The authors describe a technique involving conebeam CT performed during intrathecal contrast injection as an adjunct to digital subtraction myelography, allowing identification of some otherwise-missed CSF-venous fistulas.
SUMMARY:
Lateral decubitus digital subtraction myelography is an effective technique for precisely localizing CSF-venous fistulas, a common cause of spontaneous intracranial hypotension. However, despite an optimal imaging technique, digital subtraction myelography fails to identify some CSF-venous fistulas for a variety of reasons. Here, we describe a technique involving conebeam CT performed during intrathecal contrast injection as an adjunct to digital subtraction myelography, allowing identification of some otherwise-missed CSF-venous fistulas.
Spontaneous intracranial hypotension is caused by spinal CSF leaks, which, in turn, have multiple etiologies. These include dural tears (type 1), leaking meningeal diverticula (type 2), and CSF-venous fistulas (CVFs, type 3).1 Myelographic techniques with high spatial and temporal resolution are needed to localize CSF leaks, including CVFs, which may be seen for only a short time after intrathecal contrast injection.2,3 Currently, digital subtraction myelography (DSM) and dynamic CT myelography (CTM) are among the most widely used techniques for CSF leak localization.4,5 Both examinations are performed with the patient in the lateral decubitus position when a CVF is suspected, to maximize their yield.6,7
Conebeam CT (CBCT) has been used in neuroangiography for many years as an adjunct to DSA, and it has multiple applications in this field.8 CBCT uses a rotating x-ray source and detector to acquire multiple fluoroscopic images, which are subsequently reconstructed into a 3D, cross-sectional data set. Unlike with traditional CT, the cone-shaped x-ray source covers the entire imaging FOV in each image, allowing the entire CT to be rendered with a single rotation. Modern equipment allows the user to select variable frame rates, degrees of rotation, and other imaging parameters to balance image quality with radiation dose. CBCT has multiple emerging applications. One prior report described 2 patients in whom CBCT was used to help localize a dural tear.9 To our knowledge, however, CBCT has not been previously reported in the assessment of CVFs. Here, we describe a technique for lateral decubitus DSM (LDDSM) with subsequent CBCT that can be used to identify CVFs that may not be apparent on DSM alone.
TECHNICAL REPORT
Our institution’s technique for LDDSM has been previously described in detail but has been recently modified to incorporate CBCT.4 For patients with clinically suspected spontaneous intracranial hypotension based on the International Classification of Headache Disorders Criteria and no extradural fluid on spine MR imaging that would suggest a type 1 leak, we routinely pursue 2-day LDDSM, starting with the patient in the right lateral decubitus position on the first day.
Patients are placed in the lateral decubitus position with a custom cushion under the pelvis to promote caudocranial flow of contrast. After lumbar puncture with a 20- or 22-gauge spinal needle, a straight anterior-posterior projection is obtained, imaging from C7–T1 through the lowest level that can be seen without electronic magnification. DSM is performed at 1 frame per second for about 90 seconds while injecting 5 mL of Omnipaque 300 (GE Healthcare) and flushing with 5 mL of normal saline. A second straight anterior-posterior projection is obtained from the lumbar puncture site to the highest spinal level that can be seen, and dynamic imaging is repeated during injection of 3 mL of Omnipaque 300.
The images are immediately reviewed while the patient is on the table. If a definite CVF is seen, no further imaging is done. If no definite CVF is seen, the images are further scrutinized for indeterminate findings that may warrant CBCT. These include but are not limited to the following: 1) “flickering” densities that could represent subtle venous opacification, 2) dense foci superimposed on a meningeal diverticulum that could represent a vein overlapping a diverticulum, and 3) large or irregular diverticula that may conceal an occult CVF (Figs 1–3 and Online Video). If such findings are seen, the flat panel detector is centered over the spinal level of greatest concern based on imaging review, and the machine is prepared for CBCT. Depending on the degree of geometric magnification and patient size, about 6 vertebral levels can be captured within the FOV, and we typically place the level of interest in the center of the imaging field. Another 3 mL of Omnipaque 300 is injected during continuous fluoroscopy, which allows immediate visualization of ascending contrast. Once the contrast bolus reaches the spinal level of interest, CBCT is immediately performed. To minimize motion, we obtain the CBCT at end inspiration with the patient holding his or her breath. Finally, the spinal needle is removed, and the patient is taken for immediate lateral decubitus CT myelography using a dual-energy scanner (Somatom Force; Siemens).
FIG 1.
