secondary MR subtype will escalate due to the aging of the population worldwide and the ongoing obesity and atrial fibrillation (AF) epidemic. The mortality risk of AFMR is comparable to that of degenerative MR.3 However, its mechanisms and clinical presentation are poorly defined, and in the recent European Society of Cardiology/ European Association for Cardio-Thoracic Surgery and American Heart Association/American College of Cardiology valvular heart disease guidelines, AFMR is recognized mainly in the context of AF and lacks separate recommendations for grading, follow-up, and treatment.4,5
Mechanistically, AFMR is commonly attributed to adverse LA remodeling such as increased LA and annular size. However, the impact of atrial mechanics has been scarcely described. Atrial mechanical function may be an important contributor to clinical outcome in AFMR.6 We aim to characterize LA function and remodeling patterns in a large population of patients with significant AFMR and assess the independent impact of atrial mechanics on the patients’ long-term outcome.
Methods
Study Population
Patients with AFMR examined at the Massachusetts General Hospital in the period 2001 to 2019 were identified in the institutional echocardiographic database. The definition of AFMR was significant (moderate or greater) MR with normal-sized LV and LV function (LV EF, ≥50%; Carpentier type I).6 Patients with LV wall motion abnormalities or other MR mechanisms were excluded. The echocardiographic evaluation that was the basis for inclusion is referred to as the index examination. Only patients followed up at the Massachusetts General Hospital were included in the study. MR severity was graded using a standardized and integrative method based on combined qualitative, semiquantitative, and quantitative evaluation7 performed by cardiologists with specialized expertise in echocardiography. Patients with evidence of structural mitral valve pathology, other severe valve disease, hypertrophic or dilated cardiomyopathy, congenital heart disease, pulmonary embolism, or constrictive pericarditis were excluded.
Obesity was defined as a body mass index ≥30 kg/m². Relevant comorbidities, including HFpEF, daily medication, including HF treatment, and symptoms at presentation were obtained via chart review. History of AF was assessed by manual review of all resting electrocardiograms (available in all patients), 24-hour Holter registrations (available in 11% of the study population), and charts prior to the index examination. Type of heart rhythm during echocardiography was also assessed.
The study was approved by the institutional review board and was conducted in accordance with the revised Declaration of Helsinki. Due to the retrospective nature of the study, informed consent was not required. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Echocardiographic Measurements
Comprehensive transthoracic echocardiograms were performed by experienced sonographers and analyzed on Syngo Dynamics stations (Siemens Medical Solutions Munich, Germany) equipped with 2-dimensional speckle tracking software (2D Cardiac Performance Analysis; Tomtec Arena 2.42, Unterschleissheim, Germany).
LA Size and Function
LA volume was measured in dedicated apical 4- and 2- chamber views at 3 different time points: end diastole (the frame just after mitral valve closure), onset of atrial contraction (identified by the P wave on ECG), and end systole (the frame just before mitral valve opening), rendering minimum LA volume (LAVmin), pre-LA, and maximum LA volume (LAVmax), respectively.8 LA appendage and the ostia of pulmonary veins, if visible, were excluded from the volumes. LA dilatation was assessed based on LAVmax indexed for body surface area. The increase in LA volume from minimum to maximum during the reservoir phase defined the LA reservoir volume (also referred to as the total emptying LA volume in the literature).9 The LA emptying fraction was calculated as the ratio of the LA reservoir volume and LAVmax.
LA strain was measured using specific software (2D Cardiac Performance Analysis; Tomtec Arena 2.42) and end diastole as the zero-strain reference point.9 LA reservoir function was assessed by the reservoir strain (LASr) and additionally estimated from the product LASr (as a surrogate of LA pressure change10–12)×LA reservoir volume13–15—a simplified index of LA reservoir work (LAWr).
Additionally, the LA expansion index—a previously proposed marker of reservoir function—was deducted from the ratio of LA reservoir volume to LAVmin.16 In patients in sinus rhythm at the index examination, LA strain during the atrial contraction phase was also quantified.9 In patients in AF, both LA size and function were assessed in 3 consecutive cardiac cycles during reasonably regular rhythm and averaged.9
Of note, the ratio of mitral peak E-wave velocity and mitral annular e′ velocity is not reported as it is not recommended to be used in functional analyses in patients with significant MR and preserved LV EF.17,18
LV Size and Function
LV size was evaluated by the 2-dimensional wall and cavity linear dimensions and by triplane volumes following the joint American Society of Echocardiography and European Association of Cardiovascular Imaging guidelines.19 LV hypertrophy was identified as recommended in populations with higher prevalence of obesity as LV mass indexed for height2.7 ≥49.2 g/m2.7 in men and ≥46.7 g/m2.7 in women.20–22 The ratio of LV end-diastolic posterior wall thickness/internal diameter (the relative wall thickness) indicated concentric LV geometry when ≥0.43.23
LV systolic function was assessed by both EF and global left ventricular longitudinal strain (GLS; low if less negative than −16.7% in men and <−17.8% in women) using all 3 standard apical views.19,24
Echocardiographic Control Group
To examine the individual impact of AFMR on LA function, we additionally measured LA size and reservoir function in a separate, contemporary cohort (examined between 2017 and 2022) of patients with an established HFpEF diagnosis, none or trace MR, no history of atrial arrhythmias and sinus rhythm at the index examination, and echocardiographic data suitable for speckle tracking analysis. The exclusion criteria were otherwise similar as for the main study population. A group of 100 controls with a comparable age and sex composition as the main study population was identified and used as a comparison sample in analyses of echocardiographic data.
Outcomes
We examined the prognostic value of measures of LA reservoir function in relation to all-cause mortality and HF hospitalizations. Patients were followed up beginning with the day of the index examination and up to 2021. All deaths were ascertained from medical records and the social security death index. Hospitalization for HF was ascertained from chart reviews.
Statistical Analyses
All statistical analyses were performed using IBM SPSS Statistics 28.0 (IBM Corp, Armonk, NY). Between-group differences were assessed by unpaired t tests, 1-way ANOVA with Sidak post hoc test, χ2 test, or Fisher exact test as appropriate. Due to fundamentally different atrial mechanics, atrial size and function are reported separately for patients in sinus rhythm and AF at the index examination. Findings are reported as mean±SD and median values with error bars for the 95% CIs for continuous variables and percentages for categorical variables.
