Abstract
Context
Tumor size is important in determining the range of surgery in papillary thyroid carcinomas (PTCs), especially those smaller than 1 cm.
Objective
We aimed to analyze the features of small PTCs with aggressive subtypes based on histological characteristics.
Methods
In this retrospective study, we reviewed the medical records of 11 570 patients with PTCs smaller than or equal to 1 cm who underwent thyroidectomy between January 2009 and December 2016. Aggressive subtypes included diffuse sclerosing, solid, tall cell, columnar cell, and hobnail subtypes.
Results
Among the 11 570 patients with PTCs smaller than or equal to 1 cm, 177 aggressive PTC subtypes were identified. Propensity score matching revealed 110 tumors (62.1%) with extrathyroidal extension of aggressive PTC subtypes and 451 (51.1%) nonaggressive PTC subtypes (95% CI, 0.41-0.80; P < .001). Metastatic central and lateral neck lymph nodes constituted 3.06 ± 3.67 and 3.81 ± 5.39 of aggressive PTC subtypes and 1.22 ± 2.14 and 2.85 ± 3.79 of nonaggressive PTC subtypes, respectively (central neck nodes: 95% CI, 1.42-2.26; P < .001; lateral neck nodes: 95% CI, 2.9-5.90; P < .001). Seven patients with aggressive PTC subtypes (3.95%) and 12 with nonaggressive PTC subtypes (1.7%) exhibited recurrence.
Conclusion
Aggressive subtypes of small PTC tumors smaller than or equal to 1 cm exhibited more extrathyroidal extension and neck node metastasis. This study suggests that surgeons should consider the aggressive subtypes as important factors when deciding the range of surgery in PTCs smaller than 1 cm.
Keywords: thyroid carcinoma, papillary thyroid microcarcinoma, aggressive subtypes, extrathyroidal extension, lymph node metastasis
Papillary thyroid carcinoma (PTC) is the most common histological type of differentiated cancer of the thyroid gland (1). PTC is an indolent tumor with a 10-year survival rate of approximately 93% (2). Papillary thyroid microcarcinoma (PTMC), which is defined as PTC with a size of 1 cm or less in the greatest diameter, is associated with differences in prognosis and treatment. However, PTMC was not included as a PTC subtype in the 2022 World Health Organization classification of thyroid neoplasms (3). Small PTCs sized 1 cm or less constitute approximately 50% of the increase in PTC incidence (4). However, some aggressive PTC subtypes exhibit distinct clinical, pathological, and molecular features associated with their large size (5). Generally, diffuse sclerosing, solid, tall cell, columnar cell, and hobnail subtypes are considered traditional aggressive subtypes (4).
These aggressive subtypes possess distinct features associated with their large size and present with extrathyroid extension and nodal metastases. The diffuse sclerosing subtype exhibits unique clinical features, including a higher prevalence of underlying Hashimoto thyroiditis, higher female-to-male ratio, younger age, high vascular invasion, extrathyroid extension, and lymph node metastasis (6, 7). The tall cell subtype occurs in older patients and presents with larger tumor size and more frequent extrathyroidal involvement (8). The columnar cell subtype exhibits a rapid growth rate, local invasion, early development of lymph node metastasis, extrathyroidal extension, and high recurrence rate (9).
PTC subtypes with small tumor sizes (≤ 1 cm) may exhibit aggressiveness, but there is a paucity of studies in this regard, thus preventing optimal decision-making in treating small PTCs with aggressive subtypes (10). Thus, our study aimed to analyze the features of small PTCs with aggressive subtypes based on histological characteristics.
Materials and Methods
This retrospective study reviewed the medical records of 11 570 patients (age, 19-81 years) with PTCs smaller than or equal to 1 cm who underwent thyroidectomy at the Thyroid Cancer Center, Gangnam Severance Hospital, Yonsei University College of Medicine, Korea, between January 2009 and December 2016. Patients were included if their tumor was pathologically confirmed to be PTC before surgery using fine-needle aspiration (FNA) and if the surgery was performed at the Thyroid Cancer Center, Gangnam Severance Hospital. All included patients had no other thyroidal malignancy and underwent regular postoperative follow-ups for 5 years at the outpatient clinic.
