Abstract
The unusual behavior of the mutation ami36, which generates hyperrecombination in two point crosses, was previously attributed to a localized conversion process changing A/G mispairs into CG pairs. Although the mechanism was found to be dependent on the DNA polymerase I, the specific function responsible for this correction was still unknown. Analysis of the pneumococcal genome sequence has revealed the presence of an open reading frame homologous to the gene mutY of Escherichia coli. The gene mutY encodes an adenine glycosylase active on A/G and A/7,8-dihydro-8-oxoguanine (8-OxoG) mismatches, inducing their repair to CG and C/8-OxoG, respectively. Here we report that disrupting the pneumococcal mutY homologue abolishes the hyperrecombination induced by ami36 and leads to a mutator phenotype specifically enhancing AT-to-CG transversions. The deduced amino acid sequence of the pneumococcal MutY protein reveals the absence of four cysteines, highly conserved in the endonuclease III/MutY glycosylase family, which ligate a [4Fe-4S]2+ cluster. The actual function of this cluster is still intriguing, inasmuch as we show that the pneumococcal gene complements a mutY strain of E. coli.
In transformation of Streptococcus pneumoniae, double-stranded DNA binds to the membrane and is randomly cleaved (see reference 23 for a review). Then, single-stranded segments enter the cell from a 3′ end while the complementary strands are degraded to oligonucleotides with the opposite polarity (32). About half of the entering segments integrate into the chromosome by homologous recombination (21). The recombination process is RecA dependent (37), exchanges strands from 5′ to 3′ relative to the donor (43), and forms a donor-recipient structure which is heteroduplex when the donor and the recipient sequences are not identical (11). Both strands of the donor DNA have the same probability of entering a cell so that two complementary heteroduplexes are generated in equal frequency among the recipient bacteria (7). Pneumococcal transformation therefore allows study of the in vivo processing of heteroduplexes such as base-base mismatches. In particular, the variability observed in the transformation efficiencies of point mutations led to the discovery of a mismatch repair system (10, 20, 55). This system, called Hex, recognizes the different mismatches with varying efficiencies and induces the complete excision of the donor strand (31). Base mismatches are ranked as a function of decreasing repair efficiency by Hex as follows: G/T = A/C = G/G > C/T > A/A > T/T > A/G > C/C (6). The deletions and the additions of one or two nucleotides lead to mismatches that are very efficiently recognized by Hex (13, 14). The heterologies longer than 2 bases lead to heteroduplexes which are poorly recognized by Hex, and for those longer than 5 bases, there is no repair at all by Hex (13, 22) nor by any bacterial repair or conversion system (42).
A Hex-independent repair, specific for A/G mismatches, was found in S. pneumoniae. The existence of such a system was signalled by the hyperrecombination—i.e., the abnormally high frequency of wild-type transformants—shown by the mutation ami36 when involved in two point crosses (24). The mutation ami36 results from a CG-to-AT transversion and, consequently, forms upon transformation the mismatches A36/G+ and C+/T36. It was shown that only A/G triggers hyperrecombination (51), leading to an excess of wild-type but not double-mutant transformants (38). In addition, we know that this hyperrecombination requires the pneumococcal polA product, which is a functional homolog of the Escherichia coli PolI protein, and that upon transformation, about 50% of the A36/G+ mismatches lead to CG independently of the replication (41). These results have suggested the existence of a repair specifically changing A/G mismatches to CG pairs in S. pneumoniae. In contrast to Hex, this repair seems specific for A/G mismatches, changes A/G into CG irrespective of the recipient strand, and is closely localized around the mismatch (12).
Shortly after the proposal of an A/G-to-CG conversion in S. pneumoniae, a similar repair system was found in E. coli, depending on the gene mutY (2, 26). In vitro analyses have identified MutY as an adenine glycosylase specific for A/G mispairs (3). The complete A/G-to-CG repair requires a short patch resynthesis by the DNA polymerase I (47, 56). Further studies have suggested that the major in vivo substrate for MutY is the adenine from A/7,8-dihydro-8-oxoguanine (8-OxoG) mismatches (34). This finding indicates that MutY, as the 8-OxoG glycosylase MutM and the 8-OxodGTPase MutT, should antagonize the mutagenicity of 8-OxoG, a major product of oxidative damage of DNA (see references 17 and 35 for reviews). The gene mutY is partly homologous to the gene nth of E. coli, which encodes the DNA glycosylase endonuclease III (EndoIII) involved in the removal of oxidatively damaged pyrimidines (36). In particular, MutY and EndoIII display a four-cysteine domain which ligate a [4Fe-4S]2+ cluster presumably involved in specific DNA recognition (16, 44).
Based on the conserved domains shared by MutY, EndoIII, and a third related protein (40), we designed degenerate oligonucleotides to probe by Southern and PCR analysis the pneumococcal genome to identify a mutY homologue. Such investigations being unsuccessful we left this question, until most of the pneumococcal genome sequence became available, revealing the presence of a putative mutY homologue. However, this gene lacks the conserved cysteine domain, which may account for our previous failure to identify it by DNA probing. The aim of this work was to characterize the pneumococcal mutY homologue, mainly to analyze its involvement in the hyperrecombination induced by ami36 and its functional relatedness with the MutY protein of E. coli.
MATERIALS AND METHODS
Strains, plasmids, media, transformation procedures, and transformation efficiencies.
Strains and plasmids are listed in Table 1. The pneumococcal strains were cultured at 37°C in CAT medium (46). The selection of ami+ bacteria was carried out on synthetic medium containing excess isoleucine (50). S. pneumoniae was transformed as described (43). The transformation efficiency of a chromosomal marker is the number of transformants for this marker divided by the number of transformants for a reference marker also carried on the chromosome, in order to correct for fluctuations in competence. Usually the point mutation str41 which confers resistance to streptomycin is the reference marker.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or characteristics | Source or reference |
|---|---|---|
| S. pneumoniae | ||
| R800 | R6 derivative, ami+ hex+ | 29 |
| R801 | R800 hexB | 29 |
| 553 | R801 ami36 | Michel Sicarda |
| 800T51 | R800 mutY::pRT51 | This work |
| 801T51 | R801 mutY::pRT51 | This work |
| 553T51 | 553 mutY::pRT51 | This work |
| E. coli | ||
| DH5α | supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Bethesda Research Laboratories |
| AB1157 | thr-1 leu-6 thi-1 lacY1 galK2 ara-14 xyl-5 mtl-1 kdgK51 proA2 his-4 argE3 str-31 tsx33 supE44 | 2 |
| AB1157-Y11 | AB1157 mutY zgd::Tn10 | 2 |
| Plasmids | ||
| pR350 | pSK+ derivative, Apr Spr | 8 |
| pRT51 | pR350 with 229 bp internal to the pneumococcal mutY | This work |
| pAM238 | pGB2 derivative allowing lacZΔM15 α-complementation, Spr | Kaymeuang Cama |
| pAMY2 | pAM238 with the pneumococcal mutY on a 1,400-bp fragment | This work |
| pAMΔY | pAMY2 with deletion of the 600-bp upstream region of mutY | This work |
Laboratoire de Microbiologie et Génétique Moléculaires, Université Paul Sabatier, Toulouse, France.
