Abstract
Extracellular vesicles (EVs) are gaining increasing attention for diagnostic and therapeutic applications in various diseases. These natural nanoparticles benefit from favorable safety profiles and unique biodistribution capabilities, rendering them attractive drug-delivery modalities over synthetic analogs. However, the widespread use of EVs is limited by technological shortcomings and biological knowledge gaps that fail to unravel their heterogeneity. An in-depth understanding of their biogenesis is crucial to unlocking their full therapeutic potential. Here, we explore how knowledge about EV biogenesis can be exploited for EV bioengineering to load therapeutic protein or nucleic acid cargos into or onto EVs. We summarize more than 75 articles and discuss their findings on the formation and composition of exosomes and microvesicles, revealing multiple pathways that may be stimulation and/or cargo dependent. Our analysis further identifies key regulators of natural EV cargo loading and we discuss how this knowledge is integrated to develop engineered EV biotherapeutics.
Keywords: exosomes, microvesicles, drug delivery, EV heterogeneity, EV engineering
Graphical abstract
A lack of biological knowledge and technical challenges limits the widespread therapeutic application of extracellular vesicles. This review summarizes current knowledge on the biogenesis and composition of exosomes and microvesicles. Moreover, it extends into natural cargo sorting and discusses how this knowledge can be harnessed to develop engineered EV biotherapeutics.
Introduction
Extracellular vesicles (EVs) are natural membrane-enclosed nanoparticles secreted by all cells. They are important mediators of intercellular communication that convey messages to surrounding or distant cells in the form of proteins, lipids, nucleic acids,1,2 and even organelles.3,4,5,6 Thus, EVs exert not only physiological but also pathological functions, which renders them attractive therapeutic targets.7,8 Correspondingly, they serve as diagnostic biomarkers, mainly for cancer and neurodegenerative diseases.8 Last, owing to their unique biodistribution capabilities, versatility, and immune tolerance, EVs are being increasingly exploited as drug-delivery vehicles.9 However, their widespread application is partially limited by technical challenges in addressing EV heterogeneity.8 These issues encompass things from the choice of producer cell to the methods of EV isolation and characterization.9,10 Another similar concern sometimes is a lack of robust cargo loading strategies that rely on the biogenesis or composition of EVs.
Classically, EVs are divided into three types of vesicles based on their origin: (1) exosomes (50–200 nm), formed in the endolysosomal system11 or at the plasma membrane12; (2) microvesicles or ectosomes (0.1–1 μm), budding directly from the plasma membrane13,14,15; and (3) apoptotic bodies (1–5 μm) generated by dying cells.16 Since the last are recognized and removed by macrophages,17 it is mainly exosomes and microvesicles that are of therapeutic interest and thus will be the focus of this review. To date, the field has struggled to physically separate these two classes of EVs because they overlap in size and composition. They even share commonalities in their biogenesis pathways, which complicates the definition of mutually exclusive properties for characterization.18 However, owing to technological advancements, increasingly more is known about the mechanisms that govern their biogenesis and determine their composition. In addition, understanding the underlying processes is crucial for harnessing them as biotherapeutics.
Biogenesis of EVs
The biogenesis of EVs relies on intricate processes that are mediated by a complex interplay of signaling molecules and regulators (Figure 1). In Table 1 we compare 77 studies focusing on the regulators of different steps in EV biogenesis. For each study, we list the EV regulator of interest along with the examined producer cell types and, if relevant, applied stimuli. Moreover, we state the method of intervention with the EV regulator, the investigated EV population, and, ultimately, the impact the intervention had on EV biogenesis as measured predominantly by EV production (Table 1). The implications of the findings of these studies are discussed in more detail below. The overarching goal of this analysis was to provide a comprehensive overview of known regulators of EV biogenesis and, eventually, their putative role in EV bioengineering as discussed in the later sections of this review.
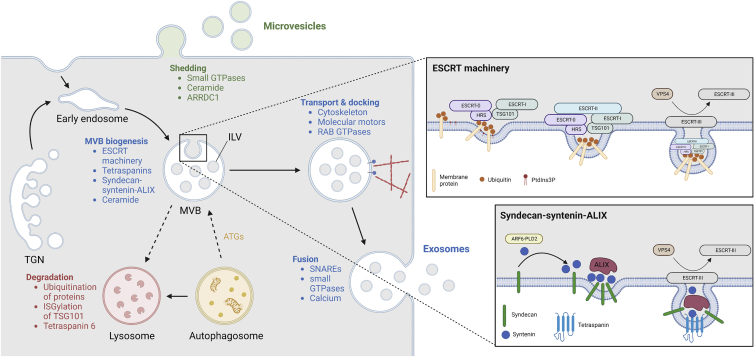
Figure 1.
EV biogenesis and its regulators
The shedding of microvesicles from the plasma membrane is controlled by small GTPases, ceramide, or ARRDC1. Exosome biogenesis is a more complex process taking place directly at the plasma membrane or in the endolysosomal system. Cargo is sorted into endosomes from the plasma membrane or the TGN. The early endosome then matures and starts forming ILVs, thereby becoming an MVB. ILV formation can be regulated by the ESCRT machinery, tetraspanins, syndecan-syntenin-ALIX, and ceramide. (Top right) ESCRT machinery: HRS (subunit of ESCRT-0) recognizes ubiquitinated proteins and PtdIns3P, leading to the recruitment of ESCRT-I by binding of its TSG101 subunit to HRS. ESCRT-II then drives the invagination and ESCRT-III the scission of the membrane. Reuse of ESCRT-III is enabled by VPS4. (Bottom right) Syndecan-syntenin-ALIX: syndecan recruits syntenin, which is regulated by ARF6 and PLD2. Subsequent interaction with ALIX promotes ILV formation, which is finalized by ESCRT-III and VPS4. This pathway commonly involves tetraspanins. After their formation, MVBs can fuse with lysosomes for degradation, which is promoted by ubiquitination of proteins, ISGylation of TSG101, and tetraspanin 6. ATGs, on the other hand, can facilitate MVB biogenesis. For secretion, MVBs are transported to the plasma membrane with the help of cytoskeletal elements, molecular motors, and RABs. Ultimately, fusion with the plasma membrane leading to the release of exosomes is mediated by SNAREs, small GTPases, and calcium. This figure was created using BioRender. ARF6, ADP ribosylation factor 6; ARRDC1, arrestin-domain-containing protein 1; ATG, autophagy-related protein; ESCRT, endosomal sorting complexes required for transport; HRS, hepatocyte growth factor-regulated tyrosine kinase substrate; ILV, intraluminal vesicle; ISG, interferon-stimulated gene; MVB, multivesicular body; PLD2, phospholipase D2; PtdIns3P, phosphatidylinositol 3-phosphate; RAB, Ras-associated binding protein; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; TGN, trans-Golgi network; TSG101, tumor susceptibility gene 101; VPS4, vacuolar protein sorting-associated protein 4.
Table 1.
Regulators of EV biogenesis
| Regulator | Cell | Intervention | EVs studied | Effect | Reference |
|---|---|---|---|---|---|
| Exosomes | |||||
| Endosome maturation | |||||
| RAB5 | MCF7 | overexpression (RAB5Q79L) | Bulk | ↓ EV secretion (syndecan, CD63, syntenin, ALIX) | Baietti et al.19 |
| mOli-neu + PLP | overexpression (RAB5Q79L) | Bulk | ↓ EV release of PLP | Trajkovic et al.20 | |
| RAB7 | MCF7 | RNAi | Bulk | ↓ EV secretion (syndecan, CD63, syntenin) | Baietti et al.19 |
| MVB formation | |||||
| ESCRT machinery | |||||
| ESCRT-0 | |||||
| - HRS | HeLa-CIITA | RNAi | CD63 (CD81+/MHCII+) | ↓ EV production | Colombo et al.21 |
| stimulated mDCs | RNAi | Bulk | ↓ EV production | Tamai et al.22 | |
| mBMDCs | RNAi | Bulk | ↓ EV production | Tamai et al.22 | |
| SCC25-H1047R | RNAi | Bulk | ↓ EV production | Hoshino et al.23 | |
| HeLa | RNAi | N/A | ↑ MVB diameter | Edgar et al.24 | |
| ↓ ILVs per MVB | |||||
| A431 | RNAi | N/A | ↓ ILVs per MVB area | Razi and Futter25 | |
| - STAM1 | HeLa-CIITA | RNAi | CD63 (CD81+/MHCII+) | ↓ EV production | Colombo et al.21 |
| ESCRT-I | |||||
| - TSG101 | HeLa-CIITA | RNAi | CD63 (CD81+/MHCII+) | ↓ EV production | Colombo et al.21 |
| HeLa | RNAi | N/A | ↑ MVB diameter | Edgar et al.24 | |
| ↓ ILVs per MVB | |||||
| A431 | RNAi | Na | ↓ MVB formation | Razi and Futter25 | |
| ESCRT-III | |||||
| - CHMP6 | HeLa GFP-CHMP4B | RNAi | bulk | ↓ EV proteins (CD9, CD63, CD81, syntenin) | Larios et al.26 |
| VPS4B | HeLa-CIITA | RNAi | CD63 (CD81+/MHCII+) | ↑ EV production | Colombo et al.21 |
| HeLa | overexpression (hVPS4E223Q) | N/A | ↓ ILVs per MVB | Sachse et al.27 | |
| Tetraspanins | |||||
| CD82/CD9 | HEK293T | overexpression (CD82/CD9) | bulk | ↑ EV production; EV β-catenin | Chairoungdua et al.28 |
| CD9 | HEK293 CD9−/−/- | overexpression (CD9YEVM)a | bulk | ↓ EV protein (CD9) | Fordjour et al.12 |
| CD63 | MNT-1 | RNAi | N/A | ↓ ILVs per MVB | van Niel et al.29 |
| HEK293 | KO | bulk | ↓ particles per cell | Hurwitz et al.30 | |
| HEK293 CD63−/− | overexpression (CD63Y235A) | bulk | ↑ EV protein (CD63) | Fordjour et al.12 | |
| Syndecan-syntenin-ALIX | |||||
| Syndecan | MCF7 | RNAi | bulk | ↓ EV production | Baietti et al.19 |
| Syntenin | MCF7 | RNAi | bulk | ↓ EV production | Baietti et al.19 |
| MCF7 | Overexpression | bulk | ↑ EV production | Baietti et al.19 | |
| - ARF6 | MCF7 | RNAi | bulk | ↓ EV proteins (syntenin, ALIX, CD63, SDC1CTF) | Ghossoub et al.31 |
| MCF7 | overexpression (ARF6T157N) | bulk | ↑ EV proteins (syntenin, ALIX, CD63) | Ghossoub et al.31 | |
| -- PLD2 | MCF7 | RNAi | bulk | ↓ EV proteins (syntenin, ALIX, CD63) | Ghossoub et al.31 |
| ALIX | HeLa-CIITA | RNAi | CD63 (CD81+/MHCII+) | ↑ MHCII on EVs | Colombo et al.21 |
| MCF7 | RNAi | bulk | ↓ EV production | Baietti et al.19 | |
| HeLa GFP-CHMP4B | overexpression (ALIXΔPRR) | bulk | ↑ EV proteins (CD9, CD63, CD81) | Larios et al.26 | |
| HeLa GFP-CHMP4B | RNAi | bulk | ↓ EV proteins (CD9, CD63, CD81, syntenin) | Larios et al.26 | |
| Ceramide | |||||
| nSMase2 | mOli-neu + PLP | GW4869/spiroepoxide/glutathione/RNAi | bulk | ↓ EV release of PLP | Trajkovic et al.20 |
| HEK293T + CD82 | GW4869 | bulk | ↓ EV proteins (flotillin, β-catenin) | Chairoungdua et al.28 | |
| SCC25-H1047R | GW4869 | bulk | ↓ EV production | Hoshino et al.23 | |
| HEK293T | GW4869/RNAi | bulk | ↓ EV production | Leidal et al.32 | |
| ↓ EV proteins (LC3, SAFB, HNRNPK) | |||||
| HeLa FLAG-RAB31Q65L | GW4869 | bulk | ↓ EV proteins (FLAG, EGFR, FLOT1, FLOT2, CD9, CD81, CD63) | Wei et al.33 | |
| ATGs | |||||
| ATG5 | MEF, MDA-MB-231 | KO | bulk | ↓ EV production | Guo et al.34 |
| ↓ EV proteins (Flotillin2, Tsg101) | |||||
| ATG12-ATG3 | MEF | overexpression (ATG3K243R) | bulk | ↓ EV proteins (total; ALIX, TSG101, GAPDH) | Murrow et al.35 |
| Transport | |||||
| Cortactin | SCC61 | RNAi | bulk | ↓ EV production | Sinha et al.36 |
| ↓ EV proteins (TSG101, CD63, Flotillin1) | |||||
| SCC61 | Overexpression | bulk | ↑ EV production | Sinha et al.36 | |
| RAB GTPases | |||||
| RAB11 | K562 | overexpression (RAB11S25N) | bulk | ↓ EV proteins (TfR, Lyn, Hsc70) | Savina et al.37 |
| ↓ EV AChE activity | |||||
| RAB35 | mOli-neu + PLP | overexpression (RAB35N120I), RNAi | bulk | ↓ EV release of PLP | Hsu et al.38 |
| RAB27A, RAB27B | HeLa | RNAi | bulk | ↓ EV production | Ostrowski et al.39 |
| ↓ EV proteins (HLA-DR, CD63, TSG101, HSC70) | |||||
| RAB27A | B16-F10,SK-Mel-28 | RNAi | bulk | ↓ EV protein (total) | Peinado et al.40 |
| RAB27A | TS/A, 4T1 | RNAi | bulk | ↓ EV proteins (total; ALIX, HSC70, CD63, TSG101) | Bobrie et al.41 |
| Fusion | |||||
| VAMP7 | K562 | overexpression (NT-VAMP7) | bulk | ↓ EV production | Fader et al.42 |
| ↓ EV AChE activity | |||||
| YKT6 | HEK293 | RNAi | bulk | ↓ EV proteins (WNT3A, CD81) | Gross et al.43 |
| A549 | RNAi | bulk | ↓ EV protein (TSG101) | Ruiz-Martinez et al.44 | |
| SNAP23 | A549 | RNAi | bulk | ↓ EV production | Wei et al.45 |
| HeLa + histamine + CD63-pHluorin | RNAi | CD63-pHluorin | ↓ fusion activity | Verweij et al.46 | |
| Syntaxin-4 | HeLa + histamine + CD63-pHluorin | RNAi | CD63-pHluorin | ↓ fusion activity | Verweij et al.46 |
| RAL1 | 4T1 | RNAi | bulk | ↓ EV production | Hyenne et al.47 |
| ↓ EV proteins (ALIX, CD63, TSG101, HSC70) | |||||
| RalA/B | 4T1 | RNAi | bulk | ↓ EV production | Ghoroghi et al.48 |
| Calcium | K562 | monensin | bulk | ↑ EV protein (TfR, Hsc70) | Savina et al.49,50 |
| ↑ EV AChE activity | |||||
| Microvesicles | |||||
| ARF6 | LOXARF6-GTP | overexpression (ARF6Q67L) | bulk | ↑ EV protein (total) | Muralidharan-Chari et al.51 |
| RhoA | HeLa + EGF | overexpression (RhoAF30L) | bulk | ↑ EV production | Li et al.52 |
| ↑ GFP release in EVs | |||||
| ARF1 | MDA-MB-231 | RNAi | bulk | ↓ EV protein (total) | Schlienger et al.53 |
| ↓ EV MMP9 activity | |||||
| ASM | astrocytes | imipramine | bulk | ↓ EV fluorescence | Bianco et al.54 |
| N9 | r-SMase | bulk | ↑ EV fluorescence | Bianco et al.54 | |
| RBCs | amitriptyline | Annexin V | ↓ EV percentage | Awojoodu et al.55 | |
| ARRDC1 | HEK293T | overexpression (ARRDC1-GFP) | bulk | ↑ GFP release in EVs | Nabhan et al.56 |
For each study, the producer cell type and, if relevant, any applied stimuli are mentioned. Moreover, the method of intervention, the investigated EV population, and the impact the intervention had on EV biogenesis are stated. EVs were predominantly studied in bulk and only a few studies looked at specific subpopulations.
