Abstract
Background/Aim: This study aimed to evaluate the toxicities and response rate of a modified TPF (docetaxel, cisplatin, and 5-fluorouracil) protocol in patients with locally advanced head and neck cancer (ECOG performance status ≤1).
Patients and Methods: Induction treatment consisted of cisplatin 25 mg/m2/day as a 90 min infusion for three consecutive days, leucovorin 20 mg/m2/day as a bolus for four consecutive days, 5-fluorouracil (5-FU) 370 mg/m2/day as a bolus for four consecutive days, and paclitaxel 60 mg/m2 as a 1-h infusion on Days 1, 8, and 15, repeated every 3-4 weeks (twelve cycles to 6 patients).
Results: The main toxicities were grade 1 neuropathy, mucositis, and fatigue. There were four episodes of severe toxicities (grade ≥3). There was one early death, and 2 patients were discontinued due to hematological toxicity. Other side effects included neutropenia, nausea, diarrhea, and vomiting.
Conclusion: Induction therapy with cisplatin, 5-fluorouracil, leucovorin, and paclitaxel in head and neck cancer is not feasible because of severe toxicity.
Keywords: Head and neck cancer, antineoplastic agents, toxicity, bone marrow, quality of life
Carcinoma of the head and neck is a major public health problem. It is estimated that every year, over 650,000 head and neck cancer cases are diagnosed, and they account for more than 330,000 deaths worldwide (1). Risk factors associated with head and neck cancer include tobacco use, alcohol consumption, exposure to human papillomavirus (HPV, oropharyngeal cancer) and Epstein–Barr virus (nasopharyngeal cancer) (2).
Head and neck cancers usually begin in the squamous cells that line the moist mucosal surfaces inside the head and neck and are known collectively as squamous cell carcinomas of the head and neck (3). The clinical presentation of this cancer varies according to where the tumor originated, including the oral cavity, pharynx (nasopharynx, oropharynx, and hypopharynx), larynx, paranasal sinuses and nasal cavity, and salivary glands (4,5). Squamous cell carcinoma makes up approximately 90% of all head and neck cancers (6).
Careful physical examination remains the primary approach for the early detection of head and neck cancer. However, a combination of clinical and imaging examinations is essential to properly stage the disease (7,8).
The choice of appropriate management depends primarily on the specific site and stage of the primary tumor at diagnosis and predicted functional outcomes following different treatment modalities (9,10). The performance status of each patient is another important aspect to take into consideration, as treatment is often very intense with multiple side effects (11).
Definitive local therapy (surgery or radiotherapy) is the key to the treatment of locally advanced (stage III/IV) squamous cell carcinoma of the head and neck, but it is associated with high rates of local recurrence and distant metastases (12,13). To increase the cure rate and reduce morbidity in those cases that are not subjected to surgery, chemotherapy is added to enhance the effect of radiotherapy and provide a treatment with effective curative intent (14).
The combined treatments can be delivered concurrently or in different temporal sequences and include induction chemotherapy with subsequent radiotherapy or surgery, chemotherapy concomitant with radiotherapy as definitive treatment, and induction chemotherapy followed by chemoradiotherapy (sequential therapy). There is currently no consensus as to which treatment modality results in better outcomes (15). The optimal timing and integration of chemotherapy with RT remain uncertain. Sequential induction followed by concurrent chemoradiation has been proposed as the optimal way to incorporate chemotherapy with locoregional therapy because of the demonstrated benefit of concurrent chemoradiation over RT alone and the decrease in distant metastases seen with induction chemotherapy (16).
Induction chemotherapy reduces the tumor size before radiotherapy or surgery, allowing for more effective local therapy. Other advantages of induction chemotherapy are the treatment of distant subclinical metastases, an increased organ preservation rate, and the provision of prognostic information, helping to select the intensity of the subsequent chemoradiotherapy (17). The benefit of neoadjuvant chemotherapy, in general, is unclear when compared with standard radiotherapy concomitant with cisplatin (18,19). The only neoadjuvant chemotherapy validated for organ preservation purposes is neoadjuvant chemotherapy in patients with laryngeal cancer (20-22). A meta-analysis of randomized trials (23) showed that the effect of neoadjuvant treatment was inferior to definitive chemoradiotherapy.
Sequential therapy can combine the benefit of induction with that of chemoradiotherapy. As a downside of sequential therapy, there is increased toxicity, which may limit patient compliance and delay definitive local therapy (24).
