Abstract
Background
Synchronous multiple primary colorectal cancer (SMPCC) involves the simultaneous occurrence of 2 or more independent primary malignant tumors in the colon or rectum. Although SMPCC is rare, it results in a higher incidence of postoperative complications and mortality compared to patients with single primary colorectal cancer (SPCRC).
Methods
The clinical factors and survival outcomes of SMPCC patients registered on the Surveillance, Epidemiology, and End Results (SEER) database between 2000 and 2017 were extracted. The patients were divided into the training and validation cohorts using a ratio of 7:3. Univariate and multivariate logistic regression analyses were used to identify the independent risk factors for early death. The performance of the nomogram was evaluated using the concordance index (C-index), calibration curves, and the area under the curve (AUC) of a receiver operating characteristics curve (ROC). A decision curve analysis (DCA) was used to evaluate the clinical utility of the nomogram and standard TNM system.
Results
A total of 4386 SMPCC patients were enrolled in the study and randomly assigned to the training (n = 3070) and validation (n = 1316) cohorts. The multivariate logistic analysis identified age, chemotherapy, radiotherapy, T stage, N stage, and M stage as independent risk factors for all-cause and cancer-specific early death. The marital status was associated with all-cause early death, and the tumor grade was associated with cancer-specific early death. In the training cohort, the nomogram achieved a C-index of 0.808 (95% CI, 0.784–0.832) and 0.843 (95% CI, 0.816–0.870) for all-cause and cancer-specific early death, respectively. Following validation, the C-index was 0.797 (95% CI, 0.758–0.837) for all-cause early death and 0.832 (95% CI, 0.789–0.875) for cancer-specific early death. The ROC and calibration curves indicated that the model had good stability and reliability. The DCA showed that the nomogram had a better clinical net value than the TNM staging system.
Conclusion
Our nomogram can provide a simple and accurate tool for clinicians to predict the risk of early death in SMPCC patients undergoing surgery and could be used to optimize the treatment according to the patient's needs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00384-023-04435-4.
Keywords: Synchronous multiple primary colorectal cancer, Early death, Nomogram, Surveillance, Epidemiology, And End Results (SEER)
Background
Colorectal cancer (CRC) is the third most common malignancy and the second cause of cancer-related mortality worldwide [1]. Synchronous multiple primary colorectal cancer (SMPCC) is a rare subtype of CRC, characterized by the presence of two or more primary CRC lesions simultaneously or within 6 months from the detection of the first lesion [2]. SMPCC accounts for about 1.1% to 8.1% of all CRC cases [3]. Although SMPCC is rare, its incidence is increasing due to improvements in diagnostic imaging techniques such as digestive endoscopy and imaging techniques have led to a decline in the missed diagnosis rate of CRC lesions [4, 5]. Numerous studies have shown that the clinical features, pathological subtypes, pathogenesis, genetic mutations, and treatment outcomes tend to vary significantly between patients diagnosed with SMPCC and those diagnosed with single primary colorectal cancer (SPCRC) [6–8]. These findings suggest that treatment applied to SPCRC may be inappropriate to apply to patients diagnosed with SMPCC patients with SMPCC may benefit from a different treatment approach. Surgery remains the primary modality for the treatment of CRC. However, patients with SMPCC, especially those diagnosed with bilateral colon tumors and synchronous colon-rectum tumors, often require extensive surgical interventions, which may involve multiple colorectal segments, two or more anastomoses, and even total colectomy or proctectomy. As a result, patients with SMPCC tend to have a higher incidence of postoperative complications and mortality than those diagnosed with SPCRC [9]. However, although numerous studies have evaluated the long-term prognosis of SMPCC, relatively few studies have assessed the short-term outcome [10–12]. As a result, there is currently no effective tool that could be used to predict short-term mortality for SMPCC patients. Therefore in this study, we aimed to investigate the incidence of early death in surgically treated SMPCC patients based on data extracted from SEER database to identify the risk factors contributing to early death. Moreover, we developed and validated a nomogram to predict early death (survival time ≤ 3 months) to enable clinicians to optimize the treatment for SMPCC patients and hence reduce the incidence of early death.
Methods
Ethical considerations
The Surveillance, Epidemiology, and Results (SEER) program of the National Cancer Institute provides cancer incidence and survival data from 18 established cancer registries which cover approximately 30 percent of the population in the United States. Since SEER is a public domain database, patient informed consent and ethical clearance were not required to conduct this study. The research complied with all relevant ethical criteria and was conducted in line with the "Declaration of Helsinki" in 1964.
Selection criteria
The SEER*Stat version 8.4.0.1 software was used to extract the demographic, clinical, and survival data of SMPCC patients registered on the SEER database between 2000 and 2017. The inclusion criteria were as follows: (1) Two or more primary CRC lesions diagnosed in the same patient; (2) Pathologically confirmed adenocarcinoma; (3) The diagnosis interval for the identification of the different primary CRC lesions of less than 6 months [13]. The exclusion criteria were; (1) Age less than 18 years old; (2) Previous history of other malignant tumors; (3) No surgical treatment; (4) Patients diagnosed only by autopsy or death certificate; (5) Diagnosed with a carcinoma-in-situ; (6) Cases with missing survival information and insufficient follow-up. In addition, the patients who did not undergo surgical interventions or had a CRC diagnosis following an autopsy were also excluded. The patient selection process is illustrated in Fig. 1.
Fig. 1.
