Abstract
Intervening sequences (IVSs) occur sporadically in several bacterial genera in the genes for 23S rRNA at relatively conserved locations. They are cleaved after transcription and lead to the presence of fragmented rRNA, which is incorporated into the ribosomes without religation but is nevertheless functional. The fragmentation of rRNA and the number of IVSs in all 72 strains of the Salmonella Reference Collection B set and 16 strains of the Salmonella Reference Collection C set, which have been established on the basis of multilocus enzyme electrophoresis (MLEE), were analyzed in the present study. Fragmentation of 23S rRNA was restricted to conserved cleavage sites located at bp 550 (helix 25) and bp 1170 (helix 45), locations where IVSs have been reported. Random cleavage at sites where IVSs could not be detected was not seen. Uncleaved IVSs were not detected in any case; thus, the IVSs invariably led to rRNA fragmentation, indicating a strong selection for maintenance of RNase III cleavage sites. The distribution of the number of IVSs carried by the different strains in the seven rrl genes is diverse, and the pattern of IVS possession could not be related to the MLEE pattern among the various Salmonella strains tested; this indicates that the IVSs are frequently exchanged between strains by lateral transfer. All eight subspecies of the genus Salmonella, including subspecies V represented by Salmonella bongori, have IVSs in both helix 25 and helix 45; this indicates that IVSs entered the genus after its divergence from Escherichia coli (more than 100 million years ago) but before separation of the genus Salmonella into many forms or that they were in the ancestor but have been lost from Escherichia.
The posttranscriptional excision by RNase III of transcribed sequences called intervening sequences (IVSs) at bp 550 and 1170 in the 23S rRNA genes of Salmonella species leads to fragmentation of 23S rRNA (8). The two sites correspond to helix 25 and helix 45 in the proposed secondary structure for the Escherichia coli rrl (ribosomal RNA large) gene (25). The tetra loops predicted at these positions in E. coli are replaced at the corresponding positions in Salmonella by an extended stem-loop structure of the IVS. The transcribed IVSs are cleaved by RNase III, an enzyme involved in the 23S rRNA maturation (8). The function of IVSs is still unclear. An RNase III-deficient strain with the IVSs intact in the rRNA is viable, although it has a reduced growth rate, presumably because RNase III is also involved in other rRNA-processing reactions (22). Transfer of a plasmid with an IVS-containing rrl gene from Salmonella into E. coli leads to the presence of fragmented 23S rRNA in E. coli, but the cell maintains a wild-type growth rate (10).
IVSs have terminal inverted repeats flanking the central region, which is a characteristic of a mobile genetic element. Thus, IVSs resemble introns; however, unlike introns, after the removal of IVSs and the disruption of the continuity of the 23S rRNA gene, the fragments are not religated. In addition, most of the IVSs do not have known coding open reading frames or terminal sequences which facilitate the splicing reactions; although open reading frames coding for a putative protein of 121 to 133 amino acids are present in IVSs from Leptospira, the protein they encode has not been detected in the cell (32). The ability of the fragmented rRNA to assemble into an active conformation, presumably by RNA-protein interactions, appears to obviate the need for exon ligation (4).
IVSs have not been reported in E. coli strains, suggesting that IVSs entered the salmonellae after the divergence of Salmonella from Escherichia or, alternatively, that they have been lost from Escherichia. The presence of IVSs, without any proven function in stable rRNA genes, is surprising and may have implications regarding the evolution of rRNA transcription units. IVSs are distributed sporadically among the bacteria, having been identified in members of the Enterobacteriaceae (8, 23, 38), Rhizobiaceae (37), Rickettsiaceae (1), and Leptospiraceae (31) families and in the genera Campylobacter (14, 19, 41) and Helicobacter (13, 18). It has also been shown that the distribution of IVSs among the several copies of rRNA operons is heterogeneous. IVSs may be spread between different bacterial genera by lateral transfer from genetic exchange and between the different rRNA genes of a cell by recombination-mediated methods (19, 32, 38).
The genus Salmonella, part of the large eubacterial family Enterobacteriaceae, was originally classified into many serotypes (each called species) by the use of somatic and flagellar antigens, but recently all strains of the genus Salmonella have been separated into two species, Salmonella enterica (for almost all strains) and Salmonella bongori (for subspecies V) (9, 16). The rRNA fragmentation pattern (designated fragmented or nonfragmented) from 77 strains of Salmonella enterica serovar Paratyphi B was used, in combination with other tests, to separate these strains into three groups (3), but later studies showed that these characters vary among isolates of the same clone as defined by multilocus enzyme genotype and thus do not give a meaningful classification (35). In this study, the Salmonella Reference Collection B (SARB) set of 72 strains representing 37 serovars of Salmonella subspecies I (6) and the Salmonella Reference Collection C (SARC) set of 16 strains representing seven subspecies of Salmonella, as well as S. bongori (subspecies V) (7), were analyzed. These sets of strains, established by multilocus enzyme electrophoresis (MLEE) in the laboratory of R. K. Selander, are available from the Salmonella Genetic Stock Centre (SGSC), University of Calgary, Calgary, Canada.
The objectives of the study were as follows: to determine if IVSs in Salmonella are invariably present at the previously reported helix 25 and helix 45 locations and if they are also present at other sites; to determine if fragmentation of rRNA can occur without the presence of IVSs; to determine if any IVSs are present but are not excised by RNase III; and to determine the diversity in distribution of IVSs within the genus Salmonella in order to see if IVSs have been distributed primarily by vertical transmission (clonal propagation) or by lateral (horizontal) transfer.
MATERIALS AND METHODS
Bacterial strains and cultivation conditions.