The most commonly used induction chemotherapy regimen is TPF (docetaxel 75 mg/m2 on Day 1, cisplatin 75 mg/m2 on Day 1, and 5-fluorouracil (5-FU) 750 mg/m2 daily, in continuous infusion for 5 days). Chemoradiation therapy shows better results with platinum-based regimens, particularly high-dose cisplatin (100 mg/m2 on Days 1, 22, and 43), but is associated with severe acute and late toxicities (25-28).
In general, concomitant cisplatin should be reserved for patients with an excellent performance status (ECOG 0 or 1). Alternative dosing schedules for cisplatin (30 to 40 mg/m2 weekly, 6 mg/m2 daily or 20 mg/m2 daily for five days a week) are sometimes used, with better patient tolerance. Two prospective randomized studies compared weekly cisplatin with cisplatin given every 3 weeks. Weekly cisplatin (30 mg/m2) was less effective compared to every 3 weeks cisplatin, albeit the trial conducted by Nononha et al. was not very well performed, especially the radiotherapy part (29). The full publication of the reported JCOG1008 trial, which compared concurrent cisplatin 40 mg/m2 weekly vs. 100 mg/m2 every three weeks, is awaited (30).
A combination of chemotherapeutic agents improved the drug response of patients with advanced head and neck cancer, but no effect on overall survival was observed. Among those selected to receive sequential therapy, there is a high mortality rate with induction chemotherapy related to toxicity, which is also one of the reasons why patients are unable to complete the radiotherapy regimen, directly impacting overall survival. The role of induction chemotherapy followed by concurrent chemoradiation (sequential therapy) versus concurrent chemoradiation alone has been assessed in several trials but remains controversial due to conflicting results (31-33).
Some of the factors that contribute to the difficulty of interpretation are differences in study designs, intensity and choice of chemotherapy regimens, and sample differences, especially in the proportions of patients associated with HPV who, theoretically, have a better prognosis and for whom, perhaps, a less aggressive regimen would be sufficient to maximize local tumor control (34-37).
Our study aimed to evaluate the toxicities and response rate of a modified TPF protocol compared to the standard TPF regimen. It is important to emphasize that the standard chemotherapy protocol (TPF) often requires the patient to be hospitalized for the continuous infusion of 5-FU, given the delay in getting a chemotherapy catheter implanted (port-a-cath), in addition to having a high toxicity. In the modified TPF, 5-FU was administered as a bolus for 4 consecutive days, avoiding hospitalization or delays in patient treatment, cisplatin was given over 3 days (25 mg/m2 D1 to D3), and docetaxel was substituted by paclitaxel that is less myelotoxic.
In addition, the significant problems associated with high toxicities as well as the resistance to current treatments and the low quality of life of patients make these efforts particularly crucial. Thus, this trial evaluated whether an induction regimen with the cisplatin, 5-FU, leucovorin, and paclitaxel combination can result in an improved response rate with better tolerance than the current standard induction regimen.
Patients and Methods
This was a pilot (feasibility) prospective single-arm study conducted with outpatients of the two university hospitals.
Ethical considerations. This study was approved by the Institutional Review Board of both institutions before study commencement and conducted according to good clinical practice and applicable regulatory guidelines (CAAE: 61767716.0.3001.0072). All patients provided written informed consent before enrollment.
Patient eligibility. Enrollment was limited to patients with measurable, previously untreated stage III or IV head and neck cancer, excluding those with metastatic disease. All subjects were required to have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of ≤1 or Karnofsky Performance Status (KPS) ≥70. Patients were required to have laboratory parameters within the normal range (hemoglobin level 12-15 g/dl, neutrophil count 2-8×103/mm3, platelet count 150-400×103/mm3), liver function (total bilirubin 0.1-1.2 md/dl), and renal function (creatinine 0.5-1.0 mg/dl). Patients with any active and decompensated comorbidity or any previous malignancy within 5 years of study entry were ineligible.
Baseline evaluation and induction chemotherapy protocol. Patients were evaluated by a medical oncologist to confirm eligibility, staging and treatment planning. Before any invasive procedure, the quality-of-life questionnaire (European Organization for the Research and Treatment of Cancer Core Quality of Life Questionnaire - EORTC QLQ-C30) was applied. All patients had a complete clinical history and physical examination, complete blood counts, and serum chemistries (liver and renal function tests). A computed tomography (CT) scan of the head and neck was evaluated before the start of induction therapy for later comparison and evaluation of the response to treatment according to the Response Evaluation Criteria in Solid Tumors (RECIST criteria version 1.1). A partial response was defined as a ≥30 percent decrease in the sum of the longest diameter of the target lesions compared with baseline. A complete response was defined as the disappearance of all target lesions and a reduction in the short axis measurement of all pathologic lymph nodes to ≤10 mm.
