Abstract
Aims
We compared diagnostic performance, costs, and association with major adverse cardiovascular events (MACE) of clinical coronary computed tomography angiography (CCTA) interpretation versus semiautomated approach that use artificial intelligence and machine learning for atherosclerosis imaging‐quantitative computed tomography (AI‐QCT) for patients being referred for nonemergent invasive coronary angiography (ICA).
Methods
CCTA data from individuals enrolled into the randomized controlled Computed Tomographic Angiography for Selective Cardiac Catheterization trial for an American College of Cardiology (ACC)/American Heart Association (AHA) guideline indication for ICA were analyzed. Site interpretation of CCTAs were compared to those analyzed by a cloud‐based software (Cleerly, Inc.) that performs AI‐QCT for stenosis determination, coronary vascular measurements and quantification and characterization of atherosclerotic plaque. CCTA interpretation and AI‐QCT guided findings were related to MACE at 1‐year follow‐up.
Results
Seven hundred forty‐seven stable patients (60 ± 12.2 years, 49% women) were included. Using AI‐QCT, 9% of patients had no CAD compared with 34% for clinical CCTA interpretation. Application of AI‐QCT to identify obstructive coronary stenosis at the ≥50% and ≥70% threshold would have reduced ICA by 87% and 95%, respectively. Clinical outcomes for patients without AI‐QCT‐identified obstructive stenosis was excellent; for 78% of patients with maximum stenosis < 50%, no cardiovascular death or acute myocardial infarction occurred. When applying an AI‐QCT referral management approach to avoid ICA in patients with <50% or <70% stenosis, overall costs were reduced by 26% and 34%, respectively.
Conclusions
In stable patients referred for ACC/AHA guideline‐indicated nonemergent ICA, application of artificial intelligence and machine learning for AI‐QCT can significantly reduce ICA rates and costs with no change in 1‐year MACE.
Keywords: artificial Intelligence, atherosclerosis, CCTA, coronary artery disease, coronary computed tomography, fractional flow reserve, quantitative coronary angiography
Application of artificial intelligence to typically acquired cardiac computed tomography angiography through atherosclerosis imaging and quantitative cardiac computed tomography through the randomized controlled Computed Tomographic Angiography for Selective Cardiac Catheterization Trial is a clinically effective (no change in 1 year major adverse cardiovascular events vs. expert reader), safe (87%−95% reduction in invasive coronary angiography [ICA]) and high value (26%−34% reduction in cost) approach to guide referral management of stable patients being considered for ICA. Clinical case example is shown.
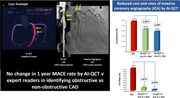
Key points
Software platform technology solutions now enable artificial intelligence guided and quantitative cardiac computed tomography angiography (CCTA) for coronary artery plaque quantification and characterization and vascular morphology assessment.
As most patients referred for invasive coronary angiography (ICA) are found not to have actionable coronary artery disease, artificial intelligence guided CCTA measures of vascular morphology may allow for improved referral management to ICA in a manner that is safe and lower in costs.
Abbreviations
- AI
artificial intelligence
- AI‐QCT
atherosclerosis imaging and quantitative cardiac computed tomography
- CAD
coronary artery disease
- CCTA
coronary computed tomography angiography
- FDA
food and drug administration
- HOPPS
hospital outpatient prospective payment
- ICA
invasive coronary angiography
- LAD
left anterior descending
- LCx
left circumflex
- LM
left main
- MPI
myocardial perfusion imaging
- RCA
right coronary artery
1. INTRODUCTION
Invasive coronary angiography (ICA) allows for evaluation of stable symptomatic patients with suspected coronary artery disease (CAD) to guide decisions of coronary revascularization. 1 , 2 While current American College of Cardiology (ACC)/American Heart Association (AHA) guidelines outline appropriate selection of patients for elective ICA, in real‐world practice, most individuals who undergo non‐emergent ICA do not have actionable CAD. 3 , 4 For these patients, ICA has been shown add to unnecessary health care system costs and increase the risk for potential procedural complications. 5 , 6
In the 2021 Updated ACC/AHA Chest Pain guideline, coronary computed tomography angiography (CCTA) has been elevated to a class IA indication to serve as a first line test for identification and exclusion for obstructive CAD with a high sensitivity of 95%−99%. 7 , 8 , 9 Evaluation of CCTA in stable symptomatic patients referred for nonemergent ICA has been done previously in the Coronary Computed Tomographic Angiography for Selective Cardiac Catheterization (CONSERVE) randomized controlled trial (RCT), which observed a selective referral strategy that incorporates a CCTA‐first approach before catheterization was associated with a 77% reduction in ICA. 10 This deferral of ICA was associated with reduced rates of coronary revascularization and downstream costs, with no differences in 12‐month rates of major adverse cardiovascular events (MACE) as compared to a direct ICA referral strategy.
