Abstract
By using mini-Tn5 transposon mutagenesis, random transcriptional fusions of promoterless bacterial luciferase, luxAB, to genes of Pseudomonas putida KT2442 were generated. Insertion mutants that responded to ammonium deficiency by induction of bioluminescence were selected. The mutant that responded most strongly was genetically analyzed and is demonstrated to bear the transposon within the assimilatory nitrate reductase gene (nasB) of P. putida KT2442. Genetic evidence as well as sequence analyses of the DNA regions flanking nasB suggest that the genes required for nitrate assimilation are not clustered. We isolated three second-site mutants in which induction of nasB expression was completely abolished under nitrogen-limiting conditions. Nucleotide sequence analysis of the chromosomal junctions revealed that in all three mutants the secondary transposon had inserted at different sites in the gltB gene of P. putida KT2442 encoding the major subunit of the glutamate synthase. A detailed physiological characterization of the gltB mutants revealed that they are unable to utilize a number of potential nitrogen sources, are defective in the ability to express nitrogen starvation proteins, display an aberrant cell morphology under nitrogen-limiting conditions, and are impaired in the capacity to survive prolonged nitrogen starvation periods.
Ammonia is for many bacteria the preferred source of nitrogen. However, since in many natural environments ammonia is not present at sufficient concentrations, most bacteria have the capacity to utilize a wide range of alternative nitrogen sources. The synthesis of proteins involved in the catabolism of nitrogenous compounds is dramatically increased when cells are grown under conditions in which ammonia is limiting. There is increasing evidence that in many different bacteria the coordinated expression of enzymes involved in nitrogen metabolism is under control of a global nitrogen regulatory system (Ntr) (37). In enteric bacteria, expression of the Ntr regulon is regulated at the transcriptional level and requires both RNA polymerase containing the sigma factor ς54, encoded by the rpoN gene, and the NtrC protein. The phosphorylated form of NtrC functions as a transcriptional activator of ς54-dependent promoters (52). The phosphorylation state of NtrC is modulated by NtrB, which is a bifunctional protein that can either transfer phosphate to NtrC or control the dephosphorylation of NtrC-phosphate (23, 41, 53). The phosphatase activity of NtrB is stimulated by the native form of the regulatory protein PII (22, 23). The modification of PII is in turn controlled by the bifunctional UTase/UR (uridylyltransferase/uridylyl-removing enzyme), which catalyzes the covalent uridylylation or deuridylylation of PII (1, 13). Since glutamine stimulates UR and 2-oxoglutarate stimulates UTase the net uridylylation of PII depends on the relative concentrations of these two metabolites, which are indicative of the nitrogen status of the cell (12).
Current knowledge of nitrogen control in bacteria is almost entirely based on research with enteric bacteria. However, it is likely that, depending on the ecological habitat of the organism, differences between the Ntr systems exist. In this study, we initiated work to characterize the Ntr system of Pseudomonas putida KT2442, a bacterium which is capable of colonizing a wide variety of niches, including freshwater, soil, and the rhizospheres of a number of agriculturally important plants (45). In P. putida KT2442, at least 43 proteins are induced during nitrogen starvation (14). Of these, 12 were found to be also induced during carbon starvation, and a core set of 8 proteins were found to be induced during starvation for phosphorus as well. Conceivably, most of the induced proteins are involved in the uptake and subsequent metabolism of alternative nitrogenous compounds. P. putida KT2442 is capable of utilizing a wide range of different nitrogen sources, including several of the amino acids, nitrate, and urea (27). The remaining proteins, especially the core proteins (generally referred to as Pex proteins), may be involved in the development of a general resistant state (15, 26, 35). Under the conditions of carbon or nitrogen starvation, P. putida exhibits markedly enhanced resistance to a variety of environmental stresses and remains fully viable for at least 1 month (15).
In this paper, we describe the isolation of a ′luxAB insertion mutant in P. putida KT2442 that is highly responsive to ammonium deprivation. This mutant is demonstrated to bear the transposon within the assimilatory nitrate reductase gene (nasB) of P. putida KT2442. Evidence is provided that the nasB gene is not clustered with the other genes required for nitrate assimilation. By the isolation and characterization of second-site mutants that no longer respond to nitrogen deprivation, we demonstrate that expression of nasB under nitrogen-limiting conditions is dependent on the presence of a functional gltB gene which encodes the major subunit of the glutamate synthase. It is shown that inactivation of gltB causes dramatic defects with respect to nitrogen utilization, expression of nitrogen starvation proteins, and starvation survival.
MATERIALS AND METHODS
Organisms, cultivation, and starvation conditions.
Bacterial strains used in this study are listed in Table 1. Escherichia coli strains were grown at 37°C in Luria-Bertani (LB) medium (4), whereas P. putida strains were routinely grown in AB minimal medium (7) supplemented with 0.3% (wt/vol) citrate. Nitrogen starvation regimens were set up after harvesting an exponentially growing culture (about 108 cells/ml) by centrifugation followed by resuspension in preheated medium depleted of ammonium. Alternatively, starvation was accomplished by exhaustion of the nitrogen source (0.3 mM NH4Cl in AB medium supplemented with 0.3% [wt/vol] citrate). Growth and starvation of P. putida cells were carried out at 30°C, and the cell mass of the cultures was measured spectrophotometrically as the optical density at 450 nm (OD450). Starvation survival of nitrogen-depleted cultures was monitored by determination of viable counts by plating 0.1-ml samples of different dilutions on LB plates. For examination of growth of P. putida on different nitrogen sources, NH4Cl in AB medium was replaced by an alternative nitrogen source such as nitrate, nitrite, urea, or different amino acids at final concentrations of 5 mM. For solid media, Bacto-Agar (Difco) was added to a final concentration of 1.5% (wt/vol). Antibiotics were added as required at the following final concentrations: ampicillin, 100 μg/ml; rifampin, 50 μg/ml; kanamycin, 50 μg/ml; gentamicin, 15 μg/ml; and carbenicillin, 400 μg/ml.
TABLE 1.
