Abstract
Background:
Sickle Cell Disease (SCD) is an inherited blood disorder which results in serious morbidity and early mortality. Novel therapies for sickle cell disease (SCD), most notably genetic therapies (GT) and HLA-mismatched donor hematopoietic cell transplantation (HCT), are in clinical trials. While potentially curative, these interventions are some of the most intensive treatments for SCD and are associated with serious and life-altering side effects which may manifest several years after treatment. Little is known about knowledge, beliefs, and attitudes of individuals with SCD, or their caregivers, towards existing and these emerging therapies.
Methods:
Patients with SCD at least 13 years of age (n=66) and caregivers (n=38) were surveyed about knowledge, attitudes, and beliefs surrounding treatments for SCD.
Results:
Only 4.8% felt “extremely knowledgeable” about GT for SCD while the majority (63.4%) reported little knowledge. Overall, health literacy was low among respondents. Most respondents had a neutral attitude regarding the safety of GT for SCD, and whether it was a good treatment for the disorder (56.7% and 58.6%, respectively). Only a few respondents endorsed the idea that GT was “unsafe” or “not a good treatment” (5.8% and 4.8% respectively). There was an association between increasing knowledge about GT and agreement that it is safe (p=0.012) and a good treatment for SCD (p=0.031).
Conclusions:
Given that very few patients with SCD feel knowledgeable about GT and a majority have neutral feelings about the safety and utility of this new approach, culturally appropriate patient-centered education is urgently needed as these treatments get regulatory approval and proceed to the clinic.
Keywords: Gene Therapy, Hematology/Oncology, Patient Education/Patient Safety/Public Education
Introduction:
Sickle Cell Disease (SCD) is the most common inherited hemoglobinopathy in the United States affecting approximately 100,000 individuals, about 40% of whom are children. SCD disproportionally impacts racial and ethnic minorities.[1] In the United States, almost 90% of patients self-report as Black, with SCD affecting 1 in 365 Black births.[1, 2] The genetic defect in SCD causes red blood cells to deform into rigid, sickle-shaped cells which occlude small blood vessels, impeding blood flow and tissue oxygenation. Most patients with SCD experience debilitating pain crises, fatigue, and significant end-organ damage, resulting in premature morbidity and mortality.[3–5] Individuals experience an average of 2 to 3 hospital admissions (lasting 5 to 6 days each) annually with a reduced lifespan (54 vs 76 years for unaffected individuals)[6]. Many patients also experience reduced social functioning secondary to missed school or work, potentially resulting in reduced income potential, higher unemployment, general disability, and lower socioeconomic status.[6]
The United States Food and Drug Administration (FDA) has recently approved three new drugs for SCD (L-glutamine [2017], voxelotor [2019], and crizanlizumab [2019]),[7] for the prevention and treatment of SCD complications. Like chronic transfusion therapy or hydroxyurea (FDA approved in 1998), none of these recently approved drugs are curative. Currently, the only potentially curative therapy for SCD is a hematopoietic cell transplant (HCT) using a human leukocyte antigen (HLA) matched donor. However, most patients with SCD lack a suitable HLA-matched donor.[8, 9] Several novel and potentially curative therapies – such as autologous genetically modified cells and HCT using alternative HLA-mismatched donors, are in clinical trials.
Despite the increased focus on novel treatments, the care of patients with SCD remains medically and socially complex. Many children with SCD still do not receive medical care consistent with current evidenced based guidelines, such as annual transcranial Doppler (TCD) ultrasound screenings or proper initiation of hydroxyurea [10, 11]. Given the fact there is even a gap between evidenced-based recommendations for well-established treatments and actual clinical practice, we hypothesized that patients may be receiving further limited information on potentially curative therapies and thus have limited knowledge and interest in novel genetic therapies (GT) or HCT for SCD. Both HCT and GT are ‘high risk-high reward’ therapies given their risk profile and curative potential. Since little is known about patient or parent/caregiver’s knowledge, attitudes, and beliefs towards HCT and GT for SCD, we sought to conduct a needs assessment of young adult patients and parental caregivers to compare responses alongside two established treatments for SCD (chronic transfusion therapy and hydroxyurea). We excluded the recently approved disease-modifying agents due to their limited approval in pediatrics at the time of survey collection.
Methods:
Patient Population:
Potential participants were pediatric patients able to assent (age 13 or above) and adults with a diagnosis of SCD (any genotype) or their caregivers. All sequential patients with SCD (any genotype), or parents/caregivers (henceforth caregiver) of patients with SCD were approached during routine SCD clinic visits at St. Jude Children’s Research Hospital and Methodist Comprehensive Sickle Cell Center in Memphis, TN and were invited to participate. Verbal consent was obtained after study explanation. The St. Jude Institutional Review Board Approved this study.
Survey Administration:
The survey instrument collected self-reported demographic information and assessed attitudes and beliefs towards common treatments for SCD (hydroxyurea, chronic transfusion therapy) and less common therapies such as HCT and GT. Questions were modified from a previous questionnaire used by the authors[12] developed through face validity[13]. No psychometric properties were evaluated for this questionnaire. Surveys were administered by two study team members (AY and YJC) using an iPad based online form. Participants entered their choices directly on the iPad unless they requested help with either navigation or reading the questions. Health literacy was assessed using The Newest Vital sign (NVS), a validated six question tool for assessing health literacy.[14] No personally identifiable information was collected. All participants received a small stipend for their participation. Since the term “bone marrow transplant” (BMT) is more familiar to the lay public than “hematopoietic cell transplant,” it was used in this survey.
Statistical Analysis:
The nominal/ordinal variables are represented by the frequency of subjects (N) and the proportion of the subjects (%) in the study. Mean (and standard deviation) or median (and interquartile range) are reported for continuous variables. McNemar’s Chi-Square test was used to assess differences in beliefs for the treatment between GT and BMT. To evaluate consistency of participants’ answers to a pair of related (GT/BMT) questions, each participant’s answers to the two questions were matched, and a Wilcoxon signed-rank test was used to analyze if a significant difference in the answers between the pair of questions exists. Pratt’s method was used to account for zeros in the Wilcoxon signed-rank test. Goodman and Kruskal’s Gamma, an ordinal measure of association, was calculated to evaluate the association between each perception question and variables of interest. P-values were calculated based on the test of whether Gamma is different from zero. The same method was also used to measure the association between GT and BMT knowledge with literacy level. The Likert scale questions are represented using diverging stacked bar charts. A few answers to Likert scales are combined into one group to simplify interpretation of the figure. All statistical analyses were performed using R software version 3.6.0.
