OBJECTIVES:
In Asian populations, the correlation between sepsis outcomes and body mass is unclear. A multicenter, prospective, observational study conducted between September 2019 and December 2020 evaluated obesity’s effects on sepsis outcomes in a national cohort.
SETTING:
Nineteen tertiary referral hospitals or university-affiliated hospitals in South Korea.
PATIENTS:
Adult patients with sepsis (n = 6,424) were classified into obese (n = 1,335) and nonobese groups (n = 5,089).
MEASUREMENTS AND RESULTS:
Obese and nonobese patients were propensity score-matched in a ratio of 1:1. Inhospital mortality was the primary outcome. After propensity score matching, the nonobese group had higher hospital mortality than the obese group (25.3% vs 36.7%; p < 0.001). The obese group had a higher home discharge rate (70.3% vs 65.2%; p < 0.001) and lower median Clinical Frailty Scale (CFS) (4 vs 5; p = 0.007) at discharge than the nonobese group, whereas the proportion of frail patients at discharge (CFS ≥ 5) was significantly higher in the nonobese group (48.7% vs 54.7%; p = 0.011). Patients were divided into four groups according to the World Health Organization body mass index (BMI) classification and performed additional analyses. The adjusted odds ratio of hospital mortality and frailty at discharge for underweight, overweight, and obese patients relative to normal BMI was 1.25 (p = 0.004), 0.58 (p < 0.001), and 0.70 (p = 0.047) and 1.53 (p < 0.001), 0.80 (p = 0.095), and 0.60 (p = 0.022), respectively.
CONCLUSIONS:
Obesity is associated with higher hospital survival and functional outcomes at discharge in Asian patients with sepsis.
Keywords: frailty, mortality, obesity, outcome, sepsis
KEY POINTS.
Question: Does obesity benefit survival and function at discharge in Asian patients with sepsis?
Findings: The obese group had a higher survival rate and a better functional status at discharge.
Meaning: Although Asian patients with sepsis have higher weight-related disease risks at a lower BMI than other races, obesity was associated not only with survival but also with better functional outcomes at discharge.
The prevalence of obesity has increased rapidly over the past few decades worldwide (1). Although obesity is a risk factor for health problems such as cardiovascular disease, it has a paradoxical effect on some diseases, especially in critically ill patients (2–4). Recently, the obesity paradox has been widely reported in patients with sepsis (5–8). Systematic reviews have suggested that patients with an increased body mass index (BMI) have improved survival following sepsis (6–9). However, these studies were conducted in Western populations, and data on Asian populations with different criteria for obesity are limited (10). Asians have a higher percentage of body fat and lower muscle mass for the same level of BMI than the Western populations (10). Therefore, the optimal cutoff for obesity in Asian populations is different from that in Western populations (10).
Several studies from East Asia have reported that patients with a low BMI had increased mortality, but no obesity paradox was reported (11–14). They demonstrated that malnutrition, and not the obesity paradox, is critical to sepsis. One possibility for such a discrepancy is the relatively lower distribution of high BMI in Asian populations than in Western populations (15). Compared with Western countries, such as the United States, Asian countries have considerably lower rates of overweight and obesity (15). When compared with the United States, the rate of obesity, defined as a BMI greater than 30 kg/m2, is nearly five to 15 times lower in Asian populations. Consequently, the effects of obesity on clinical outcomes may have been underestimated. Therefore, the obesity paradox of sepsis in Asians remains controversial and has not yet been fully investigated.
Many septic survivors experience severe weakness, weight loss, muscle loss, and functional decline upon discharge (16). Survival from sepsis remains challenging, and survivors often require long hospital stays and suffer from frailty and long-term morbidity. Therefore, obese patients may have more nutritional reserves than nonobese patients. We hypothesized that obese patients would be less vulnerable to sepsis-related outcomes than nonobese patients. In this prospective observational study, we compared the survival and postsurvival functional statuses between obese and nonobese patients using propensity score analysis.
MATERIALS AND METHODS
Study Population
Adult patients greater than or equal to 19 years old who fulfilled the diagnostic criteria for sepsis and septic shock according to the Third International Consensus Definition for Sepsis and Septic Shock (Sepsis-3) were prospectively recruited between September 2019 and December 2020 from the national multicenter registry of the Korean Sepsis Alliance (17). Overall, 19 tertiary- or university-affiliated hospitals with educational programs on sepsis bundles in South Korea participated in this study. An employee at the principal institution (Asan Medical Center) conducted regular audits to verify the quality of the reported data. This study was approved by the institutional review boards of each hospital, including the Pusan National University Yangsan Hospital Institutional Review Board (approval number: 05–2019–092; approval date: July 11, 2019). The requirement for informed consent was waived due to the minimal risk associated with a standard-of-care observational study with no interventions. The procedures were followed in accordance with the ethical standards of the responsible committee of each hospital on human experimentation and the Helsinki Declaration of 1975.
