Abstract
Study Design:
A prospective study.
Objectives:
Intervertebral disc degenerative disease is a common and frequently-occurring disease in adults and is the main cause of lower back pain. However, there is a lack of universal animal models to study disc degeneration.
Methods:
Forty-two male New Zealand white rabbits aged 12 months were used in this study. We established an endplate ischemic disc degeneration model though surgical ligation of rabbit lumbar vertebral body segment arteries. Two weeks after surgery, 6 experimental animals were randomly selected for follow-up tests. First, ischemia and lumbar disc degeneration were confirmed using imaging techniques. Then, immunohistochemical staining was performed to observe the growth of the annulus fibrosus. Finally, quantitative polymerase chain reaction, enzyme-linked immunosorbent assay, and western blotting were used to detect mRNA expression and protein content of IL-1α, TNFα, collagen II, MMP-3, aggrecan, and PLA2 in the nucleus pulposus of the disc.
Results:
Imaging examination confirmed the successful construction of a lumbar disc degeneration model. Histological analysis and biochemical analysis showed a damaged intervertebral disc structure, and collagen II and aggrecan, the key extracellular matrix components of intervertebral discs, were reduced in synthesis and content. The synthesis and expression of IL-1α, TNFα, PLA2, and MMP-3 related to disc catabolism and inflammatory response were enhanced.
Conclusions:
We successfully constructed a lumbar disc degeneration ischemia model, which provides a novel approach to study the pathological mechanisms involved in discogenic low back pain and to prevent and treat discogenic low back pain.
Keywords: intervertebral disc degeneration, low back pain, ischemia model, pathobiology, intervertebral disc
Introduction
Intervertebral disc degeneration (IDD) is the main pathological basis for intervertebral disc (IVD) protrusion and the primary cause of many vertebral degenerative diseases. 1 With changes in individual’s work and life style, the incidence of disc degeneration is increasing yearly. Lower back pain will cause individuals to lose time from work and bear additional medical expenses, thus creating a significant economic burden on society. 2 Degeneration of the IVD is a main cause of lower back pain. 3 It has been estimated that 84% of the general population will experience low back pain at some period in their lives, and many will become chronically disabled as a result. 4 To further explore the pathological processes involved IDD, it is necessary to establish animal experimental models able to mimic human IDD.
The IVD is a complex multi-component structure consisting of an outer annulus fibrosus (AF) and an inner hydrated gel-like substance called the nucleus pulposus (NP). The NP is rich in extracellular matrix (ECM) proteins, mainly consisting of hydrophilic proteoglycan (PG) Aggrecan, attached to numerous glycosaminoglycans (GAG) chains. A decisive biochemical feature of IDD is evidence of degradation of the ECM caused by an imbalance in the homeostasis mechanisms of anabolism and catabolism, which leads to the loss of PGS and collagen. 5
The blood flow to the lumbar vertebral body is largely supplied by branches of the lumbar artery and the abdominal aorta. 6 However, IVDs are tissues with no blood vessel supply, except for the outermost layer of the AF, and thus are relatively nutritionally deficient. Nutrient supply is mainly dependent on the cartilage endplate pathway for osmosis. Some researchers believe that the degeneration of the IVD begins with the degeneration of the cartilage endplate. 7 The lateral AF receives nutrients, glucose, and oxygen supplied by the small blood vessels surrounding the vertebral body starting from the spinal segmental arteries. These nutrients diffuse to IVD cells mainly through the dense ECM. 8 Therefore, the microenvironment in the IVD center is considered to have the lowest glucose and oxygen concentrations. Some studies9,10 have indirectly confirmed the relationship between IDD and peripheral blood supply, but the degree of correlation between decreased blood supply and the occurrence and development of IDD remains unclear.
With regard to the underlying pathological mechanisms of low back pain, most researchers believe that these involve (1) rupture of the NP and AF of the IVD 11 ; (2) growth of nerve fibers within the IVD through the broken fiber ring 12 ; and (3) the rupture of the NP and other tissues in the IVD that stimulate the surrounding tissue to produce inflammatory factors, of which IL-1α and TNFα play a major role.13,14 These inflammatory factors stimulate the nerve to produce pain.
In vivo animal studies have shown that impaired blood flow of the lumbar artery can directly induce IVD degeneration. Based on the results of previous studies,15,16 we hypothesized that factors reducing lumbar blood supply could cause changes in the microenvironment in lumbar IVD, leading to degenerative IVD changes. In our study, using a rabbit model, we prevented lumbar segmental vessels from reducing the blood supply to the endplate. Lack of nutrition caused metabolites not to be cleared in time, which led to degeneration of the IVD. Imaging studies confirmed the establishment of the model. Finally, evaluation of a series of inflammatory factors, enzyme levels, gene expression, and nerve fiber distribution in the IVD was performed, so as to lay the foundation for further studies on the pathological mechanisms underlying discogenic low back pain.
