Abstract
Background
Post COVID-19 syndrome (PCS) is a complex condition with partly substantial impact on patients' social and professional life and overall life quality. Currently, the underlying cause(s) of PCS are unknown. Since PCS-specific symptoms could be associated with systemic alterations in tissue oxygen supply, we aimed to investigate changes in tissue oxygenation in patients with PCS.
Methods
A case-control study including 30 PCS patients (66.6 % males, 48.6 ± 11.2 years, mean time after (first) acute infection: 324 days), 16 cardiologic patients (CVD) (65.5 % males, 56.7 ± 6.3 years) and 11 young healthy controls (55 % males, 28.5 ± 7.4 years) was conducted. Near infrared spectroscopy (NIRS) was used to assess changes in tissue oxygenation during an arterial occlusion protocol on the non-dominant forearm (brachioradialis, 760/850 nm, 5 Hz). The protocol included 10-min rest, a 2-min baseline measurement followed by a 3-min ischemic period (upper-arm cuff, 50 mmHg above resting systolic blood pressure) and a 3-min reoxygenation period. PCS patients were grouped by presence of arterial hypertension and elevated BMI to assess the impact of risk factors.
Results
No differences in mean tissue oxygenation in the pre-occlusion phase existed between groups (p ≥ 0.566). During ischemia, comparisons of linear regressions slopes revealed slower oxygen desaturation for PCS patients (−0.064 %/s) compared to CVD patients (−0.08 %/s) and healthy subjects (−0.145 %/s) (p < 0.001). After cuff release, slowest speed for reoxygenation was detected in PCS patients at 0.84 %/s compared to CVD patients (1.04 %/s) and healthy controls (CG: 2.07 %/s) (p < 0.001). The differences between PCS patients and CVD patients during ischemia remained significant also after correction for risk factors. Analyses of complications during acute infection, persistence of PCS symptoms (time after acute infection), or PCS severity (number of lead symptoms) as confounding factors did not reveal a significant effect.
Conclusions
This study provides evidence that the rate of tissue oxygen consumption is persistently altered in PCS and that PCS patients show an even slower decline in tissue oxygenation during occlusion than CVD patients. Our observations may at least partly explain PCS-specific symptoms such as physical impairment and fatigue.
Keywords: Near-infrared spectroscopy, Microcirculation, Endothelial dysfunction, COVID-19, Cardiovascular disease
1. Introduction
Post COVID-19 syndrome (PCS) and the associated broad spectrum of clinical manifestations (profound fatigue, cognitive deficits, cardio-pulmonal and physical impairment) represents a growing burden for global health and social systems (Nalbandian et al., 2021). PCS is defined by persistent symptoms ≥12 weeks after start of the acute infection and may affect up to 10 % of COVID-19 patients independent of disease severity (Venkatesan, 2021). Currently, the underlying cause of PCS remains elusive. However, different pathophysiologic mechanisms such as viral toxicity, immune dysregulation, hyperinflammation, and hypercoagulability as well as endothelial damage have been suggested (Nalbandian et al., 2021). It has been reported that COVID-19 has significant detrimental effects on the cardiovascular system and long-term cardiac sequelae have been described (Chung et al., 2021). In this regard, up to 78 % of PCS patients have a cardiac involvement (Puntmann et al., 2020), which includes symptoms such as dysrhythmias, palpitations, angina/chest pain or shortness of breath (van der Sluijs et al., 2022). In terms of impaired cardiorespiratory function, PCS patients referred to our center for medical rehabilitation report with an equally high reduction of exercise capacity compared to patients with cardiovascular disease (CVD).
It has been suggested that peripheral factors limiting O2 supply and utilization in the skeletal muscle may explain the observed physical limitations in PCS and the need for direct measurements of potential changes also including the microcirculation has been repeatedly expressed (Serviente et al., 2022). Of note, for acute COVID-19 patients, systemic microcirculatory alterations have recently been shown (Mesquida et al., 2021). However, if alterations of the peripheral microcirculation and tissue oxygenation persist in PCS patients has not been reported. Thus, we aimed to investigate changes in tissue oxygenation in patients with PCS as a potential factor contributing to PCS-specific symptoms. We hypothesized that reductions in tissue oxygenation would be comparable to CVD patients.
