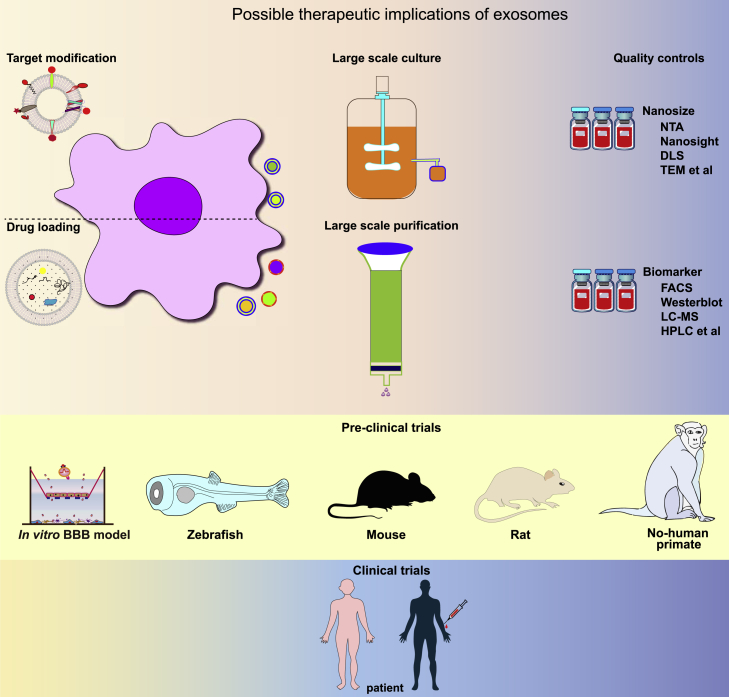
Summary
Developing strategies toward safe and effective drug delivery into the central nervous system (CNS) with improved targeting abilities and reduced off-target effects is crucial. CNS-targeted drug carriers made of synthetic molecules raise concerns about their biodegradation, clearance, immune responses, and neurotoxicity. Cell-derived nanovesicles (CDNs) have recently been applied in CNS-targeted drug delivery, because of their intrinsic stability, biocompatibility, inherent homing capability, and the ability to penetrate through biological barriers, including the blood-brain barrier. Among these CDNs, extracellular vesicles and exosomes are the most studied because their surface can be engineered and modified to cater to brain targeting. In this review, we focus on the application of CDNs in brain-targeted drug delivery to treat neurological diseases. We cover recently developed methods of exosome derivation and engineering, including exosome-like particles, hybrid exosomes, exosome-associated adeno-associated viruses, and envelope protein nanocages. Finally, we discuss the limitations and project the future development of the CDN-based brain-targeted delivery systems, and conclude that engineered CDNs hold great potential in the treatment of neurological diseases.
Keywords: drug delivery, brain, exosome, extracellular nanovesicles, central nervous system
Graphical abstract
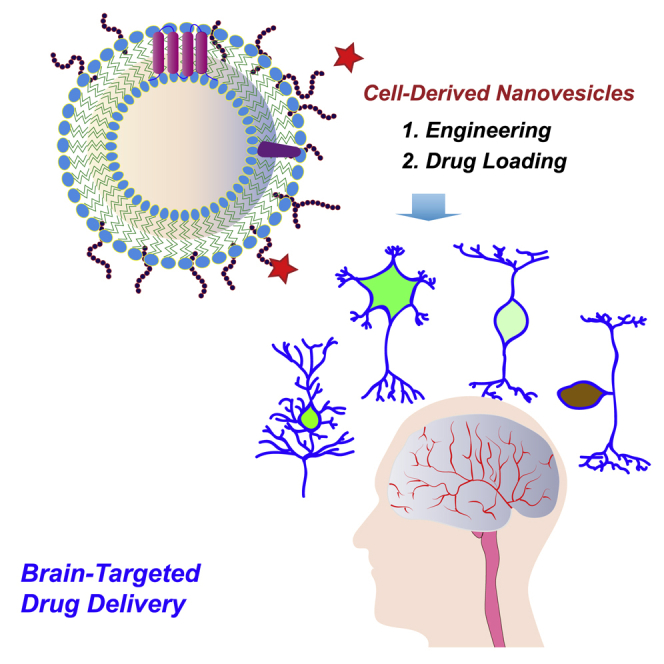
Here, we review the application of cell-derived nanovesicles in brain-targeted drug delivery to treat neurological diseases, with the focus on their derivation and engineering and transportation through the blood-brain barrier.
Introduction
The brain is the major component of the central nervous system (CNS) and the most complex part of the human body. Neurological diseases, such as brain tumors, brain infection, epilepsy, Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease are among the most expensive and devastating illnesses in medicine.1 The World Health Organization predicts that brain diseases will surpass cancer as the second leading cause of death by 2040.2 Over the past few decades, researchers have developed promising neurotherapeutic agents to target these diseases. However, because of the hindrance posed by the blood-brain barrier (BBB), the translation of these agents from bench to bedside has become difficult. Thus, contemporary research in this area is to search for new promising drug delivery vehicles and to improve the current ones for preferred pharmacokinetics and increased drug penetrability to the brain.3,4
In recent years, new strategies have been developed to facilitate the passage of drugs across the BBB, including direct intrathecal or intracerebroventricular injections and osmotic disruption of the BBB, but these techniques are highly invasive, and the recipients are susceptible to infection. The rapidly developing nanotechnology has provided new possibilities for transporting therapeutic drugs across the BBB to treat brain diseases: a variety of synthetic nanoparticles have emerged as effective non-invasive drug carriers for brain diseases, including liposomes, dendrimers, carbon quantum dots, micelles, polymeric nanoparticles, etc.5 Considering the biocompatibility, safety, and complexity of the production process, naturally occurring nanovesicles are superior to these synthetic nanocarriers. Over the past decade, cell-derived nanovesicles (CDNs), especially extracellular vesicles (EVs) and exosomes, have delineated their roles as promising drug delivery tools. EVs/exosomes have exhibited promising potential in delivering therapeutic agents across the BBB to mitigate the symptoms of neurological disorders.6,7,8,9,10 Native EVs/exosomes or EVs/exosomes loaded with small-molecule drugs or gene therapy agents have shown great potential in treating neurological diseases. The exploitation of engineered EVs/exosomes and EV/exosome derivatives for brain-targeted drug delivery further offered new possibilities.6,7,8,9,10 In this review, we start with the biogenesis, composition, and function of CDNs, provide insights into the mechanism of how exosomes transport cargos across the BBB, summarize available strategies for engineering exosomes and loading cargos, and elaborate on the current state-of-the-art research on CDNs as brain-targeting nanocarriers for the treatment of neurological disorders. In particular, we focus on how surface engineering achieves better brain-targeted delivery. Because of the ambiguity in the definitions of different types of CDNs in the literature and considering the similar roles they play in drug delivery, although we try to use CDNs as the overall term throughout the manuscript, in some scenarios the terms EVs and exosomes are used interchangeably or one of them is selected based on the choice of the original literature.
The BBB
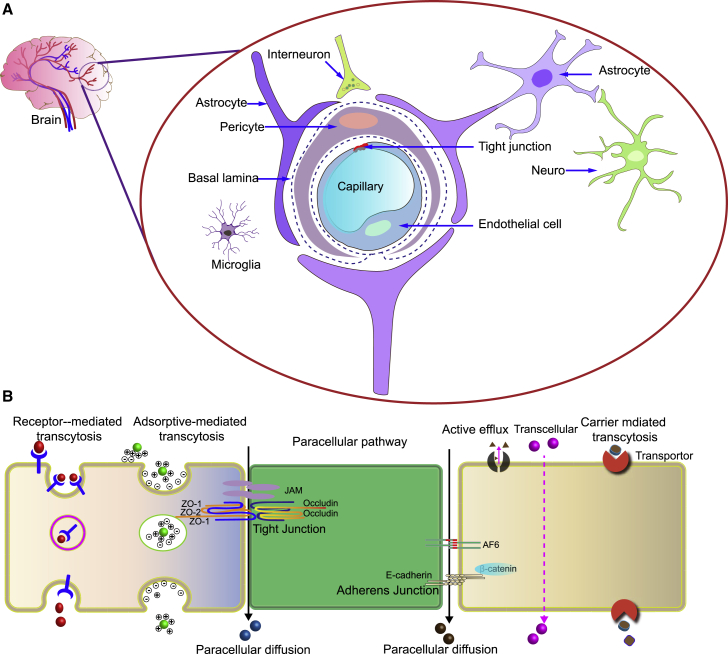
The CNS is tightly sealed from the blood environment by barriers, such as the BBB, the blood-cerebrospinal fluid barrier, and the blood-retina barrier.11 The BBB regulates material transport to the brain but also presents a major barrier in brain-targeted drug delivery. Only a few CNS diseases, such as schizophrenia, chronic pain, epilepsy, and depression are currently treatable with small-molecule drugs. The BBB thus represents a major roadblock in developing therapies for brain-specific disorders. The BBB is mainly composed of tightly connected microvascular endothelial cells, astrocytes, and pericytes. In addition to these three cells, there are other components, such as neurons and innervating cells, adjacent to microglia that also participate in the structural and functional regulation of the BBB under physiological and pathological conditions (Figure 1A). Endothelial cells (ECs) are the major functional components of the BBB, and they are connected by tight junctions, adherens junctions, and gap junctions. These endothelial junctions form a thin layer to ensure vascular stability and BBB integrity. Molecules, such as claudins, occludins, junction adhesion molecules, and zonula occludens, form the basic seal of the BBB by tightly attaching to ECs. These tight junctions keep the ECs 50–100 times more tightly connected than ECs located in the wall of peripheral microvessels, which impedes the passage of cells and small molecules into the brain.13,14 Furthermore, BBB ECs have fewer pinocytotic vesicles than peripheral ECs.15 Ions or small molecules (e.g., glucose) are transported across the BBB by active transport. The transportation routes for proteins and peptides include paracellular and transcellular transcytosis, receptor-mediated transcytosis (RMT), transporter protein-mediated transcytosis, cell-mediated transcytosis, and absorptive-mediated transcytosis. Paracellular diffusion is the passage of solute molecules through gaps between two adjacent ECs. The driving mechanism for this non-specific transport is the negative concentration gradient from the blood to the brain. Only hydrophilic small molecules (molecular weight <500 Da) can cross the BBB by paracellular diffusion.16,17 Carrier transporters, including glucose transporter 1 (GLUT1), monocarboxylic acid transporter 1 (MCT1), and L-type amino acid transporter (LAT1), are expressed at the BBB to facilitate the transport of nutrients and metabolites into or out of the brain.18,19 GLUT-1, responsible for glucose uptake, is involved in the transport of insulin or transferrin to specific receptors in the brain, whereas MCT1 selectively transports small molecules into the brain. LAT1 transports neutral amino acids with large molecular weights, such as leucine, phenylalanine, methionine, etc., but they are incapable of mediating the macromolecular drug delivery.20
Figure 1.
Structure and biological transport mechanisms of the BBB
(A) The BBB is mainly composed of vascular endothelial cells surrounded by pericytes, basal lamina, a layer of astrocyte terminal protrusions, and microglia. These cells are involved in maintaining the integrity of the BBB.
(B) Representative pathways of CDVs crossing the BBB including receptor-mediated transcytosis, adsorptive-mediated transcytosis, transporter-mediated transcytosis, and paracellular pathway. ZO-1, zonula occludens-1, also known as tight junction protein-1; ZO-2, zonula occludens-2; JAM, junctional adhesion molecules.12
Another path is through adsorptive-mediated transcytosis (AMT), which involves the binding of polycations to negative charges on the serosa.15 However, since this pathway lacks specificity, a wide range of molecules can be absorbed, and the targeting effect is thus difficult to achieve. RMT is another pathway for transporting macromolecules, which involves the binding of a specific ligand to receptors and can be harnessed to realize efficient targeted drug delivery. Receptor-mediated endocytosis involves binding macromolecules to receptor proteins, accumulation in intracellular invaginations, and entering the ECs as complexes in clathrin-coated pits through endocytosis of surface receptor transporters that are recycled to the cell surface or transported to lysosomes for degradation. RMT and AMT can effectively promote brain drug delivery, especially for macromolecular drugs.21 The pathways for crossing the BBB are summarized in Figure 1B.