Data Collection
Patients admitted to the general ward or emergency department during the study period were screened for eligibility (Appendix, http://links.lww.com/CCM/H291). Sepsis was defined according to the following criteria: a probable or confirmed diagnosis of infection and a change in the total Sequential Organ Failure Assessment (SOFA) score greater than or equal to 2 after infection. Septic shock was characterized by persistent arterial hypotension that required vasopressors to maintain a mean arterial pressure of greater than or equal to 65 mm Hg and serum lactate levels of greater than 2 mmol/L despite fluid resuscitation. All patients were followed until death or hospital discharge. The study coordinators at each participating center prospectively collected data using an electronic case report form (http://sepsis.crf.kr/). The following information was collected: demographic data, including age and sex; comorbidities and disease severity (SOFA score, hemodynamics, and laboratory variables at baseline); infection source and type (community- and hospital-acquired); multidrug-resistant pathogens in patients with positive cultures; treatment data, such as the adequacy of empirical antibiotic therapy; and ICU admissions, resource use, and outcome data, including ICU, 28-day, and hospital mortality rates. The Clinical Frailty Scale (CFS) was collected to evaluate functional status at hospitalization and discharge. CFS is a well-validated 9-point scale that ranges from 1 (very fit) to 9 (terminally ill), with higher scores indicating more severe frailty. The presence of frailty was divided according to CFS scores: frail (CFS score 5–9) versus nonfrail (CFS score 1–4). The Simplified Acute Physiology Score 3 was evaluated in ICU patients at the time of ICU admission, along with any medical events that occurred in the ICU. Multidrug resistance was defined as resistance to agents of greater than or equal to three antimicrobial categories (18). The adequacy of empirical therapy was determined based on the results of drug susceptibility testing or recommendations of relevant guidelines (19).
Data Analyses
Patients without height and weight data were excluded from the analysis. The patients were divided into two groups according to their initial BMI at admission: obese (≥25 kg/m2) and nonobese (<25 kg/m2). Clinical and laboratory variables and disease severity were compared between the groups. We used the propensity score as a balancing score to adjust for confounding variables in determining whether obesity was associated with worse clinical outcomes in patients with sepsis. Therefore, we calculated the propensity score for obesity for each patient using a multivariable logistic regression model to estimate the probability of disease assignment based on the following baseline covariates: age, sex, comorbidities, Charlson comorbidity index (CCI), CFS, SOFA score, presence of septic shock, the primary site of infection, and type of infection. Obese and nonobese patients were then paired at a ratio of 1:1 based on propensity scores using nearest neighbor matching without replacement and an optimal caliper (0.1 standard deviations of the propensity score). The primary outcome was the difference in inhospital mortality between the two groups. The secondary outcomes were differences in home discharge, CFS score at discharge, and frailty at discharge between the two groups. Furthermore, we divided the patients into four groups according to the World Health Organization BMI (WHO BMI) classification and reconfirmed the difference in primary and secondary outcomes: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2).
Statistical Analysis
Continuous variables are presented as mean ± sd or median and interquartile range depending on data distribution and were compared using Student t test or Mann-Whitney U test, as appropriate. Categorical variables are presented as frequency and percentage and were compared using the chi-square test or Fisher exact test, as appropriate. We performed a propensity score-matched (1:1) analysis to compare the obese and nonobese patients. Propensity score matching was performed based on sex, age, comorbidities, CCI, CFS, SOFA score, presence of septic shock, primary infection site, and type of infection. The risk-adjusted survival curve was plotted using the proportional hazards model and the mean of the covariate method. For comparing the four groups according to the WHO BMI classification, a 1:1 matched design by propensity score resulted in a considerable reduction in the sample size. Alternatively, we conducted logistic regression including the baseline covariates to control for potential confounding effects, where all 6,424 patients were included. To utilize the matching information by the propensity score, the logistic model also included an assignment variable indicating whether each patient was included in the set of matched samples by the propensity score. We then calculated the adjusted odds ratio (OR) of the BMI group for each outcome based on the logistic regression model, where the normal BMI group was used as the reference category. We also conducted a subgroup analysis that included only those patients admitted to the ICU. A Cox proportional hazard regression model was used to analyze the clinical factors that affected hospital mortality in the ICU subgroup. After univariate analysis, significant factors (p < 0.05) were entered into multivariate analysis with stepwise backward selection. SPSS Version 27.0 (IBM, Armonk, NY) and R software Version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) were used for all statistical analyses, with p value less than 0.05 indicating statistical significance.