Bacteria and plasmids used
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| P. putida | ||
| KT2442 | TOL plasmid-cured derivative of P. putida mt-2 | 2 |
| rpoN | RpoN− derivative of KT2440 | 27 |
| gltB | GltB− derivative of KT2442 | This work |
| N25 | nasB::Tn5 luxAB res-npt-xylE-res derivative of KT2442 | This work |
| N25Δ | Kms derivative of N25; npt removed by site-specific recombination | This work |
| D5 | gltB::Tn5 Km1 derivative of N25Δ | This work |
| D6 | gltB::Tn5 Km1 derivative of N25Δ | This work |
| D7 | gltB::Tn5 Km1 derivative of N25Δ | This work |
| E. coli | ||
| MT102 | araD139 (ara-leu)7697 Δlac thi hsdR | Laboratory collection |
| CC118(λpir) | Δ(ara-leu) araD ΔlacX74 galE galK phoA thi-1 rpsE rpoB argE(Am) recA λpir lysogen | 17 |
| Klebsiella oxytoca VJS732 | hsdR1 nasA122::Cm | 31 |
| Plasmids | ||
| pAA1 | nasB as 3.4-kb fragment in pCR2.1-TOPO; Apr, Kmr | This work |
| pAA2 | nasB as 3.4-kb fragment in pVLT33; Kmr | This work |
| pAA3 | pQF120 with 1.1-kb nasB promoter region; Apr | This work |
| pCR2.1-TOPO | Multicopy cloning vector; Kmr, Apr | Invitrogen |
| pEX18Gm | Gene replacement vector; sacB+, RP4oriT, Gmr | 19 |
| pGEM-3Zf(+) | Multicopy cloning vector; Apr | Promega |
| pJMS10 | Delivery vector for mini-Tn5 luxAB res-npt-xylE-res; Apr, Kmr | 28 |
| pMR3 | pEX18Gm derivative for inactivation of gltB; sacB+, RP4oriT, Gmr, Kmr | This work |
| pLEO141 | PstI fragment from D5 containing Km1 plus 0.6 kb of flanking DNA in pGEM-3Zf(+); Apr, Kmr | This work |
| pLEO143 | PstI fragment from D6 containing Km1 plus 2.0 kb of flanking DNA in pGEM-3Zf(+); Apr, Kmr | This work |
| pLEO145 | PstI fragment from D7 containing Km1 plus 0.9 kb of flanking DNA in pGEM-3Zf(+); Apr, Kmr | This work |
| pQF120 | luxAB-lacZ promoter probe vector; Apr | 48 |
| pRK600 | ColE1oriV RP4 tra+ RP4oriT; helper plasmid in triparental matings; Cmr | 24 |
| pVLT33 | RSF1010-lacIq/Ptac hybrid broad-host-range expression vector; Kmr | 10 |
Isolation of luxAB insertion mutants.
The hybrid transposon mini-Tn5 luxAB res-npt-xylE-res was randomly inserted into the chromosome of P. putida KT2442 by triparental mating as described previously (28). Since this hybrid transposon carries the promoterless luciferase genes from Vibrio harveyi, luminescent intensity is a measure of the strength of adjacent promoters. Bioluminescent colonies were visualized with a highly sensitive photon counting camera (ARGUS-50 system with a C2400-47 camera; Hamamatsu Photonics, Herrsching, Germany) after n-decanal was provided in the lid of the petri dish. Insertion mutants responsive to nitrogen limitation were identified upon transfer of filters bearing transconjugant colonies grown on nitrogen-rich selective plates (AB medium supplemented with 15 mM citrate, 20 mM NH4Cl, rifampin [50 μg/ml], and kanamycin [50 μg/ml]) to ones lacking the nitrogen source. Colonies of interest were identified by comparing the images of bioluminescence before and 2, 4, and 24 h after the transfer. A kanamycin-sensitive (Kms) derivative of mutant N25 was constructed by excising the res-intervening DNA fragment, which contains the kanamycin resistance gene (npt) together with the xylE gene encoding a catechol 2,3-dioxygenase (C2,3O), via site-specific recombination. This was accomplished by transferring a pUT derivative expressing the ParA resolvase of broad-host-range plasmid RP4 transiently into P. putida N25 as described previously (28). Colonies that had deleted the res-intervening DNA fragment were screened for loss of xylE activity by spraying the plates with 1% (wt/vol) catechol (C2,3O+ colonies acquire a bright yellow color from the production of hydroxymuconic semialdehyde from the meta-ring cleavage of the aromatic substrate).
Luminescence measurements.
Following addition of 1 μl of n-decanal to 200 μl of sample, bioluminescence was quantified in a Turner TD-20e (Turner Designs, Sunnyvale, Calif.) luminometer. Light outputs were integrated over 10 s and are reported as relative light units (RLU). Specific light units were defined as RLU per unit of optical density at 450 nm.
Enzyme assays.
Glutamate synthase activity was determined by the method of Meers et al. (36); specific activities are expressed as nanomoles of NADPH oxidized per minute per milligram of protein at 30°C. Protein was measured by the method of Lowry et al. (32), with bovine serum albumin as the standard.
Recombinant genetic methods and nucleotide sequence analysis.
DNA isolation, restriction analysis, and transformation of E. coli were performed according to Sambrook et al. (49). The entire nasB gene was amplified from genomic DNA of P. putida KT2442 by PCR using the primer pair NAS6 (5′-CGGAATTCCGCTCCGTAGAATGCGC-3′) and NAS8 (5′-CGGAATTCGAACCGGCAAGGTGTGC-3′) (EcoRI restriction sites are underlined). The resulting 3.4-kb DNA fragment was digested with EcoRI and inserted into the EcoRI site of pCR2.1-TOPO (Invitrogen), yielding plasmid pAA1. Likewise, pAA2 was constructed by cloning the 3.4-kb EcoRI fragment into the same site of the broad-host-range expression vector pVLT33. The PnasB::luxAB transcriptional fusion was constructed as follows. The nasB upstream region was PCR amplified using primers NAS4 (5′-GCGCGGATCCACGCTCATGACGATGCC-3′) and NAS5 (5′-GCGCGGATCCCGCTCCGTAGAATGCGC-3′) (BamHI restriction sites are underlined). Following restriction with BamHI, the 1.1-kb DNA fragment was inserted into the promoter probe vector pQF120 cut with the same enzyme. The plasmid, which contains the insert in the orientation placing PnasB upstream of the promoterless luxAB genes of the vector, was chosen, and this construct was designated pAA3. To identify the genetic loci affected by the secondary transposon, mini-Tn5 Km1, chromosomal DNA from each of the second-site mutants was isolated, subjected to digestion with PstI, and ligation into pGEM-3Zf(+) (Promega). This approach permitted the selection of recombinant plasmids containing the npt gene of mini-Tn5 Km1 plus P. putida flanking DNA. From the resulting plasmids (Table 1) the npt gene was deleted by cutting the plasmids with BamHI prior to religation and transformation into E. coli MT102. The universal primers were employed to sequence all clones by using the dideoxy-chain termination method in an ABI 373A automated sequencing station as described by the manufacturer (Applied Biosystems Inc.).
