Modified dynamic CT myelography performed with single scan acquisitions, smaller volume of contrast, and reduced scan coverage can reduce the radiation dose for type 1 and 2 CSF leak detection.
Abstract
BACKGROUND AND PURPOSE:
Dynamic CT myelography can identify spinal CSF leaks secondary to dural tears (type 1) and ruptured meningeal diverticula (type 2), but the radiation can be high secondary to multiple successive acquisitions. The purpose of this article is to discuss the procedural approach of a modified dynamic CT myelography technique with single scan acquisitions, reduced contrast volume, and condensed scan coverage and compare its radiation dose with that in traditional dynamic CT myelography.
MATERIALS AND METHODS:
Retrospective review was performed for patients with spontaneous CSF leaks showing extradural collections on spine MR imaging who underwent traditional and modified dynamic CT myelography. The radiation doses between the 2 cohorts were compared.
RESULTS:
Thirty-seven patients (25 women, 12 men) had a type 1 or 2 CSF leak on dynamic CT myelography. Thirty-one patients had a type 1 CSF leak, and 6 patients had type 2 leaks. The traditional dynamic CT myelography was performed in 25 patients, and the average number of acquisitions per dynamic CT myelography was 3.6. The mean total effective dose per dynamic CT myelography was 31.3 mSv (range, 11.3–68.4 mSv). The modified dynamic CT myelography was performed in 12 patients, and the average number of acquisitions was 2.8. The mean total effective dose per dynamic CT myelography was 15.1 mSv (range, 4.8–24.6 mSv). The effective dose and dose-length product between the cohorts were statistically significant (P < .0001 and .01, respectively).
CONCLUSIONS:
Modified dynamic CT myelography performed with single scan acquisitions, smaller volume of contrast, and reduced scan coverage can reduce the radiation dose for type 1 and 2 CSF leak detection.
Spontaneous intracranial hypotension (SIH) has 4 known causes: dural tears (type 1), ruptured meningeal diverticula (type 2), CSF-venous fistulas (type 3), and distal nerve root sleeve leaks (type 4).1,2 Type 1 and 2 CSF leaks manifest well on spine MR imaging as an extradural collection;3 however, location of the leak site is not often possible on MR imaging, and myelography is often necessary for accurate localization. Various types of myelography have been reported to identify type 1 CSF leaks, which include dynamic CT myelography (CTM),4,5 digital subtraction myelography (DSM),2,6,7 and conventional fluoroscopic myelography.8 All 3 examinations can identify the exact transition of iodinated contrast from the subarachnoid-to-extradural space, which corresponds to the leak site. While no publications have compared the sensitivity and specificity among the 3 examination techniques, CSF leak localization is often achievable with all 3 examinations.
The dynamic CTM technique has the major advantage in identifying a calcified disc, which is very commonly seen with type 1 CSF leaks and is often small and located in the upper thoracic spine.9 In fact, because of the high spatial resolution of CT, a postmyelography CT is performed after DSM or conventional fluoroscopic myelography for disc detection and treatment planning.7,8 One major limitation for dynamic CTM is the radiation dose because multiple scans of the spine, reported up to 10 times,5 are performed consecutively to ensure a high temporal resolution and to identify the leak location. Our institution initially used this technique, obtaining 3 acquisitions of the total spine from caudal-cranial, cranial-caudal, caudal-cranial, and then additional scans afterward as needed. We then observed that the CSF leak can often be identified with 3 modifications: single-scan acquisitions, reduced contrast dose, and condensed scan coverage. The purpose of this article is to discuss the procedural approach of this modified dynamic CTM technique and compare the radiation dose with that in traditional dynamic CTM.
MATERIALS AND METHODS
Patient Selection
Institutional review board approval (Kaiser Permanente IRB# 1620615–2) was obtained, which waived the requirement for informed consent. The study population consisted of all patients with spontaneous CSF leaks showing extradural collections on spine MR imaging, from August 2018 to November 2022, who underwent spinal CSF leak detection using traditional or modified dynamic CTM techniques. The spinal CSF leak levels were subdivided into cervical spine, upper thoracic spine (T1–T6 levels), lower thoracic spine (T7–T12 levels), and lumbar spine. It was noted if a calcified disc was associated with the spinal leak.
