Abstract
Mpox virus (MPXV) caused a multi-country outbreak in non-endemic areas in 2022. Following historic success of smallpox vaccination with vaccinia virus (VACV)-based vaccines, the third generation modified vaccinia Ankara (MVA)-based vaccine was used as prophylaxis for MPXV, but its effectiveness remains poorly characterized. Here, we applied two assays to quantify neutralizing antibodies (NAbs) in sera from control, MPXV-infected, or MVA-vaccinated individuals. Various levels of MVA NAbs were detected after infection, historic smallpox, or recent MVA vaccination. MPXV was minimally sensitive to neutralization. However, addition of complement enhanced detection of responsive individuals and NAb levels. Anti-MVA and -MPXV NAbs were observed in 94% and 82% of infected individuals, respectively, and 92% and 56% of MVA vaccinees, respectively. NAb titers were higher in individuals born before 1980, highlighting the impact of historic smallpox vaccination on humoral immunity. Altogether, our results indicate that MPXV neutralization is complement dependent and uncover mechanisms underlying vaccine effectiveness.
Keywords: mpox virus, neutralizing antibodies, complement, smallpox vaccination, hybrid immunity
Graphical abstract
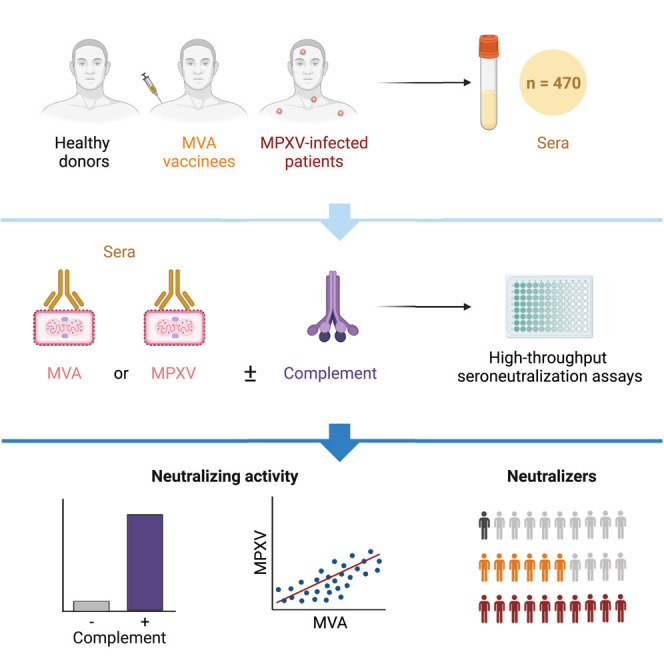
Hubert et al. developed two seroneutralization assays and measured neutralizing antibodies (NAbs) targeting MVA or MPXV in 470 sera from control, MPXV-infected, or MVA-vaccinated individuals. Efficient MPXV neutralization required complement. NAb titers were higher in individuals born before 1980, highlighting the impact of historic smallpox vaccination on humoral immunity.
Introduction
Cessation of massive vaccination in 1980 after smallpox eradication increased the risk of an Orthopoxvirus (OPXV) emergence in humans. Monkeypox virus, now termed mpox virus (MPXV), is a zoonotic virus that circulates in various animal species, mainly rodents, living in African rainforests. MPXV is endemic and in expansion in human populations from Central and West Africa.1 Two clades of MPXV were initially described. Clade 1 (ex-Central Africa clade) is associated to most of African outbreaks and causes about 11% lethality. Clade 2 (ex-West Africa clade) has a lower fatality rate (1%–6%) and is the most exported clade.2 The introduction of MPXV into non-endemic areas was successively reported in 2003 (USA3), 2018 (UK,4 Israel,5 and Singapore6) and 2021 (UK4 and USA7 , 8). In May 2022, a genotypically distinct MPXV (clade 2b) was first detected in Europe and spread all over the world.2 , 9 About 85,000 cases were reported in 110 countries, prompting the World Health Organization to claim a public health emergency of international concern. Identification of the high-risk population, men having sex with men (MSM), bisexuals, and their sexual networks, allowed adapted communication and vaccination campaigns that were associated with the end of the outbreak in late 2022. People living with HIV accounted for 38%–50% of the cases.10 The case fatality rate was low (0.1%), but most of severe and fatal cases occurred in individuals with undiagnosed advanced HIV infection.10 , 11 , 12 About 97% of cases reported during this recent outbreak were young men (median age 34), thus not previously vaccinated against smallpox. A surveillance study conducted on 760 mpox cases between 2005 and 2007 in Democratic Republic of Congo (DRC) showed that 3.8% of the infected individuals had previous smallpox vaccination compared with 26.4% of the overall population.13 Previously vaccinated individuals had a 5.2-fold lower risk of MPXV infection than unvaccinated counterparts. A 20-fold increase in mpox incidence was observed in DRC between 1980s and 2007, that may be linked to decreasing herd immunity.13 However, 21% of infected individuals reported during the 2003 surge in USA were previously vaccinated,14 indicating that childhood vaccination does not fully protect against MPXV. The historic smallpox vaccination was performed with first- and second-generation vaccinia virus (VACV)-based vaccines. A cross-protection of about 80% against mpox disease was reported.13 , 15 The protection slowly wanes over time, leading to pauci-symptomatic infection.14 , 16 , 17 The memory B cell response generated by smallpox vaccine is however long-lasting.18 , 19 Depending on studies, up to 90% of individuals vaccinated 25–75 years ago maintain substantial humoral or cellular immunity against VACV.20 , 21 , 22 Current third-generation vaccines are based on an attenuated modified vaccinia virus Ankara (MVA). The attenuated virus contains 6 major deletions compared with the ancestral VACV, representing a 10% loss of its genome.23 , 24 MVA was initially developed to serve as a safer vaccine during the last years of the World Health Organization (WHO) smallpox eradication campaign. An MVA-based vaccine produced by Bavarian Nordic (MVA-BN, commercialized under the names of IMVANEX or JYNNEOS) was approved by the Food and Drug Administration (FDA) for extended use against MPXV on the basis of challenge experiments in non-human primates (NHPs).25 , 26 , 27 Only few studies investigated MVA-based vaccine effectiveness in humans. In Israel, a short-term study estimated that one MVA dose provided as pre-exposure prophylaxis was associated with 79% reduction in infection rates.28 Another study conducted in USA during the 2022 outbreak monitored more than 5,000 mpox cases and showed a 14-fold increase in infections of unvaccinated individuals compared with JYNNEOS recipients (single dose regimen).29 The epidemiological studies estimating vaccine effectiveness are based on mpox incidence, but the underlying mechanisms remain poorly characterized. In NHPs having received a second generation vaccine, B cell depletion experiments demonstrated that protection against severe mpox disease was mediated by neutralizing antibodies (NAbs).30 Depletion of CD4+ and/or CD8+ T cells did not impact vaccine efficacy. In a late model of infection in which NHPs were infected with MPXV 3 years after vaccination, no correlation was observed between pre-challenge VACV-specific cellular immunity and peak viremia.31 In humans, acute MPXV infection triggers a rapid expansion of effector memory CD4+ and CD8+ T cells and an intensive Th1-biased response with exacerbated levels of inflammatory mediators (IL-1β, IL-6, IL-8, and TNF).32 Whether MVA-based vaccines trigger similar cellular responses remains to be fully understood. To explore the humoral responses in vaccinated or infected individuals, quantification of the levels of circulating antibodies is often assessed by home-made ELISA or seroneutralization assays directed against VACV, for reglementary reasons. Some assays are also performed with a recombinant virus carrying a GFP reporter gene (MVA-GFP) allowing easy monitoring of infection. In a recent study, low levels of MPXV-NAbs were detected after MVA vaccination in healthy individuals,33 , 34 but neutralization assays were performed in the absence of complement. The complement was previously shown to be required for VACV neutralization by monoclonal antibodies and plasma from VACV-infected mice or vaccinated humans.35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 However, its impact on MPXV neutralization by antibodies from infected patients or vaccinated individuals has not been investigated.
Here, we developed two assays for quantification of NAbs against MVA and the new clade of MPXV, in presence or absence of complement. We compared NAbs levels in sera from healthy donors having either received or not received the historical smallpox vaccine, MPXV-infected patients, and MVA-vaccinated individuals. We highlight the role of the complement for efficient MPXV neutralization.
Results
Cohorts design
Uninfected individuals
The interventional cohort clinical study (CORSER-4) was initiated in 2021, with the primary objective to study humoral responses after SARS-CoV-2 infection or vaccination in healthy individuals. A secondary objective was to analyze the basal humoral response against other viruses, including MPXV, for which no known risk of viral exposure was reported in the cohort. We randomly selected 88 individuals in two categories of age. Thirty-four individuals were born after 1980, the cessation of smallpox vaccination, and 54 were born before this year and thus most likely vaccinated against smallpox during their childhood (Table S1).
MPXV-infected individuals
A total of 66 plasma or sera was collected from 48 MPXV-infected patients diagnosed in two hospitals: 57 sera or plasma from 39 patients at Hôpital Pitié-Salpêtrière (Paris) and 9 sera from 9 patients at Hôpital Henri Mondor (Créteil). Most of patients (69%) were sampled at a single time point (3–33 days after onset of the symptoms, DOSs), while 31% were sampled from 2 to 4 times. The main characteristics of the patients are depicted in Table S2. Plasmatic MPXV genome was detectable by PCR from 2 to 25 DOS (median DOS: 5 days) (Figure S1A).
Some patients cleared the MPXV genome from the bloodstream as soon as 3 DOS and PCR-negative plasma samples were collected from 3 to 80 DOS (median DOS: 20 days) (Figure S1A). We observed that 75% and 91% of infected individuals cleared plasmatic viral DNA 3 or 4 weeks after illness onset, respectively (Figure S1B), consistently with previous observations.43 The clinical or molecular diagnosis of MPXV infection was performed from 2 to 15 DOS (Figure S1C). This timeframe may explain MPXV transmission dynamics in populations at risk.