All pathologic reports were reviewed, and the subtypes of PTCs were recategorized based on the 2022 World Health Organization Classification of Thyroid Neoplasms (3). All PTCs were classified into 11 subtypes (conventional, follicular, diffuse sclerosing, cystic, solid, oncocytic, Warthin tumor-like, tall cell, columnar cell, hobnail, and etc). The most aggressive subtypes of PTC that exhibit distinct clinical, pathological, and molecular features include the diffuse sclerosing, solid, tall cell, columnar cell, and hobnail subtypes (4).
Information on demographics, type of operation, final pathology, recurrence, and time to recurrence was examined. All diagnosed patients underwent total thyroidectomy or less-than-total thyroidectomy with prophylactic central compartment node dissection (CCND) based on the treatment policy of the institution for thyroid cancer (11). While the institution is currently conducting active surveillance for PTMC, surgery was the primary treatment for PTMC, especially before publication of the 2015 American Thyroid Association (ATA) guideline that primarily addresses active surveillance in 2016. Lateral neck node dissection was performed for cases with lateral neck node metastasis confirmed by FNA or intraoperative lymph node frozen pathology. Tumors with extrathyroidal extension were examined on the final pathologic reports. Recurrence was defined as a newly observed mass in the operation bed, and regional neck nodes within 5 years from the operation day. Multiplicity was defined as patients who had multifocal cancer nodules in one lobe or both.
Data analysis was performed using SPSS statistical software. Fisher exact or chi-square tests were used to compare categorical variables. T test was used to compare continuous variables, which were presented as mean and SD. Owing to the large differences in patient numbers for aggressive and nonaggressive subtypes (177: 11 393), we used propensity score matching to address selection bias.
The study procedures were approved by the institutional review board (IRB) of Gangnam Severance Hospital, Yonsei University College of Medicine (IRB protocol: 3-2022-0105). The study protocol was conducted in accordance with the tenets of the Declaration of Helsinki. Owing to the retrospective nature of the study, neither patient approval nor informed consent was required.
Results
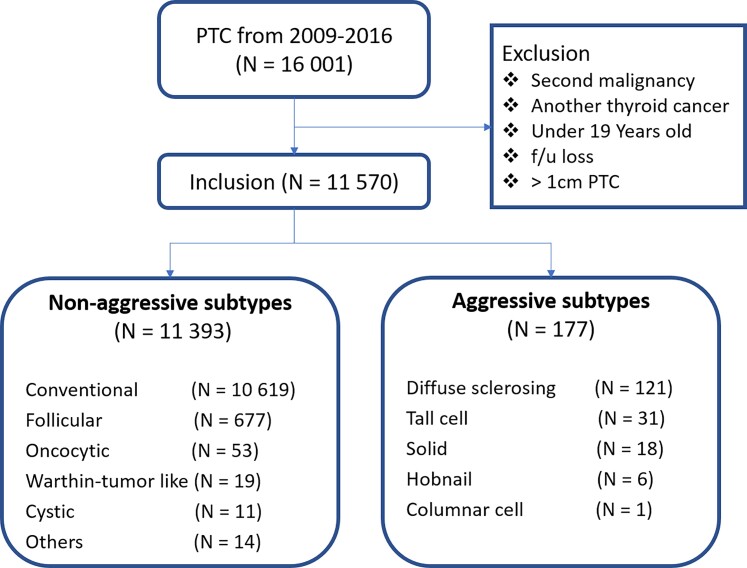
Among the 11 570 patients with PTCs smaller than or equal to 1 cm, 177 with aggressive PTC subtypes were identified: 121, 31, 18, 6, and 1 with diffuse sclerosing, tall cell, solid, hobnail, and columnar subtypes, respectively. Furthermore, a total of 10 619 patients with conventional subtypes were identified, including 677, 53, 19, and 11 with follicular, oncocytic, Warthin tumor-like, and cystic subtypes, respectively, in addition to 14 with other nonaggressive subtypes (Fig. 1). The other subtypes comprised 4 PTCs with squamous metaplasia, 5 macrofollicular subtype, 1 encapsulated PTC, 1 infiltrative PTC, 1 PTC with nodular fasciitis, and 2 without residual carcinoma. For cases without residual carcinoma, cancer was not detected in surgical pathology even though PTC was diagnosed by FNA performed before thyroidectomy. The highest number of PTC cases (1879 cases) was recorded in 2012, and the highest number of aggressive subtypes (32 cases) was recorded in 2015.
