Abstract
Study Design:
Multicenter prospective study.
Objectives:
Although intramedullary spinal cord tumor (IMSCT) and extramedullary SCT (EMSCT) surgeries carry high risk of intraoperative motor deficits (MDs), the benefits of transcranial motor evoked potential (TcMEP) monitoring are well-accepted; however, comparisons have not yet been conducted. This study aimed to clarify the efficacy of TcMEP monitoring during IMSCT and EMSCT resection surgeries.
Methods:
We prospectively reviewed TcMEP monitoring data of 81 consecutive IMSCT and 347 EMSCT patients. We compared the efficacy of interventions based on TcMEP alerts in the IMSCT and EMSCT groups. We defined our alert point as a TcMEP amplitude reduction of ≥70% from baseline.
Results:
In the IMSCT group, TcMEP monitoring revealed 20 true-positive (25%), 8 rescue (10%; rescue rate 29%), 10 false-positive, a false-negative, and 41 true-negative patients, resulting in a sensitivity of 95% and a specificity of 80%. In the EMSCT group, TcMEP monitoring revealed 20 true-positive (6%), 24 rescue (7%; rescue rate 55%), 29 false-positive, 2 false-negative, and 263 true-negative patients, resulting in a sensitivity of 91% and specificity of 90%. The most common TcMEP alert timing was during tumor resection (96% vs. 91%), and suspension surgeries with or without intravenous steroid administration were performed as intervention techniques.
Conclusions:
Postoperative MD rates in IMSCT and EMSCT surgeries using TcMEP monitoring were 25% and 6%, and rescue rates were 29% and 55%. We believe that the usage of TcMEP monitoring and appropriate intervention techniques during SCT surgeries might have predicted and prevented the occurrence of intraoperative MDs.
Keywords: transcranial motor evoked potentials, spinal cord tumor surgery, intramedullary tumor, extramedullary tumor
Introduction
Postoperative motor deficit (MD) is one of the most serious perioperative complications after spinal cord tumor (SCT) resection surgery, and multimodal intraoperative neuromonitoring (IONM), especially transcranial motor evoked potential (TcMEP) monitoring, is expected to reduce the risk of intraoperative MDs.1-4 As patients with SCT are often associated with spinal cord compression or infiltration, SCT resection surgeries increase the risk of intraoperative MDs due to gross total resection or sacrifice of nerve roots.3,5 Thus, to predict and avoid neurological complications during SCT resection surgery, it is essential to perform proper and stable TcMEP monitoring. 6
As intramedullary SCT (IMSCT) and extramedullary SCT (EMSCT) resection surgeries have high risk of intraoperative MDs, the benefits and safety of TcMEP monitoring are well accepted.3,6 To the best of our knowledge, only a few reports have considered the timing of IONM alert and the efficacy of intervention techniques, such as IMSCT and EMSCT.3,7 Therefore, the purpose of this study was to clarify the efficacy of TcMEP monitoring during IMSCT and EMSCT resection surgeries and to investigate the timing of Tc-MEP alert and intervention techniques for the prevention of intraoperative MDs. We hypothesized that the usage of TcMEP monitoring during SCT resection surgery and the proper intervention techniques after TcMEP alerts are associated with the prevention of intraoperative MDs.
Materials and Methods
Institutional Review Board Approval
This study was approved by the institutional review board of our hospital (research approval no. 19-146), and the study’s protocol adhered to the principles of the Declaration of Helsinki.
Participants
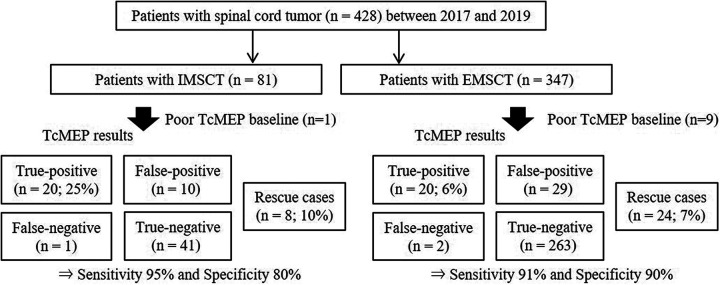
The spinal cord monitoring working group of the Japanese society for spine surgery and related research conducted a prospective nationwide multicenter survey of 3625 patients (mean age, 60.1 years old; 1,886 men and 1,739 women) who underwent spine surgery using IONM at 16 spine centers between April 2017 and March 2020. Among these, 491 patients underwent spinal tumor resection surgery after providing informed consent to participate in this study. After excluding 19 patients with metastatic tumors, 12 patients with epidural tumors, 11 patients with spine tumors, 11 patients with arachnoid cysts, 2 patients with adhesive spinal arachnoiditis, 1 patient with neurenteric cyst, 1 patient with endodermal cyst, 1 patient with intramedullary hemorrhage, 1 patient with sarcoma, 1 patient with chordoma, 1 patient with hemosiderin brain surface deposition, with 1 patient who underwent SCT resection surgery with an anterior approach, and 1 patient without intraoperative TcMEP monitoring during surgery, we examined a final study population of 428 patients with SCT who underwent resection surgery using a posterior approach (81 with IMSCT and 347 with EMSCT) (Figure 1).
Figure 1.
Study design. TcMEP indicates transcranial electrical stimulation motor evoked potential; IMSCT, intramedullary spinal cord tumor; EMSCT, extra-medullary spinal cord tumor.
