Abstract
Background/Aim: Mucinous tubular and spindle cell carcinoma (MTSCC) of the kidney is a rare histological type of renal cell carcinoma (RCC). There are few reports of MTSCC occurring in renal transplant recipients (RTRs). The aim of this study was to report a case of long-term survival of a RTR with metastatic MTSCC of the kidney with sarcomatoid changes.
Case Report: A 53-year-old male with a left retroperitoneal tumor was referred to our department. He had been receiving hemodialysis since 1991 and underwent kidney transplantation in 2015. Computed tomography (CT) revealed suspected RCC, and a radical nephrectomy was performed in June 2020. Pathological findings revealed MTSCC with sarcomatoid changes. After the surgery, multiple metastases appeared in the bilateral adrenals, skin, para-aortic lymph nodes, muscles, mesocolon, and liver. We treated the patient with metastasectomy, radiation therapy, and sequential systemic therapy with tyrosine kinase inhibitors (TKI). Two years after the initial surgery, the patient died of cancer while controlling its progression.
Conclusion: We report a RTR with aggressive and metastatic MTSCC with sarcomatoid changes, resulting in a longer survival time relative to multimodal therapy.
Keywords: Mucinous tubular and spindle cell carcinoma, renal transplant recipients, sarcomatoid changes
Mucinous tubular and spindle cell carcinoma (MTSCC) of the kidney is a rare histological type of renal cell carcinoma (RCC) that was initially defined as a low-grade neoplasm with good prognosis according to the 2004 World Health Organization (WHO) classification (1). However, several cases have been reported with aggressive clinical features and poor outcomes (2-5). Accordingly, the term indolent kidney cancer was deleted from the 2016 WHO classification.
It is well known that renal transplant recipients (RTRs) had three to seven times higher incidence of self-kidney cancer than the general population (6-8). Nevertheless, to our knowledge, there were no reports of MTSCC in RTRs.
Herein, we describe the case of RTR with metastatic MTSCC with sarcomatoid components.
Case Report
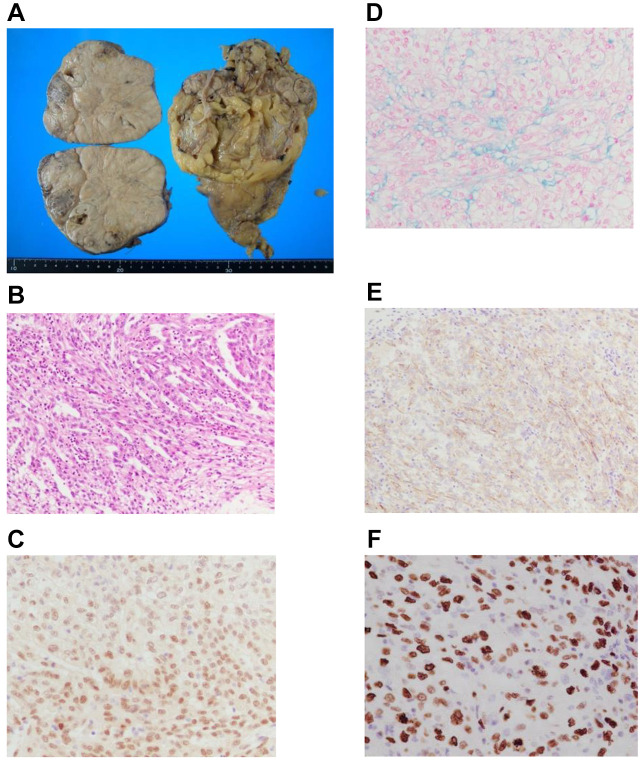
A 53-year-old Japanese male with end-stage renal disease secondary to renal sclerosis underwent living-related kidney transplantation in the right iliac fossa in 2015. His hemodialysis duration was 24 years. He received the immunosuppressive drugs tacrolimus, mycophenolate mofetil (MMF), and methylprednisolone. Five years after transplantation, follow-up computed tomography (CT) revealed a left retroperitoneal tumor and was referred to our clinic for further examination and treatment. An enhanced CT scan showed a left renal tumor (Figure 1A). The tumor was less enhanced in the early phase (Figure 1B) and more enhanced in the nephrographic phase (Figure 1C). A tumor biopsy revealed suspected kidney cancer, and we performed an open radical nephrectomy with renal hilar lymph node dissection in June 2020. Pathological findings revealed MTSCC (pT3a) with sarcomatoid changes and no nodal metastasis. The surgical specimen measured 11.5 × 9.5×7.0 cm in size, and macroscopic findings