RESULTS
Patients’ Characteristics
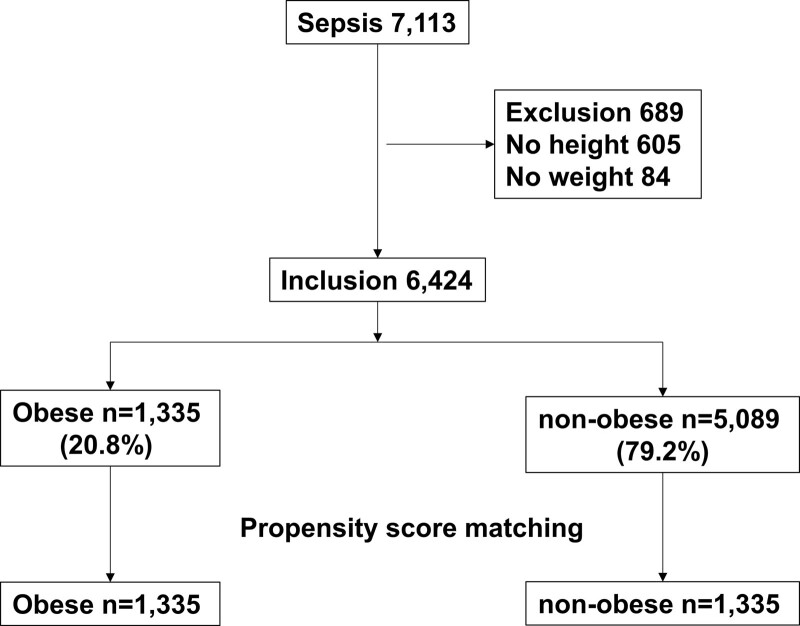
During the study period, 7,113 patients with sepsis were identified in the Korean Sepsis Alliance Registry. Of these, 689 patients without height and weight data were excluded, and 6,424 patients were included in the study (Fig. 1). Before propensity score matching, obesity was noted in 1,335 (20.8%) patients. Significant differences were noted in age, sex, and comorbidities, such as diabetes, chronic lung disease, solid malignancy, chronic neurologic disease, CCI, CFS, and Eastern Cooperative Oncology Group performance status between the obese and nonobese groups (Table 1). Additionally, the SOFA score, ICU admission, type of infection, use of combined antibiotic therapy, source control, and adjunctive corticosteroid therapy were significantly different between the groups (Table 1). After propensity score matching in a 1:1 ratio, no significant difference was found between the two groups except for BMI (Table 1). Other baseline demographic and clinical characteristics, such as physiological variables and pathogen identification, are listed in Table S1 (http://links.lww.com/CCM/H291). No significant difference was found in sepsis bundle compliance between the groups after propensity score matching (Table S2, http://links.lww.com/CCM/H291).
Figure 1.
Patient inclusion in the study. Overall, 6,424 patients were included in the study. At admission, 1,335 (20.8%) patients were obese and 5,089 (79.2%) were nonobese. Patients were divided into obese and nonobese groups according to their body mass index.
TABLE 1.
Baseline Characteristics of Patients in the Study According to Obesity
| Variables | Before Matching | After Matching | ||
|---|---|---|---|---|
| Obese (n = 1,335) | Nonobese (n = 5,089) | Obese (n = 1,335) | Nonobese (n = 1,335) | |
| Age | 70 (60–78) | 73 (62–81)a | 70 (60–78) | 70 (60–79) |
| Male | 699 (52.4) | 3,090 (60.7)a | 699 (52.4) | 726 (54.4) |
| Body mass index, kg/m2 | 27.1 (26–29.1) | 20.6 (18.4–22.5)a | 27.1 (26–29.1) | 20.9 (18.8–22.8)a |
| Comorbidities | ||||
| Cardiovascular disease | 339 (25.4) | 1,298 (25.5) | 339 (25.4) | 323 (24.2) |
| Diabetes | 566 (42.4) | 1,748 (34.3)a | 566 (42.4) | 517 (38.7) |
| Chronic lung disease | 194 (14.5) | 862 (16.9)b | 194 (14.5) | 181 (13.6) |
| Chronic kidney disease | 214 (16.0) | 730 (14.3) | 214 (16.0) | 225 (16.9) |
| Chronic liver disease | 153 (11.5) | 531 (10.4) | 153 (11.5) | 151 (11.3) |
| Solid malignancy | 439 (32.9) | 1,922 (37.8)c | 439 (32.9) | 456 (34.2) |
| Hematological malignancy | 110 (8.2) | 359 (7.1) | 110 (8.2) | 104 (7.8) |
| Connective tissue disease | 24 (1.8) | 131 (2.6) | 24 (1.8) | 29 (2.2) |
| Chronic neurologic disease | 250 (18.7) | 1,179 (23.2)c | 250 (18.7) | 256 (19.2) |
| Charlson comorbidity index | 5 (4–7) | 6 (4–7)c | 5 (4–7) | 5 (4–7) |
| Clinical Frailty Score | 4 (3–6) | 5 (3–7)a | 4 (3–6) | 4 (3–7) |
| Eastern Cooperative Oncology Group performance status | 1 (1–3) | 2 (1–3)a | 1 (1–3) | 2 (1–3) |
| Sequential Organ Failure Assessment | 6 (4–8) | 6 (4–8) | 6 (4–8) | 6 (4–8) |
| Septic shock | 257 (19.3) | 1,093 (21.5) | 257 (19.3) | 276 (20.7) |
| ICU admission | 711 (53.3) | 2,264 (44.5)a | 711 (53.3) | 723 (54.2) |
| Laboratory variables | ||||
| Lactate level, mmol/L | 2.6 (1.5–4.6) | 2.6 (1.5–4.7) | 2.6 (1.5–4.6) | 2.6 (1.6–4.8) |
| C-reactive protein, mg/dL | 11.0 (4.3–20.2) | 11.1 (4.7–19.1) | 11.0 (4.3–20.2) | 11.5 (4.5–20.0) |
| Primary site of infection | ||||
| Pulmonary | 411 (30.8) | 2,292 (45.0) | 411 (30.8) | 465 (34.8) |
| Abdominal | 454 (34.0) | 1,348 (26.5) | 454 (34.0) | 429 (32.1) |
| Urinary | 237 (17.8) | 715 (14.0) | 237 (17.8) | 231 (17.3) |
| Skin or soft tissue | 72 (5.4) | 142 (2.8) | 72 (5.4) | 49 (3.7) |
| Catheter related | 11 (0.8) | 43 (0.8) | 11 (0.8) | 13 (1.0) |
| Neurologic | 10 (0.7) | 28 (0.6) | 10 (0.7) | 6 (0.4) |
| Systemic infections without a primary site | 140 (10.5) | 521 (10.2) | 140 (10.5) | 142 (10.6) |
| Type of infection | ||||
| Community acquired | 1,003 (75.1) | 4,034 (79.3)c | 1,003 (75.1) | 997 (74.7) |
| Nosocomial | 332 (24.9) | 1,055 (20.7) | 332 (24.9) | 338 (25.3) |
| Multidrug-resistant organism | 314 (23.5) | 1,148 (22.6) | 314 (23.5) | 306 (22.9) |
| Combination antibiotic therapy | 790 (59.6) | 3,168 (62.6)b | 790 (59.6) | 827 (62.4) |
| Adequate antimicrobial therapy | 1,188 (89.0) | 4,490 (88.2) | 1,188 (89.0) | 1,178 (88.2) |
| Source control of sepsis | 208 (15.6) | 625 (12.3)c | 208 (15.6) | 207 (15.5) |
| Adjunctive corticosteroid therapy | 255 (19.1) | 891 (17.5) | 255 (19.1) | 265 (19.9) |
p < 0.001,
p < 0.05,
p < 0.01.