Regulators: ALIX, programmed cell death 6-interacting protein; ARF, ADP ribosylation factor; ARRDC1, arrestin-domain-containing protein 1; ASM, acid sphingomyelinase; ATG, autophagy-related protein; CHMP, charged multivesicular body protein; ESCRT, endosomal sorting complexes required for transport; HRS, hepatocyte growth factor-regulated tyrosine kinase substrate; nSMase2, neutral sphingomyelinase 2; PLD, phospholipase D; RAB, Ras-associated binding protein; RAL, Ras-like protein; Rho, Ras homologous protein; SNAP, synaptosomal-associated protein; STAM1, signal-transducing adaptor molecule 1; TSG101, tumor susceptibility gene 101; VAMP, vesicle-associated membrane protein; VPS4B, vacuolar protein sorting-associated protein 4B; YKT6, synaptobrevin homolog. Cells: MCF-7, human breast cancer cell line; 4T1, murine breast cancer cell line; A431, human epidermoid squamous carcinoma cell line; B16-F10, murine melanoma cell line; CIITA, major histocompatibility complex class II transactivator; HEK, human embryonic kidney cell line; HeLa, human cervical cancer cell line; K562, human myelogenous leukemia cell line; LOX, human melanoma cell line; mBMDCs, murine bone marrow-derived dendritic cells; MDA-MB-231, human breast cancer cell line; mDCs, murine dendritic cells; MEF, mouse embryonic fibroblasts; mOli-neu, murine oligodendroglial precursor cell line; N9, microglial cell line; RBCs, red blood cells; SCC, squamous cell carcinoma cell line; SK-MEL-28, human melanoma cell line; TS/A, murine mammary adenocarcinoma cell line. Others: ↓, reduction; ↑, increase; AChE, acetylcholinesterase; EGFR, epidermal growth factor receptor; FLOT, flotillin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HLA-DR, human leukocyte antigen DR; HNRNPK, heterogeneous nuclear ribonucleoprotein K; HSC70, heat-shock 70 kDa protein; ILV, intraluminal vesicle; KO, knockout; LC3, microtubule-associated protein 1A/1B-light chain 3; Lyn, tyrosine-protein kinase; MHCII, major histocompatibility class II; MMP9, metalloproteinase 9; MV, microvesicle; MVB, multivesicular body; N/A, not applicable; PLP, proteolipid protein; RNAi, RNA interference; r-SMase, recombinant sphingomyelinase; SAFB, scaffold-attachment factor B; SDC1CTF, syndecan-1 cytoplasmic fragment; TfR, transferrin receptor; WNT3A, Wnt family member 3A.
CD9 mutant that carries endosome-targeting signal.
Exosomes
Only recently, Pegtel and Gould challenged the common notion that exosome-sized vesicles stem primarily from the endolysosomal system.11 Indeed, Fordjour et al. showed experimentally that efficient budding from exosomes can occur at the plasma membrane,12 which was recently confirmed independently.57 Given the novelty of these observations, pre-existing literature considered typically only endolysosomal exosome biogenesis, which begins with the formation of multivesicular bodies (MVBs), which are then transported to the plasma membrane and, upon fusion, release exosomes into the extracellular space (Figure 1).1 MVBs are formed during endosome maturation, which is mediated by Ras-associated binding (RAB) GTPases, particularly the conversion of RAB5 to RAB7 (Table 1).58,59,60 During the transition from early to late endosomes, the limiting membrane invaginates to give rise to intraluminal vesicles (ILVs). Multiple drivers of this process have been identified, including the endosomal sorting complex required for transport (ESCRT) machinery (Table 1).61 This machinery consists of four subunits (ESCRT-0, -I, -II, and -III) that act in succession. HRS, a component of ESCRT-0, is the first to engage with the endosomal membrane, where it interacts with ubiquitinated proteins62,63 and binds to phosphoinositide (PtdIns3P).64,65,66 Next, ESCRT-I is recruited by binding of its TSG101 subunit to HRS.67,68 ESCRT-II assembly follows, and ESCRT-II, together with ESCRT-I, drives the invagination of the endosomal membrane.69,70,71 Recruitment of ESCRT-III results in membrane scission, thereby finalizing ILV formation.72,73 To enable reuse, disassociation of ESCRT-III requires VPS4 (Figure 1).74,75,76 Importantly, depletion of the ESCRT machinery does not abolish exosome production, demonstrating that MVBs can also be formed by other means.77
Alternative processes for MVB formation have been proposed; however, their cross talk and interdependence are still understudied. Tetraspanins, particularly CD63, CD81, and CD9, have emerged as key regulators of alternative MVB formation processes (Table 1). They are involved in ILV formation by clustering together and sequestering other proteins to form tetraspanin-enriched microdomains.78,79 CD63, for instance, was found to compete with HRS in ILV formation,24 implying that the production of tetraspanin-containing exosomes does not require the canonical ESCRT machinery.29,33 In fact, CD63 has been shown to regulate exosome production,29,30 and the cone-like shape of CD9 has been proposed to aid membrane curvature.80 However, increasing evidence suggests that tetraspanin-dependent exosome biogenesis requires other regulators, such as ALIX, ESCRT-III,26 or syntenin.33 This so-called syndecan-syntenin-ALIX pathway utilizes ESCRT-III and VPS4 but does not require ESCRT-0 or ubiquitination.81 Instead, syndecan recruits syntenin, which then interacts with ALIX to promote ILV biogenesis.19,82 Syntenin, in turn, was shown to be regulated by ARF6 and phospholipase D2 (PLD2) (Figure 1).31 In another study, however, exosomal secretion of β-catenin driven by CD82 or CD9 overexpression was dependent on ceramide,28 which is another known regulator of ILV formation. Briefly, neutral sphingomyelinase (nSMase) 2 hydrolyzes sphingomyelin to ceramide, which initiates membrane curvature. Multiple studies have shown that inhibition of sphingomyelinase reduces exosome release,20,23,28,32,83,84,85 while induction with ceramide causes an increase (Table 1).20,85,86 Recently, non-conventional exosome production at the nuclear envelope of activated neutrophils was found to require nSMase1 and ceramide.87 In addition, interaction of nSMase activation-associated factor (NSMAF), a regulator of nSMase2, with microtubule-associated protein 1A/1B-light chain 3 (LC3) is pivotal for its loading and secretion in ceramide-dependent exosomes.32 Also, ceramide-dependent formation of EGFR-containing exosomes requires RAB31 to engage flotillin proteins.33 Intriguingly, Wei et al. hypothesize that RAB31-flotillin regulates tetraspanin sorting into exosomes, which upon stimulation (e.g., EGFR) predominates over the basal syndecan-syntenin-ALIX pathway.33 Clearly, MVB formation is a highly complex process that appears cargo and stimulation dependent and involves redundant pathways.
After their formation, MVBs either are destined for degradation in lysosomes or fuse with the plasma membrane, leading to the release of ILVs as exosomes (Figure 1). The drivers of this decision are largely unknown, but are believed to be determined already during MVB formation. For instance, ubiquitination of proteins,88,89 ISGylation of TSG101,90 or tetraspanin-6, a negative regulator of syntenin,91 have been shown to lead MVBs down the degradative pathway. This supports the notion that ESCRT-dependent formation of MVBs is commonly associated with degenerative and syndecan-syntenin-ALIX with secretory MVBs.19,92 Also, lysobisphosphatidic acid (LBPA), a partner of ALIX, has been proposed to regulate the fate of ILVs.26,81,93,94 Aside from that, unconventional protein secretion from autophagosomes can involve MVBs.95 In fact, autophagy-related (ATG) proteins have been implicated in exosome production (Table 1) by decreasing the acidification of MVBs34 or by interacting with and stimulating ALIX.35
The transport of MVBs to the plasma membrane is largely unknown but shares mechanisms of conventional vesicular trafficking along cytoskeletal elements. Hence, it is an active process driven by molecular motors and directed by small GTPases (i.e., RAB GTPases). The involvement of cytoskeletal proteins was exemplified in a study where exosome-mediated transfer of CD63-GFP from polarized T cells to antigen-presenting cells (APCs) was abolished in the presence of actin cytoskeleton inhibitors.96 Furthermore, cortactin, as a regulator of branched actin dynamics, was found to control MVB docking at the plasma membrane.36 Also, ALIX has been suggested to play a role in actin-dependent intracellular distribution of endosomes.97 Apart from that, RAB GTPases have been identified as spatiotemporal coordinators of MVB traffic (Table 1). Initially, RAB11 was found to regulate exosome release in K562 cells37 and has since been shown to do so by influencing plasma membrane docking of MVBs,49 similar to RAB35 in oligodendroglial cells.38 In addition, RAB27 isoforms have been observed to function differently depending on the cell type (Table 1). Similarly, in contrast to K562 cells,37 RAB11 inhibition did not affect the production of exosomes in HeLa cells,39 supporting the overall notion that RAB GTPases regulate exosome secretion in a cell-type-dependent manner.
The fusion of MVBs with the plasma membrane and release of exosomes are driven by soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins. Here, vesicle-associated membrane proteins (VAMPs; or v-SNAREs) interact with syntaxin and SNAP (or t-SNAREs) on the plasma membrane to form trans-SNARE complexes, which provide proximity and the necessary mechanical force.98 SNAREs involved in exosome release include VAMP7,99,42 YTK6,43,44 SNAP23,45,46 and syntaxin-4 (Table 1).46 Their downregulation negatively affects exosome release rates, but in the case of VAMP7, only in K56242 and not in MDCK cells.99 Other proteins involved in exosome secretion include V-ATPases100 and small GTPases. Intriguingly, Verweij et al. developed a pH-sensitive system using CD63-pHluorin to directly visualize fusion of MVBs with the plasma membrane.46,101 Using this technology, the same group showed recently that dynamic endoplasmic reticulum-late endosome membrane contact sites can modulate exosome secretion by regulating certain MVB fusion events to the plasma membrane, showing crucial involvement of RAB27.102 Moreover, the small GTPase RAL1 was found to control exosome production in 4T1 cells and was further demonstrated to recruit syntaxin 5 (t-SNARE) in C. elegans, thereby promoting MVB fusion with the plasma membrane.47 Since then, other Ral GTPases, specifically RalA/B, have been implicated, not necessarily in the fusion step, but in MVB homeostasis and exosome secretion by acting directly through PLD1.48 Furthermore, to overcome electrostatic repulsion between membranes, the fusion event can be facilitated by bivalent ions.98 In fact, MVB fusion with the plasma membrane was demonstrated to depend on calcium in K562 cells.49,50
Microvesicles
Considerably less is known about the formation of microvesicles (MVs) or ectosomes. Only a few regulators of MV shedding from the plasma membrane have been identified and studied (Table 1). Increasing evidence points to the involvement of small GTPases, such as the Rho family103 and ARFs,51,52,53 by regulating cytoskeletal elements. Activation of ARF6, for example, was found to enhance MV production in melanoma cells, which was further shown to depend on the recruitment or activation of its downstream effectors PLD, ERK, and myosin II light chain kinase (MLCK).51 Another study was unable to observe the same effect of ARF6 in HeLa cells, but instead demonstrated that RhoA and its effectors (ROCK, LIMK, cofilin) are involved in MV formation.52 Moreover, it is not surprising that, as an upstream regulator of the Rho family, ARF1 was found to govern MV shedding in MDA-MB-231 cells.53 Apart from that, acid sphingomyelinase (ASM), as another regulator of ceramide in addition to nSMase, which is involved in exosome biogenesis, has been implicated in MV biogenesis. Initially, it was found that ATP stimulates the production of MVs in microglia,104 which relies on the activation of the ionotropic ATP receptor P2X7,105 phosphorylation of p38, and ultimately ASM.54 Similarly, MV generation in red blood cells was linked to ASM.55 Last, the formation of adaptor protein arrestin domain-containing protein 1 (ARRDC1)-mediated MVs was shown to share commonalities with viral gag-induced membrane shedding. Both require the help of TSG101 and VPS4 as well as localization to the plasma membrane, which for ARRDC1 is mediated by its arrestin domain.56
Cargo sorting into EVs
EVs are gaining ground as drug-delivery modalities for a wide range of diseases. They benefit from favorable safety profiles as well as the ability to cross biological barriers and protect their contents from degradation.8,9 While some applications exploit the inherent therapeutic properties of EVs, others manipulate them to deliver a specific cargo.8,9,106,107,108 This manipulation can be realized by endogenous or exogenous means, referring to methods performed pre- or post-EV isolation, respectively.108 Exogenous techniques show high efficiencies but may compromise EV integrity and are restricted to smaller payloads (extensively reviewed elsewhere108,109). Endogenous approaches are generally more labor intensive, yet suitable for larger macromolecular cargos.108,109 Endogenous loading techniques mainly encompass genetic engineering of the source cells to overexpress a protein of interest fused to proteins involved in EV biogenesis, consequently exploiting their inherent capabilities to be incorporated into EVs.106,108,110 In addition, non-genetic source cell manipulation approaches have been successfully established.110 In the following section, we will first briefly illustrate the natural loading of macromolecular cargo during EV biogenesis, focusing on protein and nucleic acid cargo. Next, we will discuss current strategies for endogenous bioengineering of source cells to specifically sort therapeutic cargo molecules into or onto EVs by modifying EV biogenesis pathways.
Protein cargo
EVs carry a broad range of transmembrane proteins, membrane-associated proteins, and luminally loaded soluble proteins. For instance, Hurwitz et al. performed proteomic characterization of EVs derived from 60 different cancer cell lines and identified 6,071 proteins in total, of which 213 were common to all cell types, and only a minority were exclusive to a specific cell source.111 Similarly, Kugeratski et al. identified 3,759 proteins in EVs derived from 14 cell lines, of which 642 proteins were unique to different cell types.112 Furthermore, Hoshino et al. analyzed 497 EV preparations from cell lines, tissue explants, and plasma, from both mice and humans, and identified homologies in the protein signature of these EVs.113 These studies at large reflect the fact that the majority of the EV proteomes are similar and enriched in proteins involved in EV biogenesis. In addition, only a minority of the proteome reflects the cell-type specificity. Importantly, the mechanisms involved in sorting or loading of EV-associated proteins are yet to be determined, but most of these proteins are cell-surface receptors, which could indicate that they originate from plasma membrane budding or shedding.
