Summary
Background
Pulmonary tuberculosis (PTB) can result in long-term health consequences, even after successful treatment. We conducted a systematic review and meta-analysis to estimate the occurrence of respiratory impairment, other disability states, and respiratory complications following successful PTB treatment.
Methods
We identified studies from January 1, 1960, to December 6, 2022, describing populations of all ages that successfully completed treatment for active PTB and had been assessed for at least one of the following outcomes: occurrence of respiratory impairment, other disability states, or respiratory complications following PTB treatment. Studies were excluded if they reported on participants with self-reported TB, extra-pulmonary TB, inactive TB, latent TB, or if participants had been selected on the basis of having more advanced disease. Study characteristics and outcome-related data were abstracted. Meta-analysis was performed using a random effects model. We adapted the Newcastle Ottawa Scale to evaluate the methodological quality of the included studies. Heterogeneity was assessed using the I2 statistic and prediction intervals. Publication bias was assessed using Doi plots and LFK indices. This study is registered with PROSPERO (CRD42021276327).
Findings
61 studies with 41,014 participants with PTB were included. In 42 studies reporting post-treatment lung function measurements, 59.1% (I2 = 98.3%) of participants with PTB had abnormal spirometry compared to 5.4% (I2 = 97.4%) of controls. Specifically, 17.8% (I2 = 96.6%) had obstruction, 21.3% (I2 = 95.4%) restriction, and 12.7% (I2 = 93.2%) a mixed pattern. Among 13 studies with 3179 participants with PTB, 72.6% (I2 = 92.8%) of participants with PTB had a Medical Research Council dyspnoea score of 1–2 and 24.7% (I2 = 92.2%) a score of 3–5. Mean 6-min walk distance in 13 studies was 440.5 m (I2 = 99.0%) in all participants (78.9% predicted, I2 = 98.9%) and 403.0 m (I2 = 95.1%) among MDR-TB participants in 3 studies (70.5% predicted, I2 = 97.6%). Four studies reported data on incidence of lung cancer, with an incidence rate ratio of 4.0 (95% CI 2.1–7.6) and incidence rate difference of 2.7 per 1000 person-years (95% CI 1.2–4.2) when compared to controls. Quality assessment indicated overall low-quality evidence in this field, heterogeneity was high for pooled estimates of nearly all outcomes of interest, and publication bias was considered likely for almost all outcomes.
Interpretation
The occurrence of post-PTB respiratory impairment, other disability states, and respiratory complications is high, adding to the potential benefits of disease prevention, and highlighting the need for optimised management after successful treatment.
Funding
Canadian Institutes of Health Research Foundation Grant.
Keywords: Tuberculosis, Tuberculosis treatment, Disability, Impairment, Complications
Research in context.
Evidence before this study
We searched four databases between Jan 1, 1960, and Dec 6, 2022, with no restriction by language, for studies of any design evaluating respiratory impairment, disability states, and respiratory complications following treatment completion for pulmonary tuberculosis (PTB). Our search comprised comprehensive terms for “tuberculosis”, “impairment”, “disability”, and “complication”. Previous studies have assessed, to some degree, the prevalence of disability, respiratory impairment, and certain respiratory complications after pulmonary tuberculosis (PTB) treatment. A meta-analysis in 2015 revealed that patients with previous TB had threefold higher odds of developing chronic obstructive pulmonary disease (COPD), a review in 2021 estimated that approximately 1 in every 4 TB survivors develop some type of respiratory impairment, four reviews found a significant association between TB and subsequent lung cancer, and three reviews showed that post-TB participants generally had lower quality-of-life. Previous reviews included participants with self-reported TB, extra-pulmonary TB, inactive TB, and even latent TB. This has the potential to alter the true prevalence of respiratory impairment and complications due to pulmonary TB. Importantly, the extent to which post-PTB sequalae lead to health-related quality of life and long-term disability has not been well-defined, as previous reviews have not summarised activity-limiting dyspnoea based on the Medical Research Council (MRC) dyspnoea scale or exercise capacity using the 6-min walk test (6MWT). Additionally, previous studies have not estimated the occurrence of all three of these important post-PTB outcomes (post-PTB respiratory impairment, other disability states, and respiratory complications) in one comprehensive review.
Added value of this study
We did a systematic review and meta-analysis of 61 studies with 41,014 participants with PTB in health-care settings in 29 countries and 5 continents. We demonstrated that the occurrence of post-PTB respiratory impairment, other disability states, and complications is substantial. While the pattern of abnormalities differed between studies (17.8% obstruction, 21.3% restriction, and 12.7% a mixed pattern), over half of all persons had abnormal spirometry despite successful treatment for PTB. With regards to functional disability, post-TB participants had markedly lower-than-predicted scores on measures of exercise capacity (6MWT) and physical disability due to breathlessness (MRC dyspnoea scale) compared to controls. Our review found a fourfold higher risk of developing lung cancer in post-PTB participants compared to controls. Furthermore, persistent cavitation, fibrosis, bronchiectasis, and aspergilloma were common complications post-TB.
Implications of all the available evidence
The findings of this review highlight the important morbidity caused by PTB and demonstrate that PTB is a major cause of worldwide disability. This, in turn, emphasises the importance of prevention, including the greater potential benefits of TB preventive therapy (TPT) beyond simply mortality. To estimate the health burden that may remain after PTB treatment, future studies should translate measures of disability into quality-adjusted life years. Studies assessing the determinants of disability could lead to the development of strategies to limit or prevent disability, as well as to identify optimal management for this outcome.
Introduction
In 2021, WHO estimated that 10.6 million individuals worldwide developed tuberculosis (TB) and 1.6 million died, making TB the second leading cause of death among all infectious diseases, after COVID-19.1 There are an estimated 155 million TB survivors globally, translating to 1 out of every 50 persons alive in 2020.2 Although pulmonary tuberculosis (PTB) disease is curable when an effective regimen is used, the health burden of PTB does not end with successful treatment. In fact, after successful completion of PTB treatment, many individuals develop chronic respiratory complications such as abnormal lung function,3, 4, 5, 6, 7, 8, 9, 10 bronchiectasis,11,12 and lung cancer.13, 14, 15, 16, 17, 18 These complications may result in disability and reduced health-related quality of life.
Recent TB treatment guidelines have suggested that long-term health consequences should be assessed, but robust evidence to support specific recommendations is lacking.19 While previous systematic reviews have assessed the prevalence of respiratory impairment and certain respiratory complications after treatment of PTB, broader inclusion criteria were commonly used, such as including participants with self-reported TB, extra-pulmonary TB, inactive TB, and even latent TB.3, 4, 5, 6,13, 14, 15, 16, 17, 18 A previous systematic review demonstrated higher odds of developing lung cancer post-TB when studies of self-reported TB were excluded (OR 2.26 compared to 2.09).15 Therefore, it is unclear if the estimation of the occurrence of respiratory impairment and complications would be different with more stringent PTB inclusion criteria. Additionally, three previous reviews have demonstrated that persons post-TB generally score lower on health-related quality of life questionnaires,20, 21, 22 but previous reviews have not assessed the impact of TB on more objective measures of functional disability, such as activity-limiting dyspnoea based on the Medical Research Council (MRC) dyspnoea scale and exercise capacity using the 6-min walk test. Therefore, the extent to which post-TB respiratory impairments lead to limitations in function has not been well-defined. A comprehensive understanding of the health burden that may remain after treatment of PTB is needed to develop clinical guidance and inform clinical research in post-PTB management. We conducted a comprehensive systematic review and meta-analysis with strict post-PTB inclusion criteria to estimate the occurrence of respiratory impairment, other disability states, and respiratory complications following successful treatment of PTB.
Methods
Objectives
Our systematic review and meta-analysis are reported according to PRISMA guidelines (S1 PRISMA Checklist), and follows a protocol registered in PROSPERO (CRD42021276327). The overall objective of this systematic review was to estimate the occurrence of respiratory impairment, other disability states, and respiratory complications following PTB treatment.
Search strategy and study selection
We searched MEDLINE, Embase, Health Star, and Cochrane from January 1, 1960, to December 6, 2022, to identify studies describing populations that successfully completed treatment for PTB disease, in whom the development of long-term respiratory impairment, other disability states and/or complications were described. We chose to restrict our search from 1960 onwards because medical treatment for tuberculosis was often sub-optimal before then (e.g., Rifampin was approved by the FDA in 1971, and PZA was widely used only after 1980). Detailed information on the search strategy and search outcomes can be found in the supplementary material.
We included all languages in our search, as well as at the stage of title and abstract review, to identify studies for full text review. Studies published in English, French, Portuguese, Spanish, Korean, Japanese, Mandarin and Cantonese were eligible for inclusion. We did not have the translation resources available to critically assess articles written in other languages. The studies had to report on participants that had successfully completed PTB treatment, and who had been assessed for at least one of the outcomes of interest (i.e., respiratory impairment and other disability states, or complications, as defined below). Furthermore, included studies reported that the study participants or a subset of the participants had successfully completed treatment for PTB disease. PTB could be diagnosed either microbiologically or clinically based on history and/or radiology. For studies reporting post-TB complications, only those that reported sufficient information to permit the calculation of prevalence or incidence of post-TB complications were included. If more than one study reported results from the same group of participants, we included the information from the most relevant publication.
Studies were excluded if they reported on a sample size of less than 25 participants to try to avoid selection bias as well as possible publication bias in studies with extremely small sample sizes. We also excluded studies that reported on participants who self-reported a history of TB diagnosis and/or TB treatment (e.g., in population-based questionnaires). Participants with extra-pulmonary TB (including pleural TB or concomitant pleural involvement) were excluded from analysis. A study was excluded if it included participants with extra-pulmonary TB and did not stratify results separately for extra-pulmonary and pulmonary TB participants. We excluded extra-pulmonary TB as our aim was to identify respiratory impairment and disability, as well as pulmonary complications. Studies were excluded if participants were a sub-set of all persons with TB disease at that centre, and were selected on the basis of: (i) having more advanced or extensive disease, (such as those with destroyed lung on the basis of imaging, typically bilateral disease or cavitary disease); or, (ii) had respiratory impairment at baseline (e.g. a study which only included persons with abnormal spirometry), or (iii) were “author defined” as having severe disease. These were excluded to avoid selection bias for severe impairment. Studies were excluded if participants had PTB disease that had spontaneously resolved (i.e., resolved without treatment, or “inactive TB”). These diagnoses are largely based on radiological findings, which are non-specific, so there is no way to confirm that the participants actually had tuberculosis.23,24 Review articles, editorials, opinion letters, conference abstracts and convenience samples were also excluded. Two reviewers (JT and SL) independently screened titles, abstracts, and full text. When a consensus was not achieved, a third reviewer was consulted (MB).