Figure 1.
Flow diagram of patients who underwent thyroidectomy and number of papillary thyroid carcinoma (PTC) subtypes smaller than or equal to 1 cm.
Among the patients with small PTCs, 9307 were women (80.4%), and 2263 were men (19.6%) (Table 1). Among the patients with aggressive PTC subtypes, 132 were women (74.6%), and 45 were men (25.4%). Among the patients with nonaggressive subtypes, 9175 were women (80.5%), and 2218 were men (19.5%). The mean patient ages were 40.9 and 45.7 years for aggressive and nonaggressive subtypes, respectively. Mean patient age was significantly lower for aggressive than for nonaggressive subtypes (P < .047). Among patients with aggressive subtypes, 127 (71.8%) underwent total thyroidectomy with CCND, and 50 (28.2%) underwent less-than-total thyroidectomy with CCND. Among patients with nonaggressive PTC subtypes, 6263 (54.7%) underwent total thyroidectomy with CCND, and 5157 (45.3%) underwent less-than-total thyroidectomy with CCND. More patients with aggressive subtypes underwent total thyroidectomy than patients with nonaggressive subtypes (95% CI, 0.34-0.66; P < .001). Among the patients who underwent total thyroidectomy, 112 (88.2%) and 5110 (81.6%) received radioactive iodine (RAI) therapy for aggressive and nonaggressive subtypes, respectively. Extrathyroidal extension was observed in 110 (62.1%) patients with aggressive PTC and 5226 (45.9%) patients with nonaggressive PTC, suggesting that aggressive PTC subtypes had more significant capsular invasion than nonaggressive PTC subtypes (95% CI, 0.38-0.70; P < .001) With regard to multiplicity, 133 solitary cancer was the most common cancer in both groups (57.6% vs 73.1%), and multifocal 134 cancers constituted 75 (42.4%) cases of aggressive PTC subtypes and 3067 (26.9%) cases of 135 nonaggressive PTC subtypes (Table 2).
Table 1.
Clinicopathologic characteristics of patients
| PTC ≤ 1 cm (n = 11 570) | |
|---|---|
| Sex, n (%) | |
| ȃMale | 2623 (19.6) |
| ȃFemale | 9307 (80.4) |
| Age, mean ± SD, y | 45.4 ± 10.9 |
| Operation type, n (%) | |
| ȃLess than total thyroidectomy | 5207 (45.0) |
| ȃTotal thyroidectomy | 6363 (55.0) |
| Positive central neck nodes, n (%) | 3794 (32.8) |
| Central neck node dissection, mean ± SD, No. | |
| ȃObtained central neck lymph nodes | 5.32 ± 4.49 |
| ȃMetastatic central neck lymph nodes | 0.84 ± 1.18 |
| Lateral neck node dissection, n (%) | 1113 (9.6) |
| Lateral neck node dissection, mean ± SD, No. | |
| ȃObtained lateral neck lymph nodes | 21.31 ± 19.67 |
| ȃMetastatic lateral neck lymph nodes | 2.44 ± 3.72 |
| Extrathyroidal extension, n (%) | 5336 (46.1) |
| Multiplicity, n (%) | 3142 (27.1) |
Abbreviation: PTC, papillary thyroid carcinoma.
Table 2.
Pathology and recurrence of aggressive papillary thyroid carcinoma subtypes smaller than or equal to 1 cm
| Aggressive subtypes (n = 177) | Nonaggressive subtypes (n = 11 393) | P | |
|---|---|---|---|
| Positive central neck nodes, n (%) | 124 (70.1) | 3670 (32.2) | < .001a |
| Central neck node dissection, mean ± SD, No. | |||
| ȃObtained central neck lymph nodes | 6.79 ± 5.34 | 5.30 ± 4.48 | < .001a |
| ȃMetastatic central neck lymph nodes | 3.06 ± 3.69 | 0.8 ± 1.71 | < .001a |
| Lateral neck node dissection, n (%) | 54 (30.1) | 1012 (8.9) | < .001a |
| Lateral neck node dissection, mean ± SD, No. | |||
| ȃObtained lateral neck lymph nodes | 19.24 ± 24.30 | 21.51 ± 19.15 | .267 |
| ȃMetastatic lateral neck lymph nodes | 3.81 ± 5.39 | 2.30 ± 3.48 | < .001a |
| Extrathyroidal extension, n (%) | 110 (62.1) | 5226 (45.9) | < .001a |
| Multiplicity, n (%) | 75 (42.4) | 3067 (26.9) | < .001a |
| RAI, n (%) | 112/127 (88.2) | 5110/6263 (81.6) | .057 |
| Recurrence, n (%) | 7 (3.95) | 98 (0.86) | < .001a |
| Time to recurrence, mean ± SD, mo | 58.64 ± 0.53 | 59.74 ± 0.03 | < .001a |
RAI was counted based on the patients who underwent total thyroidectomy.