IMVANEX recipients
Sera from IMVANEX vaccinees were collected at the Centre Hospitalier Universitaire d’Orléans and at Hôpital Henri Mondor. The characteristics of the patients are indicated in Table S3. We analyzed 147 sera from 86 individuals that were not infected by MPXV at the time of inclusion. Most of older individuals (born before 1980) received a single dose of IMVANEX except 4 who received a second dose. The 32 younger individuals (born after 1980) were not previously vaccinated and received two doses within 28–42 days of each other.
MVA-HIV recipients
We analyzed 169 sera from 67 participants of the VRI01 clinical trial performed in 2014–2015. Participants received two doses of an MVA-HIV vaccine candidate separated by 8 weeks.44 In total, 66 sera were collected at day 0 to measure pre-existing or basal neutralization levels, 42 sera were collected 2 weeks after the 1st dose and 61 after the 2nd dose (Table S4).
Development of MVA and MPXV neutralization assays
We set up two assays to measure the neutralizing activity of sera or plasma against MVA and MPXV. Samples were heated 30 min at 56°C to inactivate the complement and interferons that may be potentially present.
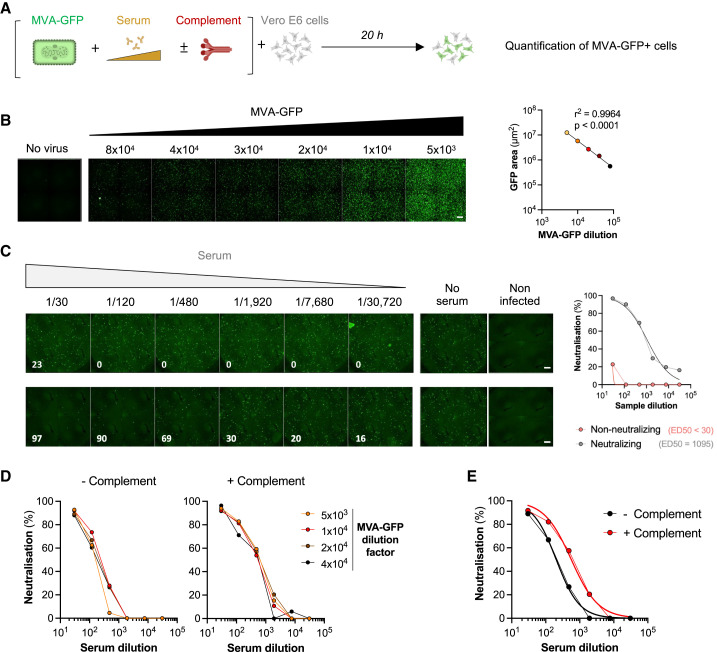
The first assay was based on the use of a MVA virus expressing GFP upon infection (MVA-GFP),45 allowing a rapid quantification of viral infection. We selected Vero E6 cells as target cells because they are naturally sensitive to MVA and widely used in neutralization assays. The principle of the neutralization assay is outlined in Figure 1 A. MVA-GFP particles were exposed for 2 h at 37°C to serial dilutions of the sera, with or without 10% guinea pig serum as a source of complement (GPC).35 , 42 , 46 The mixture was then added onto Vero E6 cells. After 20 h, the area of GFP+ cells was scored with an automated microscope. We first determined the optimal viral input by exposing Vero E6 cells to increasing doses of MVA-GFP. The number of GFP+ cells correlated with the viral inoculum (Figure 1B). We show two examples of a non-neutralizing serum and a neutralizing serum collected from one MPXV-infected patient at days 2 and 56 DOS, respectively (Figure 1C). We then calculated the effective dose of serum required to inhibit 50% of infection (ED50) and demonstrated an increase in ED50 overtime (Figure 1C). Sera from uninfected individuals did not neutralize MVA (see below). Neutralization across the different viral inocula was similar, indicating that variations in the number of infected cells, at least in this selected range of infection, did not impact the calculation of ED50 (Figure 1D). Neutralization was about 2.5-fold more efficient in the presence of complement, independently of the viral inoculum (Figure 1E). Heat-inactivated guinea pig sera did not enhance neutralization titers of the tested sera (not shown).
Figure 1.
The MVA-GFP seroneutralization assay
(A) A Modified vaccinia virus Ankara expressing GFP (MVA-GFP) was mixed to serial dilutions of sera to be tested in the absence or the presence of 10% guinea pig serum as a source of complement. After 30 min to 2 h, the mixture was added onto Vero E6 target cells. After 20 h, GFP+ infected cells were quantified by high-content confocal microscopy and the percentage of neutralization was calculated.
(B) Vero E6 cells were exposed to serially diluted MVA-GFP. After 20 h, the GFP+ area was quantified. Left: representative images of MVA-GFP-infected cells. The numbers indicate the dilution factor of the viral inoculum. Scale bars: 500 μm. Right: standard curve of the GFP signal.
(C) Examples of MVA-GFP neutralization by 4-fold serial dilutions of two plasma from the same MPXV-infected patient at 2 and 56 days after the onset of symptoms. Left: examples of micrographs. The numbers indicate the percentage of neutralization by considering the no serum and non-infected conditions as 0% and 100% neutralization, respectively. Scale bars: 500 μm. Right: activity of the non-neutralizing (red) and neutralizing plasma (gray). The median effective dose (ED50) represents the plasma dilution reducing infection by 50%.
(D) Neutralization of a serum from an IMVANEX vaccinee tested with several MVA-GFP inocula, in the absence (left) or the presence (right panel) of complement. Representative graphs are shown.
(E) Example of neutralization of MVA-GFP (dilution factor: 2 × 104) by a serum from an IMVANEX vaccinee.
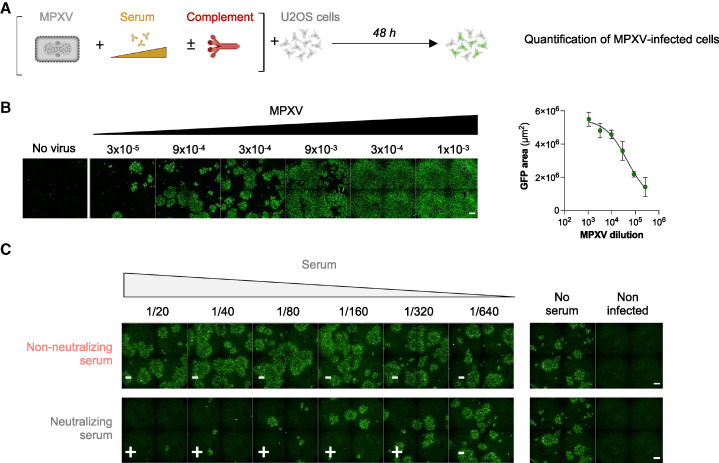
The second assay used an authentic clinical MPXV strain isolated on Vero E6 cells from a typical lesion of a French patient infected in June 2022 (GenBank accession number OQ249661).47 The virus was cytopathic and formed plaques in Vero E6 cells, with titers reaching 5×107 plaque-forming units (PFUs)/mL. For the neutralization assay, we tested different cell lines and observed that the U2OS human osteosarcoma-derived cell line was particularly sensitive to MPXV infection. The flat shape of U2OS cells facilitated automated imaging.48 Clusters of infected cells, revealed after immunostaining with a rabbit polyclonal anti-VACV antibody, were readily detectable 2 days after infection. The MPXV neutralization assay is thus roughly similar to the MVA assay, except that target cells and incubation times are different, as outlined in Figure 2 A. In the absence of serum, the number of infected cells correlated with the viral inoculum (Figure 2B). We selected a non-saturating inoculum for neutralizing experiments. Infected cells formed foci of different size, reflecting the known direct cell-cell transmission of poxvirus.49 Contrary to the abortive single-round MVA infection in mammalian cells, MPXV infection leads to continuous production of infectious particles over time, introducing a variability in the number of infected cells and size of the foci. The automated scoring of infection based on the surface of positive cells was thus unsuitable to accurately calculate a neutralization activity. We thus defined a binary MPXV neutralization index for each serum dilution, by considering the “no serum” infection signal as negative (non-neutralizing), whereas wells in which the infection signal was reduced were considered as positive (neutralizing). The neutralization titer was thus calculated as the highest sample dilution in which neutralization was detected. Examples of non-neutralizing and neutralizing sera are presented in Figure 2C. With both MVA and MPXV assays, we arbitrarily defined a positive threshold (limit of detection [LOD]) for samples displaying a neutralizing activity at 1/40 dilution, 2-fold above the first dilution of sera tested. We selected this cutoff to avoid unspecific neutralization results that may be caused by high concentrations of human sera. The same threshold was used for experiments performed with or without complement. The proportion of neutralizers was then evaluated by calculating the percentage of individuals exhibiting a neutralizing activity > LOD.
Figure 2.
The MPXV seroneutralization assay
(A) Mpox virus (MPXV) was mixed to serial dilutions of sera to be tested, in the absence or the presence of 10% guinea pig serum as a source of complement. After 30 min to 2 h, the mixture was added to U2OS target cells. After 48 h, the cells were fixed. MPXV infection was monitored by immunofluorescence using a rabbit polyclonal anti-VACV antibody. The percentage of neutralization was calculated.
(B) U2OS cells were exposed to serially diluted MPXV. After 48 h, cells were fixed and stained. Left: representative images of MPXV-infected cells. The numbers indicate the dilution factor of the viral inoculum. Scale bars: 500 μm. Right: standard curve of the signal.
(C) Examples of MPXV neutralization by 2-fold serial dilutions of a non-neutralizing serum (uninfected individual) and a neutralizing serum (MPXV-infected patient). The white symbols show the presence (+) or absence (−) of neutralization. Scale bars: 500 μm. The neutralizing titer was calculated by selecting the highest dilution in which neutralization was observed.
We used the two assays to measure the neutralizing activity of 470 sera from healthy controls and from different categories of MPXV-infected or MVA-vaccinated individuals. Neutralization titers were calculated with or without complement supplementation.
