Abstract
Introduction
The diagnostic delay in nail melanoma (NM) has been repeatedly emphasized. It may be related to both clinical misinterpretations and to errors in the bioptic procedure.
Objectives
To assess the efficacy of histopathologic examination in different diagnostic biopsies in NM.
Methods
We retrospectively investigated the diagnostic procedures and histopathologic specimens referred to the Laboratory of Dermatopathology for the clinical suspicion of NM from January 2006 to January 2016.
Results
Eighty-six nail histopathologic specimens were analyzed consisting in 60 longitudinal, 23 punch and 3 tangential biopsies. A diagnosis of NM was performed in 20 cases, benign melanocytic activation in 51 cases and melanocytic nevi in 15 patients. Longitudinal and tangential biopsy were diagnostic in all cases, regardless of the clinical suspicion. Nail matrix punch biopsy instead was not diagnostic in most of the cases (13/23 specimens).
Conclusions
In the presence of an NM clinical suspicion, longitudinal biopsy is recommended (lateral or median) because it provides exhaustive information on the characteristics of melanocytes morphology and distribution in all the components of the nail unit. Tangential biopsy, recently encouraged by expert authors due to the optimal surgical outcome, in our experience gives incomplete information on tumor extension. Punch matrix biopsy gives limited evidence in the diagnosis of NM.
Keywords: nail melanoma, subungual melanoma, pathology, surgical excision, biopsy
Introduction
Nail melanoma (NM) prevalence in Caucasians is estimated to range from 0.3 to 2.8 % of all melanomas. NM clinical presentation may be heterogeneous, and misdiagnoses are frequent [1–8].
The most frequent clinical misdiagnoses of NM are benign inflammatory diseases or benign neoplasms that cause nail plate pigmentation. Non-pigmented NM, also called amelanotic NM could be difficult to differentiate from squamous cell carcinoma of the nail (NSCC) or other conditions. Misdiagnoses in NM may be also due to inappropriate surgical sampling and subsequent difficulties in the interpretation of histopathologic specimens.
Because of the particular anatomic location and the scarce number of cases in Caucasians, in the clinical suspicion of NM, no absolute consensus exists regarding the optimal diagnostic procedures.
Bioptic techniques have been adequately described by nail experts [9–23], but the two main problems in the correct choice and performance of the biopsy are probably related to the difficulty in performing surgery in the nail unit (insufficient familiarity with nail apparatus anatomy among dermatologic surgeons) and the risk of permanent nail dystrophy when an excessive amount of nail matrix is excised.
Objectives
The aim of the study was to assess the efficacy of histopathologic examination in different diagnostic biopsies in NM.
Methods
We retrospectively investigated the diagnostic procedures administered in patients referring for a clinical suspicion of NM. Histopathologic data were obtained from the archive of Dermatopathology, University of Bologna, from January 2006 to January 2016. Since our Laboratory is a referral center for nail pathology, specimens are prevenient from our institution, but also from other centers or private practices. The key words for the database search were: “melanocytic activation”, “melanocytic hyperplasia”, “melanoma” and “nevi”.
The histopathologic charts containing the selected key words were reviewed for the following: 1) clinical query 2) the type of biopsy, and 3) histopathologic diagnosis.
The type of bioptic procedures assessed were: 1) punch biopsy (PB) 2) longitudinal biopsy (LB) considering both types lateral and median, and 3) tangential biopsy (TB).
Transverse matrix biopsy was not considered among the evaluated procedures since it is not widely used in the surgical practice in our institution and no cases were referred in our laboratory (9).
Finally, the appropriateness of the bioptic procedures was evaluated in correlation to the final histopathologic responses. All NM histopathologic specimens were assessed by two dermatopathologists (PAF and CM) respecting the American Joint Committee on Cancer (AJCC) guidelines [24].
Results
Eight-six cases were selected (54 females/32 males). Demographic data are presented in Table 1.
Table 1.
Patients, clinical presentation and diagnostic procedures of the nail unit.
| Diagnosis (cases) | Gender (Female/ Male) | Age in years (median) | Site (hands/ feet) | Clinical presentation | Bioptic procedure |
|---|---|---|---|---|---|
| Total (86) | 54/32 | 46 | 56/27 | 81 melanonychia/ 5 nodular neoplasms | 60 longitudinal biopsy/ 23 punch biopsy/ 3 tangential biopsy |
| Nail Melanoma (20) | 14/6 | 61 | 14/3 | 16 longitudinal melanonychia/ 4 nodular neoplasms | 12 longitudinal biopsy/ 5 punch biopsy/ 3 tangential biopsy |
| Benign melanocytic activation (51) | 33/18 | 52 | 32/19 | 50 nail pigmentation/ 1 nodular mass of the nail bed | 34 longitudinal biopsy/ 17 punch biopsy |
| Nevus (15) | 8 / 7 | 25 | 10 / 3 | 15 nail pigmentations | 14 longitudinal biopsy/ 1 punch biopsy |
The most frequent queries from clinicians (first endpoint) were NM in 55 patients (64%); followed by NM versus melanocytic nevus in 9 cases (10%) and NM versus friction/ post-traumatic melanonychia in 22 patients (26%).
