Abstract
Purpose of Review
Patients with seizure disorders commonly suffer shoulder dislocations and subsequent instability. Due to high rates of recurrence and bone loss, management of this instability and associated pathology has proven to be more complex than that of patients without seizure disorders. The ultimate goal of this review is to outline the various treatment modalities and their respective outcomes in this complex patient population.
Recent Findings
Optimization of medical management of seizure disorders is imperative. However, despite these efforts, the incidence of post-operative seizure activity continues to be a concern. These subsequent episodes increase the risk of further instability and failure of surgical procedures. Overall, the use of soft tissue procedures has proven to result in increased recurrence of instability compared to bone-block augmenting and grafting procedures. There are a variety of bone-block procedures that have been described for anterior and posterior instability. Despite their success in decreasing further instability, they are associated with several complications that patients should be informed of.
Summary
There is no consensus regarding the optimal surgical management of shoulder instability in patients with seizure activity. A multidisciplinary approach to the management of the seizure activity is paramount to the success of their treatment. Further studies are required to evaluate the optimal timing and type of surgical intervention for individualized cases.
Keywords: Shoulder instability, Shoulder dislocation, Seizure, Epilepsy, Bone graft, Shoulder
Introduction
Patients with epilepsy and other complex seizure disorders unfortunately suffer from several other medical pathologies as a result of seizures, and this includes shoulder instability [1–4]. This can present as a first-time dislocation, recurrent instability, chronic locked dislocation, and/or fracture dislocation [1]. Although shoulder dislocations after seizure activity may be underdiagnosed [5], they have been reported to occur at a rate of 0.6% after each seizure event [6]. The most common direction of instability in this patient population continues to be debated as there are conflicting conclusions within the literature [1, 3, 7••]. There have been many described procedures for the management of shoulder instability that include soft tissue, bone block, and arthroplasty procedures. Compared to patients without epilepsy, those who have epilepsy have been found to have a staggeringly higher recurrence rate of dislocations after surgical management of shoulder instability [8]. This may be due to further seizure activity post-operatively or more significant bone loss prior to surgical intervention.
Within the orthopedic literature, no consensus or definitive guidelines have been developed for the management of shoulder instability in the setting of seizure disorders. The purpose of this article is to review the workup, treatment choices, and associated clinical outcomes of shoulder instability in the setting of seizure disorders.
Pathomechanics
During seizure activity, there is an imbalance of forces applied to the glenohumeral joint through the surrounding musculature. The internal rotators have been known to be stronger than the external rotators of the shoulder [9, 10]. During a generalized seizure, the pectoralis major and latissimus dorsi overpower the external rotators and force the humerus into an adducted, flexed, and internally rotated position [1, 9, 10]. This position and contraction imbalance forces the humeral head posteriorly and results in a posterior dislocation. This motion has been found to occur in the tonic extensor phase of generalized tonic–clonic seizures [11]. An anterior dislocation may occur if a seizure occurs while the arm is an abducted and externally rotated position that forces the humeral head anteriorly. Versive partial–type seizures that cause a fencing type posture are more likely to cause anterior dislocations [11]. Furthermore, seizure activity may lead patients to suffering traumatic falls that directly cause the shoulder dislocation.
Clinical Assessment
It is imperative that the management of shoulder instability in patients with seizure disorders follow a multidisciplinary approach. Since the cause of shoulder instability and its associated pathology in these patients is the seizure itself, the cause of seizure activity should be evaluated by medical and neurological teams. The most agreed-upon definition of epilepsy that also initiates therapy is as follows: two unprovoked seizures that are greater than 24 h apart [12]. This is due to the risk of a third seizure event occurring after two prior events being greater than 60% [12]. Therefore, patients with a first-time seizure require significant workup to assess the cause and treatment of this abnormal neurological activity. Often, if a cause is discovered and treated, patients do not have further seizure activity [12].
In patients with epilepsy, a neurological consultation is required in order to treat the seizure disorder and alter the threshold of further seizure activity [4, 12, 13]. Maximally decreasing the risk of subsequent seizure activity through medical management is imperative prior to considering surgical intervention for shoulder instability. The likelihood of being permanently seizure-free decreases with each failed treatment attempt [14, 15]. Furthermore, it has been well documented that non-compliance with anti-seizure medication continues to be a significant factor in the recurrence of seizure activity [14, 15]. Counselling from surgical and medical teams regarding the importance of adherence to medications is of strong significance in the overall treatment of these patients.
With regard to shoulder instability, a detailed history documenting the frequency, direction of instability, associated neurovascular compromise, and impairment associated with this pathology should be completed. Physical examination should include the assessment of an active and passive range of motion of the shoulder, strength testing to rule out associated rotator cuff pathology, and specific tests that assess the direction of the instability. Anterior/posterior load and shift tests as well as apprehension tests will provide surgeons with an understanding of the clinical direction of instability [16].
Radiographic Assessment
Patients with seizure disorders are commonly found to have associated bone loss in the setting of shoulder instability due to the large force applied by seizure activity and the number of events that subsequently occur [8, 13, 15]. Radiographic assessment should include Grashey, Scapular Y, and axillary views to assess congruity of the joint and potential glenoid and/or humeral bone loss [16]. The anteroposterior views allow for the assessment of potential inferior glenoid bone loss to be visualized. The axillary view allows for confirmation of a reduced joint as well as assessment of anterior/posterior glenoid defects and glenoid version [16]. An additional Stryker notch view (anteroposterior view with arm in internal rotation) has been found to allow for a better appreciation of Hill-Sachs defects [17, 18].
