Abstract
Inflammation is a hallmark in severe diseases such as atherosclerosis and non-alcohol-induced steatohepatitis (NASH). In the development of inflammation, prostaglandins, especially prostaglandin E2 (PGE2), are major players alongside with chemo- and cytokines, like tumor-necrosis-factor alpha (TNFα) and interleukin-1 beta (IL-1β). During inflammation, PGE2 synthesis can be increased by the transcriptional induction of the two key enzymes: cyclooxygenase 2 (COX-2), which converts arachidonic acid to PGH2, and microsomal prostaglandin E2 synthase 1 (mPGES-1), which synthesizes PGE2 from PGH2. Both COX-2 and mPGES-2 were induced by a dietary intervention where mice were fed a fatty acid-rich and, more importantly, cholesterol-rich diet, leading to the development of NASH. Since macrophages are the main source of PGE2 synthesis and cholesterol is predominantly transported as LDL, the regulation of COX-2 and mPGES-1 expression by native LDL was analyzed in human macrophage cell lines. THP-1 and U937 monocytes were differentiated into macrophages, through which TNFα and PGE-2 induced COX-2 and mPGES-1 expression by LDL could be analyzed on both mRNA and protein levels. In addition, the interaction of LDL- and EP receptor signal chains in COX-2/mPGES-1 expression and PGE2-synthesis were analyzed in more detail using EP receptor specific agonists. Furthermore, the LDL-mediated signal transduction in THP-1 macrophages was analyzed by measuring ERK and Akt phosphorylation as well as transcriptional regulation of transcription factor Egr-1. COX-2 and mPGES-1 were induced in both THP-1 and U937 macrophages by the combination of TNFα and PGE2. Surprisingly, LDL dose-dependently increased the expression of mPGES-1 but repressed the expression of COX-2 on mRNA and protein levels in both cell lines. The interaction of LDL and PGE2 signal chains in mPGES-1 induction as well as PGE2-synthesis could be mimicked by through simultaneous stimulation with EP2 and EP4 agonists. In THP-1 macrophages, LDL induced Akt-phosphorylation, which could be blocked by a PI3 kinase inhibitor. Alongside blocking Akt-phosphorylation, the PI3K inhibitor inhibited LDL-mediated mPGES-1 induction; however, it did not attenuate the repression of COX-2 expression. LDL repressed basal ERK phosphorylation and expression of downstream transcription factor Egr-1, which might lead to inhibition of COX-2 expression. These findings suggest that simultaneous stimulation with a combination of TNFα, PGE2, and native LDL-activated signal chains in macrophage cell lines leads to maximal mPGES-1 activity, as well repression of COX-2 expression, by activating PI3K as well as repression of ERK/Egr-1 signal chains.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10753-022-01778-y.
KEY WORDS: Prostaglandin E2, LDL, PI3K, Signal transduction
INTRODUCTION
There is increasing evidence that inflammatory disorders are a hallmark of severe chronic diseases such as atherosclerosis, non-alcohol-induced steatohepatitis (NASH), inflammatory bowel diseases, and rheumatoid arthritis [1–3]. Inflammation is a complex process with a broad spectrum of mediators been involved such as cytokines, chemokines, and as major players, the prostaglandins [4]. Prostaglandins are formed when the C-20 unsaturated fatty acid arachidonic acid is released from the plasma membrane by phospholipases (PLA2s). Arachidonic acid is then metabolized by the sequential actions of prostaglandin G/H synthase (cyclooxygenase, COX) and the respective synthases. The cyclooxygenase exists as two distinct enzymes referred to as COX-1 and COX-2. Among these bioactive lipids, PGE2 is a pivotal prostaglandin that regulates multiple biological processes both under normal and pathological conditions [5]. In addition to its function as key mediator in inflammation, PGE2 plays a key role in physiologic cellular events such as female reproduction, regulation of vascular tension, kidney and neuronal functions, and gastric mucosal protection. In inflammation, PGE2 and its specific G protein-coupled EP receptors (EP1-EP4) are involved in the fine tuning of the inflammatory response [6].
Proteins involved in PGE2 synthesis and signalling have been shown to be increased in inflammation [7]. Thus, the expression of COX-2, mPGES-1, and PGE2 receptors (EP-R) was increased in inflammatory regions of atherosclerotic plaques of patients with carotid stenosis and the proteins were colocalized in the plaque cells [8]. Dietary lipids, especially cholesterol, are important factors in the progress of inflammation and PGE2 synthesis. In mice, a high fat/high cholesterol containing diet fat induced a “non-alcohol-induced-steato-hepatitis (NASH)” whereas a high fat diet without cholesterol did not [9–11]. In the liver of NASH-mice the number of infiltrating macrophages was increased whereas the expression of both COX-2 and mPGES-1 was induced [12]. Dietary cholesterol is transported to the liver from peripheral organs as chylomicron remnants, which are converted to VLDL, IDL, and finally to cholesterol-rich low-density-lipoprotein (LDL). After leaving the liver, LDL may serve as a source for cholesterol in any cell of the body. In addition, LDL can be oxidatively modified in vivo in oxidation events that include phospholipids, fatty acids, and Apo B modifications [13]. Oxidation of LDL can increase its proinflammatory and proatherogenic properties as oxLDL can be taken up by macrophages via scavenger receptors A and B (SR-A and SR-B (CD36) or lectin-like oxLDL receptor (LOX-1) leading to foam cell formation [14]. The knowledge regarding the regulation of COX-2 and mPGES-1 in macrophages by native or oxLDL is limited and sometimes controversial. In primary human macrophages, ox-LDL repressed LPS-induced COX-2 expression [15], whereas in THP-1 macrophages, oxLDL increased COX-2 expression [16]. In the U937 macrophage cell line, the cholesterol oxidation product 27-hydroxycholesterol induced both the expression of COX-2 and mPGES-1 [17]. Little to nothing is known about the regulation of COX-2/mPGES-1 expression by native LDL under inflammatory conditions.
The aim of the current study was to analyze the role of native LDL as a modulator of TNFα/PGE2-induced COX-2/mPGES-1 expression in human macrophage cell lines.
Our data indicates that native LDL enhanced the TNFα/PGE2-elicited induction of mPGES-1 in macrophages by an activation of PI3K whereas COX-2 expression was repressed, perhaps due to a reduction of ERK phosphorylation and subsequent repression of Egr-1 expression.
MATERIALS AND METHODS
Materials
All chemicals were purchased from commercial sources indicated throughout the text. Oligonucleotides were custom-synthesized by Biolegio (Nijmegen, Netherlands).
EP receptor specific agonists 19(R)-hydroxy prostaglandin E2 (EP2 agonist), CAY10598 (EP4 agonist), and the PGE2 ELISA were from Cayman chemicals (Ann Arbor, USA). Antibodies used were GAPDH (sc-2578) and mPGES-1 (sc-365844) from Santa Cruz Biotechnology (Heidelberg, Germany), COX-2 (12282), Akt (9272) and pAkt Ser-473 (4060), ERK (9102), pERK (9106), pIKK α/β Ser176/180 (2694), and cleaved caspase-3 Asp175 (9661) from Cell Signalling (Frankfurt, Germany). Human LDL was purchased from LEE Biosolutions (Maryland Heights, USA).
Cell Culture and Treatment
THP-1 und U937 monocytes were cultured in very low endotoxin RPMI1640 containing 10% heat-inactivated FCS and antibiotics. Monocytes were seeded in 35 mm dishes (106 cells/plate) in culture medium and differentiated to macrophages by the addition of 100 ng/ml PMA for 24 h. Cells were washed two times with serum-free medium and incubated with medium containing 0.5% FCS for another 24 h. Cells were then treated for 24 h, washed two times with PBS and then transferred to liquid nitrogen.
Cell Viability Assay (Alamar Blu)
Cell viability was analyzed by measuring the conversion of non-fluorescent resazurin to fluorescent resorufin by viable THP-1 or U937 macrophages (Alamar Blue assay) [18]. Cells were plated in 96-well plates (105 cells/well) and differentiated to macrophages as described above. After serum-starvation macrophages were stimulated with 50 ng/ml TNFα + 1 µM PGE2 and increasing LDL-concentrations for 24 h. Then, cells were washed and incubated with 0.1 mg/ml resazurin in RPMI 1640 for 2 h. Resorufin fluorescence was measured every 30 min (Em 544 nm;Exc 590 nm).
MDA Assay
Differentiated THP-1 cells were incubated in culture medium (RPMI 16,040 + 0.5% (v/v) FCS) with or without 500 µg/ml native LDL for 0 h or 24 h at 37 °C. Then, malondialdehyde as a product of lipid peroxidation [19] was measured in cell culture supernatants using a commercial TBARS assay kit (Cayman chemicals, item 10009055) according to the manufacturer’s instructions.
