Abstract
Inhaled nitric oxide (iNO) acts as a selective pulmonary vasodilator and it is currently approved by the FDA for the treatment of persistent pulmonary hypertension of the newborn. iNO has been demonstrated to effectively decrease pulmonary artery pressure and improve oxygenation, while decreasing extracorporeal life support use in hypoxic newborns affected by persistent pulmonary hypertension. Also, iNO seems a safe treatment with limited side effects. Despite the promising beneficial effects of NO in the preclinical literature, there is still a lack of high quality evidence for the use of iNO in clinical settings. A variety of clinical applications have been suggested in and out of the critical care environment, aiming to use iNO in respiratory failure and pulmonary hypertension of adults or as a preventative measure of hemolysis-induced vasoconstriction, ischemia/reperfusion injury and as a potential treatment of renal failure associated with cardiopulmonary bypass. In this narrative review we aim to present a comprehensive summary of the potential use of iNO in several clinical conditions with its suggested benefits, including its recent application in the scenario of the COVID-19 pandemic. Randomized controlled trials, meta-analyses, guidelines, observational studies and case-series were reported and the main findings summarized. Furthermore, we will describe the toxicity profile of NO and discuss an innovative proposed strategy to produce iNO. Overall, iNO exhibits a wide range of potential clinical benefits, that certainly warrants further efforts with randomized clinical trials to determine specific therapeutic roles of iNO.
Keywords: Nitric oxide, Critically ill, Clinical applications, Pulmonary hypertension, Ischemia/reperfusion injury, Toxicology, COVID-19
1. Introduction
Nitric oxide (NO) is an endogenous molecule produced by Nitric Oxide Synthases (NOS), through the oxidation of L-citrulline and L-arginine [1]. It is secreted tonically and in response to shear stress by healthy endothelial cells, where it acts as a major mediator of vascular relaxation [2]. This effect is determined by the NO-mediated activation of soluble guanylate cyclase, which catalyzes the formation of the second messenger cyclic guanosine monophosphate and eventually leads to vasodilation [3]. In addition, NO reduces smooth muscle cells proliferation [4], platelet aggregation [5] and endothelial leukocyte binding [6]. Consequently, it exerts a protective role on blood vessels and participates in vascular homeostasis [7]. NO bioavailability may be decreased, for example, by L-arginine deficiency and depletion of tetrahydrobiopterin, an NOS cofactor [8]. This determines a disproportion between antiproliferative, antithrombotic and vasodilatory effects and proliferative, prothrombotic and vasoconstrictive substances. This imbalance contributes to a pathological condition known as endothelial dysfunction, which has been involved in the pathogenesis of cardiovascular diseases [9].
As a therapeutic agent, NO is available as inhaled NO (iNO) or carried by NO donors, like sodium nitroprusside or organic nitrates administered via parenteral route [10]. iNO is a selective pulmonary vasodilator which exhibited no systemic vasodilating effects [11], even when inhaled at concentrations as high as 160 ppm [12], while, on the contrary, NO donors induce both pulmonary and systemic blood vessels relaxation [10]. Currently, iNO requires storage in tanks and its use is limited to short period treatment and inpatient settings.
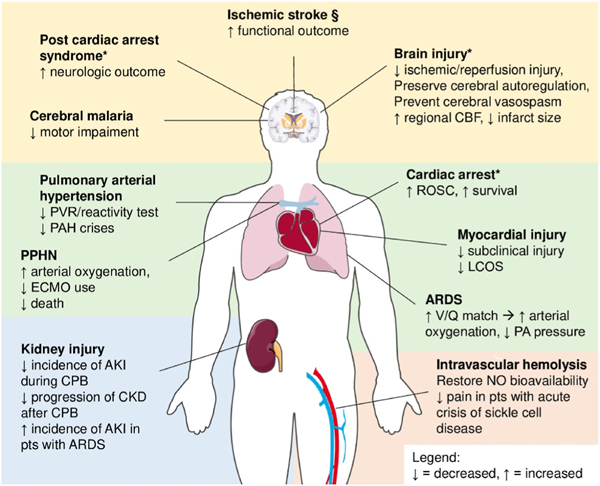
At present, the only indication for iNO use approved by the Food and Drug Administration (FDA) is the treatment of persistent pulmonary hypertension of newborns (PPHN) [13]. Along with this indication, iNO has been considered as a potential treatment for other diseases, ranging from the pulmonary arterial hypertension (PAH) of the adults [14] to the acute respiratory distress syndrome (ARDS) [15]. Moreover, recent data investigated the use of iNO to prevent hemolysis-induced vasoconstriction [16], decrease the ischemia/reperfusion injury [17] and prevent the renal failure associated with cardiopulmonary bypass (CPB) [18]. In this review, we aim to describe the current evidence on the use of iNO in these various clinical situations, along with its toxicity profile. Moreover, a novel strategy to produce iNO will be discussed. A summary of the performed and ongoing randomized controlled trials (RCTs) administering NO therapy is presented in Table 1 and Table 2, respectively. A concise representation of the potential benefits of NO in humans is depicted in Fig. 1.
Table 1.
Randomized controlled trials on nitric oxide administration for the treatment of various clinical conditions.
| Trial | Aim/hypothesis | Population (N) | Treatment group | Control group | NO dose | Primary endpoint | Main results |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Respiratory failure | |||||||
|
| |||||||
| Wu, 2016 [19] | Efficacy in PPHN | Newborns (86) | iNO + HFOV | HFOV | 20–80 ppm | PAP, FiO2, OI, duration of MV and O2 therapy, mortality | All endpoints better in the iNO than in control group |
| Bronicki, 2015 [20] | iNO ↓ MV duration in HRF | Pediatric (55) | iNO until death or 28 d or ventilator free | Placebo until death or 28 d or ventilator free | 5 ppm | VFD at 28 d | VFD greater in the iNO group (p = 0.05) |
| González, 2010 [21] | Early iNO prevents severe HRF in moderate HRF and PAH | Newborns (56) | iNO + conventional MV | Conventional MV | 20 ppm | Rate of progression to OI > 40 | 25% iNO vs 61% control group (p < 0.05) |
| Su, 2008 [22] | iNO ↓ OI in HRF | Preterm infants (65) | iNO + conventional care | Conventional care | 5–20 ppm | Mean OI at 24 h | OI ↓ in the iNO group (p < 0.01) |
| Dani, 2007 [23] | iNO ↓ BPD and/or death in HRF | Preterm infants (40) | iNO at 10 ppm for 4 h, then 6 ppm until extubation or FiO2 <30% w/MAP <8 cm H2O + conventional care | Conventional care | 6–10 ppm | BPD incidence and death | 50% iNO vs 90% control group (p = 0.016) |
| Lindwall, 2005 [24] | Effect of iNO on oxygenation in HRF | Preterm infants (15) | iNO for 30 min + nasal CPAP | Nasal CPAP + placebo | 10 ppm | Changes in oxygenation measured as aAPO2 | aAPO2 ↑ 20% in the iNO group (p = 0.006) |
| Schreiber, 2003 [25] | iNO ↓ incidence of chronic lung disease/ death | Preterm infants (207) | iNO 10 ppm day 1, then 5 ppm for 6 d | Placebo | 5–10 ppm | Incidence of chronic lung disease/death | 48,6% iNO vs 63.7% control group (p = 0.03) |
| Sadiq, 2003 [26] | iNO prevents worsening of PAH in PPHN | near-term and term infants (80) | iNO + conventional care | Conventional care | Up to 80 ppm | Development of severe PPHN | 15% iNO vs 58% control group (p < 0.0005) |
| Srisuparp, 2002 [27] | Safety and effect of iNO on oxygenation in mild to moderate HRF | Preterm infants (34) | iNO 20 ppm, then weaned to 5 ppm over 1–2 d | Conventional care | 5–20 ppm | IVH incidence, OI, paO2 | No differences in IVH incidence between groups. OI ↓ 15% and paO2 ↑ by 50% in iNO group (p = 0.04 and 0.02, respectively) |
| Baxter, 2002 [28] | Efficacy of iNO on oxygenation, shunt and PVRI in HRF | Adults (14) | iNO + 100% O2 for 30 min, then 30 min wash-out, then no iNO + 100% O2 for 30 min. | Randomized cross-over | 5–25 ppm | Shunt, PVRI, oxygenation | iNO ↓ pulmonary shunt (p = 0.002). Other endpoints: ns |
| Christou, 2000 [29] | iNO ↓ mortality and ↓ ECMO use in PPHN | near-term and term newborns (40) | iNO + HFOV | HFOV | Up to 40 ppm | Mortality, ECMO use | ECMO use: 14% iNO vs 55% control group (p = 0.007), Mortality: ns. |
| Clark, 2000 [30] | Low-dose iNO ↓ ECMO use in PPHN | near-term and term newborns (248) | iNO at 20 ppm up to 1 d, then 5 ppm up to 4 d | Placebo | 5–20 ppm | ECMO use | ECMO use: 38% iNO vs 64% control group (p = 0.001) |
| The Franco- Belgium Collaborative NO Trail Group, 1999 [31] | iNO ↑ oxygenation in HRF | preterm and near-term newborns (204) | iNO | Placebo | 10 ppm | OI change at 2 h | OI ↓ 6.2 iNO vs 2.9 control group (p = 0.005) |
| Dobyns, 1999 [32] | iNO effect on oxygenation in HRF | Children (108) | iNO for minimum 3 d + MV | MV | 10 ppm | OI | OI ↓ 10.2 iNO vs 2.7 control group (p < 0.05) |
| Troncy, 1998 [33] | iNO effect on lung function in ARDS | Adults (30) | iNO + conventional care | Conventional care | 0.5–40 ppm | P/F, alveolar dead space, lung compliance, venous admixture | P/F ↑ 59% in INO vs 9.3% control group (p = 0.02). Other endpoints: ns |
| Michael, 1998 [34] | iNO effect on oxygenation in ARDS | Adults (40) | iNO + conventional care up to 3 d | Conventional care | 5–20 ppm | P/F | P/F t in the first 24 h in the iNO group. |
| Dellinger, 1998 [35] | iNO effect on oxygenation in ARDS | Adults (117) | iNO | Placebo | 1.25–80 ppm | paO2 ↑ >20% | ~60% responders in iNO vs 24% control group in the first 4 h (p ≤ 0.021) |
| The NINOS Group, 1997 [36] | iNO ↓ ECMO use and/ or death in HRF | near-term and term newborns (235) | iNO | 100% O2 | 20 ppm | ECMO use and/or death | 64% iNO vs 46% vs control group (p = 0.006) |
| Wessel, 1997 [37] | iNO effect on mortality, ECMO use and oxygenation in PPHN | near-term and term Newborns (49) | iNO + conventional care | Conventional care | 5–80 ppm | Mortality, ECMO use, OI | OI ↓ 31% iNO vs control group.Other endpoints: ns |
| Roberts Jr, 1997 [38] | iNO effect on oxygenation in PPHN | Term infants (58) | iNO + conventional care | Placebo + conventional care | 80 ppm | Doubling rate of oxygenation | 53% iNO vs 7% control group (p = 0.002) |
| NCT00240487 | Determine iNO treatment timing in pediatric ARDS | Children (52) | iNO for the first 4 h, then no iNO for 4 h | No iNO for the first 4 h, then iNO for 4 h | 10 ppm | Changes in P/F ratio | ns |
| Van Meurs, 2007 [39] | iNO ↓ BPD and/or death in HRF | Preterm infants (29) | iNO + conventional care up to 14 d | Conventional care | 5–10 ppm | BPD incidence and death | ns |
| Field, 2007 [40] | Assess clinical and cost-effectiveness of iNO in HRF | Infants (60) | iNO + conventional care | Conventional care | 5–20 ppm | Death and severe disability | ns |
| Kinsella, 2006 [41] | iNO ↓ BPD and/or death in HRF | Preterm infants (793) | iNO for 21 d or until extubation + conventional care | Conventional care | 5 ppm | BPD incidence and death | ns |
| Meurs, 2005 [42] | iNO ↓ BPD and/or death in HRF | Preterm infants (420) | iNO | Conventional care | 5–10 ppm | BPD incidence and death | ns |
| Hascoet, 2005 [43] | Safety and efficacy in infants w/HRF | Preterm infants (860) | iNO | Placebo | 5 ppm | Intact survival at 28 d | ns |
| Taylor, 2004 [44] | Efficacy of iNO in ARDS | Adults (385) | iNO until 28 d or discontinuation of assisted breathing or death | Placebo until 28 d or discontinuation of assisted breathing or death | 5 ppm | Days alive and off assisted breathing | ns |
| Konduri, 2004 [45] | iNO ↓ incidence of ECMO/death in HRF | near-term and term infants (299) | iNO | Placebo | 5–20 ppm | Incidence of ECMO/ death | ns |
| Finer, 2001 [46] | No differences in oxygenation improvement between low and high dose of iNO in pts w/ HRF | near-term and term infants (36) | Low-dose iNO (LD) | High-dose iNO (HD) | LD: 1–2 ppm HD: 10–20 ppm | paO2, OI | ns |
| Cornfield, 1999 [47] | iNO ↑ oxygenation in PPHN | near-term and term infants (38) | iNO 2 ppm, 20 ppm if worsening oxygenation | Placebo, 20 ppm if worsening oxygenation | 2–20 ppm | OI | ns |
