Abstract
Context
Prospective studies have demonstrated the efficacy of osilodrostat in Cushing disease. No study has evaluated osilodrostat in a series of patients with paraneoplastic Cushing syndrome/ectopic adrenocorticotropin syndrome (PNCS/EAS).
Objective
This work aimed to evaluate in France the real-world efficacy and safety of osilodrostat in patients with PNCS/EAS.
Methods
A total of 33 patients with PNCS/EAS with intense/severe hypercortisolism were involved in this retrospective, multicenter, real-world study. Patients received osilodrostat between May 2019 and March 2022 at a median initial dose (range) of 4 mg/day (1-60) and maximum dose, 20 mg/day (4-100), first under patient then cohort temporary authorizations and after marketing authorization. Regimens used titration (n = 6), block and replace (n = 16), or titration followed by block and replace (n = 11).
Results
In 11 patients receiving osilodrostat as first-line monotherapy, median 24-hour urinary free cortisol (24h-UFC) decreased dramatically (from 26 × upper limit of normal [ULN; 2.9-659] to 0.11 × ULN [0.08-14.9]; P < .001). In 9 of them, 24h-UFC normalization was achieved in 2 weeks (median). Thirteen additional patients were previously treated with classic steroidogenesis inhibitors but 10 of these 13 were not controlled. In these patients, osilodrostat monotherapy, used as second line, induced a significantly decreased of 24h-UFC (from 2.6 × ULN [1.1-144] to 0.22 × ULN [0.12-0.66]; P < .01). Nine additional patients received osilodrostat in combination with another anticortisolic drug, decreasing 24h-UFC from 11.8 × ULN (0.3-247) to 0.43 × ULN (0.33-2.4) (P < .01). In parallel, major clinical symptoms/comorbidities improved dramatically with improvement in blood pressure, hyperglycemia, and hypokalemia, allowing the discontinuation or dose reduction of patient treatments. Adrenal insufficiency (grade 3-4) was reported in 8 of 33 patients.
Conclusion
Osilodrostat is a rapidly efficient therapy for PNCS/EAS with severe/intense hypercortisolism. Osilodrostat was generally well tolerated; adrenal insufficiency was the main side effect.
Keywords: Cushing syndrome, ectopic adrenocorticotropic hormone syndrome, corticotropin, paraneoplastic Cushing syndrome, neuroendocrine tumors, steroidogenesis inhibitors, osilodrostat, hypokalemia, adrenal insufficiency
Paraneoplastic Cushing syndrome (PNCS) is the cause of a minority of cases of adrenocorticotropic hormone (ACTH)-dependent CS (1-4). PNCS is related to ectopic secretion of ACTH by an extrapituitary neuroendocrine tumor, and is also referred to as ectopic ACTH syndrome (EAS) (1-4). PNCS/EAS is associated with recent onset of generally very intense and severe hypercortisolism, often leading to life-threatening clinical manifestations (2, 4, 5-7). These life-threatening complications include metabolic disorders such as hypokalemia (2, 4, 5) and diabetes; vascular pathologies such as arterial hypertension (HTN); thromboembolic events (2, 4, 6-8); and catabolic changes that may lead to myopathy, skin lesions, and fractures (2-5, 6-9). Severe hypercortisolism caused by PNCS/EAS is also associated with the development of viral, bacterial, or parasitic infections (2, 4, 9, 10) and acute psychiatric disorders (2-4, 6, 7, 11).
In this instance, interruption or substantial reduction of intense cortisol production is urgently needed to improve all of these pathologies and prevent further life-threatening complications (2, 4, 9, 11, 12). Resection of the primary tumor may not possible in a number of cases such as occult and covert or metastatic ACTH-secreting tumors (2, 4, 11, 12). Bilateral adrenalectomy has been proposed to control severe and intense CS (13-15); however, both general anesthesia and surgery are not always feasible or risk free in patients with multiple complications (2, 11, 12, 15, 16). For these patients, in agreement with Endocrine Society recommendations, first-line combinations therapy with steroid synthesis inhibitors such as metyrapone and ketoconazole have been shown to control hypercortisolism and are preferred by other teams to avoid lifelong adrenal insufficiency (17-21). This goal is particularly important when ACTH-secreting neuroendocrine tumors (NETs) are small and localized or occult since they are associated with an excellent long-term prognosis.
Osilodrostat, initially known as LCI-699, is a recently developed anticortisolic inhibitor of steroidogenesis that acts mainly on both adrenal 11-hydroxylase and aldosterone synthase (22, 23). Its anticortisolic efficacy, mainly in patients with Cushing disease, has been clearly demonstrated in phase 1 to 3 studies (24-26) and was the basis for marketing authorization in Europe in January 2020 and in the United States in March 2020 for endogenous CS (orphan drug designation) (https://www.ema.europa.eu/en/medicines/human/EPAR/isturisa; https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-adults-cushings-disease). Since its authorization, effective cortisol inhibition has also been reported in a limited number of cases of CS associated with cortisol secretion by malignant adrenocortical cancer (27). However, osilodrostat has been evaluated in only a handful of patients with PNCS/EAS (28-30), and the preliminary results of these case reports suggest effective control of hypercortisolism.
In France, osilodrostat received a temporary authorization for use (TAU) from the French National Agency for Medicines and Health Products Safety (ANSM, Agence nationale de sécurité du médicament et des produits de santé) in April 2019 (nominative TAU) followed by a cohort TAU (https://ansm.sante.fr/tableau-acces-derogatoire/osilodrostat-1-mg-comprime-pellicule-isturisa). As a result, osilodrostat use was possible in France for CS of all causes, including for patients with PNCS/EAS.
The purpose of this study was to report real-world clinical experience with the use of osilodrostat in a large number (n = 33) of patients with severe PNCS/EAS who were treated between May 2019 and March 2022 by French teams. The data we compiled in this real-life observational study are to our knowledge the largest reported series of PNCS that received this medical treatment. The data we present here indicate that osilodrostat is an effective drug in patients with intense and severe hypercortisolism caused by PNCS/EAS.
Materials and Methods
This study includes items recommended by the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement (https://journals.plos.org/plosmedicine/article? id = 10.1371/journal.pmed.0040296).
Data from 33 patients with PNCS/EAS treated with osilodrostat from May 2019 and March 2022 were included. In all participating centers the same form was used to collect data that were part of routine patient management, and were then anonymized. Ethics committee approval was obtained for the analysis (from the French National Agency for Medicines, ANSM No. 2022-AO1139-34 and Paris-Saclay University No. 09122648 as well as approval by each local ethics committee). Patients were eligible for the study if they had poor initial clinical status due to intense hypercortisolism and/or severe comorbidities related to underlying PNCS/EAS.
The patient population included 15 women and 18 men, with a median age of 65 years (range, 24-85 years). Clinical and hormonal characteristics at diagnosis and causes of PNCS/EAS are detailed in Table 1. The ACTH-secreting NETs were gastrointestinal NETs (n = 4); small-cell lung neuroendocrine carcinoma (n = 8); bronchial carcinoid tumors/well-differentiated NETs located in the bronchi (n = 7); pancreatic well-differentiated NET (n = 5); ear nose throat NETs (n = 1); parotid NETs (n = 1); occult tumors (n = 2); NETs of unknown origin (n = 2); medullary thyroid carcinoma (n = 2); and neuroendocrine prostate cancer (n = 1).
Table 1.