Abbreviation: RAI, radioactive iodine.
P less than .001.
To avoid selection bias, we conducted propensity score matching for sex, age, and operation type at a 1:4 ratio and analyzed 177 patients with aggressive subtypes and 705 patients with nonaggressive subtypes (Table 3). The number of patients with metastatic central neck lymph nodes was 124 (70.1%) among those with aggressive PTC subtypes and 286 (40.6%) among those with nonaggressive PTC subtypes based on propensity score matching. Metastatic central neck lymph nodes were observed in 3.06 ± 3.67 of aggressive and 1.22 ± 2.14 of nonaggressive PTC subtypes (95% CI, 2.00-2.52; P = .001). The number of metastatic central neck lymph nodes in aggressive PTC subtypes was 0.8 ± 1.71. The number of obtained central neck lymph nodes did not differ between aggressive and nonaggressive PTC subtypes (6.79 ± 5.34 vs 578 ± 4.44; P = .022). In total, 54 (30.1%) of 177 patients with aggressive subtypes and 102 (14.5%) of 705 patients with nonaggressive subtypes underwent lateral neck node dissection based on propensity score matching. Approximately twice the percentage of aggressive PTC subtypes tended to metastasize to lateral neck nodes (30.1% vs 14.5%) (see Table 3). Aggressive PTC subtypes exhibited a more aggressive trend (3.81 ± 5.39) than nonaggressive PTC subtypes (2.30 ± 3.48) for metastatic lateral neck lymph nodes (see Table 3). With regard to multiplicity, solitary cancer was the most common cancer in both groups (57.6% vs 66.2%), and bilateral multifocal cancers constituted 48 (27.1%) cases of aggressive PTC subtypes and 173 (19.6%) cases of nonaggressive PTC subtypes (see Table 3).
Table 3.
Pathology and recurrence of aggressive papillary thyroid carcinoma subtypes smaller than or equal to 1 cm (propensity score matching, 1:4)
| Aggressive subtypes (n = 177) | Nonaggressive subtypes (n = 705) | P | |
|---|---|---|---|
| Positive central neck nodes, n (%) | 124 (70.1) | 286 (40.6) | < .001c |
| Central neck node dissection, mean ± SD, No. | |||
| ȃObtained central neck lymph nodes | 6.79 ± 5.34 | 5.78 ± 4.44 | .022a |
| ȃMetastatic central neck lymph nodes | 3.06 ± 3.69 | 1.22 ± 2.14 | .001b |
| Lateral neck node dissection, n (%) | 54 (30.1) | 102 (14.5) | < .001c |
| Lateral neck node dissection, mean ± SD, No. | |||
| ȃObtained lateral neck lymph nodes | 19.24 ± 24.30 | 25.01 ± 20.99 | .002b |
| ȃMetastatic lateral neck lymph nodes | 3.81 ± 5.39 | 2.85 ± 3.79 | < .001c |
| Extrathyroidal extension, n (%) | 110 (62.1) | 451 (51.1) | < .001c |
| Multiplicity, n (%) | 75 (42.4) | 298 (33.8) | .011a |
| RAI, n (%) | 112/127 (88.2) | 432/492 (87.8) | .906 |
| Recurrence, n (%) | 7 (3.95) | 12 (1.7) | .065 |
| Time to recurrence, mean ± SD, mo | 58.64 ± 0.53 | 59.30 ± 0.21 | .066 |
RAI was counted based on the patients who underwent total thyroidectomy.
Abbreviation: RAI, radioactive iodine.