Results:
Sixty-six individuals with SCD (median age 23 years) and 38 caregivers (median age 36 years) completed the survey, the majority of whom identified as Black (96.2%). The homozygous SS genotype was most common (61.5%). Respondent demographics are listed in Table 1 alongside self-reported assessment of health and disease control. Health literacy was limited in this population with mean and median NVS scores of 2.3 (SD 1.7) and 2 (IQR 1,3), respectively: a score of less than 4 indicates the possibility of limited literacy on this scale. Only 21% scored 4 or above on the NVS (suggesting adequate health literacy). There was no association between health literacy levels and knowledge about GT or BMT (p=0.12 and 0.52 respectively).
Table 1:
Demographics and Self-Reported Disease Assessment Among Study Participant.
| Overall (N=104) % | |
|---|---|
| Sickle cell disease patient sex (N = 66) | |
| Male | 32 (48.5%) |
| Female | 34 (51.5%) |
| Parent/Caregiver sex (N = 38) | |
| Male | 6 (15.8%) |
| Female | 32 (84.2%) |
| Race | |
| Black or African American | 100 (96.2%) |
| White (not Hispanic or Latino) | 0 |
| Hispanic or Latino | 4 (3.8%) |
| Age of parent/ caregiver respondents (N = 38) | |
| Median (IQR) | 36 (30, 39) |
| Age of Sickle Cell Disease Patient respondents (N = 66) | |
| Median (IQR) | 23 (18, 29) |
| Type of Sickle cell disease | |
| Hb SS | 64 (61.5%) |
| Hb SC | 25 (24%) |
| Hb Sβ0 thalassemia | 6 (5.8%) |
| Hb Sβ+ thalassemia | 6 (5.8%) |
| Other | 2 (1.9%) |
| Don’t know | 1 (1%) |
| Health Literacy score (The Newest Vital Sign) 1 | |
| Mean (SD) | 2.3 (1.7) |
| Median (IQR) | 2 (1, 3) |
| Other than you/your child, does anybody else in your family have sickle cell disease | |
| Yes | 39 (37.5%) |
| No | 65 (62.5%) |
| How would you describe you/your child’s overall health? | |
| Poor | 5 (4.8%) |
| Fair | 23 (22.1%) |
| Good | 47 (45.2%) |
| Very good | 20 (19.2%) |
| Excellent | 9 (8.7%) |
| How you think you/your child’s disease has changed over time? | |
| Worsened | 22 (21.2%) |
| Stabilized | 64 (61.5%) |
| Improved | 18 (17.3%) |
| My/my child’s treatment is controlling my disease satisfactorily | |
| Strongly Disagree | 3 (2.9%) |
| Disagree | 7 (6.7%) |
| Neither agree nor disagree | 22 (21.2%) |
| Agree | 55 (52.9%) |
| Strongly agree | 17 (16.4%) |
| Have you/your child been offered the following treatments before? 1 | |
| Blood transfusions | 57 (54.8%) |
| Hydroxyurea | 69 (66.3%) |
| Bone marrow transplant | 19 (18.3%) |
| Gene therapy | 6 (5.8%) |
| None of the above | 20 (19.2%) |
| Do you / your child 1 : | |
| Currently take Hydroxyurea | 64 (61.5%) |
| Taken Hydroxyurea in the past | 14 (13.5%) |
| Currently receive chronic blood transfusions | 13 (12.5%) |
| Has received chronic blood transfusions in the past | 11 (10.6%) |
| None of the above | 26 (25%) |
| Do you know anyone with sickle cell disease who has had a bone marrow transplant? | |
| Yes | 20 (19.2%) |
| No | 84 (80.8%) |
| Which of the following treatments can cure sickle cell disease? 2 | |
| Blood transfusions | 20 (19.2%) |
| Hydroxyurea | 31 (29.8%) |
| Bone marrow transplant | 62 (59.6%) |
| Gene therapy | 23 (22.1%) |
| There is no cure for sickle cell disease | 11 (10.6%) |
A score of less than for indicates the possibility of limited literacy on The Newest Vital Sign.
Does not sum to 104, participants could select multiple choices.
While 59.6% of respondents recognized BMT as a curative therapy for SCD, only 22.1% recognized GT as potentially curative (Table 1). As shown in Figure 1, 55.8% of participants felt moderately/extremely knowledgeable about hydroxyurea for SCD compared to only 20.2% for either BMT or GT. More people agreed/strongly agreed that hydroxyurea and blood transfusions were good and safe treatments for SCD, while most neither agreed nor disagreed (i.e. felt neutral) about these statements for BMT and GT (Figure 2).
Figure 1: Self-Reported Knowledge About Gene Therapy, Bone Marrow Transplantation, and Hydroxyurea as Potential Treatments for Sickle Cell Disease.
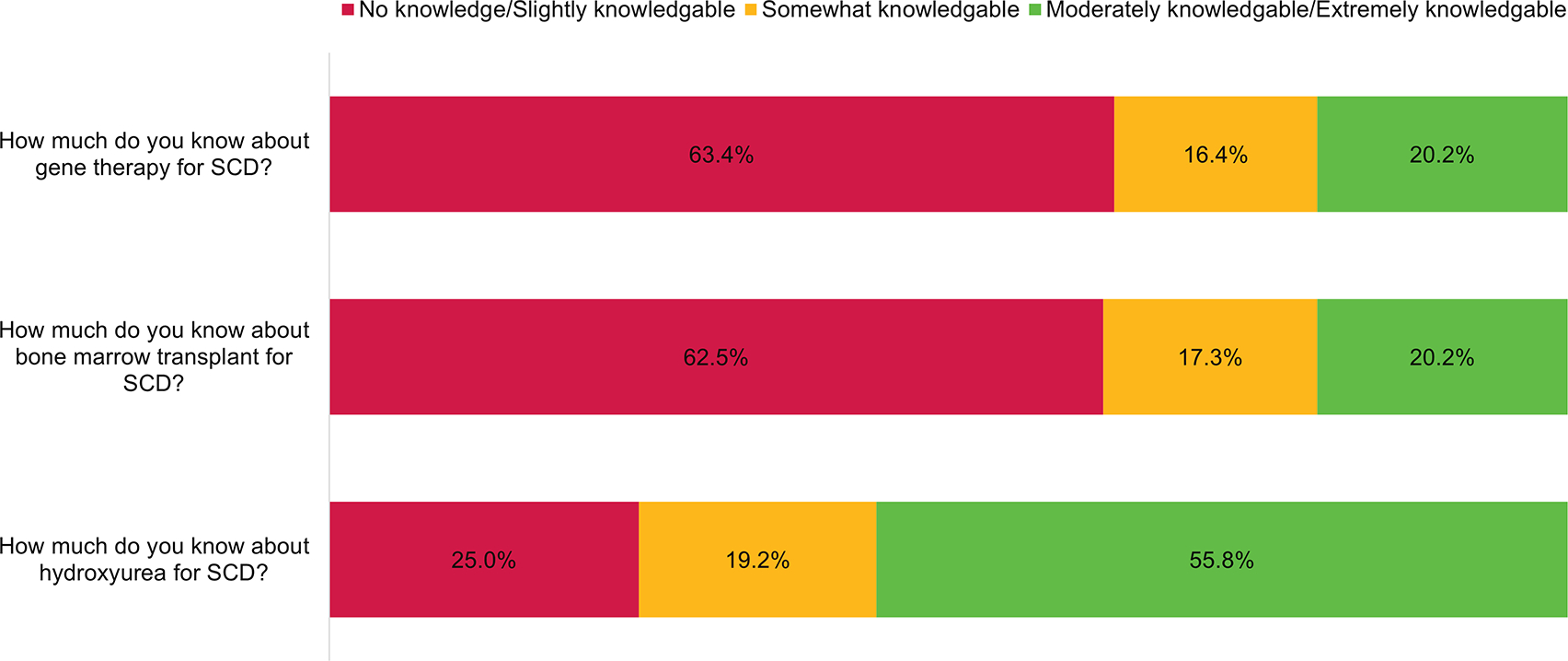
Using a five-point Likert Scale, participants were asked to report their knowledge of two potentially curative treatments for Sickle Cell Disease (SCD) alongside a commonly available disease-modifying therapy (Hydroxyurea). Self-reported knowledge about Hydroxyurea therapy for SCD was significantly greater than for either bone marrow transplantation or gene therapy (P < 0.0001, for both comparisons).