2. Materials and methods
2.1. Study design
A case-control study was conducted at medical rehabilitation Clinic Königsfeld, Ennepetal, Germany between 11/2021 and 05/2022. Patients with an indication for medical rehabilitation based on a PCS diagnosis were consecutively recruited. Patients with a CVD diagnosis and no signs or symptoms of PCS were used as control group based on comparable reductions in exercise capacity (i.e. ∼70 % of age- and sex-adjusted reference). Young healthy controls were included for comparison to unimpaired tissue oxygenation. All participants gave their written informed consent. The study was approved by the local ethics committee (University of Witten/Herdecke, 195/2021) and was conducted in accordance with the Declaration of Helsinki.
2.2. Measurements and analysis
Near infrared spectroscopy (NIRS) was used to assess changes in tissue oxygenation based on the Beer–Lambert law and wavelengths of 760 and 850 nm (sampling rate 5 Hz) using an established vascular occlusion protocol (VOT) as described (Mesquida et al., 2021; McManus et al., 2018; Iannetta et al., 2019). In brief, a NIRS sensor (PortaMon, Artinis Medical Systems, The Netherlands) was applied to the non-dominant forearm in a circumferential orientation over the brachioradialis muscle, 5–10 cm distal to the proximal head of the radius. After 10 min of rest, the arterial occlusion protocol was performed including a 2 min baseline measurement followed by a 3 min ischemic period (upper-arm cuff, 50 mmHg above resting systolic blood pressure) and a 3 min reoxygenation period (Mesquida et al., 2021). All measurements were performed in the morning, in a quiet room (20 ± 2 °C) in supine position after an overnight fast. Participants were asked to refrain from caffeine, nicotine, and exercise on the day of measurement (Soares et al., 2020). Bioimpedance analysis (InBody 770, InBody Europe B.V., Germany) were performed to assess body fat mass and fat mass of the investigated arm. The absolute concentration of tissue oxyhemo(+myo)globin (O2Hb), deoxyhemo(+myo)globin (HHb), and total hemo(+myo)globin (tHb) was determined and expressed as tissue oxygen saturation (TSI %) calculated as O2Hb/(O2Hb + HHb) × 100 (Puntmann et al., 2020). TSI thus reflects the dynamic balance between O2 supply and consumption.
2.3. Statistical analysis
Prism 9 (GraphPad Software, USA) was used for statistical analysis and data presentation. Data is presented as mean and SD, n (%) or 95 % CI as indicated. Group means were compared using one-way ANOVA corrected for multiple comparison (Tukey's test), unpaired two-sided t-test or ANCOVA (for sex as covariate). Linear regression analysis and calculation of regression slopes were performed to compare changes in TSI over time during occlusion and reoxygenation. Slopes were calculated for the first minute after occlusion as well as during the entire exponential period of ischemia. Time between the (second) rapid increase and maximum was considered for the reperfusion slope. Normalization was conducted in case of non-normal distribution. Pearson's correlation coefficient was used for correlation analysis. To assess the impact of risk factors, PCS patients were grouped by presence of arterial hypertension (HPT) or BMI (≤/> median BMI = 31.0 kg ∗ m−2).
3. Results
Thirty patients diagnosed with PCS were recruited during their first week of inpatient medical rehabilitation and NIRS was applied to assess tissue oxygenation. Mean time after (first) acute infection was 324 days (range: 75–774 days). Sixteen patients with CVD (mainly coronary artery disease) and eleven healthy individuals (CG) served as controls (Table 1 ).
Table 1.