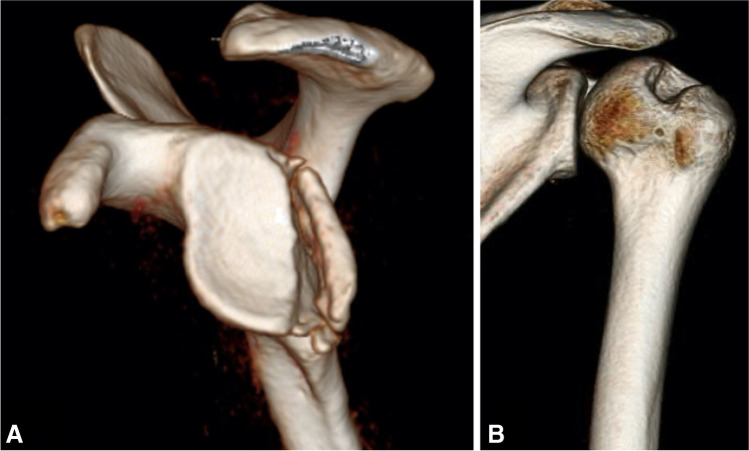
Computer tomography (CT) imaging of the shoulder has been considered the gold standard in determining the presence of and quantifying glenoid and/or humeral bone loss [17]. The use of 3D CT scans allows for the isolation of the scapula or humerus to allow for better visualization of the respective bony lesions in isolation (Fig. 1). Glenoid bone loss quantification can be achieved through linear or area measurements. Linear measurement techniques use the glenoid width as the reference to which the defect is compared. Numerous techniques have been described that utilize linear measurement and these include the glenoid index [19], width-to-length ratio [20], and the ratio method [21]. Of the many methods described, the most popular technique is the “Pico” method [22]. In this method, a circle of best fit is placed overlaying the posteroinferior aspect of the glenoid. The uncovered bony area within this circle is considered a bone loss and a percentage is thereby calculated [22]. Bois et al. demonstrated that using the Pico method in combination with 3D CT resulted in the smallest margin of error [23]. Furthermore, linear methods have been found to overestimate bone loss compared to area methods [23]. Recently, numerous studies have demonstrated the efficacy of 3D-MRI as an alternative modality to calculate bone loss with the advantage of decreased radiation to patients [24–26].
Fig. 1.
3D reconstruction of CT scan (two different shoulders) of A isolated scapular view depicting posterior glenoid bone loss. B Isolated humeral reconstruction depicting a Hill-Sachs lesion
As the incidence of Hill-Sachs lesions increases with recurrent instability [27], it is important to assess and quantify their presence in patients with seizure disorders. The “glenoid track” concept was originally introduced by Yamamoto et al. to summarize the effects of both humeral and glenoid bone loss in a singular concept that may predict subsequent dislocation [28]. The glenoid track is defined as the contact area between the glenoid and humeral head when the arm is taken through a range of motion. In this cadaveric-based study, Yamamoto et al. discovered that the medial margin of the glenoid track is at a distance that is proportional to approximately 84% of the glenoid width. If the humeral lesion extends medially beyond the glenoid track, the lesion may engage with the glenoid rim and risk dislocation [28]. Subsequent studies have recognized that the orientation and location of the humeral defect also affect the risk of its engagement against the glenoid rim [7••]. Similar to these concepts that have been utilized in the treatment of traumatic shoulder dislocations, it is imperative to assess global bone loss in patients with seizure disorders.
Treatment Options
Overall, the management of shoulder instability in patients with seizure disorders requires a multidisciplinary effort to first treat the seizure disorder and decrease the odds of further seizure activity. Since the surgical intervention is a stress that may decrease the threshold for further seizure activity, medical optimization with anticonvulsive agents by a neurological team is needed. Often, these medications require slow titration to a therapeutic dosage that may vary between patients. Since the complete resolution of seizure activity is not guaranteed, surgical intervention is often delayed until optimal control of the seizure disorder is achieved. No recommended timeline for seizure-free activity has been defined in the literature. Since poor compliance with anticonvulsive medications is the main risk factor for recurrent seizure activity, it is important for all teams involved in the care of the patient to monitor medication compliance during this period [2]. At times, it may be difficult to delay surgical intervention for a specific time as patients may have already suffered a significant bone loss that predisposes further instability events despite conservative measures. Therefore, the optimal seizure-free time period pre-operatively may differ for each patient depending on their instability pathology. Surgical interventions that have been utilized in this patient population resemble the options available to those without seizure activity. Non-operative management, soft tissue procedures, bony procedures, arthroplasty, and arthrodesis interventions may be utilized in certain cases. While the authors generally prefer patients to be seizure free for 6 months prior to surgery, we will occasionally decrease this period by up to 3 months in cases where the bone loss is relatively severe.
Soft Tissue Repair or Bony Reconstruction
Debate continues regarding the optimal management of traumatic shoulder dislocations based on a variety of risk factors such as age, activity level, and amount of bone loss [29]. Studies assessing the choice between soft tissue repair versus bony reconstruction in patients with seizure disorders are even more limited. One of the earliest studied cohorts by Buhler and Gerber included 34 seizure-induced unstable shoulders with a mean age of 43.5 years and were followed for at least 10 years [2]. Revision procedures were common within this cohort as attempts to initially stabilize with soft tissue procedures were common. Anterior shoulder dislocations that were subsequently treated with a bony reconstruction procedure were successful in 12 of 13 patients. Initial failures were attributed to medication non-compliance as well as initial bony defects that were not addressed with bony procedures. This success of bony procedures was in agreement with a smaller cohort (14 shoulders) of patients that underwent a bony reconstruction for seizure-induced anterior shoulder instability and resulted in no further recurrence of dislocations [30].