Figure 2: Participant Assessment of Safety and Utility of Various Treatments for Sickle Cell Disease.
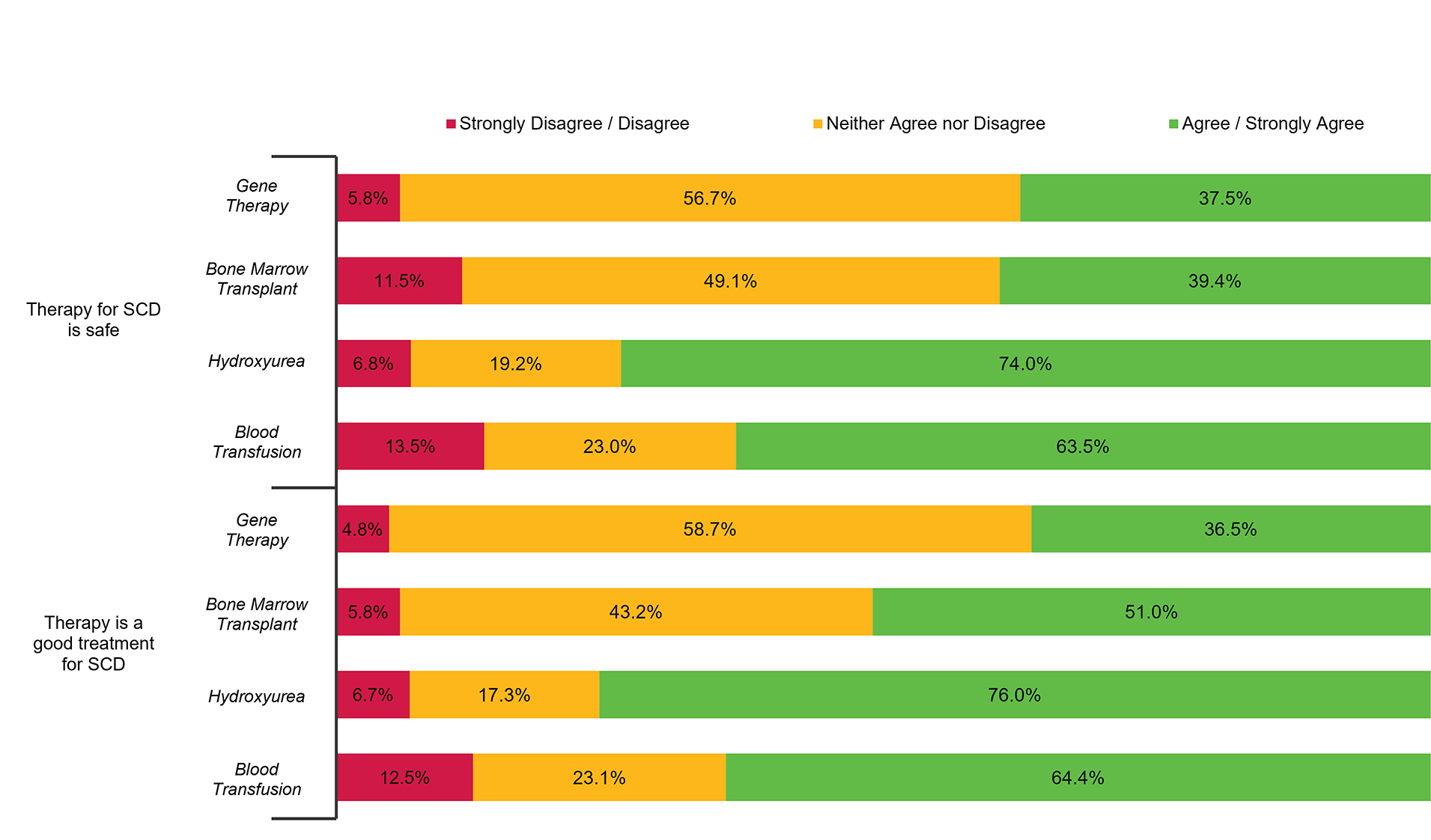
Using a five-point Likert Scale, participants were asked to rate the safety and utility of two potentially curative treatments for Sickle Cell Disease (SCD) (Gene Therapy, Bone Marrow Treatment) alongside two commonly utilized disease-modifying interventions (Hydroxyurea and Chronic Blood Transfusion Therapy).
When GT is compared to BMT, people are more likely to endorse that BMT can result in a ‘forever cure’ (11.5% vs 20.2%, p = 0.04), otherwise there were no significant differences in beliefs between these two treatments (Supplemental Table 1). For factors influencing participation in a BMT or GT clinical trial, we found no significant difference based on assessment treatment or risks or benefits (Figure 3a) and attitudes about treatment outcomes (Figure 3b). A respondent’s assessment of their (their child’s) overall health status (i.e. assessment of how health or ill one was) was more likely to influence decision making around GT versus BMT (p = 0.040), otherwise no significant differences existed influencing decision-making for psychosocial factors (Figure 4).