| Kinsella, 1999 [48] | iNO ↑ survival in HRF | Preterm infants (80) | iNO | Placebo | 5 ppm | Survival to discharge | ns |
| Lundin, 1999 [49] | iNO ↑ reversal of ALI in pts previously responder to iNO | Adults (268) | iNO up to 30 days or endpoint reached | Conventional care | 1–40 ppm | Rate of ALI reversal | ns |
| Davidson, 1998 [50] | Efficacy of iNO on PPHN | Term infants (155) | iNO + conventional care | Conventional care | 5–80 ppm | Major Sequelae Index (incidence of death, ECMO, neurologic | ns |
| The NINOS Group, 1997 [51] | iNO ↓ ECMO use and/ or death in HRF and congenital diaphragmatic hernia | near-term and term infants (53) | iNO | 100% O2 | 20 ppm | injury, BPD) ECMO use/death | ns |
| Barefield, 1996 [52] | iNO ↓ ECMO use in PPHN | near-term and term infants (17) | iNO + conventional MV | Conventional MV | 20–80 ppm | ECMO use | ns |
|
|
|
|
|
|
|
|
|
| Pulmonary Arterial Hypertension | |||||||
|
| |||||||
| Nathan, 2020 [53] | iNO ↑ physical activity in PAH + pulmonary fibrosis | Adults (41) | Pulsed iNO for 8w | Placebo | 30 μg/kg IBW/h | moderate/vigorous physical activity improvement | ↑ in the iNO vs control group |
| Vonbank, 2003 [54] | iNO safety in pts w/ PAH due to COPD | Adults (40) | Pulsed iNO + O2 over 3 months | O2 | 20 ppm | Pulmonary and systemic hemodynamics. Arterial oxygenation. NO2 | ↑ in pulmonary hemodynamics. Other endpoints: ns |
| Hasuda, 2000 [55] | iNO ↑ exercise capacity in precapillary PAH | Adults (14) | iNO + exercise on a cycle ergometer | Exercise on a cycle ergometer (Randomized cross-over) | 20 ppm | Peak exercise load, anaerobic threshold, VO2 | Only VO2 ↑ in the iNO vs control group |
| Van Meurs, 1997 [56] | iNO effect on oxygenation HRF | Preterm (11) | iNO | Placebo, (Randomized, cross-over) | 1–20 ppm | P/F | P/F ↑ >25% in 10 out of 11 participants |
|
|
|
|
|
|
|
|
|
| AKI prevention after CPB | |||||||
|
| |||||||
| Lei, 2018 [18] | NO ↓ AKI after CPB | Adults (244) | NO for 24 h | Placebo | 80 ppm | AKI occurrence | |
|
|
|
|
|
|
|
|
|
| 50% NO vs 64% control group (p = 0.014) | |||||||
|
|
|
|
|
|
|
|
|
| NO during CPB for CHD | |||||||
|
| |||||||
| Elzein, 2020 [57] | NO ↓ ischemia/ reperfusion injury | Newborns (24) | NO | Placebo | 40 ppm | Multiple markers of organ injury | Only Tn lower in the iNO vs control group (p = 0.009) |
| James, 2016 [58] | NO ↓ LCOS | Children (198) | NO | Placebo | 20 ppm | LCOS incidence | 15% iNO vs 31% control group (p = 0.007) |
| Checchia, 2013 [59] | NO ↓ ischemia/ reperfusion injury | Children (16) | NO | Placebo | 20 ppm | Duration MV, ICU LOS, TnI and BNP levels | All endpoints better in the iNO group (p < 0.05) |
| Miller, 2000 [60] | NO ↓ PAH crises | Infants (124) | NO | Placebo | 10 ppm | Number of PAH crises | 4 PAH crises NO vs 7 control group (p < 0.001) |
| Day, 2000 [61] | NO ↓ PAH crises | Infants (40) | NO | Placebo | 20 ppm | Number of PAH crises | ns |
| Niebler, 2021 [62] | NO ↓ activation and depletion of PLTs | Infants (40) | NO | Placebo | 20 ppm | PLTs count | ns |
|
|
|
|
|
|
|
|
|
| Resuscitation | |||||||
|
| |||||||
| Sekar, 2020 [63] | Prevention of O2 exposure during resuscitation | Preterm infants needing help with breathing (28) | iNO for 17 min | Placebo for 17 min | 20 ppm | Cumulative FiO2, time w/FiO2 >60%, pre/postductal saturation, heart rate, need for intubation | Cumulative FiO2 (p = 0.001) and time w/FiO2 >60% (p < 0.0001) lower in the iNO group |
|
|
|
|
|
|
|
|
|
| Transfusions | |||||||
|
| |||||||
| Berra, 2014 [16] | Blood transfusions older than 40 d ↑ PAP | Obese adults (14) | Transfusion with 3-d, 40-d, and 40-d old blood with iNO | Randomized cross-over | 80 ppm | PAP | 40 d old blood ↑ PAP. iNO prevents it |
|
|
|
|
|
|
|
|
|
| Sickle cell disease | |||||||
|
| |||||||
| Head, 2010 [64] | iNO ↓ intensity of painful crisis | Adults (23) | iNO for 4 h | Room air | 80 ppm | Mean change of pain score after 4 h of iNO | iNO ↓ pain scores (p = 0.02) |
| Maitre, 2015 [65] | iNO ↓ treatment failure in pts with acute chest syndrome | Adults (100) | iNO for 3 d | Placebo | 80 ppm | Treatment failure rate at 3 d | ns |
| Gladwin, 2011 [66] | iNO ↓ duration of painful crisis | >10 years old (150) | iNO up to 3 d | Placebo | 40–80 ppm | Time to resolution of painful crisis | ns |
|
|
|
|
|
|
|
|
|
| Severe malaria | |||||||
|
| |||||||
| Bangirana, 2018 [67] | Neuroprotective role of iNO | Children (130) | iNO up to 72 h | Room air | 80 ppm | Neurocognitive outcomes | ↓ fine motor impairment in iNO vs control group (RR, 95% CI: 0.36, 0.14–0.96) |
| Conroy, 2016 [68] | Safety of iNO | Children (180) | iNO for 3 d | Room air | 80 ppm | MetHb levels >10% mandate treatment interruption | MetHb >10% in 5.7% patients in iNO group. Authors conclude iNO is safe if MetHb is measured during administration |
| Hawkes, 2015 [69] | iNO ↑ severe malaria outcome | Children (180) | iNO up to 3 d | Room air | 80 ppm | Ang-2 levels in the first 3 d | ns |
| Mwanga- Amumpaire, 2015 [70] | iNO ↑ Ang-1 | Children (92) | iNO for at least 2 d | Room air | 80 ppm | Ang-1 levels at 2 d | ns |
Legend:
decreases/decreased
increases/increased.
Abbreviations: aAPO2, alveolar-arterial oxygen tension difference; AKI, acute kidney injury; ALI, acute lung injury; Ang-1/Ang-2, angiotensin 1 and 2; ARDS, acute respiratory distress syndrome; BNP, B-type natriuretic peptide; BPD, bronchopulmonary dysplasia; CI, confidence interval; cmH2O, centimeters of water; COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; d, day(s); d, day(s); ECMO, extracorporeal membrane oxygenation; FiO2, fraction of inspired oxygen; h, hour(s); h, hour; HFOV, high frequency oscillatory ventilation; HRF, hypoxic respiratory failure; IBW, ideal body weight; iNO, inhaled nitric oxide; IVH, intraventricular hemorrhage; LCOS: low cardiac output syndrome; LOS, length of stay; MAP, mean arterial pressure; MetHb, methemoglobin; MV, mechanical ventilation; n.a., not available; NO, nitric oxide; NO2, nitrogen dioxide; ns, not significant; O2, oxygen; OI, oxygenation index; P/F, partial oxygen pressure-to-fraction of inspired oxygen ratio; PAH, pulmonary artery hypertension; paO2, partial pressure of oxygen; PAP, pulmonary artery pressure; PLTs, platelets; PPHN, persistent pulmonary hypertension of the newborn; ppm, part per million; PVRI, pulmonary vascular resistances index; RR, relative risk; Tn, troponin; VFD, ventilator- free days; VO2, oxygen consumption; w, week(s); w/, with.
Table 2.
Ongoing randomized controlled trials on nitric oxide for the treatment of various clinical conditions.
| Trial | Aim/hypothesis | Population (N) | Treatment group | Control group | NO dose | Primary endpoint |
|---|---|---|---|---|---|---|
|
| ||||||
| Respiratory failure NCT04776408 | Measure dead space or shunt fraction before and after iNO | Adults (100) | Lung recruitment maneuver + iNO | Lung recruitment maneuver | n.a. | V/Q mismatch through EIT |
|
|
|
|
|
|
|
|
| Covid19 | ||||||
|
| ||||||
| NO-COVID-19 NCT04388683 | iNO ↓ progression to advanced disease | Adults (42) | iNO | Conventional care | 20 ppm | Progression to ↑ FiO2, ventilatory support or death |
| NCT05012319 | NO ↓ need of urgent care | Adults (500) | NO nasal spray | Placebo | n.a. | Need of urgent care |
| NCT04397692 | Safety | Adults (20) | iNO | Conventional care | 80 ppm | Time to deterioration |
| Lei, 2020 [71] | iNO ↑ oxygenation | Adults (200) | iNO 80 ppm for 2 d, then 40 ppm, then weaning | Conventional care | 40 ppm | Change in P/F at 2 d |
| Lei, 2020 [72] | iNO prevents progression of mild/moderate Covid19 | Adults (70) | iNO for 20–30 min bid for 14 d + conventional care | Conventional care | 140–180 ppm | Need for intubation and MV |
| NCT04601077 | Safety | Adults (100) | NO lozenges | Placebo | 30 mg | Low BP and dizziness incidence |
| NCT04383002 | iNO safety, antiviral effect and prevention of progression to HRF | Adults (20) | iNO for 6 h/d for 2 days + standard of care | Standard of care | 160 ppm | Covid19 PCR status at 7 d |
| NO COV-ED NCT04338828 | iNO ↑ respiratory status, prevent hospitalization and t clinical course in ED | Adults (47) | iNO for 20–30 min | O2 2 l/min | 140–300 ppm | Rate of return to ED w/in 28 d |
| COViNOX NCT04421508 | Efficacy and safety of iNO | Adults (500) | Pulsed iNO | Pulsed placebo | 125mcg/kg IBW/h | Death or HRF at 28 d |
| NICOR NCT04476992 | Safety | Adults (20) | iNO high dose 30 min bid for 14 d | iNO high dose 30min bid + continuous iNO low dose for 14 d | High dose: 200 ppm | Change in MetHb level at 2 d |
| Low dose: 20 ppm | ||||||
| NCT04443868 | Efficacy of NO to ↓ infection duration | Adults (50) | NO nasal irrigation daily (240 ml) | Normal saline nasal irrigation (240 ml) | 14 ppm | Sars-Cov-2 viral load baseline through 6 d |
| NCT04460183 | Efficacy of RESP301 (NO generating solution) in ↓ progression of Covid19 and safety | Adults (300) | Inhaled RESP301 tid up to 10 d + conventional care | Conventional care | n.a. | Progression on the modified WHO ordinal scale by 14 d |
|
|
|
|
|
|
|
|
| Pulmonary Arterial Hypertension | ||||||
|
| ||||||
| NCT01457781 | Efficacy and safety | Adults (80) | Pulsed iNO up to 24h/ day for 16 w | Placebo | 0.025–0.075 mg/kg IBW/h | Change in PVR |
| NCT03267108 | Efficacy and safety | Adults (300) | Pulsed iNO | Placebo | 0.045 mg/kg IBW/h | Change in moderate to vigorous activity, adverse events |
| NCT03576885 | iNO ↓ death, PAH and BPD incidence | Newborns (138) | iNO for 14 d or resolution of PAH | Placebo | 20 ppm | Death, BPD incidence |
|
|
|
|
|
|
|
|
| NO during CPB | ||||||
|
| ||||||
| Marrazzo, 2019 [73] | NO ↓ AKI in pts w/endothelial dysfunction | Adults (250) | NO | Placebo | 80 ppm | AKI occurrence |
| NCT03946462 | NO ↓ AKI | Newborns (24) | NO | Placebo | 20 ppm | NGAL/Cystatin-C levels |
| NCT04216927 | NO ↓ AKI after CPB | Newborns (40) | NO | Placebo | 20 ppm | AKI incidence |
| Schlapbach, 2019 | NO ↓ MV duration |
Infants (1320) | NO | Placebo | 20 ppm | VFD at 28 d |
| NASO NCT03661385 | NO ↓ post-operative MAEs | Children, Adults (900) | NO | Standard care | 20 ppm | MAEs: cardiac arrest, emergency chest |
| NCT05101746 | NO is neuro and renal protective | Children (50) | NO | Standard care | 20 ppm | opening, ECMO, death GFAP and NGAL levels |
|
|
|
|
|
|
|
|
| Brain Injury | ||||||
|
| ||||||
| NCT03260569 | Effect of iNO on gas exchange in TBI | Adults (38) | iNO | Placebo | 20 ppm | Change in paO2 |
Legend:
decreases/decreased
increases/increased.
Abbreviations: AKI, acute kidney injury; bid, bis in die; BP, blood pressure; BPD, bronchopulmonary dysplasia; CPB, cardiopulmonary bypass; d, day(s), ECMO, extracorporeal membrane oxygenation; EIT, electrical impedance tomography; FiO2, fraction of inspired oxygen; GFAP, glial fibrillary acid protein; h, hour(s); HRF, hypoxic respiratory failure; IBW, ideal body weight; iNO, inhaled nitric oxide; MAE, major adverse event; MetHb, methemoglobin; min, minutes; MV, mechanical ventilation; n.a., not available; NGAL, neutrophil gelatinase-associated lipocalin; NO, nitric oxide; P/F, partial oxygen pressure-to-fraction of inspired oxygen ratio; PAH, pulmonary arterial hypertension; paO2, partial pressure of oxygen; PCR, polymerase chain reaction; ppm, part per million; PVR, pulmonary vascular resistances; tid, tris in die; VFD, ventilator-free days; w/with; w/in, within.
Fig. 1.