Basal neutralizing activity of samples from uninfected individuals
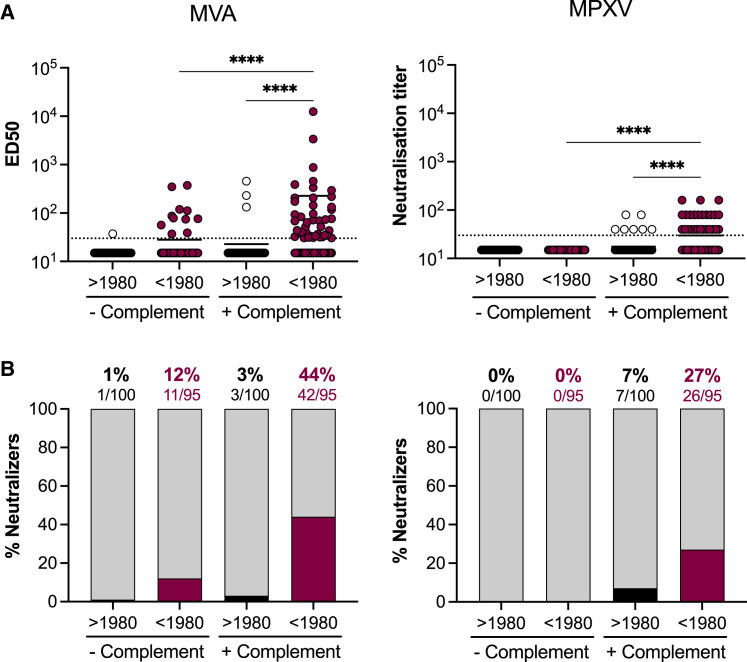
We first assessed the basal neutralization levels of sera from uninfected donors (Figure 3 ). We took advantage of the different cohorts to analyze a total of 195 sera. We combined 88 sera from the CORSER-4 cohort of uninfected donors, 41 sera of IMVANEX vaccinees collected before vaccination, and 66 sera of MVA-HIV vaccinated individuals collected before administration of the candidate vaccine. We separated the donors by age: 95 donors born before 1980 were likely vaccinated in their childhood and were designated “older” participants, whereas 100 donors born after 1980, or “younger” participants, were considered unvaccinated. Figure 3A displays the neutralization titers for each individual, and Figure 3B the percentage of individuals with a detectable neutralizing activity. In the absence of complement, we did not detect anti-MVA NAbs in 99% (99/100) of younger donors. Low levels of NAbs (mean ED50: 127) were measured in 12% (11/95) of older donors, suggesting a residual humoral response to historical smallpox vaccination. Addition of 10% GPC enhanced neutralization titers to a mean ED50 of 273 in 3% (3/100) and a mean of 494 in 44% (42/96) of younger and older individuals, respectively. Thus, the percentage of both younger and older individuals was approximatively 3-fold higher in the presence of complement. With MPXV, we did not detect any neutralization with these sera in the absence of complement. Addition of complement enhanced neutralization titers to a mean of 51 in 7% (7/100) of younger participants and a mean of 70 in 27% (26/95) of older participants. In 5/7 younger individuals that neutralized MPXV, titers remained low (equal to 40) and may thus represent a non-specific neutralization.
Figure 3.
Basal levels of neutralizing antibodies in uninfected individuals
(A) Seroneutralization of MVA-GFP (left) and MPXV (right) by sera from uninfected individuals in the absence and the presence of 10% guinea pig serum as a source of complement. Sera from individuals born before and after 1980 were analyzed. See also Table S1. The dotted lines represent the limit of detection (LOD). Each dot represents an individual and data are mean of 2–6 independent experiments. Bars indicate mean values. Statistical analysis: Kruskal-Wallis tests with Dunn’s multiple comparisons correction (∗∗∗∗p < 0.0001).
(B) The proportion of neutralizers was estimated as the percentage of individuals exhibiting a neutralizing activity > LOD.
Altogether, these results indicate that uninfected and unvaccinated individuals born after 1980 did neither neutralize MVA nor MPXV in absence of complement. Based on the results obtained on younger individuals in the presence of complement, the specificity of the MVA and MPXV assays was estimated to be 97% and 93%, respectively. The complement enhanced the sensitivity of the two assays.
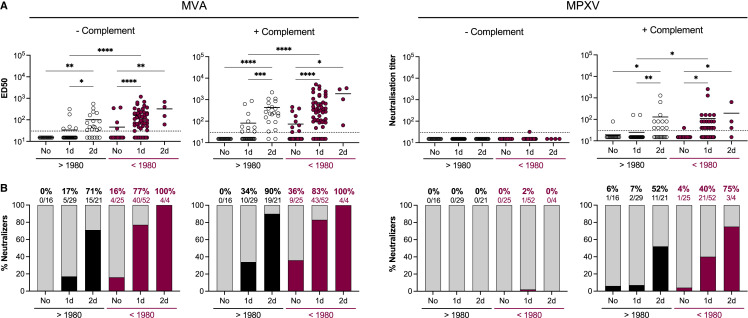
Neutralizing antibodies induced by MPXV infection
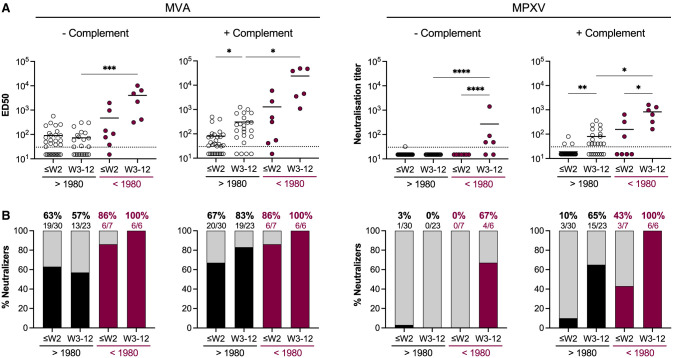
We then measured the neutralizing activity of sera from MPXV-infected individuals collected at different time points after onset of symptoms (Figure 4 ). We classified the individuals in the two same categories of age to analyze the influence of potential smallpox childhood vaccination on MPXV infection-elicited antibody titers. With the MVA assay performed without complement, we detected anti-MVA NAbs in approximatively 60% of patients regardless of the time after the onset of symptoms (mean ED50: 134). Higher levels of NAbs (mean ED50: 1,522) were detected in older individuals, suggesting a potential boost effect of MPXV infection on memory B cell populations previously generated by smallpox vaccination. Anti-MVA neutralizing titers increased over time. In the presence of complement, up to 83% of the younger individuals and 100% of the older individuals displayed NAbs 2–12 weeks after the onset of symptoms, with higher neutralization titers in older (mean ED50: 14,486) than in younger (mean ED50: 302) patients. With the MPXV assay, we did not detect NAbs in the absence of complement in the younger individuals. In the older individuals, 67% (4/6) displayed NAbs, 2 weeks after onset of symptoms. Addition of complement increased anti-MPXV activity of the sera, with respectively 65% and 100% of the younger and older individuals harboring NAbs 2–12 weeks after onset of symptoms (mean neutralization titers: 71 in younger and 600 in older individuals). With both MVA and MPXV assays, NAb titers were significantly higher in older individuals. We then analyzed the evolution of NAb titers over time regardless of the age of the patients, by mixing the results obtained with the two age categories (Figures S2A and S2B). Both MVA- and MPXV-neutralizing titers increased over time. The sensitivity of the MVA and MPXV complement-based assays, assuming that all infected individuals have developed a neutralizing response when assessed ≥4 weeks after onset of symptoms, was estimated to be 94% and 82%, respectively. Alternatively, these numbers may represent the true rates of positivity at near 100% sensitivity. NAbs were detected in a majority (61%) of infected individuals as soon as 1 week after onset of symptoms.
Figure 4.
Neutralizing antibodies induced by MPXV infection
(A) Seroneutralization of MVA-GFP (left) and MPXV (right) by sera from MPXV-infected patients in the absence and the presence of 10% guinea pig serum as a source of complement. Sera from individuals born before and after 1980 were analyzed. The weeks of sample collection are shown (≤W2: samples collected during the two first weeks after onset of symptom; W3–12: samples collected between the 3rd and 12th weeks). See also Table S2. The dotted lines represent the limit of detection (LOD). Each dot represents an individual and data are mean of two to six independent experiments. Bars indicate mean values. Statistical analysis: Kruskal-Wallis tests with Dunn’s multiple comparisons correction (∗p < 0.03; ∗∗p < 0.002; ∗∗∗p < 0.0002; ∗∗∗∗p < 0.0001).
(B) The proportion of neutralizers was estimated as the percentage of individuals exhibiting a neutralizing activity > LOD.
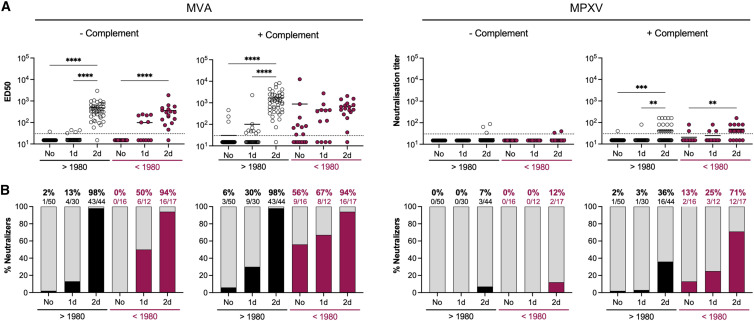
Neutralizing antibodies elicited by IMVANEX vaccination
We next examined the ability of IMVANEX vaccination to elicit MVA- NAbs (Figure 5 ). The recent IMVANEX vaccination scheme in France consisted of two doses with an interval of 28–35 days in individuals born after 1980, or one single dose in individuals having received the historic smallpox vaccine. However, 4 older individuals (born before 1980) received two doses of IMVANEX and were also included in the analysis. With the MVA assay, we observed a progressive increase of anti-MVA NAbs after one and two vaccine doses. After 1 dose, the proportion of neutralizers was 17% and 77% among younger and older participants, respectively. After 2 doses, the proportion reached 71% and 100% of the younger and older individuals, respectively, highlighting the importance of the second dose. A single dose of MVA vaccine elicited similar levels of NAbs and a similar proportion of neutralizers in vaccinees that received the historic smallpox vaccine (mean ED50 of 209 in 77% of neutralizers) when compared with a two-dose regimen in the naive individuals (mean ED50 of 136 in 71% of neutralizers). Addition of complement largely increased NAb titers, with 90% of neutralizers detected among the young individuals (mean ED50: 470) and 100% among the older individuals (mean ED50: 1,890) 2–8 weeks after full vaccination. After administration of one vaccine dose, NAb titers were significantly higher in older than in younger individuals. In younger individuals, anti-MVA neutralizing titers were 1.5-fold higher in vaccine recipients than in MPXV-infected patients. The same sera were tested in the MPXV-based assay. In the presence of complement, 52% of younger individuals having received two doses of IMVANEX, and 40% of older individuals having received one dose of IMVANEX displayed detectable and similar levels of anti-MPXV NAbs (mean neutralization titers: 233 and 253, respectively). Anti-MPXV-neutralizing titers were 3-fold higher in vaccinated individuals than in MPXV-infected patients.