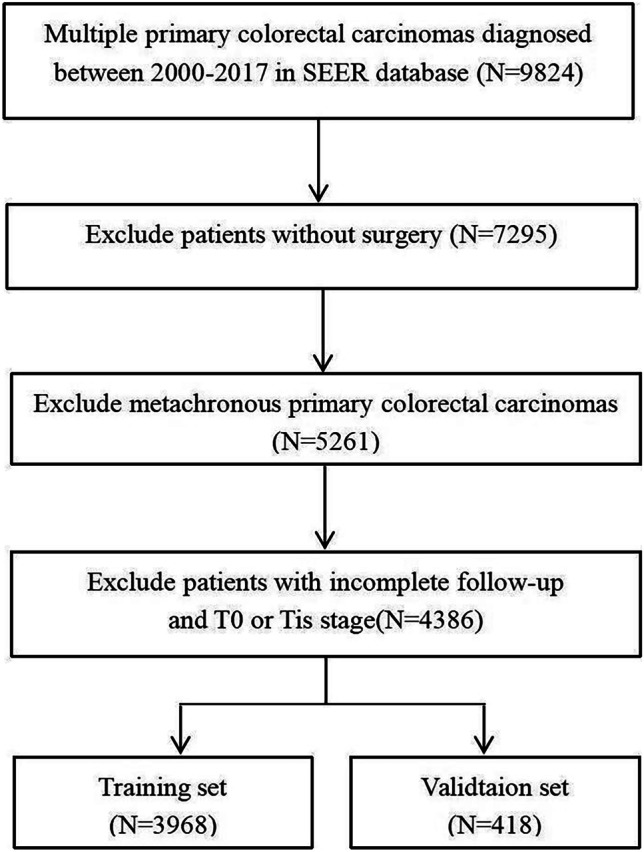
Flowchart illustrating the patient selection process
Data extraction
The CRC were coded as defined by the International Classification of Cancer Diseases (ICD-O-3). Codes C18.0 to C18.9 refer to colon tumors, C19.9 to rectosigmoid tumors and C20.9 to rectal tumors. All tumors were divided into 3 groups; right colon, left colon, and rectum. Tumors located between the cecum to the transverse colon were classified as right colon, while those located between the splenic flexure and the sigmoid colon were classified as left colon. Tumors encompassing the rectosigmoid junction and the rectum were classified as rectum. Patients were divided into 3 groups in accordance to the positional relationship of the multiple tumor lesions: unilateral group, bilateral group, and rectum-colon synchronous group. The unilateral group included patients with synchronous tumors located on one side (right-right colon, rectum-rectum, left-left colon), the bilateral group included patients with synchronous tumors on both sides (right-left colon, left–right colon), and the rectum-colon synchronous group included patients with synchronous tumors affecting the colon and the rectum (rectum-right colon; rectum-left colon). The lesion with the most advanced stage or size among the multiple lesions was used as the index tumor for analysis.
Statistical analysis
All the patients were randomly assigned to the training and validation cohorts using a ratio of 7:3. The primary outcome measures for this study were early all-cause and cancer-specific early death within 3 months of diagnosis [14]. The categorical variables were expressed as numbers and percentages (n,%), and the differences in the distribution of the variables between the training and validation cohorts were assessed using Pearson's chi-square test. Univariate logistic regression analysis was performed on the training cohorts to identify the risk factors for all-cause and cancer-specific early death. The significant risk factors were included in the multivariate logistic regression analysis to identify the independent risk factors. The independent risk factors were then used to construct predictive nomograms for all-cause and cancer-specific early death. By mapping the value of each factor to the "points" axis, the points for early death probability for each variable were obtained. The total points can be calculated by summing them up [15].
The performance of the nomogram in the training and validation cohorts was evaluated as follows. The concordance index (C-index) was used to evaluate the nomogram's predictive performance, and a calibration curve with a 1000-times bootstrapping was plotted to evaluate the consistency between the actual and predicted probabilities. The area under the curve (AUC) with the 95% confidence interval (CI) of a receiver operating characteristic (ROC) curve was calculated to evaluate the discrimination ability of the nomogram. An area under the roc curve (AUC) value above 0.7 was considered to have good predictive capabilities [16]. Finally, a decision curve analysis (DCA) was performed to compare the clinical utility of the nomogram and standard AJCC TNM staging system. All statistical analyzes were carried out using the R software (version 4.1.2), and a two-sided p-value below 0.05 was deemed statistically significant.
Results
Patient characteristics
A total of 4386 SMPCC patients were enrolled in the study, of whom 53.92% (n = 2365) were males, and the rest were females (46.08%, n = 2021). Most patients were Caucasian (79.30%, n = 3478) and aged above 60 years (79.41%, n = 3483). The majority of the patients (66.53%, n = 2918) had bilateral tumors or rectum-colon synchronous tumors. Out of the 4386 SMPCC patients, 14.48% (n = 635) developed distant metastases, 34.72% (n = 1523) received chemotherapy, and 10.3% (n = 452) received radiotherapy. All-cause early death occurred in 9.07% (n = 398) of patients, while cancer-specific early death occurred in 6.00% (n = 263). The characteristics of the patients according to the early death are summarized in Supplementary Table 1.
The patients were randomly divided into the training (n = 3070) and validation (n = 1316) cohorts. The demographic and clinical features of the SMPCC patients in the training and validation cohorts are summarized in Table 1. There were no significant differences in demographic and clinical characteristics between the training and validation cohorts.
Table 1.
