Abstract
Objective.
To describe anthropometric, sensory, and neurodevelopmental outcomes of children who were Zika virus–exposed from birth to 36 months.
Study design.
The study cohort included 114 children born to mothers with confirmed and probable Zika virus pregnancy infection in 2016–2017. Children attending study visits from May 2017 through February 2020 underwent physical/neurologic, sensory examinations, and neurodevelopmental assessments with the Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III) and Ages and Stages Questionnaires, Third Edition (ASQ-3).
Results.
Three of the 114 children (2.6%) had microcephaly (z-score for head circumference ≤ −2) at birth, 19 of 35 (54.3%) had posterior eye abnormalities in retinal images, and 11 of 109 (10.1%) had nonspecific findings on brain ultrasound. Three of 107 children (2.8%) failed hearing screening at birth. Of those children with follow-up data, 17 of 97 (17.5%) failed age-appropriate vision screening. The BSID-III identified developmental delay in at least 1 domain in at least one-third of children, with higher prevalence in the language domain. ASQ-3 screen positive delay peaked at around 24 or 36 months, with some domains showing a decrease at older ages. Correlations among BSID-III and ASQ-3 scores were observed, representing professional and parental perspectives at 24 and 36 months (r = 0.32-0.78; P < .05).
Conclusion.
The presence of neurodevelopmental sequelae in early childhood suggests that identification of long-term impairment remains critical to attaining optimal child development. Long-term follow-up highlights vulnerability in the language domain, which likely could be influenced by early intervention, promoting cognitive development and school readiness in exposed children.
Keywords: congenital Zika virus exposure, prenatal Zika virus exposure, children without microcephaly/asymptomatic at birth, neurodevelopmental outcomes, developmental delay
Introduction
Zika virus, a member of the Flaviviridae family, became manifest as a pathogen during an epidemic of febrile rash illness in northeastern Brazil, followed by an increase in infants with microcephaly born to mothers who lived in or visited in the same region during pregnancy.1–4 Although 4–7% of Zika virus infections during pregnancy result in fetal loss, a critical outcome of Zika virus infection during pregnancy, characterized as congenital Zika syndrome,5 is identified in 5%–14% of neonates, including 4%–6% with microcephaly.6 Therefore, 79%–91% are asymptomatic infected neonates without clinically evident birth defects, who can be at risk for medium and long-term sequelae.6, 7 An array of adverse outcomes have been recognized in asymptomatic infected children over time, including postnatal microcephaly,7–10 developmental delay,7, 11–15 autism spectrum disorder,8 and poor visual function,16, 17 supporting expert recommendations for follow-up of all exposed children with developmental monitoring and screening.7, 18–20,21 For pediatric providers in at-risk regions of the world, full knowledge of the spectrum of disease and a high index of suspicion are essential, because most maternal infections are asymptomatic and Zika virus transmission can occur without identifiable outbreaks.6
Puerto Rico, a US territory with an estimated population of 3.2 million and a primarily Hispanic ethnic composition (98.7%),22 experienced the Zika epidemic from January 2016 through December 2017, with a total of 40,630 confirmed cases, including 4,134 pregnant women.23 The Pediatric Outcomes of Prenatal Zika Exposure cohort study, active since May 2017, aims to describe the spectrum of disease in children born to mothers with confirmed and probable Zika virus infection during pregnancy. Here we report the longitudinal prevalence of anthropometric, sensory and neurodevelopmental outcomes from birth to 36 months in this group of Hispanic children from Puerto Rico, most of whom were asymptomatic at birth.
Methods
This prospective cohort includes 114 children of mothers meeting the enrollment criteria born at 2 academic hospital sites in the city of Ponce that serve an estimated population of 477,603 living in the southern region of Puerto Rico.24 The Centers for Disease Control and Prevention (CDC) reviewed and the Ponce Medical School Foundation Institutional Review Board approved this study. Parental informed consent was obtained in writing. During the Zika epidemic, the Puerto Rico Department of Health Zika Active Pregnancy Surveillance System, in collaboration with the CDC, identified and actively monitored pregnant women for infection during each trimester, as well as their exposed children. Mothers with confirmed and probable Zika virus infection during pregnancy were included after confirmation of their infection status in the birth record and the Zika Active Pregnancy Surveillance System Registry. Confirmed infection was defined as presence of Zika virus RNA in maternal serum on the CDC’s Trioplex real-time reverse transcriptase-polymerase chain reaction (PCR) assay. Probable cases were defined by maternal serum positive or equivocal Zika virus immunoglobulin M (IgM) antibody capture enzyme-linked immunosorbent assay (Zika virus IgM antibody capture ELISA [MAC-ELISA]) or maternal recent flavivirus infection as evidenced by positive anti-Zika virus IgM MAC-ELISA and positive anti-dengue virus IgM ELISA, based on epidemiologic considerations.25 For symptomatic pregnant women, the timing of infection was determined from the week of gestation26 or the trimester coincident with symptom onset as documented in the birth record. Maternal education attainment and other sociodemographic characteristics were obtained through direct interview by trained study personnel.
Enrollment of children in the dynamic cohort occurred at birth or at any age-specific study visit every 6 months. Sixty-two children were enrolled in the neonatal period and 52 were enrolled at a study visit between May 1, 2017, and December 2019 (Figure 1. Assessments were conducted until February 28, 2020. Newborn diagnostic procedures at both study sites followed Puerto Rico Department of Health mandates and CDC guidelines.18, 19 Trained study personnel performed record extraction of sociodemographic information, medical, obstetric, and delivery history; and childbirth outcomes, including results of automated auditory brainstem response testing, brain ultrasonography, and retinal images with the RetCam3 visualization system (Clarity Medical Systems, Inc), mandated on all exposed newborns. Hospital radiology technicians performed brain ultrasonography in 109 of 114 (95.6%), and a nurse trained in RetCam3 system obtained retinal images in 35 of these114 (30.7%). Pediatric subspecialty experts in radiology and ophthalmology provided the readings to 92 of 109 ultrasound images (84.4%) and 32 of 35 retinal images (91.4%), respectively. Their findings were documented in standardized study forms that specified Zika virus-associated birth defects and allowed for relevant observations that could contribute to the spectrum of disease. Along with microcephaly, these clinically evident retinal imaging and brain ultrasound findings constitute the case definition for symptomatic children at birth.
Figure 1. Dynamic cohort enrollment and compliance with study procedures and assessments, Pediatric Outcomes of Prenatal Zika Exposure study, Puerto Rico 2017–2020.
