Highlights
-
•
SBRT fore rare oligoprogressive (70%)/oligorecurrent SCLC.
-
•
The median LC/DR/OS were NR, 4.5 and 17.2 months, respectively.
-
•
Initial LS associated with decreased post-SBRT OS and DR.
Keywords: Lung cancer, Stereotactic body radiotherapy (SBRT), Local radical treatment, Oligometastases
Abstract
Introduction
The role of local ablative treatments, including stereotactic body radiotherapy (SBRT), is an area of active research in oligometastatic patients. Small cell lung cancer (SCLC) has a poor prognosis, with common diffuse metastatic evolution. We evaluated the outcomes after SBRT in uncommon oligoprogressive/oligorecurrent SCLC presentation.
Methods
Data of SCLC patients who received SBRT for oligoprogressive/oligorecurrent metastatic disease at four centers were retrospectively analyzed. Patients with synchronous oligometastatic disease, SBRT for primary lung tumor and brain radiosurgery were not included. Relapse and survival rates were defined as the time between the date of SBRT and the first event.
Results
Twenty patients (60% with initially limited-disease [LD]) presenting 24 lesions were identified. Oligoprogression and oligorecurrence were observed in 6/20 (30%) and 14/20 (70%) patients, respectively. SBRT was delivered to one (n = 16) to two (n = 4) lesions (median size, 26 mm), mainly to lung [n = 17/24] metastases. At a median follow-up of 2.9 years, no local relapse was observed and 15/20 patients experienced a distant relapse (DR). The median DR and OS were 4.5 months (95 %CI: 2.9–13.7 months) and 17.2 months (95 %CI: 7.5–65.2 months), respectively. The 3-year distant control and OS rates were 25% (95 %CI: 6–44%) and 37% (95 %CI: 15–59%), respectively. Initial LD (vs extensive-disease) was the only prognosis factor associated with a lower risk of post-SBRT DR (HR: 0.3; 95% CI: 0–0.88; p = 0.03). There was no severe observed SBRT-related toxicities.
Conclusion
Prognosis was poor, with DR occurring in most patients. However, local control was excellent and long term response after SBRT may rarely occur in patients with oligoprogressive/oligorecurrent SCLC. Local ablative treatments should be discussed in a multidisciplinary setting on well-selected cases.
Introduction
Metastatic small-cell lung cancer (SCLC) patients account for two thirds of initial presentation and display a poor prognosis. Despite recent advances with the incorporation of newer systemic treatments delivered in first (anti-PD-L1 to platinum-based doublet) or second line (lurbinectedin), the median survival is respectively of one year and of six months [1]. Several newer compounds are currently being tested but current standard systemic treatment options remain limited [1]. If polymetastatic evolution is the most frequent pattern of relapse in SCLC, there are some rare cases of limited “oligometastatic” recurrence or progression.
The oligometastatic disease concept suggests that patients with a limited number of metastases have a favorable prognosis [2]. Recent prospective trials reported clinical improvements with the addition of ablative local treatments (mainly stereotactic body radiotherapy [SBRT]) in oligometastatic patients [3]. There have also been recent efforts in refining the definition/classification of the oligometastatic disease, including for non-small cell lung cancer (NSCLC) [4], [5], [6], [7], [8]. Several phase II/III trials are ongoing, including oligoprogressive (secondary resistance while receiving systemic treatment; e.g. HALT [NCT03256981] and CURB [NCT03808662] trials) and oligorecurrent presentations (e.g. in various histologies: SABR-COMET-3 [NCT03862911], and for rare histologies: EORTC-1945 OLIGO-RARE [NCT04498767] trials). However, metastatic SCLC are not included in the trials because of high recurrence rates and poor outcomes overall. Stereotactic cranial radiosurgery (SRS) has been more often proposed in SCLC patients with limited brain progression with randomized trials ongoing in this setting [9]. In uncommon cases of SCLC patients showing extra-cerebral oligometastatic recurrence, SBRT might also be discussed. This work aimed at evaluating the role of SBRT as a possible therapeutic option in SCLC with oligoprogressive/oligorecurrent presentations.