Variables, Data Sources, and Bias
Patient characteristics and surgical data were collected by reviewing medical and anesthetic records, that included age, sex, height, body weight, body mass index (BMI), preoperative MD, postoperative MD, tumor level (classified by cervical, thoracic, and lumbar level), duration of surgery, estimated blood loss, pathology of SCT, the timing of alert, and the intervention techniques.
Anesthesia Management, Intraoperative Monitoring and Surgical Intervention Techniques
Anesthesia was induced and maintained as described previously. 8 Total intravenous anesthesia was maintained with pump-controlled intravenous infusion of remifentanil (1 mg/kg/h) and propofol (100–150 mg/kg/min with target-controlled infusion) based on the bispectral index (BIS) (>40 and <60) of each patient during IONM.
Multimodal IONM, including TcMEP, somatosensory evoked potential (SSEP), and free-run electromyography (EMG) monitoring, were performed as described previously. 8 TcMEPs were recorded using a Neuromaster MEE 1232 Stimulator (Nihon Kohden, Tokyo, Japan). The evoked muscles were selected from the sternocleidomastoid, trapezius, deltoid, biceps brachii, triceps brachii, abductor digit minim, and abductor pollicis brevis in the upper extremities, from the quadriceps, hamstrings, tibial anterior, gastrocnemius, flexor hallucis brevis, and abductor hallucis muscles in the lower extremities, and at least 1 proximal and 1 distal muscle. Fewer than 20 transcranial stimuli were delivered in trains of 5 to 10 stimuli with stimulation intensities of 100–200 mA, an inter-stimulus interval of 2 ms, a 50–1000 Hz filter, and a recording time of 100 ms. Corkscrew electrodes were placed symmetrically 2 cm anterior and 5–7 cm to the Cz point (international 10–20 system) over the motor cortex.
In response to the TcMEP alert, the spine surgeons performed rescue interventions to promote the recovery of TcMEP amplitude, such as suspension of surgery, warm saline irrigation of the spinal cord, control of systematic hypotension, and/or intravenous steroid injection. If the amplitudes did not show sufficient recovery, the surgeons decided whether or not to continue tumor resection surgery, considering the entire circumstances and condition of the patient.
Definition of the Alert Point and Grouping
The cut-off (alert) point was defined as described previously.8,9 The baseline control TcMEP amplitude was classified as that observed at the time of incision or prior to decompression, depending on the circumstances of each case. Patients with poor TcMEP baseline waveform derivation were defined as those without waveforms from all evoked muscles. We then defined our alert point as an amplitude reduction of ≥70% from baseline, based on a previous study. 9 We defined postoperative MD as the muscle strength of the patient decreasing by 1 or more grades on the manual muscle test compared with the preoperative score. Neurological examination was performed soon after surgery or at 1 day postoperatively. Postoperative MDs were further classified as transient (absent at 3 months postoperatively) or persistent (present at 3 months postoperatively). The delayed onset of paralysis was negative. In this study, rescue case was defined as a patient with recovered TcMEP amplitudes after certain procedures and without a de novo MD. During statistical analysis, rescue cases were excluded from the analysis of accuracy, because we were not convinced that the temporal decrease in amplitude indicated real MD without a wake-up test. The alert occurrence rate was set as the number of patients with TcMEP alert (true-positive [TP] + rescue + false-positive [FP] patients) divided by the total group number. We defined both TP and rescue patients as possible MD patients. The rescue rate was set as the number of rescue patients divided by the number of possible MD patients.
Statistical Analysis
Categorical variables are expressed as absolute numbers and percentages and were analyzed using chi-square tests or Fisher’s exact test, as appropriate. Continuous variables with normal distributions are expressed as the mean ± standard deviation and were analyzed using unpaired t-tests. Statistical analyses were conducted using SPSS Version 23.0 (IBM, Armonk, NY, USA). P-values of <0.05 were considered statistically significant.
Results
Among the IMSCT group, except for 1 patient with poor TcMEP baseline waveform (TcMEP derivation rate 98.8%; Figure 1), TcMEP monitoring revealed 38 patients with TcMEP alerts, including 20 TP patients (24.7%), 10 FP patients, and 8 rescue patients (9.9%; rescue rate 28.6%). Furthermore, TcMEP monitoring revealed 42 patients without TcMEP alerts, including 1 FN patient, and 41 TN patients. Eight rescue patients were excluded from the analysis of accuracy. Therefore, the sensitivity, specificity, positive predictive value, and negative predictive value were 95.2%, 80.4%, 66.7%, and 97.6%, respectively. Among patients in the EMSCT group, except for 9 patients with poor TcMEP baseline waveform (TcMEP derivation rate 97.4%; Figure 1), TcMEP monitoring revealed 73 patients with TcMEP alerts, including 20 TP patients (5.8%), 29 FP patients, and 24 rescue patients (6.9%; rescue rate 54.5%). Furthermore, TcMEP monitoring revealed 265 patients without TcMEP alerts, including 2 FN patient, and 263 TN patients. Twenty-four rescue patients were excluded from the analysis of accuracy. Therefore, the sensitivity, specificity, positive predictive value, and negative predictive value were 90.9%, 90.1%, 40.8%, and 99.2%, respectively. The baseline and surgical factors between the IMSCT and EMSCT groups are provided in Table 1. There were significant differences in age, sex, and tumor level between the groups (P < 0.001, P = 0.006, and P = 0.002, respectively). Compared to the group with EMSCT, the group with IMSCT had a significantly longer mean duration of surgery (358 min vs. 248 min, P < 0.001). Among 21 patients with postoperative MDs in the IMSCT group, 5 patients (5/81, 6.2%) had persistent MDs at 3 months postoperatively. Among 22 patients with postoperative MDs in the EMSCT group, 5 patients (5/347, 1.4%) had persistent MDs at 3 months postoperatively.