Clinical and demographic characteristics of patients with Post COVID-19 Syndrome (PCS), cardiovascular disease patients (CVD) and control group (CG).
| PCS (n = 30) | CVD (n = 16) | CG (n = 11) | P | |
|---|---|---|---|---|
| Age, years | 48.6 ± 11.2 | 56.7 ± 6.3 | 28.5b ± 7.5 | <0.001 |
| Sex, n (%) | 0.774 | |||
| Female | 10 (33.3) | 6 (37.5) | 5 (45) | |
| Male | 20 (66.6) | 10 (62.5) | 6 (55) | |
| Height, cm | 174.3 ± 8.6 | 174.9 ± 9.7 | 174.4 ± 5.4 | 0.973 |
| Weight, kg | 101.1 ± 22.5 | 93.3 ± 17.1 | 70.9a ± 12.3 | <0.001 |
| BMI, kg ∗ m−2 | 33.1 ± 6.3 | 30.5 ± 4.9 | 23.2a ± 3.6 | <0.001 |
| Fatmass investigated armc, % | 31.1 ± 24.8 | 26.3 ± 16.3 | 7.1a ± 5.3 | 0.009 |
| O2peakd, mL·kg−1·min−1 | 18.5 ± 4.6 | 20.7 ± 3.4 | – | 0.154 |
| Complications during acute SARS-CoV-2 infection, n (%) |
||||
| Hospitalization | 7 (23.3) | – | – | |
| Invasive mechanical ventilation | 7 (23.3) | – | – | |
| Pneumonia | 6 (20) | – | – | |
| Pulmonary embolism | 3 (10) | – | – | |
| PCS lead symptomse, n (%) | ||||
| Physical impairment | 21 (70) | – | – | |
| Dyspnea | 23 (76.7) | – | – | |
| Cognitive impairment | 11 (36.7) | – | – | |
| Arterial hypertension, n (%) | 18 (60) | 13 (81.3) | – | 0.143 |
| Coronary artery disease, n (%) | 2 (6.6) | 14 (87.5) | – | <0.001 |
| Non-hemodynamically stenosis | 1 (3.3) | – | – | 0.460 |
| One vessel disease | 1 (3.3) | 6 (37.5) | – | 0.002 |
| Two vessel disease | – | 6 (37.5) | – | <0.001 |
| Three vessel disease | – | 2 (12.5) | – | 0.480 |
| STEMI/NSTEMI, n (%) | 0 (0) | 6 (37.5) | – | <0.001 |
| Treatment, n (%) | ||||
| PCI performed | 1 (3.3) | 13 (75) | – | <0.001 |
| Bypass performed | – | 1 (6.3) | – | 0.166 |
| LVEF, n (%) | ||||
| Slightly reduced (41–50 %) | – | 3 (18.8) | – | 0.014 |
| Medication, n (%) | ||||
| ACE-inhibitor | 11 (36.7) | 4 (25) | – | 0.421 |
| Anticoagulant | 7 (23.3) | 12 (75) | – | <0.001 |
| Statin | 7 (23.3) | 13 (81.3) | – | <0.001 |
| Beta blocker | 15 (50) | 15 (93.8) | – | 0.003 |
| Angiotensin II receptor blocker | 5 (16.7) | 11 (68.8) | – | <0.001 |
| Calcium channel blocker | 7 (23.3) | 7 (43.8) | – | 0.152 |
| Diuretic | 9 (30) | 7 (43.8) | – | 0.351 |
| Glucocorticoid | 5 (16.7) | 0 (0) | – | 0.084 |
| Analgesic drug | 12 (40) | 14 (87.5) | – | 0.002 |
| Antidepressant | 7 (23.3) | 2 (12.5) | – | 0.378 |
| Active smoker, n (%) | 4 (13.3) | 2 (12.5) | – | 0.936 |
| Elixhauser Comorbidity Index (ECI) | −0.1 ± 3.4 | −0.9 ± 3.8 | – | 0.515 |
| Multidimensional fatigue inventory (MFI) | ||||
| Overall score | 70 ± 10.7 | 50.3 ± 12.8 | – | <0.001 |
| Physical fatigue | 76.1 ± 12.8 | 59.5 ± 19.4 | – | 0.007 |
| Mental fatigue | 64.1 ± 20.7 | 47 ± 11.1 | – | 0.020 |
Data is presented as n (%) or mean ± SD. P values were calculated using independent t-test, Chi-squared test or one-way ANOVA corrected for multiple comparison if indicated. ECI: according to the modified Van Walraven comorbidity index (van Walraven et al., 2009). The 20-item self-report MFI was used to assess fatigue (Smets et al., 1995).