Data extraction
Two reviewers (JT and SL) extracted 20% of the data using a standardised data form, then findings were checked for concordance. The agreement was high (95%); thus, the remaining data was extracted individually. Data extracted included study design, country, level of care of the study site (primary, secondary, or tertiary), inclusion criteria, and follow-up period. Given the retrospective nature of assessing post-TB outcomes in post-TB cross-sectional study designs, we considered these studies to be retrospective cohort studies. We collected information on the characteristics of the study populations including age, sex, height, weight, BMI, education level, smoking history, HIV status, and medical co-morbidities. Extracted TB-related data consisted of the method of TB diagnosis, treatment regimen, drug susceptibility, number of TB treatment episodes, sputum AFB smear grade, culture positivity, and time from onset of symptoms to diagnosis and treatment.
Mean spirometry data was extracted as well as the number of participants with different categories of lung impairment (obstructive, restrictive, or mixed patterns). Symptom assessment scores, values on disability measures, and all related reference values/ranges for these disability outcomes were extracted. For each type of post-TB complication, the diagnostic modality, history of the same complication prior to PTB, and the number of participants who did and did not develop the complication following TB treatment completion were extracted.
Quality assessment
We adapted the Newcastle Ottawa Scale for observational cohort studies to evaluate the methodological quality of the included studies. These included questions related to the ascertainment of exposure, outcome assessments, and follow-up period. Detailed information is displayed in Fig. S1. Two reviewers (JT and SL) independently assessed the risks of bias, and any disagreements were solved through consensus. Each domain was scored as either “yes” or “no”, and each “yes” domain was 1 point. Risk was categorised as either “low risk” or “some concerns”. To be deemed “low risk”, a study had to score a minimum of 5 points: 3 points from the three critical questions (questions #1, #6, and #7), and 2 points from the four remaining questions. All studies not meeting the criteria for “low risk” were deemed to have “some concerns” (Table S1). Of note, we chose to not use the Joanna Briggs Institute Critical Appraisal tool for Prevalence Studies because this tool guides whether to include or exclude an article based on quality assessment25; we believed that excluding articles based on quality would have led to the exclusion of many papers with valuable and pertinent findings.
Definitions of outcomes and measurement strategy
The outcomes considered and terminology used in this review were informed by WHO's International Classification of Function, Disability and Health (ICF).26 This classification recognises disability as a global construct encompassing impairment, activity limitations, and participation restrictions. We defined post-TB respiratory impairment based on lung function measurements (e.g., by spirometry), and post-TB disability was defined on the basis of exercise capacity measured by the 6-min walk test, and physical disability due to breathlessness as measured by the MRC dyspnoea scale. Health conditions that arise as complications of TB were also assessed.
Post-TB impairments were identified from pre-bronchodilator spirometry. Most studies did not measure lung volumes or diffusion capacity thus this information could not be used for classification of impairments. Of the studies reporting spirometric results, some reported the actual spirometric values (means or medians), other studies reported the number of persons with abnormal results classified by type, and other studies reported both spirometry values and classifications. Impairments were classified into three main types by the authors as a) obstruction, b) restriction, or c) mixed patterns.27 Most commonly, Obstruction was defined as a forced expiratory volume in 1 s/forced vital capacity ratio (FEV1/FVC) less than 70% or less than the lower limit of normal (LLN); Restriction was defined as FEV1/FVC ≥70% with FVC <80% predicted or < LLN; Mixed pattern was defined as FEV1/FVC <70% with FVC <80% predicted. Exceptions to these definitions are outlined in the appendix. It is well-established that a reduced FVC by itself does not prove a restrictive impairment and that lung volumes are needed to accurately classify restriction.27 However, included studies did not measure lung volumes, possibly because of the greater complexity and additional equipment required for such measurements (i.e., body plethysmograph). Therefore, restrictive impairments were defined based on spirometry alone.
Other post-TB disability states included the impact of shortness of breath on function, low functional exercise capacity, and poor health-related quality of life. The Medical Research Council (MRC) dyspnoea scale classifies, on a 1 to 5 scale (original) or 0 to 4 scale (modified) the extent to which breathlessness limits walking and activities of daily living with the highest value representing the most severe limitation.28 The Six Minute Walk Test (6MWT) is a measure of functional walking capacity and was originally developed for use with people with pulmonary conditions.29 The St. George Respiratory Questionnaire (SGRQ) is a standardised, airways disease specific respiratory questionnaire that can be used to measure health-related quality of life. It has been validated for use in the TB patient population and has been translated into 77 languages.30,31 The questionnaire comprises 50 items which are grouped under three components, namely “symptoms” (8 items) which measures respiratory symptoms, “activity” (16 items) which measures impairment of mobility or physical activity, and “impact” (26 items) which measures the psychosocial impact of disease. Total scores range from 0 to 100; higher scores correspond to worse health-related quality of life.
After the successful completion of treatment for PTB, respiratory complications included the persistence of cavitation, and the development of fibrosis, bronchiectasis, aspergilloma and lung cancer. Apart from lung cancer, all other complications were diagnosed based on radiological interpretation alone. Note that chronic pulmonary aspergillosis (CPA) is an umbrella term for a spectrum of diseases caused by various species of Aspergillus and may be further categorised into five subtypes, one of which is aspergilloma, defined as a radiographic image of a ball or mass within a cavity.32 The diagnosis of CPA ideally involves a combination of clinical presentation, radiological findings, positive cultures for Aspergillus, and serological tests (i.e., Aspergillus specific IgG). However, we accepted authors’ definition of aspergilloma based on radiological findings alone.
Statistical analysis
Post-TB respiratory impairment
A meta-analysis of the spirometry results was performed. First, we pooled the proportion of patients categorised by the original studies into three main patterns of spirometry abnormalities: obstruction, restriction, and mixed, as defined above. Generalised linear mixed models with random effects with a binomial distribution and logit link were used for the analysis. Pooled estimates were back transformed into proportions.33 We used the “metaprop” function from package “meta” (version 5.2.0) in R.
We pooled the following spirometric values: mean/median absolute FEV1 (L), percent predicted FEV1, absolute FVC (L), percent predicted FVC and ratio of FEV1/FVC. Studies reported mean or median spirometry results, so we first harmonised the data by transforming medians into means using the Box–Cox method34 and the package “estmeansd” (version 0.2.1) in R. We then used random-effects models and the inverse-variance weighting method to pool the mean spirometry results from each study using the “metamean” function from package “meta” in R (version 5.2.0).
To assess possible sources of heterogeneity, pre-specified analyses were performed and stratified by smoking status, HIV status, drug-susceptible or multidrug-resistant TB, microbiological confirmation of TB (yes/no), and timing of spirometry (e.g., at the time of diagnosis, at end-of-treatment, or after end-of-treatment). In post hoc analysis, we performed analyses stratified by income setting (high-income versus low- and middle-income) and by study level mean age of included participants. Age was stratified into the following categories: <18 years, 18–34 years, 35–49 years, 50–65 years, and >65 years.
Other post-TB disability outcomes
Three different measures of other disability states were synthesised in our review: the MRC scale, 6MWT, and the SGRQ.
Due to large and unexplained differences in SGRQ scores between studies, we felt pooling these would not have provided a meaningful summary. Instead, the individual scores were displayed for each study and for each time point to demonstrate the trend over time during and post-TB treatment.
For the measures using the MRC scale, we meta-analysed the proportions of patients with moderate to severe disability due to dyspnoea (i.e., walks slower than other people or stops after 100 m or unable to leave the house). The same model described above to pool proportions was used. Data was stratified by HIV status, drug resistance, and by pattern of spirometry abnormality to address possible heterogeneity. We also performed stratified analyses by mean age but not by country-level income because all studies reporting MRC data were from low- or middle-income countries.
Finally, 6MWT results were pooled. Equations described elsewhere35 were used to calculate the lower limit of normal for each population group based on their mean height, age, sex, and weight. To estimate the percent predicted, the mean distance reported in the study was divided by their respective calculated lower limit of normal. Random-effects models and inverse-variance weighting were then used to pool mean distances and mean predicted values. We also performed stratified analyses by drug sensitivity (MDR vs drug-sensitive) and age, but not by country-level income because all studies reporting 6MWT data were from low- or middle-income countries.
Post-TB respiratory complications
For five complications after successful PTB treatment: (i) the presence of persistent cavitation, (ii) fibrosis, (iii) bronchiectasis, (iv) aspergilloma and (v) lung cancer, we pooled the cumulative incidence as proportions using generalised linear mixed models with random effects with a binomial distribution and logit link. From studies of post-TB lung cancer that included non-TB controls and provided follow-up time in person-years, we estimated pooled risk ratios and risk differences. For this analysis, individual study outcome estimates were log or logit transformed and random effects meta-analysed using generalised linear mixed models with a Poisson distribution. Pooled estimates were again back transformed. For this analysis, we used function “matainc” from package “meta” (version 5.2.0) in R. We also performed stratified analyses by age and country-level income.
Meta-regression
We conducted seven meta-regression models. In the first model, we fit a logistic mixed effects regression model. The dependent variable was the proportion of patients with abnormal spirometry. The independent variable was categorical, where cohorts were classified as only patients with drug-susceptible or multidrug-resistant TB. Coefficients were exponentiated to obtain odds ratios. In the remaining models, we fitted a linear mixed effects regression model, in which the dependent variables were the study level mean of measurements of lung function (FEV1 (L), FVC (L), FEV1% predicted, FVC % predicted, FEV1/FVC ratio. The same independent variable described above was used. We reported the linear coefficients and their confidence intervals. We used the package meta in R (version 5; 2) using the metareg function.