Real-Time RT-PCR
Cells were stimulated with 1 µM PGE2, 1 µM EP-R agonists and/or 50 ng/ml TNFα in the presence of native human LDL for the time indicated and washed with PBS. Total RNA was isolated from treated cells using Peqgold total RNA kit (Peqlab, Germany). One microgram total RNA were reverse transcribed into cDNA using oligo dT primers and an M-MuLV Reverse Transcriptase (Thermo Scientific, Germany). Hot start real- time PCR for the quantification of each transcript was carried using 2 × Maxima SybrGreen qPCR mix (Thermo Scientific), 0.25 µM of each primer and 2.5 µL of cDNA, which was diluted 1:10. PCR was performed with an initial enzyme activation step at 95 °C for 10 min, followed by 42 cycles of denaturation at 95 °C for 30 s, annealing at 57 °C for 30 s, and extension at 72 °C for 30 s in a real-time DNA thermal cycler (CFX96™, 10 µl reaction volume, BIO-RAD; Munich). The oligonucleotides used are listed in Table 1. The expression level was calculated as an n-fold induction of the gene of interest (int) in treated versus control cells with GAPDH, actin, or HPRT as reference genes (ref). The calculation is based on the differences in the threshold cycles between control (c) and treated (t) groups according to the formula: . For the final N-fold calculation, the mean of n-fold calculations using different reference genes was determined. For the calculation of EP-R or COX-1/2 copy numbers of plasmids with cloned cDNAs coding for EP-R, COX-1/2 and GAPDH were used as templates to prepare standard curves with defined copy numbers.
Table 1.
Oligonucleotide Primers Used for Real-Time qPCR
| Gene | Forward | Reverse |
|---|---|---|
| GAPDH | 5′-TGATGACATCAAGAAGGTGG | 5′-TTACTCCTTGGAGGCCATGT |
| Actin | 5′-CCCAGCCATGTACGTTGCTAT | 5′-GGGTGGCTTTTAGGATGGCAA |
| HPRT | 5′-AGGGACTGAACGTCTTGCTCG | 5′-ATCCAACACTTCGTGGGGTC |
| COX-1 | 5′-CTCCGGTTCTTGCTGTTCCT | 5′-GTCACACTGGTAGCGGTCAA |
| COX-2 | 5′-TGTGCCTGATGATTGCCCGA CTCC | 5′-TGTTGTGTTCCCGCAGCCAGATTG |
| mPGES-1 | 5′-GAAGAAGGCCTTTGCCAACCC | 5′-GTGCATCCAGGCGACAAAAG |
| EP1 | 5′-TCGCTTCGGCCTCCACCTTCTTTG | 5′-CGTTGGGCCTCTGGTTGTGCTTAG |
| EP2 | 5′-CGAGACGCGACAGTGGCTTCC | 5′-CGAGACGCGGCGCTGGTAGA |
| EP3 | 5′-CGGGGCTACGGAGGGGATGC | 5′-ATGGCGCTGGCGATGAACAACGAG |
| EP4 | 5′-TCGCGCAAGGAGCAGAAGGAGACG | 5′-GGACGGTGGCGAGAATGAGGAAGG |
PGE2 ELISA
Cells were stimulated with 1 µM EP2 + EP4 agonists + 50 ng/ml TNFα in the absence or presence of 500 µg/ml native human LDL for 24 h. Then, supernatants were collected and processed for PGE2 quantification by competitive sandwich ELISA according to the manufacturer’s instructions.
Western Blot Analysis
THP-1 and U937 cells were stimulated with 1 µM PGE2, 1 µM EP2/EP4-R agonist, and/or 50 ng/ml TNFα in the presence of native human LDL for the time indicated. Cells were lysed in lysis buffer (20 mM Tris pH 7.4, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM ethylene glycol tetraacetic acid (EGTA), 1% (v/v) Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM ß-glycerolphosphate, 50 mM NaF, protease inhibitors, and 1 mM sodium orthovanadate), homogenized by sonication and insoluble material was removed by centrifugation (10,000 × g, 15 min, 4 °C). Protein content was determined using Bradford assay [20]. Proteins were resolved by SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane. Membranes were blocked in 5% non-fat dry milk in 20 mM Tris, 136 mM NaCl, and 0.1% (v/v) Tween (TBS/Tween) for 1 h at room temperature and incubated with the first antibody in TBS/Tween containing 5% bovine serum albumin overnight at 4 °C and a horseradish-peroxidase-conjugated anti-rabbit or anti-mouse IgG for 2 h at room temperature. Visualization of immune complexes was performed using a Clarity chemiluminescence reagent (BioRad, München).
Statistical Analysis
To correct for differences in sensitivity of different cell charges towards the stimuli, values were normalized to average inducibility defined as the mean of values obtained for as indicated: control, TNFα + PGE2 and TNFα + PGE2 + 500 µg/ml LDL-treated cells. Results were analyzed by one- or two-way ANOVA and Tukey’s multicomparison test as indicated in the figure legends.
RESULTS
Regulation of COX-2 and mPGES-1 mRNA and Protein Expression by a Combination of TNFα and PGE2 and Increasing Concentrations of Native LDL
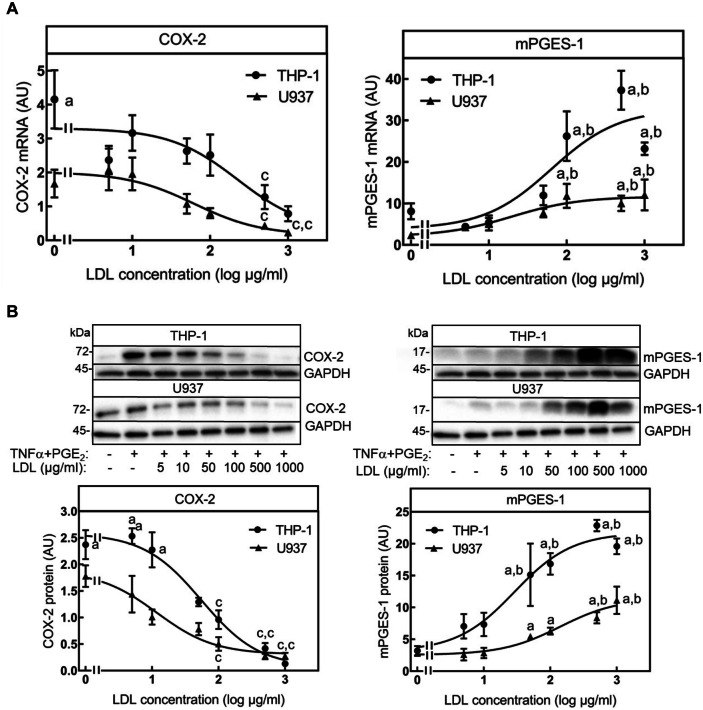
THP-1 and U937 monocytes were differentiated into macrophages, serum starved for 24 h, and cultured with a combination of TNFα and PGE2 with increasing concentrations of native LDL for 24 h. PGE2 and TNFα were used as a proinflammatory stimulus as both molecules are upregulated in inflammatory processes at the same time and in former studies the combination of TNFα and PGE2 led to maximal induction of the proinflammatory chemokine IL-8 in monocytes [21]. COX-2 and mPGES-1 mRNA expression was measured by real-time PCR (A) and protein expression by Western blot (B). The combination of TNFα and PGE2 induced COX-2 mRNA expression in THP-1 (4-fold) but only slightly in U937 macrophages (2-fold) (Fig. 1A). Both inductions were statistically not significant. Stimulation with native LDL repressed TNFα + PGE2-induced COX-2 mRNA expression slightly starting at 10 µg/ml and significantly at concentrations of 500–1000 µg/ml in both cell lines (Fig. 1A). mPGES-1 mRNA expression was induced by a combination of TNFα and PGE2 in both cell lines (THP-1: 8-fold; U937: 2-fold). Both inductions were not significant. Surprisingly, and in contrast to COX-2 mRNA expression, native LDL increased mPGES-1 mRNA expression in both cell lines significantly at concentrations greater than or equal to 100 µg/ml. The maximal induction was observed at 500 µg/ml (Fig. 1A).
Fig. 1.
Dose-dependent modulation of TNFα/PGE2 induced COX-2 and mPGES-1 mRNA and protein expression by native LDL in THP-1 and U937 macrophages. THP-1 and U937 monocytes were differentiated to macrophages with 100 ng/ml PMA for 24 h and then incubated in culture medium containing 0.5% (v/v) FCS for another 24 h. Macrophages were then stimulated with 50 ng/ml TNFα and 1 µM PGE2 (TE) and increasing concentrations of native LDL for 24 h. A COX-2 and mPGES-1 mRNA content was measured by qPCR as described in the method section with GAPDH, actin, and HPRT as reference genes. B Proteins were extracted from cells with lysis buffer, and expression of COX-2 and mPGES-1 protein was measured by Western Blot using specific antibodies for COX-2 and mPGES-1, GAPDH as a housekeeping protein, peroxidase-coupled secondary antibodies, and a luminogenic substrate. Band intensity was quantified luminometrically. COX-2 and mPGES-1 protein was expressed as ratio between enzyme and GAPDH. Data shown are means ± SEM of at least five independent experiments performed in triplicate. Statistics: one-way ANOVA with Tukey’s multicomparison test: a, significantly higher than control; b, significantly higher than TE; c, significantly lower than TE (p < 0.05).
Similar to the regulation of COX-2 mRNA, the combination of TNFα and PGE2 induced COX-2 protein slightly but not significant in THP-1 and U937 macrophages (2-fold) (Fig. 1B). In line with COX-2 mRNA native LDL dose-dependently decreased COX-2 protein expression both in THP-1 and U937 macrophages. The inhibition was significant with concentrations of 100 µg/ml and above. Maximal inhibition was achieved with 500 µg/ml native LDL. Similar to COX-2, the expression of mPGES-1 was slightly but not significantly induced by the combination of TNFα and PGE2 in THP-1 and U937 cells (3-fold). In line with the regulation of mPGES-1 mRNA native LDL dose-dependently enhanced mPGES-1 protein expression in both cell lines being maximal at 500 g/ml LDL (THP-1: 2010-fold and U937: 10-fold) (Fig. 1B). In summary, native LDL regulates COX-2 and mPGES-1 in an opposite manner in the two macrophage cell lines: Whereas COX-2 expression was repressed on both the mRNA and the protein levels, mPGES-1 expression was enhanced by native LDL.