In this analysis of the CONSERVE RCT, we hypothesized that application of atherosclerosis imaging and quantitative cardiac computed tomography (AI‐QCT) would allow for better determination of patients with and without obstructive CAD who may benefit from ICA, and that this approach would be associated with reduced ICA and lower costs without added risk of MACE.
2. METHODS
This study evaluated patients from the Coronary Computed Tomographic Angiography for Selective Cardiac Catheterization (CONSERVE; NCT01810198) RCT who underwent CCTA. For each participant, after receiving written informed consent, eligible patients were randomly assigned to a selective referral or direct referral strategy. This study was a post hoc analysis of the selective referral arm. The original study protocol was approved at each enrolling site by the local institutional review board or ethics committee. Full study details can be found in the landmark publication. 10 Briefly, CONSERVE was a 1:1 randomized, controlled, open‐label, international, multicenter trial at 22 hospitals and cardiology practices in North America, East Asia, Europe, and India. A selective referral strategy was defined by initial performance of CCTA, with ICA performed at the discretion of the local physician informed by the CCTA findings. The study participants were stable patients with suspected but without known CAD referred for non‐emergent ICA based upon American College of Cardiology/American Heart Association (ACC/AHA) guidelines for ICA, and included indications based on abnormal stress testing or suspected CAD symptoms. 1 , 11 Exclusion criteria included known history of CAD, ACC/AHA Class I or III indication for ICA, known complex congenital heart disease, or planned ICA for reasons other than CAD evaluation. Among 784 patients undergoing CCTA in the index study, 37 CCTA studies were not present due to image file corruption. No available CCTA study (0%) was excluded from analysis by AI‐QCT for poor CCTA image quality, with 747 patients included in the final study cohort.
The primary composite endpoint for MACE included death, nonfatal myocardial infarction, unstable angina, stroke, urgent or emergent coronary revascularization, and cardiovascular hospitalization. Further data were collected for downstream invasive and noninvasive coronary procedures, as well as cardiovascular and all cause hospitalizations. The primary endpoint was analyzed at 1 year of follow‐up. Secondary endpoints included evaluation of downstream coronary revascularization, invasive and noninvasive CAD diagnostic testing, and hospitalizations. If a patient had an independent clinical events committee, blinded to randomization assignment, adjudicated all clinical endpoints under the guidance of a Data Safety and Monitoring Board.
CCTA was performed using a single‐ or dual‐source CT scanner with ≥64 detector rows and a detector row width of ≤75 mm in accordance with Society of Cardiovascular Computed Tomography (SCCT) guidelines. 12 For both ICA and CCTA, presence or absence of angiographic stenoses ≥ 50% and ≥70% was recorded by local site physicians meeting a minimum of Level II or Level III Certification for CCTA interpretation, 13 , 14 and the maximum perpatient % stenosis was used to identify the presence or absence of obstructive CAD. Normal ICAs were considered as those that demonstrated no obstructive stenosis ≥ 50% or ≥70%. ICAs was performed in agreement with clinical indications and imaging standards by certified and experienced interventional cardiologists.
AI‐QCT was performed using a commercially available software platform (Cleerly Labs, Cleerly, Inc.) that performs atherosclerosis imaging quantitative CCTA (AI‐QCT) analysis 15 , 16 , 17 using a series of validated convolutional neural network models for quantitative image quality assessment, coronary segmentation and labeling, vascular morphology measurements, and atherosclerotic plaque characterization. 15 Hundred percent of studies were analyzable by AI‐QCT and included in the study results. A case example with invasive angiography correlation is shown in Figure 1. Prior validation of AI‐QCT has been reported in 2 multicenter trials. 15 , 17 Study analysis was performed in‐kind for this investigator‐initiated study.