Heterogeneity
Heterogeneity was assessed using the I2 statistic36 and prediction intervals.37
Publication bias
Publication bias was assessed visually using Doi plots and quantitatively using LFK indices.38 Publication bias was interpreted as unlikely if there was symmetry in the Doi plots and if LFK indices fell between −1.0 and + 1.0. Conversely, publication bias was considered likely if there was asymmetry in the Doi plots and if LFK indices were greater than 1.0 or less than −1.0. As previously recommended by Cochrane, we only assessed publication bias for outcomes reported in at least 10 cohorts.39
Ethics
Specific ethics approval was not required due to the study design.
Role of the funding source
The funding source had no role in study design, data collection, analysis, interpretation, or manuscript preparation.
Results
11,582 titles were identified in our search for titles published between Jan 1, 1960, and Dec 6, 2022 (Fig. 1). After excluding duplicates, 7385 abstracts were screened, of which 464 full texts were reviewed. Among those, 16 were excluded due to not having the translation resources available to critically assess articles in 7 languages. We included 60 studies and identified three additional studies from reference lists of the included studies. In total, 63 studies with 41,014 patients with PTB were included40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80,81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102. Of the 63 studies, 61 were unique study populations (two pairs of studies reported different post-TB assessments in the same study population52,53,73,99). For convenience, the 61 “study populations” will be referred to as “studies” throughout this manuscript. 14 of the 61 studies included control groups; 4 studies with 816,079 controls assessed post-TB lung cancer and 10 studies with 45,829 controls assessed post-TB lung impairment. Using the quality assessment tool shown in Fig. S1, overall, 22 studies were deemed "low risk” of bias, and 39 studies “some concerns” of bias (Table S1). Information on how control groups were matched to TB groups can be found in Table S2.
Fig. 1.
PRISMA flowchart of selected studies. PTB, pulmonary tuberculosis; TB, tuberculosis.
20 studies were prospective cohorts, and forty-one were retrospective cohorts (Table 1). No randomised trials or case–control studies were included. The majority of studies (50/61, 82%) were from low- or middle-income countries. Twelve studies assessed only patients with DS-TB (drug-sensitive TB), four assessed only patients with multidrug-resistant TB (MDR-TB), 11 included patients with DS-TB and MDR-TB, and 34 did not report data on drug susceptibility. In the 45 studies reporting age, the mean age was 41.0 (SD 11.0). In the 17 studies reporting BMI, the mean BMI was 22.1 (SD 2.0). In the 13 studies that reported smoking pack-years, the mean pack-year was 12.0 (SD 11.1). Additional patient and study characteristics are described in Tables S2 and S3.
Table 1.
Summary of characteristics of included studies and participants.
| Characteristics | Study populations (n = 61) | TB participants (n = 41,014) |
|---|---|---|
| Study characteristics | ||
| Study design | ||
| Retrospective | 41 | 37,555 |
| Prospective | 20 | 3459 |
| Inclusion of comparator group | ||
| No: Only TB participants | 48 | 19,900 |
| Yes: TB patients and controlsa | 13 | 21,114 |
| World Bank income category for country of study | ||
| High income | 10 | 30,355 |
| Low or middle income | 50 | 10,598 |
| Both low and high income (multicentric study) | 1 | 61 |
| Level of care at study site | ||
| Primary care | 4 | 485 |
| Secondary care | 21 | 3793 |
| Tertiary care | 23 | 5288 |
| Mixed care | 3 | 6536 |
| Not reported | 10 | 24,912 |
| Participant characteristics | ||
| Demographic | ||
| Male sex | ||
| ≥60% of population | 28 | 13,950 |
| <60% of population | 24 | 12,111 |
| Female sex | ||
| ≥60% of population | 3 | 465 |
| <60% of population | 49 | 25,596 |
| Sex not reported | 9 | 14,953 |
| Mean age (SD)c | 45 | 41.0 (11.0) |
| Mean BMI (SD)c | 17 | 22.1 (2.0) |
| DST status | ||
| Only DS-TB | 12 | 3071 |
| Only MDR-TB | 4 | 225 |
| Mixed DS-TB and MDR-TB | 11 | 1168 |
| Not reported | 34 | 36,550 |
| Method of confirmation of TB diagnosis | ||
| Microbiologically confirmed | 26 | 6271 |
| Mixed (clinical, radiological, microbiological)b | 7 | 1833 |
| Not reported | 28 | 32,910 |
| Smoking status | ||
| All never-smokers | 5 | 1760 |
| All ever-smokers | 1 | 44 |
| Mixed | 44 | 15,640 |
| Not reported | 11 | 23,570 |
| Mean pack/year (SD)c | 13 | 12.0 (11.1) |
| HIV status | ||
| All HIV negative | 7 | 691 |
| All HIV positive | 2 | 216 |
| Mixed | 23 | 9419 |
| Not reported | 29 | 30,688 |
| Outcomes | ||
| Pulmonary function | 51 | |
| Only reported spirometry measures | 9 | 3955 |
| Reported only classification of abnormality | 13 | 2870 |
| Reported both spirometry measures and classification of abnormality | 29 | 3952 |
| Post-TB complications | 20 | |
| Lung cancer | 5 | 32,951 |
| Extra-pulmonary cancer | 2 | 11,988 |
| Bronchiectasis | 7 | 2547 |
| Aspergilloma | 4 | 1710 |
| Cavitation | 11 | 1664 |
| Fibrosis | 5 | 1403 |
| Measures of disability | 30 | |
| Medical Research Council (MRC) dyspnoea scale | 13 | 3179 |
| Six-minute walk test (6MWT) | 16 | 1491 |
| St. George's Respiratory Questionnaire (SGRQ) | 8 | 1212 |
TB, tuberculosis; DST, drug sensitivity; DS-TB, drug susceptible tuberculosis; MDR-TB, multidrug-resistant tuberculosis; SD, standard deviation; BMI, body mass index.
Total Control participants included = 861,908.
Of the studies that confirmed TB with mixed methods, the proportion of diagnoses confirmed microbiologically ranged from 40% to 80%.
Shown as the number of studies in the population which reported on the characteristic, followed by the mean (SD).
Among 38 studies reporting actual results of spirometry (Table 2 and Table S4), the pooled mean FEV1 was 71.1% predicted, pooled mean FVC was 77.8% predicted, and pooled mean FEV1/FVC ratio was 0.76. Heterogeneity, based on the estimated I squared values, was high for most of these pooled analyses. Ever-smokers had lower mean values of all three parameters than never-smokers. Participants treated for MDR-TB had overall lower mean values than participants treated for DS-TB. When results were stratified by country level income, there was a notable decrease in the percent predicted FEV1 and FVC in participants in low- or middle-income countries compared to high-income countries, but no difference when stratified by participant age or study risk of bias.
Table 2.
Lung function abnormalities: Pooled spirometry results among studies that reported actual measurements of lung function.
| Meta-analysis | Number of study populations | Pooled estimate (95% CI) | Prediction interval | I2 |
|---|---|---|---|---|
| All TB patients | ||||
| FEV1 (L) | 24 | 2.2 (2.0–2.3) | 1.4 to 3.0 | 97.0% |
| FEV1% pred | 32 | 71.1 (66.5–75.7) | 44.3 to 97.9 | 97.2% |
| FVC (L) | 23 | 3.0 (2.8–3.1) | 2.2 to 3.7 | 95.8% |
| FVC % pred | 28 | 77.8 (73.5–82.1) | 54.3 to 101.4 | 97.1% |
| FEV1/FVC ratio | 31 | 0.76 (0.73–0.79) | 0.6 to 0.9 | 97.8% |
| Controls | ||||
| FEV1 (L) | 3 | 2.9 (2.6–3.1) | – | 92.3% |
| FEV1% pred | 2 | 89.1 (84.4–93.9) | – | 79.2% |
| FVC (L) | 2 | 3.2 (3.2–3.3) | – | 20.3% |
| FVC % pred | 2 | 83.9 (68.4–99.3) | – | 97.5% |
| FEV1/FVC ratio | 2 | 0.89 (0.76–1.02) | – | 99.5% |
| DS-TB | ||||
| FEV1 (L) | 6 | 2.2 (1.9–2.6) | 1.0 to 3.5 | 97.7% |
| FEV1% pred | 6 | 74.3 (70.6–78) | 62.3 to 86.3 | 77.2% |
| FVC (L) | 5 | 3.2 (2.9–3.5) | 2.1 to 4.2 | 92.8% |
| FVC % pred | 6 | 79.4 (72.9–85.9) | 56.0 to 102.7 | 90.8% |
| FEV1/FVC ratio | 7 | 0.77 (0.72–0.83) | 0.6 to 1.0 | 99.0% |
| MDR-TB | ||||
| FEV1 (L) | 4 | 2.1 (1.8–2.4) | 0.6 to 3.6 | 85.5% |
| FEV1% pred | 5 | 57.6 (43.2–72) | 1.4 to 100.0 | 96.9% |
| FVC (L) | 4 | 2.8 (2.6–3.1) | 1.9 to 3.8 | 61.0% |
| FVC % pred | 4 | 68.0 (51.6–84.4) | 0.0 to 100.0 | 95.0% |
| FEV1/FVC ratio | 5 | 0.71 (0.70–0.80) | 0.5 to 0.9 | 84.1% |
Pooled estimates included all persons at risk for respiratory impairment (I.e., the denominator in these estimates was all persons post-TB in the studies reporting on post-TB respiratory impairment).
“-“ Denotes prediction interval was not able to be estimated.
TB, tuberculosis; HIV, human immunodeficiency virus; DS-TB, drug susceptible tuberculosis; MDR-TB, multidrug-resistant tuberculosis; FEV1, forced vital capacity in 1 second; FVC, forced vital capacity; %pred., percentage of predicted value; L, litres; CI, confidence interval.
Among 42 studies which reported spirometry abnormalities classified by type, the pooled proportion of TB participants with abnormal spirometry was 59.1% compared to 5.4% of controls (Table 3 and Fig. S2). The precise definition of spirometry abnormality varied by study (Table S5). The pooled proportions of type of spirometry abnormalities were: 17.8% obstruction, 21.3% restriction, and 12.7% mixed pattern. When stratified by smoking history, a higher proportion of ever-smokers had obstruction compared to never-smokers. When results were stratified by country level income, there was a notable decrease in the pooled proportion of participants with abnormal spirometry and obstruction in participants from low-or middle-income countries, but no difference when stratified by participant age or study risk of bias (Table S6). When stratified by time since diagnosis (Tables S7 and S8), the proportions of participants with any spirometry abnormalities – or types of abnormalities – were different at different time points, but without any clear pattern of worsening or resolution over time; the measurements at different time-points were made in different studies.