Figure 3: Impact of Potential Treatment Risks and Benefits on Participant Willingness to Undergo Gene Therapy or Bone Marrow Transplant.
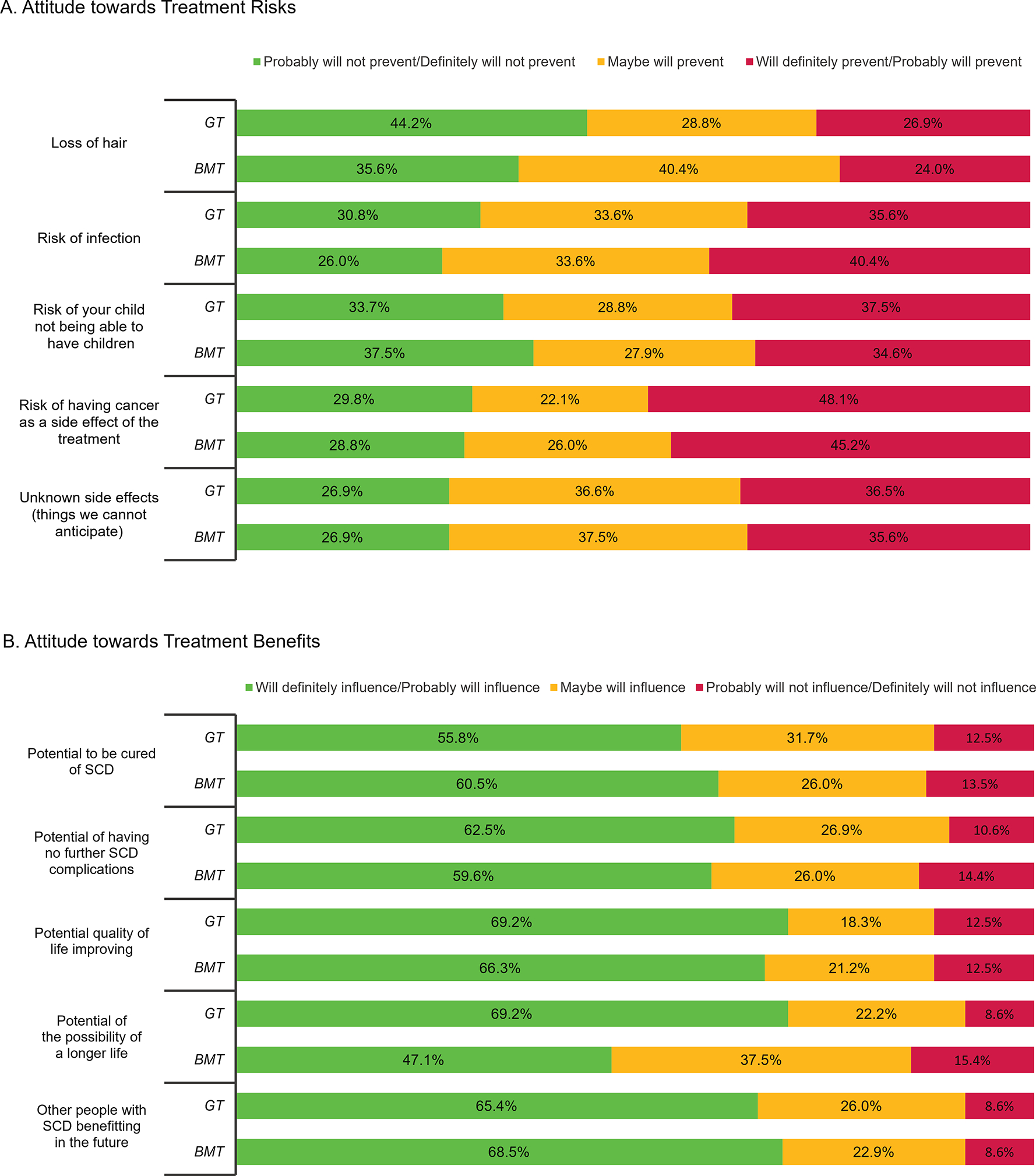
Figure 3A depicts the likelihood of potential therapy-related side effects on one’s decision to undergo gene therapy (GT) or bone marrow transplant (BMT), while Figure 3B depicts the impact of potential treatment benefits on one’s decision to undergo GT or BMT. Significant p-values (P < 0.05) are included when applicable.
Figure 4: Impact of Psychosocial Factors on Participant Willingness to Undergo Gene Therapy or Bone Marrow Transplant.
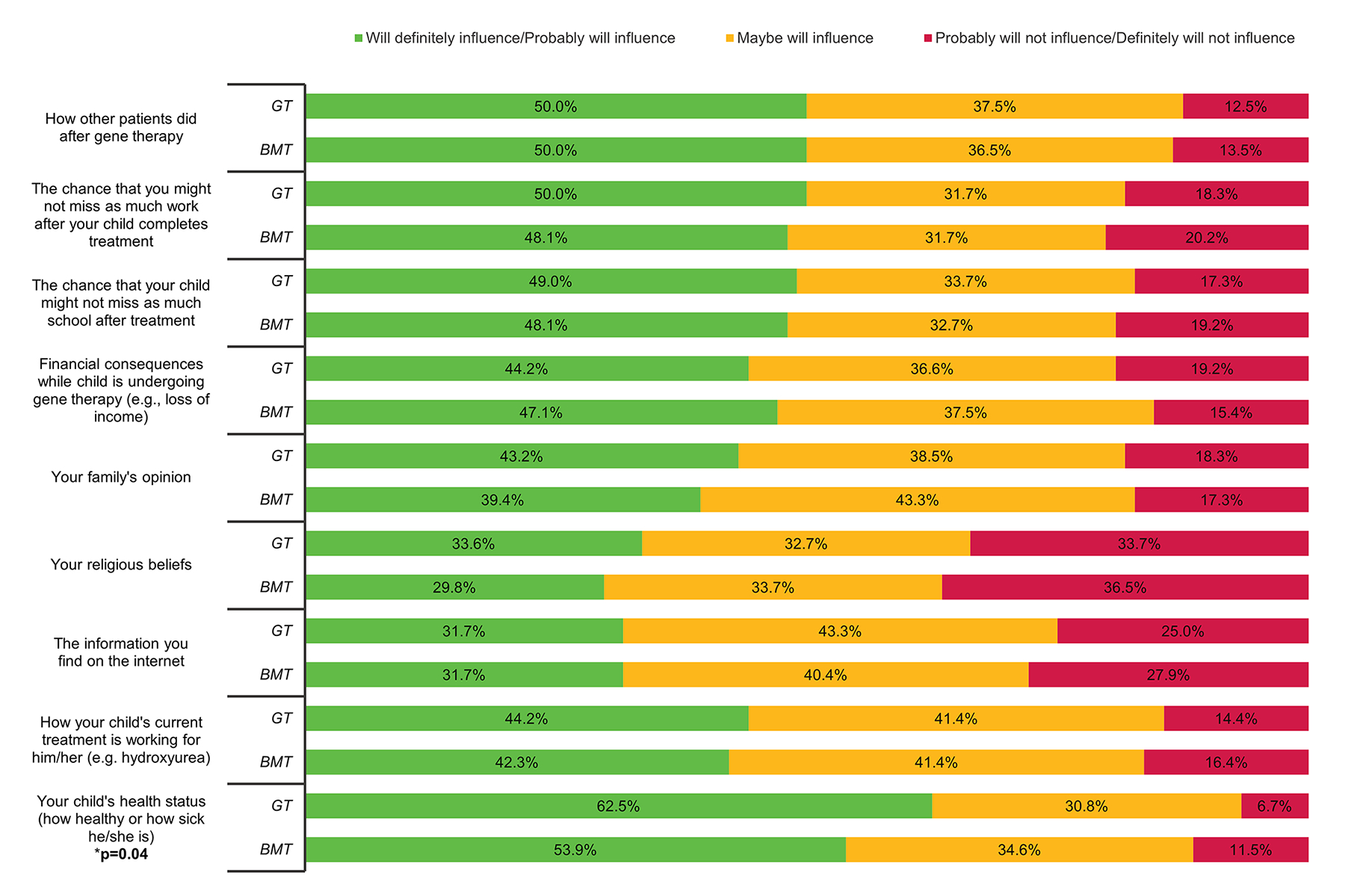
The potential impact of external psycho-social factors influencing a respondent’s decision to undergo gene therapy (GT) or bone marrow transplant (BMT). Significant p-values (p < 0.05) are included when applicable.
