Abstract
Background
Colorectal cancer causes the majority of large bowel obstructions and surgical resection remains the gold standard for curative treatment. There is evidence that a deviating stoma as a bridge to surgery can reduce postoperative mortality rate; however, the optimal stoma type is unclear. The aim of this study was to compare outcomes between ileostomy and colostomy as a bridge to surgery in left-sided obstructive colon cancer.
Methods
This was a national, retrospective population-based cohort study with 75 contributing hospitals. Patients with radiological left-sided obstructive colon cancer between 2009 and 2016, where a deviating stoma was used as a bridge to surgery, were included. Exclusion criteria were palliative treatment intent, perforation at presentation, emergency resection, and multivisceral resection.
Results
A total of 321 patients underwent a deviating stoma; 41 (12.7 per cent) ileostomies and 280 (87.2 per cent) colostomies. The ileostomy group had longer length of stay (median 13 (interquartile range (i.q.r.) 10–16) versus 9 (i.q.r. 6–14) days, P = 0.003) and more nutritional support during the bridging interval. Both groups showed similar complication rates in the bridging interval and after primary resection, including anastomotic leakage. Stoma reversal during resection was more common in the colostomy group (9 (22.0 per cent) versus 129 (46.1 per cent) for ileostomy and colostomy respectively, P = 0.006).
Conclusion
This study demonstrated that patients having a colostomy as a bridge to surgery in left-sided obstructive colon cancer had a shorter length of stay and lower need for nutritional support. No difference in postoperative complications were found.
To the best of our knowledge, this is the first article providing a head-to-head comparison in a nationwide study of the use of ileostomy versus colostomy as a bridge to surgery in colonic cancer. Results favour a colostomy as a bridge to surgery.
Introduction
Colorectal cancer is one of the leading causes of cancer-related death worldwide1. Large bowel obstruction is a common consequence of locally advanced colorectal tumours, with reported incidence rates ranging between 10 and 30 per cent2. Emergency resection in patients with acute large bowel obstruction has proven to be a high-risk procedure, with reported mortality rates as high as 41 per cent3. Recently, it has been shown that a deviating stoma as a bridge to (elective) surgery reduces 90-day mortality and permanent stoma rates compared with emergency surgery4. In addition, this approach has been shown to be oncologically safe in terms of disease-free survival and loco-regional recurrence5,6. For left-sided obstructive colon carcinoma, either a colostomy or an ileostomy can be made to deviate the faecal stream. Both techniques pose advantages and disadvantages. An advantage of an ileostomy could be that it is generally easier and quicker to create due to the longer and more mobile mesentery. The proposed benefits of a colostomy compared with an ileostomy are prevention of caecal perforation due to decompression distal to the ileocecal valve and a lower risk of a high-output stoma. However, due to anatomical reasons, a colostomy is not always easy or even feasible without major dissection or conversion to laparotomy and parastomal herniation is more prevalent7. Debate remains about whether a diverting colostomy increases anastomotic leakage when performing primary anastomosis. Previously, ileostomy and colostomy have been compared in postoperative diversion after low anterior resection or defunctioning of colorectal anastomosis7,8. However, a diverting enterostomy as a bridge to surgery is being used for a different purpose with a different timeframe, which may influence outcomes and the incidence of complications. Comparisons of colostomy versus ileostomy during the bridging interval of obstructive colon cancer in (international) practice are lacking and the choice of stoma is mostly dependent on surgeons’ preference in current daily practice. The aim of this study was to retrospectively compare the use of ileostomy with colostomy as a bridge to surgery in left-sided obstructive colon cancer in the Netherlands.
Methods
This is a retrospective cohort selected from a population-based cohort study, which was led by the Dutch Snapshot Research Group conforming to the STROBE guidelines for reporting cohort studies (supplementary material). This nationwide cohort study was performed at 75 hospitals in the Netherlands according to a predefined protocol, including patients treated for left-sided obstructive colon cancer treated from 1 January 2009 to 31 December 20169. The cohort identified patients who had resection of left-sided obstructive colonic carcinoma through the Dutch Colorectal Audit (DCRA). Patients were included when a deviating stoma was created as a bridge to surgery for a radiologically confirmed symptomatic colonic obstruction (for example abdominal distention, nausea, and/or vomiting) caused by a malignant tumour in the distal colon (sigmoid, descending colon, or splenic flexure). Exclusion criteria were: treatment with palliative treatment intent, perforation at initial presentation, emergency resection of the malignant tumour, and multivisceral en bloc resections6.
Study endpoints
The primary endpoint was the total length of hospital stay during the interval between stoma formation and primary resection. Secondary endpoints were: complications in the interval until resection (such as stoma necrosis (percentage), high-output stoma (percentage), abscess formation (percentage), perforation (percentage), incidence of readmission (percentage), and re-intervention (percentage)), duration of the interval between stoma formation and primary resection, complications during resection, conversion rate, incidence of primary anastomosis, reversal of a deviating stoma during primary resection, complications after resection, anastomotic leakage of anastomosis after primary resection, and hospital stay. Long-term outcomes included permanent stoma rates. The specific reason behind the choice of stoma was not recorded, due to the retrospective nature of this study.
Statistical analysis
Categorical or dichotomous variables are presented as absolute numbers with percentages and were compared using the χ2 test. Continuous variables are shown as mean(s.d.) or median (interquartile range (i.q.r.)) and were compared using an independent Student’s t test or a Mann–Whitney U test, according to their distribution. Univariable logistic regression was used to assess for possible confounding and the odds ratio was used to depict associations. Log rank transformation was used to correct for positive skew and unequal variances. A P value of <0.050 was considered to be significant. All analyses were performed using R studio version 3.1.