Thangarajah and Lambert evaluated 49 consecutive patients with epilepsy that were treated (36 treated surgically) for recurrent shoulder instability over a 15-year period [8]. This cohort included 36 anterior, 8 posterior, and 5 multidirectional unstable shoulders. The overall recurrence rate of those treated surgically was 69%. Furthermore, 60% of those who had recurrence subsequently underwent revision surgery [8]. The index operations consisted of 23 soft tissue procedures, 11 glenoid bony reconstructions, and 2 humeral head bony reconstructions. The recurrence rate after soft tissue procedures was 71% compared to 28% in those who underwent bony reconstruction. On average, approximately 2.6 procedures were required to achieve stabilization within this cohort [8]. Despite demonstrating an increased failure rate of soft tissue procedures, this study did not clearly outline the degree of bone loss that these patients had at the time of these procedures as this may contribute to the failure rate.
Soft Tissue Procedures
In the USA, the most commonly performed surgical procedure to address shoulder instability in non-epileptic patients is an arthroscopic labral repair [31]. Its advantages include shorter recovery time, improved visualization of intra-articular pathology, and improved cosmesis. Risk factors for failure of Bankart repair include an insufficient number of anchors used, number of pre-operative dislocations, time interval from initial dislocation to operative intervention, off-track Hill-Sachs lesions, and extent of associated glenoid bone loss [32]. Purchase et al. first described the arthroscopic remplissage technique that involves filling a Hill-Sachs lesion with an infraspinatus tenodesis along with a posterior capsulodesis [33]. This technique grew in popularity and has been increasingly used in the setting of off-track Hill-Sachs lesions [34]. The addition of a remplissage procedure to a Bankart repair was studied in a randomized controlled trial of 108 patients with anterior instability, less than 15% glenoid bone loss, and an off-track Hill-Sachs lesion. At 24-month follow up, the odds ratio of recurrent instability in the Bankart repair–only group relative to the group with additional remplissage was 5.49. Furthermore, if the Hill-Sachs lesion was > 15% of the humeral head or > 20 mm, the odds ratio increased to 11.5 [35].
In patients with seizure disorders, only one study has been reported to assess the outcomes of arthroscopic Bankart repair combined with remplissage. This retrospective review of 29 recurrent anterior shoulder dislocations included patients with a Hill-Sachs defect and excluded those with more than 20% of glenoid bone loss. This cohort had a mean age of 28.3 years and had a mean follow-up of 3.1 years. The overall rate of post-operative recurrent instability was 17.2% [7••]. Importantly, recurrent instability did not occur in patients who did not suffer post-operative seizure activity [7••]. Therefore, this study demonstrated that with minimal bone loss, this procedure is likely to be effective in patients who do not have any further seizure activity.
Bone Reconstruction Procedures
Historically, the use of glenoid bony reconstruction procedures has been reserved for cases with greater than 20–25% bone loss as Boileau et al. demonstrated 75% failure of arthroscopic stabilization procedures in such cases [36]. Subsequent studies in a high level of activity patients demonstrated that the critical cutoff may be approximately 13.5% as patients with bone loss above that threshold had worse outcome scores after arthroscopic Bankart repair [37]. Numerous bony reconstruction procedures have been described to combat significant bone loss on the glenoid side. The procedures vary from their use of autograft at the same site, different site, or allograft options.
In the setting of anterior dislocations, the Latarjet procedure involves the transfer of the coracoid to the anteroinferior aspect of the glenoid [38]. This procedure not only extends the glenoid articular track but also provides an anterior soft tissue restraint with the associated advancement of the conjoint tendon. Although minor modifications of this procedure have been described, it is generally completed through a deltopectoral approach where the coracoid is entirely exposed with visualization of the coracoclavicular (CC) ligaments. The pectoralis minor insertion is dissected off the medial aspect of the coracoid, and the coracoacromial ligament is dissected off the lateral aspect. While preserving the CC ligaments and protecting the brachial plexus, a coracoid osteotomy is completed. The subscapularis is next split to expose the joint capsule and subsequently, the glenoid. The coracoid graft is then secured to the anteroinferior aspect of the glenoid after the defect is prepared for grafting [38].
In a small study of anterior instability by Ersen et al., the Latarjet procedure was performed on 11 shoulders of patients with epilepsy and 54 shoulders without a seizure disorder. Patient-reported outcome scores did not differ between the two groups, but the dislocation rate was higher in the epilepsy group (9%) compared to the non-epilepsy group (1.8%) [39]. Three of nine patients with epilepsy suffered a post-operative seizure. One patient suffered this seizure in the early post-operative period (< 6 weeks) and suffered screw fixation failure that required a revision procedure. The two other patients suffered their repeat seizure activity 6 six weeks and did not have a recurrent dislocation [39]. Another study that assessed the Latarjet procedure for anterior instability compared 10 shoulders in patients with a seizure disorder and 44 without a seizure disorder [40••]. The study demonstrated that 50% of shoulders with a seizure disorder had recurrent seizure activity and these post-operative seizures increased the risk of having a recurrent dislocation by 39.9 times [40••]. Interestingly, within the seizure group, no recurrent dislocations occurred in those who did not suffer a post-operative seizure [40••]. Meanwhile, the recurrence of instability in the control group was only 2.3% [40••].
Conversely, a recent study that evaluated the outcomes of an arthroscopic Latarjet procedure compared 21 shoulders in patients with a seizure disorder with a matched cohort of 21 patients without such a disorder. These patients were matched for age, sex, handedness, activity level, and size of the bone defect [41••]. Both groups demonstrated significantly improved post-operative clinical outcomes scores and range of motion. No differences in these outcomes were observed between the two groups [41••]. Interestingly, despite the fact that 33% of cases with seizure disorder suffered post-operative seizures, no recurrent glenohumeral dislocations occurred [41••].