Modified Dynamic CTM Technique
In patients with a high clinical suspicion for SIH and/or intracranial imaging findings consistent with SIH, a noncontrast total spine MR imaging is performed with fat-suppressed T2-weighted sequences. In our experience, as well as another study,10 MR imaging can reliably detect extradural fluid collections similar to conventional CT myelography. Thus, we do not perform conventional CT myelography simply to identify the presence or absence of an extradural collection.
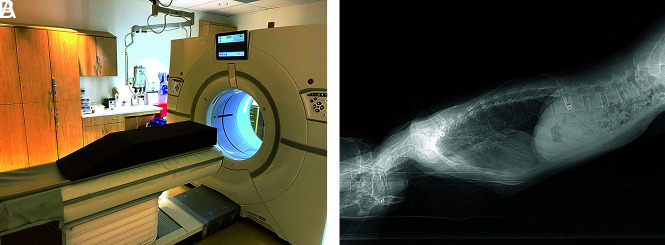
The patient position for the dynamic CTM is predicated on the location of the extradural collection on spine MR imaging. If the collection is ventral or both ventral and dorsal, these collections are often secondary to ventral CSF leaks (type 1). If the extradural collection is predominantly lateral and centered along the meningeal diverticula, a ruptured meningeal diverticulum is often the cause (type 2). On the basis of these findings, we place the patient Trendelenburg prone for type 1 leaks and Trendelenburg decubitus for type 2 leaks using a custom-made wedge with a 15° angle (Fig 1A). Depending on the patient body habitus, if the angle is still not adequate, we will often place blankets or pillows under the hips to ensure an adequate angle of 15°–20°, because this is one of the key requisites to ensure that the contrast bolus adequately coats the upper thoracic and cervical spine.
FIG 1.
Patient positioning for modified dynamic CTM. A, A custom-made firm wedge with a 15° slope is placed on the CT gantry. The hips are placed at the apex of the wedge, with the feet closest to the scanner. The patient is positioned Trendelenburg prone for presumed ventral dural tears (type 1 CSF leaks) and positioned Trendelenburg decubitus for presumed ruptured meningeal diverticula (type 2 CSF leaks). B, A lateral scout radiograph shows an adequate angle for contrast to flow from the lumbar puncture site to the cervical spine.
Frontal and lateral scout images of the total spine are obtained, and the angle is evaluated again to ensure that the patient is adequately Trendelenburg (Fig 1B) or to determine if adjustments are needed. After satisfactory positioning, a lumbar puncture is typically performed at the L3–L4 level. To ensure that the needle is subarachnoid, we perform a test dose of 0.2 mL of preservative-free iohexol contrast (Omnipaque 300; GE Healthcare). After confirmation of contrast within the subarachnoid space, image planning with the CT technologist is performed to obtain 1 caudal-to-cranial acquisition from the needle entry site to the C2 vertebral body using a standard kernel, 0.625-mm section thickness, and automatic exposure control radiation setting. The proceduralist will then inject 3 mL of the aforementioned contrast, exit the CT suite, and the CT technologist will perform the preset scanning acquisition.
The proceduralist will evaluate the axial images on the CT scanner console for the contrast transition from the subarachnoid-to-extradural space. If additional scrutiny is needed, the proceduralist will evaluate the axial and sagittal images on a dedicated radiology workstation located near the CT suite. The proceduralist is afforded this time to review the scans because a small volume of contrast is administered. If the images are satisfactory and the leak site and cause are identified, the myelogram is complete. If confirmation is needed, only the scan plane of the area of interest is prepared—not the total spine—to minimize radiation, followed by an additional 2–3 mL of contrast injection; then the scan is performed. If the contrast has not migrated cranially, additional contrast is administered up to a maximum of 10 mL. If the more caudal aspect of the spine does not show the CSF leak, a portion can be omitted in the subsequent scanning acquisition to conserve the radiation dose. A small volume of contrast is intentionally used initially. If a large volume (eg, 10 mL) is administered initially, exact leak localization may not be possible because the extradural contrast can diffuse throughout the spine, akin to spine MR imaging or a conventional CTM. If there is a suspicion of a lower thoracic CSF leak on the spine MR imaging, an initial dose of 1–2 mL of iodinated contrast can be used on the first scan acquisition. In case a follow-up dynamic CTM is needed for treatment planning, the scan coverage can be restricted to the spinal leak location to minimize the radiation dose.