Clinical and hormonal characteristics of patients at diagnosis
| No. | Sex | Age, y | Tumor | Metastasis | UFC | Serum cortisol, ng/mLb | ACTH, pg/mL | K+, mmol/L | Hypertension | Diabetes | Complications | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg/24 ha | × ULN | |||||||||||
| 1 | M | 66 | GI-NET | Yes | 32 950 | 659 | 980 | 126 | 1.7 | Yes | Yes | S |
| 2 | M | 59 | SCLC | Yes | 21 448 | 247 | 834 | 763 | 2.2 | Yes | Yes | F |
| 3 | F | 60 | SCLC | Yes | 10 000 | 154 | 1150 | 116 | 2.2 | Yes | No | HF |
| 4 | M | 63 | SCLC | Yes | 9550 | 147 | 1350 | 495 | 2.7 | Yes | Yes | S |
| 5 | M | 70 | SCLC | Yes | 2640 | 59 | 700 | 250 | 2.7 | Yes | No | HF |
| 6 | F | 77 | Bca | No | 5445 | 84 | 348 | 67 | 2.1 | Yes | Yes | S; HF |
| 7 | M | 60 | Pca | Yes | 25 382 | 292 | 700 | 554 | 2.4 | Yes | Yes | — |
| 8 | F | 45 | GI-NET | Yes | 3940 | 79 | 500 | 165 | 2.9 | Yes | Yes | — |
| 9 | F | 65 | ENT | No | 3842 | 77 | 700 | 380 | 1.9 | Yes | Yes | S; P |
| 10 | M | 64 | GI-NET | Yes | 1930 | 24 | 743 | 220 | 1.9 | Yes | Yes | S; P |
| 11 | F | 46 | Parotid | Yes | 2157 | 27 | 405 | 345 | 2.5 | No | No | DVT |
| 12 | M | 85 | SCLC | Yes | 1678 | 26 | 617 | 125 | 2.1 | Yes | Yes | HF |
| 13 | M | 59 | GI-NET | No | 2560 | 32 | 1189 | 232 | 2.1 | Yes | Yes | — |
| 14 | F | 71 | Bca | No | 633 | 13 | 250 | 137 | 2.1 | Yes | Yes | — |
| 15 | F | 68 | Bca | Yes | 588 | 12 | 203 | 49 | 2.5 | Yes | Yes | HF |
| 16 | M | 74 | Occult | No | 500 | 11 | 200 | 180 | 2.5 | Yes | Yes | S; P |
| 17 | F | 72 | Bca | No | 475 | 7 | 357 | 78 | 2.9 | Yes | Yes | — |
| 18 | M | 53 | Bca | Yes | 468 | 7 | 250 | 122 | 2.7 | Yes | No | DVT; P; F |
| 19 | F | 57 | Occult | No | 1300 | 20 | 435 | 196 | 2.9 | Yes | Yes | S; F |
| 20 | F | 24 | Bca | No | 1803 | 28 | 197 | 102 | 4.2 | Yes | No | — |
| 21 | F | 57 | Pca | Yes | 2974 | 39 | 578 | 139 | 2.3 | Yes | No | — |
| 22 | F | 71 | Unk | Yes | 116 | 2 | 241 | 123 | 3.0 | Yes | No | S; DVT |
| 23 | M | 71 | MTC | Yes | 190 | 3 | 141 | 26 | 4.4 | Yes | No | P; F |
| 24 | M | 74 | NEPC | Yes | 369 | 5 | 362 | 65 | 1.5 | Yes | No | P |
| 25 | M | 56 | Unk | Yes | 4092 | 47 | 467 | 181 | 2.5 | Yes | Yes | — |
| 26 | M | 76 | MTC | Yes | 80 | 2 | 187 | 31 | 4.4 | Yes | Yes | — |
| 27 | F | 71 | Pca | Yes | 555 | 7 | 329 | 233 | 2.5 | Yes | Yes | S; HF |
| 28 | M | 58 | SCLC | No | 835 | 10 | 873 | 250 | 2.4 | Yes | No | — |
| 29 | M | 45 | Pca | Yes | 27 273 | 420 | 747 | 159 | 2.4 | No | Yes | S |
| 30 | M | 74 | Bca | No | 1322 | 17 | 919 | 241 | 3.1 | Yes | Yes | — |
| 31 | M | 71 | SCLC | Yes | 8120 | 102 | 711 | 90 | 2.2 | Yes | No | — |
| 32 | F | 51 | SCLC | Yes | NA | NA | > 1000 | 164 | 2.5 | No | No | P |
| 33 | F | 71 | Pca | No | 770 | 10 | 267 | 169 | 3.0 | Yes | Yes | — |
| Median | 65 | 1867 | 26 | 484 | 164 | 2.5 | ||||||
Abbreviations: ACTH, adrenocorticotropin; Bca, bronchial carcinoid tumors (well-differentiated NETs located in the bronchi); ENT, ear, nose, and throat; F, female; GI-NET, gastrointestinal neuroendocrine tumor; K+, serum potassium; M, male; MTC, medullary thyroid carcinoma; NEPC, neuroendocrine prostate cancer; NET, neuroendocrine tumor; Pca, pancreatic carcinoid tumors (pancreatic NETs); SCLC, small-cell lung carcinoma; UFC, urinary free cortisol; ULN, upper limit of normal; Unk, unknown.
Conversion factor for UFC: 1 µg/24 hours = 2.76 nmol/24 hours.
Conversion factor for serum cortisol: 1 ng/mL = 2.76 nmol/L; ULN, upper limit of normal of the local hormonology laboratories. ULN for UFC was between 45 and 80 µg/24 hours (124 and 220 nmol/24 h). ULN for cortisol serum is between 180 and 220 ng/mL (496 and 607 nmol/L). ULN for ACTH is between 49 and 63 pg/mL. Hypokalemia is defined as K+ less than or equal to 3.5 mmol/L. Hypercorticism-related complications: DVT, deep vein thrombosis; F, fracture; HF, heart failure; P, psychiatric disorders; S, sepsis.
Of the 33 patients included, 30 (91%) had intense hypercortisolism at diagnosis defined as an increase in 24-hour urinary free cortisol (24h-UFC) greater than 5 times the upper limit of normal [ULN]). Of note, 25 of 33 (76%) patients had very intense hypercortisolism (11, 12) with 24h-UFC equal to or greater than 10 × ULN.
Median 24h-UFC was 1867 µg/24 hours (range, 80-32 950 µg/24 h) (5152 nmol/24 hours [220-90 942 nmol/24 h] (see individual values in Table 1).
At diagnosis, serum cortisol (see Table 1) was also significantly increased (median 484 ng/mL [range, 141-1350 ng/mL]) (1335 nmol/L [389-3726 nmol/L]) and circulating ACTH was above the ULN of the local hormonal laboratory in 91% (30/33) of patients and in the normal (inappropriate) range in 3 patients. Median serum ACTH was 164 pg/mL (26-763 pg/mL) (see Table 1).
Main comorbidities related to hypercortisolism at diagnosis are indicated in Table 1. Briefly, 30 of 33 (91%) had hypokalemia (kalemia ≤ 3.5 mmol/L), 29 of 33 (88%) had HTN, and 21 of 33 (64%) had diabetes. In addition, 20 of 33 (61%) patients had at least 1 other severe PNCS/EAS-related comorbidity (sepsis, fracture, heart failure, severe psychiatric disorders, or deep vein thrombosis) (2, 4, 6-11).
Interventions
Eleven (33%) of the 33 included patients with PNCS/EAS received osilodrostat as first-line treatment in a monotherapy regimen (patients 1, 3, 6, 8, 11, 12, 14, 16, 17, 18, and 23; Table 2). In this group, the median starting dose of osilodrostat was 10 mg/day (range, 2-40 mg/d) and the maximum dose was 20 mg/day (10-100/mg/d).
Table 2.
Time course of urinary free cortisol, comorbidities, and associated therapies in ectopic adrenocorticotropin syndrome patients receiving osilodrostat
| No. | Other anticortisolic used before osilodrostat | Other anticortisolic associated, with osilodrostat | Initial dose mg/d | Maximum dose, mg/d | Strategy | UFC, µg/24 ha | Time for UFC nadir (weeks) | Morning serum cortisol (ng/mL)b | SBP, mm Hg | DBP, mm Hg | No. of antihypertensive drugs | K+, mmol/L | Spironolactone, mg/d | Potassium supplem., g/d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | Nadir | Before | Under | Before | Under | Before | Under | Before | Under | Before | Under | Before | Under | Before | Under | |||||||
| 1 | 4 | 14 | T&B | 32 950 | 743 | 12 | 980 | 528 | 187 | 110 | 100 | 60 | 2 | 6 | 3.9 | 4.0 | 100 | 50 | 7.6 | 5.4 | ||
| 2 | M | 60 | 60 | B | 21 448 | 46 | 1 | 834 | 109 | 180 | 139 | 96 | 72 | 5 | 4 | 3.5 | 3.8 | 150 | 150 | 9.6 | 2.4 | |
| 3 | 40 | 80 | B | 10 000 | 5 | 2 | 1150 | 10 | 150 | 126 | 80 | 85 | 4 | 1 | 3.1 | 4.2 | 150 | 150 | 7.6 | 0 | ||
| 4 | K | 60 | 80 | T | 9550 | 158 | 2 | 1350 | 710 | 168 | 144 | 101 | 76 | 3 | 3 | 3.9 | 4.4 | 150 | 100 | 3.6 | 0 | |
| 5 | M | 20 | 60 | B | 6500 | 5 | 2 | 900 | 10 | 160 | 110 | 80 | 70 | 5 | 1 | 3.2 | 4.2 | 25 | 0 | 5.4 | 0 | |
| 6 | 4 | 20 | B | 5445 | 5 | 2 | 348 | 10 | 176 | 135 | 85 | 69 | 3 | 0 | 3.3 | 4.4 | 150 | 0 | 3 | 0 | ||
| 7 | K + M | 4 | 60 | T | 5419 | 57 | 10 | 519 | 85 | 150 | 119 | 90 | 69 | 1 | 2 | 2.6 | 3.5 | 150 | 0 | 0 | 0 | |
| 8 | 4 | 100 | T&B | 3940 | 88 | 40 | 500 | 138 | 170 | 121 | 70 | 58 | 2 | 2 | 3.2 | 4.2 | 25 | 0 | 3.6 | 0 | ||
| 9 | K | 2 | 20 | T | 3842 | 30 | 22 | 700 | 153 | 150 | 136 | 95 | 52 | 4 | 0 | 4.0 | 3.5 | 75 | 0 | 7.2 | 1.5 | |
| 10 | K + M | K | 10 | 40 | T&B | 2223 | 7 | 9 | 180 | 63 | 142 | 134 | 66 | 63 | 3 | 0 | 3.3 | 4.3 | 0 | 0 | 5.4 | 1.8 |