Transporting exosomes across the BBB
Some CDVs, including exosomes (30–150 nm), are known to enter the brain and serve as delivery vehicles for brain-targeted drug delivery.22 For example, Yang and co-workers explored how exosomes mitigated across the BBB and reported that bEND.3 cell-derived exosomes loaded with rhodamine 123 could be delivered to the brain through the brain endothelium region, while rhodamine 123 alone remained in the blood vessels, indicating that exosomes can pass the BBB and increase the ability of drugs to cross the BBB. They then utilized bEND.3 cell-derived exosomes to deliver anticancer drugs for the treatment of neuronal glioblastoma (GBM), which showed a significant cytotoxic effect.23
Although many findings report that exosomes could deliver drugs across the BBB, how exosomes interact with the BBB and enter brain regions remains elusive. Chen and co-workers revealed that exosomes were internalized by brain microvascular ECs (BMECs) via endocytosis. Using HEK293T-derived exosomes and in vitro BBB monolayers as model systems, they demonstrated that exosomes passed across the BBB primarily through active endocytosis of BMECs using the transcellular pathway rather than the passive paracellular pathway.24 The binding of exosome surface ligands to brain EC receptors is currently considered the primary mechanism for exosomes to cross the BBB. Yuan et al. revealed that modification with brain homing peptides is not required to penetrate the BBB.11 Macrophage (Mφ) exosomes crossed the BBB through the interaction between the intercellular adhesion molecule-1 and integrin lymphocyte function-associated antigen 1.25 Blood-derived exosomes also exhibited natural brain targeting capability via interaction with transferrin receptors (TfRs).26 CD46 has been found to be the main receptor of BBB cells for the uptake of exosomes derived from brain metastatic tumor cells.27
Certain types of exosomes have been reported to harness various receptors or cellular pathways to cross the BBB. For example, under brain inflammation, upregulation of intracellular adhesion molecule 1 (ICAM-1) in brain inflammation correlated with the enhanced uptake of Mφ exosomes by BBB cells.25 Exosomes can also achieve BBB crossing through endocytosis of BMECs by means of special adhesion proteins on the surface of exosomes, such as integrins, tetraspanins, CD11b and CD18 receptors, major histocompatibility complex, etc. Exosomes released from neuronal and non-neuronal cells mediate the information exchange within CNS. Furthermore, most exosomes cross the BMEC monolayer through the transcellular pathway, endocytosis, multivesicular body (MVB) formation, and exocytosis, while only a few exosomes pass through the BBB via passive diffusion, i.e., paracellular path.24 Intuitively, under the condition of BBB breakdown due to inflammation or immune responses, the paracellular diffusion route may increase.28 The strategies to achieve drug delivery across the BBB generally involve modulation of physiological barriers, opening tight junctions, and inhibiting efflux pumps. Using an in vitro model, Banks et al. tested the ability of 10 exosome populations derived from mouse, human, cancerous, and non-cancerous cell lines to cross the BBB. The authors discovered a vast rate difference of over 10-fold, and different exosome populations acted differently to modulators suggesting heterogeneity in the transport mechanism.29 Thus, deciphering the mechanism of this highly selective barrier will further facilitate the development of new approaches to aid the crossing of the BBB by rationally designing effective exosome-based brain delivery systems.30
Biogenesis of exosomes
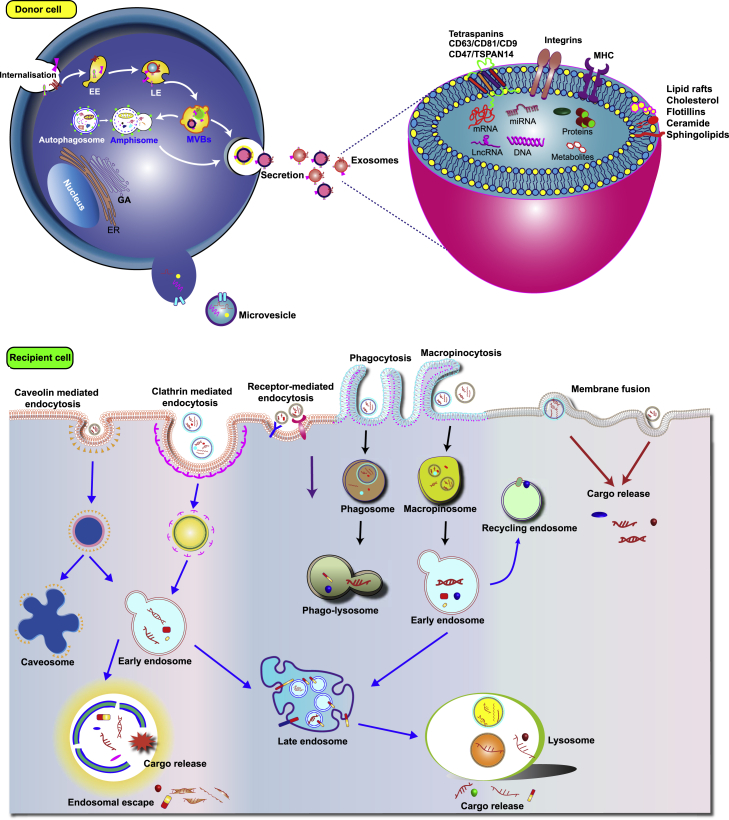
Exosomes originate from early endosomes that mature and become late sorted endosomes (LEs) (Figure 2). LEs, also referred to as MVBs, are formed upon the invagination and budding of portions of the endosome membrane into the lumen.31,32,33 Intraluminal vesicles (ILVs) are released into the extracellular space by fusion of the LE membrane with the plasma membrane. Microvesicles are larger than exosomes and are formed through outward budding of the plasma membrane. Within exosomes, nucleic acids (DNA, RNA, and miRNAs), lipid rafts (cholesterol and flotillin-1), proteins (adhesion molecules, tetraspanins, cytoplasmic proteins, and endosomal sorting complex required for transport [ESCRT]) can be found. After the ILVs are released into the extracellular space as exosomes, they can be internalized by recipient cells via different endocytic pathways, such as clathrin-dependent and clathrin-independent endocytosis, phagocytosis, and micropinocytosis.31,32,33 Exosomes interact with the target cells through different mechanisms. They can fuse with the target cell membrane and release their cargo directly into the cytoplasm of the target cell. Or, without being internalized, they can activate downstream signaling cascades by attaching to target cell membranes through ligand-receptor interactions.31,32,33
Figure 2.
Schematic illustrations showing the biogenesis and secretion of exosomes and their cellular update pathways
GA, Golgi apparatus; MVB, multivesicular body; EE, early endosome; LE, late endosome; MHC, major histocompatibility complex; CD, cluster of differentiation; TSPAN, tetraspanins.31,32,33
Exosomes play key roles in intercellular communications in regulating normal physiological processes as well as contributing to pathological conditions. In the nervous system, exosomes are involved in maintaining myelin sheaths, immune defense, inflammatory response, neurogenesis, and eliminating waste products.34 Also, exosomes in the brain play a role in CNS diseases, such as stroke, AD, PD, prion disease, and chronic traumatic encephalopathy. Containing multiple biologically active substances, exosomes are viewed as miniature compartments of the intracellular environment and encode information about the disease progression. Exosomes from the CNS can be found in CSF and peripheral body fluids, and the change in their contents with neurological conditions may serve as collectable samples for early diagnosis of brain diseases. For example, increased exosomal content of glutaminase C has been reported to be associated with the early pathogenesis of AD.35 On the other hand, exosomes enriched with growth factors or miRNA are released by neural stem/progenitor cells during neural stem cell (NSC) differentiation.36,37
Native CDVs for brain-targeted drug delivery
Some native CDVs can cross the BBB. For example, tumor-derived exosomes show organ-specific tropism through the action of integrin.38 Morad and co-workers confirmed that tumor-derived exosomes cross the BBB via transcytosis in several models of BBB, including the cell-based in vitro model, zebrafish model, and mouse model.39 Mechanistic studies elucidated that tumor-derived CDVs have the intrinsic ability to circumvent the reduced rate of transcytosis in the BBB by decreasing rab7 expression in brain ECs and enhancing their uptake efficiency by astrocytes.39 Exosomes derived from gliomas can upregulate Lipocalin-2 expression in BMECs, thereby increasing endothelial membrane fluidity in the BBB.40 Such BBB-crossing glioma-derived exosomes offer an effective therapeutic strategy for drug delivery to the brain. Tumor cell-derived exosomes, however, may increase the risk of tumorigenicity and immunogenicity in the host cells. Their use in clinical applications, therefore, raises concerns about the safety issue.
Microglia are the resident Mφs of the central nervous system. Microglia-derived nanovesicles mediate cell-cell communications and regulate neuronal inflammation and immune responses. Li et al. injected CDVs derived from M2 microglia into mice (M2-EVs) via tail vein injection, and found M2-EVs reduced brain atrophy volume, promoted functional recovery, oligodendrogenesis and white matter repair in vivo, increased oligodendrocyte precursor cell proliferation, survival, and differentiation in vitro. Further studies showed that M2-EVs promote white matter repair via miR-23a-5p, possibly by directly targeting Olig3 after ischemic stroke.41 Reynolds et al. utilized microglia-derived exosomes as a vehicle to deliver small interfering RNA (siRNA) across the BBB to target human telomerase reverse transcriptase-immortalized human microglial cells (HTHU) latently infected by HIV (HTHU-HIV). The result showed that HTHU exosomes could cross the BBB and efficiently deliver the cargo to the CNS.42 Microglial EVs can deliver to recipient cells not only miRNAs but also cytokines, such as TNF and IL-4,43,44 bioactive lipids,45,46 and growth factors, such as TGF-beta.47 Therefore, engineered microglial EVs may also represent a multimodal drug delivery platform to target different CNS cells to promote regeneration.
Mφ-derived exosomes can also bypass the BBB. Mφs loaded with catalase could facilitate drug delivery to cells in neurovascular units, such as neurons, astrocytes, and BMECs.48 A considerable number of Mφ-derived exosomes loaded with the antioxidant catalase were detected in PD mouse brains after intranasal administration and offered a remarkable neuroprotective effect.49 Moreover, the pathology of the brain also promotes the selective uptake of exosomes by BBB cells. Upregulation of the inflammatory factor ICAM-1 enhanced the cellular uptake of Mφ exosomes by BBB cells. Mφ exosomes accumulate in the inflamed brain 5.8-fold higher than that in the healthy brain.25 Overall, Mφ-derived exosomes loaded with catalase have potential in the treatment of inflammatory and neurodegenerative diseases. These findings have important implications for the use of Mφ-derived exosomes as nanocarriers to deliver therapeutic proteins across the BBB. T cell-derived exosomes loaded with curcumin (Cur) exhibited a high affinity with microglial cells (∼60%) and subsequently induced microglial apoptosis.50
Brain EC-derived exosomes have the inherent ability to bypass the BBB for brain delivery or they may protect neurons from hypoxic injury. For example, cerebral EC (bEnd.3 cell)-derived exosomes (EC-Exos) were used to treat acute ischemic brain injury.51 EC-Exos promoted functional motor recovery in the model of ischemic stroke produced by temporary middle cerebral artery occlusion and reperfusion. The regulation of apical transmission, synaptic plasticity, and synaptic vesicle cycle was significantly improved after exosome treatment. In addition, EC-Exo-mediated delivery of microRNA-126-3p could protect nerve cells from apoptosis and increased neurite outgrowth. bEnd.3 cell-derived exosomes decreased the infarct volume and improved neurobehavioral recovery after ischemic stroke. Furthermore, neural progenitor cell proliferation and migration were activated after exosome treatment. These findings demonstrated the mechanism by which EC-Exos play a role in the remodeling of neuronal and vascular units and altered brain plasticity in acute brain ischemic injury conditions.51 Other studies have also exploited this BBB crossing ability of the EC-Exo carrier for targeted drug delivery in brain cancer treatment.23
Besides, mesenchymal stem cell (MSC)-derived exosomes have also found use in the treatment of stroke, AD, and neurodevelopment disorder.52,53,54 MSC-derived exosomes can reduce inflammation at the injury site, thereby improving recovery from brain injury by enhancing neurogenesis and angiogenesis.55,56 Studies found that the administration of MSC-derived exosomes induces neuronal recovery and improved motor and cognitive deficits after traumatic brain injury. Moreover, exosomes can effectively improve brain functional outcomes after stroke. Intravenous administration of MSC-derived exosomes improved functional recovery and enhanced neuritis remodeling, neurogenesis, neurovascular plasticity, and behavioral and neurological performance.57,58 Table 1 summarizes the preclinical studies of treating neurodegenerative diseases with EVs/exosomes. For example, MSC-derived exosomes inhibited the expression of pro-inflammatory protein-inducible nitric oxide synthase to alleviate nerve damage, repair synaptic transmission, and long-term potentiation in AD mice.73 MSC-derived exosomes alleviate the symptoms of PD by enhancing angiogenesis and neuroprotective activity.74,75 MSC-derived exosomes may also treat brain diseases by transferring miRNAs (miRs). MSC-derived EVs ameliorate AD by delivering miR-29c-3p into neurons to target BACE1 and activate the Wnt/β-catenin pathway.76 In other examples, MSC-derived exosome-mediated delivery of miR-133b promoted neurite outgrowth and functional recovery in stroke.77,78 Engineered exosomes derived from MSCs enriched with miR-17-92 promoted neurogenesis, oligodendrogenesis, and neurite plasticity, and enhanced neurological recovery after stroke.62 Because miR-124 has been shown to exert a therapeutic effect in acute ischemic stroke by promoting neurogenesis, a recent clinical trial (NCT03384433) evaluated the potential of miR-124-loaded MSC-derived exosomes in reversing the disability of patients with acute ischemic stroke. Other ongoing clinical trials are summarized in Table 2.