Sets of strains from the SARB (Table 1) (6) and SARC (Table 2) (7), obtained from R. K. Selander, are maintained at the SGSC (www.ucalgary.ca/∼kesander). Strains are stored at −70°C in 15% glycerol. Single colonies isolated from the stocks were used in all experiments. All cultures were grown in Luria broth base, supplied by Gibco BRL (Luria broth base is equivalent to Millers LB broth base); it is hereafter called LB broth. It contains peptone (10 g/liter), yeast extract (5 g/liter), and NaCl (10 g/liter). 1.5% Bacto Agar (Difco) was added when solid medium was used.
TABLE 1.
Strains of Salmonella from SARBa
| Electrophoretic type | SARB no.b | RKS strain no.c | S. enterica serovar |
|---|---|---|---|
| Ag1 | 1 | 1701 | Agona |
| An1 | 2 | 2403 | Anatum |
| Ba2 | 3 | 4231 | Brandenburg |
| Cs1 | 4 | 1280 | Choleraesuis |
| Cs6 | 5 | 1239 | Choleraesuis |
| Cs11 | 6 | 3169 | Choleraesuis |
| Cs13 | 7 | 4640 | Choleraesuis |
| Dt1 | 8 | 4647 | Decatur |
| De1 | 9 | 246 | Derby |
| De13 | 10 | 241 | Derby |
| De31 | 11 | 243 | Derby |
| Du1 | 12 | 1518 | Dublin |
| Du3 | 13 | 4717 | Dublin |
| Du2 | 14 | 1550 | Dublin |
| Di1 | 15 | 4239 | Duisburg |
| En1 | 16 | 53 | Enteritidis |
| En2 | 17 | 761 | Enteritidis |
| En3 | 18 | 69 | Enteritidis |
| En7 | 19 | 1208 | Enteritidis |
| Em1 | 20 | 1216 | Emek |
| Ga2 | 21 | 2962 | Gallinarum |
| Ha1 | 22 | 4241 | Haifa |
| He1 | 23 | 539 | Heidelberg |
| He3 | 24 | 1391 | Heidelberg |
| Id1 | 25 | 4250 | Indiana |
| In1 | 26 | 1490 | Infantis |
| In3 | 27 | 1452 | Infantis |
| Mi1 | 28 | 2833 | Miami |
| Mi5 | 29 | 4381 | Miami |
| Mo1 | 30 | 1762 | Montevideo |
| Mo6 | 31 | 1740 | Montevideo |
| Mu1 | 32 | 3121 | Muenchen |
| Mu2 | 33 | 4288 | Muenchen |
| Mu3 | 34 | 4300 | Muenchen |
| Mu4 | 35 | 4272 | Muenchen |
| Np8 | 36 | 2016 | Newport |
| Np11 | 37 | 1915 | Newport |
| Np15 | 38 | 1956 | Newport |
| Pn1 | 39 | 1793 | Panama |
| Pn2 | 40 | 1776 | Panama |
| Pn12 | 41 | 1779 | Panama |
| Pa1 | 42 | 4993 | Paratyphi A |
| Pb1 | 43 | 3222 | Paratyphi B |
| Pb3 | 44 | 3202 | Paratyphi B |
| Pb4 | 45 | 3201 | Paratyphi B |
| Pb5 | 46 | 3274 | Paratyphi B |
| Pb7 | 47 | 3215 | Paratyphi B |
| Pc1 | 48 | 4587 | Paratyphi C |
| Pc2 | 49 | 4594 | Paratyphi C |
| Pc4 | 50 | 4620 | Paratyphi C |
| Pu3 | 51 | 2266 | Pullorum |
| Pu4 | 52 | 2246 | Pullorum |
| Re1 | 53 | 4256 | Reading |
| Ru1 | 54 | 4938 | Rubislaw |
| Sp3 | 55 | 1690 | Saintpaul |
| Sp4 | 56 | 1686 | Saintpaul |
| Sw1 | 57 | 4261 | Schwarzengrund |
| Se1 | 58 | 2866 | Sendai |
| Sf1 | 59 | 2358 | Senftenberg |
| St1 | 60 | 4264 | Stanley |
| Sv2 | 61 | 4267 | Stanleyville |
| Th1 | 62 | 1767 | Thompson |
| Tp1 | 63 | 3333 | Typhi |
| Tp2 | 64 | 3320 | Typhi |
| Tm1 | 65 | 284 | Typhimurium |
| Tm7 | 66 | 203 | Typhimurium |
| Tm12 | 67 | 837 | Typhimurium |
| Tm23 | 68 | 4535 | Typhimurium |
| Ts1 | 69 | 3134 | Typhisuis |
| Ts3 | 70 | 3133 | Typhissuis |
| Wi1 | 71 | 4000 | Wien |
| Wi2 | 72 | 3998 | Wien |
TABLE 2.
Strains of Salmonella from SARCa
| Group | SARC no.b | RKS no.c | Salmonella serovar or species |
|---|---|---|---|
| I | 1 | s4194 | Serovar Typhimurium |
| 2 | s3333 | Serovar Typhi | |
| II | 3 | s2985 | |
| 4 | s2993 | ||
| IIIa | 5 | s2980 | |
| 6 | s2983 | ||
| IIIb | 7 | s2978 | |
| 8 | s2979 | ||
| IV | 9 | s3015 | |
| 10 | s3027 | ||
| V | 11 | s3041 | S. bongori |
| 12 | s3044 | S. bongori | |
| VI | 13 | s2995 | |
| 14 | s3057 | ||
| VII | 15 | s3013 | |
| 16 | s3014 |
The SARC sets of strains was obtained from R. K. Selander (7) and is circulated by the Salmonella Genetic Stock Center, University of Calgary.
The SARC number is the number assigned to each of the 16 strains of Salmonella representing subgenus I.
The RKS number is the number assigned by R. K. Selander to stock strains.
Enzymes and chemicals.