Modulation of Cell Viability and Induction of Apoptosis by Increasing LDL Concentrations
As repression of COX-2 and induction of mPGES-1 expression was achieved predominantly at higher LDL-concentrations, cell viability under these conditions was analyzed by cell-dependent resazurin to resorufin conversion (Alamar Blue assay). Induction of apoptosis was determined by capase-3 cleavage in lysates of treated cells. As shown in supplemental Fig. 1, LDL in combination with TNFα and PGE2 decreased resazurin to resorufin conversion in viable cells at 500 µg/ml and 1000 µg/ml by 20–30% in THP-1 and U937 macrophages. However, this reduction was statistically not significant. Stimulation of THP-1 macrophages with concentration ≥ 100 µg/ml induced caspase-3 cleavage slightly but not significantly (Supplemental Fig. 2). No cleavage was observed in U937 cells. In conclusion, LDL affected COX-2 and mPGES-1 expression already at concentrations that caused no significant reduction of cell viability.
Interaction of Native LDL, TNFα, and PGE2 in the Regulation of COX-2 and mPGES-1 Expression
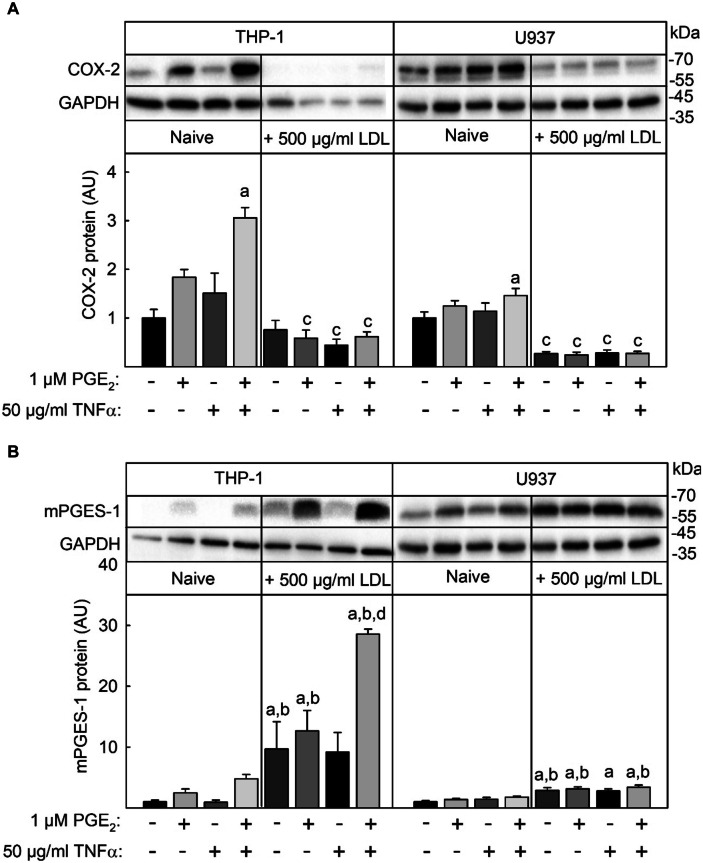
To analyze the interaction of native LDL, TNFα, and PGE2 in the regulation of COX-2 and mPGES-1 expression in more detail, THP-1 and U937 macrophages were stimulated wit 50 µg/ml TNFα and 1 µM PGE2 alone or in combination in the absence or presence of 500 µg/ml native LDL. Then, COX-2 and mPGES-1 expression was analyzed on the protein level. In THP-1 and U937 macrophages, only the combination of TNFα and PGE2 significantly induced COX-2 expression whereas mPGES-1 expression by TNFα and PGE2 was not significant in both cell lines (Fig. 2A, B). Induction of COX-2 protein expression was repressed by 500 µg/ml native LDL in both cell lines. Native LDL alone induced mPGES-1 protein expression significantly in both cell lines (THP-1: 10-fold, U937: 3-fold). This induction was enhanced only by the combination of TNFα and PGE2 in THP-1 macrophages but not in U937 cells.
Fig. 2.
Modulation of TNFα, PGE2, or TNFα and PGE2 induced COX-2 and mPGES-1 mRNA and protein expression by native LDL in THP-1 and U937 macrophages. THP-1 and U937 monocytes were differentiated and cultured described in the legend of Fig. 1. Macrophages were then stimulated with 50 ng/ml TNFα, 1 µM PGE2, or 50 ng/ml TNFα + 1 µM PGE2 in the absence or presence of 500 µg/ml native LDL for 24 h. COX-2 (A) and mPGES-1 (B) protein content was measured as described in the legend of Fig. 1B. Data shown are means ± SEM of at least five independent experiments performed in triplicate. Statistics: two-way ANOVA with Tukey’s multicomparison test: a, significantly higher than control; b, significantly higher than without 500 µg/ml LDL; c, significantly lower than without 500 µg/ml LDL; d, significantly higher than TNFα + 500 µg/ml LDL or PGE2 + 500 µg/ml LDL (p < 0.05).
In summary, the induction of both enzymes which are important in the production of PGE2 was maximal by the simultaneous stimulation with TNFα and PGE2. In THP-1 but not U937 cells, induction of COX-2 and mPGES-1 by PGE2 seems to be more important than by TNFα. Native LDL modulates this TNFα + PGE2-mediated enzyme induction in an opposite way: LDL repressed basal and TNFα + PGE2 induced expression of COX-2 and enhanced basal and induced expression of mPGES-1.
THP-1 and U937 Macrophages Predominantly Expressed EP2 and EP4 Subtype
PGE2 acts upon a family of four different G-protein-coupled receptors called EP1 to EP4. To analyze which EP-R subtype might be involved in the induction of the LDL-modulated COX-2 and mPGES-1 induction by PGE2 in the macrophage cell lines, EP-R mRNA in these cells was quantified by real-time RT-PCR. To estimate exact EP-R mRNA copies, standard curves with plasmids containing defined copies of EP-R or GAPDH cDNA were prepared. EP2 mRNA was most abundant in both cell lines and was twice the expression of EP4 (Table 2). EP1 and EP3 mRNAs expression levels were very low in both macrophage cell lines. All EP mRNAs were expressed in THP-1 and U937 macrophages to a comparable level. It was, therefore, most likely that the PGE2-dependent induction of COX-2 and mPGES-1 expression was mediated via EP2 and EP4 receptors.
Table 2.
EP-R mRNA Profile in THP-1 and U937 Macrophages
| Cells | EP1 | EP2 | EP3 | EP4 |
|---|---|---|---|---|
| THP-1 | 0.03 ± 0.03 | 144.91 ± 66.97 | 5.62 ± 2.1 | 68.29 ± 17.36 |
| U937 | 0.001 ± 0.0001 | 121 ± 35.95 | 2.49 ± 0.63 | 54.63 ± 14.35 |
THP-1 and U937 cells were differentiated and cultured as described in the legend to Fig. 1. EP-R mRNA and GAPDH mRNA of control cells was measured by real-time RT-qPCR as described in the “MATERIALS AND METHODS” section. Plasmids (102–108 copies) containing EP-R or GAPDH cDNAs were used for preparing standard curves for the calculation of EP-R or GAPDH mRNA copy numbers. Data represent the mean ± SEM of at least five independent RNA preparations. EP-R mRNA contents are expressed as copy number EP-R mRNA × 1000/copy number GAPDH mRNA.