| 11 | 10 | 10 | B | 2157 | 157 | 1 | 405 | 179 | 116 | NA | 56 | NA | 0 | 1 | 3.8 | 4.1 | 0 | 25 | 15 | 2.4 | ||
| 12 | 40 | 60 | B | 1678 | 5 | 1 | 617 | 10 | 167 | 124 | 91 | 60 | 2 | 0 | 3.0 | 4.2 | 200 | 0 | 5.4 | 0 | ||
| 13 | M | 15 | 20 | B | 895 | 7 | 1 | 364 | 34 | 112 | 100 | 68 | 70 | 1 | 0 | 3.7 | 4.9 | 0 | 0 | 3.6 | 0 | |
| 14 | 2 | 10 | T&B | 633 | 14 | 56 | 250 | 31 | 170 | 125 | 90 | 65 | 2 | 1 | 3.2 | 4.2 | 50 | 50 | 1.2 | 0 | ||
| 15 | K | 4 | 4 | B | 588 | 18 | 2 | 203 | 10 | 143 | 130 | 63 | 70 | 2 | 1 | 3.9 | 4.2 | 0 | 0 | 3.6 | 0 | |
| 16 | 10 | 20 | B | 500 | 5 | 2 | 200 | 130 | 176 | 140 | 79 | 90 | 3 | 2 | 3.5 | 4.5 | 50 | 0 | NA | 0 | ||
| 17 | 4 | 20 | B | 475 | 23 | 2 | 357 | 10 | 181 | 168 | 85 | 82 | 3 | 2 | 3.4 | 3.1 | 75 | 0 | 0 | 0 | ||
| 18 | 10 | 12 | B | 468 | 5 | 2 | 176 | 10 | 140 | 129 | 98 | 75 | 2 | 2 | 3.9 | 4.5 | 0 | 0 | 0 | 0 | ||
| 19 | K + M | K + M | 10 | 60 | T&B | 356 | 5 | 6 | 174 | 10 | 126 | 126 | 89 | 80 | 4 | 0 | 3.9 | 4.5 | 50 | 0 | 6 | 0 |
| 20 | K | K | 4 | 20 | T | 340 | 123 | 7 | 206 | 117 | 152 | 109 | 75 | 49 | 3 | 3 | 4.6 | 4.4 | 150 | 75 | 0 | 0 |
| 21 | K | 20 | 30 | T&B | 283 | 10 | 41 | 241 | 16 | 172 | 116 | 92 | 68 | 0 | 0 | 3.7 | 3.5 | 0 | 0 | 0 | 0 | |
| 22 | K | 4 | 30 | T&B | 197 | 22 | 7 | 229 | 18 | 160 | 155 | 70 | 76 | 5 | 1 | 3.3 | 4.1 | 150 | 0 | 3.6 | 0 | |
| 23 | 10 | 20 | B | 190 | 5 | 1 | 141 | 10 | 140 | 130 | 77 | 61 | 1 | 0 | 4.4 | 4.0 | 0 | 0 | 0 | 0 | ||
| 24 | M | 20 | 20 | T&B | 119 | 36 | 8 | 181 | 29 | 137 | 105 | 68 | 61 | 2 | 2 | 4.1 | 5.0 | 0 | 0 | 0 | 0 | |
| 25 | M | 4 | 4 | B | 111 | 30 | 6 | 199 | 100 | 111 | 100 | 69 | 60 | 1 | 0 | 4.9 | 4.1 | 25 | 0 | 6 | 0 | |
| 26 | K | 4 | 10 | B | 105 | 15 | 2 | 147 | 196 | 124 | 110 | 65 | 51 | 3 | 2 | 4.4 | 4.1 | 0 | 0 | 0 | 0 | |
| 27 | M | 2 | 30 | B | 90 | 10 | 1 | 145 | 15 | 123 | 134 | 72 | 62 | 3 | 1 | 4.6 | 4.2 | 0 | 75 | 0 | 0 | |
| 28 | M | 10 | 10 | B | 85 | 10 | 4 | 127 | 36 | 112 | 113 | 68 | 62 | 1 | 0 | 4.0 | 4.6 | 100 | 0 | 0 | 0 | |
| 29 | M + C | C | 4 | 40 | T&B | 57 | 28 | — | 88 | 90 | 102 | 100 | 60 | 60 | 0 | 0 | 3.5 | 4.1 | 0 | 0 | 0 | 0 |
| 30 | K + M | 10 | 40 | T&B | 40 | 38 | — | 124 | 66 | 110 | 140 | 63 | 85 | 0 | 0 | 3.6 | 4.4 | 0 | 0 | 3.6 | 0 | |
| 31 | K + M | K | 1 | 7 | T | 20 | 27 | — | 163 | 72 | 105 | 140 | 60 | 70 | 2 | 2 | 4.5 | 4.2 | 50 | 50 | 0 | 0 |
| 32 | K + M | 1 | 25 | T | 16 | 45 | — | 145 | 134 | 104 | NA | 66 | NA | 0 | 0 | 4.3 | 4.6 | 0 | 0 | 0 | 0 | |
| 33 | M | 3 | 20 | T&B | 13 | 12 | — | 135 | 48 | 130 | 140 | 85 | 90 | 1 | 1 | 4.3 | 4.1 | 0 | 0 | 0 | 0 | |
| Median | 4 | 20 | 475 | 18 | 2.4 | 206 | 48 | 143 | 126 | 77 | 69 | 2.0 | 1.0 | 3.8 | 4.2 | 25.0 | 0.0 | 3.3 | 0.0 | |||
Time for UFC nadir has not been indicated for patients with normal UFC at the time of osilodrostat initiation.
Abbreviations: B, block and replace from the outset; Before, last measure before osilodrostat initiation; C, cabergoline strategy; DBP, diastolic blood pressure; K, ketoconazole; M, metyrapone; NA, not available; K+, serum potassium; SBP, systolic blood pressure; T&B, initial titration followed by block and replace; T, titration; under, last evaluation under osilodrostat. UFC, urinary free cortisol; ULN, upper limit of normal.
Conversion factor for UFC: 1 µg/24 hours = 2.76 nmol/24 hours.
Conversion factor for serum cortisol: 1 ng/mL = 2.76 nmol/L.
Thirteen (39%) of the included patients had been previously treated with other steroidogenesis inhibitor therapies, which had to be stopped and replaced by osilodrostat monotherapy used as second-line treatment (see Table 2). In this group, replacement of the previous first-line steroidogenesis inhibitors by osilodrostat (see Table 2) was decided for 1 or more of the following reasons: (a) a break in the supply of metyrapone in France (https://www.sfendocrino.org/rupture-de-production-de-la-metopirone/) in 4 patients; (b) insufficient efficacy of first-line therapy motivated the introduction of osilodrostat in 9 patients; (c), intolerance (cutaneous, digestive, hepatic) (31) to first-line anticortisolic therapy in 5 patients; and (d), replacement was related to a drug interaction with chemotherapies used in 1 patient. In these 13 patients, the median initial dose of osilodrostat used as second line was 4 mg/day (range, 1-20 mg/d) and the median maximum dose was 25 mg/day (4-60 mg/d).
Additionally, a group of 9 (27%) patients received osilodrostat in combination with another anticortisolic drug (bitherapy) (patients 2, 4, 9, 10, 15, 19, 20, 29, and 31; see Table 2). In this group, the initial dose of osilodrostat was 4 mg/day (range, 1-60 mg/d), and the maximum median dose was 40 mg/day (range, 4-80 mg/d). Prior treatments with steroidogenesis inhibitors and anticortisolic drugs used in combination with osilodrostat are shown in Table 2.
The individual duration and doses of prior treatments by metyrapone and ketoconazole are detailed in Supplementary Table S1 (32). The median daily dose of metyrapone prescribed before the introduction of osilodrostat was 2875 mg (range, 1500-4500 mg) and its median length was 14 weeks (range, 2-97 wk). The median daily dose of ketoconazole prescribed before the introduction of osilodrostat was 800 mg (range, 200-1200 mg) and its median length was 5 weeks (range, 1-103 wk).
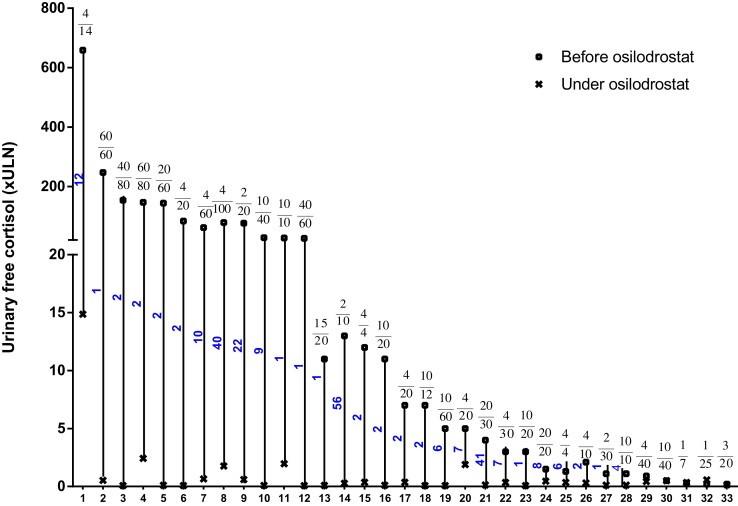
The individual initial doses of osilodrostat received by all 33 PNCS/EAS patients included in this study are shown in Fig. 1 and Table 2.
Figure 1.
Urinary free cortisol (24-h [24h-UFC], × upper limit of normal [ULN]) before and during osilodrostat treatment in 33 patients with paraneoplastic Cushing syndrome/ectopic adrenocorticotropin syndrome. Each vertical set of data points is for 1 patient. Patients are shown in order of decreasing baseline 24h-UFC concentration. For each patient the time elapsed (wk) between the introduction of the osilodrostat and the 24h-UFC nadir is shown. Patients who received osilodrostat as first-line or second line monotherapy or those who received osilodrostat in combination with another anticortisolic drug (bitherapy) are detailed in Table 2. The numbers above vertical lines indicate both initial (upper) osilodrostat dosages (mg/d), and maximum dosages (lower number) either at nadir or closest to nadir of 24h-UFC.
The treatment strategy used was either titration, without combination with hydrocortisone or another glucocorticoid replacement therapy (n = 6), a block and replace regimen upfront (n = 16), or initial titration followed by block and replace secondarily (n = 11).
The patients were followed by French endocrinologists in expert endocrinology departments of participating hospitals.
Follow-up, recommended by the French drug agency (ANSM) after granting nominative TAU followed by a cohort TAU, included systematic evaluation of clinical status, routine biochemical parameters, and hormonal evaluation to collect data to better assess the efficacy and safety of the drug in real-life use. All patients included were hospitalized and received symptomatic treatment of comorbidities including potassium supplementation, antimineralocorticoid spironolactone, antihypertensive drugs (see Table 2), and oral antidiabetes drugs or insulin.
The practical use of osilodrostat in this real-life observational study is summarized in Table 3.
Table 3.