Materials and Methods
Experimental Animals
Forty-two male New Zealand white rabbits, 2.5 ± 0.5 kg in body weight, were used in this study. They were all approximately 12 months old. The animals were provided by the Hubei Experimental Animal Research Center. Spinal deformity was excluded by imaging studies. The study plan was reviewed and approved by the Examination Committee and Ethics Committee of Experimental Animal Welfare of our Hospital. The animal studies were also approved by the Experimental Animal Welfare Review Committee and the Animal Care Committee of our Hospital.
Surgical Procedure
A dose of 3.0% sodium pentobarbital (30 mg/kg) was injected intraperitoneally. Following anesthesia, the right limbs were fixed in the supine position. The surgical area was sheared and disinfected with iodophor, and the L2-L5 IVD segment was exposed through the left posterior peritoneal approach. An incision was made from under the 12th rib to the upper edge of the iliac crest, about 8-cm in length. The tissues of each layer were cut open in turn, the retroperitoneum and adipose tissues were exposed, and the paraspinal fasciculus psoas major muscle was blunt dissected, exposing the anterior margin of 4 consecutive vertebral bodies (L2/3, L3/4, L4/5, and L5/6). The iliac crest horizontal line marked the location of the L5/6 disc. At the same time, L4 and L5 segmental arteries were completely ligated (Supplementary Figure 1). The L4/5 IVDs were used as ischemic bilateral IVDs. The L3/4 IVDs were used as IVDs in the unilateral ischemia test group. The L2/3 IVD served as the normal control. The wounds were sutured layer by layer after the procedure. Intramuscular injection of penicillin (200,000 units/day) was administered for 3 consecutive days to prevent infection. Following the operation, the animals were put back into their cage for observation and routine feeding.
640-Slice Spiral Computed Tomography Analysis
Two weeks after surgery, 6 experimental animals were randomly selected. Rabbits were fixed in the supine position in the rabbit cases. Computed tomography (CT) was performed using a Toshiba Aquilion 1 TSX-301A 320 (640-slice spiral CT) for CT-digital subtraction angiography (DSA) of the segmental blood vessels.
Transmission Electron Microscopy Analysis
Two weeks after the operation, 6 experimental animals with segments of vascular occlusion confirmed by 640-slice CT were euthanized by air embolization. L2 to L5 IVDs were completely removed and decalcified. The tissue mass after sectioning was soaked in phosphate buffer solution and thoroughly rinsed with water for 10 min. Tissue blocks were then placed in a solution of 2% osmium acid and fixed at room temperature for 1 h. Finally, blocks were immersed in 10 ethanol solutions with an increasing concentration gradient from 50% to 100% for 10 min in each solution. After dehydration, blocks were embedded in an epoxy resin. Next, blocks were sliced and stained with toluidine blue and were observed under an optical microscope. The specimens were cut into ultra-thin sections and were evaluated using transmission electron microscopy (Hitachi S-3000 N).
X-Ray Analysis
Digital X-ray photographic cameras were used for capturing lumbar lateral X-rays. Three rabbits were randomly selected at various time points after the first, second, and third months of the operation, and the height of the IVD and the vertebral body were measured. According to the method described by An et al 17 the Disc Height Index (DHI) was calculated to indicate changes in the vertebral body clearance. The method was as follows: the average IVD height was measured dividing by the heights of the adjacent vertebrae, DHI = 2 × (D+E+F)/(A+B+C+G+H+I). Individual rabbits with significant differences in DHI were identified. The percentage DHI was used to assess the postoperative change in disc height: DHI percentage = postoperative DHI/ preoperative DHI.
Magnetic Resonance Imaging Analysis
At each timepoint (i.e., 1, 2, and 3 months after surgery), 6 specimens were randomly selected and magnetic resonance imaging (MRI) was performed using a Siemens 3.0 T MAGNETIC resonance scanner. The IVD L1-L6 was positioned at the T2 phase coronal scan, and the signal changes of postoperative degeneration were observed via a midsagittal scan. According to the Thompson classification, the changes in T2 signal strength were classified into 1-4 levels: Level 1, normal; Level 2, slightly weakened and high signal area significantly reduced; Level 3, moderate weakening; and Level 4, severe weakening.
Sampling and Specimen Handling
Animals were randomly selected at 1, 2, and 3 months after surgery and euthanized by air embolization. L2 to L5 IVDs were completely removed and samples were divided into 2 parts: one was quickly immerged into liquid nitrogen for evaluation of protein and mRNA expression; the other was fixed in 10% formaldehyde solution for further histological staining.
Histological Analysis
The specimens were fixed in 10% neutral formalin solution for 24 h, then rinsed and decalcified in decalcification solution for 48 h. The residual soft tissue was removed, split along the midline of the sagittal plane of the vertebral body, and the specimen was packed in a section embedding box. Paraffin-embedded sections were prepared at a thickness of 4 μm.