The impact of LA function on outcomes was tested in Kaplan-Meier survival analyses with log-rank test for the overall analysis, as well as in univariable and multivariable Cox regression analyses. Additionally, the strength of the relationship between different indexes of LA function and death was assessed in the receiver operating characteristic analysis with comparison between the areas under the curve of the different parameters. For survival analyses, patients were grouped according to the median value of LASr and LAWr, and the association with all-cause death was assessed univariably in the whole AFMR population and in the 3 AFMR subgroups: HFpEF, AF, and HFpEF+AF. Multivariable Cox regression models were additionally run in the whole AFMR population and covariates selected based both on clinical relevance and a P<0.1 level of significance for the association with the dependent variables in univariable analyses. Results are reported as hazard ratios with 95% CIs. A 2-tailed P≤0.05 was considered significant both in univariable and multivariable analyses.
Results
Study Population
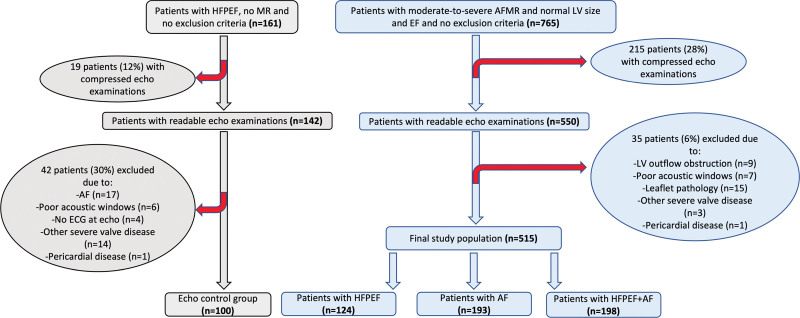
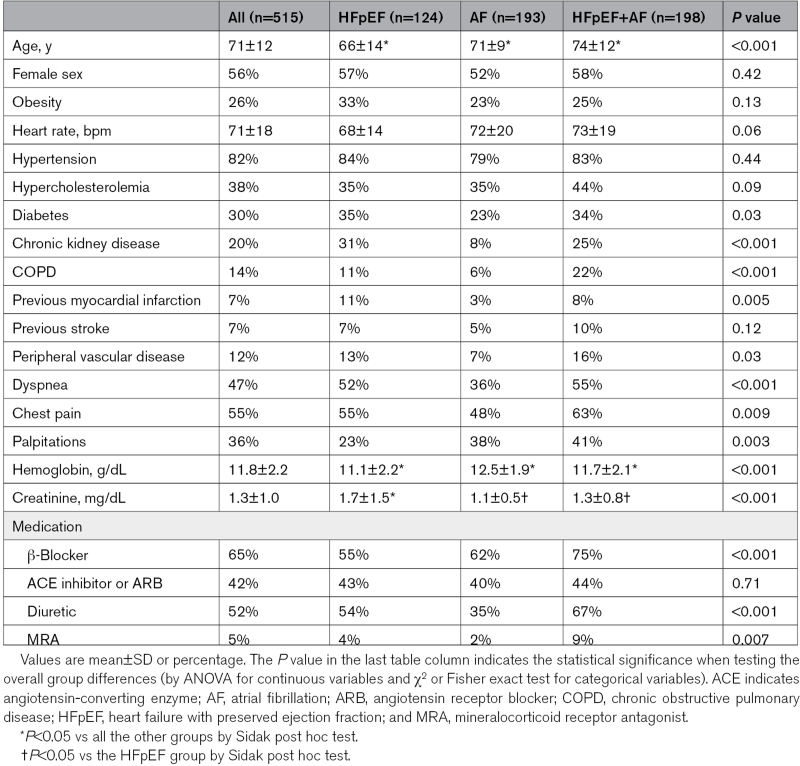
A total of 515 patients with significant AFMR (97% with moderate and 3% with severe AFMR) were included (Figure 1). Among them, 124 (24%) had previously documented HFpEF but no atrial arrythmias, 193 (38%) had a history of AF, and 198 (38%) had both HFpEF and AF (HFpEF+AF). While the groups had comparable distribution of sexes and equally high prevalence of hypertension, patients with HFpEF+AF were significantly older and had higher prevalence of chronic obstructive pulmonary disease and peripheral vascular disease (Table 1). The AF group had less comorbidities including less diabetes, chronic kidney disease, and clinical vascular disease, slightly higher hemoglobin, and lower creatinine than the other 2 groups. Combined HFpEF+AF was associated with a higher prevalence of symptoms at the index examination, particularly dyspnea and chest pain, and these patients used more often β-blockers, diuretics, and mineralocorticoid receptor antagonists (Table 1).
Figure 1.
Study enrollment flowchart. AF indicates atrial fibrillation; AFMR, atrial functional mitral regurgitation; EF, ejection fraction; HFpEF, heart failure with preserved ejection fraction; LV, left ventricle; and MR, mitral regurgitation.
Table 1.
Demographics and Clinical Characteristics
Ventricular Size and Function
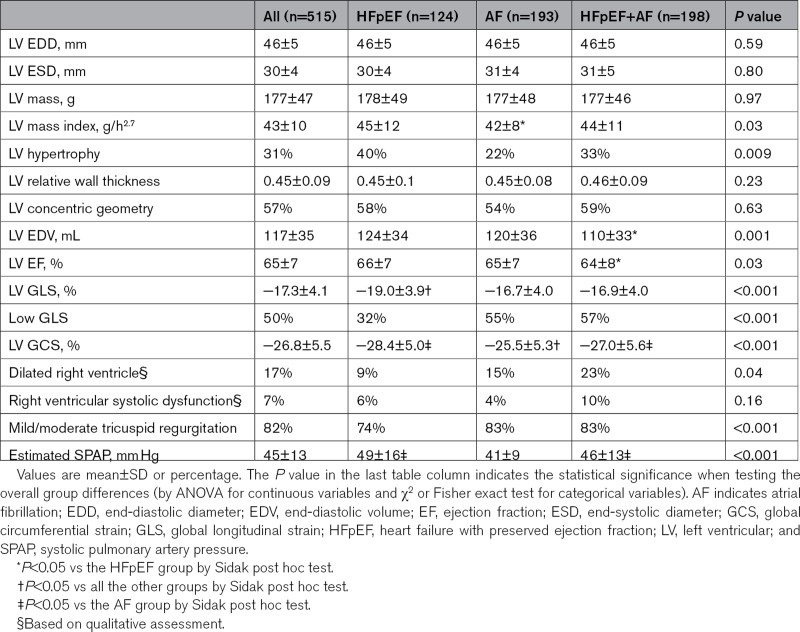
Patients had on average normal LV cavity size, mass, and EF with no between-group differences (Table 2). Concentric LV remodeling was a frequent finding in this study population but with an even distribution among the 3 groups. Of note, LV mass index was similar in HFpEF patients with and without AF, underlining the multifactorial nature of increased LV filling pressure in HFpEF.