Figure 1.
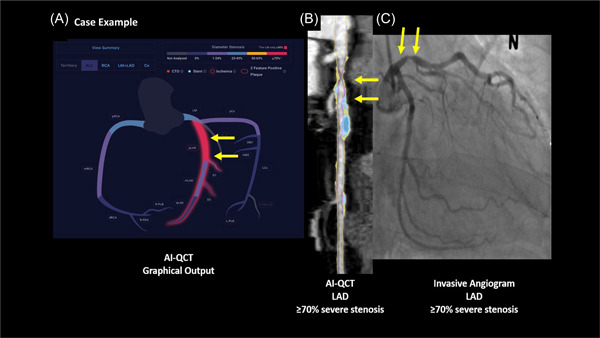
Example of a patient with AI‐QCT analysis demonstrating severe obstructive (>70%) luminal stenosis in the proximal LAD with invasive cath correlation. AI‐QCT total plaque volume, calcified and noncalcified plaque is also shown. AI‐QCT, atherosclerosis imaging and quantitative cardiac computed tomography; LAD, left anterior descending.
Coronary segments with a diameter ≥ 2 mm were included in the analysis using a modified 18‐segment SCCT model. 10 , 16 Each segment was evaluated for the presence or absence of coronary atherosclerosis, defined as any tissue structure > 1 mm3 within the coronary artery wall that was differentiated from the surrounding epicardial tissue, epicardial fat or the vessel lumen itself. The following CAD features were evaluated:
Stenosis: Utilizing a normal proximal reference vessel cross‐sectional slice, the start and the end of the lesion were identified, and from the cross‐sectional slice that demonstrated the greatest absolute narrowing, % diameter stenosis severity was automatically calculated. Obstructive stenosis was defined at ≥50% and ≥70% stenosis thresholds. All vessels with 0% stenosis were defined as having no CAD.
Atherosclerosis: Atherosclerosis characterization was performed by AI‐QCT for every coronary artery and its branches. Plaque volumes (PVs) (mm3) were calculated for each coronary lesion and then summated to compute the total PV at the patient level. Plaque with a minimum volume of ≥3mm3 was included for analysis. This provided data for analysis on both the per‐lesion and per‐patient level. PV was further categorized using Hounsfield unit (HU) ranges with noncalcified plaque (NCP) defined as HU between −30 and +350; low density‐NCP (LD‐NCP) defined as plaques < 30 HU; and calcified plaque (CP) defined as >350 HU. 17
All statistical analyses were performed using SAS version 9.4 (SAS). Continuous data are reported as mean ± standard deviation, and categorical variables are presented as absolute numbers with corresponding percentages. The rates of stenosis, 0%, 1%−24%, 25%−49%, ≥50% and ≥70% were compared individually between AI‐QCT and Level II/III site readers on a per patient and per vessel basis. The per‐patient differences were evaluated using McNemar's test of the paired data. The per‐vessel rates were compared using the logistic Generalized Estimating Equations method to account for the correlation of the multiple vessels from the same patient. The ability of AI‐QCT and stenosis level II/III site readers to predict the occurrence of a MACE event was compared by generating Receiver Operating Characteristic (ROC) curves for each approach, with stenosis categorized as 0%, 1%−24%, 25%−49%, 50%−69% and 70%−100%. The differences in the predictive ability of each method were compared by calculating and comparing the area under the ROC curves (AUC).
Resource utilization and cost models were established to estimate the rate of downstream ICA using an AI‐QCT‐first approach set at a ≥ 50% and ≥70% to define severe stenosis. The costs of CCTA, invasive angiography and stress testing were determined based on recently published Hospital Outpatient Prospective Payment (HOPPS) standards. 18 AI‐QCT costs were set at $1500 USD.