Significantly difference with PCS and CVD.
Significantly difference with PCS.
Assessed with Bioelectrical impedance analysis.
PCS: n = 26; CVD: n = 11.
Four PCS patients reported other disease-specific symptoms.
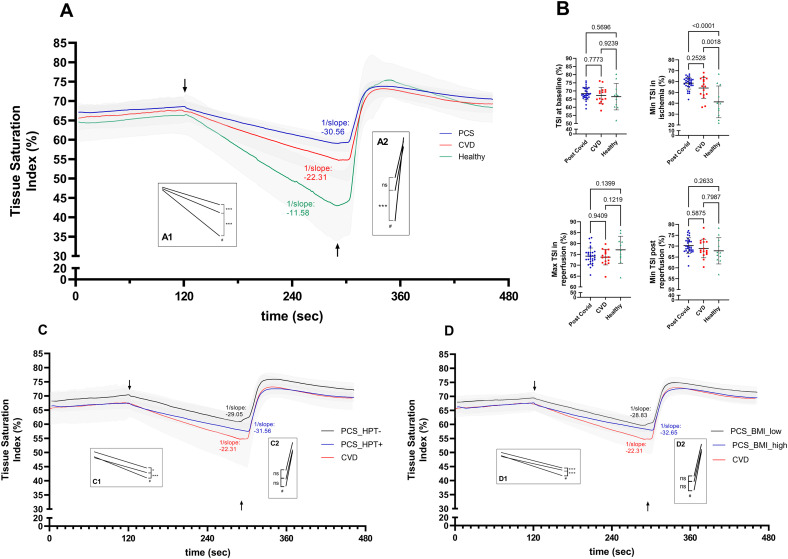
At rest, participants showed similar mean tissue oxygenation in terms of TSI (pre-occlusion phase, PCS: 68.3 ± 3.7 %; CVD: 67.3 ± 4.7 %; CG: 66.5 ± 7.8 %; p ≥ 0.566) (Fig. 1A/B). Comparisons of linear regressions slopes during arterial occlusion showed significant differences between the three groups (p < 0.001), indicating the smallest decrease in oxygen saturation for PCS patients at −0.064 %/s compared to CVD patients at −0.08 %/s and healthy subjects at −0.145 %/s (Fig. 1A). While healthy subjects showed a significantly lower minimum O2 saturation in ischemia (p ≤ 0.002), no significant difference (p = 0.253) was detected between PCS and CVD patients (CG: 41.3 ± 14 %; PCS: 58.5 ± 5.4 %; CVD: 54.1 ± 9.2 %) (Fig. 1B). Of note, results were consistent between analysis of the initial 1-min period or the entire exponential ischemic phase. Comparisons of linear regressions slopes after cuff release also indicated significant differences between the three groups (p < 0.001) with the slowest speed for reoxygenation in PCS patients at 0.84 %/s compared to CVD patients (1.04 %/s) and healthy controls (2.07 %/s) (Fig. 1A). Reperfusion during the first 10 s after cuff release was also lower in PCS (0.78 %/s) compared to CVD (0.91 %/s) but lacked significance (p = 0.821), while reperfusion was highest in the CG (1.76 %/s, p ≤ 0.007). Mean maximum TSI after reperfusion suggested no significant difference between the groups (PCS: 74.2 ± 3.7 %; CVD: 73.7 ± 3.5 %; CG: 77.1 ± 6.0 %) (Fig. 1B). Analyses of the impact of risk factors revealed significantly slower oxygen consumption in ischemia in PCS patients with HPT or elevated BMI compared to PCS patients without HPT or lower BMI, respectively (p < 0.05) (Fig. 1C/D). However, the significant difference between PCS patients and CVD patients was unaffected by risk factors.
Fig. 1.