Table 2.
Cardiac Structure and Function
LV GLS was on average at the lower limit of normal in the whole population (Table 2). In patients in sinus rhythm during the index echocardiography, the mean LV GLS value was normal and comparable between groups: −18.9±3.9%/−19.1±3.5%/−18.5±3.8% in the HFpEF/AF/HFpEF+AF group, respectively. Considering only patients with AF at the index examination, LV GLS was similarly impaired in the AF and HFpEF+AF groups: −15.2±3.6% versus −15.7±3.7%. Furthermore, patients with combined HFpEF+AF had more frequently a dilated right ventricle with impaired global systolic function (Table 2).
LA Function and Phenotypes of LA Remodeling
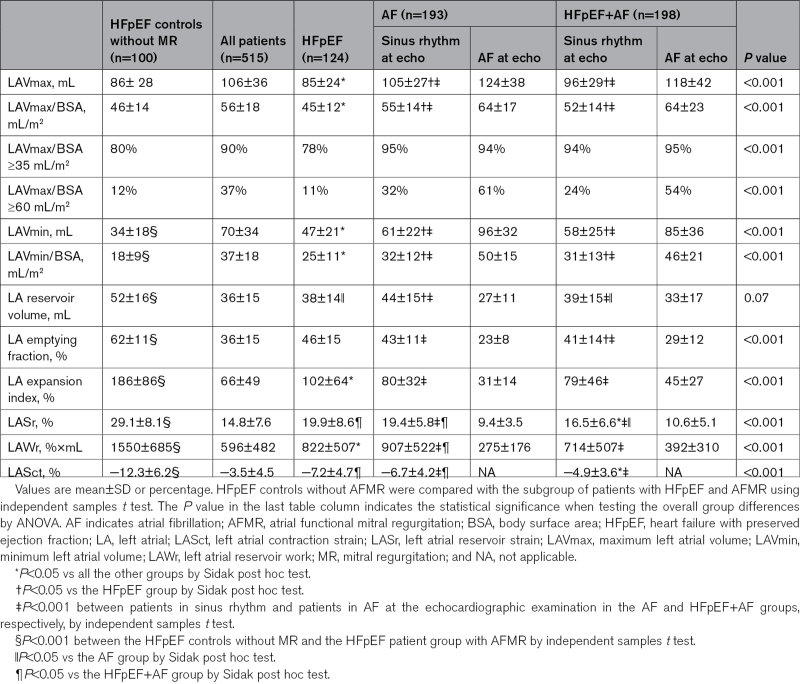
In the whole study population, LAVmax was increased (LAVmax/body surface area, ≥35 mL/m2) in 90% of patients and severely increased (LAVmax/body surface area, ≥60 mL/m2) in 37%. LASr and LAWr were both severely impaired (Table 3). Patients with LASr and LAWr below the median values were older, had a longer AF history, had a higher heart rate, used more often a diuretic, had larger LAVmax and LAVmin, and more often combined HFpEF+AF.
Table 3.
LA Size and Function
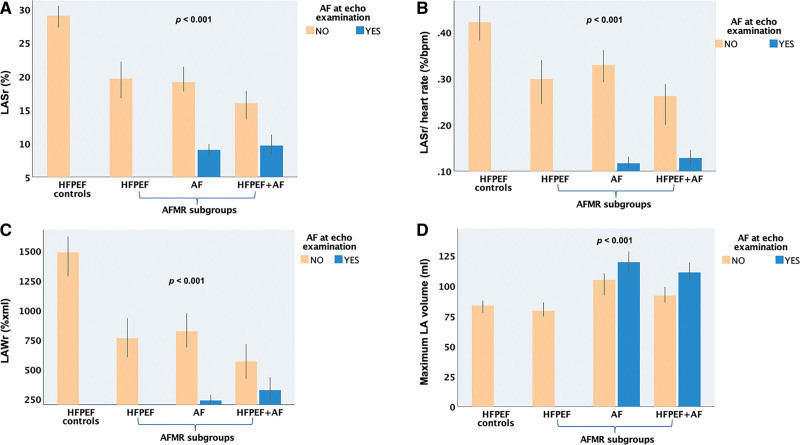
The pattern of LA remodeling differed between the 3 AFMR groups. In the AF and HFpEF+AF groups, patients with AF at the index examination (61% and 59% of the AF and HFpEF+AF groups, respectively) had the largest LAVs of the whole population and concomitantly severely impaired reservoir function by either LASr, LAWr, and LA expansion index (Table 3; Figure 2). When comparing only patients in sinus rhythm at echocardiography, the HFpEF and AF groups had significant differences in LAVmax, LAVmin, and LA reservoir volume, with the HFpEF patients having the smallest LA volumes and the AF group the largest ones. Moreover HFpEF patients had comparable LASr, but lower LASr normalized for heart rate and lower LAWr than the AF group. The HFpEF+AF patients had intermediate LA size but the most severe atrial dysfunction by either LASr or LAWr (Table 3; Figure 2).
Figure 2.
Comparison of atrial function and size between heart failure with preserved ejection fraction (HFpEF) controls and the 3 atrial functional mitral regurgitation (AFMR) subgroups: HFpEF, atrial fibrillation (AF), and HFpEF+AF. A, Left atrial reservoir strain (LASr). B, LASr normalized for heart rate. C, Left atrial reservoir work (LAWr). D, Maximum left atrial (LA) volume. P value for comparison between groups by 1-way ANOVA with Sidak post hoc test.
To assess the contribution of AFMR to atrial remodeling, the AFMR patients were compared to a contemporary cohort of HFpEF subjects with similar sex and age distribution, but with no or only trace MR. LAVmax was comparable between patients with HFpEF with and without MR, but subjects with HFpEF and no MR had smaller LAVmin, larger reservoir volume, and significantly higher LA function by either LASr, LAWr, LA emptying fraction, and expansion index (Table 3; Figure 2).
Impact of Atrial Function on Outcomes
During the median follow-up of 5 (1–17) years, the AFMR patients experienced a high number of adverse events, including 197 (38%) deaths and 153 (30%) first-time HF hospitalizations. Only 14 patients (2.7%) underwent mitral valve surgery. The AF group had lower mortality (24%) and hospitalizations for HF symptoms (20%), while adverse events were most common in the HFpEF+AF group with 49% mortality and 33% first-time HF hospitalizations during the study period (P<0.001).