Table 1.
Comparison of Baseline and Surgical Factors Between Groups With IMSCT and EMSCT.a
| Variable | Total (n = 428) | IMSCT (n = 81) | EMSCT (n = 347) | P valueb |
|---|---|---|---|---|
| Age, years | 56.0 ± 17.9 | 48.7 ± 18.7 | 57.7 ± 17.3 | <0.001 |
| Female sex | 233 (54.4%) | 33 (40.7%) | 200 (57.6%) | 0.006 |
| Height, cm | 160.4 ± 10.1 | 162.1 ± 11.3 | 160.0 ± 9.8 | 0.093 |
| Body weight, kg | 60.0 ± 12.9 | 62.7 ± 16.0 | 59.4 ± 12.0 | 0.089 |
| Body mass index, kg/m2 | 23.2 ± 3.7 | 23.7 ± 4.3 | 23.1 ± 3.5 | 0.281 |
| Preoperative MD | 150 (35.1%) | 35 (43.2%) | 115 (33.1%) | 0.087 |
| Postoperative de novo MD | 44 (10.3%) | 21 (25.9%) | 23 (6.6%) | <0.001 |
| Tumor level | 0.002 | |||
| Cervical | 110 (25.7%) | 24 (29.6%) | 86 (24.8%) | |
| Thoracic | 189 (44.2%) | 46 (56.8%) | 143 (41.2%) | |
| Lumbar | 125 (29.2%) | 11 (13.6%) | 114 (32.9%) | |
| Unknown | 4 (0.9%) | 0 (0%) | 4 (1.2%) | |
| Surgical factors | ||||
| Duration of surgery, mins | 269 ± 118 | 358 ± 132 | 248 ± 105 | <0.001 |
| Estimated blood loss, mL | 173 ± 222 | 159 ± 195 | 176 ± 228 | 0.524 |
Abbreviations: IMSCT, intramedullary spinal cord tumor; EMSCT, extra-medullary spinal cord tumor; MD, motor deficit.
a Values are expressed as number of patients (%), or mean ± SD.
b Indicates IMSCT vs. EMSCT.
Baseline, Surgical Factors, and TcMEP Amplitude Between TP and Rescue Patients With IMSCT (Table 2) and EMSCT (Table 3)
Table 2.
Comparison of Baseline, Surgical Factors, and Tcmep Amplitude Between True-Positive and Rescue Patients With Imsct.a
| Variable (IMSCT group) | True-positive (n = 20) | Rescue (n = 8) | P value |
|---|---|---|---|
| Age, years | 53.0 ± 17.3 | 45.8 ± 19.2 | 0.339 |
| Female sex | 9 (45.0%) | 2 (25.0%) | 0.296 |
| Height, cm | 161.1 ± 8.3 | 167.4 ± 8.0 | 0.078 |
| Body weight, kg | 64.0 ± 14.2 | 69.2 ± 22.1 | 0.463 |
| Body mass index, kg/m2 | 24.4 ± 3.9 | 24.5 ± 6.8 | 0.979 |
| Preoperative MD | 8 (40.0%) | 5 (62.5%) | 0.255 |
| Persistent MD | 5 (25.0%) | - | - |
| Tumor level | 0.033 | ||
| Cervical | 3 (15.0%) | 2 (25.0%) | |
| Thoracic | 16 (80.0%) | 3 (37.5%) | |
| Lumbar | 1 (5.0%) | 3 (37.5%) | |
| Type of IMSCT | 0.254 | ||
| Ependymoma | 6 (30.0%) | 2 (25.0%) | |
| Hemangioma | 2 (10.0%) | 3 (37.5%) | |
| Hemangioblastoma | 4 (20.0%) | 0 (0%) | |
| Astrocytoma | 0 (0%) | 1 (12.5%) | |
| Glioma | 1 (5.0%) | 0 (0%) | |
| Others and unknown | 7 (35.0%) | 2 (25.0%) | |
| Surgical factors | |||
| Duration of surgery, mins | 377 ± 150 | 403 ± 143 | 0.681 |
| Estimated blood loss, mL | 199 ± 182 | 332 ± 467 | 0.455 |
| TcMEP amplitude | |||
| Alert timing, % | 15.2 ± 11.0 | 12.4 ± 11.9 | 0.586 |
| Final, % | 9.6 ± 8.3 | 39.1 ± 31.0 | 0.045 |
Abbreviations: TcMEP, transcranial motor-evoked potential; IMSCT, intramedullary spinal cord tumor; MD, motor deficit.
a Values are expressed as number of patients (%), or mean ± SD.
Table 3.