showed this tumor was a nodular mass with a greyish-white cut surface accompanied by necrosis (Figure 2A). Histologically, this tumor had tubular architecture and was composed of fascicles of spindle cells (Figure 2B). Immunohistochemically, the tumor cells were positive for PAX8 (Figure 2C) and alcian-blue (Figure 2D), partially positive for alpha-methylacyl-CoA (AMACR) (Figure 2E) and epithelial membrane antigen (EMA), and negative for cytokeratin 7, GATA3, calretinin, uroplakin-II, and WT-1. The Ki-67 index was 53.2% (Figure 2F). After diagnosis, we changed the MMF to the mammalian target of rapamycin (mTOR) inhibitor everolimus. CT detected left adrenal metastases two months after surgery, and we performed left adrenalectomy in August 2020. The pathological findings revealed metastatic MTSCC. Six months after the initial surgery, right adrenal and back skin metastases appeared, and pazopanib (400 mg/day) was started. In January 2021, his back skin metastasis was extensive, and a metastasectomy of his back skin tumor was performed. Pathological examination revealed a metastatic MTSCC. The right adrenal metastasis was larger, and para-aortic LN swelling and tumor mass on the right hip muscle appeared on positron emission tomography-CT (PET-CT) in March 2021. As a result, we changed his medication to axitinib 10 mg per day, and radiation therapy targeting all metastases was performed (45 Gy/total dose). Although the size of the metastatic tumors (right adrenal, para-aortic LN, and right hip muscle) decreased, sigmoid mesocolon metastasis was found as a new lesion on PET-CT in May 2021. Radiation therapy for sigmoid mesocolon metastasis was administered (total dose: 45 Gy) in June 2021. The size of the right adrenal and sigmoid mesocolon metastases decreased, and the right hip muscle metastasis disappeared; however, the para-aortic LN was larger, and a new lesion appeared in the right shoulder muscle in September 2021. In November 2021, he received radiation therapy with a total dose of 30 Gy targeted to the right shoulder muscle. Although the right shoulder muscle was stable disease (SD), the paraaortic LN and sigmoid mesocolon were progressive disease (PD), and he received radiation therapy to the paraaortic LN with a total dose of 45 Gy in January 2022. Axitinib was discontinued for every radiation therapy duration. In April 2022, enhanced CT revealed multiple new metastases in the right trapezius, left axillary soft tissue, and liver, and metastasis in the sigmoid mesocolon and LN were PD; his medication was changed to cabozantinib 60 mg per day. However, his condition gradually worsened, and he died of cancer in June 2022.
Figure 1. Computed tomography imaging findings. Enhanced computed tomography coronal section in early phase (A). Horizontal section in early phase (B), and in nephrographic phase (C).
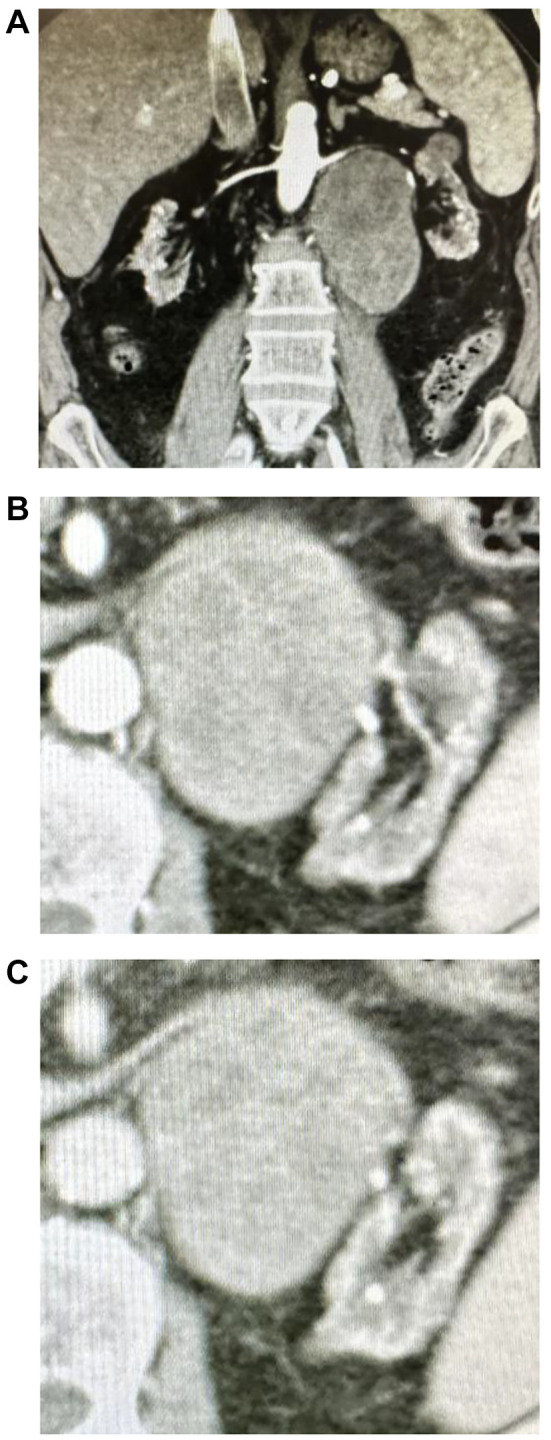
Figure 2. Histopathological features of mucinous tubular and spindle cell carcinoma. Macroscopic findings (A). Haematoxylin and eosin stain (B). PAX8 (C). Alcian-blue (D). AMACR (E). Ki-67 index (F).
Discussion