Figure 5.
Neutralizing antibodies induced by IMVANEX vaccination
(A) Seroneutralization of MVA-GFP (left) and MPXV (right) by sera from IMVANEX vaccinees in the absence and the presence of 10% guinea pig serum as a source of complement. Sera from individuals born before and after 1980 were analyzed. The vaccination status at the time of sample collection is indicated (no: samples collected before vaccination; 1d: samples collected after the 1st dose; 2d: samples collected after the 2nd dose). See also Table S3. The dotted lines represent the limit of detection (LOD). Each dot represents an individual, and data are mean of two to six independent experiments. Bars indicate mean values. Statistical analysis: Kruskal-Wallis tests with Dunn’s multiple comparisons correction (∗p < 0.03; ∗∗p < 0.002; ∗∗∗p < 0.0002; ∗∗∗∗p < 0.0001).
(B) The proportion of neutralizers was estimated as the percentage of individuals exhibiting a neutralizing activity > LOD.
These results indicate that IMVANEX elicited NAbs in most of vaccine recipients, with higher titers detected in older individuals. When combining the results obtained with the younger and older individuals in the two assays, we detected a proportion of 92% and 56% of vaccine recipients displaying anti-MVA and -MPXV NAbs, respectively. The sera neutralized more efficiently MVA than MPXV, reflecting the higher sensitivity of the MVA assay and/or a better anti-MVA immune response generated with an MVA-based vaccine.
Neutralizing antibodies elicited by an experimental MVA-HIV vaccine
To further assess MVA immunogenicity, we measured NAb levels in sera from participants of a clinical trial (Figure 6 ) that aimed at characterizing a candidate MVA vaccine carrying HIV antigens (MVA-HIV).44 Participants received two doses of the MVA-HIV vaccine candidate separated by 8 weeks. Sera were longitudinally collected before the first injection and 2 weeks after the first and second doses. In the two age categories, levels of anti-MVA NAbs increased after each injection and 94%–98% of neutralizers were detected after 2 doses, regardless of the presence of complement. The proportion of neutralizers among MVA-HIV recipients was roughly similar to that of IMVANEX. In the younger individuals, anti-MVA neutralizing titers were about 3.6-fold higher 2 weeks after the second dose of MVA-HIV than 2–8 weeks after the second dose of IMVANEX. In the older individuals, the titers were similar after one dose of MVA-HIV or IMVANEX. When analyzed in the MPXV-based assay, NAb levels and the proportion of neutralizers were lower than those measured in the MVA-based assay. After two doses, 36% of younger and 71% of older individuals mildly neutralized MPXV (neutralization titers of 88 and 63, respectively).
Figure 6.
Neutralizing antibodies induced by an experimental MVA-HIV vaccine
(A) Seroneutralization of MVA-GFP (left) and MPXV (right) by sera from IMVANEX vaccinees in the absence and the presence of 10% guinea pig serum as a source of complement. Sera from individuals born before and after 1980 were analyzed. The vaccination status at the time of sample collection is indicated. (No: samples collected before vaccination; 1d: samples collected after the 1st dose; 2d: samples collected after the 2nd dose.) See also Table S4. The dotted lines represent the limit of detection (LOD). Each dot represents an individual and data are mean of two to six independent experiments. Bars indicate mean values. Statistical analysis: Kruskal-Wallis tests with Dunn’s multiple comparisons correction (∗p < 0.03; ∗∗p < 0.002; ∗∗∗p < 0.0002; ∗∗∗∗p < 0.0001).
(B) The proportion of neutralizers was estimated as the percentage of individuals exhibiting a neutralizing activity > LOD.
Therefore, despite potential differences in the MVA strain, preparation methods and injected doses, both IMVANEX and an experimental MVA-HIV vaccine triggered a similar and strong humoral response, mostly detectable in the presence of complement. NAbs titers were lower against MPXV than MVA.
Correlation between MVA- and MPXV-based neutralization assays
We examined the links that may exist between anti-MVA and anti-MPXV NAb titers (Figure S2C). There was a significant correlation between the two assays in infected individuals. The correlation was less marked but also significant in vaccine recipients. These data highlight the interest to use the more sensitive MVA-GFP system to monitor levels of poxvirus-specific antibodies.
Discussion
The efficacy of currently available third generation MVA-based vaccines against mpox and the nature of the humoral response generated after MPXV infection remain poorly characterized. We established two cell-based assays to measure the levels of NAbs targeting MVA or MPXV in previously smallpox-vaccinated individuals, MPXV-infected patients, and IMVANEX or MVA-HIV vaccine recipients. We show that about 44% and 27% of French individuals born before 1980 carry detectable anti-MVA and anti-MPXV NAbs, respectively, confirming the existence of long-term cross-NAbs elicited by childhood vaccination. Whether this residual neutralization protects against mpox disease could be assessed by comparing the clinical features of younger and older infected patients, if their historical vaccination status is known. Anti-MVA NAbs were detected in 94% of MPXV-infected patients up to 12 weeks after onset of illness (83% and 100% of younger and older patients, respectively), 92% of IMVANEX recipients (90% and 100% of younger and older individuals, respectively), and 97% of MVA-HIV recipients (98% and 94% of younger and older individuals, respectively) when assays were performed in the presence of complement. Addition of complement also enhanced anti-MPXV NAbs levels and the proportion of NAb-positive individuals, which reached 82% of MPXV-infected patients (65% and 100% of younger and older patients, respectively), 56% of IMVANEX recipients (52% and 75% of younger and older patients, respectively), and 46% of MVA-HIV recipients (36% and 71% of younger and older individuals, respectively). The differences in neutralization titers observed in younger and older individuals likely reflect the impact of childhood smallpox vaccination. Anti-MVA and MPXV NAb titers reached higher levels in older than in younger individuals, both after MPXV infection or first and second IMVANEX injection, strongly suggesting a reactivation of a long-term B cell memory response.21 It is also possible that a hybrid immunity,50 , 51 generated by a combination of vaccination and infection, triggers an efficient humoral response. The highest anti-MVA and anti-MPXV NAb titers were measured in previously smallpox-vaccinated MPXV-infected patients, highlighting this potential optimal cross-protection mediated by hybrid immunity. The relatively low antibody levels with poor neutralizing capacities recently reported in Dutch cohorts of infected or vaccinated individuals might be due to the absence of use of complement in neutralizing assays.33 Our work highlights the potential role of complement in improving the sensitivity of the assays and in more accurately reflecting the anti-poxvirus humoral response in infected or vaccinated individuals.
The mechanisms underlying anti-MPXV activity of the complement remain to be elucidated. The complement enhances VACV neutralization by monoclonal antibodies or sera from VACV-infected mice or vaccinated individuals.35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 Two infectious forms of viral particles are generated during the life cycle of poxviruses. Mature virions (MVs) consist of a viral core containing the dsDNA genome, two lateral bodies and one lipid bilayer (envelope). MV virions accumulate in the interior of infected cells and are released upon cell lysis. Enveloped virions (EVs) are wrapped MV surrounded by a second membrane and are secreted in the extracellular medium by exocytosis.52 Due to their different morphogenesis, MV and EV express distinct sets of proteins at their surface. Twenty viral proteins decorate the surface of MV, including the entry-fusion complex composed of 11 viral proteins (A21, A28, G3, H2, O3, A16, F9, G9, J5, L1, and L5),53 whereas EV contain 6 viral proteins (including A33, A34, and B5)54 anchored in the outer membrane. The fusogenic properties of MV make this form responsible for viral entry into target cells and more sensitive to NAbs than EV. Immunodominant viral proteins are found in either MV (H3, A27, D8, and L1) and EV (A33 and B5).46 , 55 , 56 , 57 , 58 , 59 , 60 A recent study demonstrated that MPXV infection elicited strong antibody and B cell responses against A35 and H3 antigens,61 which exhibit 95% and 93% sequence similarity with A33 and H3 VACV homologs, respectively. Although neutralization of MV is easy to achieve, neutralization of EV often requires complement. As described for instance for retroviruses,62 the mechanisms of complement-mediated enhancement of neutralization vary between viral proteins. Anti-A33 antibodies induce complement-dependent virolysis of EV and subsequent release of MV.41 Anti-B5 antibodies successively bind C1q and C3 in an isotype-dependent manner, leading to virion opsonization and sterically preventing EV attachment to target cells.35 , 42 Binding of anti-B5 antibodies to VACV-infected cells leads to complement-dependent cytotoxicity (CDC).35 , 42 In a mouse model, an optimal protection against VACV challenge was mediated by a mixture of monoclonal antibodies that target 4 viral antigens.46 Antibodies targeting A33, L1, A27, and H3 proteins exhibited the broadest cross-protection against various OPXV including VACV, cowpox virus (CPXV), and MPXV.46 Whether these antibodies act by neutralizing viral particles or by mediating other effector functions remains to be explored. The complement can also impair viral infectivity by itself, either by binding to the virion surface and sterically preventing its attachment to the receptor63 , 64 , 65 but also by inducing the formation of the membrane attack complex (MAC) onto viral particles,66 , 67 resulting in their destruction. Future studies are warranted to characterize the role of the complement pathway and its potential additive or synergic effects on MPXV neutralization by polyclonal and monoclonal antibodies.
The titers of NAbs and the proportion of reactive individuals were globally higher with the MVA assay than with the MPXV assay. The greater sensitivity of the MVA assay is probably in part due to technical differences with the MPXV assay. MVA is a single-cycle virus in most mammalian cells, including the Vero E6 cells used here, and is thus likely more sensitive to neutralization than the fully replicative MPXV isolate. A direct cell-to-cell viral transfer,49 visualized by clusters of MPXV-infected cells with heterogeneous sizes in our cultures, may also introduce a variability in the readout and may decrease NAb efficiency. It would have been of interest to measure the overall levels of anti-poxvirus antibodies in the different samples analyzed here. The lack of a validated commercially available ELISA assay at the initiation of this study, and the limited volumes of the sera precluded this analysis. It will be worth comparing our assays with ELISA tests or other serological assays in various groups of vaccinated or infected individuals.