Taq polymerase, deoxynucleoside triphosphates, and DNase I were obtained from Pharmacia. RNasin was purchased from Promega, and diethyl pyrocarbonate was purchased from Sigma. Most other chemicals, including agarose, were purchased from Gibco BRL.
Primers.
Amplicon A (for amplicon identification, see Fig. 1) containing helix 25 was amplified with primers P1 and P2. Amplicon B containing helix 45 was amplified with primers P3 and P4. All primers were synthesized by the University Core DNA Services (Health Science Centre, University of Calgary): P1 (5′ GCGTCGGTAAGGTGATATG 3′), P2 (5′ GCTATCTCCCGGTTTGATTG 3′), P3 (5′ CCGATGCAAACTGCCAATAC 3′), and P4 (5′ TTCTCTACCTGACCACCTG 3′) (21). These primers are located at E. coli rrlB bp 74 to 92, 786 to 805, 901 to 920, and 1616 to 1634, respectively (25).
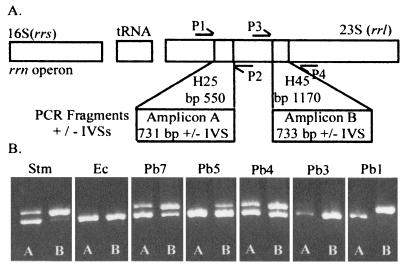
FIG. 1.
(A) Structure of the rrn operon. A single rRNA operon comprises one 16S rRNA (rrs gene), one or two tRNAs, and one 23S rRNA (rrl gene). The rrl gene may include IVSs at H25 (helix 25 of the proposed secondary structure of the rRNA at bp 550) and/or H45 (helix 45 at bp 1170). Primers P1 and P2 were used for amplification of a 731-bp region containing helix 25 (amplicon A), and primers P3 and P4 were used for amplification of a 733-bp region containing helix 45 (amplicon B); these amplicons may contain IVSs. (B) PCR products which were obtained using templates from the following strains: Stm, serovar Typhimurium LT2; Ec, E. coli K-12; Pb7; Pb5; Pb4; Pb3; and Pb1. A, amplicon A; B, amplicon B. Each strain has seven copies of the rrl gene. The lower band in each lane (approximately 730 bp) represents PCR product from an rrl gene which has no IVS; the upper band represents PCR product from an rrl gene which has an IVS. See the text for an interpretation of the number of rrl genes with IVSs in each strain.
PCR and agarose gel electrophoresis.
PCR was carried out according to the instructions accompanying the Taq polymerase on a Techne Gene E thermal cycler. Templates were prepared by boiling bacterial cells (obtained on the tip of a toothpick from a single colony) in 500 μl of water for 5 min and then rapidly cooling on ice. Two microliters of the template was used for each reaction. The thermal profile consisted of 30 cycles of 1 min of denaturation (94°C), 1 min of annealing (56°C), and 1 min of extension (72°C). A final extension step for 10 min at 72°C was performed. All PCR products were electrophoresed on 1.5% agarose gels in 0.5× Tris-borate-EDTA (TBE) buffer (1× TBE buffer contains 90 mM Tris-HCl, 90 mM boric acid, and 2 mM EDTA [pH 8.0]) at 8 V/cm in the presence of 0.5 μg of ethidium bromide per ml.
RNA isolation, electrophoresis, Northern blotting, and methylene blue staining.
Bacteria were grown overnight in LB broth with shaking at 37°C. One milliliter of this culture was inoculated into 20 ml of fresh broth and grown for 4 h under similar conditions. Eight milliliters of the culture was used for the isolation of rRNA by the method described previously (21).
The RNA was quantitated spectrophotometrically and 10 to 20 μg of RNA was electrophoresed on a 1.5% agarose gel in 10 mM sodium phosphate buffer (pH 6.8) at 5 V/cm by the glyoxal-dimethyl sulfoxide denaturation method (34) with constant recirculation from anode to cathode. The RNA was blotted to a Hybond-N+ membrane (Amersham) by capillary action overnight and stained using 0.04% methylene blue in 0.5 M sodium acetate, pH 5.2. The blot was then washed several times with water and photographed using the Diamed photodocumentation system or scanned using Scan Jet 6100C (Hewlett-Packard).
RESULTS
Diversity of distribution of IVSs in the SARB and SARC strains by PCR analysis.
Using whole genomic DNA from the bacterial strains as the template, primers P1 and P2 were used for the amplification of the region of DNA including helix 25 of the rrl gene (amplicon A), and primers P3 and P4 were used for the helix 45 region (amplicon B) (Fig. 1A). The PCR products were electrophoresed to determine the number of rrl genes with IVS insertions (Fig. 1B). The PCR amplicon is smaller (faster running) and is expected to be 731 bp (helix 25) or 733 bp (helix 45) when there is no IVS present (based on E. coli rrl gene numbering) (25); when an IVS is present, the PCR product is larger (slower running). On the basis of the relative intensities of the slow- and fast-running bands, the number of rrl genes with IVSs out of the total seven rrl genes was determined; this is dependent on the assumption that amplification of all rrl genes is equivalent.
Both amplicons A and B in E. coli K-12 are fast running, showing that E. coli does not have IVSs. In S. enterica serovar Typhimurium LT2, the intensity of the slow-running band for amplicon A (helix 25) corresponds to five copies of rrl genes without IVSs and the intensity of the fast-running band corresponds to two copies of rrl genes with IVSs. Similarly, the result for amplicon B indicates one rrl gene without an IVS and six genes with helix 45 IVSs; these data are consistent with the earlier report (21). This reveals the presence of two helix 25 and six helix 45 IVSs (see Fig. 3). Similar analysis leads to the conclusion that S. enterica serovar Paratyphi B electrophoretic type 1 (Pb1) has no insertions in helix 25 and seven insertions in helix 45. Strain Pb3 has one insertion in helix 25 and no insertions in helix 45. Strain Pb4 has two helix 25 and four helix 45 IVSs, while strains Pb5 and Pb7 have ratios of 0:1 and 2:4 helix 25 to helix 45 IVSs, respectively (see Fig. 3).