Patients and methods
Oligometastatic disease definitions were derived from prior consensus [4], [5], [6]. Patients eligible in this retrospective multicentre analysis included all patients with histologically confirmed limited- (LD) or extensive-disease (ED) SCLC showing an oligoprogressive (i.e. while on active systemic treatment) or oligorecurrent (i.e. no active systemic therapy) disease (limited number of radically treatable lesions) [4], [5], [6], [7], [8]. A biopsy was recommended but not mandatory at the time of progression. In the subset of patients with oligoprogression, SBRT was delivered to the site(s) of progression with continuation of the same systemic therapy (no washout if chemotherapy or immunotherapy). Baseline characteristics were determined by retrospective collection after ethical approval by the local Institutional Review Boards and all patients had initial staging including a whole body computed tomography (CT). Synchronous oligometastatic disease, SBRT for primary lung tumor and SRS for isolated brain oligometastatic diseasepatients were not included in this analysis. Patient with prior history of treated brain metastases were included. Patients with poorly diffuse serosal metastases, more than three sites of extra-cerebral progression, poor performance status (Eastern Cooperative Oncology Group performance status [PS] > 2) were not eligible to SBRT. SBRT was approved for all patients at the multidisciplinary tumor board (MTB). SBRT was performed as per international guidelines recommendations (including immobilisation system, image guidance and respiration management) and the total dose was delivered to the planning target volume (PTV) according a risk-adapted approach. All patients had a baseline whole body CT, and 18-F-fluorodeoxyglucose positron emission tomography (FDG-PET) and brain magnetic resonance imaging (MRI) were strongly encouraged. All patients had a whole body CT-scan 3 months after SBRT.
The primary endpoint was subsequent distant relapse (DR) after SBRT. Secondary endpoints were: Secondary endpoints were: local relapse, progression-free survival (PFS), overall survival (OS), and toxicity. Response was evaluated according to RECIST 1.1. SBRT-related toxicity was graded according to NCI-CTC v5.0. Follow-up was estimated using the reverse Kaplan–Meier method. OS and distant control rates were estimated using the Kaplan–Meier method. Events were defined as the time between the beginning of SBRT and the first event. Survival curves were compared using the log-rank test for the univariate analysis. Statistical analyses were performed using SPSS software, version 29 (SPSS Inc., USA). All reported p values are two-sided, and p values less than 0.05 were considered significant.
Results
Twenty patients presenting 24 treated lesions were identified from 19/10/2021 to 07/03/2022 at four Centers (Table 1). Most patients were male (60%; n = 12/20), the median age was 67.7 years (range, 43.1–76.4 years) and all patients were long-term smokers (median of 40 pack year, range 20–80; and 14/20 were former smokers). Tumor initial presentation (Time period of initial diagnoses: 2010–2019) consisted of LD for 12/20 (60%) patients and two of those patients (2/12) had a subsequent polymetastatic relapse before induced oligoprogression/oligorecurrence. All patients received prior initial standard treatments (LD: thoracic chemoradiotherapy [n = 12/12] +/- prophylactic cranial irradiation [PCI, n = 10/12]; ED: etoposide-platinum-based doublet chemotherapy [n = 8/8, only one received a combination of chemotherapy and immunotherapy] +/- PCI [n = 1] +/- palliative thoracic irradiation [n = 2]). Subsequent treatments before oligoprogression/oligorecurrence presentation included whole brain radiotherapy (WBRT, n = 5); SRS (n = 1) and systemic treatments (n = 7, median number of one line; range, 1–3 lines). One patient also received local ablative treatments (adrenal thermal ablation and lung surgery) for a prior oligorecurrence (repeat oligometastatic scenario).
Table 1.