3. RESULTS
3.1. Clinical characteristics of the study population
Demographic and clinical characteristics of the study cohort (60 ± 12 years, 49% women) are listed in Table 1. There was a high prevalence of CAD risk factors, including: 57% hypertension, 33% hyperlipidemia and 30% smokers. 88% of patients experienced symptoms suggestive of CAD, with the majority (70%) having typical or atypical angina.
Table 1.
Baseline demographics and clinical characteristics.
| Variable (% or mean ± SD) | All Patients (N = 747) |
|---|---|
| Age, years | 60 ± 12.2 |
| Women | 49% (363) |
| Body mass index, kg/m2 | 25.6 ± 4.0 |
| Race/Ethnicitiy | |
| African American | 0.5% (4) |
| Asian | 86% (639) |
| Hispanic | 0.5% (4) |
| White | 13% (98) |
| Hypertension | 57% (427) |
| Dyslipidemia | 33% (249) |
| Diabetes | 26% (193) |
| Family history of CAD | 9% (67) |
| Current smoker or history of smoking ≤ 1 year | 30% (224) |
| Symptoms | |
| Typical angina | 30% (224) |
| Atypical angina | 40% (300) |
| Noncardiac chest pain | 2% (17) |
| Asymptomatic | 12% (90) |
| Other | 15% (115) |
3.2. Comparison of an AI‐QCT approach to clinical CCTA interpretation
Application of AI‐QCT identified 87% and 95% patients without stenosis ≥ 50% and ≥70%, respectively, who would be eligible for ICA deferral (Table 2). For intermediate stenoses 50‐69%, AI‐QCT identified 8% of patients (n = 60/747). By comparison, site interpretation by Level II/III readers identified 27% (n = 205/747) with ≥50% and 16% (n = 117/747) with ≥70% stenosis (p < .001), and 12% (n = 88/787) patients with intermediate (50%−69%) stenoses who would be eligible for post‐CCTA stress testing after randomization and CCTA.
Table 2.
Downstream ICA and stress testing after AI‐QCT Applied to CCTA.
| Downstream test | Stenosis % | % (Number) by AI‐QCT | % Number by site read (level II/III readers) | p Value |
|---|---|---|---|---|
| ICA (Per vessel) | 0% | 21% (477/2237) | 56% (1253/2237) | <.0001 |
| 1%−24% | 55% (1222/2237) | 15% (326/2237) | <.0001 | |
| 25%−49% | 18% (411/2237) | 15% (340/2237) | .0113 | |
| ≥50% | 6% (127/2237) | 14% (318/2237) | <.0001 | |
| ≥70% | 2.1% (47/2237) | 7% (163/2237) | <.0001 | |
| ICA (Per patient) | 0% | 9% (67/747) | 35% (260/747) | <.0001 |
| 1%−24% | 49% (365/747) | 16% (117/747) | <.0001 | |
| 25%−49% | 29% (218/747) | 22% (165/747) | <.001 | |
| ≥50% | 13% (97/747) | 28% (208/747) | <.0001 | |
| ≥70% | 5% (37/747) | 16% (117/747) | <.0001 | |
| Stress testing (per‐patient) | 50%−69% | 8% (60/747) | 12% (88/747) | <.019 |
3.3. MACE rates
During mean follow‐up of 1.1 ± 0.4 years, 4.3% (n = 32) patients experienced MACE (3.8% [n = 29]) for cardiac hospitalization. When stratified by AI‐QCT measures of coronary stenosis (Table 3), amongst the 97 patients with obstructive (≥50%) stenosis, 1 patient (1.0%) suffered cardiovascular death and 1 patient (1.0%) had an acute myocardial infarction. No deaths or myocardial infarctions occurred in 78% (n = 583) patients with nonobstructive ≤ 50%. In addition, for nonobstructive ≤ 50%. patients (n = 583), 1 (1.5%) patient by AI‐QCT 0% stenosis had a cardiac hospitalization. 24 (4.1%) had MACE excluding cardiovascular death or acute myocardial infarction including unstable angina (6, 1.0%), cardiac hospitalization (22, 3.8%) and/or stroke (2, 0.3%). When categorizing stenosis severity as 0%, 1%−24%, 26%−49%, 50%−69%, >70%, stenosis severity to predict MACE events was similar between AI‐QCT (AUC of 0.61; 95% CI 0.52−0.70) and Level II/III CCTA interpretation (AUC of 0.63; 95% CI 0.53−0.73; p = .64). AI‐QCT‐based quantification of atherosclerotic plaque demonstrated a linear and significant association between the absolute PV and MACE with a hazard ratio for each PV category of 2.0 (95% CI 1.3−3.0; p = .0012). For patients with PV between 0 and 300 mm3 (n = 509), 301−750 mm3 (n = 174) and ≥750 mm3 (n = 64), there was an observed MACE rate of 2.6%, 7.0%, and 9.4%, respectively, (p = .001).