Interaction of EP2/EP4 Agonists, TNFα, and Native LDL, in the Regulation of COX-2 and mPGES-1 Expression and PGE2-synthesis in THP-1 Macrophages
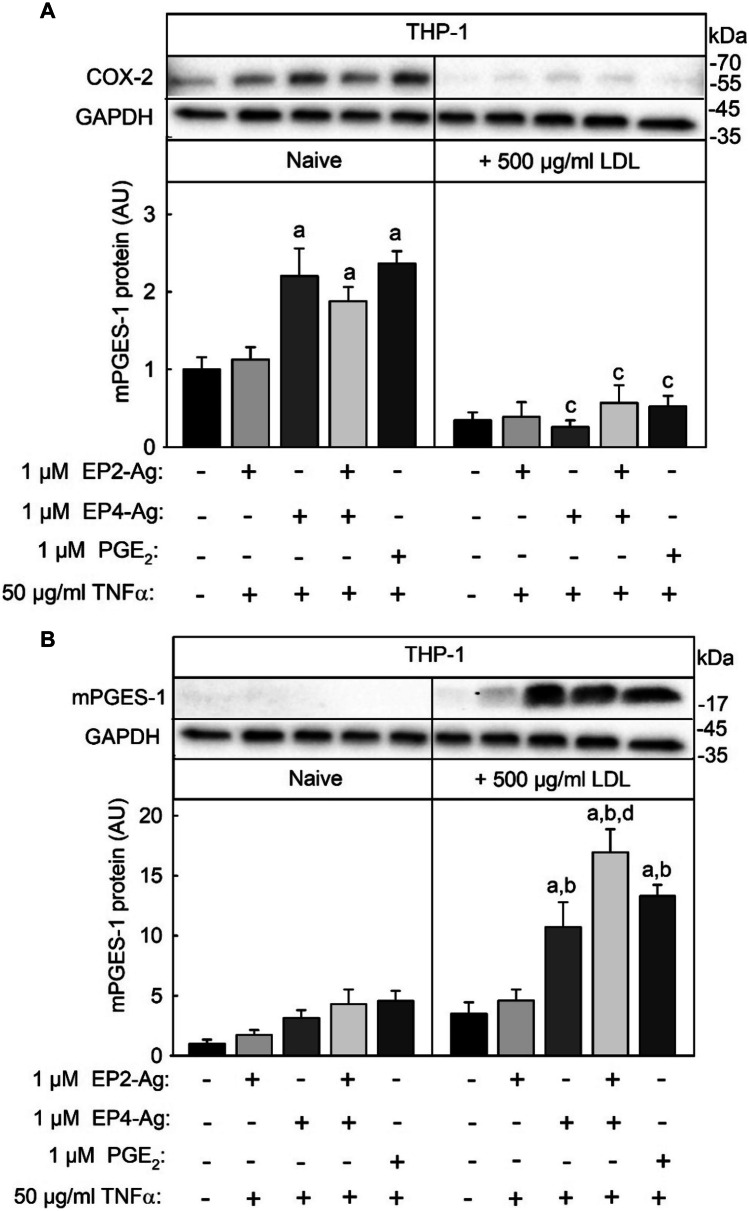
Since the regulation of COX-2 and mPGES-1 protein expression by TNFα and PGE2 and native LDL, as well EP-R expression was similar in THP-1 and U937 macrophages, all further experiments were performed solely on THP-1 cells. As the expression of EP1 and EP3 was very low in THP-1 cells, it was analyzed whether EP2 and or EP4 receptor agonists can mimic the effect of PGE2 in the regulation of COX-2 and mPGES-1 expression. THP-1 macrophages were stimulated with 50 µg/ml TNFα and either 1 µM of EP2 and EP4-agonist alone or in combination or 1 µM PGE2 and 50 ng/ml TNFα in the absence or presence of 500 µg/ml native LDL for 24 h. Then, COX-2 and mPGES-1 protein expression was analyzed. COX-2 expression was significantly induced to a similar level by TNFα in combination with the EP4-agonist alone, the combination of EP2/EP4-agonists and PGE2 (2-fold), but not in combination with the EP2-agonist alone (Fig. 3A). COX-2 expression, induced by the different combinations of stimuli, was significantly repressed by native LDL. The expression of mPGES-1 was also induced by TNFα in combination with the EP4-agonist alone, the combination of EP2/EP4-agonists and PGE2 (twofold) but to a lower level in combination with the EP2-agonist alone (Fig. 3B). However, these inductions were not significant. In combination with native LDL, TNFα and the EP4- but not the EP2-agonist significantly induced mPGES-1 expression to a comparable level as the stimulation with TNFα and PGE2. However, maximal mPGES-1 expression was induced with the combination of native LDL, TNFα, and both EP2/EP4-agonist.
Fig. 3.
Modulation of EP2/EP-4 agonist, PGE2, and TNFα induced COX-2 and mPGES-1 protein expression by native LDL in THP-1 macrophages. THP-1 monocytes were differentiated and cultured described in the legend of Fig. 1. THP-1 macrophages were then stimulated with either 1 µM EP-2 or 1 µM EP-4 agonist alone or in combination + 50 ng/ml TNFα or 1 µM PGE2 + 50 ng/ml TNFα in the absence or presence of 500 µg/ml native LDL for 24 h. COX-2 (A) and mPGES-1 protein (B) was measured as described in the legend of Fig. 2. Data shown are means ± SEM of at least five independent experiments performed in triplicate. Statistics: two-way ANOVA with Tukey’s multicomparison test: a, significantly higher than control; b, significantly higher than without 500 µg/ml LDL; c, significantly lower than without 500 µg/ml LDL; d, significantly higher than EP4-agonist + TNFα + 500 µg/ml LDL (p < 0.05).
The results show that COX-2 and mPGES-1 expression were induced in THP-1 cells by PGE2 mainly via EP4 receptor signal chains and that LDL can repress the signal chains leading to COX-2 expression and enhance the signal chains leading to mPGES-1 expression.
Next, it was analyzed how PGE2-synthesis was regulated by EP2/EP4-agonists, TNFα, and native LDL. As observed for mPGES-1 mRNA and protein expression stimulation of THP-1 macrophages with EP2/EP4-agonists and TNFα as well as 500 µg/ml native LDL induced PGE2-synthesis to a comparable level (3-fold, Fig. 4). PGE2-synthesis was highest after simultaneous stimulation with EP2/4-agonist and TNFα and native LDL (6-fold). Therefore, PGE2-synthesis showed the same regulation pattern as mPGES-1 expression.
Fig. 4.
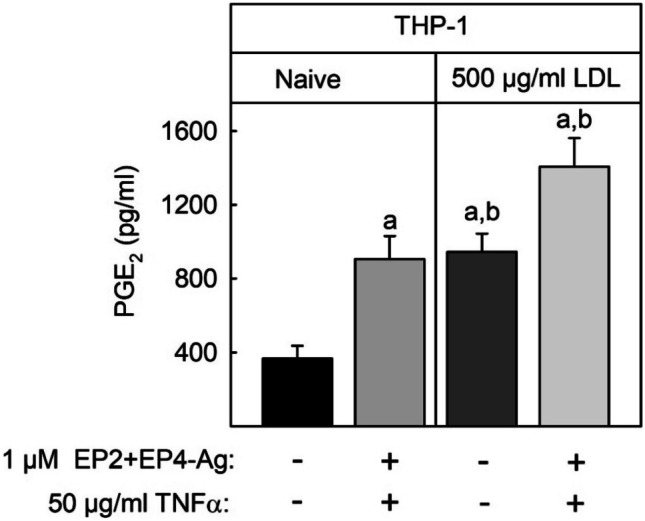
Modulation of PGE2 synthesis by EP-2/4 agonist, TNFα, and native LDL in THP-1 macrophages. THP-1 monocytes were differentiated, cultured, and stimulated as described in the legend of Fig. 3. PGE2 concentration in the supernatant of the cells was measured by competitive sandwich ELISA. Data shown are means ± SEM of at least five independent experiments performed in triplicate. Statistics: two-way ANOVA with Tukey’s multicomparison test: a, significantly higher than control; b, significantly higher than without 500 µg/ml LDL (p < 0.05).
Inhibition of LDL-Mediated Induction of mPGES-1 Expression by the PI3K Inhibitor LY294004
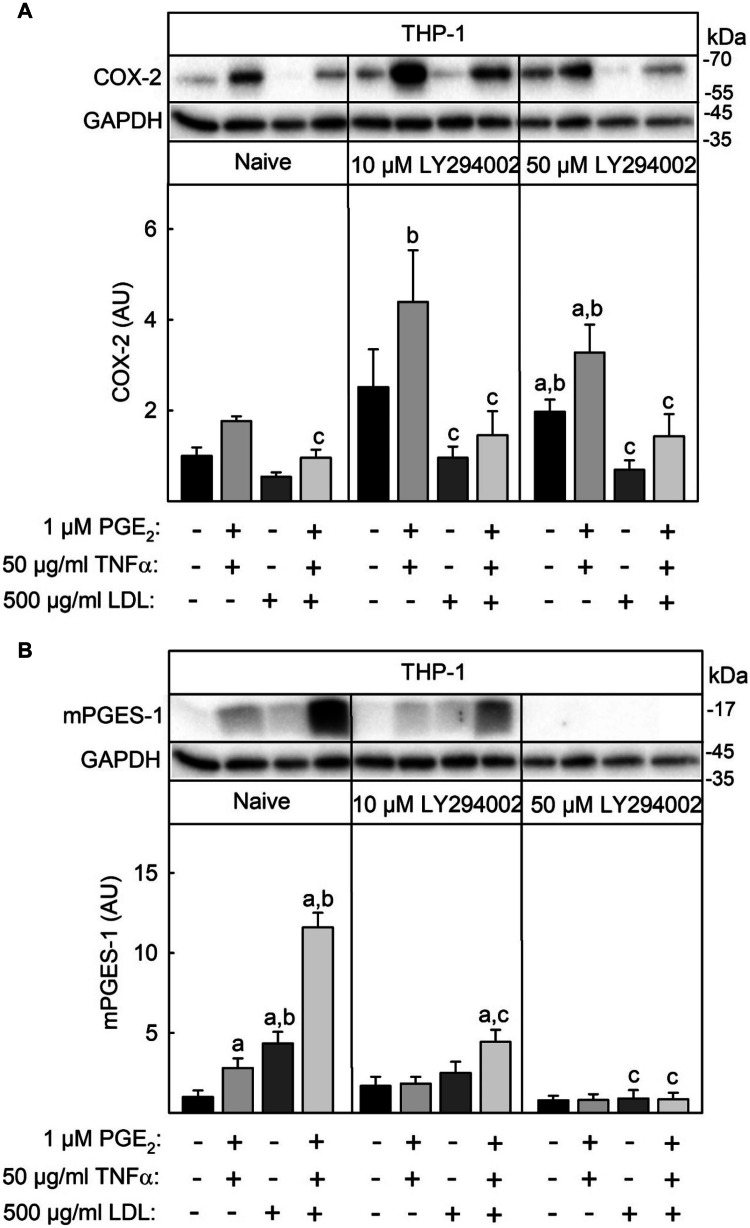
Expression of COX-2 and mPGES-1, which are the key enzymes in inflammation-induced PGE2 synthesis, is usually induced in concert by proinflammatory stimuli such as LPS and IL-1β by activation of transcription factor NFκB [22, 23]. An uncoupled expression of the two enzymes was described in primary rat microglia cells. In these cells, the PI3K inhibitors LY294002 or NVP-BEZ235 inhibited LPS-induced mPGES-1 expression and enhanced LPS-induced COX-2 expression [24, 25]. To analyze whether PI3K is also involved in the LDL-mediated repression of COX-2 vs. induction of mPGES-1, THP-1 macrophages were incubated with 10 µM or 50 µM LY294002 30 min before and during the stimulation with the combination of 50 ng/ml TNFα and 1 µM PGE2 in the absence or presence of 500 µM native LDL. Then, COX-2 and mPGES-1 expression was determined by Western blot. Preincubation with 10 µM LY294002 significantly reduced induction of mPGES-1 expression by the combination of TNFα and PGE2 in the presence of LDL (Fig. 5B). By contrast, LY294002 had no effect on the LDL-induced repression of COX-2 expression (Fig. 5A). In conclusion, PI3K may be involved in the LDL-mediated induction of mPGES-1 but not in the repression of COX-2.