P less than or equal to .05.
P less than or equal to .01.
P less than or equal to .001.
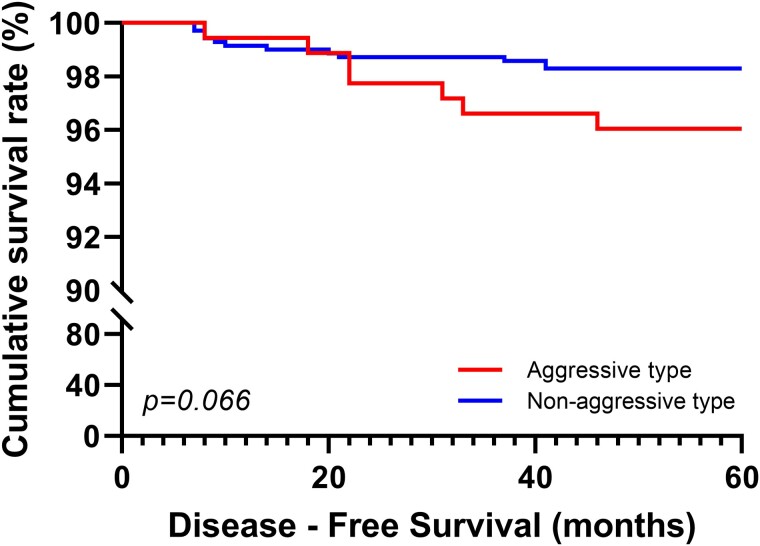
With regard to 5-year disease-free survival, 7 (3.95%) of 177 patients with aggressive PTC subtypes and 12 (1.7%) of 705 patients with nonaggressive PTC subtypes exhibited cancer recurrence in the central or lateral neck nodes or the operation bed. All recurring cancers were the conventional PTC subtype in the nonaggressive PTC subtypes and the diffuse sclerosing subtype in the aggressive subtype. The average time to recurrence for aggressive and nonaggressive subtypes were 58.6 and 59.3 months, respectively, with no statistically significant difference (see Table 3). Despite a small tumor size of 1 cm, aggressive PTC subtypes tended to relapse with P equal to .66 based on the Kaplan-Meier curve for 5-year disease-free survival (Fig. 2). The odds ratio for aggressive PTC subtypes was 2.38. In total, 98 (0.86%) of 11 393 patients with nonaggressive subtypes exhibited recurrence, with a statistically significant difference between aggressive and nonaggressive PTC subtypes.
Figure 2.
Kaplan-Meier analysis of disease-free survival rates for 5 years according to aggressive and nonaggressive papillary thyroid carcinoma subtypes.
Discussion
A subset of small-sized PTCs display aggressive pathologic and clinical features, including regional metastasis and structural recurrence post surgery (12-15). The 2015 guidelines of the ATA recommended thyroid lobectomy as the optimal treatment for patients with small PTC (ie, papillary thyroid microcarcinoma) that lacks features to indicate the removal of the contralateral lobe, such as nodal metastasis or a family history of thyroid cancer (16). The ATA guidelines also recommended considering a less aggressive approach involving active surveillance with serial ultrasound as an alternative management strategy for low-risk tumors without evidence of metastasis or local invasion and without cytologic evidence of aggressive disease (16, 17). Nevertheless, such aggressive subtypes of PTCs are difficult to diagnose based on FNA in patients, and almost all instances of aggressive PTC subtypes are diagnosed after surgery. Therefore, surgical pathologic confirmation is critical. In the present study, none of the patients were diagnosed with aggressive PTC subtypes before surgery despite strongly suspected tumors on imaging, such as diffuse sclerosing subtype with enlarged heterogeneous parenchyma and diffuse scattered microcalcifications exhibiting a “snow storm” appearance on ultrasonography (6).