We report the first case of RTR with metastatic MTSCC with sarcomatoid changes treated with sequential TKI, metastasectomy, and radiotherapy in a situation where immune checkpoint inhibitors (ICIs) could not be used, resulting in a 2-year survival time.
MTSCC of the kidney is an extremely rare histological type, with fewer than 100 cases reported since it was recognized as a discrete pathological entity in 2004 (9,10). Generally, MTSCC follows an indolent course, and most cases are managed successfully with surgical removal (11). However, several cases of metastatic MTSCC with an aggressive course and poor outcome have been reported recently (2), especially in patients with MTSCC with sarcomatoid components, with a survival time of less than 1 year (12-14). There is no recommended systemic therapy for metastatic MTSCC because it is very rare. The cases of metastatic and aggressive MTSCC are summarized in Table I. In 2010, Larkin et al. reported that sunitinib is an effective treatment for patients with MTSCC (15). However, another study showed that pazopanib was ineffective for MTSCC (16). Ivey et al. reported that aggressive surgical resection may be effective for patients with MTSCC with only LN metastases (10). Takahashi et al. reported that a patient with metastatic MTSCC who received nivolumab showed a complete response (17). Fuchizawa et al. (18) reported that the disease of a 69- year-old male with MTSCC of the left kidney and multiple bone metastases was controlled by administering of ipilimumab and nivolumab combination therapy after cytoreductive nephrectomy, and the patient remained disease-free without immunotherapy. In addition, this study suggested that the immune-checkpoint inhibitors (ICIs) were effective in metastatic MTSCC, similar to other RCCs (19,20). However, regarding RTRs, there are two problems: using immunosuppressive drugs to prevent graft loss may support the increase in cancer (6), and using ICIs may induce rejection and graft loss. A systematic review of the literature found that ICIs may lead to improved cancer outcomes. However, 42% of RTRs with cancer treated with ICIs developed acute graft rejection, and 65.5% of them did not recover and required dialysis (21). In contrast, Murakami et al. suggested that mTOR inhibitor therapy helped maintain graft tolerance and achieve tumor immunity simultaneously for ICI-treated RTRs (22). Because our case study showed that nivolumab-ipilimumab combination therapy could be feasible with acceptable complications and is considered effective in hemodialysis patients with mRCC (23), it was one of the treatment choices to use ICIs. We treated him with TKI only because he wanted to avoid rejection, graft loss, and physical and psychological distress. Additionally, we treated the patient with metastasectomy and radiotherapy; nevertheless, he died of cancer two years after the initial surgery. Most patients with MTSCC with sarcomatoid changes died of cancer after less than one year; our patient had 2 years survival time possibly because of the multidisciplinary therapy. Because a treatment strategy for metastatic MTSCC has not been established, it is necessary to accumulate metastatic MTSCC cases in the future.