Comparison of Baseline, Surgical Factors, and Tcmep Amplitude Between True-Positive and Rescue Patients With Emsct.a
| Variable (EMSCT group) | True-positive (n = 20) | Rescue (n = 24) | P value |
|---|---|---|---|
| Age, years | 59.3 ± 14.0 | 59.9 ± 16.3 | 0.886 |
| Female sex | 14 (70.0%) | 12 (50.0%) | 0.179 |
| Height, cm | 158.2 ± 11.7 | 164.1 ± 11.0 | 0.092 |
| Body weight, kg | 58.5 ± 11.0 | 63.7 ± 14.4 | 0.196 |
| Body mass index, kg/m2 | 22.9 ± 3.5 | 23.5 ± 3.8 | 0.588 |
| Preoperative MD | 16 (80.0%) | 12 (50.0%) | 0.039 |
| Persistent MD | 4 (20.0%) | - | - |
| Tumor level | 0.364 | ||
| Cervical | 4 (20.0%) | 9 (37.5%) | |
| Thoracic | 10 (50.0%) | 11 (45.8%) | |
| Lumbar | 6 (30.0%) | 4 (16.7%) | |
| Type of EMSCT | 0.525 | ||
| Schwannoma | 5 (25.0%) | 10 (41.7%) | |
| Meningioma | 8 (40.0%) | 8 (33.3%) | |
| Neurofibroma | 6 (30.0%) | 6 (25.0%) | |
| Others and unknown | 1 (5.0%) | 0 (0%) | |
| Surgical factors | |||
| Duration of surgery, mins | 351 ± 135 | 293 ± 112 | 0.124 |
| Estimated blood loss, mL | 367 ± 394 | 247 ± 263 | 0.236 |
| TcMEP amplitude | |||
| Alert timing, % | 15.4 ± 11.5 | 17.0 ± 9.0 | 0.595 |
| Final, % | 16.2 ± 9.7 | 64.3 ± 26.9 | <0.001 |
Abbreviations: TcMEP, transcranial motor-evoked potential; EMSCT, extra-medullary spinal cord tumor; MD, motor deficit.
a Values are expressed as number of patients (%), or mean ± SD.
Among patients in the IMSCT group, there was significant differences in tumor level between TP and rescue patients (P = 0.033). Among patients in the EMSCT group, there was significant difference in preoperative MD between TP and rescue patients (P = 0.039).
Timing of TcMEP Alert Among Patients With TcMEP Alert (Table 4)
Table 4.
Timing of TcMEP Alert Among Patients With TcMEP Alert.a
| Timing of TcMEP alert | (A) Alert N (%) | (B) FP alert N (%) |
(A-B) Possible MD alert N (%) | (C) Postop MD N (%) | Postop MD rate (100×C/A) |
|---|---|---|---|---|---|
| During IMSCT surgery | |||||
| Dura opening | 1 (3%) | 0 (0%) | 1 (4%) | 1 (5%) | 100% |
| Tumor resection | 31 (82%) | 4 (40%) | 27 (96%) | 19 (95%) | 61% |
| Other/surgery unrelated | 2 (5%) | 2 (20%) | 0 (0%) | 0 (0%) | 0% |
| Unknown | 4 (11%) | 4 (40%) | 0 (0%) | 0 (0%) | 0% |
| Overall | 38 (100%) | 10 (100%) | 28 (100%) | 20 (100%) | 55% |
| During EMSCT surgery | |||||
| Exposure | 4 (5%) | 3 (10%) | 1 (2%) | 1 (5%) | 25% |
| Lamina opening | 2 (3%) | 1 (3%) | 1 (2%) | 1 (5%) | 50% |
| Dura opening | 1 (1%) | 1 (3%) | 0 (0%) | 0 (0%) | 0% |
| Tumor resection | 51 (70%) | 11 (38%) | 40 (91%) | 17 (85%) | 33% |
| Root sacrifice | 1 (1%) | 0 (0%) | 1 (2%) | 1 (5%) | 100% |
| Osteotomy | 1 (1%) | 0 (0%) | 1 (2%) | 0 (0%) | 0% |
| Unknown | 13 (18%) | 13 (45%) | 0 (0%) | 0 (0%) | 0% |
| Overall | 73 (100%) | 29 (100%) | 44 (100%) | 20 (100%) | 33% |
Abbreviations: TcMEP, transcranial motor-evoked potential; IMSCT, intramedullary spinal cord tumor; EMSCT, extra-medullary spinal cord tumor; Postop, postoperative; MD, motor deficit; FP, false-positive.
a Values are expressed as number of patients (%).
The most common timing of TcMEP alerts was during tumor resection (IMSCT vs. EMSCT, 82% vs. 70%), and the incidence of postoperative MD for those alerts was 61% vs. 33%, respectively.
Intervention Techniques After TcMEP Alerts Related to Possible MD (Table 5)
Table 5.
Rescue Rate After Each Intervention Technique Among Patients With Possible MD Alerts.a
| Timing of TcMEP alert | Intervention technique | (A) Intervention to possible MD alert, N | (B) Rescue, N | Rescue rate (100×B/A) |
|---|---|---|---|---|
| During IMSCT surgery | ||||
| Dura opening | Steroid injection | 1 | 0 | 0% |
| Tumor resection | Suspension of surgery, with steroid injection | 19 | 4 | 21% |
| Suspension of surgery | 8 | 4 | 50% | |
| Overall | 28 | 8 | 29% | |
| During EMSCT surgery | ||||
| Exposure | Suspension of surgery, with steroid injection | 1 | 0 | 0% |
| Lamina opening | No intervention | 1 | 0 | 0% |
| Tumor resection | Suspension of surgery, with steroid injection | 6 | 3 | 50% |
| Suspension surgery | 32 | 19 | 59% | |
| Additional decompression | 2 | 1 | 50% | |
| Root sacrifice | Steroid injection | 1 | 0 | 0% |
| Osteotomy | Suspension of surgery | 1 | 1 | 100% |
| Overall | 44 | 24 | 55% | |
Abbreviations: MD, motor deficit; TcMEP, transcranial motor-evoked potential; IMSCT, intramedullary spinal cord tumor; EMSCT, extra-medullary spinal cord tumor.
a Values are expressed as number of patients (%).