Fig. 5.
Inhibition of LDL-induced mPGES-1 expression by the PI3K inhibitor LY294002 in THP-1 macrophages. THP-1 macrophages were treated with 10 µM or 50 µM of the PI3K inhibitor Y294002 30 min before and during they were stimulated with 50 ng/ml TNFα + 1 µM PGE2 in the presence or absence of 500 µg/ml native LDL for 24 h. COX-2 (A) and mPGES-1 (B) were determined by Western blot as described in the legend of Fig. 2. Data shown are means ± SEM of at least five independent experiments performed in triplicate. Statistics: two-way ANOVA with Tukey’s multicomparison test: a, significantly higher than unstimulated control cells; b, significant higher than without LDL; c, significantly lower than naive, p < 0.05.
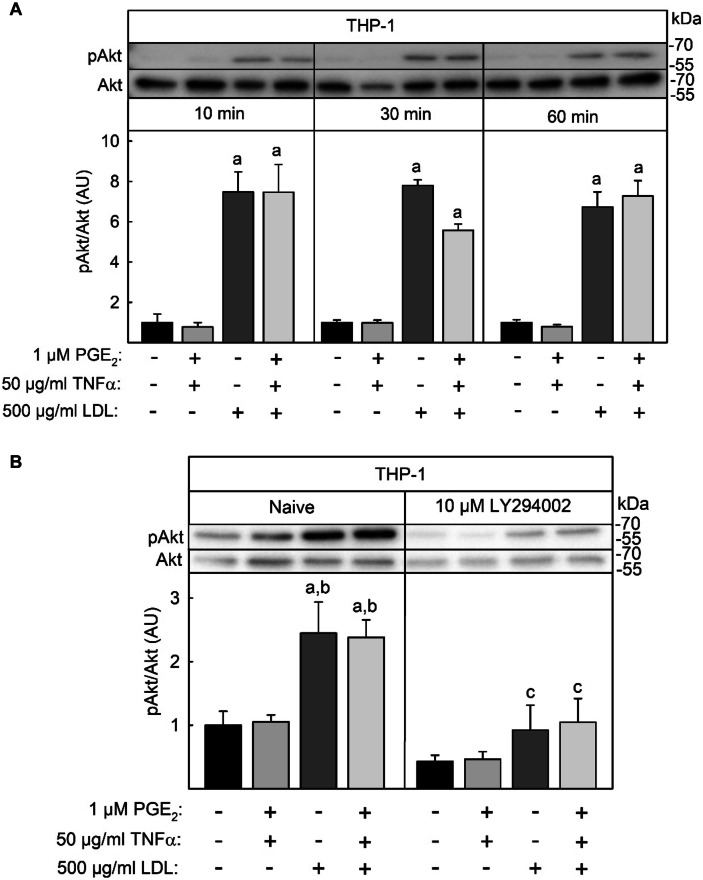
LDL-Mediated Activation of Akt-Kinase in THP-1 Macrophages
As inhibition of PI3K repressed LDL-mediated induction of mPGES-1 in THP-1 macrophages, this point to a direct activation of PI3K by native LDL. A down-stream target substrate of activated PI3K is the Akt-kinase, which is activated by PI3K-mediated phosphorylation. Therefore, LDL-mediated Akt phosphorylation was analyzed by Western blot using a phospho-specific Akt antibody. Stimulation of THP-1 macrophages with 500 µg/ml native LDL for 10 min led to significant Akt-phosphorylation which was constant at 30 and 60 min and was not effected by simultaneous stimulation with TNFα and 1 µM PGE2 (Fig. 6A). In addition, a significant LDL-mediated Akt phosphorylation was also measured at the end of the 24 h LDL stimulation period and was suppressed by the PI3K inhibitor LY294002 (Fig. 6B). In conclusion, LDL-mediated induction of mPGES-1 expression is most likely a result of LDL-mediated PI3K activation.
Fig. 6.
LDL-induced Akt kinase phosphorylation in THP-1 macrophages. A THP-1 macrophages were stimulated with 50 ng/ml TNFα + 1 µM PGE2 in the presence or absence of 500 µg/ml native LDL for the times indicated. B THP-1 macrophages were treated the PI3K inhibitor Y294002 30 min before and during they were stimulated with 50 ng/ml TNFα + 1 µM PGE2 in the presence or absence of 500 µg/ml native LDL for 24 h as described in the legend of Fig. 5. Proteins were extracted from cells with lysis buffer containing fluoride and vanadate to inhibit phosphatases. Phosphorylated and total Akt kinase were determined by Western blot using specific antibodies, peroxidase-coupled secondary antibodies, and a luminogenic substrate. Band intensity was quantified luminometrically and expressed as ratio between phosphorylated and total protein. Data shown are means ± SEM of at least five independent experiments performed in triplicate. Statistics: two-way ANOVA with Tukey’s multicomparison test: a, significantly higher than unstimulated control cells; b, significantly higher than without LDL; c, significantly lower than naive, p < 0.05.
LDL-Mediated Inhibition of Egr-1 Expression and ERK-Phosphorylation
In contrast to rat microglia cells, where PI3K inhibitors simultaneously induced COX-2 and repressed mPGES-1 expression, LY294002 did not reverse LDL-mediated repression of COX-2 expression. So, there is some evidence that native LDL repressed COX-2 through other signal chains. An important transcription factor which positively controls both COX-2 and mPGES-1 expression is the transcription factor early growth response protein 1 (Egr-1) [23, 26]. Egr-1 is induced by proinflammatory stimuli such as LPS or IL-1β [23, 27]. So, next it was examined whether native LDL can modulate Egr-1 expression. Egr-1 mRNA expression was not modulated by stimulation with the combination of TNFα and PGE2. By contrast, 500 µg/ml native LDL significantly repressed Egr-1 mRNA expression both in control and TNFα and 1 µM PGE2 stimulated THP-1 macrophages (Fig. 7A). Besides the induction of Egr-1 expression by proinflammatory stimuli, Egr-1 expression is also controlled by extracellular-signal regulated kinase ERK which is activated by phosphorylation and deactivated through dephosphorylation. Through Western blotting with a phospho-secific ERK antibody, it was determined that native LDL can modulate the phosphorylation status of ERK. Similar to Egr-1 mRNA expression, the basal phosphorylation of ERK, which was uninspected high in PMA-treated, serum-starved THP-1 cells, was not influenced by stimulation with the combination of TNFα and PGE2 but was significantly repressed by stimulation with 500 µg/ml native LDL for 24 h (Fig. 7B). Therefore, it was assumed that LDL-mediated repression of COX-2 was a result of LDL-mediated repression of ERK phosphorylation and Egr-1 expression.
Fig. 7.