Table 3.
Lung function abnormalities: Pooled proportions of TB participants with different types of spirometry abnormalities.a
| Meta-analysis | Number of study populations | Proportion of participants (n/N) | Pooled estimate (95% CI) | Prediction intervals | I2 |
|---|---|---|---|---|---|
| All TB patients | |||||
| Abnormal | 42 | 4082/9864 | 59.1% (48.8%–68.7%) | 8.4%–95.8% | 98.3% |
| Obstruction | 41 | 1882/9803 | 17.8% (13.4%–23.1%) | 2.5%–64.5% | 96.6% |
| Restriction | 35 | 1339/5822 | 21.3% (15.3%–28.9%) | 2.3%–75.7% | 95.4% |
| Mixed pattern | 30 | 861/5095 | 12.7% (8.2%–19.2%) | 1.0%–68.9% | 93.2% |
| Controls | |||||
| Abnormal | 4 | 794/21,804 | 5.4% (2.6%–10.8%) | 0.2%–67.7% | 97.4% |
| Obstruction | 4 | 794/21,804 | 5.4% (2.6%–10.8%) | 0.2%–67.7% | 97.4% |
| Restriction/Mixed | 0 | – | – | – | – |
| DS-TB | |||||
| Abnormal | 12 | 1677/2900 | 54.4% (41.8%–66.5%) | 13.7%–89.9% | 96.3% |
| Obstruction | 12 | 777/2900 | 16.8% (9.6%–27.5%) | 1.7%–70.6% | 94.2% |
| Restriction | 11 | 493/2771 | 16.1% (6.8%–33.8%) | 0.5%–89.0% | 96.9% |
| Mixed pattern | 7 | 407/2086 | 14.5% (6.5%–29.4%) | 0.7%–80.4% | 96.4% |
| MDR-TB | |||||
| Abnormal | 7 | 178/286 | 85.9% (49.5%–97.4%) | 1.1%–100.0% | 93.3% |
| Obstruction | 7 | 57/286 | 18.8% (11.3%–29.6%) | 3.5%–59.9% | 71.9% |
| Restriction | 5 | 41/161 | 24.2% (15.5%–35.9%) | 6.0%–61.7% | 27.% |
| Mixed pattern | 4 | 80/134 | 60.1% (47.3%–71.6%) | 18.0%–91.1% | 60.5% |
| HIV positive | |||||
| Abnormal | 3 | 111/398 | 27.8% (19.4%–38.0%) | 0.2%–98.8% | 79.6% |
| Obstruction | 3 | 54/398 | 13.9% (10.1%–18.8%) | 0.9%–75.1% | 44.3% |
| Restriction | 2 | 54/316 | 17.1% (13.3%–21.6%) | – | – |
| Mixed pattern | 1 | 3/85 | 3.5% (1.1%–10.4%) | – | – |
| HIV negative | |||||
| Abnormal | 7 | 739/985 | 82.6% (65%–92.4%) | 14.9%–99.2% | 95.2% |
| Obstruction | 7 | 396/985 | 30.1% (17.1%–47.3%) | 3.0%–85.5% | 94.3% |
| Restriction | 6 | 123/728 | 17.3% (14.0%–21.3%) | 10.4%–27.4% | 35.7% |
| Mixed pattern | 6 | 220/826 | 30.3% (15.2%–51.4%) | 1.7%–91.7% | 92.6% |
Pooled estimates included all persons at risk for respiratory impairment (I.e., the denominator in these estimates was all persons post-TB in the studies reporting on post-TB respiratory impairment).
“-“ Denotes prediction interval was not able to be estimated.
TB, tuberculosis; HIV, human immunodeficiency virus; DS-TB, drug susceptible tuberculosis; MDR-TB, multidrug-resistant tuberculosis; FEV1, forced vital capacity in 1 s; FVC, forced vital capacity; %pred., percentage of predicted value; L, litres; CI, confidence interval; LLN, lower limit of normal.
Definitions of spirometry abnormalities: Obstruction pattern = FEV1/FVC < LLN or <70%; Restriction pattern = FEV1/FVC ratio >70% and FVC <80% or < LLN; Mixed pattern = FEV1/FVC ratio <70% and FVC <80% pred. Exceptions to these definitions are outlined in the appendix (Table S5).
In meta-regression, there was a statistically significant reduction in percent predicted FEV1 in MDR-TB versus DS-TB participants (−16.16, 95% CI: −29.95 to −2.37). Similarly, MDR-TB participants had four-fold higher odds of spirometry abnormalities than DS-TB participants OR 3.97 (95% CI 0.98 to 16.19), but this was of borderline statistical significance (Table 4).
Table 4.
Meta-regression of spirometry results.
| Linear meta-regression of spirometry measurements in MDR compared to DS-TB | ||
|---|---|---|
| Meta-regression | Number of study populations | Coefficients (95% CI) |
| FEV1 (L) | 10 | −0.17 (−0.66 to 0.33) |
| FEV1% predicted | 11 | −16.16 (−29.95 to −2.37)a |
| FVC (L) | 9 | −0.33 (−0.73 to 0.06) |
| FVC % predicted | 10 | −10.98 (−26.13 to 4.17) |
| FV1/FVC ratio | 12 | −0.07 (−0.15 to 0.02) |
| Logistic meta-regression of odds of abnormala spirometry results | ||
|---|---|---|
| Meta-regression | Number of study populations | Odds ratio (95% CI) |
| DS-TB | 19 | Reference |
| MDR-TB | 19 | 3.97 (0.98–16.19) |
Pooled estimates included all persons at risk for respiratory impairment (i.e., the denominator in these estimates was all persons post-TB in the studies reporting on post-TB respiratory impairment).
DS-TB, drug susceptible tuberculosis; MDR-TB, multidrug-resistant tuberculosis; FEV1, forced vital capacity in 1 s; FVC, forced vital capacity; %pred., percentage of predicted value; L, litres; CI, Confidence Interval.
Any type of spirometry abnormality including obstruction, restriction, or mixed pattern.
Tables S9 and S10 summarise the post-TB disability and post-TB complication outcomes assessed by individual studies. As seen in Table 5, among 13 studies with 3179 participants with PTB, at the end of treatment, 72.6% had an MRC score of 1–2 and 24.7% had a score of 3–5. A greater proportion of participants with restriction had MRC scores >2, compared to participants with obstruction (see also Table S11). When results were stratified by participant age and study risk of bias, no notable differences were observed (Table S12).
Table 5.
Pooled estimates of Medical Research Council (MRC) Dyspnoea score.a
| Population | Outcome | Number of study populations | Proportion of participants (n/N) | Pooled estimate (95% CI) | Prediction interval | I2 |
|---|---|---|---|---|---|---|
| All TB patients | MRC 1–2b | 13 | 2439/3179 | 72.6% (64.4%–79.6%) | 35.8%–92.7% | 92.8% |
| MRC 3–5b | 13 | 706/3179 | 24.7% (18.8%–31.7%) | 7.6%–56.8% | 92.2% | |
| MDR-TB | MRC 1–2 | 1 | 24/32 | 75.0% (57.4%–87.0%) | – | – |
| MRC 3–5 | 1 | 8/32 | 25.0% (13.0%–42.6%) | – | – | |
| DS-TB | MRC 1–2 | 1 | 119/144 | 82.6% (75.6%–88.0%) | – | – |
| MRC 3–5 | 1 | 25/144 | 17.4% (12.0%–24.4%) | – | – | |
| HIV positive | MRC 1–2 | 2 | 336/443 | 75.8% (71.6%–79.6%) | – | 21.5% |
| MRC 3–5 | 2 | 107/443 | 24.2% (20.4%–28.4%) | – | 21.5% | |
| HIV negative | MRC 1–2 | 2 | 266/358 | 74.3% (69.5%–78.6%) | – | 1.3% |
| MRC 3–5 | 2 | 92/358 | 25.7% (21.4%–30.5%) | – | 1.3% | |
| Obstructionc | MRC 1–2 | 2 | 185/227 | 81.5% (75.9%–86.0%) | – | 63.1% |
| MRC 3–5 | 2 | 42/227 | 18.5% (14.0%–24.1%) | – | 63.1% | |
| Restrictionc | MRC 1–2 | 2 | 129/196 | 69.6% (52.1%–82.8%) | – | 88.7% |
| MRC 3–5 | 2 | 67/196 | 30.4% (17.2%–47.9%) | – | 88.7% | |
| Mixed patternc | MRC 1–2 | 2 | 98/170 | 56.6% (38.4%–73.3%) | – | 90.8% |
| MRC 3–5 | 2 | 72/170 | 43.4% (26.7%–61.6%) | – | 90.8% |
Pooled estimates included all persons at risk for disability (I.e., the denominator in these estimates was all persons post-TB in the studies reporting on post-TB MRC scores).
MRC Scale: 1 = no dyspnoea except with strenuous exercise, 2 = dyspnoea when hurrying on the level or walking up a slight hill, 3 = walks slower than people of the same age on the level because of dyspnoea or has to stop for breath when walking at own pace on the level, 4 = stops for breath after walking 100 m or after a few minutes on the level, 5 = too dyspneic to leave the house or breathless when dressing.
“-“ Denotes prediction interval was not able to be estimated.
TB, tuberculosis; HIV, human immunodeficiency virus; DS-TB, drug susceptible tuberculosis; MDR-TB, multidrug-resistant tuberculosis; FEV1, forced vital capacity in 1 s; FVC, forced vital capacity; CI, confidence interval.
Due to pooling, estimates (%) do not always add up to 100%. MRC was measured at the end of treatment or after treatment completion.
Or an equivalent score on the Modified Medical Research Council (mMRC) scale. An MRC score of 1–2 is equivalent to an mMRC score of 0–1. An MRC score of 3–5 is equivalent to an mMRC score of 2–4.
As measured by spirometry after 6 months of treatment. Manji [70], performed spirometry after 5 months of treatment. Only measures at end of treatment were used for this table. Definitions of spirometry abnormalities: Obstruction pattern = FEV1/FVC < LLN or <70%; Restriction pattern = FEV1/FVC ratio >70% and FVC <80% or < LLN; Mixed pattern = FEV1/FVC ratio <70% and FVC <80% pred. Exceptions to these definitions are outlined in the appendix (Table S5).