Data are presented as median (interquartile range) or number (%).
Primary and Secondary Outcomes in Propensity-Matched Patients
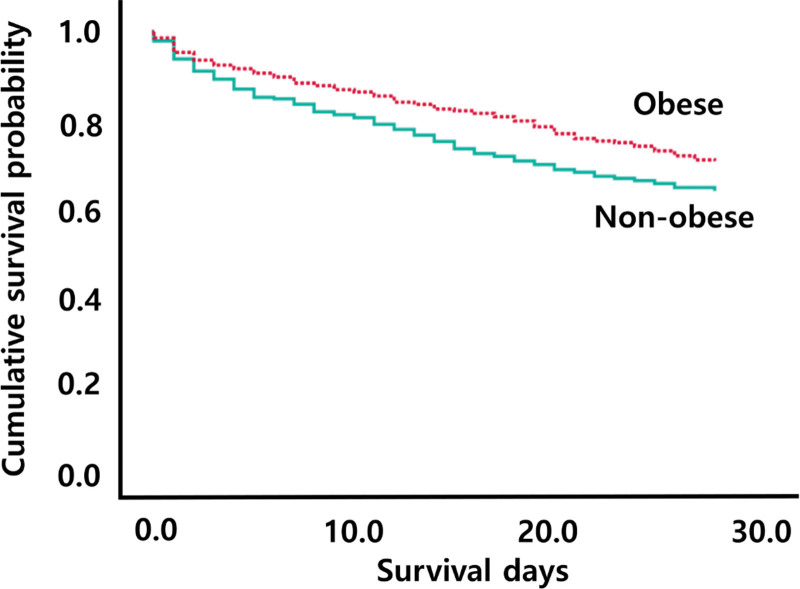
As the primary outcome, inhospital mortality was significantly lower in the obese group than in the nonobese group (25.3% vs 36.7%; p < 0.001) (Table 2). In multivariable Cox regression analysis, obesity was associated with a decreased risk of death (adjusted hazard ratio [HR], 0.78; 95% CI, 0.68–0.90; p = 0.001) (Table S3, http://links.lww.com/CCM/H291). The Kaplan-Meier (KM) curve revealed that mortality at 28 days was significantly lower in obese patients (χ2 = 16.94; p < 0.001; Fig. 2) than in nonobese patients.
TABLE 2.
Clinical Outcomes After Propensity Matching
| Variables | Obese (n = 1,335) | Nonobese (n = 1,335) | p |
|---|---|---|---|
| Primary outcomes | |||
| Hospital mortality | 338 (25.3) | 490 (36.7) | < 0.001 |
| Hospital days | 13 (6–25) | 15 (7–29) | 0.072 |
| Secondary outcomes | |||
| ICU mortality | 178 (24.9) | 223 (30.8) | 0.043 |
| ICU days | 5 (2–11) | 5 (2–12) | 0.162 |
| Home discharge | 701 (70.3) | 551 (65.2) | < 0.001 |
| CFS at discharge | 4 (3–7) | 5 (3–7) | 0.007 |
| Frail (CFS ≥ 5) | 486 (48.7) | 462 (54.7) | 0.011 |
CFS = Clinical Frailty Score.
Data are presented as median (interquartile range) or number (%).
Figure 2.
Kaplan-Meier analysis. Mortality at 28 d according to obesity (χ2 = 16.94; p < 0.001).
Among the secondary outcomes, the home discharge rate was significantly higher in the obese group than in the nonobese group (70.3% vs 65.2%; p < 0.001). The median CFS at discharge was significantly lower in the obese group than in the nonobese group (4 [3–7] vs 5 [3–7], respectively; p = 0.007). The proportion of frail patients at discharge (CFS ≥5) was significantly lower in the obese group than in the nonobese group (48.7% vs 54.7%; p = 0.011).