Baseline SCLC patient clinical characteristics (n = 20) and lesions (n = 24) treatments.
| n (%) | |
|---|---|
| Median age (years, range) | 67.7 (43.1–76.4) |
| Gender | |
| Male | 12 (60) |
| Female | 8 (40) |
| Initial stage | |
| Limited | 12 (60) |
| Extensive | 8 (40) |
| Prior treatments | |
| Thoracic chemoradiotherapy | 12 (60) |
| PCI | 11 (55) |
| First line doublet-chemotherapy | 8 (40) |
| Chemo-immunotherapy | 1 (5) |
| Palliative thoracic irradiation | 2 (10) |
| WBRT | 5 (25) |
| Subsequent line(s) of chemotherapy | 7 (35) |
| Brain SRS | 1 (5) |
| Local ablative treatments | 1 (5) |
| Type of oligometastatic disease | |
| Oligoprogression | 6 (30) |
| Oligorecurrence | 14 (70) |
| ECOG PS before SBRT | |
| 0 | 4 (20) |
| 1 | 16 (80) |
| SBRT (n = 24 lesions) | |
| Median dose (Gy, range) | 48 (30–60) |
| Median EQD2 (Gy, range) | 83.3 (40–110) |
| Median number of fractions (range) | 5 (3–10) |
| Median duration (days, range) | 9 (3–17) |
| Median lesion size (mm, range) | 26 (7–57) |
| Techniques | |
| Static 3D (4D-CT, free breathing) | 10 (42) |
| Static 3D (DIBH) | 2 (8) |
| VMAT (4D-CT, free breathing) | 7 (29) |
| Tracking CyberKnife® | 5 (21) |
| Locations | |
| Lung | 17 (71) |
| Adrenal | 5 (21) |
| Spine | 1 (4) |
| Pancreas | 1 (4) |
SCLC, Small cell lung cancer; PCI, prophylactic cerebral irradiation; WBRT, whole brain radiation therapy; SBRT, stereotactic body radiation therapy; SRS, stereotactic radiosurgery; EQD2: equivalent dose in 2-Gy fractions; 4D-CT, four-dimension computed-tomography; DIBH, Deep inspiration breath hold.
Oligoprogression and oligorecurrence were observed in 6/20 (30%) and 14/20 (70%) patients, respectively. This consisted of the de-novo metachronous metastatic relapse for 10/12 LD patients (median time from the end of initial standard treatment: 1.7 years, range 0.7–4.4 years). For the 10 remaining patients with prior metastatic diagnosis, the oligometastatic presentation occurred at a median time of 12 months (range, 3.7–22.2 months) after initial metastatic diagnosis.
Before SBRT, ECOG PS was 0 (4/20) or 1 (n = 16/20). A biopsy of the irradiated target was performed in 4 patients (3 lung lesions and one bone lesion). SBRT (Time period of SBRT: 2013–2021) was delivered to 24 metastatic lesions (mostly one site per patient: 16/20 = 80%; two lesions for 4/20 patients), at a median dose of 48 Gy (range, 30–60 Gy; median equivalent dose in 2-Gy fractions [EQD2]: 83.3 Gy [range, 40–110 Gy]) in 5 fractions (range, 3–10 fractions; median duration of 9 days, range 3–17 days). The median lesion size was 26 mm (range, 7–57 mm) and mostly located in the lung (n = 17/24; 71%) followed by adrenal (n = 5/24), spine (n = 1) and pancreas (n = 1). Image-guided SBRT techniques included static 3D (free breathing or deep inspiration breath hold) or VMAT (free breathing) beams and Tracking CyberKnife® for 12/24 (50%) or 7/24 (29%) and 5/24 (21%) lesions, respectively.
The best local responses according to RECIST 1.1 criteria were: partial (n = 17/24; 71%) or complete response (n = 3/24) and stable disease (n = 4/24). Pre- and post-SBRT FDG-PET were performed in 11/20 (13/24 lesions) and 7/20 patients (7/24 lesions), respectively. Among 6/20 patients with both pre- and post-FDG-PET available data, the irradiated lesions SUVmax median value decreased was of 59% (range, 44–100%).
