Abstract
Background/Aim: Liver cancer is one of the malignancies with the highest mortality-to-incidence ratio worldwide. Therefore, novel therapeutic approaches are urgently needed. Combination therapy and drug repurposing can improve the response of the patients to therapy in several cancers. The aim of the present study was to merge these two strategies and evaluate whether the two-drug- or three-drug- combination of sorafenib, raloxifene, and loratadine improves the antineoplastic effect on human liver cancer cells in comparison to the single-drug effect.
Materials and Methods: The human liver cancer cell lines HepG2 and HuH7 were studied. The effect of sorafenib, raloxifene, and loratadine on the metabolic activity was determined using the MTT assay. The inhibitory concentrations (IC20 and IC50) were calculated from these results and used in the drug-combination experiments. Apoptosis and cell survival were studied by flow cytometry and using the colony formation assay, respectively.
Results: In both cell lines, sorafenib, raloxifene, and loratadine in two-drug and three-drug combinations significantly reduced metabolic activity and significantly increased the percentage of apoptotic cells compared to the single-drug effect. In addition, all the combinations significantly reduced the colony-forming capacity in the HepG2 cell line. Surprisingly, the effect of raloxifene on apoptosis was similar to that observed using the combinations.
Conclusion: The triple combination sorafenib-raloxifene-loratadine may be a novel promising approach in the treatment of liver cancer patients.
Keywords: Raloxifene, loratadine, hepatocellular carcinoma, drug combination, drug repurposing
Liver cancer is a poor prognosis malignancy that ranks seventh in incidence and fourth in mortality worldwide, reaching a mortality-to-incidence ratio up to 91.6% (1). Unfortunately, it is diagnosed when the patient is at an advanced stage, when the tumor already extends over a large part of the organ (2). The recommended treatment option is systemic drug therapy, which generally employs multi-kinase inhibitors such as sorafenib or regorafenib (3,4). However, these therapies are ineffective in most cases due to late diagnosis, or the development of tumor resistance, and recurrence rates are unfortunately estimated to be 15% to 30% within one year and 70% over the next 5 years after treatment (5). Combination therapy is a strategy that has resulted in better pharmacological treatments; it is intended to improve the therapeutic effect and reduce toxicity, as well as lowering the probability of resistance. In addition, repurposing of approved drugs offer alternative therapeutic options in a relatively fast and in some cases less expensive manner. We demonstrated that the combination of the antihistamine astemizole with gefitinib had a superior antineoplastic effect on human lung cancer cell lines in comparison to the single-drug treatment (6). Additionally, Ellegaard et al. found that the use of loratadine in patients diagnosed with lung cancer was associated with a significant reduction in mortality (7). Besides, some selective estrogen receptor-modulators (SERMs) such as raloxifene have antineoplastic activity in both in vitro and in vivo assays (8,9). Here, we evaluated whether the two- or three- drug combination of sorafenib, raloxifene, and loratadine possess significant antineoplastic effect on human liver cancer cell lines.
Materials and Methods
Cell lines and reagents. The human liver cancer cell lines HepG2 and HuH7 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured according to the manufacturer’s instructions. Cells were seeded and cultured for 24 h and then exposed to the different drugs. Sorafenib, raloxifene, and loratadine were purchased from Sigma Aldrich Co. (St Louis, MO, USA).
Metabolic activity. Metabolic activity was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric method. Cells were seeded in 96-well plates (4,000 cells per well) and incubated for 72 h at 37˚C either in culture medium-only, or in the presence of each drug or two- or three-drug combination, or in the presence of the vehicle (DMSO). MTT was added 4 h before the end of the incubation period. Subsequently, acid-SDS was added and incubated overnight. The next day the absorbances were read in a Thermo Fisher MultiskanSkyHigh microplate spectrophotometer (Waltham, MA, USA) using two filters (595 nm and 690 nm). The inhibitory concentration (IC) decreasing cellular metabolic activity at 20% and 50% of the maximum effect (IC20 and IC50, respectively) were obtained by analyzing the concentration-response curves of each drug in this MTT assays and used in the combination experiments.