A defined P. putida KT2442 gltB mutant was constructed by the gene replacement method described by Hoang et al. (19). First, two DNA fragments homologous to sequences in the 5′ and 3′ coding regions of the gltB gene were amplified by PCR. Using the primer pair GLTB98 (5′-AAGCTTGACCACTACATCTGCAGC-3′) and GLTB173 (5′-TCTAGAGCGCGACTTCAGGCGG-3′), a 750-bp fragment with HindIII and XbaI restriction sites (restriction sites are underlined) introduced on the respective ends was generated and cloned into the vector pCR2.1-TOPO yielding pTOPO98. Similarly, using the primer pair GLTB317 (5′-ACTAGTCGAAGATCAAGCAGGTGG-3′) and GLTB424 (5′-GGTACCGCCCTGGTTGCCGTGC-3′), a 1.1-kb fragment with BamHI and SpeI restriction sites (restriction sites are underlined) introduced on the respective ends was generated and cloned into the vector pCR2.1-TOPO, yielding pTOPO317. Next, plasmid pTOPO98 was digested with HindIII and XbaI, and the resulting 750-bp gltB fragment was inserted into the compatible sites of the gene replacement vector pEX18Gm (19), giving rise to plasmid pMR1. Plasmid pMR2 was constructed by inserting a 4.0-kb BamHI-XbaI DNA cassette containing the promoterless luxAB genes together with the npt resistance marker into the BamHI-XbaI sites of pMR1. Finally, the second gltB fragment was inserted as a 1.1-kb BamHI-XbaI fragment from pTOPO317 into the BamHI-SpeI sites of pMR2. The resulting plasmid, pMR3, was then transferred to P. putida KT2442 by triparental mating, and plasmid integrants were selected on LB medium containing kanamycin, gentamicin, and rifampin. Merodiploids were resolved by plating on LB medium containing 5% sucrose. The genetic structure of the Kanr Gms gltB mutant was confirmed by Southern blotting using part of the gltB gene as probe as well as by PCR analysis.
Resolution of cell proteins on two-dimensional polyacrylamide gels.
Two hours after shift to nitrogen-free medium, 2-ml culture samples (with an OD450 of approximately 0.5) were removed and labeled with 2 μl of [35S]methionine (15 mCi/ml; Amersham SJ235) for 10 min at 30°C. Following a 1-min chase with unlabeled methionine at a final concentration of 1 μg/ml, chloramphenicol was added to 0.5 mg/ml and the cells were harvested by centrifugation at 0°C (10,000 × g). The preparation of cell extracts and two-dimensional polyacrylamide gel electrophoresis separation were performed as described by Kilstrup et al. (25).
RESULTS
Transposon mutagenesis and isolation of P. putida KT2442 mutants responding to nitrogen limitation.
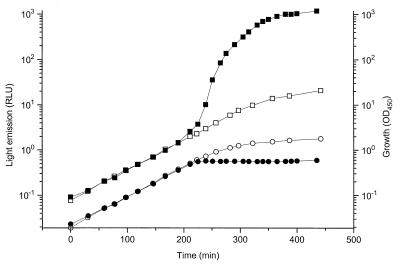
The suicide vector pUT was used to deliver the hybrid transposon mini-Tn5 luxAB res-npt-xylE-res (11, 28) into the P. putida KT2442 chromosome. Approximately 25,000 Kmr Rifr transconjugants were screened for increased bioluminescence upon transfer of colony-bearing filters to AB minimal medium lacking ammonium (see Materials and Methods for details). A total of 21 mutants presumptively carrying insertions in nitrogen starvation genes were obtained. When these mutants were grown in liquid AB medium, light levels were low during exponential growth but increased dramatically upon depletion of the nitrogen source. Southern blot analysis of the 21 mutants using a DNA fragment containing the luxB gene as a probe demonstrated that single copies of the hybrid transposon had inserted at different chromosomal locations (data not shown). One mutant, designated N25, was found to produce extremely high levels of bioluminescence under nitrogen-limited conditions but to be tightly repressed during growth in medium containing excess ammonium (Fig. 1).
FIG. 1.
Induction of bioluminescence in the mini-Tn5 luxAB res-npt-xylE-res insertion mutant N25 upon nitrogen deprivation. The bacterial culture was grown on AB medium containing 20 mM citrate as carbon source and either limiting amounts of nitrogen (0.3 mM ammonium; closed symbols) or excess ammonium (20 mM; open symbols). OD450 (●, ○) and bioluminescence (measured in RLU; ■, □) were determined along the growth curve. The data presented are representative for at least three separate experiments.
Characterization of P. putida N25.
Since many genes that are derepressed in the absence of high ammonium concentrations are involved in the utilization of alternative nitrogen sources (47), we examined the ability of mutant N25 to grow in ammonium-free minimal medium supplemented with different nitrogen sources. While the wild type grew well in AB medium containing 5 mM nitrate as a sole nitrogen source, strain N25 was unable to utilize nitrate (see below). The fact that the mutant was able to grow on nitrite indicated that either the uptake system for nitrate or the assimilatory nitrate reductase, which catalyzes the conversion from nitrate to nitrite, is defective in P. putida N25. Apart from the difference in the ability to utilize nitrate as sole nitrogen source, wild-type and mutant strains were indistinguishable.
To further characterize the nature of the transposon insertion in strain N25, the mutated locus was cloned into the vector pUC18 as a BamHI fragment, selecting for transposon-encoded kanamycin resistance. The resulting plasmid contains part of the transposon and approximately 15 kb of chromosomal DNA downstream of the transposon insertion point. Nucleotide sequence analysis of the flanking DNA revealed an open reading frame (ORF) with high homology to the C-terminal regions of nitrate reductases from various organisms. Furthermore, a cosmid library of P. putida KT2442 (46) was transferred to mutant N25, and cosmids that enabled the mutant to grow on medium containing nitrate as sole nitrogen source were isolated. Southern blotting using a DNA fragment containing part of the cloned nitrate reductase as a probe confirmed the presence of the gene on each of the cosmids (data not shown). Following restriction of the cosmids with PstI, a common, approximately 2 kb DNA fragment was cloned into pGEM-3Zf(+). Nucleotide sequence analysis revealed an ORF with high homology to the N-terminal regions of various nitrate reductases. On the basis of these sequence data, we designed a specific primer pair to PCR amplify the complete gene (2,175 bp), which was designated nasB. The deduced amino acid sequence of nasB showed highest amino acid identity to the assimilatory nitrate reductases of Synechococcus sp. (41% identity) and Oscillatoria chalybea (37% identity) (Fig. 2). The nasB gene was also cloned into the broad-host-range expression vector pVLT33 (10), and the resulting plasmid, pAA2, was transferred into P. putida N25Δ, a Kms derivative of N25 (see Materials and Methods for its construction) as well as into Klebsiella oxytoca VJSK732, a defined K. oxytoca M5al nitrate reductase mutant (31). Neither P. putida N25 nor K. oxytoca VJSK732 cells harboring plasmid pVLT33 grew on media containing nitrate as sole nitrogen source, while cells of both strains harboring pAA2 were able to grow (data not shown). In conclusion, these results clearly demonstrate that P. putida N25 bears the mini-Tn5 luxAB transposon within the assimilatory nitrate reductase.
FIG. 2.
Comparison of the assimilatory nitrate reductases from P. putida KT2442 (Pp; GenBank accession no. AF203789), Oscillatoria chalybea (Oc; 51), and Synechococcus sp. strain PCC 7942 (Ss; GenBank accession no. X74597). Amino acids common to all three sequences are indicated by asterisks. Residues that are identical in at least two sequences are shown in boldface. Gaps have been introduced to improve the alignment.
The genes required for nitrate assimilation are not clustered in one operon.