Clinical applications and effects of nitric oxide. Nitric oxide is currently approved by the FDA, with the sole indication for administration in patients with PPHN. iNO is sometimes used as a rescue therapy in severe ARDS. The other disease named in the figure represent potential target for iNO treatment. Clinical data are still poor. * diseases where only preclinical studies are available or clinical trials are ongoing. § conflicting data, see text for further details. Abbreviations: PVR, pulmonary vascular resistances; PPHN, persistent pulmonary hypertension of the newborn; ECMO, extracorporeal membrane oxygenation; ROSC, return of spontaneous circulation; LCOS, low cardiac output syndrome; ARDS, acute respiratory distress syndrome; V/Q, ventilation-to- perfusion ratio; PA, pulmonary artery; AKI, acute kidney injury; CPB, cardiopulmonary bypass; CKD, chronic kidney disease; pts, patients; NO, nitric oxide; PAH, pulmonary arterial hypertension.
2. Methods
A search of the literature of Medline database published until January 19, 2022 was performed to detect publications evaluating the use of NO in clinical context. Priority was given to RCTs, meta-analysis and guidelines. When this evidence was not available, observational studies and case series were considered. The Mesh term “Nitric Oxide” was used along with the Mesh Terms that identified the conditions and diseases treated in this review. Ongoing clinical trials were identified through search on clinicaltrials.gov, being RCT preferred over other study designs. “Nitric Oxide” was entered as drug name and descriptors of the conditions or diseases related to this review were used (see Supplement 1 for the complete list of search terms). Other relevant articles not included in our original search were identified through snowballing.
3. Clinical applications of NO
3.1. iNO for persistent pulmonary hypertension of the newborn
iNO is approved by the FDA for the treatment of PPHN at the dose of 20 ppm for up to 14 days [13]. iNO improves oxygenation and decreases extracorporeal membrane oxygenation (ECMO) use in term and late preterm newborns with PPHN. It was Kinsella et al. [74] and Roberts et al. [75], who first described the benefit of iNO up to 80 ppm on oxygenation in newborns with PPHN. Since then, many trials confirmed these results and found a reduction in ECMO use with iNO treatment [30,38,50,74,75]. More widely, the beneficial effect of iNO in newborns was confirmed by a Cochrane systematic review and meta-analysis in 2017 [76]. Indeed, iNO reduced the incidence of the combined endpoint of death or use of ECMO in term or near-term newborns with hypoxemic respiratory failure. However, this reduction was due to a decrease in the use of ECMO, while mortality was not affected. Moreover, oxygenation was improved. These beneficial effects occurred regardless of whether or not there was echocardiographic evidence of PPHN.
3.2. iNO for chronic pulmonary arterial hypertension
iNO is commonly used for vasoreactivity testing to identify patients with PAH primarily caused by increased pulmonary vascular resistances (PVR), in the absence of severe vascular remodeling. Only patients with idiopathic, heritable or drug-induced PAH are tested as they are the most likely to show vasoreactivity [77]. In the other forms of PAH the test is not indicated as iNO may exacerbate pulmonary edema due to left heart failure and in other types of PAH evidence is lacking [77]. As suggested by current guidelines this test is performed before the initiation of any PAH-specific treatment to identify potential responders to calcium channel blockers [78]. Although vasoreactivity testing is performed in those with idiopathic, heritable, or drug-induced PAH to determine candidacy for calcium channel blocker therapy, observed decreases in PVR and mean pulmonary arterial pressure (PAP) with iNO are independent predictors of survival across the broad range of etiologies for PAH [79,80]. Chronic vasodilatory therapy may precipitate pulmonary edema, if pulmonary venous hypertension coexists. In such circumstances, short acting iNO can be used to establish whether pulmonary arterial vasodilator might be detrimental [81].
There are few studies evaluating the indication of iNO for the treatment of PAH, due to lack of accessibility of iNO in the out-patient settings. Data from case reports and observational studies showed that long term iNO improved PAH and sometimes relieved symptoms [14, 82–84]. Results from a randomized controlled trial are awaited (NCT01457781), albeit a beneficial effect on mortality was not reported so far. Moreover, a recent meta-analysis reported that perioperative administration of NO in patients with PAH undergoing cardiac surgery had no clinical benefits in term of ICU stay, mortality, duration of mechanical ventilation and reduction of PAP [85].
3.3. iNO for the treatment of ARDS in children and adult patients
Historically, a low dose of iNO (e.g. 5–20 ppm) was demonstrated to improve arterial oxygenation and reduce PAP, in patients with severe ARDS [44]. iNO is a potent selective pulmonary vasodilator. Indeed, it has a half-life of 2–6 s [86] as it is rapidly scavenged by hemoglobin [87] and is also metabolized to more stable nitrite dioxide and nitrite trioxide, which lack the vasodilatory properties of NO. Moreover, it is delivered locally by inhalation. These properties make iNO able to dilate selectively the pulmonary vessels of ventilated lung units, consequently improving ventilation/perfusion matching, without causing systemic hypotension [3]. Despite this physiologic effect, iNO is not part of routine therapy in ARDS, while it is suggested as one of the therapeutic “rescue” strategies for severe hypoxemia. This is because of its transient effects lost after 24–48 h and because no benefit on survival or other clinical outcomes (e.g., ventilator-free days (VFD), ICU length of stay) was demonstrated. Moreover, in some studies, iNO seemed to worsen renal function [88–90]. For these reasons, the United Kingdom Faculty of Intensive Care Medicine guidelines give a weak recommendation against the use of iNO in ARDS [91]. The current guideline of the American Thoracic Society for ARDS treatment does not make any recommendation about the use of iNO, however they consider inhaled vasodilators as an issue to be addressed in the future iterations of the guideline [15]. Nevertheless, although no clear clinical benefit was observed, iNO is still administered in up to 13% of severe ARDS patients worldwide [92].
Interestingly, the sepsis-associated ARDS deserves a particular mention. Indeed, it is well-known that endogenous NO is increased in sepsis due to upregulation of inducible NOS and plays a pivotal role in the sepsis-induced hypotension. Although hypotension is detrimental, NO vasodilation could have a role in keeping microvessels patent and thus preserve the microcirculatory perfusion [93,94]. A preclinical study showed that sepsis-associated endogenous NO upregulation may decrease iNO effect on pulmonary circulation [95]. Other evidence suggested that iNO diminished lung inflammation and injury and decreased endogenous NO upregulation at the lungs [96,97]. In this case, iNO would act as negative feedback on endogenous NO production. Data are limited and the effects of iNO in this subtype of ARDS have to be investigated to clarify whether iNO could improve the management of this disease.
3.4. iNO in Covid19 patients
The Covid19 pandemic has been challenging healthcare systems all over the world, due to the high hospitalization rate and the high incidence of ARDS, requiring ICU admission [98,99]. Severely hypoxemic patients may require ECMO, however due to limited resources this option might not be applicable. For this reason, iNO, due to its property to improve V/Q matching, may serve as an alternative or as a bridge to gain time for availability of resources or lung healing. Moreover, iNO may have a direct antimicrobial effect against Sars-CoV-2, as suggested by an in vitro study, in which Sars-CoV-infected cells had higher survival when exposed to S-nitroso-N-acetylpenicillamine (SNAP), an NO donor [100]. Similarly, also Sars-CoV-2-infected cells were exposed to SNAP and a dose-dependent inhibitory effect on replication of Sars-CoV-2 was observed. In addition, SNAP delayed or completely prevented the development of the viral cytopathic effect [101]. Other proposed potential benefits of iNO are a bronchodilatory [102] and anti-inflammatory effect [103]. To date, data are limited and mainly consist of case series and results are contrasting. iNO is administered at doses ranging from 10 to 80 ppm in mechanically ventilated patients. A report of 16 patients with Covid19-related refractory hypoxemia (defined as P/F ratio <100, despite PEEP ≥10 cmH2O and prone position) showed iNO at 20–30 ppm did not improve oxygenation. However, a trend towards a better response was observed in patients with concomitant Covid19 pneumonia and right ventricular dysfunction [104]. Similarly, a pilot study on 10 patients with severe hypoxemia (FiO2 80%, PEEP 15 cmH2O) showed no benefit on oxygenation after a trial of iNO at 20 ppm for 30 min [105]. In contrast, a study on 34 patients, showed that iNO at 10 ppm administered when P/F ratio was under 150 improved P/F ratio from a median of 70 to 144 in 65% of the subjects included in the study [106]. The responders had a lower P/F ratio compared to non-responders (70 vs 134, P < 0.0001). Similarly, another study on 12 patients demonstrated that iNO 20–80 ppm resulted in a P/F ratio change from 136 to 170 in the supine position and a decrease in the dead-space-to-tidal-volume ratio from 0.54 to 0.46. Subsequent prone positioning, further increased the P/F ratio from 145 to 205 [107]. However, differences in the iNO dose and the timing of iNO administration (early vs late-rescue therapy after intubation) might explain these different results. Moreover, combined iNO at 10 ppm and almitrine supplementation significantly increased P/F ratio from 102 at baseline to 180. As almitrine acts as a pulmonary vasoconstrictor, the iNO vasodilatory effect might have been enhanced by flow diversion towards better ventilated lung areas, thus improving V/Q matching [108].
iNO has also been suggested as adjuvant treatment in spontaneously breathing patients with severe Covid19. Twenty-nine patients with Sars-CoV-2 infection confirmed and cough or tachypnea (respiratory rate above 24 breaths/minute) received high dose iNO (160 ppm) twice a day for 30 min up to 14 days via a face mask, until resolution of symptoms, discharge, intubation, or transition to palliative care. iNO decreased respiratory rate in tachypneic patients and improved oxygenation when hypoxemia was present. Moreover, iNO was well tolerated in spontaneously breathing patients and it was safe as MetHb and nitrogen dioxide levels were below the safety threshold [109,110]. High dose iNO has also been tested on six pregnant patients with Covid19 hypoxic respiratory failure. iNO improved oxygenation and was well tolerated, suggesting a possible benefit of this treatment [103].
Thus far, no efficacy of iNO in Covid19 patients can be inferred, due to the lack of data and their limited quality. Randomized controlled trials could elucidate the role of iNO in this specific disease [71,72].
3.5. iNO and its role on hemolysis
Intravascular hemolysis increases levels of free hemoglobin in the blood. This causes a depletion of endogenous NO bioavailability and oxidative stress. This inflammatory state leads to endothelial dysfunction, impairment of the microvascular flow and vaso-occlusive organ damage [111]. Intravascular hemolysis is common to several diseases, such as severe malaria and sickle cell disease. These have been considered for adjunctive treatment with iNO. Indeed, severe malaria is characterized by decreased NO bioavailability and has a mortality rate of 10–30% [112], despite the availability of effective anti-malarial medications. Increasing evidence suggested that iNO may dampen endothelial activation, reduce injury in the pulmonary vessels and exert neuroprotective properties [113,114]. Moreover, in clinical trials, iNO did not affect levels of Angiopoietin 2, a biomarker of endothelial activation and malaria severity [69,70]. However, iNO administration up to 80 ppm was safe [68,70] and reduced the risk of fine motor impairment in patients affected by cerebral malaria [67]. No study evaluated the effect of iNO on survival as the primary outcome in patients with malaria.
Also sickle cell disease is associated with impaired NO metabolism [115], thus iNO was administered as possible adjuvant to improve clinical outcomes in patients with sickle cell disease complicated by veno-occlusive disease, presented as acute chest syndrome or acute painful crises. One small RCT found iNO to effectively decrease mean pain scores referred by the patients [64]. On the contrary, two more recent trials found iNO did not decrease either the duration of painful crisis [66], or the treatment failure rate of acute chest syndrome, defined as (1) death from any cause, or (2) need for endotracheal intubation, or (3) decrease of PaO2/FiO2 ≥ 15 mmHg between days 1 and 3, or (4) augmented therapy defined as new transfusion or phlebotomy [65]. An ongoing study is evaluating whether iNO can improve PAH in patients with sickle cell disease (NCT00023296).
Another potential application of iNO is during transfusions of stored red blood cells (RBC). Stored RBC undergo hemolysis and thus have high concentrations of microparticles, free hemoglobin, heme and iron, which are then released into circulation when transfused [116]. Hemoglobin free in plasma and contained in microparticle rapidly depletes endogenous NO through its scavenging. This induces endothelial cells dysfunction and may result in pulmonary and systemic hypertension [16,117–120]. Risbano et al. demonstrated that arginase-1 and free hemoglobin levels increased in healthy volunteers who received an intraarterial transfusion of autologous 42-day-old RBC. Both arginase-1 and free hemoglobin have been associated with endothelial dysfunction. Moreover, they demonstrated that 42-day-old RBC decreased the expected vasodilatory response to intraarterial infusion of acetylcholine, probably by scavenging of NO or oxidative inactivation of endogenous NO. The administration of iNO proved to be effective in preventing these detrimental effects of stored RBC transfusion both preclinically and clinically [16,121]. Of note, Berra and colleagues demonstrated that transfusing 40-day old blood increased mean pulmonary artery pressure (18 ± 2 to 23 ± 2 mmHg; P < 0.05) in obese adults with endothelial dysfunction, estimated through cardiac ultrasound. Whereas, breathing NO at 80 ppm during transfusion avoided the increase in pulmonary artery pressure (17 ± 2 to 12 ± 1 mmHg; P < 0.05), while transfusing aged stored RBC [16].