Table 3.
MACE rate by AI‐QCT stenosis measurements.
| MACE Endpoints | ALL (n = 747) | 0% (N = 67) | 1%−49% (N = 583) | ≥50% (N = 97) | ≥70% (N = 37) |
|---|---|---|---|---|---|
| CV Death | 1 (0.1%) | 0 (0%) | 0 | 1 (1.0%) | 0 (0%) |
| Acute myocardial infarction | 1 (0.1%) | 0 (0%) | 0 (0%) | 1 (1.0%) | 0 (0%) |
| Unstable angina | 6 (0.8%) | 0 (0%) | 6 (1.0%) | 0 (0%) | 0 (0%) |
| Cardiac hospitalization | 29 (3.8%) | 1 (1.5%) | 22 (3.8%) | 5 (5.2%) | 1 (2.7%) |
| Stroke | 2 (0.3%) | 0 (0%) | 2 (0.3%) | 0 (0%) | 0 (0%) |
3.4. Cost‐analysis
Results of an AI‐QCT‐based strategy for referral management of only patients with high‐grade stenosis to ICA are listed in Table 4. At a ≥ 50% and ≥70% stenosis threshold, application of AI‐QCT would have resulted in 87% and 95% patients, respectively, avoiding unnecessary ICA at a 26% and 34% cost‐savings, respectively.
Table 4.
Diagnostic cost of strategies for direct ICA referral and referral to ICA based upon AI‐QCT.
| Scenario | N | Cost | Cost/Patient | Change |
|---|---|---|---|---|
| Straight to ICA | ||||
| ICA | 784 | $2, 175, 600 | $2775 | |
| AI‐QCT, followed by ICA if ≥ 50% | ||||
| CCTA | 747 | $135, 954 | ||
| AI‐QCT | 747 | $1, 120, 500 | ||
| ICA | 97 | $269, 175 | ||
| Total | $1, 525, 629 | $2042 | ‐26% | |
| AI‐QCT, followed by ICA if ≥ 70% | ||||
| CCTA | 747 | $135 954 | ||
| AI‐QCT | 747 | $1 120 500 | ||
| ICA | 37 | $102 675 | ||
| Total | $1 359 129 | $1819 | ‐34% | |
4. DISCUSSION
In this present study, we evaluated for the first time an AI‐QCT strategy to guide judicious referral to nonemergent ICA for patients with an ACC/AHA Guideline indication and determined that adoption of an AI‐QCT approach could reduce unnecessary ICA by 87%−95% based upon stenosis severity thresholds. The rates of safe ICA deferral from AI‐QCT were significantly higher than those based upon Level II/III reader interpretation of CCTA. Further, the AI‐QCT approach was safe, with no patient experiencing MACE during the length of the follow‐up period who had been quantified as having non‐severe stenosis by AI‐QCT. Finally, an AI‐QCT approach was cost‐efficient compared to standard of care Level II/III CCTA interpretation, with a 26‐34% reduction in costs by AI‐QCT‐based ICA deferral.
To our knowledge, these present study results represent the first to evaluate within a multicenter RCT the clinic‐economic feasibility of an AI‐QCT approach for comprehensive assessment of atherosclerosis, stenosis and other vascular morphology features for determining appropriateness of ICA for patients with guideline indications for nonemergent catheterization. Our findings provide strong evidence that integration of leading‐edge machine intelligence tools applied to CCTA can have large implications in the proper selection of patients for ICA versus those who can safely avoid unnecessary invasive, expensive, and potentially harmful procedures. The additional prognostic utility of quantified atherosclerotic burden by AI‐QCT for robust identification of individuals at risk of future MACE, as was observed in this study, provides significant incremental value for the widespread use of AI‐QCT in clinical practice.