Apoptosis. Apoptosis was determined using the Annexin V-FITC kit (Thermo Fisher Scientific, Waltham, MA, USA). HepG2 and HuH7 cells were seeded in 60 mm Petri dishes (50,000 cells per plate) for 72 h at 37˚C either in culture medium alone, or in the presence of the individual drugs or selected combinations, or DMSO. Camptothecin (apoptosis inducer) and methanol (necrosis inducer) were used as controls, which were added to the cells 24 h before the end of the incubation period. The samples were then read on the CYAN ADP flow cytometer (Dako, Glostrup, Denmark). Percentages of viable (FITC-negative and PI-negative), early apoptotic (FITC-positive and PI-negative), late apoptotic (FITC-positive and PI-positive), and necrotic (FITC-negative and PI-positive) cells were obtained by quadrant analysis using the Kaluza version 2.1 software.
Colony formation assay. HepG2 cells were seeded in 60 mm Petri dishes (400 cells per plate) to allow colony growth from single and separated cells. Twenty four hours later, the cells were incubated for 72 h at 37˚C either in culture medium alone, or in the presence of the individual drugs or selected combinations or DMSO. Subsequently, the cells were allowed to grow in the absence of the drugs for 7 days. Then, the cells were fixed with ethanol (absolute grade) for 15 min and stained with crystal violet (1%) for 15 min, washed with water, and counted.
Statistical analysis. Data statistical analysis was performed using the GraphPad Prism software (La Jolla, CA, USA). One-way ANOVA analysis, followed by Tukey’s test was performed. p-Values <0.05 were considered as statistically significant.
Results
Determination of the IC20 and IC50 of sorafenib, raloxifene, and loratadine on the human liver cancer cell lines. Concentration-response curves of the three drugs were obtained using the MTT assay. Ten different concentrations of each drug were evaluated and compared against the effect of DMSO (data not shown). From these curves, the IC20 and IC50 were calculated (Table I) and used in the subsequent combination experiments.
Table I. Inhibitory concentrations [IC (μM)] obtained for each drug and cell line.
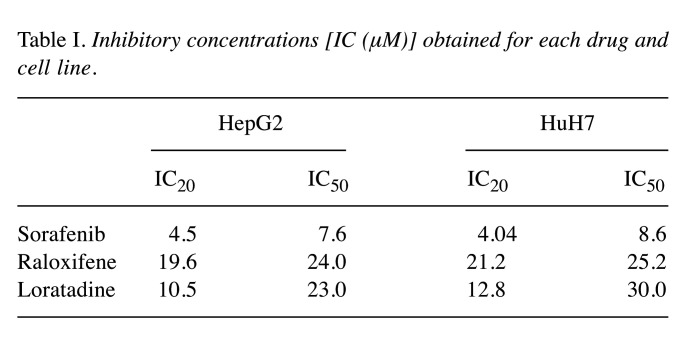
The drug combinations exhibit strong anti-metabolic activity on both cell lines. Figure 1A shows the quantitative determination of the effect of the single drugs on the metabolic activity of HepG2 and HuH7 cells at their corresponding ICs. Some of the two-drug combinations (Figure 1B) resulted in significant higher inhibition of the metabolic activity in both cell lines in comparison to the single-drug effect. Two-drug combinations at their IC20 inhibited around 60% of the metabolic activity. In accordance, three-drug combination at their IC20 inhibited up to 75% of such activity. The higher the concentration used, the stronger the inhibitory effect. In some cases, almost total inhibition of the metabolic activity was achieved when the cells were treated with three-drug combinations (Figure 1C).