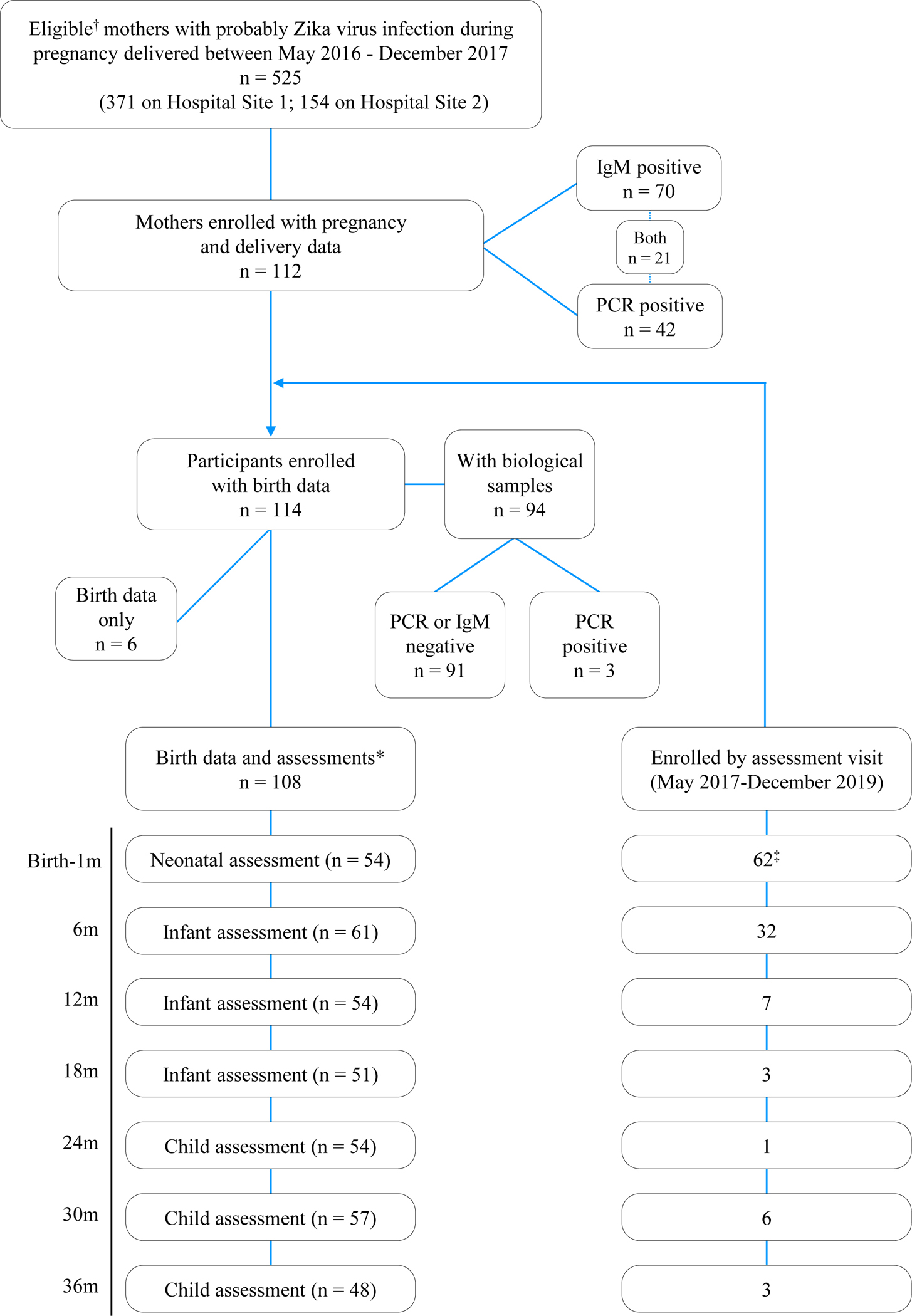
†Eligibility: Mothers with positive Zika virus PCR or immunoglobulin M (IgM) antibody at any trimester during pregnancy delivered at 1 of 2 hospital study sites during the Zika epidemic.
*Participants with birth data extracted from neonatal record and assessments performed in the neonatal period and at any of the follow-up-age-specific study visits.
‡Two participants with birth data completed assessments at the 6-month visit.
Study pediatricians performed comprehensive physical and neurologic examinations following structured formats, and trained study personnel obtained anthropometric measures and instrument-based vision screening following American Academy of Pediatrics-recommended procedures.27 Repeat automated auditory brainstem response testing was performes in 27 children during the first year of life. Analysis of birth head circumference (HC), weight, and length by gestational age was based on INTERGROWTH-21st standards for term infants’ and pre-term infants’ postnatal growth until 42 weeks.28 After birth, growth analysis was based on World Health Organization standards for children, correcting for <40 weeks of gestational age, until 24 months.29 Microcephaly at birth was defined as an HC z-score ≤ −2.00 for sex and gestational age based on INTERGROWTH-21st standards. Zika virus-associated birth defects and neurodevelopmental abnormalities were based on CDC surveillance classification for children (birth to 2 years) born to mothers with any evidence of infection.7
Experienced, licensed clinical psychologists administered the Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III)30 in Spanish, the primary language of the families, to assess children’s neurodevelopment at 24 months and 36 months. Scores <85 (at least 1 SD below the average) on cognitive, language, or motor composite scores indicate neurodevelopmental delay.
Parents completed the Ages and Stages Questionnaire, Third Edition (ASQ-3)31 to screen for developmental delays in communication, gross motor skills, fine motor skill, problem solving, and personal-social skills at each study visit from age 6 months. The ASQ-3 provides valuable parental perspectives to help identify developmental risks conducive to early intervention. Scores at or below the age-based cutoff score suggest that a child is at risk for developmental delay. Scores in the monitoring zone suggest that the child can benefit from follow-up developmental assessment.
Statistical Analysis
Descriptive statistics were calculated for sociodemographic and clinical characteristics of the study population. Mean (SD) and percentage were calculated for continuous and categorical variables, respectively. The prevalence of developmental risk or delay as assessed by the BSID-III and ASQ-3, respectively, was calculated at each assessment age. The chi-square test was used to assess whether the prevalence of developmental delay differed significantly within a domain. Summary variables indicating the presence of developmental problems at any time between 6 and 36 months were derived for each domain. Poisson regression with robust error variance was used to test the adjusted associations between maternal age, education, and child sex with developmental problems. Maternal age was dichotomized at the mean, and education was dichotomized as high school or less vs more than high school as the highest level of education attained. Consistency between the parent-reported ASQ-3 and directly assessed BSID-III measures were examined via Pearson correlations. A P-value < .05 denoted statistical significance. Analyses were performed with SAS version 9.4 (SAS Institute). Sample sizes for descriptive analyses reflect the number of people who completed each assessment, ranging from 48 to 114 (Figure 1). Correlations and regression analyses were separate complete case analyses according to age group and developmental domain examined.
Results
Mother and child clinical and sociodemographic characteristics are presented in Table 1. The cohort comprised 114 children born of 112 mothers with a mean age of 26 years (Figure 1). Forty-two pregnant women 42 (37.5%) had a laboratory-confirmed infection by PCR and 70 (62.5%) had probable infection with Zika virus IgM-positive results; 21 (18.8%) were positive for both. Fifty-two mothers (46.4%) had symptoms, including 28 infected during the first trimester. Most mothers had public health insurance (n = 90, 80.4%) and an annual household income <$15,000 (n = 80, 71.4%). Forty-eight mothers (42.9%) had an educational level of high school or less. Children were born at a mean gestational age of 38.1 weeks (range, 31–40 weeks); 13 (11.4%) were premature births (31-36 weeks of gestational age). Sixty children (52.6%) were female. Neonatal assessment revealed 3 of 114 children (2.6%) with microcephaly at birth (HC z-score ≤ −2), 3 of 107 (2.8%) with abnormal hearing screening, 19 of 35 (54.3%) with retinal imaging abnormalities, 11 of 109 (10.1%) with nonspecific brain ultrasound findings, and 7 of 114 (6.1%) with neuromotor (movement or tone) abnormalities. Three children with microcephaly had normal brain ultrasound images at birth, 1 child with congenital Zika syndrome was assessed at 2 years by magnetic resonance imaging, which showed a brachycephalic skull configuration with a hypoplastic inferior cerebellar vermix, enlarged ventricular occipital horns, and abnormal (diffusely heterogeneous) white matter. At 30 months, this child had severe microcephaly (HC z-score, −4.57) and serious neurodevelopmental, sensory, and musculoskeletal impairments. A second child had normal HC and development at 24 months, and the third child was lost to follow-up soon after enrollment. Among those without microcephaly who had retinal imaging and brain ultrasound, 34 (97.1%) had no abnormality and thus were considered asymptomatic.