Summary of osilodrostat use in clinical practice in 33 patients with paraneoplastic Cushing syndrome/ectopic adrenocorticotropin syndrome
| First-line monotherapy | Second-line monotherapy | Combined therapy | |
|---|---|---|---|
| No. of patients (%) | 11 (33%) | 13 (39%) | 9 (27%) |
| Previous therapiesa/associated therapiesb | 0/0 | M, K, K + M, Ca | M, K,K + M, M + C a/M, K,K + M, M + Cb |
| Starting osilodrostat dose median (range), mg/db | 10 (2-40) | 4 (1-20) | 4 (1-20) |
| Treatment strategy (n)b | T (0) | T (2) | T (4) |
| T then B (3) | T then B (5) | T then B (3) | |
| B upfront (8) | B upfront (6) | B upfront (2) | |
| Patients with 24h-UFC normalization, n (%) | 9 (82%) | 13 (100%) | 6 (67%) |
| Time (wk) required to normalize 24h-UFC, median, rangeb | 2 (1-56) | 5 (1-41) | 4 (1-22) |
Hormonal Evaluation
Hormone concentrations were measured in each center by accredited (COFRAC, France; www.cofrac.fr) clinical endocrinology laboratories, all of which participate in the French national quality control program for steroid and peptide hormone immunoassays (Pro-BioQual, France; www.probioqual.com).
Various immunoassays or liquid chromatography–mass spectrometry assays were used at the different centers to measure serum and urinary cortisol. Serum ACTH levels were measured using sandwich immunoassays (immunoradiometric assay, enzyme immunoassay) available in France. In brief, for 24h-UFC, the ULN was between 45 and 80 µg/24 hours. For the morning serum cortisol, the ULN was between 180 and 220 ng/mL. For the morning ACTH, the ULN was between 49 and 63 pg/mL.
Clinical Evaluation
Clinical assessment of CS was retrospectively evaluated from data collected from medical charts and forms using a semiquantitative arbitrary clinical score adapted from Ferriere et al (33) and including relevant clinical signs also evaluated in the LINC 3 and LINC 4 trials (25, 26). The score included the following 7 items: obesity of the face and trunk; purple stretch marks; myopathy; supraclavicular fat pad; buffalo hump; facial erythrosis; and facial plethora. In premenopausal women, the score included the same previous items as well as 2 additional items: hirsutism and amenorrhea. In postmenopausal women, the same items as previously were used, with the exception of amenorrhea.
Each of the clinical items was scored as 0 (absent), 1 (low), or 2 (high), resulting in a total score of 14, 18, and 16, respectively, in men, premenopausal women, and postmenopausal women. To standardize these scores, the value 1.0 corresponded to the maximum in each aforementioned patient category.
Statistical Analysis
The results are expressed as medians and ranges [min-max]. The Wilcoxon matched pairs test was used for comparison of UFC, serum cortisol, clinical score, systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI), and the biological parameters kalemia, glycemia, and glycated hemoglobin A1c (HbA1c) in PNCS/EAS before and during osilodrostat therapy. P values less than .05 were considered statistically significant. GraphPad Prism Software (version 9.4.0) was used for statistical analysis.
Results
Hormonal Efficacy
In the 11 patients treated with osilodrostat monotherapy, prescribed as first-line therapy (group 1, patients 1, 3, 6, 8, 11, 12, 14, 16, 17, 18, and 23 in Table 2), 24h-UFC decreased dramatically, from a preintervention median of 1678 µg/24 hours (190-32 950µg/24 h) (4631 nmol/24 h [524-90 942 nmol/24 h]) to 5 µg/24 hours (5-743µg/24 h) (13.8 nmol/24 h [13.8-2050 nmol/24 h]) (P < .001). Normalization of 24h-UFC was achieved in 9 of these 11 patients with a median delay of 2 weeks (range, 1-56 week) (see Fig. 1 and Table 2). In the 2 remaining patients, 24h-UFC decreased dramatically (44-fold for patient 1 and 14-fold for patient 11). In parallel, serum cortisol also decreased significantly, from 357 ng/mL (141-1150 ng/mL) (985 nmol/L [389-3174 nmol/L]) to 10 ng/mL (10-528 ng/mL) (27.6 ng/mL [27.6-1457 nmol/L]) (P < .001).
Among the 13 patients who were previously treated with other anticortisolic therapies (group 2), 10 were not controlled (24h-UFC > ULN) before the introduction of osilodrostat. They received osilodrostat used as monotherapy (patients 5, 7, 13, 21, 22, 24, 25, 26, 27, 28; see Fig. 1 and Table 2). During osilodrostat monotherapy, 24h-UFC was normalized in all 10 patients, with a decrease from 158 µg/24 hours (85-6500 µg/24 h) (436 nmol/24 h [234-17 940 nmol/24 h]) to 12 µg/24 hours (5-57 µg/24 h) (33 nmol/24 h [13.8-157 nmol/24 h]) (P < .01). In the remaining 3 patients whose 24h-UFC was already normalized at the time of osilodrostat initiation, cortisol control (24h-UFC < ULN) was maintained (patients 30, 32, and 33; see Table 2).
Finally, in the 9 patients who were treated with osilodrostat in combination with another anticortisolic therapy (group 3, patients 2, 4, 9, 10, 15, 19, 20, 29, and 31; see Fig. 1 and Table 2), 24h-UFC decreased from a median of 588 µg/24 hours (20-21 448 µg/24 h) (1622 nmol/L [55-59 196 nmol/L]) to 28 µg/24 hours (5-158 µg/24 h) (77 nmol/L [13.8-436 nmol/L]) (P < .01).
For all 33 patients included, 24h-UFC individual changes on osilodrostat therapy (before introduction and nadir achieved under this drug) are detailed in Table 2.
Clinical Efficacy and Effect on Cushing Syndrome–related Comorbidities
Hypertension
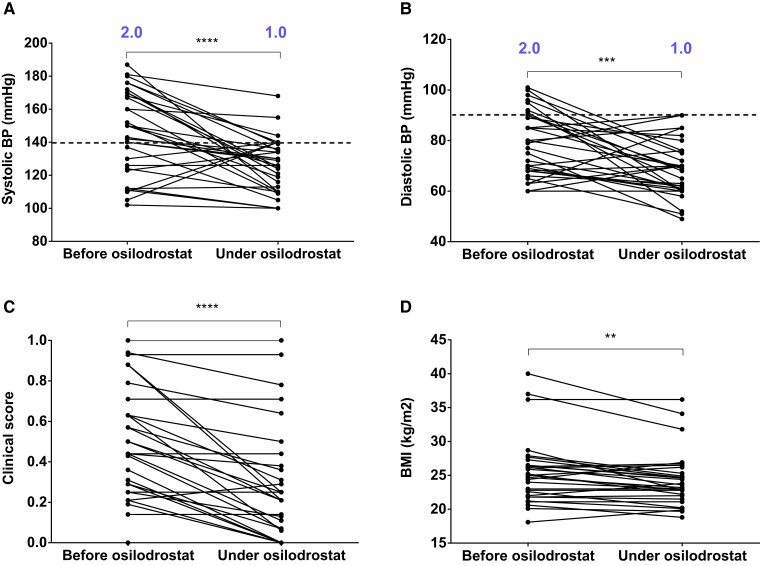
Thirty of the 33 patients (91%) had HTN at the time of PNCS/EAS diagnosis (see Table 2), and 28 had already received an antihypertensive treatment at the time of osilodrostat initiation. In addition, 18 of 33 (54.5%) patients had SBP greater than 140 mm Hg and 7 of 33 (21%) had DBP greater than 90 mm Hg when osilodrostat was initiated. Only 3 of 33 patients had SBP that remained greater than 140 mm Hg and none had DBP greater than 90 mm Hg during osilodrostat treatment.
Median SBP decreased from 143 mm Hg (102-187 mm Hg) to 126 mm Hg (100-168 mm Hg) with osilodrostat treatment (P < .0001) (Fig. 2A and Table 2). Median DBP decreased from 77 mm Hg (56-101 mm Hg) to 69 mm Hg (49-90 mm Hg) with osilodrostat treatment (P < .001) (Fig. 2B and Table 2). This improvement both in SBP and DBP was paralleled by a decrease in the number of antihypertensive treatments (see Table 2).
Figure 2.
Time course of clinical parameters/comorbidities associated with hypercortisolism in patients with paraneoplastic Cushing syndrome/ectopic adrenocorticotropin syndrome treated by osilodrostat. A and B, Systolic (SBP) and diastolic blood pressure (DBP) at the time of osilodrostat introduction and during osilodrostat treatment after control of hypercortisolism, respectively. The median number of antihypertensive medications are indicated in blue. Dotted line indicates the upper limit of normal (European Society of Hypertension Guidelines). C, Evolution of the arbitrary clinical score of Cushing syndrome at the time of the introduction of osilodrostat and during osilodrostat treatment after control of hypercortisolism (see text). D, Change in body mass index (BMI) at the time of osilodrostat introduction and during osilodrostat treatment after control of hypercortisolism. **P less than .01; ***P less than .001; ****P less than .0001 (Wilcoxon matched-pairs signed rank test).
Clinical signs of hypercortisolism
The arbitrary clinical score reflecting CS severity (Fig. 2C) decreased significantly with osilodrostat treatment, from 0.44 to 0.21 (P < .0001). In addition, BMI significantly decreased with osilodrostat treatment, from 25.0 (18-40) to 23.4 (19-36) (P < .01) (Fig. 2D).
Myopathy
Of note, before osilodrostat therapy, 3 patients (6, 19, and 23; see Table 1) were hospitalized with functional impotence resulting in a bedridden state for several months due to major myopathy. After 3 to 6 months of osilodrostat therapy, these patients were able to ambulate again, move around independently, and were able to return to their home.