In-Depth Analysis According to WHO BMI Classification
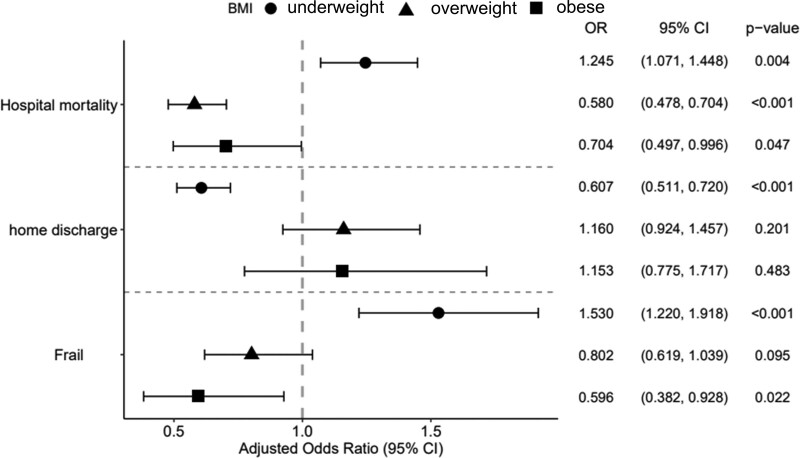
We divided the patients into four groups according to the WHO BMI classification. The adjusted OR of each group for the three study outcomes (hospital mortality, home discharge, and frailty at discharge) is shown in Figure 3. The adjusted OR of hospital mortality was 1.25 (p = 0.004) for underweight, 0.58 (p < 0.001) for overweight, and 0.70 (p = 0.047) for obese relative to normal BMI. The adjusted OR of frailty at discharge was 1.53 (p < 0.001) for underweight, 0.80 (p = 0.095) for overweight, and 0.60 (p = 0.022) for obesity relative to normal BMI. The adjusted OR of home discharge was significantly lower in the underweight group (adjusted OR, 0.61; p < 0.001) relative to normal BMI.
Figure 3.
Adjusted odds ratios according to WHO body mass index (BMI) classification for the three clinical outcomes. The adjusted odds ratio (OR) was calculated from the logistic regression model of the BMI group for each clinical outcome with baseline covariates.
Subgroup Analysis of Propensity-Matched Patients Admitted to the ICU
After propensity score matching, 711 (53.3%) patients in the obese group and 723 (54.2%) patients in the nonobese group were admitted to the ICU. No significant differences were noted in the baseline characteristics between the groups, except for BMI (Table S4, http://links.lww.com/CCM/H291).
The inhospital mortality was significantly lower in the obese group than in the nonobese group (33.2% vs 43.3%; p < 0.001) (Table S5, http://links.lww.com/CCM/H291). In the multivariate Cox regression analysis, obesity was significantly associated with hospital mortality (Table S6, http://links.lww.com/CCM/H291; adjusted HR, 0.83; 95% CI, 0.70–0.98; p = 0.031). The KM curve revealed that the mortality at 28 days was significantly lower in obese patients (χ2 = 8.83; p = 0.003; Fig. S1A, http://links.lww.com/CCM/H291) than in nonobese patients.
The home discharge rate was significantly higher in the obese group than in the nonobese group (70.7% vs 64.1%; p < 0.001). The proportion of frail patients at discharge was significantly lower in the obese group than in the nonobese group (52.2% vs 60.2%; p = 0.016). In the multivariate Cox regression analysis, obesity was significantly associated with home discharge (Table S7, http://links.lww.com/CCM/H291) (adjusted HR, 1.36; 95% CI, 1.15–1.60; p < 0.001). The KM curve revealed that home discharge was significantly higher in obese patients (χ2 = 17.43; p < 0.001; Fig. S1B, http://links.lww.com/CCM/H291) than in nonobese patients.
DISCUSSION
This study demonstrated a paradoxically positive association between obesity and clinical outcomes in patients with sepsis. After adjusting for potential confounders using the propensity score, the obese group had significantly higher survival than the nonobese group. Among the patients who survived, those in the obese group were more likely to be discharged to their home than those in other hospitals or nursing facilities compared with those in the nonobese group. The obese group had lower frailty at discharge than the nonobese group. The obesity paradox phenomenon exists in sepsis, not only in terms of survival but also in functional outcomes at discharge.
Obesity is known to reduce overall life expectancy and results in chronic inflammation, which leads to many health problems and chronic diseases (20). It is also associated with an increased risk of infection and sepsis (21–25). In Western population-based studies, the obesity paradox has been widely evaluated, and it has been reported that obesity plays a protective role in sepsis (5–9, 26). However, it has not been fully evaluated in the Asian populations, and there are conflicting data on the relationship between obesity and sepsis outcomes (11–14, 27, 28). Most Asian studies on the effects of BMI on sepsis outcomes have focused on the underweight population (11–14). They suggested that a low BMI negatively affected sepsis outcomes. However, critically ill obese individuals have not been fully evaluated in terms of their health outcomes due to sepsis. Given the growing trend of obesity in Asia, further investigations are warranted (29).