For the treatment of these aggressive subtypes, the ATA recommends lobectomy for small unifocal intrathyroidal tumors, total thyroidectomy with therapeutic neck dissection if nodes are involved, or prophylactic CCND for T3 and T4 tumors (16). Following total thyroidectomy, the need for RAI therapy should be considered. In this study, patients with more aggressive subtypes tended to undergo total thyroidectomy (71.8%) instead of less-than-total thyroidectomy (28.2%) as almost all the surgical treatments were performed before the release of the latest ATA 2015 guidelines. In the case of central lymph node dissection, total thyroidectomy is more likely than lobectomy to harvest more central lymph nodes. In this study, the aggressive subtypes of PTCs had a statistically significantly higher number of central lymph nodes harvested at the initial procedure. Of the 177 patients with aggressive subtypes of PTC, 127 (72%) underwent total thyroidectomy, while 6236 patients (55%) of the 11 393 patients with nonaggressive subtypes PTC underwent total thyroidectomy. If similar rates of operation types were observed, one primary reason could be an inherent surgical bias influenced by preoperative ultrasound features such as diffuse sclerosing subtypes or a cytology report via FNA. However, it is difficult to expect to diagnose a subtype of PTC before final surgical pathology because of its small tumor size and the innate limitation of FNA. Wang et al (18) suggested that radical treatment approaches should be considered because of the poor prognosis of the columnar cell subtype. It is essential to identify indolent cases because they have better outcomes with a low incidence of recurrences or metastasis (19-21). Approximately 20% of tall cell subtype cases are refractory to RAI. Gunalp et al (22) reported that tall cell subtype histology implies a higher incidence of extrathyroidal and lymphovascular invasion. These factors underpin the higher recurrence rates and poorer prognosis of tall cell subtypes compared with those of conventional PTCs. As such, aggressive treatment is recommended, particularly in the early stages of the disease. Given that most pathologists are familiar with tall cell subtype features and generously sample the thyroid gland, it is common to identify small (subcentimeter) tall cell subtypes confined to the thyroid without vascular invasion.
Several issues pertaining to aggressive subtypes warrant further investigation. It remains unclear how to treat patients with a tall cell component that does not reach the “official” diagnostic percentage cutoff. The tall cell subtype is defined as a tumor composed predominantly of neoplastic cells with a 3:1 height-to-width ratio and exhibits abundant eosinophilic (oncocytic-like) cytoplasm. The diagnosis of the tall cell subtype requires tall cells to account for 30% or more of all tumor cells (3). A tumor with tall cells that account for less than 30% is considered the conventional subtype; however, having a treatment plan is difficult for the conventional subtype of PTCs. With regard to the columnar cell subtype, identifying indolent encapsulated cases is critical because they have better outcomes with a low incidence of recurrence or metastasis (19-21). Studies have recommended strict treatment and follow-up for the solid subtype because this subtype has higher rates of vascular invasion and recurrence than conventional PTC (23). These tumors may be treated using targeted molecular therapy in the future (24). One aspect that must be considered is that the diffuse sclerosing subtype, which is more prevalent in children and young patients, tends to be iodine avid, and even patients with distant metastases have a good prognosis (25, 26). By contrast, the other subtypes, which predominantly affect older patients and exhibit locally invasive or recurrent disease, are RAI refractory and have a poor prognosis. In general, the prognosis for small (T1 or T2) tumors that are intrathyroidal at presentation is excellent, similar to that for PTC. However, there is a paucity of studies on the aggressiveness of small tumors, which precludes optimal treatment decisions for aggressive PTC subtypes smaller than or equal to 1 cm. As such, this study analyzed the features of aggressive PTCs with tumor sizes 1 cm or smaller based on histological characteristics, with the goal of defining treatment directions for small PTCs. Our analysis revealed that aggressive PTC subtypes exhibited aggressiveness with substantial extrathyroidal extension and central and lateral neck node metastasis compared with nonaggressive PTC subtypes (see Tables 2 and 3). Despite the lack of statistical significance, recurrence rates and disease-free survival tended to be shorter and were associated with more frequent relapse.
One limitation of this study is its retrospective single-center design, which may not be representative of all PTC cases. Furthermore, the criteria for classifying PTC subtypes have been revised periodically, thus precluding the accurate classification of PTC subtypes.
In conclusion, small (≤ 1 cm) aggressive PTC subtypes exhibited more aggressive features, alongside extrathyroidal extension and neck node metastasis. Despite the lack of statistical significance, aggressive subtypes of small PTCs tended to exhibit recurrence. This study suggests that surgeons should consider the aggressive subtypes as important factors when deciding the range of surgery in PTCs smaller than 1 cm.
Financial Support
No funding or sponsorship was received for this study or the publication of this article.