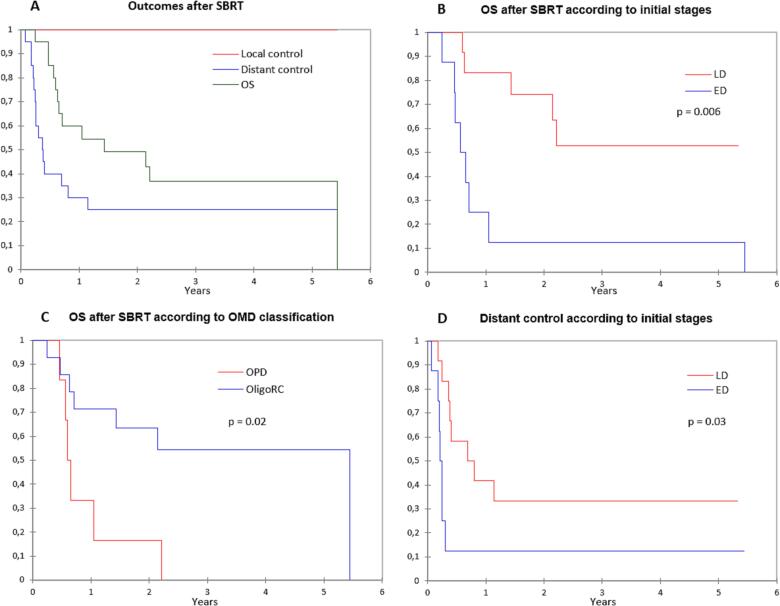
At a median follow-up of 2.9 years (95% CI: 2.3–5.3 years), seven patients remained alive. All other patients died from disease evolution, except one from another cause (traumatic brain injury). No local relapse was observed and 5/20 patients did not demonstrated subsequent distant relapse (DR). The median DR and OS were 4.5 months (95% CI: 2.9–13.7 months) and 17.2 months (95% CI: 7.5–65.2 months), respectively (Fig. 1A). The 3-year OS, PFS and distant control rates were 37% (95% CI: 15–59%), 25% (95% CI: 6–44%) and 25% (95% CI: 6–44%), respectively.
Fig. 1.
(A) Outcomes from SBRT for oligoprogressive/oligorecurrent SCLC. (B) Overall survival according to initial stages (C) and (D) to oligoprogression/oligorecurrence presentation Distant control according to initial stages.
Abbreviation: SBRT, stereotactic body radiation therapy; OS, overall survival; OPD, oligoprogressive disease; OligoRC, Oligorecurrent; LD, limited disease; ED, extensive disease; OMD, oligometastatic disease.
Patient with initial LD-SCLC (vs ED-SCLC; HR: 0.2; 95% CI: 0–0.7; p = 0.006; Fig. 1B), oligorecurrence (vs oligoprogression; HR: 0.3; 95% CI: 0–0.8; p = 0.02;; Fig. 1C) and non-systemic metastasis location (i.e. lung and/or prior brain vs systemic location, HR: 0.3; 95% CI: 0–0.95; p = 0.04) had a longer post-SBRT OS (HR: 0.2; 95% CI: 0–0.7; p = 0.01; Table 2). Age, gender, smoking status, the number of prior lines of treatment, prior WBRT, ECOG PS, and center of treatment did not correlate with OS. Patient with initial LD-SCLC (vs ED-SCLC) had a lower risk of post-SBRT DR (HR: 0.3; 95% CI: 0–0.88; p = 0.03; Fig. 1D). Although not significant, patients with oligorecurrent disease (vs oligoprogression; p = 0.1), longer diagnosis-oligometastatic presentation delay (>1years vs less than 1 year, p = 0.2) and systemic metastasis location (vs lung and/or history of prior brain location, p = 0.1) had lower DR rates. Age, gender, smoking status, the number of prior lines of treatment, prior WBRT, ECOG PS, and center of treatment did not correlate with DR occurrence (Table 2). There was no observed severe SBRT-related toxicities (Grade 1 chest wall pain; n = 1; G2 hemoptysis: n = 1).