Demographic and clinical characteristics of the training and validation cohorts
| Variables | Training cohort (n = 3070) |
Validation cohort (n = 1316) |
p-value |
|---|---|---|---|
| Sex | |||
| Female | 1408 (45.86%) | 613 (46.58%) | 0.686 |
| Male | 1662 (54.14%) | 703 (53.42%) | |
| Age(years) | |||
| < 50 | 226 (7.36%) | 113 (8.59%) | 0.733 |
| 50–59 | 397 (12.93%) | 167 (12.69%) | |
| 60–69 | 667 (21.73%) | 285 (21.66%) | |
| 70–79 | 891 (29.02%) | 390 (29.64%) | |
| 80–89 | 793 (25.83%) | 324 (24.62%) | |
| ≥ 90 | 96 (3.13%) | 37 (2.81%) | |
| Race | |||
| White | 2424 (78.96%) | 1054 (80.09%) | 0.640 |
| Black | 259 (8.44%) | 109 (8.28%) | |
| Other | 387 (12.61%) | 153 (11.63%) | |
| Marital | |||
| Married | 1595 (51.95%) | 677 (51.44%) | 0.942 |
| UnMarried | 1367 (44.53%) | 591 (44.91%) | |
| Unknown | 108 (3.52%) | 48 (3.65%) | |
| Tumor number | |||
| 2 | 2867 (93.42%) | 1220 (92.78%) | 0.478 |
| ≥ 3 | 202 (6.58%) | 95 (7.22%) | |
| Tumor position | |||
| Unilateral group | 1013 (33.02%) | 453 (34.42%) | 0.385 |
|
Bilateral/ rectum-colon synchronous group |
2055 (66.98%) | 863 (65.58%) | |
| Histology | |||
| AC | 2644 (86.12%) | 1118 (84.95%) | 0.333 |
| MAC/SRCC | 426 (13.88%) | 198 (15.05%) | |
| Grade | |||
| Well/moderately | 2223 (72.41%) | 934 (70.97%) | 0.554 |
| Poorly/undifferentiated | 753 (24.53%) | 336 (25.53%) | |
| Unknown | 94 (3.06%) | 46 (3.50%) | |
| T stage | |||
| T1 | 680 (22.15%) | 285 (21.66%) | 0.563 |
| T3 | 1836 (59.80%) | 799 (60.71%) | |
| T4 | 428 (13.94%) | 169 (12.84%) | |
| TX | 126 (4.10%) | 63 (4.79%) | |
| N stage | |||
| N0 | 1659 (54.04%) | 687 (52.20%) | 0.327 |
| N1 | 854 (27.82%) | 385 (29.26%) | |
| N2 | 536 (17.46%) | 222 (16.87%) | |
| NX | 21 (0.68%) | 22 (1.67%) | |
| M stage | |||
| M0 | 2626 (85.54%) | 1125 (85.49%) | 1.000 |
| M1 | 444 (14.46%) | 191 (14.51%) | |
| Tumor size(mm) | |||
| ≤ 50 | 1641 (53.45%) | 714 (54.26%) | 0.533 |
| > 50 | 1135 (36.97%) | 490 (37.23%) | |
| Unknown | 294 (9.58%) | 112 (8.51%) | |
| Chemotherapy | |||
| Yes | 1066 (34.72%) | 457 (34.73%) | 1.000 |
| No/Unknown | 2004 (65.28%) | 859 (65.27%) | |
| Radiotherapy | |||
| Yes | 334 (10.88%) | 118 (8.97%) | 0.064 |
| No/Unknown | 2736 (89.12%) | 1198 (91.03%) | |
| Surgery | |||
| Non-extensive excision | 2707 (88.18%) | 1161 (88.22%) | 1.000 |
| Extensive resection | 363 (11.82%) | 155 (11.78%) |
Extensive resection: total colectomy or total proctocolectomy; non-extensive excision: segmental rection, subtotal colectomy, left and right hemicolectomy, total proctectomy
AC adenocarcinoma, MAC mucinous or mucin-producing adenocarcinoma, SRCC signet ring cell carcinoma.
Risk factors for early all-cause and cancer-specific early death
The univariate logistic regression showed that age, chemotherapy, radiotherapy, histologic type, differentiation grade, T stage, N stage, M stage, and tumor size were associated with early all-cause and cancer-specific early death. Marital status was associated with cancer-specific early death. After performing multivariate logistic regression analysis on the above variables, age chemotherapy, radiotherapy, T stage, N stage, and M stage were identified as independent risk factors for all-cause and cancer-specific early death. In addition, marital status was identified as an independent risk factor for all-cause early death, while the histological grade was identified as an independent risk factor for cancer-specific early death (Tables 2 and 3).
Table 2.
The univariable and multivariate logistic regression analysis of all-cause early death
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Sex | ||||||
| Female | Reference | |||||
| Male | 0.68 | 0.53–0.88 | 0.003 | 1.01 | 0.76–1.36 | 0.92 |
| Age(years) | ||||||
| < 50 | Reference | |||||
| 50–59 | 2.12 | 0.59–7.67 | 0.253 | 2.15 | 0.57–8.03 | 0.256 |
| 60–69 | 4.24 | 1.29–13.9 | 0.017 | 4.12 | 1.22–13.9 | 0.023 |
| 70–79 | 7.43 | 2.33–23.74 | 0.001 | 6.4 | 1.95–21.06 | 0.002 |
| 80–89 | 14.98 | 4.73–47.47 | < 0.001 | 9.67 | 2.95–31.73 | < 0.001 |
| ≥ 90 | 12.69 | 3.56–45.26 | < 0.001 | 6.11 | 1.63–22.96 | 0.007 |
| Race | ||||||
| White | Reference | |||||
| Black | 0.95 | 0.61–1.49 | 0.832 | |||
| Other | 0.82 | 0.55–1.22 | 0.331 | |||
| Marital | ||||||
| Married | Reference | |||||
| UnMarried | 1.83 | 1.42–2.36 | < 0.001 | 1.35 | 1.01–1.81 | 0.043 |
| Unknown | 0.94 | 0.43–2.08 | 0.888 | 0.75 | 0.31–1.77 | 0.505 |
| Tumor number | ||||||