Traditional Dynamic CTM Technique
The patient positioning and lumbar puncture technique were the same. Intrathecal contrast was injected with 3–10 mL, depending on the preference of the radiologist, and 3 scans of the total spine were obtained from caudal-cranial, cranial-caudal, and caudal-cranial, followed by additional single-scan acquisitions as necessary until the leak site was identified.
Image Review and Reporting
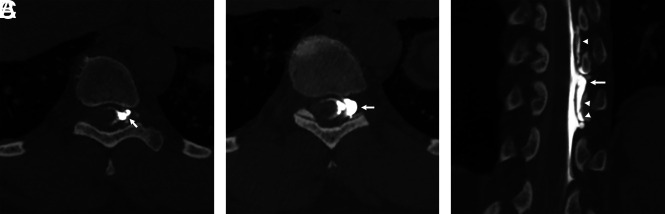
The dynamic CTM is evaluated for the presence of a type 1 or 2 CSF leak. The transition point where the contrast media flows from subarachnoid to extradural in location corresponds to the leak site. If there is an associated calcified disc, the location and size are reported (Fig 2). Type 1 leaks may occasionally be posterior or lateral in location, and we report the leak location and whether there is an associated dorsal osteophyte.11 For type 2 leaks, we evaluate for CSF leakage centered at a meningeal diverticulum.
FIG 2.
Detection of type 1 CSF leak with modified dynamic CTM in 2 scan acquisitions. A, Coronal contrast-enhanced fat-suppressed T1-weighted image shows dural enhancement (arrows) consistent with spontaneous intracranial hypotension. B, Axial fat-suppressed T2-weighted image shows ventral and dorsal extradural fluid (arrows) consistent with a CSF leak. C, Sagittal image from dynamic CTM after 3 mL of intrathecal iodinated contrast shows coating of the entire ventral spine, with a transition point at the T1–T2 level (arrow). D, Sagittal image from dynamic CTM after additional injection of 2 mL of contrast and scanning of the cervicothoracic junction only confirms a leak at the T1–T2 level, where there is a split in the contrast column (arrow) and extradural contrast layering dependently with gravity in the cervical spine (arrowheads). E, Axial image from the dynamic CTM shows that the CSF leak (arrowheads) is secondary to a ventral midline calcified disc (arrow).
Radiation Data
The number of dynamic CTM examinations was recorded until the CSF leak level was identified, and the number of scan acquisitions per CTM examination was tallied. The CT dose index and dose-length product were obtained for each acquisition, and an average per CTM examination was calculated. The effective dose per scan acquisition was obtained using a commercial software DoseWatch (GE Healthcare), and the total effective dose was calculated for each CTM examination.
Statistical Analysis
To identify any differences in CT dose index, dose-length product, and total effective dose between the traditional and modified dynamic CTM techniques, we performed homoscedastic t-tests (Excel; Microsoft), and P values < .05 were considered statistically significant.
RESULTS
There were 38 patients with a spontaneous extradural collection on spine MR imaging who underwent dynamic CTM. One patient was excluded because the dynamic CTM was negative for an extradural collection. We presumed that this was related to spontaneous patching of the spinal leak because the patient’s symptoms resolved. There were 37 patients (25 women, 12 men) with a type 1 or 2 CSF leak on dynamic CTM with a mean age of 44.1 years and a mean Bern SIH score of 5.7 (Table 1). Thirty-one patients had a type 1 CSF leak, and the leak locations were 2 cervical, 22 upper thoracic (T1–T6), 7 lower thoracic (T7–T12), and no lumbar spine. Thirty of 31 patients with type 1 CSF leaks had a ventral tear, with 24 calcified discs identified as the leak cause, while 1/31 patients had a lateral tear. Six patients had type 2 leaks with ruptured meningeal diverticula, and they were all in the lower thoracic (T7–T12) spine. The specific leak location was identified on the first dynamic CTM in 35 patients and on the second dynamic CTM in 2 patients: One patient had a traditional CTM, and the other patient had a modified CTM.