Immunohistochemistry
The tissue was dewaxed and hydrated. Next, the primary antibody (mouse anti-rat monoclonal antibody GAP 43 or rabbit anti-human polyclonal antibody PGP9.5) was added and specimens were incubated at 4°C overnight. Next, the secondary antibody (general-purpose EnVision TM kit, Built Bioengineering Research Institute, Nanjing, China) was added and the specimens were placed at 37°C for 20 min. The samples were developed by incubation in DAB solution (ZSGB-BIO Corporation, Beijing, China). Hematoxylin staining was performed for 30 s and the slides were visualized using A BX51 microscope (Olympus, Tokyo, Japan) to observe the positive expression of nerve fibers in the annulus of the degenerative disc.
Quantitative PCR
We first extracted total tissue RNA using the Trizol (Invitrogen Life Technologies, USA) method. Quantitative PCR (QPCR) was performed with the First Strand cDNA Synthesis Kit (TOYOBO, Tokyo, Japan). PCR reactions and dissociation stage was achieved using the StepOne™ Real-Time PCR (Life Technologies, Shanghai, China). Thermocycling conditions were as follows: 95°C for 1 min, followed by 40 cycles of 95°C for 20 s, 58°C for 15 s, and 72°C for 20 s, and end extension at 72°C for 5 min. The relative expression level was calculated using the 2-ΔΔCt method and levels were normalized using β-actin expression. The primer sequences are shown in Table 1.
Table 1.
The Sequence of All the Primers Used for Real-Time PCR.
| Forward (5’-3’) | Reverse (5’-3’) | |
|---|---|---|
| IL-1α | CAACAAGTGGTGTTCTCCAT | GAGGTGCTGATGTACCAGT |
| TNFα | AGATGGTCACCCTCAGATCAG | GAAGAGAACCTGGGAGTAGATGAG |
| MMP-3 | AGCCAATGGAAATGAAAACTCTTC | CCAGTGGATAGGCTGAGCAAA |
| Col II | GCACCCATGGACATTGGAGGG | GACACGGAGTAGCACCATCG |
| agreccan | GGTCGTGGTGAAAGGTGTTG | GGTGGAAGCCATCCTCGTA |
| PLA2 | GCCTTGCATTCCGGTAAAGAACATG | GGGAACAGCAGATGATAAGTCAGAGCTAG |
| GAPDH | CTGCCGCCTGGAGAAAG | CGACCTGGTCCTCGGTGTA |
Enzyme-Linked Immunosorbent Assay
Radio-Immunoprecipitation Assay (RIPA) buffer was used to extract total proteins from the IVD annulus and NP, and the expression of inflammatory factors IL-1α and TNFα were detected by enzyme-linked immunosorbent assay (ELISA). The collagen II content was determined using the Collagen II Assay Kit (Built Bioengineering Research Institute, Nanjing, China), and the secretion of GAG was determined by the GAG Assay Kit (Aspen, Shanghai, China). The protocol was performed according to manufacturer’s instructions.
Western Blotting
The tissues were lysed with a RIPA buffer containing a complete mix of protease inhibitors (Aspen, Shanghai, China). The Bicinchoninic acid (BCA) Protein Concentration Assay Kit (Aspen, Shanghai, China) was then used to determine the protein concentration of the samples. Equivalent sample proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (Aspen, Shanghai, China) and transferred to PVDF membrane (Merck Millipore). Primary and secondary antibodies were used for incubation as follows: mouse anti-MMP-3 antibody (1:500, LS-C8751, LCBio), anti-PLA2 (1:500, ab180469, Abcam), and HRP-Goat Mouse (1:10,000 074-1806, KPL). Finally, chemiluminescence was detected by exposure in a dark room and films were scanned and archived. AlphaEaseFC 4.0 software was used to process and analyze the optical density (OD) value of the target bands. 18
Statistical Methods
The one-way analysis of variance and minimum difference post-hoc test were used to analyze the differences in the percentage DHI for each time period. The data collection of the new lumbar IVD model for ischemia and degeneration was established and improved. A P-value < .05 was considered as statistically significant.
Results
640-Slice Spiral Computed Tomography Analysis
Among the 6 animals randomly selected, 1 animal showed no occlusion of the L4 and L5 vessels, 1 animal had slight occlusion of L4 and L5 segments, and in 4 animals the L4 and L5 segments were completely occluded, indicating the successful establishment of ischemic lumbar disc degeneration model (Figure 1A).
Figure 1.