During IMSCT and EMSCT surgery, most of the possible MD alerts occurred during tumor resection, and the intervention techniques by the surgeon consisted of the following procedures: suspension surgery with intravenous steroid injection, suspension surgery without intravenous steroid injection, or additional decompression. Among patients with possible MD alerts during IMSCT resection, 21% were rescued by suspension surgery with intravenous steroid injection, and 50% were rescued by suspension surgery without intravenous steroid injection. Among patients with possible MD alerts during EMSCT resection, 50% were rescued by suspension surgery with intravenous steroid injection, and 59% were rescued by suspension surgery without intravenous steroid injection.
Patient Data and Clinical Results of 3 False-Negative (FN) Patients (Table 6)
Table 6.
Patient Data and Clinical Results of 3 False-Negative Patients.
| Patient | Pathology | Level | Preop MD | Postop MD | Final follow-up |
|---|---|---|---|---|---|
| 75, F | Astrocytoma | T12 | None | Rt. Quad (4) | Recovery within 1 month |
| 51, F | Schwannoma | T12/L1 | None | Rt. TA (4), EHL (4) | Recovery within 1 month |
| 61, M | Schwannoma | T11/12 | Rt. IP (2) | Bil. IP (Rt./Lt., 0/2), Quad (1/4) | Persistent MD |
Abbreviations: Preop, preoperative; Postop, postoperative; MD, motor deficit; Rt., right; Lt., left; Bil., bilateral; IP, iliopsoas; Quad, quadriceps; TA, tibialis anterior; EHL, extensor hallucis longus.
There was 1 patient with an astrocytoma in the IMSCT group and 2 patients with schwannomas in the EMSCT group. The level of surgery in all patients was the thoracolumbar level; that is, epi-conus level. Among 3 FN patients, 2 patients had no preoperative MDs and postoperative MDs due to spinal segmental injuries at epi-conus level. One of 3 FN patients had ≤3 preoperative MDs on the muscle motor testing, and the postoperative persistent MDs were due to bilateral spinal segment injuries rather than spinal tract injuries.
Subgroup Analysis
The results of TcMEP monitoring according to each type and level of SCT are provided in Table 7. Among the 23 patients with ependymoma, TcMEP monitoring revealed 6 TP patients (26%), 2 rescue patients (9%), and 3 FP patients. Therefore, the alert and rescue rates were 48% and 25%, respectively. Classified by each level of IMSCT (cervical vs. thoracic vs. lumbar), TcMEP results showed that the alert and rescue rates were 29% vs. 60% vs. 36%, and 40% vs. 16% vs. 75%, respectively. Among the 139 patients with schwannomas, TcMEP monitoring revealed 6 TP patients (4%), 10 rescue patients (7%), and 11 FP patients. Therefore, the alert and rescue rates were 19% and 63%, respectively. Classified by each level of EMSCT (cervical vs. thoracic vs. lumbar), TcMEP results showed that the alert and rescue rates were 24% vs. 26% vs. 15%, and 69% vs. 52% vs. 40%, respectively.
Table 7.
Results of Subgroup Analyses for Tcmep Monitoring by Each Type and Level of Spinal Cord Tumor.a
| Subgroup | N | TP | Rescue | FP | FN | TN | Alert rate | Rescue rate |
|---|---|---|---|---|---|---|---|---|
| Type of IMSCT | ||||||||
| Ependymoma | 23 | 6 (26%) | 2 (9%) | 3 | 0 | 12 | 48% | 25% |
| Hemangioma | 13 | 2 (15%) | 3 (23%) | 4 | 0 | 4 | 69% | 60% |
| Hemangioblastoma | 12 | 4 (33%) | 0 (0%) | 1 | 0 | 7 | 42% | 0% |
| Astrocytoma | 5 | 0 (0%) | 1 (20%) | 1 | 1 | 2 | 40% | 100% |
| Glioma | 3 | 1 (33%) | 0 (0%) | 0 | 0 | 2 | 33% | 0% |
| Others and unknown | 24 | 7 (29%) | 2 (8%) | 1 | 0 | 14 | 42% | 22% |
| Level of IMSCT | ||||||||
| Cervical | 24 | 3 (13%) | 2 (8%) | 2 | 0 | 17 | 29% | 40% |
| Thoracic | 45 | 16 (36%) | 3 (7%) | 8 | 1 | 17 | 60% | 16% |
| Lumbar | 11 | 1 (9%) | 3 (27%) | 0 | 0 | 7 | 36% | 75% |
| Preoperative MD (+) | 34 | 8 (24%) | 5 (15%) | 6 | 0 | 15 | 56% | 38% |
| Preoperative MD (-) | 46 | 12 (26%) | 3 (7%) | 4 | 1 | 26 | 41% | 20% |
| Overall in IMSCT | 80 | 20 (25%) | 8 (10%) | 10 | 1 | 41 | 48% | 29% |
| Type of EMSCT | ||||||||
| Schwannoma | 139 | 6 (4%) | 10 (7%) | 11 | 2 | 110 | 19% | 63% |
| Meningioma | 55 | 8 (15%) | 8 (15%) | 4 | 0 | 32 | 36% | 50% |
| Neurofibroma | 4 | 0 (0%) | 0 (0%) | 0 | 0 | 4 | 0% | - |
| Others and unknown | 145 | 6 (4%) | 6 (4%) | 14 | 0 | 117 | 18% | 50% |
| Level of EMSCT | ||||||||
| Cervical | 85 | 4 (5%) | 9 (11%) | 7 | 0 | 65 | 24% | 69% |
| Thoracic | 136 | 10 (7%) | 11 (8%) | 15 | 2 | 98 | 26% | 52% |
| Lumbar | 113 | 6 (5%) | 4 (4%) | 7 | 0 | 96 | 15% | 40% |
| Unknown | 4 | 0 (0%) | 0 (0%) | 0 | 0 | 4 | 0% | - |
| Preoperative MD (+) | 109 | 16 (15%) | 12 (11%) | 9 | 1 | 71 | 34% | 43% |
| Preoperative MD (-) | 229 | 4 (2%) | 12 (5%) | 20 | 1 | 192 | 16% | 75% |
| Overall | 338 | 20 (6%) | 24 (7%) | 29 | 2 | 263 | 22% | 55% |
Abbreviations: TcMEP, transcranial motor-evoked potential; TP, true-positive; FP, false-positive; FN, false-negative; TN, true-negative; MD, motor deficit; IMSCT, intramedullary spinal cord tumor; EMSCT, extra-medullary spinal cord tumor.