The different sensitivities of the MVA and MPXV assays may also reflect antigenic diversity among the OPXV genus. Anti-MPXV NAb titers were about 2-fold higher in MPXV-infected individuals than in MVA-based vaccine recipients, suggesting that the repertoire of cross-reactive antibodies generated upon MPXV infection may be different than after vaccination. However, anti-MVA NAb titers were of similar level in infected and vaccinated individuals. The MVA-based assay thus represents a sensitive and convenient tool to assess the anti-pox neutralizing response in immunized persons.
MVA-based vaccination (IMVANEX or JYNNEOS) is currently recommended as pre-exposure prophylaxis in high-risk populations or as post-exposure prophylaxis, even if breakthrough infections were observed after post-exposure vaccination.68 Mpox incidence was between 7.4- and 9.6-times lower in individuals having received on one or two doses of JYNEOS, respectively,69 suggesting an impact of vaccination on mpox outbreak decline. The clinical forms of mpox were also milder in recently vaccinated than in unvaccinated patients.70 Associating NAbs levels to clinical vaccine efficacy will help defining correlates of protection against MPXV infection or disease severity. MVA is also currently used as a candidate vaccine platform against several viruses, bacteria, and parasites.23 The utilization of data from clinical trials with these candidates is of interest to define optimal vaccinal schemes to induce cross-neutralizing MPXV antibodies. For instance, we show that in the VRI01 trial, participants that received 2 doses of an MVA-based HIV vaccine candidate (MVA-HIV) elicited somewhat higher levels of anti-MVA NAbs than after IMVANEX administration. The differences might be due to various vaccine formulation, preparation, or administrated doses. An anti-OPXV cross-neutralizing response was also reported with other MVA-based vaccine candidates.33 , 71
Our study has several limitations. We did not have access to the historical smallpox vaccination status of the older individuals. However, the vaccination was obligatory in France until 1979, and it is likely that most of the participants were vaccinated. We did not measure the overall levels of anti-OPXV antibodies in the samples but focused on their neutralizing activity. We did not analyze the correlations that may exist between disease severity, HIV status, and NAb levels, even if a preliminary survey did not reveal major differences between individuals, apart from the age effect reported here. It will be also of interest analyzing the long-term duration of the humoral response in the different categories of vaccinated or infected individuals.
In summary, we have analyzed the neutralizing humoral response elicited by MVA vaccination or MPXV infection. The sensitive neutralization assays that we implemented may help defining correlates of protection against infection or disease severity. The assays can be used for epidemiological surveys, assessment of the duration of protection conferred by previous infection or by authorized and candidate vaccines, and for analysis of any immunotherapeutic intervention.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-VACV antibody | Invitrogen | Cat#PA1-7258; RRID:AB_561894 |
| Alexa Fluor 488-coupled goat anti-rabbit antibody | Invitrogen | A11008 ; RRID:AB_143165 |
| Bacterial and virus strains | ||
| MVA-GFP | ANRS-MIE | Sagoo et al.45 |
| MPXV/2022/FR/CMIP | Cellule d’Intervention Biologique d’Urgence of Institut Pasteur, Paris, France | GISAID: EPI_ISL_16260402 Balière et al.47 Batéjat et al.72 |
| Biological samples | ||
| Human sera samples from healthy donors | Medical Center of Institut Pasteur, Paris, France | NCT04325646 |
| Human plasma samples from MPXV-infected patients | Pitié-Salpêtrière hospital, Paris, France | Palich et al.43 Nouchi et al.73 |
| Human sera samples from MPXV-infected patients | Henri-Mondor hospital, Créteil, France | CPP Ile-de-France VII and IX n°10–023 |
| Human sera samples from IMVANEX vaccinees | Henri-Mondor hospital, Créteil, France | IDRCB: 2018-A01610-55 |
| Human sera samples from IMVANEX vaccinees | CHU Orléans, France | NCT05315583 |
| Human sera samples from MVA-HIV recipients | Vaccine Research Institute, Créteil, France |
NCT02038842 Richert et al.44 |
| Chemicals, peptides, and recombinant proteins | ||
| Hoechst 33342 | Invitrogen | Cat#H3570 |
| Paraformaldehyde | Electron Microscopy Sciences | Cat#15714 |
| Deposited data | ||
| MPXV/2022/FR/CMIP | GISAID EpiPox database | GenBank:Q249661 Balière et al.47 Batéjat et al.72 |
| Experimental models: Cell lines | ||
| Vero E6 | ATCC | Cat#CRL-1586; RRID:CVCL_0574 |
| U2OS | ATCC | Cat#HTB-96; RRID:CVCL_0042 |
| Software and algorithms | ||
| Harmony High-Content Imaging and Analysis Software | PerkinElmer | Cat#HH17000012 |
| Excel 365 | Microsoft | https://www.microsoft/com/en-ca/microsoft-365/excel |
| Prism 9 | Graphpad | https://www.graphpad.com/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Pr. Olivier Schwartz (olivier.schwartz@pasteur.fr).
Materials availability
All unique/stable reagents generated in this study are available from the lead contact with a completed Materials Transfer Agreement (MTA).
Experimental model and subject details
Cohort of uninfected donors
A prospective, monocentric, longitudinal, interventional cohort clinical study (CORSER-4) is conducted since March 2020, with the objective to study the humoral responses after SARS-CoV-2 infection or vaccination in healthy individuals. This study was approved by the ethical committee “Comité de Protection des Personnes Ile de France III, on February 19, 2020. The cohort is registered with ClinicalTrials.gov (NCT04325646). At enrollment, written informed consent was collected from each individual, authorizing the analysis of seroneutralization against different viral species. We randomly selected 88 sera from 22- to 69-years old participants (Table S1). Smallpox vaccination was interrupted in 1980, the date of official smallpox eradication. Thus, we used 1980 as a birth year cut-off to distinguish “younger” and “older” healthy donors: 39% (34/88) were born after 1980 and were not vaccinated against smallpox (“younger”), whereas 61% (54/88) were born before 1980 (“older”) and probably received a historic smallpox vaccine.
Cohorts of MPXV-infected patients
In May 2022, a clinical study enrolling 70 individuals infected with MPXV was initiated at Pitié-Salpêtrière hospital (Paris, France).43 , 73 Persons with PCR-confirmed mpox were invited to contribute to the case series by their health care provider. Written informed consent was obtained in accordance with local standards and maintained in the participants’ clinical file.43 , 73 We obtained 57 sera or plasma collected from 39 patients (Table S2). All patients were male. Eighteen (7/39) and 82% (32/39) were born before or after 1980, respectively. Samples were collected from 2 to 80 days after onset of symptoms, with a majority collected during the first two weeks of symptoms. 58% (33/57) were positive for MPXV by PCR and were collected from 2 to 25 days after onset of symptoms (median DOS: 5 days). Most of these samples were collected during the 1st and 2nd weeks of illness. 42% (24/57) were PCR-negative and were collected at a median DOS of 18 days. Three samples were collected later, at 47, 56 and 80 days post-symptoms.
In July 2022, a prospective, monocentric cohort clinical study enrolling 9 individuals infected with MPXV was initiated at Henri-Mondor hospital (Créteil, France). The study protocol was approved by the regional investigational review board (CPP Ile-de-France VII and IX) with approval reference 10–023. At enrollment, written informed consent was collected from each patient. For the present study, we obtained 9 sera from 9 patients (Table S2). All patients were male, 22% (2/9) and 78% (7/9) were born before and after 1980, respectively. Samples were collected from 18 to 33 days after the onset of symptoms and were negative for MPXV by PCR. Most of the samples (8/9) were collected at least 4 weeks after onset of symptoms.
Cohorts of IMVANEX vaccinees
In April 2022, a prospective, monocentric, longitudinal, interventional cohort study enrolling up to 400 individuals was launched at Orléans hospital (France) to explore humoral responses induced by either natural infection, vaccines, or therapeutic monoclonal antibodies. This cohort, firstly dedicated to COVID-19, was extended to various infectious diseases, and included persons vaccinated with IMVANEX. This study was approved by the “Comité de Protection des Personnes Est II” ethical committee under the reference 2022-A00177-36 (NCT05315583). At enrollment, written informed consent was collected from each patient. Forty-two patients were included in this study: 40% (17/42) were born after 1980 and were not previously vaccinated against smallpox. They received two doses of IMVANEX vaccine with ≈30 days between each dose. Longitudinal samples were collected before vaccination, about 30 days after the 1st dose and 35 days after the 2nd dose (Table S3). Twenty nine percent (5/17) were treated for HIV and 59 % (10/17) were under antiretroviral pre-exposure prophylaxis (PrEP). Sixty percent (25/42) of the patients were born after 1980 and probably vaccinated against smallpox during childhood. The majority (22/25) received a single dose of IMVANEX whereas 12% (3/25) received a second dose. Sera were collected 34 days and 23 days after first and second doses, respectively. Sixty percent (15/25) were treated for HIV and 40 % (10/25) were under anti-retroviral PrEP.
In July 2022, a prospective, monocentric, longitudinal cohort clinical study enrolling 43 individuals vaccinated with a third generation MVA-based vaccine (IMVANEX) was initiated at Henri-Mondor hospital (Créteil, France). This study was approved by the appropriate ethical committee under the reference IDRCB: 2018-A01610-55. At enrollment, written informed consent was collected from each patient. For the present study, 43 patients were included: 35% (15/43) were born after 1980 and not previously vaccinated against smallpox. They received two doses of IMVANEX within 28 days of interval. Sera were collected 28 days after the 1st dose and 15 days after the 2nd dose (Table S3). Twenty-seven percent (4/15) were treated for HIV and 47% (7/15) were under pre-exposure prophylaxis (PrEP). Sixty five percent of the patients (28/43) were born before 1980 and probably vaccinated against smallpox during childhood. All received a single dose of IMVANEX and 3% (1/29) received two doses. Sera were collected 15 days after each dose. Among the older patients, 36% (10/28) were treated for HIV and 32% (9/28) were under pre-exposure prophylaxis (PrEP).