Results
Patient characteristics
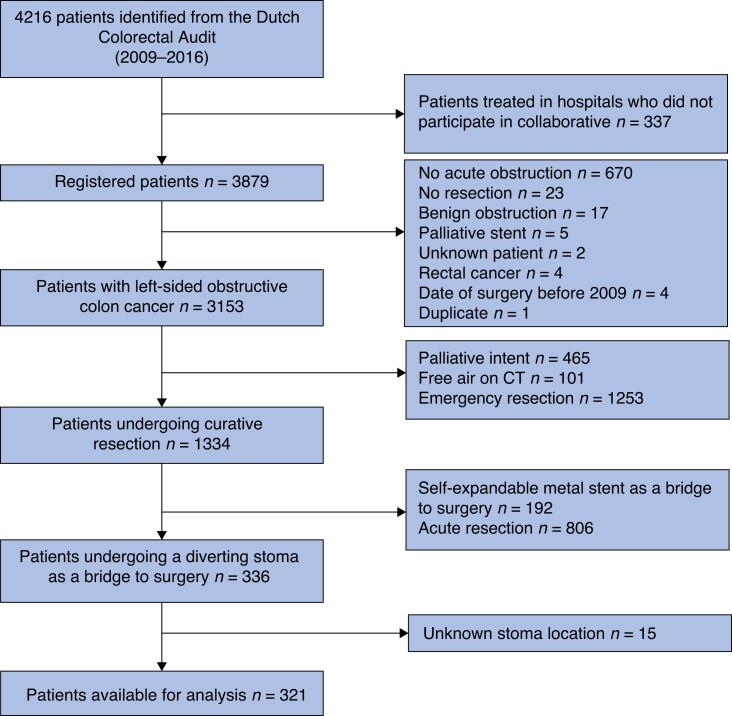
From the total cohort of 3153 patients, 321 patients who met the inclusion criteria were included; 41 (12.7 per cent) underwent an ileostomy and 280 (87.2 per cent) underwent a colostomy (Fig. 1). A total of 75 hospital centres contributed to this cohort; ileostomy patients were included from 24 different centres and colostomy patients from 58 centres. Only three centres solely performed deviating ileostomies. Centres who performed an ileostomy ranged from large university hospitals to small regional hospitals. The median follow-up was 32 (i.q.r. 15–57) months6. In this data set less than 2 per cent of data were missing per variable, except for body mass index (4.5 per cent) and cT staging (66 per cent). Therefore pT staging was used in baseline characteristics instead of cT staging. Baseline variables, clinical presentation, and pathology are summarized in Table 1. Baseline and pathology-related characteristics were not significantly different between the two groups. The percentage of patients with a high ASA classification of III and IV was 20.0 per cent in the colostomy group versus 34.1 per cent in the ileostomy group (P = 0.065).
Fig. 1.
Flow chart of patient inclusion
Table 1.
Baseline characteristics, clinical presentation, and pathology outcomes
| Ileostomy (n = 41) | Colostomy(n = 280) | P | |
|---|---|---|---|
| Age (years), mean(s.d.) | 69(10.4) | 67(11.8) | 0.347 |
| Male | 25 (61.0) | 162 (57.9) | 0.835 |
| BMI (kg/m2), mean(s.d.) | 24.8(5.3) | 25.2(3.9) | 0.549 |
| ASA classification III–IV | 14 (34.1) | 56 (20.0) | 0.065 |
| Tumour location | |||
| Splenic flexure | 6 (14.6) | 46 (16.4) | 0.522 |
| Descending colon | 9 (22.0) | 42 (15.0) | |
| Sigmoidal colon | 26 (63.4) | 192 (68.6) | |
| pT stage | |||
| 2 | 2 (4.9) | 13 (4.7) | 0.994 |
| 3 | 25 (61.0) | 172 (61.9) | |
| 4 | 14 (34.1) | 93 (33.5) | |
| Synchronous tumour | 3 (7.3) | 6 (2.1) | 0.171 |
| cM1 stage | 5 (13.2) | 27 (9.7) | 0.703 |
| Previous abdominal surgery | 18 (43.9) | 103 (36.8) | 0.480 |
| Interval from presentation to enterostomy (days), median (i.q.r.) | 1 (0–4) | 1 (0–2) | 0.063 |
| Abdominal pain at presentation | 23 (76.7) | 217 (84.8) | 0.379 |
| Vomiting at presentation | 16 (57.1) | 176 (67.7) | 0.361 |
| Patient reported weight loss >10% | 7 (25.9) | 45 (19.2) | 0.568 |
| Time since no oral intake at presentation (days), median (i.q.r.) | 0 (0–1) | 0 (0–1) | 0.423 |
| Time since not passing a stool at presentation (days), median (i.q.r.) | 3 (1–5) | 3 (1–6) | 0.630 |
| Ileus at CT imaging at presentation | 18 (62.1) | 175 (71.7) | 0.388 |
| Max diameter of colon at presentation (cm), median (i.q.r.) | 9 (8–10) | 9 (8–10) | 0.955 |
| Leucocyte level at presentation (109/l), median (i.q.r.) | 10.2 (7.9–13.5) | 11.2 (8.6–13.7) | 0.425 |
| C-reactive protein level at presentation (mg/l), median (i.q.r.) | 20.0 (9.5–48.0) | 14.0 (6.0–36.0) | 0.246 |
Values are n (%) unless otherwise indicated. i.q.r., interquartile range.
First presentation and nutritional status
Both groups presented with similar symptoms and nutritional status (Table 1). There were no significant differences in maximum bowel diameter (median of 9 (i.q.r. 8–10) versus 9 (i.q.r. 8–10) cm for ileostomy and colostomy respectively, P = 0.955), presence of vomiting (57 versus 67 per cent for ileostomy and colostomy respectively, P = 0.361), abdominal pain (77 versus 85 per cent for ileostomy and colostomy respectively, P = 0.379), or greater than 10 per cent weight loss (26 versus 19 per cent for ileostomy and colostomy respectively, P = 0.568).