The following antibiotics and concentrations were used for the selection of S. pneumoniae transformants or mutants: 5 μM methotrexate, rifampin (1 μg/ml), streptomycin (200 μg/ml), and spectinomycin (200 μg/ml). E. coli was grown in Luria-Bertani medium (48). For the detection of mutY-pAM238 recombinant clones, the white-blue screen was used by adding 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (40 μg/ml) and isopropyl-β-d-thiogalactoside (IPTG) (40 μg/ml). M9 minimal medium prepared as described (48) and supplemented with 0.2% Casamino acids was used with AB1157/pAMY2 strains for induction or repression of the lacZ promoter. Induction was achieved by adding 2 mM IPTG and 0.4% glycerol; repression was achieved by adding 0.4% glucose. Ligation products and plasmids were introduced in E. coli strains by electrotransformation. The antibiotics and concentrations used for the selection of E. coli transformants or mutants were ampicillin (100 μg/ml), rifampin (100 μg/ml), and spectinomycin (100 μg/ml).
DNA techniques.
Plasmids were extracted from E. coli by the alkaline lysis method (48). Chromosomal extraction of S. pneumoniae was performed as described (5). PCR amplifications were done with the Hot Tub DNA polymerase (Amersham). The oligonucleotides used for the amplification of the pneumococcal rpoB region involved in the resistance to rifampin were upstream, 5′-CGCTTCTTTGACCCACGTCG, and downstream, 5′-CCGTCAGCGATGAAATCGCC. Sequencing of the Rifr mutations was performed directly on the PCR products, with the CircumVent Thermal Cycle DNA Sequencing Kit (New England Biolabs) and using the following internal primer: 5′-GACAATGAAGTCTTGACACC. Sequencing the cloned mutY gene in the region of the expected cysteines was performed on the plasmid pAMY2 using a CEQ 2000 apparatus and a CEQ dye terminator cycle sequencing kit (Beckman). The oligonucleotide used to prime this sequence was: 5′-TGCGGGTCTTGGCGCGTCTG.
Construction of strains disrupted for the mutY homologue.
A 575-bp fragment, internal to the pneumococcal mutY homologue, was amplified from chromosomal DNA of the strain R800, using the following primers: upstream, 5′-TTGCCTTGGAGGAGAAGTAA, and downstream, 5′-ATTCTGATATGCCGCACTAA. The PCR product was digested with HindIII and HincII, generating an HindIII-HincII fragment of 227 bp and two flanking fragments. The 229-bp fragment was cloned in pR350 digested with HindIII and HincII. The resulting recombinant plasmid pRT51 was used to transform the S. pneumoniae strains R800, R801, and 553, in order to inactivate by homology-directed insertion the mutY-like gene (30). The transformants resistant to spectinomycin were selected.
Cloning of the pneumococcal mutY homologue.
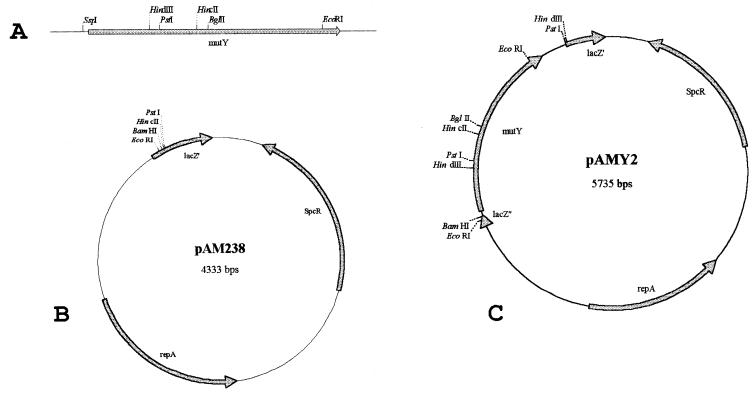
A DNA segment of 1,561 bp including the open reading frame (ORF) containing the mutY homologue was obtained by PCR amplification, using chromosomal DNA of the strain R800 as a template, and the following oligonucleotides: up, 5′-CAGCTTCACCTTGCCGTAGG, and down, 5′-TGCTCTAGCGCTTCACGACC. Further cloning is described in Fig. 1.
FIG. 1.
The 1,561-bp fragment (A) containing the pneumococcal mutY was cut with Ssp1, leaving 17 bp before the −35 region of the putative promoter. The resulting 1,402-bp fragment was directly ligated into pAM238 (B) linearized with the blunt cutting enzyme HincII. The ligation mix was used to transform the E. coli strain DH5α. Spectinomycin-resistant transformants leading to white colonies on IPTG–X-gal-containing medium were cultured, and their plasmids were extracted and analyzed by restriction digests, EcoRI, PstI, EcoRI/PstI, and HindIII. One recombinant clone, called pAMY2 (C), displaying the 1,402-bp fragment with the mutY gene oriented downstream of the lac promoter, was kept for further analysis.
Homology searches, genome analysis, and multiple sequence alignments.
Finding sequences sharing homology with the proteins MutY, EndoIII, and MutM of E. coli was performed with the TBLASTN search on microbial genomes, finished and unfinished (http://www.ncbi.nlm.nih.gov/BLAST /unfinishedgenome.html). Finding the complete sequence of the mutY gene of S. pneumoniae was possible due to the generosity of The Institute for Genomic Research (TIGR) allowing us to access the unfinished pneumococcal genome. Alignments of the protein sequences were performed using the Multalin program (http://www.toulouse.inra.fr/multalin.html).
Nucleotide sequence accession number.
The sequence of the internal region of the mutY gene of the pneumococcal strain R800 has been assigned EMBL accession no. AJ271596.
RESULTS
Presence of an atypical mutY homologue in the pneumococcal genome.