| 2 | Reference | |||||
| ≥ 3 | 1.53 | 1–2.36 | 0.052 | |||
| Tumor position | ||||||
| Unilateral group | Reference | |||||
| Bilateral / rectum -colon synchronous group | 1.01 | 0.78–1.32 | 0.916 | |||
| Histology | ||||||
| AC | Reference | |||||
| MAC/ SRCC | 1.49 | 1.08–2.05 | 0.015 | 1.25 | 0.87–1.78 | 0.224 |
| Grade | ||||||
| Well/moderately | Reference | |||||
| Poorly/undifferentiated | 1.56 | 1.19–2.04 | 0.001 | 1.21 | 0.89–1.64 | 0.218 |
| Unknown | 1.37 | 0.7–2.68 | 0.362 | 1.62 | 0.75–3.51 | 0.218 |
| T stage | ||||||
| T1/2 | Reference | |||||
| T3 | 1.52 | 1.06–2.2 | 0.024 | 1.33 | 0.88–2.01 | 0.176 |
| T4 | 2.65 | 1.73–4.07 | < 0.001 | 2.02 | 1.21–3.39 | 0.007 |
| TX | 5.28 | 3.12–8.92 | < 0.001 | 1.73 | 0.86–3.49 | 0.124 |
| N stage | ||||||
| N0 | Reference | |||||
| N1 | 1.62 | 1.21–2.16 | 0.001 | 1.81 | 1.31–2.51 | < 0.001 |
| N2 | 2.1 | 1.54–2.88 | < 0.001 | 2.44 | 1.65–3.61 | < 0.001 |
| NX | 0.68 | 0.09–5.09 | 0.705 | 0.35 | 0.04–2.91 | 0.331 |
| M stage | ||||||
| M0 | Reference | |||||
| M1 | 3.17 | 2.4–4.18 | < 0.001 | 3.61 | 2.44–5.35 | < 0.001 |
| Tumor size(mm) | ||||||
| ≤ 50 | Reference | |||||
| > 50 | 1.48 | 1.14–1.91 | 0.003 | 1.26 | 0.94–1.68 | 0.123 |
| Unknown | 0.95 | 0.59–1.52 | 0.824 | 1.45 | 0.86–2.46 | 0.165 |
| Chemotherapy | ||||||
| Yes | Reference | |||||
| No/Unknown | 6.02 | 3.96–9.14 | < 0.001 | 7.11 | 4.45–11.36 | < 0.001 |
| Radiotherapy | ||||||
| Yes | Reference | |||||
| No/Unknown | 12.33 | 3.93–38.69 | < 0.001 | 4.45 | 1.33–14.87 | 0.015 |
| Surgery | ||||||
| Non-extensive excision | Reference | |||||
| Extensive resection | 1.24 | 0.87–1.78 | 0.233 |
Extensive resection: total colectomy or total proctocolectomy; non-extensive excision: segmental rection, subtotal colectomy, left and right hemicolectomy, total proctectomy
AC adenocarcinoma, MAC mucinous or mucin-producing adenocarcinoma, SRCC signet ring cell carcinoma.
Table 3.
The univariable and multivariate logistic regression analysis of cancer-specific early death
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Sex | ||||||
| Female | Reference | |||||
| Male | 0.64 | 0.48–0.87 | 0.005 | 0.99 | 0.69–1.42 | 0.963 |
| Age(years) | ||||||
| < 50 | Reference | |||||
| 50–59 | 2.88 | 0.63–13.27 | 0.174 | 3.11 | 0.65–14.94 | 0.157 |
| 60–69 | 4.4 | 1.03–18.71 | 0.045 | 4.65 | 1.05–20.58 | 0.043 |
| 70–79 | 6.39 | 1.54–26.51 | 0.011 | 6.31 | 1.46–27.26 | 0.014 |
| 80–89 | 14.19 | 3.46–58.13 | < 0.001 | 10.69 | 2.49–45.94 | 0.001 |
| ≥ 90 | 14.96 | 3.25–68.92 | 0.001 | 8.21 | 1.65–40.76 | 0.010 |
| Race | ||||||
| White | Reference | |||||
| Black | 1.1 | 0.66–1.85 | 0.713 | |||
| Other | 0.77 | 0.46–1.27 | 0.301 | |||
| Marital | ||||||
| Married | Reference | |||||
| UnMarried | 1.85 | 1.35–2.52 | < 0.001 | 1.31 | 0.91–1.89 | 0.142 |
| Unknown | 1.05 | 0.41–2.65 | 0.922 | 0.77 | 0.27–2.21 | 0.626 |
| Tumor number | ||||||
| 2 | Reference | |||||
| ≥ 3 | 1.54 | 0.92–2.6 | 0.103 | |||
| Tumor position | ||||||
| Unilateral group | Reference | |||||
| Bilateral group/ rectum-colon synchronous group | 1.12 | 0.81–1.55 | 0.505 | |||
| Histology | ||||||
| AC | Reference | |||||
| MAC/ SRCC | 1.6 | 1.09–2.34 | 0.016 | 1.28 | 0.83–1.96 | 0.265 |
| Grade | ||||||
| Well/moderately | Reference | |||||
| Poorly/undifferentiated | 2.15 | 1.57–2.94 | < 0.001 | 1.6 | 1.12–2.29 | 0.010 |
| Unknown | 1.66 | 0.75–3.69 | 0.21 | 2.11 | 0.85–5.23 | 0.105 |
| T stage | ||||||
| T1/2 | Reference | |||||
| T3 | 1.84 | 1.11–3.04 | 0.017 | 1.3 | 0.74–2.27 | 0.362 |
| T4 | 4.11 | 2.37–7.14 | < 0.001 | 2.23 | 1.16–4.29 | 0.016 |
| TX | 8.41 | 4.44–15.93 | < 0.001 | 1.57 | 0.69–3.59 | 0.280 |
| N stage | ||||||
| N0 | Reference | |||||
| N1 | 2.12 | 1.47–3.06 | < 0.001 | 2.11 | 1.4–3.17 | < 0.001 |
| N2 | 3.26 | 2.24–4.75 | < 0.001 | 2.9 | 1.82–4.63 | < 0.001 |
| NX | 1.32 | 0.17–9.99 | 0.789 | 0.56 | 0.06–4.82 | 0.596 |
| M stage | ||||||
| M0 | Reference | |||||
| M1 | 5 | 3.64–6.85 | < 0.001 | 5.22 | 3.38–8.07 | < 0.001 |
| Tumor size(mm) | ||||||
| ≤ 50 | Reference | |||||
| > 50 | 1.75 | 1.28–2.39 | 0.001 | 1.42 | 0.99–2.02 | 0.054 |
| Unknown | 0.94 | 0.51–1.71 | 0.834 | 1.47 | 0.75–2.89 | 0.264 |
| Chemotherapy | ||||||
| Yes | Reference | |||||
| No/Unknown | 4.73 | 2.95–7.57 | < 0.001 | 6.7 | 3.93–11.42 | < 0.001 |
| Radiotherapy | ||||||
| Yes | Reference | |||||
| No/Unknown | 11.86 | 2.93–48.02 | 0.001 | 5.05 | 1.14–22.34 | 0.033 |
| Surgery | ||||||