Other Graft Options
Due to conflicting outcomes about the efficacy of the Latarjet procedure for anterior instability in the setting of seizure disorders, other graft options have been increasingly used. The risk of further seizure activity has led some surgeons to utilize larger graft options to decrease the odds of further instability events. Furthermore, these graft options may also be utilized primarily in the setting of posterior instability.
In terms of autograft sources, the use of the distal clavicle and iliac crest has been described for shoulder instability. The advantages of the autologous distal clavicle osteoarticular graft include its local availability and the presence of articular cartilage. Tokish et al. first described the arthroscopic distal clavicle autograft for shoulder instability with the rationale of providing an osteochondral surface to reduce the risk for subsequent arthritis [42]. Nonetheless, a cadaveric-based study demonstrated that the mean percentage of glenoid surface area that the distal clavicle graft may restore is similar to that of the Latarjet procedure [43]. Therefore, the use of the distal clavicle is less likely to be utilized in patients with seizure disorders as a larger bone graft may be needed.
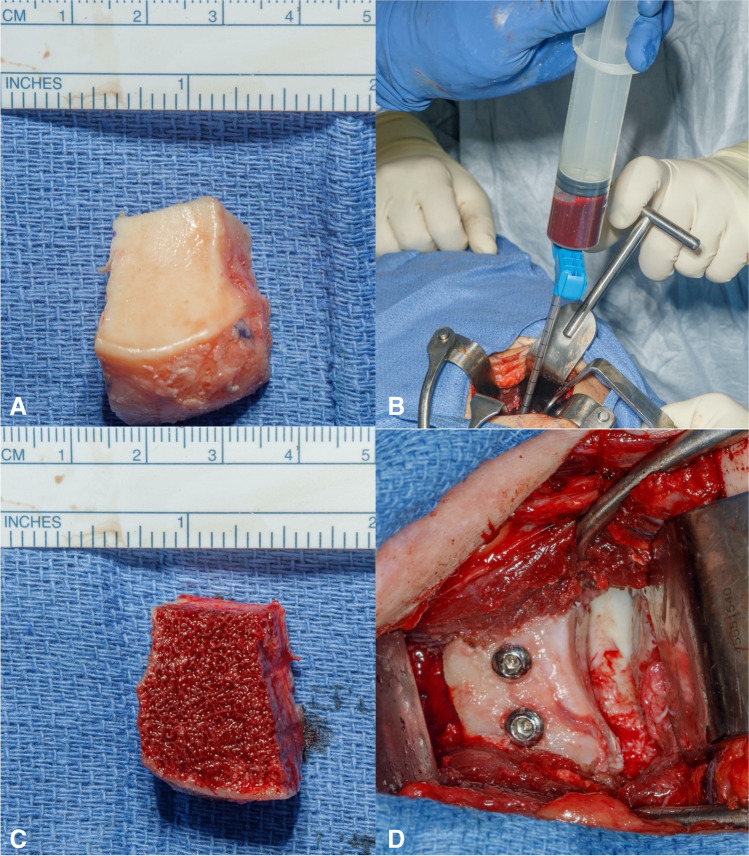
In terms of allograft use, fresh distal tibial allograft (DTA) had initially been described for use in revision settings and has now become a primary option for some surgeons [44]. There have been concerns regarding the potential complications of a Latarjet procedure and its nonanatomic geometry. The DTA has been found to have an identical radius of curvature as a native glenoid and therefore offer great congruency to a humeral head [44]. Its benefits include the lack of donor site morbidity, the presence of an articular surface, and the flexibility to contour a larger graft as needed to match the shape of the defect [44, 45]. This procedure is carried out through a standard deltopectoral approach that is followed by a subscapularis split and capsulotomy. A Fukuda retractor is then placed into the glenohumeral joint to retract the humeral head and expose the glenoid articular surface. The glenoid defect is then cleared of any scar tissue and measured to estimate the needed size of the DTA. On a back table, the DTA is prepared and cut to the dimensions of the defect. Next, a pulse lavage is used to clear the graft of any bone marrow. The graft is then soaked in bone marrow aspirate obtained from the native glenoid vault. The use of fully threaded, non-cannulated screws is preferred for optimal strength of graft fixation (Fig. 2). It is important to maintain visualization of the articular surface prior to fixing the graft to ensure adequate graft placement for stability and congruity.
Fig. 2.
Steps for preparation of distal tibial allograft. A DTA cut to appropriate dimension per size of glenoid defect. B Glenoid aspirate using a commercially provided device. C After the allograft is cleaned of any marrow remnants, it is soaked with the glenoid aspirate. D Final position and fixation of the DTA to the anterior glenoid defect
Frank et al. assessed a balanced cohort of patients who had either undergone a Latarjet or DTA procedure for shoulder instability or had a minimum of 2-year follow-up. There were no significant differences in the post-operative clinical outcome scores and complication rates between the two cohorts [46]. To date, no studies have specifically outlined the outcomes of DTA as a primary procedure in the setting of seizure disorders. Heterogenous studies have combined the use of many allograft and autograft techniques to demonstrate success in these patients [4, 8].