Cohort of MVA-HIV recipients
In 2014-2015, a clinical trial conducted by the Vaccine Research Institute (VRI, Créteil, France) was performed in 92 individuals to evaluate the effectiveness of MVA-HIV as a vaccine candidate. The protocol was approved by an ethics committee (Comité de Protection des Personnes Ile-de-France V, Paris, France) and the competent French health authority (Agence Nationale de Sécurité du Médicament et des Produits de Santé) and was conducted in accordance with the declaration of Helsinki. All volunteers provided written and signed informed consent for the trial. The trial was registered with ClinicalTrials.gov (NCT02038842) and EudraCT (2012-002456-17).44 At enrollment, written informed consent was collected and each participant was exposed to two doses of MVA-HIV within 8 weeks of interval. Sera were collected before the initiation of the trial, 2 weeks after the 1st dose, and 2 weeks after the 2nd dose. We randomly selected 66 participants. Seventy-six percent (50/66) were born after 1980 and 24% (16/66) were older and probably vaccinated against smallpox during their childhood. A total of 169 sera was collected: 39% (66/169) before initiation of vaccination, 25% (42/169) after the first dose and 36% (61/169) after the second dose (Table S4).
Cells and viruses
Vero E6 and U2OS cells74 were grown in Dulbecco’s Modified Eagle Medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco). All cells were maintained at 37°C and 5% CO2 for culture. Cells were routinely tested negative for mycoplasma. A Modified Vaccinia virus Ankara carrying a GFP reporter gene (MVA-GFP)45 was provided by ANRS-MIE.
A MPXV strain (MPXV/2022/FR/CMIP) was isolated from a pustular lesion of a 36-year-old French man who consulted at the Medical Center of Institut Pasteur (CMIP), in June 2022. The clinical specimen was inoculated on Vero E6 cells, whose supernatant was harvested after 3 days and tested positive for the presence of MPXV by PCR.47 , 72
Biosafety
All experiments with infectious MPXV were conducted under strict BSL3 conditions. Manipulations involving inactivated or non-inactivated MPXV were performed according to the French regulations on dual use pathogens.
Method details
MVA neutralization assay
Vero E6 cells were plated at 2 × 104 cells per well in a μClear 96-well plate (Greiner Bio-One). Indicated concentrations of MVA-GFP were mixed (ratio 1:1) with serial dilutions (from 1/30 to 1/30,000) of previously heat-inactivated (30 min at 56°C) plasma or serum in the presence or absence of 10% guinea pig serum as a source of complement (GPC; Rockland). After incubation for 2 h at 37°C, the mixture was added onto Vero E6 cell monolayers. Twenty hours later, cells were fixed for 30 min at room temperature (RT) with 4% paraformaldehyde (PFA, Electron Microscopy Sciences), washed and stained with Hoechst (1:1,000 dilution; Invitrogen). Images were acquired with an Opera Phenix high-content confocal microscope (Perkin Elmer). The GFP area and the number of nuclei were quantified using the Harmony software (Perkin Elmer). The percentage of neutralization was calculated using the GFP area as the value with the following formula: 100 × (1 − (value with serum − value in “noninfected”)/(value in “no serum” − value in “noninfected”)). Neutralizing activity of each plasma or serum was expressed as the ED50 (effective dose inhibiting 50% of infection). ED50 were calculated using a reconstructed curve with the percentage of neutralization at the different serum concentrations.
MPXV neutralization assay
U2OS cells were plated at 2 × 104 cells per well in a μClear 96-well plate (Greiner Bio-One). Indicated concentrations of MPXV were mixed (ratio 1:1) in a BSL-3 facility with serial dilutions of previously heat-inactivated (30 min at 56°C) plasma or serum in the presence or absence of 10% GPC. After incubation for 2 h at 37°C, the mixture was added onto U2OS cells monolayers. Forty-eight hours later, cells were fixed for 30 min at RT with 4% paraformaldehyde, washed and immunostained for MPXV antigens with rabbit polyclonal anti-VACV antibodies (PA1-7258, Invitrogen), and an Alexa Fluor 488-coupled goat anti-rabbit antibody (Invitrogen). Nuclei were stained with Hoechst. Images were acquired with an Opera Phenix high-content confocal microscope (PerkinElmer). The MPXV+ area and the number of nuclei were quantified using the Harmony software (PerkinElmer). The neutralization titer was determined as the highest plasma or serum dilution in which the MPXV+ area was inferior to that of the “No serum” condition.
Quantification and statistical analysis
Reproducibility of the neutralization assays
Each serum or plasma sample was tested at 6 dilutions in 2-4 independent experiments. Data are presented as the mean ED50 or neutralization titers obtained in the independent experiments. The intra-sample variability was below 5% in >95% of the samples. Samples were retested in case of discrepant results.
Statistical analysis
Calculations were performed using Excel 365 (Microsoft). Figures and statistical analyses were conducted using GraphPad Prism 9. Statistical significance between different groups was calculated using the tests indicated in each figure legend. No statistical methods were used to predetermine sample size.
Acknowledgments
The authors thank the individuals and patients who participated in this study; the members of the Virus and Immunity Unit (Institut Pasteur) and other teams for discussion and help; Bruno Hoen and Pierre Buffet (Institut Pasteur) for their support; Quentin Grassin, Damien Hoinard, and Maxence Feher (Institut Pasteur) for technical assistance and biosafety support; Nathalie Aulner and the staff at the UtechS Photonic BioImaging (UPBI) core facility (Institut Pasteur), a member of the France BioImaging network, for image acquisition and analysis; Emmanuel Roux, Marie-Charlotte Hallouin-Bernard (Institut Pasteur), Fabienne Peira, Vanessa Legros, Aurelie Theillay, Sandra Pallay, Daniela Pires Roteia (CHU Orléans), Agathe Nouchi, Vincent Berot, Yara Wakim, Cécile Brin, Ariane Gavaud, Yanis Tamzali, Alexandre Bleibtreu (Hôpital Pitié Salpêtrière, APHP), and Véronique Rieux (ANRS-MIE) for their help with the cohorts and William Henry Bolland for critical reading of the manuscript.
Work in the O.S. lab is funded by Institut Pasteur, Urgence COVID-19 Fundraising Campaign of Institut Pasteur, Fondation pour la Recherche Médicale (FRM), ANRS-MIE, the Vaccine Research Institute (ANR-10-LABX-77), Labex IBEID (ANR-10-LABX-62-IBEID), ANR / FRM Flash Covid PROTEO-SARS-CoV-2, ANR Coronamito, HERA european funding, Sanofi and IDISCOVR. D.P. is supported by the Vaccine Research Institute. Work in the A.G.M. lab is funded by ANRS-MIE, Emergen program (Ministère de la Santé et Ministère de l’Enseignement Supérieur et de la Recherche), ANR / FRM Flash Covid (COVID-NeuroResp). The Opera system was co-funded by Institut Pasteur and the Région ile de France (DIM1Health). The funders of this study had no role in study design, data collection, analysis, and interpretation, or writing of the article.
Author contributions
Conceptualization, M.H., F.G.-B., T.B., F.P., D.P., L.H., V.P., T.P., A.-G.M., J.-D.L., C.B., Y.L., and J.-C.M., and O.S.; methodology: M.H., F.G.-B., F.P., D.P., T.B., and O.S.; validation: M.H., F.G.-B., F.P., D.P., T.B., and O.S.; formal analysis: M.H., D.P., and T.B.; investigation: M.H., F.G.-B., F.P., D.P., T.B., and O.S.; data collection and analysis: M.H., F.G.-B., F.P., D.P., and T.B.; resources: J.V., A.W., S.B., S.M., R.P., G.M., H.D., S.G., J.L.L.-Z., W.V., F.T., S.F.-P., M.D., H.L., L.A., M.-N.U., L.H., V.P., T.P., A.-G.M., J.-D.L., C.B., Y.L., and J.-C.M.; writing – original draft: M.H. and O.S.; writing – review & editing: M.H., F.G.-B., T.B., A.W., S.B., R.P., L.H., V.P., T.P., A.-G.M., J.-D.L., C.B., Y.L., J.-C.M., and O.S.; visualization: M.H., F.G.-B., F.P., D.P., T.B., and O.S.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: May 5, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.chom.2023.05.001.
Supplemental information
Data and code availability
-
•
MPXV genome has been deposited (GISAID: EPI_ISL_16260402) and is publicly available as of the date of publication. Accession number is listed in the key resources table.
-
•
This study did not generate any new codes.