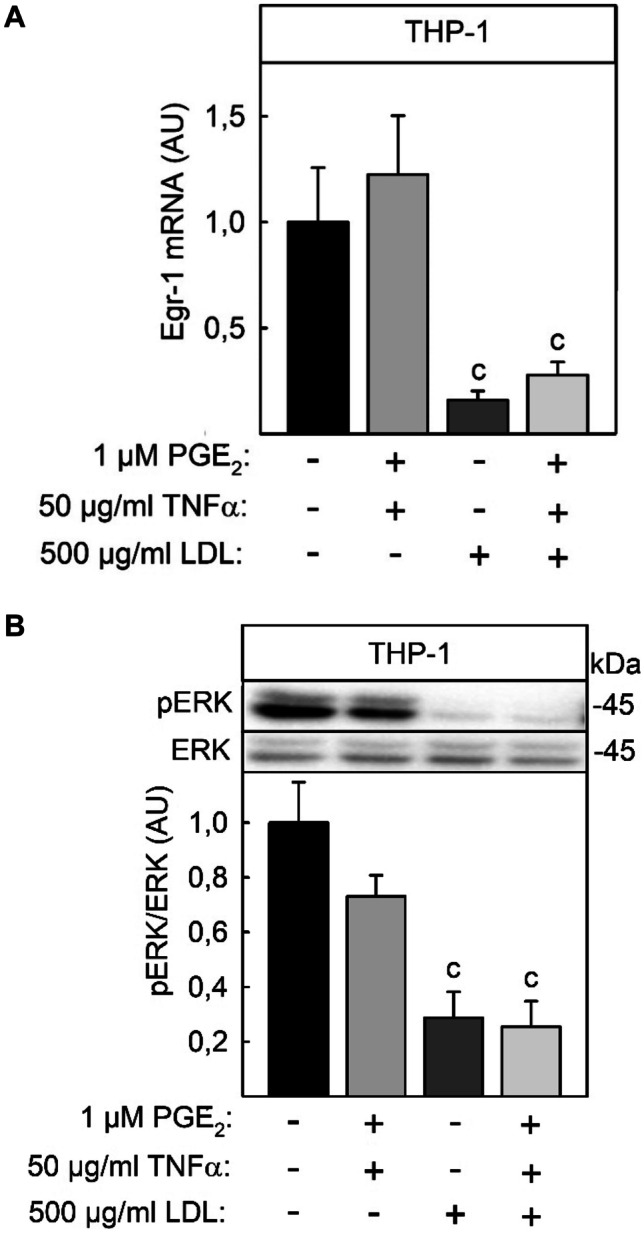
LDL-repressed Egr-1 expression and reduction of ERK kinase phosphorylation in THP-1 macrophages. THP-1 macrophages were stimulated with 50 ng/ml TNFα + 1 µM PGE2 in the presence or absence of 500 µg/ml native LDL for the times indicated. A Phosphorylated and total ERK kinase were determined by Western blot as described in the legend of Fig. 7. B Egr-1 mRNA content was measured as described in the legend of Fig. 1. Data shown are means ± SEM of at least five independent experiments performed in triplicate. Statistics: two-way ANOVA with Tukey’s multicomparison test: c, significant lower than without LDL, p < 0.05.
LDL-Mediated Modulation of NFκB Signalling
Coordinated expression of COX-2 and mPGES-1 by LPS and IL-1β is mainly controlled by transcription factor NFκB [23]. One of the important steps in NFκB activation is the activation of IKK by phosphorylation. Then, phosphorylated IKK can phosphorylate IκB which leads to its ubiquitinylation and proteasomal degradation and NFκB activation. Therefore, we measured IKK phosphorylation by TNFα + PGE2 and increasing concentrations of LDL in lysates of THP-1 macrophages by western blot. As shown in Supplemental Fig. 3 treatment with TNα + PGE2 increased the amount of pIKK slightly but not significantly. IKK phosphorylation was marginally and also not significantly repressed by increasing LDL concentrations. So, activation of NFκB signal chain seems not to be involved in the LDL-mediated regulation of COX-2 and mPGES-1 expression.
DISCUSSION
This current study showed that simultaneous activation of TNFα-R and EP2/EP4 signal chains induced COX-2 and mPGES-1 mRNA and protein expression in the macrophage cell lines THP-1 and U937. Surprisingly, native LDL repressed COX-2 expression and markedly enhanced mPGES-1 as well as PGE2 synthesis. In THP-1 cells, LDL-mediated induction of mPGES-1 expression was most likely induced by PI3K activation, whereas COX-2 repression may be a functional consequence of reduced ERK phosphorylation and expression of transcription factor Egr-1.
Role of Native LDL in the Inflammatory Response
A hallmark of the development of atherosclerosis is the uptake of modified LDL, which undergoes spontaneous oxidation or modification, by macrophages via scavenger receptors such as CD36 or SR-A, resulting in the formation of foam cells. Beside foam cell formation, oxidized LDL also triggers a chronic inflammatory response, which is a confirmed main cause of plaque rupture and thrombosis [28]. Macrophages in atherosclerotic lesions are key players in the inflammatory process as they produce proinflammatory cytokines and eicosanoids [29]. Only little is known about the function of native LDL in atherosclerotic inflammatory response. Different from oxidized LDL, native LDL is bound and internalized by the LDL-R. Its expression is under tight control of the intracellular cholesterol concentration: an increase in cellular cholesterol downregulates LDL-R expression via a negative feedback loop [30].
Interestingly, most studies that analyzed the influence of native LDL on the inflammatory response in macrophages showed that native LDL rather inhibited than enhanced inflammation. In THP-1 macrophages, native LDL dose-dependently inhibited the serum amyloid A (SAA), induced IL-1β and TNFα expression, most likely by inhibiting NLRP3 inflammosome activation by SAA [31]. A mixture of native LDL, VLDL, and HDL (VLR) also inhibited the basal expression of several inflammatory genes such as IL-6, IL1β, and IL-23A [32]. By contrast to the inhibition of SAA-induced inflammation, the authors of this study postulated that native LDL inhibited expression of inflammatory genes by hypermethylation of their promotor region, whereas LDL had no effect on histone acetylation. In line with these two reports, degradation of the LDL-R in THP-1 macrophages by PCSK9 led to a proinflammatory response, inducing the expression of the proinflammatory cytokines IL-1β, TNFα, MCP-1, and CXCL2 [33].
The aim of the current study was to analyze whether native LDL can modulate the expression of COX-2 and mPGES-1. However, although the experiments were performed with purified, native LDL, spontaneous oxidation during the experiment cannot be entirely excluded. Thus, we determined the oxidation status of the native LDL using malondialdehyde (MDA) assay as an indicator of lipid peroxidation (Supplemental Fig. 4). Whereas MDA was not detectable in the culture medium, the addition of 500 µg/ml LDL, which was not actively modified by chemical oxidation, significantly increased MDA concentration. MDA concentration was markedly enhanced by 24-h incubation with THP-1 macrophages (5-fold). It can, therefore, not be excluded that oxidized LDL also contributed to the regulation of COX-2 and mPGES-1.
The effect of mPGES-1 expression on the development of inflammatory disease as atherosclerosis was analyzed using mPGES-1 knockout mice. Global deletion of mPGES-1 in an LDL-R (-/-), fat-fed model slows atherogenesis, which is indicated by a reduced plaque burden and a reduced number of macrophage foam cells in atherosclerotic lesions [34]. Cell-specific deletion of the mPGES-1 revealed that PGE2 formation in macrophages appears to be essential in this process: Mice lacking mPGES-1 expression in myeloid cells showed markedly reduced atherogenesis in an LDL-R (-/-), high fat-fed model, whereas depletion of mPGES-1 in vascular-smooth muscle or endothelial cells did not alter development of atherosclerosis in this mouse model [35]. These results implicate, that the LDL-mediated induction of mPGES-1 expression (Fig. 1) and enhanced PGE2-secretion (Fig. 4) observed in the current study might increase development of atherosclerosis, especially in combination with other inflammatory mediators such as TNFα and PGE2 which induced maximal expression of the chemotactic cytokine IL-8 in monocytic cell lines and PBMC [21].
Increase of PGE2-synthesis After LDL-Mediated Concomitant mPGES-1 Induction and COX-2 Repression
For an effective synthesis of PGE2, in most cases, the expression of mPGES-1, which generate PGE2 from PGH2, is functionally coupled to expression of COX-2, which provide PGH2 by conversion of arachidonic acid. Coordinated induction of expression of COX-2 and mPGES-1 by pro-inflammatory stimuli such as IL-1β, TNFα, or LPS, activating transcription factor NFκB or PGE2, via activation of transcription factor CREB was observed in macrophages [36]. In the current study, native LDL markedly decreased COX-2 but enhanced mPGES-1 expression, both induced by the combination of TNFα and PGE2. Native LDL stimulated PGE2 synthesis and enhanced PGE2-synthesis induced by the combination of TNFα andEP2/EP4 agonists. Since LDL repressed COX-2 the question arose, which pathway might furnish PGH2 as substrate for the subsequent mPGES-1 reaction? One possible explanation might be the high constitutive expression of COX-1 in THP-1 cells (Table 3). In the current study, the COX-1 mRNA copy numbers in naive or TNFα and PGE2 stimulated THP-1 macrophages were comparable to COX-2 mRNA (Table 3). A robust COX-1 expression in THP-1 macrophages was also confirmed in other publications [37, 38]. Therefore, COX-1 rather than COX-2 may generate PGH2 in THP-1 macrophages, which was converted to PGE2 by LDL-induced mPGES-1.
Table 3.
COX mRNA Profile in THP-1 Macrophages
| COX-1 Control | COX-1 TNFα + PGE2 | COX-2 Control | COX-2 TNFα + PGE2 |
|---|---|---|---|
| 3.14 ± 1.27 | 6.09 ± 1.97 | 2.48 ± 1.57 | 11.9 ± 4.29 |
THP-1 cells were differentiated and cultured as described in the legend to Fig. 1. COX mRNA and GAPDH mRNA of control and TNFα + PGE2-stimulated cells was measured by real-time RT-qPCR as described in the “MATERIALS AND METHODS” section. Plasmids (102–108 copies) containing COX or GAPDH cDNAs were used for preparing standard curves for the calculation of COX or GAPDH mRNA copy numbers. Data represent the mean ± SEM of at least five independent RNA preparations. COX mRNA contents are expressed as copy number COX/PGES mRNA × 1000/copy number GAPDH mRNA.