EVs are highly enriched in various tetraspanins such as CD81, CD63, CD9, CD82, and CD37.114,115 The tetraspanin family comprises proteins that are neither enzyme-linked receptors nor catalytically active receptors, but that may promote the sorting of protein cargos, especially tetraspanin-interacting proteins such as integrins,116 ICAM-1,117 IGFS-8,118 major histocompatibility complex (MHC) class II proteins,115,119 and syndecan.19 However, various reports failed to see differences in EV numbers or EV proteomes upon overexpression or silencing of CD63, CD81, or CD9.120,121 Furthermore, based on the cellular localization of tetraspanins, it has been speculated that tetraspanins such as CD63 are exclusively present on EVs of MVB origin, whereas CD81, which is primarily localized on the cell surface, is preferentially sorted into MVs.12,57,122 This is reflected by the fact that CD63-positive vesicles are CD81 low or negative and vice versa.12 In addition to tetraspanins, there are also other scaffolding transmembrane proteins that are associated with EVs, such as flotillin 1 and 2,123 IL-6R,124 EGFR,125 T cell receptor,126 chimeric antigen receptor,127 Notch receptors,128 GPCR receptors,129,130 PD-L1,131 TGFB,132 and ADAM proteases,133 on the surface of EVs. Apart from transmembrane proteins, the surface is also rich in membrane-interacting proteins, especially proteins with glycosylphosphatidylinositol (GPI) anchors, for example, complement-inhibiting proteins DAF and MAC-IP,134 as well as cell-surface proteoglycan glypican-1.135 In addition, on the inner leaflet, a range of proteins have been identified, including small GTPases, which are involved in the biogenesis and adhere to the inner leaflet by post-translational prenylation.38,39,136 In addition to prenylated proteins, myristoylated proteins such as BASP-1137 and Src signaling kinases136 also interact with the inner leaflet and sort into EVs. Similarly, lentiviral gag uses N-terminal myristoylation for loading into viruses or EVs.138 Other posttranslational modifications that have been shown to drive cargo sorting are ubiquitination, SUMOlyation,139,140 and phosphorylation.141 Other proteins that are abundant in EVs interact with, or are part of, ESCRT complexes such as ALIX, TSG101, and syntenin.71 Apart from biogenesis-related proteins, EVs are also enriched with molecular chaperones such as Hsp70, Hsp90, and Hsp20.142,143,144 Finally, cytosolic proteins, such as actin and tubulin, are also sorted into EVs, most likely upon MV shedding from the plasma membrane.111
RNA cargo
More than a decade ago, multiple studies showed the EV-mediated functional intercellular transfer of RNA.145,146,147,148,149,150 According to numerous reports using next-generation sequencing and microarray technologies to characterize RNA content in EVs derived from cell culture, tissues, or biological fluids, EVs contain both coding and non-coding RNA species.151 Apart from mRNA, EVs are enriched in small RNA species, such as transfer RNAs (tRNAs), microRNAs (miRNAs), small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), mitochondrial RNAs (mtRNAs), piwi-interacting RNAs (piRNAs), vault RNAs (vtRNAs), and Y RNAs.96,151,152,153,154,155,156 Circular RNAs (circRNAs) and fragments of ribosomal RNAs (rRNAs) and long non-coding RNAs (lncRNAs) have also been identified in EVs.157,158,159,160,161,162,163
The molecular mechanisms of RNA sorting into EVs are still not fully understood. Current literature indicates that factors like RNA abundance, sequence length, cellular location, or the ability to associate with certain proteins or membrane lipids regulate RNA sorting into EVs.164,165 Generally, sorting mechanisms are classified as active or passive RNA-loading processes.166 Passive loading into EVs strongly depends on the intracellular concentration of a certain RNA, and its enrichment in EVs is exclusively source cell conditional.157,167 An active, or selective, loading mechanism of RNA into EVs, on the other hand, is indicated by an enrichment for a certain RNA in EVs that is not necessarily mirrored in the overall RNA content of the source cell.96,146,168,169 For instance, preferential enrichment in EVs was reported for 3′ UTR mRNA fragments with regulatory elements and recurring motifs, such as variations of a stem-loop-forming 25-nt “zip code” sequence encompassing a GTGCC core motif and a single miRNA-1289 binding site.160,170 This was also shown specifically for miRNA containing so-called EXOmotifs, with the strongest being CGGGAG, while miRNAs with CELLmotifs, such as AGAAC, CAGU, or AUUA, were retained in the source cells.171 Furthermore, 40 miRNA seed sequences were identified as motifs enriched in lncRNA associated with prostate cancer EVs.172 Overall, these studies suggest an intriguing mechanism for sorting certain miRNAs into EVs: by using mRNA fragments or lncRNAs as RNA sponges.170,172 Correspondingly, the enrichment of certain sequence motifs in EV RNA could point to a sorting mechanism involving RNA-binding proteins (RBPs) with differential sequence affinities. As there are more than 4,000 RBPs annotated in the human genome at the time of this writing,173 and RBPs comprise 25% of the EV protein content,157 it can be safely assumed that they play a key role in the active sorting of RNA into EVs. In fact, several RBPs and their selective RNA cargo have been linked to EVs, such as YBX1,174,175,176,177 SYNCRIP,178,179 Arc1,180 AGO2,181,182 ALIX,183 MVP,184,185 hnRNPU,186 and ANXA2.187,188 Apart from specific binding of RNA sequence motifs, additional EV sorting signals include RNA or RBP modifications, such as SUMOylation, as reported for miRNA loading into EVs by hnRNPAB1140; phosphorylation, as shown for exosomal 5′pppRNA in latent EBV infection189; or LC3 conjugation, as described for hnRNPK and SAFB-mediated loading of small ncRNA species during the secretory autophagy pathway.32 In addition, RNA association with different membrane lipids during vesicle formation was proposed as another distinct mechanism for selective RNA sorting into EVs.190,191,192,193
Even with years’ worth of outstanding research, the genuine complexity of RNA loading mechanisms during EV biogenesis pathways is by far not completely understood. A recent study clearly demonstrated the heterogeneity of RNA content in different EV subpopulations, indicating distinct preferences and, possibly, limitations in loading mechanisms and EV capacity depending on the EV biogenesis pathway.151 Therefore, additional efforts to understand EV heterogeneity, especially considering vesicular RNA cargo sorting pathways, are essential. Moreover, recent reports questioned the consensual knowledge of the true biological impact of EV-derived RNA, in particular low-abundant miRNA.194 Similarly, awareness was raised in the field to reflect on EV isolation methodologies and the limitations of certain functional assays, especially when working with RNA from EVs produced by transient transfection.195,196,197 Thus, careful assessment of biological claims and ample use of proper controls is key to unravel the true role and functional impact of EV-derived RNA.
DNA cargo
While RNA as a cargo nucleic acid in EVs has been extensively studied, there are substantially fewer studies on the biogenesis or clinical significance of EV-associated DNA. To date, DNA species reported associated with EVs include genomic double-stranded DNA (dsDNA),198,199,200,201 along with dsDNA-binding histone proteins,202 single-stranded DNA (ssDNA),203 mitochondrial DNA (mtDNA),204,205 and viral DNA.206 Predominantly, EV-associated DNA has been proposed as a putative biomarker in liquid biopsies of cancer patients198,207,208,209,210 or as a tool for non-invasive prenatal diagnostics.210,211 However, EV-associated DNA is mostly sensitive to enzymatic treatment and thus detected mainly on the outside of certain EV subpopulations.212 Interestingly, no chromosomal regions have been found to be overrepresented in remaining EV-enclosed DNA, hinting at the absence of a specific loading mechanism for genomic DNA into EVs.212 Yet, a nucleosome-associated pattern212 of long genomic DNA fragments as well as chromatinized DNA structures209 in EVs was observed, suggesting a still elusive mechanism for genomic DNA loading during EV biogenesis.
Conversely, despite evident reports of EV-associated DNA, its presence and biological impact are still highly disputable, as the field often considers EV-associated DNA a contaminant from improper EV purification.122 Thus, the authentic role of DNA in EV biogenesis remains to be elucidated.210
Hijacking the EV biogenesis pathway for biotherapeutic cargo loading
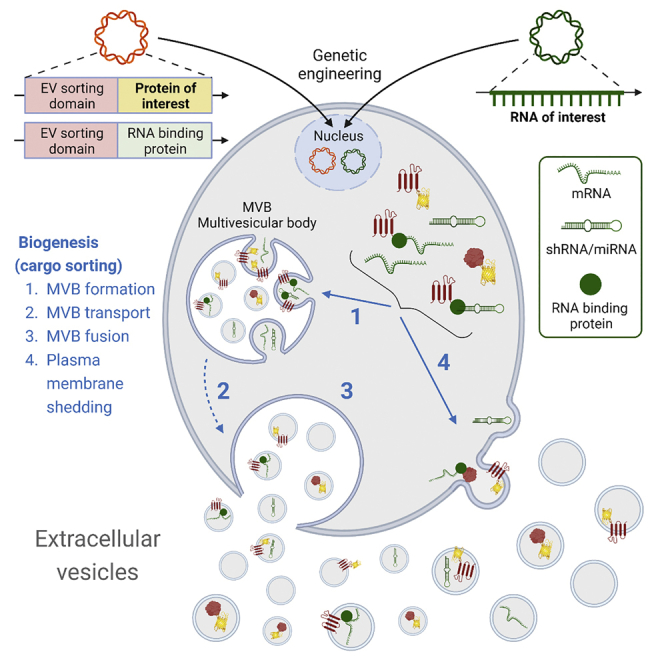
EVs are enriched with a variety of biomolecular cargo, including lipids, proteins, and nucleic acids. With the application of new genetic tools, such as RNAi, CRISPR-Cas9, and recombinant DNA technology, in combination with the recent technological advances in EV characterization, the biological phenomena involved in the sorting of cargo molecules into EVs are starting to unravel (Table 1). Thus, by employing current state-of-the-art methods of synthetic biology, we and others have exploited the known biological mechanisms to engineer EVs with a variety of therapeutic cargo.
Protein cargo loading into EVs
Protein sorting into EVs is a highly regulated process, and the majority of these proteins are ubiquitously enriched in EVs irrespective of the source cell due to their involvement in EV biogenesis. Therefore, hitchhiking on proteins involved in EV biogenesis serves as an efficient mean of endogenously bioengineering EVs with biotherapeutics. For endogenous loading, the parental cells are genetically engineered to overexpress the desired protein fused to an EV scaffold, which is then incorporated into the secreted vesicles during EV biogenesis (Figure 2).108 Myriads of endogenous engineering scaffolds have been tested in the past decade for EV luminal and surface engineering (Figure 2). However, with such a vast diversity of proteins involved in EV biogenesis and cargo loading, identifying versatile strategies to load biotherapeutic cargo into EVs is highly challenging. Importantly, fusion of a certain cargo to one regulator of EV biogenesis may lead to suboptimal loading into EVs, while fusion to another could result in superior engineering performance. Our group has shown this for eGFP, where fusion to ALIX yielded several orders of magnitude lower cargo encapsulation into EVs compared with a fusion to CD63.121 Thus, adapting known molecular mechanisms involved in EV engineering is ambitious, owing to their extraordinary complexity, but mindful design and further deepening of our knowledge will result in the development of successful strategies. In the following sections, we discuss current strategies for endogenous EV bioengineering and cargo loading based on native EV biogenesis and touch upon emerging developments and future directions of the EV bioengineering field.
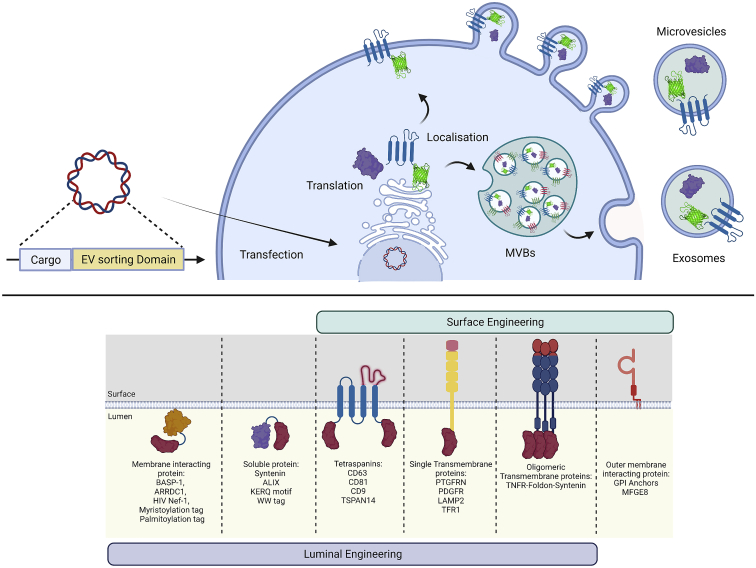
Figure 2.
Endogenous EV engineering strategies for therapeutic cargo loading
The top illustrates the general principle of producer cell engineering. A genetic expression construct encoding the therapeutic cargo connected to an EV sorting domain is introduced into and expressed by EV producer cells. The therapeutic cargo is sorted into EVs during EV biogenesis. Depending on the nature of the EV sorting domain, engineered EVs originate from the MVB-exosome pathway or microvesicle pathway. The bottom lists means of luminal or surface engineering of EVs, including examples for EV sorting domains successfully used in the field. This figure was created using BioRender.
Luminal-protein loading
EVs are enriched with numerous luminal proteins; however, not all EV proteins can be used for the endogenous engineering of EVs. This is primarily due to loss of functionality upon fusion of the protein of interest to an EV sorting scaffold.121 Ideally, the EV engineering scaffold should allow for efficient packaging of multiple copies of proteins without interfering with the EV biogenesis pathway and luminal content of the EVs. Therefore, various studies have systematically compared different EV sorting domains for endogenous engineering using the latest innovations in EV characterization to determine the efficiency at a single-EV level. For instance, we previously systematically compared 12 endogenous EV engineering scaffolds for encapsulating EGFP into EVs and identified the tetraspanins CD63, CD81, and CD9 as the most efficient loading scaffolds.121 For instance, CD63 could load 40–60 EGFP molecules per vesicle. Moreover, a recent study by Silva et al. achieved loading of 150 molecules of EGFP per vesicle using TSPAN14 as the EV scaffold.213 Interestingly, the subcellular location and resulting EV proteome were largely unaltered upon engineering EVs endogenously with CD63-EGFP fusion proteins.12,121 In addition to tetraspanins, other identified EV sorting domains include syntenin,121 ARRDC1,214 BASP-1,137 syndecan-1,121 and HIV-I Nef protein.215 Importantly, a direct fusion of the therapeutic protein, to either the N or the C terminus of an EV scaffold, may affect the functionality of the therapeutic protein in the recipient cells.216 Therefore, various sophisticated systems have been developed, such as the light-induced dimerization system216 and small-molecule-controlled protein association,217 which allow for the release of biotherapeutic cargo from the EV scaffold post-encapsulation. Also, the fusion of certain tags to the protein of interest can mediate EV sorting. For instance, insertion of the KFERQ motif can drive loading into Lamp2A-positive EVs.218 Similarly, WW-domain-tagged proteins are ubiquitinated upon recognition by Ndfip1 and are efficiently packaged into EVs.219 Based on these novel developments in endogenous EV engineering, EVs have been bioengineered for the delivery of CRISPR Cas9,217,220 IκBα superrepressor,221 Cre recombinase,214,216,219 and lysosomal enzymes.222 Importantly, all these endogenous EV engineering strategies achieve the labeling of certain EV subpopulations only, as one sorting domain mainly makes use of one of multiple EV biogenesis pathways.1 Therefore, exploring different combinations of EV sorting domains to achieve the engineering of a broader EV population will aid in enhancing the therapeutic efficacy of EVs.