Examination of the nucleotide sequence upstream of the nasB gene for promoter-like sequences revealed a −24 TGGC/−12 TTGC sequence, characteristic of ς54-dependent promoters (38, 50). For further analysis, a 1.1-kb DNA fragment containing the putative promoter region was PCR amplified and cloned upstream of the promoterless luxAB genes of the promoter probe vector pQF120 (48). The resulting plasmid, pAA3, was then transferred to the P. putida wild type as well as to a rpoN-deficient mutant (27). Measurements of bioluminescence revealed that in both strains transcription of nasB was strongly repressed in the presence of ammonia (11 ± 3 [wild type] and 13 ± 4 [rpoN mutant] RLU/OD450). However, upon shift to nitrogen-free medium, bioluminescence increased about 900-fold (to 10,400 ± 450 RLU/OD450) in the wild type whereas no induction was observed with the rpoN mutant, indicating that expression of nasB is dependent on ς54. Moreover, since the putative promoter region is only 140 bp upstream of the translational start site of the nasB gene (GenBank accession no. AF203789), this result also indicates that nasB is the first gene of this transcription unit. The fact that P. putida N25 is able to utilize nitrite as sole nitrogen source suggests that the other genes which are required for the assimilation of nitrate (the nitrate/nitrite transport system and the assimilatory nitrite reductase) are unlikely to be located downstream of nasB in one operon. This hypothesis was confirmed by sequence analysis of the nasB downstream region, which revealed the existence of an ORF with significant homology to assimilatory sulfite reductases from various organisms (GenBank accession no. AF240673).
Isolation of second-site mutants defective in PnasB::luxAB expression.
To identify genes affecting expression of the nitrate reductase, we mutagenized P. putida N25Δ with the hybrid transposon mini-Tn5 Km1 (11, 17) and selected transconjugants on AB plates containing kanamycin (50 μg/ml) plus rifampin (50 μg/ml). Approximately 10,000 transconjugants were screened for the inability to induce bioluminescence on medium lacking ammonium. Five mutants, designated D1, D4, D5, D6, and D7, were isolated. Southern blot experiments using the npt gene as a probe revealed that the mini-Tn5 Km1 transposon had inserted at separate locations in the P. putida N25 chromosome (data not shown). Similar to the parent strain N25, these mutants exhibited very low light levels when grown in the presence of 20 mM ammonium. Upon shift to nitrogen-free medium, however, light production increased 750-fold in the primary mutant N25 but only approximately 10-fold in D1 and D4, and no induction was observed in D5, D6, and D7 (Table 2). We also investigated whether the presence of nitrate or nitrite would affect expression of nasB by shifting cultures to AB medium containing either nitrate or nitrite. However, neither with N25 nor with the second-site mutants did we observe significant differences in the levels of bioluminescence compared to those obtained in medium containing no nitrogen. Since the most dramatic effects were seen with mutants D5 to D7, further investigations focused on the analysis of these three mutants.
TABLE 2.
Induction of luxAB gene expression in P. putida N25 and the different second-site mutantsa
| Strain(s) | Specific bioluminescenceb (RLU/OD450)
|
|||
|---|---|---|---|---|
| NH4+ | −N | NO3− | NO2− | |
| N25 | 2.3 ± 0.8 | 2,131 ± 110 | 2,060 ± 152 | 1,891 ± 98 |
| D1 | 1.7 ± 0.8 | 21 ± 3 | 24 ± 4 | 25 ± 4 |
| D4 | 2.0 ± 0.3 | 28 ± 3 | 26 ± 3 | 28 ± 5 |
| D5, D6, D7 | 1.8 ± 0.5 | 2.0 ± 0.7 | 1.5 ± 0.9 | 2.2 ± 0.7 |
Bacterial cultures were grown exponentially in AB medium containing 20 mM ammonium. At an OD450 of approximately 0.5, the cells were harvested and resuspended in prewarmed AB medium containing either 20 mM NH4+, 5 mM NO3−, 5 mM NO2−, or no nitrogen (−N). Cell density and bioluminescence were monitored for 4 h after the shift.
Mean and standard deviation for multiple samples taken after shift to fresh medium from at least three independent experiments.
D5, D6, and D7 harbor a defective gltB gene.
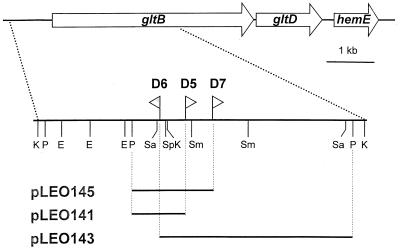
To ascertain the gene(s) mutated in the three second-site mutants by the insertion of mini-Tn5 Km1, the flanking DNAs were cloned. This was accomplished by digesting the chromosomal DNAs with PstI, a restriction enzyme that cuts downstream of the npt gene of the mini-Tn5 Km1 element, and ligating these DNA fragments into the cloning vector pGEM-3Zf(+) cut with the same enzyme. Following transformation of E. coli MT102, Kmr clones were isolated. Sequencing of plasmids pLEO141, pLEO143, and pLEO145, containing the flanking DNA regions of mutants D5, D6, and D7, respectively, revealed that in all three cases the transposon had inserted in the same gene (Fig. 3). The deduced amino acid sequences were found to be highly homologous to regions of the major subunits of the glutamate synthases (GltB) of Pseudomonas aeruginosa (at least 84% identical residues) and E. coli (at least 61% identical residues) (GenBank accession no. AF203790 and AF203791). In agreement with the genetic analysis, it was found that specific glutamate synthase (GOGAT [glutamine 2-oxoglutarate amidotransferase]; EC 1.4.1.13) activities were much lower in the three mutant strains than in the wild type (<5 versus 120 nmol of NADPH oxidized/min/mg of protein). These results clearly demonstrate that the mini-Tn5 Km1 insertions in D5, D6, and D7 have interrupted the P. putida KT2442 gltB gene encoding the major subunit of the glutamate synthase.
FIG. 3.
Physical and genetic map of the gltB gene region of P. putida KT2442. The locations and orientations of the mini-Tn5 Km1 elements are indicated by triangles. Key restriction enzyme sites (E, EcoRI; K, KpnI; P, PstI; Sa, SacI; Sm, SmaI; Sp, SphI) are shown. The allocation of genes downstream of gltB is based on partial sequence analyses of the 3′ DNA region. Recombinant clones containing the npt gene, I end of mini-Tn5, plus flanking chromosomal DNA were generated by cloning PstI-digested chromosomal DNA isolated from the three second-site mutants into the vector pGEM-3Zf(+). Plasmid pLEO141 contains the flanking DNA region from P. putida D5, pLEO143 from D7, and pLEO145 from D6.
Physiological characterization of the gltB mutants.