In 23 studies, the mean 6MWT performed at the end of – or after – treatment was 431.5 m in all participants (Table 6 and Table S13). In the 13 studies for which the percent predicted could be calculated, the pooled mean distance was 440.5 m which corresponded to 78.9% predicted. In 5 studies of only DS-TB participants, the pooled mean 6MWT was 474.6 m (84,3% predicted) compared to 403.0 m in 3 studies which included MDR-TB or DR-TB participants (70.5% predicted). When results were stratified by participant age and study risk of bias, no notable differences were observed (Table S14).
Table 6.
Pooled mean estimates of 6-min walk test (6MWT).a
| Population | All studies |
Only studies in which percent predicted could be calculated |
|
|---|---|---|---|
| Pooled estimate (m) | Pooled estimate (m) | Pooled percent predicted (%) | |
| Allb | |||
| Number of study populations | 23 | 13 | 13 |
| Outcome (95% CI) | 431.5 (390.4–472.6) | 440.5 (395.2–485.8) | 78.9 (69.2–90.0) |
| Prediction interval | 220.2 to 642.8 | 253.5 to 627.4 | 45.8 to 100.0 |
| I2 | 99.4% | 99.0% | 98.9% |
| MDR-TB or DR-TB | |||
| Number of study populations | 7 | 3 | 3 |
| Outcome (95% CI) | 372.4 (270.7–474.1) | 403.0 (294.2–511.7) | 70.5 (49.9–99.6) |
| Prediction interval | 0.0 to 747.4 | – | – |
| I2 | 99.6% | 95.1% | 97.6% |
| Only DS-TB | |||
| Number of study populations | 10 | 5 | 5 |
| Outcome (95% CI) | 465.2 (422.7–507.7) | 474.6 (396–553.3) | 84.3 (70.8–100.3) |
| Prediction interval | 303.8 to 626.6 | 166.0 to 783.3 | 42.6 to 100.0 |
| I2 | 99.2% | 99.6% | 99.4% |
Pooled estimates included all persons at risk for disability (I.e., all persons post-TB who underwent 6MWT).
“-“ Denotes prediction interval was not able to be estimated.
TB, tuberculosis; MDR-TB, multidrug-resistant tuberculosis; DS-TB, drug sensitive tuberculosis; EOT, end of treatment; m, metres; CI, confidence interval.
6MWT was measured at end of treatment or after treatment completion.
Includes both MDR-TB and DS-TB studies, and studies that either reported mixed populations (MDR and DS). Note also that some studies reported 6MWT results for multiple cohorts.
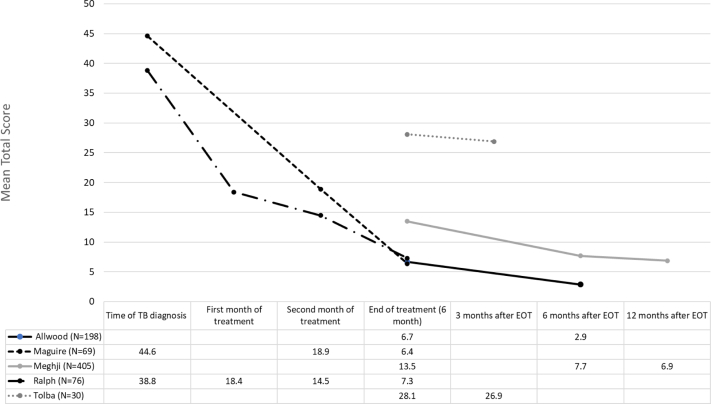
SGRQ scores varied widely, without clear reasons for the large differences between studies. However, a more consistent finding was that scores were worse at the time of TB diagnosis and improved during treatment and thereafter (Fig. 2, Tables S15 and S16).
Fig. 2.
Mean total St. George's Respiratory Questionnaire scores at different time points. TB, tuberculosis; EOT, end of treatment.
The occurrence of several post-TB complications ranged from 1.9% developing aspergillomas to 37.4% developing fibrosis, 16.8% bronchiectasis, and 31.4% persistent cavitation (Tables 7 and S17). Four studies reported data on incidence of lung cancer, with an incidence rate ratio of 4.0 (95% CI: 2.1–7.6) and an incidence rate difference of 2.7 per 1000 person-years (95% CI: 1.2–4.2) when compared to controls (Table 8). As summarised in Table S18, two studies adjusted for cigarette smoking, and the other two adjusted for incidence of chronic obstructive pulmonary disease (COPD) – a proxy of smoking exposure.
Table 7.
Incidence of complications: Pooled estimates of incidence of complications after successful TB treatment.
| Population | Outcome | n | Cavitation | Fibrosis | Bronchiectasis | Aspergilloma |
|---|---|---|---|---|---|---|
| TB | Number of study populations | 5 | 11 | 5 | 7 | 4 |
| Proportion of participants (n/N) | 680/32,951 | 438/1479 | 402/1403 | 423/2547 | 32/1710 | |
| Pooled estimate (95% CI) | 2.1% (1.2%–3.9%) | 31.4% (18.6%–47.7%) | 37.4% (21.0%–57.5%) | 16.8% (5.1%–43.4%) | 1.9% (0.9%–4.1%) | |
| I2 | 98.1% | 93.4% | 98.3% | 98.9% | 82.9% | |
| Prediction interval | 0.2%–19.8% | 3.0%–87.0% | 2.4%–93.6% | 0.2%–96.4% | 0.1%–39.9% |
Pooled estimates included all persons at risk for complications (I.e., the denominator in these estimates was all persons post-TB in the studies reporting on post-TB complications).
TB, tuberculosis; CI, confidence interval.
Table 8.
Incidence of complications: Pooled estimates of incidence of lung cancer after successful TB treatment in studies also reporting incidence in non-TB controls.
| Population | Number of study populations | Proportion of participants with lung cancer (n/N) | Lung cancer per 1000 PY (95% CI) | Incidence rate ratio (95% CI) | Incidence Rate Difference per 1000 PY (95% CI) |
|---|---|---|---|---|---|
| TB | 4 | 290/89,429 | 3.9 (2.5–6)a | 4 (2.1–7.6) | 2.7 (1.2–4.2) |
| Controls | 4 | 3197/7,288,122 | 1 (0.4–2.4)b | NA | NA |
Pooled estimates included all persons at risk for complications (I.e., the denominator in these estimates was all persons post-TB in the studies reporting on post-TB complications).
TB, tuberculosis; PY, person-years; CI, confidence interval; NA, not applicable.
Prediction interval: 0.49 to 30.64, I2 = 92.3%.
Prediction interval: 0 to 7.4, I2 = 99.9%.
Estimates of heterogeneity for most of the pooled estimates of outcomes were significant (i.e., I2 50–80%) or substantial (i.e., I2 >80%) with wide prediction intervals, as seen in Table 2, Table 3, Table 5, Table 6, Table 7, Table 8, and Tables S4, S6–8, S12, S14, S17. Publication bias was considered unlikely for studies reporting the mean post-TB FVC percent predicted (LFK index −0.7) but likely for all other outcomes (Fig. S3).
Discussion
In this systematic review and meta-analysis, the occurrence of post-PTB impairment, functional disability and complications was high. While the pattern of abnormalities differed between studies, over half of all persons had abnormal spirometry following successful treatment for PTB. With regards to functional disability, a considerable proportion (24.7%) of post-PTB participants had activity-limiting dyspnoea (i.e., an MRC score of greater than 2), and patients achieved only 78.9% of predicted on the 6-min walk test. As for post-PTB complications, a striking finding in our review was the fourfold higher risk of developing lung cancer in post-PTB participants compared to controls.
As summarised in Table S19, a systematic review in 2011 reported an odds ratio range of 2.6–8.9 for developing COPD and estimated an annual decline in FEV1 and FVC of 30–45 mL.4 A systematic review from 2013 reported that nearly 90% of studies reported a positive association between PTB and chronic airflow obstruction,5 and a meta-analysis in 2015 demonstrated a threefold higher odds of developing COPD after TB.6,10 A more recent 2021 meta-analysis reported the pooled prevalence of respiratory impairment (defined as the development of any type of abnormal lung function or respiratory complication) to be 23.1% overall, and higher in DR-TB than DS-TB participants.3 Using more strict TB-related inclusion criteria, in our review the proportion of persons with abnormal spirometry was higher at 59.1%, with 62.3% vs 9.3% of persons with abnormal spirometry in studies in low-middle compared to high-income countries. We also found a four-fold increase in odds of spirometry abnormalities in MDR-TB compared to DS-TB participants. We did not assess changes in lung function parameters over time, but we found that post-TB participants had significantly lower lung function parameters than controls.
Our review found that lung cancer incidence was substantially higher among post-PTB participants than among controls (IRR 4.0, 95% CI 2.1–7.6), as has been reported in six previous meta-analyses, although measures of association varied. A review published in 2009 estimated a relative risk of 2.9 (95% CI 1.6–5.3) after controlling for lifetime tobacco smoke exposure,18 a relative risk of 1.69 (95% CI 1.46–1.95) in a 2020 review,13 a pooled summary estimate of 1.85 (95% CI 1.52–2.25) in a 2021 review,16 a relative risk of 2.17 (95% CI 1.83–2.57) in a 2022 review,14 an odds ratio of 2.09 (95% CI 1.62–2.69) in another 2022 review,15 and finally an odds ratio of 1.74 (95% CI 1.42–2.13) in the most recent review after adjusting for age and smoking.17
Our review found the occurrence of several other post-TB complications to be high, which compares similarly to Meghji et al. who reported high prevalence of cavitation (8.3–83.7% on CXR versus 7.4–34.6% on CT), fibrosis (25.0–70.4% on CXR versus 70.0–92.6% on CT), and bronchiectasis (4.3–11.2% on CXR versus 35.0–86.0% on CT).12 We did not specifically examine if the incidence of these complications differed by the imaging modality utilised. However, of the 15 included studies which reported the incidence of post-TB complications based on imaging, 9 used CXR, 2 used CT, 2 used a combination of both CXR and CT, and 2 did not report the imaging modality used (Table S9). Therefore, Meghji's findings raise the possibility that the true incidence of certain complications, such as fibrosis and bronchiectasis, could be higher given that the sensitivity for these radiological diagnoses is higher on CT scan compared to CXR,103 and many centres in TB-endemic settings globally do not have access to CT imaging.