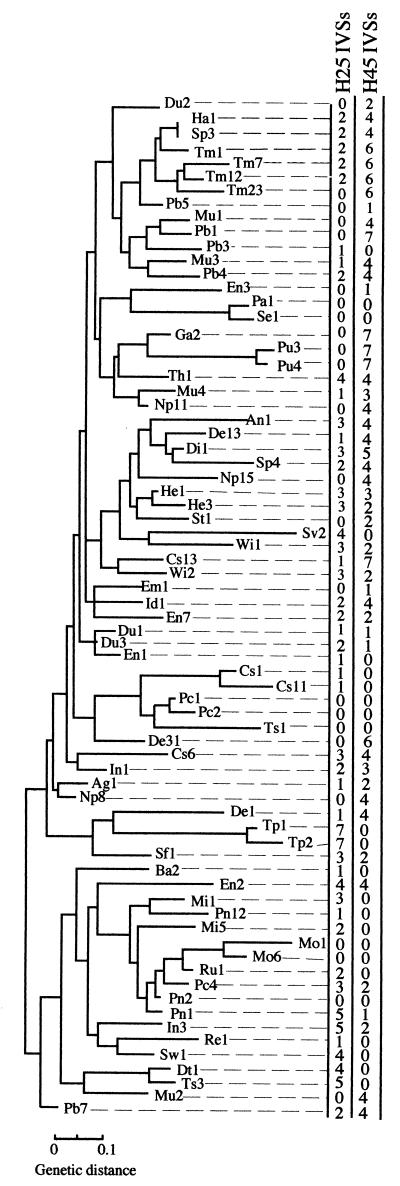
FIG. 3.
The presence of IVSs in 72 strains of Salmonella subgenus I of the SARB set (6). The relationships of the 72 strains shown on the left as electrophoretic types were determined by MLEE. The Salmonella species and SARB numbers are listed in Table 1. The total numbers of IVS insertions in H25 (helix 25) and H45 (helix 45) in the seven rrl genes for 23S rRNA were determined by PCR to detect IVSs and also by rRNA cleavage; the inferred number of IVSs, which was the same for both methods in all 72 strains, is shown.
Diversity of distribution of IVSs in the SARB and SARC strains by RNA analysis.
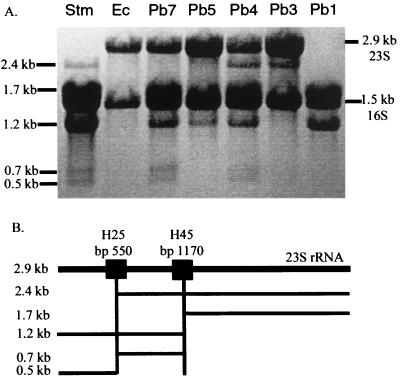
The extent of fragmentation of the 23S rRNA was examined in order to determine the site(s) of cleavage in the rRNA and the proportion of the rRNA molecules in which cleavage has occurred. Figure 2B shows the known sites of the IVSs previously determined in Salmonella rrl genes and the sizes of the rRNA fragments which would result if cleavage occurred at these sites. Figure 2A shows the rRNA fragmentation patterns for the same strains for which PCR data reveal the presence of IVSs in Fig. 1B.
FIG. 2.
(A) rRNA from bacterial strains, detected by electrophoresis, Northern blotting, and methylene blue staining. The same seven strains were tested, in the same order, as in Fig. 1. See Table 1 for a list of the strains tested. Normal rRNA sizes are indicated on the right (23S and 16S). Sizes, in kilobases, on the left indicate fragmented rRNA. (B) The heavy line at the top indicates the full-length rrl gene for 23S rRNA; when transcribed, this produces rRNA of 2.9 kb. The boxes at helix 25 and helix 45 indicate IVSs which result in posttranscriptional cleavage of the rRNA by RNase III. 23S rRNA fragments of 2.4 and 0.5 kb indicate that one rrl gene carries an IVS in helix 25. 23S rRNA fragments of 1.7 and 1.2 kb indicate that one rrl gene carries an IVS in helix 45. 23S rRNA fragments of 1.7, 0.7, and 0.5 kb indicate that one rrl gene carries IVSs in both helices. See the text for an interpretation of the number of rrl genes with IVSs in each strain.
The extent and pattern of fragmentation were studied by analyzing the different band sizes and intensities. The band intensities stoichiometrically add up to reflect the cleavage products resulting from seven copies of rrn operons after the removal of the IVSs. An intact 23S RNA fragment is 2.9 kb in length. 23S rRNA fragments of 2.4 and 0.5 kb indicate that one rrl gene carries an IVS in helix 25, fragments of 1.7 and 1.2 kb indicate an IVS in helix 45, and fragments of 1.7, 0.7, and 0.5 kb indicate IVSs in both helices.
E. coli K-12 gives an intact 2.9-kb band (23S rRNA) and an intact 1.5-kb band (16S rRNA), indicating no fragmentation; as expected, the genome of E. coli K-12 reveals an absence of IVSs in all seven rrl genes (5). In the case of strain Pb1 the 2.9-kb band is absent, and all rRNA is present as 1.7- and 1.2-kb bands; this indicates the excision of helix 45 IVSs from all seven rrl genes. Pb3 shows a 2.4- and a 0.5-kb band (barely visible in Fig. 2), indicating the excision of one helix 25 IVS, while the uncleaved 2.9-kb band and the absence of 1.7-, 1.2-, and 0.7-kb bands indicates no IVSs in helix 45. By similar analysis, it can be seen that Pb5 has no helix 25 IVSs and one helix 45 IVS; Pb3, Pb4, and other SARB and SARC strains were similarly analyzed. All conclusions are listed in Fig. 3.