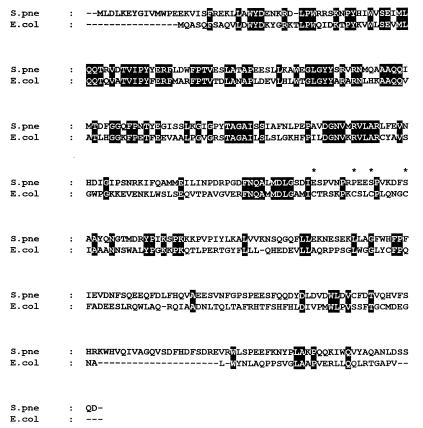
The hyperrecombination induced by ami36 in pneumococcus relies on an A/G-to-CG repair (51). Such a repair might be mediated by a protein related to MutY, the adenine glycosylase of E. coli which removes A from A/G mispairs, allowing their repair to CG pairs. Inasmuch as more than 90% of the pneumococcal genome sequence was available, we have looked for a mutY-like gene in this sequence. A BLAST search revealed the presence of a putative ORF whose deduced translation leads to a protein containing 391 amino acids and displaying 30% identity with MutY of E. coli (Fig. 2). This ORF lies in the contiguous region Sp80 of the pneumococcal genome sequenced by TIGR. The presence of putative promoter and terminator sequences upstream and downstream of the ORF suggests that it can be transcribed as a single gene. As shown (Fig. 2), the deduced amino acids sequence of the putative pneumococcal mutY gene does not display four cysteines, characteristic of the MutY/EndoIII family, and which are involved in the formation of a [4Fe-4S]2+ cluster. We have sequenced the internal region of the cloned mutY gene of our wild-type strain R800 and confirmed this atypical feature.
FIG. 2.
Alignment of the MutY amino acid sequence of E. coli (E.col) (GI accession number 1789331) and the 391-amino-acid sequence deduced from a putative ORF found in the S. pneumoniae (S.pne) genome sequence. Shading highlights identical amino acids in both sequences. The asterisks indicate the positions of the four cysteines present in the E. coli protein but lacking in the ORF of S. pneumoniae.
The mutY gene controls hyperrecombination.
In crosses involving two linked ami mutations, one donor and one recipient, breaks or recombinations occur between the two sites involved in the cross. Such breaks generate transformants which are either double ami mutants or ami+. The mutations of the ami locus confer resistance to methotrexate, but double mutants cannot be distinguished by this procedure, while ami+ bacteria are selectable in synthetic medium containing excess isoleucine (Iler) (50). The number of breaks and recombination events occurring between two closely linked sites, thus the number of Iler transformants, is proportional to the physical distance separating the two sites (4). With regard to this rule of proportionality, ami36 generates an abnormally high frequency of ami+ transformants. For example, crosses between ami36 and ami6, 27 bp apart, produce 20 to 25% ami+ bacteria while about 1% is predicted by the distance (24). The pneumococcal strains 553 (hexB ami36 mutY+) and 553T51 (hexB ami36 mutY::pRT51), were transformed with a chromosomal DNA carrying the mutations ami6 and str41 in order to test whether the hyperrecombination induced by ami36 was affected by the inactivation of mutY. Hex-deficient strains were used as the recipients to avoid interferences between the Hex system and the hyperrecombination process (24). Hyperrecombination was abolished in the strain 553T51 (Table 2). The proportion of double transformants, ami+ str41, which lead to bigger colonies than single str41 transformants, was 20 times lower in the strain 553T51, confirming that hyperrecombination was suppressed.
TABLE 2.
Inactivation of mutY abolishes hyperrecombination
| Recipient strain | Donor DNA | No. of transformants/ml
|
Recombination indexa | |
|---|---|---|---|---|
| Ami+ | Smr | |||
| 553 (ami36 hexB mutY+) | ami6 str41 | 7.9 × 105 | 3.8 × 106 | 21 |
| 553T51 (ami36 hexB mutY) | ami6 str41 | 3.5 × 104 | 3.6 × 106 | 1 |
The ratio of Ami+ to Smr transformants, given as a percentage.
Disruption of the pneumococcal mutY confers a mutator phenotype.
Hyperrecombination depends on the repair to CG of an A/G mismatch formed upon transformation. If this repair is also active on the mismatches which form spontaneously within the chromosome, the mutants affected for this function might display a high mutation frequency. The mutation frequencies of resistance to streptomycin (Smr), rifampin (Rifr), and methotrexate (Mtxr) of the pneumococcal strains R800 and 800T51 were investigated (Tables 3 and 4). Single colonies were picked and used to inoculate liquid cultures that were then grown to stationary phase and plated on selective media (Table 3). Alternatively, the colonies were picked and directly streaked on solid selective media to score the mutants appeared within the colonies (Table 4). While the rate of Smr mutants remained unchanged (not shown), both methods indicate that the proportions of Mtxr and Rifr mutants were enhanced in 800T51 compared with R800. Streaking the colonies on selective media and scoring the mutants grown on the streaks appears to be an easy way to estimate accurately the mutation frequency.
TABLE 3.
Mutator phenotype of pneumococcal strain 800T51 as shown by plating liquid culturesa
| Strain | No. of cultures with mutants/total no. of cultures
|
Avg no. of mutant colonies/109 bacteria
|
Frequency of mutant colonies relative to R800
|
|||
|---|---|---|---|---|---|---|
| Mtxr | Rifr | Mtxr | Rifr | Mtxr | Rifr | |
| R800 (hex+ mutY+) | 10/10 | 22/42 | 4,700 | 16 | 1 | 1 |
| 800T51 (hex+ mutY) | 10/10 | 39/39 | 21,000 | 530 | 4 | 33 |
Liquid cultures inoculated from single colonies were grown to stationary phase and plated on antibiotic agar plates (40 μl was plated for Mtxr; 400 μl was plated for Rifr).
TABLE 4.
Mutator phenotype of pneumococcal strain 800T51 as shown by streaking coloniesa
| Strain | No. of streaks with mutants/total no. of streaks
|
Avg no. of mutant colonies/streak
|
Frequency of mutant colonies relative to R800
|
|||
|---|---|---|---|---|---|---|
| Mtxr | Rifr | Mtxr | Rifr | Mtxr | Rifr | |
| R800 (hex+ mutY+) | 41/57 | 28/420 | 1.2 | 0.069 | 1 | 1 |
| 800T51 (hex+ mutY) | 71/73 | 138/469 | 3.6 | 0.35 | 3 | 5 |
Single colonies were picked and directly inoculated on antibiotic agar plates by a single streak.
The pneumococcal mutY gene specifically prevents CG-to-AT transversions.