We performed a nationwide prospective observational study and found that obesity was associated with better hospital survival and functional outcomes in sepsis. Several factors should be considered when investigating the effects of obesity on sepsis outcomes in Asia. First, compared with the U.S. population (BMI <18.5 kg/m2, 5.8%; BMI ≥25 kg/m2, 62.8%), the underweight ratio is high, and the obesity ratio is low in Asians (30). Particularly, BMI greater than or equal to 30 kg/m2 accounted for 3.6% of our cohort, and morbid obesity was rare. Another point to note is that the criteria for obesity differ from those in the Western population. Asians have a higher percentage of body fat and low muscle mass for the same level of BMI than other races (10). Asians have 3–5% higher total body fat than Western populations with the same BMI (31). South Asians have particularly high levels of body fat and are more prone to developing abdominal obesity, which may account for their high risk of type 2 diabetes and cardiovascular diseases (32). In other words, Asians have a higher weight-related disease risk at a lower BMI than other races. Increases in weight over time are more harmful in Asians than in other ethnic groups (33). Confirming whether obesity has a paradoxical beneficial effect on Asians has important implications.
In this study, the organ failure state of the patients admitted to the ICU revealed an interesting course over time (Table S8, http://links.lww.com/CCM/H291). Although there was no difference between the initial SOFA score and serum lactic acid level, the degree of organ failure after 3 days in the ICU was more severe in the nonobese group than in the obese group. Notably, we found a significant difference in the requirement for mechanical ventilator support during the ICU stay between the two groups (51.3% vs 61%; p < 0.001; Table S9, http://links.lww.com/CCM/H291). Although not statistically significant, the duration of mechanical ventilation (6 vs 7 d; p = 0.550; Table S9, http://links.lww.com/CCM/H291) and the rate of tracheostomy at discharge from the ICU were lower in the obese group than in the nonobese group (8.1% vs 12%; p = 0.035; Table S9, http://links.lww.com/CCM/H291). This may be closely related to the low frailty scores at the discharge of obese patients. Many patients with sepsis are at risk of severe loss of muscle mass and quality (16, 34). Patients who become frail after sepsis may have an increased risk of extubation failure, may more frequently require a tracheostomy, and may have difficulties weaning from a ventilator (35, 36). Obesity can prevent muscle wasting and weakness in sepsis and demonstrates a different metabolic response to sepsis in comparison with lean patients by preserving muscle mass while losing fat mass (37, 38). Excess body fat may serve as a protective reservoir of energy to prevent muscle loss secondary to a highly catabolic state during sepsis (39). Additionally, obesity may modulate immune responses during sepsis through differential expression of adipokines and may protect patients from sepsis by increasing the sequestration of lipopolysaccharides from adipose tissue through very low-density lipoprotein receptors (40, 41).
Obesity has recently been associated with a high risk of mortality in COVID-19 patients (42, 43). However, results concerning the association between BMI and mortality from COVID-19 are conflicting (44–47). Although studies at the beginning of this pandemic suggested a negative impact of obesity on COVID-19 disease, the results did not show significant differences over time (44). Additionally, previous meta-analyses have some limitations that can be generalized and compared with our results (42, 43). These studies have methodological limitations, such as the heterogeneity of some analyses, selection bias, and inadequate adjustment for confounding variables. Furthermore, the data from those studies were collected during the early pandemic, when the critical care system was not fully provided to COVID-19 sepsis patients. Therefore, the role of obesity in COVID-19 remains controversial. Further research is required to analyze the effects of obesity on COVID-19 outcomes.
Our study had some limitations. First, the nature of this subject did not allow for a prospective and controlled design to evaluate the actual impact of obesity on the clinical outcomes of sepsis. However, we adjusted for all potential confounding variables, including age, sex, comorbidity, CCI, CFS, severity, site of infection, and treatment strategy, which could affect prognosis. Additional analysis was performed by further subdividing the patients into four groups according to the WHO BMI classification, and the primary and secondary outcomes were reconfirmed. Second, this study did not include information regarding long-term outcomes such as quality of life, disability, readmission, and 1-year mortality. These outcomes may serve as indirect surrogates for the protective role of obesity in sepsis. Finally, because our analysis utilized baseline BMI measurements, we could not evaluate the effects of BMI changes over time.
CONCLUSIONS
Although the outcomes of sepsis continue to improve with advances in critical care support, sepsis still affects daily life. Obesity is closely associated not only with survival but also with functional outcomes. Sepsis survivors, even those who return home, frequently experience postintensive care syndrome and chronic physical disabilities. Further studies are warranted to determine the long-term effects of obesity, including on the survivor’s quality of life.
ACKNOWLEDGMENTS
The following persons and institutions participated in the Korean Sepsis Alliance (KSA): Steering Committee—Chae-Man Lim (Chair), Sang-Bum Hong, Dong Kyu oh, Gee Young Suh, Kyeongman Jeon, Ryoung-Eun Ko, Young-Jae Cho, Yeon Joo Lee, Sung Yoon Lim, and Sunghoon Park; Participated Persons and Centers—Kangwon National University Hospital—JeongwonHeo; Korea University Anam Hospital—Jae-myeong Lee; Daegu Catholic University Hospital—Kyung Chan Kim; Seoul National University Bundang Hospital—Yeon Joo Lee; Inje University Sanggye Paik Hospital—Youjin Chang; Samsung Medical Center—Kyeongman Jeon; Seoul National University Hospital—Sang-Min Lee; Asan Medical Center—Chae-Man Lim, Suk-Kyung Hong; Pusan National University Yangsan Hospital—Woo Hyun Cho; Chonnam National University Hospital—Sang Hyun Kwak; Jeonbuk National University Hospital—Heung Bum Lee; Ulsan University Hospital—Jong-Joon Ahn; Jeju National University Hospital—Gil MyeongSeong; Chungnam National University Hospital—Jae Young Moon; Hallym University Sacred Heart Hospital—Sunghoon Park; Hanyang University Guri Hospital—Tai Sun Park.