Figure 1. Three-drug combinations of sorafenib (S), raloxifene (R), and loratadine (L) abolish the metabolic activity of HepG2 and HUH7 cell lines. A) Effect of single drugs; B) effect of two-drug combinations; C) effect of three-drug combinations. The combination of two or three drugs significantly inhibited metabolic activity in both cell lines compared to the effect of individual drugs. Cells were cultured in the presence of the drug combinations for 72 h and results are shown as the mean±S.D. of eight replicates for each group and from three different experiments. Statistically significant differences in (A) vs. the DMSO group (*); in (B) vs. S IC20 (●), S IC50 (◆), L IC20 (□), L IC50 (▲), R IC20 (§), and R IC50 (φ); in (C) vs. groups using S IC20 (△) in the two-drug combination assay, and vs. groups using S IC50 (θ) also in the two-drug combination assay. p<0.05 in all cases.
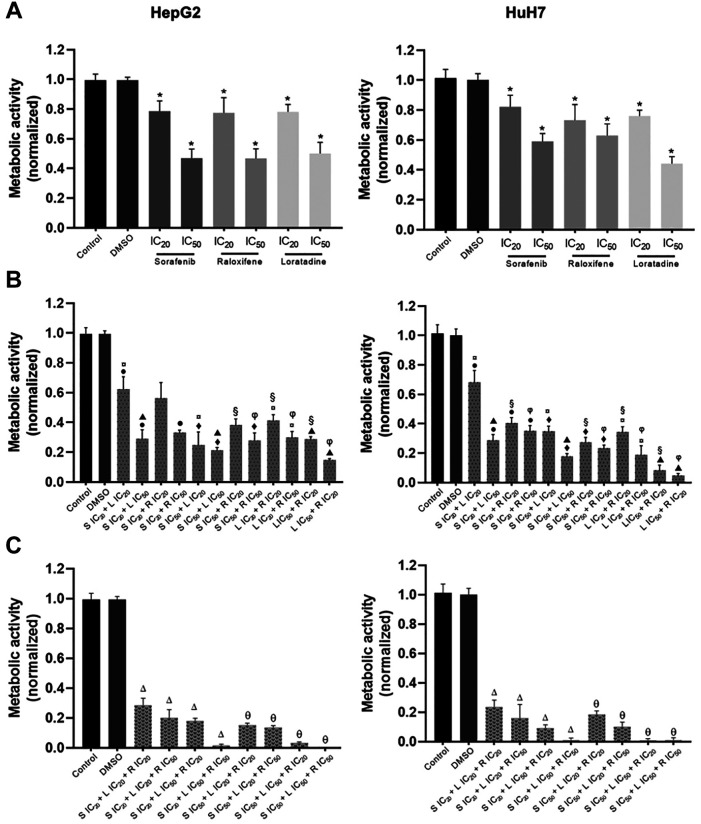
Apoptosis is substantially increased by the drug combinations in both cell lines. Considering the metabolic activity results, the effect on apoptosis of the single-drug and selected drug combinations was studied. In most cases, single-drug treatments significantly decreased the percentage of viable cells and increased late apoptosis in HepG2 cells (Figure 2A) or early apoptosis in HuH7 cells (Figure 2B) in comparison to the corresponding DMSO-treated cells. Noteworthy, raloxifene displayed the strongest single-drug induction of apoptosis even at its IC20. The combination loratadine-raloxifene also showed potent pro-apoptotic effect on both cell lines, as well as the three-drug combinations. Interestingly, the effect of the drug combinations tested was alike to that produced by raloxifene alone at its IC50.
Figure 2. Induction of apoptosis by sorafenib (S), raloxifene (R), and loratadine (L), and their combinations in the human liver cancer cell lines. A) HepG2 and B) HuH7 cells. The combinations of the two and three drugs significantly increased the percentage of cells in apoptosis compared to the activity of the individual drugs, except for raloxifene at its IC50. Cells were cultured in the presence of the combinations or individual drugs for 72 h. Apoptosis was studied by flow cytometry using the annexin V-FITC kit and propidium iodide (PI) staining, and results are shown as the mean±S.D. of three different experiments. Representative plots from the flow-cytometry data for each combination studied are shown. Statistically significant difference vs. the DMSO group (*), S IC20 (●), S IC50 (◆), R IC20 (○), R IC50 (φ), and L IC50 (▲). p<0.05 in all cases.