The second endpoint of the study was the evaluation of the bioptic procedures:
LB was performed in 60 patients;
A 3 or 4 mm PB was performed in 23 cases;
A TB was performed in 3 cases.
On histopathologic evaluation a diagnosis of NM was performed in 20 cases, benign melanocytic activation in 51 patients and melanocytic nevi in 15 cases, respectively.
Regarding the final endpoint of the study, on the appropriateness of the bioptic procedure:
-
12/20 patients affected by NM had undergone a LB; 5/20 a PB and 3/20 a TB.
LB was diagnostic in all cases. PB was diagnostic only in 2/5 of melanoma cases. In both diagnosed cases of NM, PB was performed at the level of nail matrix. In the remaining 3 cases (in all them, PB was performed at the nail bad), dermatopathologists had requested clinical patient re-evaluation and a second LB was performed in 2 cases and en-block tumor excision in 1 case (the clinical features were strongly suggestive of NM). The histopathologic interpretation of TB was consistent with the diagnosis of melanoma in all the 3 cases.
A LB was diagnostic in 34/51 cases that were finally diagnosed with benign melanocytic activation and in the remaining 17 cases a PB was performed. PB was not diagnostic in 10/17 cases, all performed at the nail bed level. A second biopsy was requested in all cases together with a clinical re-evaluation. The remaining 7 cases of PB were diagnostic and were performed at the nail matrix level.
A LB was performed in 14/15 cases that were diagnosed as melanocytic nevi and a PB at the nail matrix level was performed in 1/15 cases. All cases were correctly diagnosed.
All NM patients underwent therapeutic surgery (conservative approach or phalanx disarticulation) and are still undergoing regular clinical and instrumental controls. Patients with a different diagnosis (nevi or benign melanocytic activation) are undergoing clinical follow up where necessary.
Conclusions
Misdiagnoses of NM are continuously reported in the scientific literature [25,26].
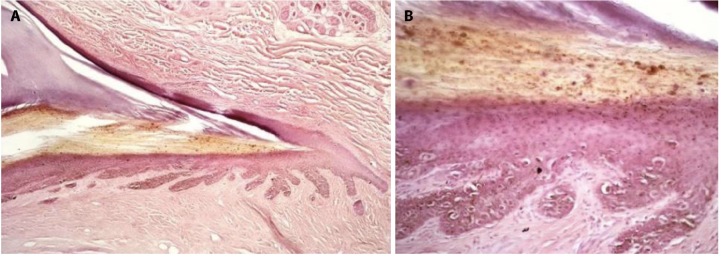
The most common clinical presentation of NM is longitudinal melanonychia, which is a brown to black band of the nail plate, starting from the proximal nail fold and extending to the distal margin of the nail. Nail plate pigmentation is due to melanin granules incorporated in the nail plate during the process of nail matrix keratinization. In NM, both melanin granules and atypical (pagetoid) melanocytes (Figure 1, A and B) can be observed (authors experience).
Figure 1.
(A, B) Nail melanoma: histopathologic aspects of a nail matrix biopsy (H&E original magnification 10X; 25X) showing proliferation of atypical melanocytes in the basal and supra-basal layers of the nail matrix.
The process of keratinization in the nail unit is an evolving process. It initiates at the nail matrix level and is deduced clinically with the expansion of the plate. Melanocytes are present among matrix keratinocytes, but they are usually quiescent and not produce pigment. Melanin synthesis may occur in benign and malignant conditions, and it is characterized by incorporation of melanin in nail matrix keratinocytes during nail plate production. The pigmentation therefore initially starts in the proximal nail plate and occupies the whole length of it during growth. This process is estimated to last from 4–6 to 10–12 months (hands and feet). When we observe a pigmented band, we therefore only see the degree and distribution of melanin within the nail plate and not the site of origin of it, as the matrix lies below the proximal nail folds and only its distal part, the lunula, is visible from the outside.