In an association analysis measured by Goodman and Kruskal’s gamma (γ), we found several significant associations between participant self-reported characteristics and beliefs about gene therapy, bone marrow transplantation, hydroxyurea, and chronic transfusion as treatments for sickle cell disease (all shown in Figure 5). A significant positive association was discovered between increasing knowledge about GT and perceptions of GT being a safe or good treatment for SCD (p=0.01 and p=0.03 respectively). Correspondingly, increasing knowledge of BMT was found to be significantly associated with a perception that BMT is a good treatment (p=0.02). Higher health literacy level was also positively associated with the perception that BMT is a safe (p=0.05) or good (p=0.04) treatment. Having a family history of SCD had no significant influence on participants’ beliefs about any of treatment strategies. However, having known someone with a SCD who has had a BMT was found to be significantly associated with an agreement that BMT is a good treatment for SCD (p=0.029). Males were less likely to agree that BMT is safe (p=0.04). Those who experienced improvement in their/ (their child’s) health condition over time strongly believed that BMT is a good treatment (p=0.02), or the received treatment is controlling disease satisfactorily (p=0.002). Additionally, perception of hydroxyurea as a safe or good treatment for SCD was significantly prevalent amongst those reporting improvement in their health over the past year (p=0.001 and 0.011 respectively). Hydroxyurea was also perceived as a safe treatment by parents/caregivers of a child participant with SCD (p=0.02). ‘Overall healthy’ participants (who described their overall health as good, very good, or excellent on a survey) strongly agreed that hydroxyurea is a safe (p<0.001) or good (p=0.03) treatment. Self-reported healthy participants also perceived that their treatment is controlling their SCD disease satisfactorily (p<0.001).
Figure 5: Association Between Participant Characteristics and Beliefs about Gene Therapy, Bone Marrow Transplantation, and Hydroxyurea as Treatment for Sickle Cell Disease Measured by Goodman and Kruskal’s Gamma coefficient (γ).
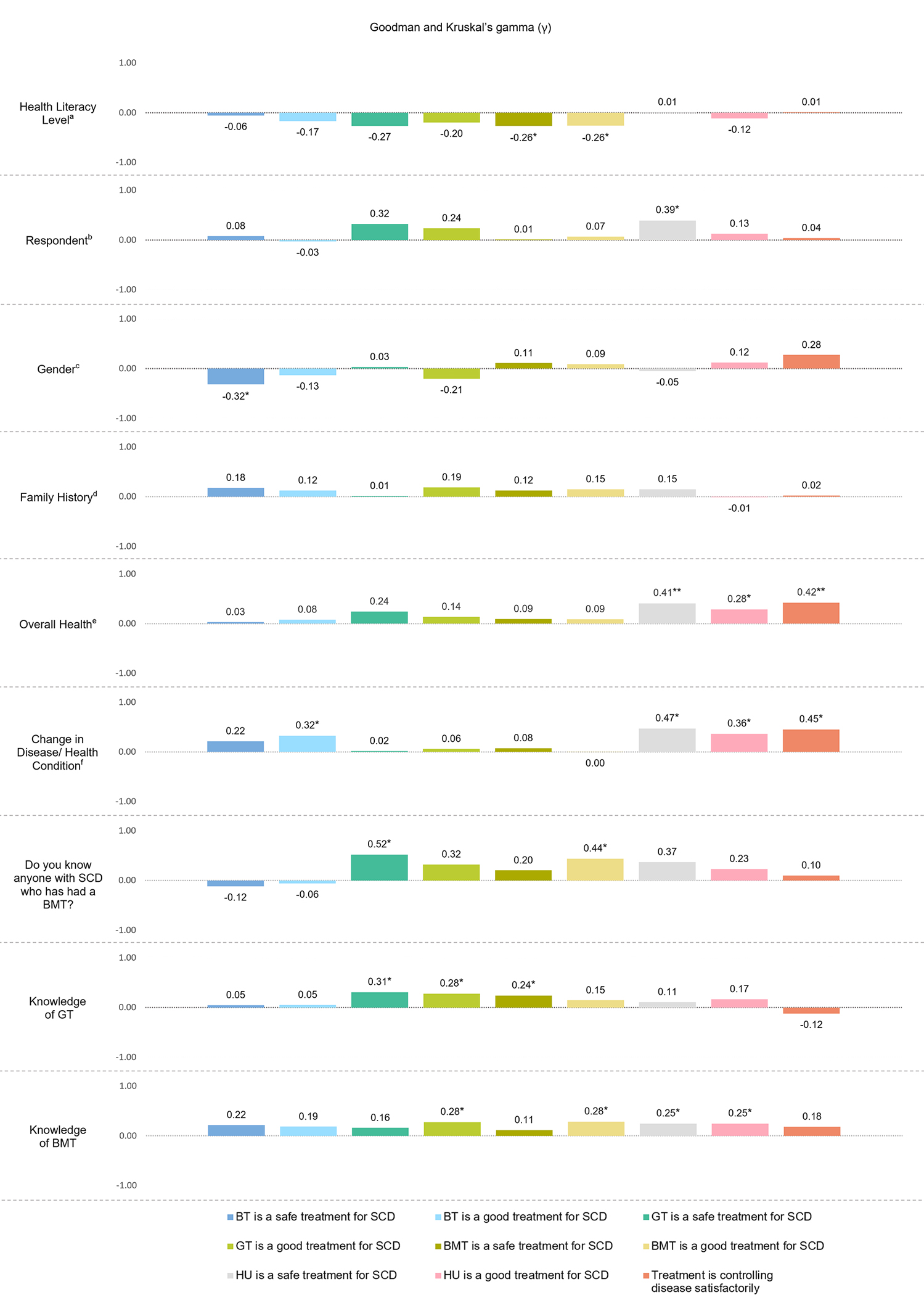
The figure displays the results of an association analysis (Goodman and Kruskal’s Gamma) looking at the relationship between participant characteristics and their beliefs about gene therapy (GT), bone marrow transplant (BMT), and Hydroxyurea (HU) as interventions for Sickle Cell Disease (SCD). Significant associations are indicated with one asterisk (*) for p < 0.05 and two asterisks (**) for p < 0.001. See results for full explanation of the significant associations.