Mtxr mutations, which inactivate the ami operon, may arise throughout within the 6,000 bp of the ami locus. The sequence determination of the mutations appearing in such a long locus requires at first the localization of these mutations. By contrast, the Rifr mutations are usually located in the gene rpoB, which encodes the β-subunit of the RNA polymerase, and map mostly in a region of about 300 bases, called cluster I in E. coli (19). The determination of the DNA changes which arise in independent Rifr mutants should reveal the mutator specificity of the strain 800T51. Searching the pneumococcal genome sequence (with the TIGR database) indicates that cluster I is almost identical in pneumococcus and other bacteria, as confirmed recently by Enright et al. (9). A 1,500-bp segment including cluster I was amplified in independent Rifr mutants. All the amplified fragments carried the Rifr mutation as verified by their ability to transform a Rifs strain to the Rifr phenotype. A third oligonucleotide located 50 bp upstream of cluster I was used to prime the sequencing reactions directly on the PCR products. Out of 23 independent Rifr mutants, the mutations were found on three codons of cluster I, among which was the codon for serine 495 (Table 5). Rifr mutations in this codon were previously reported in E. coli but not in S. pneumoniae (9, 19). We have detected GC-to-TA (and CG-to-AT) transversions in three types of Rifr mutants out of five types characterized. Such transversions represent 3 mutations out of 9 sequenced in R800, and 13 mutations out of 14 sequenced in 800T51. CG-to-AT transversions are therefore specifically enhanced in the strain disrupted for the mutY homologue.
TABLE 5.
Sequences of the Rifr mutationsa
| Strain and Rif type | Triplet 1465–1467 (a.a. 489) | Triplet 1483–1485 (a.a. 495) | Triplet 1465–1467 (a.a. 499) | No. of mutants |
|---|---|---|---|---|
| R800 (hex+ mutY+) | ||||
| Wild-type Rifs | GAC (Asp) | TCT (Ser) | CAC (His) | |
| Rifr type 1 | TAC (Tyr) | 1 | ||
| Rifr type 2 | TTT (Phe) | 2 | ||
| Rifr type 3 | AAC (Asn) | 2 | ||
| Rifr type 4 | TAC (Tyr) | 4 | ||
| 800T51 (hex+ mutY) | ||||
| Rifr type 1 | TAC (Tyr) | 3 | ||
| Rifr type 5 | TAT (Tyr) | 4 | ||
| Rifr type 3 | AAC (Asn) | 6 | ||
| Rifr type 4 | TAC (Tyr) | 1 |
Triplets and amino acid (a.a.) numbers are relative to the pneumococcal rpoB gene. Asp 489, Ser 495, and His 499 correspond to Asp 516, Ser 522, and His 526, respectively, in the E. coli rpoB gene. Only the mutated codons are indicated in the rows of data for Rifr types.
Transformation efficiencies of CG-to-AT transversions.
Crosses between ami36 and ami6 produce about 20% ami+ transformants instead of the 1% predicted on the basis of the 27 bp separating the two mutations. The correction of A36/G+ mismatches to C+G+ pairs by MutY is responsible for this excess of ami+ transformants. In transformations of an ami+ strain with ami36 DNA, the MutY correction must occur similarly, changing the same amount of A/G mismatches to CG pairs. In a mutY+ strain, the transformation efficiency of ami36 should display a decrease of about 20% compared with the transformation efficiency in a mutY strain. If we assume that in a hexB mutY background, i.e., without any potential repair, the transformation efficiency of ami36 is 1, then a 20% decrease in a hexB mutY+ should lead to an efficiency of 0.8. Despite the normal fluctuation occurring in the measurements of the transformation efficiencies, an efficiency of 0.8 should be distinct from an efficiency of 1. We have constructed a hexB mutY strain by disrupting the mutY gene of the hexB strain R801, leading to the strain 801T51. Chromosomal DNA containing the mutations ami36 and str41 was used to transform R801 (hexB mutY+ ami+) and 801T51 (hexB mutY ami+). The same transformations were carried out with chromosomal DNA containing the mutations ami6 and str41. ami6 is a spontaneous mutation corresponding to a GC-to-AT transition (6), which generates upon transformation the mismatches A/C and G/T. On average (Table 6) and in a reproducible way, ami36 displays a transformation efficiency reduced by 0.2 in the mutY+ background, while ami6 remains mostly unaffected.
TABLE 6.
Transformation efficiencies of ami and Rifr mutations
| Cross (straina × DNAb) | Transformation efficienciesc measured in independent crossesd | Avg efficiency |
|---|---|---|
| R801 × ami36 | 0.62, 0.77, 0.82, 0.88, 0.88, 0.88, 0.91, 0.91, 0.92, 1.03, 1.19 | 0.89 |
| 801T51 × ami36 | 0.86, 0.9, 0.95, 1.03, 1.06, 1.10, 1.10, 1.13, 1.35, 1.38 | 1.09 |
| R801 × ami6 | 0.73, 0.77, 1.07, 1.10, 1.10, 1.17, 1.22, 1.28 | 1.06 |
| 801T51 × ami6 | 0.83, 0.92, 0.96, 0.96, 0.98, 1.04, 1.1, 1.19, 1.25, 1.25 | 1.05 |
| R801 × Rifr type 1 | 0.85, 0.97, 1.15 | 0.99 |
| 801T51 × Rifr type 1 | 0.78, 1, 1.05 | 0.94 |
| R801 × Rifr type 3 | 0.91, 0.93, 1.01 | 0.95 |
| 801T51 × Rifr type 3 | 0.94, 1.09, 1.13 | 1.05 |
| R801 × Rifr type 5 | 0.92, 0.94 | 0.93 |
| 801T51 × Rifr type 5 | 1.06, 1.23 | 1.14 |
| R801 × Rifr type 4 | 0.81, 0.93, 1.01 | 0.92 |
| 801T51 × Rifr type 4 | 1.03, 1.05, 1.06 | 1.05 |
R801 is hexB mutY+ and 801T51 is hexB mutY.
Each DNA carries the mutation str41 in addition to the indicated ami or rif mutation.
Ratios of Mtxr to Smr transformants for ami36 and ami6 as donor DNAs and of Rifr to Smr transformants for Rifr type 1, 3, 4, and 5 as donor DNAs.
In each cross two independent platings were done for each phenotype (Mtxr, Rifr, or Smr), to give the averaged indices indicated.
To test the same question with CG-to-AT mutations other than ami36, we have measured the transformation efficiencies of different Rifr mutations previously sequenced (Table 5). The DNAs extracted from the Rifr mutants were used to transform a strain with the mutation str41. The Rifr transformants were selected, and their DNAs, carrying both a Rifr mutation and str41, were used to transform the strains R801 and 801T51. The ratios of Rifr to Smr transformants were measured as transformation efficiencies. On average, the differences in transformation efficiency observed between a mutY and a mutY+ background were −0.05, 0.1, and 0.21 for CG-to-AT mutations type 1, 3, and 5, respectively. These values are weak and are close to 0.13, the efficiency measured for the Rifr mutation type 4, which does not generate an A/G mismatch upon transformation. Although generating a donor-recipient A/G mismatch, these Rifr mutations are globally not, or not strongly, affected by the MutY repair. However, we cannot exclude the possibility that Rifr type 5 has undergone a significant repair, close to the one observed for ami36.