Hypokalemia
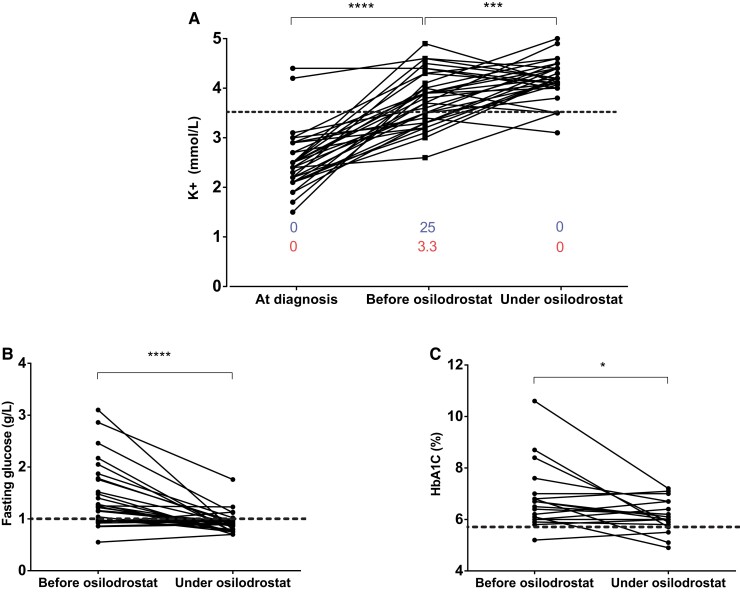
At the time of initial diagnosis of PNCS/EAS and before any potassium supplementation, hypokalemia (kalemia ≤ 3.5 mmol/L) was detected in 30 of 33 (91%) patients (see Table 1). Median kalemia at this time was 2.5 mmol/L (minimum 1.5 mmol/L) (see Table 1). At osilodrostat treatment initiation, that is, after potassium supplementation and/or spironolactone treatment, 13 of 33 (39%) patients were still hypokalemic with a median kalemia of 3.8 mmol/L (range, 2.6-4.9 mmol/L). After osilodrostat treatment, only 4 of 33 (12%) patients remained hypokalemic and circulating potassium increased to 4.2 mmol/L (range, 3.1-5.0 mmol/L) (P < .001). Notably, this increase in circulating potassium occurred in parallel with a decrease in symptomatic treatments (potassium and spironolactone; Fig. 3A and Table 2).
Figure 3.
Evolution of main biological disturbances associated with hypercortisolism in patients with paraneoplastic Cushing syndrome/ectopic adrenocorticotropin syndrome (PNCS/EAS) treated with osilodrostat. A, Individual circulating potassium levels in patients with PNCS/EAS, measured at diagnosis, at the time of introduction of osilodrostat (before osilodrostat) and after reaching nadir of urinary free cortisol (24h-UFC) (during osilodrostat) (see also Tables 1 and 2). Blue numbers indicate median spironolactone dosage (mg/d) and red numbers indicate median potassium dosage (g/d). Red dotted line indicates the lower limits of normal. B and C, Evolution of fasting blood glucose and glycated hemoglobin A1c (HbA1c), respectively, at time of the introduction of osilodrostat and after nadir of UFC. Dotted line indicates upper limit of normal in a nondiabetic French population. *P less than .05; ***P less than .001; ****P less than .0001 (Wilcoxon matched-pairs signed rank test).
Hyperglycemia and diabetes
During osilodrostat therapy, available fasting blood glucose levels decreased very significantly, from 1.2 g/L (range, 0.6-3.1 g/L) before treatment to 0.9 g/L (range, 0.7-1.8 g/L) (P < .0001) (Fig. 3B). Similarly, available HbA1c decreased significantly from 6.7% (range, 4.2%-10.6%) before osilodrostat treatment to 6.1% (range, 4.9%-7.2%) on therapy (P < .05) (Fig. 3C). This improvement in fasting blood glucose and HbA1c was associated with a reduction in the number of patients requiring insulin (from 11 to 8). Moreover, in diabetic patients still requiring insulin, the median insulin dose decreased from 33 IU/day (range, 11-60 IU/d) to 14 IU/day (range, 7-30 IU/d) (P < .05).
Follow-up and Safety
Of the 33 PNCS/EAS patients evaluated in this study, 18 of the 22 with metastatic disease (see Table 1) died during follow-up, between 0.4 and 140 months after the initial diagnosis. All but one patient died of progression of their metastatic disease.
Of the 33 patients treated with osilodrostat, 8 (24%) had at least 1 episode of adrenal insufficiency (grade 3-4) revealed by clinical and biological signs (hypotension and/or hyponatremia with hyperkalemia). Of these patients, 7 who did not receive hydrocortisone before hospitalization had low plasma cortisol levels (from nondetectable to 53 ng/mL [nondetectable-146 nmol/L]).
Of note, one patient died after control of hypercortisolism (patient 10; see Table 2) of a probable acute adrenal crisis associating malaise with hypotension (SBP/DBP: 108/63 mm Hg), hyponatremia (135 mmol/L), and hyperkalemia (5.2 mmol/L). This patient was treated for PNCS/EAS secondary to metastatic gastrinoma. He went to the local emergency department for hematuria and dizziness. However, despite the existence of hypotension, hyponatremia, and hyperkaliemia, the diagnosis of acute adrenal insufficiency was not identified and no appropriate hydrocortisone therapy was started. It should be emphasized that a hormonal evaluation carried out while on osilodrostat therapy, 3 months before this patient’s death, had already shown a low morning serum cortisol (63 ng/mL; normal range, 85.0-210 ng/mL), a very low UFC at 7 μg/24 hours (normal range, 22-65 µg/24 h) associated with very high circulating ACTH levels (ACTH 4959 pg/mL [normal range, 24-60 pg/mL]), whereas before initiating osilodrostat therapy circulating ACTH levels were 220 pg/mL. These previous hormonal data associated with the clinical features preceding the death reinforce the hypothesis of an acute adrenal insufficiency.
Liver function abnormalities leading to drug discontinuation or reduction were not reported for any patients receiving osilodrostat as monotherapy, either as first-line or second-line therapy.
One patient receiving a combination of osilodrostat and ketoconazole who had liver metastases experienced an increase in liver enzymes of 3 to 5 times the ULN.
No alteration in the corrected QT interval leading to treatment discontinuation was documented in any of the 33 patients who received osilodrostat. Notably, even among patients who received the highest doses (≥ 20 mg/d), there was no statistically significant increase of the corrected QT interval (median of 412 ms before treatment and 408 ms on osilodrostat).
Discussion
The effective cortisol inhibition achieved by osilodrostat therapy in Cushing disease has been demonstrated by several prospective studies published in recent years (24-26). The efficacy of this drug has also been demonstrated in the rarer etiologies of CS such as adrenocortical carcinoma, although only in isolated clinical case reports or small series (27-29). In the most severe and intense cause of CS—secondary to PNCS/EAS—(2, 4, 5, 7, 9, 11, 12, 17, 18), which are much rarer than Cushing disease (1, 3), the efficacy of cortisol inhibition by osilodrostat has been suggested by only a few clinical case reports (28-30). The objective of this study was to evaluate in France the efficacy of cortisol inhibition by osilodrostat in a real-world setting in a large series that included 33 patients with PNCS/EAS. To our knowledge, this is one of the 2 larger worldwide-reported series of PNCS/EAS patients with intense and/or severe hypercortisolism who received medical therapy with an adrenal steroidogenesis inhibitor (19). In addition, contrary to historical series (19, 34), all PNCS/EAS patients included in our study were evaluated very recently with more homogeneous and modern approaches in terms of hormonal and imaging assessment.
In nearly one-third of patients with PNCS/EAS studied here, osilodrostat was used as monotherapy and as first-line anticortisol treatment. In 82% of these patients, normalization of 24h-UFC was obtained, confirming osilodrostat efficacy. The efficacy of osilodrostat as monotherapy in these patients was even more remarkable because almost half of the patients in this group had very intense hypercortisolism with 24h-UFC greater than 10 times the ULN when osilodrostat was introduced.
Another one-third of the patients PNCS/EAS in this study had received previous first-line treatment with other adrenal steroidogenesis inhibitors, mainly metyrapone or ketoconazole, prescribed either as monotherapy or as combined therapy. These previous treatments were discontinued for various, sometimes interrelated, reasons such as a shortage of supply (discussed later), drug intolerance, or lack of efficacy or partial efficacy. In the majority (10/13) of the patients of this group, who were not previously controlled with these steroidogenesis inhibitors, a switch to osilodrostat as monotherapy led to control of hypercortisolism.
In a third group, including 9 patients, osilodrostat was used in combination with another inhibitor of steroidogenesis, mainly metyrapone or ketoconazole, with good efficacy and satisfactory tolerance, specifically hepatic tolerance. These results indicate that a combination of osilodrostat with these classic drugs is possible in practice and may be an effective option, particularly in situations of very intense and severe hypercortisolism or when intolerance occurs when increasing the dose of one of the drugs.
The existence of this new, effective steroidogenesis inhibitor, therefore, allows for expansion of the therapeutic tools available to endocrinologists managing PNCS/EAS patients, who are often in a serious condition. In particular, osilodrostat offers useful therapeutic alternatives in cases of intolerance, unavailability, or even insufficient efficacy of the older steroidogenesis inhibitors. One point that should also be emphasized is that, in France, before the marketing authorization, the French drug agency (ANSM) very quickly granted TAU for osilodrostat following a supply interruption of metyrapone in Europe and France (https://www.nve.nl/content/uploads/2019/03/letter-for-Dutch-Society-of-Endocrinology.pdf; https://www.sfendocrino.org/rupture-de-production-de-la-metopirone/). The availability of this drug has, therefore, made it possible to avoid leaving patients with severe hypercortisolism without medical treatment.