(A/B) Tissue oxygenation is altered in patients with Post COVID-19 Syndrome.
(A) Changes of tissue oxygenation determined by near infrared spectroscopy (NIRS) before, during, and after standardized arterial occlusion. Inserts (A1) and (A2) show statistical comparison of linear regression slopes between patients with Post COVID-19 Syndrome (PCS), cardiovascular disease patients (CVD) and healthy controls during ischemia (3 min) and reperfusion. Respective linear regression slopes during ischemia are given as 1/slope for visualization indicating significantly slower oxygen consumption in PCS patients. (B) Comparison of tissue oxygenation before and after ischemia and reperfusion by tissue saturation index (TSI) between PCS and CVD patients and healthy controls. Individual data is presented with group mean and standard deviation. (C/D) Analyses of the effect of comorbidities on altered tissue oxygenation in PCS patients. (C) PCS patients were grouped by presence of arterial hypertension (HPT). Inserts (C1) and (C2) show statistical comparison of linear regression slopes between PCS patients (+) with (n = 18) and without (−) HPT (n = 12), and CVD patients during ischemia. Respective linear regression slopes during ischemia indicated significantly slower oxygen consumption in PCS patients with HPT compared to PCS patients without HPT and CVD patients. (D) PCS patients were grouped by BMI (≤/> median BMI = 31.0 kg ∗ m−2). Inserts (D1) and (D2) show statistical comparison of linear regression slopes between PCS patients with higher BMI compared to PCS patients with lower BMI and CVD patients during ischemia. Respective linear regression slopes during ischemia indicated significantly slower oxygen consumption in PCS patients with HPT compared to PCS patients without HPT and CVD patients. Data is presented as group mean values with 95 % confidence interval. Tissue saturation index was calculated as O2Hb/(O2Hb + HHb) × 100. P-values were calculated by comparison of linear regression slopes (in A, C, D) or one-way ANOVA corrected for multiple comparison (in B). ↓ start of ischemic phase (3 min), ↑ end of occlusion. #p < 0.05 for three-group comparison; *p = 0.01, ***p < 0.0001 for two-group comparison; ns, not significant.
Of the analyzed PCS patient group, ten PCS patients had experienced complications such as pneumonia, pulmonary embolism and/or needed mechanical ventilation during acute infection (Table 1). These patients had comparable desaturation (−0.066 %/s) and reperfusion slopes (0.891 %/s) to the group without complications (−0.063 %/s and 0.809 %/s) (p ≥ 0.62). Analysis of the severity of PCS, assessed by the number of lead symptoms, on tissue oxygenation revealed no differences. Correlation analyses of the desaturation and reperfusion slope with the time after acute infection (persistence of PCS symptoms) did not reveal a significant association. Of note, PCS patients were significantly younger than CVD patients (p = 0.012), which is of relevance since TSI has been reported to decrease with age (Mesquida et al., 2013; Rosenberry and Nelson, 2020). No significant difference in the number of females existed between the three groups (p = 0.774) and results were unaffected by sex. There were no differences in the fat mass of the investigated arm between PCS and CVD patients (PCS: 3.6 ± 2.5 kg; CVD: 2.6 ± 1.6 kg; p = 0.170).
4. Discussion
This study provides evidence that the rate of tissue oxygen consumption is altered in PCS patients. Using a standardized arterial occlusion protocol and continuous assessment of oxygenated hemoglobin by NIRS revealed, that PCS patients show an even slower decline in tissue oxygenation during ischemia at rest, compared to CVD patients. Our data suggest a persistently impaired systemic tissue oxygen consumption beyond an acute COVID-19 infection which may contribute to the described PCS symptoms, predominantly the severely reduced cardiovascular fitness and muscular weakness.