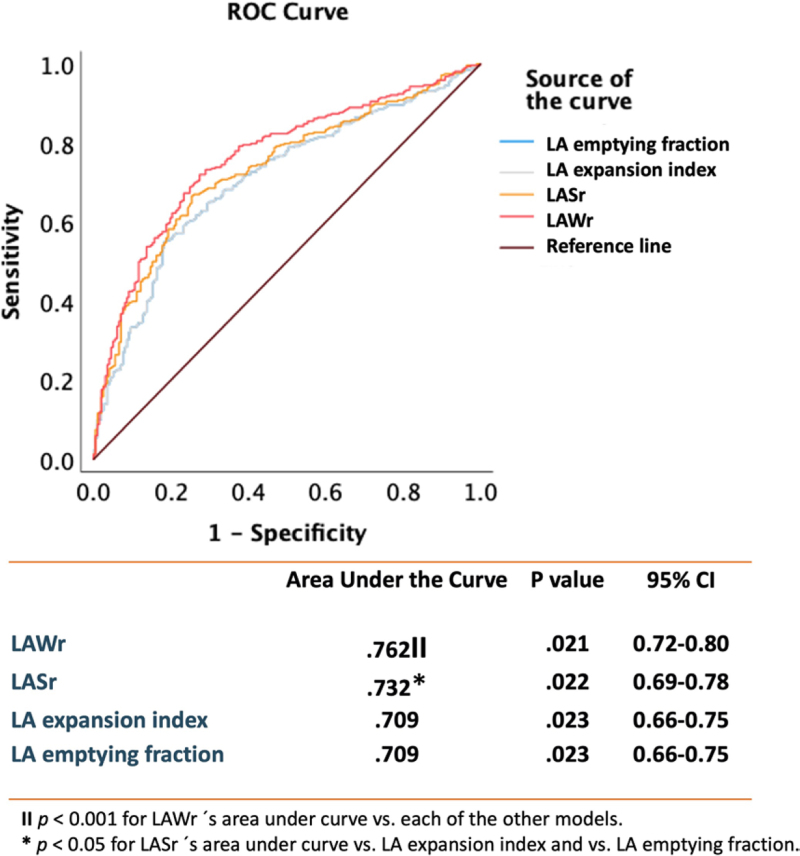
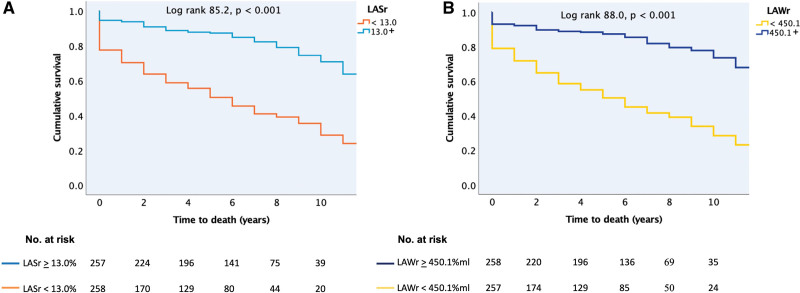
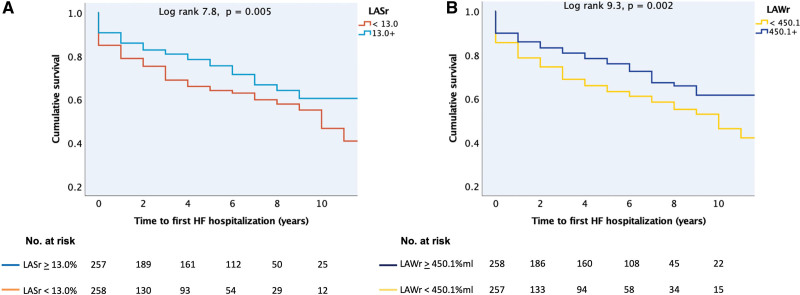
Of all the atrial functional indexes, LAWr followed by LASr had the strongest association with all-cause death (Figure 3). When the whole patient population was grouped according to the median value of either LASr or LAWr, low atrial function by either parameter was strongly associated with higher risk of death over time (Figure 4) and with a higher probability of HF hospitalization (Figure 5).
Figure 3.
Receiver operating characteristic (ROC) analysis showing the strength of the univariable relationship between different indexes of left atrial (LA) reservoir function (LA reservoir work [LAWr], LA reservoir strain [LASr], LA emptying fraction, and LA expansion index) and all-cause death. The table shows the areas under the curve (column 2) with the P value for the association between each index of LA reservoir function and all-cause death (column 3) and the 95% CIs (column 4). Additionally, the P values for the comparison between the areas under the curve of the 4 indexes of LA reservoir function are indicated by the symbols ‖ and *. ‖P<0.001 for LAWr area under the curve vs each of the other models. *P<0.05 for LASr area under the curve vs LA expansion index and vs LA emptying fraction.
Figure 4.
Kaplan-Meier curves for time to all-cause death. A, In patients with left atrial reservoir strain (LASr) less than/greater than or equal to the median value of 13.0%. B, In patients with left atrial reservoir work (LAWr) less than/greater than or equal to the median value of 450.1%×mL. The value of the log-rank test is given for the overall analysis with the respective P value.
Figure 5.
Kaplan-Meier curves for time to first heart failure (HF) hospitalization after inclusion in the study. A, In patients with left atrial reservoir strain (LASr) less than/greater than or equal to the median value of 13.0%. B, In patients with left atrial reservoir work (LAWr) less than/greater than or equal to the median value of 450.1%×mL. The value of the log-rank test is given for the overall analysis with the respective P value.