Complementation of a mutY strain of E. coli.
To investigate whether the mutY gene of S. pneumoniae could complement a mutY mutant of E. coli, we cloned it into pAM238, a low-copy-number plasmid. In the recombinant plasmid pAMY2, the pneumococcal gene, which nevertheless keeps its own putative promoter, is oriented so that transcription could also occur from the lacZ promoter. The control plasmid pAMΔY derives from pAMY2 by BamHI-BglII digestion (Fig. 1), leading to a 599-bp deletion, including the promoter and the 545 adjacent base pairs of the pneumococcal mutY gene. The strains AB1157 and AB1157-Y11 containing pAMY2 or pAMΔY were grown on Luria-Bertani agar plates. Independent colonies were streaked on rifampin-containing medium to analyze the mutator phenotype (Table 7). Suppression of the mutY mutator phenotype was observed with pAMY2, not with pAMΔY, suggesting that the pneumococcal mutY gene encodes a protein functionally similar to the adenine glycosylase MutY of E. coli. The complementation was confirmed from liquid cultures and from colonies grown on minimal medium allowing or not allowing gene induction from the lacZ promoter (not shown). The repression of the lacZ promoter had no influence on the complementation by pAMY2, suggesting that the cloned gene is transcribed from its own promoter.
TABLE 7.
The pneumococcal mutY+ gene complements an E. coli mutY strain
| E. coli strain (genotype) | Plasmid | No. of streaks with mutant coloniesa/total no. of streaks | Avg no. of mutants/streak |
|---|---|---|---|
| AB1157 (mutY+) | pAMΔY | 5/40 | 0.2 |
| AB1157 (mutY+) | pAMY2 | 5/34 | 0.3 |
| AB1157Y11 (mutY) | pAMΔY | 27/39 | 3 |
| AB1157Y11 (mutY) | pAMY2 | 7/38 | 0.3 |
Single colonies of the indicated strain containing the indicated plasmid were picked and inoculated on rifampin agar plates by a single streak.
DISCUSSION
The hyperrecombination induced by the mutation ami36 is triggered by an A/G-to-CG correction system requiring the DNA polymerase I of pneumococcus. However, the genetic control of this repair was still unknown. As the sequence flanking ami36 looked important for this hyperrecombination (12, 24), it was actually unclear whether this pathway was more related to MutY, which repairs A/G to CG, or to the VSP pathway, which repairs G/T to GC in defined sequence environments (reviewed in reference 25). As we show here that the disruption of a pneumococcal mutY homologue abolishes hyperrecombination, we can assume that the main protein controlling the A/G-to-CG repair involved in this hyperrecombination is a MutY-like adenine glycosylase. We have found that mutY strains are mutators, suggesting that this pathway is active not only on the mismatches occurring upon transformation, but also on the spontaneous ones. The Rifr mutation pattern reveals an enhancement of CG-to-AT transversions in the pneumococcal mutY background, which is expected if MutY repairs A/G mismatches to CG pairs. Also confirming the specialized action of mutY is the fact that no increase in the Smr mutant frequency was detected in a mutY background. Indeed, only AT-to-CG transversions in the str gene, which encodes the ribosomal protein S12, have been found to confer the Smr phenotype (33).
A comparison between the spontaneous mutation frequencies and the transformation efficiencies of the Rifr mutations characterized in this work is relevant. In a mutY background, we have observed at least a fivefold increase of the frequency of Rifr mutants (Table 4). These mutants are mostly due to CG-to-AT mutations (Table 5), and we can estimate at least a 10-fold increase of these transversions. Such an increase is observed in a mutY-deficient strain, suggesting that, in a mutY+ background, at least 90% of the A/G mismatches are corrected to CG pairs if the pneumococcal MutY is specific for A/G mispairs. Transforming Rifr bacteria with a DNA mutated to Rifr by a CG-to-AT change generates either A/G or C/T mismatches in the recipient cells. Assuming that MutY repairs at least 90% of the A/G mismatches to CG pairs and has no effect on C/T mismatches, the transformation efficiency of such Rifr mutations should be reduced by at least 45% in a mutY+ strain compared with a mutY strain. Such a reduction was not observed. The best decrease we have detected is a 20% decrease with Rifr type 5. The transformation efficiencies of the Rifr mutations rather suggest that the MutY system has a weak effect on A/G mismatches created upon transformation. To render this observation compatible with the high and specific mutagenicity of mutY strains, we propose that the A/G mismatches which form upon transformation might be structurally different from those which form spontaneously, in particular because upon transformation a three-stranded DNA could be formed.
Alternatively, the best substrate for the pneumococcal system might not be A/G but A/8-OxoG mismatches. In E. coli, A/8-OxoG is thought to be the primary in vivo substrate for the adenine glycosylase MutY and the major cause of CG to-AT-transversions observed in the mutY strains (34). As the pneumococcal wild-type gene cloned on a low-copy-number plasmid suppresses the mutator phenotype of an E. coli mutY mutant, we can assume that the pneumococcal function is similar to that of the E. coli MutY glycosylase and must be able to trigger the change A/8-OxoG to C/8-OxoG. In addition, it is well established in E. coli that MutY cooperates with the proteins MutT and MutM to form an antimutator system specialized against the mutagenic potential of 8-OxoG, which relies mainly on its capability to pair with adenine (53). MutT, first identified as a dGTPase, is mainly involved in the hydrolysis of oxodGTP to oxodGMP (27), and MutM is a DNA glycosylase specialized for the removal of 8-OxoG from C/8-OxoG mispairs (54). Interestingly, the pneumococcal mutX gene was found to be functionally similar to mutT (33), and searching the pneumococcal genome reveals a putative mutM-like gene. The presence of three genes in S. pneumoniae, homologous to mutY, mutT, and mutM, strongly suggests that the same antimutator system exists in S. pneumoniae. The oxidative stress and the mutagenicity it generates must be actual problems for this nonrespiratory bacterium.