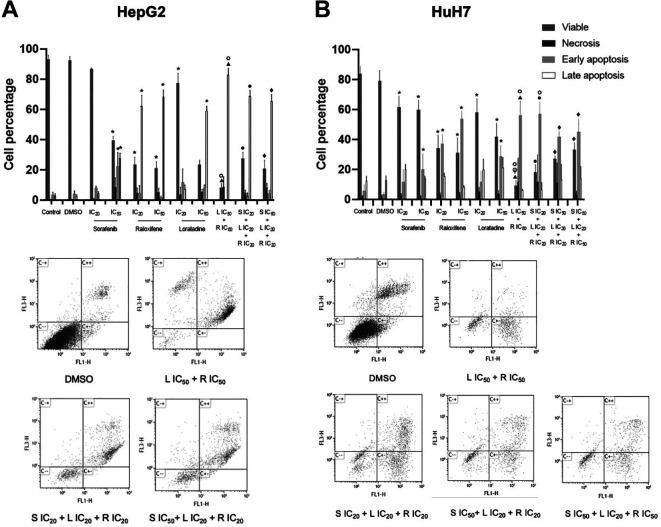
The combinations tested abolished the colony-forming capacity of HepG2 cells. Cell survival was assessed by the colony formation assay. Single-drug treatment inhibited colony formation in a very clear and potent manner in all cases. The combinations tested fully suppressed colony formation (Figure 3). Under our experimental conditions (see Materials and Methods) it was not possible to grow colonies from single cells in the case of the HuH7 cell line, probably due to the low number of cultured cells necessary to guarantee growth from individual separated cells.
Figure 3. Drug combinations of sorafenib (S), raloxifene (R), and loratadine (L) suppress the colony-forming capability of HepG2 cells. The combinations tested in this assay were able to completely inhibit the formation of colonies. Cells were cultured in the presence of the individual drugs or their combinations or DMSO for 72 h and then allowed to grow for additional 7 days in the absence of the drugs. Results are shown as the mean±S.D. from three different assays. A) Representative examples of colony formation in Petri dishes, cells were stained with crystal violet (1%), B) percentage of colonies formed under each condition. Statistically significant difference vs. the DMSO group (*), and S IC20 (●). p<0.05 in all cases.
Discussion
Here, we demonstrate that both loratadine and raloxifene and their combinations with sorafenib have significant activity against human liver cancer cells.
Several targets of raloxifene have been identified as being involved in the development of HCC, either dependent or independent of its action as a SERM. Raloxifene has affinity for ER-α and ER-β, both of which promote liver carcinogenesis when its expression is reduced (10,11). One of the target genes of ER-α is the protein tyrosine phosphatase receptor type O (PTPRO), a membrane protein that has tumor suppressor activity in various cancers including HCC (12) via kinases like JAK2 and PI3K, leading to inhibition of the transcription factor STAT3 which has relevant activity in cancer cell growth and survival (13,14). ER-α interacts with NF-kB and inhibits the secretion of IL-6, one of the most relevant interleukins in HCC development; thus, raloxifene by its inhibitory action on IL-6 secretion interferes in tumor progression via ER-α (9,15). In addition, raloxifene suppresses TGF-α-induced HCC cell migration through ERβ-mediated inhibition of the AKT signaling pathway (8), which is also involved in tumor development including liver cancer (16).
Moreover, raloxifene activation of the transmembrane G-protein-coupled estrogen receptor (GPER) has protective effect against tumorigenesis in HCC through modulation of the inflammatory response (17). Qui et al. showed that GPER activation in human liver cancer cell lines led to sustained activation of the ERK pathway, decreasing cell viability and inducing apoptosis (18). Interestingly, Wang et al. proposed raloxifene use in HCC, demonstrating that it is a potent inhibitor of the IL-6/GP130 signaling pathway, which is involved in HCC oncogenesis (9). The aryl hydrocarbon receptor (AhR) is another pharmacological target of raloxifene independently of its effects on estrogen receptors. O’Donnell et al. reported that raloxifene-AhR activation induces apoptosis in a triple-negative human breast cancer cell line and in human hepatoma cells (19).