The successful construction of a rabbit lumbar intervertebral disc ischemia model and the presence of intervertebral disc degeneration were confirmed. A, Lumbar segmental artery ischemia was confirmed by 640 spiral CT-DSA: (1) The arterial vessels at the left segment of L4 and L5 were slightly occluded; (2, 4, 5, 6) The arterial vessels at the left segment of L4 and L5 were completely occluded. (3) Arterial vessels are visible at the left segment of L4 and L5. B, Transmission electron microscopy (TEM) was used to detect the capillary buds in the cartilage endplate. There were very few capillary buds in the L3-L5 endplate, and no capillary buds in the L5 upper endplate, confirming endplate ischemia. C, X-ray images of the lumbar spine showing that the height of the intervertebral disc decreased with increasing ischemia of the intervertebral disc (*P < .05, compared with NC). D, At 1, 2, and 3 months after surgery, disc signals in both the unilateral ischemic group and the bilateral ischemic group were reduced; the reduction in the bilateral ischemic group was more significant than in the unilateral ischemic group.
Transmission Electron Microscopy Analysis
The results of endplate ischemia of IVD by electron microscopy revealed the presence of complete vascular buds growing in the lower endplate of L3, abundant vascular sinuses in the endplate, and adequate blood supply in the endplate. The vascular buds of L4 upper and lower endplates and L5 upper endplates were significantly reduced, and the pattern of soap bubbles around the blood vessel buds had changed (Figure 1B).
X-Ray Analysis
No statistical differences were observed in the preoperative disc height between the L2/3 normal control group and the L3/4 unilateral ischemic group or L4/5 bilateral ischemic group. Changes in IVD height in the L2/3 months before and 1, 2, and 3 months after the intervention were not significantly different. However, there were statistically significant differences between the L3/4 unilateral ischemic group and the L4/5 bilateral ischemic group in terms of reduction in disc height 1, 2, and 3 months after surgery in both groups and components, especially in the bilateral ischemic group (Figure 1C).
Magnetic Resonance Imaging Analysis
Preoperative MRI imaging showed strong signals of normal IVD. One month postoperatively, the MRI images showed no significant decrease in IVD signals at L3/4 in the unilateral ischemic group and a slight decrease in the IVD signals at L4/5 in the bilateral ischemic group. MRI obtained 2 months after surgery showed a slight decrease in IVD signal in the unilateral ischemic group and a moderate decrease in the bilateral ischemic group. Three months after surgery, images showed moderate reduction of IVD signals in the unilateral ischemic group and severe reduction in the bilateral ischemic group. In the control group (L2/3), the signal intensity did not change significantly, uniformly showing high signals (Figure 1D). The degeneration of L2/3, L3/4, and L4/5 IVDs were evaluated according to the Thompson classification (Table 2).
Table 2.
Thompson Grading Statistical Table at Different Time Points After Operation in Each Group.
| Classification | L2/3 (Control) | L3/4 (Unilateral) | L4/5 (Bilateral) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Scoring | 1M | 2M | 3M | 1M | 2M | 3M | 1M | 2M | 3M |
| I | 6 | 5 | 4 | 4 | 2 | 1 | 0 | 0 | 0 |
| II | 0 | 1 | 2 | 2 | 3 | 4 | 4 | 0 | 0 |
| III | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 1 |
| IV | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 5 |
Histological Findings and Scoring
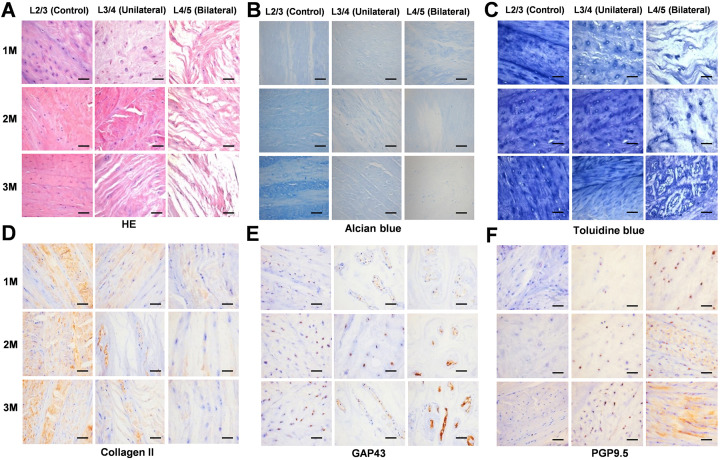
HE staining performed to evaluate structure of the IVD, showed that in the normal control group, the chondrocyte nucleus was blue, the cytoplasm and cartilage surface layer were pink, and the structure of lumbar disc was intact. In the unilateral ischemia group, chondrocytes showed partial degeneration and necrosis, and the structure of the lumbar vertebral disc was destroyed. In the bilateral ischemia group, HE staining revealed significant necrosis of chondrocytes and the structure of the lumbar disc was severely damaged. With increasing postoperative time, except for the normal control group, the tissue morphology revealed a trend of becoming increasingly incomplete over time (Figure 2A).
Figure 2.
