Hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) splice donor (rs72613567) variant is associated with a lower risk of inflammation and fibrosis among patients with non-alcoholic fatty liver disease (NAFLD).1, 2 The mechanism whereby rs72613567 protects against fibrosis development is unclear, but it could be a direct effect and/or indirectly through intermediate histological lesions such as lobular inflammation, ballooning, and portal inflammation. Understanding the pathways through rs72613567 might lead to fibrosis is a critical step for the development of more precise and targeted therapies that could halt the disease progression. We conducted a causal mediation analysis to investigate whether protects against fibrosis by reducing the severity of intermediate histological lesions and/or by a direct effect on fibrosis.
Our study population consisted of 1153 non-Hispanic Whites (NHW) selected from 1697 adults with biopsy-confirmed NAFLD without a history of excessive alcohol intake registered prospectively in different studies (PIVENS and FLINT trials as well as NAFLD Adult Databases 1 and 2 [clinicaltrial.gov identifier: NCT01030484]) conducted in the NASH Clinical Research Network (NASH CRN) through December 2019.2–4 We have previously examined the association between rs72613567 and liver histology severity using this NHW population.2 More details on participants’ selection, clinical phenotypes, liver histology, and HSD17B13 rs72613567 genotyping can be found elsewhere.2 We used the NASH CRN Scoring System to assess the NAFLD histology severity, and it was classified as follows: steatosis (0–3), lobular inflammation (0–2), ballooning degeneration (0–2), and overall fibrosis (0–4).5 HSD17B13 rs72613567 was successfully genotyped in all DNA samples.
A causal mediation analysis examined the effect of rs72613567 (additive genetic model: −/−, −/A, A/A) on overall fibrosis (0–4) explained (indirect effect) or unexplained (direct effect) by intermediate histology lesions (steatosis [0–3], lobular inflammation [0–2], ballooning [0–2], portal inflammation [0–2]. β coefficients were estimated using 95% bootstrap bias-corrected confidence intervals (CIs) based on 10,000 bootstrap samples. β coefficients provide an index of the magnitude of the effect size between exposure and outcome, and it is considered significant if the upper and lower bounds of the 95% bias-corrected CIs do not contain zero. The analysis was implemented by the PROCESS macro (model 4 “parallel mediation model”) in SPSS (Chicago, IL, USA).6 All analyses were adjusted for age, sex, body mass index (kg/m2), type 2 diabetes, and selected genetic variants (PNPLA3 rs738409, TM6SF2 rs58542926, MBOAT7 rs641738).
The prevalence of rs72613567 A-allele was 36%. Supplemental Table 1 shows the baseline characteristics of the study population. Paths a in Figure 1, represent relationships between rs72613567 and histology traits. We detected negative relationships of rs72613567 with ballooning (β= −0.09, 95% CI: −0.17 to −0.01), portal inflammation (β= −0.04, 95% CI: −0.09 to −0.001) and lobular inflammation (β= −0.09, 95% CI: −0.17 to −0.01). rs72613567 was positively correlated with steatosis (β=0.13, 95% CI: 0.05–0.21). Paths b in Figure 1 represent correlations between histology traits and fibrosis, and results have been previously reported by our group.7 Briefly, both ballooning (β=0.54, 95% CI: 0.46–0.62) and portal inflammation (β=0.77, 95% CI: 0.67–0.88) were positively associated with fibrosis severity. Lobular inflammation (β=0.08, 95% CI: −0.01 to 0.17) tended to be positively associated with fibrosis but did not reach statistical significance. Steatosis (β= −0.11, 95% CI: −0.18 to −0.04) was negatively associated with fibrosis.
Figure 1. Statistical diagram of a parallel mediation model including 4 mediators.