In addition to assessing outcomes of post-TB complications and impairment, this systematic review performed a comprehensive evaluation of post-TB functional disability. This review has demonstrated that the occurrence of post-PTB impairment, other disability states, and complications is substantial, with marked decrement in predicted scores across all outcomes. This highlights the negative impact of respiratory impairment and complications on the quality of life of TB-survivors. Furthermore, the scarcity of high-quality studies assessing post-TB disability and complete lack of intervention studies are important gaps in the literature.
There are several important limitations to consider. Notably, there was significant clinical heterogeneity among the included studies, related to differences in drug susceptibility results, smoking history, HIV status and age of participants. Based on the calculated I squared values and prediction intervals, heterogeneity was high for pooled estimates of nearly all outcomes of interest. Publication bias was considered likely for the majority of outcomes in which this could be assessed. Quality assessment also indicated overall low-quality evidence in this field. Hence, inferences should be made with caution. Reporting of clinical data was limited in many studies, making it challenging to assess the impact of clinical characteristics such as co-morbidities on measured outcomes. Studies differed in methods of ascertaining outcomes. For example, outcomes were measured at differing time points post-PTB treatment. Additionally, differing definitions were reported for the classification of spirometry abnormalities, and the classification of spirometry abnormalities was based on spirometry measures alone without considering measures of lung volume or diffusion capacity. This is especially problematic when defining restrictive impairments, as a reduced FVC by itself does not prove a restrictive impairment and is associated with a low total lung capacity (TLC) less than half the time.104,105 Therefore, classifying restriction based on spirometry alone likely overestimates its occurrence. Absolute spirometry measurements were typically not stratified by the pattern of abnormality, thus making it difficult to meta-analyse this data. Post-TB complications were diagnosed based on radiological interpretation alone and were therefore subject to inter-reader variability. There were relatively few prospective studies, and these tended to have small study populations. Finally, we could not identify any studies that calculated measures of burden of disease (e.g., QALYs or DALYs).
The strengths of this review include the large number of cohorts meta-analysed (N = 61) and the large population of participants with PTB (N = 41,014), which allowed us to perform more detailed stratified analyses. We also evaluated cohorts from different countries, with a wide range of socioeconomic status and resource availability, enhancing the generalizability of our findings. In this review, a wide range of outcomes were assessed, including spirometry abnormalities, functional disability, and respiratory complications. Moreover, these outcomes were assessed only in participants who had already successfully completed treatment, thus identifying participants who still had negative outcomes despite apparent cure.
In order to develop a comprehensive understanding of the total health burden that may remain after treatment of PTB, future studies should have more standardised methods in how they assess outcomes pertaining to health-related quality of life. Outcomes should be assessed at similar time points, both before treatment completion (to establish baselines) and far enough after treatment (e.g., one year post-treatment) to understand the dynamics of post-TB disability over time. Cohorts should include detailed demographic and clinical data related to participants’ other comorbidities in order to estimate the impact of PTB more precisely on post-TB outcomes. Future systematic reviews can also consider performing individual patient data meta-analyses to better adjust for confounding variables at the individual level. In addition, it will be important for future studies to translate measures of disability into quality-adjusted life years, similar to what has been done in the COPD population.106
The mechanisms that lead to respiratory impairments and disability after successful treatment of PTB are unclear. We suggest that careful identification of determinants of these outcomes might be a helpful way to generate hypotheses about these mechanisms. However, in this review, relatively few studies reported associations of disability or impairment with participant characteristics, limiting our ability to generate hypotheses. In the few studies assessing determinants, lung impairment was greater in persons treated in low- and middle-income settings, or with MDR-TB. These are both markers of greater extent of disease which may lead to more lung destruction. We suggest that future studies assess potential determinants of disability, including extent of disease, cavitation, age, cigarette smoking, obesity, and comorbid illnesses such as HIV and diabetes. In addition to generating hypotheses regarding pathogenesis, understanding the determinants of disability could guide future studies of strategies to limit or prevent disability, as well as identify optimal management for post-TB complications.
In conclusion, the findings of this review underscore the important morbidity caused by PTB and that PTB is a major cause of disability globally. This, in turn, emphasises the importance of prevention, including TB Preventive Therapy; by preventing TB disease with its immediate consequences, these strategies will also prevent long term post-PTB impairment, functional disability, and complications.
Contributors
JT, MB, NM, JJ, and DM were involved in the design of the study. MB ran the literature search. JT and SL screened studies, extracted data, and assessed risk of bias. JT, SL, MB, and DM accessed and verified the underlying data. MB and DM did statistical analyses. JT wrote the initial draft of the manuscript. All authors provided critical conceptual input, interpreted data, and critically revised the manuscript. All authors had full access to all study data, and the corresponding author made the final decision to submit for publication.
Data sharing statement
The datasets that support the findings of this systematic review and meta-analysis, including all extracted data and the code used to analyse data for this study, are available from the corresponding author upon request.
Declaration of interests
We declare no competing interests.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (CIHR) Foundation Grant (FRD #143350).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101979.
Contributor Information
Mayara Lisboa Bastos, Email: mayara.bastos@mail.mcgill.ca.
Dick Menzies, Email: dick.menzies@mcgill.ca.
Appendix A. Supplementary data
References
- 1.Global tuberculosis report. World Health Organization; Geneva: 2022. https://wwwwhoint/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 Available at: [Google Scholar]
- 2.Dodd P.J., Yuen C.M., Jayasooriya S.M., van der Zalm M.M., Seddon J.A. Quantifying the global number of tuberculosis survivors: a modelling study. Lancet Infect Dis. 2021;21(7):984–992. doi: 10.1016/S1473-3099(20)30919-1. [DOI] [PubMed] [Google Scholar]
- 3.Alene K.A., Wangdi K., Colquhoun S., et al. Tuberculosis related disability: a systematic review and meta-analysis. BMC Med. 2021;19(1):1–19. doi: 10.1186/s12916-021-02063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrlich R., Adams S., Baatjies R., Jeebhay M.F. Chronic airflow obstruction and respiratory symptoms following tuberculosis: a review of South African studies. Int J Tubercul Lung Dis. 2011;15(7):886–891. doi: 10.5588/ijtld.10.0526. [DOI] [PubMed] [Google Scholar]
- 5.Allwood B.W., Myer L., Bateman E.D. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration. 2013;86(1):76–85. doi: 10.1159/000350917. [DOI] [PubMed] [Google Scholar]
- 6.Byrne A.L., Marais B.J., Mitnick C.D., Lecca L., Marks G.B. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis. 2015:138–146. doi: 10.1016/j.ijid.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Pasipanodya J.G., Miller T.L., Vecino M., et al. Pulmonary impairment after tuberculosis. Chest. 2007;131(6):1817–1824. doi: 10.1378/chest.06-2949. [DOI] [PubMed] [Google Scholar]
- 8.Vecino M., Pasipanodya J.G., Slocum P., et al. Evidence for chronic lung impairment in patients treated for pulmonary tuberculosis. J Infect Public Health. 2011;4(5–6):244–252. doi: 10.1016/j.jiph.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Migliori G.B., Marx F., Ambrosino N., et al. Clinical standards for the assessment, management and rehabilitation of post-TB lung disease. Int J Tubercul Lung Dis. 2021;25(10):797–813. doi: 10.5588/ijtld.21.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrne A.L., Marais B.J., Mitnick C.D., Lecca L., Marks G.B. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis. 2015:138–146. doi: 10.1016/j.ijid.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Dhar R., Singh S., Talwar D., et al. Bronchiectasis in India: results from the European multicentre bronchiectasis audit and research collaboration (EMBARC) and respiratory research network of India registry. Lancet Global Health. 2019;7(9):e1269–e1279. doi: 10.1016/S2214-109X(19)30327-4. [DOI] [PubMed] [Google Scholar]
- 12.Meghji J., Simpson H., Squire S.B., Mortimer K. A systematic review of the prevalence and pattern of imaging defined post-TB lung disease. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0161176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung C.Y., Huang H.-L., Rahman M., et al. Cancer incidence attributable to tuberculosis in 2015: global, regional, and national estimates. BMC Cancer. 2020;20(1):1–13. doi: 10.1186/s12885-020-06891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdeahad H., Salehi M., Yaghoubi A., Aalami A.H., Aalami F., Soleimanpour S. Previous pulmonary tuberculosis enhances the risk of lung cancer: systematic reviews and meta-analysis. Infectious Diseases. 2022;54(4):255–268. doi: 10.1080/23744235.2021.2006772. [DOI] [PubMed] [Google Scholar]
