Dear Editor,
Chinese guidelines grant the use of Azvudine and Paxlovid in COVID-19 patients, especially those with pre-existing comorbidities.1, 2 Recently, Gao Y et al. reported that Paxlovid appears to be superior to Azvudine in the virus clearance among general COVID-19 patients.3 However, a multicenter randomized controlled study demonstrated that Paxlovid showed no significant reduction in the risk of all-cause mortality on day 28 and the duration of virus clearance in hospitalized adult COVID-19 patients with pre-existing comorbidities.4 Several studies demonstrated that Azvudine could reduce the duration of virus clearance and improve the clinical prognosis in COVID-19 patients including those with pre-existing comorbidities.5, 6, 7, 8 Therefore, concerns arise about the clinical effectiveness of Azvudine versus Paxlovid in COVID-19 patients with pre-existing comorbidities on admission.
Here, we conducted a single-center, retrospective cohort study during the outbreak caused by the omicron from December 5, 2022 to January 31, 2023 in Xiangya Hospital of Central South University. The study included hospitalized patients with pre-existing comorbidities and confirmed diagnosis of SARS-CoV-2 infection who received Paxlovid or Azvudine. The patients with these conditions were excluded: 1) younger than 18 years; 2) received oxygen support or mechanical ventilation on the date of the admission; 3) not received any antiviral agents; 4) received both Azvudine and Paxlovid. The study was approved by the institutional review board of Xiangya Hospital of Central South University, and all the patients were anonymous and no need for individual informed consent.
The primary endpoint was a composite disease progression outcome which was defined as any of the following events: 1) non-invasive respiratory support; 2) initiation of endotracheal intubation; 3) intensive care unit admission; 4) all-cause death. The secondary endpoints were each of these individual disease progression outcomes. Patients were observed from the date of admission until discharge, occurrence of outcome event or death, whichever came first. We used propensity-score models conditional on baseline characteristics, and the probability of receiving Azvudine was estimated in an approach of calliper matching without replacement, with a calliper width of 0.2. The baseline covariates included age, sex, time from symptom onset to hospitalization, COVID-19 vaccination status, severity of COVID-19 on admission (severe cases were defined as having respiratory rate ≥30, or oxygen saturation ≤93%, or PaO2/FiO2 ≤300 mmHg, or lung infiltrates>50%), and concomitant treatments initiated at admission (systemic steroid and antibiotics). The standard mean differences (SMDs) were used to assess the balance of each baseline covariates between the groups before and after propensity-score matching which less than 0.1 indicating covariate was balanced.9 The incidence rates of outcome events were calculated as the number of outcome events / (sum of person × hospital days). Univariate Cox regression model was used to estimate a hazard ratio (HR) with 95% confidence interval (CI) for each result between the groups and subgroup analyses were conducted in each stratum of the aforementioned baseline characteristics to evaluate the robustness of the estimates. All statistical analyses were conducted with R version 4.2.1. P value less than 0.05 was statistically significant.