Table 1.
Summary of native EVs/exosomes for brain drug delivery and therapy in preclinical studies
| Exosomes/MVs source | Cargo | Administration route | Brain disease (animal model) | Reference |
|---|---|---|---|---|
| Macrophages | catalase | intranasal or intravenously injected | Parkinson’s disease | Haney et al.49 |
| EL-4 T cells | curcumin | intranasal administration | brain inflammation | Zhuang et al.50 |
| bEnd.3 cells | doxorubicin | intravenous injection | glioblastoma | Zhang et al.59 |
| HEK293T | Cre recombinase | intranasal delivery | across the BBB | Sterzenbach et al.60 |
| Human bone marrow-derived mesenchymal stem cells (hBM-MSCs) | glucose-coated gold nanoparticle (GNP) | intranasal administration | stroke model | Betzer et al.61 |
| Human adipose mesenchymal stem cell (hADSC) | miR-124 | intracerebroventricular | ischemic stroke | Wang et al.55 |
| MSCs | miRNA-17-92 | intravenous | ischemic stroke | Xin et al.62 |
| Gingival MSC | TNF-α-stimulated | intraocular injection | retinal ischemia-reperfusion injury | Yu et al.63 |
| Macrophages | lipopolysaccharide (LPS)-stimulated | intravenous injection | ischemic stroke | Zheng et al.64 |
| Macrophages | curcumin | tail vein injection | ischemia-reperfusion injury | He et al.65 |
| MSC | – | tail vein injection | intracerebral hemorrhage | Han et al.66 |
| Blood | dopamine | intravenous injection | Parkinson’s disease | Qu et al.26 |
| Plasma | edaravone | intravenous injected into rats via tail vein | ischemic stroke | Guo et al.67 |
| MSC | – | intravenous | Parkinson’s disease | Chen et al.68 |
| Bone marrow-derived MSCs (BM-MSCs) | SDF-1alpha | intravenous | PD rat model | Cova et al.69 |
| BM-MSCs | – | intravenously injected by tail vein | stroke | Xin et al.57 |
| BM-MSCs | – | tail vein injection | traumatic brain injury | Zhang et al.70 |
| Human umbilical cord mesenchymal stem cells (hUC-MSCs) | – | injection | Alzheimer’s disease | Ding et al.71 |
| hUC-MSCs hBM-MSCs |
– | intranasally administered | autism mouse model | Liang et al.53; Perets et al.72 |
Table 2.
Ongoing clinical trials of EVs/exosomes for treatment of brain diseases
| EVs/Exosomes | Sponsor/organization | Administration route | Brain disease | Reference |
|---|---|---|---|---|
| miR-124-Loaded MSC-Exos | Saeed Oraei Yazdani | intranasal | acute ischemic stroke |
NCT03384433 phase 1 phase 2 |
| Adipose MSC-exos | Ruijin Hospital, Shanghai Jiao Tong University | stereotaxis/intraparenchymal | Alzheimer’s disease |
NCT04388982 phase 1 phase 2 |
| Neonatal stem cell-Exos | Neurological Associates of West Los Angeles | intravenously, epineural using ultrasound guidance | craniofacial neuralgia | NCT04202783 |
| Allogenic exosomes | Neurological Associates of West Los Angeles | ultrasound delivery of exosomes | refractory depression, anxiety disorders, neurodegenerative diseases | NCT04202770 |
EVs released from NSCs also provide therapeutic effects by modulating the function of neurons and glia in the brain. NSC-derived EVs exhibited better brain repair in a mouse stroke model compared with MSC-EVs.79 hNSC-derived EVs by intravenous administration attenuated AD-related behavioral and molecular neuropathology in the AD mice model.80 These results suggest that EVs from native sources can improve brain function by enhancing neurite remodeling and neurogenesis, inhibiting brain cell apoptosis, improving the brain microenvironment, mediating immune responses, inducing vascular remodeling, and alleviating inflammation. Although native EVs or exosomes can cross the BBB under normal conditions, they may accumulate in the liver and spleen at the condition of brain injury. Increasing the enrichment of EVs and exosomes in the brain by developing new engineering strategies is thereby a valuable research direction. For example, EVs can be engineered to target dopaminergic neurons in PD, medium spiny interneurons in Huntington’s disease, and motor neurons in amyotrophic lateral sclerosis.81
Surface engineering for brain-targeted drug delivery
Although native EVs/exosomes can cross the BBB, recent studies have shown that systemically injected unmodified EVs/exosomes from various cell types mainly accumulate in the liver and spleen, with only <1% reaching the brain.82,83 Endowing EVs/exosomes with brain-targeting capability will increase the accumulation in the brain.
Some exosomal surface proteins can be the points of modification. Lysosome-associated membrane glycoprotein 2b (Lamp2b) on the surface of the exosomes can be modified by genetic engineering without compromising the structural integrity and properties of the exosomes.84 In one example, a 29-amino-acid peptide derived from rabies virus glycoprotein (RVG) that specifically binds to nicotinic acetylcholine receptor was engineered onto the surface of exosomes through genetic fusion with Lamp2b. Dendritic cell-derived exosomes carrying Lamp2b-RVG could deliver siRNA to the mouse brain. The Lamp2b-RVG-modified exosomes (RVG-Exos) were loaded with exogenous siRNA targeting BACE1, an important regulatory gene related to the pathogenesis of AD, and were injected intravenously. BACE1 siRNA was delivered to neurons, microglia, and oligodendrocytes in the brain, resulting in the knockdown of BACE1.85 In another report, RVG-modified exosomes from MSCs were delivered to the brain region, attenuated the neuroinflammation induced by amyloid-beta, and improved learning and memory dysfunction in a mouse model of AD.86 Modification with the RVG sequence resulted in a 3-fold increase of exosomes in the cortex and hippocampus. Also, loading miR-124 in RVG-modified MSC-derived exosomes effectively delivered miRNA to the infarct site and ameliorated brain injury by promoting neural progenitor cells to acquire a neuronal phenotype, thereby protecting against ischemic injury.87 Intravenous delivery of HMGB1 siRNA into the ischemic brain using RVG peptide-modified exosomes also reduced HMGB1 levels and infarct size.88 These studies show that Lamp2b-RVG-Exos can be used for targeted delivery of gene drugs to the brain and may find use in the treatment of brain-related diseases. This vehicle can also find use in treating viral infection. Zhang et al. reported the use of RVG-modified small EVs for targeted delivery of antiviral siRNA to the head of fetal mice, which significantly inhibited Zika virus infection and alleviated fetal microcephaly caused by virus infection.89
Regarding the cargo, RVG-Exos have been used to deliver siRNA, miRNA, circRNAs, mRNA, and proteins to the brain and showed therapeutic efficacy. RVG-Exos delivered circDYM to the brain through intravenous injection-inhibited microglial activation, and astrocyte dysfunction, and improved depression-like behavior, bringing hope to patients with mental disorders.90 In the first nonhuman primate study, RVG-Exo-mediated delivery of circSCMH1-EVs improved motor function recovery, especially finger-grasping abilities after stroke in the monkey.91 Systemic administration of recombinant human NGF protein and its mRNA by RVG-Exos efficiently delivered the cargo into the ischemic cortex and translated directly into recipient cells to reduce ischemic injury. A functional study exhibited that brain-targeted RVG-Exo-mediated delivery of NGF reduces ischemic injury by remodeling microglial polarization, promoting inflammatory cell survival, and increasing the number of doublecortin-positive cells.92 In another report, Ren et al. packaged aptamer F5R2, which recognizes the fibrillar α-synuclein into RVG-Exos, and observed that this treatment cleared α-synuclein aggregates in cultured neurons, prevented synaptic protein loss and neuronal death, and improved the associated motor dysfunction.93
Modifying exosomes with a ligand specific to GBM could lead to GBM-targeted gene therapy. Installing a TfR-binding peptide T7 on the surface of exosomes produces T7-Lamp2b-Exo for the treatment of GBM.94 T7-Lamp2b-Exo targeted TfR-rich C6 GBM cells more efficiently than unmodified exosomes or RVG-modified exosomes. Intravenous administration of T7-Lamp2b-Exo-loaded anti-miR-21 effectively reduced miR-21 levels in GBM and led to higher tumor suppression and reduced side effects.95 Kim et al. reported the comparison of exosomes decorated by a TfR-binding peptide T7 (T7-exo) and exosomes decorated by the RVG-Exo in the delivery of antisense miRNA oligonucleotides against miR-21 (AMO-21) to the brain for the treatment of GBM. The authors showed that T7-exo had a higher delivery efficiency both in vitro and in vivo, but that T7-exo/AMO-21 and RVG-exo/AMO-21 showed comparable therapeutic effects on the brain tumor as a result of the systemic injection. This result is perplexing and awaits further clarification.95 T7 peptide has been generally regarded as a superior tumor-targeting peptide because of the tight binding affinity between T7 and TfR (KD around 10 nM) and the high expression levels of TfRs in the brain tumor.96,97,98
An increase in the expression level of stromal cell-derived factor (SDF-1) was observed in ischemic stroke. Among all the chemokines, CXCR4 is a major cell surface receptor that orchestrates cell migration upon binding to its ligand SDF-1. CXCR4-overexpressing cell membranes functionalized nanocarrier presented efficient delivery of drugs in the ischemic brain or stroke.99,100 Further studies showed that overexpression of CXCR4 enhanced the migration of exosomes to the ischemic brain. Exosomes derived from BMSCs overexpressing CXCR4 can promote the proliferation and tube formation of microvascular ECs, and enhance the recovery of vascular function and nerve repair after ischemic cerebral infarction.101 Furthermore, as the SDF-1/CXCR4 axis plays an important role in tumor cell metastasis, exosomes expressing CXCR4 together with chemotherapeutic drugs exerted remarkable activity against brain metastasis of breast cancer in vivo.102
Besides, the tetraspanin superfamily proteins CD63/CD9/CD81 can also be used for surface functionalization and protein fusion. Tetraspanins can be modified to display cell-targeting sequences by fusing targeting moieties into the extracellular loops of the tetraspanins. For example, the low-density lipoprotein receptor (LDLR)-mediated transcytosis pathway can be harnessed for exosome delivery by engineering ApoB-expressing exosomes through ApoB and tetraspanin CD9 conjugation. The accumulation of CD9-ApoB exosomes in cortical blood vessels extended the retention time in the brain for up to 24 h103 These findings reveal that expressing specific ligands that bind receptors, e.g., TfR, LDLR, and GLUT1, on exosomal surface proteins can enhance CNS targeting capabilities.
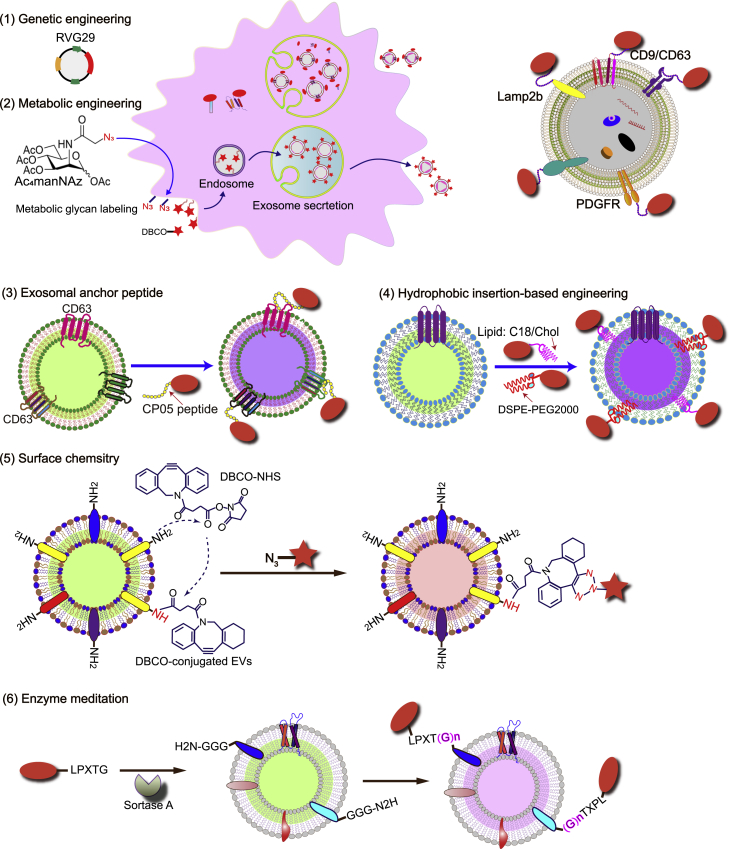
Altogether, these studies demonstrate the feasibility of genetic engineering of exosomal surface proteins for brain-targeted drug delivery and disease treatment. Genetic engineering of the exosomal surface proteins is a viable method to introduce targeting peptides, but this method has several pitfalls. First, genetic engineering is laborious and expensive in clinical applications. Second, this method cannot be applied to pre-separated CDVs or CDVs collected from tissue or body fluids, or hard-to-transfect cells, including red blood cells and stem cells. Also, the step of gene transfer incurs additional risk. In addition to genetic engineering of brain-targeted exosomes, other strategies for surface modification of exosomes include covalent conjugation reactions (click reaction, enzymatic reactions, and metabolic engineering of exosome parent cells) and non-covalent modifications (multivalent electrostatic interactions, aptamer-based modifications, ligand-receptor interactions, hydrophobic interactions, and modifications with CP05 anchor peptide) (summarized in Figure 3 and Table 3). Chemical modification, instead, avoids the risk of gene transfer and the multi-step manipulation. But the key is to form a stable linkage between the targeting sequence and CDVs and the removal of the excess chemical entities.