Analysis of IVSs in the 72 SARB set strains and 16 SARC set strains of Salmonella.
The number of rrl genes containing IVSs in helix 25 and/or helix 45 regions were determined by PCR as described above (Fig. 1) for all 86 strains of the SARB and SARC sets, and the data are recorded in Fig. 3 and 4. Similarly, the pattern of fragmentation of the rRNA was used to determine the number of rrl genes that produced rRNA that was cleaved; these data too are recorded in Fig. 3 and 4. In some cases, the assignment of numbers of IVSs was immediately clear, but in others several independent PCR or rRNA runs were needed.
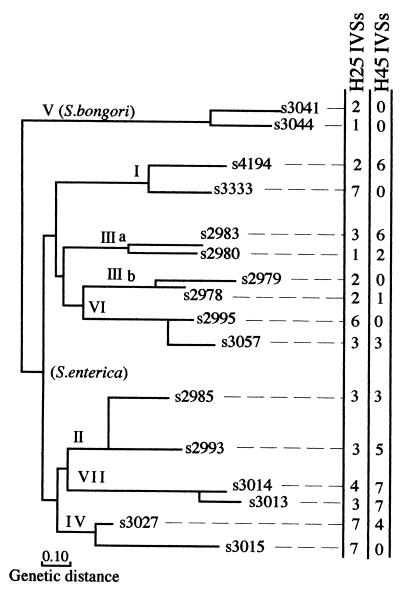
FIG. 4.
The presence of IVSs for the 16 SARC strains representing the eight Salmonella subgroups; genetic distance was determined as described in the legend to Fig. 3 (7). The total number of IVSs in helix 25 and helix 45 was determined by PCR to detect IVSs and also by analysis of RNA to show fragmentation; the inferred number of IVSs from both methods was the same for each strain. Species and SARC numbers are listed in Table 2.
The number of IVSs (detected by PCR) corresponded to the number of IVSs calculated from the rRNA fragmentation (determined from Northern blot analyses of the rRNA) in all 72 strains of the SARB set and all 16 strains of the SARC set. The strains portray extensive diversity in the possession of IVSs, leading to the following conclusions. (i) Possession of IVSs is common, but there are some strains (8 of 72 in SARB and 0 of 16 in SARC) that have no IVSs. (ii) No strains are saturated with the presence of IVSs in all the helix 25 and helix 45 locations; the maximum number of IVSs present in a strain was a 7:4 distribution in helix 25 and helix 45, respectively, in the case of SARC strains 10 and 16. (iii) Closely related strains (according to MLEE) often show identical or very similar patterns of distribution of IVSs, for example, strains Pa1 and Se1 and Pc1, Pc2, and Ts1. (iv) However, there is no strong overall pattern of possession of IVSs on the trees in either helix 25 or helix 45.
DISCUSSION
The number of IVSs in the seven rrl genes (detected by PCR) and the number of cleavage sites in the rRNA (detected by the fragmentation pattern of the rRNA) from 72 Salmonella strains of the SARB set (representing subspecies I) and 16 strains of the SARC set (representing all eight subspecies of Salmonella) were determined; this led to three conclusions.
Firstly, PCR and RNA fragmentation data indicate that all IVSs in Salmonella occur in or close to helix 25 (bp 550) or helix 45 (bp 1170) regions of the 23S rRNA. IVSs in both helix 25 and helix 45 have been previously reported in Helicobacter (13), Haemophilus (39), Proteus, and Providencia (23). IVSs around bp 1170 have been reported in Actinobacillus (11), Campylobacter (14), Leptospira (31), Yersinia (38), Rhodobacter (13), and Coxiella (1). IVSs have also been found at other locations, at bp 135 and 400 of the rrl gene in Rhizobiaceae (37) as well as in the 16S rRNA of Campylobacter helveticus (19), Helicobacter canis (18, 33), Clostridium spp. (30), Rhizobium tropici (43), and Caedibacter caryophila (40). Thus, the positions where IVSs can occur are diverse but by no means random, and in Salmonella all the IVSs are at the two most common sites. Insertion of IVSs and excision from the transcripts are thus tolerated at these locations in the rRNA, suggesting that continuity of the rrl gene in these regions of the 23S rRNA is not required for ribosome function. These IVSs in Salmonella occur at positions homologous to those in which expansion elements occur in eukaryotes such as Drosophila (reviewed by Burgin et al. [8]). These expansion elements are extremely variable in sequence and position (15); some, which have acquired functions and are removed during rRNA processing, are called transcribed spacers or fragmentation spacers (15).
Secondly, fragmentation of the rRNA in Salmonella is always due to the presence of an IVS at the cleavage sites (Fig. 1 to 4). Fragmentation in other genera can result from other causes; in the central region of the 23S rRNA at sites where no IVSs were present, fragmentation was seen in Agrobacterium and Rhizobium strains (36).
Thirdly, uncleaved IVSs were not detected in any of the strains tested, indicating that IVSs when present are always cleaved by RNase III. RNase III-deficient mutants of serovar Typhimurium, which retain IVSs in all their ribosomes, are known to be viable in culture (22), yet uncleaved IVSs were not detected in wild-type strains of Salmonella. This indicates that these strains must have a strong selective pressure for the maintenance of sequences which form a base-paired stem at the site of the IVS in the rRNA transcript (this stem is the substrate for RNase III excision of the IVS); otherwise, unexcised IVSs would occasionally be detected. This suggests that the continued presence of the IVS in the rRNA hinders optimal functioning of the ribosome. The regions at the ends of the 23S rRNA form a stable stem, essential for RNase III-mediated 23S rRNA maturation (17); we propose that there must be similar conservation of the stem region of the IVS to ensure their removal. This suggests that although strains with intact IVSs are viable in culture (22), they would not survive in nature; removal of IVSs confers selective advantage.
