Abstract
Cancer cachexia is a multifactorial syndrome characterized by a significant loss of skeletal muscle, which negatively affects the quality of life. Inhibition of myostatin (Mstn), a negative regulator of skeletal muscle growth and differentiation, has been proven to preserve muscle mass in muscle atrophy diseases, including cachexia. However, myostatin inhibitors have repeatedly failed clinical trials because of modest therapeutic effects and side effects due to the poor efficiency and toxicity of existing delivery methods. Here, we describe a novel method for delivering Mstn siRNA to skeletal muscles using red blood cell-derived extracellular vesicles (RBCEVs) in a cancer cachectic mouse model. Our data show that RBCEVs are taken up by myofibers via intramuscular administration. Repeated intramuscular administrations with RBCEVs allowed the delivery of siRNAs, thereby inhibiting Mstn, increasing muscle growth, and preventing cachexia in cancer-bearing mice. We observed the same therapeutic effects when delivering siRNAs against malonyl-CoA decarboxylase, an enzyme driving dysfunctional fatty acid metabolism in skeletal muscles during cancer cachexia. We demonstrate that intramuscular siRNA delivery by RBCEVs is safe and non-inflammatory. Hence, this method is useful to reduce the therapeutic dose of siRNAs, to avoid toxicity and off-target effects caused by systemic administration of naked siRNAs at high doses.
Keywords: extracellular vesicles, cancer cachexia, siRNA, RNA delivery, myostatin, malonyl-CoA decarboxylase
Graphical abstract

Le and colleagues demonstrate that repeated intramuscular administrations with Mstn siRNA or Mlycd siRNA loaded into red blood cell extracellular vesicles result in inhibition of Mstn or Mlycd gene expression, increased muscle growth, and prevention of cachexia in cancer-bearing mice without considerably systemic toxicity and inflammation.
Introduction
Cancer cachexia is a multifactorial complication that affects ∼80% of patients with advanced cancer.1 The hallmarks of cancer cachexia are ongoing weight loss with progressive atrophy of skeletal muscle. The mechanisms underlying cancer cachexia are complex and intertwined. Various inflammatory cytokines and pro-cachectic factors secreted by primary tumor, metastases, or activated immune cells are involved in muscle catabolism and convey pro-atrophic signals that ultimately promote cancer cachexia.2 Cachexia causes frailty in patients and is often exacerbated by surgery and chemotherapies.2,3 Importantly, it adversely impinges on the quality and longevity of life, thus representing the primary cause of death in ∼20%–30% of cancer patients.1 Despite the high prevalence of cancer cachexia, there is no standard cure available. Contrary to popular belief among clinicians, experimental models have shown that cachexia is not completely irreversible.3 However, in practice, conventional nutritional support, appetite stimulants, and physical exercise have only had minor effects in partially restoring skeletal muscle mass.3 To date, a substantial proportion of cancer patients still succumb to cachexia, due to the lack of safe and effective pharmacological treatments. Indeed, a number of trials with novel therapeutics have failed in various stages of clinical development due to inadequate efficacy and the risk of incapacitating side effects.4,5,6 Hence, cancer cachexia remains an unmet medical need. Involuntary loss of skeletal muscle mass and/or strength is the major manifestation of cancer cachexia and has been demonstrated in many cancer types, including melanoma, pancreatic, liver, lung, and colorectal cancers.7 Interestingly, a series of preclinical evidence demonstrate that reversing skeletal muscle loss in animal models of cancer cachexia leads to prolonged survival, despite the ongoing tumor burden.8,9,10 Reversal of the loss of body weight and muscle mass represents the ultimate management target for cachexia, offering symptomatic relief and supportive care for cancer patients.
Myostatin (MSTN, also termed growth and differentiation factor 8) is a member of the transforming growth factor β (TGF-β) superfamily.11 It is highly expressed in skeletal muscle and functions as a negative regulator of skeletal muscle growth and differentiation. Inhibition of MSTN signaling or mutations in the MSTN gene have been shown to counteract the loss of muscle mass and strength and extend survival without affecting tumor growth in animal models of cancer cachexia.8,10,12,13 Extensive efforts have been expended on developing agents capable of modulating MSTN signaling for clinical applications. However, the MSTN inhibitors tested in clinical trials thus far are limited to protein drugs, such as neutralizing antibody, peptibody, monobody, or decoy receptors,12 which may bring difficulties to clinical development and applications because of the higher complexity and expenses in production compared with small-molecule compounds.14 Most MSTN inhibitors manifest inconsistent functional benefits, non-muscle-related adverse effects including telangiectasia and hemorrhages, and, more notably, off-target effects in the heart and other tissues due to the cross-reactivity between MSTN-targeting agents and other TGF-β (or receptor) family members,12,15 thus underscoring the need for highly specific agents and modalities that only target MSTN in the skeletal muscles. Moreover, given the complex pathogenesis of cancer cachexia and other muscle atrophy diseases, multi-target approaches that are able to target MSTN and other genes specifically in tandem might show superior therapeutic windows.
A variety of evidence has pinpointed an important role of excessive lipid catabolism in driving skeletal muscle atrophy.16,17 Fat loss usually precedes muscle loss and arises as a result of elevated lipolysis coupled with excessive fatty acid oxidation in cancer cachexia models.16,17 Pharmacological blockade or genetic inhibition of lipid catabolism effectively prevents skeletal muscle wasting.16,17 In fatty acid biosynthesis and oxidation, malonyl-CoA decarboxylase (Mcd or Mlycd) is a major regulator that catalyzes the depletion of malonyl-CoA to produce acetyl-CoA, thus activating fatty acid oxidation by relieving the malonyl-CoA-mediated inhibition of carnitine palmitoyltransferases, especially in the skeletal muscles.18 Interestingly, it has been shown that the amount of malonyl-CoA inversely correlates with fatty acid β-oxidation in mouse models of cancer cachexia.19 As such, inhibition of the malonyl-CoA decarboxylase gene (Mlycd) affords an alternative therapeutic strategy to rapidly and selectively increase malonyl-CoA levels in skeletal muscles, thereby decreasing excessive fatty acid oxidation and muscle atrophy in cancer cachexia.
Gene therapy based on small interfering RNA (siRNA) is a promising therapeutic modality as it targets disease-related genes in a sequence-specific manner, which enables more precise and personalized treatment of various diseases. After a two-decade journey since its discovery, siRNA therapy has finally reached an extraordinary milestone and currently constitutes the major modality under clinical evaluation.20 Despite the therapeutic prospects of siRNAs, the intracellular and extracellular permeability, activity, and stability of naked unmodified siRNAs hamper its extensive clinical applications. The biggest limitation of using siRNAs lies in the necessity of achieving efficient and safe delivery to desired cells and tissues. To surmount these obstacles, a number of delivery systems, such as liposomes, cationic polymers, and cell-penetrating peptides (CPPs), have been extensively used.21 However, their safety, toxicity and specificity have always been a cause for concern. Cationic liposomes and polymers tend to induce DNA damage and increase pro-inflammatory gene expression in multiple organs.22 CPPs with low tissue selectivity deliver cargos all over the body via non-specific cellular internalization, resulting in unexpected toxicity caused by cargos.23
Recently, extracellular vesicles (EVs) have attracted attention for clinical applications because of their biosafety and biocompatibility as nanovehicles. EVs are naturally secreted membranous vesicles comprising exosomes and microvesicles.24 They are widely distributed in bodily fluids and play essential roles in intercellular communication via the transport of bioactive nucleic acids, proteins, and lipids to adjacent and distant cells.24 Given their intrinsic ability to pass through tissue and cellular lipid membrane barriers, EVs are emerging as novel and promising drug delivery vehicles.24,25 With respect to accessibility, scalability, cost-effectiveness, non-immunogenicity, and non-oncogenicity, the red blood cell-derived EVs (RBCEVs) we have developed are superior to conventional synthetic vehicles and EVs from other sources.26 In recent studies, we have developed an advanced delivery platform for anti-cancer therapeutics utilizing RBCEVs. We also highlighted the versatile range of loads for RBCEVs, from antisense oligonucleotide and immunomodulatory RNA (hairpin loop RNA) to hydrophobic molecules such as paclitaxel.26,27,28 RBCEV-delivered therapeutic cargos were shown to have a high propensity for tumor cells in both proximal and distal sites where they functionally suppressed tumor growth and progression.26,27,28 Moreover, the higher tumor bioavailability of RBCEVs whose surface proteins were covalently conjugated with receptor-specific peptides/nanobodies enabled considerably lower drug dosage and thus enhanced efficacy and safety.27,28
Thus, we hypothesized that RBCEVs can deliver therapeutic siRNAs to manipulate gene expression in skeletal muscles upon intramuscular administration for the treatment of cancer cachexia. Here, we demonstrated that RBCEVs can be robustly taken up by muscle cells via endocytosis in culture and in vivo. To explore their therapeutic potential, we administered Mstn siRNA- and Mlycd siRNA-loaded RBCEVs intramuscularly to specifically and efficiently abrogate Mstn and Mlycd expression to increase skeletal muscle growth in wild-type mice. Furthermore, Mstn siRNA- and Mlycd siRNA-loaded RBCEVs alleviated skeletal muscle loss and prolonged survival rate in mouse models of cancer cachexia. Finally, RBCEVs showed no evidence of systemic toxicity and inflammatory effects at therapeutically effective doses, affording a safe and efficient siRNA drug delivery strategy for muscle degenerative diseases.
Results
RBCEVs are internalized by myofibers via intramuscular administration
RBCEVs were purified from healthy donors as described previously.26 Western blot analysis confirmed that EV common markers (ALIX and TSG101), RBCEV surface marker (glycophorin A [GPA]), and RBC major protein (hemoglobin A [HBA]) were enriched in RBCEVs, whereas the cytoskeleton protein β-actin was absent (Figure S1A). Single-EV flow cytometric analysis revealed that approximately ∼83% of RBCEVs were positive for GPA (Figure S1B).