Surface-protein engineering
As mentioned earlier, the EV surface is enriched with various transmembrane proteins or GPI-anchored proteins. These proteins have a variety of effector functions, such as ligands for target cell recognition223 and signaling131 or receptors for decoying toxins, biologics, and viruses.133,224 For developing advanced therapies using EVs, surface engineering is crucial for achieving targeted delivery, signaling, and decoy function. Similar to the luminal engineering of EVs, various surface engineering scaffolds have been identified through a systematic comparison of EV-associated transmembrane proteins. One of the most widely used scaffolds for the surface display of ligands is Lamp2, a transmembrane protein associated with the endolysosomal pathway.225 Importantly, Lamp2b labels vesicle populations originating from the endolysosomal pathway, and engineering efficiency is highly dependent on the producer cell.121,226 Therefore, novel surface engineering scaffolds are much needed to target a broader range of EV subpopulations and ensure high engineering efficiencies independent of their cell sources. In addition to their application in luminal engineering of EVs, tetraspanins can be exploited for the display of protein biologics on the EV surface. The second extracellular loop of tetraspanins is highly modular and facilitates the insertion of ligands for the surface engineering of EVs.227 With this strategy we recently dramatically increased EV circulation times in vivo by displaying an albumin-binding domain on their surface.227
Notably, the use of tetraspanins for surface engineering should be done with caution, as the display of ligands in a closed-conformation extracellular loop may affect the functionality. Furthermore, the display of large ligands in the extracellular loop can have a negative impact on the sorting of the fusion protein into EVs.121 To overcome this limitation, a fifth transmembrane domain has been added to either the N or the C terminus of tetraspanins, or the fourth transmembrane domain is deleted to facilitate the display of larger ligands on the EV surface.226,228 Apart from the tetraspanin protein family, PTGFRN was identified as a scaffold for surface engineering of EVs and achieved loading of up to 1,000 engineered molecules per EV.137 Importantly, PTGFRN interacts with CD9 and CD81, and the use of this scaffold may target CD9 and CD81 EV subpopulations.229 Similarly, PDGF, another EV-associated transmembrane protein, has been used for EV engineering.230 Apart from transmembrane proteins, GPI anchors231 and phosphatidylserine-binding proteins such as MFGE8232 can also facilitate surface engineering of EVs. Notably, the association of these scaffolds with an EV surface is reversible and dependent on phospholipid composition, which may limit the engineering efficacy. EV-associated luminal proteins have also been used for surface engineering of EVs. We recently described one such approach in which transmembrane proteins, upon fusion with syntenin and an oligomerization domain, enabled efficient EV surface engineering, while the EV surface proteome was largely unaltered.226 Importantly, this strategy proved to be more efficient for the surface display of cytokine receptors than for the use of other scaffolds, such as PTGFRN, MFGE8, PDGF, or CD63.226
Nucleic acid cargo loading
After the discovery of functional RNA transfer via EVs,145 tremendous efforts were invested to create bioengineered EVs as nanosized biomimetic delivery agents for therapeutic nucleic acid cargo. While small RNA species can nowadays be efficiently and successfully encapsulated into post-purified EVs by exogenous loading approaches,225,233,234 the development of efficient and robust endogenous loading required for larger nucleic acid cargos has proven significantly more difficult. As exact sorting mechanisms of any nucleic acid species into EVs remained unclear, the first approaches to endogenous EV bioengineering for nucleic acid loading were based on overexpression of sequence-optimized miRNAs, small interfering RNAs (siRNAs), and other small RNAs,190,235,236,237 as well as therapeutic mRNAs (Figure 3).238,239 However, as knowledge about specific RNA sorting mechanisms deepened, the resulting exploitation of EV biogenesis pathways has greatly accelerated the development of EV bioengineering strategies. For instance, siRNA sequences incorporated into a Dicer-independent pre-miRNA stem loop (pre-miR-451) showed enhanced loading efficiency using the miRNA sorting machinery into EVs.240 Also, this approach of siRNA delivery dramatically decreased the siRNA dose needed in target cells for effective gene silencing.240 For EV loading of nucleic acid cargo, the endogenous loading strategies for therapeutic proteins as discussed previously have been adapted by fusing a nucleic acid-binding protein to an EV-sorting protein. Transient or stable co-expression of the fusion protein with the nucleic acid displaying the compatible binding motif leads to nucleic acid binding and efficient loading into the EVs during biogenesis (Figure 3). This methodology has been successfully employed for small RNAs,230,241,242 as well as longer RNA species, such as mRNA.214,243,244 Target therapeutic mRNA expression in EV source cells also leads to therapeutic protein expression and passive protein loading into EVs.245 Thus, special care needs to be applied when evaluating cargo mRNA functionality upon delivery of engineered EVs to recipient cells.245 Consequently, in an experimental setting, the co-delivery of passively loaded protein needs to be properly controlled for, while in a therapeutic setting, co-delivery of mRNA and therapeutic protein can be favorable. A recent approach addressing this methodological issue elegantly combined endogenous and exogenous EV loading strategies.246 Here, they expressed a DNA aptamer sealing target mRNA translation in producer cells while being efficiently loaded into EVs by a CD9-zinc finger fusion protein.246 To disable DNA aptamer binding, purified mRNA/DNA aptamer-loaded EVs were electroporated to encapsulate a Klenow fragment exonuclease, degrade the aptamer, and enable mRNA translation in recipient cells in vitro and in vivo.246
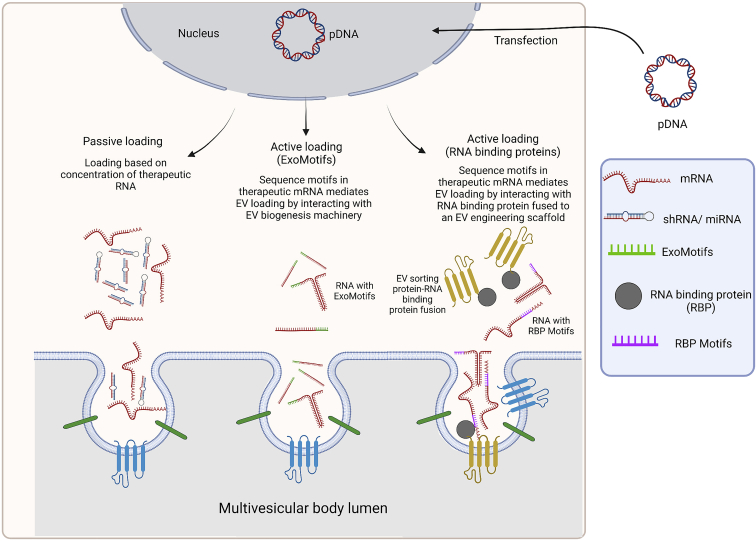
Figure 3.
Strategies for nucleic acid loading into EVs
RNA species are sorted into EVs either passively, triggered by high local abundance and membrane proximity, or specifically, by means of active sorting. Active sorting processes are initiated by the presence of defined motifs in the RNA sequence, such as EXOmotifs or RBP motifs, that either associate with luminal membranes during MVB formation or are recognized by RNA-binding proteins (RBPs), which in turn are incorporated into EVs during biogenesis. These natural sorting pathways during EV biogenesis can be exploited for bioengineering strategies to achieve targeted loading of therapeutic RNA cargos into EVs. This figure was created using BioRender.
As increasing numbers of strategies for EV endogenous engineering platforms for therapeutic RNA delivery emerge, the scientific discussion about capacity limitations of EVs as a drug-delivery tool becomes more pressing. For instance, naturally sorted rRNA and snRNA have been identified at more than one copy per EV, while full-length mRNA copies have been reported at a maximum figure of 1 in 1,000 EVs.151,165 Moreover, full-length mRNA transcripts longer than 1,000 nt are almost absent in small EVs and detectable only in larger vesicles.151 Thus, the question arises as to which of the biologically very heterogeneous EV populations have the physical capacity and means to be endogenously loaded with full-length, functional longer RNA species? Furthermore, once acquired, how can the field translate this knowledge into technology development? Currently, there are neither binding official agreements on nucleic acid quantification or reporting methods nor a gold standard in EV purification methodology.18,164,247 EV production and purification are subject to informed methodological decisions of the respective researcher, and thus a fair comparison of RNA loading figures from the literature seems nearly impossible. However, efficiencies of up to one selectively loaded mRNA copy per EV have been reported when passive loading of protein, attached to the mRNA or translated from it, was suppressed.246 This observation points to a promising avenue toward improving RNA loading strategies. In addition, and highly remarkably, as biomimetic vehicles for RNA delivery, EVs performed much more efficiently than state-of-the-art lipid formulations.237,240,248 Thus, further unlocking mechanisms of EV biogenesis for the development of safe, elegant, and efficient engineering strategies for nucleic acid delivery via EVs will tremendously accelerate the nanomedicine and gene therapy fields for a plethora of applications.
Non-genetic source cell modifications for endogenous EV loading
Genetic engineering for endogenous cargo loading into bioengineered EVs bears certain safety risks for their clinical applicability.110,249 Thus, non-genetic means of source cell modifications exploiting EV biogenesis pathways are valuable to the field. Current methodologies include cellular metabolic labeling and intrinsic cell membrane modifications.110,249 For instance, glycans of EV producer cells were metabolically engineered to incorporate active azides into the membranes of secreted EVs, which were then combined with bioorthogonal click conjugation to modulate the EV characteristics after EV purification.250,251 Furthermore, the treatment of endothelial cells with ethanol led to increased sorting of pro-angiogenic miRNAs and lncRNAs into secreted EVs.252,253 Another non-genetic loading technique is the recycling of externally provided EV cargos. Interestingly, phagocytic cells have been reported to incorporate superparamagnetic iron oxide nanoparticles along with therapeutic agents into their EVs by phagocytosis when supplied with the EV cargo through simple addition to the growth medium.254,255 Alternatively, fusing the EV source cell membrane with synthetic membrane fusogenic liposomes containing hydrophobic compounds was described as another method to load cargo into secreted EVs.256,257 As large-scale EV production is still a significant bottleneck in clinical translation of EV therapeutics, non-genetic approaches to produce sufficient yields of therapeutically active EVs can be indispensable additions to existing EV bioengineering strategies.239,258,259,260 Methodologies to boost EV production and secretion include, but are not limited to, metabolic changes in the source cells by defined medium compositions,258,259,260 3D cultures in bioreactors,253,258,261,262 and physical stimulation.239,263,264
Conclusions
The field of EVs has seen an immense transformation in the past few decades, from being their regarded as garbage bags to now being regarded as essential mediators in intercellular communication. Owing to their unique ability to transfer macromolecules across cells and biological barriers, EVs are considered a rising star in the field of drug delivery. Notably, EVs outcompete the majority of the synthetic delivery vectors in terms of better efficacy, extrahepatic delivery, and much lower toxicity. Overall, these developments have led to the initiation of various clinical trials using EVs as a therapeutic intervention. Intriguingly, several trials report therapeutic activity and, most importantly, no toxicity has been observed overall in the study participants. These reports on ongoing phase 1 clinical trials are highly promising, and it is hoped they will lead to a progression to phase 2/3 placebo-controlled settings soon. However, although technologies for large-scale and Good Manufacturing Practice (GMP)-grade culturing of cells exist, clinical manufacturing of EVs is still an unaddressed territory. This is primarily hindered by a lack of technologies that allow for large-scale production of highly pure EVs without affecting their integrity and biophysical properties. A particular challenge for the purification of EVs from the extracellular environment is its enrichment in apoptotic bodies, protein aggregates, and ribonucleic complexes, which display features similar to those of EVs, such as size or density, and thus potentially co-purify with EVs using current technologies. Another obstacle, which is largely unaddressed, is the GMP/Good Clinical Practice (GCP)-grade storage while conserving the therapeutic effect of EVs. Acquisition of more in-depth knowledge about EV stability and development of purification technologies for enriching specific EV populations will be key to achieve a smooth translation from bench to bedside in the future.
Funding
This project has received funding from the European Research Council (ERC) under the consolidator grant (proposal n° 101001374) and the European Union's Horizon 2020 research and innovation programme (grant agreement n° 825828). The project was further supported by the Swedish Research Council (agreement n° 4-258/2021), Cancerfonden (agreement n° 4-511/2022), and The Strategical Research Foundation (SSF) Industrial Research Centre (agreement n° IRC15-0065).
Acknowledgments
We thank Dirk Michiel Pegtel (UMC Amsterdam, the Netherlands) and Antonin de Fougerolles (Evox Therapeutics Ltd, Oxford, UK) for invaluable input on the conception of this article.
Author contributions
J.R., S.E.-A., D.G., and A.Z. were all involved in drafting and writing the manuscript.
Declaration of interests
D.G. is a consultant and S.E.-A. is a co-founder and shareholder of Evox Therapeutics Ltd, Oxford, United Kingdom. Evox Therapeutics Ltd is a company developing engineered exosomes to deliver protein- and nucleic acid-based therapeutics.