Trees showing relationships among the 72 strains of the SARB set (6) and 16 strains of the SARC set (7) of Salmonella were previously constructed, based on MLEE. We determined the number of IVSs in the seven rrl genes for each strain. The data show that very closely related strains often show identical or very similar patterns of distribution of IVSs (Fig. 3 and 4); for example, of eight strains with no IVSs, seven are members of three groups found at different locations on the SARB tree (Fig. 3) (Pa1 and Se1; Pc1, Pc2, and Ts1; and Mo1 and Mo6). This indicates some degree of stability of IVSs, indicating vertical transmission. However, there is also much evidence for lateral transfer of IVSs. IVSs are often found in strains which are unrelated according to MLEE criteria; IVSs seem widely distributed through the various divisions of the SARB set and are usually present in each of the eight subspecies of the SARC set (Fig. 3 and 4). Thus, there is no strong overall pattern of possession of IVSs on the tree either in helix 25 or in helix 45. This indicates that the possession of IVSs is, to a large extent, a random phenomenon and that the process of acquisition through lateral transfer and spread among the seven rrl genes is continuous among the Salmonella spp. An apparent exception to this is subgenus V (S. bongori) which has no helix 45 IVSs in the two strains tested (Fig. 4), suggesting the possibility that helix 45 entered after the divergence of S. enterica from S. bongori, but this apparent exception is not real, for IVSs were detected by PCR in helix 45 in 2 of 13 strains of S. bongori which were tested (data not shown).
It was earlier reported that E. coli strains do not have IVSs (8). We confirmed this; all 72 strains from the ECOR (E. coli Reference) set (26) were tested by PCR for the possession of IVSs at the helix 25 and helix 45 regions, and none were detected (data not shown). We propose that Salmonella acquired IVSs, presumably by a lateral transfer event from an external source, after the divergence of Salmonella from Escherichia, said to have occurred more than 100 million years ago (27). The more or less random distribution of IVSs in the different bacterial genera, as shown by their presence in Salmonella and absence from Escherichia and by the random distribution of the number of IVSs in the seven rrl genes of the different Salmonella species, indicates that they are not essential for survival. Ribosomes with fragmented 23S rRNA are functional, but fragmentation of Salmonella rRNA is not necessary, since 8 of 72 Salmonella strains in the SARB set have no IVSs and no fragmentation yet are viable. It is hence not clear if the IVSs have been recently acquired in evolution or if they were present in the ancestors of Salmonella and Escherichia and were lost from some strains in the course of evolution.
Helix 25 IVSs from Providencia rettgeri show 76% nucleotide identity to helix 25 IVSs from the rrlH operon of serovar Typhimurium (21, 23). Blocks of similar sequences are shared between the helix 45 IVSs from Proteus, Providencia (23), Salmonella (8), and Yersinia (38). Thus, some of the IVSs from these four genera of Enterobacteriaceae in which IVSs have been detected share sequence homology. Advanced BLAST searches with increased E values of the nonredundant nucleotide database at the National Centre for Biotechnology Information (2) do not reveal homology between the enterobacterial IVSs and IVSs from other genera of bacteria. This indicates lateral transfer of IVSs between the four different enterobacterial genera but no equivalent transfer between the enterobacteria and other bacteria. Yet even when there is no homology, IVSs in different bacterial genera occur at relatively conserved locations, usually in helix 25 and helix 45.
Lateral gene transfer has been seen in other regions of the rRNA. Sequences of the 16S to 23S spacer regions of rrnE from S. enterica subspecies indicate that subspecies I is closer to E. coli K-12 than to other Salmonella subspecies, suggesting lateral transfer events (28). Similarly, lateral transfer is indicated by conserved sequence blocks in the 16S to 23S spacer region among unrelated isolates of Haemophilus parainfluenzae, Haemophilus influenzae, Haemophilus ducreyi, and Actinobacillus spp. (29). The internal transcribed sequence 1 (ITS1) of Enterococcus faecium contained an additional 115-nucleotide stem-loop structure as compared to the ITS1s of other enterococci, and one of the three ITSs of Enterococcus hirae contained a similar 107-nucleotide stem-loop; these data suggest that insertion occurred by a lateral transfer event (24). The driving force for heterogeneity in the hypervariable α region of the rrs gene from Streptomyces is thought to be lateral transfer of conjugative plasmids that can mobilize the host chromosome at a high frequency (42).
These observations indicate that IVSs are transferred among the bacterial species by lateral transfer using phage-mediated transduction, transformation, or conjugation. The spread between the different rrl genes of an individual cell could be a result of reciprocal recombination (RecA mediated) or by gene conversion (nonreciprocal) (20). However, rather than a continuous process of IVS transfer between the species of Salmonella, an alternative hypothesis suggests that the progenitor Salmonella obtained an IVS (probably by lateral transfer) but that subsequent variation between different species was simply due to the loss of IVSs from some strains. In order to test these hypotheses, we are sequencing the DNA of representative IVSs.
IVSs thus seem to be mobile genetic elements distributed sporadically at relatively conserved locations in the rRNA genes among the different bacterial species. The number of IVSs present in a strain does not seem to be important; however, no strains were observed that had all 14 locations in helix 25 and helix 45 saturated with IVSs (Fig. 3 and 4). IVSs lead to the fragmentation of the rRNA without any apparent effect on the functioning of the ribosome. Speculated functions for the IVS include protection against bacteriocins (38) and adaptive changes in stationary-phase cultures (12).
ACKNOWLEDGMENTS
We greatly appreciate the work of the following who provided data on some of the strains reported here: Samer Elkassem, Albert Tam, and Teresa Wong.
The work was supported by an operating grant from the Natural Sciences and Engineering Research Council of Canada and by grants 34829-09A1 and WU-98-53 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, Bethesda, Md.