Some antihistamines have gained enormous interest as potential anticancer drugs. In retrospective studies with patients diagnosed with different types of cancer including breast cancer and melanoma, Fritz et al. found that the use of the antihistamine loratadine and its metabolite desloratadine, was associated with improved overall survival (20,21). Noteworthy, Ellegaard et al. found that the use of loratadine in patients diagnosed with lung cancer was associated with a significant reduction in mortality, but desloratadine had no a significant effect (7). They suggested that the strong sensitization of the lysosomal membrane in cancer cells allows the entry of cationic amphiphilic drugs (CADs), including loratadine, to this organelle leading to cell death (7,22). Interestingly, the reduction in mortality was greater in patients with a prior use of chemotherapy (7). Furthermore, Adly evaluated the cytotoxic effect of the combination of loratadine and cisplatin in HCC cell lines, finding that both drugs act synergistically (23). Adly also observed that loratadine alone was able to induce cell cycle arrest in the G2/M phase in SNU449 cells, and that this effect remained when the cells were co-incubated with cisplatin plus loratadine. Interestingly, Kim et al. found a very similar effect on the vincristine-resistant cell subline KBV20C. They reported that loratadine sensitized KBV20C cells to the effect of vincristine and that this combination also induced G2-arrest (24). The possible G2-arrest induced by the combination studied in our work deserves further investigation.
Loratadine is an antagonist of the histamine type 1 receptor (H1HR), which is over-expressed in HCC tissues and related to cancer progression. In accordance, Zhao et al. reported that inhibiting this receptor significantly suppressed tumor growth and metastasis in a mouse xenograft model of HCC (25). The calcium channel TRPV2, seems to be involved in the progression of HCC since its mRNA and protein levels are increased in well-differentiated HCC compared to undifferentiated tissue (26). TRPV2 is related to endometrial cancer progression and loratadine, astemizole, and clemizole were identified as TRPV2 blockers, with loratadine being the most potent antagonist, leading to inhibition of cell proliferation and migration of HEK293 cells (27). The potential effect of loratadine on TRPV2 channels in HCC cells deserves further investigation.
The anti-inflammatory activity of loratadine by suppressing NF-kB has also been shown (28) and involves the reduction in proinflammatory component levels like IL-6 and TNF-α. In addition, loratadine, by inhibiting TAK1 suppresses the activator protein 1 (AP-1) signaling pathway, which enables the transcription of inflammation-related enzymes including MMPs (29), and has an important role in cell proliferation, survival, differentiation, apoptosis, and migration (30). Very recently, it was described that desloratadine possesses potential antineoplastic activity through the blockade of N-myristoyl transferase 1 (NMT1) activity, an enzyme that contributes to HCC progression (31). In the same work, the authors showed that the concomitant treatment of desloratadine and sorafenib improved its anticancer effects in comparison to the single-drug treatment. Whether loratadine has a similar effect on NMT1 remains elusive.
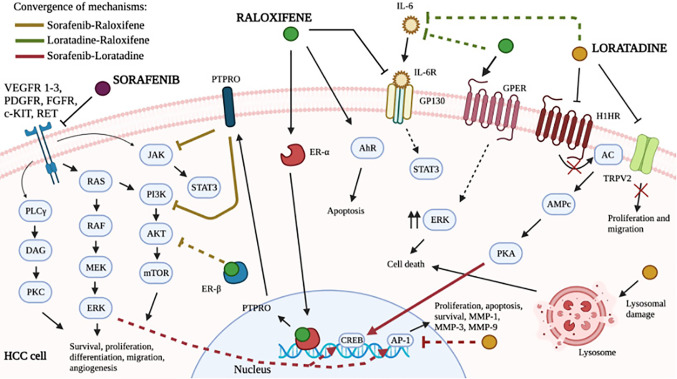
Here, we demonstrate that the three-drug combination sorafenib-loratadine-raloxifene is a potential approach in HCC therapy. The diverse possible mechanisms explaining the strong effect observed are depicted in Figure 4. Shared signaling pathways between two drugs may be potentiated: for example the PI3K/Akt/mTOR pathway in the case of sorafenib and raloxifene, or inhibition of IL-6 secretion in the case of raloxifene and loratadine, or the Ras or TAK1/MEK/ERK and AP-1 in the case of sorafenib and loratadine (32). Notably, the shared pathways described have been associated with the development of resistance to sorafenib in liver cancer (33). Thus, the major clinical problem of sorafenib resistance could be evaded using the three-drug combination here studied, improving the clinical response to sorafenib. Definitely, in vivo studies as well as several “omic” approaches analyzing the effect of the three-drug combination on gene and protein expression are needed and may help to uncover new pathways associated with HCC progression or sorafenib resistance.