Table 1.
Sociodemographic and clinical characteristics at birth of study population, Pediatric Outcomes of Prenatal Zika Exposure study, Puerto Rico, 2017–2020.
| Characteristics | Values |
|---|---|
|
| |
| Maternal | |
| Age, y, mean (SD) | 26.11 (5.39) |
| Age 13–25 y, n/N (%) | 58/112 (51.79) |
| Age 26–40 y, n/N (%) | 54/112 (48.21) |
| Married/living with partner, n/N (%) | 92/112 (82.14) |
| Health insurance, public, n/N (%) | 90/112 (80.36) |
| Educational attainment, n/N (%) | |
| LTHS or HS | 48/112 (42.86) |
| More than HS | 64/112 (57.14) |
| Unemployed, n/N (%) | 69/112 (61.61) |
| Household income < $15,000, n/N (%) | 80/112 (71.43) |
| Prenatal diagnosis, n/N (%) | |
| PCR positive | 42/112 (37.50) |
| IgM positive | 70/112 (62.50) |
| Both | 21/112 (18.75) |
| Symptomatic infection, n/N (%) | 52/112 (46.43) |
| Trimester of symptomatic infection, n/N (%)* | |
| First | 28/52 (53.85) |
| Second | 15/52 (28.85) |
| Third | 8/52 (15.38) |
| Not identified | 1/52 (1.92) |
| Prenatal drug/alcohol/tobacco use, n/N (%) | 1/112 (0.89) |
| Infant | |
| Female sex, n/N (%) | 60/114 (52.63) |
| Gestational age, wk, mean (SD) | 38.06 (1.58) |
| Premature birth (<37 wk), n/N (%) | 13/114 (11.40) |
| Birth weight, kg, mean (SD) | 3.09 (0.51) |
| Birth length, cm, mean (SD) | 49.13 (3.20) |
| Weight for gestational age classification, n/N (%) | |
| PreTSGA | 2/114 (1.75) |
| TSGA | 9/114 (7.89) |
| HC, cm, mean (SD) | 33.50 (1.56) |
| HC z-score −1 to −2, n/N (%) | 12/114 (10.53) |
| HC z-score −2 to −3, n/N (%) | 3/114 (2.63) |
| ABR, n/N (%) | 3/107 (2.80) |
| Ophthalmologic abnormalities (RetCam)† images | 19/35 (54.29) |
| Nonspecific brain ultrasound findings‡ | 11/109 (10.09) |
| Germinolytic or subependymal cyst | 7/109 (6.42) |
| Choroid plexus cyst | 4/109 (3.67) |
| Lenticulostriate vasculopathy | 1/109 (0.92) |
| Abnormal neuromotor§ | 7/114 (6.14) |
| Asymptomatic¶ | 34/35 (97.1) |
ABR, abnormal; HS, high school; LTHS, less than high school; PreTSGA, preterm small for gestational age; TSGA, term small for gestational age.
One case missing trimester of symptoms.
Optic nerve dysplasia, optic nerve pallor, optic disc hypoplasia, optic disc cupping, and macular pigment mottling.
Associated to developmental risk.
Tremors, hypotonia, and hypertonia.
HC z-score > −2 and no abnormalities in retinal image and/or no brain ultrasound findings among those with complete testing (n = 35).
Of the 97 children with follow-up after birth, 3 (3.1%) developed a seizure disorder, and 23 (23.7%) had neuromotor (movement or tone) abnormalities that persisted in 1 or more follow-up visits (Table 2). Seventeen of 97 children (17.5%) who underwent clinical evaluation or instrument-based vision screening had possible visual impairment, including 14 with abnormal retinal images at birth. Twelve of these children were referred for subspecialty confirmation, and 4 were evaluated. All 4 had a confirmatory diagnosis, including hyperopia in 3 and astigmatism in 1. Over time, only 2 of 97 (2.1%) had failure to thrive at any visit. At the end of the study period, 47 participants were considered lost to follow-up (ie, absent on 2 consecutive visits and/or unable to be contacted). Twelve (25.5%) were lost to emigration after the Hurricane María island-wide natural disaster of September 2017 or the 6.4 magnitude earthquake of January 2020 in southern Puerto Rico. Inactive participants were more likely to have private health insurance and mothers with probable (ie, Zika virus IgM) infection (Table 3).
Table 2.
Structural and functional abnormalities at any time after birth, Pediatric Outcomes of Prenatal Zika Exposure study, Puerto Rico, 2017–2020.
| Abnormalities | n/N (%) |
|---|---|
|
| |
| Microcephaly | 1/97 (1.03) |
| Auditory brainstem response | 0/31 (0) |
| Failure to thrive* | 2/97 (2.06) |
| Possible visual impairment | 17/97 (17.53) |
| Pediatric assessment | 3/97 (3.09) |
| Instrument-based screening | 14/69 (20.29) |
| Contractures | 1/97 (1.03) |
| Seizures | 3/97 (3.09) |
| Motor tone & function | |
| Hypotonia | 32/97 (32.99) |
| Hypertonia | 8/97 (8.25) |
| Both hypotonia/hypertonia | 2/97 (2.06) |
| Abnormal movements† | 7/97 (7.22) |
| Tremors† | 14/97 (14.43) |
| Abnormal posturing† | 7/97 (7.22) |
| Swallowing difficulties† | 8/97 (8.25) |
| Developmental risk or delay | |
| Parental report (ASQ-3), any domain in the first year | 16/74 (21.62) |
| Parental report (ASQ-3), any domain in the second year | 29/60 (48.33) |
| Parental report (ASQ-3), any domain in the third year | 34/61 (55.74) |
| Professional assessment (BSID-III), any domain in the second year | 33/53 (62.26) |
| Professional assessment (BSID-III), any domain in the third year | 17/47 (36.17) |
Height z-scores < −2 and weight or weight/height z-scores < −2.
Parental report.
Table 3.
Sociodemographic characteristics of active and inactive study participants by 2020, Pediatric Outcomes of Prenatal Zika Exposure study, Puerto Rico, 2017–2020
| Active (n = 65) | Inactive (n = 47) | |||||
|---|---|---|---|---|---|---|
| Characteristics | N | n | % | n | % | P value* |
|
| ||||||
| Maternal characteristics | ||||||
| Zika virus diagnosis | ||||||
| Immunoglobulin M positive | 70 | 35 | 53.8 | 35 | 74.5 | .026 |
| PCR pos | 42 | 30 | 46.2 | 12 | 25.5 | |
| Municipality | ||||||
| Ponce | 51 | 33 | 50.8 | 18 | 38.3 | .191 |
| Other | 61 | 32 | 49.2 | 29 | 61.7 | |
| Area of residence | ||||||
| Rural | 47 | 29 | 44.6 | 18 | 38.3 | .504 |
| Urban | 65 | 36 | 55.4 | 29 | 61.7 | |
| Educational attainment | ||||||
| LTHS or HS | 48 | 26 | 40.0 | 22 | 46.8 | .472 |
| More than HS | 64 | 39 | 60.0 | 25 | 53.2 | |
| Household income | ||||||
| < $15,000 | 83 | 49 | 75.8 | 34 | 72.3 | .717 |
| ≥$15,000 | 29 | 16 | 24.6 | 13 | 27.7 | |
| Health insurance | ||||||
| Private | 22 | 7 | 10.8 | 15 | 31.9 | .005 |
| Public | 90 | 58 | 89.2 | 32 | 68.1 | |
| Child characteristics | ||||||
| Sex | ||||||
| Female | 60 | 39 | 58.2 | 21 | 44.7 | .154 |
| Male | 54 | 28 | 41.8 | 26 | 55.3 | |
Significant P values are in bold type.