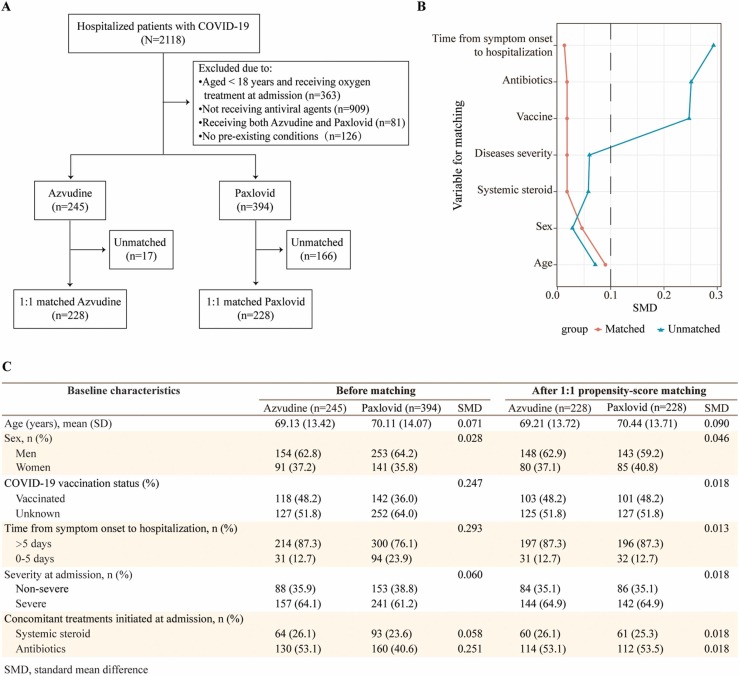
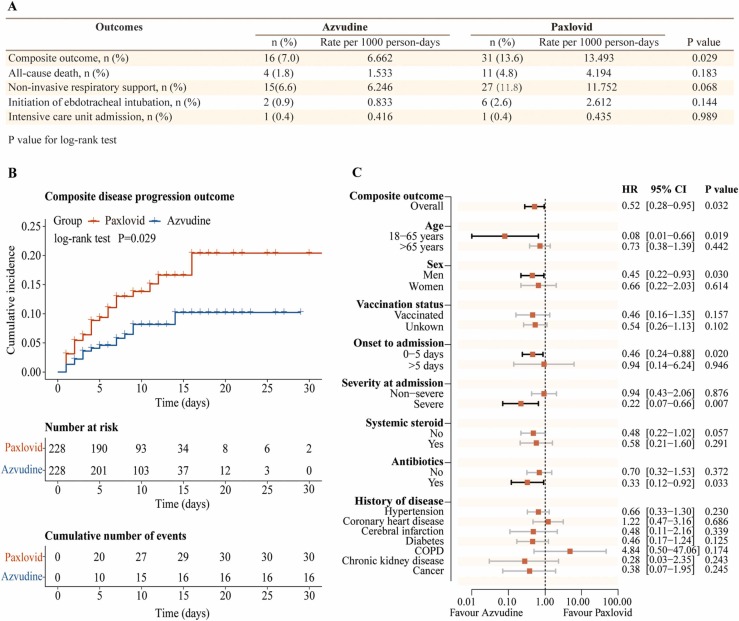
Among 2118 hospitalized COVID-19 patients, 245 Azvudine recipients and 394 Paxlovid recipients with pre-existing comorbidities were eligible for inclusion ( Fig. 1A). After propensity-score matching, we finally included 228 Azvudine recipients and 228 Paxlovid recipients, and the baseline characteristics of patients were balanced between the two groups, with SMDs lower than 0.1 (Fig. 1B, C). Frequencies of symptoms and laboratory parameters were comparable between the groups in general (Tables S1, S2). The crude incidence rate of composite disease progression outcome was 6.662 per 1000 person-days in patients treated with Azvudine versus 13.493 per 1000 person-days in the Paxlovid group (P = 0.029). There were no statistical differences between these two groups in the rates of all-cause death (P = 0.183), non-invasive respiratory support (P = 0.068), initiation of endotracheal intubation (P = 0.144), intensive care unit admission (P = 0.989) ( Fig. 2A). Cumulative hazard analysis demonstrated that patients treated with Azvudine had lower risk of composite disease progression outcome than those treated with Paxlovid (hazard ratio [HR]: 0.51; 95% CI: 0.28–0.95, P = 0.029) (Fig. 2B). Subgroup analyses indicated the robustness of the composite disease progression outcomes, with point estimates of HRs ranging from 0.28–0.95, and the results retained significance among male recipients, patients under the age of 65 years, those receiving the hospitalization beyond 5 days since onset, those with severe COVID-19 at admission and those received antibiotic treatment at admission (Fig. 2C).
Fig. 1.
Identification of Azvudine and Paxlovid recipients through 1:1 propensity score-matching. A) Flow chart showed the inclusion and exclusion of COVID-19 hospitalized patients during the study period. B) Standard mean differences between the groups before and after 1:1 propensity score-matching. C) Baseline characteristics of the included patients before and after 1:1 propensity score-matching.
Fig. 2.
Azvudine versus Paxlovid for oral treatment of COVID-19 patients with pre-existing comorbidities. A) Composite and individual outcomes in Azvudine and Paxlovid groups. B) Cumulative incidence of composite disease progression outcomes for Azvudine versus Paxlovid recipients. C) The effectiveness of Azvudine versus Paxlovid in reducing the risk of composite disease progression outcome by subgroups of selected baseline characteristics. Abbreviation, COPD, chronic obstructive pulmonary disease.