Table I. Previous case reports of patients with aggressive and metastatic mucinous tubular and spindle cell carcinoma.
*TNM at initial diagnosis or after initial surgery. CN: Cytoreductive nephrectomy; LN: lymph node; LND: lymph node dissection; PN: partial nephrectomy; RN: radical nephrectomy; F: female; M: male.
Conclusion
We first observed that the RTR of metastatic MTSCC with sarcomatoid changes treated with sequential TKI therapy, metastasectomy, radiotherapy, and a switch from MMF to everolimus, resulted in a 2-year survival time.
Conflicts of Interest
All Authors have no conflicts of interest to declare in relation to this study.
Authors’ Contributions
YK wrote the manuscript and provided the figures. YK, KY, RM, HF, KH, KU, JI, HI, and TT cared for the patients and administered the systemic therapy. YN pathologically diagnosed this tumor as MTSCC. All Authors have read and approved the final manuscript.
References
- 1.Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49(5):798–805. doi: 10.1016/j.eururo.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 2.Isono M, Seguchi K, Yamanaka M, Miyai K, Okubo K, Ito K. Rapid progression of mucinous tubular and spindle cell carcinoma of the kidney without sarcomatoid changes: A case report. Urol Case Rep. 2020;31:101162. doi: 10.1016/j.eucr.2020.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang J, Dong B, Zhang J, Chen Y, Wu X, Kong W, Jin D, Xue W, Liu D, Huang Y. [Clinicopathological characteristics and prognosis of 11 patients with renal mucinous tubular and spindle cell carcinoma] Zhonghua Zhong Liu Za Zhi. 2014;36(9):693–696. [PubMed] [Google Scholar]
- 4.Wang H, Xie J, Lu C, Zhang D, Jiang J. Renal mucinous tubular and spindle cell carcinoma: report of four cases and literature review. Int J Clin Exp Pathol. 2015;8(3):3122–3126. [PMC free article] [PubMed] [Google Scholar]
- 5.Pillay N, Ramdial PK, Cooper K, Batuule D. Mucinous tubular and spindle cell carcinoma with aggressive histomorphology—a sarcomatoid variant. Hum Pathol. 2008;39(6):966–969. doi: 10.1016/j.humpath.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 8.de Fijter JW. Cancer and mTOR inhibitors in transplant recipients. Transplantation. 2017;101(1):45–55. doi: 10.1097/TP.0000000000001447. [DOI] [PubMed] [Google Scholar]
- 9.Gaafar A, Valentí C, Echevarria C, Laforga JB, López JI. Renal mucinous and tubular spindle cell carcinoma: a clinicopathological study of 4 cases. Ann Saudi Med. 2006;26(6):466–470. doi: 10.5144/0256-4947.2006.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivey JA 3rd, Cortese C, Baird BA, Thiel DD, Lyon TD. Mucinous tubular and spindle cell carcinoma of the kidney with nodal metastasis managed with surgical resection. Eur Urol Open Sci. 2021;29:10–14. doi: 10.1016/j.euros.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miura K, Adachi Y, Shirahase T, Nagashima Y, Suemune K, Sakaida N, Nakano Y, Sakai Y, Shimizu S, Ikehara S. A case of high-grade mucinous tubular and spindle cell carcinoma. J Surg Case Rep. 2020;2020(2):rjaa014. doi: 10.1093/jscr/rjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhillon J, Amin MB, Selbs E, Turi GK, Paner GP, Reuter VE. Mucinous tubular and spindle cell carcinoma of the kidney with sarcomatoid change. Am J Surg Pathol. 2009;33(1):44–49. doi: 10.1097/PAS.0b013e3181829ed5. [DOI] [PubMed] [Google Scholar]
- 13.Arafah M, Zaidi SN. Mucinous tubular and spindle cell carcinoma of the kidney with sarcomatoid transformation. Saudi J Kidney Dis Transpl. 2013;24(3):557–560. doi: 10.4103/1319-2442.111066. [DOI] [PubMed] [Google Scholar]