To assess whether inactivation of gltB also affects expression of other genes known to be controlled by the Ntr, we tested the mutants for growth on various nitrogen sources. We also included the P. putida KT2440 rpoN mutant, which is deficient in all Ntr-regulated functions (27). The primary insertion mutant, N25, differed from P. putida KT2442 only in its inability to grow on nitrate as the sole nitrogen source (Table 3). The gltB mutants were found to be unable to grow on nitrite, urea, alanine, glycine, isoleucine, leucine, serine, and low levels of ammonium (1 mM or less). The range of nitrogen sources that could be used by the rpoN mutant was identical to that of the gltB mutants except that the rpoN mutant was unable to utilize glutamate or aspartate as nitrogen source but was able to grow on low levels of ammonium. Noteworthy is the fact that when the rpoN mutant was initially characterized (27), it was, in contrast to our observations, reported to grow on aspartate and glutamate. Since different carbon sources were used in the two studies (glucose versus citrate), we tested whether the rpoN mutant is able to grow on AB minimal medium supplemented with 0.2% glucose (instead of 15 mM citrate) and aspartate or glutamate as sole nitrogen source. Both media supported growth of the rpoN mutant, suggesting that the ability to utilize these two amino acids as nitrogen sources is linked to carbon metabolism.
TABLE 3.
Utilization of various nitrogen sources by P. putida KT2442 and different mutant strainsa
| Nitrogen source(s) | Utilization of nitrogen
|
|||
|---|---|---|---|---|
| Wild type | N25 | rpoN mutant | gltB mutants D5, D6, and D7 | |
| 10 mM NH4+ | + | + | + | + |
| 1 mM NH4+ | + | + | + | − |
| NO3− | + | − | − | − |
| NO2−, urea, Ala, Gly, Ile, Leu, Ser | + | + | − | − |
| Asp, Glub | + | + | − | − |
| Asn, Gln, His, Lys, Pro | + | + | + | + |
Growth was assessed by measuring OD450 after incubation in liquid AB minimal medium supplemented with 0.3% citrate and nitrogen sources at final concentrations of 5 mM for 24 h. +, utilization (OD450 > 0.3); −, no utilization (OD450 < 0.3).
The rpoN mutant is able to utilize aspartate and glutamate as nitrogen sources when the medium is supplemented with 0.2% glucose instead of 15 mM citrate.
Since the P. putida rpoN mutant was shown to be nonmotile and unable to utilize C4-dicarboxylates except malate (27), we also tested the different mutants for motility in swimming agar and for growth on succinate and fumarate as carbon sources. However, in contrast to the rpoN mutant, all second-site mutants were motile and grew on both substrates.
The gltB mutants are impaired in their starvation survival capacities.
Since the inactivation of a key enzyme in the nitrogen regulon can be expected to reduce the ability to survive prolonged nitrogen starvation periods, we next tested the starvation survival capacity of the gltB mutants. In agreement with previous findings (15), we found that both wild-type and primary mutant, N25, remained fully viable for at least 1 month of nitrogen starvation. In contrast, the three gltB mutants were found to be severely impaired in the ability to survive prolonged incubation in nitrogen-free medium. After 24 days of nitrogen starvation, less than 0.001% of the initial populations remained viable, suggesting that gltB plays an essential role for the survival of nitrogen starvation periods.
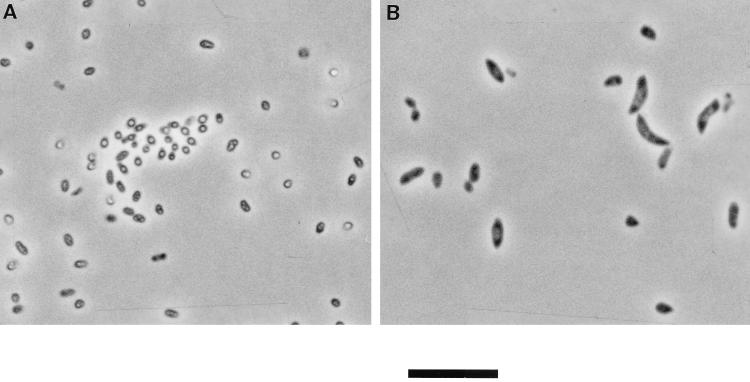
The gltB mutants exhibit aberrant cell morphologies during nitrogen starvation.
Under nitrogen-limited conditions, P. putida KT2442 accumulates large amounts of the biopolymer poly-3-hydroxyalkanoate (20, 21). Three days after a shift to nitrogen-free medium, cells from wild-type and N25 cultures contained one to several coalescent granules characteristic of poly-3-hydroxyalkanoate when inspected microscopically (Fig. 4A). In contrast, the gltB mutants did not contain any visible granules. Moreover, the mutant cells were found to be significantly swollen compared with the wild type, and often cells were banana shaped and contained condensed material at the cell poles (Fig. 4B). Interestingly, this aberrant cell shape of the mutants was observed only under the condition of nitrogen starvation. During growth in the presence of excess ammonium (5 mM or more), mutant and wild-type cells were completely indistinguishable.
FIG. 4.
Phase-contrast micrographs of (A) P. putida KT2442 and (B) the secondary mutant D7 after 3 days of nitrogen starvation. Cells were examined in a Carl Zeiss Axioplan phase contrast microscope with a 100× objective lens. Bar represent 5 μm.
The gltB mutants exhibit marked defects in nitrogen starvation-induced gene expression.
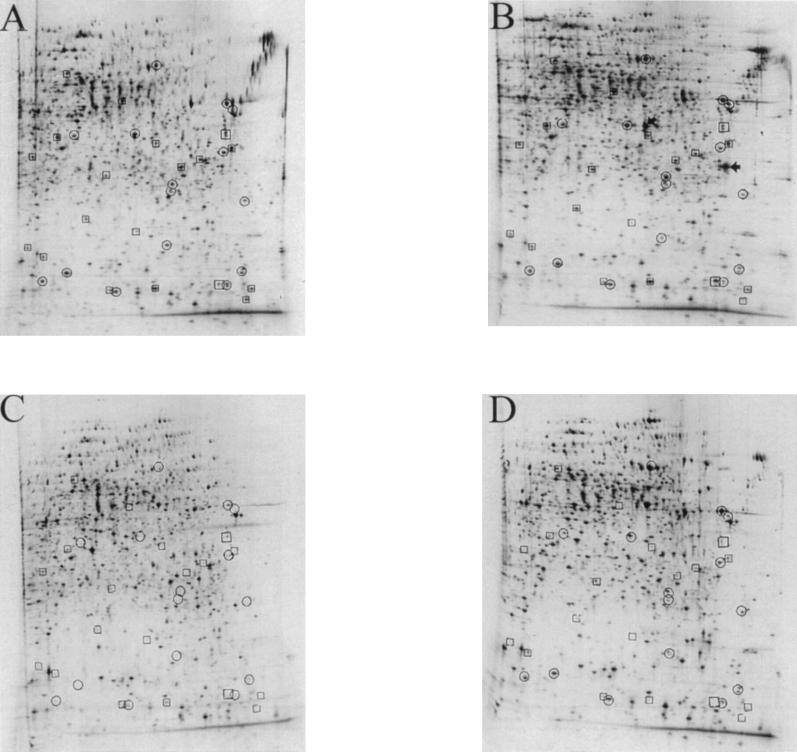
To investigate the effects of the gltB mutation on global nitrogen regulation, we performed two-dimensional gel electrophoresis of the wild-type strain, the primary mutant N25, the three gltB mutants, and the rpoN mutant. Two hours after removal of the nitrogen source, induction of bioluminescence in N25 was maximal; at the same time, we labeled the cells of the various cultures with radioactive methionine. Apart from two proteins which were highly induced in N25 (on the basis of the molecular weights and the pIs of the spots, it appears likely that they represent the LuxAB proteins), we detected no differences between the primary mutant and the wild type (Fig. 5A and B). The two-dimensional protein patterns of D5, D6, and D7 were, as expected, virtually identical (data not shown). However, the three gltB mutants failed to express a number of proteins that were induced in both wild-type and parent strains (Fig. 5D). The same set of proteins missing in the secondary mutants were also absent in the rpoN mutant strain, indicating that these are under Ntr control (Fig. 5C). In addition, the rpoN mutant lacked several proteins that were expressed by all of the other strains.