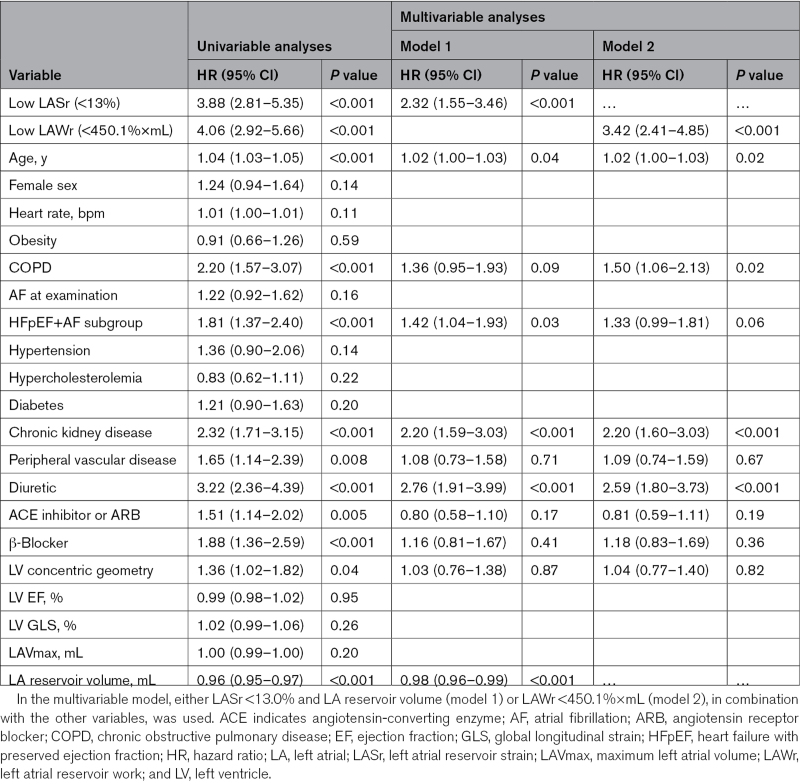
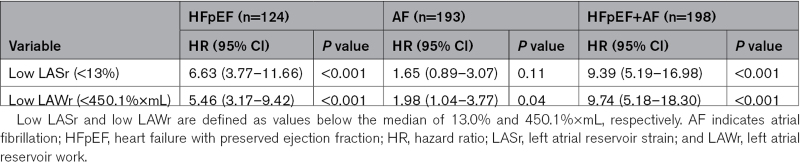
In univariable Cox regression analyses, low LAWr and low LASr predicted a 4.1-fold and 3.9-fold higher risk of all-cause death (Table 4). In subgroup analyses, low LAWr and low LASr were strongly associated with death in the HFpEF+AF group (Table 5). Concentric LV geometry and lower LA reservoir volume, as well as higher age, chronic obstructive pulmonary disease, chronic kidney disease, peripheral vascular disease, HF treatment, or being in the HFpEF+AF subgroup predicted also higher risk of death. Importantly, neither LV function nor LAVmax was associated with outcome in patients with significant AFMR. Having AF at index examination did not predict outcome. After adjustment for these confounders in multivariable models, LAWr below the median value of 450.1%×mL remained the strongest independent predictor caring a 3.4-fold higher risk of death. LARs under the median value of 13% translated into an independent 2.3-fold higher risk of all-cause death (Table 4). When LAVmax was forced into the multivariable models, the results remained unchanged.
Table 4.
Predictors of All-Cause Death in Patients With Moderate and Greater Atrial Functional Mitral Regurgitation
Table 5.
Univariable Cox Analyses of the Association Between Low LASr and Low LAWr and All-Cause Death in Subgroups of Patients With Significant Atrial Functional Mitral Regurgitation
Discussion
In a large cohort of patients with moderate and greater AFMR, we found that AFMR is associated with either AF alone or HFpEF with or without AF. We demonstrate that these commonly AFMR-linked pathologies are associated with partially different patterns of LA remodeling, with more severely dilated atria with higher storage capacity in AFMR patients with AF alone and smaller, stiffer atria in HFpEF. LA function assessed by LASr or LAWr—a simplified index of the passive LA work in the reservoir phase—had a strong impact on patients’ mortality risk after correction for multiple confounders and was a particularly powerful predictor of death in the HFpEF groups. On the contrary, LAVmax—a guideline-recommended criterion for grading and considering intervention in chronic MR—had no impact on outcome in patients with significant AFMR. Finally, LAVmax did not distinguish between HFpEF subjects with or without AFMR, in contrast to atrial function, which was significantly lower in the presence of AFMR.
AFMR Clinical Presentation
Functional MR in patients with normal LV size and EF but dilated LA has received increased clinical attention during the last years due to its escalating prevalence and recognition. AFMR commonly occurs in patients with a history of AF.1,25 Recent American Heart Association/American College of Cardiology and European Society of Cardiology/ European Association for Cardio-Thoracic Surgery guidelines for the management of valvular heart disease describe AFMR as an MR subtype associated with long-standing AF and attribute its etiology to atrial annular dilatation.4,5 However, AFMR has also been described previously in HFpEF patients albeit in a limited number of studies and mostly in patients with mild MR.6,26 In the present cohort, we show that HFpEF is highly associated with AFMR being present in 63% of our patients. Interestingly, LA was rarely severely dilated in the AFMR patients with HFpEF but no history of AF (11% of this group), implying that annular dilation is not the sole mechanisms behind significant MR in this clinical setting.
Phenotypes of Atrial Remodeling in AFMR
AF and HFpEF increase in prevalence with age, have similar risk factors and clinical features, and are physiopathologically linked in complex and still incompletely understood ways.27 In 285 patients with HFpEF, Reddy et al28 described a continuum of disease with increasing AF burden, from none to paroxysmal and then permanent, in parallel with increasing LA myopathy assessed by LASr or LA compliance. In contrast, in AFMR, we did not see a continuum pattern with development of AF signaling an end-stage process but rather distinct phenotypes with important mechanistic insights about the interplay between atrial function and atrial structural remodeling. The AF-alone patients had larger LA reservoir volumes suggesting more compliant atria with higher storage reserves and hence greater accommodation of the AFMR-related LA volume overload. In contrast, the HFpEF-alone patients had lower LA reservoir volumes suggesting smaller, stiffer atria.29 This was associated with higher estimated pulmonary pressures and worse outcomes, denoting the adverse prognostic impact of a noncompliant LA. By cardiac magnetic resonance imaging, myocardial fibrosis has previously been shown to be common in HFpEF patients,30 but its distribution in the cardiac chambers has been less characterized. Our findings suggest that HFpEF patients who develop significant AFMR might be an HFpEF subgroup with excessive fibrosis of the LA.29 This requires, however, further elucidation by histopathological and imaging studies.
Atrial mechanical function was significantly lower in our AFMR patients than in previously published healthy individuals of similar age.31 Additionally, in HFpEF patients without AF, atrial function was lower in those with AFMR than in those without MR from our control group, consistent with previously described cohorts.2
Of note, assessment of both LA and LV strain was significantly influenced by the presence of AF, with impaired LV GLS in patients with AF compared with normal values in those in sinus rhythm at the index examination and concomitantly severely impaired LASr and LAWr. AF causes both loss of atrial contraction and atrial relaxation, and LA function should ideally be assessed in sinus rhythm in patients with still paroxysmal AF and otherwise reported separately for patients in different types of cardiac rhythms at echocardiography.