Posterior Bone-Block Options
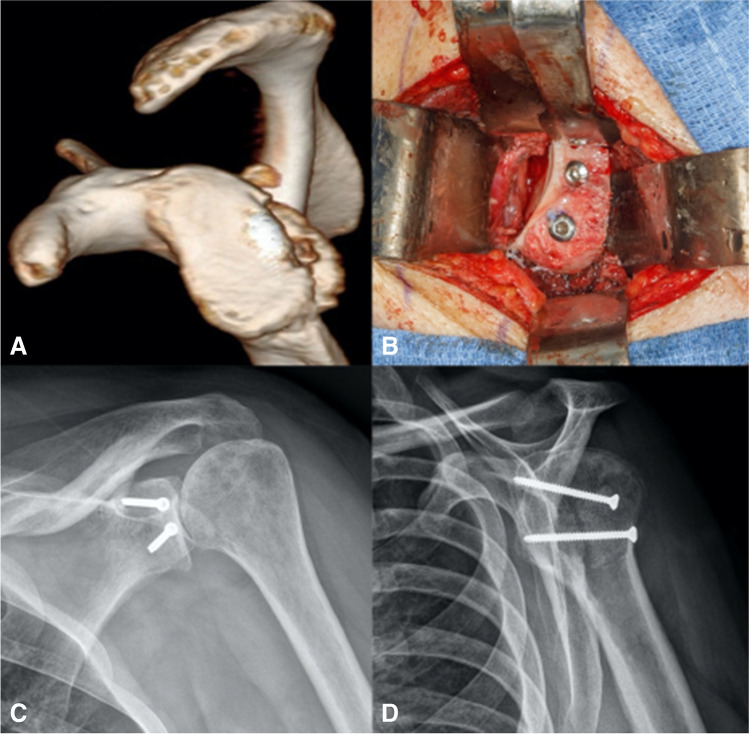
In posterior instability, several procedures have been historically described and include the following: posterior labral and capsular repair, posterior capsular shift, rotator interval closure, lesser tuberosity transfer, glenoid osteotomy, bone-block procedures, and arthroplasty [47]. For bone-block procedures, similar graft options have been described and largely include the DTA (Fig. 3) and iliac crest autograft. Generally, a modified or standard Judet approach is utilized for these cases. Often, the posterior capsulolabral tissue is compromised, and the posterior aspect of the glenoid can be visualized. Camenzind et al. demonstrated that the arthroscopically assisted iliac crest grafting for posterior instability results in a reliable placement of the graft and improved clinical outcomes. Meanwhile, resorption was common in all patients as longer radiographic follow-up was obtained [48]. Furthermore, 47% of cases required the removal of hardware due to prominent screw heads irritating the posterior soft tissue [48]. Barbier et al. reported on 8 patients who underwent iliac crest bone block for posterior instability and demonstrated improved postoperative constant scores and no recurrence of instability. Nonetheless, patients had remained in the hospital for a mean of 6 days. Three patients required reoperations for hardware removal and one patient suffered a hematoma at the donor site [49]. Other smaller studies demonstrated up to 36% recurrence of posterior instability with a similar procedure [50].
Fig. 3.
A Case example of posterior instability with associated posterior glenoid bone deficiency. B Intraoperative image depicting use of DTA that is fixed with fully threaded cortical screws. Postoperative C anteroposterior and D lateral radiographs demonstrating fixation of posteriorly based DTA
In a study that included ten patients (7 with prior stabilization procedures) who underwent DTA for posterior instability, two of these patients had a known seizure disorder. One patient demonstrated subjective recurrence of instability while two patients underwent revision operations related to screw prominence [47]. The proposed advantages to a DTA in posterior instability are similar to those mentioned earlier with regard to anterior instability. Despite no consensus on the optimal graft choice, proponents of the DTA over iliac crest for posterior instability strongly oppose the risks associated with donor site morbidity. These include risks of infection at a secondary site, a secondary scar, gait disturbance in the first few weeks after surgery, and the risk to the lateral femoral cutaneous nerve [51].
At present time, no studies have directly compared DTA and autologous iliac crest bone grafting options for posterior instability in patients with seizure disorders. Prior literature in the non-seizure population demonstrates similar outcomes for both procedures. Therefore, to avoid the risks of donor site morbidity, the use of DTA may be favored as it also provides improved articular congruence as seen in anteriorly based cases.
Humeral Head Bone Augmentation
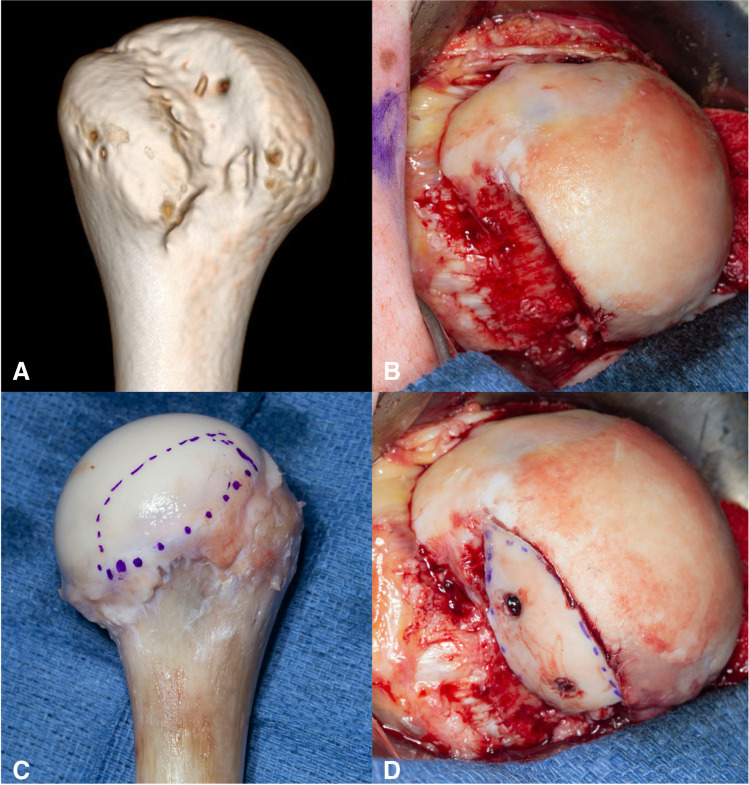
To date, only one study has been published that specifically evaluates the role of humeral head bony augmentation in addition to a glenoid procedure for shoulder instability in patients with seizure disorders. Roach et al. retrospectively reviewed 15 shoulders that were treated with humeral head bone augmentation procedures (Fig. 4) for shoulder instability. All but one case underwent glenoid and humeral-sided procedures. The average humeral head defect was 20 × 15 × 24 mm while the average glenoid bone loss was 20.8%. All patients reported post-operative seizure activity, and only one suffered a recurrent dislocation. At a mean follow-up of 4.8 years, self-reported satisfaction was “better” or “much better” in 92% of cases [52••]. Nonetheless, this is a relatively small study; therefore, definitive practice-changing conclusions cannot be made.