Table 1:
Patient demographics of spinal CSF leaks
| Demographics | |
| No. | 37 |
| Mean age (SD) at symptom onset (yr) | 44.1 (10.2) |
| Sex | |
| Female | 25 |
| Male | 12 |
| Type 1 leak location (n = 31) | |
| Cervical | 2 |
| Upper thoracic (T1–T6) | 22 |
| Lower thoracic (T7–T12) | 7 |
| Lumbar | 0 |
| Type 2 leak location (n = 6) | |
| Lower thoracic (T7–T12) | 6 |
The traditional dynamic CTM was performed in 25 patients, and the average number of acquisitions per dynamic CTM was 3.6 (range, 1–7). The mean CT dose index, dose-length product, and total effective dose per dynamic CTM were 13.4 mGy (range, 6.6–31.6 mGy), 647.2 mGy cm (range, 259.1–727.2 mGy cm), and 31.3 mSv (range, 11.3–68.4 mSv), respectively (Table 2). The radiation dose was not available in 2 patients.
Table 2:
Radiation doses of traditional and modified dynamic CTM
| Traditional Dynamic CTM Technique | Modified Dynamic CTM Technique | P Value | |
|---|---|---|---|
| No of patients | 25 | 12 | |
| Mean radiation dose | |||
| CTDI | 13.4 (range, 6.6–31.6) mGy | 10.6 (range, 4.0–28.3) mGy | .24 |
| DLP | 647.2 (range, 259.1–727.2) mGy cm | 406.7 (range, 165.7–747.5) mGy cm | .01 |
| Total effective dose | 31.3 (range, 11.3–68.4) mSv | 15.1 (range, 4.8–24.6) mSv | <.0001 |
Note:—CTDI indicates CT dose index; DLP, dose-length product.
Modified dynamic CTM was performed in 12 patients, and the average number of acquisitions per dynamic CTM was 2.8 (range, 2–4). The mean CT dose index, dose-length product, and total effective dose per dynamic CTM were 10.6 mGy (range, 4.0–28.3 mGy), 406.7 mGy cm (range, 165.7–747.5 mGy cm), and 15.1 mSv (range, 4.8–24.6 mSv), respectively.
The modified dynamic CTM mean effective dose and dose-length product were statistically significant compared with the traditional technique (P < .0001 and .01, respectively). The CT dose index was not statistically significant (P = .24).
In all patients with ≥4 scan acquisitions per dynamic CTM (traditional and modified), there was an average of 2.6 acquisitions in which the injected intrathecal contrast did not extend to the cervical spine. In patients with <4 scan acquisitions per dynamic CTM examination, there was an average of 0.21 acquisitions in which the injected intrathecal contrast did not extend to the cervical spine.
DISCUSSION
Dynamic CTM is a valuable tool to identify the precise leak site in patients with type 1 (dural tear) and type 2 (ruptured meningeal diverticula) CSF leaks. The technique was originally devised by performing multiple consecutive scan acquisitions of the total spine to identify the leak site. In 1 report by Thielen et al4 with 14 patients, a noncontrast CT of the spine was performed, followed by the operator injecting contrast, while up to 6 acquisitions of the total spine were performed with alternating caudal-cranial and cranial-caudal scans. The mean effective dose per study was 70.64 mSV (range, 21.5–182. 9 mSV). Another study by Dobrocky et al5 with 14 patients used a similar technique of obtaining multiple acquisitions (mean, 7; range, 3–10 acquisitions) but had a smaller volume of coverage (mean, 8 vertebral body levels; range, 4–12 levels). The mean effective dose per study was 24.4 mSv (range, 9.8–67.5 mSv). Early in our practice, we performed a similar technique of obtaining multiple successive scans, and the mean effective dose per study was 31.3 mSv (range, 11.3–68.4 mSv). After we switched to our modified dynamic CTM technique, the mean effective dose per study was 15.1 mSv (range, 4.8–24.6 mSv). This is a radiation savings of approximately 50% effective dose compared with our older technique, 40% compared with the dose of Dobrocky et al, and 80% compared with Thielen et al. Thus, radiation savings is possible with a modified dynamic CTM technique, and this modified technique may be beneficial in all patients but particularly young adults and children with spinal CSF leaks. In addition to our lower radiation dose, we had an average of 2.8 acquisitions with our new technique, which is also less than those in both aforementioned studies.
For type 1 CSF leaks, most leaks were ventral in location (30/31 patients) and were often secondary to calcified discs (24/31 patients) in the upper thoracic spine (22/31 patients). One tip that we found helpful either during the myelogram or afterward is to scrutinize the sagittal reformatted images of the CTM rotated in a horizontal plane (Fig 2) and observe this transition point, because the extradural collection can be easier to identify in its entirety as opposed to 1 section on the axial imaging. For type 2 leaks, all of these were in the lower thoracic spine. We scrutinize the ruptured meningeal diverticulum predominantly on axial imaging but have also found the coronal plane helpful in characterization.