a Values are number of patients (%) unless otherwise indicated.
Discussion
New Onset Rates of MD During IMSCT and EMSCT Surgeries
The postoperative MD rates after IMSCT resection surgery were 7.4–43.7% in previous articles.2-4,7,10-13 Garces-Ambrossi et al., reported that 34% of patients had postoperative MDs after IMSCT resection surgery, and 41% of patients with postoperative MDs showed recovery of MDs within 1 month postoperatively. 4 Transient MD rate after IMSCT surgeries is understudied, but a few reports have shown that less than two-thirds of patients with postoperative MDs showed recovery of MDs within 6 weeks postoperatively. 4 Meanwhile, the postoperative MD rates after EMSCT resection surgery were 1.5–19.5% in previous articles.3,14-16 In this multicenter prospective study between 2017 and 2019, 25.9% and 6.3% of patients had postoperative MDs after IMSCT and EMSCT resection surgeries, and 6.2% and 1.4% of patients had persistent MDs, respectively. Altogether, among patients with MDs who received IMSCT and EMSCT resection surgeries, 76% and 78%, respectively, showed recovery of MDs within 3 months postoperatively. In this study, MD rates after IMSCT and EMSCT resection surgeries were similar to the rates in previous reports; however, our results showed relatively lower persistent MD rates compared to those of previous reports. Even with the use of IONM, SCT resection surgery is demanding and carries a significant risk of transient or permanent MDs. In contrast, we believe that the usage of TcMEP monitoring with high sensitivity and specificity and the intervention techniques used during SCT surgeries might have decreased the permanent MD rates.
Alert Criteria and Rescue Procedures During SCT Surgery
As patients with SCT resection surgery had various neurologic dysfunctions postoperatively, we routinely performed multimodal IONM, including TcMEP, SSEP, and free-run EMG monitoring. The efficacy of TcMEP monitoring during SCT resection surgery is controversial due to the presence of postoperative motor and/or sensory deficits. 17 Focusing on postoperative MDs as outcomes in this multicenter study, results of TcMEP monitoring during IMSCT and EMSCT surgeries showed high sensitivity and specificity (a sensitivity of 91–95% and a specificity of 80–90%) compared with those in a recent review article (a sensitivity of 84% and specificity of 83%). 2 As TcMEP alerts (an TcMEP amplitude reduction of ≥70% from baseline) gave us necessary information about the presence of damage to the spinal cord, we had to interpret these to perform appropriate intervention techniques. Its value varies depending on the timing of the alert and how the surgeon interprets the information during the resection surgery.
Patients with TcMEP alerts during SCT resection surgery undergo treatment using various kinds of intervention techniques, including suspension of surgery, warm saline irrigation of the surgical area, control of systematic hypotension, and/or usage of intravenous steroid injection for the recovery of TcMEP amplitude.18-20 Although facilitating the proper interventions after TcMEP alerts can prevent permanent MDs, that is, irreversible neural damage, only a few studies have evaluated intraoperative waveform recovery after interventions during SCT resection surgery.3,18,19 Yoshida et al., revealed that the alert timing and adequate interventions are important to prevent neurological complications. 3 Our results were in line with these findings. Among the EMSCT group, the overall rescue rate was 55%, and permanent MD rate was 1.4%. In contrast, among the IMSCT group, the overall rescue rate remained at only 29%, and the permanent MD rate was 6.2%. This showed that IMSCT resection was risky. At this timing, suspension of surgery with intravenous steroid injection was ineffective, and the rescue rate was 21%. Yoshida et al., stated that intervention techniques, including intravenous steroid injection or control of blood pressure, may need to be attempted prior to tumor resection to prevent further cord damage due to edema or ischemia after further spinal cord injury. 3 Kim et al., reported improvement in TcMEP waveform after intravenous steroid injection; however, the efficacy and the proper dosage of the steroids remain unclear. 21 These intervention techniques are not always appropriate for waveform recovery because we need to consider the FP alerts due to non-surgical factors, including anesthetic fade or systemic change.22,23 We paid attention to the management of anesthesia and confirmed the reproducibility of TcMEP alerts. We believe that the analysis and examination of the rescue cases, and found that the rates are important to improve surgical skills and rescue techniques.