Our study results are in direct accordance with recent data published from RCTs have established a utility of CCTA to guide decisions of ICA referral. As an example, in the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) Trial, use of CCTA was associated with fewer catheterizations showing no obstructive CAD than was functional testing (3.4% vs. 4.3%, p = .02). 19 In a comparison to expert core laboratory, clinical site readers demonstrated significant overestimation of stenosis, with a 68% increased erroneous rate of severe stenosis. This overestimation may have influenced the higher rates of ICA in study, and is keeping with our current findings wherein clinical CCTA interpretation was associated with a significantly higher rate of false positive severe stenoses compared to a validated AI‐QCT platform.
The importance of these findings stems from prior information reported from the National Cardiovascular Data Registry which demonstrated that nearly 2/3 patients referred for ICA will not, in fact, be found to have actionable CAD. 4 While some of this ICA normalcy may be attributed to inappropriate referral for non‐guideline‐indicated reasons, the current study restricted enrollment to those patients with specific ACC/AHA recommended indication, and still identified the majority of patients to not, in fact, have any stenosis ≥ 50% or ≥70%. These data have important ramifications not only to the use of ICA as a diagnostic modality but also percutaneous coronary intervention (PCI) as a therapeutic modality. Prior studies have exhibited a strong relationship between ICA and PCI, particularly when they are performed at the same setting. 20 This so‐called “diagnostic‐therapeutic cascade,” if broken, may reduce unnecessary PCI for patients who will not benefit from its performance. 21 In the original CONSERVE trial, PCI rates were reduced by ~50% and, based upon the current study findings, could be further reduced by application of an AI‐QCT strategy.
5. LIMITATIONS
The present study is not without limitations. The current analyses were performed in post hoc fashion from an international, multicenter, RCT. Further, AI‐QCT was compared to clinical site interpretation by expert readers, but no blinded CCTA core laboratory was employed. Similarly, as the CONSERVE trial evaluation of ICA was done in pragmatic fashion, no blinded quantitative coronary angiography (QCA) analysis was performed and AI‐QCT could not be directly compared to QCA for diagnostic accuracy measures. However, in prior multicenter clinical trials, AI‐QCT has been previously demonstrated as having robust diagnostic performance compared to expert readers and QCA. The present decision model assumed that all severe stenoses would trigger referral to ICA and that ICA holds perfect sensitivity and specificity.
6. CONCLUSIONS
Application of AI to typically acquired CCTA is a clinically effective, safe and cost savings approach to guide referral management of patients being considered for ICA.
CONFLICT OF INTEREST STATEMENT
J. P. E. is an employee and retains equity in Cleerly Inc. J. K. M. serves as an employee and reports equity interest in Cleerly Inc.; serves on the Scientific Advisory Board for Arineta; reports equity interest in Upside Foods; and receives research funding from the National Institutes of Health. T. C. is an employee of Cleerly. A. D. C. reports grant funding from G. W. Heart and Vascular Institute, and modest equity in Cleerly and is a consultant for Siemens Healthineers. The remaining authors declare no conflict of interest.
7. ACKNOWLEDGMENTS
The authors wish to thank Ms. Catherine Cantlay for her assistance in preparation of the manuscript.
Kim Y, Choi AD, Telluri A, et al. Atherosclerosis Imaging Quantitative Computed Tomography (AI‐QCT) to guide referral to invasive coronary angiography in the randomized controlled CONSERVE trial. Clin Cardiol. 2023;46:477‐483. 10.1002/clc.23995
DATA AVAILABILITY STATEMENT
Data may be obtained from a third party and are not publicly available. Index data for the CONSERVE trial has been previously published. Deidentified patient data are not publicly available, except if necessary to confirm study results; requests for data may be made by contacting Drs. Hyuk‐Jae Chang or James Min.