- 15.Hwang S.Y., Kim J.Y., Lee H.S., et al. Pulmonary tuberculosis and risk of lung cancer: a systematic review and meta-analysis. J Clin Med. 2022;11(3):765. doi: 10.3390/jcm11030765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ang L., Ghosh P., Seow W.J. Association between previous lung diseases and lung cancer risk: a systematic review and meta-analysis. Carcinogenesis. 2021;42(12):1461–1474. doi: 10.1093/carcin/bgab082. [DOI] [PubMed] [Google Scholar]
- 17.Cabrera-Sanchez J., Cuba V., Vega V., Van der Stuyft P., Otero L. Lung cancer occurrence after an episode of tuberculosis: a systematic review and meta-analysis. Eur Respir Rev. 2022;31(165) doi: 10.1183/16000617.0025-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang H.Y., Li X.L., Yu X.S., et al. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: a systematic review. Int J Cancer. 2009;125(12):2936–2944. doi: 10.1002/ijc.24636. [DOI] [PubMed] [Google Scholar]
- 19.Johnston J.C., Cooper R., Menzies D. Chapter 5: treatment of tuberculosis disease. Can J Respir Crit Care Sleep Med. 2022;6(sup1):66–76. [Google Scholar]
- 20.Guo N., Marra F., Marra C.A. Measuring health-related quality of life in tuberculosis: a systematic review. Health Qual Life Outcome. 2009;7:1–10. doi: 10.1186/1477-7525-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer M., Leavens A., Schwartzman K. A systematic review and meta-analysis of the impact of tuberculosis on health-related quality of life. Qual Life Res. 2013;22:2213–2235. doi: 10.1007/s11136-012-0329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aggarwal A.N. Quality of life with tuberculosis. J Clin Tubercul Mycobacterial Dis. 2019;17 doi: 10.1016/j.jctube.2019.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toman K. World Health Organization; 2004. Toman's tuberculosis: case detection, treatment, and monitoring: questions and answers. [Google Scholar]
- 24.Organization W.H. World Health Organization; 2016. Chest radiography in tuberculosis detection: summary of current WHO recommendations and guidance on programmatic approaches. [Google Scholar]
- 25.Munn Z.M.S., Lisy K., Riitano D., Tufanaru C. In: JBI manual for evidence synthesis. Aromataris E., Munn Z., editors. JBI; 2020. Chapter 5: systematic reviews of prevalence and incidence. [Google Scholar]
- 26.World Health Organization . World Health Organization; 2001. IFC: International classification of functioning, disability and health. [Google Scholar]
- 27.Stanojevic S., Kaminsky D.A., Miller M.R., et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1) doi: 10.1183/13993003.01499-2021. [DOI] [PubMed] [Google Scholar]
- 28.Bestall J., Paul E., Garrod R., Garnham R., Jones P., Wedzicha J. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ACoPSfCPF Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 30.Pasipanodya J.G., Miller T.L., Vecino M., et al. Using the St. George respiratory questionnaire to ascertain health quality in persons with treated pulmonary tuberculosis. Chest. 2007;132(5):1591–1598. doi: 10.1378/chest.07-0755. [DOI] [PubMed] [Google Scholar]
- 31.Jones P. 2016. St George ‘S respiratory questionnaire for Copd patients (Sgrq-C) [Google Scholar]
- 32.Zarif A., Thomas A., Vayro A. Focus: rare disease: chronic pulmonary aspergillosis: a brief review. Yale J Biol Med. 2021;94(4):673. [PMC free article] [PubMed] [Google Scholar]
- 33.Hamza T.H., van Houwelingen H.C., Stijnen T. The binomial distribution of meta-analysis was preferred to model within-study variability. J Clin Epidemiol. 2008;61(1):41–51. doi: 10.1016/j.jclinepi.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 34.McGrath S., Zhao X., Steele R., Thombs B.D., Benedetti A., Collaboration D.S.D. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29(9):2520–2537. doi: 10.1177/0962280219889080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enright P.L., Sherrill D.L. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 36.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 37.IntHout J., Ioannidis J.P., Rovers M.M., Goeman J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7) doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furuya-Kanamori L., Barendregt J.J., Doi S.A. A new improved graphical and quantitative method for detecting bias in meta-analysis. JBI Evidence Implement. 2018;16(4):195–203. doi: 10.1097/XEB.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 39.Sterne J.A., Sutton A.J., Ioannidis J.P., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343 doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 40.Akanbi M.O., Taiwo B.O., Achenbach C.J., et al. HIV associated chronic obstructive pulmonary disease in Nigeria. J AIDS Clin Res. 2015;6(5) doi: 10.4172/2155-6113.1000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akkara S.A., Shah A.D., Adalja M., Akkara A.G., Rathi A., Shah D.N. Pulmonary tuberculosis: the day after. Int J Tubercul Lung Dis. 2013;17(6):810–813. doi: 10.5588/ijtld.12.0317. [DOI] [PubMed] [Google Scholar]
- 42.Allwood B.W., Maasdorp E., Kim G.J., et al. Transition from restrictive to obstructive lung function impairment during treatment and follow-up of active tuberculosis. Int J Chronic Obstr Pulm Dis. 2020;15:1039–1047. doi: 10.2147/COPD.S219731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allwood B.W., Stolbrink M., Baines N., et al. Persistent chronic respiratory symptoms despite TB cure is poorly correlated with lung function. Int J Tubercul Lung Dis. 2021;25(4):262–270. doi: 10.5588/ijtld.20.0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.An S.J., Kim Y.J., Han S.S., Heo J. Effects of age on the association between pulmonary tuberculosis and lung cancer in a South Korean cohort. J Thorac Dis. 2020;12(3):375–382. doi: 10.21037/jtd.2020.01.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auld S.C., Kornfeld H., Maenetje P., et al. Pulmonary restriction predicts long-term pulmonary impairment in people with HIV and tuberculosis. BMC Pulm Med. 2021;21(1):19. doi: 10.1186/s12890-020-01368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badivuku S., Pavlovic M., Plavec D., Turk R. Secondary inactive pulmonary tuberculosis and non specific bronchial reactivity. Rev Med Chile. 1995;123(8):967–973. [PubMed] [Google Scholar]
- 47.Baez-Saldana R., Lopez-Arteaga Y., Bizarron-Muro A., et al. A novel scoring system to measure radiographic abnormalities and related spirometric values in cured pulmonary tuberculosis. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0078926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banu Rekha V., Ramachandran R., Kuppurao K., et al. Assessment of long term status of sputum positive pulmonary TB patients successfully treated with short course chemotherapy. Indian J Tubercul. 2009;56(3):132–140. [PubMed] [Google Scholar]
- 49.Bemba E.L.P., Moyikoua R., Ouedraogo A.R., et al. Spirometric and radiographic profile of patients with pulmonary tuberculosis treated and cured at the Department of Pulmonology of Brazzaville University Hospital. Rev Pneumol Clin. 2017;73(5):217–224. doi: 10.1016/j.pneumo.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Binegdie A.B., Getachew M., Sherman C.B., Schluger N.W. Prevalence of chronic obstructive pulmonary disease (COPD) among patients successfully treated for pulmonary tuberculosis in Ethiopia. Ethiop Med J. 2020;58(2) [Google Scholar]
- 51.Binegdie A.B., Parekh M., Tolessa T.B., et al. Sequelae of patients treated for pulmonary tuberculosis in chest clinic, Tikur Anbessa specialized hospital (Tash), Addis Ababa, Ethiopia. Ethiop Med J. 2015;53(4):167–171. [PubMed] [Google Scholar]
- 52.Byrne A.L., Marais B.J., Mitnick C.D., et al. Chronic airflow obstruction after successful treatment of multidrug-resistant tuberculosis. ERJ Open Res. 2017;3(3) doi: 10.1183/23120541.00026-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Byrne A.L., Marais B.J., Mitnick C.D., et al. Asthma and atopy prevalence are not reduced among former tuberculosis patients compared with controls in Lima, Peru. BMC Pulm Med. 2019;19(1):1–9. doi: 10.1186/s12890-019-0804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chushkin M.I., Ots O.N. Impaired pulmonary function after treatment for tuberculosis: the end of the disease? J Bras Pneumol. 2017;43(1):38–43. doi: 10.1590/S1806-37562016000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daniels K.J., Irusen E., Pharaoh H., Hanekom S. Post-tuberculosis health-related quality of life, lung function and exercise capacity in a cured pulmonary tuberculosis population in the Breede Valley District, South Africa. S Afr J Physiother. 2019;75(1):1319. doi: 10.4102/sajp.v75i1.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de la Mora I.L., Martinez-Oceguera D., Laniado-Laborin R. Chronic airway obstruction after successful treatment of tuberculosis and its impact on quality of life. Int J Tubercul Lung Dis. 2015;19(7):808–810. doi: 10.5588/ijtld.14.0983. [DOI] [PubMed] [Google Scholar]
- 57.De Valliere S., Barker R. Residual lung damage after completion of treatment for multidrug-resistant tuberculosis. Int J Tubercul Lung Dis. 2004;8(6):767–771. [PubMed] [Google Scholar]
- 58.Di Naso F.C., Pereira J.S., Schuh S.J., Unis G. Functional evaluation in patients with pulmonary tuberculosis sequelae. Rev Port Pneumol. 2011;17(5):216–221. doi: 10.1016/j.rppneu.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Gandhi K., Gupta S., Singla R. Risk factors associated with development of pulmonary impairment after tuberculosis. Indian J Tubercul. 2016;63(1):34–38. doi: 10.1016/j.ijtb.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Gupte A.N., Paradkar M., Selvaraju S., et al. Assessment of lung function in successfully treated tuberculosis reveals high burden of ventilatory defects and COPD. PLoS One. 2019;14(5) doi: 10.1371/journal.pone.0217289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hnizdo E., Singh T., Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000;55(1):32–38. doi: 10.1136/thorax.55.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kavya S., Priya J., Gangadharan V. A study to assess the clinico-radiological and spirometric profile of post tuberculosis patients in a tertiary care centre. Int J Res Pharm Sci. 2020;11(SPL4):1350–1356. [Google Scholar]
- 63.Khosa C., Bhatt N., Massango I., et al. Development of chronic lung impairment in Mozambican TB patients and associated risks. BMC Pulm Med. 2020;20(1):127. doi: 10.1186/s12890-020-1167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ko Y., Lee Y.M., Lee H.Y., et al. Changes in lung function according to disease extent before and after pulmonary tuberculosis. Int J Tubercul Lung Dis. 2015;19(5):589–595. doi: 10.5588/ijtld.14.0454. [DOI] [PubMed] [Google Scholar]