Table 2.
Univariate analyses for overall survival and distant relapse.
|
Death from SBRT |
Distant relapse from SBRT |
|||
|---|---|---|---|---|
|
Univariate |
Univariate |
|||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| LD (vs ED) | 0.2 (0–0.7) | 0.006 | 0.3 (0–0.9) | 0.03 |
| Oligorecurrent (vs OPD) | 0.3 (0–0.8) | 0.02 | 0.5 (0.2–1.3) | 0.1 |
| Lungs or prior brain mets (vs systemic mets) | 0.3 (0–0.95) | 0.04 | 0.5 (0.2–1.3) | 0.1 |
| Diagnosis to OMD delay > 1 year (vs < 1 year) | 0.1 (0.1–1.2) | 0.1 | 0.5 (0.2–1.5) | 0.2 |
LD, limited disease as initial presentation; ED, extensive disease as initial presentation; OPD, oligoprogressive disease; mets, metastases; OMD, oligometastatic disease; SBRT, stereotactic body radiation therapy; HR, hazard ratio.
Discussion
SCLC is an aggressive disease and therefore, a limited “oligometastatic” extra-cranial relapse presentation is rare. Radical local treatment of oligometastatic patients has increased given developments in imaging (mainly FDG-PET and brain MRI) and access to effective and better-tolerated treatments such as SBRT. Few randomized phase 2 data have been reported in NSCLC [3]. The SABR-COMET phase 2 trial included oligometastatic patients (but only 18% (n = 18) NSCLC) and showed an OS advantage with SBRT (50 months vs 28 months in the control arm) [10]. However, SCLC patients are generally not included in such trials.
There are some reports suggesting that ED-synchronous oligometastatic SCLC have better outcomes as compared to polymetastatic presentation [11], [12]. There has been a randomized phase II study on oligometastatic SCLC where consolidative conformal radiation therapy (45 Gy in 15 fractions both to locoregional disease and residual metastases after chemotherapy) delayed time to progression (HR = 0.53) [13]. For patients with a single brain metastasis, data from a large, international, retrospective study reported an encouraging median OS of 11 months after SRS [9], [14]. There is however no such data available for extra-cranial SBRT. To our knowledge, our work is the first describing SBRT results for oligometastatic progression/recurrence in SCLC patients. In our analysis, the prognosis appeared encouraging (3-year OS: 37%, median OS: 17.2 months), with however disease recurrence occurring in most patients (3-year distant control and PFS: 25%). However, expected OS in this situation is around 6 months, highlighting a potential benefit of this strategy, although in highly selected patients. Local control was excellent and long-term response after SBRT occurred, with 6/20 patients still alive more than two years after SBRT. In two recent analyses assessing SBRT in oligoprogressive/oligo-resistant NSCLC patients receiving anti-PD-(L)1, the median OS was doubled (∼36 months) as compared to our series but the proportion of progression-free patients>2 years after local therapy was also less than 30% [15], [16].
Interpretation of this retrospective work is limited given the small sample size, and the absence of systematic FDG-PET and histological proof. The population is also heterogeneous because it included a mix of initial stages and various oligometastatic scenarios (induced/de-novo/repeat) [4]. SCLC transformation of NSCLC may also occur [17] but the opposite is generally exceptional. Another primary lung cancer occurrence may also exist, but most often after years of follow-up. It should also be highlighted that immunotherapy was not widely adopted at the time of our analysis or earlier reports [11] and that further synergistic effects with SBRT could hopefully happen [18], [19].
Identifying patients that will not ultimately develop an aggressive polymetastatic disease is crucial to propose such local ablative strategy. Here, only patients with initial LD-SCLC (vs ED-SCLC) had a lower risk of post-SBRT DR (HR: 0.3; p = 0.03). More insight in SCLC biology and the integration of liquid biopsies (molecular profiling, molecular residual disease…) could be of interest in selecting patients for personalized strategies [20], [21]. Meanwhile, local ablative treatments should be discussed in a multidisciplinary setting on well-selected cases.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
Footnotes
This work was presented at ESTRO 2022, Copenhagen, Denmark.