FIG. 5.
Autoradiograms of two-dimensional polyacrylamide gels of total cellular protein of P. putida KT2442 (A), the parental lux fusion strain N25 (B), the rpoN mutant (C), and the second-site mutant D5 (D). Cells were shifted to nitrogen starvation regimes as described in Materials and Methods. Thirty minutes after removal of the nitrogen source (at this time point, maximal lux gene expression was observed in P. putida N25), the cells were labeled with l-[35S]-methionine. Boxed spots denote proteins that exhibit a markedly decreased rate of expression in both the rpoN mutant and the three second-site mutants D5, D6, and D7 compared with the wild-type or the primary mutant N25. Circled spots represent proteins that are missing only in the rpoN mutant. The two proteins that were found in N25 but not in the wild type or any of the mutant strains are marked by arrows.
Construction of a defined gltB mutant.
To clone the entire gltB gene, we isolated recombinant cosmids containing large DNA fragments of the P. putida genome that (i) enabled the second-site mutants to grow on AB medium containing 15 mM citrate as carbon source and 5 mM nitrite as sole nitrogen source and that (ii) restored their ability to respond to nitrogen limitation as assessed by measurements of bioluminescence under nitrogen-limiting conditions. As expected, these cosmids contained the entire gltB gene plus several kilobases of flanking DNA. The nucleotide sequence of the gltB downstream region was partially determined (Fig. 3). This analysis revealed that the gltB gene is succeeded by the gltD gene encoding the small subunit of the glutamate synthase. Further downstream we identified an ORF the deduced amino acid sequence of which shows strong similarity to the uroporphyrinogen III decarboxylase (HemE) from P. aeruginosa and E. coli (Fig. 3). Interestingly, in P. aeruginosa hemE is also located downstream of the gltBD operon (GenBank accession no. PAU81261). By using a gene replacement method, we constructed a defined gltB mutant in the P. putida KT2442 wild-type background (see Materials and Methods for details). This mutant was physiologically characterized with respect to nitrogen utilization and starvation survival and was found to be virtually indistinguishable from the three second-site mutants (Table 3 and data not shown). These results clearly demonstrate that the phenotypes observed with the second-site mutants are independent of the nasB mutation.
DISCUSSION
We have isolated 21 independent luxAB insertion mutants of P. putida KT2442 that respond to nitrogen deprivation with increased bioluminescence. It is demonstrated that in the mutant that responded most strongly, P. putida N25, the lux-bearing transposon had inactivated the nasB gene encoding the assimilatory nitrate reductase. The genes involved in nitrate and nitrite assimilation are in many bacteria organized in clusters that encode all of the enzymes required for uptake and reduction of nitrate and nitrite (39). Sequence inspection of the 5′ DNA region of nasB revealed the presence of a sequence bearing strong homology to the ς54 promoter −12/−24 consensus sequence. A transcriptional fusion of this presumptive promoter to luxAB was used to show that transcription of nasB is in fact ς54 dependent. Analysis of the nasB downstream region did not reveal the presence of additional nitrate assimilation genes. These results demonstrate that in contrast to many other bacteria, the P. putida KT2442 genes for the utilization of nitrate are not clustered.
By employing second-site mutagenesis, three mini-Tn5 Km1 insertion mutants of P. putida N25 that no longer responded to nitrogen deprivation were isolated. In all three mutants, the secondary transposon was found to interrupt the gltB gene encoding the major subunit of the glutamate synthase (GOGAT). In E. coli, gltB mutations affecting biosynthesis of glutamate synthase result in a Ntr− phenotype (44), i.e., the inability to utilize a number of amino acids as sources for nitrogen. Furthermore, GOGAT-deficient mutants have also been described for Klebsiella pneumoniae (40), Azospirillum brasilense (3, 34), Bradyrhizobium japonicum (42), Rhizobium meliloti (30, 43), and Azorhizobium sesbaniae ORS571 (18). In all of these cases, GOGAT deficiency caused pleiotropic nitrogen assimilation defects, the so-called Asm− (nonassimilatory) phenotype. In accordance with these data, the P. putida gltB mutants were found to be unable to grow on nitrite, urea, low levels of ammonium (below 1 mM), and several of the amino acids as nitrogen sources. These results together with a detailed analysis of the patterns of proteins synthesized under nitrogen starvation conditions by the wild type and the different mutants clearly established that inactivation of the gltB gene in P. putida KT2442 renders the strain Ntr−. Hence, the inability of the gltB mutants to induce expression of nasB under nitrogen-limiting conditions appears to be due to a failure in the global Ntr.
The three gltB mutants are severely impaired in the ability to survive prolonged periods of nitrogen starvation compared to the parent strain N25. To our knowledge, it has not been shown earlier that gltB mutants are sensitive to nitrogen starvation. However, it has been reported recently that GOGAT-defective mutants of Salmonella enterica serovar Typhimurium are sensitive to osmotic stress when grown under ammonia-limited conditions (8). The authors suggest that increased l-glutamate synthesis is necessary for growth in hyperosmotic media. E. coli also responds to osmotic upshifts by accumulating K+ and synthesizing glutamate as a counterion to restore the cell's turgor pressure (9). Whether the accumulation of l-glutamate also plays a role for the survival during prolonged periods nitrogen starvation remains to be elucidated. The aberrant cell morphology observed with the three P. putida gltB mutants under nitrogen-limiting conditions may be an indication that the cells were indeed osmotically stressed due to the lack of GOGAT activity.
At present there are two views concerning the function of GOGAT during nitrogen limitation (47). One view holds that GOGAT is essential for induction of the Ntr response because it removes the glutamine that is produced during ammonia assimilation under nitrogen-limiting conditions. A high cellular ratio of glutamine to α-ketoglutarate indicates that nitrogen is not limiting growth and consequently switches off the Ntr response. An alternative hypothesis proposes that the Ntr− phenotype conferred by insertion mutations in the gltB genes of E. coli and K. pneumoniae is not due to the absence of GOGAT activity but rather due to polar effects of the insertion on transcription of the downstream genes gltD (encoding the minor subunit of GOGAT) and gltF (5, 6, 29). It has been speculated that the gltF gene product interacts in a hitherto unknown manner with components of the Ntr network. This speculation is supported by the finding that the Ntr− phenotype of E. coli gltB mutants can be suppressed by mutations in ntrB. These suppressor mutants exhibit high constitutive expression of glutamine synthetase even under conditions of nitrogen excess, presumably due to changes in the configuration of the NtrB protein such that it no longer is able to dephosphorylate NtrC, thereby leaving the entire nitrogen regulon permanently activated (33, 44).