Fig. 4.
A Case example of humeral head defect in the setting of recurrent instability. B Intraoperative image depicting extent of humeral defect. C Proximal humeral allograft was utilized, D and the native defect was templated and outlined prior to preparing the graft for fixation to fill the void
Shoulder Arthroplasty
In a study that followed 49 unstable shoulders in patients with epilepsy, glenohumeral arthrosis was found in 45% of cases [8]. The authors concluded that the damage caused by repetitive high-energy dislocations, the number of pre-operative dislocations, and the subsequent recurrence rate contribute to the increased rate of arthritis found in this patient population [8]. The use of arthroplasty is of particular concern in this patient population due to their relatively younger age and risk of subsequent seizures. In another study, eight patients with epilepsy that were treated with an arthroplasty procedure for recurrent instability were followed for a mean of 4.7 years [53]. Three patients underwent an anatomic total shoulder arthroplasty and five underwent a hemiarthroplasty procedure. While all patients reported experiencing post-operative seizure activity, no further shoulder instability was reported. Two patients who underwent a hemiarthroplasty required revision to a total shoulder arthroplasty due to glenoid erosion [53].
Conclusion
The management of shoulder instability in the setting of epilepsy continues to be a significant challenge. Due to heterogeneity and a small number of studies, a definitive consensus on their treatment continues to be debated. Overall, bone block procedures appear to be favored over soft tissue procedures due to the prevalence of post-operative seizure activity that appears to result in increased recurrence of shoulder instability. A variety of bone graft options has been described and appears to be successful in the management of anterior and posterior instability. Most importantly, optimal and strict medical management of seizure disorders is required to decrease recurrent seizure activity and subsequent shoulder instability.
Declarations
Conflict of Interest
No author has any financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Goudie EB, Murray IR, Robinson CM. Instability of the shoulder following seizures. J Bone Joint Surg Br Vol. 2012;94(6):721–728. doi: 10.1302/0301-620X.94B6.28259. [DOI] [PubMed] [Google Scholar]
- 2.Buhler M, Gerber C. Shoulder instability related to epileptic seizures. J Shoulder Elbow Surg. 2002;11(4):339–344. doi: 10.1067/mse.2002.124524. [DOI] [PubMed] [Google Scholar]
- 3.Thangarajah T, Lambert S. Management of recurrent shoulder instability in patients with epilepsy. J Shoulder Elbow Surg. 2015;24(11):1723–1727. doi: 10.1016/j.jse.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Thangarajah T, Lambert SM. Management of recurrent shoulder instability in patients with epilepsy. J Shoulder Elbow Surg. 2016;25(8):1376–1384. doi: 10.1016/j.jse.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Schulz TJ, Jacobs B, Patterson RL., Jr Unrecognized dislocations of the shoulder. J Trauma. 1969;9(12):1009–1023. doi: 10.1097/00005373-196912000-00005. [DOI] [PubMed] [Google Scholar]
- 6.DeToledo JC, Lowe MR. Seizures, lateral decubitus, aspiration, and shoulder dislocation: time to change the guidelines? Neurology. 2001;56(3):290–291. doi: 10.1212/wnl.56.3.290. [DOI] [PubMed] [Google Scholar]
- 7.Guity MR, Sobhani EA. Mid-term results of arthroscopic Bankart repair and remplissage for recurrent anterior shoulder instability in patients with a history of seizures. BMC Musculoskelet Disord. 2022;23(1):12. doi: 10.1186/s12891-021-04960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thangarajah T, Lambert S. The management of recurrent shoulder instability in patients with epilepsy: a 15-year experience. J Shoulder Elbow Surg. 2015;24(11):1723–1727. doi: 10.1016/j.jse.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Hinton RY. Isokinetic evaluation of shoulder rotational strength in high school baseball pitchers. Am J Sports Med. 1988;16(3):274–279. doi: 10.1177/036354658801600314. [DOI] [PubMed] [Google Scholar]
- 10.Shaw JL. Bilateral posterior fracture-dislocation of the shoulder and other trauma caused by convulsive seizures. J Bone Joint Surg Am. 1971;53(7):1437–1440. doi: 10.2106/00004623-197153070-00023. [DOI] [PubMed] [Google Scholar]
- 11.DeToledo JC, Lowe MR, Ramsay RE. Restraining patients and shoulder dislocations during seizures. J Shoulder Elbow Surg. 1999;8(4):300–302. doi: 10.1016/s1058-2746(99)90149-0. [DOI] [PubMed] [Google Scholar]
- 12.Falco-Walter JJ, Scheffer IE, Fisher RS. The new definition and classification of seizures and epilepsy. Epilepsy Res. 2018;139:73–79. doi: 10.1016/j.eplepsyres.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Langenbruch L, Rickert C, Gosheger G, Schorn D, Schliemann B, Brix T, et al. Seizure-induced shoulder dislocations - case series and review of the literature. Seizure. 2019;70:38–42. doi: 10.1016/j.seizure.2019.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Brodtkorb E, Samsonsen C, Sund JK, Bråthen G, Helde G, Reimers A. Treatment non-adherence in pseudo-refractory epilepsy. Epilepsy Res. 2016;122:1–6. doi: 10.1016/j.eplepsyres.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Beghi E, Giussani G, Sander JW. The natural history and prognosis of epilepsy. Epileptic Disord. 2015;17(3):243–253. doi: 10.1684/epd.2015.0751. [DOI] [PubMed] [Google Scholar]