Abbreviations
- ATA
American Thyroid Association
- CCND
central compartment node dissection
- FNA
fine-needle aspiration
- PTC
papillary thyroid carcinoma
- PTMC
papillary thyroid microcarcinoma
- RAI
radioactive iodine
Contributor Information
Jin Seok Lee, Department of Surgery, Thyroid Cancer Center, Gangnam Severance Hospital, Institute of Refractory Thyroid Cancer, Yonsei University College of Medicine, Seoul 06273, Korea.
Jun Sung Lee, Department of Surgery, Thyroid Cancer Center, Gangnam Severance Hospital, Institute of Refractory Thyroid Cancer, Yonsei University College of Medicine, Seoul 06273, Korea.
Hyeok Jun Yun, Department of Surgery, Thyroid Cancer Center, Gangnam Severance Hospital, Institute of Refractory Thyroid Cancer, Yonsei University College of Medicine, Seoul 06273, Korea.
Seok Mo Kim, Department of Surgery, Thyroid Cancer Center, Gangnam Severance Hospital, Institute of Refractory Thyroid Cancer, Yonsei University College of Medicine, Seoul 06273, Korea.
Hojin Chang, Department of Surgery, Thyroid Cancer Center, Gangnam Severance Hospital, Institute of Refractory Thyroid Cancer, Yonsei University College of Medicine, Seoul 06273, Korea.
Yong Sang Lee, Department of Surgery, Thyroid Cancer Center, Gangnam Severance Hospital, Institute of Refractory Thyroid Cancer, Yonsei University College of Medicine, Seoul 06273, Korea.
Hang-Seok Chang, Department of Surgery, Thyroid Cancer Center, Gangnam Severance Hospital, Institute of Refractory Thyroid Cancer, Yonsei University College of Medicine, Seoul 06273, Korea.
Cheong Soo Park, Department of Surgery, CHA Ilsan Medical Center, Goyang-si 10414, Korea.
Author Contributions
Study concept and design: Y.L. Data acquisition, analysis, and interpretation: J.L. Drafting of the manuscript: J.L., H.Y., Y.L., and H.S.C. Statistical analysis: J.L. All authors contributed to the article and approved the submitted version.
Disclosures
The authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Lloyd RV, Osamura RY, Klöppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. Vol 10, 4th ed. International Agency for Research on Cancer; 2017. [Google Scholar]
- 2. Clark OH. Thyroid cancer and lymph node metastases. J Surg Oncol. 2011;103(6):615‐618. [DOI] [PubMed] [Google Scholar]
- 3. Baloch ZW, Asa SL, Barletta JA, et al. . Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022;33(1):27‐63. [DOI] [PubMed] [Google Scholar]
- 4. A Snapshot of Thyroid Cancer: Incidence and mortality. National Cancer Institute; 2014. Accessed June 17, 2022.https://www.cancer.gov/research/progress/snapshots/thyroid
- 5. Silver CE, Owen RP, Rodrigo JP, Rinaldo A, Devaney KO, Ferlito A. Aggressive variants of papillary thyroid carcinoma. Head Neck. 2011;33(7):1052‐1059. [DOI] [PubMed] [Google Scholar]
- 6. Kwak JY, Kim EK, Hong SW, et al. . Diffuse sclerosing variant of papillary carcinoma of the thyroid: ultrasound features with histopathological correlation. Clin Radiol. 2007;62(4):382‐386. [DOI] [PubMed] [Google Scholar]
- 7. Vuong HG, Kondo T, Pham TQ, et al. . Prognostic significance of diffuse sclerosing variant papillary thyroid carcinoma: a systematic review and meta-analysis. Eur J Endocrinol. 2017;176(4):433‐441. [DOI] [PubMed] [Google Scholar]
- 8. Nath MC, Erickson LA. Aggressive variants of papillary thyroid carcinoma: hobnail, tall cell, columnar, and solid. Adv Anatomic Pathol. 2018;25(3):172‐179. [DOI] [PubMed] [Google Scholar]
- 9. Gaertner EM, Davidson M, Wenig BM. The columnar cell variant of thyroid papillary carcinoma. Case report and discussion of an unusually aggressive thyroid papillary carcinoma. Am J Surg Pathol. 1995;19(8):940‐947. [DOI] [PubMed] [Google Scholar]