In patients treated with osilodrostat as monotherapy, either as first-line treatment or when switching from a different anticortisolic drug, the time required to control hypercortisolism varied. This variability was possibly related to several factors, including the prior intensity of the hypercortisolism, the dose of osilodrostat used at the start of this therapy, and the speed with which a dose increase was implemented during titration (discussed later). It could also be related to the therapeutic approach used (titration vs block and replace), which could also affect the doses used and the speed with which the daily dosage is increased.
Regardless of the particular reasons for the chosen dosage, it is interesting to note that in several patients with PNCS/EAS and very intense hypercortisolism, 24h-UFC normalization could be achieved within 1 to 2 weeks, which means that osilodrostat monotherapy is suitable for emergency situations associated with severe hypercortisolism, as we have already demonstrated with a combination of other steroidogenesis inhibitors (17, 18). Given the severity of PNCS/EAS, a rapid decrease in cortisol levels is mandatory (2, 4, 11, 12, 20, 21) and it is therefore important to consider that this is an achievable goal using osilodrostat, assuming that a medical therapy strategy approach is used.
Another point that should be emphasized is the considerable variability in the daily dosage of osilodrostat used by French endocrinologists. Initial dosage was possibly partly dictated by the intensity of the hypercortisolism. Of note, the highest initial daily doses of osilodrostat (40-60 mg/d) were used in patients with very intense hypercortisolism and the lowest doses (1-2 mg/d) in patients with milder hypercortisolism. However, at some centers, patients with very intense hypercortisolism were treated with relatively low initial dosages, which indicates heterogeneous prescribing practices between centers addressing hypercortisolism of similar intensity.
Interestingly, the potency of osilodrostat and the dosage of the pills made it possible to administer high doses of the drug with a reduced number of pills. This compares favorably with metyrapone in monotherapy (19, 34) and with ketoconazole and metyrapone combination therapy (17, 18). This point of tolerance is not trivial considering the number of treatments that these patients in poor condition may receive.
In published studies that evaluated the efficacy of osilodrostat in Cushing disease, the therapeutic strategy used was exclusively titration (24-26), that is, a gradual increase of the daily dosage of osilodrostat to control hypercortisolism. Real-world practical-use strategies for PNCS/EAS implemented in this study were variable. Only a minority of patients (6/33) were treated throughout follow-up using exclusively a titration strategy. In 11 patients initially treated with a titration strategy, the therapeutic approach was switched during follow-up to a block and replace strategy. This change was motivated in some cases by the onset of adrenal insufficiency, sometimes acute, and the need to prevent its recurrence. In other cases, when it became more difficult to monitor the clinical course of hypercortisolism on osilodrostat during the 2020 to 2022 period lockdown due to the COVID pandemic, and/or a substantial distance from the patient's home to the hospital, prescribers also replaced the titration strategy with that of block and replace. In almost half of the cases, patients with PNCS/EAS were treated immediately using a block and replace strategy. The disclosed motivation of prescribers was essentially to prevent acute adrenal insufficiency.
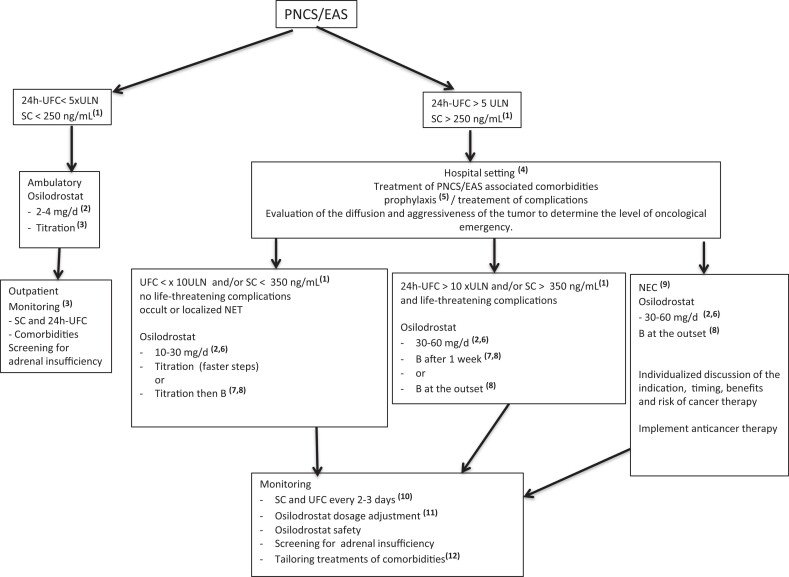
The place and strategies used as well as effectiveness of osilodrostat therapy in this real-life observational study are summarized in Table 3. On the basis of these collected data we extracted several therapeutic scenarios that take into account the intensity of hypercortisolism, the associated complications, and the tumor status of the patients. For each of these scenarios we propose a use of osilodrostat systematized in Fig. 4: In PNCS/EAS patients with moderate hypercortisolism without severe complications and without locoregional invasion or metastatic spread of the NET, we propose a titration approach with low initial doses of osilodrostat and with steps, monitoring, and follow-up similar those used in Cushing disease described in the LINC 2 to LINC 4 trials (24-26). In PNCS/EAS patients with more intense hypercortisolism (UFC > 5 but < 10 × ULN) but no life-threatening complications, we propose hospitalization and initiating osilodrostat therapy with higher doses from the start (10-30 mg/d) with close monitoring of serum cortisol and/or 24-UFC levels (every 3 d) to adapt the dose (see Fig. 4). In this situation, titration still seems possible if osilodrostat initial doses used are in the lower limit of the proposed range (ie, 10-15 mg/d) but with faster titration steps. However, a strategy of block and replace after normalization of serum and/or urinary cortisol could also be relevant in these patients given the higher risk of adrenal insufficiency and especially if a return to domicile is considered in the short or medium term. In PNCS/EAS patients with very intense hypercortisolism (24h-UFC > 10 × ULN) associated with serious complication(s) that represent a medical emergency (2), we propose mandatory hospitalization and the use of very high doses of osilodrostat from the start (30-60 mg/d) as we showed here they can rapidly control very intense and severe hypercortisolism (see Fig. 4).
Figure 4.
Proposed algorithm for practical use of osilodrostat in patients with paraneoplastic Cushing syndrome/ectopic adrenocorticotropin syndrome (PNCS/EAS): proposal in scenarios depending on the intensity of hypercortisolism, associated complications, and oncological status. These propositions are “expert advice” rather than being evidence based. (1)To convert ng/mL to nmol/L × 2.759 (1 ng/mL = 2.759 nmol/L); (2)initial dose administered orally in 2 daily doses; (3) as reported in LINC studies ((24-26)); (4)hospitalization so as to allow close monitoring and perform checklist as reported in (2) that allow to identification of major comorbidities/complications (severe hypokalemia, pulmonary embolism, heart failure, deep infection and/or sepsis, vertebral fractures);(5)prophylaxis for pneumocystis (trimethoprim/sulfamethoxazole) and thromboembolism (low-molecular-weight heparin); (6)initial dose of osilodrostat can be personalized within the proposed range according to the intensity of hypercortisolism and/or severity of comorbidities and complications as well as the general condition and age of the patient; (7)block and replace (B) strategy as soon as SC and/or 24h-UFC reach values below the upper limits of normal; (8)B: hydrocortisone: 20 to 30 mg/d or prednisone: 4 to 7 mg/d or dexamethasone: 0.5 mg/d; (9)locoregional and/or metastatic spread of responsible neuroendocrine cancer (NEC). (10)In case of replacement therapy with hydrocortisone, stop this steroid 24 hours before and replace with dexamethasone or prednisolone before measuring SC and/or 24h-UFC; (11)if SC and 24h-UFC are still high, increase osilodrostat daily dose. In this case a combined therapy associating metyrapone or with ketoconazole can also be discussed. In case of confirmed low SC and/or UFC, a decrease of daily dose of osilodrostat can also be proposed. (12)Potassium, spironolactone, insulin, oral antidiabetic, and antihypertensive drugs; 24h-UFC: 24-hour urinary free cortisol; SC: serum cortisol.
Given the higher doses of osilodrostat used in these 2 last scenarios, it seems appropriate to start glucocorticoid substitution rapidly and according to the rapidity of the fall in urinary and/or serum cortisol (see Fig. 4).
In a number of PNCS/EAS patients, management of intense/very intense hypercortisolism is even more complex because of the association of locoregional and/or metastatic NET spread. In these settings we propose the use of high doses of osilodrostat from the outset (see Fig. 4). This approach allows us to rapidly control hypercortisolism and to avoid the appearance of or worsening of the patient's condition by the onset of additional acute complications. In these severe neoplastic and endocrinological contexts, we also believe that a block and replace strategy from the outset simplifies monitoring while minimizing the risk of acute adrenal insufficiency (see Fig. 4). In this way, the anticancer treatment can be started without delay and in a less acute and unstable endocrinological context. Moreover, the block and replace procedure will enable us to avoid adrenal insufficiency that could appear in patients treated with high doses of osilodrostat in whom the secretion of tumoral ACTH (and of cortisol) would strongly decrease following the antitumor effect of the anticancer treatments.
We are aware that the aforementioned therapeutic proposals are not based on prospective controlled studies. They reflect only an “expert opinion” arising from the real-life data presented here. They should therefore not be considered rigidly, but as benchmarks pending additional studies and adapted to the particular situation of each patient.
In this study, we also provide data demonstrating that treatment with osilodrostat not only significantly reduced circulating cortisol and 24h-UFC but also improved clinical signs and metabolic disturbances related to intense hypercortisolism. Thus, in parallel with the decrease in 24h-UFC, a significant decrease in BMI and a very significant improvement in the clinical index of hypercortisolism were observed. Similarly, 3 comorbidities frequently associated with CS (6) were improved by osilodrostat therapy. Thus, in the majority of patients, SBP and DBP were normalized, while decreasing the number of antihypertensive treatments that patients were receiving before the administration of osilodrostat. Similarly, fasting hyperglycemia and HbA1c significantly improved while decreasing or even stopping the insulin therapy prescribed in some patients who had diabetes associated with CS secondary to PNCS/EAS.