REFERENCES
- 1.Afseth G, Mo Y-Y, Mallavia L P. Characterization of the 23S and 5S rRNA genes of Coxiella burnetti and identification of an intervening sequence within the 23S rRNA gene. J Bacteriol. 1995;177:2946–2949. doi: 10.1128/jb.177.10.2946-2949.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker R M, Kearney G M, Nicholson P, Blair A L, Porter R C, Crichton P B. Types of Salmonella paratyphi B and their phylogenetic significance. J Med Microbiol. 1988;26:285–293. doi: 10.1099/00222615-26-4-285. [DOI] [PubMed] [Google Scholar]
- 4.Belfort M, Raeban M E, Coetzee T, Dalgaard J Z. Prokaryotic introns and inteins: a panoply of form and function. J Bacteriol. 1995;177:3897–3903. doi: 10.1128/jb.177.14.3897-3903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blattner F, Plunkett G, Bloch C, Perna N, Burland V, Riley M, Collado-Vides J, Glasner J, Rode C, Mayhew G, Greyor J, Davis N, Kirkpatrick H, Goeden M, Rose D, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 6.Boyd E F, Wang F, Beltran P, Plock S A, Nelson K, Selander R K. Salmonella Reference Collection B (SARB): strains of 37 serovars of subspecies I. J Gen Microbiol. 1993;139:1125–1132. doi: 10.1099/00221287-139-6-1125. [DOI] [PubMed] [Google Scholar]
- 7.Boyd E F, Wang F S, Whittam S T, Selander R K. Molecular genetic relationships of the salmonellae. Appl Environ Microbiol. 1996;62:804–808. doi: 10.1128/aem.62.3.804-808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgin A B, Parodos K, Lane D J, Pace N R. The excision of intervening sequences from Salmonella 23S rRNA. Cell. 1990;60:405–414. doi: 10.1016/0092-8674(90)90592-3. [DOI] [PubMed] [Google Scholar]
- 9.Crosa J H, Brenner D J, Ewing W H, Falkow S. Molecular relationships among the salmonellae. J Bacteriol. 1973;115:307–315. doi: 10.1128/jb.115.1.307-315.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregory S T, O'Connor M, Dahlberg A E. Functional Escherichia coli 23S rRNA containing processed and unprocessed intervening sequences from Salmonella typhimurium. Nucleic Acids Res. 1996;24:4918–4923. doi: 10.1093/nar/24.24.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haraszthy V I, Sunday G J, Bobek L A, Motley T S, Preus H, Zambon J J. Identification and analysis of the gap region in the 23S ribosomal RNA from Actinobacillus actinomycetemcomitans. J Dent Res. 1992;71:1561–1568. doi: 10.1177/00220345920710090401. [DOI] [PubMed] [Google Scholar]
- 12.Hsu D, Shih L M, Zee Y C. Degradation of ribosomal RNA in Salmonella strains: a novel mechanism to regulate the concentration of ribosomal RNA and ribosomes. J Bacteriol. 1994;176:4761–4765. doi: 10.1128/jb.176.15.4761-4765.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurtado A, Clewley J P, Linton D, Owen R J, Stanley J. Sequence similarities between large subunit ribosomal RNA gene intervening sequences from different Helicobacter species. Gene. 1997;194:69–75. doi: 10.1016/s0378-1119(97)00158-3. [DOI] [PubMed] [Google Scholar]
- 14.Konkel M E, Marconi R T, Meda D J, Ceiplak W. Identification and characterization of an intervening sequence within the 23S ribosomal RNA genes of Campylobacter jejuni. Mol Microbiol. 1994;14:235–241. doi: 10.1111/j.1365-2958.1994.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 15.Lanversin G, Jacq B. Sequence and secondary structure of the central domain of Drosophila 26S rRNA: a universal model for central domain of the large rRNA containing the region in which the central break may happen. J Mol Evol. 1989;28:403–417. doi: 10.1007/BF02603076. [DOI] [PubMed] [Google Scholar]
- 16.Le Minor L. Genus III. Salmonella Lignières 1900, 389AL. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams and Wilkins; 1984. pp. 427–458. [Google Scholar]
- 17.Liiv A, Remme J. Base-pairing of 23S rRNA ends is essential for ribosomal large subunit assembly. J Mol Biol. 1998;276:537–545. doi: 10.1006/jmbi.1997.1532. [DOI] [PubMed] [Google Scholar]
- 18.Linton D, Clewely P J, Burnens A, Owen J R, Stanley J. An intervening sequence (IVS) in the 16S rRNA gene of the eubacterium Helicobacter canis. Nucleic Acids Res. 1994;22:1954–1958. doi: 10.1093/nar/22.11.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linton D, Dewhirst F E, Clewley J P, Owen R J. Two types of 16S ribosomal RNA genes are found in Campylobacter helveticus: analysis, application and characterization of the intervening sequence found in some strains. Microbiology. 1994;140:847–855. doi: 10.1099/00221287-140-4-847. [DOI] [PubMed] [Google Scholar]
- 20.Mattatall N R, Daines D A, Sanderson K E. Salmonella typhi contains identical intervening sequences in all seven rrl genes. J Bacteriol. 1996;178:5323–5326. doi: 10.1128/jb.178.17.5323-5326.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattatall N R, Sanderson K E. Salmonella typhimurium LT2 possesses three distinct 23S rRNA intervening sequences. J Bacteriol. 1996;178:2272–2278. doi: 10.1128/jb.178.8.2272-2278.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattatall N R, Sanderson K E. RNase III deficient Salmonella typhimurium LT2 contains intervening sequences in the 23S rRNA. FEMS Microbiol Lett. 1998;159:179–185. doi: 10.1111/j.1574-6968.1998.tb12858.x. [DOI] [PubMed] [Google Scholar]