To examine the uptake of RBCEVs by myotubes in vitro, we differentiated C2C12 myoblasts into myotubes, and incubated them with RBCEVs that were labeled with carboxyfluorescein succinimidyl ester (CFSE). To investigate the mechanism of RBCEV uptake, we utilized wortmannin, an endocytosis inhibitor, and cytochalasin D, a broad inhibitor of endocytosis, phagocytosis, and macropinocytosis. Specifically, C2C12 myotubes were pre-treated with 10 μM wortmannin or 0.5 μM cytochalasin D 1 h prior to the 6-h incubation with CFSE-labeled RBCEVs. Flowthrough (FT) of the last EV wash and free dye processed in the same way as EV samples were used as controls. Upon incubation with CFSE-labeled EVs, a punctate staining pattern of internalized EVs was observed throughout the cytosol of C2C12 cells by confocal imaging, indicating endocytosis of EVs by the cells (Figure 1A). Indeed, inhibition of endocytosis through the use of wortmannin or cytochalasin D resulted in a decrease in EV uptake (Figure 1A). Moreover, we did not observe CFSE signals in C2C12 cells treated with free dye and FT controls, suggesting that our EV washing protocol is sufficient to remove the free dye. These data were further confirmed by flow cytometric analysis. RBCEVs labeled with MemGlow 640 probe (MG640-EVs) were incubated with inhibitor pre-treated myotubes for 6 and 24 h. As a result, MG640-EVs substantially increased the MG640 fluorescent signals in myotubes as early as 6 h compared with the FT control (Figure 1B). The endocytotic activity of cells, albeit nearly ∼100% positive for MG640, was inhibited by wortmannin and cytochalasin D shown by ∼60.8% and ∼33.3% decreases in the MG640 intensity, respectively, at 6 h, compared with the non-inhibitor-treated control (Figures 1B and S1C). The inhibitory effects of wortmannin and cytochalasin D lasted until 24 h, demonstrating ∼55.9% and ∼45.2% reductions in fluorescent intensity, respectively (Figures 1B and S1C). Consistently, fluorescent signals were barely detectable in the cells treated with FT and free dye controls, implying that the observed cellular fluorescence was not caused by carryover of unbound dye. Together, these data suggest that RBCEVs can be internalized by myotubes via endocytosis.
Figure 1.
RBCEVs carrying siRNAs are internalized by myofibers via intramuscular administration
(A) Representative immunofluorescent images of RBCEVs uptake in C2C12 myotubes pre-treated with 10 μM wortmannin or 0.5 μM cytochalasin D. Nuclei were stained with Hoechst (blue). RBCEVs were labeled with CFSE (green) and co-stained with anti-GPA antibody (tangerine). Plasma membrane was stained with MemGlow 640 (MG640) fluorogenic cell membrane probe (red). Flowthrough of the last EV wash and free dye were used as controls. Scale bar, 20 μm. (B) Flow cytometric analysis of uptake of MemGlow 640-labeled RBCEVs (MG640-EVs) by C2C12 myotubes pre-treated with 10 μM wortmannin or 0.5 μM cytochalasin D (n = 3 biological replicates). Flowthrough of the last EV wash and free dye were used as controls. (C) Representative DiR fluorescent images of nude mice and their organs 6 or 24 h after intramuscular injection of DiR-labeled RBCEVs, flowthrough of the RBCEV wash (FT), or free dye control (FD). Br, brain; Lu, lung; He, heart; Li, liver; Sp, spleen; Pa, pancreas; Ki, kidney; Bo, bone; Qa, quadriceps. (D) Representative immunofluorescent images of transverse sections of muscles 24 h after intramuscular injection of CFSE-labeled RBCEVs, flowthrough of EV wash, or free dye control. Nuclei were stained with DAPI (blue). RBCEVs were labeled with CFSE (green). Myofibers were stained with Phalloidin-iFluor 532 fluorescent dye (red). (E) Schematics of in vivo siRNA delivery. siRNAs targeting Mstn were loaded in RBCEVs and injected into the quadriceps of C57BL/6 mice. After 24 h, a biopsy of each injection site was collected for qRT-PCR. qPCR comparison of Mstn knockdown by four siRNAs loaded in RBCEVs (n = 3 biological replicates). qPCR comparison of Mstn knockdown by naked siMstn-4 and siMstn-4 loaded in RBCEVs (n = 3 biological replicates). All bar graphs represent mean ± SEM. ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 determined by Student’s two-tailed t test, ns, not significant.
To visualize EV uptake in mouse muscle tissue, we labeled RBCEVs with DiR fluorescent dye and washed them thoroughly using size-exclusion chromatography (SEC) according to our previous study in which we demonstrated that DiR-labeled EVs could be separated from DiR micelles using SEC.27 As a control, free DiR dye was also washed side-by-side in the same way. DiR-labeled EVs were injected into the quadriceps of nude mice (Figure 1C). DiR fluorescence was solely detected in the EV-injected quadriceps at 6 h, while no fluorescence was detectable in the quadriceps injected with the FT of EV wash or free dye control (Figure 1C). The fluorescence remained in the quadriceps after 24 h, indicating that RBCEVs were taken up primarily by quadriceps myofibers. The absence of fluorescence in other organs was attributed to the minimal leakage of EVs to the circulation, below the detection limit. Immunostaining of the myofiber cross-sections confirmed that CFSE-labeled RBCEVs were internalized by multiple myofibers in the quadriceps of C57BL/6 mice (Figure 1D). These data suggest that the intramuscular injection of EVs is an ideal method for muscle-specific therapeutic delivery.
RBCEVs deliver siRNAs into myofibers for knockdown of Mstn
To assess siRNA loading in EVs, RBCEVs were loaded with FAM-conjugated siRNAs using REG1 and subjected to single-EV flow cytometry. REG1 is a commercial transfection reagent capable of loading nucleic acids into EVs with low toxicity.27,28 RBCEVs treated with REG1 only were used as a mock control. The data revealed that ∼93.6% RBCEVs were positive for FAM fluorescence, suggesting an efficient siRNA loading (Figure S2A).
To knock down myostatin, we designed four Mstn siRNAs (Table S1), loaded each of them into RBCEVs, and injected the EVs into quadriceps of C57BL/6 mice (Figure 1E). After 24 h, muscle biopsy at the injection site was collected and homogenized for RNA extraction. qPCR analysis revealed that Mstn siRNA-4 (siMstn-4) exhibited the highest knockdown efficiency among the four siRNAs (Figure 1E). We loaded Mstn siRNA-4 into different batches of RBCEVs purified from three independent donors and quantified the copy number of Mstn siRNA per individual RBCEV using agarose gel electrophoresis (Figures S2B–S2D). By comparing the band intensity of Mstn siRNA in RBCEVs with that of a serial dilution of input Mstn siRNA, we confirmed that there were ∼500 copies of Mstn siRNA loaded into each RBCEV on average (Figures S2C and S2D), corresponding to a loading efficiency of ∼80.8% (Figure S2H). Moreover, the loading efficiency was consistent and reproducible among different batches of RBCEVs (Figures S2B and S2D). Of note, the endogenous RNAs that may be present in RBCEVs were barely detectable (Figure S2H), suggesting that the contribution of endogenous RNAs was negligible. We further demonstrated that RBCEVs could protect Mstn siRNA from degradation, therefore facilitating functional delivery of therapeutic siRNAs to muscle cells (Figures S2H and 1E). Mstn siRNA loaded into RBCEVs exhibited a ∼10% reduction in the band intensity after RNase If treatment, whereas the level of the naked Mstn siRNA treated with RNase If reduced ∼77% compared with the untreated controls (Figure S2H). Likewise the naked Mstn siRNA delivered in vivo did not withstand extracellular RNase, thus hindering its efficient delivery and efficacy unless it was loaded into RBCEVs (Figure 1E).
The dose-dependent in vivo effect of Mstn siRNA-loaded RBCEVs was assessed at 24 h post-administration (Figure S3A). The suboptimal dose of 50 μg Mstn siRNA-loaded RBCEVs (that conferred ∼73% inhibition) was selected for subsequent experiments. Following intramuscular administration of one single dose of Mstn siRNA-loaded RBCEVs, the inhibitory effect on Mstn expression was significantly stronger on day 1 to day 3, while declining gradually until day 5 (Figure S3C).
Intramuscular treatment with Mstn siRNA-loaded RBCEVs promotes muscle growth
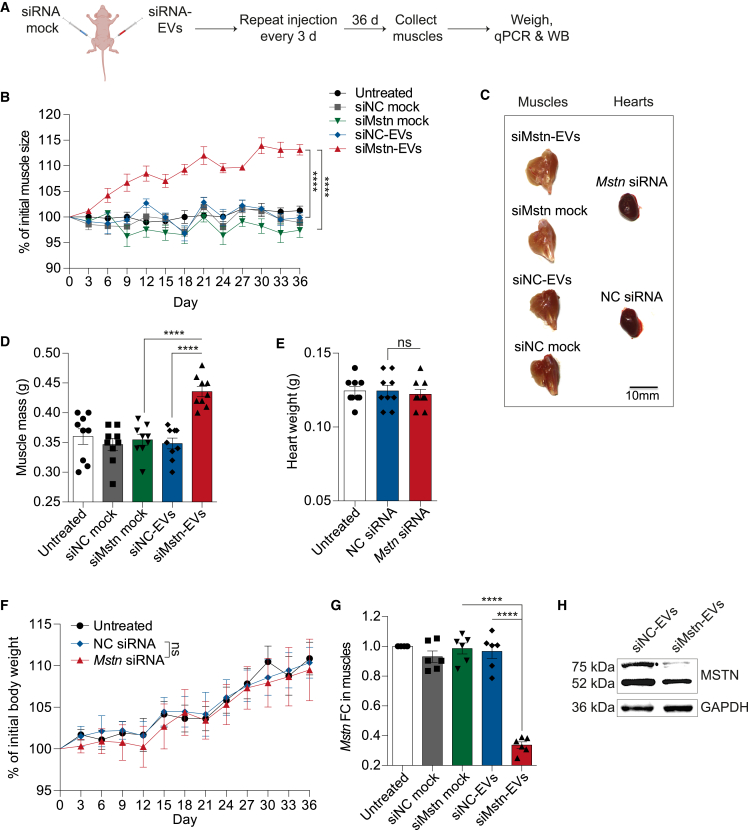
We subsequently examined the functional impact of RBCEV-mediated delivery of Mstn siRNA on muscle growth. To achieve sustained Mstn knockdown, nude mice were treated intramuscularly with 50 μg of Mstn siRNA-loaded RBCEVs (siMstn-EVs) once every 3 days for 36 days (Figure 2A). As a control, the contralateral muscle of the same mice was injected with an equivalent dose of Mstn siRNA-REG1 complex (siMstn mock without EVs, equimolar amounts of Mstn siRNA was used to complex with REG1) (Figure 2A). A scrambled siRNA was also used as a negative control (siNC). The muscle size was measured every 3 days as described in the materials and methods (Figure S4). We found that Mstn siRNA-loaded EVs significantly promoted muscle growth over time compared with the controls (Figure 2B). Mstn knockdown led to an increase in the overall size of siMstn-EVs treated muscles (Figure 2C), and a ∼24.2% increase in the weight of siMstn-EVs treated muscles compared with siNC-EVs treated muscles and a ∼21.8% increase compared with siMstn mock treated contralateral muscles (Figure 2D). The possible adverse effect of siMstn-EVs on the overall weight of the heart and the body was also assessed. As expected, local delivery of siMstn-EVs had no effect on the heart and overall body weight as shown in Figures 2E and 2F. In addition, the skin of treated nude mice did not present any sign of telangiectasia and hemorrhages (data not shown), which was found in patients treated with MSTN inhibitor.15 Mstn mRNA expression, as determined by qPCR and normalized to Gapdh, was decreased significantly by ∼65.2% in the muscle treated with Mstn siRNA-loaded RBCEVs compared with that treated with NC siRNA-loaded RBCEVs (Figure 2G). Notably, siMstn mock treatment showed no inhibitory effect on Mstn (Figure 2G), which is indicative of inefficient delivery by REG1 alone in vivo. Knockdown of Mstn protein by Mstn siRNA-loaded EVs was further confirmed by western blot analysis of the quadriceps muscle lysate (Figure 2H). Together, these data validated the robust delivery of siRNAs by RBCEVs to the muscle for inhibition of Mstn expression, thus promoting muscle growth in vivo.