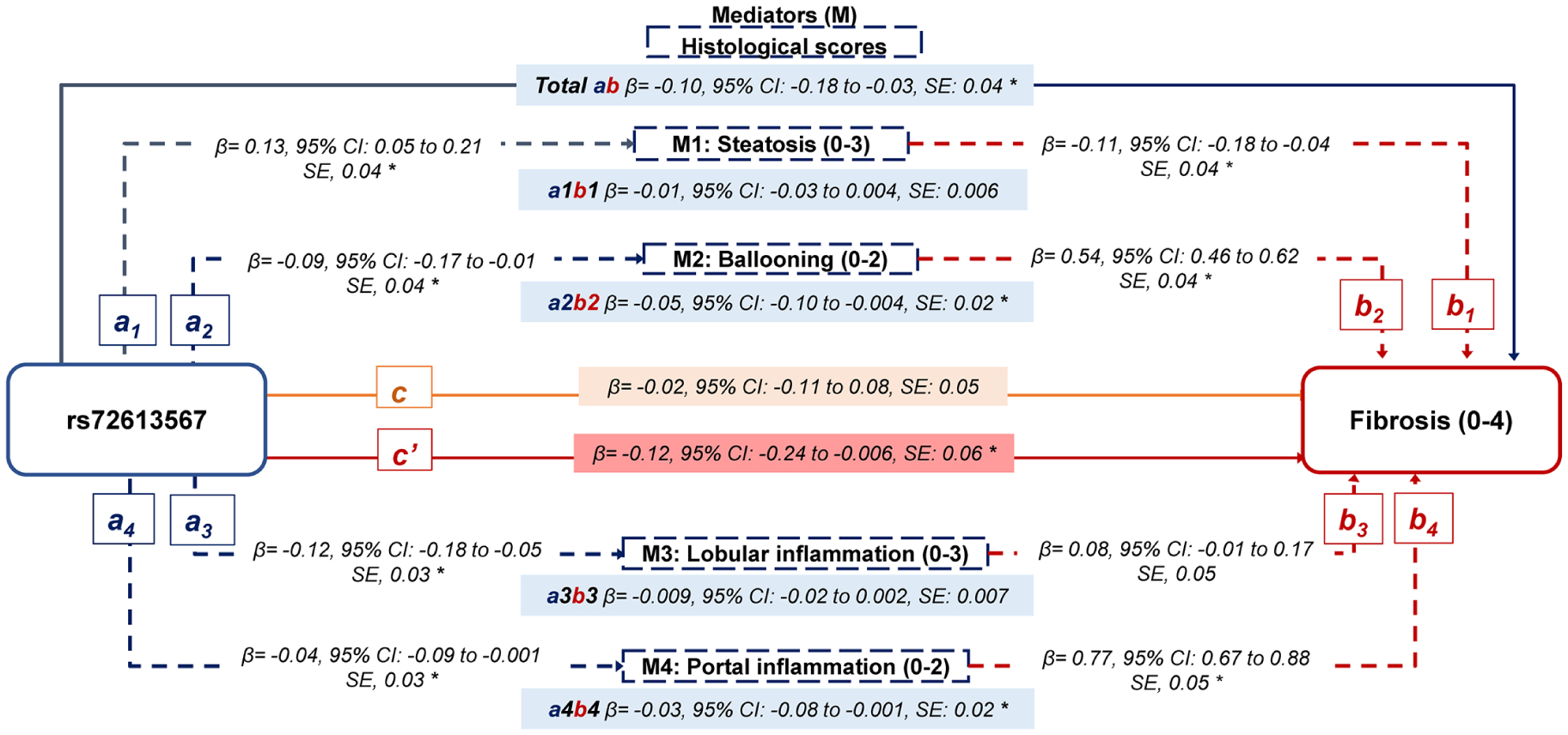
a: The effect of rs72613567 on the mediator (individual histological score)
b: The effect of the mediator (individual histological score) on the fibrosis score.
ab: The indirect effect of the rs72613567 on the fibrosis score through each mediator (individual histological score).
Total ab: The sum of all indirect effects of the rs72613567 on the fibrosis score through all mediators.
c: The direct effect of rs72613567 on the fibrosis score, not operating through mediators.
c’: The total effect of rs72613567.