Histological analysis and immunohistochemistry. A, HE staining showing the structural integrity of the intervertebral disc decreased with increasing ischemia. B, Alcian blue staining indicating that the content of proteoglycan decreases with increasing ischemia. C, Toluidine blue staining indicates that the calcified layer of the intervertebral disc thickens with increasing ischemia. D, With increasing degree of ischemia, the positive expression of collagen II decreased progressively. E, The expression of GAP43 in the intervertebral disc is enhanced with increasing ischemia. F, The expression of PGP9.5 is enhanced with increasing ischemia. The boxed regions are shown at 40 × magnification (scale bar = 100 μm).
Alcian blue staining used for the detection of PGs showed the presence of cyano-stained acidic PGs in the normal control group. The intensity of the staining of the unilateral ischemic group was weakened, indicating a reduction in acidic PGs content, while Alcian blue staining of bilateral ischemic group samples was the weakest, indicating that the content of acidic PGs was the lowest. With increasing postoperative time, the content of acidic PGs in the bilateral ischemic group continued to decrease, while differences between the other 2 groups were not significant (Figure 2B).
The calcification of IVD cartilage endplate as revealed by toluidine blue staining indicated a dark blue cartilage layer, a light blue calcification layer, and a gray bone marrow cavity in the normal control group. The staining of chondrocytes in the unilateral ischemic group and in the bilateral ischemic group was obviously weakened, the boundaries were indistinct, and the calcification layer was thickened, especially in the bilateral ischemic group. With increasing of postoperative time, there were no significant differences in the degree of coloration between each group (Figure 2C).
Immunohistochemistry
Immunohistochemistry analysis was used to evaluate collagen II levels in each sample group. In the normal control group, collagen II was positively expressed, while in the unilateral ischemia group the positive signals were weakened, and signals were significantly lower in the bilateral ischemic group. With increasing of postoperative time, there was no significant difference in collagen II expression across groups (Figure 2D).
GAP43 was weakly positively expressed in the normal control group, and expression was enhanced in the unilateral ischemic group, and more strongly expressed in the bilateral ischemic group. With increasing postoperative time, there were no significant differences in GAP43 expression across groups (Figure 2E).
PGP9.5 showed minimal or no expression in the normal control samples, although increased expression was observed in the unilateral ischemic group, and the strongest expression was found in the bilateral ischemic group. With increasing postoperative time, the expression of PGP9.5 increased across all groups (Figure 2F).
Biochemical Analysis
QPCR at different time points indicated that mRNA levels of IL-1α, TNFα, MMP-3, and PLA2 in the normal control samples were very low, while expression in the unilateral and bilateral ischemic groups was higher than that in the normal control group, and increased mRNA expression levels were more marked in the bilateral ischemic group. With the extension of postoperative time, the mRNA expression of PLA2 in the bilateral ischemic group showed no significant changes in trend, except that mRNA expression of PLA2 in the bilateral ischemic group gradually increased even further, indicating that expression of these genes was induced during the early stages of ischemia and showed irreversible and continued high expression over time. Both collagen II and aggrecan in the normal control group were very highly expressed, while mRNA expression levels in the unilateral and bilateral ischemia groups showed a significant decrease, with the expression in the bilateral ischemia group being the lowest for both genes. With increasing postoperative time, the mRNA expression of collagen II and aggrecan in the different groups showed a decreasing trend to different degrees, but in the normal control group expression was significantly higher than the unilateral or bilateral ischemia groups (Figure 3A-F).
Figure 3.
mRNA expression of IL-1α, TNFα, collagen II, MMP-3, aggrecan, PLA2 genes at 1, 2, and 3 months postoperatively. As the postoperative time is prolonged, mRNA expression of IL-1α (A), TNFα (B), MMP-3 (E), and PLA2 (F) are all higher than that expressed by the normal control group, while mRNA expression of these genes in the bilateral ischemia group is particularly increased. Collagen II (C) and aggrecan (D) mRNA in the normal control group show high levels of expression, while mRNA expression of collagen II and aggrecan of the unilateral and bilateral ischemia samples were significantly reduced, and the lowest levels are expressed by the bilateral ischemia group (*P < .05, compared with control group).
ELISA revealed that the expression of IL-1α and TNFα were lowest in the normal control and the levels of both cytokines increased in both the unilateral and bilateral ischemic groups, with the highest content measured in the bilateral ischemic group. As the postoperative time increased, IL-1α and TNFα remained consistently elevated in the unilateral and bilateral ischemic groups (Figure 4A, B). Collagen II and GAG protein levels were highly expressed in the normal control group, while in the unilateral and bilateral ischemia groups their levels in the IVD both showed a decrease in trend, with lowest levels measured in the bilateral ischemia group. Overtime, the postoperative expression of collagen II and GAG in each group remained at low levels (Figure 4C, D).
Figure 4.