Pearson’s chi-square test
Neurodevelopmental Outcomes at 24 and 36 Months
Children’s performance on the BSID-III at 24 months reflected average mean composite scores on the motor (93.4; SD, 13.7) and cognitive (90.6; SD, 12.7) domains, and below average score on language domain (83.3; SD, 15.0). Mean scores at 36 months were average on all domains, with a slight increase in the language domain scores (86.7; SD, 19.5), although on the lower borderline of the average range (Table 4). Parental reports on the ASQ-3 (n=53) revealed mean scores at 24 months in the cautionary “monitoring” zone for communication, gross motor, fine motor, and personal-social domains (Table 4), indicating that the child likely could benefit from follow up actions. Similarly, mean scores at 36 months (n=47) in problem solving and personal-social domains were in the “monitoring” zone.
Table 4.
Child neurodevelopmental assessment scores according to BSID-III and ASQ-3 domains, Pediatric Outcomes of Prenatal Zika Exposure study, Puerto Rico, 2017–2020
| Tests by age-specific visit | Domains, mean (SD) | ||||
|
| |||||
| BSID-III* | Language | Motor | Cognitive | ||
| 24 mo (n = 53) | 83.28 (15.02)† | 93.40 (13.69) | 90.58 (12.67) | ||
| 36 mo (n = 47) | 86.70 (19.46) | 90.26 (13.20) | 93.19 (8.49) | ||
| ASQ-3 | Communication | Gross Motor | Fine Motor | Problem Solving | Personal-Social |
| 6 mo (n = 61) | 54.10 (6.86) | 46.64 (10.48) | 52.62 (7.89) | 52.87 (9.38) | 53.93 (6.96) |
| 12 mo (n = 54) | 49.54 (11.34) | 46.20 (18.50) | 48.15 (10.92) | 45.46 (13.88) | 41.11 (13.86) |
| 18 mo (n = 51) | 31.57 (15.95) | 50.10 (14.23) | 42.75 (12.66)‡ | 49.22 (11.85) | 47.65 (11.15) |
| 24 mo (n = 53) | 34.34 (20.69)‡ | 44.15 (14.96)‡ | 43.87 (14.30)‡ | 44.19 (15.38) | 40.94 (12.60)‡ |
| 30 mo (n = 57) | 45.79 (19.15) | 50.79 (11.98) | 49.47 (13.68) | 35.44 (11.74)‡ | 42.28 (15.50) |
| 36 mo (n = 47) | 45.00 (18.83) | 56.30 (6.87) | 40.00 (18.77) | 36.63 (15.39)‡ | 43.70 (15.07)‡ |
Composite scores.
Scores in the developmental delay range (−1SD).
Scores within the monitoring zone.
Prevalence of Neurodevelopmental Problems
Figure 2 displays the prevalence of developmental delay and risk according to BSID-III and ASQ-3 domains, respectively, at age 6-36 months. The BSID-III assessment showed high levels of developmental delay, with some change over time. For example, cognitive delay was present in 24.5% at 24 months and in 10.6% at 36 months (P < .001); language delay was present in 50.9% at 24 months and in 31.9% at 36 months (P < .001); and motor delay was present in 21.2% at 24 months and in 27.7% at 36 months (P < .001). Among the domains, a moderate-severe (−2 SD to −3 SD) delay in language at 24 months (17.0%) and 36 months (14.9%) was more prevalent (Figure 3). A finding of interest is developmental delay in at least one domain identified by the BSID-III at 24 or 36 months in 10 of 19 children (52.6%) with abnormal retinal images and in 5 of 11 (45.5%) with nonspecific brain ultrasound findings at birth (data not shown). For the ASQ-3, the prevalence of developmental risk was low in infancy and increased with age, peaking at around 24 or 36 months, with some domains showing lower prevalence of risk at older ages. Specifically, the prevalence of communication delay risk was low at 6 months (1.6%) and increased to 45.3% by 24 months (P = .21); by 36 months, the prevalence was 36.9% (P < .001). Similarly, the prevalence of gross motor delay risk increased from 6.6% at 6 months to 43.4% at 24 months (P = .08) and was greatly reduced by 36 months (8.7%; P = .85). A similar pattern was evident for fine motor delay risk: 3.3% at 6 months, 50.9% at 24 months (P = .36), and 29.8% at 36 months (P = .01). For the problem solving and personal-social domains, developmental risk generally increased with age. For problem solving, 67.4% exhibited developmental risk at 36 months. For personal-social, 56.6% were at risk at 24 months, and 45.7% were at risk by 36 months (P = .59).
Figure 2.
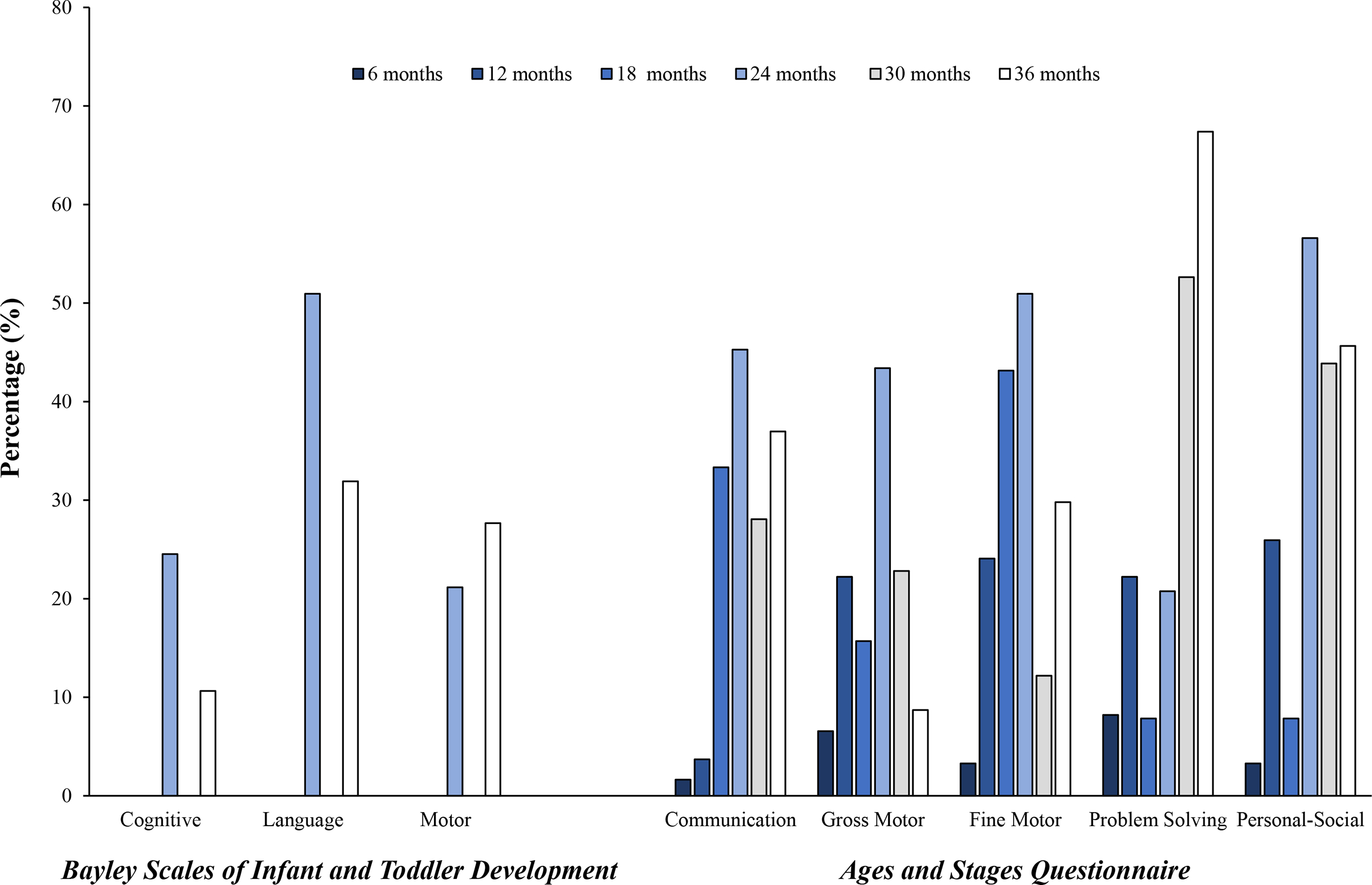
Prevalence of developmental delay or risk by BSID-III and ASQ-3 domains, Pediatric Outcomes of Prenatal Zika Exposure study, Puerto Rico, 2017–2020.