Figure 2.
RBCEV-mediated delivery of Mstn siRNA promotes muscle growth
(A) Schema of delivering Mstn siRNA or negative control (NC) siRNA to quadriceps using RBCEVs (loading mediated by REG1) or a mock control (REG1 only). Each mouse was injected with either Mstn or NC siRNA as mock on the right quadriceps and siRNA-loaded RBCEVs on the left quadriceps. The injection was repeated at different sites in the same quadriceps every 3 days. (B) Change in muscle size (relative to the initial muscle size on day 0) during the treatments (n = 9 mice for all conditions). (C) Representative images of muscles and hearts. Scale bar, 10 mm. (D) Muscle mass after all treatments (n = 9 mice for all conditions). (E) Weight of hearts after all treatments (n = 9 mice for all conditions). (F) Change in overall body weight (relative to the initial body weight on day 0) during the treatments (n = 9 mice for all conditions). (G) qPCR analysis of Mstn fold change (FC) relative to untreated control, normalized to Gapdh in quadriceps after all treatments (n = 6 mice for all conditions). (H) Western blot analysis of MSTN protein expression in quadriceps treated with siNC- or siMstn-EVs. All bar graphs represent mean ± SEM. Two-way ANOVA test (B and F) and Student’s two-tailed t test (D, E, and G), ∗∗∗∗p < 0.0001, ns, not significant.
RBCEV-mediated delivery of Mstn siRNA suppresses cachexia in lung cancer mouse model
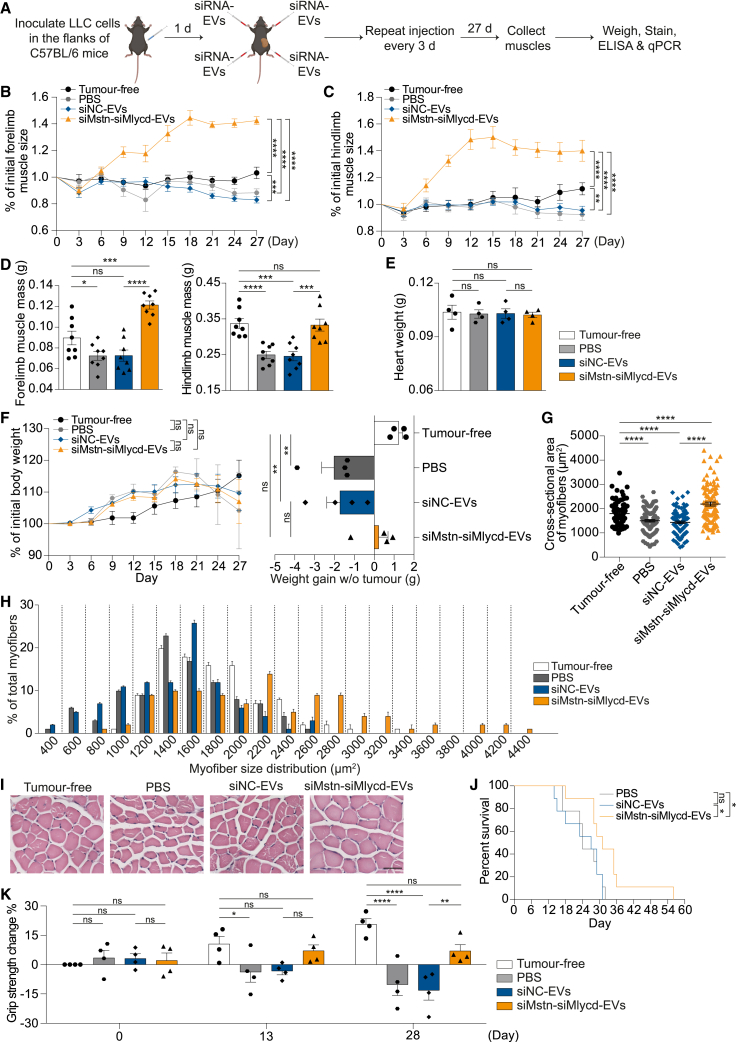
After confirming that Mstn siRNA-loaded RBCEVs could effectively promote muscle growth, we sought to apply it to treat cachectic mouse models bearing syngeneic lung carcinoma cells. We inoculated Lewis lung carcinoma (LLC) cells in the flanks of C57BL/6 mice and initiated the treatment 1 day after the inoculation (Figure 3A). We treated all four limbs of the mice with intramuscular injections of RBCEVs containing Mstn siRNA or NC siRNA every 3 days and monitored muscle growth (Figure 3A). Tumor-free C57BL/6 mice were used as a control. We observed an effective therapeutic effect of siMstn-EVs as early as 6 days after the initiation of dosing (Figure 3B). The siMstn-EVs treatment prevented skeletal muscle atrophy in the forelimbs (Figure 3B), as well as the hindlimbs to some extent, compared with tumor-free mice without any treatment (Figure 3C). In contrast, siNC-EVs treatment failed to counteract muscle atrophy in both the forelimbs and hindlimbs (Figures 3B and 3C). At the endpoint, the muscle mass of control mice treated with siNC-EVs significantly decreased by ∼28.6% and ∼14.1%, respectively, in the hindlimbs and forelimbs, compared with tumor-free mice, confirming the impact of cancer-associated cachexia (Figure 3D). In contrast, siMstn-EV treatment significantly prevented muscle mass loss in the forelimbs, and to a lesser extent in the hindlimbs (Figure 3D), possibly because the hindlimbs are in closer proximity to the tumor grafts. None of the groups showed any signs of cardiac hypertrophy or atrophy as determined by the heart weight (Figure 3E), suggesting that siMstn-EVs delivered locally to skeletal muscles did not cause systemic side effects. Although siMstn-EVs did not show an obvious effect on the overall tumor-bearing body weight, it did diminish cancer cachexia to some extent, as shown by increased carcass weight (Figure 3F). In contrast, the control siNC-EV-treated mice showed reduced weight gain due to cachexia, although the results did not reach statistical significance (Figure 3F). We performed histological staining of the transverse muscle sections and examined the cross-sectional areas of approximately 100 myofibers per muscle. Overall, the myofiber area increased by ∼18% after treatment with siMstn-EVs compared with control siNC-EVs (Figure 3G). The cross-sectional areas of myofibers were further classified by frequency distribution. The percentage of myofibers within 1,000–2,000 μm2 tended to be significantly higher in the siMstn-EV group than in the control siNC-EV group (Figure 3H). Representative micrographs showed that myofibers treated with siMstn-EVs were visibly larger than those treated with control siNC-EVs (Figure 3I). To assess the effect of siMstn-EVs on the overall survival of cancer cachectic mice, we repeated the experiment by treating tumor-bearing C57BL/6 mice with intramuscular injections of the same dose of siMstn-EVs at the same frequency. siMstn-EVs slightly but significantly extended the lifespan of cancer cachectic mice, despite 100% mortality at the endpoint (Figure 3J). These data suggest that Mstn is still a viable therapeutic target for cancer cachexia treatment, given the efficient delivery of Mstn siRNA by RBCEVs.
Figure 3.
RBCEV-mediated delivery of Mstn siRNA suppresses cancer cachexia in lung cancer mouse model
(A) Schema of delivering Mstn siRNA or negative control (NC) siRNA to muscles using RBCEVs in C57BL/6 mice bearing Lewis lung carcinoma (LLC) tumors. Each mouse was injected with either Mstn siRNA or NC siRNA-loaded RBCEVs in the muscles of all four limbs. (B) Change in forelimb muscle size (relative to the initial muscle size on day 0) during the treatments (n = 3 mice for tumor-free, 8 mice for siNC-EVs, 9 mice for siMstn-EVs). (C) Change in hindlimb muscle size (relative to the initial muscle size on day 0) during the treatments (n = 3 mice for tumor-free, 8 mice for siNC-EVs, 9 mice for siMstn-EVs). (D) Mass of forelimb and hindlimb muscles after all treatments (n = 3 mice for tumor-free, 8 mice for siNC-EVs, 9 mice for siMstn-EVs). (E) Weight of hearts after all treatments (n = 3 mice for tumor-free, 8 mice for siNC-EVs, 9 mice for siMstn-EVs). (F) Change in overall tumor-bearing body weight (relative to the initial body weight on day 0) during the treatments and carcass weight (tumors removed) after treatments (n = 3 mice for tumor-free, 8 mice for siNC-EVs, 9 mice for siMstn-EVs). (G) Average cross-sectional areas of 100 myofibers chosen at random from each condition. (H) Myofiber size distribution. (I) Representative images of transverse sections of muscles with H&E staining. Scale bar, 50 μm. (J) Survival rate of cachectic mice (n = 8 mice for both conditions). All bar graphs represent mean ± SEM. Two-way ANOVA test (B, C, and F), Student’s two-tailed t test (D–H), and log rank test (J). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant.