In our center for medical rehabilitation, PCS patients present with considerably impaired physical exercise capacity, indicated by a maximal oxygen uptake capacity of ∼70 % of the age- and sex-adjusted reference, comparable to patients with diagnosed CVD. Of note, maximal aerobic capacity has been shown to correlate with microvascular reactivity, expressed by higher reperfusion slopes (Rasica et al., 2022), which may at least partly explain this observation. Impaired oxygen consumption in tissues and in general may be based on two main and potentially synergistic physiological alterations. First, tissue concentration of microvessels may be reduced in PCS leading to a reduction in gas exchange. Second, a reduction in mitochondrial number or function could lead to a lower oxygen pressure gradient following recent findings that mitochondria represent an “oxygen sink” and that uncoupling of the mitochondrial respiratory chain leads to alterations in O2 gradients within the cellular microenvironment (Mori et al., 2021). Of note, processes during acute COVID-19 infections such as entry of viral RNA into mitochondria, have been postulated to harm mitochondrial function (Shang et al., 2021) potentially leading to mitochondrial uncoupling. The involvement of endothelial dysfunction (ED) in COVID-19 has also been discussed (Nägele et al., 2020) and reperfusion after ischemia and hemoglobin resaturation seem to depend on ED. (Mesquida et al., 2013) However, the velocity of reperfusion is influenced by a hypoxic stimulus (Mesquida et al., 2013). Higher TSI values in PCS patients at the end of ischemia suggest a lower hypoxic stimulus, which could explain the difference in the upslope but may not explain differences in oxygen saturation during ischemia. The dependency of the oxygen saturation at the end of ischemia and the reperfusion slope was recently shown (Rosenberry and Nelson, 2020). Our investigation on the impact of risk factors such as arterial hypertension or elevated BMI suggested, that these further impair the rate of tissue oxygen consumption in PCS, while PCS patients without these risk factors still presented lowered rates compared to CVD patients. Of note, complications during acute infection, time after acute infection, or PCS severity did not affect the observed alterations in our series.
Some limitations of this initial report on altered tissue oxygenation in PCS patients may apply. The study consisted of a small heterogenous sample of patients referred to medical rehabilitation based on persisting PCS-specific symptoms with large variability in the period after (first) acute infection. Any secondary analyses of possible confounding factors including severity of the acute infection, time after infection, and risk factors (hypertension, elevated BMI) should be seen as hypothesis generating. In addition, the possible pathophysiological link between altered tissue oxygenation and PCS remains a hypothesis.
We conclude that our observations may be based on changes in the systemic microcirculation and/or mitochondrial dysfunction in PCS rather than ED. Future larger studies also focusing on cellular and molecular alterations in PCS will be needed to gain detailed insight in confounding variables and underlying physiological changes. Additionally, there is the need to identify appropriate interventions including exercise-based medical rehabilitation tailored to stimulate improved microcirculation and mitochondrial function.
CRediT authorship contribution statement
Conceptualization: Hendrik Schäfer, Boris Schmitz, Frank C. Mooren.
Data collection and analysis: Hendrik Schäfer, Marc Teschler, Boris Schmitz.
Interpretation of results: Boris Schmitz, Frank C. Mooren, Hendrik Schäfer.
Writing – original draft: Hendrik Schäfer.
Writing – review & editing: Hendrik Schäfer, Boris Schmitz, Frank C. Mooren.
Funding
FS and BS are supported by the European Commission within the Horizon 2020 framework program (grant number: 101017424).
Declaration of competing interest
We confirm that there is no conflict of interest, financial or otherwise, in our submission of the manuscript.
Acknowledgments
We thank all patients for participating in this study.
Data availability
Data is available from the corresponding author upon reasonable request.