Both technicians and dermatopathologists should be familiar with the nail anatomy to achieve an optimal processing of the specimens and to avoid diagnostic misinterpretations. But, most important, the surgeon should know that a biopsy of melanonychia should excise the nail matrix, which is the source of the pigmentation.
In the present study, two main issues were considered on the suspicion of NM: 1) where to biopsy and 2) which type of biopsy offers the best diagnostic approach.
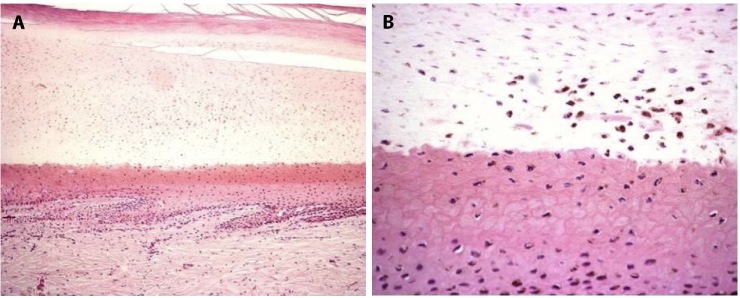
In the nail unit, biopsies can be performed on four main locations: the nail folds, bed, plate and matrix. In physiologic conditions, most melanocytes are located at the nail matrix level, while very few or no melanocytes can be observed at the nail bed [27]. Benign conditions that correlate to melanocytic activation (51 cases herein analyzed), show that the pigmentation of the nail plate is mainly due to distal matrix activated melanocytes, whereas melanocytes at the nail bed are almost absent (Figure 2, A and B).
Figure 2.
(A, B) Benign melanocytic activation: histopathologic aspects of a longitudinal nail biopsy (H&E original magnification 20X; 25X) showing proliferation of melanocytes in the nail matrix and nail bed.
Regarding the correct choice and execution of the bioptic procedure and its correlation to the definitive diagnosis, we can state, that in our experience, in the presence of a NM clinical suspicion, a longitudinal biopsy is recommended in all cases of nail pigmentation (lateral or median). In our survey, LB offered the correct diagnosis in 60/ 60 cases while PB was diagnostic in 10/23 patients. On LB, correct information is obtained regarding melanocytes morphology and distribution through the nail matrix, nail bed and hyponychium. A PB of the nail matrix is correctly performed for pigmented bands, in the clinical suspicion of a benign melanocytic activation (patients history of drug assumption, ethnicity). Nail bed biopsy should be avoided since it can often lead to misdiagnoses of NM. In our experience, a TB, recently encouraged by expert authors due to the optimal surgical outcome (less dystrophy of the nail plate), gives incomplete information on tumor extension [23]. In the present study, the final determination of Breslow thickness after en bloc excision of the nail unit was evaluated as difficult and imprecise by histopathologists (during the first TB a major portion of the distal matrix was excised in all three cases). Breslow thickness evaluation could be an issue of concern since it represents one of the most important predictive factors when dealing with NM. We believe TB could be proposed to highly collaborative and compliant patients after adequate explanations.
A limitation of this study was the retrospective evaluation of cases.
In conclusion, in the presence of an NM clinical suspicion, LB is recommended because it provides exhaustive information on the characteristics of melanocytes morphology and distribution in all the components of the nail unit.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Quinn MJ, Thompson JE, Crotty K, McCarthy WH, Coates AS. Subungual melanoma of the hand. J Hand Surg Am. 1996;21(3):506–511. doi: 10.1016/S0363-5023(96)80371-6. [DOI] [PubMed] [Google Scholar]
- 2.Banfield CC, Redburn JC, Dawber RP. The incidence and prognosis of nail apparatus melanoma. A retrospective study of 105 patients in four English regions. Br J Dermatol. 1998;139(2):276–279. doi: 10.1046/j.1365-2133.1998.02365.x. [DOI] [PubMed] [Google Scholar]
- 3.Blessing K, Kernohan NM, Park KG. Subungual malignant melanoma: Clinicopathological features of 100 cases. Histopathology. 1991;19(5):425–429. doi: 10.1111/j.1365-2559.1991.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 4.Dika E, Patrizi A, Fanti PA, et al. The Prognosis of Nail Apparatus Melanoma: 20 Years of Experience from a Single Institute. Dermatology. 2016;232(2):177–184. doi: 10.1159/000441293. [DOI] [PubMed] [Google Scholar]