Figure 3.
Surface engineering strategies of exosomes for targeted delivery
DBCO, dibenzocyclooctyne; DSPE, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine; PEG, polyethylene glycol; NHS, N-hydroxysuccinimide; LPXTG and LPX(G)n, representing amino acid sequences; CP05, a CD63 binding peptide; C18, 18 C fatty acid stearic acid; Chol, cholesterol.7,8
Table 3.
Examples of engineering exosomes for brain targeting and delivery
| Carrier proteins | Method of engineering | Cargo | Effect | Reference |
|---|---|---|---|---|
| T7-Lamp2b (HAIYPRH, T7) | genetic engineering | antisense miR-21 (AMO-21) | target delivery AMO-21 into intracranial GBM | Kim et al.95 |
| CD9-ApoB | genetic engineering | N/A | prolong retention in the brain | Tian et al.104 |
| RVG-LAMP-2B | genetic engineering | BACE1 siRNA | directing BACE1 siRNA to brain neurons with great potential for AD therapy | Cui et al.86 |
| genetic engineering | HMGB1-siRNA | higher brain accumulation, effective gene silence in ischemic strokes. | Kim et al.88 | |
| genetic engineering | miR-124 | promotes gene-drug delivery to the ischemic cortex of the brain and attenuates ischemic damage | Yang et al.87 | |
| genetic engineering | opioid receptor mu (MOR) siRNA | delivery of siRNA to the central nervous system and restrain the morphine relapse | Liu et al.105 | |
| genetic engineering | mRNA (e.g., nluc, catalase) | deliver cargo mRNA to the brain, rescues neurotoxicity and neuroinflammation in Parkinson’s disease | Kojima et al.106 | |
| genetic engineering | circDYM | ameliorates depressive-like behaviors | Yu et al.90 | |
| genetic engineering | circSCMH1 | enhance functional recovery in both rodent and nonhuman primate ischemic stroke models | Yang et al.91 | |
| genetic engineering | nerve growth factor protein and mRNA | simultaneous delivery of nerve growth factor mRNA and protein to the ischemic cortex | Yang et al.92 | |
| genetic engineering | BM-MSC exos | targeted MSC-derived exosomes to cortex and hippocampus rescue memory deficits in AD mouse | Xin et al.77 | |
| genetic engineering | aptamer F5R1 or F5R2 | decrease the α-synuclein aggregates in the PD model | Ren et al.93 | |
| genetic engineering | MSC-exosome | regulate neuroinflammatory responses in the AD brain | Cui et al.86 | |
| RVG-CP05 | anchor peptide | RVG-CP05 | exosomal anchor peptide enables direct functionalization of exosomes for brain-targeted delivery | Gao et al.107 |
| CXCR4 | genetic engineering | MSC-exosome | enhances the delivery of exosomes to the ischemic brain tissue | Li et al.101 |
| genetic engineering | TRAIL | anti-brain metastases of breast cancer | Liu et al.102 | |
| RGD-C1C2 (ACDCRGDCFC, RGD) | genetic engineering | neural progenitor cell-exosome | target the lesion region of the ischemic brain and anti-inflammatory activity | Gao et al.107 |
| c(RGDyK) peptide (Arg-Gly-Asp-D-Tyr-Lys) |
bio-orthogonal copper-free click chemistry | MSC-exosome, curcumin | target ischemic tissue, anti-inflammatory approaches to ischemic stroke | Tian et al.104 |
| c(RGDyK) peptide | bio-orthogonal copper-free click chemistry | MSC-exosome, miR-210 | targeted delivery to the ischemic brain, regulate angiogenesis in ischemic brain injury | Zhang et al.108 |
| NRP-1 targeted peptide (RGERPPR, RGE) | click chemistry | superparamagnetic iron oxide nanoparticles, curcumin | brain-targeted imaging and treatment of glioma | Jia et al.109 |
| Angiopep-2 (TFFYGGSRGKRNNFKTEEY, An2) | – | signal transducers and activators of transcription 3 siRNA | crossed the BBB and targeted to glial cells, enhanced GBM’s median survival rate | Liang et al.110 |
| Angiopep-2; CD133 RNA aptamers | amphiphilic molecule bridge | temozolomide, O6-benzylguanine | dual targeting effect of exosomes, anti- GBM properties | Liang et al.111 |
| Angiopep-2 | DSPE-PEG2000-Angiopep-2 | docetaxel | targeted brain-tumor drug delivery and therapy | Wu et al.112 |
| Iron oxide nanoparticles | physical engineering | – | accumulate to the ischemic lesion, promote anti-inflammatory response, angiogenesis, and anti-apoptosis in the ischemic brain lesion | Kim et al.113 |
Chemical modifications of exosomes for brain delivery have also shown success. For example, peptide RVG-modified poly(2-ethyl-2-oxazoline)-dioleoylphosphatidylethanolamine can insert into the lipid membrane of exosomes and display RVG peptide on exosomes.86 MSC-Exos chemically modified with RVG exhibited increased targeting to the cortex and hippocampus after intravenous administration, which resulted in reduced Aβ levels and rescued memory deficits in a mouse model of AD.86 In another example, MSC-Exos were modified with a neuropilin-1 targeting peptide by “click” reaction to achieve glioma targeting. The modified exosomes were loaded with superparamagnetic iron oxide nanoparticles and Cur for simultaneous diagnosis and brain cancer therapy.109 Tian and co-workers conjugated an RGD peptide onto exosomal surfaces via the copper-free click reaction. In a mouse stroke model, c(RGDyK) peptide-conjugated exosomes showed 11-fold higher delivery to the ischemic region of the brain compared with exosomes conjugated with a scrambled peptide.104 Furthermore, Cur-loaded cRGD-Exos showed that cRGD-Exo-Cur attenuated inflammation and neural apoptosis in the lesion area, indicating the potential use for the treatment of ischemic injury.104 The RGD-modified exosomes also delivered miR-210 to the brain and induced angiogenesis for brain tissue repair after cerebral ischemia.108 Alternatively, Tian and co-workers recombinantly expressed a fused lactadherin protein domain with RGD-4C peptide, and functionalized EVs based on the interaction between this fusion protein and EVs.114
LDLR-related protein (LRP-1) on brain capillary ECs and glioma cells can transport endogenous proteins and small molecules into the brain through transcytosis.115 This pathway can be harnessed for exosome-mediated brain delivery. The angiopep-2 peptide is a high-affinity ligand of LRP-1.116 Liang et al. developed angiopep-2-functionalized exosomes (angiopep-2-Exos), which were used to deliver signal transducers and activators of transcription 3-siRNA.110 Exosome mimics coated with angiopep-2 also enhanced the brain tumor targeting ability.112 Moreover, dual-targeting exosomes with both angiopep-2 and CD133 RNA aptamers showed efficient internalization by both U87MG and GBM stem cells.111 Collectively, these studies indicated that angiopep-2 peptide-displaying exosomes hold promise in GBM therapy.
Hybridization of exosomes with other vectors
Besides using native EVs/exosomes and surface modified exosomes for brain-targeted drug delivery, EVs/exosomes can also be hybridized or fused with other vectors or vehicles to realize brain-targeted delivery. Adeno-associated virus (AAV) vectors have shown promise in gene therapy. Recently, it was found that AAVs are also naturally secreted via EVs and exosomes; AAVs enveloped in the lipid membrane of exosomes are termed vexosomes or exo-AAVs.117 Compared with AAVs, exo-AAVs outperformed in crossing the BBB; systemic delivery of exo-AAVs enabled stable transduction of brain cells (neurons, astrocytes, ECs) with a more widespread and longer gene expression.118 Importantly, no cytotoxicity was observed in exo-AAV-transduced cells. Although exo-AAVs constructed from native exosomes are BBB-penetrating vehicles, we still can enhance their brain targeting capability by surface modification with brain targeting ligands.
Exosomes can self-assemble with protein nanostructures to form hybrid carriers. Votteler et al. designed self-assembling protein nanocages that direct their own release from human cells inside small vesicles in a manner that resembles some viruses, known as enveloped protein nanocages (EPNs).119 EPNs incorporating the vesicular stomatitis viral glycoprotein can fuse with desired cells and deliver their cargo, thus transporting contents from one cell to another. Each EPN can actively load up to 60 different therapeutic proteins, thereby greatly increasing EV capacity, and the modification of the EPN sequence is well tolerated, offering the potential for packaging specific macromolecules and delivering them to target cells for clinical applications. Ovchinnikova et al. showed that the loading of engineered EVs mediated by modified EPN-Fos nanocages improved active protein loading inside EVs and their delivery into target cells.120 This new type of EV/exosome derivatives may be harnessed for brain-specific delivery in the future.
Hybrid exosomes formed by membrane fusion of exosomes and liposomes have been shown to enhance drug delivery.121 Compared with exosomes alone, the hybrid exosomes can efficiently encapsulate large plasmids, including the CRISPR-Cas9 expression vectors. Besides, drug-loaded hybrid exosomes could be endocytosed by MSCs and release cargos inside the cells, providing a new strategy to deliver the CRISPR-Cas9 system for gene manipulation in the brain. Hybrid vesicles resulted from the fusion of EVs derived from cardiac progenitor cells (CPCs) and liposomes retained the functional regenerative properties of CPC EVs and efficiently delivered the siRNA.122 Liposome-EV hybrid vehicles showed an improved cellular delivery efficiency of a chemotherapeutic agent 3–4 times higher than the free drug or the drug-loaded liposome.123 Cheng et al. designed a hybrid therapeutic nanovesicles, named hGLV, by fusing gene-engineered exosomes with drug-loaded thermosensitive liposomes, and demonstrated that the CD47-overexpressed hGLV exhibited long blood circulation and improved the Mφ-mediated phagocytosis of tumor cells by blocking CD47 signals.124 Furthermore, a bioinspired hybrid nanoplatform, namely miR497/TP-HENPs, was developed by fusion of CD47-expressing tumor exosomes and targeting peptide cRGD modified liposomes to co-deliver the chemotherapeutic drug triptolide (TP) and miR497.125
Hybridization of exosomes with inorganic nanoparticles has also been reported. Mo and Li synthesized a core-shell hydroxychloroquine (HCQ) nanocarrier comprising hollow zinc sulfide (ZnS) nanoparticles covered by a shell of dual-stimuli responsive hybrid exosomes containing exosomes and pH- and redox-responsive iRGD-derived phosphatidylserine, named HCQ@ZnS@exo@iRGD. Functionalization of HCQ@ZnS@exo with iRGD significantly improved BBB crossing for better GBM therapy. The designed nanostructures furnished a synergistic photodynamic/chemotherapeutic nanoplatform for brain tumors.126
Cargo loading
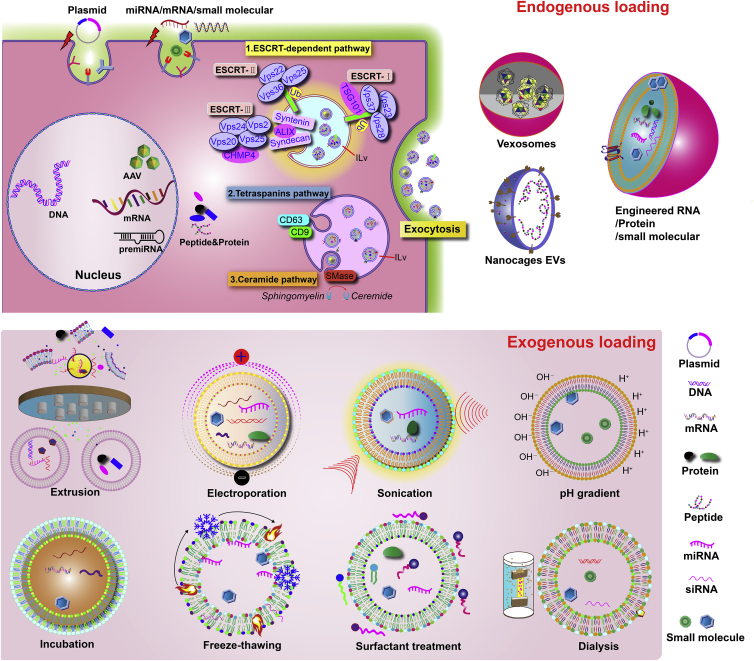
EVs/exosomes can load a range of cargos, including lipids, proteins, nucleic acids, or other cellular components. Cargos can be loaded into EVs/exosomes through various chemical and physical methods. Several mechanisms are involved in loading cargo into EVs/exosomes, e.g., ESCRT-dependent, lipid raft, and ceramide-mediated mechanisms conduct the sorting and loading of endogenous cargos.127,128,129,130 However, under native conditions, EVs/exosomes have limited efficiency in endogenous cargo loading.127,128,129,130 Therefore, multiple techniques have been employed to load therapeutic cargo into EVs/exosomes through exogenous loading methods, such as incubation of the cargo with EV/exosome-secreting cells and transfection of nucleic acid cargo into EVs/exosomes. Therapeutic cargos (proteins, nucleic acids, and small molecules) can be loaded into EVs/exosomes through packaging. Transient transfection of protein, plasmid DNA, mRNA, or miRNAs into donor cells can pack them as cargo into secreted EVs/exosomes. Co-incubating small molecules with donor cells can load them into EVs/exosomes through the endogenous exosome biogenesis pathway. Exogenous cargo can be loaded directly into EVs/exosomes by electroporation, sonication, extrusion, and freeze-thaw cycling.127,128,129,130 However, these physical methods may lead to aggregation of exosomes and disruption of exosome membrane structure, and may require excessive purification steps. In general, the cargo loading methods can be categorized as endogenous and exogenous methods as summarized in Figure 4.