Figure 4. Potential molecular mechanisms improving the antiproliferative effect of the combination of sorafenib, loratadine, and raloxifene in hepatocellular carcinoma. The scheme integrates common signaling pathways involved in cell proliferation, apoptosis, or resistance to sorafenib. Solid lines represent a direct effect, while dashed lines denote the participation of intermediate components as described in the main text.
The mortality-to-incidence ratio of HCC is worrying. Increasing evidence suggests drug repurposing (alone or in combinations) as potential treatment against different diseases including cancer (34,35). Definitely, these approaches will lead to the development of more and improved therapeutic options. Loratadine is the most commonly prescribed antihistamine (7) and sorafenib and raloxifene are FDA-approved anticancer drugs. Taken together, the major antiproliferative effects of the three-drug combination here shown, strongly warrant its study in clinical settings.
Conflicts of Interest
The Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
FV-M, CS-A and EH-G performed the experiments; FV-M, EHG, MEM-G and JC analyzed the data; FV-M and JC wrote the paper; FV-M, MEM-G and JC made substantial contributions to the manuscript; all Authors contributed to the design of the experiments, reviewed, and approved the manuscript.
Acknowledgements
FV-M received the CONACyT fellowship number 787907 supporting her M.Sc. studies.
References
- 1.Estimated age-standardized incidence and mortality rates (World) in 2020, World, both sexes, all ages, Global Cancer Observatory: Cancer Today. Lyon, France, International Agency for Research on Cancer. Available at: https://gco.iarc.fr. [Last accessed on October 14, 2022]
- 2.Attwa MH, El-Etreby SA. Guide for diagnosis and treatment of hepatocellular carcinoma. World J Hepatol. 2015;7(12):1632–1651. doi: 10.4254/wjh.v7.i12.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol. 2014;20(15):4115–4127. doi: 10.3748/wjg.v20.i15.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Likhitsup A, Razumilava N, Parikh ND. Treatment for advanced hepatocellular carcinoma: current standard and the future. Clin Liver Dis (Hoboken) 2019;13(1):13–19. doi: 10.1002/cld.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu XD, Li KS, Sun HC. Adjuvant therapies after curative treatments for hepatocellular carcinoma: Current status and prospects. Genes Dis. 2020;7(3):359–369. doi: 10.1016/j.gendis.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chávez-López MG, Zúñiga-García V, Hernández-Gallegos E, Vera E, Chasiquiza-Anchatuña CA, Viteri-Yánez M, Sanchez-Ramos J, Garrido E, Camacho J. The combination astemizole-gefitinib as a potential therapy for human lung cancer. Onco Targets Ther. 2017;10:5795–5803. doi: 10.2147/OTT.S144506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellegaard AM, Dehlendorff C, Vind AC, Anand A, Cederkvist L, Petersen NHT, Nylandsted J, Stenvang J, Mellemgaard A, Østerlind K, Friis S, Jäättelä M. Repurposing cationic amphiphilic antihistamines for cancer treatment. EBioMedicine. 2016;9:130–139. doi: 10.1016/j.ebiom.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsushima-Nishiwaki R, Yamada N, Hattori Y, Hosokawa Y, Tachi J, Hori T, Kozawa O. SERMs (selective estrogen receptor modulator), acting as estrogen receptor β agonists in hepatocellular carcinoma cells, inhibit the transforming growth factor-α-induced migration via specific inhibition of AKT signaling pathway. PLoS One. 2022;17(1):e0262485. doi: 10.1371/journal.pone.0262485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Ma H, Zhao C, Liu T, Yan D, Jou D, Li H, Zhang C, Lü J, Li C, Lin J, Li S, Lin L. Growth-suppressive activity of raloxifene on liver cancer cells by targeting IL-6/GP130 signaling. Oncotarget. 