| Non-extensive excision | Reference | |||||
| Extensive resection | 1.24 | 0.8–1.91 | 0.344 | |||
Extensive resection: total colectomy or total proctocolectomy; non-extensive excision: segmental rection, subtotal colectomy, left and right hemicolectomy, total proctectomy
AC adenocarcinoma, MAC mucinous or mucin-producing adenocarcinoma, SRCC signet ring cell carcinoma
Construction of the nomogram
The independent risk factors for all-cause and cancer-specific early death were used to construct the predictive nomograms for SPMCC (Fig. 2A, B). The nomograms show the scores corresponding to each risk factor, and the total point represents the sum of all variable scores. The risk for developing all-cause and cancer-specific early death can be found by drawing a line from the total points to the risk score.
Fig. 2.
Prediction nomogram of all-cause early death (A) and cancer-specific early death (B) for SMPCC patients with surgery
Performance of the nomogram
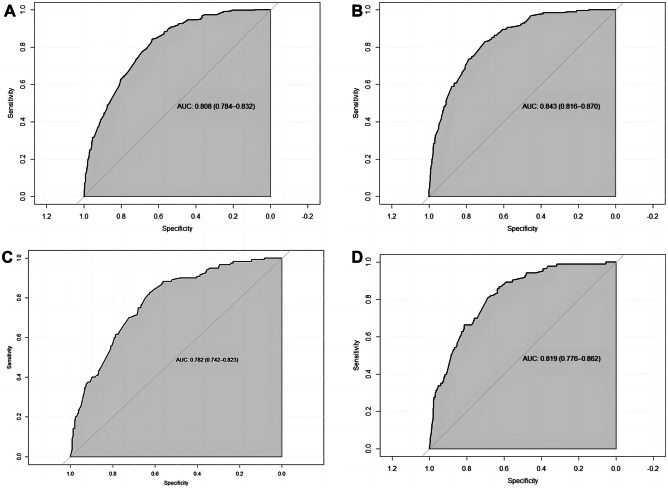
In the training cohort, the nomogram achieved a C-index of 0.808 (95% CI, 0.784–0.832) and 0.843 (95% CI, 0.816–0.870) for all-cause and cancer-specific early death, respectively. After validation, the nomogram achieved a C-index of 0.797 (95% CI, 0.758–0.837) and 0.832 (95% CI, 0.789–0.875) for all-cause and cancer-specific early death, respectively. As shown in the calibration curves, the nomogram achieved considerable agreement between the predicted and actual observations in both training and validation cohorts since the prediction curves are close to the diagonal line (Fig. 3). The AUC values in the training cohort for all-cause and cancer-specific early death were 0.808 (95% CI, 0.784–0.832, Fig. 4A) and 0.843 (95% CI, 0.816–0.870, Fig. 4B), respectively. Following validation, the nomogram achieved an AUC of 0.782 (95% CI, 0.742–0.823, Fig. 4C) and 0.816 (95% CI, 0.779–0.862, Fig. 4D) for all-cause and cancer-specific early death, respectively. The DCA showed that compared to the TNM AJCC staging system, the nomograms achieved a better net benefit for predicting all-cause and cancer-specific early death in both training and validation cohorts (Fig. 5).
Fig. 3.
Calibration curves of nomograms for early death. Red line is the performance of nomogram. Blue line corrects for any bias in nomogram. The diagonal line represents a perfect prediction by an ideal model. A Calibration curve of all-cause early death in the training cohort. B Calibration curve of cancer-specific early death in the training cohort. C Calibration curve of all-cause early death in the validation cohort. D Calibration curve of cancer-specific early death in the validation cohort
Fig. 4.
ROC curves of nomogram for predicting early death. A ROC curve of all-cause early death in the training cohort. B ROC curve of cancer-specific early death in the training cohort. C ROC curve of all-cause early death in the validation cohort. D ROC curve of cancer-specific early death in the validation cohort
Fig. 5.
The decision curve analysis (DCA) curves of nomograms for early death, the nomograms (blue line) had a better clinical net value than the TNM staging system (red line). A DCA curve of all-cause early death in the training cohort. B DCA curve of cancer-specific early death in the training cohort. C DCA curve of all-cause early death in the validation cohort. D DCA curve of cancer-specific early death in the validation cohort
Discussion
SMPCC is a rare CRC subtype characterized by multiple primary synchronous tumors within the colon and rectum. The pathogenesis of SMPCC remains unclear and tends to differ from that of SPCRC. Studies have reported that patients with inflammatory bowel diseases, high microsatellite instability (MSI-H), high CpG island methylation phenotype, hereditary non-polyposis, and familial adenomatous polyposis have a higher risk of developing SMPCC [11, 17, 18]. Surgery is considered the primary treatment option for SMPCC. However, SMPCC patients are more likely to suffer from postoperative complications and early death than SPCRC as they tend to require more extensive surgery [9, 19]. Therefore there is a need to identify survival risk factors for SMPCC to optimize the treatment for these patients. To the best of our knowledge, this is the first study to construct a prognostic prediction nomogram that could be used to predict all-cause and cancer-specific early death in patients with resected SMPCC.