Intramuscular treatment with Mlycd siRNA-loaded RBCEVs promotes muscle growth
We further used siRNAs delivered by RBCEVs to knock down malonyl-CoA dehydrogenase and prevent muscle atrophy due to excessive fatty acid oxidation. Firstly, we examined the inhibitory effect of three siRNAs against Mlycd (Table S1). RBCEVs (50 μg) containing each of the three Mlycd siRNAs were injected intramuscularly into nude mice, and siMlycd-1 exhibited the best inhibitory effect among the three Mlycd siRNAs (Figure 4A); thus, siMlycd-1 was selected in subsequent studies. Loading Mlycd siRNA into three different batches of RBCEVs consistently yielded ∼520 copies of Mlycd siRNA per RBCEV on average, confirming the reproducibility of our loading approach (Figures S2E–S2G). The corresponding loading efficiency was ∼81.7%, relative to unloaded Mlycd siRNA (Figure S2H). Furthermore, RBCEVs could protect Mlycd siRNA from RNase degradation, shown by a lesser reduction in the level of RBCEV-associated Mlycd siRNA compared with the naked Mlycd siRNA after RNase If treatment (Figure S2H). The dose- and time-dependent effect of Mlycd siRNA-loaded RBCEVs on Mlycd expression was similar to that of Mstn siRNA-loaded RBCEVs. A single dose of 50 μg Mlycd siRNA-loaded RBCEVs at the 24-h time point, which resulted in ∼70% inhibition of Mlycd expression, was selected for subsequent experiments (Figures S3B and S3D).
Figure 4.
RBCEV-mediated delivery of Mlycd siRNA promotes muscle growth
(A) qPCR comparison of Mlycd knockdown by three siRNAs loaded in RBCEVs (n = 5 biological replicates). (B) Schema of delivering Mlycd siRNA or negative control (NC) siRNA to quadriceps using RBCEVs (loading mediated by REG1) or a mock control (REG1 only). Each mouse was injected with either Mlycd or NC siRNA as mock on the right quadriceps and siRNA-loaded RBCEVs on the left quadriceps. The injection was repeated at different sites in the same quadriceps every 3 days. (C) Western blot analysis of MCD expression. (D) Change in muscle size (relative to the initial muscle size on day 0) during the treatments (n = 4 mice for untreated and siNC, 5 mice for siMlycd). (E) Muscle mass after all treatments (n = 4 mice for untreated and siNC, 5 mice for siMlycd). (F) Weight of hearts after all treatments (n = 4 mice for untreated and siNC, 5 mice for siMlycd). (G) Change in overall body weight (relative to the initial body weight on day 0) during the treatments (n = 4 mice for untreated and siNC, 5 mice for siMlycd). (H) Average cross-sectional areas of 100 myofibers chosen at random from each condition. (I) Myofiber size distribution. (J) Representative images of transverse sections of muscles with H&E staining. Scale bar, 50 μm. All bar graphs represent mean ± SEM. Two-way ANOVA test (D and G) and Student’s two-tailed t test (A, E, F, and H). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant.
To assess the functional effect of Mlycd siRNA on muscle growth, nude mice were treated intramuscularly with 50 μg of RBCEVs containing Mlycd siRNA (siMlycd-EVs) once every 3 days for 45 days (Figure 4B). Similar to the Mstn siRNA experiment, an equivalent dose of the Mlycd siRNA-REG1 complex (equimolar amounts of Mlycd siRNA was used to complex with REG1) as a mock control was injected into the contralateral muscle of the same mice (Figure 4B). Western blot analysis confirmed the knockdown of MCD protein after the treatment in vivo (Figure 4C). Interestingly, knockdown of Mlycd by RBCEV-delivered siRNA substantially promoted muscle growth (Figure 4D). Mlycd siRNA delivered by REG1 and NC siRNA did not generate any differences in muscle growth (Figure 4D). Moreover, siMlycd-EV-treated quadriceps muscle showed increased mass over other controls (Figure 4E). There was no evidence of adverse effects of siMlycd-EVs on the heart and body weight (Figures 4F and 4G). The increase in muscle size after treatment with siMlycd-EVs was also supported by histological examination of myofiber size, demonstrating up to a ∼21% increase in myofiber cross-sectional area compared with control siNC-EVs (Figure 4H). Myofiber size distribution analysis revealed a shift toward an increased frequency of larger myofibers (2,400–3,800 μm2) after treatment with siMlycd-EVs compared with control groups (Figure 4I). Representative micrographs are shown in Figure 4J. These data imply that Mlycd is an important negative regulator of muscle growth in mice.
RBCEV-mediated delivery of Mlycd siRNA alone or in combination with Mstn siRNA suppresses cancer cachexia in lung cancer mouse model
To assess the therapeutic effect of Mlycd siRNA, we delivered siMlycd-EVs intramuscularly and analyzed the treatment effects on cachexia in C57BL/6 mice bearing LLC tumors (Figure 5A). We found that siMlycd-EVs treatment could promote muscle growth in both forelimbs and hindlimbs compared with siNC-EVs (Figures 5B and 5C), thus preventing muscle atrophy in the tumor-bearing mice (Figure 5D). Intramuscular delivery of siMlycd-EVs did not change heart weight and overall tumor-bearing body weight (Figures 5E and 5F). siMlycd-EV treatment did indeed increase carcass weight compared with the mice treated with siNC-EVs (Figure 5F). However, the treatment had no effect on the survival of the mice (Figure 5G).
Figure 5.
RBCEV-mediated delivery of Mlycd siRNA suppresses cancer cachexia in lung cancer mouse model
(A) Schema of delivering Mlycd siRNA or negative control (NC) siRNA to muscles using RBCEVs in C57BL/6 mice bearing Lewis lung carcinoma (LLC) tumors. Each mouse was injected with either Mlycd siRNA or NC siRNA-loaded RBCEVs in the muscles of all four limbs. (B) Change in forelimb muscle size (relative to the initial muscle size on day 0) during the treatments (n = 3 mice for tumor-free, 8 mice for siNC-EVs, 9 mice for siMlycd-EVs). (C) Change in hindlimb muscle size (relative to the initial muscle size on day 0) during the treatments (n = 3 mice for tumor-free, 8 mice for siNC-EVs, 9 mice for siMlycd-EVs). (D) Mass of forelimb and hindlimb muscles after all treatments (n = 3 mice for tumor-free, 8 mice for siNC-EVs, 9 mice for siMlycd-EVs). (E) Weight of hearts after all treatments (n = 3 mice for tumor-free, 8 mice for siNC-EVs, 9 mice for siMlycd-EVs). (F) Change in overall tumor-bearing body weight (relative to the initial body weight on day 0) during the treatments and carcass weight (tumors removed) after treatments (n = 3 mice for tumor-free, 8 mice for siNC-EVs, 9 mice for siMlycd-EVs). (G) Survival rate of cachectic mice (n = 4 mice for both conditions). All bar graphs represent mean ± SEM. Two-way ANOVA test (B, C, and F), Student’s two-tailed t test (D–F), and log rank test (G). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant.
To enhance the therapeutic treatment of cancer cachexia, we sought to implement a combination therapy that targets Mstn and Mlycd simultaneously using RBCEVs loaded with both Mstn siRNA and Mlycd siRNA (Figure 6A). As expected, the treatment with the combined siRNA-EVs led to substantial increases in both forelimb and hindlimb muscle size compared with other controls, and better than the effects observed with single siRNAs (Figures 6B and 6C). Moreover, the combination therapy exerted an even stronger effect against muscle atrophy as shown by greater forelimb muscle mass and similar hindlimb muscle mass compared with the tumor-free mice (Figure 6D). In contrast, PBS and siNC-EV treatments failed to counteract muscle loss. Intramuscular delivery of siMstn-siMlycd-EVs did not change heart weight and the overall tumor-bearing body weight (Figures 6E and 6F). The combination therapy did increase carcass weight compared with the mice treated with control siNC-EVs, although the results did not reach statistical significance (Figure 6F). H&E staining analysis further revealed that the combination therapy significantly increased the cross-sectional myofiber areas, and increased the frequency of larger myofibers ranging from 2,200 to 4,400 μm2 compared with the other control mice (Figures 6G–6I). Furthermore, the combination therapy extended the survival of cachectic mice significantly (Figure 6J).
Figure 6.
RBCEV-mediated delivery of Mlycd siRNA in combination with Mstn siRNA suppresses cancer cachexia in lung cancer mouse model
(A) Schema of delivering Mstn and Mlycd siRNAs or negative control (NC) siRNA to muscles using RBCEVs in C57BL/6 mice bearing Lewis lung carcinoma (LLC) tumors. Each mouse was injected with either Mstn and Mlycd siRNAs or NC siRNA-loaded RBCEVs in the muscles of all four limbs. (B) Change in forelimb muscle size (relative to the initial muscle size on day 0) during the treatments (n = 4 mice for all conditions). (C) Change in hindlimb muscle size (relative to the initial muscle size on day 0) during the treatments (n = 4 mice for all conditions). (D) Mass of forelimb and hindlimb muscles after all treatments (n = 4 mice for all conditions). (E) Heart weight after all treatments (n = 4 mice for all conditions). (F) Change in overall tumor-bearing body weight (relative to the initial body weight on day 0) during the treatments and carcass weight (tumors removed) after treatments (n = 4 mice for all conditions). (G) Average cross-sectional areas of 100 myofibers chosen at random from each condition. (H) Myofiber size distribution. (I) Representative images of transverse sections of muscles with H&E staining. Scale bar, 50 μm. (J) Survival rate of cachectic mice (n = 9 mice for all conditions). (K) Change in forelimb grip strength relative to the grip strength of tumor-free mice on day 0 (n = 4 mice for all conditions). All bar graphs represent mean ± SEM. Two-way ANOVA test (B, C, and F), Student’s two-tailed t test (D–F, G, and K), and log rank test (J). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant.
To assess changes in muscle performance as a result of the combination therapy, forelimb grip strength of the treated mice was measured at the initial, interim, and final time points. The grip strength of forelimb muscles treated with control siNC-EVs differed from baseline levels by +3.1%, −3.3%, and −13.1%, respectively, on days 0, 13, and 28 (Figure 6K). In comparison, the combined siRNA-EVs increased grip strength by +7.0% over 13 days and +6.9% over 28 days. These data suggest that the decrease in muscle function in cancer cachexia could be rescued by the combination therapy effectively.