The treatment regimen consisted of paclitaxel (60 mg/m2 as a 1-h infusion on Days 1, 8, and 15), cisplatin (25 mg/m2/day as a 90-min infusion on three consecutive days on Days 1, 2, and 3), leucovorin (20 mg/m2/day on Days 1, 2, 3, and 4) and 5-FU in a bolus (370 mg/m2/day on Days 1-4). Cycles were repeated every 4 weeks. The treatment was administered on an outpatient basis for a maximum of three cycles.
Retreatment on Day 29 required a neutrophil count >1,000/mm3, a platelet count >100,000/mm3, and resolution of all other nonhematological toxicities (except alopecia) to baseline. The doses of the drugs were reduced by 30% following any episode of toxicity, grade 3-4 until toxicity regression to grade 1 or 2 according to Common Terminology Criteria for Adverse Events (CTCAE). Blood chemistries were performed before each cycle of therapy.
Restaging CT scans were scheduled to be performed during the third cycle of induction chemotherapy. Clinical response was defined for each patient according to the combined findings of CT, complete blood cell counts, and toxicity outcomes.
Study endpoints and statistical analysis. The primary endpoint of the study was the tumor response to induction chemotherapy. The secondary endpoints were toxicity and quality of life. Toxicity was graded according to the CTCAE. Descriptive statistics were used to characterize the response and toxicity rates.
Since this was a pilot study, no sample size estimation was carried out before starting. Due to the limited number of patients, a case-by-case analysis of study participants was performed for response rate, toxicities, and quality of life.
Results
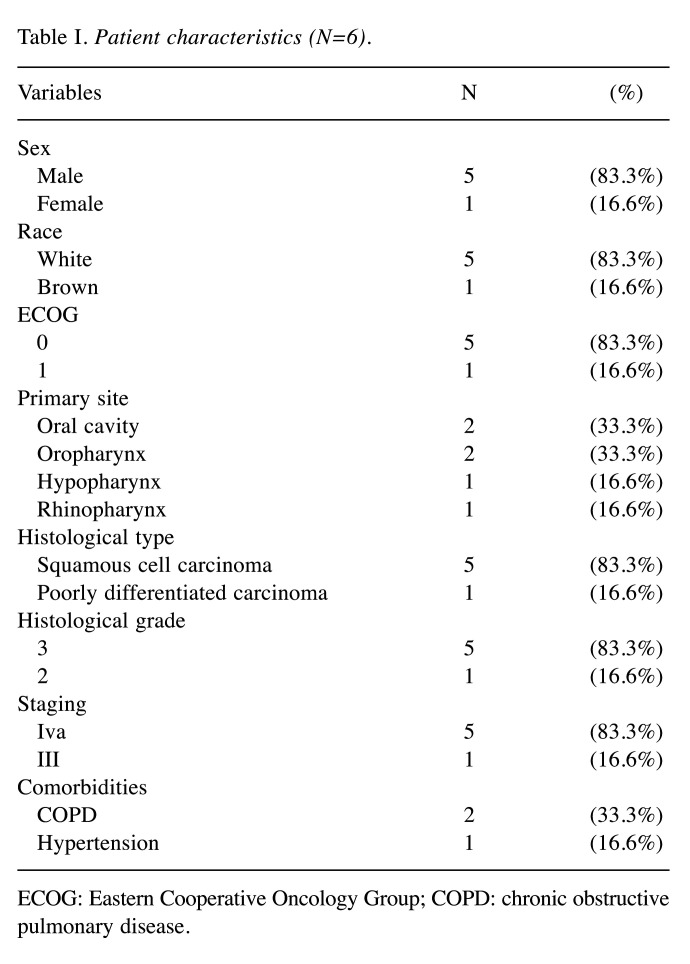
Between January and November 2017, six patients with head and neck cancer were recruited in this study. A total of 12 cycles of a combination of paclitaxel, cisplatin, leucovorin, and 5-FU were administered, with a mean of 2 per patient (ranging from 1 to 3). Among the patients, five (83.3%) patients were men and one (16.6%) was a woman. The most common histological type was squamous cell carcinoma (five cases, 83.3%), and the predominant primary sites were the oral cavity and oropharynx (one case each, 33% each). Their characteristics are shown in Table I.