3.6. iNO and its role on myocardial injury
NO may exert cardioprotective properties. Although limited data are available, a study examined the role of NO at 20 ppm on 29 patients. NO was administered for 8 h during and after CPB. In this group, biomarkers of myocardial injury, like creatine kinase MB (CK-MB) fraction, total CK, and troponin I (TnI) were significantly lower than in the control group. This suggests that NO may blunt the subclinical myocardial injury typical of CPB. Another study on 69 patients undergoing coronary artery bypass graft, showed that adding NO at 40 ppm to the oxygenator of the CPB decreased the level of CK-MB and TnI and the inotropic requirement. Also, the effect of iNO on ischemic reperfusion injury in patients revascularized after ST-elevation myocardial infarction was explored. Although iNO at 80 ppm for 4 h after reperfusion was safe, no reduction in infarct size was observed. Moreover, the Kaplan–Meier analysis for the composite of death, recurrent ischemia, stroke, or rehospitalizations showed a tendency toward lower event rates with iNO at 4 months and 1-year follow-up, warranting further investigations. In addition, iNO determined acute hemodynamic improvements in 13 adults patients with right ventricle infarction and cardiogenic shock. Indeed, iNO significantly decreased mean right arterial pressure, mean pulmonary artery pressure and pulmonary vascular resistance and increased the cardiac and stroke volume indexes. The cardioprotective effects of NO donors were also evaluated in patients with acute ST-elevation myocardial infarction undergoing percutaneous coronary intervention. In these two RCTs it has been observed no reduction of infarct size in both systemic and intracoronary administration of sodium nitrite.
3.7. Protective role of NO on cardiac output and kidney function during cardiopulmonary bypass
Cardiac surgery for CHD is characterized by a CPB-induced systemic inflammatory response [122]. This determines the low cardiac output syndrome (LCOS) [123] and increases morbidity and mortality [124]. Supplemental NO, with its anti-inflammatory properties, may dampen the negative effects of CPB. A small study on 16 children found 20 ppm of NO halved the duration of mechanical ventilation and shortened the ICU length of stay of 1 day. It also lowered troponin levels and B-type natriuretic peptide concentrations when compared to the placebo group [59]. Another larger trial on 198 infants and children showed the NO group developed fewer LCOS than the control group (15% vs 31%, p = 0.007) [58]. Also, the ICU stay was nearly halved in the NO group and ECMO was used less frequently. This trial also showed a trend towards decreased duration of mechanical ventilation, especially in children aged less than 2 years old. To further investigate this latter result, the NITRIC trial was designed and is ongoing [125]. In addition, some other trials showed NO decreased the incidence of postoperative PAH crises when administered during CPB [60,61].
Another common complication of CPB is acute kidney injury (AKI) is [126,127]. To date, no therapeutic agent is available to prevent the postoperative decline in renal function. The mechanism is multifactorial. Advanced age, female sex, preoperative comorbidities (e.g., diabetes mellitus, heart failure chronic obstructive pulmonary disease, obesity) play a pivotal role in the development of AKI. Moreover, CPB- and cardiac surgery-related conditions (e.g., duration of aortic clamping and CPB, pulsatile vs non pulsatile flow, normothermic vs hypothermic CPB, hemolysis during CPB) has been involved in the pathogenesis of this complication [128]. Indeed, patients undergoing cardiac surgery often present endothelial dysfunction, which may play a role in increasing the risk of postsurgical AKI [73]. In addition, higher levels of free hemoglobin and NO consumption were identified in patients after CPB compared to preoperative levels and hemolysis correlated with the increase in pulmonary and systemic vascular resistances [120]. Therefore, it could be suggested a beneficial effect of exogenous NO on hemodynamics during CPB, which could improve the cardiac output and consequently the renal perfusion. Moreover, hemolysis induced by the CPB releases free hemoglobin, which is filtered by the kidney, may directly determine oxidative damage [129] and may deplete endogenous NO, causing vasoconstriction and inflammation [130]. Preclinical studies showed that NO regulates major metabolic pathways in the proximal tube of the kidney. These are important to prevent oxidative stress, which eventually leads to AKI [131]. A recent systematic review and meta-analysis of 5 trials found that NO was associated with a reduced risk of AKI, particularly when administered from the beginning of CPB (RR 0.71, 95% CI 0.54–0.94, I2 = 10%). NO increased the MetHb level, but it had no clinical impact [132]. Of note, Lei et al. administered NO up to 80 ppm through the membrane lung and by inhalation during the postoperative period for 24 h to patients undergoing multiple cardiac valve replacement. NO proved to decrease AKI (RR 0.78 [95%CI 0.62–0.97]) and transition to stage 3 chronic kidney disease both at 90 days (RR 0.64 [95%CI 0.41–0.99]) and at 1 year follow-up (RR 0.59 [95%CI 0.36–0.96]). Furthermore, NO decreased major kidney adverse event (i.e. a composite outcome of loss of 25% of eGFR from baseline, end-stage renal disease requiring a continuous renal replacement therapy, and mortality) at 30 and 90 days and 1 year after ICU admission [18]. However, the study population was relatively young (mean age 48). Furthermore, rheumatic fever was the main cause of valvular heart disease. To test whether the beneficial effect of NO to prevent post cardiac surgery AKI may be widened to older patients in the presence of endothelial dysfunction, a trial is currently ongoing at Massachusetts General Hospital [73] (NCT02836899). Also, other studies are investigating whether NO prevents AKI after CPB in neonates undergoing cardiac surgery due to congenital heart diseases (CHD) (NCT04216927, NCT03946462).
3.8. iNO as a treatment during cardiac arrest
iNO has been considered as a potential therapeutic agent in patients with cardiac arrest (CA) [133]. Hypoxic-ischemic brain injury is a key component of the post-reperfusion state known as post-CA syndrome, accounting for poor neurological outcome and poor survival rates observed after return of spontaneous circulation (ROSC) [134]. Post-CA syndrome is characterized by systemic inflammation with diffuse endothelial dysfunction, increased vascular permeability, as well as platelet activation. Together with this pathophysiological alterations, endogenous NO depletion has been observed in this condition [17,135]. Among the extrapulmonary effects of iNO, protection against brain injury in the post-CA period has emerged in experimental settings. Specifically, reduction of brain inflammation and oxidative stress represent some of the potential mechanisms for neurological preservation offered by NO through a guanylyl cyclase (GC) dependent or independent pathways [135,136]. Several preclinical studies showed that NO inhalation following resuscitation improved survival rate and neurological outcome while reducing brain edema, neuronal apoptosis and cerebral inflammatory cytokines levels [136,137]. Furthermore, iNO increased the proportion of ROSC [138,139], allowing for superior hemodynamics and higher cerebral blood flow [140] when administered during cardiopulmonary resuscitation (CPR) in a pediatric pig model of shock associated-CA [139,140]. Of particular interest for translation from experimental to clinical setting, NO inhalation during CPR has been observed also in a high proportion (~80%) of pediatric in-hospital CA subjects with PH [141]. Interestingly, all patients in whom iNO was increased or initiated during CPR achieved ROSC, and 50% survived to hospital discharge.
Finally, iNO lacks the effect on systemic pressure, typical of NO donor drugs, while it improves transpulmonary blood flow and RV function during CPR performed with a ventricular assist device [142, 143], making it an appealing option for unstable CA patients undergoing ECMO. A recent propensity-matched analysis comparing 20 in-hospital CA adult patients receiving iNO with age-matched controls receiving standard care, showed that iNO is feasible and might be beneficial [144]. Indeed, inhalation of NO 40 ppm starting following resuscitation until 24 h after ROSC was associated with a higher probability of survival at discharge compared to controls (35% vs 20%, p = 0.034). Although no difference in favorable neurological outcome was observed [144], the neuroprotective effect of iNO administration during CA until 24 h post ROSC is currently under investigation in a clinical trial (NCT04134078). The primary aim of this study is the rate of ROSC and cerebral oxygenation in patients with in-hospital CA. Also neurological outcomes at hospital discharge and 6-month survival will be evaluated.
3.9. NO as a neuroprotectant
The neuroprotective properties of NO has been shown also in various neurologic disorders. Preclinical data showed that it may dampen ischemic/reperfusion brain injury [17,145], preserve cerebral autoregulation after traumatic brain injury [146], prevent from cerebral vasospasm after subarachnoid hemorrhage [147], improve regional blood flow and decrease infarct size in ischemic stroke [148,149]. An analysis of the ENOS trial showed transdermal glyceryl trinitrate (GTN), a NO donor, improved functional outcomes and decreased deaths in the subgroup of patients which received GTN within 6 h from stroke [150]. The RIGHT trial showed the improvement in functional outcome, if GTN was administered in the acute stroke [151]. However, due to the small sample size, the RIGHT 2 trial was performed and did not confirm these findings [152]. As for iNO, an ongoing clinical study is aiming to analyze variations of cerebral blood flow after iNO in patients affected by ischemic stroke and to compare them to health subjects (NCT03023449). Moreover, results are pending from a small trial which primarily aimed to evaluate whether up to 40 ppm of iNO improved refractory vasospasm (NCT04988932).
3.10. Toxicology and adverse effects of iNO
Notwithstanding the illustrated benefits of iNO, the toxicologic profile and potential adverse effects of iNO should be mentioned [153]. Indeed, NO undergoes oxidation to nitrogen dioxide (NO2) spontaneously. NO2 is an airway irritant and can determine pulmonary edema. The permissible exposure limit for NO2 is 5 ppm and doses of 20 ppm are considered as immediately dangerous to life or health by the Centers for Disease Control and Prevention [154]. However, in major clinical trials iNO up to 80 ppm was not associated with excessive levels of NO2 and evidence of NO2 intoxication [50,76]. Moreover, in patients receiving intermittent iNO at high doses (160 ppm), NO2 levels of 5.6 ppm were reported during only one iNO administration out of 343 [12]. In addition, iNO reacts with superoxide anion, which is produced during ischemia/reperfusion injury [155] and forms peroxynitrite, a highly reactive oxidant species [156]. Peroxynitrite has showed ability to interfere with lung surfactant activity [157] and may affect mitochondrial respiration [158]. These detrimental effects have not been well investigated in human population, however they may affect the outcome when iNO is used as an organ protectant following ischemia/reperfusion injury. iNO may also alter DNA, thus making it a potential mutagen, although a carcinogenic effect has never been demonstrated so far [159–162]. iNO is associated with methemoglobinemia (MetHb) [163]. MetHb is usually <1% in healthy individuals [164] and does not usually have clinical implications until concentrations of 10%. This adverse effect is unusual at iNO doses below 40 ppm [157]. Treatment with methylene blue should be instituted for levels >20% in all patients and for lower levels in symptomatic patients (e.g., end-organ dysfunction) [165]. From a hemodynamic perspective, iNO could cause systemic hypotension (>2% higher incidence compared to placebo) [153] and could worsen left heart failure in patients with left heart dysfunction [166]. In these situations, it will be reasonable to interrupt the treatment with iNO. Moreover, since a rebound of pulmonary hypertension has been described in up to 25% of patients when iNO was interrupted abruptly, gradual tapering of iNO is suggested [167]. If this measure was unsuccessful, immediate reinstitution of iNO therapy and coadministration of sildenafil could be considered to allow the weaning from iNO [168,169]. Finally, iNO may worsen renal function in patients with ARDS. A meta-analysis of 1363 patients enrolled in 10 RCTs, showed iNO was associated to an increased risk of AKI compared to placebo (RR 1.4, 95% CI, 1.06–1.83) [170]. By contrast, limited data showed iNO protected against AKI in patients receiving prolonged CPB [18].
In conclusion, although rare, should any adverse effect appear at iNO therapeutic doses (less than 80 ppm), it is suggested to disrupt iNO administration immediately and institute supportive care and specific therapy, when available.
3.11. A novel strategy to produce NO: potential for clinical applications
Despite the demonstrated and potential benefits of the NO, its widespread use is limited because NO is stocked in cylinders which are cumbersome, expensive, require a distribution network and trained healthcare professionals. Moreover, iNO is one of the most expensive drug used in neonatal departments [171]. This limited the use of NO to short periods of therapy and to the inpatient setting. To overcome these drawbacks, a lightweight, portable, economical NO generator from air has been developed recently. It uses pulsed electrical discharge and can produce therapeutic doses of NO for at least one month and can be powered with batteries [172,173]. This system has been already tested in humans and appeared safe. Indeed, two exploratory studies showed no adverse events occurred during or after the NO breathing through this novel system and MetHb and nitrogen dioxide levels remained within safety range [174,175]. Moreover, preliminary results evidenced that in patients with pulmonary hypertension, electrically generated NO induces pulmonary hemodynamics effects equivalent to NO from cylinders [174]. This device would allow the use of iNO in the ambulatory out-of-hospital setting and, as it is economical, would increase accessibility to NO treatment, including patients in developing countries. Moreover, it would permit to investigate NO as a potential chronic therapy [176].
4. Conclusions
NO has well-established clinical applications in PPHN as it decreases mortality and ECMO use. Some limited data also showed a benefit in improving oxygenation in ARDS and a reduction of the pulmonary artery pressure in patients with PAH. Limited data indicate the use of NO as a potential organ protective strategy. Altogether, NO shows a low toxicity profile at the suggested clinical doses (i.e. 1–80 ppm, up to 160 ppm for COVID-19 infections), thus randomized clinical trials should be endorsed not to overlook the potential clinical benefits of such a gas in the critically ill patients. In addition, considering the new development of a portable NO generator, which eliminates the need to stock NO and makes it more affordable compared to NO delivered by cylinders, the application of NO in chronic and out-of-hospital conditions should be further investigated.