Figure 3.

Prevalence of severity of developmental delay on BSID-III domains, Pediatric Outcomes of Prenatal Zika Exposure study, Puerto Rico, 2017–2020.
Overall, BSID-III assessments identified developmental delays in any domain in 33 of 53 children (62.3%) during the second year and 17 of 47 (36.2%) during the third year (Table 2). Results from ASQ-3 identified developmental risk in any domain in 16 of 74 children (21.6%) in the first year, in 29 of 60 (48.3%) in the second year, and in 34 of 61 (55.7%) during the third year.
Neurodevelopmental Problems According to Sociodemographic Characteristics
Some patterns in developmental delay were noted according to maternal age, education, and child sex as assessed in multivariate Poisson models (Table 5). Children of mothers aged 13–25 years had a significantly lower risk of developmental delay in ASQ-3 communication (relative risk [RR], 0.56; 95% CI, 0.3–0.96) and problem solving (RR, 0.65; 95% CI, 0.46–0.92) compared to children of mothers aged >25 years. Children of mothers with a high-school education or less tended to have a greater risk of BSID-III assessed gross motor delay compared with children of mothers with more education (RR, 2.35; 95% CI, 0.96–5.73), although the difference was not statistically significant. Compared with females, males had significantly higher BSID-III language delay (RR, 2.00; 95% CI, 1.18–3.40) and risk of ASQ-3 fine motor delay (RR, 1.63; 95% CI, 1.11–2.37). Nonsignificant associations also suggested that males may have had higher BSID-III cognitive delay (RR, 2.30; 95% CI, 0.87–6.10), and risk of ASQ-3 communication delay (RR, 1.56; 95% CI, 0.95–2.55).
Table 5.
Prevalence of any developmental risk or delay from birth to 36 months according to ASQ-3 and BSID-III assessments and multivariate associations with maternal age, education, and child sex, Pediatric Outcomes of Prenatal Zika Exposure study, Puerto Rico, 2017–2020
| Domains | Delay | No delay | Risk ratio (95% CI) |
|---|---|---|---|
|
| |||
| ASQ domains* | |||
| Communication | 40.66 (37) | 59.34 (54) | |
| Maternal age, 13–25 y | 35.14 (13) | 57.41 (31) | 0.56 (0.33 to 0.96) |
| Maternal age, 26–40 y | 64.86 (24) | 42.59 (23) | Reference |
| Education, high school or less | 43.24 (16) | 44.44 (24) | 1.09 (0.67 to 1.79) |
| Education, more than high school | 56.76 (21) | 55.56 (30) | Reference |
| Child sex, male | 54.05 (20) | 35.19 (19) | 1.56 (0.95 to 2.55) |
| Child sex, female | 45.95 (17) | 64.81 (35) | Reference |
| Gross Motor | 40.66 (37) | 59.34 (54) | |
| Maternal age, 13–25 y | 43.24 (16) | 51.85 (28) | 0.77 (0.46 to 1.30) |
| Maternal age, 26–40 y | 56.76 (21) | 48.15 (26) | Reference |
| Education, high school or less | 45.95 (17) | 42.59 (23) | 1.20 (0.71 to 2.00) |
| Education, more than high school | 54.05 (20) | 57.41 (31) | Reference |
| Child sex, male | 37.84 (14) | 46.30 (25) | 0.80 (0.48 to 1.33) |
| Child sex, female | 62.16 (23) | 53.70 (29) | Reference |
| Fine Motor | 53.85 (49) | 46.15 (42) | |
| Maternal age, 13–25 y | 40.82 (20) | 57.14 (24) | 0.71 (0.49 to 1.06) |
| Maternal age, 26–40 y | 59.18 (29) | 42.86 (18) | Reference |
| Education, high school or less | 44.90 (22) | 42.86 (18) | 1.09 (0.75 to 1.59) |
| Education, more than high school | 55.10 (27) | 57.14 (24) | Reference |
| Child sex, male | 55.10 (27) | 28.57 (12) | 1.63 (1.11 to 2.37) |
| Child sex, female | 44.90 (22) | 71.43 (30) | Reference |
| Problem Solving | 61.54 (56) | 38.46 (35) | |
| Maternal age, 13–25 y | 39.29 (22) | 62.86 (22) | 0.65 (0.46 to 0.92) |
| Maternal age, 26–40 y | 62.86 (34) | 37.14 (13) | Reference |
| Education, high school or less | 46.43 (26) | 40.00 (14) | 1.24 (0.90 to 1.70) |
| Education, more than high school | 53.57 (30) | 60.00 (21) | Reference |
| Child sex, male | 46.43 (26) | 37.14 (13) | 1.13 (0.83 to 1.55) |
| Child sex, female | 53.57 (30) | 62.86 (22) | Reference |
| Personal Social | 59.34 (54) | 40.66 (37) | |
| Maternal age, 13–25 y | 40.74 (22) | 59.46 (22) | 1.25 (0.87 to 1.78) |
| Maternal age, 26–40 y | 59.26 (32) | 40.54 (15) | Reference |
| Education, high school or less | 35.19 (19) | 56.76 (21) | 1.38 (0.94 to 2.02) |
| Education, more than high school | 64.81 (35) | 43.24 (16) | Reference |
| Child sex, male | 46.30 (25) | 37.84 (14) | 0.84 (0.61 to 1.17) |
| Child sex, female | 53.70 (29) | 62.16 (23) | Reference |
| BSID-III domains† | |||
| Cognitive | 21.88 (14) | 78.13 (50) | |
| Maternal age, 13–25 y | 28.57 (4) | 46.00 (23) | 0.54 (0.19 to 1.52) |
| Maternal age, 26–40 y | 71.43 (10) | 54.00 (27) | Reference |
| Education, high school or less | 42.86 (6) | 40.00 (20) | 1.09 (0.43 to 2.80) |
| Education, more than high school | 57.14 (8) | 60.00 (30) | Reference |
| Child sex, male | 64.29 (9) | 38.00 (19) | 2.30 (0.87 to 6.10) |
| Child sex, female | 35.71 (5) | 62.00 (31) | Reference |
| Language | 48.44 (31) | 51.56 (33) | |
| Maternal age, 13–25 y | 35.48 (11) | 48.48 (16) | 0.73 (0.44 to 1.21) |
| Maternal age, 26–40 y | 64.52 (20) | 51.52 (17) | Reference |
| Education, high school or less | 45.16 (14) | 36.36 (12) | 1.17 (0.73 to 1.89) |
| Education, more than high school | 54.84 (17) | 63.64 (21) | Reference |
| Child sex, male | 61.29 (19) | 27.27 (9) | 2.00 (1.18 to 3.40) |
| Child sex, female | 38.71 (12) | 72.73 (24) | Reference |
| Motor | 25.40 (16) | 74.60 (47) | |
| Maternal age, 13–25 y | 37.50 (6) | 44.68 (21) | 0.70 (0.31 to 1.62) |
| Maternal age, 26–40 y | 62.50 (10) | 55.32 (26) | Reference |
| Education, high school or less | 62.50 (10) | 34.04 (16) | 2.35 (0.96 to 5.73) |
| Education, more than high school | 37.50 (6) | 65.96 (31) | Reference |
| Child sex, male | 62.50 (10) | 38.30 (18) | 1.89 (0.78 to 4.59) |
| Child sex, female | 37.50 (6) | 61.70 (29) | Reference |
ASQ scores at or above age-based cutoff scores indicate possible developmental delay.