Supplementary Material
Footnotes
Dr. Yeo helped in conceptualization. Dr. Cho helped in methodology. Drs. Yeo, Kim, Jang, Park, and Oh helped in data curation and resources. Drs. Yeo and Kim helped in investigation and formal analysis. Drs. Yeo and Cho helped in writing—original draft. Dr. Lim helped in funding acquisition and project administration. Drs. Jeon and Lim helped in supervision. Dr. Cho helped in validation and visualization. Drs. Jeon, Lim, and Cho helped in writing—review and editing.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by the Research Program funded by the Korea Disease Control and Prevention Agency (fund code 2019E280500, 2021-10-026).
The authors have disclosed that they do not have any potential conflicts of interest.
This study was approved by the institutional review board of each hospital (Table S10, http://links.lww.com/CCM/H291), including the Pusan National University Yangsan Hospital Institutional Review Board (approval number: 05–2019–092, approval date: July 11, 2019, study title: Korean Sepsis Alliance Registry: A Multicenter Observational Cohort Study). The number of patients enrolled from each hospital used in the analysis is presented in Table S11 (http://links.lww.com/CCM/H291).
REFERENCES
- 1.Goda A, Masuyama T: Obesity and overweight in Asian people. Circ J 2016; 80:2425–2426 [DOI] [PubMed] [Google Scholar]
- 2.Karampela I, Chrysanthopoulou E, Christodoulatos GS, et al. : Is there an obesity paradox in critical illness? Epidemiologic and metabolic considerations. Curr Obes Rep 2020; 9:231–244 [DOI] [PubMed] [Google Scholar]
- 3.Cho WH, Oh JY, Yeo HJ, et al. : Obesity survival paradox in pneumonia supported with extracorporeal membrane oxygenation: Analysis of the national registry. J Crit Care 2018; 48:453–457 [DOI] [PubMed] [Google Scholar]
- 4.Schetz M, De Jong A, Deane AM, et al. : Obesity in the critically ill: A narrative review. Intensive Care Med 2019; 45:757–769 [DOI] [PubMed] [Google Scholar]
- 5.Jagan N, Morrow LE, Walters RW, et al. : Sepsis and the obesity paradox: Size matters in more than one way. Crit Care Med 2020; 48:e776–e782 [DOI] [PubMed] [Google Scholar]
- 6.Trivedi V, Bavishi C, Jean R: Impact of obesity on sepsis mortality: A systematic review. J Crit Care 2015; 30:518–524 [DOI] [PubMed] [Google Scholar]
- 7.Pepper DJ, Sun J, Welsh J, et al. : Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: A systematic review and meta-analysis. Crit Care 2016; 20:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pepper DJ, Demirkale CY, Sun J, et al. : Does obesity protect against death in sepsis? A retrospective cohort study of 55,038 adult patients. Crit Care Med 2019; 47:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Liu X, Chen Q, et al. : The role of increased body mass index in outcomes of sepsis: A systematic review and meta-analysis. BMC Anesthesiol 2017; 17:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization: Regional Office for the Western P. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney, Health Communications Australia, 2000 [Google Scholar]
- 11.Sato T, Kudo D, Kushimoto S, et al. : Associations between low body mass index and mortality in patients with sepsis: A retrospective analysis of a cohort study in Japan. PLoS One 2021; 16:e0252955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oami T, Karasawa S, Shimada T, et al. : Association between low body mass index and increased 28-day mortality of severe sepsis in Japanese cohorts. Sci Rep 2021; 11:1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SM, Kang JW, Jo YH, et al. : Underweight is associated with mortality in patients with severe sepsis and septic shock. Intensive Care Med Exp 2015; 3:A876 [Google Scholar]
- 14.Wei-Shun Y, Yi-Cheng C, Chia-Hsuin C, et al. : The association between body mass index and the risk of hospitalization and mortality due to infection: A prospective cohort study. Open Forum Infect Dis 2021; 8:ofaa545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramachandran A, Chamukuttan S, Shetty SA, et al. : Obesity in Asia--is it different from rest of the world. Diabetes Metab Res Rev 2012; 28:47–51 [DOI] [PubMed] [Google Scholar]
- 16.Kim T, Huh S, Kim SY, et al. : ICU rehabilitation is associated with reduced long-term mortality from sepsis in patients with low skeletal muscle mass: A case control study. Ann Transl Med 2019; 7:430–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeo HJ, Lee YS, Kim TH, et al. : Vasopressor initiation within 1 hour of fluid loading is associated with increased mortality in septic shock patients: Analysis of national registry data. Crit Care Med 2022; 50:e351–e360 [DOI] [PubMed] [Google Scholar]
- 18.Magiorakos AP, Srinivasan A, Carey RB, et al. : Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–281 [DOI] [PubMed] [Google Scholar]
- 19.Evans L, Rhodes A, Alhazzani W, et al. : Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021; 47:1181–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haslam DW, James WO: Obesity. Lancet. 2005; 366:1197–1209 [DOI] [PubMed] [Google Scholar]
- 21.Dobner J, Kaser S: Body mass index and the risk of infection - from underweight to obesity. Clin Microbiol Infect 2018; 24:24–28 [DOI] [PubMed] [Google Scholar]