-
•
Any additional information required to re-analyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Gessain A., Nakoune E., Yazdanpanah Y. Monkeypox. N. Engl. J. Med. 2022;387:1783–1793. doi: 10.1056/NEJMra2208860. [DOI] [PubMed] [Google Scholar]
- 2.Wang L., Shang J., Weng S., Aliyari S.R., Ji C., Cheng G., Wu A. Genomic annotation and molecular evolution of monkeypox virus outbreak in 2022. J. Med. Virol. 2023;95:e28036. doi: 10.1002/jmv.28036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ligon B.L. Monkeypox: a review of the history and emergence in the western hemisphere. Semin. Pediatr. Infect. Dis. 2004;15:280–287. doi: 10.1053/j.spid.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adler H., Gould S., Hine P., Snell L.B., Wong W., Houlihan C.F., Osborne J.C., Rampling T., Beadsworth M.B., Duncan C.J., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect. Dis. 2022;22:1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erez N., Achdout H., Milrot E., Schwartz Y., Wiener-Well Y., Paran N., Politi B., Tamir H., Israely T., Weiss S., et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg. Infect. Dis. 2019;25:980–983. doi: 10.3201/eid2505.190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yong S.E.F., Ng O.T., Ho Z.J.M., Mak T.M., Marimuthu K., Vasoo S., Yeo T.W., Ng Y.K., Cui L., Ferdous Z., et al. Imported monkeypox, Singapore. Emerg. Infect. Dis. 2020;26:1826–1830. doi: 10.3201/eid2608.191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao A.K., Schulte J., Chen T.H., Hughes C.M., Davidson W., Neff J.M., Markarian M., Delea K.C., Wada S., Liddell A., et al. Monkeypox in a traveler returning from Nigeria — Dallas, Texas, July 2021. MMWR Morb. Mortal. Wkly. Rep. 2022;71:509–516. doi: 10.15585/mmwr.mm7114a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costello V., Sowash M., Gaur A., Cardis M., Pasieka H., Wortmann G., Ramdeen S. Imported monkeypox from international traveler, Maryland, USA, 2021. Emerg. Infect. Dis. 2022;28:1002–1005. doi: 10.3201/eid2805.220292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isidro J., Borges V., Pinto M., Sobral D., Santos J.D., Nunes A., Mixão V., Ferreira R., Santos D., Duarte S., et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 2022;28:1569–1572. doi: 10.1038/s41591-022-01907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitjà O., Alemany A., Marks M., Mora J.I.L., Rodríguez-Aldama J.C., Silva M.S.T., Herrera E.A.C., Crabtree-Ramirez B., Blanco J.L., Girometti N., et al. Mpox in people with advanced HIV infection: a global case series. Lancet. 2023;401:939–949. doi: 10.1016/S0140-6736(23)00273-8. [DOI] [PubMed] [Google Scholar]
- 11.Alarcón J., Kim M., Terashita D., Davar K., Garrigues J.M., Guccione J.P., Evans M.G., Hemarajata P., Wald-Dickler N., Holtom P., et al. An Mpox-related death in the United States. N. Engl. J. Med. 2023;388:1246–1247. doi: 10.1056/NEJMc2214921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahase E. Mpox: clinicians identify severe form with high mortality in advanced HIV patients. BMJ. 2023;380:422. doi: 10.1136/bmj.p422. [DOI] [PubMed] [Google Scholar]
- 13.Rimoin A.W., Mulembakani P.M., Johnston S.C., Lloyd Smith J.O., Kisalu N.K., Kinkela T.L., Blumberg S., Thomassen H.A., Pike B.L., Fair J.N., et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. USA. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karem K.L., Reynolds M., Hughes C., Braden Z., Nigam P., Crotty S., Glidewell J., Ahmed R., Amara R., Damon I.K. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin. Vaccine Immunol. 2007;14:1318–1327. doi: 10.1128/CVI.00148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine P.E., Jezek Z., Grab B., Dixon H. The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 1988;17:643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 16.Jezek Z., Marennikova S.S., Mutumbo M., Nakano J.H., Paluku K.M., Szczeniowski M. Human monkeypox: a study of 2,510 contacts of 214 patients. J. Infect. Dis. 1986;154:551–555. doi: 10.1093/infdis/154.4.551. [DOI] [PubMed] [Google Scholar]
- 17.Moschese D., Farinacci D., Pozza G., Ciccullo A., Cossu M.V., Giacomelli A., Borgonovo F., Mileto D., Santangelo R., Tamburrini E., et al. Is smallpox vaccination protective against human monkeypox? J. Med. Virol. 2023;95:e28077. doi: 10.1002/jmv.28077. [DOI] [PubMed] [Google Scholar]
- 18.Bricaire F., Combadière B., Rouleau E., Bossi P., Garin D., Lebrun-Vignes B., Autran B. Evaluation of residual immune response against human pox virus before and after revaccination in healthy volunteers. Bull. Acad. Natl. Med. 2006;190:1035–1046. discussion 1046–1049. [PubMed] [Google Scholar]
- 19.Chappert P., Huetz F., Espinasse M.A., Chatonnet F., Pannetier L., Da Silva L., Goetz C., Mégret J., Sokal A., Crickx E., et al. Human anti-smallpox long-lived memory B cells are defined by dynamic interactions in the splenic niche and long-lasting germinal center imprinting. Immunity. 2022;55 doi: 10.1016/j.immuni.2022.08.019. 1872–1890.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammarlund E., Lewis M.W., Hansen S.G., Strelow L.I., Nelson J.A., Sexton G.J., Hanifin J.M., Slifka M.K. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 21.Crotty S., Felgner P., Davies H., Glidewell J., Villarreal L., Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 22.Taub D.D., Ershler W.B., Janowski M., Artz A., Key M.L., McKelvey J., Muller D., Moss B., Ferrucci L., Duffey P.L., et al. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am. J. Med. 2008;121:1058–1064. doi: 10.1016/j.amjmed.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volz A., Sutter G. Modified vaccinia virus Ankara: history, value in basic research, and current perspectives for vaccine development. Adv. Virus Res. 2017;97:187–243. doi: 10.1016/bs.aivir.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stickl H., Hochstein-Mintzel V. Intracutaneous smallpox vaccination with a weak pathogenic vaccinia virus (“MVA virus”) Munch. Med. Wochenschr. 1971;113:1149–1153. [PubMed] [Google Scholar]
- 25.Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Whitbeck J.C., Cohen G.H., Eisenberg R.J., Hartmann C.J., Jackson D.L., Kulesh D.A., et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 26.Earl P.L., Americo J.L., Wyatt L.S., Espenshade O., Bassler J., Gong K., Lin S., Peters E., Rhodes L., Spano Y.E., et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc. Natl. Acad. Sci. USA. 2008;105:10889–10894. doi: 10.1073/pnas.0804985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatch G.J., Graham V.A., Bewley K.R., Tree J.A., Dennis M., Taylor I., Funnell S.G.P., Bate S.R., Steeds K., Tipton T., et al. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J. Virol. 2013;87:7805–7815. doi: 10.1128/JVI.03481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arbel R., Sagy Y.W., Zucker R., Arieh N.G., Markovits H., Abu-Ahmad W., Battat E., Ramot N., Carmeli G., Mark-Amir A., et al. 2022. Effectiveness of a single-dose modified vaccinia Ankara in human monkeypox: an observational study. Preprint at Research Square. [DOI] [Google Scholar]
- 29.Payne A.B., Ray L.C., Kugeler K.J., Fothergill A., White E.B., Canning M., Farrar J.L., Feldstein L.R., Gundlapalli A.V., Houck K., et al. Incidence of monkeypox among unvaccinated persons compared with persons receiving ≥1 JYNNEOS vaccine dose – 32 U.S. Jurisdictions, July 31-September 3, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:1278–1282. doi: 10.15585/mmwr.mm7140e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edghill-Smith Y., Golding H., Manischewitz J., King L.R., Scott D., Bray M., Nalca A., Hooper J.W., Whitehouse C.A., Schmitz J.E., et al. Smallpox vaccine–induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 31.Nigam P., Earl P.L., Americo J.L., Sharma S., Wyatt L.S., Edghill-Spano Y., Chennareddi L.S., Silvera P., Moss B., Robinson H.L., et al. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology. 2007;366:73–83. doi: 10.1016/j.virol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agrati C., Cossarizza A., Mazzotta V., Grassi G., Casetti R., De Biasi S., Pinnetti C., Gili S., Mondi A., Cristofanelli F., et al. Immunological signature in human cases of monkeypox infection in 2022 outbreak: an observational study. Lancet Infect. Dis. 2023;23:320–330. doi: 10.1016/S1473-3099(22)00662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaeck L.M., Lamers M.M., Verstrepen B.E., Bestebroer T.M., van Royen M.E., Götz H., Shamier M.C., van Leeuwen L.P.M., Schmitz K.S., Alblas K., et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med. 2023;29:270–278. doi: 10.1038/s41591-022-02090-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cosma A., Bühler S., Nagaraj R., Staib C., Hammarin A.L., Wahren B., Goebel F.D., Erfle V., Sutter G. Neutralization assay using a modified vaccinia virus Ankara vector expressing the green fluorescent protein is a high-throughput method to monitor the humoral immune response against vaccinia virus. Clin. Diagn. Lab. Immunol. 2004;11:406–410. doi: 10.1128/CDLI.11.2.406-410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benhnia M.R.-E.-I., McCausland M.M., Laudenslager J., Granger S.W., Rickert S., Koriazova L., Tahara T., Kubo R.T., Kato S., Crotty S. Heavily isotype-dependent protective activities of human antibodies against vaccinia virus extracellular virion antigen B5. J. Virol. 2009;83:12355–12367. doi: 10.1128/JVI.01593-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen M.E., Xiao Y., Eisenberg R.J., Cohen G.H., Isaacs S.N. Antibody against extracellular vaccinia virus (EV) protects mice through complement and Fc receptors. PLoS One. 2011;6:e20597. doi: 10.1371/journal.pone.0020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishimura C., Nomura M., Kitaoka M., Takeuchi Y., Kimura M. Complement requirement of the neutralizing antibody appearing after immunization with smallpox vaccine. Jpn. J. Microbiol. 1968;12:256–259. doi: 10.1111/j.1348-0421.1968.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 38.Mucker E.M., Lindquist M., Hooper J.W. Particle-specific neutralizing activity of a monoclonal antibody targeting the poxvirus A33 protein reveals differences between cell associated and extracellular enveloped virions. Virology. 2020;544:42–54. doi: 10.1016/j.virol.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Kaever T., Meng X., Matho M.H., Schlossman A., Li S., Sela-Culang I., Ofran Y., Buller M., Crump R.W., Parker S., et al. Potent neutralization of vaccinia virus by divergent murine antibodies targeting a common site of vulnerability in L1 protein. J. Virol. 2014;88:11339–11355. doi: 10.1128/JVI.01491-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matho M.H., Schlossman A., Meng X., Benhnia M.R.