Atrial Dysfunction and Outcome in AFMR
Reduced atrial deformation in the reservoir phase has recently been identified as a predictor of worse outcome in patients with HF,32 including patients with HFpEF,2 as well as in patients with primary MR referred to mitral valve repair33,34 and patients with secondary MR of ventricular cause.35
Atrial function has not been previously characterized in relation to outcome in AFMR of more than mild degree. This study examined a large AFMR cohort with a high number of adverse events during long-term follow-up, including a 38% mortality. This confirms previous findings of comparable excess mortality in patients with primary MR and AFMR.3 We additionally found that reduced LASr was a predictor of both all-cause death and higher risk of HF hospitalization in patients with AFMR. Moreover, combining LASr and LA reservoir volume in LAWr improved both prediction of death and the distinction between the AFMR phenotypes. As such, low LAWr in AFMR was strongly related to worse survival in patients with HFpEF, highlighting the important negative prognostic impact of LA noncompliance.
Of note, LASr and LAWr performed better in risk prediction analyses than other previously proposed markers of LA function, including the LA expansion index.16
Neither maximum LA size nor LV function as assessed by LV EF and GLS had an impact on prognosis in our study population. This challenges the common belief that LA enlargement is both the cause and the determinant of outcome in AFMR patients.4,36,37
Clinical Implications
AFMR has been etiologically attributed to atrial and annular dilation, with later studies also demonstrating an additional role of incomplete leaflet remodeling and atriogenic posterior leaflet tethering.25,38–40 The role of abnormal atrial mechanical function has not been previously examined. Our study included patients with established moderate or greater AFMR so we could not address the initial cause of the regurgitation. However, in significant AFMR, it is noteworthy that LA dilation, assessed as maximum LA volume at end systole, had no impact on prognosis and that only 37% of the whole cohort had severely dilated LA by standard criteria. Moreover, LAVmax was comparable between HFpEF patients with AFMR and HFpEF controls without MR. So, even if LA and annular dilation might be the kickoff of AFMR, its progression and prognosis are more closely related to atrial dysfunction.
While our data point to the important prognostic impact of low LA function in AFMR, they do not exclude that treating AFMR per se might improve outcomes. Strategies addressing both AFMR, rhythm control, and LA dysfunction such as an atrial shunt device can be considered.41 In our study, common medications that are recommended for HF with reduced EF such as angiotensin receptor blockers, angiotensin-converting enzyme inhibitors, and β-blockers did not have independent prognostic value in AFMR, while the use of diuretics more than doubled the risk of death, perhaps reflecting, at least partially, their use in more advanced HF stages. This might suggest that optimal medical treatment for patients with HF with reduced EF, with demonstrated value in secondary MR of ventricular cause,42,43 does not have the same advantages in AFMR. Catheter AF ablation has previously been shown to reduce LA size and AFMR severity in selected AF patients.37 However, ablations can increase LA stiffness through scar formation,44 and one should probably be particularly careful in using this therapeutic strategy in patients with concomitant AF and HFpEF that have, as our data show, limited atrial expansibility and severe LA dysfunction.
Studies on the relative contribution of atrial dysfunction versus increasing MR volume to symptoms during exercise are lacking in AFMR patients. Exercise testing may unmask stiff LAs or worsening MR in symptomatic AFMR patients.
Study Limitations
The present study is a retrospective follow-up of patients with significant AFMR and has the limitations inherent to this study design. However, it is the largest AFMR cohort characterized clinically and echocardiographically up to this date, and our findings contribute to better understanding of an increasingly prevalent MR subtype. Data on AFMR effective regurgitant orifice area and regurgitation volume were not available for the present analyses; however, the vast majority of the patients (97%) had moderate AFMR based on a comprehensive evaluation done by experienced echocardiologists. History of AF was based on review of all previous electrocardiograms and clinical charts, so one cannot exclude that some HFpEF patients might have had an undocumented AF episode. However, this would only reduce the observed differences between the AFMR phenotypes (bias toward the null). Similarly, even if none of our patients with AF had previous HF hospitalizations and this subgroup had significantly less dyspnea and use of diuretics, some of the patients with AF might have had earlier stages of HFpEF.27 However, this would again only bias our results toward the null. Contemporary controls with AFMR and no MR were not followed up for the same duration of time as AFMR patients. The control group was though only selected for comparison of atrial remodeling between HFpEF with and without AFMR, as LA function in relation to outcome has been previously reported in patients with HFpEF with no significant MR.2,28 Finally, LAWr requires further validation in both experimental and clinical settings.
Conclusions
AFMR develops in patients with AF or HFpEF in whom it is associated with partially different LA remodeling patterns. LA mechanics does not parallel changes in LA size and is severely impaired in significant AFMR and particularly in patients with a history of HFpEF in whom it is a strong predictor of death. Neither maximum LA size nor LV function are significant prognosticators in AFMR. The outcome of patients with AFMR is related to the degree of LA reservoir dysfunction independent of comorbidities and medical treatment.
Article Information
Sources of Funding
Dr Cramariuc received open project support from Helse Vest (project number F-12615) during the conduct of this study. Dr van Kampen has been awarded the American Heart Association Postdoctoral Fellowship Award. Dr Hung was supported, in part, by National Institutes of Health/National Heart, Lung, and Blood Institute 2 RO1HL103723-05. Dr Hung is also the principal investigator of an institutional research grant for core laboratory adjudication from Edwards Lifesciences.
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- AFMR
- atrial functional mitral regurgitation
- EF
- ejection fraction
- GLS
- global left ventricular longitudinal strain
- HF
- heart failure
- HFpEF
- heart failure with preserved ejection fraction
- LA
- left atrium
- LASr
- left atrial reservoir strain
- LAVmax
- maximum left atrial volume
- LAVmin
- minimum left atrial volume
- LAWr
- left atrial reservoir work
- LV
- left ventricle
- MR
- mitral regurgitation
D. Cramariuc and H. Alfraidi contributed equally.
For Sources of Funding and Disclosures, see page 431.
Contributor Information
Dana Cramariuc, Email: dana.cramariuc@gmail.com.
Hassan Alfraidi, Email: haalfraidi@toh.ca.
Yasufumi Nagata, Email: nyasufumi1979@yahoo.co.jp.
Robert A. Levine, Email: rlevine@partners.org.
Antonia van Kampen, Email: AVANKAMPEN@mgh.harvard.edu.
Carl Andrews, Email: CTANDREWS@mgh.harvard.edu.