Intramuscular delivery of siRNAs using RBCEVs is non-toxic and non-inflammatory
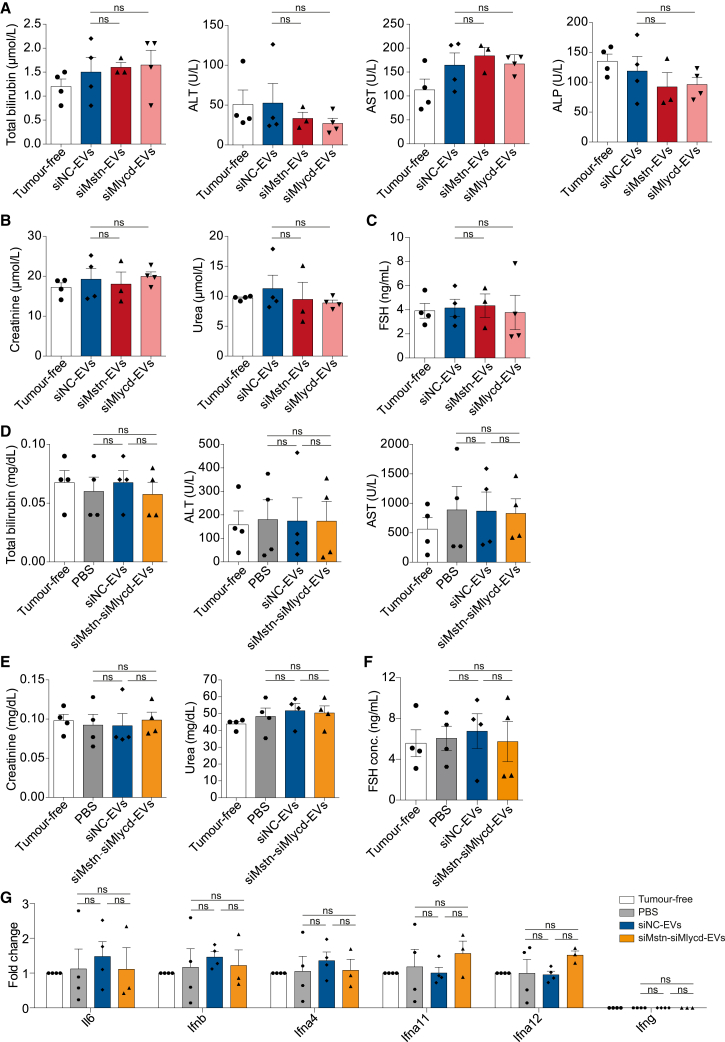
To investigate the potential toxicity and inflammatory effects of RBCEV-delivered siRNAs, we collected whole blood and serum from EV-treated mice at the endpoint. Unlike many other RNAi therapies delivered by cationic polymers and liposomes, there was no evidence of liver and kidney toxicity with RBCEV-delivered siRNAs, as determined by the concentration of serum total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), creatinine and urea (Figures 7A, 7B, 7D, and 7E). Due to the high structural similarity between MSTN and other TGF-β family members, the lack of specificity observed in many other MSTN antibodies or inhibitors raised the possibility of off-target effects in other tissues. For example, Activin, another TGF-β family member, stimulates proper follicle-stimulating hormone (FSH) release. A clinical trial report had demonstrated that healthy, postmenopausal women who received a single dose of MSTN inhibitor showed ∼43% decrease in serum FSH, an effect attributed to suppression of activin signaling.29 Therefore, we also examined the serum level of FSH in female mice after intramuscular administration of siMstn-EVs, siMlycd-EVs, or the combined siMstn-siMlycd-EVs. As shown in Figures 7C and 7F, the level of serum FSH in mice receiving siMstn-EVs, siMlycd-EVs, and siMstn-siMlycd-EVs remained unchanged compared with those receiving siNC-EVs. There were also no significant changes in the expression of inflammation-associated genes, indicating a lack of inflammatory reactions from TGF-β suppression (Figure 7G). In addition, we evaluated the presence of IgG antibodies against RBCEV surface proteins in the mouse sera using bead-assisted flow cytometry (Figure S5A). We did observe increased levels of EV-specific IgG in the sera after repeated intramuscular administration (Figures S5B and S5C). This was also corroborated by ELISA, yielding a similar pattern of IgG antibodies against RBCEV lysates in the sera (Figures S5D and S5E), indicative of immune responses to EVs from a source of xenogeneic cells after repeated intramuscular administration. Collectively, these data suggest that intramuscular delivery of siRNAs using RBCEVs represents a non-toxic and non-inflammatory therapeutic delivery strategy for cancer cachexia treatment if RBCEVs are generated in a non-xenogeneic manner.
Figure 7.
Intramuscular delivery of siRNAs using RBCEVs is safe and non-inflammatory
(A) Liver toxicity indicated by the concentration of total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) after treatment with either siMstn-EVs or siMlycd-EVs (n = 3 mice for siMstn-EVs, 4 mice for tumor-free, siNC-EVs, and siMlycd-EVs). (B) Kidney function indicated by creatinine and urea levels after treatment with either siMstn-EVs or siMlycd-EVs (n = 3 mice for siMstn-EVs, 4 mice for tumor-free, siNC-EVs, and siMlycd-EVs). (C) Function of the reproductive system indicated by follicle-stimulating hormone (FSH) after the treatment with either siMstn-EVs or siMlycd-EVs (n = 3 mice for siMstn-EVs, 4 mice for tumor-free, siNC-EVs, and siMlycd-EVs). (D) Liver toxicity indicated by the concentration of total bilirubin, ALT, and AST after a combined treatment of siMstn-siMlycd-EVs (n = 4 mice for all conditions). (E) Kidney function indicated by creatinine and urea levels after a combined treatment of siMstn-siMlycd-EVs (n = 4 mice for all conditions). (F) Function of the reproductive system indicated by FSH after the combined treatment of siMstn-siMlycd-EVs (n = 4 mice for all conditions). All parameters were measured in the blood of the C57BL/6 mice bearing LLC tumors after treatments with Mstn and/or Mlycd siRNA-loaded RBCEVs. (G) qPCR analysis of inflammation-associated genes in the muscles after the combined treatment with siMstn-siMlycd-EVs (n = 4 mice for all conditions). All bar graphs represent mean ± SEM. ns, not significant, determined by Student’s two-tailed t test.
Discussion
Cancer-induced cachexia is far too common, being present in patients suffering from a wide variety of malignancies, with detrimental effects on survival and the quality of life.1,7 To date, no treatment modalities are available to halt or reverse its progressive and debilitating muscle atrophy. In recent years, an increasing understanding of the underlying molecular mechanisms behind cachexia is shaping the building blocks of new cancer cachexia treatments. Targeting MSTN and its binding heteromeric receptor complexes has enabled preclinical studies and clinical trials to identify and develop treatments to combat cachexia. However, MSTN is highly similar in structure to other TGF-β family members. The lack of specificity of current MSTN inhibitors limits their applications and extensions.12,30 In this study, we screened for a sequence-specific siRNA against Mstn that inhibited its mRNA and protein expression with a high efficiency. Intramuscular administration of Mstn siRNA using RBCEVs effectively increased muscle mass and myofiber size in a relatively short period, attenuated muscle loss, and prolonged the survival rate of cancer cachectic mice. In line with other in vivo studies of MSTN inhibition in mice,12,13 our results support the hypothesis that counteracting Mstn expression by siRNA is an effective treatment for muscle atrophy arising from cancer cachexia and may eventually become the basis for therapy against muscle degenerative diseases.
To our knowledge, this is the first study to target Mlycd, an important enzyme driving dysfunctional fatty acid metabolism in muscles, for cancer cachexia treatment. During cancer cachexia, acceleration of fatty acid oxidation leads to an increase in reactive oxygen species, activation of p38 MAPK signaling, and progressive muscle wasting.16 Blockade of fatty acid oxidation by etomoxir is able to reduce muscle wasting,16 but etomoxir may cause adverse effects due to its non-specificity and on-target toxicity in the liver.31,32 Targeting Mlycd, which activates fatty acid oxidation, offers an alternative strategy to prevent muscle wasting. As such, we have validated that intramuscular delivery of Mlycd siRNA using RBCEVs effectively inhibited Mlycd expression and reduced cachexia in mice bearing cancer. Mlycd could be a new viable therapeutic target for cancer cachexia treatment. This study was powered on an expected attenuation of skeletal muscle atrophy. However, there were non-significant differences in secondary outcome parameters, such as muscle strength (data not shown) and survival rate. Hence, we combined Mlycd and Mstn siRNAs for an enhanced therapeutic efficacy against cancer cachexia. As expected, we observed a better effect with the combination therapy of both Mlycd and Mstn siRNA-loaded RBCEVs on muscle growth and muscle strength, as well as survival rate of cancer cachectic mice. This observation might provide new insights into the treatment of cancer cachexia.
An obvious practical problem with RNA drug delivery is the inadequate efficacy and unexpected toxicity caused by commonly used delivery systems.21 To circumvent these issues, the entirety of this study utilizes RBCEVs as siRNA delivery vehicles, because RBCEVs are easily accessible, scalable, and devoid of DNA, growth factors, and toxic substances, since human RBCs are enucleated primary cells.26 Apart from delivery vehicles, the administration route should also be taken into consideration to ensure that the most efficacious dose reaches the targeted site in the most convenient way, with the lowest risk of side effects. To this end, we inject RBCEVs carrying therapeutic siRNAs via intramuscular administration. The administered RBCEVs are delivered directly into the depth of selected muscles, and thereafter quickly internalized in muscle cells, with minimal leakage to other organs, bypassing rapid renal clearance and first-pass metabolism. Several preclinical studies have reported that myostatin silencing after systemic administration of myostatin inhibitors promoted cardiac hypertrophy and/or an increase in heart weight, which is a worrisome risk factor for heart failure.33,34 Heart tissue and other tissues’ side effects precluded myostatin inhibitors from further development. Here, we are trying to maximize skeletal muscle efficacy while avoiding potential side effects of myostatin silencing on cardiomyocytes and other tissues by avoiding systemic administration.