Supplementary Material
Acknowledgments
The authors of this review would like to dedicate this manuscript to the memory of Warren M. Zapol, M.D., a visionary scientist, a tireless mentor and a scientific father of generations of physicians. His discovery on the therapeutic use of inhaled nitric oxide gas is one of the major contribution he gave to the critical care fields which expanded tremendously since then and the focus of our present review.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
LB receives salary support from K23 HL128882/NHLBI NIH as principal investigator for his work on hemolysis and nitric oxide. LB receives technologies and devices from iNO Therapeutics LLC, Praxair Inc., Masimo Corp. LB receives grants from “Fast Grants for COVID-19 research” at Mercatus Center of George Mason University and from iNO Therapeutics LLC. Laboratory work is supported by the Reginald Jenney Endowment Chair at Harvard Medical School, by Sundry Funds at Massachusetts General Hospital, and by laboratory funds of the Anesthesia Center for Critical Care Research of the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital. RM was supported by a COVID Fast Grant (George Mason University), the National Heart, Lung, and Blood Institute (R01HL142809), and the Wild Family Foundation. ER is supported by the Bicocca Starting grant 2020 from the University of Milano-Bicocca with the project titled: “Functional Residual Capacity Assessment using a Wash-In/Wash-Out technique based on a fast main-stream O2 Sensor with nanofluorescenT geometry for severe lung injury (FAST) - COVID and beyond”. ER was supported by the International Young Investigator Award 2018 from European Society of Intensive Care Medicine (ESICM) with the project titled: “Role of the exhaled breath condensate as non-invasive monitoring of the lung inflammation during ARDS: a prospective cohort study”. ER was supported by the National Merck Sharp & Dohme Corporation Research Award 2017 from the Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva ` (SIAARTI) with the project titled: “Studio della concentrazione di ossido nitrico nell’esalato espiratorio come marcatore di danno polmonare acuto in pazienti adulti con ARDS sottoposti a ventilazione meccanica”.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.niox.2022.01.007.
References
- 1.Stuehr DJ, Santolini J, Wang Z-Q, Wei C-C, Adak S, Update on mechanism J. Biol. Chem. 279 (2004) 36167–36170, 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 2.Corson MA, James NL, Latta SE, Nerem RM, Berk BC, Harrison DG, Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress, Circ. Res 79 (1996) 984–991, 10.1161/01.res.79.5.984. [DOI] [PubMed] [Google Scholar]
- 3.Steudel W, Hurford WE, Zapol WM, Inhaled nitric oxide: basic biology and clinical applications, Anesthesiology 91 (1999) 1090–1121, 10.1097/00000542-199910000-00030. [DOI] [PubMed] [Google Scholar]
- 4.Garg UC, Hassid A, Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells, J. Clin. Invest 83 (1989) 1774–1777, 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radomski MW, Vallance P, Whitley G, Foxwell N, Moncada S, Platelet adhesion to human vascular endothelium is modulated by constitutive and cytokine induced nitric oxide, Cardiovasc. Res 27 (1993) 1380–1382, 10.1093/cvr/27.7.1380. [DOI] [PubMed] [Google Scholar]
- 6.Lefer AM, Nitric oxide: nature’s naturally occurring leukocyte inhibitor, Circulation 95 (1997) 553–554, 10.1161/01.cir.95.3.553. [DOI] [PubMed] [Google Scholar]
- 7.Deanfield JE, Halcox JP, Rabelink TJ, Endothelial function and dysfunction: testing and clinical relevance, Circulation 115 (2007) 1285–1295, 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 8.Tejero J, Shiva S, Gladwin MT, Sources of vascular nitric oxide and reactive oxygen species and their regulation, Physiol. Rev 99 (2019) 311–379, 10.1152/physrev.00036.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter M, Taddei S, et al. , The assessment of endothelial function: from research into clinical practice, Circulation 126 (2012) 753–767, 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller MR, Megson IL, Recent developments in nitric oxide donor drugs, Br. J. Pharmacol 151 (2007) 305–321, 10.1038/sj.bjp.0707224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frostell CG, Blomqvist H, Hedenstierna G, Lundberg J, Zapol WM, Inhaled nitric oxide selectively reverses human hypoxic pulmonary vasoconstriction without causing systemic vasodilation, Anesthesiology 78 (1993) 427–435, 10.1097/00000542-199303000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Goldbart A, Golan-Tripto I, Pillar G, Livnat-Levanon G, Efrati O, Spiegel R, Lubetzky R, Lavie M, Carmon L, Ghaffari A, et al. , Inhaled nitric oxide therapy in acute bronchiolitis: a multicenter randomized clinical trial, Sci. Rep 10 (2020) 9605, 10.1038/s41598-020-66433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics, Committee on fetus and newborn. Use of inhaled nitric oxide, Pediatrics 106 (2000) 344–345. [PubMed] [Google Scholar]
- 14.Pérez-Peñate G, Julià-Serdà G, Pulido-Duque JM, Górriz-Gómez E, Cabrera-Navarro P, One-year continuous inhaled nitric oxide for primary pulmonary hypertension, Chest 119 (2001) 970–973, 10.1378/chest.119.3.970. [DOI] [PubMed] [Google Scholar]
- 15.Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG, et al. , An Official American Thoracic Society/European Society of intensive care Medicine/Society of critical care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome, Am. J. Respir. Crit. Care Med 195 (2017) 1253–1263, 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 16.Berra L, Pinciroli R, Stowell CP, Wang L, Yu B, Fernandez BO, Feelisch M, Mietto C, Hod EA, Chipman D, et al. , Autologous transfusion of stored red blood cells increases pulmonary artery pressure, Am. J. Respir. Crit. Care Med 190 (2014) 800–807, 10.1164/rccm.201405-0850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyazaki Y, Ichinose F, Nitric oxide in post-cardiac arrest syndrome, J. Cardiovasc. Pharmacol 75 (2020) 508–515, 10.1097/FJC.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 18.Lei C, Berra L, Rezoagli E, Yu B, Dong H, Yu S, Hou L, Chen M, Chen W, Wang H, et al. , Nitric oxide decreases acute kidney injury and stage 3 chronic kidney disease after cardiac surgery, Am. J. Respir. Crit. Care Med 198 (2018) 1279–1287, 10.1164/rccm.201710-2150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu H-W, Li Z-G, Liu G, Lu G-Z, Liang H-Y, Effect of nitric oxide inhalation for the treatment of neonatal pulmonary hypertension, Eur. Rev. Med. Pharmacol. Sci. 20 (2016) 4607–4611. [PubMed] [Google Scholar]
- 20.Bronicki RA, Fortenberry J, Schreiber M, Checchia PA, Anas NG, Multicenter randomized controlled trial of inhaled nitric oxide for pediatric acute respiratory distress syndrome, J. Pediatr 166 (2015) 365–369, 10.1016/j.jpeds.2014.10.011,e1. [DOI] [PubMed] [Google Scholar]
- 21.González A, Fabres J, D’Apremont I, Urcelay G, Avaca M, Gandolfi C, Kattan J, Randomized controlled trial of early compared with delayed use of inhaled nitric oxide in newborns with a moderate respiratory failure and pulmonary hypertension, J. Perinatol. 30 (2010) 420–424, 10.1038/jp.2009.171. [DOI] [PubMed] [Google Scholar]
- 22.Su PH, Chen JY, Inhaled nitric oxide in the management of preterm infants with severe respiratory failure, J. Perinatol. 28 (2008) 112–116, 10.1038/sj.jp.7211881. [DOI] [PubMed] [Google Scholar]
- 23.Dani C, Bertini G, Pezzati M, Filippi L, Cecchi A, Rubaltelli FF, Inhaled nitric oxide in very preterm infants with severe respiratory distress syndrome, Acta Paediatr. 95 (2006) 1116–1123, 10.1080/08035250600702594. [DOI] [PubMed] [Google Scholar]
- 24.Lindwall R, Blennow M, Svensson M, Jonsson B, Berggren-Boström E, Flanby M, Lönnqvist P-A, Frostell C, Norman M, A pilot study of inhaled nitric oxide in preterm infants treated with nasal continuous positive airway pressure for respiratory distress syndrome, Intensive Care Med. 31 (2005) 959–964, 10.1007/s00134-005-2593-5. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P, Inhaled nitric oxide in premature infants with the respiratory distress syndrome, N. Engl. J. Med 349 (2003) 2099–2107, 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 26.Sadiq HF, Mantych G, Benawra RS, Devaskar UP, Hocker JR, Inhaled nitric oxide in the treatment of moderate persistent pulmonary hypertension of the newborn: a randomized controlled, multicenter trial, J. Perinatol. 23 (2003) 98–103, 10.1038/sj.jp.7210878. [DOI] [PubMed] [Google Scholar]
- 27.Srisuparp P, Heitschmidt M, Schreiber MD, Inhaled nitric oxide therapy in premature infants with mild to moderate respiratory distress syndrome, J. Med. Assoc. Thai. 85 (Suppl 2) (2002) S469–S478. [PubMed] [Google Scholar]
- 28.Baxter FJ, Randall J, Miller JD, Higgins DA, Powles ACP, Choi PT-L, Rescue therapy with inhaled nitric oxide in critically ill patients with severe hypoxemic respiratory failure (brief report), Can. J. Anaesth 49 (2002) 315–318, 10.1007/BF03020535. [DOI] [PubMed] [Google Scholar]
- 29.Christou H, Van Marter LJ, Wessel DL, Allred EN, Kane JW, Thompson JE, Stark AR, Kourembanas S, Inhaled nitric oxide reduces the need for extracorporeal membrane oxygenation in infants with persistent pulmonary hypertension of the newborn, Crit. Care Med. 28 (2000) 3722–3727, 10.1097/00003246-200011000-00031. [DOI] [PubMed] [Google Scholar]
- 30.Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP, Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical inhaled nitric oxide research group, N. Engl. J. Med 342 (2000) 469–474, 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 31.Early compared with delayed inhaled nitric oxide in moderately hypoxaemic neonates with respiratory failure: a randomised controlled trial. The Franco- Belgium collaborative NO trial group, Lancet 354 (1999) 1066–1071. [PubMed] [Google Scholar]
- 32.Dobyns EL, Cornfield DN, Anas NG, Fortenberry JD, Tasker RC, Lynch A, Liu P, Eells PL, Griebel J, Baier M, et al. , Multicenter randomized controlled trial of the effects of inhaled nitric oxide therapy on gas exchange in children with acute hypoxemic respiratory failure, J. Pediatr 134 (1999) 406–412, 10.1016/s0022-3476(99)70196–4. [DOI] [PubMed] [Google Scholar]
- 33.Troncy E, Collet JP, Shapiro S, Guimond JG, Blair L, Ducruet T, Francoeur M, Charbonneau M, Blaise G, Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study, Am. J. Respir. Crit. Care Med 157 (1998) 1483–1488, 10.1164/ajrccm.157.5.9707090. [DOI] [PubMed] [Google Scholar]
- 34.Michael JR, Barton RG, Saffle JR, Mone M, Markewitz BA, Hillier K, Elstad MR, Campbell EJ, Troyer BE, Whatley RE, et al. , Inhaled nitric oxide versus conventional therapy: effect on oxygenation in ARDS, Am. J. Respir. Crit. Care Med 157 (1998) 1372–1380, 10.1164/ajrccm.157.5.96-10089. [DOI] [PubMed] [Google Scholar]
- 35.Dellinger RP, Zimmerman JL, Taylor RW, Straube RC, Hauser DL, Criner GJ, Davis K, Hyers TM, Papadakos P, Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled nitric oxide in ARDS study group, Crit. Care Med. 26 (1998) 15–23, 10.1097/00003246-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Neonatal inhaled nitric oxide study group inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure, N. Engl. J. Med 336 (1997) 597–604, 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 37.[] Wessel DL, Adatia I, Van Marter LJ, Thompson JE, Kane JW, Stark AR, Kourembanas S, Improved oxygenation in a randomized trial of inhaled nitric oxide for persistent pulmonary hypertension of the newborn, Pediatrics 100 (1997) E7, 10.1542/peds.100.5.e7. [DOI] [PubMed] [Google Scholar]
- 38.Roberts JD, Fineman JR, Morin FC, Shaul PW, Rimar S, Schreiber MD, Polin RA, Zwass MS, Zayek MM, Gross I, et al. , Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The inhaled nitric oxide study group, N. Engl. J. Med 336 (1997) 605–610, 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- 39.Van Meurs KP, Hintz SR, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, Perritt R, Das A, Higgins RD, Stevenson DK, Inhaled nitric oxide in infants >1500 g and <34 Weeks gestation with severe respiratory failure, J. Perinatol. 27 (2007) 347–352, 10.1038/sj.jp.7211690. [DOI] [PubMed] [Google Scholar]
- 40.Field D, Elbourne D, Hardy P, Fenton AC, Ahluwalia J, Halliday HL, Subhedar N, Heinonen K, Aikio O, Grieve R, et al. , Neonatal ventilation with inhaled nitric oxide vs. Ventilatory support without inhaled nitric oxide for infants with severe respiratory failure born at or near term: the INNOVO multicentre randomised controlled trial, Neonatology 91 (2007) 73–82, 10.1159/000097123. [DOI] [PubMed] [Google Scholar]