References
- 1.Zugazagoitia J., Paz-Ares L. Extensive-Stage Small-Cell Lung Cancer: First-Line and Second-Line Treatment Options. J Clin Oncol. 2022;40(6):671–680. doi: 10.1200/JCO.21.01881. [DOI] [PubMed] [Google Scholar]
- 2.Weichselbaum R.R. The 46th David A. Karnofsky Memorial Award Lecture: Oligometastasis From Conception to Treatment. J Clin Oncol. 2018;36:32403250. doi: 10.1200/JCO.18.00847. [DOI] [PubMed] [Google Scholar]
- 3.Jasper K., Stiles B., McDonald F., Palma D.A. Practical Management of Oligometastatic Non-Small-Cell Lung Cancer. J Clin Oncol. 2022;40(6):635–641. doi: 10.1200/JCO.21.01719. [DOI] [PubMed] [Google Scholar]
- 4.Guckenberger M., Lievens Y., Bouma A.B., Collette L., Dekker A., deSouza N.M., et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21(1):e18–e28. doi: 10.1016/S1470-2045(19)30718-1. [DOI] [PubMed] [Google Scholar]
- 5.Dingemans A.M.C., Hendriks L.E.L., Berghmans T., Levy A., Hasan B., Faivre-Finn C., et al. Consensus paper from thoracic oncology experts about the definition of synchronous oligometastatic nonsmall cell lung cancer. J Thorac Oncol. 2019 doi: 10.1016/j.jtho.2019.07.025. pii: S15560864(19)306550. [DOI] [PubMed] [Google Scholar]
- 6.Levy A., Hendriks L.E.L., Berghmans T., Faivre-Finn C., GiajLevra M., GiajLevra N., et al. EORTC Lung Cancer Group (EORTC LCG). EORTC Lung Cancer Group survey on the definition of NSCLC synchronous oligometastatic disease. Eur J Cancer. 2019;122:109–114. doi: 10.1016/j.ejca.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Hendriks L.E.L., Dooms C., Berghmans T., Novello S., Levy A., De Ruysscher D., et al. European Organisation for Research and Treatment of Cancer (EORTC) Lung Cancer Group. Defining oligometastatic non-small cell lung cancer: A simulated multidisciplinary expert opinion. Eur J Cancer. 2019;123:28–35. doi: 10.1016/j.ejca.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Giaj-Levra N., Giaj-Levra M., Durieux V., Novello S., Besse B., Hasan B., et al. European Organization for Research and Treatment of Cancer-Lung Cancer Group. Defining Synchronous Oligometastatic Non-Small Cell Lung Cancer: A Systematic Review. J Thorac Oncol. 2019;14(12):2053–2061. doi: 10.1016/j.jtho.2019.05.037. [DOI] [PubMed] [Google Scholar]
- 9.Crockett C., Belderbos J., Levy A., McDonald F., Le Péchoux C., Faivre-Finn C. Prophylactic cranial irradiation (PCI), hippocampal avoidance (HA) whole brain radiotherapy (WBRT) and stereotactic radiosurgery (SRS) in small cell lung cancer (SCLC): Where do we stand? Lung Cancer. 2021;162:96–105. doi: 10.1016/j.lungcan.2021.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Harrow S, Palma DA, Olson R, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic Radiation for the Comprehensive Treatment of Oligometastases (SABR-COMET): Extended Long-Term Outcomes. Int J Radiat Oncol Biol Phys. 2022 26:S0360-3016(22)00412-6. Epub ahead of print. [DOI] [PubMed]