- 16.Provencher MT, Bhatia S, Ghodadra NS, Grumet RC, Bach BR, Jr, Dewing CB, et al. Recurrent shoulder instability: current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am. 2010;92(Suppl 2):133–151. doi: 10.2106/jbjs.J.00906. [DOI] [PubMed] [Google Scholar]
- 17.Fox JA, Sanchez A, Zajac TJ, Provencher MT. Understanding the Hill-Sachs lesion in its role in patients with recurrent anterior shoulder instability. Curr Rev Musculoskelet Med. 2017;10(4):469–479. doi: 10.1007/s12178-017-9437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saliken DJ, Bornes TD, Bouliane MJ, Sheps DM, Beaupre LA. Imaging methods for quantifying glenoid and Hill-Sachs bone loss in traumatic instability of the shoulder: a scoping review. BMC Musculoskelet Disord. 2015;16:164. doi: 10.1186/s12891-015-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376–382. doi: 10.1016/j.arthro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Griffith JF, Antonio GE, Tong CW, Ming CK. Anterior shoulder dislocation: quantification of glenoid bone loss with CT. AJR Am J Roentgenol. 2003;180(5):1423–1430. doi: 10.2214/ajr.180.5.1801423. [DOI] [PubMed] [Google Scholar]
- 21.Barchilon VS, Kotz E, Barchilon Ben-Av M, Glazer E, Nyska M. A simple method for quantitative evaluation of the missing area of the anterior glenoid in anterior instability of the glenohumeral joint. Skeletal Radiol. 2008;37(8):731–736. doi: 10.1007/s00256-008-0506-8. [DOI] [PubMed] [Google Scholar]
- 22.Baudi P, Righi P, Bolognesi D, Rivetta S, Rossi Urtoler E, Guicciardi N, et al. How to identify and calculate glenoid bone deficit. Chir Organi Mov. 2005;90(2):145–152. [PubMed] [Google Scholar]
- 23.Bois AJ, Fening SD, Polster J, Jones MH, Miniaci A. Quantifying glenoid bone loss in anterior shoulder instability: reliability and accuracy of 2-dimensional and 3-dimensional computed tomography measurement techniques. Am J Sports Med. 2012;40(11):2569–2577. doi: 10.1177/0363546512458247. [DOI] [PubMed] [Google Scholar]
- 24.Walter WR, Samim M, LaPolla FWZ, Gyftopoulos S. Imaging quantification of glenoid bone loss in patients with glenohumeral instability: a systematic review. AJR Am J Roentgenol. 2019:1–10. 10.2214/ajr.18.20504. [DOI] [PubMed]
- 25.Vopat BG, Cai W, Torriani M, Vopat ML, Hemma M, Harris GJ, et al. Measurement of glenoid bone loss with 3-dimensional magnetic resonance imaging: a matched computed tomography analysis. Arthroscopy. 2018;34(12):3141–3147. doi: 10.1016/j.arthro.2018.06.050. [DOI] [PubMed] [Google Scholar]
- 26.Yanke AB, Shin JJ, Pearson I, Bach BR, Jr, Romeo AA, Cole BJ, et al. Three-dimensional magnetic resonance imaging quantification of glenoid bone loss is equivalent to 3-dimensional computed tomography quantification: cadaveric study. Arthroscopy. 2017;33(4):709–715. doi: 10.1016/j.arthro.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Yiannakopoulos CK, Mataragas E, Antonogiannakis E. A comparison of the spectrum of intra-articular lesions in acute and chronic anterior shoulder instability. Arthroscopy. 2007;23(9):985–990. doi: 10.1016/j.arthro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto N, Itoi E, Abe H, Minagawa H, Seki N, Shimada Y, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16(5):649–656. doi: 10.1016/j.jse.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Dekker TJ, Peebles LA, Bernhardson AS, Golijanin P, Di Giacomo G, Hackett TR, et al. Limited predictive value of the instability severity index score: evaluation of 217 consecutive cases of recurrent anterior shoulder instability. Arthroscopy. 2021;37(5):1381–1391. doi: 10.1016/j.arthro.2020.12.185. [DOI] [PubMed] [Google Scholar]
- 30.Hutchinson JW, Neumann L, Wallace WA. Bone buttress operation for recurrent anterior shoulder dislocation in epilepsy. J Bone Joint Surg Br Vol. 1995;77(6):928–932. doi: 10.1302/0301-620X.77B6.7593109. [DOI] [PubMed] [Google Scholar]
- 31.Provencher MT, Midtgaard KS, Owens BD, Tokish JM. Diagnosis and management of traumatic anterior shoulder instability. J Am Acad Orthop Surg. 2021;29(2):e51–e61. doi: 10.5435/jaaos-d-20-00202. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Lim KH, Kim JW. Risk factors for recurrence of anterior-inferior instability of the shoulder after arthroscopic Bankart repair in patients younger than 30 years. Arthroscopy. 2018;34(9):2530–2536. doi: 10.1016/j.arthro.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 33.Purchase RJ, Wolf EM, Hobgood ER, Pollock ME, Smalley CC. Hill-Sachs “remplissage”: an arthroscopic solution for the engaging Hill-Sachs lesion. Arthroscopy. 2008;24(6):723–726. doi: 10.1016/j.arthro.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Polio W, Brolin TJ. Remplissage for anterior shoulder instability: history, indications, and outcomes. Orthop Clin North Am. 2022;53(3):327–338. doi: 10.1016/j.ocl.2022.02.005. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald P, McRae S, Old J, Marsh J, Dubberley J, Stranges G, et al. Arthroscopic Bankart repair with and without arthroscopic infraspinatus remplissage in anterior shoulder instability with a Hill-Sachs defect: a randomized controlled trial. J Shoulder Elbow Surg. 2021;30(6):1288–1298. doi: 10.1016/j.jse.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Boileau P, Villalba M, Héry JY, Balg F, Ahrens P, Neyton L. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am. 2006;88(8):1755–1763. doi: 10.2106/jbjs.E.00817. [DOI] [PubMed] [Google Scholar]