References
- Chung M.K., Zidar D.A., Bristow M.R., Cameron S.J., Chan T., Harding C.V., Kwon D.H., Singh T., Tilton J.C., Tsai E.J., Tucker N.R., Barnard J., Loscalzo J. COVID-19 and cardiovascular disease: from bench to bedside. Circ. Res. 2021;128:1214–1236. doi: 10.1161/CIRCRESAHA.121.317997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetta D., Inglis E.C., Soares R.N., McLay K.M., Pogliaghi S., Murias J.M. Reliability of microvascular responsiveness measures derived from near-infrared spectroscopy across a variety of ischemic periods in young and older individuals. Microvasc. Res. 2019;122:117–124. doi: 10.1016/j.mvr.2018.10.001. [DOI] [PubMed] [Google Scholar]
- McManus C.J., Collison J., Cooper C.E. Performance comparison of the MOXY and PortaMon near-infrared spectroscopy muscle oximeters at rest and during exercise. J. Biomed. Opt. 2018;23:1–14. doi: 10.1117/1.JBO.23.1.015007. [DOI] [PubMed] [Google Scholar]
- Mesquida J., Gruartmoner G., Espinal C. Skeletal muscle oxygen saturation (StO2) measured by near-infrared spectroscopy in the critically ill patients. Biomed. Res. Int. 2013;2013 doi: 10.1155/2013/502194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquida J., Caballer A., Cortese L., Vila C., Karadeniz U., Pagliazzi M., Zanoletti M., Pacheco A.P., Castro P., García-de-Acilu M., Mesquita R.C., Busch D.R., Durduran T. Peripheral microcirculatory alterations are associated with the severity of acute respiratory distress syndrome in COVID-19 patients admitted to intermediate respiratory and intensive care units. Crit. Care. 2021;25:381. doi: 10.1186/s13054-021-03803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M.P., Penjweini R., Knutson J.R., Wang P.-Y., Hwang P.M. Mitochondria and oxygen homeostasis. FEBS J. 2021;289:6959–6968. doi: 10.1111/febs.16115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nägele M.P., Haubner B., Tanner F.C., Ruschitzka F., Flammer A.J. Endothelial dysfunction in COVID-19: current findings and therapeutic implications. Atherosclerosis. 2020;314:58–62. doi: 10.1016/j.atherosclerosis.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., Shchendrygina A., Escher F., Vasa-Nicotera M., Zeiher A.M., Vehreschild M., Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasica L., Inglis E.C., Iannetta D., Soares R.N., Murias J.M. Fitness level- and sex-related differences in macrovascular and microvascular responses during reactive hyperemia. Med. Sci. Sports Exerc. 2022;54:497–506. doi: 10.1249/MSS.0000000000002806. [DOI] [PubMed] [Google Scholar]
- Rosenberry R., Nelson M.D. Reactive hyperemia: a review of methods, mechanisms, and considerations. Am. J. Phys. Regul. Integr. Comp. Phys. 2020;318:R605–R618. doi: 10.1152/ajpregu.00339.2019. [DOI] [PubMed] [Google Scholar]
- Serviente C., Decker S.T., Layec G. From heart to muscle: pathophysiological mechanisms underlying long-term physical sequelae from SARS-CoV-2 infection. J. Appl. Physiol. (1985) 2022;(132):581–592. doi: 10.1152/japplphysiol.00734.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C., Liu Z., Zhu Y., Lu J., Ge C., Zhang C., Li N., Jin N., Li Y., Tian M., Li X. SARS-CoV-2 causes mitochondrial dysfunction and Mitophagy impairment. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.780768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets E., Garssen B., Bonke B., de Haes J. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- Soares R.N., de Oliveira G.V., Alvares T.S., Murias J.M. The effects of the analysis strategy on the correlation between the NIRS reperfusion measures and the FMD response. Microvasc. Res. 2020;127 doi: 10.1016/j.mvr.2019.103922. [DOI] [PubMed] [Google Scholar]
- van der Sluijs K.M., Bakker E.A., Schuijt T.J., Joseph J., Kavousi M., Geersing G.-J., Rutten F.H., Hartman Y.A.W., Thijssen D.H.J., Eijsvogels T.M.H. Long-term cardiovascular health status and physical functioning of non-hospitalized COVID-19 patients compared to non-COVID-19 controls. Am. J. Physiol. Heart Circ. Physiol. 2022;324:47–56. doi: 10.1152/ajpheart.00335.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Walraven C., Austin P.C., Jennings A., Quan H., Forster A.J. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med. Care. 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- Venkatesan P. NICE guideline on long COVID. Lancet Respir. Med. 2021;9:129. doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available from the corresponding author upon reasonable request.