Figure 4.
Summary of cargo loading strategies into exosomes
ESCRT, endosomal sorting complex required for transport; Tsg101, tumor susceptibility gene 101; Vps, vacuolar protein sorting; CHMP, chromatin-modifying protein/charged multivesicular body protein family; Smase, sphingomyelinase.127,128,129,130
To address the issue of low loading efficiency, researchers took various approaches to engineer EVs/exosomes. For example, cargo molecules can be directly loaded into exosomes in situ inside the host cells by fusing or interacting with the exosomal transmembrane proteins. Yim et al. introduced a technique called exosomes for protein loading via optically reversible protein-protein interactions (EXPLORs).131 In the donor cells, the exosomal membrane protein CD9 was conjugated with CIBN, and CRY2 was fused with the cargo protein (CIBN and CRY2 are a pair of photoresponsive interactive proteins). Then continuous irradiation of blue light activated the interaction between CIBN and CRY2, which induced the loading of the cargo protein into the exosome. Ndfip1, a ubiquitin ligase adaptor protein, was able to export the bound protein into the lumen of exosomes. Integrating a protein-protein interaction module controlled by Ndfip1 and the WW tag, the fusion of target protein and the WW tag leads to interaction with NDfip, causing ubiquitination of target protein and subsequent loading into exosomes. Using this method, exosomes containing Cre recombinase were developed, and engineered exosomes could cross the BBB to receptor neurons in many brain regions via the nasal route.60 The ability of engineered exosomes to load and deliver bioactive proteins across the BBB is an important step in developing therapies to treat brain disorders. In another example, Jiang et al. first identified that a viral protein of quasi-enveloped HAV, pX, when fused with eGFP, guided eGFP into exosome-like EVs by directing eGFP into MVBs.132 Mechanistic studies of this observation revealed that apoptosis-linked gene 2-interacting protein X (ALIX) was responsible for this because of the interaction between pX and ALIX. Furthermore, the authors delineated that the C-terminal half of pX was sufficient for guiding the cargo into EVs. Therefore, this work identified a protein tag that, through genetic fusion with the cargo protein, can guide the protein cargo into exosome-like EVs.133
Loading RNA into exosomes has also been achieved. Hung and Leonard reported an approach of fusing the MS2 bacteriophage coat protein to EV-associated proteins and the cognate MS2 stem loop into cargo RNAs to realize enhanced cargo RNA loading (up to 6-fold) into EVs.133 Li et al. fused RNA binding protein HuR with the exosomal membrane protein CD9, and the CD9-HuR exosomes could enrich the functional miRNA inhibitor or CRISPR/dCas9 when the RNAs were engineered to have the AU-rich elements.134 The same group also fused HuR to the C terminus of Lamp2b, and Lamp2b-HuR exosomes drive the endogenous RNA targets recognized by the RNA-binding motif to the lysosome for degradation in the recipient cells, especially when the exosomes were acidified.135 Engineering-functionalized exosomes with RNA-binding proteins may find use in RNA-based gene therapies for CNS diseases.
Besides genetic engineering methods, physical treatments of the exosome membranes have long been explored to increase the loading capacity of exosomes. Small-molecule drugs can directly cross the lipid bilayer of parent cells and generate small-molecule drug-loaded exosomes. Hydrophobic drugs have a higher propensity to be included in the lipid bilayer of the exosome. For hydrophilic drugs, special treatment, such as electroporation, is required. Electroporation imposes an electric field in the form of short high-voltage pulses to create transient micropores on the surface of exosomes, so that the drug can easily diffuse and the integrity of the exosome membrane is restored. Electroporation has been widely used to encapsulate a variety of cargos, including proteins and mRNAs. Exosomes loaded with doxorubicin through electroporation exhibited an encapsulation efficiency up to 20%.136 On the other hand, electroporation increases the loading of siRNA or miRNAs, ranging from 30% to 60%.72,85 Notwithstanding, electroporation may induce aggregation of exosomes and cargos and optimization is often needed. Other methods include the use of surfactant molecules, such as saponins or tritons, which can increase membrane permeabilization. Saponin enhanced the loading capacity of catalase into exosomes, thus promoting the drug’s therapeutic effects in PD.49,137 Loading of porphyrins by hypotonic dialysis also achieved increased drug loading efficiency.79 Alternating freezing and thawing (usually at room temperature) in liquid nitrogen or −80°C also helped cargo loading into the exosomes.138 Sonication can induce exosome membrane reorganization, and applying sound waves to induce mechanical shear force upon the exosome membrane facilitates the cargo loading.127 Extrusion means forcing exosomes through 100–400 nm porous membranes to induce membrane deconstruction and reconstruction. Employing extrusion exhibited increased loading of catalase (22.2%).49 Altogether, although these methods disturb the membrane and could possibly lead to functional disturbance or distortion on CDNs, they are very convenient to use.
Summary and perspective
Although numerous potential neurotherapeutic agents have been developed in various disease models, the fact that 98% of small-molecule drugs and almost 100% of large-molecule drugs cannot cross the BBB impedes their clinical applications. CDNs have attracted attention due to their potential as the new-generation drug carriers for the treatment of brain diseases owing to their natural tendency to cross the BBB and their capacities of being extensively engineered. Notwithstanding such potential, challenges still remain.
Large-scale production of GMP-grade EVs/exosomes is a major bottleneck in advancing EV/exosome-based therapies into the clinic. The bioreactor-based culture system provides a possible solution.139 Watson et al. reported a hollow-fiber bioreactor culture that yielded ∼40-fold more EVs per mL conditioned medium compared with conventional cell culture.140 Haraszti et al. cultivated umbilical cord-derived MSCs in scalable microcarrier-based three-dimensional (3D) cultures. In combination with the conventional differential ultracentrifugation, 3D culture yields 20-fold more exosomes (3D-UC-Exos) than two-dimensional cultures (2D-UC-Exos). Tangential flow filtration (TFF) in combination with 3D MSC cultures further improves the yield of exosomes (3D-TFF-Exos) by 7-fold over 3D-UC-Exos. 3D-TFF-Exos are seven times more potent in siRNA transfer to neurons compared with 2D-UC-Exos.141 Currently, hollow fiber bioreactors from commercial sources have been widely used for large-scale production of EVs.142 Yang et al. compared exosomes derived from cells cultured on 3D graphene scaffolds and 2D graphene film and discovered dramatic differences in the levels of 195 kinds of miRNAs and proteins between the two. Exosomes from 3D culture also gave higher therapeutic effects on ameliorating the memory and cognitive deficits in AD mice than exosomes from 2D culture.143 Although it awaits further study about its generality, the 3D bioreactor likely provides a growth environment for cells that better mimics that under the physiological conditions, and hence CDVs more similar to the native ones.
The GMP-grade MSC-derived exosomes have been tested in in vitro and in vivo models without showing any significant side effects. However, the technology still needs further testing on different types of cells, especially in the production of engineered EVs/exosomes. Moreover, for brain-targeted delivery, strategies to effectively label EVs/exosomes and/or the cargos, together with advanced in vivo imaging tools for high-accuracy quantitative monitoring of exosome delivery to brain tissues are required, and the detailed mechanism of the BBB penetration needs to be unveiled.
Pathological conditions in the brain lead to the breakdown of the BBB, which affects the update of exosomes in the brain. BBB breakdown increases BBB permeability and is known to facilitate entry into the brain of neurotoxic blood-derived products, cells, and pathogens.144 One of the consequences of BBB breakdown is cellular infiltration: extravasation of red blood cells, peripheral Mφs, and neutrophils has been found in patients with AD, PD, and ALS, which causes microbleeds and immune responses in the brain. Besides cells, EVs containing α-synuclein have been found in the CSF and blood, suggesting bidirectional transport of CDNs.145,146 Although neurologists commonly assume that BBB breakdown facilitates the delivery of large molecules, including CDNs, to the brain, it has the opposite effect. The pathological BBB breakdown is accompanied by vascular changes, such as endothelial degeneration, reduced expression of tight junctions and adherens junctions, increased endothelial bulk flow transcytosis, disrupted BBB transporter expression, pericyte degeneration, perivascular accumulation of toxic products, inflammation, and immune responses, which adversely affect the entry of therapeutic drugs and the carriers.144 A generalizable model of how externally administered CDNs enter the brain under BBB breakdown conditions is not available in the literature, and most of the exosome-mediated delivery work focused on the therapeutic efficacy and did not compare the delivery routes or efficiency to the healthy and diseased brain. As one relevant example, Yuan et al. reported that brain inflammation enhanced the delivery of the brain-derived neurotrophic factor by naive Mφ exosomes, possibly due to the overexpression of upregulation of ICAM-1 in the inflamed brain.104,114 To better understand exosome-mediated delivery under pathological conditions, a comparison of the exosome delivery to diseased and healthy brains is needed.
Overall, EVs/exosomes have exhibited great potential in the treatment of brain diseases. To improve the effectiveness of treatments, engineering brain-targeting EVs/exosomes with higher targeting accuracy, improved circulation time, and enhanced cargo loading is required to facilitate efficient delivery of cargo to specific brain tissues or cells. Following the rapidly accumulating preclinical data, there is an urgent need to conduct in-depth evaluations of the efficacy and safety of CDNs in the treatment of brain diseases in vivo and push selected strategies to clinical trials (Figure 5).
Figure 5.
Schematic illustration showing the journal of exosomes from pre-clinical studies to clinical trials
Acknowledgments
This work was funded by the Science and Technology Innovation Committee of Shenzhen (nos. JCYJ20200109150700942, JCYJ20180306170922163, SGDX20201103095800003, and GJHZ20200731095606019), the Guangdong Basic and Applied Basic Research Foundation (2020A1515011581 and 2021A1515010985), the Key Area Research and Development Program of Guangdong Province (2019B030335001), the Guangdong International Cooperation Project (no. 2021A0505030011), the Sanming Project of Medicine (no. SZSM201612079), the Shenzhen Fund for Guangdong Provincial High Level Clinical Key Specialties (no. SZGSP013), the Shenzhen Key Medical Discipline Construction Fund (no. SZXK042), and an ITC TCFS grant (GHP/074/20SZ).