Post-deviating enterostomy outcome
All patients underwent loop stomas. The initial length of stay after a deviating enterostomy was 5 days shorter in the colostomy group (median of 13 (i.q.r. 9–15) versus 8 (i.q.r. 5–17) days for ileostomy and colostomy respectively, P = 0.008). Complication rates during the interval were 12 per cent in the ileostomy group versus 8 per cent in the colostomy group (P = 0.479). Readmission (9.8 versus 6.1 per cent for ileostomy and colostomy respectively, P = 0.589) and re-intervention (2.4 versus 3.0 per cent for ileostomy and colostomy respectively, P = 0.999) rates did not significantly differ between the two groups. Postoperative outcomes after a deviating stoma are described in Table 2.
Table 2.
Postoperative outcomes after a diverting stoma
| Ileostomy(n = 41) | Colostomy(n = 280) | P | |
|---|---|---|---|
| Initial length of stay after stoma (days), median (i.q.r.) | 13 (9–25) | 8 (5–17) | 0.008* |
| Hospital discharge before resection | 33 (84.6) | 247 (90.5) | 0.407 |
| Stoma complications during the interval | 5 (12.2) | 21 (7.5) | 0.470 |
| High-output stoma | 1 (2.4) | 4 (1.4) | 0.999 |
| Skin-complication stoma | 1 (2.4) | 1 (0.4) | 0.607 |
| Stoma herniation | 1 (2.4) | 0 (0.0) | 0.266 |
| Stoma prolapse | 0 (0.0) | 6 (2.1) | 0.738 |
| Stoma necrosis | 0 (0.0) | 3 (1.1) | 0.999 |
| Ileus caused by stoma | 2 (4.9) | 2 (0.7) | 0.138 |
| Abscess formation | 0 (0.0) | 1 (0.4) | 0.999 |
| Readmission during the interval | 4 (9.8) | 17 (6.1) | 0.589 |
| Re-intervention during the interval | 1 (2.4) | 9 (3.0) | 0.999 |
| Stoma revision due to prolapse | 0 (0.0) | 0 (0.0) | NA |
| Reoperation due to perforation | 0 (0.0) | 2 (0.7) | 0.999 |
Values are n (%) unless otherwise indicated. *Significant P value. i.q.r., interquartile range; NA, not applicable.
Interval between a deviating enterostomy and resection
The time to resection appeared to be almost 3 weeks longer in the ileostomy group (median of 56 (i.q.r. 29–107) versus 35 (i.q.r. 22–63) days for ileostomy and colostomy respectively, P = 0.020). The total length of hospital stay until resection (consisting of initial stay and adding potential readmission) was significantly longer in the ileostomy group (median of 13 (i.q.r. 10–16) versus 9 (i.q.r. 6–14) days for ileostomy and colostomy respectively, P = 0.003). The incidence of overall neoadjuvant treatment was not significantly different between the two groups (26.8 versus 14.6 per cent for ileostomy and colostomy respectively, P = 0.080). However, neoadjuvant chemotherapy was more common in the ileostomy group (26.8 versus 13.2 per cent for ileostomy and colostomy respectively, P = 0.029).
To adjust for the difference in neoadjuvant treatment, the 52 patients who received neoadjuvant treatment were excluded in a sub-analysis. The median interval from a deviating stoma to resection remained longer in the ileostomy group (44 (i.q.r. 21–75) versus 30 ( i.q.r. 20–47) days for ileostomy and colostomy respectively, P = 0.048) and the total length of stay after a deviating stoma (including readmission) also remained longer (median of 15 (i.q.r. 13–16) versus 9 (i.q.r. 6–14) days for ileostomy and colostomy respectively, P < 0.001). In addition, the need for supplementary feeding (enteral and total parenteral feeding) remained higher in the ileostomy group (36.7 versus 9.2 per cent for ileostomy and colostomy respectively, P < 0.001). Interval outcomes are described in Table 3.
Table 3.
Bridging-to-surgery interval outcomes
| Ileostomy (n = 41) | Colostomy(n = 280) | P | |
|---|---|---|---|
| Total length of stay until resection (including readmission) (days), median (i.q.r.) | 13 (10–16) | 9 (6–14) | 0.003* |
| Time to resection (days), median (i.q.r.) | 56 (29–107) | 35 (22–63) | 0.020* |
| Supplementary feeding | 11 (26.8) | 24 (8.6) | 0.001* |
| Enteral supplemental feeding | 5 (12.2) | 10 (3.6) | 0.042* |
| Parenteral supplemental feeding | 7 (17.1) | 20 (7.1)) | 0.066 |
| Neoadjuvant treatment | 11 (26.8) | 41 (14.6) | 0.080 |
| Neoadjuvant radiotherapy | 5 (12.2) | 14 (5.0) | 0.142 |
| Duration of radiotherapy (days), median (i.q.r.) | 31 (18–32) | 35 (28–36) | 0.235 |
| Neoadjuvant systemic therapy | 11 (26.8) | 37 (13.2) | 0.029* |
| Duration of systemic therapy (days), median (i.q.r.) | 64 (39–118) | 62 (36–105) | 0.658 |
| Neoadjuvant metastases resection | 3 (7.3) | 3 (1.1) | 0.033* |
Values are n (%) unless otherwise indicated. *Significant P value. i.q.r., interquartile range.
The length of stay was adjusted for right skew distribution through log transformation. Age, sex, body mass index, surgical abdominal history, ASA classification, and stoma type were included in the univariable and multivariable analyses (Table 4). Univariable linear analysis showed ASA classification and stoma type as confounders for length of stay. In the multivariable linear analysis, ASA classification remained a significant confounding factor for length of hospital stay.
Table 4.