The levels of IL-1α, TNFα, collagen II, GAG, MMP-3, and PLA2 detected using biochemical methods. Compared with the control group, levels of IL-1α (A) and TNFα (B) were consistently higher in the unilateral and bilateral ischemic groups as postoperative time increased, and the expression of collagen II (C) and GAG (D) in the unilateral and bilateral ischemic groups continued to decline as the postoperative time was prolonged. MMP-3 (E) and PLA2 (F) were consistently highly expressed in the unilateral and bilateral ischemic groups as the postoperative time increased (*P < .05, compared with control group).
The WB results show that in the normal control group, the expression of proteins related to disc catabolism, MMP-3 and PLA2, was maintained at a low level, which contributed to maintain the normal physiological function of the disc. However, the expression of MMP-3 and PLA2 in the unilateral and bilateral ischemic groups showed an increasing trend, with the highest levels observed in the bilateral ischemic group. Over time, postoperative expression of MMP-3 and PLA2 in the unilateral and bilateral ischemic groups continued to be elevated (Figure 4E, F).
Discussion
It is generally believed that IDD is the main cause of low back pain and numerous reports have evaluated biological treatment to improve degenerative disc disease using animal models.19-22 Experimentally, there are many ways to induce disc degeneration.23-26 At present, the most studied and most common method is acupuncture, 24 although this method may cause direct damage to the IVD. 27 Many studies have also investigated models of IVD ischemia; however, some directly damaged the cartilage endplate while others modified the vertebrae strength. Currently, there are no models that reflect the complexity of human discs or that can simulate natural degeneration of human IVDs. 2
Hou et al recently published a study using a rabbit model of lumbar ischemia in which pingyangmycin was injected into the endplate of the vertebra, and successfully induced IVD degeneration. 28 Importantly, they studied the anatomy of the lumbar artery in rabbits using DSA and vascular casting, and the results showed that, unlike the human anatomy, the trunk of the lumbar artery in rabbits departs directly from the aorta and is divided into right and left branches that subdivide into internal and external branches directed at several areas of the lumbar spine. Thus, L4/L5 vertebral blood flow could be almost completely impaired by dual ligation of L4 and L5. Compared with the model reported by Hou et al, the rabbit lumbar artery ligation model we propose in our study can be considered as a vertebral ischemia model, with impaired proximal lumbar blood flow.
In this study, we ligated the segment artery which led to endplate ischemia. Ligation of segmental arteries does not cause direct damage to the disc. Indeed, X-ray studies revealed a significant reduction in disc height at 1 month in both the unilateral and bilateral ischemic groups and the T2-weighted MRI scans showed a significant reduction in signal strength of the discs at the 1-month follow-up. Therefore, the findings indicated that the model had undergone significant degeneration in 1 month, which was in line with other previously described animal models, such as needle puncture or chemically induced models. Histological and immunohistochemical tests showed that the morphological structure and the expression of degeneration biomarkers of the IVDs models had changed significantly, which was very similar to the characteristics of human disc degeneration.
With the advancement of research on the etiology of disc degeneration, genetic factors have been identified to play an important role in its occurrence and progression.29,30 Moreover, the influence of genetic factors on IDD is greater than that of environmental factors.31-33 It has been recognized that catabolic and pro-inflammatory factors such as IL-1α, TNFα, PLA2, and MMP-3 play an important role in the process of IDD, and significant increases in the expression of IL-1α, TNFα, PLA2, and MMP-3 can be detected in IDD. In this study, mRNA expression, protein synthesis, and expression of IL-1α, TNFα, PLA2, and MMP-3 were increased in response to disc catabolism and the inflammatory response. Meanwhile, the above changes were most obvious in the early stage of IVD ischemia, which presented irreversible continuous changes with the prolongation of IVD ischemia, and ischemia was more significant. The present study confirmed the presence of mRNA and protein expression of inflammatory factors IL-1α and TNFα during disc degeneration. A proposal has been made to introduce related genes into IVD cells through gene therapy, but it is still in the early stages of development and related studies in vivo have not been carried out. Our study provides a suitable research model to further evaluate this theory.
However, our model has some limitations. There are some biomechanical and anatomical differences between rabbits and human spines. Studies have reported that the mechanical load may impair the diffusion of nutrients into IVDs and may accelerate the degeneration of IVDs. 34 Therefore, the biomechanical effects of rabbit models during degeneration may differ from those of humans. In this study, we did not perform biomechanical assessments such as hydrostatic pressure in degenerated discs; thus, additional studies are necessary to assess the mechanisms and characteristics of IDD due to cartilage endplate ischemia.
In summary, this study showed that ligation of lumbar segmental arteries in rabbits could induce endplate ischemia leading to disc degeneration, which to some extent simulates human disc degeneration. Because impaired flow due to atherosclerosis of the lumbar arteries is associated with lumbar disc degeneration and low back pain, 15 the ischemia model established in this study lays a foundation for further study on the pathological mechanism of low back pain.