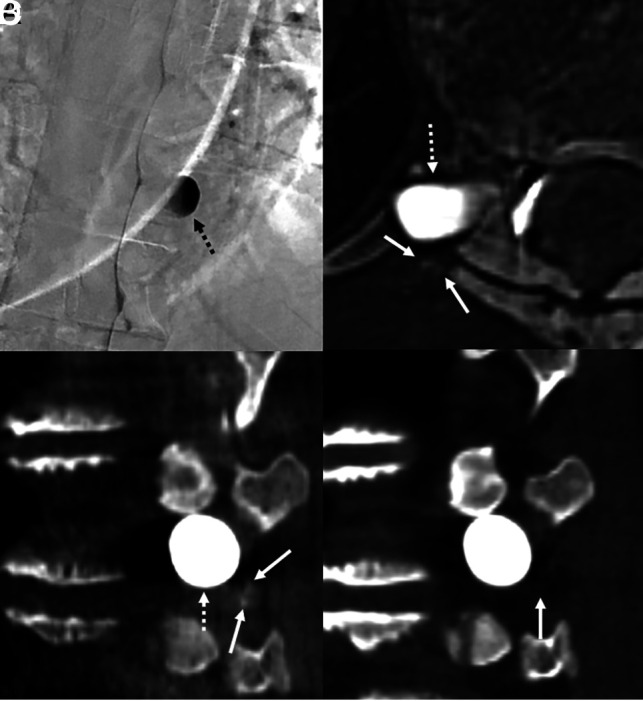
A 52-year-old woman with years of orthostatic headaches and brain MR imaging demonstrating brain sag and pachymeningeal enhancement. Right lateral decubitus DSM (A) shows a large, right T10 meningeal diverticulum (A, dashed arrow), but no venous opacification was seen on dynamic imaging. Axial (B) and sagittal (C) images from CBCT obtained during contrast injection demonstrate subtle opacification of intramuscular venous branches (B and C, arrows) adjacent to the diverticulum (B and C, dashed arrows), compatible with CSF-venous fistula. Sagittal 50-keV monoenergetic reconstruction from right lateral decubitus CT obtained 15 minutes later (D) no longer shows opacification of these veins in the same location (D, arrow). The patient was treated with transvenous Onyx embolization of the right T10 fistula, with complete resolution of symptoms in 3 months.
FIG 2.
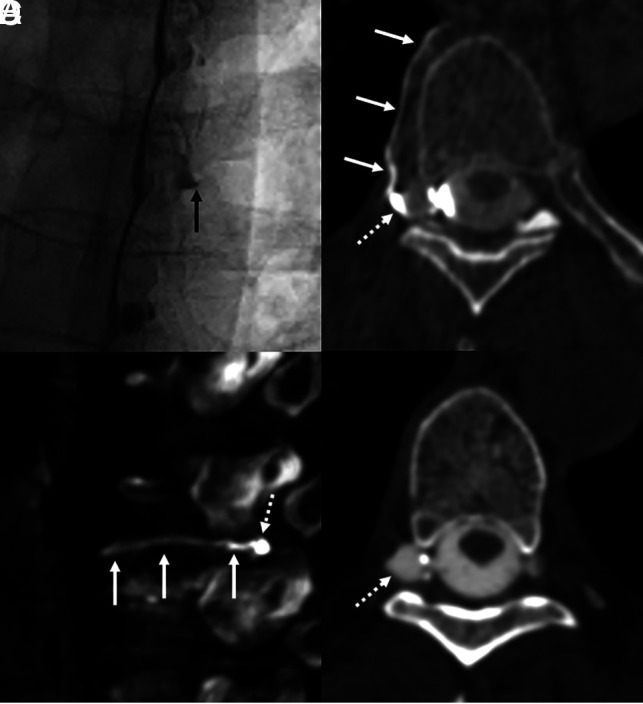
A 56-year-old woman with several months of orthostatic headaches. Brain MR imaging (not shown) demonstrated brain sag and pachymeningeal enhancement. Unsubtracted image from right lateral decubitus DSM (A) shows a prominent right T6 meningeal diverticulum (A, arrow), with subtle flickering density along the lateral edge of the diverticulum during dynamic imaging. Axial (B) and sagittal (C) images from CBCT obtained during contrast injection demonstrate opacification of the right T6 paraspinal vein (B and C, solid arrows), distinct from the diverticulum (B and C, dashed arrows), overall compatible with CSF-venous fistula. Axial 50-keV reconstruction from delayed right lateral decubitus CT obtained 15 minutes later (D) shows a prominent right T6 meningeal diverticulum (D, dashed arrow) without convincing venous contrast. Transvenous Onyx embolization of the right T6 fistula is pending.
FIG 3.