- 10. Coca-Pelaz A, Shah JP, Hernandez-Prera JC, et al. . Papillary thyroid cancer-aggressive variants and impact on management: a narrative review. Adv Ther. 2020;37(7):3112‐3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee YS, Nam KH, Chung WY, Chang HS, Park CS. Postoperative complications of thyroid cancer in a single center experience. J Korean Med Sci. 2010;25(4):541‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jin WX, Ye DR, Sun YH, et al. . Prediction of central lymph node metastasis in papillary thyroid microcarcinoma according to clinicopathologic factors and thyroid nodule sonographic features: a case-control study. Cancer Manage Res. 2018;10:3237‐3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang F, Zhong Q, Huang Z, Lian M, Fang J. Survival in papillary thyroid microcarcinoma: a comparative analysis between the 7th and 8th versions of the AJCC/UICC staging system based on the SEER database. Front Endocrinol (Lausanne). 2019;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arora N, Turbendian HK, Kato MA, Moo TA, Zarnegar R, Fahey TJ III. Papillary thyroid carcinoma and microcarcinoma: is there a need to distinguish the two? Thyroid. 2009;19(5):473‐477. [DOI] [PubMed] [Google Scholar]
- 15. Park YJ, Kim YA, Lee YJ, et al. . Papillary microcarcinoma in comparison with larger papillary thyroid carcinoma in BRAF(V600E) mutation, clinicopathological features, and immunohistochemical findings. Head Neck. 2010;32(1):38‐45. [DOI] [PubMed] [Google Scholar]
- 16. Haugen BR, Alexander EK, Bible KC, et al. . 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sugitani I, Ito Y, Takeuchi D, et al. . Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: consensus statements from the Japan Association of Endocrine Surgery Task Force on Management for Papillary Thyroid Microcarcinoma. Thyroid. 2021;31(2):183‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang S, Xiong Y, Zhao Q, Song H, Yi P, Liu C. Columnar cell papillary thyroid carcinoma prognosis: findings from the SEER database using propensity score matching analysis. Am J Transl Res. 2019;11(9):6262‐6270. [PMC free article] [PubMed] [Google Scholar]
- 19. Wenig BM, Thompson LD, Adair CF, Shmookler B, Heffess CS. Thyroid papillary carcinoma of columnar cell type: a clinicopathologic study of 16 cases. Cancer. 1998;82(4):740‐753. [PubMed] [Google Scholar]
- 20. Cho J, Shin JH, Hahn SY, Oh YL. Columnar cell variant of papillary thyroid carcinoma: ultrasonographic and clinical differentiation between the indolent and aggressive types. Korean J Radiol. 2018;19(5):1000‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang C, Cheng T, Zheng X, et al. . Clinical behaviors of rare variants of papillary thyroid carcinoma are associated with survival: a population-level analysis. Cancer Manage Res. 2018;10:465‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gunalp B, Okuyucu K, Ince S, Ayan A, Alagoz E. Impact of tall cell variant histology on predicting relapse and changing the management of papillary thyroid carcinoma patients. Hell J Nucl Med. 2017;20(2):122‐127. [DOI] [PubMed] [Google Scholar]
- 23. Vuong HG, Odate T, Duong UN, et al. . Prognostic importance of solid variant papillary thyroid carcinoma: a systematic review and meta-analysis. Head Neck. 2018;40(7):1588‐1597. [DOI] [PubMed] [Google Scholar]
- 24. Ambrosi F, Righi A, Ricci C, Erickson LA, Lloyd RV, Asioli S. Hobnail variant of papillary thyroid carcinoma: a literature review. Endocr Pathol. 2017;28(4):293‐301. [DOI] [PubMed] [Google Scholar]
- 25. Malandrino P, Russo M, Regalbuto C, et al. . Outcome of the diffuse sclerosing variant of papillary thyroid cancer: a meta-analysis. Thyroid. 2016;26(9):1285‐1292. [DOI] [PubMed] [Google Scholar]
- 26. Yamashita H, Noguchi S, Takahashi H. The diffuse sclerosing variant of papillary thyroid carcinoma is not an aggressive subtype of papillary carcinoma. J Thyroid Disord Ther. 2014;3:163. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.