Univariable and multivariable regression analysis of confounders for length of stay
| Preoperative and perioperative risk factors | Univariate linear | Multivariable linear | ||
|---|---|---|---|---|
| Odds ratio (95% c.i.) | P | Odds ratio (95% c.i.) | P | |
| Sex (male versus female) | 1.02 (0.87,1.19) | 0.718 | 0.87 (1.02,1.20) | 0.798 |
| Age (continuous, years) | 1.00 (0.99,1.01) | 0.990 | 1.59 (0.99,1.01) | 0.946 |
| BMI (continuous, per increasing kg/m2) | 0.99 (0.97,1.08) | 0.342 | 0.99 (0.97,1.01) | 0.345 |
| ASA classification (categorical, I–IV) | 8.19 (9.21,10.4) | 0.001* | 1.21 (1.06,1.21) | 0.006* |
| Surgical abdominal history (yes versus no) | 1.15 (0.98,1.35) | 0.071 | 1.09 (0.08,417.80) | 0.317 |
| Stoma type (ileostomy versus colostomy) | 0.79 (0.63,1.01) | 0.045* | 0.83 (0.92,1.29) | 0.153 |
*Significant P value.
Primary resection and postoperative outcome
No differences were observed regarding the performance of primary anastomosis (95.1 versus 84.6 per cent for ileostomy and colostomy respectively, P = 0.124). However, stoma closure during primary resection was more common in the colostomy group (22.0 versus 46.1 per cent for ileostomy and colostomy respectively, P = 0.006), with no higher rate of construction of a new stoma (4.9 versus 11.4 per cent for ileostomy and colostomy respectively, P = 0.311). The presence of a stoma after primary resection was 81 per cent in the ileostomy group and 64 per cent in the colostomy group. The length of stay after primary resection was comparable between the two groups (median of 6 (i.q.r. 5–11) versus 7 (i.q.r. 5–10) days for ileostomy and colostomy respectively, P = 0.883). As for complications, intraoperative complication rates (2.4 versus 3.6 per cent for ileostomy and colostomy respectively, P = 0.999) and conversion rates (0 versus 9.6 per cent for ileostomy and colostomy respectively, P = 0.074) did not differ significantly. Furthermore, postoperative complications were comparable between the two groups: anastomotic leakage (9.8 versus 4.3 per cent for ileostomy and colostomy respectively, P = 0.269), 30-day readmission (17.1 versus 14.7 per cent for ileostomy and colostomy respectively, P = 0.884), and 90-day surgical complications (23.1 versus 19.1 per cent for ileostomy and colostomy respectively, P = 0.731). Procedural and postoperative outcomes are summarized in Table 5.
Table 5.
Procedural and postoperative outcomes after tumour resection
| Ileostomy(n = 41) | Colostomy(n = 280) | P | |
|---|---|---|---|
| Type of surgery | 0.495 | ||
| Sigmoid resection | 22 (53.7) | 174 (62.1) | |
| Left hemicolectomy | 14 (34.1) | 84 (30.0) | |
| Subtotal colectomy | 3 (7.3) | 14 (5.0) | |
| Extended left hemicolectomy | 1 (2.4) | 7 (2.5) | |
| Transverse colectomy | 1 (2.4) | 1 (0.4) | |
| Primary anastomosis | 39 (95.1) | 236 (84.6) | 0.124 |
| Reversal of diverting stoma during resection | 9 (22.0) | 129 (46.1) | 0.006* |
| New stoma during resection | 2 (4.9) | 32 (11.4) | 0.311 |
| Stoma present directly after resection | 33 (80.5) | 1780 (63.8) | 0.061 |
| Minimally invasive approach | 12 (29.3) | 148 (52.9) | 0.008* |
| Conversion | 0 (0) | 27 (9.60) | 0.074 |
| No perioperative complications | 40 (97.6) | 270 (96.4) | 0.999 |
| Perioperative transfusion | 0 (0.0) | 1 (0.4) | |
| Perioperative iatrogenic damage | 0 (0.0) | 3 (1.1) | |
| Perioperative bladder damage | 1 (2.4) | 0 (0.0) | |
| Other operative complications | 0 (0.0) | 6 (2.1) | |
| Length of stay after resection (days), median (i.q.r.) | 6 (5–11) | 7 (5–10) | 0.883 |
| ICU days, median (i.q.r.) | 0 (0–1) | 0 (0–0) | 0.003* |
| 30-day mortality rate | 2 (4.9) | 4 (1.4) | 0.297 |
| Anastomotic leakage | 4 (9.8) | 12 (4.3) | 0.269 |
| Abscess formation | 3 (7.3) | 10 (3.6) | 0.406 |
| 90-day surgical complication | 9 (23.1) | 53 (19.1) | 0.731 |
| 30-day readmission | 7 (17.1) | 41 (14.7) | 0.884 |
| Stoma present at the end of follow-up | 11 (27.5) | 79 (28.4) | 0.999 |
| Adjuvant chemotherapy | 14 (36.8) | 116 (41.9) | 0.683 |
| R0 resection | 37 (90.2) | 259 (92.5) | 0.858 |
Values are n (%) unless otherwise indicated. *Significant P value. i.q.r., interquartile range.
Discussion
In this study, a diverting ileostomy was associated with a higher need for supplementary feeding and a longer hospital stay during the bridging-to-surgery interval. Despite the theoretical discussions on specific complications of both types of enterostomies, no differences were found in postoperative complications during the bridging interval or after resection of the primary tumour. This suggests that both approaches are safe as a bridge to surgery in left-sided obstructive colon carcinoma, but may suggest a clinical preference for a colostomy over an ileostomy in daily practice based on these results. An unexpected observation was that reversal of the enterostomy during primary resection was more common in the colostomy group.