References
- 1.van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/NRM.2017.125. [DOI] [PubMed] [Google Scholar]
- 2.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boudreau L.H., Duchez A.C., Cloutier N., Soulet D., Martin N., Bollinger J., Paré A., Rousseau M., Naika G.S., Lévesque T., et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124:2173–2183. doi: 10.1182/BLOOD-2014-05-573543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phinney D.G., di Giuseppe M., Njah J., Sala E., Shiva S., St Croix C.M., Stolz D.B., Watkins S.C., Di Y.P., Leikauf G.D., et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015;6:8472. doi: 10.1038/NCOMMS9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison T.J., Jackson M.v., Cunningham E.K., Kissenpfennig A., McAuley D.F., O’Kane C.M., Krasnodembskaya A.D. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. Care Med. 2017;196:1275–1286. doi: 10.1164/RCCM.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peruzzotti-Jametti L., Bernstock J.D., Willis C.M., Manferrari G., Rogall R., Fernandez-Vizarra E., Williamson J.C., Braga A., van den Bosch A., Leonardi T., et al. Neural stem cells traffic functional mitochondria via extracellular vesicles. Plos Biol. 2021;19:e3001166. doi: 10.1371/JOURNAL.PBIO.3001166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yates A.G., Pink R.C., Erdbrügger U., Siljander P.R.M., Dellar E.R., Pantazi P., Akbar N., Cooke W.R., Vatish M., Dias-Neto E., et al. In sickness and in health: the functional role of extracellular vesicles in physiology and pathology in vivo. J. Extracell. Vesicles. 2022;11:e12151. doi: 10.1002/JEV2.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng L., Hill A.F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 2022;21(5):379–399. doi: 10.1038/s41573-022-00410-w. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann I.K., Wood M.J.A., Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 10.Meng W., He C., Hao Y., Wang L., Li L., Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27:585–598. doi: 10.1080/10717544.2020.1748758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pegtel D.M., Gould S.J. Exosomes. Annu. Rev. Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 12.Fordjour F.K., Guo C., Ai Y., Daaboul G.G., Gould S.J. A shared, stochastic pathway mediates exosome protein budding along plasma and endosome membranes. J. Biol. Chem. 2022;298:102394. doi: 10.1016/j.jbc.2022.102394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollet H., Conrard L., Cloos A.S., Tyteca D. Plasma membrane lipid domains as platforms for vesicle biogenesis and shedding? Biomolecules. 2018;8:94. doi: 10.3390/biom8030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rilla K. Diverse plasma membrane protrusions act as platforms for extracellular vesicle shedding. J. Extracell. Vesicles. 2021;10:e12148. doi: 10.1002/jev2.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocucci E., Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364–372. doi: 10.1016/J.TCB.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Li M., Liao L., Tian W. Extracellular vesicles derived from apoptotic cells: an essential link between death and regeneration. Front. Cell Dev. Biol. 2020;8:573511. doi: 10.3389/fcell.2020.573511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segawa K., Nagata S. An apoptotic “eat me” signal: phosphatidylserine exposure. Trends Cell Biol. 2015;25:639–650. doi: 10.1016/j.tcb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baietti M.F., Zhang Z., Mortier E., Melchior A., Degeest G., Geeraerts A., Ivarsson Y., Depoortere F., Coomans C., Vermeiren E., et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 20.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B., Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 21.Colombo M., Moita C., van Niel G., Kowal J., Vigneron J., Benaroch P., Manel N., Moita L.F., Théry C., Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013;126:5553–5565. doi: 10.1242/JCS.128868/263554/AM/ANALYSIS-OF-ESCRT-FUNCTIONS-IN-EXOSOME-BIOGENESIS. [DOI] [PubMed] [Google Scholar]
- 22.Tamai K., Tanaka N., Nakano T., Kakazu E., Kondo Y., Inoue J., Shiina M., Fukushima K., Hoshino T., Sano K., et al. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem. Biophys. Res. Commun. 2010;399:384–390. doi: 10.1016/j.bbrc.2010.07.083. [DOI] [PubMed] [Google Scholar]
- 23.Hoshino D., Kirkbride K.C., Costello K., Clark E.S., Sinha S., Grega-Larson N., Tyska M.J., Weaver A.M. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5:1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgar J.R., Eden E.R., Futter C.E. Hrs- and CD63-dependent competing mechanisms make different sized endosomal intraluminal vesicles. Traffic. 2014;15:197–211. doi: 10.1111/tra.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razi M., Futter C.E. Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol. Biol. Cell. 2006;17:3469–3483. doi: 10.1091/mbc.E05-11-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larios J., Mercier V., Roux A., Gruenberg J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J. Cell Biol. 2020;219:e201904113. doi: 10.1083/jcb.201904113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachse M., Strous G.J., Klumperman J. ATPase-deficient hVPS4 impairs formation of internal endosomal vesicles and stabilizes bilayered clathrin coats on endosomal vacuoles. J. Cell Sci. 2004;117:1699–1708. doi: 10.1242/jcs.00998. [DOI] [PubMed] [Google Scholar]
- 28.Chairoungdua A., Smith D.L., Pochard P., Hull M., Caplan M.J. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J. Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Niel G., Charrin S., Simoes S., Romao M., Rochin L., Saftig P., Marks M.S., Rubinstein E., Raposo G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev. Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurwitz S.N., Conlon M.M., Rider M.A., Brownstein N.C., Meckes D.G. Nanoparticle analysis sheds budding insights into genetic drivers of extracellular vesicle biogenesis. J. Extracell. Vesicles. 2016;5:31295. doi: 10.3402/jev.v5.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghossoub R., Lembo F., Rubio A., Gaillard C.B., Bouchet J., Vitale N., Slavík J., Machala M., Zimmermann P. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat. Commun. 2014;5:3477–3512. doi: 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- 32.Leidal A.M., Huang H.H., Marsh T., Solvik T., Zhang D., Ye J., Kai F., Goldsmith J., Liu J.Y., Huang Y.H., et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat. Cell Biol. 2020;22:187–199. doi: 10.1038/s41556-019-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei D., Zhan W., Gao Y., Huang L., Gong R., Wang W., Zhang R., Wu Y., Gao S., Kang T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021;31:157–177. doi: 10.1038/s41422-020-00409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo H., Chitiprolu M., Roncevic L., Javalet C., Hemming F.J., Trung M.T., Meng L., Latreille E., Tanese de Souza C., McCulloch D., et al. Atg5 disassociates the V1V0-ATPase to promote exosome production and tumor metastasis independent of canonical macroautophagy. Dev. Cell. 2017;43:716–730.e7. doi: 10.1016/j.devcel.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Murrow L., Malhotra R., Debnath J. ATG12-ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat. Cell Biol. 2015;17:300–310. doi: 10.1038/ncb3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinha S., Hoshino D., Hong N.H., Kirkbride K.C., Grega-Larson N.E., Seiki M., Tyska M.J., Weaver A.M. Cortactin promotes exosome secretion by controlling branched actin dynamics. J. Cell Biol. 2016;214:197–213. doi: 10.1083/jcb.201601025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savina A., Vidal M., Colombo M.I. The exosome pathway in K562 cells is regulated by Rab11. J. Cell Sci. 2002;115:2505–2515. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- 38.Hsu C., Morohashi Y., Yoshimura S.I., Manrique-Hoyos N., Jung S., Lauterbach M.A., Bakhti M., Grønborg M., Möbius W., Rhee J., et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 2010;189:223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostrowski M., Carmo N.B., Krumeich S., Fanget I., Raposo G., Savina A., Moita C.F., Schauer K., Hume A.N., Freitas R.P., et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 40.Peinado H., Alečković M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., García-Santos G., Ghajar C., et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bobrie A., Krumeich S., Reyal F., Recchi C., Moita L.F., Seabra M.C., Ostrowski M., Théry C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 42.Fader C.M., Sánchez D.G., Mestre M.B., Colombo M.I. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim. Biophys. Acta. 2009;1793:1901–1916. doi: 10.1016/j.bbamcr.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Gross J.C., Chaudhary V., Bartscherer K., Boutros M. Active Wnt proteins are secreted on exosomes. Nat. Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz-Martinez M., Navarro A., Marrades R.M., Viñolas N., Santasusagna S., Muñoz C., Ramírez J., Molins L., Monzo M. YKT6 expression, exosome release, and survival in non-small cell lung cancer. Oncotarget. 2016;7:51515–51524. doi: 10.18632/ONCOTARGET.9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei Y., Wang D., Jin F., Bian Z., Li L., Liang H., Li M., Shi L., Pan C., Zhu D., et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat. Commun. 2017;8(1):14041–14112. doi: 10.1038/ncomms14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verweij F.J., Bebelman M.P., Jimenez C.R., Garcia-Vallejo J.J., Janssen H., Neefjes J., Knol J.C., de Goeij-de Haas R., Piersma S.R., Baglio S.R., et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J. Cell Biol. 2018;217:1129–1142. doi: 10.1083/JCB.201703206/VIDEO-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hyenne V., Apaydin A., Rodriguez D., Spiegelhalter C., Hoff-Yoessle S., Diem M., Tak S., Lefebvre O., Schwab Y., Goetz J.G., Labouesse M. RAL-1 controls multivesicular body biogenesis and exosome secretion. J. Cell Biol. 2015;211:27–37. doi: 10.1083/jcb.201504136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghoroghi S., Mary B., Larnicol A., Asokan N., Klein A., Osmani N., Busnelli I., Delalande F., Paul N., Halary S., et al. Ral GTPases promote breast cancer metastasis by controlling biogenesis and organ targeting of exosomes. Elife. 2021;10:1615399-29. doi: 10.7554/ELIFE.61539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savina A., Fader C.M., Damiani M.T., Colombo M.I. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 50.Savina A., Furlán M., Vidal M., Colombo M.I. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 51.Muralidharan-Chari V., Clancy J., Plou C., Romao M., Chavrier P., Raposo G., D’Souza-Schorey C. ARF6-regulated shedding of tumor-cell derived plasma membrane microvesicles. Curr. Biol. 2009;19:1875–1885. doi: 10.1016/J.CUB.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li B., Antonyak M.A., Zhang J., Cerione R.A. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene. 2012;31:4740–4749. doi: 10.1038/ONC.2011.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlienger S., Campbell S., Claing A. ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast cancer cell invasion. Mol. Biol. Cell. 2014;25:17–29. doi: 10.1091/MBC.E13-06-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bianco F., Perrotta C., Novellino L., Francolini M., Riganti L., Menna E., Saglietti L., Schuchman E.H., Furlan R., Clementi E., et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009;28:1043–1054. doi: 10.1038/EMBOJ.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Awojoodu A.O., Keegan P.M., Lane A.R., Zhang Y., Lynch K.R., Platt M.O., Botchwey E.A. Acid sphingomyelinase is activated in sickle cell erythrocytes and contributes to inflammatory microparticle generation in SCD. Blood. 2014;124:1941–1950. doi: 10.1182/BLOOD-2014-01-543652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nabhan J.F., Hu R., Oh R.S., Cohen S.N., Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl. Acad. Sci. USA. 2012;109:4146–4151. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathieu M., Névo N., Jouve M., Valenzuela J.I., Maurin M., Verweij F.J., Palmulli R., Lankar D., Dingli F., Loew D., et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun. 2021;12:4389. doi: 10.1038/s41467-021-24384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rink J., Ghigo E., Kalaidzidis Y., Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 59.Poteryaev D., Datta S., Ackema K., Zerial M., Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141:497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 60.Galvez T., Gilleron J., Zerial M., O’Sullivan G.A. SnapShot: mammalian rab proteins in endocytic trafficking. Cell. 2012;151:234–234.e2. doi: 10.1016/j.cell.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 61.Hurley J.H. ESCRT complexes and the biogenesis of multivesicular bodies. Curr. Opin. Cell Biol. 2008;20:4–11. doi: 10.1016/J.CEB.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bilodeau P.S., Urbanowski J.L., Winistorfer S.C., Piper R.C. The Vps27p-Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell Biol. 2002;4:534–539. doi: 10.1038/ncb815. [DOI] [PubMed] [Google Scholar]
- 63.Raiborg C., Bache K.G., Gillooly D.J., Madshus I.H., Stang E., Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 2002;4:394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- 64.Odorizzi G., Babst M., Emr S.D. Fab1p Ptdlns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95:847–858. doi: 10.1016/S0092-8674(00)81707-9. [DOI] [PubMed] [Google Scholar]
- 65.Wurmser A.E., Emr S.D. Phosphoinositide signaling and turnover: PtdIns(3)P, a regulator of membrane traffic, is transported to the vacuole and degraded by a process that requires lumenal vacuolar hydrolase activities. EMBO J. 1998;17:4930–4942. doi: 10.1093/emboj/17.17.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wegner C.S., Schink K.O., Stenmark H., Brech A. Monitoring phosphatidylinositol 3-phosphate in multivesicular endosome biogenesis. Methods Enzymol. 2014;534:3–23. doi: 10.1016/B978-0-12-397926-1.00001-9. [DOI] [PubMed] [Google Scholar]
- 67.Bache K.G., Brech A., Mehlum A., Stenmark H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J. Cell Biol. 2003;162:435–442. doi: 10.1083/jcb.200302131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu Q., Hope L.W., Brasch M., Reinhard C., Cohen S.N. TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc. Natl. Acad. Sci. USA. 2003;100:7626–7631. doi: 10.1073/pnas.0932599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teo H., Gill D.J., Sun J., Perisic O., Veprintsev D.B., Vallis Y., Emr S.D., Williams R.L. ESCRT-I core and ESCRT-II GLUE domain structures reveal role for GLUE in linking to ESCRT-I and membranes. Cell. 2006;125:99–111. doi: 10.1016/j.cell.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 70.Gill D.J., Teo H., Sun J., Perisic O., Veprintsev D.B., Emr S.D., Williams R.L. Structural insight into the ESCRT-I/-II link and its role in MVB trafficking. EMBO J. 2007;26:600–612. doi: 10.1038/sj.emboj.7601501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vietri M., Radulovic M., Stenmark H. The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 2020;21:25–42. doi: 10.1038/s41580-019-0177-4. [DOI] [PubMed] [Google Scholar]