Both the traditional and modified dynamic CTMs were successful in finding the exact leak site, which was identified in 35 patients on the first dynamic CTM and 2 patients on the second dynamic CTM. While dynamic CTM, in general, is helpful in characterizing the leak site, the traditional technique that uses multiple successive scans may be unnecessary. In our experience using the traditional method, we often found the leak site on the first scan. The additional acquisitions increased the radiation dose and did not necessarily provide much additional information (Fig 3). Insufficient contrast coating of the upper spine is one of the challenges that can be encountered with dynamic CTM. This is usually secondary to a shallow Trendelenburg angle, and if present, the patient can be elevated higher to ensure an approximately 15°–20° angle. This pitfall is something we encountered early in our practice and was magnified when we used to perform successive scan acquisitions in dynamic CTM, and additional scans were required to ensure that the contrast media went acceptably cranial. This pitfall was a promulgating factor to switch to the modified dynamic CTM technique with single scan acquisitions. In addition, our data show that patients with ≥4 scan acquisitions per dynamic CTM had more acquisitions in which the contrast did not extend to the cervical spine as opposed to patients with <4 scan acquisitions.
FIG 3.
Detection of type 1 CSF leak with traditional dynamic CTM in 3 scan acquisitions. A–C, Sagittal images from 3 successive acquisitions of the total spine dynamic CTM show a split of contrast at the T1–T2 level with contrast accumulating in the ventral extradural space (arrows). This was first evident on the first acquisition, and the leak site can be determined with this acquisition alone. The second and third acquisitions show slightly more contrast accumulation in the extradural space. D, Axial image of the first acquisition of the dynamic CTM shows a calcified disc (arrowhead) splitting the contrast within the subarachnoid space (arrows).
Dynamic fluoroscopic myelography and DSM are additional techniques that can identify type 1 and 2 CSF leaks. The former provides high temporal resolution, and the detector can be moved to follow the contrast media flowing cranially. DSM also has high temporal resolution but has a limited area of scan coverage and is sensitive to breathing and motion artifacts, resulting in general anesthesia in certain scenarios. DSM also has higher radiation compared with fluoroscopic myelography. Dynamic CTM has relatively less temporal resolution but has excellent spatial resolution, particularly when evaluating calcified discs in the upper thoracic, which is an area that can be obscured on planar imaging due to superimposed shoulders and ribs.8 Last, dynamic CTM can image the whole spine, and, in our experience, is a fairly easy technique to learn.
There are relatively fewer reports discussing the imaging evaluation of type 2 leaks (ruptured meningeal diverticula) compared with other types of CSF leaks, probably related to the rarity of these leaks. While type 2 leaks are less common, the same modified dynamic CTM can precisely identify the specific ruptured meningeal diverticulum with reduced radiation. One report describes decubitus dynamic CTM for lateral leaks with 6 acquisitions of the spine.12 Our modified technique was able to reduce the number of scan acquisitions and area of contrast (Fig 4). Last, owing to the rarity of these leaks, one may misinterpret the extradural collection on the spine MR imaging and position the patient in a Trendelenburg prone position and fail to identify the ruptured meningeal diverticulum. In these cases, the patient may be rotated to a decubitus position to identify the leak, or the patient may have to return on a separate day and undergo the dynamic CTM in the decubitus position.
FIG 4.
Detection of a type 2 CSF leak with a modified dynamic CTM in 2 scan acquisitions. A, Axial image from left decubitus dynamic CTM after 1 mL of intrathecal iodinated contrast shows extradural contrast leaking from a ruptured left T9–T10 meningeal diverticulum (arrow). B, Axial image after an additional 2 mL of contrast was administered shows further leakage at this site (arrow), providing additional confirmation in the leak location. C, Coronal image from the same second CTM acquisition shows the leak extent better—ruptured diverticulum (arrow) and extradural contrast cranially and caudally (arrowheads).