The rs72613567 A-allele was associated with a lower risk of fibrosis (Total effect, β= −0.12, 95% CI: −0.24 to −0.006), Path c’ in Figure 1. We did not observe a direct association between rs72613567 A-allele and fibrosis (β= −0.02, 95% CI: −0.11 to 0.08, Path c in Figure 1), indicating that intermediate histology traits fully mediate the association between rs72613567 and fibrosis (Total indirect effect: β= −0.10, 95% CI: −0.18 to −0.03), Path total ab in Figure 1. Ballooning (β= −0.05, 95% CI: −0.10 to −0.004, Path a2b2 in Figure 1) and portal inflammation (β= −0.03, 95% CI: −0.08 to −0.001, Path a4b4 in Figure 1) were found to mediate the relationship between rs72613567 and fibrosis by 50% and 30%, respectively. Associations between rs72613567 and fibrosis were not attributed to changes in steatosis (β= −0.01, 95% CI: −0.03 to 0.004, Path a1b1 in Figure 1) or lobular inflammation (β= −0.009, 95% CI: −0.02 to 0.002, Path a3b3 in Figure 1). In supplemental table 2, we examined the effect of rs72613567 on fibrosis through different sequential combinations of histology traits. This analysis showed that lobular inflammation followed by ballooning or portal inflammation significantly mediated the effect of rs72613567 on fibrosis. Portal inflammation followed by ballooning and vice versa also remained as significant pathways in the mediation analysis.
Our mediation analyses showed that rs72613567 may likely exert its protective effect on fibrosis through the amelioration of inflammatory lesions, wherein ballooning and portal inflammation may play a major role. Unlike HSD17B13 rs72613567, PNPLA3 rs738409 seems to increase the risk of fibrosis via direct pathways without the mediation of other histology factors, which suggests that rs738409 likely promotes fibrosis development by triggering specific fibrogenic pathways. However, half of the total effect of rs738409 on fibrosis severity appears to be mediated primarily through portal inflammation.7 Consistent with previous studies, we observed that only ballooning and portal inflammation were found to positively contribute to fibrosis severity,8 and interestingly the effect of rs72613567 on fibrosis was mostly explained by changes through these two histology lesions. Collectively, these findings might support the hypothesis of the central role of inflammation in promoting fibrosis. The pro-fibrotic microenvironment seen in NASH could be explained by complex interactions of parenchymal and non-parenchymal cells which drive and perpetuate NASH progression. Impaired hepatocyte replication, expansion of hepatic progenitor cells, and ductular reaction have been strongly correlated with the presence and extension of portal inflammation in NAFLD9, 10 Alternatively, it could be hypothesized that activation of the innate immune system and increased release of pro-inflammatory cytokines from portal tract inflammatory cells could lead to amplification and perpetuation of inflammation, hepatocyte injury, and hence fibrosis progression in NAFLD.11
HSD17B13 rs72613567, a loss-of-function variant, has consistently been associated with a lower risk of advanced chronic liver disease (CLD).1, 2, 12 However, little is known about the underlying mechanisms whereby rs72613567 ameliorates the development and progression of fibrosis. From the clinical perspective, our findings suggest that rs72613567 might potentially exert its protective effects on fibrosis by reducing the severity of inflammatory pathways. However, the cross-sectional nature of our study might limit our ability to confirm whether the relationship between rs72613567 and fibrosis via intermediate histology traits remains constant over time among NAFLD patients undergoing consecutive biopsies. Given that rs72613567 might also protect against fibrosis progression of alcoholic liver disease (ALD)12, it would be interesting to confirm whether the same patterns of associations between rs72613567, intermediate histology traits, and fibrosis are seen among individuals with ALD.
In conclusion, our mediation analysis suggests that rs72613567 may indirectly reduce fibrosis severity through improvements in ballooning degeneration and portal inflammation, and these findings may offer a better understanding of how rs72613567 affects disease progression and the potential benefits of a targeted-HSD17B13 intervention to reduce inflammation, and subsequently fibrosis.