- 41.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS, et al. , Early inhaled nitric oxide therapy in premature newborns with respiratory failure, N. Engl. J. Med 355 (2006) 354–364, 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 42.Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, Perritt R, Higgins RD, Oh W, Hudak ML, et al. , Inhaled nitric oxide for premature infants with severe respiratory failure, N. Engl. J. Med 353 (2005) 13–22, 10.1056/NEJMoa043927. [DOI] [PubMed] [Google Scholar]
- 43.[] Hascoet JM, Fresson J, Claris O, Hamon I, Lombet J, Liska A, Cantagrel S, Al Hosri J, Thiriez G, Valdes V, et al. , The safety and efficacy of nitric oxide therapy in premature infants, J. Pediatr 146 (2005) 318–323, 10.1016/j.jpeds.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 44.Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K, Kelly KM, Smith TC, Small RJ, Inhaled nitric oxide in ARDS study group low- dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial, JAMA 291 (2004) 1603–1609, 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 45.[] Konduri GG, Solimano A, Sokol GM, Singer J, Ehrenkranz RA, Singhal N, Wright LL, Van Meurs K, Stork E, Kirpalani H, et al. , A randomized trial of early versus standard inhaled nitric oxide therapy in term and near-term newborn infants with hypoxic respiratory failure, Pediatrics 113 (2004) 559–564, 10.1542/peds.113.3.559. [DOI] [PubMed] [Google Scholar]
- 46.[] Finer NN, Sun JW, Rich W, Knodel E, Barrington KJ, Randomized, prospective study of low-dose versus high-dose inhaled nitric oxide in the neonate with hypoxic respiratory failure, Pediatrics 108 (2001) 949–955, 10.1542/peds.108.4.949. [DOI] [PubMed] [Google Scholar]
- 47.[] Cornfield DN, Maynard RC, deRegnier RA, Guiang SF, Barbato JE, Milla CE, Randomized, controlled trial of low-dose inhaled nitric oxide in the treatment of term and near-term infants with respiratory failure and pulmonary hypertension, Pediatrics 104 (1999) 1089–1094, 10.1542/peds.104.5.1089. [DOI] [PubMed] [Google Scholar]
- 48.Kinsella JP, Walsh WF, Bose CL, Gerstmann DR, Labella JJ, Sardesai S, Walsh-Sukys MC, McCaffrey MJ, Cornfield DN, Bhutani VK, et al. , Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomised controlled trial, Lancet 354 (1999) 1061–1065, 10.1016/s0140-6736(99)03558–8. [DOI] [PubMed] [Google Scholar]
- 49.Lundin S, Mang H, Smithies M, Stenqvist O, Frostell C, Inhalation of nitric oxide in acute lung injury: results of a European multicentre study. The European study group of inhaled nitric oxide, Intensive Care Med. 25 (1999) 911–919, 10.1007/s001340050982. [DOI] [PubMed] [Google Scholar]
- 50.[] Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R, Rhines J, Chang CT, Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double-masked, placebo-controlled, dose-response, multicenter study. The I-no/PPHN study group, Pediatrics 101 (1998) 325–334, 10.1542/peds.101.3.325. [DOI] [PubMed] [Google Scholar]
- 51.[] Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. The neonatal inhaled nitric oxide study group (NINOS), Pediatrics 99 (1997) 838–845, 10.1542/peds.99.6.838. [DOI] [PubMed] [Google Scholar]
- 52.Barefield ES, Karle VA, Phillips JB, Carlo WA, Inhaled nitric oxide in term infants with hypoxemic respiratory failure, J. Pediatr 129 (1996) 279–286, 10.1016/s0022-3476(96)70255-x. [DOI] [PubMed] [Google Scholar]
- 53.Nathan SD, Flaherty KR, Glassberg MK, Raghu G, Swigris J, Alvarez R, Ettinger N, Loyd J, Fernandes P, Gillies H, et al. , A randomized, double-blind, placebo-controlled study of pulsed, inhaled nitric oxide in subjects at risk of pulmonary hypertension associated with pulmonary fibrosis, Chest 158 (2020) 637–645, 10.1016/j.chest.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 54.Vonbank K, Ziesche R, Higenbottam TW, Stiebellehner L, Petkov V, Schenk P, Germann P, Block LH, Controlled prospective randomised trial on the effects on pulmonary haemodynamics of the ambulatory long term use of nitric oxide and oxygen in patients with severe COPD, Thorax 58 (2003) 289–293, 10.1136/thorax.58.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasuda T, Satoh T, Shimouchi A, Sakamaki F, Kyotani S, Matsumoto T, Goto Y, Nakanishi N, Improvement in exercise capacity with nitric oxide inhalation in patients with precapillary pulmonary hypertension, Circulation 101 (2000) 2066–2070, 10.1161/01.cir.101.17.2066. [DOI] [PubMed] [Google Scholar]
- 56.Van Meurs KP, Rhine WD, Asselin JM, Durand DJ, Response of premature infants with severe respiratory failure to inhaled nitric oxide. Preemie NO collaborative group, Pediatr. Pulmonol. 24 (1997) 319–323, . [DOI] [PubMed] [Google Scholar]
- 57.Elzein C, Urbas C, Hughes B, Li Y, Lefaiver C, Ilbawi M, Vricella L, Efficacy of nitric oxide administration in attenuating ischemia/reperfusion injury during neonatal cardiopulmonary bypass, World J. Pediatr. Congenit. Heart Surg. 11 (2020) 417–423, 10.1177/2150135120911034. [DOI] [PubMed] [Google Scholar]
- 58.James C, Millar J, Horton S, Brizard C, Molesworth C, Butt W, Nitric oxide administration during paediatric cardiopulmonary bypass: a randomised controlled trial, Intensive Care Med. 42 (2016) 1744–1752, 10.1007/s00134-016-4420-6. [DOI] [PubMed] [Google Scholar]
- 59.Checchia PA, Bronicki RA, Muenzer JT, Dixon D, Raithel S, Gandhi SK, Huddleston CB, Nitric oxide delivery during cardiopulmonary bypass reduces postoperative morbidity in children–a randomized trial, J. Thorac. Cardiovasc. Surg. 146 (2013) 530–536, 10.1016/j.jtcvs.2012.09.100. [DOI] [PubMed] [Google Scholar]
- 60.Miller OI, Tang SF, Keech A, Pigott NB, Beller E, Celermajer DS, Inhaled nitric oxide and prevention of pulmonary hypertension after congenital heart surgery: a randomised double-blind study, Lancet 356 (2000) 1464–1469, 10.1016/S0140-6736(00)02869–5. [DOI] [PubMed] [Google Scholar]
- 61.Day RW, Hawkins JA, McGough EC, Creze KL é, G.S. Orsmond, Randomized controlled study of inhaled nitric oxide after operation for congenital heart disease, Ann. Thorac. Surg. 69 (2000) 1907–1912, 10.1016/s0003-4975(00)01312–6, discussion 1913. [DOI] [PubMed] [Google Scholar]
- 62.Niebler RA, Chiang-Ching H, Daley K, Janecke R, Jobe SM, Mitchell ME, Varner C, Woods K, Scott JP, Nitric oxide added to the sweep gas of the oxygenator during cardiopulmonary bypass in infants: a pilot randomized controlled trial, Artif. Organs 45 (2021) 22–28, 10.1111/aor.13788. [DOI] [PubMed] [Google Scholar]
- 63.Sekar K, Szyld E, McCoy M, Wlodaver A, Dannaway D, Helmbrecht A, Riley J, Manfredo A, Anderson M, Lakshminrusimha S, et al. , Inhaled nitric oxide as an adjunct to neonatal resuscitation in premature infants: a pilot, double blind, randomized controlled trial, Pediatr. Res 87 (2020) 523–528, 10.1038/s41390-019-0643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Head CA, Swerdlow P, McDade WA, Joshi RM, Ikuta T, Cooper ML, Eckman JR, Beneficial effects of nitric oxide breathing in adult patients with sickle cell crisis, Am. J. Hematol 85 (2010) 800–802, 10.1002/ajh.21832. [DOI] [PubMed] [Google Scholar]
- 65.Maitre B, Djibre M, Katsahian S, Habibi A, Stankovic Stojanovic K, Khellaf M, Bourgeon I, Lionnet F, Charles-Nelson A, Brochard L, et al. , Inhaled nitric oxide for acute chest syndrome in adult sickle cell patients: a randomized controlled study, Intensive Care Med. 41 (2015) 2121–2129, 10.1007/s00134-015-4060-2. [DOI] [PubMed] [Google Scholar]
- 66.Gladwin MT, Kato GJ, Weiner D, Onyekwere OC, Dampier C, Hsu L, Hagar RW, Howard T, Nuss R, Okam MM, et al. , Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial, JAMA 305 (2011) 893–902, 10.1001/jama.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bangirana P, Conroy AL, Opoka RO, Hawkes MT, Hermann L, Miller C, Namasopo S, Liles WC, John CC, Kain KC, Inhaled nitric oxide and cognition in pediatric severe malaria: a randomized double-blind placebo controlled trial, PLoS One 13 (2018), e0191550, 10.1371/journal.pone.0191550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conroy AL, Hawkes M, Hayford K, Hermann L, McDonald CR, Sharma S, Namasopo S, Opoka RO, John CC, Liles WC, et al. , Methemoglobin and nitric oxide therapy in Ugandan children hospitalized for febrile illness: results from a prospective cohort study and randomized double-blind placebo-controlled trial, BMC Pediatr. 16 (2016) 177, 10.1186/s12887-016-0719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hawkes MT, Conroy AL, Opoka RO, Hermann L, Thorpe KE, McDonald C, Kim H, Higgins S, Namasopo S, John C, et al. , Inhaled nitric oxide as adjunctive therapy for severe malaria: a randomized controlled trial, Malar. J. 14 (2015) 421, 10.1186/s12936-015-0946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mwanga-Amumpaire J, Carroll RW, Baudin E, Kemigisha E, Nampijja D, Mworozi K, Santorino D, Nyehangane D, Nathan DI, De Beaudrap P, et al. , Inhaled nitric oxide as an adjunctive treatment for cerebral malaria in children: a phase II randomized open-label clinical trial, Open Forum Infect. Dis. 2 (2015) ofv111, 10.1093/ofid/ofv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lei C, Su B, Dong H, Bellavia A, Di Fenza R, Safaee Fakhr B, Gianni S, Grassi LG, Kacmarek R, Araujo Morais CC, et al. , Protocol of a randomized controlled trial testing inhaled nitric oxide in mechanically ventilated patients with severe acute respiratory syndrome in COVID-19 (SARS-CoV-2), medRxiv (2020), 10.1101/2020.03.09.20033530,2020.03.09.. [DOI] [PubMed] [Google Scholar]
- 72.Lei C, Su B, Dong H, Fakhr BS, Grassi LG, Di Fenza R, Gianni S, Pinciroli R, Vassena E, Morais CCA, et al. , Protocol for a randomized controlled trial testing inhaled nitric oxide therapy in spontaneously breathing patients with COVID-19, medRxiv 10 (2020) 20033522, 10.1101/2020.03.10.20033522,2020.03. [DOI] [Google Scholar]
- 73.Marrazzo F, Spina S, Zadek F, Lama T, Xu C, Larson G, Rezoagli E, Malhotra R, Zheng H, Bittner EA, et al. , Protocol of a randomised controlled trial in cardiac surgical patients with endothelial dysfunction aimed to prevent postoperative acute kidney injury by administering nitric oxide gas, BMJ Open 9 (2019), e026848, 10.1136/bmjopen-2018-026848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kinsella JP, Neish SR, Shaffer E, Abman SH, Low-dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn, Lancet 340 (1992) 819–820, 10.1016/0140-6736(92)92687-b. [DOI] [PubMed] [Google Scholar]
- 75.Roberts JD, Polaner DM, Lang P, Zapol WM, Inhaled nitric oxide in persistent pulmonary hypertension of the newborn, Lancet 340 (1992) 818–819, 10.1016/0140-6736(92)92686-a. [DOI] [PubMed] [Google Scholar]
- 76.Barrington KJ, Finer N, Pennaforte T, Altit G, Nitric oxide for respiratory failure in infants born at or near term, Cochrane Database Syst. Rev. 1 (2017) CD000399, 10.1002/14651858.CD000399.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gali Nè, M. Humbert, J.-L. Vachiery, S. Gibbs, I. Lang, A. Torbicki, G. Simonneau, A. Peacock, A. Vonk Noordegraaf, M. Beghetti, et al. , ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European respiratory Society (ERS): endorsed by: Association for European Paediatric and congenital cardiology (AEPC), International Society for heart and lung Transplantation (ISHLT), Eur. Heart J 37 (2016) 67–119, 10.1093/eurheartj/ehv317,2015. [DOI] [PubMed] [Google Scholar]
- 78.Klinger JR, Elliott CG, Levine DJ, Bossone E, Duvall L, Fagan K, Frantsve- Hawley J, Kawut SM, Ryan JJ, Rosenzweig EB, et al. , Therapy for pulmonary arterial hypertension in adults: update of the CHEST guideline and expert panel report, Chest 155 (2019) 565–586, 10.1016/j.chest.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 79.Malhotra R, Hess D, Lewis GD, Bloch KD, Waxman AB, Semigran MJ, Vasoreactivity to inhaled nitric oxide with oxygen predicts long-term survival in pulmonary arterial hypertension, Pulm. Circ. 1 (2011) 250–258, 10.4103/2045-8932.83449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Post MC, Janssens S, Van de Werf F, Budts W, Responsiveness to inhaled nitric oxide is a predictor for mid-term survival in adult patients with congenital heart defects and pulmonary arterial hypertension, Eur. Heart J 25 (2004) 1651–1656, 10.1016/j.ehj.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 81.Creagh-Brown BC, Nicholson AG, Showkathali R, Gibbs JSR, Howard LSGE, Pulmonary veno-occlusive disease presenting with recurrent pulmonary oedema and the use of nitric oxide to predict response to sildenafil, Thorax 63 (2008) 933–934, 10.1136/thx.2007.088831. [DOI] [PubMed] [Google Scholar]
- 82.Koh E, Niimura J, Nakamura T, Yamakage H, Takahashi H, Long-term inhalation of nitric oxide for a patient with primary pulmonary hypertension, Jpn. Circ. J 62 (1998) 940–942, 10.1253/jcj.62.940. [DOI] [PubMed] [Google Scholar]
- 83.Channick RN, Newhart JW, Johnson FW, Williams PJ, Auger WR, Fedullo PF, Moser KM, Pulsed delivery of inhaled nitric oxide to patients with primary pulmonary hypertension: an ambulatory delivery system and initial clinical tests, Chest 109 (1996) 1545–1549, 10.1378/chest.109.6.1545. [DOI] [PubMed] [Google Scholar]