Table I. Patient characteristics (N=6).
ECOG: Eastern Cooperative Oncology Group; COPD: chronic obstructive pulmonary disease.
Only half of the patients were evaluable for response rate. Of these, two patients showed a partial response, with a reduction in the size of the primary lesion (26,3% and 27%), and the third had a complete response.
Three patients were excluded from the response analysis because they did not receive any complete cycles of chemotherapy due to hematological toxicity, and one patient died before the tumor status could be assessed.
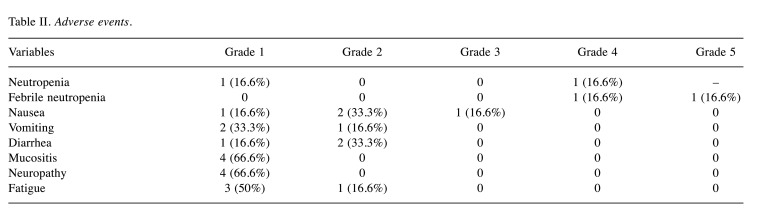
Toxicity. Table II highlights the side effects recorded for this treatment plan. The most common toxicities were hematological, mucositis, fatigue, and neuropathy. There were four episodes (66%) of severe toxicities (grade ≥3), three of which were hematological, and one of which was nausea.
Table II. Adverse events.
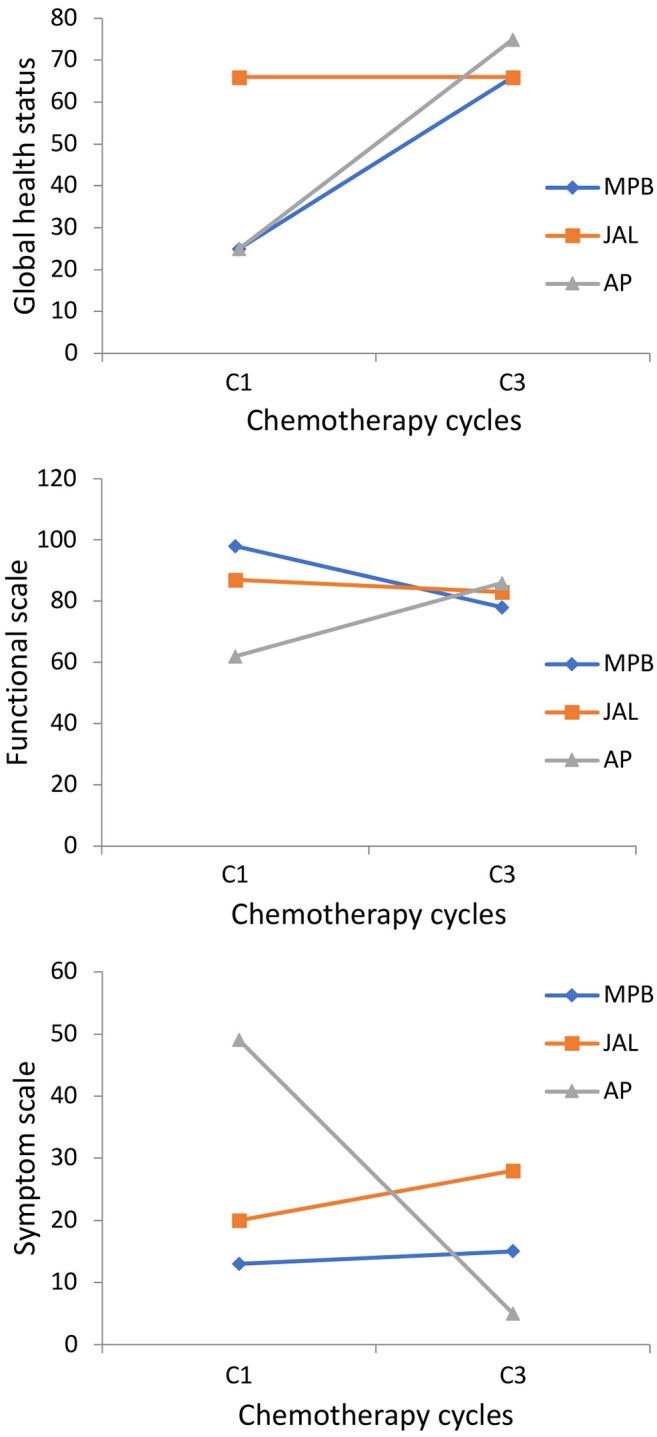
Quality of life analysis using the EORTC QLQ-30 questionnaire was also performed for the three patients who completed chemotherapy (Figure 1). Two cases showed improvement in the global health scale, and one remained stable. On the functional scale, only one patient had improvement, and when the symptom scale was evaluated, two patients had considerable worsening.