References
- 1.Muraru D, Guta AC, Ochoa-Jimenez RC, Bartos D, Aruta P, Mihaila S, Popescu BA, Iliceto S, Basso C, Badano LP. Functional regurgitation of atrioventricular valves and atrial fibrillation: an elusive pathophysiological link deserving further attention. J Am Soc Echocardiogr. 2020;33:42–53. doi: 10.1016/j.echo.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 2.Tamargo M, Obokata M, Reddy YNV, Pislaru SV, Lin G, Egbe AC, Nishimura RA, Borlaug BA. Functional mitral regurgitation and left atrial myopathy in heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22:489–498. doi: 10.1002/ejhf.1699 [DOI] [PubMed] [Google Scholar]
- 3.Dziadzko V, Dziadzko M, Medina-Inojosa JR, Benfari G, Michelena HI, Crestanello JA, Maalouf J, Thapa P, Enriquez-Sarano M. Causes and mechanisms of isolated mitral regurgitation in the community: clinical context and outcome. Eur Heart J. 2019;40:2194–2202. doi: 10.1093/eurheartj/ehz314 [DOI] [PubMed] [Google Scholar]
- 4.Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, et al. ; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395 [DOI] [PubMed] [Google Scholar]
- 5.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923 [DOI] [PubMed] [Google Scholar]
- 6.Deferm S, Bertrand PB, Verbrugge FH, Verhaert D, Rega F, Thomas JD, Vandervoort PM. Atrial functional mitral regurgitation: JACC review topic of the week. J Am Coll Cardiol. 2019;73:2465–2476. doi: 10.1016/j.jacc.2019.02.061 [DOI] [PubMed] [Google Scholar]
- 7.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 8.Thomas L, Muraru D, Popescu BA, Sitges M, Rosca M, Pedrizzetti G, Henein MY, Donal E, Badano LP. Evaluation of left atrial size and function: relevance for clinical practice. J Am Soc Echocardiogr. 2020;33:934–952. doi: 10.1016/j.echo.2020.03.021 [DOI] [PubMed] [Google Scholar]
- 9.Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, D’Hooge J, Donal E, Fraser AG, Marwick T, et al. ; Industry representatives. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19:591–600. doi: 10.1093/ehjci/jey042 [DOI] [PubMed] [Google Scholar]
- 10.Cameli M, Sparla S, Losito M, Righini FM, Menci D, Lisi M, D’Ascenzi F, Focardi M, Favilli R, Pierli C, et al. Correlation of left atrial strain and Doppler measurements with invasive measurement of left ventricular end-diastolic pressure in patients stratified for different values of ejection fraction. Echocardiogr. 2016;33:398–405. doi: 10.1111/echo.13094 [DOI] [PubMed] [Google Scholar]
- 11.Inoue K, Khan FH, Remme EW, Ohte N, Garcia-Izquierdo E, Chetrit M, Monivas-Palomero V, Mingo-Santos S, Andersen OS, Gude E, et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur Heart J Cardiovasc Imaging. 2021;23:61–70. doi: 10.1093/ehjci/jeaa415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popescu BA, Beladan CC, Nagueh SF, Smiseth OA. How to assess left ventricular filling pressures by echocardiography in clinical practice. Eur Heart J Cardiovasc Imaging. 2022;23:1127–1129. doi: 10.1093/ehjci/jeac123 [DOI] [PubMed] [Google Scholar]
- 13.Dermlim A, Osuga T, Nakamura K, Morita T, Nisa K, Sasaoka K, Leela-Arporn R, Nagata N, Tamura M, Sasaki N, et al. Effect of acute volume loading on left atrial strain values derived from two-dimensional speckle tracking echocardiography in dogs. J Vet Med Sci. 2019;81:949–957. doi: 10.1292/jvms.19-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue K, Kawakami H, Akazawa Y, Higashi H, Higaki T, Yamaguchi O. Echocardiographic assessment of atrial function: from basic mechanics to specific cardiac diseases. J Cardiovasc Dev Dis. 2022;9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonnenblick EH. Force-velocity relations in mammalian heart muscle. Am J Physiol. 1962;202:931–939. doi: 10.1152/ajplegacy.1962.202.5.931 [DOI] [PubMed] [Google Scholar]
- 16.Genovese D, Muraru D, Marra MP, Carrer A, Previtero M, Palermo C, Tarantini G, Parati G, Iliceto S, Badano LP. Left atrial expansion index for noninvasive estimation of pulmonary capillary wedge pressure: a cardiac catheterization validation study. J Am Soc Echocardiogr. 2021;34:1242–1252. doi: 10.1016/j.echo.2021.07.009 [DOI] [PubMed] [Google Scholar]
- 17.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 18.Smiseth OA, Morris DA, Cardim N, Cikes M, Delgado V, Donal E, Flachskampf FA, Galderisi M, Gerber BL, Gimelli A, et al. ; Reviewers: This document was reviewed by members of the 2018–2020 EACVI Scientific Documents Committee. Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2022;23:e34–e61. doi: 10.1093/ehjci/jeab154 [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 20.de Simone G, Izzo R, Chinali M, De Marco M, Casalnuovo G, Rozza F, Girfoglio D, Iovino GL, Trimarco B, De Luca N. Does information on systolic and diastolic function improve prediction of a cardiovascular event by left ventricular hypertrophy in arterial hypertension? Hypertension. 2010;56:99–104. doi: 10.1161/HYPERTENSIONAHA.110.150128 [DOI] [PubMed] [Google Scholar]
- 21.Grymyr LMD, Nadirpour S, Gerdts E, Nedrebo BG, Hjertaas JJ, Matre K, Cramariuc D. Left ventricular myocardial oxygen demand and subclinical dysfunction in patients with severe obesity referred for bariatric surgery. Nutr Metab Cardiovasc Dis. 2021;31:666–674. doi: 10.1016/j.numecd.2020.10.009 [DOI] [PubMed] [Google Scholar]
- 22.Grymyr LMD, Nadirpour S, Gerdts E, Nedrebo BG, Hjertaas JJ, Matre K, Cramariuc D. One-year impact of bariatric surgery on left ventricular mechanics: results from the prospective FatWest study. Eur Heart J Open. 2021;1:oeab024. doi: 10.1093/ehjopen/oeab024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto T, Dulgheru R, Bernard A, Ilardi F, Contu L, Addetia K, Caballero L, Akhaladze N, Athanassopoulos GD, Barone D, et al. Echocardiographic reference ranges for normal left ventricular 2D strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2017;18:833–840. doi: 10.1093/ehjci/jex140 [DOI] [PubMed] [Google Scholar]