BSID-III scores meeting thresholds for mild, moderate, or severe delay.
Correlations Between ASQ-3 and BSID-III Domains
There was significant agreement between clinically assessed BSID-III and parent-reported ASQ-3 development scores (Table 6). For example, strong and highly significant correlations were observed among BSID-III and ASQ-3 scores when both were assessed at 24 and 36 months (r = 0.32-0.78 P< .05).
Table 6.
Correlations among the ASQ-3 and BSID-III in cognitive, language, and motor domains, Pediatric Outcomes of Prenatal Zika Exposure study, Puerto Rico, 2017–2020
| BSID-III domains, 24 months | BSID-III domains, 36 months | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| ASQ-3 domains | Cognitive | Language | Motor | Cognitive | Language | Motor |
|
| ||||||
| Communication | ||||||
| 6 mo, r | 0.19 | 0.15 | 0.25 | 0.39 | 0.23 | 0.27 |
| P value | .25 | .35 | .13 | .03 | .21 | .14 |
| 12 mo, r | 0.15 | 0.13 | 0.09 | 0.19 | 0.10 | 0.17 |
| P value | .39 | .46 | .59 | .33 | .61 | .41 |
| 18 mo, r | 0.53 | 0.69 | 0.49 | 0.64 | 0.71 | 0.67 |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| 24 mo, r | 0.47 | 0.71 | 0.48 | 0.68 | 0.75 | 0.73 |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| 30 mo, r | 0.60 | 0.57 | 0.56 | 0.61 | 0.64 | 0.68 |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| 36 mo, r | 0.59 | 0.70 | 0.65 | 0.64 | 0.76 | 0.74 |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| Gross Motor | ||||||
| 6 mo, r | 0.23 | 0.17 | 0.17 | 0.32 | 0.34 | 0.28 |
| P value | .15 | .28 | .30 | .08 | .07 | .14 |
| 12 mo, r | 0.43 | 0.37 | 0.48 | 0.44 | 0.35 | 0.41 |
| P value | .01 | .03 | .004 | .02 | .07 | .03 |
| 18 mo, r | 0.22 | 0.08 | 0.41 | 0.40 | 0.48 | 0.50 |
| P value | .15 | .60 | .006 | .03 | .006 | .004 |
| 24 mo, r | 0.34 | 0.37 | 0.52 | 0.46 | 0.40 | 0.54 |
| P value | .01 | .008 | <.001 | .005 | .01 | <.001 |
| 30 mo, r | 0.44 | 0.50 | 0.63 | 0.55 | 0.55 | 0.60 |
| P value | .003 | .0005 | <.001 | <.001 | <.001 | <.001 |
| 36 mo, r | 0.35 | 0.36 | 0.48 | 0.32 | 0.35 | 0.51 |
| P value | .04 | .03 | .004 | .03 | .02 | <.001 |
| Fine Motor | ||||||
| 6 mo, r | 0.10 | 0.15 | 0.12 | 0.20 | −0.03 | 0.03 |
| P value | .53 | .36 | .45 | .29 | .86 | .86 |
| 12 mo, r | −0.05 | 0.15 | 0.19 | −0.18 | −0.12 | 0.01 |
| P value | .78 | .40 | .27 | .37 | .55 | .94 |
| 18 mo, r | 0.29 | 0.42 | 0.33 | 0.45 | 0.39 | 0.50 |
| P value | .05 | .004 | .03 | .01 | .03 | .004 |
| 24 mo, r | 0.42 | 0.36 | 0.63 | 0.44 | 0.55 | 0.62 |
| P value | .002 | .008 | <.001 | .008 | <.001 | <.001 |
| 30 mo, r | 0.48 | 0.42 | 0.57 | 0.59 | 0.57 | 0.71 |
| P value | <.001 | .004 | <.001 | <.001 | <.001 | <.001 |
| 36 mo, r | 0.54 | 0.56 | 0.66 | 0.56 | 0.65 | 0.75 |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| Problem Solving | ||||||
| 6 mo, r | 0.20 | 0.10 | 0.10 | 0.44 | 0.26 | 0.20 |
| P value | .23 | .53 | .54 | .01 | .17 | .28 |
| 12 mo, r | 0.32 | 0.32 | 0.29 | 0.01 | 0.27 | 0.29 |
| P value | .07 | .06 | .10 | .96 | .17 | .14 |
| 18 mo, r | 0.40 | 0.24 | 0.50 | 0.44 | 0.46 | 0.50 |
| P value | .007 | .12 | <.001 | .01 | .008 | .004 |
| 24 mo, r | 0.56 | 0.50 | 0.78 | 0.70 | 0.70 | 0.75 |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| 30 mo, r | 0.60 | 0.65 | 0.53 | 0.49 | 0.63 | 0.55 |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| 36 mo, r | 0.66 | 0.64 | 0.70 | 0.64 | 0.73 | 0.70 |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
Sample size for correlations between assessments range from n = 27 to n = 52. Only P values < .05 are presented.
Discussion
The study describes pediatric outcomes of prenatal Zika virus exposure in a cohort of 114 Hispanic children born in Ponce, Puerto Rico during and after the Zika epidemic of 2016–2017. Understanding of the neurodevelopmental outcomes associated with prenatal Zika virus exposure is facilitated by a well-characterized study sample and the implementation of specialized longitudinal assessments identifying long-term age-specific impairment, an enhancement to cross-sectional approaches, or when assessments were implemented within a wide age range.8, 11–15, 32 The high consistency between the 2 assessment tools supports the validity of neurodevelopmental problems in 1 or more domains in at least one-third of children at 36 months (36.2% in the BSID-III, 55.7% in the ASQ-3), compared with a US prevalence for emotional, developmental, and behavioral problems of 10.8% among children aged 0–5 years.33 In our study the largest proportion of exposed children (97.1%) did not have clinically evident birth defects, that is, no microcephaly or abnormalities in retinal images or brain ultrasound. This finding was similar to the experience in surveillance and prospective cohort studies that enrolled mothers with confirmed or probable Zika virus infection.7, 8 Consistent with our findings, most studies with follow-up in children without microcephaly after 12 months have reported physical, sensory, or neurodevelopmental abnormalities that become clinically evident as the children grow, leading to recommendations for long-term detailed follow-up.8, 11, 13–15 Some studies of children without microcephaly at birth failed to show developmental defects on follow-up; nevertheless, the authors suggested the possible need for larger and longer-term studies.34, 35
Birth assessments identified 3 children (2.6%) with microcephaly, and among those tested, 19 (54%) had Zika-associated abnormalities in retinal image and 11 (10%) had non-specific brain ultrasound findings that were associated with developmental risk in a study by Mulkey et al.36 The proportion of children born prematurely and with low birth weight is consistent with the 2016 Puerto Rico rates of 11.4% and 9.7%, respectively.37 In mothers with symptomatic Zika, one-half of the infections occurred during the first trimester, when the risk for microcephaly is greatest; nevertheless, the rate of microcephaly in our cohort was lower than the 6%–11% reported in the US Zika Pregnancy Register landmark study of birth defects.38 Different rates of microcephaly have been reported in surveillance and observational studies from different countries and sites, suggesting that multifactorial etiologies could be involved, including genetic influences and environmental factors not fully defined.39 Nevertheless, retinal and optic nerve abnormalities were identified in approximately one-half of those tested, and eye abnormalities in the absence of microcephaly and other brain abnormalities have been described as Zika-associated birth defects.40,7
Two studies with laboratory-confirmed infection in mothers8 and neonates13 that assessed children without microcephaly between 7 and 32 months and between 14.4 and 22 months reported a BSID-III prevalence of developmental delay in 1 or more domains similar to our study, at 34.0% and 38.4%, respectively.8,13 These results represent children with mild delay within an age range, limiting age-specific comparisons. Hearing screening identified abnormalities in only 2.8% of our tested newborns, a lower rate than reported in other cohorts of Zika-exposed children (4.6%–12%),10,15,11,8 except for 1 (0%),13 likely reflecting differences in testing and study populations. Vision screening identified 17.5% of children with possible vision impairment. Vision abnormalities that were identified on follow-up also have been documented in the medical literature.16,17 Postnatal neuromotor abnormalities were rare, and their significance requires the consideration of other neurologic examination findings.