-E.-I., Kaever T., Buller M., Doronin K., Parker S., Peters B., Crotty S., et al. Structural and functional characterization of anti-A33 antibodies reveal a potent cross-species orthopox viruses neutralizer. PLoS Pathog. 2015;11:e1005148. doi: 10.1371/journal.ppat.1005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lustig S., Fogg C., Whitbeck J.C., Moss B. Synergistic neutralizing activities of antibodies to outer membrane proteins of the two infectious forms of vaccinia virus in the presence of complement. Virology. 2004;328:30–35. doi: 10.1016/j.virol.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 42.Benhnia M.R.-E.-I., McCausland M.M., Moyron J., Laudenslager J., Granger S., Rickert S., Koriazova L., Kubo R., Kato S., Crotty S. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J. Virol. 2009;83:1201–1215. doi: 10.1128/JVI.01797-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palich R., Burrel S., Monsel G., Nouchi A., Bleibtreu A., Seang S., Bérot V., Brin C., Gavaud A., Wakim Y., et al. Viral loads in clinical samples of men with monkeypox virus infection: a French case series. Lancet Infect. Dis. 2023;23:74–80. doi: 10.1016/S1473-3099(22)00586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richert L., Lelièvre J.D., Lacabaratz C., Hardel L., Hocini H., Wiedemann A., Lucht F., Poizot-Martin I., Bauduin C., Diallo A., et al. T cell immunogenicity, gene expression profile, and safety of four heterologous prime-boost combinations of HIV vaccine candidates in healthy volunteers: results of the randomized multi-arm Phase I/II ANRS VRI01 trial. J. Immunol. 2022;208:2663–2674. doi: 10.4049/jimmunol.2101076. [DOI] [PubMed] [Google Scholar]
- 45.Sagoo P., Garcia Z., Breart B., Lemaître F., Michonneau D., Albert M.L., Levy Y., Bousso P. In vivo imaging of inflammasome activation reveals a subcapsular macrophage burst response that mobilizes innate and adaptive immunity. Nat. Med. 2016;22:64–71. doi: 10.1038/nm.4016. [DOI] [PubMed] [Google Scholar]
- 46.Gilchuk I., Gilchuk P., Sapparapu G., Lampley R., Singh V., Kose N., Blum D.L., Hughes L.J., Satheshkumar P.S., Townsend M.B., et al. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell. 2016;167 doi: 10.1016/j.cell.2016.09.049. 684–694.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balière C., Hourdel V., Kwasiborski A., Grassin Q., Feher M., Hoinard D., Vanhomwegen J., Taieb F., Consigny P.H., Manuguerra J.C., et al. Complete genome sequences of monkeypox virus from a French clinical sample and the corresponding isolated strain, obtained using nanopore sequencing. Microbiol. Resour. Announc. 2023;12:e0000923. doi: 10.1128/mra.00009-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., Planchais C., Porrot F., Robillard N., Puech J., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 49.Cifuentes-Munoz N., El Najjar F., Dutch R.E. In: Advances in Virus Research Virus Assembly and Exit Pathways. Kielian M., Mettenleiter T.C., Roossinck M.J., editors. Academic Press; 2020. Chapter three - viral cell-to-cell spread: conventional and non-conventional ways; pp. 85–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andreano E., Paciello I., Piccini G., Manganaro N., Pileri P., Hyseni I., Leonardi M., Pantano E., Abbiento V., Benincasa L., et al. Hybrid immunity improves B cells and antibodies against SARS-CoV-2 variants. Nature. 2021;600:530–535. doi: 10.1038/s41586-021-04117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crotty, S. (2021). Hybrid immunity. Science 372, 1392–1393. 10.1126/science.abj2258. [DOI]
- 52.Smith G.L., Law M. The exit of vaccinia virus from infected cells. Virus Res. 2004;106:189–197. doi: 10.1016/j.virusres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 53.Moss B. Membrane fusion during poxvirus entry. Semin. Cell Dev. Biol. 2016;60:89–96. doi: 10.1016/j.semcdb.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pickup D.J. Extracellular virions: the advance guard of poxvirus infections. PLoS Pathog. 2015;11:e1004904. doi: 10.1371/journal.ppat.1004904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zajonc D.M. Antibody recognition of immunodominant vaccinia virus envelope proteins. Subcell. Biochem. 2017;83:103–126. doi: 10.1007/978-3-319-46503-6_4. [DOI] [PubMed] [Google Scholar]
- 56.Davies D.H., Liang X., Hernandez J.E., Randall A., Hirst S., Mu Y., Romero K.M., Nguyen T.T., Kalantari-Dehaghi M., Crotty S., et al. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. USA. 2005;102:547–552. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davies D.H., Molina D.M., Wrammert J., Miller J., Hirst S., Mu Y., Pablo J., Unal B., Nakajima-Sasaki R., Liang X., et al. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics. 2007;7:1678–1686. doi: 10.1002/pmic.200600926. [DOI] [PubMed] [Google Scholar]
- 58.Lawrence S.J., Lottenbach K.R., Newman F.K., Buller R.M.L., Bellone C.J., Chen J.J., Cohen G.H., Eisenberg R.J., Belshe R.B., Stanley S.L., Jr., et al. Antibody responses to vaccinia membrane proteins after smallpox vaccination. J. Infect. Dis. 2007;196:220–229. doi: 10.1086/518793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benhnia M.R.-E.-I., McCausland M.M., Su H.P., Singh K., Hoffmann J., Davies D.H., Felgner P.L., Head S., Sette A., Garboczi D.N., et al. Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine. J. Virol. 2008;82:3751–3768. doi: 10.1128/JVI.02244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pütz M.M., Midgley C.M., Law M., Smith G.L. Quantification of antibody responses against multiple antigens of the two infectious forms of vaccinia virus provides a benchmark for smallpox vaccination. Nat. Med. 2006;12:1310–1315. doi: 10.1038/nm1457. [DOI] [PubMed] [Google Scholar]
- 61.Yefet R., Friedel N., Tamir H., Polonsky K., Mor M., Cherry-Mimran L., Taleb E., Hagin D., Sprecher E., Israely T., et al. Monkeypox infection elicits strong antibody and B cell response against A35R and H3L antigens. iScience. 2023;26:105957. doi: 10.1016/j.isci.2023.105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oroszlan S., Nowinski R.C. Lysis of retroviruses with monoclonal antibodies against viral envelope proteins. Virology. 1980;101:296–299. doi: 10.1016/0042-6822(80)90507-3. [DOI] [PubMed] [Google Scholar]
- 63.Fuchs A., Lin T.Y., Beasley D.W., Stover C.M., Schwaeble W.J., Pierson T.C., Diamond M.S. Direct complement restriction of Flavivirus infection requires glycan recognition by mannose binding lectin. Cell Host Microbe. 2010;8:186–195. doi: 10.1016/j.chom.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jayasekera J.P., Moseman E.A., Carroll M.C. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J. Virol. 2007;81:3487–3494. doi: 10.1128/JVI.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ji X., Olinger G.G., Aris S., Chen Y., Gewurz H., Spear G.T. Mannose-binding lectin binds to Ebola and Marburg envelope glycoproteins, resulting in blocking of virus interaction with DC-SIGN and complement-mediated virus neutralization. J. Gen. Virol. 2005;86:2535–2542. doi: 10.1099/vir.0.81199-0. [DOI] [PubMed] [Google Scholar]
- 66.Schiela B., Bernklau S., Malekshahi Z., Deutschmann D., Koske I., Banki Z., Thielens N.M., Würzner R., Speth C., Weiss G., et al. Active human complement reduces the Zika virus load via formation of the membrane-attack complex. Front. Immunol. 2018;9:2177. doi: 10.3389/fimmu.2018.02177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spear G.T., Sullivan B.L., Takefman D.M., Landay A.L., Lint T.F. Human immunodeficiency virus (HIV)-infected cells and free virus directly activate the classical complement pathway in rabbit, mouse and guinea-pig sera; activation results in virus neutralization by virolysis. Immunology. 1991;73:377–382. [PMC free article] [PubMed] [Google Scholar]
- 68.Thy M., Peiffer-Smadja N., Mailhe M., Kramer L., Ferré V.M., Houhou N., Tarhini H., Bertin C., Beaumont A.L., Garé M., et al. Breakthrough infections after postexposure vaccination against mpox. N. Engl. J. Med. 2022;387:2477–2479. doi: 10.1056/NEJMc2211944. [DOI] [PubMed] [Google Scholar]
- 69.Payne A.B., Ray L.C., Cole M.M., Canning M., Houck K., Shah H.J., Farrar J.L., Lewis N.M., Fothergill A., White E.B., et al. Reduced risk for mpox after receipt of 1 or 2 doses of JYNNEOS vaccine compared with risk among unvaccinated persons – 43 U.S. Jurisdictions, July 31-October 1, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:1560–1564. doi: 10.15585/mmwr.mm7149a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farrar J.L., Lewis N.M., Houck K., Canning M., Fothergill A., Payne A.B., Cohen A.L., Vance J., Brassil B., Youngkin E., et al. Demographic and clinical characteristics of mpox in persons who had previously received 1 dose of JYNNEOS vaccine and in unvaccinated persons – 29 U.S. Jurisdictions, May 22-September 3, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:1610–1615. doi: 10.15585/mmwr.mm715152a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raadsen M.P., Dahlke C., Fathi A., Lamers M.M., van den Doel P., Zaeck L.M., van Royen M.E., de Bruin E., Sikkema R., Koopmans M., et al. Brief report: monkeypox virus cross-neutralizing antibodies in clinical trial subjects vaccinated with modified vaccinia virus Ankara encoding MERS-coronavirus spike protein. J. Infect. Dis. 2023 doi: 10.1093/infdis/jiad052. Published online March 1, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Batéjat C., Grassin Q., Feher M., Hoinard D., Vanhomwegen J., Manuguerra J.C., Leclercq I. Heat inactivation of monkeypox virus. J. Biosaf. Biosecur. 2022;4:121–123. doi: 10.1016/j.jobb.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nouchi A., Brin C., Martin A., Favier M., Palich R., Wakim Y., Gavaud A., Seang S., Valantin M.A., Bleibtreu A., et al. Prospective cohort of 70 consecutive cases of human monkeypox: clinical description with focus on dermatological presentation. J. Eur. Acad. Dermatol. Venereol. 2023;37:e403–e405. doi: 10.1111/jdv.18742. [DOI] [PubMed] [Google Scholar]
- 74.Buchrieser J., Dufloo J., Hubert M., Monel B., Planas D., Rajah M.M., Planchais C., Porrot F., Guivel-Benhassine F., Van der Werf S., et al. Syncytia formation by SARS-CoV-2-infected cells. EMBO J. 2020;39:e106267. doi: 10.15252/embj.2020106267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
MPXV genome has been deposited (GISAID: EPI_ISL_16260402) and is publicly available as of the date of publication. Accession number is listed in the key resources table.
-
•
This study did not generate any new codes.
-
•
Any additional information required to re-analyze the data reported in this paper is available from the lead contact upon request.