To attain efficacy in muscle, multiple dosing was required during the treatment. Multiple administrations of unencapsulated siRNAs at low doses did not abrogate gene expression or promote muscle growth. Unexpected toxicity or off-target effects might occur when a high dose of siRNAs is applied. Repeated administrations of RBCEV-delivered siRNAs at low doses, indeed, confer a significant treatment efficacy in muscle without any considerable toxicity in the liver and kidney as well as systemic inflammation. It is noted that one single dose of siRNA-loaded RBCEVs did not increase the size of skeletal muscles, which is likely due to the relatively low stability of unmodified siRNAs. Other researchers have shown that conjugation with other chemical moieties can increase siRNA stability. At this point, chemical modifications and dosing regimen will need to be optimized to support maximal stability of siRNAs and optimal duration of effect.34,35 We do not exclude the potential immunological effects of xenogeneic RBCEVs, which have been reported in recent studies.36,37 Indeed, we detected EV-specific IgGs in the mouse sera after repeated intramuscular injections with human RBCEVs. However, note that we did not observe any significant immunological toxicity to the RBCEV-targeted muscles nor the mouse organs in general (as shown in Figure 7). Moreover, this potential issue would be immediately resolved in clinic in reality, where only human RBCEVs would be used in human-to-human injections, by using autologous or allogeneic RBCEVs, similar to human blood transfusions. Taken collectively, RBCEV-mediated delivery of therapeutic siRNAs could represent an alternative strategy for the prevention and treatment of muscle degenerative diseases.
Materials and methods
Cell culture and differentiation
Mouse myoblast C2C12 cell line was purchased from the American Type Culture Collection. C2C12 myoblasts were cultured in high glucose Dulbecco’s modified Eagle medium (DMEM) (Thermo Fisher Scientific) supplemented with 10% (v/v) fetal bovine serum (Biowest, France), 1× penicillin-streptomycin (Gibco), and 5 μg/mL Plasmocin prophylactic (InvivoGen) in a humidified incubator at 37°C with 5% CO2. When myoblasts reached 90%–95% confluency, the cells were rinsed with phosphate-buffered saline (PBS) (Gibco) and the medium was replaced with differentiation medium (DM)-high glucose DMEM containing 2% (v/v) horse serum (Gibco) to promote differentiation into myotubes. Fresh DM was changed every other day.
Purification of RBCEVs
Blood samples of healthy donors with informed consent were obtained from Innovative Research. RBCs were separated from whole blood and RBCEVs were purified from RBCs at ESCO Aster as described previously.27 In brief, RBCs were separated from plasma using centrifugation at 1,000 × g for 8 min at 4°C and washed three times with PBS (1,000 × g for 8 min at 4°C). White blood cells were completely removed by leukodepletion filters (Nigale, China). Isolated RBCs were collected in Nigale buffer (0.2 g/L citric acid, 1.5 g/L sodium citrate, 7.93 g/L glucose, 0.94 g/L sodium dihydrogen phosphate, 0.14 g/L adenine, 4.97 g/L sodium chloride, and 14.57 g/L mannitol), diluted in PBS containing 0.1 mg/mL calcium chloride, and incubated overnight with 10 μM calcium ionophore (Sigma) at 37°C with 5% CO2. RBCs and cell debris were pelleted and the supernatant was collected by centrifugation at increasing speed (600 × g for 20 min, 1,600 × g for 15 min, and 3,260 × g for 15 min). The supernatant was filtered through a 0.45-μm membrane before ultracentrifugation at 50,000 × g for 70 min at 4°C using an SW32 rotor (Beckman Coulter). The pellet was resuspended in 1 mL of PBS and subsequently loaded onto a 60% sucrose cushion and ultracentrifuged at 50,000 × g for 16 h at 4°C. RBCEVs were collected at the interface and washed with PBS at 50,000 × g for 70 min at 4°C. Purified RBCEVs were stored in PBS containing 4% trehalose at −80°C.
Western blot analysis
RBCEVs were lysed with RIPA buffer (Thermo Fisher Scientific) supplemented with protease inhibitors (Biotool) for 5 min on ice. Muscle tissues were homogenised and lysed for 30 min on ice. Protein concentration was measured by a Pierce BCA assay (Life Technologies) with BSA (New England Biolabs, UK) concentration as standards. A total of 20 μg protein lysate was loaded onto 10% polyacrylamide gels together with a Precision Plus Protein Kaleidoscope-prestained protein standard (Bio-Rad). The proteins were transferred to Immobilon-P polyvinylidene difluoride membranes (Merck Millipore), which were blocked using 5% milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 h followed by an incubation with primary antibody, anti-ALIX antibody (Santa Cruz, dilution 1:1,000), anti-TSG101 antibody (Santa Cruz, dilution 1:1,000), anti-human GAPDH antibody (Santa Cruz, dilution 1:1,000), anti-human GPA antibody (Santa Cruz, dilution 1:500), anti-human HBA antibody (Santa Cruz, dilution 1:1,000), anti-human β-actin antibody (CST, dilution 1:1,000), anti-mouse myostatin antibody (Abcam, UK, dilution 1:1,000), or anti-mouse MCD antibody (Invitrogen, dilution 1:1,000) overnight at 4°C. The membranes were washed three times with TBST and then incubated with HRP-conjugated secondary antibody (Jackson ImmunoResearch, dilution 1:1,000) for 1 h at room temperature. The blots were imaged using a ChemiDoc gel documentation system (Bio-Rad).
SiRNA loading into RBCEVs and quantification
RBCEVs (50 μg) were transfected with 1 μg of Mstn or Mlycd siRNA or FAM-conjugated siRNAs (Shanghai GenePharma, China) using REG1 transfection reagent (Carmine Therapeutics) according to the manufacturer’s protocols. In brief, 1 μg of siRNA was incubated with 7 μL of REG1 in OptiMEM I Reduced-Serum Medium (Thermo Fisher Scientific) for 15 min at room temperature. The resulting siRNA-REG1 complex was incubated with 50 μg of RBCEVs for 1 h at room temperature. Afterward, free siRNAs and transfection reagents were washed away using three rounds of centrifugation at 21,000 × g for 30 min.
For siRNA loading efficiency, 50 μg of siRNA-loaded RBCEVs were dissociated and lysed in 1% Triton X-100 for 5 min at room temperature and then subjected to 2% agarose electrophoretic separation along with a serial dilution of input siRNAs in Tris-Borate-EDTA buffer (1st BASE, Singapore) for 30 min at 80 V. Unloaded RBCEVs were used as a control to account for any endogenous RNA that may affect the downstream quantification. The gel was imaged using a ChemiDoc gel documentation system (Bio-Rad). The RNA band intensity was measured by ImageJ v.1.8.0 and the serial dilution was used to plot a standard curve, which in turn was used to calculate the copy number of siRNA loaded into each individual RBCEV. RBCEV particle number was determined by a ZetaView nanoparticle tracking analyzer (Particle Metrix, Germany). For RNase treatment, 50 μg of siRNA-loaded RBCEVs was incubated with 20 U of RNase If (New England Biolabs, UK) at 37°C for 30 min and inactivated at 75°C for 5 min prior to incubation with 1% Triton X-100 and 2% agarose electrophoretic separation.
Single EV flow cytometry
For analysis of single EVs using flow cytometry, EVs were stained with biotinylated anti-human GPA (BioLegend) followed by streptavidin-Alexa Fluor 488 (Abcam) antibody. EVs stained with streptavidin-Alexa Fluor 488 antibody only were compared with unstained EVs, to eliminate any contribution by non-specific binding of antibody to EVs. For assessing siRNA loading, FAM-conjugated siRNAs were loaded onto EVs and the FAM signal was acquired using the 488-nm laser. EVs were washed twice in PBS, diluted 10,000-fold and analyzed using a NanoFCM system (NanoFCM, UK). The laser power was fixed at 8 mW and the SS decay at 10%. The sampling pressure was fixed at 1.0 kPa prior to acquisition and events were recorded for a duration of 1 min for each sample. All samples diluted accordingly in filtered PBS were compared with PBS only to eliminate any contribution by aggregated antibodies/proteins. Flow cytometric plots were generated using FlowJo v10.0.7.
Labeling of RBCEVs with fluorogenic dye
A total of 1 mg of RBCEVs was incubated with 100 nM MemGlow 640 fluorogenic membrane probe (Cytoskeleton) for 2 h at room temperature, 20 μM CFSE (Life Technologies) for 1 h at 37°C, or 2 μM DiR (Thermo Fisher Scientific) for 20 min at room temperature. Following labeling, RBCEVs were spun down at 21,000 × g. The resulting pellet was resuspended in 500 μL PBS and passed through a qEV-original SEC column (Izon, New Zealand). The fractions containing RBCEVs (fractions 7–9) were collected and washed twice at 21,000 × g for 20 min using centrifugation. The FT of the last EV wash was used as a negative control. A free dye control (100 nM MemGlow 640 in PBS, 20 μM CFSE in PBS, or 2 μM DiR in PBS) was processed in the same way as EV samples side-by-side to ensure that the observed recipient cell fluorescence uptake is not caused by carryover of unbound dye.
In vitro uptake of RBCEVs in myotubes
The differentiated C2C12 myotubes in a 24-well plate were pre-treated with 10 μM wortmannin or 0.5 μM cytochalasin D for 1 h followed by incubation with 0.2 μg/μL CFSE-RBCEVs or MG640-RBCEVs for 6–24 h in a humidified incubator at 37°C with 5% CO2. For immunofluorescent staining, the cells were rinsed twice with PBS and fixed for 10 min using 4% paraformaldehyde (Alfa Aesar) in PBS. Blocking buffer (5% donkey serum and 0.1% Triton X-100 in PBS) was applied for 1 h at room temperature. After incubation with mouse anti-human GPA antibody (BioLegend) for 2 h at 4°C, the cells were incubated with donkey anti-mouse secondary antibody conjugated with Alex Fluor 594 fluorophore (Jackson ImmunoResearch) for 1 h at room temperature. The cells were then rinsed in PBS and stained with Hoechst 33342 (Abcam) and MemGlow 640 for 10 min at room temperature. The cells were subsequently washed three times with PBS. Images were acquired using an Olympus FV3000 confocal microscope (Olympus, Japan). Image acquisition was conducted using FluoView software. For flow cytometry analysis, the cells were harvested and washed with PBS twice and re-suspended in FACS buffer. The data were analyzed using a FACS LSRII cytometer (BD BioSciences). Flow cytometric plots were generated using FlowJo v.10.0.7.
In vivo uptake of RBCEVs in myofibers
A total of 100 μg DiR-labeled RBCEVs were injected into the quadriceps of nude mice. Free dye processed in the same way as EV samples and the FT of the last EV wash were used as controls. After 6 and 24 h, the mice were sacrificed and DiR fluorescence was measured in the organs using IVIS Lumina II (PerkinElmer).
A total of 100 μg CFSE-labeled RBCEVs were injected into the quadriceps of C57BL/6 mice. After 24 h, the mice were anesthetized and perfused with formalin. The quadriceps were then excised and fixed in formalin overnight followed by snap freezing in OCT. Frozen tissues were sectioned at a thickness of 5 μM, blocked with 5% BSA and 0.1% Triton X-100, and stained with Phalloidin-iFluor 532 fluorescein dye (Cayman Chemical). Sections were counterstained with DAPI (Thermo Fisher Scientific), mounted with anti-fade mounting medium (Abcam), and imaged using an Olympus FV3000 microscope.