- 23.Miller W L, Pabbaraju K, Sanderson K E. Fragmentation of 23S rRNA in strains of Proteus and Providencia results from intervening sequences in the rrn (rRNA) genes. J Bacteriol. 2000;182:1109–1117. doi: 10.1128/jb.182.4.1109-1117.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naimi A, Beck G, Monique M, Lefebvre G, Branlanti C. Determination of the nucleotide sequence of the 23S ribosomal RNA and flanking spacers of an Enterococcus faecium strain reveals insertion-deletion events in the ribosomal spacer 1 of enterococci. Syst Appl Microbiol. 1999;22:9–21. doi: 10.1016/S0723-2020(99)80023-X. [DOI] [PubMed] [Google Scholar]
- 25.Noller H F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- 26.Ochman H, Selander R K. Standard reference strains of Escherichia coli from natural populations. J Bacteriol. 1984;157:690–693. doi: 10.1128/jb.157.2.690-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochman H, Wilson A C. Evolutionary history of enteric bacteria. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society of Microbiology; 1987. pp. 1649–1654. [Google Scholar]
- 28.Perez-Luz S, Rodriguez-Valera F, Lan R, Reeves P R. Variation in the ribosomal operon 16S-23S gene spacer region in representatives of Salmonella enterica subspecies. J Bacteriol. 1998;180:2144–2151. doi: 10.1128/jb.180.8.2144-2151.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pritivera A, Rappazzo G, Sangari P, Giannino V, Licciardello L, Stefani S. Cloning and sequencing of a 16S/23S ribosomal spacer from Haemophilus parainfluenzae reveals an invariant, mosiac-like organisation of sequence blocks. FEMS Microbiol Lett. 1998;164:289–294. doi: 10.1111/j.1574-6968.1998.tb13100.x. [DOI] [PubMed] [Google Scholar]
- 30.Rainey F A, Ward-Rainey N L, Janssen P H, Hippe H, Stackebrandt E. Clostridium paradoxum DSM 7308 contains multiple 16S ribosomal RNA genes with heterogenous intervening sequences. Microbiology. 1996;142:2087–2095. doi: 10.1099/13500872-142-8-2087. [DOI] [PubMed] [Google Scholar]
- 31.Ralph D, McClelland M. Intervening sequences with a conserved open reading frame in eubacterial 23S rRNA genes. Proc Natl Acad Sci USA. 1993;90:6864–6868. doi: 10.1073/pnas.90.14.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ralph D, McClelland M. Phylogenetic evidence for horizontal transfer of an intervening sequence between species in a spirochete genus. J Bacteriol. 1994;176:5982–5987. doi: 10.1128/jb.176.19.5982-5987.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redburn A C, Patel B K C. Phylogenetic analysis of Desulfotomaculum thermobenzoicum using polymerase chain reaction amplified 16S rRNA-specific DNA. FEMS Microbiol Lett. 1993;113:81–86. doi: 10.1111/j.1574-6968.1993.tb06492.x. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Selander R K, Beltran P, Smith N H, Barker R M, Crichton P B, Old D C, Musser J M, Whittam T S. Genetic population structure, clonal phylogeny, and pathogenicity of Salmonella paratyphi B. Infect Immun. 1990;58:1891–1901. doi: 10.1128/iai.58.6.1891-1901.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selenska-Pobell S, Doring H. Sequences around the fragmentation sites of the large subunit ribosomal RNA in the family Rhizobiaceae. Antonie Leeuwenhoek. 1998;73:55–67. doi: 10.1023/a:1000540023194. [DOI] [PubMed] [Google Scholar]
- 37.Selenska-Pobell S, Evguenieva-Hackenberg E. Fragmentation of the large-subunit rRNA in the family Rhizobiaceae. J Bacteriol. 1995;177:6993–6998. doi: 10.1128/jb.177.23.6993-6998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skurnik M, Toivanen P. Intervening sequences (IVSs) in the 23S ribosomal RNA genes of pathogenic Yersinia enterocolitica strains. The intervening sequences in Y. enterocolitica and Salmonella typhimurium have a common origin. Mol Microbiol. 1991;5:585–593. doi: 10.1111/j.1365-2958.1991.tb00729.x. [DOI] [PubMed] [Google Scholar]
- 39.Song X-M, Forsgren A, Janson H. Fragmentation heterogeneity of 23S ribosomal RNA in Haemophilus species. Gene. 1999;230:287–293. doi: 10.1016/s0378-1119(99)00063-3. [DOI] [PubMed] [Google Scholar]
- 40.Springer N, Ludwig W, Amann R, Schmidt H J, Goertz H D, Schleifer K H. Occurrence of fragmented 16S rRNA in an obligate bacterial symbiont of Paramecium caudatum. Proc Natl Acad Sci USA. 1993;90:9892–9895. doi: 10.1073/pnas.90.21.9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trust T J, Logan S M, Gustafson C E, Romaniuk P J, Kim N W, Chan V L, Ragan M A, Guerry P, Gutell R R. Phylogenetic and molecular characterization of a 23S rRNA gene positions the genus Campylobacter in the epsilon subdivision of Proteobacteria and shows that the presence of transcribed spacers is common in Campylobacter spp. J Bacteriol. 1994;176:4597–4609. doi: 10.1128/jb.176.15.4597-4609.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ueda K, Seki T, Kodo T, Yoshida T, Kataoka M. Two distinct mechanisms cause heterogeneity of 16S rRNA. J Bacteriol. 1999;181:78–82. doi: 10.1128/jb.181.1.78-82.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willems A, Collins M D. Phylogenetic analysis of rhizobia and agrobacteria based on 16S ribosomal RNA gene sequences. Int J Syst Bacteriol. 1993;43:305–313. doi: 10.1099/00207713-43-2-305. [DOI] [PubMed] [Google Scholar]