Author contributions
Y.L. and J.X. conceived and designed the review. Y.L. drew the figure and wrote the manuscript. Z.I. and J.X. revised the manuscript. J.X. gave final approval of the manuscript. J.P., D.L., J.H., and C.X. contributed to the constructive discussions. All authors have read and agreed to the submitted version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Li Duan, Email: duanl@szu.edu.cn.
Jiang Xia, Email: jiangxia@cuhk.edu.hk.
References
- 1.Naz F., Siddique Y.H. Human brain disorders: a review. Open Biol. J. 2020;8:6–21. [Google Scholar]
- 2.Gammon K. Neurodegenerative disease: brain windfall. Nature. 2014;515:299–300. doi: 10.1038/nj7526-299a. [DOI] [PubMed] [Google Scholar]
- 3.Pardridge W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks W.A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009;9:S3. doi: 10.1186/1471-2377-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khawli L.A., Prubhu S. 2013. Drug Delivery across the Blood–Brain Barrier. [DOI] [PubMed] [Google Scholar]
- 6.Liang Y., Iqbal Z., Wang J., Xu L., Xu X., Ouyang K., Zhang H., Lu J., Duan L., Xia J. Cell-derived extracellular vesicles for CRISPR/Cas9 delivery: engineering strategies for cargo packaging and loading. Biomater. Sci. 2022;10:4095–4106. doi: 10.1039/d2bm00480a. [DOI] [PubMed] [Google Scholar]
- 7.Duan L., Ouyang K., Wang J., Xu L., Xu X., Wen C., Xie Y., Liang Y., Xia J. Exosomes as targeted delivery platform of CRISPR/Cas9 for therapeutic genome editing. Chembiochem. 2021;22:3360–3368. doi: 10.1002/cbic.202100359. [DOI] [PubMed] [Google Scholar]
- 8.Liang Y., Duan L., Lu J., Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan L., Xu L., Xu X., Qin Z., Zhou X., Xiao Y., Liang Y., Xia J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2021;13:1387–1397. doi: 10.1039/d0nr07622h. [DOI] [PubMed] [Google Scholar]
- 10.Duan L., Xu X., Xu L., Chen H., Li X., Alahdal M., Xiao Y., Liang Y., Xia J. Exosome-mediated drug delivery for cell-free therapy of osteoarthritis. Curr. Med. Chem. 2021;28:6458–6483. doi: 10.2174/0929867327666201118161232. [DOI] [PubMed] [Google Scholar]
- 11.Engelhardt B., Sorokin L. The blood–brain and the blood–cerebrospinal fluid barriers: function and dysfunction. Semin. Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 12.Abbott N.J., Rönnbäck L., Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 13.Kadry H., Noorani B., Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17:69. doi: 10.1186/s12987-020-00230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daneman R., Prat A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preston J.E., Joan Abbott N., Begley D.J. Transcytosis of macromolecules at the blood–brain barrier. Adv. Pharmacol. 2014;71:147–163. doi: 10.1016/bs.apha.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Garg T., Bhandari S., Rath G., Goyal A.K. Current strategies for targeted delivery of bio-active drug molecules in the treatment of brain tumor. J. Drug Target. 2015;23:865–887. doi: 10.3109/1061186X.2015.1029930. [DOI] [PubMed] [Google Scholar]
- 17.Mulvihill J.J., Cunnane E.M., Ross A.M., Duskey J.T., Tosi G., Grabrucker A.M. Drug delivery across the blood-brain barrier: recent advances in the use of nanocarriers. Nanomedicine (Lond) 2020;15:205–214. doi: 10.2217/nnm-2019-0367. [DOI] [PubMed] [Google Scholar]
- 18.Guerin C., Laterra J., Hruban R.H., Brem H., Drewes L.R., Goldstein G.W. The glucose transporter and blood-brain barrier of human brain tumors. Ann. Neurol. 1990;28:758–765. doi: 10.1002/ana.410280606. [DOI] [PubMed] [Google Scholar]
- 19.Pérez-Escuredo J., Van Hée V.F., Sboarina M., Falces J., Payen V.L., Pellerin L., Sonveaux P. Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta. 2016;1863:2481–2497. doi: 10.1016/j.bbamcr.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duelli R., Enerson B.E., Gerhart D.Z., Drewes L.R. Expression of large amino acid transporter LAT1 in rat brain endothelium. J. Cereb. Blood Flow Metab. 2000;20:1557–1562. doi: 10.1097/00004647-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Zhu X., Jin K., Huang Y., Pang Z. Brain Targeted Drug Delivery System. Elsevier; 2019. Brain drug delivery by adsorption-mediated transcytosis; pp. 159–183. [Google Scholar]
- 22.Bashyal S., Thapa C., Lee S. Recent progresses in exosome-based systems for targeted drug delivery to the brain. J. Control Release. 2022;348:723–744. doi: 10.1016/j.jconrel.2022.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Yang T., Martin P., Fogarty B., Brown A., Schurman K., Phipps R., Yin V.P., Lockman P., Bai S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015;32:2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C.C., Liu L., Ma F., Wong C.W., Guo X.E., Chacko J.V., Farhoodi H.P., Zhang S.X., Zimak J., Ségaliny A., et al. Elucidation of exosome migration across the blood–brain barrier model in vitro. Cell. Mol. Bioeng. 2016;9:509–529. doi: 10.1007/s12195-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan D., Zhao Y., Banks W.A., Bullock K.M., Haney M., Batrakova E., Kabanov A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. doi: 10.1016/j.biomaterials.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu M., Lin Q., Huang L., Fu Y., Wang L., He S., Fu Y., Yang S., Zhang Z., Zhang L., Sun X. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson's disease. J. Control Release. 2018;287:156–166. doi: 10.1016/j.jconrel.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 27.Kuroda H., Tachikawa M., Yagi Y., Umetsu M., Nurdin A., Miyauchi E., Watanabe M., Uchida Y., Terasaki T. Cluster of differentiation 46 Is the major receptor in human blood–brain barrier endothelial cells for uptake of exosomes derived from brain-metastatic melanoma cells (SK-Mel-28) Mol. Pharm. 2019;16:292–304. doi: 10.1021/acs.molpharmaceut.8b00985. [DOI] [PubMed] [Google Scholar]
- 28.Bernardo-Castro S., Sousa J.A., Brás A., Cecília C., Rodrigues B., Almendra L., Machado C., Santo G., Silva F., Ferreira L., et al. Pathophysiology of blood-brain barrier permeability throughout the different stages of ischemic stroke and its implication on hemorrhagic transformation and recovery. Front. Neurol. 2020;11:594672. doi: 10.3389/fneur.2020.594672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banks W.A., Sharma P., Bullock K.M., Hansen K.M., Ludwig N., Whiteside T.L. Transport of extracellular vesicles across the blood-brain barrier: brain pharmacokinetics and effects of inflammation. Int. J. Mol. Sci. 2020;21:4407. doi: 10.3390/ijms21124407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heidarzadeh M., Gürsoy-Özdemir Y., Kaya M., Eslami Abriz A., Zarebkohan A., Rahbarghazi R., Sokullu E. Exosomal delivery of therapeutic modulators through the blood-brain barrier, promise and pitfalls. Cell Biosci. 2021;11:142. doi: 10.1186/s13578-021-00650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hessvik N.P., Llorente A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie S., Zhang Q., Jiang L. Current knowledge on exosome biogenesis, cargo-sorting mechanism and therapeutic implications. Membranes (Basel) 2022;12:498. doi: 10.3390/membranes12050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akers J.C., Gonda D., Kim R., Carter B.S., Chen C.C. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W., Bai X., Zhang A., Huang J., Xu S., Zhang J. Role of exosomes in central nervous system diseases. Front. Mol. Neurosci. 2019;12:240. doi: 10.3389/fnmol.2019.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao G., Zhao S., Xia X., Li C., Li C., Ji C., Sheng S., Tang Y., Zhu J., Wang Y., et al. Glutaminase C regulates microglial activation and pro-inflammatory exosome release: relevance to the pathogenesis of Alzheimer’s disease. Front. Cell. Neurosci. 2019;13:264. doi: 10.3389/fncel.2019.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Y., Li C., Huang Y., Wang Y., Xia X., Zheng J.C. Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun. Signal. 2019;17:96. doi: 10.1186/s12964-019-0418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan P., Ding L., Chen H., Wang Y., Li C., Zhao S., Yang X., Ma Y., Zhu J., Qi X., et al. Neural stem cell-derived exosomes regulate neural stem cell differentiation through miR-9-Hes1 axis. Front. Cell Dev. Biol. 2021;9:601600. doi: 10.3389/fcell.2021.601600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoshino A., Costa-Silva B., Shen T.-L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morad G., Carman C.V., Hagedorn E.J., Perlin J.R., Zon L.I., Mustafaoglu N., Park T.-E., Ingber D.E., Daisy C.C., Moses M.A. Tumor-derived extracellular vesicles breach the intact blood–brain barrier via transcytosis. ACS Nano. 2019;13:13853–13865. doi: 10.1021/acsnano.9b04397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang C., Wu Y., Wang L., Li S., Zhou J., Tan Y., Song J., Xing H., Yi K., Zhan Q., et al. Glioma-derived exosomes hijack the blood–brain barrier to facilitate nanocapsule delivery via LCN2. J. Control Release. 2022;345:537–548. doi: 10.1016/j.jconrel.2022.03.038. [DOI] [PubMed] [Google Scholar]
- 41.Li Y., Liu Z., Song Y., Pan J.J., Jiang Y., Shi X., Liu C., Ma Y., Luo L., Mamtilahun M., et al. M2 microglia-derived extracellular vesicles promote white matter repair and functional recovery via miR-23a-5p after cerebral ischemia in mice. Theranostics. 2022;12:3553–3573. doi: 10.7150/thno.68895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds J.L., Mahajan S.D. Transmigration of tetraspanin 2 (Tspan2) siRNA via microglia derived exosomes across the blood brain barrier modifies the production of immune mediators by microglia cells. J. Neuroimmune Pharmacol. 2020;15:554–563. doi: 10.1007/s11481-019-09895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casella G., Colombo F., Finardi A., Descamps H., Ill-Raga G., Spinelli A., Podini P., Bastoni M., Martino G., Muzio L., Furlan R. Extracellular vesicles containing IL-4 modulate neuroinflammation in a mouse model of multiple sclerosis. Mol. Ther. 2018;26:2107–2118. doi: 10.1016/j.ymthe.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raffaele S., Gelosa P., Bonfanti E., Lombardi M., Castiglioni L., Cimino M., Sironi L., Abbracchio M.P., Verderio C., Fumagalli M. Microglial vesicles improve post-stroke recovery by preventing immune cell senescence and favoring oligodendrogenesis. Mol. Ther. 2021;29:1439–1458. doi: 10.1016/j.ymthe.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gabrielli M., Battista N., Riganti L., Prada I., Antonucci F., Cantone L., Matteoli M., Maccarrone M., Verderio C. Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep. 2015;16:213–220. doi: 10.15252/embr.201439668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lombardi M., Parolisi R., Scaroni F., Bonfanti E., Gualerzi A., Gabrielli M., Kerlero de Rosbo N., Uccelli A., Giussani P., Viani P., et al. Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure. Acta Neuropathol. 2019;138:987–1012. doi: 10.1007/s00401-019-02049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang D., Huang J., Wang F., Ding H., Cui Y., Yang Y., Xu J., Luo H., Gao Y., Pan L., et al. BMI1 regulates multiple myeloma-associated macrophage's pro-myeloma functions. Cell Death Dis. 2021;12:495. doi: 10.1038/s41419-021-03748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haney M.J., Suresh P., Zhao Y., Kanmogne G.D., Kadiu I., Sokolsky-Papkov M., Klyachko N.L., Mosley R.L., Kabanov A.V., Gendelman H.E., Batrakova E.V. Blood-borne macrophage–neural cell interactions hitchhike on endosome networks for cell-based nanozyme brain delivery. Nanomedicine. 2012;7:815–833. doi: 10.2217/nnm.11.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., Patel T., Piroyan A., Sokolsky M., Kabanov A.V., Batrakova E.V. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J. Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhuang X., Xiang X., Grizzle W., Sun D., Zhang S., Axtell R.C., Ju S., Mu J., Zhang L., Steinman L., et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou S., Gao B., Sun C., Bai Y., Cheng D., Zhang Y., Li X., Zhao J., Xu D. Vascular endothelial cell-derived exosomes protect neural stem cells against ischemia/reperfusion injury. Neuroscience. 2020;441:184–196. doi: 10.1016/j.neuroscience.2020.05.046. [DOI] [PubMed] [Google Scholar]