- 25.Kagiyama N, Mondillo S, Yoshida K, Mandoli GE, Cameli M. Subtypes of atrial functional mitral regurgitation: imaging insights into their mechanisms and therapeutic implications. JACC Cardiovasc Imaging. 2020;13:820–835. doi: 10.1016/j.jcmg.2019.01.040 [DOI] [PubMed] [Google Scholar]
- 26.Ennezat PV, Marechaux S, Pibarot P, Le Jemtel TH. Secondary mitral regurgitation in heart failure with reduced or preserved left ventricular ejection fraction. Cardiology. 2013;125:110–117. doi: 10.1159/000350356 [DOI] [PubMed] [Google Scholar]
- 27.Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. 2016;68:2217–2228. doi: 10.1016/j.jacc.2016.08.048 [DOI] [PubMed] [Google Scholar]
- 28.Reddy YNV, Obokata M, Verbrugge FH, Lin G, Borlaug BA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol. 2020;76:1051–1064. doi: 10.1016/j.jacc.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cameli M, Lisi M, Righini FM, Massoni A, Natali BM, Focardi M, Tacchini D, Geyer A, Curci V, Di Tommaso C, et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. 2013;111:595–601. doi: 10.1016/j.amjcard.2012.10.049 [DOI] [PubMed] [Google Scholar]
- 30.Sweeney M, Corden B, Cook SA. Targeting cardiac fibrosis in heart failure with preserved ejection fraction: mirage or miracle? EMBO Mol Med. 2020;12:e10865. doi: 10.15252/emmm.201910865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen AB, Skaarup KG, Hauser R, Johansen ND, Lassen MCH, Jensen GB, Schnohr P, Mogelvang R, Biering-Sorensen T. Normal values and reference ranges for left atrial strain by speckle-tracking echocardiography: the Copenhagen City Heart Study. Eur Heart J Cardiovasc Imaging. 2021;23:42–51. doi: 10.1093/ehjci/jeab201 [DOI] [PubMed] [Google Scholar]
- 32.Bouwmeester S, van der Stam JA, van Loon SLM, van Riel NAW, Boer AK, Dekker LR, Scharnhorst V, Houthuizen P. Left atrial reservoir strain as a predictor of cardiac outcome in patients with heart failure: the HaFaC cohort study. BMC Cardiovasc Disord. 2022;22:104. doi: 10.1186/s12872-022-02545-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stassen J, van Wijngaarden AL, Butcher SC, Palmen M, Herbots L, Bax JJ, Delgado V, Ajmone Marsan N. Prognostic value of left atrial reservoir function in patients with severe primary mitral regurgitation undergoing mitral valve repair. Eur Heart J Cardiovasc Imaging. 2022;24:142–151. doi: 10.1093/ehjci/jeac058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Debonnaire P, Leong DP, Witkowski TG, Al Amri I, Joyce E, Katsanos S, Schalij MJ, Bax JJ, Delgado V, Marsan NA. Left atrial function by two-dimensional speckle-tracking echocardiography in patients with severe organic mitral regurgitation: association with guidelines-based surgical indication and postoperative (long-term) survival. J Am Soc Echocardiogr. 2013;26:1053–1062. doi: 10.1016/j.echo.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 35.Stassen J, Namazi F, van der Bijl P, van Wijngaarden SE, Kamperidis V, Marsan NA, Delgado V, Bax JJ. Left atrial reservoir function and outcomes in secondary mitral regurgitation. J Am Soc Echocardiogr. 2022;35:477–485.e3. doi: 10.1016/j.echo.2022.01.007 [DOI] [PubMed] [Google Scholar]
- 36.Gertz ZM, Raina A, Mountantonakis SE, Zado ES, Callans DJ, Marchlinski FE, Keane MG, Silvestry FE. The impact of mitral regurgitation on patients undergoing catheter ablation of atrial fibrillation. Europace. 2011;13:1127–1132. doi: 10.1093/europace/eur098 [DOI] [PubMed] [Google Scholar]
- 37.Gertz ZM, Raina A, Saghy L, Zado ES, Callans DJ, Marchlinski FE, Keane MG, Silvestry FE. Evidence of atrial functional mitral regurgitation due to atrial fibrillation: reversal with arrhythmia control. J Am Coll Cardiol. 2011;58:1474–1481. doi: 10.1016/j.jacc.2011.06.032 [DOI] [PubMed] [Google Scholar]
- 38.Dal-Bianco JP, Aikawa E, Bischoff J, Guerrero JL, Handschumacher MD, Sullivan S, Johnson B, Titus JS, Iwamoto Y, Wylie-Sears J, et al. Active adaptation of the tethered mitral valve: insights into a compensatory mechanism for functional mitral regurgitation. Circulation. 2009;120:334–342. doi: 10.1161/CIRCULATIONAHA.108.846782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silbiger JJ. Does left atrial enlargement contribute to mitral leaflet tethering in patients with functional mitral regurgitation? Proposed role of atriogenic leaflet tethering. Echocardiogr. 2014;31:1310–1311. doi: 10.1111/echo.12629 [DOI] [PubMed] [Google Scholar]
- 40.Mesi O, Gad MM, Crane AD, Ramchand J, Puri R, Layoun H, Miyasaka R, Gillinov MA, Wierup P, Griffin BP, et al. Severe atrial functional mitral regurgitation: clinical and echocardiographic characteristics, management and outcomes. JACC Cardiovasc Imaging. 2021;14:797–808. doi: 10.1016/j.jcmg.2021.02.008 [DOI] [PubMed] [Google Scholar]
- 41.Shah SJ, Borlaug BA, Chung ES, Cutlip DE, Debonnaire P, Fail PS, Gao Q, Hasenfuss G, Kahwash R, Kaye DM, et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet. 2022;399:1130–1140. doi: 10.1016/S0140-6736(22)00016-2 [DOI] [PubMed] [Google Scholar]
- 42.Solomon SD, Skali H, Anavekar NS, Bourgoun M, Barvik S, Ghali JK, Warnica JW, Khrakovskaya M, Arnold JM, Schwartz Y, et al. Changes in ventricular size and function in patients treated with valsartan, captopril, or both after myocardial infarction. Circulation. 2005;111:3411–3419. doi: 10.1161/CIRCULATIONAHA.104.508093 [DOI] [PubMed] [Google Scholar]
- 43.Kang DH, Park SJ, Shin SH, Hong GR, Lee S, Kim MS, Yun SC, Song JM, Park SW, Kim JJ. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. 2019;139:1354–1365. doi: 10.1161/CIRCULATIONAHA.118.037077 [DOI] [PubMed] [Google Scholar]
- 44.Packer M. Effect of catheter ablation on pre-existing abnormalities of left atrial systolic, diastolic, and neurohormonal functions in patients with chronic heart failure and atrial fibrillation. Eur Heart J. 2019;40:1873–1879. doi: 10.1093/eurheartj/ehz284 [DOI] [PMC free article] [PubMed] [Google Scholar]