Supplemental Material
Supplemental Material, sj-jpg-1-gsj-10.1177_21925682211012323 for Establishment of a New Model of Lumbar Intervertebral Disc Degeneration With Pathological Characteristics by Yongming Jin, Guangfeng Mao, Chen Yang, Chen Xia, Chuyong Chen, Fangfang Shi and Qi Chen in Global Spine Journal
Footnotes
Authors’ Note: Yongming Jin and Guangfeng Mao are contributed equally to this work.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Application Research of Public Technology of Science and Technology Department of Zhejiang Province (No. 2012C33069) and the Health Science and Technology Plan Project of Zhejiang Province (No. 2021KY495).
ORCID iD: Fangfang Shi, MD, PhD  https://orcid.org/0000-0002-9017-2695
https://orcid.org/0000-0002-9017-2695
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Chan DD, Khan SN, Ye X, et al. Mechanical deformation and glycosaminoglycan content changes in a rabbit annular puncture disc degeneration model. Spine. 2011;36(18):1438–1445. doi:10.1097/BRS.0b013e3181f8be52 [DOI] [PubMed] [Google Scholar]
- 2.Daly C, Ghosh P, Jenkin G, Oehme D, Goldschlager T. A review of animal models of intervertebral disc degeneration: pathophysiology, regeneration, and translation to the clinic. Biomed Res Int. 2016;2016:5952165. doi:10.1155/2016/5952165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vergroesen PP, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23(7):1057–1070. doi:10.1016/j.joca.2015.03.028 [DOI] [PubMed] [Google Scholar]
- 4.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi:10.1038/nrrheum.2013.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vo NV, Hartman RA, Patil PR, et al. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res. 2016;34(8):1289–1306. doi:10.1002/jor.23195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crock HV, Yoshizawa H. The blood supply of the lumbar vertebral column. Clin Orthop Relat Res. 1976;(115):6–21. [PubMed] [Google Scholar]
- 7.Battié MC, Videman T, Gill K, et al. 1991 Volvo award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine. 1991;16(9):1015–1021. [PubMed] [Google Scholar]
- 8.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29(23):2700–2709. doi:10.1097/01.brs.0000146499.97948.52 [DOI] [PubMed] [Google Scholar]
- 9.Robertson PA, Nicholson OR. ACC and back injuries: the relevance of pre-existing asymptomatic conditions revisited. N Z Med J. 2011;124(1335):65–72. [PubMed] [Google Scholar]
- 10.Videman T, Battié MC, Parent E, Gibbons LE, Vainio P, Kaprio J. Progression and determinants of quantitative magnetic resonance imaging measures of lumbar disc degeneration: a five-year follow-up of adult male monozygotic twins. Spine. 2008;33(13):1484–1490. doi:10.1097/BRS.0b013e3181753bb1 [DOI] [PubMed] [Google Scholar]
- 11.Harris-Hayes M, Sahrmann SA, Van Dillen LR. Relationship between the hip and low back pain in athletes who participate in rotation-related sports. J Sport Rehabil. 2009;18(1):60–75. doi:10.1123/jsr.18.1.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freemont AJ, Watkins A, Le Maitre C, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197(3):286–292. doi:10.1002/path.1108 [DOI] [PubMed] [Google Scholar]
- 13.Richardson SM, Doyle P, Minogue BM, Gnanalingham K, Hoyland JA. Increased expression of matrix metalloproteinase-10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res Ther. 2009;11(4): R126. doi:10.1186/ar2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solovieva S, Kouhia S, Leino-Arjas P, et al. Interleukin 1 polymorphisms and intervertebral disc degeneration. Epidemiology (Cambridge, Mass). 2004;15(5):626–633. doi:10.1097/01.ede.0000135179.04563.35 [DOI] [PubMed] [Google Scholar]
- 15.Kauppila LI. Atherosclerosis and disc degeneration/low-back pain—a systematic review. Eur J Vasc Endovasc Surg. 2009;37(6):661–670. doi:10.1016/j.ejvs.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 16.Kauppila LI, McAlindon T, Evans S, Wilson PW, Kiel D, Felson DT. Disc degeneration/back pain and calcification of the abdominal aorta. A 25-year follow-up study in Framingham. Spine. 1997;22(14):1642–1647. doi:10.1097/00007632-199707150-00023 [DOI] [PubMed] [Google Scholar]
- 17.An HS, Takegami K, Kamada H, et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine. 2005;30(1):25–31. doi:10.1097/01.brs.0000148002.68656.4d [DOI] [PubMed] [Google Scholar]