The fact that no gltF homologue is present downstream of gltB in P. putida KT2442 strongly favors the hypothesis that GOGAT enzymatic activity is needed for Ntr response. This result is fully consistent with a recent study which demonstrated that in E. coli GltF is in fact a periplasmatic protein that is apparently not involved in regulation of nitrogen catabolism (16). Whether GOGAT enzymatic activity is required for the removal of glutamine, which may accumulate during nitrogen starvation and consequently abolishes the Ntr response in P. putida KT2442, has yet to be investigated.
ACKNOWLEDGMENTS
We thank A. Nielsen, L. Stabell, and B. Schumacher for excellent technical assistance, J.-L. Ramos, H. P. Schweizer, and V. Stewart for supplying bacterial strains and plasmids, and F. Bruno for helpful discussions.
This work was supported by grants from the Swedish Environmental Protection Agency and the Danish Center for Microbial Ecology. L.E. was in part supported by the Research Training Program of the EU (no. BIO4CT965025).
REFERENCES
- 1.Adler S P, Purich D, Stadtman E R. Cascade control of Escherichia coli glutamine synthetase. Properties of the PII regulatory protein and the uridylyltransferase-uridylyl-removing enzyme. J Biol Chem. 1975;250:6264–6272. [PubMed] [Google Scholar]
- 2.Bagdasarian M, Lurz B, Ruckert B, Franklin F C H, Bagdasarian M M, Frey J, Timmis K N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors for gene cloning in Pseudomonas. Gene. 1981;16:237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- 3.Bani D, Berberio C, Bazzicalupo M, Favilli F, Gallori E, Polsinelli M. Isolation and characterization of glutamate synthase mutants of Azospirillum brasiliense. J Gen Microbiol. 1980;119:239–244. [Google Scholar]
- 4.Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castaño, I., F. Bastarrachea, and A. A. Covarrubias. 1988. gltBDF operon of Escherichia coli. 170:821–827. [DOI] [PMC free article] [PubMed]
- 6.Castaño I, Flores N, Valle F, Covarrubias A A, Bolivar F. gltF, a member of the gltBDF operon of Escherichia coli, is involved in nitrogen-regulated gene expression. Mol Microbiol. 1992;6:2733–2741. doi: 10.1111/j.1365-2958.1992.tb01450.x. [DOI] [PubMed] [Google Scholar]
- 7.Clark J D, Maaløe O. DNA replication and the cell division cycle in Escherichia coli. J Mol Biol. 1967;23:99–112. [Google Scholar]
- 8.Csonka L N, Ikeda T P, Fletcher S A, Kustu S. The accumulation of glutamate is necessary for optimal growth of Salmonella typhimurium in media of high osmolarity but not induction of the proU operon. J Bacteriol. 1994;176:6324–6333. doi: 10.1128/jb.176.20.6324-6333.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csonka L N, Hanson A D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 10.de Lorenzo V, Eltis L, Kessler B, Timmis K N. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123:17–24. doi: 10.1016/0378-1119(93)90533-9. [DOI] [PubMed] [Google Scholar]
- 11.de Lorenzo V, Timmis K N. Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5 and Tn10-derived mini-transposons. Methods Enzymol. 1994;235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 12.Engleman E, Francis S. Cascade control of E. coli glutamine synthetase. II. Metabolite regulation of the enzymes in the cascade. Arch Biochem Biophys. 1978;191:602–612. doi: 10.1016/0003-9861(78)90398-3. [DOI] [PubMed] [Google Scholar]
- 13.Garcia E, Rhee S G. Cascade control of Escherichia coli glutamine synthetase: purification and properties of PII uridylyltransferase and uridylyl-removing enzyme. J Biol Chem. 1983;258:2246–2253. [PubMed] [Google Scholar]
- 14.Givskov M, Eberl L, Molin S. Responses to nutrient starvation in Pseudomonas putida KT2442: two-dimensional electrophoretic analysis of starvation- and stress-induced proteins. J Bacteriol. 1994;176:4816–4824. doi: 10.1128/jb.176.16.4816-4824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Givskov M, Eberl L, Møller S, Poulsen L K, Molin S. Responses to nutrient starvation in Pseudomonas putida KT2442: analysis of general cross-protection, cell shape, and macromolar content. J Bacteriol. 1994;176:7–14. doi: 10.1128/jb.176.1.7-14.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grassl G, Bufe B, Müller B, Rösel M, Kleiner D. Characterization of the gltF gene product of Escherichia coli. FEMS Microbiol Lett. 1999;179:79–84. doi: 10.1111/j.1574-6968.1999.tb08711.x. [DOI] [PubMed] [Google Scholar]
- 17.Herrero M, de Lorenzo V, Timmis K. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilgert U, Schell J, de Bruijn F J. Isolation and characterization of Tn5-induced NADPH-glutamate synthase (GOGAT−) mutants of Azorhizobium sesbaniae ORS571 and cloning of the corresponding glt locus. Mol Gen Genet. 1987;210:195–202. [Google Scholar]
- 19.Hoang T T, Karkhoff-Schweizer R R, Kutchma A J, Schweizer H P. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212:77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 20.Huijberts G N M, Eggink G, de Waard P, Huisman G W, Withold B. Pseudomonas putida KT2442 cultivated on glucose accumulates poly(3-hydroxyalkanoate) consisting of saturated and unsaturated monomers. Appl Environ Microbiol. 1992;58:536–544. doi: 10.1128/aem.58.2.536-544.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huisman G W, de Leeuw O, Eggink G, Witholt B. Synthesis of poly-3-hydroxyalkanoates is a common feature of fluorescent pseudomonads. Appl Environ Microbiol. 1989;55:1949–1954. doi: 10.1128/aem.55.8.1949-1954.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamberov E S, Atkinson M R, Chandarn P, Ninfa A J. Effect of mutations in Escherichia coli glnL (ntrB), encoding nitrogen regulator II (NRII or NTRB), on the phosphatase activity involved in bacterial nitrogen regulation. J Biol Chem. 1994;269:28294–28299. [PubMed] [Google Scholar]
- 23.Keener J, Kustu S. Protein kinase and phosphoprotein phosphatase activities of nitrogen regulatory proteins NTRB and NTRC of enteric bacteria: roles of the conserved amino-terminal domain of NTRC. Proc Natl Acad Sci USA. 1988;85:4976–4980. doi: 10.1073/pnas.85.14.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessler B, de Lorenzo V, Timmis K N. A general system to integrate lacZ fusions into the chromosomes of Gram-negative eubacteria: regulation of the Pm promoter of the TOL plasmid studied with all controlling elements in monocopy. Mol Gen Genet. 1992;233:293–301. doi: 10.1007/BF00587591. [DOI] [PubMed] [Google Scholar]