- 72.Teis D., Saksena S., Emr S.D. Ordered assembly of the ESCRT-III complex on endosomes is required to sequester cargo during MVB formation. Dev. Cell. 2008;15:578–589. doi: 10.1016/j.devcel.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 73.Wollert T., Wunder C., Lippincott-Schwartz J., Hurley J.H. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lata S., Schoehn G., Jain A., Pires R., Piehler J., Gottlinger H.G., Weissenhorn W. Helical structures of ESCRT-III are disassembled by VPS4. Science. 1979;321:1354–1357. doi: 10.1126/science.1161070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saksena S., Wahlman J., Teis D., Johnson A.E., Emr S.D. Functional reconstitution of ESCRT-III assembly and disassembly. Cell. 2009;136:97–109. doi: 10.1016/j.cell.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jackson C.E., Scruggs B.S., Schaffer J.E., Hanson P.I. Effects of inhibiting VPS4 support a general role for ESCRTs in extracellular vesicle biogenesis. Biophys. J. 2017;113:1342–1352. doi: 10.1016/j.bpj.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stuffers S., Sem Wegner C., Stenmark H., Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10:925–937. doi: 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- 78.Perez-Hernandez D., Gutiérrez-Vázquez C., Jorge I., López-Martín S., Ursa A., Sánchez-Madrid F., Vázquez J., Yáñez-Mó M. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J. Biol. Chem. 2013;288:11649–11661. doi: 10.1074/jbc.M112.445304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jankovičová J., Sečová P., Michalková K., Antalíková J. Tetraspanins, more than markers of extracellular vesicles in reproduction. Int. J. Mol. Sci. 2020;21:7568–7630. doi: 10.3390/ijms21207568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Umeda R., Satouh Y., Takemoto M., Nakada-Nakura Y., Liu K., Yokoyama T., Shirouzu M., Iwata S., Nomura N., Sato K., et al. Structural insights into tetraspanin CD9 function. Nat. Commun. 2020;11:1606. doi: 10.1038/s41467-020-15459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gruenberg J. Life in the lumen: the multivesicular endosome. Traffic. 2020;21:76–93. doi: 10.1111/tra.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsui T., Osaki F., Hiragi S., Sakamaki Y., Fukuda M. ALIX and ceramide differentially control polarized small extracellular vesicle release from epithelial cells. EMBO Rep. 2021;22:e51475. doi: 10.15252/embr.202051475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang Y., Li C.W., Chan L.C., Wei Y., Hsu J.M., Xia W., Cha J.H., Hou J., Hsu J.L., Sun L., Hung M.C. Exosomal PD-L1 harbors active defense function to suppress t cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28:862–864. doi: 10.1038/s41422-018-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Madeo M., Colbert P.L., Vermeer D.W., Lucido C.T., Cain J.T., Vichaya E.G., Grossberg A.J., Muirhead D., Rickel A.P., Hong Z., et al. Cancer exosomes induce tumor innervation. Nat. Commun. 2018;9:4284–4315. doi: 10.1038/s41467-018-06640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng Q., Li X., Wang Y., Dong M., Zhan F.H., Liu J. The ceramide pathway is involved in the survival, apoptosis and exosome functions of human multiple myeloma cells in vitro. Acta Pharmacol. Sin. 2018;39:561–568. doi: 10.1038/aps.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li R., Blanchette-Mackie E.J., Ladisch S. Induction of endocytic vesicles by exogenous C6-ceramide. J. Biol. Chem. 1999;274:21121–21127. doi: 10.1074/jbc.274.30.21121. [DOI] [PubMed] [Google Scholar]
- 87.Arya S.B., Chen S., Jordan-Javed F., Parent C.A. Ceramide-rich microdomains facilitate nuclear envelope budding for non-conventional exosome formation. Nat. Cell Biol. 2022;24:1019–1028. doi: 10.1038/s41556-022-00934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Niel G., Wubbolts R., ten Broeke T., Buschow S.I., Ossendorp F.A., Melief C.J., Raposo G., van Balkom B.W., Stoorvogel W. Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity. 2006;25:885–894. doi: 10.1016/j.immuni.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 89.Buschow S.I., Nolte-’t Hoen E.N.M., van Niel G., Pols M.S., ten Broeke T., Lauwen M., Ossendorp F., Melief C.J.M., Raposo G., Wubbolts R., et al. MHC II in dendritic cells is targeted to lysosomes or t cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10:1528–1542. doi: 10.1111/j.1600-0854.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 90.Villarroya-Beltri C., Baixauli F., Mittelbrunn M., Fernández-Delgado I., Torralba D., Moreno-Gonzalo O., Baldanta S., Enrich C., Guerra S., Sánchez-Madrid F. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat. Commun. 2016;7:13588. doi: 10.1038/ncomms13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghossoub R., Chéry M., Audebert S., Leblanc R., Egea-Jimenez A.L., Lembo F., Mammar S., le Dez F., Camoin L., Borg J.P., et al. Tetraspanin-6 negatively regulates exosome production. Proc. Natl. Acad. Sci. USA. 2020;117:5913–5922. doi: 10.1073/pnas.1922447117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fan W., Guo J., Gao B., Zhang W., Ling L., Xu T., Pan C., Li L., Chen S., Wang H., et al. Flotillin-mediated endocytosis and ALIX-syntenin-1-mediated exocytosis protect the cell membrane from damage caused by necroptosis. Sci. Signal. 2019;12:eaaw3423. doi: 10.1126/scisignal.aaw3423. [DOI] [PubMed] [Google Scholar]
- 93.Kobayashi T., Stang E., Fang K.S., de Moerloose P., Parton R.G., Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–197. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- 94.White I.J., Bailey L.M., Aghakhani M.R., Moss S.E., Futter C.E. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2006;25:1–12. doi: 10.1038/sj.emboj.7600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Noh S.H., Kim Y.J., Lee M.G. Autophagy-related pathways in vesicular unconventional protein secretion. Front. Cell Dev. Biol. 2022;10:892450. doi: 10.3389/FCELL.2022.892450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mittelbrunn M., Gutiérrez-Vázquez C., Villarroya-Beltri C., González S., Sánchez-Cabo F., González M.Á., Bernad A., Sánchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cabezas A., Bache K.G., Brech A., Stenmark H. Alix regulates cortical actin and the spatial distribution of endosomes. J. Cell Sci. 2005;118:2625–2635. doi: 10.1242/jcs.02382. [DOI] [PubMed] [Google Scholar]
- 98.Han J., Pluhackova K., Böckmann R.A. The multifaceted role of SNARE proteins in membrane fusion. Front. Physiol. 2017;8:5. doi: 10.3389/fphys.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Proux-Gillardeaux V., Raposo G., Irinopoulou T., Galli T. Expression of the Longin domain of TI-VAMP impairs lysosomal secretion and epithelial cell migration. Biol. Cell. 2007;99:261–271. doi: 10.1042/bc20060097. [DOI] [PubMed] [Google Scholar]
- 100.Cashikar A.G., Hanson P.I. A cell-based assay for CD63-containing extracellular vesicles. PLoS One. 2019;14:e0220007. doi: 10.1371/JOURNAL.PONE.0220007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bebelman M.P., Bun P., Huveneers S., van Niel G., Pegtel D.M., Verweij F.J. Real-time imaging of multivesicular body-plasma membrane fusion to quantify exosome release from single cells. Nat. Protoc. 2020;15:102–121. doi: 10.1038/S41596-019-0245-4. [DOI] [PubMed] [Google Scholar]
- 102.Verweij F.J., Bebelman M.P., George A.E., Couty M., Bécot A., Palmulli R., Heiligenstein X., Sirés-Campos J., Raposo G., Pegtel D.M., van Niel G. ER membrane contact sites support endosomal small GTPase conversion for exosome secretion. J. Cell Biol. 2022;221:e202112032. doi: 10.1083/JCB.202112032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Antonyak M.A., Wilson K.F., Cerione R.A. R(h)oads to microvesicles. Small GTPases. 2012;3:219–224. doi: 10.4161/SGTP.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bianco F., Pravettoni E., Colombo A., Schenk U., Möller T., Matteoli M., Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1β release from microglia. J. Immunol. 2005;174:7268–7277. doi: 10.4049/JIMMUNOL.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 105.Peña-Altamira L.E., Polazzi E., Giuliani P., Beraudi A., Massenzio F., Mengoni I., Poli A., Zuccarini M., Ciccarelli R., di Iorio P., et al. Release of soluble and vesicular purine nucleoside phosphorylase from rat astrocytes and microglia induced by pro-inflammatory stimulation with extracellular ATP via P2X7 receptors. Neurochem. Int. 2018;115:37–49. doi: 10.1016/J.NEUINT.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 106.Teng F., Fussenegger M. Shedding light on extracellular vesicle biogenesis and bioengineering. Adv. Sci. 2020;8:2003505. doi: 10.1002/advs.202003505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.EL-Andaloussi S., Mäger I., Breakefield X.O., Wood M.J.A. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 108.Wiklander O.P.B., Brennan M.Á., Lötvall J., Breakefield X.O., El Andaloussi S., Lotvall J., Breakefield X.O., el Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019;11:eaav8521. doi: 10.1126/scitranslmed.aav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fu S., Wang Y., Xia X., Zheng J.C. Exosome engineering: current progress in cargo loading and targeted delivery. NanoImpact. 2020;20:100261. doi: 10.1016/j.impact.2020.100261. [DOI] [Google Scholar]
- 110.Armstrong J.P.K., Holme M.N., Stevens M.M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11:69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hurwitz S.N., Rider M.A., Bundy J.L., Liu X., Singh R.K., Meckes D.G., Jr. Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget. 2016;7:86999–87015. doi: 10.18632/oncotarget.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kugeratski F.G., Hodge K., Lilla S., McAndrews K.M., Zhou X., Hwang R.F., Zanivan S., Kalluri R. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat. Cell Biol. 2021;23:631–641. doi: 10.1038/s41556-021-00693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hoshino A., Kim H.S., Bojmar L., Gyan K.E., Cioffi M., Hernandez J., Zambirinis C.P., Rodrigues G., Molina H., Heissel S., et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182:1044–1061.e18. doi: 10.1016/j.cell.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Andreu Z., Yáñez-Mó M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014;5:442. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Escola J.M., Kleijmeer M.J., Stoorvogel W., Griffith J.M., Yoshie O., Geuze H.J. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 116.Rieu S., Géminard C., Rabesandratana H., Sainte-Marie J., Vidal M. Exosomes released during reticulocyte maturation bind to fibronectin via integrin α4β1. Eur. J. Biochem. 2000;267:583–590. doi: 10.1046/j.1432-1327.2000.01036.x. [DOI] [PubMed] [Google Scholar]
- 117.Segura E., Nicco C., Lombard B., Véron P., Raposo G., Batteux F., Amigorena S., Théry C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 118.Liang Y., Eng W.S., Colquhoun D.R., Dinglasan R.R., Graham D.R., Mahal L.K. Complex N-linked glycans serve as a determinant for exosome/microvesicle cargo recruitment. J. Biol. Chem. 2014;289:32526–32537. doi: 10.1074/jbc.M114.606269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Raposo G., Nijman H.W., Stoorvogel W., Liejendekker R., Harding C.v., Melief C.J., Geuze H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Petersen S.H., Odintsova E., Haigh T.A., Rickinson A.B., Taylor G.S., Berditchevski F. The role of tetraspanin CD63 in antigen presentation via MHC class II. Eur. J. Immunol. 2011;41:2556–2561. doi: 10.1002/eji.201141438. [DOI] [PubMed] [Google Scholar]
- 121.Corso G., Heusermann W., Trojer D., Görgens A., Steib E., Voshol J., Graff A., Genoud C., Lee Y., Hean J., et al. Systematic characterization of extracellular vesicle sorting domains and quantification at the single molecule – single vesicle level by fluorescence correlation spectroscopy and single particle imaging. J. Extracell. Vesicles. 2019;8:1663043. doi: 10.1080/20013078.2019.1663043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jeppesen D.K., Fenix A.M., Franklin J.L., Higginbotham J.N., Zhang Q., Zimmerman L.J., Liebler D.C., Ping J., Liu Q., Evans R., et al. Reassessment of exosome composition. Cell. 2019;177:428–445.e18. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Phuyal S., Hessvik N.P., Skotland T., Sandvig K., Llorente A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. 2014;281:2214–2227. doi: 10.1111/febs.12775. [DOI] [PubMed] [Google Scholar]
- 124.Arnold P., Lückstädt W., Li W., Boll I., Lokau J., Garbers C., Lucius R., Rose-John S., Becker-Pauly C. Joint reconstituted signaling of the IL-6 receptor via extracellular vesicles. Cells. 2020;9:1307. doi: 10.3390/cells9051307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Adamczyk K.A., Klein-Scory S., Tehrani M.M., Warnken U., Schmiegel W., Schnölzer M., Schwarte-Waldhoff I., Schnölzer M., Schwarte-Waldhoff I. Characterization of soluble and exosomal forms of the EGFR released from pancreatic cancer cells. Life Sci. 2011;89:304–312. doi: 10.1016/j.lfs.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 126.Blanchard N., Lankar D., Faure F., Regnault A., Dumont C., Raposo G., Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/ζ complex. J. Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 127.Fu W., Lei C., Liu S., Cui Y., Wang C., Qian K., Li T., Shen Y., Fan X., Lin F., et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat. Commun. 2019;10:4355. doi: 10.1038/s41467-019-12321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sheldon H., Heikamp E., Turley H., Dragovic R., Thomas P., Oon C.E., Leek R., Edelmann M., Kessler B., Sainson R.C.A., et al. New mechanism for Notch signaling to endothelium at a distance by delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–2394. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- 129.Li M., Lu Y., Xu Y., Wang J., Zhang C., Du Y., Wang L., Li L., Wang B., Shen J., et al. Horizontal transfer of exosomal CXCR4 promotes murine hepatocarcinoma cell migration, invasion and lymphangiogenesis. Gene. 2018;676:101–109. doi: 10.1016/j.gene.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 130.Nager A.R., Goldstein J.S., Herranz-Pérez V., Portran D., Ye F., Garcia-Verdugo J.M., Nachury M.V. An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling. Cell. 2017;168:252–263.e14. doi: 10.1016/j.cell.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen G., Huang A.C., Zhang W., Zhang G., Wu M., Xu W., Yu Z., Yang J., Wang B., Sun H., et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shelke G.V., Yin Y., Jang S.C., Lässer C., Wennmalm S., Hoffmann H.J., Li L., Gho Y.S., Nilsson J.A., Lötvall J. Endosomal signalling via exosome surface TGFβ-1. J. Extracell. Vesicles. 2019;8:1650458. doi: 10.1080/20013078.2019.1650458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Keller M.D., Ching K.L., Liang F.X., Dhabaria A., Tam K., Ueberheide B.M., Unutmaz D., Torres V.J., Cadwell K. Decoy exosomes provide protection against bacterial toxins. Nature. 2020;579:260–264. doi: 10.1038/s41586-020-2066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rabesandratana H., Toutant J.P., Reggio H., Vidal M. Decay-accelerating factor (CD55) and membrane inhibitor of reactive lysis (CD59) are released within exosomes during in vitro maturation of reticulocytes. Blood. 1998;91:2573–2580. doi: 10.1182/blood.v91.7.2573. [DOI] [PubMed] [Google Scholar]
- 135.Diamandis E.P., Plebani M. Glypican-1 as a highly sensitive and specific pancreatic cancer biomarker. Clin. Chem. Lab. Med. 2016;54:e1–e2. doi: 10.1515/cclm-2015-0773. [DOI] [PubMed] [Google Scholar]
- 136.Demory Beckler M., Higginbotham J.N., Franklin J.L., Ham A.J., Halvey P.J., Imasuen I.E., Whitwell C., Li M., Liebler D.C., Coffey R.J. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol. Cell. Proteomics. 2013;12:343–355. doi: 10.1074/mcp.M112.022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dooley K., McConnell R.E., Xu K., Lewis N.D., Haupt S., Youniss M.R., Martin S., Sia C.L., McCoy C., Moniz R.J., et al. A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol. Ther. 2021;29:1729–1743. doi: 10.1016/j.ymthe.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fang Y., Wu N., Gan X., Yan W., Morrell J.C., Gould S.J. Higher-order oligomerization targets plasma membrane proteins and HIV Gag to exosomes. Walter P. PLoS Biol. 2007;5:e158–e1283. doi: 10.1371/journal.pbio.0050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kunadt M., Eckermann K., Stuendl A., Gong J., Russo B., Strauss K., Rai S., Kügler S., Falomir Lockhart L., Schwalbe M., et al. Extracellular vesicle sorting of α-Synuclein is regulated by sumoylation. Acta Neuropathol. 2015;129:695–713. doi: 10.1007/s00401-015-1408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Villarroya-Beltri C., Gutiérrez-Vázquez C., Sánchez-Cabo F., Pérez-Hernández D., Vázquez J., Martin-Cofreces N., Martinez-Herrera D.J., Pascual-Montano A., Mittelbrunn M., Sánchez-Madrid F., et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen I.H., Xue L., Hsu C.C., Paez J.S.P., Pan L., Andaluz H., Wendt M.K., Iliuk A.B., Zhu J.K., Tao W.A. Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proc. Natl. Acad. Sci. USA. 2017;114:3175–3180. doi: 10.1073/pnas.1618088114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Merendino A.M., Bucchieri F., Campanella C., Marcianò V., Ribbene A., David S., Zummo G., Burgio G., Corona D.F.V., Conway de Macario E., et al. Hsp60 is actively secreted by human tumor cells. PLoS One. 2010;5:e9247. doi: 10.1371/journal.pone.0009247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang X., Gu H., Huang W., Peng J., Li Y., Yang L., Qin D., Essandoh K., Wang Y., Peng T., Fan G.C. Hsp20-mediated activation of exosome biogenesis in cardiomyocytes improves cardiac function and angiogenesis in diabetic mice. Diabetes. 2016;65:3111–3128. doi: 10.2337/db15-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Takeuchi T., Suzuki M., Fujikake N., Popiel H.A., Kikuchi H., Futaki S., Wada K., Nagai Y. Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc. Natl. Acad. Sci. USA. 2015;112:E2497–E2506. doi: 10.1073/pnas.1412651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 146.Skog J., Würdinger T., van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M., Curry W.T., Jr., Carter B.S., Krichevsky A.M., Breakefield X.O., et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Deregibus M.C., Cantaluppi V., Calogero R., lo Iacono M., Tetta C., Biancone L., Bruno S., Bussolati B., Camussi G. Endothelial progenitor cell-derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. J. Am. Soc. Hematol. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 148.Stoorvogel W. Functional transfer of microRNA by exosomes. Blood. 2012;119:646–648. doi: 10.1182/blood-2011-11-389478. [DOI] [PubMed] [Google Scholar]