In this study, we extracted the clinical data of 4386 SMPCC patients from the SEER database. Of these patients, 9.07% and 6.00% died due to all-cause and cancer-specific cause. The univariate and multivariate logistic regression analysis identified older age, no or unknown chemotherapy, no or unknown radiotherapy, and higher TNM stage as predictive risk factors for all-cause and cancer-specific early death. While the risk of early death from all-cause was higher in unmarried patients, the risk of early death from cancer-specific cause was higher for patients diagnosed with poorly or undifferentiated grade.
Our predictive nomograms based on the above risk factors achieved good predictive performance for early death in both training and validation cohorts. In addition, our nomogram achieved a higher clinical net benefit than the standard AJCC TNM staging system, thus confirming the clinical value of the nomogram. The AJCC TNM staging system is widely used to predict the prognosis of CRC. Previous studies have shown that tumor invasion depth, lymph node metastasis, and distant organ metastasis are associated with early death in patients with CRC undergoing surgery [20]. Consistent with these studies, our research has identified these three factors as predictive of early death in SMPCC patients. Consistent with previous studies, 79.14% of SMPCC patients in our study were aged 60 years or more and had a higher mean age than SPCC patients [21]. Similarly to our findings, previous studies have shown that advanced age is a risk factor for poor short-term and long-term prognosis in CRC patients [22, 23]. Older adults are more likely to present with comorbidities, poor physical status, and a higher incidence of preoperative intestinal obstruction and perforation than younger patients. Moreover, older adults are also more likely to require emergency surgery [24, 25]. As a result, elderly patients are more at risk of developing postoperative complications and mortality than younger patients [26, 27]. In addition, older patients are less likely to tolerate radiotherapy and chemotherapy due to their poor physical status. Chronic diseases such as heart failure, diabetes, and chronic obstructive pulmonary disease are more common in elderly patients [28, 29]. Therefore, elderly patients have an increased risk of dying from non-cancer-specific causes. Therefore, the treatment of elderly SMPCC patients undergoing surgery needs to be optimized to reduce the risk of early death.
There is still a lack of consensus on the optimal postoperative adjuvant therapy for SMPCC patients, particularly for stage II disease. Some studies suggest that SMPCC patients are more at risk of developing micrometastases than SPCRC patients and hence are more likely to benefit from postoperative adjuvant therapy [30]. On the other hand, some studies argue that since SMPCC patients are more likely to exhibit MSI-H (high microsatellite instability) or dMMR (deficient MMR) than SPCRC patients, they are less likely to benefit from fluorouracil-based chemotherapy [31]. Our study showed that both chemotherapy and radiotherapy reduced the risk of early death in SMPCC patients. For SMPCC patients undergoing surgery at risk of early death, adjuvant therapy such as radiotherapy and chemotherapy should be considered depending on the individual circumstance. However, larger clinical randomized controlled studies are required to identify the optimal adjuvant therapy for SMPCC patients.
Similar to previous studies, married patients were less at risk of developing early death [32]. Married patients are more likely to receive physical and financial support from their partners to cope with the disease, and, therefore, they are less likely to suffer from early death. Moreover, consistent with previous studies, we also found that poorly differentiated or undifferentiated tumors are more at risk of developing early mortality due to a higher risk of metastasis [33].
Our findings suggested that extensive resection (total colectomy or proctocolectomy) did not increase the risk of early death in SMPCC patients. Currently, there is no consensus on the extent of surgical resection for SMPCC patients. Hemicolectomy or extended hemicolectomy should be considered for tumors located in the same or adjacent segment. However, extensive resection (total colectomy or proctocolectomy) or multiple segmental resections with synchronous bowel anastomoses are recommended if the tumors were localized in distant segments [19]. Some studies have demonstrated that extensive resection can improve prognosis in SMPCC patients compared with multiple.segmental resections. However, due to the small sample size in this study, the conclusions need to be further verified [34, 35]. In addition, extensive resection for SMPCC patients with high-risk factors, including; familial adenomatous polyposis, inflammatory bowel disease, or hereditary non-adenomatous colorectal cancer was recommended by most studies [36, 37].
This study has several limitations that have to be acknowledged. The data were retrospectively extracted from the SEER database. The lack of quality control in the data included in the SEER database may have biased our results. In addition, we could not explore the association of other potential risk factors for early death in SMPCC, such as nutritional status, carcinoembryonic antigen (CEA), and susceptibility factors (inflammatory bowel disease, familial adenomatous polyposis, and hereditary non-adenomatous colorectal cancer) as this information was not reported in the SEER database. Finally, since the data was collected from a single database, further research is required to validate the generalizability of the nomogram in multiple centers.
Conclusion
In this study, we developed a novel risk prediction nomogram for early all-cause and CS survival in patients with resected SMPCC using data extracted from the SEER database. The nomogram achieved high prediction accuracy and consistency in both training and validation cohorts. The DCA showed that the nomograms had a better clinical net value than the TNM staging system. This model can provide a simple and accurate tool for clinicians to predict the risk of early death in SMPCC patients undergoing surgery and could be used to optimize the treatment according to the patient's needs.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Conceptualization: Xiangyu Zhang. Data curation: Liang Zhao, Yanpeng Hu. Formal analysis: Xiangyu Zhang, Wanbo Ren. Writing—original draft: Kai Deng, Liang Zhao. Writing—review and editing: Xiangyu Zhang, Wanbo Ren.