LDL-Stimulated Signal Chains Leading to Induction of mPGES-1 Expression and Repression of COX-2 Expression
In the current study, native LDL repressed the induction of TNFα + PGE2-induced COX-2 expression and simultaneously enhanced the expression of mPGES-1 (Fig. 1). Most studies analyzing the regulation of COX-2 and mPGES-1 expression show a coordinated expression of both enzymes. Expression of both enzymes in macrophages were induced by LPS or IL-1β mediated activation of transcription NFκB as well as PGE2-mediated CREB activation [23, 36]. A simultaneous induction of COX-2 and mPGES-1 was also found by activation of the NLRP3 inflammasome [39] and by LPS [40]. By contrast, COX-2 and mPGES-1 expression in rat glia cells were regulated in an uncoupled way using inhibitors of PI3K. Whereas LPS-induced COX-2 expression was enhanced by stimulation of PI3K, LPS-induced expression of mPGES-1 was repressed, leading to an overall reduction of PGE2 and an induction of PGD2-release [24, 25]. To prove whether LDL-mediated repression of COX-2 and enhanced expression of mPGS-1 was due to an activation of PI3K, activation of the kinase by LDL was analyzed measuring phosphorylation of the PI3K substrate Akt kinase (Fig. 6) as well as using a PI3K inhibitor (Fig. 5). In line with this hypothesis first, stimulation of THP-1 macrophages led to a phosphorylation of Akt kinase (Fig. 6) and secondly, the LDL-mediated enhancement of mPGES-1 expression was completely blocked in THP-1 cells using the pan PI3K inhibitor LY294002 (Fig. 5). These data strongly indicate, for the first time, that native LDL enhanced mPGES1 expression in activated macrophages by a PI3K-dependent signal chain. An LDL-mediated activation of Akt kinase was also described in human endothelial cells [41]. In these cells, native LDL activated a PI3K-Akt-mTOr signalling pathway most likely by the formation of an LDL-R/ insulin receptor complex. Activation of this signal chain led to an LDL-mediated, insulin-mimetic glucose uptake in the endothelial cells.
By contrast to mPGES-1 expression, the PI3K inhibitor did not influence LDL-mediated repression of COX-2 expression in activated THP-1 macrophages. One of the key factors in the induction of COX-2 expression is the transcription factor Egr-1, which is transcriptionally controlled by an activated ERK kinase [42, 43]. The current study indicated that native LDL repressed Egr-1 expression and abolished ERK phosphorylation in THP-1 macrophages (Fig. 7) which may explain the repression of COX-2 expression by LDL.
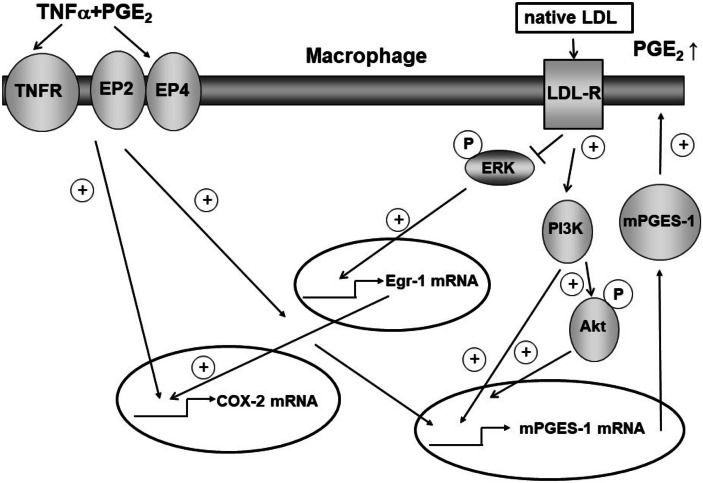
These findings suggest, for the first, time that in human macrophage cell lines LDL simultaneously repressed COX-2 and enhanced mPGES-1 expression induced by TNFα and PGE2 signal chains, most likely by inactivating pERK as well as activating PI3K (Fig. 8).
Fig. 8.
Model of the TNFα/PGE2/LDL-mediated regulation of COX-2 and mPGES1 expression in macrophage cell lines.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- COX
Cyclooxygenase
- GAPDH
Glycerinaldehyde 3-phosphate dehydrogenase
- G protein
Guanine nucleotide-binding protein
- EP1-4
Prostaglandin E2 receptor subtype 1–4
- I-κB
Inhibitor of kappaB
- IKK
Inhibitor of kappaB kinase
- LPS
Lipopolysaccharid
- mPGES-1
Microsomal prostaglandin E2 synthase 1
- NF-κB
Nuclear factor-kappa B
- PGE2
Prostaglandin E2
- PKA
Protein kinase A
- PKC
Protein kinase C
- PI3K
Phosphoinositid 3 Kinase
- TNFα
Tumor Necrosis Factor-α
- TNFα-R
Tumor Necrosis Factor-α receptor
Author Contribution
Frank Neuschäfer-Rube, Theresa Schön, and Ines Kahnt performed the experiments and analyzed data. Frank Neuschäfer-Rube and Gerhard Paul Püschel planned the study and wrote the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of Data and Materials
All the data supporting the findings of the study were shown in this paper and are available upon reasonable request.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, W. Gilroy D, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM, Chronic inflammation in the etiology of disease across the life span. Nature Medicine. 2019 doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett, J.M., G. Reeves, G.E. Billman, and J.P. Sturmberg. 2018. Inflammation-nature’s way to efficiently respond to all types of challenges: Implications for understanding and managing “the epidemic” of chronic diseases. Frontiers in Medicine (Lausanne). [DOI] [PMC free article] [PubMed]
- 3.Kotas, M.E., and R. Medzhitov. 2015. Homeostasis, inflammation, and disease susceptibility. Cell.10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed]
- 4.Yao, C., and S. Narumiya. 2019. Prostaglandin‐cytokine crosstalk in chronic inflammation. British Journal of Pharmacology. 10.1111/bph.14530. [DOI] [PMC free article] [PubMed]
- 5.Narumiya, S. 2007. Physiology and pathophysiology of prostanoid receptors. Proceedings of the Japan Academy Series B: Physical and Biological Sciences. 10.2183/pjab/83.296. [DOI] [PMC free article] [PubMed]
- 6.Tsuge, K., T. Inazumi, A. Shimamoto, and Y. Sugimoto. 2019. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. International Immunology. 10.1093/intimm/dxz021. [DOI] [PubMed]
- 7.Linton, M.F., and S. Fazio. 2004. Cyclooxygenase-2 and inflammation in atherosclerosis Current Opinion in Pharmacology 4: 116–123. 10.1016/j.coph.2003.12.003. [DOI] [PubMed]
- 8.Gómez-Hernández A, Martín-Ventura JL, Sánchez-Galán E, Vidal C, Ortego M, Blanco-Colio LM, Ortega L, Tuñón L, Egido J. Overexpression of COX-2, prostaglandin E synthase-1 and prostaglandin E receptors in blood mononuclear cells and plaque of patients with carotid atherosclerosis: Regulation by nuclear factor-kappaB. Atherosclerosis. 2006;187:139–149. doi: 10.1016/j.atherosclerosis.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Henkel, J., C.D. Coleman, A. Schraplau, K. Jöhrens, D. Weber, J.P. Castro, M. Hugo, T.J. Schulz, S. Krämer, A. Schürmann, and G.P. Püschel. 2017. Induction of steatohepatitis (NASH) with insulin resistance in wild-type B6 mice by a Western-type diet containing soybean oil and cholesterol. Molecular Medicine 23: 70–82. 10.2119/molmed.2016.00203. [DOI] [PMC free article] [PubMed]
- 10.Henkel H, Alfine E, Saín J, Jöhrens K, Weber D, Castro JP, König J, Stuhlmann C, Vahrenbrink M, Jonas W, Kleinridders A, Püschel GP. Soybean oil-derived poly-unsaturated fatty acids enhance liver damage in NAFLD induced by dietary cholesterol. Nutrients. 2018;10:1326. doi: 10.3390/nu10091326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Püschel GP, Henkel J. Dietary cholesterol does not break your heart but kills your liver. Porto Biomedical Journal. 2019;29:12. doi: 10.1016/j.pbj.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henkel J, Coleman CD, Schraplau Jöhrens K, Weiss TS, Jonas W, Schürmann A, Püschel GP. Augmented liver inflammation in a microsomal prostaglandin E synthase 1 (mPGES-1)-deficient diet-induced mouse NASH model. Science and Reports. 2018;8:16127. doi: 10.1038/s41598-018-34633-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N. Oxidized low-density lipoprotein. Methods in Molecular Biology. 2010;610:403–417. doi: 10.1007/978-1-60327-029-8_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lara-Guzmán OJ, Gil-Izquierdo Á, Medina S, Osorio E, Álvarez-Quintero R, Zuluaga N, Oger C, Galano JM, Durand T, Muñoz-Durango K. Oxidized LDL triggers changes in oxidative stress and inflammatory biomarkers in human macrophages. Redox Biology. 2018;15:1–11. doi: 10.1016/j.redox.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eligini S, Colli S, Basso F, Sironi L, Tremoli E. Oxidized low density lipoprotein suppresses expression of inducible cyclooxygenase in human macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:1719–1722. doi: 10.1161/01.atv.19.7.1719. [DOI] [PubMed] [Google Scholar]
- 16.Wang, L., J.W. Xia, Z.P. Ke, and B.H. Zhang. 2019. Blockade of NEAT1 represses inflammation response and lipid uptake via modulating miR-342–3p in human macrophages THP-1 cells. Journal of Cellular Physiology 234: 5319–5326. 10.1002/jcp.27340. [DOI] [PubMed]