Figure 1. Score of the EORTC-C30 questionnaire for three patients who completed chemotherapy.
Discussion
In this study, six patients were evaluated, and only half managed to complete three cycles of chemotherapy with cisplatin, 5-FU in bolus and paclitaxel (CFP) due to hematological toxicity. Of the severe toxicities (≥3), hematological was the most prevalent (three cases, 50%), with 16.6% (one case) of neutropenia and 33.3% (two cases) of febrile neutropenia. Our results showed that two patients (33%) had a partial response, and one (16.6%) had a complete response.
Two randomized phase III trials using the TPF regimen (TAX 323 and TAX 324) confirmed the superiority of TPF over the PF regimen in terms of a response (15,16).
In the TAX 324 study, some patients in the TPF group discontinued treatment (27%) because of progressive disease (7%) and adverse events (7%). Regarding the response rate after induction chemotherapy, 55% of the patients in the TPF group had a partial response and 17% had a complete response. The rate of febrile neutropenia was 12% in the TPF group, with 1 (<1%) death due to toxicity (15).
In TAX 323, a total of 358 patients were randomly assigned to the TPF group or the PF group. In the TPF group, 38 patients discontinued the treatment before the scheduled completion of the study, and the most frequent reasons for discontinuation were progressive disease (7.9%) and adverse events (6.2%). 5.2% of patients in the TPF group had febrile neutropenia, and deaths associated with toxic effects occurred in 4 patients (2.3%). Partial response to chemotherapy was found in 59.3% of patients treated with TPF and a complete response in 8.5% (16).
Studies have indicated that toxic effects could decrease the patient’s tolerance to chemotherapy, leading to treatment interruption (17-24). In this study, we found that toxicity from the combination of drugs was a limiting factor, although the patients enrolled in this study who completed treatment presented a pathological response. Before the end of the first chemotherapy cycle, two patients were admitted to the ICU due to febrile neutropenia with pulmonary focus. One patient died due to septic shock, and the other patient declined in performance, was unable to receive further chemotherapy, and was monitored exclusively by the palliative care team.
Preliminary studies that evaluated the feasibility and activity of an outpatient chemotherapy regimen consisting of cisplatin, 5-FU, and leucovorin (CFL) in patients with advanced head and neck (H/N) and esophageal squamous cell carcinoma also reported that 54% of the patients presented with grade 3 or higher neutropenia (25).
Neutropenia is a major dose-limiting toxicity of myelosuppressive chemotherapy that predisposes patients to serious infections and is seen most often during the initial cycles of therapy (26,29). Several studies have confirmed that prophylactic treatment with filgrastim could be used to reduce the risk of chemotherapy-induced neutropenia (26,29,30). Thus, in the current study, prophylactic filgrastim schedules were used on Days 5, 6, and 7 of the chemotherapy cycles and levofloxacin for 10 days in each cycle. However, two events of grade 4 febrile neutropenia occurred during the first cycle.
In this study, among the patients who completed the chemotherapy protocol, one had good tolerance and presented grade 1 neuropathy at the end of the third cycle, but when starting cisplatin concomitant with radiotherapy (21 days after the third cycle) as a definitive treatment, he developed grade 4 neuropathy. This suggested that the residual neuropathy of paclitaxel caused during induction may have been exacerbated by cisplatin concomitant with radiotherapy. As described in the literature, chemotherapy-induced neuropathy is a common, dose-dependent adverse effect of several antineoplastics and is most commonly reported for paclitaxel when given alone or in combination with other neurotoxic antineoplastic agents, such as cisplatin (31,32).
Previous studies confirmed that induction chemotherapy was a good alternative if the chemotherapy regimen included taxanes, particularly the TPF regimen (15,16). Nevertheless, in the current study, the addition of paclitaxel was probably responsible for the higher toxicity. In this context, if more studies are performed with similar schemes, it would be prudent to start with an even lower dose of paclitaxel. Because of the high rates of febrile neutropenia with pulmonary focus, colony stimulating factor and levofloxacin should be prophylactically administered.