Dose- and time-dependent effects of RBCEVs on gene expression
For dose-dependent response, mouse quadriceps received a single dose of 12.5, 25, 50, or 100 μg Mstn/Mlycd siRNA-loaded RBCEVs via intramuscular administration. A corresponding amount of NC siRNA-loaded RBCEVs was used as a negative control. After 24 h, quadriceps at the injection site were collected and subjected to RNA extraction followed by qPCR analysis of gene expression.
For time-dependent response, a single dose of 50 μg Mstn/Mlycd siRNA-loaded RBCEVs were injected in the quadriceps via intramuscular administration. NC siRNA-loaded RBCEVs (50 μg) were used as a negative control. At 24, 48, 72, 96, and 120 h post administration, quadriceps at the injection site were collected and subjected to RNA extraction followed by qPCR analysis of gene expression.
In vivo generation of cancer cachectic model and treatment with RBCEVs
All mouse experiments were performed according to experimental protocols approved by the Institutional Animal Care and Use Committee of the National University of Singapore. Mice of similar ages were tagged and grouped randomly for control and test treatments. Experiments were performed in a blinded manner. Exclusion was applied to the mice that accidentally died due to anesthesia. C57BL/6NTac mice were purchased from InVivos (Singapore). BALB/c nude mice (strain NU/JInv) were purchased from the Jackson Laboratory.
A total of 5 million LLC cells were inoculated in the flanks of 6-week-old C57BL/6 mice. After 1 day, mice were grouped randomly and injected with 2.5 mg/kg (50 μg) siRNA-loaded RBCEVs intramuscularly at multiple sites in the four limbs. Intramuscular injections were repeated nine times at intervals of 3 days. Muscle size, particularly the distance between the quadriceps femoris (triceps brachii) muscle and femur (or humerus) including surrounding skin and subcutaneous adipose tissue at 50% proximal of the distance between the quadriceps tendon (or triceps tendon) and the femoral greater trochanter (or humeral greater tubercle) of the hindlimb (or forelimb), was measured using an electronic digital caliper every 3 days following anesthesia and the removal of fur to expose the skin. Overall body weight was measured using an electronic balance machine every 3 days. Mice were sacrificed when tumors approached a lethal size. Muscles and hearts were collected for analysis.
Measurement of grip strength
We optimized a protocol for grip strength measurement based on a previous protocol.38 The apparatus consisted of several metal weights. To measure grip strength in the forelimbs, the mice were held gently by the base of the tail and allowed to grasp the metal weights with their forelimbs. The heaviest weights lifted by the mice for 3 s were recorded and normalized to their overall body weight. The measurements were conducted at three time points (0, 13, and 28 days post administration of siRNA-EVs).
H&E staining
Following overnight fixation in 10% neutral buffered formalin (Sigma), muscle tissues were sequentially dehydrated in 70%, 80%, 90%, and 100% ethanol at 37°C using a Leica TP1020 tissue processor (Leica, Germany). Samples were cleared in three baths of Histo-Clear (National Diagnostics), each for 1.5 h at 37°C, and impregnated in three baths of paraffin wax (Thermo Fisher Scientific), each for 1 h at 62°C, respectively. The paraffin blocks were sectioned at 4 μm using a Leica RM2255 rotary microtome. Sections were dried at 37°C and serially dewaxed in three baths of Histo-Clear, then immersed in one bath of absolute ethanol, each for 10 min. Sections were rehydrated in 90%, 75%, and 50% ethanol, each for 10 dips, and distilled water for 10 dips. Subsequently, sections were stained with Mayer’s hematoxylin (Abcam) for 1 min. After washing with water, the sections were blued with 2% ammonia (Sigma). Sections were subsequently immersed in 70% ethanol for 10 dips and stained with alcoholic eosin (Abcam) for 30 s. After washing with three baths of 90% ethanol, sections were dehydrated in absolute ethanol, cleared in Histo-Clear then mounted using DPX Mounting solution (Sigma). Images were acquired using a TissueFAXS PLUS slide scanner (TissueGnostics, Austria). Image acquisition was conducted using TissueFAXS viewer software while further analysis and quantification were conducted using ImageJ v.1.8.0.
RNA extraction and qRT-PCR
Total RNA was extracted from tissues using TRIzol (Invitrogen) according to the manufacturer’s manuals. RNA was converted to cDNA using a high-capacity cDNA reverse-transcription kit (Applied Biosystems) following the manufacturer’s protocol. mRNA levels were quantified using SsoAdvanced universal SYBR Green qPCR kit (Bio-Rad), normalized to Gapdh (for primer sequences, see Table S2). All qPCR reactions were performed using a QuantStudio 6 Flex Real-Time PCR System (Life Technologies).
Toxicity and immunogenicity assessment
The mice were regularly monitored during the study for visible signs of toxicity or stress. Mouse blood was harvested by cardiac puncture at the endpoint of in vivo treatments. The whole blood was clotted for 30 min at 37°C and the sera were isolated following centrifugation at 5,000 rpm for 20 min. Biochemistry parameters including the concentration of total bilirubin, ALT, AST, ALP, creatinine, and urea were analyzed by Comparative Medicine Diagnostics Lab at the National University of Singapore. FSH concentration in the blood was measured using an ELISA kit (Elabscience, China) according to the manufacturer’s instructions. RNA samples were isolated from the muscle tissues for qRT-PCR analysis of immune related genes including Il6, Ifnb, Ifna4, Ifna11, Ifna12, and Ifng.
EV-specific antibody measurements
For bead-assisted flow cytometry, a total of 50 μg RBCEVs were hybridized with 2.5 μg aldehyde/sulfate latex beads (4% w/v, 4 μm, Thermo Fisher Scientific) overnight at 4°C on a shaker. The resulting EV-bead hybrids were blocked with 1% BSA in PBS for 1 h at room temperature. After three washes with PBST (0.05% Tween 20 in PBS), the EV-bead hybrids were incubated with mouse sera (30× dilution in 1% BSA) for 2 h at 37°C followed by anti-mouse IgG conjugated with Alexa Fluor 488 (200 ng/mL in 1% BSA, Jackson ImmunoResearch) for 2 h at 37°C, and then washed three times with PBST. Anti-human GPA antibody was used as a positive control. The data were analyzed by FACS LSRII cytometer (BD BioSciences). Flow cytometric plots were generated using FlowJo v.10.0.7.
EV-specific antibody levels were also measured by ELISA. RBCEVs were lysed in RIPA buffer supplemented with protease inhibitors for 5 min on ice. ELISA plates were coated with 1 μg/mL RBCEV lysates in 10 mM phosphate buffer overnight at 4°C. Plates were aspirated and blocked with 1% BSA in PBS for 1 h at room temperature. After four washes with PBST, 100 μL of mouse sera (30× dilution in 1% BSA) was added to the wells and incubated for 2 h at 37°C followed by anti-mouse IgG conjugated with HRP (200 ng/mL in 1% BSA, Jackson ImmunoResearch) for 2 h at 37°C. Anti-human GPA antibody was used as a positive control. After four washes with PBST, 100 μL of TMB substrate solution (Thermo Fisher Scientific) was added and incubated in the dark for 10 min, after which the reaction was stopped using 0.16 M sulfuric acid. The plate was measured at 450 nm on a microplate reader (Tecan, Switzerland).
Statistical analysis
All statistical analysis was performed using Student’s two-tailed t tests in GraphPad Prism 8 (GraphPad Software, CA) to determine significant differences between treated samples and control. p values less than 0.05 were considered significant, based on at least three independent replicates. In all the graphs, data are presented as median or mean and standard error of the mean. Animal experiments were repeated in groups of five to six mice. The minimum sample size of three was determined using G∗Power analysis which compares the mean difference of two independent groups with α error prob = 0.05, effect size d = 5, and power = 0.95.
Acknowledgments
We would like to thank Desy Silviana and Xiangliang Lin at ESCO Aster (Singapore) for obtaining and processing blood samples. We also wish to thank the groups of Dr. Jiahai Shi, Prof. Fred Wong, and A/Prof. Lina Lim (National University of Singapore), Dr. William Cho and Victor Ma (Queen Elizabeth Hospital, Hong Kong), A/Prof. Kenneth Witwer (Johns Hopkins University), and Prof. Judy Lieberman (Harvard Medical School) for providing various reagents and advice. We also appreciate Carmine Therapeutics for providing the REG-1 reagent. We also acknowledge the help from Xuan Dang, Wen Xiu Loh, Phuong Nguyen, Trinh Tran, Yudi Wisantoso, Annie Hsu, and Poonam Rai (National University of Singapore), and Weixi Wang and Bono Gong (City University of Hong Kong). Schematics were generated using BioRender.com. This project is supported by the National University of Singapore (grant no. NUHSRO/2019/076/STARTUP/02), the National Natural Science Foundation of China (91957202), the Singapore Ministry of Health’s National Medical Research Council under its Open Fund – Individual Research Grant (OFIRG20nov-0049), the Singapore Ministry of Education (MOE-T2EP30121-0016), the National Key R&D Program (2019YFA0801701, 2021YFE0111800), the Strategic Priority Research Program of the CAS (XDA16010109), the CAS Project for Young Scientists in Basic Research (YSBR-012), the State Key Laboratory of Stem Cell and Reproductive Biology, and the Howard Hughes Medical Institute. Z.W. is supported by the Resilient & Growth fellowship (NRF-MP-2020-0004), funded by the National Science Foundation of Singapore. B.P. is supported by the NUSMed postdoctoral fellowship (NUSMED/2021/PDF/05), funded by the Singapore Ministry of Education.
Author contributions
Conceptualization, B.P., Y.Y., S.-C.N., and M.T.N.L.; data curation, B.P. and M.T.N.L.; formal analysis, B.P. and Y.Y.; investigation, B.P., Y.Y., Z.W., R.T., T.T.P., E.Y.M.Y., M.P., M.K.J., and T.C.P.; methodology, B.P.; validation, B.P., Y.Y., Z.W., R.T., T.T.P., M.K.J., and T.C.P.; visualization, B.P. and M.T.N.L.; writing – original draft, B.P.; writing – review & editing, B.P., Y.Y., Z.W., R.T., M.P., E.Y.M.Y., T.C.P., S.-C.N., and M.T.N.L.; resources, T.T.P., K.L., and S.-C.N.; funding acquisition, S.-C.N. and M.T.N.L.; supervision, S.-C.N. and M.T.N.L; project administration, S.-C.N. and M.T.N.L.