Discussion:
We show that patients and caregivers have very limited knowledge of the two potentially curative treatments for SCD (BMT and GT). While the first clinical trials of BMT in SCD began over 25 years ago[15–17], and HLA-matched BMT is a well-established curative therapy for SCD, in this assessment nearly 40% of affected individuals failed to recognize BMT as a potentially curative therapy for SCD. As part of our structured patient education procedure, our SCD clinic routinely discusses curative therapies, such as BMT and GT, together when we introduce potentially curative treatments for SCD. Our practice is to recommend curative treatments to all patients who meet eligibility (e.g., higher stroke risk) and introduce them (but not recommend) to all other patients, so they become acquainted with the available treatment options. Given our clinical practice, these results were surprising. These results emphasize that additional educational efforts specifically dedicated to GT and BMT are needed to increase patient knowledge about these potentially curative treatment options. Given that haploidentical BMT and GT are presently available to relatively few individuals who qualify for a clinical trial, it is important for SCD providers to review and discuss eligibility criteria with patients who are interested or may qualify for such trials. As at least two GT products for SCD with distinct mechanisms of action are likely to be considered by the FDA for approval soon, it is even more important for patients and their providers to know about these treatments so that they can make informed decisions. It may be helpful for SCD clinics to build time into appointments to discuss these treatment options.
Participants in this needs assessment had significantly more knowledge about HU than either BMT or GT, with the majority (74%-76%) identifying HU as good and safe treatment for SCD. It is unknown how this belief might influence one’s consideration of BMT or GT as alternative interventions for SCD. It seems likely that an individual who believes HU is a good and safe treatment, as compared to BMT or GT, may choose HU over these more intensive “high-risk, high-reward” interventions, even though the former does not have a curative intent. Ultimately, it may be an individual’s assessment of how ill one is from SCD and how well (or poorly) the disease is controlled by current disease-modifying therapies. As noted in Figure 4, self-assessment of health status was very likely to influence decision-making around GT, even more than for BMT. It is likely that other contextual factors (such as familiarity with BMT or existing beliefs) ultimately impact an individual’s interest in pursuing a given treatment option for SCD. Further research into patient decision-making around disease-modifying versus potentially curative options is needed to better understand the decision-making process and to best support patients facing such decisions in the future.
In this cross-sectional survey of patients and caregivers with SCD only 21% of individuals demonstrated adequate literacy (Table 1) as measured by a validated literacy screener (The Newest Vital Sign[14]). This has important implications for the development of patient-facing materials and educational content for patients with SCD. Multi-modal communication tools that incorporate audio/video aids to augment written communication are likely to be beneficial. In our previous work on informed consent communication in a pediatric oncology setting, a structured multi-visit process and informational cover sheet were recommended to be used for communication by parents.[18] Increased time discussing options was also associated with better participant understanding and[19] in another study regarding discussions of genomic-sequencing, use of a structured, multi-modal two-visit approach significantly increased parental knowledge and understanding around important scientific concepts.[20] This suggests that repetition and reinforcement of complex information using pictorial cues, in ways accessible to different adult-learning styles, is beneficial and may be particularly helpful among adults with limited literacy Clinicians should also tailor their discussions about potentially curative therapies in a way that in meets the unique informational needs of individual families. These approaches can promote understanding and enhance informed decision-making among patients receiving complex medical treatments; or in circumstances where multiple treatment options are being considered. Furthermore, individuals with SCD who have experienced neurovascular events may require additional tailored communication support if they have neurocognitive deficits.[21] Other groups have shown that SCD patients and caregivers prefer one-on-one conversations with providers and augmentation of information sharing through use of electronic media.[22] Although this finding is consistent with our ongoing stakeholder research, further implementation research is needed to establish best practices for education about GT or BMT and informed consent communication in patients with SCD.
We found no relationship between health literacy and self-reported knowledge of BMT or GT. This is consistent with our work in parents of children with cancer which found no relationship between self-reported literacy or parental educational attainment and improvements in their genetic knowledge following an educational intervention.[23] We postulate that neither higher educational attainment nor greater baseline health literacy adequately prepares patients to readily understand unfamiliar or complex medical concepts and that repetition is likely necessary to facilitate understanding. Our results did find a positive association between greater knowledge of BMT or GT and perceptions that it was a good treatment. We also found a positive association between knowing someone who had a BMT and belief that it was a good treatment. It may be that greater familiarity with the intervention and its potential benefits (as measured by self-reported knowledge or experience) reduce uncertainty and fear and increase one’s belief that it is a good and/or safe treatment for SCD. Greater education, including the opportunity to interact with others who have undergone BMT or GT, may be effective in increasing the acceptability of these potentially curative treatments among at-risk patients.
Although recent data by Booth et al found that eight of nine focus group participants with SCD would select GT as a curative option, the informational sheet shared with patients did not disclose detailed information about potential side effects[24]. As indicated by our results, detailed information about risk is important to patients and may influence decision-making around potentially curative high risk-high reward therapies for SCD. For example, in our assessment almost half of all respondents (48%) said a potential risk of development of cancer after GT would “definitely/probably would preclude their participation” in a clinical trial. Given the recent reports of patients enrolled in a SCD GT clinical trial developing leukemia/myelodysplastic syndrome and the subsequent temporary suspension of these trials,[25] it is critical that providers honestly and transparently discuss what is known and not known about this potential risk. The risk of developing infertility from the conditioning regimen is another important barrier in acceptability of these novel treatments as it will “definitely prevent” one-third of patients from pursuing GT or BMT and “may prevent” another one-third from pursuing these potentially curative interventions. Although future research is needed about effects of GT and BMT on fertility, increased access to fertility preservation services may ameliorate this barrier for some patients. Relatedly, advocacy efforts are necessary to improve insurance coverage and ensure these, as well as other supportive care services, are accessible to interested patients.