Limitations
This study had several limitations. First, among multimodal IONM, only TcMEP monitoring was analyzed. Previous reports showed that multimodal IONM, including TcMEP, SSEP, D-wave, and free-run EMG, was essential for SCT resection surgery.18,24 In this prospective multicenter study, we only analyzed TcMEP monitoring and the presence of postoperative MDs to unify the methodology. Second, the cut-off of alert was unified as a TcMEP amplitude reduction ≥70% from baseline amplitude. Furthermore, the selection of evoked muscles was not standardized, and this may have resulted in an FP rate of approximately 9.1% and the inclusion of 3 FN patients, and we may need to reconsider the making the alert point to depend on each evoked muscle using a multi-stage monitoring alert strategy according to the pathology or level of SCT in future studies. 25 Finally, we included various surgical procedures for SCT resection surgery with multiple pathologies, and the results of TcMEP monitoring may vary depending on different surgical procedures and various pathologies. These heterogeneities may have affected the clinical outcomes. A multicenter prospective study with a larger number of patients is required to further clarify the characteristics of various alerts, diagnostic accuracy on multimodal IONM using wake-up test, and the proper intervention techniques during SCT resection surgeries.
Conclusions
The postoperative MD rates after IMSCT and EMSCT surgeries using TcMEP monitoring with high sensitivity and specificity were 25% and 6%, respectively, and the rescue rates were 29% and 55%, respectively. TcMEP monitoring is a highly sensitive and specific means of detecting neurological injury during IMSCT and EMSCT surgeries, and can be used in future trials of appropriate intervention techniques to minimize postoperative MD in patients with detected intraoperative injuries or TcMEP alerts.
Acknowledgments
Intraoperative monitoring was performed with the help of medical engineers and anesthesiologists in multiple centers. S. Dr. Matsuyama supervised the study. Dr. Yoshida was responsible for the study’s conception and design. Dr. Ushirozako acquired, analyzed, and interpreted data, drafted the article, and approved the final version on behalf of all authors. All authors have critically revised the article and reviewed the submitted version. We would like to thank Editage (www.editage.com) for English language editing.
Authors’ Note: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. All study participants provided informed consent, and the study protocol was approved by the Institutional Review Board of our university hospital (No. 19-146).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Hiroki Ushirozako, MD, PhD  https://orcid.org/0000-0002-7722-2205
https://orcid.org/0000-0002-7722-2205
Shiro Imagama, MD, PhD  https://orcid.org/0000-0003-1721-9626
https://orcid.org/0000-0003-1721-9626
Kazuyoshi Kobayashi, MD, PhD  https://orcid.org/0000-0003-1533-3476
https://orcid.org/0000-0003-1533-3476
Kei Ando, MD, PhD  https://orcid.org/0000-0002-1088-2903
https://orcid.org/0000-0002-1088-2903
Hideki Shigematsu, MD, PhD  https://orcid.org/0000-0002-0892-3382
https://orcid.org/0000-0002-0892-3382
References
- 1.Hohenberger C, Gugg C, Schmidt NO, Zeman F, Schebesch KM. Functional outcome after surgical treatment of spinal meningioma. J Clin Neurosci. 2020;77:62–66. doi:10.1016/j.jocn.2020.05.042 [DOI] [PubMed] [Google Scholar]
- 2.Rijs K, Klimek M, Scheltens-de Boer M, Biesheuvel K, Harhangi BS. Intraoperative neuromonitoring in patients with intramedullary spinal cord tumor: a systematic review, meta-analysis, and case series. World Neurosurg. 2019;125:498–510e2. doi:10.1016/j.wneu.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 3.Yoshida G, Ando M, Imagama S, et al. Alert timing and corresponding intervention with intraoperative spinal cord monitoring for high-risk spinal surgery. Spine (Phila Pa 1976). 2019;44(8):470–479. doi:10.1097/BRS.0000000000002900 [DOI] [PubMed] [Google Scholar]
- 4.Garces-Ambrossi GL, McGirt MJ, Mehta VA, et al. Factors associated with progression-free survival and long-term neurological outcome after resection of intramedullary spinal cord tumors: analysis of 101 consecutive cases. J Neurosurg Spine. 2009;11(5):591–599. doi:10.3171/2009.4.SPINE08159 [DOI] [PubMed] [Google Scholar]
- 5.Sutter M, Eggspuehler A, Grob D, et al. The validity of multimodal intraoperative monitoring (MIOM) in surgery of 109 spine and spinal cord tumors. Eur Spine J. 2007;16(Suppl 2):197–208. doi:10.1007/s00586-007-0422-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velayutham P, Rajshekhar V, Chacko AG, Krothapalli Babu S. Influence of tumor location and other variables on predictive value of intraoperative myogenic motor-evoked potentials in spinal cord tumor surgery. World Neurosurg. 2016;92:264–272. doi:10.1016/j.wneu.2016.04.117 [DOI] [PubMed] [Google Scholar]