Data availability
The data that support the findings of this study are available from the Surveillance, Epidemiology, and End Results (SEER) database at http://www.seer.cancer.gov.
Declarations
Conflict of interest
The authors have declared no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics 2022. CA: Cancer J Clin 72(1):7–33. 10.3322/caac.21708 [DOI] [PubMed]
- 2.Rennert G, Robinson E, Rennert HS, Neugut AI. Clinical characteristics of metachronous colorectal tumors. Int J Cancer. 1995;60:743–747. doi: 10.1002/ijc.2910600602. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Wang D, Wei Y, Liu P, Xu J (2017) Clinical outcomes of laparoscopic-assisted synchronous bowel anastomoses for synchronous colorectal cancer: initial clinical experience. Oncotarget, 8(6), 10741–10747. 10.18632/oncotarget.12899 [DOI] [PMC free article] [PubMed]
- 4.Flor N, Zanchetta E, Di Leo G, Mezzanzanica Met al (2018) Synchronous colorectal cancer using CT colonography vs. other means: a systematic review and meta-analysis. Abdominal radiology, 43(12), 3241–3249. 10.1007/s00261-018-1658-1 [DOI] [PubMed]
- 5.Achiam MP, Holst Andersen LP, Klein M, Chabanova E, Thomsen HS, Rosenberg J. Preoperative evaluation of synchronous colorectal cancer using MR colonography. Acad Radiol. 2009;16(7):790–797. doi: 10.1016/j.acra.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Di J, Yang H, Jiang B, Wang Z, Ji J, Su X. Whole exome sequencing reveals intertumor heterogeneity and distinct genetic origins of sporadic synchronous colorectal cancer. Int J Cancer. 2018;142(5):927–939. doi: 10.1002/ijc.31140. [DOI] [PubMed] [Google Scholar]
- 7.Jung YS, Park JH, Park CH. Serrated Polyps and the Risk of Metachronous Colorectal Advanced Neoplasia: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2022;20(1):31–43.e1. doi: 10.1016/j.cgh.2020.09.051. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalo V, Lozano JJ, Muñoz J, Balaguer F, Pellisé M, Miguel CR et al (2010) Aberrant gene promoter methylation associated with sporadic multiple colorectal cancer. PLoS One 5(1):e8777. 10.1371/journal.pone.0008777 [DOI] [PMC free article] [PubMed]
- 9.Warps AK, Detering R, Dekker JWT, Tollenaar RAEM, Tanis PJ. A 10-Year Evaluation of Short-Term Outcomes After Synchronous Colorectal Cancer Surgery: a Dutch Population-Based Study. J Gastrointest Surg. 2021;25(10):2637–2648. doi: 10.1007/s11605-021-05036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He W, Zheng C, Wang Y, Dan J, Zhu M, Wei M, Wang J, Wang Z. Prognosis of synchronous colorectal carcinoma compared to solitary colorectal carcinoma: a matched pair analysis. Eur J Gastroenterol Hepatol. 2019;31(12):1489–1495. doi: 10.1097/MEG.0000000000001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin CC, Kuo YH, Chiang JM. Synchronous colorectal carcinoma: predisposing factors and characteristics. Colorectal Dis. 2019;21(4):432–440. doi: 10.1111/codi.14539. [DOI] [PubMed] [Google Scholar]
- 12.Thiels CA, Naik ND, Bergquist JR, Spindler BA, Habermann EB, Kelley SR, et al. Survival following synchronous colon cancer resection. J Surg Oncol. 2016;114(1):80–85. doi: 10.1002/jso.24258. [DOI] [PubMed] [Google Scholar]
- 13.Yang XB, Zhang LH, Xue JN, Wang YC, Yang X, Zhang N, et al. High incidence combination of multiple primary malignant tumors of the digestive system. World J Gastroenterol. 2022;28(41):5982–5992. doi: 10.3748/wjg.v28.i41.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feliu J, Espinosa E, Basterretxea L, Paredero I, Llabrés E, Jiménez-Munárriz B, et al. Prediction of Chemotoxicity, Unplanned Hospitalizations and Early Death in Older Patients with Colorectal Cancer Treated with Chemotherapy. Cancers. 2021;14(1):127. doi: 10.3390/cancers14010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen XL, Wei YA, Ren XH, Zhang X, Li GY, Lu ZW, et al. Predictive factors for successful sperm retrieval by microdissection testicular sperm extraction in men with nonobstructive azoospermia and a history of cryptorchidism. Asian journal of andrology. 2022;24(5):503–508. doi: 10.4103/aja2021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Wang J, Li L, Qin H, Wei Y, Zhang X et al (2022) AC010973.2 promotes cell proliferation and is one of six stemness-related genes that predict overall survival of renal clear cell carcinoma. Scientific Rep 12(1):4272. 10.1038/s41598-022-07070-1 [DOI] [PMC free article] [PubMed]
- 17.Lam AK, Chan SS, Leung M (2014) Synchronous colorectal cancer: clinical, pathological and molecular implications. World J Gastroenterol 20(22):6815–6820. 10.3748/wjg.v20.i22.6815 [DOI] [PMC free article] [PubMed]
- 18.Gonzalo V, Lozano JJ, Muñoz J, Balaguer F, Pellisé M, Rodríguez de Miguel C (2010) Aberrant gene promoter methylation associated with sporadic multiple colorectal cancer. PloS One 5(1):e8777.10.1371/journal.pone.0008777 [DOI] [PMC free article] [PubMed]