- 65.Kumar Rai D., Kumar R. Identification of risk factors for radiological sequelae in patients treated for pulmonary tuberculosis: prospective observational cohort study. Indian J Tubercul. 2020;67(4):534–538. doi: 10.1016/j.ijtb.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 66.Lee C.H., Lee M.C., Lin H.H., et al. Pulmonary tuberculosis and delay in anti-tuberculous treatment are important risk factors for chronic obstructive pulmonary disease. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leung C.C., Hui L., Lee R.S., et al. Tuberculosis is associated with increased lung cancer mortality. Int J Tubercul Lung Dis. 2013;17(5):687–692. doi: 10.5588/ijtld.12.0816. [DOI] [PubMed] [Google Scholar]
- 68.Maguire G., Anstey N.M., Ardian M., et al. Pulmonary tuberculosis, impaired lung function, disability and quality of life in a high-burden setting. Int J Tubercul Lung Dis. 2009;13(12):1500–1506. [PubMed] [Google Scholar]
- 69.Mancuzo E.V., Martins Netto E., Sulmonett N., et al. Spirometry results after treatment for pulmonary tuberculosis: comparison between patients with and without previous lung disease: a multicenter study. J Bras Pneumol. 2020;46(2) doi: 10.36416/1806-3756/e20180198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manji M., Shayo G., Mamuya S., Mpembeni R., Jusabani A., Mugusi F. Lung functions among patients with pulmonary tuberculosis in Dar es Salaam - a cross-sectional study. BMC Pulm Med. 2016;16(1):58. doi: 10.1186/s12890-016-0213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mattila T., Heliovaara M., Rissanen H., Knekt P., Puukka P., Vasankari T. Tuberculosis, airway obstruction and mortality in a Finnish population. COPD. 2017;14(2):143–149. doi: 10.1080/15412555.2016.1250253. [DOI] [PubMed] [Google Scholar]
- 72.Mbatchou Ngahane B.H., Nouyep J., Nganda Motto M., et al. Post-tuberculous lung function impairment in a tuberculosis reference clinic in Cameroon. Respir Med. 2016;114:67–71. doi: 10.1016/j.rmed.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 73.Meghji J., Lesosky M., Joekes E., et al. Patient outcomes associated with post-tuberculosis lung damage in Malawi: a prospective cohort study. Thorax. 2020;75(3):269–278. doi: 10.1136/thoraxjnl-2019-213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Munoz-Torrico M., Cid-Juarez S., Gochicoa-Rangel L., et al. Functional impact of sequelae in drug-susceptible and multidrug-resistant tuberculosis. Int J Tubercul Lung Dis. 2020;24(7):700–705. doi: 10.5588/ijtld.19.0809. [DOI] [PubMed] [Google Scholar]
- 75.Musafiri S.D.V., Munganyinka B.C., Manzi O., Kalisa L., Rutayisire P.C. The aftermath of pulmonary tuberculosis: predictors of severe pulmonary sequelae and quality of life of patients visiting a tertiary level of care in Rwanda, East Africa. Austin J Pulm Respir Med. 2015;2(2):1027. [Google Scholar]
- 76.Nihues Sde S., Mancuzo E.V., Sulmonetti N., et al. Chronic symptoms and pulmonary dysfunction in post-tuberculosis Brazilian patients. Braz J Infect Dis. 2015;19(5):492–497. doi: 10.1016/j.bjid.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nuwagira E., Stadelman A., Baluku J.B., et al. Obstructive lung disease and quality of life after cure of multi-drug-resistant tuberculosis in Uganda: a cross-sectional study. Trop Med Health. 2020;48:34. doi: 10.1186/s41182-020-00221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ojuawo O.B., Fawibe A.E., Desalu O.O., et al. Spirometric abnormalities following treatment for pulmonary tuberculosis in Ilorin, Nigeria. Niger Postgrad Med J. 2020;27(3):163–170. doi: 10.4103/npmj.npmj_18_20. [DOI] [PubMed] [Google Scholar]
- 79.Osman R.K., Mortimer K., Bjune G., El Sony A.I. Chronic respiratory disease in adults treated for tuberculosis in Khartoum, Sudan. Public Health Action. 2016;6(3):199–204. doi: 10.5588/pha.16.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ouedraogo A.R., Adjoh K.S., Fiogbe A.A., et al. Functional evaluation and quality of life in patients treated for pulmonary tuberculosis: an underestimated issue in countries with limited resources. Rev Mal Respir. 2016;33(3):267–268. doi: 10.1016/j.rmr.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 81.Page I.D., Byanyima R., Hosmane S., et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J. 2019;53(3) doi: 10.1183/13993003.01184-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patil P., Patil S. A six-month follow-up study to evaluate changes of pulmonary function test in Category I pulmonary tuberculosis treatment completed patient. Natl J Physiol Pharm Pharmacol. 2017;8(1) [Google Scholar]
- 83.Patil S., Patil R., Jadhav A. Pulmonary functions' assessment in post-tuberculosis cases by spirometry: obstructive pattern is predominant and needs cautious evaluation in all treated cases irrespective of symptoms. Int J Mycobacteriol. 2018;7(2):128. doi: 10.4103/ijmy.ijmy_56_18. [DOI] [PubMed] [Google Scholar]
- 84.Pefura-Yone E.W., Kengne A.P., Tagne-Kamdem P.E., Afane-Ze E. Clinical significance of low forced expiratory flow between 25% and 75% of vital capacity following treated pulmonary tuberculosis: a cross-sectional study. BMJ Open. 2014;4(7) doi: 10.1136/bmjopen-2014-005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Plit M.L., Anderson R., Van Rensburg C.E., et al. Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. Eur Respir J. 1998;12(2):351–356. doi: 10.1183/09031936.98.12020351. [DOI] [PubMed] [Google Scholar]
- 86.Ralph A.P., Kenangalem E., Waramori G., et al. High morbidity during treatment and residual pulmonary disability in pulmonary tuberculosis: under-recognised phenomena. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramos L.M.M., Sulmonett N., Ferreira C.S., Henriques J.F., Miranda S.S.d. Functional profile of patients with tuberculosis sequelae in a university hospital. J Bras Pneumol. 2006;32:43–47. doi: 10.1590/s1806-37132006000100010. [DOI] [PubMed] [Google Scholar]
- 88.Simonsen D.F., Farkas D.K., Sogaard M., Horsburgh C.R., Sorensen H.T., Thomsen R.W. Tuberculosis and risk of cancer: a Danish nationwide cohort study. Int J Tubercul Lung Dis. 2014;18(10):1211–1219. doi: 10.5588/ijtld.14.0161. [DOI] [PubMed] [Google Scholar]
- 89.Singla N., Singla R., Fernandes S., Behera D. Post treatment sequelae of multi-drug resistant tuberculosis patients. Indian J Tubercul. 2009;56(4):206–212. [PubMed] [Google Scholar]
- 90.Singla R., Mallick M., Mrigpuri P., Singla N., Gupta A. Sequelae of pulmonary multidrug-resistant tuberculosis at the completion of treatment. Lung India. 2018;35(1):4–8. doi: 10.4103/lungindia.lungindia_269_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tolba S.K., Abd El-Hady A., Moussa H., Abd Alaal M.E.M., Amin W., Aboelmagd F. Efficacy of pulmonary rehabilitation program in patients with treated pulmonary tuberculosis. Sys Rev Pharm. 2021;12(3):516–521. [Google Scholar]
- 92.Wu C.Y., Hu H.Y., Pu C.Y., et al. Pulmonary tuberculosis increases the risk of lung cancer: a population-based cohort study. Cancer. 2011;117(3):618–624. doi: 10.1002/cncr.25616. [DOI] [PubMed] [Google Scholar]
- 93.Wu H., Asad U.K., Wu J., Zhang G., Zhang G., Lu X. CT findings of TB in diabetic and non-diabetic patients: a comparison before and after anti-tuberculous therapy. Radiol Infect Dis. 2016;3(1):15–22. [Google Scholar]
- 94.Yu Y.-H., Liao C.-C., Hsu W.-H., et al. Increased lung cancer risk among patients with pulmonary tuberculosis: a population cohort study. J Thorac Oncol. 2011;6(1):32–37. doi: 10.1097/JTO.0b013e3181fb4fcc. [DOI] [PubMed] [Google Scholar]
- 95.Ahmed S., Ahmed Z., Baneen U., Masood I., Bhargava R. Bronchial hyper-responsiveness in post-tubercular patients: a case-control study. J Clin Diagn Res. 2022;16(1) [Google Scholar]
- 96.Kim T., Lee H., Sim Y.S., et al. Respiratory symptoms and health-related quality of life in post-tuberculosis subjects with physician-diagnosed bronchiectasis: a cross-sectional study. J Thorac Dis. 2021;13(8):4894. doi: 10.21037/jtd-20-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mpagama S., Msaji K., Kaswaga O., et al. The burden and determinants of post-TB lung disease. Int J Tubercul Lung Dis. 2021;25(10):846–853. doi: 10.5588/ijtld.21.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishi M.P., Mancuzo E.V., Sulmonett N., Almeida I.N.d., César A.L.A., Miranda S.S.d. vol. 63. Revista do Instituto de Medicina Tropical de São Paulo; 2021. (Pulmonary functional assessment: longitudinal study after treatment of pulmonary tuberculosis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nightingale R., Chinoko B., Lesosky M., et al. Respiratory symptoms and lung function in patients treated for pulmonary tuberculosis in Malawi: a prospective cohort study. Thorax. 2022;77(11):1131–1139. doi: 10.1136/thoraxjnl-2021-217190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nkereuwem E., Agbla S., Sallahdeen A., et al. Reduced lung function and health-related quality of life after treatment for pulmonary tuberculosis in Gambian children: a cross-sectional comparative study. Thorax. 2023;78(3):281–287. doi: 10.1136/thorax-2022-219085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pydipalli M., Chinnakali P., Rajaram M., Sundaram S.P., Roy G. Lung function impairment in patients treated for pulmonary tuberculosis and associated factors in Puducherry, South India. Indian J Community Med. 2022;47(1):111. doi: 10.4103/ijcm.ijcm_564_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Silva D.R., Freitas A.A., Guimarães A.R., et al. Post-tuberculosis lung disease: a comparison of Brazilian, Italian, and Mexican cohorts. J Bras Pneumol. 2022:48. doi: 10.36416/1806-3756/e20210515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grenier P., Maurice F., Musset D., Menu Y., Nahum H. Bronchiectasis: assessment by thin-section CT. Radiology. 1986;161(1):95–99. doi: 10.1148/radiology.161.1.3763889. [DOI] [PubMed] [Google Scholar]
- 104.Glady C.A., Aaron S.D., Lunau M., Clinch J., Dales R.E. A spirometry-based algorithm to direct lung function testing in the pulmonary function laboratory. Chest. 2003;123(6):1939–1946. doi: 10.1378/chest.123.6.1939. [DOI] [PubMed] [Google Scholar]
- 105.Aaron S.D., Dales R.E., Cardinal P. How accurate is spirometry at predicting restrictive pulmonary impairment? Chest. 1999;115(3):869–873. doi: 10.1378/chest.115.3.869. [DOI] [PubMed] [Google Scholar]
- 106.Miravitlles M., Huerta A., Valle M., et al. Clinical variables impacting on the estimation of utilities in chronic obstructive pulmonary disease. Int J Chronic Obstr Pulm Dis. 2015;10:367. doi: 10.2147/COPD.S76397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.