There was longer length of stay and longer interval to primary resection in the ileostomy group. The shortened length of stay suggests a possible advantage for a colostomy as a bridge to surgery from financial and logistical perspectives, but also from a patient perspective. A possible reason for the elongated length of stay in the ileostomy group could be the significantly higher incidence of systemic neoadjuvant treatment. The optimal duration of neoadjuvant treatment is not consistent internationally, which may contribute to a longer hospitalization or interval to resection10,11. Nevertheless, the observed longer length of stay and the longer interval between a deviating enterostomy and primary resection remained significant after exclusion of neoadjuvant treatment. The incidence of neoadjuvant treatment alone does not seem to provide sufficient cause for differences in length of stay and interval between the two groups. Another possible alternative reason for the longer length of stay in the ileostomy group could be due to a higher proportion of co-morbidities in the ileostomy group12. In this study, ASA classification was indeed defined as a confounding factor for extended stay, although baseline characteristics did not significantly differ.
There was a significantly higher need for nutritional support in the ileostomy group, which remained significantly higher in the ileostomy group after exclusion of neoadjuvant treatment. This suggests slower recovery in the ileostomy group in terms of intake and maintenance of weight, requiring nutritional support. This supports the previous literature, which describes an increased risk for nutritional deficiencies in ileostomy patients, especially in the first interval after ileostomy placement13–15.
Outcomes of this study suggest that both approaches can be used safely in all patients with left-sided obstructive colon cancer in terms of complications during the bridging interval and after primary resection. Prior studies reporting on complications in both types of enterostomy in a permanent or long-term setting show conflicting results16,17. However, this study showed a lower incidence of stoma prolapse in the colostomy group compared with previous studies17,18. A possible explanation could be that the current literature reports on permanent and long-term stomas, compared with the bridge-to-surgery stomas reported in this study (35-day interval to surgery). Finally, stoma reversal during resection was more common in the colostomy group.
Although the longer length of stay in the ileostomy group is notable, there is a need for cautious interpretation of this study because of the risk of bias, the retrospective nature of the study, and the small number of ileostomy patients. Futhermore, the reason for specific choice of a type of enterostomy was not recorded, due to the retrospective nature of the study, which poses a risk of selection bias. The authors tried to minimize this risk by comparing baseline and clinical symptoms at first presentation, which showed no significant differences between the groups. Another limitation of this study is that it only reported outcomes in the first interval from diagnosis up to 90 days after primary resection. To address the lack of evidence in this area, it would be interesting for future research to assess the differences between both enterostomy types in an international, prospective setting to confirm the outcomes of this study19,20.
A shorter length of stay during the bridging interval and a lower need for nutritional support were observed in the colostomy group compared with the ileostomy group. This may suggest a preference for a colostomy as a bridge to surgery in left-sided obstructive colon cancer from logistical, financial, and patient perspectives. This study shows equal complication rates in the ileostomy and colostomy groups in the bridging interval and after resection. However, as the sample size of the ileostomy group is especially small, there is a need for good-quality, international, higher-volume, prospective studies in the future to strengthen the results of this study.
Collaborators
The Dutch Snapshot Research Group
F. J. Amelung, E. C. J. Consten, T. A. Burghgraef (Meander Medisch Centrum, Amersfoort, the Netherlands); D. A. Hess, R. Roukema (Antonius Ziekenhuis, Sneek, the Netherlands); A. Demirkiran, M. Tenhagen, J. Straatman (Beverwijk, the Netherlands); G. Nieuwenhuijzen, H. J. T. Rutten, G. Vugts (Catharina Ziekenhuis, Eindhoven, the Netherlands); B. Inberg, A. Kreiter, S. Scheurs (Koningin Beatrix ziekenhuis, Winterswijk, the Netherlands); M. F. Gerhards, R. L. G. M. Blom, M. J. A. M. Russchen, A. van den Berg (OLVG, Amsterdam, the Netherlands); J. W. T. Dekker, H. P. Versteegh (Reinier de Graaf, Delft, the Netherlands); F. W. H. Kloppenberg, I. S. Bakker, J. T. H. Hamminga (Treant Drenthe en Groningen, Bethesda, Refaja, Scheper, the Netherlands); J. L. M. Konsten, M. van Heinsbergen (VieCurie, the Netherlands); S. T. van Vugt, J. E. Bouwman (Wilhelmina Ziekenhuis, Assen, the Netherlands); J. T. Heikens, A. van den Berg, M. Takkenberg, L. Graat (Rivierenland, Tiel, the Netherlands); A. J. N. M. Bastiaansen, E. A. Gorter, J. W. S. Merkus (Haga Ziekenhuis, den Haag, the Netherlands); E. G. Boerma, L. Koolen, D. Jean Pierre (Zuyderland (Atrium + Orbis), Heerlen, the Netherlands); E. van der Harst, W. Hogendoorn, L. H. Wijngaarden (Maasstad Ziekenhuis, Rotterdam, the