2017;8(20):33683–33693. doi: 10.18632/oncotarget.16898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sukocheva OA. Estrogen, estrogen receptors, and hepatocellular carcinoma: Are we there yet. World J Gastroenterol. 2018;24(1):1–4. doi: 10.3748/wjg.v24.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi L, Feng Y, Lin H, Ma R, Cai X. Role of estrogen in hepatocellular carcinoma: is inflammation the key. J Transl Med. 2014;12:93. doi: 10.1186/1479-5876-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie F, Dong H, Zhang H. Regulatory functions of protein tyrosine phosphatase receptor type O in immune cells. Front Immunol. 2021;12:783370. doi: 10.3389/fimmu.2021.783370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svinka J, Mikulits W, Eferl R. STAT3 in hepatocellular carcinoma: new perspectives. Hepat Oncol. 2014;1(1):107–120. doi: 10.2217/hep.13.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng X, Liu X. Therapeutic value of estrogen receptor α in hepatocellular carcinoma based on molecular mechanisms. J Clin Transl Hepatol. 2022;10(1):140–146. doi: 10.14218/JCTH.2021.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Lin H, Wu G, Zhu M, Li M. IL-6/STAT3 is a promising therapeutic target for hepatocellular carcinoma. Front Oncol. 2021;11:760971. doi: 10.3389/fonc.2021.760971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun EJ, Wankell M, Palamuthusingam P, McFarlane C, Hebbard L. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomedicines. 2021;9(11):1639. doi: 10.3390/biomedicines9111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei T, Chen W, Wen L, Zhang J, Zhang Q, Yang J, Liu H, Chen BW, Zhou Y, Feng X, Yang Q, Bai X, Liang T. G protein-coupled estrogen receptor deficiency accelerates liver tumorigenesis by enhancing inflammation and fibrosis. Cancer Lett. 2016;382(2):195–202. doi: 10.1016/j.canlet.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Qiu YA, Xiong J, Fu Q, Dong Y, Liu M, Peng M, Jin W, Zhou L, Xu X, Huang X, Fu A, Xu G, Tu G, Yu T. GPER-induced ERK signaling decreases cell viability of hepatocellular carcinoma. Front Oncol. 2021;11:638171. doi: 10.3389/fonc.2021.638171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donnell EF, Koch DC, Bisson WH, Jang HS, Kolluri SK. The aryl hydrocarbon receptor mediates raloxifene-induced apoptosis in estrogen receptor-negative hepatoma and breast cancer cells. Cell Death Dis. 2014;5(1):e1038. doi: 10.1038/cddis.2013.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritz I, Wagner P, Broberg P, Einefors R, Olsson H. Desloratadine and loratadine stand out among common H(1)-antihistamines for association with improved breast cancer survival. Acta Oncol. 2020;59(9):1103–1109. doi: 10.1080/0284186X.2020.1769185. [DOI] [PubMed] [Google Scholar]
- 21.Fritz I, Wagner P, Olsson H. Improved survival in several cancers with use of H(1)-antihistamines desloratadine and loratadine. Transl Oncol. 2021;14(4):101029. doi: 10.1016/j.tranon.2021.101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kallunki T, Olsen OD, Jäättelä M. Cancer-associated lysosomal changes: friends or foes. Oncogene. 2013;32(16):1995–2004. doi: 10.1038/onc.2012.292. [DOI] [PubMed] [Google Scholar]