ASQ-3 results present a tendency of increased prevalence of developmental risk by age, consistent with the paradigm in which the full extent of consequences of early brain insult might not be evident until years have passed and in response to increasing developmental demands,41 underscoring the effectiveness of developmental screening using appropriate instruments.42 Of interest are the BSID-III adverse findings by domain and age that peaked at 24 months with some improvement at 36 months, most notably in the communication domain. Our study and others8, 13 have identified language and communication problems as a common sequela of intrauterine Zika virus exposure, a finding that merits in-depth research to elucidate underlying pathophysiologic mechanisms and their clinical correlates. Language delay can improve with early intervention, home literacy, and a nurturing environment with long-lasting effects on academic, social, and emotional development and well-being. Early intervention (mandated for all exposed children in Puerto Rico) and family responses to study findings during a sensitive period of child development could explain the improvement noted in the older age group, a reassuring prospect that depends on the timeliness and effectiveness of the interventions.
The prevalence of developmental delay in any domain decreased from approximately two-thirds at 24 months to approximately one-third at 36 months. Interpretation of these findings is compounded by several factors that influence outcomes of early brain insult, including brain plasticity compensatory mechanisms, presence or absence of health, good nutrition, security and safety, a nurturing home environment, and timely implementation or not of early education, therapeutic, and rehabilitative interventions.43,44,45 Nurturing care in particular has been found to influence child development and could attenuate the effects of adversity.44
Strengths of this study include standardized assessments on a reasonably sized population resulting in developmental outcomes at 2 time points for the BSID-III and 6 time points for the ASQ-3 that represent longitudinal and systematic follow-up of complex developmental processes. The validity and comparability of developmental tools representing expert professional and parental perspectives add value to these findings and highlight the importance of parents’ observations. In addition, the study design allowed for identification of patterns in developmental delay according to maternal age and education and child sex in the multivariate model that contribute to the published evidence.11–15 These patterns warrant further exploration and the inclusion of a comparison group to better understand the risk factors involved.
Our study has limitations in maternal diagnosis not confirmed by virus detection but rather by IgM serology in the majority of study participants, and one-half of the mothers had a symptomatic infection. The absence of a control group limited the assessment of developmental risks and precluded measuring the influence of other health and environmental mediators of child development. Although experienced clinical professionals implemented structured assessments, they could not be blinded to the exposure and information bias was possible. Another limitation is that the BSID-III and ASQ-3 testing lack normative data for Puerto Rican children. In addition, some families of children with abnormal retinal images and/or vision screening could not access the subspecialist to confirm the diagnosis for the purposes of this study. The high attrition rate was caused in part by 2 major disasters that forced family mobility and emigration. Among the reasons for emigrating, families reported economic distress and the need for specialized pediatric services. These factors could have affected the ability to enroll and retain children with more severe outcomes.
The recognition of language and communication developmental risks among children without microcephaly alerts pediatric healthcare professionals and families and can contribute to early identification and timely intervention. Furthermore, our study and others suggest that identifying long-term impairment related to early brain injury from Zika virus remains critical and will require follow-up to inform support needs and explore the long-term consequences of prenatal exposure. Future research must elucidate the risks to school readiness and identify effective preventive measures that can improve the health and well-being of children and families.
Funding/support:
Funding for this study was provided by the National Institutes of Health (NIH), National Institute of Minority Health and Health Disparities (NIMHD) (Grant U54MD007579) and CDC (Grant 3U01CK000437-02S1). The funding from the NIH was built on the foundation of a CDC cooperative agreement supplement. The NIH-NIMHD had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the CDC.
Abbreviations:
- ASQ-3
Ages and Stages Questionnaire, Third Edition
- CDC
Centers for Disease Control and Prevention
- BSID-III
Bayley Scales of Infant and Toddler Development, Third Edition
- ELISA
Enzyme-linked immunosorbent assay
- HC
Head circumference
- IgM
Immunoglobulin M
- PCR
Polymerase chain reaction
- RR
Relative risk
Footnotes
Conflicts of interest disclosure: The authors have no conflicts of interest relevant to this article to disclose.