Precise localization of all CSF leaks is important for treatment planning. At our institution, we perform targeted blood and fibrin glue patches for types 1 and 2 CSF leaks via a foraminal and/or interlaminar approach, trying to place the needle as close to the leak site as possible. We have witnessed symptomatic improvement in all patients. We refer patients with incomplete resolution of symptoms for neurosurgical treatment. Thus, identifying the precise leak site is paramount for surgical referrals.
Our study has limitations, including its retrospective nature and is subject to bias. Second, our study sample size with the modified dynamic CTM technique is small. Nonetheless, this sample size is comparable with that in 2 other previously mentioned studies on traditional dynamic CTM.4,5
CONCLUSIONS
Dynamic CTM is a useful technique to help identify the exact spinal leak location and cause in patients with type 1 (dural tears) and 2 (ruptured meningeal diverticula) CSF leaks. A modified version of this technique by performing single scan acquisitions with a smaller volume of contrast and reduced scan coverage can help reduce the radiation dose.
ABBREVIATIONS:
- CTM
CT myelography
- DSM
digital subtraction myelography
- SIH
spontaneous intracranial hypotension
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Schievink WI, Maya MM, Jean-Pierre S, et al. A classification system of spontaneous spinal CSF leaks. Neurology 2016;87:673–79 10.1212/WNL.0000000000002986 [DOI] [PubMed] [Google Scholar]
- 2.Farb RI, Nicholson PJ, Peng PW, et al. Spontaneous intracranial hypotension: a systematic imaging approach for CSF leak localization and management based on MRI and digital subtraction myelography. AJNR Am J Neuroradiol 2019;40:745–53 10.3174/ajnr.A6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobrocky T, Winklehner A, Breiding PS, et al. Spine MRI in spontaneous intracranial hypotension for CSF leak detection: nonsuperiority of intrathecal gadolinium to heavily T2-weighted fat-saturated sequences. AJNR Am J Neuroradiol 2020;41:1309–15 10.3174/ajnr.A6592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thielen KR, Sillery JC, Morris JM, et al. Ultrafast dynamic computed tomography myelography for the precise identification of high-flow cerebrospinal fluid leaks caused by spiculated spinal osteophytes. J Neurosurg Spine 2015;22:324–31 10.3171/2014.10.SPINE14209 [DOI] [PubMed] [Google Scholar]
- 5.Dobrocky T, Mosimann PJ, Zibold F, et al. Cryptogenic cerebrospinal fluid leaks in spontaneous intracranial hypotension: role of dynamic CT myelography. Radiology 2018;289:766–72 10.1148/radiol.2018180732 [DOI] [PubMed] [Google Scholar]
- 6.Hoxworth JM, Patel AC, Bosch EP, et al. Localization of a rapid CSF leak with digital subtraction myelography. AJNR Am J Neuroradiol 2009;30:516–19 10.3174/ajnr.A1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoxworth JM, Trentman TL, Kotsenas AL, et al. The role of digital subtraction myelography in the diagnosis and localization of spontaneous spinal CSF leaks. AJR Am J Roentgenol 2012;199:649–53 10.2214/AJR.11.8238 [DOI] [PubMed] [Google Scholar]
- 8.Piechowiak EI, Pospieszny K, Haeni L, et al. Role of conventional dynamic myelography for detection of high-flow cerebrospinal fluid leaks: optimizing the technique. Clin Neuroradiol 2021;31:633–41 10.1007/s00062-020-00943-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mamlouk MD, Shen PY, Jun P, et al. Spontaneous spinal CSF leaks stratified by age, body mass index, and spinal level. AJNR Am J Neuroradiol 2022;43:1068–72 10.3174/ajnr.A7548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tay ASS, Maya M, Moser FG, et al. Computed tomography vs heavily T2-weighted magnetic resonance myelography for the initial evaluation of patients with spontaneous intracranial hypotension. JAMA Neurol 2021;78:1275–76 10.1001/jamaneurol.2021.2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madhavan AA, Shlapak DP, Benson JC, et al. Osseous spicules of the posterior elements causing fast cerebrospinal fluid leaks. Neuroradiology 2022;64:1689–93 10.1007/s00234-022-02943-8 [DOI] [PubMed] [Google Scholar]
- 12.Madhavan AA, Verdoorn JT, Shlapak DP, et al. Lateral decubitus dynamic CT myelography for fast cerebrospinal fluid leak localization. Neuroradiology 2022;64:1897–903 10.1007/s00234-022-02985-y [DOI] [PubMed] [Google Scholar]