- 19.Bos ACRK, Matthijsen RA, van Erning FN, van Oijen MGH, Rutten HJT, Lemmens VEPP. Treatment and Outcome of Synchronous Colorectal Carcinomas: A Nationwide Study. Ann Surg Oncol. 2018;25(2):414–421. doi: 10.1245/s10434-017-6255-y. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Mao M, Xu G, Lin F, Sun P, Baklaushev VP. The incidence, associated factors, and predictive nomogram for early death in stage IV colorectal cancer. Int J Colorectal Dis. 2019;34(7):1189–1201. doi: 10.1007/s00384-019-03306-1. [DOI] [PubMed] [Google Scholar]
- 21.Lam AK, Carmichael R, Gertraud Buettner P, Gopalan V, Ho YH, Siu S. Clinicopathological significance of synchronous carcinoma in colorectal cancer. Am J Surg. 2011;202(1):39–44. doi: 10.1016/j.amjsurg.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Gross CP, Guo Z, McAvay GJ, Allore HG, Young M, Tinetti ME. Multimorbidity and survival in older persons with colorectal cancer. J Am Geriatr Soc. 2006;54(12):1898–1904. doi: 10.1111/j.1532-5415.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- 23.Verweij NM, Schiphorst AH, Maas HA, Zimmerman DD, van den Bos F, Pronk A. Colorectal Cancer Resections in the Oldest Old Between 2011 and 2012 in The Netherlands. Ann Surg Oncol. 2016;23(6):1875–1882. doi: 10.1245/s10434-015-5085-z. [DOI] [PubMed] [Google Scholar]
- 24.Dekker JW, Gooiker GA, Bastiaannet E, van den Broek CB, van der Geest LG, van de Velde CJ. Cause of death the first year after curative colorectal cancer surgery; a prolonged impact of the surgery in elderly colorectal cancer patients. Eur J Surg Oncol. 2014;40(11):1481–1487. doi: 10.1016/j.ejso.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Gomes A, Rocha R, Marinho R, Sousa M, Pignatelli N, Carneiro C, et al. Colorectal surgical mortality and morbidity in elderly patients: comparison of POSSUM, P-POSSUM, CR-POSSUM, and CR-BHOM. Int J Colorectal Dis. 2015;30(2):173–179. doi: 10.1007/s00384-014-2071-z. [DOI] [PubMed] [Google Scholar]
- 26.Panis Y, Maggiori L, Caranhac G, Bretagnol F, Vicaut E. Mortality after colorectal cancer surgery: a French survey of more than 84,000 patients. Ann Surg. 2011;254(5):738–744. doi: 10.1097/SLA.0b013e31823604ac. [DOI] [PubMed] [Google Scholar]
- 27.Schellerer VS, Merkel S, Schumann SC, Schlabrakowski A, Förtsch T, Schildberg C et al (2012) Despite aggressive histopathology survival is not impaired in young patients with colorectal cancer: CRC in patients under 50 years of age. Int J Colorectal Dis 27(1):71–79. 10.1007/s00384-011-1291-8 [DOI] [PubMed]
- 28.Chan TY, Foo CC, Law WL, Lo O (2019) Outcomes of colorectal cancer surgery in the nonagenarians: 20-year result from a tertiary center. BMC Surg 19(1):155. 10.1186/s12893-019-0623-4 [DOI] [PMC free article] [PubMed]
- 29.Fagard K, Casaer J, Wolthuis A, Flamaing J, Milisen K, Lobelle JP, et al. Postoperative complications in individuals aged 70 and over undergoing elective surgery for colorectal cancer. Colorectal Dis. 2017;19(9):O329–O338. doi: 10.1111/codi.13821. [DOI] [PubMed] [Google Scholar]
- 30.Guo Z, Hu H, Wang G (2019) New progress in dignosis and treatment of synchronous colorectal cancer. J Color Anal Surg 25(06):640–644. 10.19668/j.cnki.issn1674-0491.2019.06.003
- 31.Zhao Y, Wu J, Pei F, Zhang Y, Bai S, Shi L et al (2022) Molecular Typing and Clinical Characteristics of Synchronous Multiple Primary Colorectal Cancer. JAMA Netw Open 5(11):e2243457. 10.1001/jamanetworkopen.2022.43457 [DOI] [PMC free article] [PubMed]
- 32.Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(31):3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahmoudi L, Fallah R, Roshanaei G, Asghari-Jafarabadi M. A bayesian approach to model the underlying predictors of early recurrence and postoperative death in patients with colorectal Cancer. BMC Med Res Methodol. 2022;22(1):269. doi: 10.1186/s12874-022-01746-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Easson AM, Cotterchio M, Crosby JA, Sutherland H, Dale D, Aronson M et al (2002) A population-based study of the extent of surgical resection of potentially curable colon cancer. Ann Surg Oncol 9(4):380–387.10.1007/BF02573873 [DOI] [PubMed]
- 35.Nguyen J, Lefèvre JH, Bouchet-Doumenq C, Creavin B, Voron T, et al. Surgery for synchronous and metachronous colorectal cancer: segmental or extensive colectomy? Surg Today. 2023;53(3):338–346. doi: 10.1007/s00595-022-02624-2. [DOI] [PubMed] [Google Scholar]
- 36.Oya M, Takahashi S, Okuyama T, Yamaguchi M, Ueda Y. Synchronous colorectal carcinoma: clinico-pathological features and prognosis. Jpn J Clin Oncol. 2003;33(1):38–43. doi: 10.1093/jjco/hyg010. [DOI] [PubMed] [Google Scholar]
- 37.Easson AM, Cotterchio M, Crosby JA, Sutherland H, Dale D, Aronson M, et al. A population-based study of the extent of surgical resection of potentially curable colon cancer. Ann Surg Oncol. 2002;9(4):380–387. doi: 10.1007/BF02573873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Surveillance, Epidemiology, and End Results (SEER) database at http://www.seer.cancer.gov.