- 25.Kilstrup M, Jacobsen S J, Hammer K, Vogens F K. Induction of heat shock proteins DnaK, GroEL, and GroES by salt stress in Lactococcus lactis. Appl Environ Microbiol. 1997;63:1826–1837. doi: 10.1128/aem.63.5.1826-1837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y, Waltrud L S, Matin A. A carbon starvation survival gene of Pseudomonas putida is regulated by ς54. J Bacteriol. 1995;177:1850–1859. doi: 10.1128/jb.177.7.1850-1859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Köhler T, Harayama S, Ramos J-L, Timmis K N. Involvement of Pseudomonas putida RpoN ς factor in regulation of various metabolic functions. J Bacteriol. 1989;171:4326–4333. doi: 10.1128/jb.171.8.4326-4333.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristensen C S, Eberl L, Sanches-Romero J M, Givskov M, Molin S, de Lorenzo V. Site-specific deletions of chromosomally located DNA segments with the multimer resolution system of broad-host-range plasmid RP4. J Bacteriol. 1995;177:52–58. doi: 10.1128/jb.177.1.52-58.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuczius T, Eitinger T, D'Ari R, Castroph H, Kleiner D. The gltF gene of Klebsiella pneumoniae: cloning and characterization. Mol Gen Genet. 1991;229:479–482. doi: 10.1007/BF00267472. [DOI] [PubMed] [Google Scholar]
- 30.Lewis T A, Gonzalez R, Bostford J L. Rhizobium meliloti glutamate synthase: cloning and initial characterization of the glt locus. J Bacteriol. 1990;172:2413–2420. doi: 10.1128/jb.172.5.2413-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J T, Goldman B S, Stewart V. Structures of genes nasA and nasB, encoding assimilatory nitrate and nitrite reductases in Klebsiella pneumoniae M5al. J Bacteriol. 1993;175:2370–2378. doi: 10.1128/jb.175.8.2370-2378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin reagent. J Biol Chem. 1951;193:265–270. [PubMed] [Google Scholar]
- 33.Magasanik B. Genetic control in nitrogen assimilation in bacteria. Annu Rev Genet. 1982;16:135–168. doi: 10.1146/annurev.ge.16.120182.001031. [DOI] [PubMed] [Google Scholar]
- 34.Mandal A K, Ghosh S. Isolation of a glutamate synthase (GOGAT)-negative, pleiotropically N utilization-defective mutant of Azospirillum brasilense: cloning and partial characterization of the structural gene. J Bacteriol. 1993;175:8024–8029. doi: 10.1128/jb.175.24.8024-8029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matin A. The molecular basis of carbon starvation-induced general resistance in Escherichia coli. Mol Microbiol. 1991;5:3–11. doi: 10.1111/j.1365-2958.1991.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 36.Meers J L, Tempest D W, Brown C M. Glutamine(amide):2-oxoglutarate amino transferase oxido-reductase (NADP), an enzyme involved in the synthesis of glutamate by some bacteria. J Gen Microbiol. 1970;64:187–194. doi: 10.1099/00221287-64-2-187. [DOI] [PubMed] [Google Scholar]
- 37.Merrick M J, Edwards R A. Nitrogen control in bacteria. Microbiol Rev. 1995;59:604–622. doi: 10.1128/mr.59.4.604-622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merrick M J. In a class of its own—the RNA polymerase sigma factor ς54 (ςN) Mol Microbiol. 1993;10:903–909. doi: 10.1111/j.1365-2958.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 39.Moreno-Vivián C, Cabello P, Martínez-Luque M, Blasco R, Castillo F. Prokaryotic nitrate reduction: molecular properties and functional distinction among bacterial nitrate reductases. J Bacteriol. 1999;181:6573–6584. doi: 10.1128/jb.181.21.6573-6584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagati H, Shimizu M, Valentine R C. The mechanism of ammonia assimilation in nitrogen fixing bacteria. Arch Microbiol. 1971;79:164–175. doi: 10.1007/BF00424923. [DOI] [PubMed] [Google Scholar]
- 41.Ninfa A J, Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci USA. 1986;83:5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Gara F, Manian S, Meade J. Isolation of an Asm− mutant of Rhizobium japonicum defective in symbiotic nitrogen fixation. FEMS Microbiol Lett. 1984;24:241–245. [Google Scholar]
- 43.Osbourne M S, Singer E R. Ammonia assimilation in Rhizobium meliloti. J Bacteriol. 1980;143:1234–1240. doi: 10.1128/jb.143.3.1234-1240.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pahel G, Zelentz A D, Tyler B M. gltB gene and regulation of nitrogen metabolism by glutamine synthetase in Escherichia coli. J Bacteriol. 1978;133:139–148. doi: 10.1128/jb.133.1.139-148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos-Díaz M A, Ramos J L. Combined physical and genetic map of the Pseudomonas putida KT2440 chromosome. J Bacteriol. 1998;180:6352–6363. doi: 10.1128/jb.180.23.6352-6363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramos-González M I, Molin S. Cloning, sequencing, and phenotypic characterization of the rpoS gene from Pseudomonas putida KT2440. J Bacteriol. 1998;180:3421–3431. doi: 10.1128/jb.180.13.3421-3431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reitzer J L. Sources of nitrogen and their utilization. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W, Riley M, Schaechter M, Umbarger A E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 380–390. [Google Scholar]
- 48.Ronald S L, Kropinski A M, Farinha M A. Construction of broad host-range vectors for the selection of divergent promoters. Gene. 1990;90:145–148. doi: 10.1016/0378-1119(90)90451-v. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 50.Shingler V. Signal sensing by ς54-dependent regulators: derepression as a control mechanism. Mol Microbiol. 1996;19:409–416. doi: 10.1046/j.1365-2958.1996.388920.x. [DOI] [PubMed] [Google Scholar]
- 51.Unthan M, Klipp W, Schmid G H. Nucleotide sequence of the nasB gene encoding assimilatory nitrate reductase from the cyanobacterium Oscillatoria chalybea. Biochim Biophys Acta. 1996;1305:19–24. doi: 10.1016/0167-4781(95)00210-3. [DOI] [PubMed] [Google Scholar]
- 52.Weiss D S, Batut J, Klose K E, Keener J, Kustu S. The phosphorylated form of the enhancer-binding protein NTRC has an ATPase activity that is essential for activation of transcription. Cell. 1991;67:155–167. doi: 10.1016/0092-8674(91)90579-n. [DOI] [PubMed] [Google Scholar]
- 53.Weiss V, Magasanik B. Phosphorylation of nitrogen regulator I (NRI) of Escherichia coli. Proc Natl Acad Sci USA. 1988;85:8919–8923. doi: 10.1073/pnas.85.23.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]