REFERENCES
- 1. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American college of cardiology Foundation/American heart association task force on practice guidelines, and the American college of physicians, American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. JACC. 2012;60:2564‐2603. [DOI] [PubMed] [Google Scholar]
- 2. Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST‐Elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST‐Elevation myocardial infarction: A report of the American college of Cardiology/American heart association task force on clinical practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 2016;133:1135‐1147. [DOI] [PubMed] [Google Scholar]
- 3. Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886‐895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel MR, Dai D, Hernandez AF, et al. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am Heart J. 2014;167:846‐852. [DOI] [PubMed] [Google Scholar]
- 5. Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503‐1516. [DOI] [PubMed] [Google Scholar]
- 6. Al‐Lamee R, Thompson D, Dehbi HM, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double‐blind, randomised controlled trial. Lancet. 2018;391:31‐40. [DOI] [PubMed] [Google Scholar]
- 7. Writing Committee M, Gulati M, Levy PD, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: executive summary: a report of the American college of Cardiology/American heart association joint committee on clinical practice guidelines. JACC. 2021;78(22):2218‐2261. [DOI] [PubMed] [Google Scholar]
- 8. Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64‐multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. JACC. 2008;52:1724‐1732. [DOI] [PubMed] [Google Scholar]
- 9. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407‐477. [DOI] [PubMed] [Google Scholar]
- 10. Chang HJ, Lin FY, Gebow D, et al. Selective referral using CCTA versus direct referral for individuals referred to invasive coronary angiography for suspected CAD. JACC. 2019;12:1303‐1312. [DOI] [PubMed] [Google Scholar]
- 11. Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography. A report of the American college of Cardiology/American heart association task force on practice guidelines (Committee on Coronary Angiography). developed in collaboration with the society for cardiac angiography and interventions. JACC. 1999;33:1756‐1824. [DOI] [PubMed] [Google Scholar]
- 12. Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of cardiovascular computed tomography guidelines committee. J Cardiovasc Comput Tomogr. 2016;10:435‐449. [DOI] [PubMed] [Google Scholar]
- 13. Choi AD, Parwani P, Michos ED, et al. The global social media response to the 14th annual society of cardiovascular computed tomography scientific sessions. J Cardiovasc Comput Tomogr. 2020;14:124‐130. [DOI] [PubMed] [Google Scholar]
- 14. Choi AD, Thomas DM, Lee J, et al. 2020 SCCT guideline for training cardiology and radiology trainees as independent practitioners (Level II) and advanced practitioners (Level III) in cardiovascular computed tomography: a statement from the society of cardiovascular computed tomography. Radiolo Cardioth Imag. 2021;3:e200480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi AD, Marques H, Kumar V, et al. CT EvaLuation by ARtificial intelligence for atherosclerosis, stenosis and vascular MorphologY (CLARIFY): A multi‐center, international study. J Cardiovasc. Comput Tomogr. 2021;15(6):470‐476. [DOI] [PubMed] [Google Scholar]
- 16. Williams MC, Earls JP, Hecht H. Quantitative assessment of atherosclerotic plaque, recent progress and current limitations. J Cardiovasc Comput Tomogr. 2022;16(2):124‐137. [DOI] [PubMed] [Google Scholar]
- 17. Griffin WF, Choi AD, Riess J, Marques H, Chang HJ, CREDENCE Investigators , Earls J.P. AI evaluation of coronary stenosis on CT coronary angiography, comparison with quantitative coronary angiography and fractional flow reserve; A CREDENCE trial sub‐study. JACC Cardiovasc Imaging. 2023;16(2):193‐205. [DOI] [PubMed] [Google Scholar]
- 18. American Society of Nuclear Cardiology . Reimbursement and coding procedures. 2020. https://www.asnc.org/coding_reimbursement
- 19. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marwick TH, Cho I, ó Hartaigh B, Min JK. Finding the gatekeeper to the cardiac catheterization laboratory. JACC. 2015;65:2747‐2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lucas FL, Siewers AE, Malenka DJ, Wennberg DE. Diagnostic‐therapeutic cascade revisited: coronary angiography, coronary artery bypass graft surgery, and percutaneous coronary intervention in the modern era. Circulation. 2008;118:2797‐2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Index data for the CONSERVE trial has been previously published. Deidentified patient data are not publicly available, except if necessary to confirm study results; requests for data may be made by contacting Drs. Hyuk‐Jae Chang or James Min.