- 37.Shaha JS, Cook JB, Song DJ, Rowles DJ, Bottoni CR, Shaha SH, et al. Redefining “Critical” bone loss in shoulder instability: functional outcomes worsen with “subcritical” bone loss. Am J Sports Med. 2015;43(7):1719–1725. doi: 10.1177/0363546515578250. [DOI] [PubMed] [Google Scholar]
- 38.Burkhart SS, De Beer JF, Barth JR, Cresswell T, Roberts C, Richards DP. Results of modified Latarjet reconstruction in patients with anteroinferior instability and significant bone loss. Arthroscopy. 2007;23(10):1033–1041. doi: 10.1016/j.arthro.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Ersen A, Bayram S, Birisik F, Atalar AC, Demirhan M. The effectiveness of the Latarjet procedure for shoulder instability in patients with epilepsy. Orthop Traumatol Surg Res. 2017;103(8):1277–1282. doi: 10.1016/j.otsr.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 40.Thon SG, Branche K, Houck DA, Didinger T, Vidal AF, Frank RM, et al. Effectiveness of Latarjet for anterior shoulder instability in patients with seizure disorder. JSES Int. 2021;5(2):171–174. doi: 10.1016/j.jseint.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dzidzishvili L, Calvo C, Valencia M, Calvo E. Outcomes of arthroscopic Latarjet procedure for anterior glenohumeral instability in patients with epilepsy: a case-control study. Am J Sports Med. 2022;50(3):708–716. doi: 10.1177/03635465211067531. [DOI] [PubMed] [Google Scholar]
- 42.Tokish JM, Fitzpatrick K, Cook JB, Mallon WJ. Arthroscopic distal clavicular autograft for treating shoulder instability with glenoid bone loss. Arthrosc Tech. 2014;3(4):e475–e481. doi: 10.1016/j.eats.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larouche M, Knowles N, Ferreira L, Tokish JM, Athwal GS. Osteoarticular distal clavicle autograft for the management of instability-related glenoid bone loss: an anatomic and cadaveric study. J Shoulder Elbow Surg. 2020;29(8):1615–1620. doi: 10.1016/j.jse.2019.12.027. [DOI] [PubMed] [Google Scholar]
- 44.Provencher MT, Ghodadra N, LeClere L, Solomon DJ, Romeo AA. Anatomic osteochondral glenoid reconstruction for recurrent glenohumeral instability with glenoid deficiency using a distal tibia allograft. Arthroscopy. 2009;25(4):446–452. doi: 10.1016/j.arthro.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 45.Provencher MT, Frank RM, Golijanin P, Gross D, Cole BJ, Verma NN, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891–897. doi: 10.1016/j.arthro.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 46.Frank RM, Romeo AA, Richardson C, Sumner S, Verma NN, Cole BJ, et al. Outcomes of Latarjet versus distal tibia allograft for anterior shoulder instability repair: a matched cohort analysis. Am J Sports Med. 2018;46(5):1030–1038. doi: 10.1177/0363546517744203. [DOI] [PubMed] [Google Scholar]
- 47.Gilat R, Haunschild ED, Tauro T, Evuarherhe A, Fu MC, Romeo A, et al. Distal tibial allograft augmentation for posterior shoulder instability associated with glenoid bony deficiency: a case series. Arthrosc Sports Med Rehabil. 2020;2(6):e743–e752. doi: 10.1016/j.asmr.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Camenzind RS, Gossing L, Martin Becerra J, Ernstbrunner L, Serane-Fresnel J, Lafosse L. Restoration of the posterior glenoid in recurrent posterior shoulder instability using an arthroscopically placed iliac crest bone graft: a computed tomography-based analysis. Orthop J Sports Med. 2021;9(1):2325967120976378. doi: 10.1177/2325967120976378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barbier O, Ollat D, Marchaland JP, Versier G. Iliac bone-block autograft for posterior shoulder instability. Orthop Traumatol Surg Res. 2009;95(2):100–107. doi: 10.1016/j.otsr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Hachem AI, Molina-Creixell A, Rius X, Rodriguez-Bascones K, Cabo Cabo FJ, Agullo JL, et al. Comprehensive management of posterior shoulder instability: diagnosis, indications, and technique for arthroscopic bone block augmentation. EFORT Open Rev. 2022;7(8):576–586. doi: 10.1530/EOR-22-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costa Mendes L, Sauvigné T, Guiol J. Morbidity of autologous bone harvesting in implantology: literature review from 1990 to 2015. Rev Stomatol Chir Maxillofac Chir Orale. 2016;117(6):388–402. doi: 10.1016/j.revsto.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Roach RP, Crozier MW, Moser MW, Struk AM, Wright TW. Management of bipolar shoulder injuries with humeral head allograft in patients with active, uncontrolled seizure disorder: case series and review of literature. JSES Int. 2022;6(1):132–136. doi: 10.1016/j.jseint.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thangarajah T, Falworth M, Lambert SM. Anatomical shoulder arthroplasty in epileptic patients with instability arthropathy and persistent seizures. J Orthop Surg (Hong Kong) 2017;25(2):2309499017717198. doi: 10.1177/2309499017717198. [DOI] [PubMed] [Google Scholar]