Data sharing statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- [1].Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, et al. Possible association between Zika virus infection and microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016;65:59–62. [DOI] [PubMed] [Google Scholar]
- [2].Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT, do Carmo GM, Henriques CM, Coelho GE, et al. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy - Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016;65:242–7. [DOI] [PubMed] [Google Scholar]
- [3].de Araujo TVB, Rodrigues LC, de Alencar Ximenes RA, de Barros Miranda-Filho D, Montarroyos UR, de Melo APL, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis 2016;16:1356–63. [DOI] [PubMed] [Google Scholar]
- [4].Brasil P, Pereira JP Jr., Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 2016;375:2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, Fonseca EB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr 2017;171:288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Musso D, Ko AI, Baud D. Zika virus infection - after the pandemic. N Engl J Med 2019;381:1444–57. [DOI] [PubMed] [Google Scholar]
- [7].Rice ME, Galang RR, Roth NM, Ellington SR, Moore CA, Valencia-Prado M, et al. Vital signs: Zika-associated birth defects and neurodevelopmental abnormalities possibly associated with congenital Zika virus infection - US territories and freely associated states, 2018. MMWR Morb Mortal Wkly Rep 2018;67:858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nielsen-Saines K, Brasil P, Kerin T, Vasconcelos Z, Gabaglia CR, Damasceno L, et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat Med 2019;25:1213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aragao M, Holanda AC, Brainer-Lima AM, Petribu NCL, Castillo M, van der Linden V, et al. Nonmicrocephalic infants with congenital Zika syndrome suspected only after neuroimaging evaluation compared with those with microcephaly at birth and postnatally: how large is the Zika virus “iceberg”? AJNR Am J Neuroradiol 2017;38:1427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vianna RAO, Lovero KL, Oliveira SA, Fernandes AR, Santos T, Lima L, et al. Children born to mothers with rash during Zika virus epidemic in Brazil: first 18 months of life. J Trop Pediatr 2019;65:592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lopes Moreira ME, Nielsen-Saines K, Brasil P, Kerin T, Damasceno L, Pone M, et al. Neurodevelopment in infants exposed to Zika virus in utero. N Engl J Med 2018;379:2377–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cardoso TF Jr., Dos Santos RS, Correa RM, Campos JV, Silva RB, Tobias CC, et al. Congenital Zika infection: neurology can occur without microcephaly. Arch Dis Child 2019;104:199–200. [DOI] [PubMed] [Google Scholar]
- [13].Faical AV, de Oliveira JC, Oliveira JVV, de Almeida BL, Agra IA, Alcantara LCJ, et al. Neurodevelopmental delay in normocephalic children with in utero exposure to Zika virus. BMJ Paediatr Open 2019;3:e000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mulkey SB, Arroyave-Wessel M, Peyton C, Bulas DI, Fourzali Y, Jiang J, et al. Neurodevelopmental abnormalities in children with in utero Zika virus exposure without congenital Zika syndrome. JAMA Pediatr 2020;174:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cranston JS, Tiene SF, Nielsen-Saines K, Vasconcelos Z, Pone MV, Pone S, et al. Association between antenatal exposure to Zika virus and anatomical and neurodevelopmental abnormalities in children. JAMA Netw Open 2020;3:e209303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zin AA, Tsui I, Rossetto JD, Gaw SL, Neves LM, Zin OA, et al. Visual function in infants with antenatal Zika virus exposure. J AAPOS 2018;22:452–6 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Portnoi Baran LC, Fernades da Costa M, Summer Vidal K, Damico FM, Salgueiro Barboni MT, da Silva Lima D, et al. Alterations in visual acuity and visual development in infants 1–24 months old either exposed to or infected by Zika virus during gestation, with and without microcephaly. J AAPOS 2019;23:215 e1–e7. [DOI] [PubMed] [Google Scholar]
- [18].Staples JE, Dziuban EJ, Fischer M, Cragan JD, Rasmussen SA, Cannon MJ, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection - United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:63–7. [DOI] [PubMed] [Google Scholar]
- [19].Adebanjo T, Godfred-Cato S, Viens L, Fischer M, Staples JE, Kuhnert-Tallman W, et al. Update: Interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep 2017;66:1089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Honein MA, Jamieson DJ. Revealing the effects of Zika-detection of brain abnormalities and other disabilities associated with congenital infection. JAMA Pediatr 2019;173:16–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kapogiannis BG, Chakhtoura N, Hazra R, Spong CY. Bridging knowledge gaps to understand how Zika virus exposure and infection affect child development. JAMA Pediatr 2017;171:478–85. [DOI] [PubMed] [Google Scholar]
- [22].United States Census Bureau. Quick Facts: Puerto Rico 2019. Accessed March 1, 2021. https://www.census.gov/quickfacts/fact/table/PR
- [23].Puerto Rico Department of Health. Informe Semanal de Enfermedades Arbovirales (ArboV): Semana: 52 - 2017. Estadisticas, Registros y Publicaciones; 2018. Accessed February 16, 2021. http://www.salud.gov.pr/Estadisticas-Registros-y-Publicaciones/Informes%20Arbovirales/Reporte%20ArboV%20semana%2052-2017.pdf [Google Scholar]
- [24].United States Census Bureau. Puerto Rico municipios population totals: 2010–2019. Accessed January 20, 2021. https://www.census.gov/data/tables/time-series/demo/popest/2010s-total-puerto-rico.html
- [25].Sharp TM, Fischer M, Muñoz-Jordan JL, Paz-Bailey G, Staples JE, Gregory CJ, et al. Dengue and Zika virus diagnostic testing for patients with a clinically compatible illness and risk for infection with both viruses. MMWR Recomm Rep 2019;68:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].American College of Obstetricians and Gynecologists. Committee Opinion No. 700: methods for estimating the due date. Obstet Gynecol 2017; 129:e150–4. [DOI] [PubMed] [Google Scholar]
- [27].Donahue SP, Baker CN, Committee on Practice, Ambulatory Medicine AAP, Section on Ophthalmology AAP, American Association of Certified Orthoptists, American Association for Pediatric Ophtalmology and Strabismus, et al. Procedures for the evaluation of the visual system by pediatricians. Pediatrics 2016. 10.1542/peds.2015-3597 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [28].Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014;384:857–68. [DOI] [PubMed] [Google Scholar]
- [29].A health professional’s guide for using the new WHO growth charts. Paediatr Child Health 2010;15:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bayley N Bayley Scales of Infant and Toddler Development: Third edition. Administration Manual. San Antonio (TX): Psychorp; 2006. [Google Scholar]
- [31].Squires J, Bricker D, Twombley E, Potter L. Ages and Stages Questionnaires (ASQ): a parent-completed child monitoring system. 3rd ed. Baltimore (MD): Paul H. Brookes; 2009. [Google Scholar]
- [32].Valdes V, Zorrilla CD, Gabard-Durnam L, Muler-Mendez N, Rahman ZI, Rivera D, et al. Cognitive development of infants exposed to the Zika virus in Puerto Rico. JAMA Netw Open 2019;2:e1914061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Child and Adolescent Health Measurement Initiative. 2018–2019 National Survey of Children’s Health (NSCH) data query. Accessed April 28, 2021. https://www.childhealthdata.org/browse/survey/results?q=7965&r=1&g=812
- [34].Subissi L, Dub T, Besnard M, Mariteragi-Helle T, Nhan T, Lutringer-Magnin D, et al. Zika virus infection during pregnancy and effects on early childhood development, French Polynesia, 2013–2016. Emerg Infect Dis 2018;24:1850–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Golin V, Mimica IM, Mimica LM. Oropharynx microbiota among alcoholics and non-alcoholics. Sao Paulo Med J 1998;116:1727–33. [DOI] [PubMed] [Google Scholar]
- [36].Mulkey SB, Vezina G, Bulas DI, Khademian Z, Blask A, Kousa Y, et al. Neuroimaging findings in normocephalic newborns with intrauterine Zika virus exposure. Pediatr Neurol 2018;78:75–8. [DOI] [PubMed] [Google Scholar]
- [37].Departamento de Salud de Puerto Rico. Informe Anual de Estadisticas Vitales: Nacimientos años 2015 y 2016; 2019. Accessed January 21, 2021. http://www.salud.gov.pr/Estadisticas-Registros-y-Publicaciones/Informes%20Arbovirales/Reporte%20ArboV%20semana%2052-2017.pdf
- [38].Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, et al. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA 2017;317:59–68. [DOI] [PubMed] [Google Scholar]
- [39].Barbeito-Andrés J, Schuler-Faccini L, Garcez PP. Why is congenital Zika syndrome asymmetrically distributed among human populations? PLoS Biol 2018;16:e2006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ventura CV, Ventura LO. Ophthalmologic manifestations associated with Zika virus infection. Pediatrics 2018;141:S161–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain 2011;134:2197–221. [DOI] [PubMed] [Google Scholar]
- [42].Glascoe FP. Evidence-based early detection of developmental-behavioral problems in primary care: what to expect and how to do it. J Pediatr Health Care 2015;29:46–53. [DOI] [PubMed] [Google Scholar]
- [43].Kolb B, Harker A, Gibb R. Principles of plasticity in the developing brain. Dev Med Child Neurol 2017;59:1218–23. [DOI] [PubMed] [Google Scholar]
- [44].Black MM, Walker SP, Fernald LCH, Andersen CT, DiGirolamo AM, Lu C, et al. Early childhood development coming of age: science through the life course. Lancet 2017;389:77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Magai DN, Karyotaki E, Mutua AM, Chongwo E, Nasambu C, Ssewanyana D, et al. Long-term outcomes of survivors of neonatal insults: a systematic review and meta-analysis. PLoS One 2020;15:e0231947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