- 17.Gargiulo S, Rossin D, Testa G, Gamba P, Staurenghi E, Biasi F, Poli G, Leonarduzzi G. Up-regulation of COX-2 and mPGES-1 by 27-hydroxycholesterol and 4-hydroxynonenal: A crucial role in atherosclerotic plaque instability. Free Radical Biology & Medicine. 2018;129:354–363. doi: 10.1016/j.freeradbiomed.2018.09.046. [DOI] [PubMed] [Google Scholar]
- 18.Rampersad SN. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors (Basel) 2012;12:12347–12360. doi: 10.3390/s120912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods in Molecular Biology. 1998;108:101–106. doi: 10.1385/0-89603-472-0:101. [DOI] [PubMed] [Google Scholar]
- 20.Kielkopf, C.L., W. Bauer, and I.L. Urbatsch. 2020. Bradford assay for determining protein concentration. Cold Spring Harbor Protocols 4: 102269. 10.1101/pdb.prot102269. [DOI] [PubMed]
- 21.Neuschäfer-Rube F, Pathe-Neuschäfer-Rube A, Hippenstiel S, Püschel GP. PGE2 enhanced TNFα-mediated IL-8 induction in monocytic cell lines and PBMC. Cytokine. 2019;113:105–111. doi: 10.1016/j.cyto.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Farley J, Sirois J, MacFarlane PH, Kombé A, Laverty S. Evaluation of coexpression of microsomal prostaglandin E synthase-1 and cyclooxygenase-2 in interleukin-1-stimulated equine articular chondrocytes. American Journal of Veterinary Research. 2005;66:1985–1989. doi: 10.2460/ajvr.2005.66.1985. [DOI] [PubMed] [Google Scholar]
- 23.Díaz-Muñoz MD, Osma-García IC, Cacheiro-Llaguno C, Fresno M, Iñiguez MA. Coordinated up-regulation of cyclooxygenase-2 and microsomal prostaglandin E synthase 1 transcription by nuclear factor kappa B and early growth response-1 in macrophages. Cellular Signalling. 2010;22:1427–1436. doi: 10.1016/j.cellsig.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 24.de Oliveira, A.C., E. Candelario-Jalil, H.S. Bhatia, K. Lieb, M. Hüll, and B.L. Fiebich. 2008. Regulation of prostaglandin E2 synthase expression in activated primary rat microglia: evidence for uncoupled regulation of mPGES-1 and COX-2. Glia 56: 844–55. 10.1002/glia.20658. [DOI] [PubMed]
- 25.de Oliveira AC, Candelario-Jalil E, Langbein J, Wendeburg L, Bhatia HS, Schlachetzki JC, Biber K, Fiebich BL. Pharmacological inhibition of Akt and downstream pathways modulates the expression of COX-2 and mPGES-1 in activated microglia. Journal of Neuroinflammation. 2012;3:2. doi: 10.1186/1742-2094-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillem-Llobat P, Íñiguez MA. Inhibition of lipopolysaccharide-induced gene expression by liver X receptor ligands in macrophages involves interference with early growth response factor 1. Prostaglandins Leukotrienes and Essential Fatty Acids. 2015;96:37–49. doi: 10.1016/j.plefa.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Guerrero E, Chen E, Kockx M, An SW, Chong BH, Khachigian LM. IL-1beta signals through the EGF receptor and activates Egr-1 through MMP-ADAM. PLoS ONE. 2012;7:e39811. doi: 10.1371/journal.pone.0039811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. Journal of Internal Medicine. 2015;278:483–493. doi: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nature Immunology. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 30.Dawson PA, Hofmann SL, van der Westhuyzen DR, Südhof TC, Brown MS, Goldstein JL. Sterol-dependent repression of low density lipoprotein receptor promoter mediated by 16-base pair sequence adjacent to binding site for transcription factor Sp1. Journal of Biological Chemistry. 1988;263:3372–3379. doi: 10.1016/S0021-9258(18)69081-7. [DOI] [PubMed] [Google Scholar]
- 31.Nurmi, K., K. Niemi, I. Kareinen, K. Silventoinen, M.B. Lorey, Y. Chen, V.P. Kouri, J. Parantainen, T. Juutilainen, K. Öörni, P.T. Kovanen, D. Nordström, S. Matikainen, and K.K. Eklund. 2011. Native and oxidised lipoproteins negatively regulate the serum amyloid A-induced NLRP3 inflammasome activation in human macrophages. Clinical & Translational Immunology 10. 10.1002/cti2.1323. [DOI] [PMC free article] [PubMed]
- 32.Rangel-Salazar R, Wickström-Lindholm M, Aguilar-Salinas CA, Alvarado-Caudillo Y, Døssing K, Esteller M, Labourier E, Lund G, Nielsen FC, Rodríguez-Ríos D, Solís-Martínez MO, Wrobel K, Wrobel K, Zaina S. Human native lipoprotein-induced de novo DNA methylation is associated with repression of inflammatory genes in THP-1 macrophages. BMC Genomics. 2021;25:582. doi: 10.1186/1471-2164-12-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricci C, Ruscica M, Camera M, Rossetti L, Macchi C, Colciago A, Zanotti I, Lupo MG, Adorni MP, Cicero AFG, Fogacci F, Corsini A, Ferri N. PCSK9 induces a pro-inflammatory response in macrophages. Science and Reports. 2018;8:2267. doi: 10.1038/s41598-018-20425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M, Zukas A, Hui Y, Ricciotti E, Puré E, FitzGerald GA. Deletion of microsomal prostaglandin E synthase-1 augments prostacyclin and retards atherogenesis. Proceedings of the National Academy of Sciences. 2006;103:14507–14512. doi: 10.1073/pnas.0606586103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen L, Yang G, Monslow J, Todd L, Cormode DP, Tang J, Grant GR, DeLong JH, Tang SY, Lawson JA, Pure E, Fitzgerald GA. Myeloid cell microsomal prostaglandin E synthase-1 fosters atherogenesis in mice. Proceedings of the National Academy of Sciences U S A. 2014;111:6828–6833. doi: 10.1073/pnas.1401797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Díaz-Muñoz MD, Osma-García IC, Fresno M, Iñiguez MA. Involvement of PGE2 and the cAMP signalling pathway in the up-regulation of COX-2 and mPGES-1 expression in LPS-activated macrophages. The Biochemical Journal. 2012;443:451–461. doi: 10.1042/BJ20111052. [DOI] [PubMed] [Google Scholar]
- 37.Smith CJ, Morrow JD, Roberts LJ, 2nd, Marnett LJ. Differentiation of monocytoid THP-1 cells with phorbol ester induces expression of prostaglandin endoperoxide synthase-1 (COX-1) Biochemical and Biophysical Research Communications. 1993;192:787–793. doi: 10.1006/bbrc.1993.1483. [DOI] [PubMed] [Google Scholar]
- 38.Korbecki J, Baranowska-Bosiacka I, Gutowska I, Piotrowska K, Chlubek D. Cyclooxygenase-1 as the main source of proinflammatory factors after sodium orthovanadate treatment. Biological Trace Element Research. 2015;163:103–111. doi: 10.1007/s12011-014-0176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuang Y, Zhao F, Liang J, Deng X, Zhang Y, Ding G, Zhang A, Jia Z, Huang S. Activation of COX-2/mPGES-1/PGE2 cascade via NLRP3 inflammasome contributes to albumin-induced proximal tubule cell injury. Cellular Physiology and Biochemistry. 2017;42:797–807. doi: 10.1159/000478070. [DOI] [PubMed] [Google Scholar]
- 40.Font-Nieves M, Sans-Fons MG, Gorina R, Bonfill-Teixidor E, Salas-Pérdomo A, Márquez-Kisinousky L, Santalucia T, Planas AM. Induction of COX-2 enzyme and down-regulation of COX-1 expression by lipopolysaccharide (LPS) control prostaglandin E2 production in astrocytes. Journal of Biological Chemistry. 2012;287:6454–6456. doi: 10.1074/jbc.M111.327874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu L, Wu G, Yang X, Jia X, Li J, Bai X, Li W, Zhao Y, Li Y, Cheng W, Liu S, Jin S. Low density lipoprotein mimics insulin action on autophagy and glucose uptake in endothelial cells. Science and Reports. 2019;28:3020. doi: 10.1038/s41598-019-39559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takuya T, Zhang F, Sun L, Hao F, Schmitz-Peiffer C, Xu X, Cui MZ. Lysophosphatidic acid induces early growth response-1 (Egr-1) protein expression via protein kinase Cδ-regulated extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) activation in vascular smooth muscle cells. Journal of Biological Chemistry. 2012;287:22635–22642. doi: 10.1074/jbc.M111.335695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pagel JI, Deindl E. Disease progression mediated by egr-1 associated signaling in response to oxidative stress. International Journal of Molecular Sciences. 2012;13:13104–13117. doi: 10.3390/ijms131013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data supporting the findings of the study were shown in this paper and are available upon reasonable request.