- 149.Ratajczak J., Miekus K., Kucia M., Zhang J., Reca R., Dvorak P., Ratajczak M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 150.Pegtel D.M., Cosmopoulos K., Thorley-Lawson D.A., van Eijndhoven M.A.J., Hopmans E.S., Lindenberg J.L., de Gruijl T.D., Würdinger T., Middeldorp J.M. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. USA. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wei Z., Batagov A.O., Schinelli S., Wang J., Wang Y., el Fatimy R., Rabinovsky R., Balaj L., Chen C.C., Hochberg F., et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat. Commun. 2017;8:1145. doi: 10.1038/s41467-017-01196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van Balkom B.W.M., Eisele A.S., Pegtel D.M., Bervoets S., Verhaar M.C. Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting. J. Extracell. Vesicles. 2015;4:26760–26814. doi: 10.3402/JEV.V4.26760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Montecalvo A., Larregina A.T., Shufesky W.J., Stolz D.B., Sullivan M.L.G., Karlsson J.M., Baty C.J., Gibson G.A., Erdos G., Wang Z., et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Thomou T., Mori M.A., Dreyfuss J.M., Konishi M., Sakaguchi M., Wolfrum C., Rao T.N., Winnay J.N., Garcia-Martin R., Grinspoon S.K., et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450–455. doi: 10.1038/NATURE21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yun B., Kim Y., Park D.J., Oh S. Comparative analysis of dietary exosome-derived microRNAs from human, bovine and caprine colostrum and mature milk. J. Anim. Sci. Technol. 2021;63:593–602. doi: 10.5187/jast.2021.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Michael A., Bajracharya S.D., Yuen P.S.T., Zhou H., Star R.A., Illei G.G., Alevizos I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34–38. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sork H., Corso G., Krjutskov K., Johansson H.J., Nordin J.Z., Wiklander O.P.B., Lee Y.X.F., Westholm J.O., Lehtiö J., Wood M.J.A., et al. Heterogeneity and interplay of the extracellular vesicle small RNA transcriptome and proteome. Sci. Rep. 2018;8:10813. doi: 10.1038/s41598-018-28485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jiang L., Vader P., Schiffelers R.M. Extracellular vesicles for nucleic acid delivery: progress and prospects for safe RNA-based gene therapy. Gene Ther. 2017;24:157–166. doi: 10.1038/gt.2017.8. [DOI] [PubMed] [Google Scholar]
- 159.Cambier L., de Couto G., Ibrahim A., Echavez A.K., Valle J., Liu W., Kreke M., Smith R.R., Marbán L., Marbán E. RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL -10 expression and secretion. EMBO Mol. Med. 2017;9:337–352. doi: 10.15252/emmm.201606924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Batagov A.O., Kurochkin I.V. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3′-untranslated regions. Biol. Direct. 2013;8:12. doi: 10.1186/1745-6150-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Akers J.C., Ramakrishnan V., Kim R., Phillips S., Kaimal V., Mao Y., Hua W., Yang I., Fu C.C., Nolan J., et al. miRNA contents of cerebrospinal fluid extracellular vesicles in glioblastoma patients. J. Neurooncol. 2015;123:205–216. doi: 10.1007/s11060-015-1784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim K.M., Abdelmohsen K., Mustapic M., Kapogiannis D., Gorospe M. RNA in extracellular vesicles. Wires. RNA. 2017;8:1413. doi: 10.1002/wrna.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Léveillé N., Baglio S.R. Exosome-transferred lncRNAs at the core of cancer bone lesions. Crit. Rev. Oncol. Hematol. 2019;139:125–127. doi: 10.1016/J.CRITREVONC.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 164.Das S., Extracellular RNA Communication Consortium. Ansel K.M., Bitzer M., Breakefield X.O., Charest A., Galas D.J., Gerstein M.B., Gupta M., Milosavljevic A., et al. The extracellular RNA communication consortium: establishing foundational knowledge and technologies for extracellular RNA research. Cell. 2019;177:231–242. doi: 10.1016/J.CELL.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.O’Brien K., Breyne K., Ughetto S., Laurent L.C., Breakefield X.O., O’Brien K., Breyne K., Ughetto S., Laurent L.C., Breakefield X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020;21:585–606. doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fabbiano F., Corsi J., Gurrieri E., Trevisan C., Notarangelo M., D’Agostino V.G. RNA packaging into extracellular vesicles: an orchestra of RNA-binding proteins? J. Extracell. Vesicles. 2020;10:e12043. doi: 10.1002/jev2.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brinkman K., Meyer L., Bickel A., Enderle D., Berking C., Skog J., Noerholm M. Extracellular vesicles from plasma have higher tumour RNA fraction than platelets. J. Extracell. Vesicles. 2020;9:1741176. doi: 10.1080/20013078.2020.1741176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Guduric-Fuchs J., O’connor A., Camp B., O’neill C.L., Medina R.J., Simpson D.A. Selective extracellular vesicle-mediated export of an overlapping set of MicroRNAs from multiple cell types. BMC genomics. 2012;13:1–14. doi: 10.1186/1471-2164-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Baglio S.R., Rooijers K., Koppers-Lalic D., Verweij F.J., Pérez Lanzón M., Zini N., Naaijkens B., Perut F., Niessen H.W.M., Baldini N., Pegtel D.M. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015;6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bolukbasi M.F., Mizrak A., Ozdener G.B., Madlener S., Ströbel T., Erkan E.P., Fan J.B., Breakefield X.O., Saydam O. MiR-1289 and “zipcode”-like sequence enrich mRNAs in microvesicles. Mol. Ther. Nucleic Acids. 2012;1:e10. doi: 10.1038/mtna.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Garcia-Martin R., Wang G., Brandão B.B., Zanotto T.M., Shah S., Kumar Patel S., Schilling B., Kahn C.R. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601:446–451. doi: 10.1038/s41586-021-04234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ahadi A., Brennan S., Kennedy P.J., Hutvagner G., Tran N. Long non-coding RNAs harboring miRNA seed regions are enriched in prostate cancer exosomes. Sci. Rep. 2016;6:24922. doi: 10.1038/srep24922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schwarzl T. EMBL RBPbase; 2016. RBPbase v0.2.0 Alpha.https://rbpbase.shiny.embl.de/ [Google Scholar]
- 174.Yanshina D.D., Kossinova O.A., Gopanenko A.V., Krasheninina O.A., Malygin A.A., Venyaminova A.G., Karpova G.G. Structural features of the interaction of the 3’-untranslated region of mRNA containing exosomal RNA-specific motifs with YB-1, a potential mediator of mRNA sorting. Biochimie. 2018;144:134–143. doi: 10.1016/j.biochi.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 175.Shurtleff M.J., Temoche-Diaz M.M., Karfilis K.v., Ri S., Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016;5:e19276. doi: 10.7554/eLife.19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shurtleff M.J., Yao J., Qin Y., Nottingham R.M., Temoche-Diaz M.M., Schekman R., Lambowitz A.M. Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc. Natl. Acad. Sci. USA. 2017;114:E8987–E8995. doi: 10.1073/pnas.1712108114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kossinova O.A., Gopanenko A.v., Tamkovich S.N., Krasheninina O.A., Tupikin A.E., Kiseleva E., Yanshina D.D., Malygin A.A., Ven’yaminova A.G., Kabilov M.R., Karpova G.G. Cytosolic YB-1 and NSUN2 are the only proteins recognizing specific motifs present in mRNAs enriched in exosomes. Biochim. Biophys. Acta Proteins Proteom. 2017;1865:664–673. doi: 10.1016/j.bbapap.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 178.Hobor F., Dallmann A., Ball N.J., Cicchini C., Battistelli C., Ogrodowicz R.W., Christodoulou E., Martin S.R., Castello A., Tripodi M., et al. A cryptic RNA-binding domain mediates Syncrip recognition and exosomal partitioning of miRNA targets. Nat. Commun. 2018;9:831. doi: 10.1038/s41467-018-03182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Santangelo L., Giurato G., Cicchini C., Montaldo C., Mancone C., Tarallo R., Battistelli C., Alonzi T., Weisz A., Tripodi M. The RNA-binding protein SYNCRIP is a component of the hepatocyte exosomal machinery controlling MicroRNA sorting. Cell Rep. 2016;17:799–808. doi: 10.1016/j.celrep.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 180.Ashley J., Cordy B., Lucia D., Fradkin L.G., Budnik V., Thomson T. Retrovirus-like gag protein Arc1 binds RNA and traffics across synaptic boutons. Cell. 2018;172:262–274.e11. doi: 10.1016/j.cell.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McKenzie A.J., Hoshino D., Hong N.H., Cha D.J., Franklin J.L., Coffey R.J., Patton J.G., Weaver A.M. KRAS-MEK signaling controls Ago2 sorting into exosomes. Cell Rep. 2016;15:978–987. doi: 10.1016/j.celrep.2016.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Weaver A.M., Patton J.G. Argonautes in extracellular vesicles: artifact or selected cargo? Cancer Res. 2020;80:379–381. doi: 10.1158/0008-5472.CAN-19-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Iavello A., Frech V.S.L., Gai C., Deregibus M.C., Quesenberry P.J., Camussi G. Role of Alix in miRNA packaging during extracellular vesicle biogenesis. Int. J. Mol. Med. 2016;37:958–966. doi: 10.3892/ijmm.2016.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Teng Y., Ren Y., Hu X., Mu J., Samykutty A., Zhuang X., Deng Z., Kumar A., Zhang L., Merchant M.L., et al. MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nat. Commun. 2017;8:14448. doi: 10.1038/ncomms14448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Statello L., Maugeri M., Garre E., Nawaz M., Wahlgren J., Papadimitriou A., Lundqvist C., Lindfors L., Collén A., Sunnerhagen P., et al. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PLoS One. 2018;13:e0195969. doi: 10.1371/JOURNAL.PONE.0195969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zietzer A., Hosen M.R., Wang H., Goody P.R., Sylvester M., Latz E., Nickenig G., Werner N., Jansen F. The RNA-binding protein hnRNPU regulates the sorting of microRNA-30c-5p into large extracellular vesicles. J. Extracell. Vesicles. 2020;9:1786967. doi: 10.1080/20013078.2020.1786967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hagiwara K., Katsuda T., Gailhouste L., Kosaka N., Ochiya T. Commitment of Annexin A2 in recruitment of microRNAs into extracellular vesicles. FEBS Lett. 2015;589(24 Pt B):4071–4078. doi: 10.1016/j.febslet.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 188.Otake K., Kamiguchi H., Hirozane Y. Identification of biomarkers for amyotrophic lateral sclerosis by comprehensive analysis of exosomal mRNAs in human cerebrospinal fluid. BMC Med. Genomics. 2019;12:7. doi: 10.1186/s12920-019-0473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Baglio S.R., van Eijndhoven M.A.J., Koppers-Lalic D., Berenguer J., Lougheed S.M., Gibbs S., Léveillé N., Rinkel R.N.P.M., Hopmans E.S., Swaminathan S., et al. Sensing of latent EBV infection through exosomal transfer of 5’pppRNA. Proc. Natl. Acad. Sci. USA. 2016;113:E587–E596. doi: 10.1073/pnas.1518130113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kosaka N., Iguchi H., Yoshioka Y., Takeshita F., Matsuki Y., Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Janas T., Janas M.M., Sapoń K., Janas T. Mechanisms of RNA loading into exosomes. FEBS Lett. 2015;589:1391–1398. doi: 10.1016/j.febslet.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 192.Khvorova A., Kwak Y.G., Tamkun M., Majerfeld I., Yarus M. RNAs that bind and change the permeability of phospholipid membranes. Proc. Natl. Acad. Sci. USA. 1999;96:10649–10654. doi: 10.1073/pnas.96.19.10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Barman B., Sung B.H., Krystofiak E., Ping J., Ramirez M., Millis B., Allen R., Prasad N., Chetyrkin S., Calcutt M.W., et al. VAP-A and its binding partner CERT drive biogenesis of RNA-containing extracellular vesicles at ER membrane contact sites. Dev. Cell. 2022;57:974–994.e8. doi: 10.1016/J.DEVCEL.2022.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Albanese M., Chen Y.F.A., Hüls C., Gärtner K., Tagawa T., Mejias-Perez E., Keppler O.T., Göbel C., Zeidler R., Shein M., et al. MicroRNAs are minor constituents of extracellular vesicles that are rarely delivered to target cells. Plos Genet. 2021;17:e1009951. doi: 10.1371/JOURNAL.PGEN.1009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nordin J.Z. Transfection reagents affect Extracellular Vesicle cargo transfer to recipient cells: the importance of appropriate controls in EV research. J. Extracell. Vesicles. 2022;11:e12227. doi: 10.1002/jev2.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McConnell R.E., Youniss M., Gnanasambandam B., Shah P., Zhang W., Finn J.D. Transfection reagent artefact likely accounts for some reports of extracellular vesicle function. J. Extracell. Vesicles. 2022;11:e12253. doi: 10.1002/jev2.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.McCann J., Sosa-Miranda C.D., Guo H., Reshke R., Savard A., Zardini Buzatto A., Taylor J.A., Li L., Gibbings D.J. Contaminating transfection complexes can masquerade as small extracellular vesicles and impair their delivery of RNA. J. Extracell. Vesicles. 2022;11:e12220. doi: 10.1002/jev2.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Thakur B.K., Zhang H., Becker A., Matei I., Huang Y., Costa-Silva B., Zheng Y., Hoshino A., Brazier H., Xiang J., et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.García-Romero N., Carrión-Navarro J., Esteban-Rubio S., Lázaro-Ibáñez E., Peris-Celda M., Alonso M.M., Guzmán-De-Villoria J., Fernández-Carballal C., de Mendivil A.O., García-Duque S., et al. DNA sequences within glioma-derived extracellular vesicles can cross the intact blood-brain barrier and be detected in peripheral blood of patients. Oncotarget. 2017;8:1416–1428. doi: 10.18632/oncotarget.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kahlert C., Melo S.A., Protopopov A., Tang J., Seth S., Koch M., Zhang J., Weitz J., Chin L., Futreal A., Kalluri R. Identification of doublestranded genomic dna spanning all chromosomes with mutated KRAS and P53 DNA in the serum exosomes of patients with pancreatic cancer. J. Biol. Chem. 2014;289:3869–3875. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fernando M.R., Jiang C., Krzyzanowski G.D., Ryan W.L. New evidence that a large proportion of human blood plasma cell-free DNA is localized in exosomes. PLoS One. 2017;12:e0183915. doi: 10.1371/journal.pone.0183915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Takahashi A., Okada R., Nagao K., Kawamata Y., Hanyu A., Yoshimoto S., Takasugi M., Watanabe S., Kanemaki M.T., Obuse C., Hara E. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017;8:15287. doi: 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Balaj L., Lessard R., Dai L., Cho Y.J., Pomeroy S.L., Breakefield X.O., Skog J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011;2:180. doi: 10.1038/NCOMMS1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Guescini M., Genedani S., Stocchi V., Agnati L.F. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm. 2010;117:1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 205.Cai J., Wu G., Jose P.A., Zeng C. Functional transferred DNA within extracellular vesicles. Exp. Cell Res. 2016;349:179–183. doi: 10.1016/J.YEXCR.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 206.Kouwaki T., Fukushima Y., Daito T., Sanada T., Yamamoto N., Mifsud E.J., Leong C.R., Tsukiyama-Kohara K., Kohara M., Matsumoto M., et al. Extracellular vesicles including exosomes regulate innate immune responses to hepatitis B virus infection. Front. Immunol. 2016;7:335. doi: 10.3389/FIMMU.2016.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hagey D.W., Kordes M., Görgens A., Mowoe M.O., Nordin J.Z., Moro C.F., Löhr J.M., El Andaloussi S. Löhr JMM, Andaloussi S el, Kordes con- M, Moro CF, et al. Extracellular vesicles are the primary source of blood-borne tumour-derived mutant KRAS DNA early in pancreatic cancer. J. Extracell. Vesicles. 2021;10:e12142. doi: 10.1002/JEV2.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Costa-Silva B., Aiello N.M., Ocean A.J., Singh S., Zhang H., Thakur B.K., Becker A., Hoshino A., Mark M.T., Molina H., et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Vagner T., Spinelli C., Minciacchi V.R., Balaj L., Zandian M., Conley A., Zijlstra A., Freeman M.R., Demichelis F., De S., et al. Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma. J. Extracell. Vesicles. 2018;7:1505403. doi: 10.1080/20013078.2018.1505403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Malkin E.Z., Bratman S.V. Bioactive DNA from extracellular vesicles and particles. Cell Death Dis. 2020;11:584–613. doi: 10.1038/s41419-020-02803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yaşa B., Şahin O., Öcüt E., Seven M., Sözer S. Assessment of fetal rhesus D and gender with cell-free DNA and exosomes from maternal blood. Reprod. Sci. 2021;28:562–569. doi: 10.1007/S43032-020-00321-4. [DOI] [PubMed] [Google Scholar]