Netherlands); R. T. J. kortekaas, M. C. Struijs, N. Heuchemer (Franciscus Gasthuis & Vlietland, Rotterdam, the Netherlands); P. Fockens, E. E. van Halsema, W. A. A. Borstlap, P. J. Tanis, J. Veld, W. A. Bemelman, D. D. Wisselink (Amsterdam Medical Center (AMC), Amsterdam, the Netherlands); A. C. H. M. Jongen, V. N. E. Schuermans, N. D. Bouvy (Maastricht Universitair Medisch Centrum, Maastricht, the Netherlands); C. S. Andeweg, J. W. Foppen (St Jansdal, Harderwijk, the Netherlands); J. Heemskerk, J. Scheerhoorn (Laurentius Ziekenhuis, Roermond, the Netherlands); P. van der Sluis, N. Smakman (Diakonessenhuis, Utrecht, the Netherlands); E. R. J. Bruns, E. S. van der Zaag, H. J. Schuiten, T. Argillander (Gelre, Ziekenhuizen, the Netherlands); K. Parry, D. Lips, H. Algera (Jeroen Bosch ziekenhuis, den Bosch, the Netherlands); P. Poortman, C. Steur (Waterland, Purmerend, the Netherlands); H. A. Swank, B. Lamme (Albert Schweizer ziekenhuis, Dordrecht, the Netherlands); M. N. N. J. Arron, D. van Uden, P. D. Siersema, J. H. W. de Wilt (Radboud UMC, Nijmegen, the Netherlands); L. Daniels, D. J. A. Sonneveld, K. Nielsen (Westfries Gasthuis, Hoorn, the Netherlands); I. Masselink, L. M. Lutke Holzik, G. Lo (Ziekenhuisgroep Twente, Twente, the Netherlands); A. G. Menon, J. F. Lange (Havenziekenhuis, Rotterdam, the Netherlands); B. J. van Wely, A. van Esch (Bernhoven, Ziekenhuis, the Netherlands); D. E. Moes, B. M. M. Reuber (Slotervaart Ziekenhuis, Amsterdam, the Netherlands); B. H. M. Heijnen, I. de Groot-van Veen (Lange Land Ziekenhuis, Zoetermeer, the Netherlands); A. W. H. van de Ven, C. C. M. Marres, H. E. Haak (Flevo Ziekenhuis, Almere, the Netherlands); M. Vermaas, P. van Hagen (Ijsselland Ziekenhuis, Capelle aan den Ijssel, the Netherlands); H. L. van Westreenen, J. W. A. de Haas (Isala Ziekenhuis, Zwolle, the Netherlands); J. M. Klaase, M. J. F. van Veen (Medisch Spectrum Twente, Twente, the Netherlands); A. Mearadji, J. Heeren (Bravis Ziekenhuis, Bergen op Zoom, the Netherlands); R. Silvis, J. A. M. G. Tol, C. J. L. Molenaar (Spaarne Gasthuis, Hoofddorp, the Netherlands); J. A. van Essen, T. Lettinga, L. Verkoele (Sint Jans Gasthuis, Weert, the Netherlands); G. L. Beets (Antoni van Leeuwenhoek, Amsterdam, the Netherlands); D. D. E. Zimmerman, Y. T. van Loon, P. Oomen, H. S. de Vries (Twee Steden Ziekenhuis, Tilburg, the Netherlands); J. E. van Hooft, K. C. M. J. Peeters, N. D. A. Boye (Leiden Universitair Medisch Center, Leiden, the Netherlands); F. ter Borg, A. K. Talsma, A. A. Wijkmans (Deventer Ziekenhuis, Deventer, the Netherlands); A. A. W. van Geloven, N. van Oorschot, B. Blomberg (Ter Gooi Ziekenhuis, Hilversum, the Netherlands); W. M. U. van Grevenstein, J. L. Tolenaar (Universitair Medisch Centrum Utrecht, Utrecht, the Netherlands); F. C. den Boer, J. C. Sierink, T. Paulides (Zaans Medisch Centrum, Zaandam, the Netherlands); B. M. M. Reiber, B. van de Beukel, J. B. Tuynman, H. T. Bransma (Vrije Universiteit Medisch Centrum, Amsterdam, the Netherlands); A. R. M. Brandt-Kerkhof, M. E. E. Bröker (Erasmus Medical Center, Rotterdam, the Netherlands); R. M. P. H. Crolla, J. van der Slegt, T. L. Janssen (Amphia Ziekenhuis, Breda, the Netherlands); C. Werker, H. J. Schuijt, M. J. Wiezer (Sint Antonius ziekenhuis, Nieuwegein, the Netherlands); K. van Dongen, V. Kornmann (Maasziekenhuis Pantein, Boxmeer, the Netherlands); L. Tseng, D. Smit (Groene Hart Ziekenhuis, Gouda, the Netherlands); C. Sietses, T. Visser (Gelderse Vallei, Ede, the Netherlands); G. D. Algie, M. J. Nieboer (MC Zuiderzee, Lelystad, the Netherlands); P. A. Neijenhuis, S. F. Durmaz (Alrijne Ziekenhuis, Leiden, the Netherlands); T. H. J. Aufenacker, N. Hugen, M. van Basten Batenburg (Rijnstate ziekenhuis, Arnhem, the Netherlands); M. Westerterp, J. van Groningen, W. J. de Jong (Medisch Centrum Haaglanden, den Haag, the Netherlands); R. J. Renger, F. Logeman (Rivas ziekenhuis, Gorinchem, the Netherlands); G. Slooter, K. Arts (Maxima Medisch Centrum, Utrecht, the Netherlands); J. Wegdam, G. Meisen (Elkerliek, the Netherlands); B. Wiering (Slingeland Ziekenhuis, Doetinchem, the Netherlands); H. C. J. van der Mijle, I. Paulusma, M. van der Sluis (Nij Smellinghe, Drachten, the Netherlands); K. Havenga, J. P. M. Burbach, E. J. B. Furnee (Universitair Medisch Centrum Groningen, the Netherlands); B. Polle (Canisius Wilhelmina Ziekenhuis Nijmegen, the Netherlands); C. Hoff, F. Poelmann, T. L. R. Zwols (MC Leeuwarden, Leeuwarden, the Netherlands); T. C. van Sprundel (Ommelander, Groningen, the Netherlands); S. C. Veltkamp, M. van de Wilt (Amstelland Ziekenhuis, Amstelveen, the Netherlands); W. J. Vles, A. Kamman, H. Schippers (Ikazia Ziekenhuis, Rotterdam, the Netherlands); R. L. van der Hul, A. Breijer (Van Weel Bethesda Ziekenhuis, Hoogeveen, the Netherlands); W. Kelder, B. van den Hengel (Martini Ziekenhuis Groningen, the Netherlands); R. Klicks, E. F. Kelling (BovenIJ Ziekenhuis, Amsterdam, the Netherlands); A. P. J. Houdijk, L. Heijnen (MC Alkmaar, Alkmaar, the Netherlands); F. Wit, M. Dam (Tjongerschans Heerenveen, the Netherlands); M. Raber (Ropcke Zweers Ziekenhuis, Hardenberg, the Netherlands); D. J. L. M. de Mey (Zorgsaam, Terneuzen, the Netherlands); W. van den Broek (St Anna Geldrop, Eindhoven, the Netherlands); L. Verslijs, G. W. de Klein (Diaconessenhuis, Meppel, the Netherlands); W. M. J. de Ruijter, R. de Vos tot Nederveen Cappel (Admiraal de Ruyter ziekenhuis, Vlissingen, the Netherlands).