In this retrospective cohort study, we found that Azvudine was associated with a significantly reduced risk of composite disease progression outcome compared with Paxlovid in the COVID-19 patients with pre-existing comorbidities. Notably, Azvudine showed substantial clinical benefit than Paxlovid among men, patients under the age of 65 years, those receiving the hospitalization beyond 5 days since onset, those with severe COVID-19 on admission and those received antibiotic treatment on admission. Paxlovid has been reported to significantly reduce the hospitalization and death rates in patients over 65 years of age but not those under 65 years of age, compared with matched controls.10 Our results support the use of Azvudine in those under 65 years of age.
To our knowledge, this is the first study to evaluate the clinical effectiveness of Azvudine versus Paxlovid in COVID-19 patients with pre-existing comorbidities on admission. Admittedly, our study also has some unavoidable limitations. Firstly, although the data from all patients with COVID-19 were collected continuously and adjusted for possibility of selection bias and confounding factors associated with a high risk of severe COVID-19, we could not rule out the possibility of selection bias or confounding of indications. Secondly, this is a retrospective cohort study with a single center and small sample in Hunan province, and whether our findings are applicable to other regions and different ethnic groups remains to be verified.
In summary, our findings, to some extent, indicate that Azvudine is more effective than Paxlovid in treating hospitalized COVID-19 patients with pre-existing comorbidities.
Funding
This research is supported by the National Natural Science Foundation of China (Nos. 82103183 to F.Z., 82102803, 82272849 to G.D.), and Natural Science Foundation of Hunan Province (Nos. 2022JJ40767 to F.Z., 2021JJ40976 to G.D.).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Xiangya Hospital of Central South University. The authors thank all the doctors and nurses who fight for the COVID-19 in Xiangya Hospital, Central South University.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jinf.2023.05.012.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.General Office of the National Health Commission. Notice on the issuance of Diagnosis and Treatment Protocol for novel coronavirus infection (trial version 9); 2022. Accessed on: 〈http://www.gov.cn/zhengce/zhengceku/2022-03/15/content_5679257.htm〉.
- 2.General Office of the National Health Commission. Notice on the issuance of Diagnosis and Treatment Protocol for novel coronavirus infection (trial version 10); 2023. Accessed on: 〈http://www.gov.cn/zhengce/zhengceku/2023-01/06/content_5735343.htm〉.
- 3.Gao Y., Luo Z., Ren S., Duan Z., Han Y., Liu H., et al. Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19. J Infect. 2023;86(6):e158–e160. doi: 10.1016/j.jinf.2023.03.023. Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J., Pan X., Zhang S., Li M., Ma K., Fan C., et al. Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomized controlled study. Lancet Reg Health West Pac. 2023;33 doi: 10.1016/j.lanwpc.2023.100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J.L., Li Y.H., Wang L.L., Liu H.Q., Lu S.Y., Liu Y., et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct Target Ther. 2021;6(1):414. doi: 10.1038/s41392-021-00835-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren Z., Luo H., Yu Z., Song J., Liang L., Wang L., et al. A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv Sci. 2020;7(19) doi: 10.1002/advs.202001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W., Xu H., Hong L., Yang R., Peng C., Wang G., et al. Oral azvudine (FNC) tablets in patients infected with SARS-CoV-2 omicron variant: a retrospective cohort study. medRxiv. 2023 doi: 10.1101/2023.01.05.23284180. [DOI] [Google Scholar]
- 8.Shen M., Xiao C., Sun Y., Li D., Wu P., Jin L., et al. Real-world effectiveness of Azvudine in hospitalized patients with COVID-19: a retrospective cohort study. medRxiv. 2023 doi: 10.1101/2023.01.23.23284899. [DOI] [PubMed] [Google Scholar]
- 9.Wong C.K.H., Au I.C.H., Lau K.T.K., Lau E.H.Y., Cowling B.J., Leung G.M. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis. 2022;22(12):1681–1693. doi: 10.1016/s1473-3099(22)00507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbel R., Wolff Sagy Y., Hoshen M., Battat E., Lavie G., Sergienko R., et al. Nirmatrelvir use and severe covid-19 outcomes during the omicron surge. N Engl J Med. 2022;387(9):790–798. doi: 10.1056/NEJMoa2204919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material