Hypokalemia is a common and potentially serious complication related to intense CS caused by PNCS/EAS (2, 4, 6, 11, 20, 21) that can lead to life-threatening cardiac arrythmias. Severe hypokalemia usually requires nonspecific urgent treatments such as the administration of potassium and mineralocorticoid receptor antagonists to prevent arrhythmias (2, 5, 11). A specific effective treatment is also rapid control of hypercortisolism, which limits the mineralocorticoid effect of cortisol secreted in great excess (2, 5, 6, 35). In the patients studied here, nonspecific treatment with potassium and spironolactone made it possible to partially improve serum potassium levels in many patients before the introduction of osilodrostat. Notably, on osilodrostat, the number of patients in whom hypokalemia was corrected increased while the daily doses of potassium and spironolactone were reduced or possibly even discontinued. These data in conjunction with a decrease in 24h-UFC thus reinforce the argument for the use of osilodrostat in patients with intense hypercortisolism secondary to PNCS/EAS associated with very low serum potassium levels.
The main side effect observed in this study was adrenal insufficiency. The diagnosis of adrenal insufficiency (grade 3-4) was triggered by suggestive clinical signs and confirmed by serum cortisol measurements. In one case, death probably caused by undiagnosed adrenal insufficiency was reported. This serious side effect highlights the importance of active prevention of acute adrenal insufficiency in patients treated with osilodrostat. We believe that patients receiving osilodrostat and their relatives should undergo active therapeutic education to prevent acute adrenal insufficiency. Carrying a medical card on which the potent anticortisolic effect of osilodrostat and the risk of acute adrenal insufficiency are clearly indicated would also be helpful. We systematically recommend that the patient have access to “stress doses” of glucocorticoid tablets (eg, 20 mg hydrocortisone 4×/d) if unwell and also an emergency injection kit for intramuscular or subcutaneous injection of 100 mg hydrocortisone hemisuccinate when faced with any situation suggesting decompensation or in case of serious intercurrent complication.
It should be also remembered here that some nonspecific direct cortisol immunoassays can lead to a underestimation of the cortisol decrease and therefore prevent hormonal confirmation of the diagnosis of adrenal insufficiency in patients with CS receiving a potent 11-hydroxylase inhibitor like osilodrostat. This pitfall has already been identified in patients treated with metyrapone (36) and is linked to the detection by certain direct cortisol immunoassays of the increased circulating levels of the cortisol precursor 11-deoxycortisol and erroneously recognized as circulating cortisol. It should be noted that of the 8 patients who had an episode of adrenal insufficiency leading to hospitalization, 7 without previous hydrocortisone therapy also had very low serum cortisol levels. These data indicate that in these patients the serum cortisol assays were sufficiently specific to avoid interference by an increase in 11-deoxycortisol levels and therefore to prevent the misdiagnosis of adrenal insufficiency. Similarly, we must also emphasize that in the patients with PNCS/EAS and intense hypercortisolism (CFU > 5 × ULN; see Table 2) included here and who were treated with osilodrostat monotherapy, we observed a dramatic decrease (> 92%) in UFC regardless of the technique (liquid chromatography–mass spectrometry or immunoassays) used for UFC measurements. These data also suggest that the more specific modern immunoassays used by participating centers (with a cross-reactivity of 11-deoxycortisol on urinary cortisol assays reported by manufacturers < 2%) has largely limited these immunoassay artifacts.
In conclusion, we have provided real-world data that clearly demonstrate the efficacy of cortisol synthesis inhibition by osilodrostat monotherapy in a large series of CS with PNCS/EAS, including patients with very intense hypercortisolism. We also demonstrate that osilodrostat can be used in combination with other steroidogenesis inhibitors. Our data also indicate good tolerability in the majority of patients. However, the onset of adrenal insufficiency is common and must be actively prevented by incisive therapeutic education to avoid the onset of acute adrenal insufficiency, the outcome of which may be fatal. In addition, when immediate etiological treatment of severe PNCS/EAS is not feasible, osilodrostat therapy could be an effective alternative to emergency bilateral adrenalectomy, a procedure often associated with considerable morbidity and always with permanent hypoadrenalism (2).
Osilodrostat therapy also proved to be useful during palliative care of patients with diffuse malignant NET by limiting or delaying the deterioration of the patients’ general condition. Our data also demonstrated that in real life flexible therapeutic strategies, adapted to the intensity and severity of CS, are possible. Finally, we learned from this real-life study that in practical care of intense forms of CS, French endocrinologists do not necessarily use a systematic titration approach that may be considered more suited for less intense forms of CS as usually observed in most patients with Cushing disease.
Abbreviations
- ACTH
adrenocorticotropin
- BMI
body mass index
- DBP
diastolic blood pressure
- EAS
ectopic adrenocorticotropin syndrome
- HbA1c
glycated hemoglobin A1c
- HTN
hypertension
- NET
neuroendocrine tumor
- PNCS
paraneoplastic Cushing syndrome
- SBP
systolic blood pressure
- TAU
temporary authorization for use
- UFC
urinary free cortisol
- ULN
upper limit of normal
Contributor Information
Alexandre Dormoy, Paris-Saclay University; Assistance Publique-Hôpitaux de Paris, Department of Endocrinology, Reference Centre for Rare Pituitary Diseases HYPO, Bicêtre Hospital, Le Kremlin-Bicêtre, F-94275, France.
Magalie Haissaguerre, Bordeaux University, Department of Endocrinology, Haut-Lévêque Hospital, F-33600, Pessac, France.
Géraldine Vitellius, Department of Endocrinology, Robert Debré University Hospital, F-51100, Reims, France.
Christine Do Cao, Department of Endocrinology, Centre Hospitalier Régional Universitaire de Lille, F-59037, Lille, France.
Aurore Geslot, Department of Endocrinology and Metabolic Diseases, Larrey University Hospital, F-31059, Toulouse, France.
Delphine Drui, Department of Endocrinology, Institut du Thorax, CHU de Nantes, and Nantes Université, Hôpital Nord, F-44000 Nantes, France.
Hélène Lasolle, Endocrinology Department, Reference Centre for Rare Pituitary Diseases HYPO, “Groupement Hospitalier Est” Hospices Civils de Lyon, F-69500 Bron, France.
Oceana Vieira-Pinto, Paris-Saclay University; Assistance Publique-Hôpitaux de Paris, Department of Endocrinology, Reference Centre for Rare Pituitary Diseases HYPO, Bicêtre Hospital, Le Kremlin-Bicêtre, F-94275, France.
Sylvie Salenave, Paris-Saclay University; Assistance Publique-Hôpitaux de Paris, Department of Endocrinology, Reference Centre for Rare Pituitary Diseases HYPO, Bicêtre Hospital, Le Kremlin-Bicêtre, F-94275, France.
Maud François, Department of Endocrinology, Robert Debré University Hospital, F-51100, Reims, France.
Marie Puerto, Bordeaux University, Department of Endocrinology, Haut-Lévêque Hospital, F-33600, Pessac, France.
Hélène Du Boullay, Department of Endocrinology, Savoie CHMS Hospital, F-73000 Chambéry, France.
Anne Mayer, Department of Endocrinology, Savoie CHMS Hospital, F-73000 Chambéry, France.
Anne Rod, Department of Endocrinology, CH de Niort, F-79000, Niort, France.
Claire Laurent, Department of Endocrinology, CH de Niort, F-79000, Niort, France.
Philippe Chanson, Paris-Saclay University; Assistance Publique-Hôpitaux de Paris, Department of Endocrinology, Reference Centre for Rare Pituitary Diseases HYPO, Bicêtre Hospital, Le Kremlin-Bicêtre, F-94275, France; Paris-Saclay Neuroendocrine Tumors Working Group, F-94800 Villejuif, France; INSERM UMR_S 1185, Paris-Saclay Medical School, Le Kremlin-Bicêtre, F-94275, France.
Yves Reznik, Department of Endocrinology and Diabetology, CHU Côte de Nacre, F-14033 Caen Cedex, France.
Frédéric Castinetti, Department of Endocrinology, Assistance Publique-Hopitaux de Marseille, French Reference Center for Rare Pituitary Diseases, Endo-European Reference Network and EURACAN European Expert Center on Rare Pituitary Tumors, La Conception Hospital, Aix Marseille University, F-13385, Marseille, France.
Olivier Chabre, University Grenoble Alpes, UMR 1292 INSERM-CEA-UGA, Endocrinologie CHU Grenoble Alpes, F-38000 Grenoble, France.
Eric Baudin, Paris-Saclay Neuroendocrine Tumors Working Group, F-94800 Villejuif, France; INSERM UMR_S 1185, Paris-Saclay Medical School, Le Kremlin-Bicêtre, F-94275, France; Gustave Roussy Cancer Institute; Paris-Saclay University, Endocrine Oncology and Nuclear Medicine Department, F-94800 Villejuif, France.
Gérald Raverot, Endocrinology Department, Reference Centre for Rare Pituitary Diseases HYPO, “Groupement Hospitalier Est” Hospices Civils de Lyon, F-69500 Bron, France.
Antoine Tabarin, Bordeaux University, Department of Endocrinology, Haut-Lévêque Hospital, F-33600, Pessac, France.
Jacques Young, Paris-Saclay University; Assistance Publique-Hôpitaux de Paris, Department of Endocrinology, Reference Centre for Rare Pituitary Diseases HYPO, Bicêtre Hospital, Le Kremlin-Bicêtre, F-94275, France; Paris-Saclay Neuroendocrine Tumors Working Group, F-94800 Villejuif, France; INSERM UMR_S 1185, Paris-Saclay Medical School, Le Kremlin-Bicêtre, F-94275, France.