- 5.Dika E, Piraccini BM, Fanti PA. A gray pigmented band of the third fingernail with distal splitting. JAMA Dermatol. 2014;150(2):199–200. doi: 10.1001/jamadermatol.2013.5731. [DOI] [PubMed] [Google Scholar]
- 6.Boyer A. Fungus hematode de petit doights. Gaz Med Par. 1834;2:212. [Google Scholar]
- 7.Hutchinson J. Melanosis often not black: melanotic whitlow. Br Med J. 1886;1:491. [Google Scholar]
- 8.Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33(2):185–195. doi: 10.1016/j.det.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Braun PR, Baran R, Le Gal AF, et al. Diagnosis and management of nail pigmentations. J Am Acad Dermatol. 2007;56(5):835–847. doi: 10.1016/j.jaad.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Haneke E, Baran R. Longitudinal melanonychia. Dermatol Surg. 2001;27(6):580–584. [PubMed] [Google Scholar]
- 11.Baran R, Haneke E. Diagnose und Behandlung von longitudinalen Nagel pigmentierungen. Hautarzt. 1984;35:359–365. [Google Scholar]
- 12.Baran R, Kechijian P. Longitudinal melanonychia (melanonychia striata): diagnosis and management. J Am Acad Dermatol. 1989;21(6):1165–1175. doi: 10.1016/s0190-9622(89)70324-8. [DOI] [PubMed] [Google Scholar]
- 13.Rich P. Nail biopsy. Indications and methods. J Dermatol Surg Oncol. 1992;18(8):673–682. doi: 10.1111/j.1524-4725.1992.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 14.Jellinek N. Nail biopsy. Indications and methods. J Am Acad Dermatol. 2007;56(5):803–810. doi: 10.1111/j.1524-4725.1992.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 15.Banfield CC, Dawber RP. Nail melanoma: a review of the literature with recommendations to improve patient management. Br J Dermatol. 1999;141(4):628–632. doi: 10.1046/j.1365-2133.1999.03099.x. [DOI] [PubMed] [Google Scholar]
- 16.Glat PM, Shapiro RL, Roses DF, Harris MN, Grossman JA. Management considerations for melanonychia striata and melanoma of the hand. Hand Clin. 1995;11:183–189. [PubMed] [Google Scholar]
- 17.Ruben BS. Pigmented lesions of the nail unit. Semin Cutan Med Surg. 2015 Jun;34(2):101–108. doi: 10.12788/j.sder.2015.0146. [DOI] [PubMed] [Google Scholar]
- 18.Jellinek NJ, Vélez NF. Dermatologic Manifestations of the Lower Extremity: Nail Surgery. Clin Podiatr Med Surg. 2016;33(3):319–336. doi: 10.1016/j.cpm.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Vélez NF, Jellinek NJ. Nailing it: promoting nail procedural training in residency and beyond. Dermatol Surg. 2015;41(3):424–426. doi: 10.1097/DSS.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 20.Jellinek NJ, Rubin AI. Lateral longitudinal excision of the nail unit. Dermatol Surg. 2011;37(12):1781–1785. doi: 10.1111/j.1524-4725.2011.02167.x. [DOI] [PubMed] [Google Scholar]
- 21.Jellinek N. Nail matrix biopsy of longitudinal melanonychia: diagnostic algorithm including the matrix shave biopsy. J Am Acad Dermatol. 2007;56(5):803–810. doi: 10.1016/j.jaad.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Cogrel O, Haneke E. Tangential excision for a longitudinal melanonychia. Ann Dermatol Venereol. 2015;142(6–7):450–451. doi: 10.1016/j.annder.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Di Chiacchio N, Loureiro WR, Michalany NS, Kezam Gabriel FV. Tangential Biopsy Thickness versus Lesion Depth in Longitudinal Melanonychia: A Pilot Study. Dermatol Res Pract. 2012;2012:353864. doi: 10.1155/2012/353864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levit EK, Kagen MH, Scher RK, Grossman M, Altman E. The ABC rule for clinical detection of subungual melanoma. J Am Acad Dermatol. 2000;42(2):269–274. doi: 10.1016/S0190-9622(00)90137-3. [DOI] [PubMed] [Google Scholar]
- 26.Metzger S, Ellwanger U, Stroebel W, et al. Extent and consequences of physician delay in the diagnosis of acral melanoma. Melanoma Res. 1998;8(2):181–186. doi: 10.1097/00008390-199804000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Kazi R, Moghaddam S, Chu P, Marghoob A. A Histologic Evidence of Melanocytes Isolated to the Nail Matrix. JAMA Dermatol. 2016;152(5):573–575. doi: 10.1001/jamadermatol.2015.5869. [DOI] [PubMed] [Google Scholar]