Based on all these facts, since the patients had unexpected severe toxicity, the study was closed for ethical and safety reasons. Thus, the risk of toxicity observed does not allow us to recommend this induction regimen.
Conclusion
The results showed that the combination of paclitaxel, cisplatin, leucovorin, and 5-FU at the current doses is not feasible, especially because of myelotoxicity.
Conflicts of Interest
The Authors declare that they have no conflicts of interest in relation to this study.
Authors’ Contributions
CECS: Conceptualization, data curation, formal analysis, methodology, project administration, writing – original draft, writing – review & editing. DIGC, REARC, RP, FJSMC, CVMS, EAS: Conceptualization, data curation, formal analysis, methodology, project administration, writing – original draft. AG: Conceptualization, data curation, formal analysis, methodology, project administration, writing – review & editing. All Authors have read and approved the final draft.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Dhull AK, Atri R, Dhankhar R, Chauhan AK, Kaushal V. Major risk factors in head and neck cancer: a retrospective analysis of 12-year experiences. World J Oncol. 2018;9(3):80–84. doi: 10.14740/wjon1104w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6(1):92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilyoma JM, Rambau PF, Masalu N, Kayange NM, Chalya PL. Head and neck cancers: a clinico-pathological profile and management challenges in a resource-limited setting. BMC Res Notes. 2015;8:772. doi: 10.1186/s13104-015-1773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Annu Rev Pathol. 2009;4:49–70. doi: 10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oppel F, Shao S, Schürmann M, Goon P, Albers AE, Sudhoff H. An effective primary head and neck squamous cell carcinoma in vitro model. Cells. 2019;8(6):555. doi: 10.3390/cells8060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Didkowska J, Wojciechowska U. Malignant tumors in Poland in 2013. Warsaw, Poland, National Cancer Registry. 2015:pp. 52–54. [Google Scholar]
- 8.Pałasz P, Adamski Ł, Górska-Chrząstek M, Starzyńska A, Studniarek M. Contemporary diagnostic imaging of oral squamous cell carcinoma - a review of literature. Pol J Radiol. 2017;82:193–202. doi: 10.12659/PJR.900892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Felice F, Polimeni A, Valentini V, Brugnoletti O, Cassoni A, Greco A, de Vincentiis M, Tombolini V. Radiotherapy controversies and prospective in head and neck cancer: a literature-based critical review. Neoplasia. 2018;20(3):227–232. doi: 10.1016/j.neo.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puram SV, Rocco JW. Molecular aspects of head and neck cancer therapy. Hematol Oncol Clin North Am. 2015;29(6):971–992. doi: 10.1016/j.hoc.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkashty OA, Ashry R, Tran SD. Head and neck cancer management and cancer stem cells implication. Saudi Dent J. 2019;31(4):395–416. doi: 10.1016/j.sdentj.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elicin O, Cihoric N, Vlaskou Badra E, Ozsahin M. Emerging patient-specific treatment modalities in head and neck cancer - a systematic review. Expert Opin Investig Drugs. 2019;28(4):365–376. doi: 10.1080/13543784.2019.1582642. [DOI] [PubMed] [Google Scholar]
- 13.Alsahafi E, Begg K, Amelio I, Raulf N, Lucarelli P, Sauter T, Tavassoli M. Clinical update on head and neck cancer: molecular biology and ongoing challenges. Cell Death Dis. 2019;10(8):540. doi: 10.1038/s41419-019-1769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lima JM, Bonan PR, da Cruz Perez DE, Hier M, Alaoui-Jamali MA, da Silva SD. Nanoparticle-based chemotherapy formulations for head and neck cancer: a systematic review and perspectives. Nanomaterials (Basel) 2020;10(10):1938. doi: 10.3390/nano10101938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM, Cullen K, Ervin TJ, Murphy BA, Raez LE, Cohen RB, Spaulding M, Tishler RB, Roth B, Viroglio Rdel C, Venkatesan V, Romanov I, Agarwala S, Harter KW, Dugan M, Cmelak A, Markoe AM, Read PW, Steinbrenner L, Colevas AD, Norris CM Jr, Haddad RI, TAX 324 Study Group Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 16.Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss JH, van den Weyngaert D, Awada A, Cupissol D, Kienzer HR, Rey A, Desaunois I, Bernier J, Lefebvre JL, EORTC 24971/TAX 323 Study Group Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 17.Hoek J, Bloemendal KM, van der Velden LA, van Diessen JN, van Werkhoven E, Klop WM, Tesselaar ME. Nephrotoxicity as a dose-limiting factor in a high-dose cisplatin-based chemoradiotherapy regimen for head and neck carcinomas. Cancers (Basel) 2016;8(2):21. doi: 10.3390/cancers8020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, Brockstein BE, Agulnik MB, Mittal BB, Yunus F, Samant S, Raez LE, Mehra R, Kumar P, Ondrey F, Marchand P, Braegas B, Seiwert TY, Villaflor VM, Haraf DJ, Vokes EE. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32(25):2735–2743. doi: 10.1200/JCO.2013.54.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddad R, O’Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, Clark J, Sarlis N, Lorch J, Beitler JJ, Limaye S, Riley S, Posner M. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14(3):257–264. doi: 10.1016/S1470-2045(13)70011-1. [DOI] [PubMed] [Google Scholar]
- 20.Janoray G, Pointreau Y, Garaud P, Chapet S, Alfonsi M, Sire C, Jadaud E, Calais G. Long-term results of a multicenter randomized phase III trial of induction chemotherapy with cisplatin, 5-fluorouracil, ±docetaxel for larynx preservation. J Natl Cancer Inst. 2015;108(4):djv368. doi: 10.1093/jnci/djv368. [DOI] [PubMed] [Google Scholar]
- 21.Henriques De Figueiredo B, Fortpied C, Menis J, Lefebvre JL, Barzan L, de Raucourt D, Geoffrois L, Giurgea L, Hupperets P, Leemans CR, Licitra L, Rolland F, Tesselaar M, Vermorken JB, Grégoire V, EORTC Head and Neck Cancer and Radiation Oncology Cooperative Groups Long-term update of the 24954 EORTC phase III trial on larynx preservation. Eur J Cancer. 2016;65:109–112. doi: 10.1016/j.ejca.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Lefebvre JL, Andry G, Chevalier D, Luboinski B, Collette L, Traissac L, de Raucourt D, Langendijk JA, EORTC Head and Neck Cancer Group Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann Oncol. 2012;23(10):2708–2714. doi: 10.1093/annonc/mds065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dauzier E, Lacas B, Blanchard P, Le QT, Simon C, Wolf G, Janot F, Horiuchi M, Tobias JS, Moon J, Simes J, Deshmane V, Mazeron JJ, Mehta S, Zaktonik B, Tamura M, Moyal E, Licitra L, Fortpied C, Haffty BG, Ghi MG, Gregoire V, Harris J, Bourhis J, Aupérin A, Pignon JP, MACH-NC Collaborative Group Role of chemotherapy in 5000 patients with head and neck cancer treated by curative surgery: A subgroup analysis of the meta-analysis of chemotherapy in head and neck cancer. Oral Oncol. 2019;95:106–114. doi: 10.1016/j.oraloncology.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcu LG. Improving therapeutic ratio in head and neck cancer with adjuvant and cisplatin-based treatments. Biomed Res Int. 2013;2013:817279. doi: 10.1155/2013/817279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fabrício Vde C, Amado F, Del Giglio A. Low-cost outpatient chemotherapy regimen of cisplatin, 5-fluorouracil and leucovorin for advanced head and neck and esophageal carcinomas. Sao Paulo Med J. 2008;126(1):63–66. doi: 10.1590/s1516-31802008000100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C, European Organisation for Research and Treatment of Cancer 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Kusaka T, Shiga K, Katagiri K, Saito D, Oikawa SI, Ikeda A, Tsuchida K, Miyaguchi J, Ohashi YU, Ariga H, Tanno K. Treatment outcomes and prognostic factors of concurrent chemoradiotherapy with docetaxel, cisplatin, and fluorouracil in advanced head and neck cancer. Anticancer Res. 2022;42(12):6047–6056. doi: 10.21873/anticanres.16116. [DOI] [PubMed] [Google Scholar]