- 84.Ivy DD, Parker D, Doran A, Parker D, Kinsella JP, Abman SH, Acute hemodynamic effects and home therapy using a novel pulsed nasal nitric oxide delivery system in children and young adults with pulmonary hypertension, Am. J. Cardiol. 92 (2003) 886–890, 10.1016/s0002-9149(03)00910-x. [DOI] [PubMed] [Google Scholar]
- 85.Sardo S, Osawa EA, Finco G, Gomes Galas FRB, de Almeida JP, Cutuli SL, Frassanito C, Landoni G, Hajjar LA, Nitric oxide in cardiac surgery: a meta- analysis of randomized controlled trials, J. Cardiothorac. Vasc. Anesth. 32 (2018) 2512–2519, 10.1053/j.jvca.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 86.Gibson QH, Roughton FJW, The kinetics J. Physiol. 136 (1957) 507–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rimar S, Gillis CN, Selective pulmonary vasodilation by inhaled nitric oxide is due to hemoglobin inactivation, Circulation 88 (1993) 2884–2887, 10.1161/01.cir.88.6.2884. [DOI] [PubMed] [Google Scholar]
- 88.Gebistorf F, Karam O, Wetterslev J, Afshari A, Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults, Cochrane Database Syst. Rev. (2016) CD002787, 10.1002/14651858.CD002787.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Adhikari NKJ, Burns KEA, Friedrich JO, Granton JT, Cook DJ, Meade MO, Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis, BMJ 334 (2007) 779, 10.1136/bmj.39139.716794.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Afshari A, Brok J, Møller AM, Wetterslev J, Inhaled nitric oxide for acute respiratory distress syndrome and acute lung injury in adults and children: a systematic review with meta-analysis and trial sequential analysis, Anesth. Analg. 112 (2011) 1411–1421, 10.1213/ANE.0b013e31820bd185. [DOI] [PubMed] [Google Scholar]
- 91.Griffiths MJD, McAuley DF, Perkins GD, Barrett N, Blackwood B, Boyle A, Chee N, Connolly B, Dark P, Finney S, et al. , Guidelines on the management of acute respiratory distress syndrome, BMJ Open Resp. Res 6 (2019), e000420, 10.1136/bmjresp-2019-000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. , Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries, JAMA 315 (2016) 788–800, 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 93.Trzeciak S, Cinel I, Dellinger RP, Shapiro NI, Arnold RC, Parrillo JE, Hollenberg SM, Resuscitating the microcirculation in sepsis: the central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials, Acad. Emerg. Med. 15 (2008) 399–413, 10.1111/j.1553-2712.2008.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lambden S, Bench to bedside review: therapeutic modulation of nitric oxide in sepsis—an update, Intens. Care Med. Exp. 7 (2019) 64, 10.1186/s40635-019-0274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holzmann A, Manktelow C, Taut FJ, Bloch KD, Zapol WM, Inhibition of nitric oxide synthase prevents hyporesponsiveness to inhaled nitric oxide in lungs from endotoxin-challenged rats, Anesthesiology 91 (1999) 215–221, 10.1097/00000542-199907000-00030. [DOI] [PubMed] [Google Scholar]
- 96.Webert KE, Vanderzwan J, Duggan M, Scott JA, McCormack DG, Lewis JF, Mehta S, Effects of inhaled nitric oxide in a rat model of Pseudomonas Aeruginosa pneumonia, Crit. Care Med. 28 (2000) 2397–2405, 10.1097/00003246-200007000-00035. [DOI] [PubMed] [Google Scholar]
- 97.Razavi HM, Werhun R, Scott JA, Weicker S, Wang LF, McCormack DG, Mehta S, Effects of inhaled nitric oxide in a mouse model of sepsis-induced acute lung injury, Crit. Care Med. 30 (2002) 868–873, 10.1097/00003246-200204000-00026. [DOI] [PubMed] [Google Scholar]
- 98.Rezoagli E, Magliocca A, Bellani G, Pesenti A, Grasselli G, Development of a critical care response - experiences from Italy during the coronavirus disease 2019 pandemic, Anesthesiol. Clin. 39 (2021) 265–284, 10.1016/j.anclin.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nicholson CJ, Wooster L, Sigurslid HH, Li RH, Jiang W, Tian W, Lino Cardenas CL, Malhotra R, Estimating risk of mechanical ventilation and in-hospital mortality among adult COVID-19 patients admitted to mass general Brigham: the VICE and DICE scores, EClinicalMedicine 33 (2021) 100765, 10.1016/j.eclinm.2021.100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Keyaerts E, Vijgen L, Chen L, Maes P, Hedenstierna G, Van Ranst M, Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound, Int. J. Infect. Dis 8 (2004) 223–226, 10.1016/j.ijid.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Akaberi D, Krambrich J, Ling J, Luni C, Hedenstierna G, Järhult JD, Lennerstrand J, Lundkvist Å, Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro, Redox Biol. 37 (2020) 101734, 10.1016/j.redox.2020.101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kacmarek RM, Ripple R, Cockrill BA, Bloch KJ, Zapol WM, Johnson DC, Inhaled nitric oxide. A bronchodilator in mild asthmatics with methacholine-induced bronchospasm, Am. J. Respir. Crit. Care Med 153 (1996) 128–135, 10.1164/ajrccm.153.1.8542105. [DOI] [PubMed] [Google Scholar]
- 103.Safaee Fakhr B, Wiegand SB, Pinciroli R, Gianni S, Morais CCA, Ikeda T, Miyazaki Y, Marutani E, Di Fenza R, Larson GM, et al. , High concentrations of nitric oxide inhalation therapy in pregnant patients with severe coronavirus disease 2019 (COVID-19), Obstet. Gynecol. 136 (2020) 1109–1113, 10.1097/AOG.0000000000004128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tavazzi G, Marco P, Mongodi S, Dammassa V, Romito G, Mojoli F, Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia, Crit. Care 24 (2020) 508, 10.1186/s13054-020-03222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferrari M, Santini A, Protti A, Andreis DT, Iapichino G, Castellani G, Rendiniello V, Costantini E, Cecconi M, Inhaled nitric oxide in mechanically ventilated patients with COVID-19, J. Crit. Care 60 (2020) 159–160, 10.1016/j.jcrc.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abou-Arab O, Huette P, Debouvries F, Dupont H, Jounieaux V, Mahjoub Y, Inhaled nitric oxide for critically ill covid-19 patients: a prospective study, Crit. Care 24 (2020) 645, 10.1186/s13054-020-03371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ziehr DR, Alladina J, Wolf ME, Brait KL, Malhotra A, La Vita C, Berra L, Hibbert KA, Hardin CC, Respiratory physiology of prone positioning with and without inhaled nitric oxide across the coronavirus disease 2019 acute respiratory distress syndrome severity spectrum, Crit. Care Explor. 3 (2021), e0471, 10.1097/CCE.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bagate F, Tuffet S, Masi P, Perier F, Razazi K, de Prost N, Carteaux G, Payen D, Mekontso Dessap A, Rescue therapy with inhaled nitric oxide and almitrine in COVID-19 patients with severe acute respiratory distress syndrome, Ann. Intensive Care 10 (2020) 151, 10.1186/s13613-020-00769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wiegand SB, Safaee Fakhr B, Carroll RW, Zapol WM, Kacmarek RM, Berra L, Rescue treatment with high-dose gaseous nitric oxide in spontaneously breathing patients with severe coronavirus disease 2019, Crit. Care Explor. 2 (2020), e0277, 10.1097/CCE.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Safaee Fakhr B, Di Fenza R, Gianni S, Wiegand SB, Miyazaki Y, Araujo Morais CC, Gibson LE, Chang MG, Mueller AL, Rodriguez-Lopez JM, et al. , Inhaled high dose nitric oxide is a safe and effective respiratory treatment in spontaneous breathing hospitalized patients with COVID-19 pneumonia, Nitric Oxide 116 (2021) 7–13, 10.1016/j.niox.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Graw JA, Yu B, Rezoagli E, Warren HS, Buys ES, Bloch DB, Zapol WM, Endothelial dysfunction inhibits the ability of haptoglobin to prevent hemoglobin-induced hypertension, Am. J. Physiol. Heart Circ. Physiol 312 (2017) H1120–H1127, 10.1152/ajpheart.00851.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kyu HH, Fernandez E, Artemisinin derivatives versus quinine for cerebral ´ malaria in African children: a systematic review, Bull. World Health Organ 87 (2009) 896–904, 10.2471/BLT.08.060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hawkes M, Opoka RO, Namasopo S, Miller C, Conroy AL, Serghides L, Kim H, Thampi N, Liles WC, John CC, et al. , Nitric oxide for the adjunctive treatment OF severe malaria: hypothesis and rationale, Med. Hypotheses 77 (2011) 437–444, 10.1016/j.mehy.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bergmark B, Bergmark R, Beaudrap PD, Boum Y, Mwanga-Amumpaire J, Carroll R, Zapol W, Inhaled nitric oxide and cerebral malaria: basis of a strategy for buying time for pharmacotherapy, Pediatr. Infect. Dis. J. 31 (2012) e250–254, 10.1097/INF.0b013e318266c113. [DOI] [PubMed] [Google Scholar]
- 115.Morris CR, Kuypers FA, Larkin S, Vichinsky EP, Styles LA, Patterns of arginine J. Pediatr. Hematol. Oncol 22 (2000) 515–520, 10.1097/00043426-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 116.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, et al. , Evolution of adverse changes in stored RBCs, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 17063–17068, 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM, Inhaled nitric oxide enables artificial blood transfusion without hypertension, Circulation 117 (2008) 1982–1990, 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Risbano MG, Kanias T, Triulzi D, Donadee C, Barge S, Badlam J, Jain S, Belanger AM, Kim-Shapiro DB, Gladwin MT, Effects of aged stored autologous red blood cells on human endothelial function, Am. J. Respir. Crit. Care Med 192 (2015) 1223–1233, 10.1164/rccm.201501-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baron DM, Beloiartsev A, Nakagawa A, Martyn T, Stowell CP, Malhotra R, Mayeur C, Bloch KD, Zapol WM, Adverse effects of hemorrhagic shock resuscitation with stored blood are ameliorated by inhaled nitric oxide in lambs, Crit. Care Med. 41 (2013) 2492–2501, 10.1097/CCM.0b013e31828cf456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rezoagli E, Ichinose F, Strelow S, Roy N, Shelton K, Matsumine R, Chen L, Bittner EA, Bloch DB, Zapol WM, et al. , Pulmonary and systemic vascular resistances after cardiopulmonary bypass: role of hemolysis, J. Cardiothorac. Vasc. Anesth 31 (2017) 505–515, 10.1053/j.jvca.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 121.Baron DM, Yu B, Lei C, Bagchi A, Beloiartsev A, Stowell CP, Steinbicker AU, Malhotra R, Bloch KD, Zapol WM, Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide, Anesthesiology 116 (2012) 637–647, 10.1097/ALN.0b013e318246ef77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Levy JH, Tanaka KA, Inflammatory response to cardiopulmonary bypass, Ann. Thorac. Surg 75 (2003) S715–S720, 10.1016/s0003-4975(02)04701-x. [DOI] [PubMed] [Google Scholar]
- 123.Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, Bailey JM, Akbary A, Kocsis JF, Kaczmarek R, et al. , Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease, Circulation 107 (2003) 996–1002, 10.1161/01.cir.0000051365.81920.28. [DOI] [PubMed] [Google Scholar]
- 124.Kaltman JR, Andropoulos DB, Checchia PA, Gaynor JW, Hoffman TM, Laussen PC, Ohye RG, Pearson GD, Pigula F, Tweddell J, et al. , Report of the pediatric heart network and National heart, lung, and blood institute working group on the perioperative management of congenital heart disease, Circulation 121 (2010) 2766–2772, 10.1161/CIRCULATIONAHA.109.913129. [DOI] [PubMed] [Google Scholar]
- 125.Schlapbach LJ, Horton SB, Long DA, Beca J, Erickson S, Festa M, d’Udekem Y, Alphonso N, Winlaw D, Johnson K, et al. , Study protocol: NITric oxide during cardiopulmonary bypass to improve recovery in infants with congenital heart defects (nitric trial): a randomised controlled trial, BMJ Open 9 (2019), e026664, 10.1136/bmjopen-2018-026664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mao H, Katz N, Ariyanon W, Blanca-Martos L, Adýbelli Z, Giuliani A, Danesi TH, Kim JC, Nayak A, Neri M, et al. , Cardiac surgery-associated acute kidney injury, Cardiorenal Med. 3 (2013) 178–199, 10.1159/000353134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu J, Rezoagli E, Zadek F, Bittner EA, Lei C, Berra L, Free hemoglobin ratio as a novel biomarker of acute kidney injury after on-pump cardiac surgery: secondary analysis of a randomized controlled trial, Anesth. Analg. 132 (2021) 1548–1558, 10.1213/ANE.0000000000005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Y, Bellomo R, Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment, Nat. Rev. Nephrol 13 (2017) 697–711, 10.1038/nrneph.2017.119. [DOI] [PubMed] [Google Scholar]