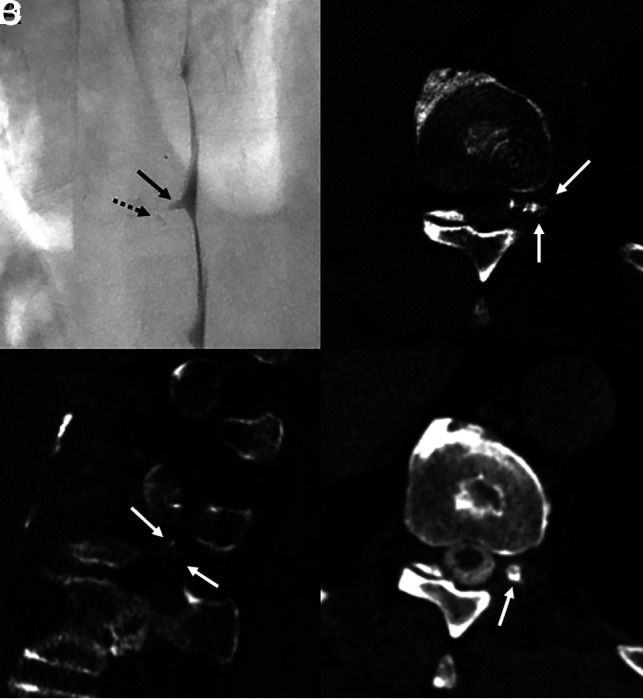
A 66-year-old man with many years of orthostatic headaches that improved after surgical treatment of a left T10 CSF-venous fistula and then recurred. Left lateral decubitus DSM (A) shows a mildly prominent left T10 meningeal diverticulum (A, arrow), with no definite venous contrast on dynamic imaging. Minimal hyperdensity seen adjacent to the diverticulum (A, dashed arrow) was initially thought to be artifactual, secondary to motion or a multilobed diverticulum. CBCT was performed to further investigate the finding. Axial (B) and sagittal (C) images from CBCT obtained during contrast injection demonstrate opacification of numerous left T10 foraminal veins (B and C, arrows), compatible with CSF-venous fistula. Axial 50-keV reconstruction from a delayed left lateral decubitus CT obtained 20 minutes later (D) shows a prominent left T10 meningeal diverticulum (D, arrow) without convincing venous contrast. The patient was treated with transvenous Onyx embolization of the left T10 fistula, with complete resolution of symptoms in 3 months.
We use an Allura Xper FD 20/20 x-ray system (Philips Healthcare). Specific parameters for CBCT include 117 kV, 132 mA, 60 frames per second, and rotation time of 8 seconds with a single rotation (thus, the total acquisition time is 8 seconds as well). This procedure provides images with a section thickness of 0.6 mm. The effective radiation dose varies depending on patient factors and the portion of the body in the FOV, but the median dose in 15 consecutive patients at our institution was estimated to be 8.7 mSv. By comparison, the median effective radiation doses for DSM and CTM alone were 13 mSv and 19.7 mSv, respectively, in 1 recent study.10
DISCUSSION
We have described a technique involving the use of CBCT as an adjunct to LDDSM for localization of CVFs in patients with spontaneous intracranial hypotension. Although LDDSM is an excellent technique for CVF localization, it has limitations that result in missed CVFs in some cases. Some of these can be found using CBCT.
The limitations of LDDSM are varied. First, although LDDSM can be performed using biplane fluoroscopy, the lateral view provides limited information in patients with a large body habitus. Therefore, we routinely use only a single anterior-posterior projection.4 As a result, venous opacification that overlaps meningeal diverticula or other opacified structures can be missed on the single anterior-posterior view. CBCT allows identification of these veins (Fig 1). Second, tiny flickering densities seen on DSM can be caused by pulmonary markings, motion, or subtle opacifying veins, leading to uncertainty in image interpretation. This dilemma can be obviated by performing DSM with the patient under general anesthesia, but that is challenging at some institutions and increases the procedural risk to patients. CBCT can help clarify such DSM findings and determine whether they represent true venous opacification (Fig 2). Finally, since DSM does not provide cross-sectional anatomic information, it is sometimes difficult to differentiate complex, multilobed meningeal diverticula from CVFs. CBCT provides 3D anatomic information that allows a more accurate determination (Fig 3). Additionally, characterization of the anatomic drainage of CVFs can be helpful for subsequent neurointerventional procedures, such as transvenous Onyx (Medtronic) embolization of CVFs.11
Many institutions, including ours, also use lateral decubitus dynamic CTM to localize CVFs. While this technique overcomes many of the limitations of DSM, it has substantially less temporal resolution than DSM. While DSM provides continuous imaging at a high frame rate, lateral decubitus dynamic CTM allows imaging at only a few separate points in time and, as a result, may miss CVFs that opacify only intermittently after contrast injection. However, further study is needed to determine how frequently CVFs have such transient or intermittent opacification, particularly accounting for factors such as the respiratory phase during imaging and layering contrast density. Nonetheless, 1 theoretic advantage of LDDSM with CBCT is that the former provides the temporal resolution of dynamic myelography needed to identify CVFs that may only opacify intermittently, while the latter provides an option to visualize potential CVFs in 3D to clarify indeterminate DSM findings. Additionally, CBCT may have advantages over delayed decubitus CTM. We have found that CVFs seen using CBCT may not be apparent on a delayed CTM obtained as early as 15 minutes later (Figs 1–3). We speculate that delayed decubitus CTM no longer has layering contrast that is sufficiently dense to visualize CVFs, even with 50-keV monoenergetic reconstructions (Figs 2–3).