Declaration of interests
M.T.N.L. is a scientific co-founder, and advisor of Carmine Therapeutics, a start-up company that develops gene therapies. Other authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.03.036.
Contributor Information
Ng Shyh-Chang, Email: huangsq@ioz.ac.cn.
Minh T.N. Le, Email: phcltnm@nus.edu.sg.
Supplemental information
Data availability
The data that support the findings of this study are available from the corresponding author Dr. Minh TN Le upon reasonable request.
References
- 1.Marceca G.P., Londhe P., Calore F. Management of cancer cachexia: attempting to develop new pharmacological agents for new effective therapeutic options. Front. Oncol. 2020;10:298. doi: 10.3389/fonc.2020.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rausch V., Sala V., Penna F., Porporato P.E., Ghigo A. Understanding the common mechanisms of heart and skeletal muscle wasting in cancer cachexia. Oncogenesis. 2021;10:1–13. doi: 10.1038/s41389-020-00288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fearon K., Arends J., Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 2013;10:90–99. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani G., Madeddu C. Cancer cachexia: medical management. Support. Care Cancer. 2010;18:1–9. doi: 10.1007/s00520-009-0722-3. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H., Asakawa A., Amitani H., Nakamura N., Inui A. Cancer cachexia—pathophysiology and management. J. Gastroenterol. 2013;48:574–594. doi: 10.1007/s00535-013-0787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Haehling S., Anker S.D. Treatment of cachexia: an overview of recent developments. Int. J. Cardiol. 2015;184:736–742. doi: 10.1016/j.ijcard.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Anker M.S., Holcomb R., Muscaritoli M., von Haehling S., Haverkamp W., Jatoi A., Morley J.E., Strasser F., Landmesser U., Coats A.J.S., Anker S.D. Orphan disease status of cancer cachexia in the USA and in the European Union: a systematic review. J. Cachexia Sarcopenia Muscle. 2019;10:22–34. doi: 10.1002/jcsm.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatakeyama S., Summermatter S., Jourdain M., Melly S., Minetti G.C., Lach-Trifilieff E. ActRII blockade protects mice from cancer cachexia and prolongs survival in the presence of anti-cancer treatments. Skelet. Muscle. 2016;6:26. doi: 10.1186/s13395-016-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toledo M., Busquets S., Penna F., Zhou X., Marmonti E., Betancourt A., Massa D., López-Soriano F.J., Han H.Q., Argilés J.M. Complete reversal of muscle wasting in experimental cancer cachexia: additive effects of activin type II receptor inhibition and β-2 agonist. Int. J. Cancer. 2016;138:2021–2029. doi: 10.1002/ijc.29930. [DOI] [PubMed] [Google Scholar]
- 10.Zhou X., Wang J.L., Lu J., Song Y., Kwak K.S., Jiao Q., Rosenfeld R., Chen Q., Boone T., Simonet W.S., et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–543. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 11.McPherron A.C., Lawler A.M., Lee S.J. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 12.Lee S.J. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J. Clin. Invest. 2021;131 doi: 10.1172/JCI148372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C.M., Yang Z., Liu C.W., Wang R., Tien P., Dale R., Sun L.Q. Myostatin antisense RNA-mediated muscle growth in normal and cancer cachexia mice. Gene Ther. 2008;15:155–160. doi: 10.1038/sj.gt.3303016. [DOI] [PubMed] [Google Scholar]
- 14.Makurvet F.D. Biologics vs. small molecules: drug costs and patient access. Med. Drug Discov. 2021;9 [Google Scholar]
- 15.Campbell C., McMillan H.J., Mah J.K., Tarnopolsky M., Selby K., McClure T., Wilson D.M., Sherman M.L., Escolar D., Attie K.M. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: results of a randomized, placebo-controlled clinical trial. Muscle Nerve. 2017;55:458–464. doi: 10.1002/mus.25268. [DOI] [PubMed] [Google Scholar]
- 16.Fukawa T., Yan-Jiang B.C., Min-Wen J.C., Jun-Hao E.T., Huang D., Qian C.N., Ong P., Li Z., Chen S., Mak S.Y., et al. Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nat. Med. 2016;22:666–671. doi: 10.1038/nm.4093. [DOI] [PubMed] [Google Scholar]
- 17.Das S.K., Eder S., Schauer S., Diwoky C., Temmel H., Guertl B., Gorkiewicz G., Tamilarasan K.P., Kumari P., Trauner M., et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 2011;333:233–238. doi: 10.1126/science.1198973. [DOI] [PubMed] [Google Scholar]
- 18.Dyck J.R.B., Cheng J.F., Stanley W.C., Barr R., Chandler M.P., Brown S., Wallace D., Arrhenius T., Harmon C., Yang G., et al. Malonyl coenzyme a decarboxylase inhibition protects the ischemic heart by inhibiting fatty acid oxidation and stimulating glucose oxidation. Circ. Res. 2004;94:e78–e84. doi: 10.1161/01.RES.0000129255.19569.8f. [DOI] [PubMed] [Google Scholar]
- 19.Celik A., Kano Y., Tsujinaka S., Okada S., Takao K., Takagi M., Chohnan S., Soda K., Kawakami M., Konishi F. Decrease in malonyl-CoA and its background metabolic alterations in murine model of cancer cachexia. Oncol. Rep. 2009;21:1105–1111. doi: 10.3892/or_00000330. [DOI] [PubMed] [Google Scholar]
- 20.Peer D. Harnessing RNAi nanomedicine for precision therapy. Mol. Cell. Ther. 2014;2:5–11. doi: 10.1186/2052-8426-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasir J.K., Labhasetwar V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv. Drug Deliv. Rev. 2007;59:718–728. doi: 10.1016/j.addr.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knudsen K.B., Northeved H., Kumar P.E.K., Permin A., Gjetting T., Andresen T.L., Larsen S., Wegener K.M., Lykkesfeldt J., Jantzen K., et al. In vivo toxicity of cationic micelles and liposomes. Nanomedicine. 2015;11:467–477. doi: 10.1016/j.nano.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Schwarze S.R., Ho A., Vocero-Akbani A., Dowdy S.F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 24.Pitt J.M., Kroemer G., Zitvogel L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J. Clin. Invest. 2016;126:1139–1143. doi: 10.1172/JCI87316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.EL Andaloussi S., Mäger I., Breakefield X.O., Wood M.J.A. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 26.Usman W.M., Pham T.C., Kwok Y.Y., Vu L.T., Ma V., Peng B., Chan Y.S., Wei L., Chin S.M., Azad A., et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018;9:2359–2415. doi: 10.1038/s41467-018-04791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pham T.C., Jayasinghe M.K., Pham T.T., Yang Y., Wei L., Usman W.M., Chen H., Pirisinu M., Gong J., Kim S., et al. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. J. Extracell. Vesicles. 2021;10 doi: 10.1002/jev2.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng B., Nguyen T.M., Jayasinghe M.K., Gao C., Pham T.T., Vu L.T., Yeo E.Y.M., Yap G., Wang L., Goh B.C., et al. Robust delivery of RIG-I agonists using extracellular vesicles for anti-cancer immunotherapy. J. Extracell. Vesicles. 2022;11 doi: 10.1002/jev2.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attie K.M., Borgstein N.G., Yang Y., Condon C.H., Wilson D.M., Pearsall A.E., Kumar R., Willins D.A., Seehra J.S., Sherman M.L. A single ascending dose study of muscle regulator ACE-031 in healthy volunteers. Muscle Nerve. 2013;47:416–423. doi: 10.1002/mus.23539. [DOI] [PubMed] [Google Scholar]
- 30.Smith R.C., Lin B.K. Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr. Opin. Support. Palliat. Care. 2013;7:352–360. doi: 10.1097/SPC.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Connor R.S., Guo L., Ghassemi S., Snyder N.W., Worth A.J., Weng L., Kam Y., Philipson B., Trefely S., Nunez-Cruz S., et al. The CPT1a inhibitor, etomoxir induces severe oxidative stress at commonly used concentrations. Sci. Rep. 2018;8:6289. doi: 10.1038/s41598-018-24676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao C.H., Liu G.Y., Wang R., Moon S.H., Gross R.W., Patti G.J. Identifying off-target effects of etomoxir reveals that carnitine palmitoyltransferase I is essential for cancer cell proliferation independent of β-oxidation. Plos Biol. 2018;16 doi: 10.1371/journal.pbio.2003782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morine K.J., Bish L.T., Pendrak K., Sleeper M.M., Barton E.R., Sweeney H.L. Systemic myostatin inhibition via liver-targeted gene transfer in normal and dystrophic mice. PloS one. 2010;5 doi: 10.1371/journal.pone.0009176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan T., Weber H., DiMuzio J., Matter A., Dogdas B., Shah T., Thankappan A., Disa J., Jadhav V., Lubbers L., et al. Silencing myostatin using cholesterol-conjugated siRNAs induces muscle growth. Mol. Ther. Nucleic Acids. 2016;5:e342. doi: 10.1038/mtna.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biscans A., Caiazzi J., McHugh N., Hariharan V., Muhuri M., Khvorova A. Docosanoic acid conjugation to siRNA enables functional and safe delivery to skeletal and cardiac muscles. Mol. Ther. 2021;29:1382–1394. doi: 10.1016/j.ymthe.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Driedonks T., Jiang L., Carlson B., Han Z., Liu G., Queen S.E., Shirk E.N., Gololobova O., Liao Z., Nyberg L.H., et al. Pharmacokinetics and biodistribution of extracellular vesicles administered intravenously and intranasally to Macaca nemestrina. J. Extracell. Biol. 2022;1:e59. doi: 10.1002/jex2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saleh A.F., Lázaro-Ibáñez E., Forsgard M.A.M., Shatnyeva O., Osteikoetxea X., Karlsson F., Heath N., Ingelsten M., Rose J., Harris J., et al. Extracellular vesicles induce minimal hepatotoxicity and immunogenicity. Nanoscale. 2019;11:6990–7001. doi: 10.1039/c8nr08720b. [DOI] [PubMed] [Google Scholar]
- 38.Deacon R.M. Measuring the strength of mice. J. Vis. Exp. 2013;76 doi: 10.3791/2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author Dr. Minh TN Le upon reasonable request.