- 22.Rogne T, Solligård E, Burgess S, et al. : Body mass index and risk of dying from a bloodstream infection: A Mendelian randomization study. PLoS Med 2020; 17:e1003413–e1003413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang HE, Griffin R, Judd S, et al. : Obesity and risk of sepsis: A population-based cohort study. Obesity (Silver Spring) 2013; 21:E762–E769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghilotti F, Bellocco R, Ye W, et al. : Obesity and risk of infections: Results from men and women in the Swedish National March Cohort. Int J Epidemiol 2019; 48:1783–1794 [DOI] [PubMed] [Google Scholar]
- 25.Winter-Jensen M, Afzal S, Jess T, et al. : Body mass index and risk of infections: A Mendelian randomization study of 101,447 individuals. Eur J Epidemiol 2020; 35:347–354 [DOI] [PubMed] [Google Scholar]
- 26.Danninger T, Rezar R, Mamandipoor B, et al. : Underweight but not overweight is associated with excess mortality in septic ICU patients. Wien Klin Wochenschr 2022; 134:139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Q, Wang M, Li S, et al. : Impact of body mass index on survival of medical patients with sepsis: A prospective cohort study in a university hospital in China. BMJ Open 2018; 8:e021979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giles KA, Hamdan AD, Pomposelli FB, et al. : Body mass index: Surgical site infections and mortality after lower extremity bypass from the National Surgical Quality Improvement Program 2005-2007. Ann Vasc Surg 2010; 24:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Health at a Glance: Asia/Pacific Measuring Progress Towards Universal Health Coverage. 2020. Available at: https://www.oecd-ilibrary.org/sites/a47d0cd2-en/index.html?itemId=/content/component/a47d0cd2-en. Accessed July 13, 2022
- 30.Mewes C, Böhnke C, Alexander T, et al. : Favorable 90-day mortality in obese Caucasian patients with septic shock according to the sepsis-3 definition. J Clin Med 2019; 9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deurenberg P, Deurenberg-Yap M, Guricci S: Asians are different from Caucasians and from each other in their body mass index/body fat percent relationship. Obes Rev 2002; 3:141–146 [DOI] [PubMed] [Google Scholar]
- 32.Misra A, Vikram NK: Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians: Evidence and implications. Nutrition 2004; 20:482–491 [DOI] [PubMed] [Google Scholar]
- 33.Pan WH, Flegal KM, Chang HY, et al. : Body mass index and obesity-related metabolic disorders in Taiwanese and US whites and blacks: Implications for definitions of overweight and obesity for Asians. Am J Clin Nutr 2004; 79:31–39 [DOI] [PubMed] [Google Scholar]
- 34.Mankowski RT, Laitano O, Clanton TL, et al. : Pathophysiology and treatment strategies of acute myopathy and muscle wasting after sepsis. J Clin Med 2021; 10:1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuda W, Uemura T, Yamamoto M, et al. : Impact of frailty on protocol-based weaning from mechanical ventilation in patients with sepsis: A retrospective cohort study. Acute Med Surg 2020; 7:e608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernando SM, McIsaac DI, Rochwerg B, et al. : Frailty and invasive mechanical ventilation: Association with outcomes, extubation failure, and tracheostomy. Intensive Care Med 2019; 45:1742–1752 [DOI] [PubMed] [Google Scholar]
- 37.Goossens C, Weckx R, Derde S, et al. : Adipose tissue protects against sepsis-induced muscle weakness in mice: From lipolysis to ketones. Crit Care 2019; 23:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goossens C, Marques MB, Derde S, et al. : Premorbid obesity, but not nutrition, prevents critical illness-induced muscle wasting and weakness. J Cachexia Sarcopenia Muscle 2017; 8:89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng PY, Eikermann M: The obesity conundrum in sepsis. BMC Anesthesiol 2017; 17:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loosen SH, Koch A, Tacke F, et al. : The role of adipokines as circulating biomarkers in critical illness and sepsis. Int J Mol Sci 2019; 20:4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimada T, Topchiy E, Leung AKK, et al. : Very low density lipoprotein receptor sequesters lipopolysaccharide into adipose tissue during sepsis. Crit Care Med 2020; 48:41–48 [DOI] [PubMed] [Google Scholar]
- 42.Singh R, Rathore SS, Khan H, et al. : Association of obesity with COVID-19 severity and mortality: An updated systemic review, meta-analysis, and meta-regression. Front Endocrinol (Lausanne) 2022; 13:780872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawadogo W, Tsegaye M, Gizaw A, et al. : Overweight and obesity as risk factors for COVID-19-associated hospitalisations and death: Systematic review and meta-analysis. BMJ Nutr Prev Health 2022; 5:10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vulturar DM, Crivii CB, Orăsan OH, et al. : Obesity impact on SARS-CoV-2 infection: Pros and Cons “Obesity Paradox”-a systematic review. J Clin Med 2022; 11:3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cummings MJ, Baldwin MR, Abrams D, et al. : Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020; 395:1763–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goyal P, Ringel JB, Rajan M, et al. : Obesity and COVID-19 in New York City: A retrospective cohort study. Ann Intern Med 2020; 173:855–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dana R, Bannay A, Bourst P, et al. : Obesity and mortality in critically ill COVID-19 patients with respiratory failure. Int J Obes 2021; 45:2028–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]