The deduced amino acid sequence of the pneumococcal MutY does not display the highly conserved stretch of four cysteines, Cys-X6-Cys-X2-Cys-X5-Cys, which coordinates a [4Fe-4S]2+ cluster loop (36), called FCL. Searching the genome sequences reveals no eukaryotic MutY lacking this domain (not shown) and just two bacterial MutY proteins lacking the cysteine domain, those of Treponema pallidum and Streptococcus pyogenes (Fig. 3). The MutY of S. pyogenes is similar to the pneumococcal one, in particular in the region where the cysteines were expected (Fig. 3), suggesting that the FCL domain was absent before divergence of the two species. In E. coli this domain is reported to be crucial for the specific DNA substrate recognition by MutY (16, 44). As the pneumococcal mutY displays a CG-to-AT antimutator action which complements the MutY deficiency in E. coli, the absence of an FCL domain in the pneumococcal protein is intriguing. Structural differences in the protein of S. pneumoniae might compensate for the absence of an iron-sulfur cluster. The C-terminal domain of the pneumococcal protein, which is reported in E. coli to be more specifically involved in 8-OxoG recognition (15, 39), is more divergent than the rest of the protein (Fig. 2) and could lead to structural differences. Nevertheless, despite its antimutator effect complementing in vivo the MutY deficiency of E. coli, the pneumococcal glycosylase might display a weaker activity than the E. coli glycosylase especially toward the A/G mismatch. In E. coli, although A/G is not the main in vivo substrate of MutY, in vitro studies indicate that the relative glycosylase activity of MutY toward A/G is not weaker than toward A/8-Oxo-G (34), although the kinetic and the mechanism of the reaction are not identical for A/G and A/8-oxoG (45). In S. pneumoniae, the correction by MutY of A/G mismatches is undetected upon transformation of Rifr mutations types 1 and 3 (Table 6). It is detected but weak upon transformation of ami36 and, less significantly, of Rifr mutation type 5 (Table 6). Previous studies have indicated that the sequence adjacent to the A/G mismatch is important for hyperrecombination and should be related to the structure generated by ami36: 5′ATTAAT-3′TAAGTA (12). The heteroduplex structures generated by the Rifr mutations are as follows, for type 1, 3, and 5, respectively: 5′CCAAGT-3′GGTGCA, 5′TCTAAC-3′AGAGTG, and 5′TTTATG-3′AAAGAC. Out of the three tested Rifr mutations, type 5 only might have undergone a detectable repair by MutY. Interestingly, the heteroduplex structure generated by Rifr mutation type 5 is quite related to the one generated by ami36. In particular three AT pairs are localized 5′ to the mismatched adenine in both ami36 and Rifr type 5. An attractive hypothesis is that the absence of an FCL domain on the pneumococcal MutY is specifically detrimental to the repair of A/G mismatches, which consequently might occur in a few sequence contexts only. Although the biological importance of A/G mismatches arising in vivo is unknown, they may represent a minor source of CG-to-AT transversions. The relatively high AT content of the pneumococcal chromosome, 60% compared with 50% in E. coli, might originate in part in the inefficient repair of A/G mismatches by a handicapped MutY protein.
FIG. 3.
Alignment of the cysteine-containing region in some bacterial MutY homologues. Asterisks indicate the position of the cysteines. Shading highlights amino acid identity in at least 6 sequences out of 10. Origins of the proteins and their GI accession numbers (in parentheses) are as follows: H.pyl, Helicobacter pylori (4154640); B.sub, Bacillus subtilis (2633186); H.inf, Haemophilus influenzae (1573768); E.col, E. coli (1789331); N.men, Neisseria meningitidis (3860539); S.pne, S. pneumoniae; S.pyo, Streptococcus pyogenes; M.tub, Mycobacterium tuberculosis (2950412); S.coe, Streptomyces coelicolor (4585587); T.pal., T. pallidum (3322622).
The unusual lack of the FCL domain in the pneumococcal MutY made us wonder whether similar features are also absent from related proteins in S. pneumoniae. We have detected in the pneumococcal genome a putative MutM homologue and a putative EndoIII homologue. MutM is an oxoG glycosylase specific for A/8-OxoG mismatches and displays a zinc finger motif coordinated by four cysteines (54). The same structure was found in the putative MutM homologue of S. pneumoniae. EndoIII has a glycosylase activity specific for damaged pyrimidines and usually displays a four-cysteine domain identical to the MutY one, also ligating a [4Fe-4S]2+ cluster. Interestingly, the putative EndoIII found in S. pneumoniae displays only the two first cysteines of a putative four-cysteine domain (not shown). To support this observation, homologues of the pneumococcal EndoIII protein were searched for in the microbial genomes. An amino acid sequence sharing the same half-cysteine domain was found in Streptococcus mutans only, a streptococcal species strongly related to S. pneumoniae. This observation suggests that the EndoIII of some streptococcal species has lost the carboxy-terminal part of an ancestral cysteine domain and should lack the iron-sulfur cluster. That an iron-sulfur cluster is lacking in EndoIII should not indicate that the activity is lost. Two EndoIII activities have been characterized in Saccharomyces cerevisiae, one of which lacks the [4Fe-4S]2+ cluster. Both are active but display some differences in the substrate specificities (1, 49). Concerning S. pneumoniae, the absence of a [4Fe-4S]2+ ligating domain in both MutY and the putative EndoIII suggests that both proteins have evolved so that the iron-sulfur cluster is not required. It is tempting to speculate that the scarcity of iron in the natural environment of S. pneumoniae and its importance for bacterial growth and virulence (18, 52), have partly oriented such an evolution.
ACKNOWLEDGMENTS
We are very grateful to Paul Modrich for giving us the strain AB1157-Y11 and for editing the manuscript and to Kaymeuang Cam for the gift of the plasmid pAM238. We of course thank Michel Sicard for giving us the virus of hyperrecombination.