Supplementary Material
Acknowledgements
Author contributions: substantial contributions to the conception and design of the work, B. P. Smalbroek and T. J. Weijs; drafting the article, B. P. Smalbroek; revising the article critically for important intellectual content: T. J. Weijs, B. P. Smalbroek, F. B. Poelmann, L. Goense, L. M. Dijksman, N. A. T. Wijffels, and D. Boerma; final approval of the version to be published: B. P. Smalbroek, T. J. Weijs, F. B. Poelmann, L. Goense, . L. M. Dijksman, R. Dijkstra, L. J. Graat, M. J. Wiezer, M. Takkenberg, J. T. Heikens, N. A. T. Wijffels, D. Boerma, A. B. Smits, and the Dutch Snapshot Research Group. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Contributor Information
Bo P Smalbroek, Department of Surgery, St Antonius Hospital, Nieuwegein, The Netherlands; Valued Based Healthcare, St Antonius Hospital, Nieuwegein, The Netherlands.
Teus J Weijs, Department of Surgery, St Antonius Hospital, Nieuwegein, The Netherlands.
Lea M Dijksman, Valued Based Healthcare, St Antonius Hospital, Nieuwegein, The Netherlands.
Floris B Poelmann, Department of Surgery, St Antonius Hospital, Nieuwegein, The Netherlands.
Lucas Goense, Department of Surgery, St Antonius Hospital, Nieuwegein, The Netherlands.
Robert R Dijkstra, Department of Surgery, St Antonius Hospital, Nieuwegein, The Netherlands.
Niels A T Wijffels, Department of Surgery, St Antonius Hospital, Nieuwegein, The Netherlands.
Djamila Boerma, Department of Surgery, St Antonius Hospital, Nieuwegein, The Netherlands.
Anke B Smits, Department of Surgery, St Antonius Hospital, Nieuwegein, The Netherlands.
the Dutch Snapshot Research Group:
F J Amelung, E C J Consten, T A Burghgraef, D A Hess, R Roukema, A Demirkiran, M Tenhagen, J Straatman, G Nieuwenhuijzen, H J T Rutten, G Vugts, B Inberg, A Kreiter, S Scheurs, M F Gerhards, R L G M Blom, M J A M Russchen, A van den Berg, J W T Dekker, H P Versteegh, F W H Kloppenberg, I S Bakker, J T H Hamminga, J L M Konsten, M van Heinsbergen, S T van Vugt, J E Bouwman, J T Heikens, A van den Berg, M Takkenberg, L Graat, A J N M Bastiaansen, E A Gorter, J W S Merkus, E G Boerma, L Koolen, D Jean Pierre, E van der Harst, W Hogendoorn, L H Wijngaarden, R T J kortekaas, M C Struijs, N Heuchemer, P Fockens, E E van Halsema, W A A Borstlap, P J Tanis, J Veld, W A Bemelman, D D Wisselink, A C H M Jongen, V N E Schuermans, N D Bouvy, C S Andeweg, J W Foppen, J Heemskerk, J Scheerhoorn, P van der Sluis, N Smakman, E R J Bruns, E S van der Zaag, H J Schuiten, T Argillander, K Parry, D Lips, H Algera, P Poortman, C Steur, H A Swank, B Lamme, M N N J Arron, D van Uden, P D Siersema, J H W de Wilt, L Daniels, D J A Sonneveld, K Nielsen, I Masselink, L M Lutke Holzik, G Lo, A G Menon, J F Lange, B J van Wely, A van Esch, D E Moes, B M M Reuber, B H M Heijnen, I de Groot-van Veen, A W H van de Ven, C C M Marres, H E Haak, M Vermaas, P van Hagen, H L van Westreenen, J W A de Haas, J M Klaase, M J F van Veen, A Mearadji, J Heeren, R Silvis, J A M G Tol, C J L Molenaar, J A van Essen, T Lettinga, L Verkoele, G L Beets, D D E Zimmerman, Y T van Loon, P Oomen, H S de Vries, J E van Hooft, K C M J Peeters, N D A Boye, F ter Borg, A K Talsma, A A Wijkmans, A A W van Geloven, N van Oorschot, B Blomberg, W M U van Grevenstein, J L Tolenaar, F C den Boer, J C Sierink, T Paulides, B M M Reiber, B van de Beukel, J B Tuynman, H T Bransma, A R M Brandt-Kerkhof, M E E Bröker, R M P H Crolla, J van der Slegt, T L Janssen, C Werker, H J Schuijt, M J Wiezer, K van Dongen, V Kornmann, L Tseng, D Smit, C Sietses, T Visser, G D Algie, M J Nieboer, P A Neijenhuis, S F Durmaz, T H J Aufenacker, N Hugen, M van Basten Batenburg, M Westerterp, J van Groningen, W J de Jong, R J Renger, F Logeman, G Slooter, K Arts, J Wegdam, G Meisen, B Wiering, H C J van der Mijle, I Paulusma, M van der Sluis, K Havenga, J P M Burbach, E J B Furnee, B Polle, C Hoff, F Poelmann, T L R Zwols, T C van Sprundel, S C Veltkamp, M van de Wilt, W J Vles, A Kamman, H Schippers, R L van der Hul, A Breijer, W Kelder, B van den Hengel, R Klicks, E F Kelling, A P J Houdijk, L Heijnen, F Wit, M Dam, M Raber, D J L M de Mey, W van den Broek, L Verslijs, G W de Klein, W M J de Ruijter, and R de Vos tot Nederveen Cappel
Funding
The authors have no funding to declare.