This study, surveying patients and caregivers from both a pediatric and adult SCD clinics adds important information to the literature as attitudes toward GT or BMT, in comparison to standard treatments such as transfusion or HU are poorly described to date. This is a cross-sectional survey of patients/ caregivers in the Southeastern United States. It is possible that patients/caregivers in other regions may have different literacy levels or varying knowledge, attitudes, and beliefs regarding potential medical interventions for SCD. Hence the generalizability of these data may be limited, but the emergent themes remain valid. Another limitation is that the survey did not assess patient attitudes and beliefs towards the more recently approved disease modifying agents as they were not widely used by patients at the time of study initiation but are being increasingly accessed by patients. Further research is needed to understand how patients consider these newer disease-modifying agents, particularly in comparison to hydroxyurea, and potentially curative interventions such as GT and BMT. Further qualitative data is needed to better understand why individuals choose or decline a particular treatment, as well as to elicit preferences for the informational content that patient stakeholders wish to see in educational materials about SCD treatments. The latter area is the focus of our ongoing research work.
Conclusion:
In summary, there exists a great unmet need to communicate more effectively about potentially curative treatment options for serious monogenic disorders, particularly as new treatments are emerging. As evidenced by the association between increasing knowledge of GT and the belief that it is a good and safe therapy, there is a tremendous opportunity to develop educational materials which are broadly accessible to members of the SCD disease community. Community engagement[26] is highly encouraged to democratize the process and foster the development of trial designs and educational materials most acceptable to the patients and caregivers. As evidenced elsewhere in healthcare, failing to effectively partner and communicate with underrepresented patient groups may perpetuate mistrust, foster a lack of interest, and ultimately result in low uptake of new interventions. To avoid this undesirable outcome, we must also partner with patient stakeholders to identify interventions and endpoints that are most desirable and meet their needs.
Supplementary Material
Previous Abstract Presentation and Publication:
-
American Society of Hematology (ASH), Annual Meeting, December 2021
Sharma, A., Young, A., Carroll, Y., Mandrell, B., Caples, M., Gattuso, J., ... & Johnson, L. M. (2021). Patient and Caregiver Attitudes Towards Gene Therapy for Sickle Cell Disease: A Need for Partnership and Education. Blood, 138, 918.
Acknowledgements:
We thank Rushil Acharya BDS, MPH for his review of the manuscript and assistance with figures
Funding Support:
This study was partly supported by an unrestricted educational grant from CRISPR Therapeutics. The funding agency had no role in the design of the study, data collection or analysis, manuscript preparation or decision to submit the manuscript. This study was partially funded by the American Society of Hematology scholar award to AS. Effort for this study (JSH, LMJ) was partially funded by NHLBI U01HL133996–04.
Role of Funder/Sponsor (if any):
The NIH had no role in the design and conduct of the study.
Abbreviations (in order of manuscript appearance):
- SCD
Sickle cell disease
- FDA
Food and Drug Administration
- GT
Gene/genetic therapy
- NVS
Newest Vital Sign
- HCT
Hematopoietic stem-cell transplantation
- BMT
Bone marrow transplant
Footnotes
Conflict of Interest Disclosures:
Dr. Akshay Sharma (AS) is the St. Jude Children’s Research Hospital site principal investigator of clinical trials for genome editing of sickle cell disease sponsored by Vertex Pharmaceuticals / CRISPR Therapeutics (NCT03745287), Novartis (NCT04443907) and Beam Therapeutics (NCT05456880). The industry sponsors provided funding for the clinical trial, which includes salary support paid to AS’s institution. AS has received consultant fee from Spotlight Therapeutics, Medexus Inc. Vertex Pharmaceuticals, Sangamo Therapeutics and Editas Medicine. AS has also received research funding from CRISPR Therapeutics and honoraria from Vindico Medical Education.
Dr. Jane Hankins receives Consultancy fees for Global Blood Therapeutics and CVS Health.
The remaining authors have no relevant conflicts of interests to disclose.
References:
- 1.Brousseau DC, Panepinto JA, Nimmer M, Hoffmann RG. The number of people with sickle-cell disease in the United States: national and state estimates. Am J Hematol, 2010. 85: 77–78. [DOI] [PubMed] [Google Scholar]
- 2.Hassell KL. Population Estimates of Sickle Cell Disease in the U.S. Am J Prev Med, 2010. 38: S512–S521. [DOI] [PubMed] [Google Scholar]
- 3.Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med, 2017. 376: 1561–1573. [DOI] [PubMed] [Google Scholar]
- 4.Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999–2009). Pediatr Blood Cancer, 2013. 60: 1482–1486. [DOI] [PubMed] [Google Scholar]
- 5.Lanzkron S, Patrick Carroll C, Haywood C. Mortality rates and age at death from sickle cell disease: U.S., 1979–2005 [Internet]. Public Health Rep, 2013. 128: 110–116. [cited 2021 Dec 19] Available from: 10.1177/003335491312800206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lubeck D, Agodoa I, Bhakta N, Danese M, Pappu K, Howard R, Gleeson M, Halperin M, Lanzkron S. Estimated Life Expectancy and Income of Patients With Sickle Cell Disease Compared With Those Without Sickle Cell Disease [Internet], 2019. 2. [cited 2021 Aug 23] Available from: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2755485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali MA, Ahmad A, Chaudry H, Aiman W, Aamir S, Yasir Anwar M, Khan A. Efficacy and safety of recently approved drugs for sickle cell disease: a review of clinical trials [Internet], 2020. [cited 2021 Dec 19] Available from: 10.1016/j.exphem.2020.08.008 [DOI] [PMC free article] [PubMed]