REFERENCES
- 1.Alseth I, Eide L, Pirovano M, Rognes T, Seeberg E, Bjoras M. The Saccharomyces cerevisiae homologues of endonuclease III from Escherichia coli, Ntg1 and Ntg2, are both required for efficient repair of spontaneous and induced oxidative DNA damage in yeast. Mol Cell Biol. 1999;19:3779–3787. doi: 10.1128/mcb.19.5.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Au K G, Cabrera M, Miller J H, Modrich P. Escherichia coli mutY gene product is required for specific A-G→C · G mismatch correction. Proc Natl Acad Sci USA. 1988;85:9163–9166. doi: 10.1073/pnas.85.23.9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Au K G, Clark S, Miller J H, Modrich P. Escherichia coli mutY gene encodes an adenine glycosylase active on G-A mispairs. Proc Natl Acad Sci USA. 1989;86:8877–8881. doi: 10.1073/pnas.86.22.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claverys J P, Lataste H, Sicard A M. Localization of two EcoRI restriction sites within the amiA locus in pneumococcus: relationship between the physical and the genetic map. In: Glover S W, Butler L O, editors. Transformation. Oxford, United Kingdom: Cotswold; 1979. pp. 161–169. [Google Scholar]
- 5.Claverys J P, Louarn J M, Sicard A M. Cloning of Streptococcus pneumoniae DNA: its use in pneumococcal transformation and in studies of mismatch repair. Gene. 1981;13:65–73. doi: 10.1016/0378-1119(81)90044-5. [DOI] [PubMed] [Google Scholar]
- 6.Claverys J P, Méjean V, Gasc A M, Sicard A M. Mismatch repair in Streptococcus pneumoniae: relationship between base mismatches and transformation efficiencies. Proc Natl Acad Sci USA. 1983;80:5956–5960. doi: 10.1073/pnas.80.19.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claverys J P, Roger M, Sicard A M. Excision and repair of mismatched base pairs in transformation of Streptococcus pneumoniae. Mol Gen Genet. 1980;178:191–201. doi: 10.1007/BF00267229. [DOI] [PubMed] [Google Scholar]
- 8.Dintilhac A, Alloing G, Granadel C, Claverys J P. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol. 1997;25:727–739. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- 9.Enright M, Zawadski P, Pickerill P, Dowson C G. Molecular evolution of rifampicin resistance in Streptococcus pneumoniae. Microb Drug Resist. 1998;4:65–70. doi: 10.1089/mdr.1998.4.65. [DOI] [PubMed] [Google Scholar]
- 10.Ephrussi-Taylor H, Gray T C. Genetic studies of recombining DNA in pneumococcal transformation. J Gen Physiol. 1966;49:211–231. doi: 10.1085/jgp.49.6.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox M S, Allen M K. On the mechanism of deoxyribonucleate integration in pneumococcal transformation. Proc Natl Acad Sci USA. 1964;52:412–419. doi: 10.1073/pnas.52.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia P, Gasc A M, Kyriakidis X, Baty D, Sicard M. DNA sequences required to induce localized conversion in Streptococcus pneumoniae transformation. Mol Gen Genet. 1988;214:509–513. doi: 10.1007/BF00330488. [DOI] [PubMed] [Google Scholar]
- 13.Gasc A M, Garcia P, Baty D, Sicard A M. Mismatch repair during pneumococcal transformation of small deletions produced by site-directed mutagenesis. Mol Gen Genet. 1987;210:369–372. doi: 10.1007/BF00325708. [DOI] [PubMed] [Google Scholar]
- 14.Gasc A M, Sicard A M. Frame-shift mutants induced by quinacrine are recognized by the mismatch repair system in Streptococcus pneumoniae. Mol Gen Genet. 1986;203:269–273. doi: 10.1007/BF00333965. [DOI] [PubMed] [Google Scholar]
- 15.Gogos A, Cillo J, Clarke N D, Lu A L. Specific recognition of A/G and A/7,8-dihydro-8-oxoguanine (8-oxoG) mismatches by Escherichia coli MutY: removal of the C-terminal domain preferentially affects A/8-oxoG recognition. Biochemistry. 1996;35:16665–16671. doi: 10.1021/bi960843w. [DOI] [PubMed] [Google Scholar]
- 16.Golinelli M P, Chmiel N H, David S S. Site-directed mutagenesis of the cysteine ligands to the [4Fe-4S] cluster of Escherichia coli MutY. Biochemistry. 1999;38:6997–7007. doi: 10.1021/bi982300n. [DOI] [PubMed] [Google Scholar]
- 17.Grollman A P, Moriya M. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 1993;9:246–249. doi: 10.1016/0168-9525(93)90089-z. [DOI] [PubMed] [Google Scholar]
- 18.Hammerschmidt S, Bethe G H, Remane P, Chhatwal G S. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect Immun. 1999;67:1683–1687. doi: 10.1128/iai.67.4.1683-1687.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin D J, Gross C A. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J Mol Biol. 1988;202:45–58. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- 20.Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970;101:373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacks S, Greenberg B, Carlson K. Fate of donor DNA in pneumococcal transformation. J Mol Biol. 1967;29:327–347. [Google Scholar]
- 22.Lacks S A, Dunn J J, Greenberg B. Identification of base mismatches recognized by the heteroduplex-DNA-repair system of Streptococcus pneumoniae. Cell. 1982;31:327–336. doi: 10.1016/0092-8674(82)90126-x. [DOI] [PubMed] [Google Scholar]
- 23.Lacks S A. Binding and entry of DNA in bacterial transformation. In: Reissig J L, editor. Microbial interactions. London, United Kingdom: Chapman and Hall; 1977. pp. 179–232. [Google Scholar]
- 24.Lefevre J C, Gasc A M, Burger A C, Mostachfi P, Sicard A M. Hyperrecombination at a specific DNA sequence in pneumococcal transformation. Proc Natl Acad Sci USA. 1984;81:5184–5188. doi: 10.1073/pnas.81.16.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieb M, Bhagwat A S. Very short patch repair: reducing the cost of cytosine methylation. Mol Microbiol. 1996;20:467–473. doi: 10.1046/j.1365-2958.1996.5291066.x. [DOI] [PubMed] [Google Scholar]
- 26.Lu A L, Chang D Y. A novel nucleotide excision repair for the conversion of an A/G mismatch to C/G base pair in E. coli. Cell. 1988;9:805–812. doi: 10.1016/s0092-8674(88)91109-9. [DOI] [PubMed] [Google Scholar]
- 27.Maki H, Sekiguchi M. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature. 1992;355:273–275. doi: 10.1038/355273a0. [DOI] [PubMed] [Google Scholar]
- 28.Manuel R C, Czerwinski E W, Lloyd R S. Identification of the structural and functional domains of MutY, an Escherichia coli DNA mismatch repair enzyme. J Biol Chem. 1996;271:16218–16226. doi: 10.1074/jbc.271.27.16218. [DOI] [PubMed] [Google Scholar]
- 29.Martin B, Prats H, Claverys J P. Cloning of the hexA mismatch-repair gene of Streptococcus pneumoniae and identification of the product. Gene. 1985;34:293–303. doi: 10.1016/0378-1119(85)90138-6. [DOI] [PubMed] [Google Scholar]