- 14.Sugimoto R, Uesugi N, Yamada N, Osakabe M, Fujita Y, Eizuka M, Kato R, Ishida K, Obara W, Nagashima Y, Sugai T. Sarcomatoid change associated with epithelial-mesenchymal transition in mucinous tubular and spindle cell carcinoma of the kidney: a case report. Int J Clin Exp Pathol. 2019;12(7):2767–2771. [PMC free article] [PubMed] [Google Scholar]
- 15.Larkin J, Fisher R, Pickering L, Thway K, Livni N, Fisher C, Gore M. Metastatic mucinous tubular and spindle cell carcinoma of the kidney responding to sunitinib. J Clin Oncol. 2010;28(28):e539–e540. doi: 10.1200/JCO.2010.30.1457. [DOI] [PubMed] [Google Scholar]
- 16.Sokolakis I, Kalogirou C, Frey L, Oelschläger M, Krebs M, Riedmiller H, Kübler H, Vergho D. Mucin-poor mucinous tubular and spindle cell carcinoma of the kidney presented with multiple metastases two years after nephrectomy: an atypical behaviour of a rare, indolent tumour. Case Rep Urol. 2017;2017:6597592. doi: 10.1155/2017/6597592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi Y, Numakura K, Aoyama Y, Okada S, Saito T, Muto Y, Koizumi A, Nara T, Chiba S, Kanda S, Saito M, Narita S, Inoue T, Satoh S, Nanjo H, Habuchi T. [Metastatic mucinous tubular and spindle cell carcinoma treated with nivolumab successfully: a case report] Hinyokika Kiyo. 2019;65(9):363–367. doi: 10.14989/ActaUrolJap_65_9_363. [DOI] [PubMed] [Google Scholar]
- 18.Fuchizawa H, Kijima T, Takada-Owada A, Nagashima Y, Okazaki A, Yokoyama M, Nishihara D, Ishida K, Kamai T. Metastatic mucinous tubular and spindle cell carcinoma of the kidney responding to nivolumab plus ipilimumab. IJU Case Rep. 2021;4(5):333–337. doi: 10.1002/iju5.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koshkin VS, Barata PC, Zhang T, George DJ, Atkins MB, Kelly WJ, Vogelzang NJ, Pal SK, Hsu J, Appleman LJ, Ornstein MC, Gilligan T, Grivas P, Garcia JA, Rini BI. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J Immunother Cancer. 2018;6(1):9. doi: 10.1186/s40425-018-0319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chahoud J, Msaouel P, Campbell MT, Bathala T, Xiao L, Gao J, Zurita AJ, Shah AY, Jonasch E, Sharma P, Tannir NM. Nivolumab for the treatment of patients with metastatic non-clear cell renal cell carcinoma (nccRCC): a single-institutional experience and literature meta-analysis. Oncologist. 2020;25(3):252–258. doi: 10.1634/theoncologist.2019-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami N, Mulvaney P, Danesh M, Abudayyeh A, Diab A, Abdel-Wahab N, Abdelrahim M, Khairallah P, Shirazian S, Kukla A, Owoyemi IO, Alhamad T, Husami S, Menon M, Santeusanio A, Blosser CD, Zuniga SC, Soler MJ, Moreso F, Mithani Z, Ortiz-Melo D, Jaimes EA, Gutgarts V, Lum E, Danovitch GM, Cardarelli F, Drews RE, Bassil C, Swank JL, Westphal S, Mannon RB, Shirai K, Kitchlu A, Ong S, Machado SM, Mothi SS, Ott PA, Rahma O, Hodi FS, Sise ME, Gupta S, Leaf DE, Devoe CE, Wanchoo R, Nair VV, Schmults CD, Hanna GJ, Sprangers B, Riella LV, Jhaveri KD, Immune Checkpoint Inhibitors in Solid Organ Transplant Consortium A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int. 2021;100(1):196–205. doi: 10.1016/j.kint.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esfahani K, Al-Aubodah TA, Thebault P, Lapointe R, Hudson M, Johnson NA, Baran D, Bhulaiga N, Takano T, Cailhier JF, Piccirillo CA, Miller WH. Targeting the mTOR pathway uncouples the efficacy and toxicity of PD-1 blockade in renal transplantation. Nat Commun. 2019;10(1):4712. doi: 10.1038/s41467-019-12628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobari Y, Yoshida K, Iizuka J, Kondo T, Ishida H, Tanabe K, Takagi T. Three cases of nivolumab plus ipilimumab therapy in haemodialysis patients with metastatic renal cell carcinoma. In Vivo. 2021;35(6):3585–3589. doi: 10.21873/invivo.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]