- 18.Beierfuß A, Dietrich H, Kremser C, et al. Knockout of Apolipoprotein E in rabbit promotes premature intervertebral disc degeneration: a new in vivo model for therapeutic approaches of spinal disc disorders. PLoS One. 2017;12(11):e0187564. doi:10.1371/journal.pone.0187564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang SZ, Rui YF, Tan Q, Wang C. Enhancing intervertebral disc repair and regeneration through biology: platelet-rich plasma as an alternative strategy. Arthritis Res Ther. 2013;15(5):220. doi:10.1186/ar4353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benneker LM, Andersson G, Iatridis JC, et al. Cell therapy for intervertebral disc repair: advancing cell therapy from bench to clinics. Eur Cells Mater. 2014;27:5–11. doi:10.22203/ecm.v027sa02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren S, Liu Y, Ma J, et al. Treatment of rabbit intervertebral disc degeneration with co-transfection by adeno-associated virus-mediated SOX9 and osteogenic protein-1 double genes in vivo. Int J Mol Med. 2013;32(5):1063–1068. doi:10.3892/ijmm.2013.1497 [DOI] [PubMed] [Google Scholar]
- 22.Carragee EJ, Don AS, Hurwitz EL, Cuellar JM, Carrino JA, Herzog R. 2009 ISSLS Prize Winner: does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine. 2009;34(21):2338–2345. doi:10.1097/BRS.0b013e3181ab5432 [DOI] [PubMed] [Google Scholar]
- 23.Xi Y, Kong J, Liu Y, et al. Minimally invasive induction of an early lumbar disc degeneration model in rhesus monkeys. Spine. 2013;38(10):E579–E586. doi:10.1097/BRS.0b013e31828b695b [DOI] [PubMed] [Google Scholar]
- 24.Rudnik-Jansen I, Tellegen A, Beukers M, et al. Safety of intradiscal delivery of triamcinolone acetonide by a poly(esteramide) microsphere platform in a large animal model of intervertebral disc degeneration. Spine J. 2019;19(5):905–919. doi:10.1016/j.spinee.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 25.Yuan W, Che W, Jiang YQ, et al. Establishment of intervertebral disc degeneration model induced by ischemic sub-endplate in rat tail. Spine J. 2015;15(5):1050–1059. doi:10.1016/j.spinee.2015.01.026 [DOI] [PubMed] [Google Scholar]
- 26.Wei F, Zhong R, Pan X, et al. Computed tomography-guided sub-end plate injection of pingyangmycin for a novel rabbit model of slowly progressive disc degeneration. Spine J. 2019;19(2):e6–e18. doi:10.1016/j.spinee.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 27.Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30(1):5–14. doi:10.1097/01.brs.0000148152.04401.20 [DOI] [PubMed] [Google Scholar]
- 28.Hou C, Tan G, Zhuang W, Yang J. Establishment of a new animal model for ischemic lumbar vertebrae. Int J Clin Exp Med. 2015;8(7):10646–10656. [PMC free article] [PubMed] [Google Scholar]
- 29.Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Jt Surg. 2006;88(Suppl 2):3–9. doi:10.2106/jbjs.e.01313 [DOI] [PubMed] [Google Scholar]
- 30.Kao PY, Chan D, Samartzis D, Sham PC, Song YQ. Genetics of lumbar disk degeneration: technology, study designs, and risk factors. Orthop Clin North Am. 2011;42(4):479–486, vii. doi:10.1016/j.ocl.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 31.Battié MC, Videman T, Kaprio J, et al. The Twin Spine Study: contributions to a changing view of disc degeneration. Spine J. 2009;9(1):47–59. doi:10.1016/j.spinee.2008.11.011 [DOI] [PubMed] [Google Scholar]
- 32.Takeda K, Kou I, Hosogane N, et al. Association of susceptibility genes for adolescent idiopathic scoliosis and intervertebral disc degeneration with adult spinal deformity. Spine. 2019;44(23):1623–1629. doi:10.1097/brs.0000000000003179 [DOI] [PubMed] [Google Scholar]
- 33.Battié MC, Ortega-Alonso A, Niemelainen R, et al. Lumbar spinal stenosis is a highly genetic condition partly mediated by disc degeneration. Arthritis Rheum. 2014;66(12):3505–3510. doi:10.1002/art.38823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arun R, Freeman BJ, Scammell BE, McNally DS, Cox E, Gowland P. 2009 ISSLS Prize Winner: what influence does sustained mechanical load have on diffusion in the human intervertebral disc? An in vivo study using serial postcontrast magnetic resonance imaging. Spine. 2009;34(21):2324–2337. doi:10.1097/BRS.0b013e3181b4df92 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-jpg-1-gsj-10.1177_21925682211012323 for Establishment of a New Model of Lumbar Intervertebral Disc Degeneration With Pathological Characteristics by Yongming Jin, Guangfeng Mao, Chen Yang, Chen Xia, Chuyong Chen, Fangfang Shi and Qi Chen in Global Spine Journal