- 129.Deuel JW, Schaer CA, Boretti FS, Opitz L, Garcia-Rubio I, Baek JH, Spahn DR, Buehler PW, Schaer DJ, Hemoglobinuria-related acute kidney injury is driven by intrarenal oxidative reactions triggering a heme toxicity response, Cell Death Dis. 7 (2016), e2064, 10.1038/cddis.2015.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meyer C, Heiss C, Drexhage C, Kehmeier ES, Balzer J, Mühlfeld A, Merx MW, Lauer T, Kühl H, Floege J, et al. , Hemodialysis-induced release of hemoglobin limits nitric oxide bioavailability and impairs vascular function, J. Am. Coll. Cardiol 55 (2010) 454–459, 10.1016/j.jacc.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 131.Martin P-Y, de Seigneux S, Nitric oxide protects against AKI by reprogramming metabolism, Nat. Rev. Nephrol. 15 (2019) 195–196, 10.1038/s41581-019-0113-z. [DOI] [PubMed] [Google Scholar]
- 132.Hu J, Spina S, Zadek F, Kamenshchikov NO, Bittner EA, Pedemonte J, Berra L, Effect of nitric oxide on postoperative acute kidney injury in patients who underwent cardiopulmonary bypass: a systematic review and meta-analysis with trial sequential analysis, Ann. Intensive Care 9 (2019) 129, 10.1186/s13613-019-0605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Magliocca A, Fries M, Inhaled gases as novel neuroprotective therapies in the postcardiac arrest period, Curr. Opin. Crit. Care 27 (2021) 255–260, 10.1097/MCC.0000000000000820. [DOI] [PubMed] [Google Scholar]
- 134.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. , Heart disease and stroke statistics-2021 update: a report from the American heart association, Circulation 143 (2021) e254–e743, 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 135.Hayashida K, Bagchi A, Miyazaki Y, Hirai S, Seth D, Silverman MG, Rezoagli E, Marutani E, Mori N, Magliocca A, et al. , Improvement in outcomes after cardiac arrest and resuscitation by inhibition of S-nitrosoglutathione reductase, Circulation 139 (2019) 815–827, 10.1161/CIRCULATIONAHA.117.032488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Minamishima S, Kida K, Tokuda K, Wang H, Sips PY, Kosugi S, Mandeville JB, Buys ES, Brouckaert P, Liu PK, et al. , Inhaled nitric oxide improves outcomes after successful cardiopulmonary resuscitation in mice, Circulation 124 (2011) 1645–1653, 10.1161/CIRCULATIONAHA.111.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kida K, Shirozu K, Yu B, Mandeville JB, Bloch KD, Ichinose F, Beneficial effects of nitric oxide on outcomes after cardiac arrest and cardiopulmonary resuscitation in hypothermia-treated mice, Anesthesiology 120 (2014) 880–889, 10.1097/ALN.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brücken A, Derwall M, Bleilevens C, Stoppe C, Götzenich A, Gaisa NT, Weis J, Nolte KW, Rossaint R, Ichinose F, et al. , Brief inhalation of nitric oxide increases resuscitation success and improves 7-day-survival after cardiac arrest in rats: a randomized controlled animal study, Crit. Care 19 (2015) 408, 10.1186/s13054-015-1128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Morgan RW, Sutton RM, Karlsson M, Lautz AJ, Mavroudis CD, Landis WP, Lin Y, Jeong S, Craig N, Nadkarni VM, et al. , Pulmonary vasodilator therapy in shock-associated cardiac arrest, Am. J. Respir. Crit. Care Med 197 (2018) 905–912, 10.1164/rccm.201709-1818OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morgan RW, Sutton RM, Himebauch AS, Roberts AL, Landis WP, Lin Y, Starr J, Ranganathan A, Delso N, Mavroudis CD, et al. , A randomized and blinded trial of inhaled nitric oxide in a piglet model of pediatric cardiopulmonary resuscitation, Resuscitation 162 (2021) 274–283, 10.1016/j.resuscitation.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morgan RW, Topjian AA, Wang Y, Atkin NJ, Kilbaugh TJ, McGowan FX, Berg RA, Mercer-Rosa L, Sutton RM, Himebauch AS, Prevalence and outcomes of pediatric in-hospital cardiac arrest associated with pulmonary hypertension, Pediatr. Crit. Care Med. 21 (2020) 305–313, 10.1097/PCC.0000000000002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nix C, Zayat R, Ebeling A, Goetzenich A, Chandrasekaran U, Rossaint R, Hatam N, Derwall M, Inhaled nitric oxide preserves ventricular function during resuscitation using a percutaneous mechanical circulatory support device in a porcine cardiac arrest model: an echocardiographic myocardial work analysis, BMC Cardiovasc. Disord 21 (2021) 189, 10.1186/s12872-021-01992-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Derwall M, Ebeling A, Nolte KW, Weis J, Rossaint R, Ichinose F, Nix C, Fries M, Brücken A, Inhaled nitric oxide improves transpulmonary blood flow and clinical outcomes after prolonged cardiac arrest: a large animal study, Crit. Care 19 (2015) 328, 10.1186/s13054-015-1050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Patel JK, Schoenfeld E, Hou W, Singer A, Rakowski E, Ahmad S, Patel R, Parikh PB, Smaldone G, Inhaled nitric oxide in adults with in-hospital cardiac arrest: a feasibility study, Nitric Oxide 115 (2021) 30–33, 10.1016/j.niox.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 145.Jiang S, Dandu C, Geng X, Clinical application of nitric oxide in ischemia and reperfusion injury: a literature review, Brain Circ. 6 (2020) 248–253, 10.4103/bc.bc_69_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pastor P, Curvello V, Hekierski H, Armstead WM, Inhaled nitric oxide protects cerebral autoregulation through prevention of impairment of ATP and calcium sensitive K channel mediated cerebrovasodilation after traumatic brain injury, Brain Res. 1711 (2019) 1–6, 10.1016/j.brainres.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 147.Lenz IJ, Plesnila N, Terpolilli NA, Role of endothelial nitric oxide synthase for early brain injury after subarachnoid hemorrhage in mice, J. Cerebr. Blood Flow Metabol. 41 (2021) 1669–1681, 10.1177/0271678X20973787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial, Lancet 385 (2015) 617–628, 10.1016/S0140-6736(14)61121–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Willmot M, Gray L, Gibson C, Murphy S, Bath PMW, A systematic review of nitric oxide donors and L-arginine in experimental stroke; effects on infarct size and cerebral blood flow, Nitric Oxide 12 (2005) 141–149, 10.1016/j.niox.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 150.Woodhouse L, Scutt P, Krishnan K, Berge E, Gommans J, Ntaios G, Wardlaw J, Sprigg N, Bath PM, ENOS investigators effect of hyperacute administration (within 6 hours) of transdermal glyceryl trinitrate, a nitric oxide donor, on outcome after stroke: subgroup Analysis of the efficacy of nitric oxide in stroke (ENOS) trial, Stroke 46 (2015) 3194–3201, 10.1161/STROKEAHA.115.009647. [DOI] [PubMed] [Google Scholar]
- 151.Ankolekar S, Fuller M, Cross I, Renton C, Cox P, Sprigg N, Siriwardena AN, Bath PM, Feasibility of an ambulance-based stroke trial, and safety of glyceryl trinitrate in ultra-acute stroke: the rapid intervention with glyceryl trinitrate in hypertensive stroke trial (RIGHT, ISRCTN66434824), Stroke 44 (2013) 3120–3128, 10.1161/STROKEAHA.113.001301. [DOI] [PubMed] [Google Scholar]
- 152.RIGHT-2 investigators prehospital transdermal glyceryl trinitrate in patients with ultra-acute presumed stroke (RIGHT-2): an ambulance-based, randomised, sham-controlled, blinded, phase 3 trial, Lancet 393 (2019) 1009–1020, 10.1016/S0140-6736(19)30194–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Approval Package for: INOMax, https://Www.Accessdata.Fda.Gov/Drugsatfda_docs/Nda/2013/020845Orig1s014.Pdf. [Google Scholar]
- 154.[] Medical Management Guidelines for Nitrogen Oxides, fttps://Wwwn.Cdc.Gov/TSP/MMG/MMGDetails.Aspx?Mmgid=394&toxid=69.
- 155.Zweier JL, Talukder MAH, The role of oxidants and free radicals in reperfusion injury, Cardiovasc. Res 70 (2006) 181–190, 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 156.Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS, Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide, Chem. Res. Toxicol 5 (1992) 834–842, 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- 157.Weinberger B, Laskin DL, Heck DE, Laskin JD, The toxicology of inhaled nitric oxide, Toxicol. Sci 59 (2001) 5–16, 10.1093/toxsci/59.1.5. [DOI] [PubMed] [Google Scholar]
- 158.Liaudet L, Soriano FG, Szabo C, Biology of nitric oxide signaling, Crit. Care ´ Med 28 (2000) N37–N52, 10.1097/00003246-200004001-00005. [DOI] [PubMed] [Google Scholar]
- 159.Arroyo PL, Hatch-Pigott V, Mower HF, Cooney RV, Mutagenicity of nitric oxide and its inhibition by antioxidants, Mutat. Res 281 (1992) 193–202, 10.1016/0165-7992(92)90008–6. [DOI] [PubMed] [Google Scholar]
- 160.Isomura K, Chikahira M, Teranishi K, Hamada K, Induction of mutations and chromosome aberrations in lung cells following in vivo exposure of rats to nitrogen oxides, Mutat. Res 136 (1984) 119–125, 10.1016/0165-1218(84)90153–8. [DOI] [PubMed] [Google Scholar]
- 161.Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR, DNA damage and mutation in human cells exposed to nitric oxide in vitro, Proc. Natl. Acad. Sci. U. S. A 89 (1992) 3030–3034, 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wink DA, Kasprzak KS, Maragos CM, Elespuru RK, Misra M, Dunams TM, Cebula TA, Koch WH, Andrews AW, Allen JS, DNA deaminating ability and genotoxicity of nitric oxide and its progenitors, Science 254 (1991) 1001–1003, 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- 163.Finer NN, Barrington KJ, Nitric oxide therapy for the newborn infant, Semin. Perinatol 24 (2000) 59–65, 10.1016/s0146-0005(00)80058–0. [DOI] [PubMed] [Google Scholar]
- 164.[] Cooling L, The RBC as a physiological object, in: McManus LM, Mitchell RN (Eds.), Pathobiology of Human Disease, Academic Press, San Diego, 2014, ISBN 978–0-12–386457-4, pp. 3049–3067. [Google Scholar]
- 165.Cefalu JN, Joshi TV, Spalitta MJ, Kadi CJ, Diaz JH, Eskander JP, Cornett EM, Kaye AD, Methemoglobinemia in the operating room and intensive care unit: early recognition, pathophysiology, and management, Adv. Ther 37 (2020) 1714–1723, 10.1007/s12325-020-01282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Loh E, Stamler JS, Hare JM, Loscalzo J, Colucci WS, Cardiovascular effects of inhaled nitric oxide in patients with left ventricular dysfunction, Circulation 90 (1994) 2780–2785, 10.1161/01.cir.90.6.2780. [DOI] [PubMed] [Google Scholar]
- 167.Christenson J, Lavoie A, O’Connor M, Bhorade S, Pohlman A, Hall JB, The incidence and pathogenesis of cardiopulmonary deterioration after abrupt withdrawal of inhaled nitric oxide, Am. J. Respir. Crit. Care Med 161 (2000) 1443–1449, 10.1164/ajrccm.161.5.9806138. [DOI] [PubMed] [Google Scholar]
- 168.Mychaskiw G, Sachdev V, Heath BJ, Sildenafil (Viagra) facilitates weaning of inhaled nitric oxide following placement of a biventricular-assist device, J. Clin. Anesth 13 (2001) 218–220, 10.1016/s0952-8180(01)00252–5. [DOI] [PubMed] [Google Scholar]
- 169.[] Sildenafil Ameliorates Effects of Inhaled Nitric Oxide Withdrawal. - PubMed Available online: https://pubmed-ncbi-nlm-nih-gov.proxy.unimib.it/10422958/ (accessed on 24 October 2021).
- 170.Ruan S-Y, Huang T-M, Wu H-Y, Wu H-D, Yu C-J, Lai M-S, Inhaled nitric oxide therapy and risk of renal dysfunction: a systematic review and meta-analysis of randomized trials, Crit. Care 19 (2015) 137, 10.1186/s13054-015-0880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yu B, Blaesi AH, Casey N, Raykhtsaum G, Zazzeron L, Jones R, Morrese A, Dobrynin D, Malhotra R, Bloch DB, et al. , Detection and removal of impurities in nitric oxide generated from air by pulsed electrical discharge, Nitric Oxide 60 (2016) 16–23, 10.1016/j.niox.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yu B, Muenster S, Blaesi AH, Bloch DB, Zapol WM, Producing nitric oxide by pulsed electrical discharge in air for portable inhalation therapy, Sci. Transl. Med 7 (2015) 294ra107, 10.1126/scitranslmed.aaa3097. [DOI] [PubMed] [Google Scholar]
- 173.Yu B, Ferrari M, Schleifer G, Blaesi AH, Wepler M, Zapol WM, Bloch DB, Development of a portable mini-generator to safely produce nitric oxide for the treatment of infants with pulmonary hypertension, Nitric Oxide 75 (2018) 70–76, 10.1016/j.niox.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Berra L, Rodriguez-Lopez J, Rezoagli E, Yu B, Fisher DF, Semigran MJ, Bloch DB, Channick RN, Zapol WM, Electric plasma–generated nitric oxide: hemodynamic effects in patients with pulmonary hypertension, Am. J. Respir. Crit. Care Med 194 (2016) 1168–1170, 10.1164/rccm.201604-0834LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gianni S, Di Fenza R, Araujo Morais CC, Fakhr BS, Mueller AL, Yu B, Carroll RW, Ichinose F, Zapol WM, Berra L, A comparison between high dose nitric oxide delivered from pressurized cylinders and nitric oxide produced by an electric generator from air. A safety pilot study, Respir. Care (2021), 09308, 10.4187/respcare.09308respcare. [DOI] [PubMed] [Google Scholar]
- 176.Yu B, Zapol WM, Berra L, Electrically generated nitric oxide from air: a safe and economical treatment for pulmonary hypertension, Intensive Care Med. 45 (2019) 1612–1614, 10.1007/s00134-019-05756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.