- 28.Zwaan I, Soror T, Bruchhage KL, Hakim SG, Schild SE, Rades D. Comparison of two cisplatin regimens for chemoradiation in patients with squamous-cell carcinoma of the head and neck. Anticancer Res. 2023;43(2):795–800. doi: 10.21873/anticanres.16220. [DOI] [PubMed] [Google Scholar]
- 29.Noronha V, Joshi A, Patil VM, Agarwal J, Ghosh-Laskar S, Budrukkar A, Murthy V, Gupta T, D’Cruz AK, Banavali S, Pai PS, Chaturvedi P, Chaukar D, Pande N, Chandrasekharan A, Talreja V, Vallathol DH, Mathrudev V, Manjrekar A, Maske K, Bhelekar AS, Nawale K, Kannan S, Gota V, Bhattacharjee A, Kane S, Juvekar SL, Prabhash K. Once-a-week versus once-every-3-weeks cisplatin chemoradiation for locally advanced head and neck cancer: a phase III randomized noninferiority trial. J Clin Oncol. 2018;36(11):1064–1072. doi: 10.1200/JCO.2017.74.9457. [DOI] [PubMed] [Google Scholar]
- 30.Kunieda F, Kiyota N, Tahara M, Kodaira T, Hayashi R, Ishikura S, Mizusawa J, Nakamura K, Fukuda H, Fujii M, Head and Neck Cancer Study Group of the Japan Clinical Oncology Group Randomized phase II/III trial of post-operative chemoradiotherapy comparing 3-weekly cisplatin with weekly cisplatin in high-risk patients with squamous cell carcinoma of head and neck: Japan Clinical Oncology Group Study (JCOG1008) Jpn J Clin Oncol. 2014;44(8):770–774. doi: 10.1093/jjco/hyu067. [DOI] [PubMed] [Google Scholar]
- 31.Starobova H, Vetter I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front Mol Neurosci. 2017;10:174. doi: 10.3389/fnmol.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scripture CD, Figg WD, Sparreboom A. Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr Neuropharmacol. 2006;4(2):165–172. doi: 10.2174/157015906776359568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haddad R, O’Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, Clark J, Sarlis N, Lorch J, Beitler JJ, Limaye S, Riley S, Posner M. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14(3):257–264. doi: 10.1016/S1470-2045(13)70011-1. [DOI] [PubMed] [Google Scholar]
- 34.Hitt R, Grau JJ, López-Pousa A, Berrocal A, García-Girón C, Irigoyen A, Sastre J, Martínez-Trufero J, Brandariz Castelo JA, Verger E, Cruz-Hernández JJ, Spanish Head and Neck Cancer Cooperative Group (TTCC) A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. 2014;25(1):216–225. doi: 10.1093/annonc/mdt461. [DOI] [PubMed] [Google Scholar]
- 35.Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, Brockstein BE, Agulnik MB, Mittal BB, Yunus F, Samant S, Raez LE, Mehra R, Kumar P, Ondrey F, Marchand P, Braegas B, Seiwert TY, Villaflor VM, Haraf DJ, Vokes EE. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32(25):2735–2743. doi: 10.1200/JCO.2013.54.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghi MG, Paccagnella A, Ferrari D, Foa P, Alterio D, Codecà C, Nolè F, Verri E, Orecchia R, Morelli F, Parisi S, Mastromauro C, Mione CA, Rossetto C, Polsinelli M, Koussis H, Loreggian L, Bonetti A, Campostrini F, Azzarello G, D’Ambrosio C, Bertoni F, Casanova C, Emiliani E, Guaraldi M, Bunkheila F, Bidoli P, Niespolo RM, Gava A, Massa E, Frattegiani A, Valduga F, Pieri G, Cipani T, Da Corte D, Chiappa F, Rulli E, GSTTC (Gruppo di Studio Tumori della Testa e del Collo) Italian Study Group Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II-III trial. Ann Oncol. 2017;28(9):2206–2212. doi: 10.1093/annonc/mdx299. [DOI] [PubMed] [Google Scholar]
- 37.Geoffrois L, Martin L, De Raucourt D, Sun XS, Tao Y, Maingon P, Buffet J, Pointreau Y, Sire C, Tuchais C, Babin E, Coutte A, Rolland F, Kaminsky MC, Alfonsi M, Lapeyre M, Saliou M, Lafond C, Jadaud E, Gery B, Zawadi A, Tourani JM, Khoury C, Henry AR, Hasbini A, Guichard F, Borel C, Meert N, Guillet P, Calais MH, Garaud P, Bourhis J. Induction chemotherapy followed by cetuximab radiotherapy is not superior to concurrent chemoradiotherapy for head and neck carcinomas: results of the GORTEC 2007-02 phase III randomized trial. J Clin Oncol. 2018;36(31):3077–3083. doi: 10.1200/JCO.2017.76.2591. [DOI] [PubMed] [Google Scholar]