Disclosure
Regarding financial disclosures, A. B. Smits has a position as an independent contractor as a robotic surgeon proctor at Intuitive Surgical Inc. and B. P. Smalbroek has received a grant from Intuitive Surgical Inc. for another study. The authors declare no other conflict of interest. No preregistration exists for this study.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The Dutch Snapshot Research Group has full access to all the data in the study and takes responsibility for the integrity of the data. The data that support the findings of this study are available from the Dutch Snapshot Research Group upon reasonable request.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal Aet al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249 [DOI] [PubMed] [Google Scholar]
- 2. Frago R, Ramirez E, Millan M, Kreisler E, del Valle E, Biondo S. Current management of acute malignant large bowel obstruction: a systematic review. Am J Surg 2014;207:127–138 [DOI] [PubMed] [Google Scholar]
- 3. Kolfschoten NE, Wouters MWJM, Gooiker GA, Eddes EH, Kievit J, Tollenaar RAEMet al. Nonelective colon cancer resections in elderly patients: results from the Dutch surgical colorectal audit. Dig Surg 2012;29:412–419 [DOI] [PubMed] [Google Scholar]
- 4. Veld JV, Amelung FJ, Borstlap WAA, van Halsema EE, Consten ECJ, Dekker JWTet al. Decompressing stoma a s bridge to elective surgery is an effective strategy for left-sided obstructive colon cancer: a national, propensity-score matched study. Ann Surg 2020;272:738–743 [DOI] [PubMed] [Google Scholar]
- 5. Amelung FJ, Burghgraef TA, Tanis PJ, van Hooft JE, Ter Borg F, Siersema PDet al. Critical appraisal of oncological safety of stent as bridge to surgery in left-sided obstructing colon cancer; a systematic review and meta-analysis. Crit Rev Oncol Hematol 2018;131:66–75 [DOI] [PubMed] [Google Scholar]
- 6. Veld JV, Amelung FJ, Borstlap WAA, van Halsema EE, Consten ECJ, Siersema PDet al. Comparison of decompressing stoma vs. stent as a bridge to surgery for left-sided obstructive colon cancer. JAMA Surg 2020;155:206–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gavriilidis P, Azoulay D, Taflampas P. Loop transverse colostomy versus loop ileostomy for defunctioning of colorectal anastomosis: a systematic review, updated conventional meta-analysis, and cumulative meta-analysis. Surg Today 2019;49:108–117 [DOI] [PubMed] [Google Scholar]
- 8. Prassas D, Vossos V, Rehders A, Knoefel WT, Krieg A. Loop ileostomy versus loop colostomy as temporary deviation after anterior resection for rectal cancer. Langenbecks Arch Surg 2020;405:1147–1153 [DOI] [PubMed] [Google Scholar]
- 9. Dutch Snapshot Research Group . Benchmarking recent national practice in rectal cancer treatment with landmark randomized controlled trials. Colorectal Dis 2017;19:O219–O231 [DOI] [PubMed] [Google Scholar]
- 10. Wasserberg N. Interval to surgery after neoadjuvant treatment for colorectal cancer. World J Gastroenterol 2014;20:4256–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mei S-W, Liu Z, Wei F-Z, Chen J-N, Wang Z-J, Shen H-Yet al. Impact of interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer patients. World J Gastroenterol 2020;26:4624–4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Assaf D, Hazzan D, Ben-Yaacov A, Laks S, Zippel D, Segev L. Predisposing factors for high output stoma in patients with a diverting loop ileostomy after colorectal surgeries. Ann Coloproctol 2021; 10.3393/ac.2021.00241.0034[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Oliveira AL, Boroni Moreira AP, Pereira Netto M, Gonçalves Leite IC. A cross-sectional study of nutritional status, diet, and dietary restrictions among persons with an ileostomy or colostomy. Ostomy Wound Manage 2018;64:18–29 [PubMed] [Google Scholar]
- 14. Vasilopoulos G, Makrigianni P, Polikandrioti M, Tsiampouris I, Karayiannis D, Margari Net al. Pre- and post-operative nutrition assessment in patients with colon cancer undergoing ileostomy. Int J Environ Res Public Health 2020;17:6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Migdanis A, Koukoulis G, Mamaloudis I, Baloyiannis I, Migdanis I, Vagena Xet al. The effect of a diverting ileostomy formation on nutritional status and energy intake of patients undergoing colorectal surgery. Clin Nutr ESPEN 2020;40:357–362 [DOI] [PubMed] [Google Scholar]
- 16. Borucki JP, Schlaeger S, Crane J, Hernon JM, Stearns AT. Risk and consequences of dehydration following colorectal cancer resection with diverting ileostomy. A systematic review and meta-analysis. Colorectal Dis 2021;23:1721–1732 [DOI] [PubMed] [Google Scholar]
- 17. Tsujinaka S, Tan K-Y, Miyakura Y, Fukano R, Oshima M, Konishi Fet al. Current management of intestinal stomas and their complications. J Anus Rectum Colon 2020;4:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krishnamurty DM, Blatnik J, Mutch M. Stoma complications. Clin Colon Rectal Surg 2017;30:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Persson E, Berndtsson I, Carlsson E, Hallén A-M, Lindholm E. Stoma-related complications and stoma size—a 2-year follow up. Colorectal Dis 2010;12:971–976 [DOI] [PubMed] [Google Scholar]
- 20. Robertson I, Leung E, Hughes D, Spiers M, Donnelly L, Mackenzie Iet al. Prospective analysis of stoma-related complications. Colorectal Dis 2005;7:279–285 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Dutch Snapshot Research Group has full access to all the data in the study and takes responsibility for the integrity of the data. The data that support the findings of this study are available from the Dutch Snapshot Research Group upon reasonable request.