- 52.Xian P., Hei Y., Wang R., Wang T., Yang J., Li J., Di Z., Liu Z., Baskys A., Liu W., et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019;9:5956–5975. doi: 10.7150/thno.33872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang Y., Duan L., Xu X., Li X., Liu M., Chen H., Lu J., Xia J. Mesenchymal stem cell-derived exosomes for treatment of autism spectrum disorder. ACS Appl. Bio Mater. 2020;3:6384–6393. doi: 10.1021/acsabm.0c00831. [DOI] [PubMed] [Google Scholar]
- 54.Guo M., Yin Z., Chen F., Lei P. Mesenchymal stem cell-derived exosome: a promising alternative in the therapy of Alzheimer’s disease. Alz. Res. Ther. 2020;12:109–114. doi: 10.1186/s13195-020-00670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y., Wang D., Guo D. MiR-124 promote neurogenic transdifferentiation of adipose derived mesenchymal stromal cells partly through RhoA/ROCK1, but not ROCK2 signaling pathway. PLoS One. 2016;11:e0146646. doi: 10.1371/journal.pone.0146646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mot Y.Y., Moses E.J., Mohd Yusoff N., Ling K.-H., Yong Y.K., Tan J.J. Mesenchymal stromal cells-derived exosome and the roles in the treatment of traumatic brain injury. Cell. Mol. Neurobiol. 2022 doi: 10.1007/s10571-022-01201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xin H., Li Y., Cui Y., Yang J.J., Zhang Z.G., Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doeppner T.R., Herz J., Görgens A., Schlechter J., Ludwig A.-K., Radtke S., de Miroschedji K., Horn P.A., Giebel B., Hermann D.M. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl. Med. 2015;4:1131–1143. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang C., Song J., Lou L., Qi X., Zhao L., Fan B., Sun G., Lv Z., Fan Z., Jiao B., Yang J. Doxorubicin-loaded nanoparticle coated with endothelial cells-derived exosomes for immunogenic chemotherapy of glioblastoma. Bioeng. Transl. Med. 2021;6:e10203. doi: 10.1002/btm2.10203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sterzenbach U., Putz U., Low L.-H., Silke J., Tan S.-S., Howitt J. Engineered exosomes as vehicles for biologically active proteins. Mol. Ther. 2017;25:1269–1278. doi: 10.1016/j.ymthe.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Betzer O., Perets N., Angel A., Motiei M., Sadan T., Yadid G., Offen D., Popovtzer R. In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano. 2017;11:10883–10893. doi: 10.1021/acsnano.7b04495. [DOI] [PubMed] [Google Scholar]
- 62.Xin H., Liu Z., Buller B., Li Y., Golembieski W., Gan X., Wang F., Lu M., Ali M.M., Zhang Z.G., Chopp M. MiR-17-92 enriched exosomes derived from multipotent mesenchymal stromal cells enhance axon-myelin remodeling and motor electrophysiological recovery after stroke. J. Cereb. Blood Flow Metab. 2021;41:1131–1144. doi: 10.1177/0271678X20950489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu Z., Wen Y., Jiang N., Li Z., Guan J., Zhang Y., Deng C., Zhao L., Zheng S.G., Zhu Y., et al. TNF-α stimulation enhances the neuroprotective effects of gingival MSCs derived exosomes in retinal ischemia-reperfusion injury via the MEG3/miR-21a-5p axis. Biomaterials. 2022;284:121484. doi: 10.1016/j.biomaterials.2022.121484. [DOI] [PubMed] [Google Scholar]
- 64.Zheng Y., He R., Wang P., Shi Y., Zhao L., Liang J. Exosomes from LPS-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization. Biomater. Sci. 2019;7:2037–2049. doi: 10.1039/c8bm01449c. [DOI] [PubMed] [Google Scholar]
- 65.He R., Jiang Y., Shi Y., Liang J., Zhao L. Curcumin-laden exosomes target ischemic brain tissue and alleviate cerebral ischemia-reperfusion injury by inhibiting ROS-mediated mitochondrial apoptosis. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;117:111314. doi: 10.1016/j.msec.2020.111314. [DOI] [PubMed] [Google Scholar]
- 66.Han Y., Seyfried D., Meng Y., Yang D., Schultz L., Chopp M., Seyfried D. Multipotent mesenchymal stromal cell–derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. J. Neurosurg. 2018;131:290–300. doi: 10.3171/2018.2.JNS171475. [DOI] [PubMed] [Google Scholar]
- 67.Guo L., Pan J., Li F., Zhao L., Shi Y. A novel brain targeted plasma exosomes enhance the neuroprotective efficacy of edaravone in ischemic stroke. IET Nanobiotechnol. 2021;15:107–116. doi: 10.1049/nbt2.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen H.-X., Liang F.-C., Gu P., Xu B.-L., Xu H.-J., Wang W.-T., Hou J.-Y., Xie D.-X., Chai X.-Q., An S.-J. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis. 2020;11:288. doi: 10.1038/s41419-020-2473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cova L., Armentero M.-T., Zennaro E., Calzarossa C., Bossolasco P., Busca G., Lambertenghi Deliliers G., Polli E., Nappi G., Silani V., Blandini F. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson's disease. Brain Res. 2010;1311:12–27. doi: 10.1016/j.brainres.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y., Chopp M., Meng Y., Katakowski M., Xin H., Mahmood A., Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015;122:856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ding M., Shen Y., Wang P., Xie Z., Xu S., Zhu Z., Wang Y., Lyu Y., Wang D., Xu L., et al. Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer’s disease. Neurochem. Res. 2018;43:2165–2177. doi: 10.1007/s11064-018-2641-5. [DOI] [PubMed] [Google Scholar]
- 72.Perets N., Oron O., Herman S., Elliott E., Offen D. Exosomes derived from mesenchymal stem cells improved core symptoms of genetically modified mouse model of autism Shank3B. Mol. Autism. 2020;11:65. doi: 10.1186/s13229-020-00366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang S.-S., Jia J., Wang Z. Mesenchymal stem cell-derived extracellular vesicles suppresses iNOS expression and ameliorates neural impairment in Alzheimer’s disease mice. J. Alzheimers Dis. 2018;61:1005–1013. doi: 10.3233/JAD-170848. [DOI] [PubMed] [Google Scholar]
- 74.Parga J.A., García-Garrote M., Martínez S., Raya Á., Labandeira-García J.L., Rodríguez-Pallares J. Prostaglandin EP2 receptors mediate mesenchymal stromal cell-neuroprotective effects on dopaminergic neurons. Mol. Neurobiol. 2018;55:4763–4776. doi: 10.1007/s12035-017-0681-5. [DOI] [PubMed] [Google Scholar]
- 75.Xue C., Li X., Ba L., Zhang M., Yang Y., Gao Y., Sun Z., Han Q., Zhao R.C. MSC-derived exosomes can enhance the angiogenesis of human brain MECs and show therapeutic potential in a mouse model of Parkinson's disease. Aging Dis. 2021;12:1211–1222. doi: 10.14336/AD.2020.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sha S., Shen X., Cao Y., Qu L. Mesenchymal stem cells-derived extracellular vesicles ameliorate Alzheimer’s disease in rat models via the microRNA-29c-3p/BACE1 axis and the Wnt/β-catenin pathway. Aging (Albany N. Y.) 2021;13:15285–15306. doi: 10.18632/aging.203088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xin H., Li Y., Buller B., Katakowski M., Zhang Y., Wang X., Shang X., Zhang Z.G., Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xin H., Li Y., Liu Z., Wang X., Shang X., Cui Y., Zhang Z.G., Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Webb R.L., Kaiser E.E., Scoville S.L., Thompson T.A., Fatima S., Pandya C., Sriram K., Swetenburg R.L., Vaibhav K., Arbab A.S., et al. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl. Stroke Res. 2018;9:530–539. doi: 10.1007/s12975-017-0599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Apodaca L.A., Baddour A.A.D., Garcia C., Alikhani L., Giedzinski E., Ru N., Agrawal A., Acharya M.M., Baulch J.E. Human neural stem cell-derived extracellular vesicles mitigate hallmarks of Alzheimer’s disease. Alzheimers Res. Ther. 2021;13:57. doi: 10.1186/s13195-021-00791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vogel A.D., Upadhya R., Shetty A.K. Neural stem cell derived extracellular vesicles: attributes and prospects for treating neurodegenerative disorders. EBioMedicine. 2018;38:273–282. doi: 10.1016/j.ebiom.2018.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wiklander O.P.B., Nordin J.Z., O'Loughlin A., Gustafsson Y., Corso G., Mäger I., Vader P., Lee Y., Sork H., Seow Y., et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mirzaaghasi A., Han Y., Ahn S.-H., Choi C., Park J.-H. Biodistribution and pharmacokinectics of liposomes and exosomes in a mouse model of sepsis. Pharmaceutics. 2021;13:427. doi: 10.3390/pharmaceutics13030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liang Y., Xu X., Li X., Xiong J., Li B., Duan L., Wang D., Xia J. Chondrocyte-targeted microRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl. Mater. Interfaces. 2020;12:36938–36947. doi: 10.1021/acsami.0c10458. [DOI] [PubMed] [Google Scholar]
- 85.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 86.Cui G.-h., Guo H.-d., Li H., Zhai Y., Gong Z.-b., Wu J., Liu J.-s., Dong Y.-r., Hou S.-x., Liu J.-r. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun. Ageing. 2019;16:10. doi: 10.1186/s12979-019-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang J., Zhang X., Chen X., Wang L., Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol. Ther. Nucleic Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim M., Kim G., Hwang D.W., Lee M. Delivery of high mobility group box-1 siRNA using brain-targeting exosomes for ischemic stroke therapy. J. Biomed. Nanotechnol. 2019;15:2401–2412. doi: 10.1166/jbn.2019.2866. [DOI] [PubMed] [Google Scholar]
- 89.Zhang R., Fu Y., Cheng M., Ma W., Zheng N., Wang Y., Wu Z. sEVsRVG selectively delivers antiviral siRNA to fetus brain, inhibits ZIKV infection and mitigates ZIKV-induced microcephaly in mouse model. Mol. Ther. 2022;30:2078–2091. doi: 10.1016/j.ymthe.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu X., Bai Y., Han B., Ju M., Tang T., Shen L., Li M., Yang L., Zhang Z., Hu G., et al. Extracellular vesicle-mediated delivery of circDYM alleviates CUS-induced depressive-like behaviours. J. Extracell. Vesicles. 2022;11:e12185. doi: 10.1002/jev2.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang L., Han B., Zhang Z., Wang S., Bai Y., Zhang Y., Tang Y., Du L., Xu L., Wu F., et al. Extracellular vesicle–mediated delivery of circular RNA SCMH1 promotes functional recovery in rodent and nonhuman primate ischemic stroke models. Circulation. 2020;142:556–574. doi: 10.1161/CIRCULATIONAHA.120.045765. [DOI] [PubMed] [Google Scholar]
- 92.Yang J., Wu S., Hou L., Zhu D., Yin S., Yang G., Wang Y. Therapeutic effects of simultaneous delivery of nerve growth factor mRNA and protein via exosomes on cerebral ischemia. Mol. Ther. Nucleic Acids. 2020;21:512–522. doi: 10.1016/j.omtn.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ren X., Zhao Y., Xue F., Zheng Y., Huang H., Wang W., Chang Y., Yang H., Zhang J. Exosomal DNA aptamer targeting α-synuclein aggregates reduced neuropathological deficits in a mouse Parkinson’s disease model. Mol. Ther. Nucleic Acids. 2019;17:726–740. doi: 10.1016/j.omtn.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han L., Huang R., Liu S., Huang S., Jiang C. Peptide-conjugated PAMAM for targeted doxorubicin delivery to transferrin receptor overexpressed tumors. Mol. Pharm. 2010;7:2156–2165. doi: 10.1021/mp100185f. [DOI] [PubMed] [Google Scholar]
- 95.Kim G., Kim M., Lee Y., Byun J.W., Hwang D.W., Lee M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J. Control Release. 2020;317:273–281. doi: 10.1016/j.jconrel.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 96.Lee J.H., Engler J.A., Collawn J.F., Moore B.A. Receptor mediated uptake of peptides that bind the human transferrin receptor. Eur. J. Biochem. 2001;268:2004–2012. doi: 10.1046/j.1432-1327.2001.02073.x. [DOI] [PubMed] [Google Scholar]