- 23.Adly N. Evaluation of cytotoxic potential of loratadine and the combination of loratadine and cisplatin on hepatocellular carcinoma cell lines. American University in Cairo (AUC), 2018. Available at: https://fount.aucegypt.edu/etds/418. [Last accessed on April 1, 2023] [Google Scholar]
- 24.Kim JY, Kim KS, Kim IS, Yoon S. Histamine receptor antagonists, loratadine and azelastine, sensitize P-gp-overexpressing antimitotic drug-resistant KBV20C cells through different molecular mechanisms. Anticancer Res. 2019;39(7):3767–3775. doi: 10.21873/anticanres.13525. [DOI] [PubMed] [Google Scholar]
- 25.Zhao J, Hou Y, Yin C, Hu J, Gao T, Huang X, Zhang X, Xing J, An J, Wan S, Li J. Upregulation of histamine receptor H1 promotes tumor progression and contributes to poor prognosis in hepatocellular carcinoma. Oncogene. 2020;39(8):1724–1738. doi: 10.1038/s41388-019-1093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G, Xie C, Sun F, Xu X, Yang Y, Zhang T, Deng Y, Wang D, Huang Z, Yang L, Huang S, Wang Q, Liu G, Zhong D, Miao X. Clinical significance of transient receptor potential vanilloid 2 expression in human hepatocellular carcinoma. Cancer Genet Cytogenet. 2010;197(1):54–59. doi: 10.1016/j.cancergencyto.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Van den Eynde C, Held K, Ciprietti M, De Clercq K, Kerselaers S, Marchand A, Chaltin P, Voets T, Vriens J. Loratadine, an antihistaminic drug, suppresses the proliferation of endometrial stromal cells by inhibition of TRPV2. Eur J Pharmacol. 2022;928:175086. doi: 10.1016/j.ejphar.2022.175086. [DOI] [PubMed] [Google Scholar]
- 28.Hunto ST, Kim HG, Baek KS, Jeong D, Kim E, Kim JH, Cho JY. Loratadine, an antihistamine drug, exhibits anti-inflammatory activity through suppression of the NF-(k)B pathway. Biochem Pharmacol. 2020;177:113949. doi: 10.1016/j.bcp.2020.113949. [DOI] [PubMed] [Google Scholar]
- 29.Jang J, Hunto ST, Kim JW, Lee HP, Kim HG, Cho JY. Anti-inflammatory activities of an anti-histamine drug, loratadine, by suppressing TAK1 in AP-1 pathway. Int J Mol Sci. 2022;23(7):3986. doi: 10.3390/ijms23073986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye N, Ding Y, Wild C, Shen Q, Zhou J. Small molecule inhibitors targeting activator protein 1 (AP-1) J Med Chem. 2014;57(16):6930–6948. doi: 10.1021/jm5004733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan XP, He Y, Yang J, Wei X, Fan YL, Zhang GG, Zhu YD, Li ZQ, Liao HX, Qin DJ, Guan XY, Li B. Blockade of NMT1 enzymatic activity inhibits N-myristoylation of VILIP3 protein and suppresses liver cancer progression. Signal Transduct Target Ther. 2023;8(1):14. doi: 10.1038/s41392-022-01248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17(7):1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- 33.Jiang L, Li L, Liu Y, Lu L, Zhan M, Yuan S, Liu Y. Drug resistance mechanism of kinase inhibitors in the treatment of hepatocellular carcinoma. Front Pharmacol. 2023;14:1097277. doi: 10.3389/fphar.2023.1097277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turabi KS, Deshmukh A, Paul S, Swami D, Siddiqui S, Kumar U, Naikar S, Devarajan S, Basu S, Paul MK, Aich J. Drug repurposing-an emerging strategy in cancer therapeutics. Naunyn Schmiedebergs Arch Pharmacol. 2022;395(10):1139–1158. doi: 10.1007/s00210-022-02263-x. [DOI] [PubMed] [Google Scholar]
- 35.Yoon S, Kim HS. Drug repositioning with an anticancer effect: Contributions to reduced cancer incidence in susceptible individuals. In Vivo. 2021;35(6):3039–3044. doi: 10.21873/invivo.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]