- 212.Lázaro-Ibáñez E., Lässer C., Shelke G.V., Crescitelli R., Jang S.C., Cvjetkovic A., García-Rodríguez A., Lötvall J. DNA analysis of low- and high-density fractions defines heterogeneous subpopulations of small extracellular vesicles based on their DNA cargo and topology. J. Extracell Vesicles. 2019;8:1656993. doi: 10.1080/20013078.2019.1656993/SUPPL_FILE/ZJEV_A_1656993_SM9781.ZIP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Silva A.M., Lázaro-Ibáñez E., Gunnarsson A., Dhande A., Daaboul G., Peacock B., Osteikoetxea X., Salmond N., Friis K.P., Shatnyeva O., Dekker N. Quantification of protein cargo loading into engineered extracellular vesicles at single-vesicle and single-molecule resolution. J. Extracell. Vesicles. 2021;10:e12130. doi: 10.1002/JEV2.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang Q., Yu J., Kadungure T., Beyene J., Zhang H., Lu Q. ARMMs as a versatile platform for intracellular delivery of macromolecules. Nat. Commun. 2018;9:960. doi: 10.1038/s41467-018-03390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Manfredi F., di Bonito P., Arenaccio C., Anticoli S., Federico M. Incorporation of heterologous proteins in engineered exosomes. Methods Mol. Biol. 2016;1448:249–260. doi: 10.1007/978-1-4939-3753-0_18. [DOI] [PubMed] [Google Scholar]
- 216.Yim N., Ryu S.W., Choi K., Lee K.R., Lee S., Choi H., Kim J., Shaker M.R., Sun W., Park J.H., et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat. Commun. 2016;7:12277. doi: 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gee P., Lung M.S.Y., Okuzaki Y., Sasakawa N., Iguchi T., Makita Y., Hozumi H., Miura Y., Yang L.F., Iwasaki M., et al. Extracellular nanovesicles for packaging of CRISPR-Cas9 protein and sgRNA to induce therapeutic exon skipping. Nat. Commun. 2020;11:1334. doi: 10.1038/s41467-020-14957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ferreira J.V., da Rosa Soares A., Ramalho J., Carvalho C.M., Cardoso M.H., Pintado P., Carvalho A.S., Beck H.C., Matthiesen R., Zuzarte M., et al. LAMP2A regulates the loading of proteins into exosomes. Sci. Adv. 2022;8:1140. doi: 10.1126/SCIADV.ABM1140/SUPPL_FILE/SCIADV.ABM1140_TABLES_S1_TO_S3.ZIP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sterzenbach U., Putz U., Low L.H., Silke J., Tan S.S., Howitt J. Engineered exosomes as vehicles for biologically active proteins. Mol. Ther. 2017;25:1269–1278. doi: 10.1016/j.ymthe.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ye Y., Zhang X., Xie F., Xu B., Xie P., Yang T., Shi Q., Zhang C.Y., Zhang Y., Chen J., et al. An engineered exosome for delivering sgRNA:Cas9 ribonucleoprotein complex and genome editing in recipient cells. Biomater. Sci. 2020;8:2966–2976. doi: 10.1039/D0BM00427H. [DOI] [PubMed] [Google Scholar]
- 221.Choi H., Kim Y., Mirzaaghasi A., Heo J., Kim Y.N., Shin J.H., Kim S., Kim N.H., Cho E.S., In Yook J., et al. Exosome-based delivery of super-repressor IκBα relieves sepsis-associated organ damage and mortality. Sci. Adv. 2020;6:eaaz6980. doi: 10.1126/sciadv.aaz6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Do M.A., Levy D., Brown A., Marriott G., Lu B. Targeted delivery of lysosomal enzymes to the endocytic compartment in human cells using engineered extracellular vesicles. Sci. Rep. 2019;9:17274. doi: 10.1038/s41598-019-53844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., di Giannatale A., Ceder S., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.de Carvalho J.V., de Castro R.O., da Silva E.Z.M., Silveira P.P., da Silva-Januário M.E., Arruda E., Jamur M.C., Oliver C., Aguiar R.S., daSilva L.L.P. Nef neutralizes the ability of exosomes from CD4+ T cells to act as decoys during HIV-1 infection. PLoS One. 2014;9:e113691. doi: 10.1371/journal.pone.0113691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 226.Gupta D., Wiklander O.P.B., Görgens A., Conceição M., Corso G., Liang X., Seow Y., Balusu S., Feldin U., Bostancioglu B., et al. Amelioration of systemic inflammation via the display of two different decoy protein receptors on extracellular vesicles. Nat. Biomed. Eng. 2021;5:1084–1098. doi: 10.1038/s41551-021-00792-z. [DOI] [PubMed] [Google Scholar]
- 227.Liang X., Niu Z., Galli V., Howe N., Zhao Y., Wiklander O.P.B., Zheng W., Wiklander R.J., Corso G., Davies C., et al. Extracellular vesicles engineered to bind albumin demonstrate extended circulation time and lymph node accumulation in mouse models. J. Extracell. Vesicles. 2022;11:e12248. doi: 10.1002/jev2.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kim H.K., Cho J., Kim E., Kim J., Yang J.S., Kim K.C., Lee J.Y., Shin Y., Palomera L.F., Park J., et al. Engineered small extracellular vesicles displaying ACE2 variants on the surface protect against SARS-CoV-2 infection. J. Extracell. Vesicles. 2022;11:e12179. doi: 10.1002/JEV2.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Stipp C.S., Orlicky D., Hemler M.E. FPRP, a major, highly stoichiometric, highly specific CD81- and CD9-associated protein. J. Biol. Chem. 2001;276:4853–4862. doi: 10.1074/JBC.M009859200. [DOI] [PubMed] [Google Scholar]
- 230.Ohno S.I., Takanashi M., Sudo K., Ueda S., Ishikawa A., Matsuyama N., Fujita K., Mizutani T., Ohgi T., Ochiya T., et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013;21:185–191. doi: 10.1038/MT.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kooijmans S.A.A., Aleza C.G., Roffler S.R., van Solinge W.W., Vader P., Schiffelers R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles. 2016;5:31053. doi: 10.3402/jev.v5.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Vorselen D., van Dommelen S.M., Sorkin R., Piontek M.C., Schiller J., Döpp S.T., Kooijmans S.A.A., van Oirschot B.A., Versluijs B.A., Bierings M.B., et al. The fluid membrane determines mechanics of erythrocyte extracellular vesicles and is softened in hereditary spherocytosis. Nat. Commun. 2018;9:4960. doi: 10.1038/s41467-018-07445-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.El-Andaloussi S., Lee Y., Lakhal-Littleton S., Li J., Seow Y., Gardiner C., Alvarez-Erviti L., Sargent I.L., Wood M.J.A. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat. Protoc. 2012;7:2112–2126. doi: 10.1038/nprot.2012.131. [DOI] [PubMed] [Google Scholar]
- 234.Usman W.M., Pham T.C., Kwok Y.Y., Vu L.T., Ma V., Peng B., Chan Y.S., Wei L., Chin S.M., Azad A., et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018;9:2359–2415. doi: 10.1038/s41467-018-04791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pan Q., Ramakrishnaiah V., Henry S., Fouraschen S., de Ruiter P.E., Kwekkeboom J., Tilanus H.W., Janssen H.L.A., van der Laan L.J.W. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi) Gut. 2012;61:1330–1339. doi: 10.1136/GUTJNL-2011-300449. [DOI] [PubMed] [Google Scholar]
- 236.Kosaka N., Iguchi H., Yoshioka Y., Hagiwara K., Takeshita F., Ochiya T. Competitive interactions of cancer cells and normal cells via secretory microRNAs. J. Biol. Chem. 2012;287:1397–1405. doi: 10.1074/JBC.M111.288662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Murphy D.E., de Jong O.G., Evers M.J.W., Nurazizah M., Schiffelers R.M., Vader P. Natural or synthetic RNA delivery: a stoichiometric comparison of extracellular vesicles and synthetic nanoparticles. Nano Lett. 2021;21:1888–1895. doi: 10.1021/acs.nanolett.1c00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mizrak A., Bolukbasi M.F., Ozdener G.B., Brenner G.J., Madlener S., Erkan E.P., Ströbel T., Breakefield X.O., Saydam O. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol. Ther. 2013;21:101–108. doi: 10.1038/mt.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yang Z., Shi J., Xie J., Wang Y., Sun J., Liu T., Zhao Y., Zhao X., Wang X., Ma Y., et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2020;4:69–83. doi: 10.1038/s41551-019-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Reshke R., Taylor J.A., Savard A., Guo H., Rhym L.H., Kowalski P.S., Trung M.T., Campbell C., Little W., Anderson D.G., Gibbings D. Reduction of the therapeutic dose of silencing RNA by packaging it in extracellular vesicles via a pre-microRNA backbone. Nat. Biomed. Eng. 2020;4:52–68. doi: 10.1038/s41551-019-0502-4. [DOI] [PubMed] [Google Scholar]
- 241.Li X., Yu Q., Zhao R., Guo X., Liu C., Zhang K., Zhang W., Liu J., Yu J., Wang S., et al. Designer exosomes for targeted delivery of a novel therapeutic cargo to enhance sorafenib-mediated ferroptosis in hepatocellular carcinoma. Front. Oncol. 2022;12:2900. doi: 10.3389/FONC.2022.898156/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Li Z., Zhou X., Wei M., Gao X., Zhao L., Shi R., Sun W., Duan Y., Yang G., Yuan L. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019;19:19–28. doi: 10.1021/ACS.NANOLETT.8B02689. [DOI] [PubMed] [Google Scholar]
- 243.Hung M.E., Leonard J.N. A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery. J. Extracell. Vesicles. 2016;5:31027. doi: 10.3402/jev.v5.31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kojima R., Bojar D., Rizzi G., Hamri G.C.E., El-Baba M.D., Saxena P., Ausländer S., Tan K.R., Fussenegger M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018;9:1305. doi: 10.1038/s41467-018-03733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zickler A.M., el Andaloussi S. Functional extracellular vesicles aplenty. Nat. Biomed. Eng. 2020;4:9–11. doi: 10.1038/s41551-019-0507-z. [DOI] [PubMed] [Google Scholar]
- 246.Zhang S., Dong Y., Wang Y., Sun W., Wei M., Yuan L., Yang G. Selective encapsulation of therapeutic mRNA in engineered extracellular vesicles by DNA aptamer. Nano Lett. 2021;21:8563–8570. doi: 10.1021/acs.nanolett.1c01817. [DOI] [PubMed] [Google Scholar]
- 247.Mateescu B., Kowal E.J.K., van Balkom B.W.M., Bartel S., Bhattacharyya S.N., Buzás E.I., Buck A.H., de Candia P., Chow F.W.N., Das S., et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA - an ISEV position paper. J. Extracell. Vesicles. 2017;6:1286095. doi: 10.1080/20013078.2017.1286095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tsai S.J., Atai N.A., Cacciottolo M., Nice J., Salehi A., Guo C., Sedgwick A., Kanagavelu S., Gould S.J. Exosome-mediated mRNA delivery in vivo is safe and can be used to induce SARS-CoV-2 immunity. J. Biol. Chem. 2021;297:101266. doi: 10.1016/j.jbc.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Man K., Brunet M.Y., Jones M.C., Cox S.C. Engineered extracellular vesicles: tailored-made nanomaterials for medical applications. Nanomaterials (Basel) 2020;10:1838–1930. doi: 10.3390/NANO10091838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang M., Altinoglu S., Takeda Y.S., Xu Q. Integrating protein engineering and bioorthogonal click conjugation for extracellular vesicle modulation and intracellular delivery. PLoS One. 2015;10:e0141860. doi: 10.1371/journal.pone.0141860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lee T.S., Kim Y., Zhang W., Song I.H., Tung C.H. Facile metabolic glycan labeling strategy for exosome tracking. Biochim. Biophys. Acta Gen. Subj. 2018;1862:1091–1100. doi: 10.1016/j.bbagen.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lamichhane T.N., Leung C.A., Douti L.Y., Jay S.M. Ethanol induces enhanced vascularization bioactivity of endothelial cell-derived extracellular vesicles via regulation of MicroRNAs and long non-coding RNAs. Sci. Rep. 2017;7:13794. doi: 10.1038/s41598-017-14356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Patel D.B., Luthers C.R., Lerman M.J., Fisher J.P., Jay S.M. Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system. Acta Biomater. 2019;95:236–244. doi: 10.1016/j.actbio.2018.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Silva A.K.A., Luciani N., Gazeau F., Aubertin K., Bonneau S., Chauvierre C., Letourneur D., Wilhelm C. Combining magnetic nanoparticles with cell derived microvesicles for drug loading and targeting. Nanomedicine. 2015;11:645–655. doi: 10.1016/J.NANO.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 255.Neubert J., Glumm J. Promoting neuronal regeneration using extracellular vesicles loaded with superparamagnetic iron oxide nanoparticles. Neural Regen. Res. 2016;11:61–63. doi: 10.4103/1673-5374.175043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lee J., Kim J., Jeong M., Lee H., Goh U., Kim H., Kim B., Park J.H. Liposome-based engineering of cells to package hydrophobic compounds in membrane vesicles for tumor penetration. Nano Lett. 2015;15:2938–2944. doi: 10.1021/nl5047494. [DOI] [PubMed] [Google Scholar]
- 257.Lee J., Lee H., Goh U., Kim J., Jeong M., Lee J., Park J.H. Cellular engineering with membrane fusogenic liposomes to produce functionalized extracellular vesicles. ACS Appl. Mater. Inter. 2016;8:6790–6795. doi: 10.1021/ACSAMI.6B01315. [DOI] [PubMed] [Google Scholar]
- 258.Palviainen M., Saari H., Kärkkäinen O., Pekkinen J., Auriola S., Yliperttula M., Puhka M., Hanhineva K., Siljander P.R.M. Metabolic signature of extracellular vesicles depends on the cell culture conditions. J. Extracell. Vesicles. 2019;8:1596669. doi: 10.1080/20013078.2019.1596669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Li J., Lee Y., Johansson H.J., Mäger I., Vader P., Nordin J.Z., Wiklander O.P.B., Lehtiö J., Wood M.J.A., Andaloussi S.E. Serum-free culture alters the quantity and protein composition of neuroblastoma-derived extracellular vesicles. J. Extracell. Vesicles. 2015;4:26883–26912. doi: 10.3402/JEV.V4.26883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bost J.P., Saher O., Hagey D., Mamand D.R., Liang X., Zheng W., Corso G., Gustafsson O., Görgens A., Smith C.E., et al. Growth media conditions influence the secretion route and release levels of engineered extracellular vesicles. Adv. Healthc. Mater. 2022;11:e2101658. doi: 10.1002/adhm.202101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mendt M., Kamerkar S., Sugimoto H., McAndrews K.M., Wu C.C., Gagea M., Yang S., Blanko E.V.R., Peng Q., Ma X., et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3:e99263. doi: 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Watson D.C., Bayik D., Srivatsan A., Bergamaschi C., Valentin A., Niu G., Bear J., Monninger M., Sun M., Morales-Kastresana A., et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. doi: 10.1016/j.biomaterials.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Bagheri H.S., Mousavi M., Rezabakhsh A., Rezaie J., Rasta S.H., Nourazarian A., Avci Ç.B., Tajalli H., Talebi M., Oryan A., et al. Low-level laser irradiation at a high power intensity increased human endothelial cell exosome secretion via Wnt signaling. Lasers Med. Sci. 2018;33:1131–1145. doi: 10.1007/S10103-018-2495-8. [DOI] [PubMed] [Google Scholar]
- 264.Zhao Z., Qu L., Shuang T., Wu S., Su Y., Lu F., Wang D., Chen B., Hao Q. Low-intensity ultrasound radiation increases exosome yield for efficient drug delivery. J. Drug Deliv. Sci. Technol. 2020;57:101713. doi: 10.1016/J.JDDST.2020.101713. [DOI] [Google Scholar]