- 30.Méjean V, Claverys J P, Vasseghi H, Sicard A M. Rapid cloning of specific DNA fragments of Streptococcus pneumoniae by vector integration into the chromosome followed by endonucleolytic excision. Gene. 1981;15:289–293. doi: 10.1016/0378-1119(81)90139-6. [DOI] [PubMed] [Google Scholar]
- 31.Méjean V, Claverys J P. Effect of mismatched base pairs on the fate of donor DNA in transformation of Streptococcus pneumoniae. Mol Gen Genet. 1984;197:467–471. doi: 10.1007/BF00329944. [DOI] [PubMed] [Google Scholar]
- 32.Méjean V, Claverys J P. DNA processing during entry in transformation of Streptococcus pneumoniae. J Biol Chem. 1993;268:5594–5599. [PubMed] [Google Scholar]
- 33.Méjean V, Salles C, Bullions L C, Bessman M J, Claverys J P. Characterization of the mutX gene of Streptococcus pneumoniae as a homologue of Escherichia coli mutT, and tentative definition of a catalytic domain of the dGTP pyrophosphohydrolases. Mol Microbiol. 1994;11:323–303. doi: 10.1111/j.1365-2958.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 34.Michaels M L, Cruz C, Grollman A P, Miller J H. Evidence that MutY and MutM combine to prevent mutations by an oxidatively damaged form of guanine in DNA. Proc Natl Acad Sci USA. 1992;89:7022–7025. doi: 10.1073/pnas.89.15.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michaels M L, Miller J H. The GO system protects organisms from the mutagenic effect of the spontaneous lesion 8-hydroxyguanine (7,8-dihydro-8-oxoguanine) J Bacteriol. 1992;174:6321–6325. doi: 10.1128/jb.174.20.6321-6325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michaels M L, Pham L, Nghiem Y, Cruz C, Miller J H. MutY, an adenine glycosylase active on G-A mispairs, has homology to endonuclease III. Nucleic Acids Res. 1990;18:3841–3845. doi: 10.1093/nar/18.13.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mortier-Barriere I, de Saizieu A, Claverys J P, Martin B. Competence-specific induction of recA is required for full recombination proficiency during transformation in Streptococcus pneumoniae. Mol Microbiol. 1998;27:159–170. doi: 10.1046/j.1365-2958.1998.00668.x. [DOI] [PubMed] [Google Scholar]
- 38.Mostachfi P, Sicard A M. Polarity of localised conversion in Streptococcus pneumoniae transformation. Mol Gen Genet. 1987;208:361–363. doi: 10.1007/BF00330467. [DOI] [PubMed] [Google Scholar]
- 39.Noll D M, Gogos A, Granek J A, Clarke N D. The C-terminal domain of the adenine-DNA glycosylase MutY confers specificity for 8-oxoguanine adenine mispairs and may have evolved from MutT, an 8-oxodGTPase. Biochemistry. 1999;38:6374–6379. doi: 10.1021/bi990335x. [DOI] [PubMed] [Google Scholar]
- 40.Nolling J, van Eeden F J, Eggen R I, de Vos W M. Modular organization of related Archaeal plasmids encoding different restriction-modification systems in Methanobacterium thermoformicicum. Nucleic Acids Res. 1992;20:6501–6507. doi: 10.1093/nar/20.24.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasta F, Sicard M A. Hyperrecombination in pneumococcus: A/G to C · G repair and requirement for DNA polymerase I. Mutat Res. 1994;315:113–122. doi: 10.1016/0921-8777(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 42.Pasta F, Sicard A M. Exclusion of long heterologous insertions and deletions from the pairing synapsis in pneumococcal transformation. Microbiology. 1996;142:695–705. doi: 10.1099/13500872-142-3-695. [DOI] [PubMed] [Google Scholar]
- 43.Pasta F, Sicard A M. Polarity of recombination in transformation of Streptococcus pneumoniae. Proc Natl Acad Sci USA. 1999;96:2943–2948. doi: 10.1073/pnas.96.6.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porello S L, Cannon M J, David S S. A substrate recognition role for the [4Fe-4S]2+ cluster of the DNA repair glycosylase MutY. Biochemistry. 1998;37:6465–6475. doi: 10.1021/bi972433t. [DOI] [PubMed] [Google Scholar]
- 45.Porello S L, Leyes A E, David S S. Single-turnover and pre-steady-state kinetics of the reaction of the adenine glycosylase MutY with mismatch-containing DNA substrates. Biochemistry. 1998;37:14756–14764. doi: 10.1021/bi981594+. [DOI] [PubMed] [Google Scholar]
- 46.Porter R D, Guild W R. Characterization of some pneumococcal bacteriophages. J Virol. 1976;19:659–667. doi: 10.1128/jvi.19.2.659-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radicella J P, Clark E A, Chen S, Fox M S. Patch length of localized repair events: role of DNA polymerase I in mutY-dependent mismatch repair. J Bacteriol. 1993;175:7732–7736. doi: 10.1128/jb.175.23.7732-7736.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 49.Senturker S, Auffret van der Kemp P, You H J, Doetsch P W, Dizdaroglu M, Boiteux S. Substrate specificities of the ntg1 and ntg2 proteins of Saccharomyces cerevisiae for oxidized DNA bases are not identical. Nucleic Acids Res. 1998;26:5270–5276. doi: 10.1093/nar/26.23.5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sicard A M. A new synthetic medium for Diplococcus pneumoniae and its use for the study of reciprocal transformation at the ami locus. Genetics. 1964;50:31–44. doi: 10.1093/genetics/50.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sicard M, Lefevre J C, Mostachfi P, Gasc A M, Sarda C. Localized conversion in Streptococcus pneumoniae recombination: heteroduplex preference. Genetics. 1985;110:557–568. doi: 10.1093/genetics/110.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tai S S, Lee C J, Winter R E. Hemin utilization is related to virulence of Streptococcus pneumoniae. Infect Immun. 1993;61:5401–5405. doi: 10.1128/iai.61.12.5401-5405.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tajiri T, Maki H, Sekiguchi M. Functional cooperation of MutT, MutM and MutY proteins in preventing mutations caused by spontaneous oxidation of guanine nucleotide in Escherichia coli. Mutat Res. 1995;336:257–267. doi: 10.1016/0921-8777(94)00062-b. [DOI] [PubMed] [Google Scholar]
- 54.Tchou J, Kasai H, Shibutani S, Chung M H, Laval J, Grollman A P, Nishimura S. 8-oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specificity. Proc Natl Acad Sci USA. 1991;88:4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tiraby J G, Fox M S. Marker discrimination in transformation and mutation of pneumococcus. Proc Natl Acad Sci USA. 1973;70:3541–3545. doi: 10.1073/pnas.70.12.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsai-Wu J J, Lu A L. Escherichia coli mutY-dependent mismatch repair involves DNA polymerase I and a short repair tract. Mol Gen Genet. 1994;244:444–450. doi: 10.1007/BF00286698. [DOI] [PubMed] [Google Scholar]