- 7.Forster MT, Marquardt G, Seifert V, Szelenyi A. Spinal cord tumor surgery—importance of continuous intraoperative neurophysiological monitoring after tumor resection. Spine (Phila Pa 1976). 2012;37(16):1001–1008. doi:10.1097/BRS.0b013e31824c76a8 [DOI] [PubMed] [Google Scholar]
- 8.Ushirozako H, Yoshida G, Kobayashi S, et al. Impact of total propofol dose during spinal surgery: anesthetic fade on transcranial motor evoked potentials. J Neurosurg Spine. 2019:1–9. doi:10.3171/2018.10.SPINE18322 [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi S, Matsuyama Y, Shinomiya K, et al. A new alarm point of transcranial electrical stimulation motor evoked potentials for intraoperative spinal cord monitoring: a prospective multicenter study from the spinal cord monitoring working group of the Japanese society for spine surgery and related research. J Neurosurg Spine. 2014;20(1):102–107. doi:10.3171/2013.10.SPINE12944 [DOI] [PubMed] [Google Scholar]
- 10.Matsuyama Y, Sakai Y, Katayama Y, et al. Surgical results of intramedullary spinal cord tumor with spinal cord monitoring to guide extent of resection. J Neurosurg Spine. 2009;10(5):404–413. doi:10.3171/2009.2.SPINE08698 [DOI] [PubMed] [Google Scholar]
- 11.Muramoto A, Imagama S, Ito Z, et al. The cutoff amplitude of transcranial motor evoked potentials for transient postoperative motor deficits in intramedullary spinal cord tumor surgery. Spine (Phila Pa 1976). 2014;39(18):1086–1094. doi:10.1097/BRS.0000000000000421 [DOI] [PubMed] [Google Scholar]
- 12.Li TY, Chu JS, Xu YL, et al. Surgical strategies and outcomes of spinal ependymomas of different lengths: analysis of 210 patients: clinical article. J Neurosurg Spine. 2014;21(2):249–259. doi:10.3171/2014.3.SPINE13481 [DOI] [PubMed] [Google Scholar]
- 13.Tiruchelvarayan R, Tang M, Perera S, Lo Y. Outcomes following aggressive surgical resection of intra-medullary spinal cord tumours with intraoperative neuro-monitoring. Proc Singapore Healthc. 2013;22:183–190. [Google Scholar]
- 14.Rajshekhar V, Velayutham P, Joseph M, Babu KS. Factors predicting the feasibility of monitoring lower-limb muscle motor evoked potentials in patients undergoing excision of spinal cord tumors. J Neurosurg Spine. 2011;14(6):748–753. doi:10.3171/2011.1.SPINE10310 [DOI] [PubMed] [Google Scholar]
- 15.Gilard V, Goia A, Ferracci FX, et al. Spinal meningioma and factors predictive of post-operative deterioration. J Neurooncol. 2018;140(1):49–54. doi:10.1007/s11060-018-2929-y [DOI] [PubMed] [Google Scholar]
- 16.Safaee MM, Lyon R, Barbaro NM, et al. Neurological outcomes and surgical complications in 221 spinal nerve sheath tumors. J Neurosurg Spine. 2017;26(1):103–111. doi:10.3171/2016.5.SPINE15974 [DOI] [PubMed] [Google Scholar]
- 17.Lakomkin N, Mistry AM, Zuckerman SL, et al. Utility of intraoperative monitoring in the resection of spinal cord tumors: an analysis by tumor location and anatomical region. Spine (Phila Pa 1976). 2018;43(4):287–294. doi:10.1097/BRS.0000000000002300 [DOI] [PubMed] [Google Scholar]
- 18.Costa P, Peretta P, Faccani G. Relevance of intraoperative D wave in spine and spinal cord surgeries. Eur Spine J. 2013;22(4):840–848. doi:10.1007/s00586-012-2576-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyun SJ, Rhim SC. Combined motor and somatosensory evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in 17 consecutive procedures. Br J Neurosurg. 2009;23(4):393–400. doi:10.1080/02688690902964744 [DOI] [PubMed] [Google Scholar]
- 20.Sala F, Palandri G, Basso E, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery. 2006;58(6):1129–1143; discussion 1129-1143. doi:10.1227/01.NEU.0000215948.97195.58 [DOI] [PubMed] [Google Scholar]
- 21.Kim DG, Son YR, Park YS, et al. Differences in multimodality intraoperative neurophysiological monitoring changes between spinal intramedullary ependymoma and hemangioblastoma. J Clin Neurophysiol. 2016;33(2):120–126. doi:10.1097/WNP.0000000000000247 [DOI] [PubMed] [Google Scholar]
- 22.Ushirozako H, Yoshida G, Hasegawa T, et al. Characteristics of false-positive alerts on transcranial motor evoked potential monitoring during pediatric scoliosis and adult spinal deformity surgery: an “anesthetic fade” phenomenon. J Neurosurg Spine. 2019:1–9. doi:10.3171/2019.9.SPINE19814 [DOI] [PubMed] [Google Scholar]
- 23.Shigematsu H, Yoshida G, Kobayashi K, et al. Understanding the effect of non-surgical factors in a transcranial motor-evoked potential alert: a retrospective cohort study. J Orthop Sci. 2020;S0949-2658(20)30197-4. doi:10.1016/j.jos.2020.07.008 [DOI] [PubMed] [Google Scholar]
- 24.Sala F, Bricolo A, Faccioli F, Lanteri P, Gerosa M. Surgery for intramedullary spinal cord tumors: the role of intraoperative (neurophysiological) monitoring. Eur Spine J. 2007;16(Suppl 2):130–139. doi:10.1007/s00586-007-0423-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujiwara Y, Matsuyama Y, Kobayashi S, et al. Two stage alarm strategy for intramedullary spinal cord tumors based on the transcranial electrically stimulated muscle evoked potential monitoring: The JSSR prospective multi-center study. J Spine Res. 2016;7(9):1343–1351. [Google Scholar]