Supplementary Material
Acknowledgments
The authors thank the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) investigators and the Ancillary Studies Committee for providing clinical samples and relevant data from the Nonalcoholic Fatty Liver Disease (NAFLD) Databases 1 and 2, PIVENS trial, and FLINT trial. The authors also thank the participants involved in the NASH CRN studies.
We gratefully acknowledge the contributions of Drs. David E. Kleiner from the Laboratory of Pathology, National Cancer Institute, Bethesda, MD, USA, and Arun J Sanyal from Division of Gastroenterology and Hepatology, Virginia Commonwealth University, Richmond, VA, USA for their critical analysis and interpretation of data as well as critical revision of the manuscript for intellectual content.
Financial support:
This study was approved by the Nonalcoholic Steatohepatitis Clinical Research Network as an Ancillary Study (NASH CRN AS # 93). The NASH CRN is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, and U01DK061713). No funding was received from the NASH CRN for conducting this ancillary study. This study was supported, in part, by the Intramural Research Program of the National Institutes of Health, National Cancer Institute to David E. Kleiner as well as David W Crabb Endowed Professorship funds to Dr. Chalasani.
Conflicts of Interests
Dr. Chalasani has ongoing paid consulting activities (or had in the preceding 12 months) with Abbvie, Madrigal, Zydus, Galectin, Altimmune, Boehringer-Ingelheim, Lilly, and Foresite. These consulting activities are generally in the areas of nonalcoholic fatty liver disease and drug hepatotoxicity. Dr. Chalasani receives research grant support from Exact Sciences, DSM, and Galectin Therapeutics where his institution receives the funding.
Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institution received research grants from Arrowhead Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Cofounder of LipoNexus Inc.
Drs. Vilar-Gomez, Wilson, Yates, and Liang have nothing to disclose.
Abbreviations:
- NAFLD
non-alcoholic fatty liver disease
- PIVENS
Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis
- FLINT
The Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment
- NASH CRN
The Nonalcoholic Steatohepatitis Research Network
- CI
confidence interval
- HSD17B13
17-β Hydroxysteroid Dehydrogenase 13
- PNPLA3
Patatin-like Phospholipase Domain-containing Protein 3
- TM6SF2
Transmembrane 6 Superfamily Member 2
- MBOAT7
Membrane-Bound O-Acyltransferase Domain Containing 7
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abul-Husn NS, Cheng X, Li AH, et al. A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N Engl J Med 2018;378:1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vilar-Gomez E, Pirola CJ, Sookoian S, et al. The Protection Conferred by HSD17B13 rs72613567 Polymorphism on Risk of Steatohepatitis and Fibrosis May Be Limited to Selected Subgroups of Patients With NAFLD. Clin Transl Gastroenterol 2021;12:e00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385:956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 6.Hayes AF, Rockwood NJ. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav Res Ther 2017;98:39–57. [DOI] [PubMed] [Google Scholar]
- 7.Vilar-Gomez E, Pirola CJ, Sookoian S, et al. PNPLA3 rs738409 and risk of fibrosis in NAFLD: Exploring mediation pathways through intermediate histological features. Hepatology 2022;76:1482–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Argo CK, Northup PG, Al-Osaimi AM, et al. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol 2009;51:371–9. [DOI] [PubMed] [Google Scholar]
- 9.Richardson MM, Jonsson JR, Powell EE, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology 2007;133:80–90. [DOI] [PubMed] [Google Scholar]
- 10.Sato K, Marzioni M, Meng F, et al. Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances. Hepatology 2019;69:420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arrese M, Cabrera D, Kalergis AM, et al. Innate Immunity and Inflammation in NAFLD/NASH. Dig Dis Sci 2016;61:1294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stickel F, Lutz P, Buch S, et al. Genetic Variation in HSD17B13 Reduces the Risk of Developing Cirrhosis and Hepatocellular Carcinoma in Alcohol Misusers. Hepatology 2020;72:88–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