- 8.Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, Hartzman R, Rizzo JD, Horowitz M, Confer D, Maiers M. HLA Match Likelihoods for Hematopoietic Stem-Cell Grafts in the U.S. Registry A BS TR AC T. N Engl J Med, 2014. 371: 339–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dehn J, Chitphakdithai P, therapy BS-… and cellular, 2021 undefined. Likelihood of Proceeding to Allogeneic Hematopoietic Cell Transplantation in the United States after Search Activation in the National Registry: Impact of Patient Age [Internet]. Elsevier; [cited 2022 Jan 24] Available from: https://www.sciencedirect.com/science/article/pii/S1083879120306595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanter J, Phillips S, Schlenz AM, Mueller M, Dooley M, Sirline L, Nickel R, Brown RC, Hilliard L, Melvin CL, Adams RJ. Transcranial doppler screening in a current cohort of children with sickle cell anemia: Results from the DISPLACE Study [Internet]. J Pediatr Hematol Oncol, 2021. [cited 2023 Feb 15] Available from: https://journals.lww.com/jpho-online/Fulltext/2021/11000/Transcranial_Doppler_Screening_in_a_Current_Cohort.10.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu LL, Hooper WC, Schieve LA. Prioritizing Sickle Cell Disease [Internet]. Pediatrics, 2022. 150. [cited 2023 Feb 15] Available from: /pediatrics/article/150/6/e2022059491/189548/Prioritizing-Sickle-Cell-Disease [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hankins J, Hinds P, Day S, Carroll Y, Li CS, Garvie P, Wang W. Therapy preference and decision-making among patients with severe sickle cell anemia and their families& [Internet]. Pediatr Blood Cancer, 2007. 48: 705–710. [cited 2022 May 2] Available from: 10.1002/pbc.20903 [DOI] [PubMed] [Google Scholar]
- 13.Reliability Fitzner K. and validity: A quick review. Diabetes Educ, 2007. 33: 775–780. [DOI] [PubMed] [Google Scholar]
- 14.Weiss BD, Mays MZ, Martz W, Castro KM, DeWalt DA, Pignone MP, Mockbee J, Hale FA. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med, 2005. 3: 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vermylen C, Cornu G, Ferster A, Brichard B, Ninane J, Ferrant A, Zenebergh A, Macs P, Dhooge C, Benoit Y, Beguin Y, Dresse MF, Sariban E. Haematopoietic stem cell transplantation for sickle cell anaemia: the first 50 patients transplanted in Belgium [Internet]. Bone Marrow Transplant 1998. 221, 1998 22: 1–6. [cited 2022 Mar 28] Available from: https://www.nature.com/articles/1701291 [DOI] [PubMed] [Google Scholar]
- 16.Johnson FL, Look AT, Gockerman J, Ruggiero MR, Dalla-Pozza L, Billings FTI. Bone-Marrow Transplantation in a Patient with Sickle-Cell Anemia [Internet]. 10.1056/NEJM198409203111207, 2010. 311: 780–783. [DOI] [PubMed] [Google Scholar]
- 17.Walters MC, Patience M, Leisenring W, Eckman JR, Scott JP, Mentzer WC, Davies SC, Ohene-Frempong K, Bernaudin F, Matthews DC, Storb R, Sullivan KM. Bone Marrow Transplantation for Sickle Cell Disease [Internet]. N Engl J Med, 1996. 335: 369–376. Available from: 10.1056/NEJM199608083350601 [DOI] [PubMed] [Google Scholar]
- 18.Johnson L-MLM, Leek ACAC, Drotar D, Noll RB, Rheingold SRSR, Kodish EDED, Baker JNJN. Practical communication guidance to improve phase 1 informed consent conversations and decision-making in pediatric oncology. Cancer, 2015. 121: 2439–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Béranger A, Bouazza N, De Haut De Sigy A, Foubert-Wenc A-C, Davous D, Aerts I, Geoerger B, Auvrignon A, Brethon B, Leblond P, Corradini N, André N, Martinez H, Dupont J-CK, Doz F, Chappuy H. Parents’ and children’s comprehension and decision in a paediatric early phase oncology trial: a prospective study [Internet]. adc.bmj.com, 2019. 104: 947–952. [cited 2022 Mar 8] Available from: https://adc.bmj.com/content/104/10/947.abstract?casa_token=xF6mi9hb7vkAAAAA:NDMcGmEsITrcqwMEd3Q-6nqxReiP0JavufP-vJklemwd0MqYCMc8cSXRvVbAKuoewHRoovw8_KqjQA [DOI] [PubMed] [Google Scholar]
- 20.Johnson L-MLM, Sykes ADAD, Lu Z, Valdez JMJM, Gattuso J, Gerhardt E, Hamilton KVKV, Harrison LWLW, Hines-Dowell SJSJ, Jurbergs N, McGee RBRB, Nuccio R, Ouma AAA, Pritchard M, Quinn EAEA, Baker JNJN, Mandrell BNBN, Nichols KEKE. Speaking genomics to parents offered germline testing for cancer predisposition: Use of a 2-visit consent model [Internet]. Cancer, 2019. 125: 2455–2464. [cited 2022 Mar 8] Available from: 10.1002/cncr.32071 [DOI] [PubMed] [Google Scholar]
- 21.Jegede T, Rawle H. Informed consent for exchange blood transfusions in sickle cell disease. Nurs Stand, 2008. 23. [DOI] [PubMed] [Google Scholar]
- 22.Omondi NA, Ferguson SES, Majhail NS, Denzen EM, Buchanan GR, Haight AE, Labotka RJ, Rizzo JD, Murphy EA. Barriers to hematopoietic cell transplantation clinical trial participation of African American and black youth with sickle cell disease and their parents. J Pediatr Hematol Oncol, 2013. 35: 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson LM, Sykes AD, Lu Z, Valdez JM, Gattuso J, Gerhardt E, Hamilton KV., Harrison LW, Hines-Dowell SJ, Jurbergs N, McGee RB, Nuccio R, Ouma AA, Pritchard M, Quinn EA, Baker JN, Mandrell BN, Nichols KE. Speaking genomics to parents offered germline testing for cancer predisposition: Use of a 2-visit consent model [Internet]. Cancer, 2019. 125: 2455–2464. [cited 2022 May 30] Available from: 10.1002/cncr.32071 [DOI] [PubMed] [Google Scholar]
- 24.Booth A, Bonham V, Porteus M, Ormond KE. Treatment decision-making in sickle cell disease patients [Internet]. J Community Genet, 2022. 13: 143. [cited 2022 May 2] Available from: /pmc/articles/PMC8799810/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.FDA Places Clinical Hold on LentiGlobin Gene Therapy Trials - ASH Clinical News [Internet] [cited 2021 May 5] Available from: https://www.ashclinicalnews.org/online-exclusives/fda-places-clinical-hold-lentiglobin-gene-therapy-trials/
- 26.Holzer JK, Ellis L, Merritt MW. Why We Need Community Engagement in Medical Research [Internet]. J Investig Med, 2014. 62: 851–855. [cited 2022 Mar 28] Available from: https://jim.bmj.com/content/62/6/851 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