- 11.Xu L.-M., Cheng C., Kang M., Luo J., Gong L.-L., Pang Q.-S., et al. Thoracic radiotherapy (TRT) improved survival in both oligo- and polymetastatic extensive stage small cell lung cancer. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-09775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirasawa M., Fukui T., Kusuhara S., Harada S., Nishinarita N., Hiyoshi Y., et al. Prognostic differences between oligometastatic and polymetastatic extensive disease-small cell lung cancer. PLoS One. 2019;14(4):e0214599. doi: 10.1371/journal.pone.0214599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gore E.M., Hu C., Sun A.Y., Grimm D.F., Ramalingam S.S., Dunlap N.E., et al. Randomized Phase II Study Comparing Prophylactic Cranial Irradiation Alone to Prophylactic Cranial Irradiation and Consolidative Extracranial Irradiation for Extensive-Disease Small Cell Lung Cancer (ED SCLC): NRG Oncology RTOG 0937. J Thorac Oncol. 2017;12(10):1561–1570. doi: 10.1016/j.jtho.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rusthoven C.G., Yamamoto M., Bernhardt D., Smith D.E., Gao D., Serizawa T., et al. Evaluation of First-line Radiosurgery vs Whole-Brain Radiotherapy for Small Cell Lung Cancer Brain Metastases: The FIRE-SCLC Cohort Study. JAMA Oncol. 2020;6(7):1028. doi: 10.1001/jamaoncol.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoenfeld AJ, Rizvi HA, Memon D, Shaverdian N, Bott MJ, Sauter JL, Tsai CJ, Lihm J, Hoyos D, Plodkowski AJ, Perez-Johnston R, Sawan P, Egger JV, Greenbaum BD, Rimner A, Riely GJ, Rudin CM, Rusch VW, Gomez DR, Hellmann MD. Systemic and Oligo-Acquired Resistance to PD-(L)1 Blockade in Lung Cancer. Clin Cancer Res. 2022;28:3797-3803. [DOI] [PMC free article] [PubMed]
- 16.Chicas-Sett R, Zafra J, Rodriguez-Abreu D, Castilla-Martinez J, Benitez G, Salas B, Hernandez S, Lloret M, Onieva JL, Barragan I, Lara PC. Combination of SABR With Anti-PD-1 in Oligoprogressive Non-Small Cell Lung Cancer and Melanoma: Results of a Prospective Multicenter Observational Study. Int J Radiat Oncol Biol Phys. 18:S0360-3016(22)00421-7. [DOI] [PubMed]
- 17.Pignataro D., Bertaglia V., Bironzo P., Olmetto E., Pisano C., Napoli V.M., et al. Oligoprogressive Disease With SCLC Transformation in EGFR-Mutated NSCLC: How Biology Knowledge Can Change the Game Rules. J Thorac Oncol. 2020;15(10):e170–e172. doi: 10.1016/j.jtho.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Doyen J., Besse B., Texier M., Bonnet N., Levy A. PD-1 iNhibitor and chemotherapy with concurrent IRradiation at VAried tumor sites in advanced Non-small cell lung cAncer: the Prospective Randomized Phase 3 NIRVANA-Lung Trial. Clin Lung Cancer. 2022;23(3):e252–e256. doi: 10.1016/j.cllc.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Remon J., Menis J., Levy A., De Ruysscher D.K.M., Hendriks L.E.L. How to optimize the incorporation of immunotherapy in trials for oligometastatic non-small cell lung cancer: a narrative review. Transl Lung Cancer Res. 2021;10(7):3486–3502. doi: 10.21037/tlcr-20-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maynard A., McCoach C.E., Rotow J.K., Harris L., Haderk F., Kerr D.L., et al. Therapy-Induced Evolution of Human Lung Cancer Revealed by Single-Cell RNA Sequencing. Cell. 2020;182(5):1232–1251.e22. doi: 10.1016/j.cell.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohan S., Foy V., Ayub M., Leong H.S., Schofield P., Sahoo S., et al. Profiling of Circulating Free DNA Using Targeted and Genome-wide Sequencing in Patients with SCLC. J Thorac Oncol. 2020;15(2):216–230. doi: 10.1016/j.jtho.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]