Our technical report has limitations. We have only performed LDDSM with CBCT in a small number of patients and are not yet able to determine how frequently it provides additional value. To date, we have used CBCT in 15 patients who had indeterminate findings on initial review of their LDDSM. In 7 cases, a CVF was found using CBCT. Five of these patients have undergone transvenous Onyx embolization for treatment, and 4 have had clinical follow-up documenting resolution of symptoms (1 patient has not yet been seen for follow-up). As more data are accrued, further study will be needed to better elucidate the yield of CBCT. Additionally, other modifications to our typical LDDSM technique, such as the use of biplane fluoroscopy, may sometimes be a better alternative to CBCT. Comparison between CBCT and lateral decubitus dynamic CTM will also be helpful. Nonetheless, we have encountered cases in which CBCT is an invaluable adjunctive tool to identify CVFs and currently consider it a worthwhile technique.
Supplementary Material
ABBREVIATIONS:
- CBCT
conebeam CT
- CTM
CT myelography
- CVF
CSF-venous fistula
- DSM
digital subtraction myelography
- LDDSM
lateral decubitus digital subtraction myelography
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
references
- 1.Schievink WI, Maya MM, Jean-Pierre S, et al. A classification system of spontaneous spinal CSF leaks. Neurology 2016;87:673–79 10.1212/WNL.0000000000002986 [DOI] [PubMed] [Google Scholar]
- 2.Hoxworth JM, Patel AC, Bosch EP, et al. Localization of a rapid CSF leak with digital subtraction myelography. AJNR Am J Neuroradiol 2009;30:516–19 10.3174/ajnr.A1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoxworth JM, Trentman TL, Kotsenas AL, et al. The role of digital subtraction myelography in the diagnosis and localization of spontaneous spinal CSF leaks. AJR Am J Roentgenol 2012;199:649–53 10.2214/AJR.11.8238 [DOI] [PubMed] [Google Scholar]
- 4.Kim DK, Brinjikji W, Morris PP, et al. Lateral decubitus digital subtraction myelography: tips, tricks, and pitfalls. AJNR Am J Neuroradiol 2020;41:21–28 10.3174/ajnr.A6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DK, Carr CM, Benson JC, et al. Diagnostic yield of lateral decubitus digital subtraction myelogram stratified by brain MRI findings. Neurology 2021;96:e1312–18 10.1212/WNL.0000000000011522 [DOI] [PubMed] [Google Scholar]
- 6.Kranz PG, Gray L, Amrhein TJ. Decubitus CT myelography for detecting subtle CSF leaks in spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2019;40:754–56 10.3174/ajnr.A5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamlouk MD, Ochi RP, Jun P, et al. Decubitus CT myelography for CSF-venous fistulas: a procedural approach. AJNR Am J Neuroradiol 2021;42:32–36 10.3174/ajnr.A6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angle JF. Cone-beam CT: vascular applications. Tech Vasc Interv Radiol 2013;16:144–49 10.1053/j.tvir.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 9.Chu E, McAuliffe W. Use of flat panel DynaCT myelography to locate the site of CSF leak. J Med Imaging Radiat Oncol 2013;57:455–59 10.1111/1754-9485.12072 [DOI] [PubMed] [Google Scholar]
- 10.Nicholson PJ, Guest WC, van Prooijen M, et al. Digital subtraction myelography is associated with less radiation dose than CT-based techniques. Clin Neuroradiol 2021;31:627–31 10.1007/s00062-020-00942-x [DOI] [PubMed] [Google Scholar]
- 11.Borg N, Cutsforth-Gregory J, Oushy S, et al. Anatomy of spinal venous drainage for the neurointerventionalist: from puncture site to intervertebral foramen. AJNR Am J Neuroradiol 2022;43:517–25 10.3174/ajnr.A7409 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