- 97.Oh S., Kim B.J., Singh N.P., Lai H., Sasaki T. Synthesis and anti-cancer activity of covalent conjugates of artemisinin and a transferrin-receptor targeting peptide. Cancer Lett. 2009;274:33–39. doi: 10.1016/j.canlet.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 98.Han L., Li J., Huang S., Huang R., Liu S., Hu X., Yi P., Shan D., Wang X., Lei H., Jiang C. Peptide-conjugated polyamidoamine dendrimer as a nanoscale tumor-targeted T1 magnetic resonance imaging contrast agent. Biomaterials. 2011;32:2989–2998. doi: 10.1016/j.biomaterials.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 99.Luo L., Zang G., Liu B., Qin X., Zhang Y., Chen Y., Zhang H., Wu W., Wang G. Bioengineering CXCR4-overexpressing cell membrane functionalized ROS-responsive nanotherapeutics for targeting cerebral ischemia-reperfusion injury. Theranostics. 2021;11:8043–8056. doi: 10.7150/thno.60785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma J., Zhang S., Liu J., Liu F., Du F., Li M., Chen A.T., Bao Y., Suh H.W., Avery J., et al. Targeted drug delivery to stroke via chemotactic recruitment of nanoparticles coated with membrane of engineered neural stem cells. Small. 2019;15:1902011. doi: 10.1002/smll.201902011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li X., Zhang Y., Wang Y., Zhao D., Sun C., Zhou S., Xu D., Zhao J. Exosomes derived from CXCR4-overexpressing BMSC promoted activation of microvascular endothelial cells in cerebral ischemia/reperfusion injury. Neural Plast. 2020;2020:8814239. doi: 10.1155/2020/8814239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu M., Hu Y., Chen G. The antitumor effect of gene-engineered exosomes in the treatment of brain metastasis of breast cancer. Front. Oncol. 2020;10:1453. doi: 10.3389/fonc.2020.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khan H., Pan J.-J., Li Y., Zhang Z., Yang G.-Y. Native and bioengineered exosomes for ischemic stroke therapy. Front. Cell Dev. Biol. 2021;9:619565. doi: 10.3389/fcell.2021.619565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tian T., Zhang H.-X., He C.-P., Fan S., Zhu Y.-L., Qi C., Huang N.-P., Xiao Z.-D., Lu Z.-H., Tannous B.A., Gao J. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y., Li D., Liu Z., Zhou Y., Chu D., Li X., Jiang X., Hou D., Chen X., Chen Y., et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci. Rep. 2015;5:17543–17610. doi: 10.1038/srep17543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kojima R., Bojar D., Rizzi G., Hamri G.C.-E., El-Baba M.D., Saxena P., Ausländer S., Tan K.R., Fussenegger M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018;9:1305–1310. doi: 10.1038/s41467-018-03733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gao X., Ran N., Dong X., Zuo B., Yang R., Zhou Q., Moulton H.M., Seow Y., Yin H. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci. Transl. Med. 2018;10:eaat0195. doi: 10.1126/scitranslmed.aat0195. [DOI] [PubMed] [Google Scholar]
- 108.Zhang H., Wu J., Wu J., Fan Q., Zhou J., Wu J., Liu S., Zang J., Ye J., Xiao M., et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J. Nanobiotechnology. 2019;17:29. doi: 10.1186/s12951-019-0461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jia G., Han Y., An Y., Ding Y., He C., Wang X., Tang Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–316. doi: 10.1016/j.biomaterials.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 110.Liang S.F., Zuo F.-F., Yin B.-C., Ye B.-C. Delivery of siRNA based on engineered exosomes for glioblastoma therapy by targeting STAT3. Biomater. Sci. 2022;10:1582–1590. doi: 10.1039/d1bm01723c. [DOI] [PubMed] [Google Scholar]
- 111.Liang S., Xu H., Ye B.-C. Membrane-decorated exosomes for combination drug delivery and improved glioma therapy. Langmuir. 2022;38:299–308. doi: 10.1021/acs.langmuir.1c02500. [DOI] [PubMed] [Google Scholar]
- 112.Wu J.-Y., Li Y.-J., Wang J., Hu X.-B., Huang S., Luo S., Xiang D.-X. Multifunctional exosome-mimetics for targeted anti-glioblastoma therapy by manipulating protein corona. J. Nanobiotechnology. 2021;19:405–415. doi: 10.1186/s12951-021-01153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim H.Y., Kim T.J., Kang L., Kim Y.-J., Kang M.K., Kim J., Ryu J.H., Hyeon T., Yoon B.-W., Ko S.-B., Kim B.S. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials. 2020;243:119942. doi: 10.1016/j.biomaterials.2020.119942. [DOI] [PubMed] [Google Scholar]
- 114.Tian T., Cao L., He C., Ye Q., Liang R., You W., Zhang H., Wu J., Ye J., Tannous B.A., Gao J. Targeted delivery of neural progenitor cell-derived extracellular vesicles for anti-inflammation after cerebral ischemia. Theranostics. 2021;11:6507–6521. doi: 10.7150/thno.56367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xin H., Sha X., Jiang X., Chen L., Law K., Gu J., Chen Y., Wang X., Fang X. The brain targeting mechanism of Angiopep-conjugated poly (ethylene glycol)-co-poly (ϵ-caprolactone) nanoparticles. Biomaterials. 2012;33:1673–1681. doi: 10.1016/j.biomaterials.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 116.Maletínská L., Blakely E.A., Bjornstad K.A., Deen D.F., Knoff L.J., Forte T.M. Human glioblastoma cell lines: levels of low-density lipoprotein receptor and low-density lipoprotein receptor-related protein. Cancer Res. 2000;60:2300–2303. [PubMed] [Google Scholar]
- 117.Orefice N.S., Souchet B., Braudeau J., Alves S., Piguet F., Collaud F., Ronzitti G., Tada S., Hantraye P., Mingozzi F., et al. Real-time monitoring of exosome enveloped-AAV spreading by endomicroscopy approach: a new tool for gene delivery in the brain. Mol. Ther. Methods Clin. Dev. 2019;14:237–251. doi: 10.1016/j.omtm.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hudry E., Martin C., Gandhi S., György B., Scheffer D.I., Mu D., Merkel S.F., Mingozzi F., Fitzpatrick Z., Dimant H., et al. Exosome-associated AAV vector as a robust and convenient neuroscience tool. Gene Ther. 2016;23:819. doi: 10.1038/gt.2016.65. [DOI] [PubMed] [Google Scholar]
- 119.Votteler J., Ogohara C., Yi S., Hsia Y., Nattermann U., Belnap D.M., King N.P., Sundquist W.I. Designed proteins induce the formation of nanocage-containing extracellular vesicles. Nature. 2016;540:292–295. doi: 10.1038/nature20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ovchinnikova L.A., Terekhov S.S., Ziganshin R.H., Bagrov D.V., Filimonova I.N., Zalevsky A.O., Lomakin Y.A. Reprogramming extracellular vesicles for protein therapeutics delivery. Pharmaceutics. 2021;13:768. doi: 10.3390/pharmaceutics13060768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin Y., Wu J., Gu W., Huang Y., Tong Z., Huang L., Tan J. Exosome–liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv. Sci. 2018;5:1700611. doi: 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Evers M.J.W., van de Wakker S.I., de Groot E.M., de Jong O.G., Gitz-François J.J.J., Seinen C.S., Sluijter J.P.G., Schiffelers R.M., Vader P. Functional siRNA delivery by extracellular vesicle–liposome hybrid nanoparticles. Adv. Healthc. Mater. 2022;11:2101202. doi: 10.1002/adhm.202101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Piffoux M., Silva A.K.A., Wilhelm C., Gazeau F., Tareste D. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS nano. 2018;12:6830–6842. doi: 10.1021/acsnano.8b02053. [DOI] [PubMed] [Google Scholar]
- 124.Cheng L., Zhang X., Tang J., Lv Q., Liu J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials. 2021;275:120964. doi: 10.1016/j.biomaterials.2021.120964. [DOI] [PubMed] [Google Scholar]
- 125.Li L., He D., Guo Q., Zhang Z., Ru D., Wang L., Gong K., Liu F., Duan Y., Li H. Exosome-liposome hybrid nanoparticle codelivery of TP and miR497 conspicuously overcomes chemoresistant ovarian cancer. J. Nanobiotechnology. 2022;20:50. doi: 10.1186/s12951-022-01264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mo J., Li M. Zinc sulfide-based hybrid exosome-coated autophagic flux-mediated hydrogen sulfide-sensitized PDT/chemotherapeutic synergistic nanoplatform for targeted treatment of glioblastoma stem-like cells in orthotopic mouse glioblastoma model. bioRxiv. 2020 Preprint at. [Google Scholar]
- 127.Fu S., Wang Y., Xia X., Zheng J.C. Exosome engineering: current progress in cargo loading and targeted delivery. NanoImpact. 2020;20:100261. [Google Scholar]
- 128.Han Y., Jones T.W., Dutta S., Zhu Y., Wang X., Narayanan S.P., Fagan S.C., Zhang D. Overview and update on methods for cargo loading into extracellular vesicles. Processes (Basel) 2021;9:356. doi: 10.3390/pr9020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen C., Sun M., Wang J., Su L., Lin J., Yan X. Active cargo loading into extracellular vesicles: highlights the heterogeneous encapsulation behaviour. J. Extracell. Vesicles. 2021;10:e12163. doi: 10.1002/jev2.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li S.P., Lin Z.X., Jiang X.Y., Yu X.Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 2018;39:542–551. doi: 10.1038/aps.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yim N., Ryu S.-W., Choi K., Lee K.R., Lee S., Choi H., Kim J., Shaker M.R., Sun W., Park J.-H., et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat. Commun. 2016;7:12277–12279. doi: 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jiang W., Ma P., Deng L., Liu Z., Wang X., Liu X., Long G. Hepatitis A virus structural protein pX interacts with ALIX and promotes the secretion of virions and foreign proteins through exosome-like vesicles. J. Extracell. Vesicles. 2020;9:1716513. doi: 10.1080/20013078.2020.1716513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hung M.E., Leonard J.N. A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery. J. Extracell. Vesicles. 2016;5:31027. doi: 10.3402/jev.v5.31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Z., Zhou X., Wei M., Gao X., Zhao L., Shi R., Sun W., Duan Y., Yang G., Yuan L. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019;19:19–28. doi: 10.1021/acs.nanolett.8b02689. [DOI] [PubMed] [Google Scholar]
- 135.Li Z., Zhou X., Gao X., Bai D., Dong Y., Sun W., Zhao L., Wei M., Yang X., Yang G., Yuan L. Fusion protein engineered exosomes for targeted degradation of specific RNAs in lysosomes: a proof-of-concept study. J. Extracell. Vesicles. 2020;9:1816710. doi: 10.1080/20013078.2020.1816710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tian Y., Li S., Song J., Ji T., Zhu M., Anderson G.J., Wei J., Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 137.Goh W.J., Lee C.K., Zou S., Woon E.C., Czarny B., Pastorin G. Doxorubicin-loaded cell-derived nanovesicles: an alternative targeted approach for anti-tumor therapy. Int. J. Nanomedicine. 2017;12:2759–2767. doi: 10.2147/IJN.S131786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim M.S., Haney M.J., Zhao Y., Mahajan V., Deygen I., Klyachko N.L., Inskoe E., Piroyan A., Sokolsky M., Okolie O., et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12:655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mendt M., Kamerkar S., Sugimoto H., McAndrews K.M., Wu C.-C., Gagea M., Yang S., Blanko E.V.R., Peng Q., Ma X., et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI insight. 2018;3:e99263. doi: 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Watson D.C., Bayik D., Srivatsan A., Bergamaschi C., Valentin A., Niu G., Bear J., Monninger M., Sun M., Morales-Kastresana A., et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. doi: 10.1016/j.biomaterials.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Haraszti R.A., Miller R., Stoppato M., Sere Y.Y., Coles A., Didiot M.C., Wollacott R., Sapp E., Dubuke M.L., Li X., et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol. Ther. 2018;26:2838–2847. doi: 10.1016/j.ymthe.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yan I.K., Shukla N., Borrelli D.A., Patel T. Use of a hollow fiber bioreactor to collect extracellular vesicles from cells in culture. Methods Mol. Biol. 2018;1740:35–41. doi: 10.1007/978-1-4939-7652-2_4. [DOI] [PubMed] [Google Scholar]
- 143.Yang L., Zhai Y., Hao Y., Zhu Z., Cheng G. The regulatory functionality of exosomes derived from hUMSCs in 3D culture for Alzheimer's disease therapy. Small. 2020;16:e1906273. doi: 10.1002/smll.201906273. [DOI] [PubMed] [Google Scholar]
- 144.Sweeney M.D., Sagare A.P., Zlokovic B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Matsumoto J., Stewart T., Sheng L., Li N., Bullock K., Song N., Shi M., Banks W.A., Zhang J. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood–brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson's disease? Acta Neuropathol. Commun. 2017;5:71. doi: 10.1186/s40478-017-0470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shi M., Liu C., Cook T.J., Bullock K.M., Zhao Y., Ginghina C., Li Y., Aro P., Dator R., He C., et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson's disease. Acta Neuropathol. 2014;128:639–650. doi: 10.1007/s00401-014-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]