Financial Support
This work was supported in part by the French Association of Patients with Adrenal Diseases (grant “Association surrénales” to J.Y.) and by Novartis and Recordati Pharma (independent Investigator Research Grants to J.Y.). The sponsors were not involved in any part of the study, including the design, data collection, analysis, or interpretation of the results.
Disclosures
The authors have nothing to disclose.
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in “References.” The study forms used for this project are available on request from the corresponding author.
References
- 1. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing's syndrome. Lancet. 2015;386(9996) 913‐927. [DOI] [PubMed] [Google Scholar]
- 2. Young J, Haissaguerre M, Viera-Pinto O, Chabre O, Baudin E, Tabarin A. Management of endocrine disease; Cushing’s syndrome due to ectopic ACTH secretion: an expert operational opinion. Eur J Endocrinol. 2020;182(4):R29‐R58. [DOI] [PubMed] [Google Scholar]
- 3. Sharma ST, Nieman LK, Feelders RA. Cushing's syndrome: epidemiology and developments in disease management. Clin Epidemiol. 2015;7:281‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hayes AR, Grossman AB. The ectopic adrenocorticotropic hormone syndrome: rarely easy, always challenging. Endocrinol Metab Clin North Am. 2018;47(2):409‐425. [DOI] [PubMed] [Google Scholar]
- 5. Torpy DJ, Mullen N, Ilias I, Nieman LK. Association of hypertension and hypokalemia with Cushing's syndrome caused by ectopic ACTH secretion: a series of 58 cases. Ann N Y Acad Sci. 2002;970(1):134‐144. [DOI] [PubMed] [Google Scholar]
- 6. Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BMK, Colao A. Complications of Cushing's syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4(7):611‐629. [DOI] [PubMed] [Google Scholar]
- 7. Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing's syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90(8):4955‐4962. [DOI] [PubMed] [Google Scholar]
- 8. Feelders RA, Nieman LK. Hypercoagulability in Cushing's syndrome: incidence, pathogenesis and need for thromboprophylaxis protocols. Pituitary. 2022;25:746‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarlis NJ, Chanock SJ, Nieman LK. Cortisolemic indices predict severe infections in Cushing syndrome due to ectopic production of adrenocorticotropin. J Clin Endocrinol Metab. 2000;85(1):42‐47. [DOI] [PubMed] [Google Scholar]
- 10. Cristante J, Lepelley M, Mallaret M, et al. Pneumocystis pneumonia can complicate medical treatment of hypercortisolism even in outpatients with Cushing's disease. Ann Endocrinol (Paris). 2020;81(6):551‐560. [DOI] [PubMed] [Google Scholar]
- 11. Feelders RA, Newell-Price J, Pivonello R, et al. Advances in the medical treatment of Cushing's syndrome. Lancet Diabetes Endocrinol. 2019;7(4):300‐312. [DOI] [PubMed] [Google Scholar]
- 12. Zandee WT, Kamp K, van Adrichem RC, et al. Effect of hormone secretory syndromes on neuroendocrine tumor prognosis. Endocr Relat Cancer. 2017;24(7):R261‐R274. [DOI] [PubMed] [Google Scholar]
- 13. Lase I, Grönberg M, Norlén O, et al. Adrenalectomy in ectopic Cushing's syndrome: a retrospective cohort study from a tertiary care centre. J Neuroendocrinol. 2021;33(12):e13030. [DOI] [PubMed] [Google Scholar]
- 14. Landry JP, Clemente-Gutierrez U, Pieterman CRC, et al. Management of adrenocorticotropic hormone-secreting neuroendocrine tumors and the role of bilateral adrenalectomy in ectopic Cushing syndrome. Surgery. 2022;172(2):559‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reincke M, Ritzel K, Oßwald A, et al. A critical reappraisal of bilateral adrenalectomy for ACTH-dependent Cushing's syndrome. Eur J Endocrinol. 2015;173(4):M23‐M32. [DOI] [PubMed] [Google Scholar]
- 16. Reibetanz J, Kelm M, Uttinger KL, et al. Differences in morbidity and mortality between unilateral adrenalectomy for adrenal Cushing's syndrome and bilateral adrenalectomy for therapy refractory extra-adrenal Cushing's syndrome. Langenbeck Arch Surg. 2022;407(6):2481‐2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamenický P, Droumaguet C, Salenave S, et al. Mitotane, metyrapone, and ketoconazole combination therapy as an alternative to rescue adrenalectomy for severe ACTH-dependent Cushing's syndrome. J Clin Endocrinol Metab. 2011;96(9):2796‐2804. [DOI] [PubMed] [Google Scholar]
- 18. Corcuff JB, Young J, Masquefa-Giraud P, et al. Rapid control of severe neoplastic hypercortisolism with metyrapone and ketoconazole. Eur J Endocrinol. 2015;172(4):473‐481. [DOI] [PubMed] [Google Scholar]
- 19. Daniel E, Aylwin S, Mustafa O, et al. Effectiveness of metyrapone in treating Cushing's syndrome: a retrospective multicenter study in 195 patients. J Clin Endocrinol Metab. 2015;100(11):4146‐4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alexandraki KI, Grossman AB. Therapeutic strategies for the treatment of severe Cushing's syndrome. Drugs. 2016;76(4):447‐458. [DOI] [PubMed] [Google Scholar]
- 21. Nieman LK, Biller BMK, Findling JW, et al. Treatment of Cushing's syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807‐2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amar L, Azizi M, Menard J, et al. Aldosterone synthase inhibition with LCI699: a proof-of-concept study in patients with primary aldosteronism. Hypertension. 2010;56(5):831‐838. [DOI] [PubMed] [Google Scholar]
- 23. Bertagna X, Pivonello R, Fleseriu M, et al. Lci699, a potent 11β-hydroxylase inhibitor, normalizes urinary cortisol in patients with Cushing's disease: results from a multicenter, proof-of-concept study. J Clin Endocrinol Metab. 2014;99(4):1375‐1383. [DOI] [PubMed] [Google Scholar]
- 24. Fleseriu M, Pivonello R, Young J, et al. Osilodrostat, a potent oral 11β-hydroxylase inhibitor: 22-week, prospective, phase II study in Cushing's disease. Pituitary. 2016;19(2):138‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pivonello R, Fleseriu M, Newell-Price J, et al. ; LINC 3 Investigators . Efficacy and safety of osilodrostat in patients with Cushing's disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase. Lancet Diabetes Endocrinol. 2020;8(9):748‐761. [DOI] [PubMed] [Google Scholar]
- 26. Gadelha M, Bex M, Feelders RA, et al. Randomized trial of osilodrostat for the treatment of Cushing disease. J Clin Endocrinol Metab. 2022;107(7):e2882‐2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tabarin A, Haissaguerre M, Lassole H, et al. Efficacy and tolerance of osilodrostat in patients with Cushing's syndrome due to adrenocortical carcinomas. Eur J Endocrinol. 2022;186(2):K1‐K4. [DOI] [PubMed] [Google Scholar]
- 28. Tanaka T, Satoh F, Ujihara M, et al. A multicenter, phase 2 study to evaluate the efficacy and safety of osilodrostat, a new 11β-hydroxylase inhibitor, in Japanese patients with endogenous Cushing's syndrome other than Cushing's disease. Endocr J. 2020;67(8):841‐852. [DOI] [PubMed] [Google Scholar]
- 29. Haissaguerre M, Puerto M, Nunes ML, Tabarin A. Efficacy and tolerance of osilodrostat in patients with severe Cushing's syndrome due to non-pituitary cancers. Eur J Endocrinol. 2020;183(4):L7‐L9. [DOI] [PubMed] [Google Scholar]
- 30. Bessiène L, Bonnet F, Tenenbaum F, Jozwiak M, Corchia A, Bertherat GL. Rapid control of severe ectopic Cushing's syndrome by oral osilodrostat monotherapy. Eur J Endocrinol. 2021;184(5):L13‐L15. [DOI] [PubMed] [Google Scholar]
- 31. Young J, Bertherat J, Vantyghem MC, et al. Hepatic safety of ketoconazole in Cushing's syndrome: results of a compassionate use programme in France. Compassionalte use programme. Eur J Endocrinol. 2018;178(5):447‐458. [DOI] [PubMed] [Google Scholar]
- 32. Dormoy A, Haissaguerre M, Vitellius G, et al. Supplementary data for: “Efficacy and safety of osilodrostat in paraneoplastic Cushing's syndrome: a real-world multicenter study in France.” Deposited November 1, 2022. 10.6084/m9.figshare.21444357.v2 [DOI] [PMC free article] [PubMed]
- 33. Ferriere A, Cortet C, Chanson P, et al. Cabergoline for Cushing's disease: a large retrospective multicenter study. Eur J Endocrinol. 2017;176(3):305‐314. [DOI] [PubMed] [Google Scholar]
- 34. Verhelst JA, Trainer PJ, Howlett TA, et al. Short and long-term responses to metyrapone in the medical management of 91 patients with Cushing's syndrome. Clin Endocrinol (Oxf). 1991;35(2):169‐178. [DOI] [PubMed] [Google Scholar]
- 35. Stewart PM, Walker BR, Holder G, O’Halloran D, Shackleton CH. 11 beta-Hydroxysteroid dehydrogenase activity in Cushing's syndrome: explaining the mineralocorticoid excess state of the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1995;80(12):3617‐3620. [DOI] [PubMed] [Google Scholar]
- 36. Monaghan PJ, Owen LJ, Trainer PJ, et al. Comparison of serum cortisol measurement by immunoassay and liquid chromatography-tandem mass spectrometry in patients receiving the 11β-hydroxylase inhibitor metyrapone. Ann Clin Biochem. 2011;48(5):441‐446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in “References.” The study forms used for this project are available on request from the corresponding author.