Highlights
-
•
Total polyphenol content in the human diet is underestimated due to inefficient extraction methodology.
-
•
Entrapment of NEPP in the food matrix meshwork makes it unavailable for digestion and absorption.
-
•
NEPP, EPP along with dietary fibre contributes to the growth of gut microbial ecology.
-
•
The microbial metabolites of NEPP shows immunomodulatory effect.
Keywords: Non-extractable polyphenols, Monomeric and polymeric polyphenols, Food-matrix polysaccharide, Colonic fermentation, Prebiotics, Gut immune-modulation
Abstract
Most of the pertinent research which aims at exploring the therapeutic effects of polyphenols usually misapprehends a large fraction of non-extractable polyphenols due to their poor aqueous-organic solvent extractability. These polymeric polyphenols (i.e., proanthocyanins, hydrolysable tannins and phenolic acids) possess a unique property to adhere to the food matrix polysaccharides and protein sowing to their structural complexity with high glycosylation, degree of polymerization, and plenty of hydroxyl groups. Surprisingly resistance to intestinal absorption does not hinder its bioactivity but accelerates its functionality manifolds due to the colonic microbial catabolism in the gastrointestinal tract, thereby protecting the body from local and systemic inflammatory diseases. This review highlights not only the chemistry, digestion, colonic metabolism of non-extractable polyphenols (NEPP) but also summarises the synergistic effect of matrix-bound NEPP exerting local as well as systemic health benefits.
Introduction
Plants produce more than 8000 phenolic compounds, usually called (poly)-phenols, which are the most efficient immune-protective components. The advanced scientific research has clearly established the significant preventive action of dietary polyphenolic antioxidants against chronic metabolic diseases due to their anti-inflammatory, antioxidant, anti-cancerous, and immune-modulatory properties (de Souza et al., 2019, Hossen et al., 2017, McClements et al., 2021). As most of the compounds arise owing to aromatic rings and hydroxyl groups of phenolics to form polymeric structures and remain in a bound and polymeric form as glycosides or esters, these are recognized as xenobiotics in the human body, having apparently low bioavailability in the human gastrointestinal system (de Souza et al., 2019, Leri et al., 2020). These secondary metabolites of plant with diversified chemical characteristics and varying solubility in the aqueous-organic solvent medium is analysed extensively by experimental and clinical trial methods focusing mainly on its bioavailability, metabolism, and mechanism of action (Pérez-Jiménez & Torres, 2011).
Till date a myriad of research regarding polyphenol chemistry and bioactivity only covered the polyphenol fraction into consideration that were extracted in aqueous-organic solvents (i.e., extractable polyphenols). Therefore, the data regarding the overall polyphenol content in food is misjudged and miscalculated because of inefficient aqueous-organic extraction methods, which are incapable of extracting the polymeric polyphenols (flavan-3-ol polymer- condensed tannin, hydrolysable tannin and bound phenolic acids) bound to the food matrix residue after extraction (Carboni Martins et al., 2022, Ding et al., 2020). These are termed as non-extractable polyphenol or macromolecular antioxidants present in bulk fraction in physiological as well as food milieu with three basic characteristics that make them different from extractable polyphenols: a) They are not extractable in conventional aqueous-organic solvents extraction method; b) They remain in a complex polymeric form being linked to the food matrix components, and c) Though these are not bio-accessible in the small intestine they become so only in the large intestine and colon after skipping small intestinal digestion (Martínez-Meza et al., 2020, Pérez-Jiménez et al., 2013, Saura-Calixto, 2012).
Although NEPP are surplus in the daily diet, 60 mg/day of daily intake in the United States with a degree of polymerization greater than 2 (Gu et al., 2004) and 450 mg/day with a higher degree of polymerization in the Spanish population (Saura-Calixto et al., 2007), their bioavailability is strongly affected by the complex structure, higher degree of polymerization, association with food matrix polysaccharides, and many other factors, the chemistry being the main player. The scientific evidence available till date indicates that the association of these polymeric polyphenols to the food matrix may help bind the complex polysaccharide thus aiding their journey to the colon unaltered (Renard et al., 2017, Zhu, 2018). The scientific testimonial gathered till date also stipulates that the health benefits of these phytochemicals might be accredited to the bio-transformed metabolites synthesized by microbial catabolism in the colon, rather than its native conformation found in foods (Huang et al., 2022).
Detailed knowledge about the overall chemistry, its mechanism of digestion and absorption of NEPP is lacking making the area of research vague. Besides there is a knowledge gap regarding the pathways and mechanism of action used by these phenolics and their metabolites as well (Carboni Martins et al., 2022). However, there are only a few studies available on the NEPP-polysaccharide cross-talk and the two-way interaction of NEPP and gut microbiome to validate the synergistic effect realistically (Tomás-Barberán & Espín, 2019). This review aims to reveal the precise updated information regarding the absorption, bioaccessibility and interaction between polysaccharide and NEPP to establish a concise clear and concept of the underlying mechanism hampering extractability followed by its efficient delivery to large intestine and colon. The thorough literature review and compilation of the information has been done to explore the synergistic role of NEPP with gut microorganisms in exerting local and systemic therapeutic effects. This review also highlighted the immunomodulatory mechanism of these metabolites, which have profound therapeutic effect against metabolic disorder and modulation of gut microbial ecology preventing dysbiosis as well.
Chemistry of non-extractable polyphenols
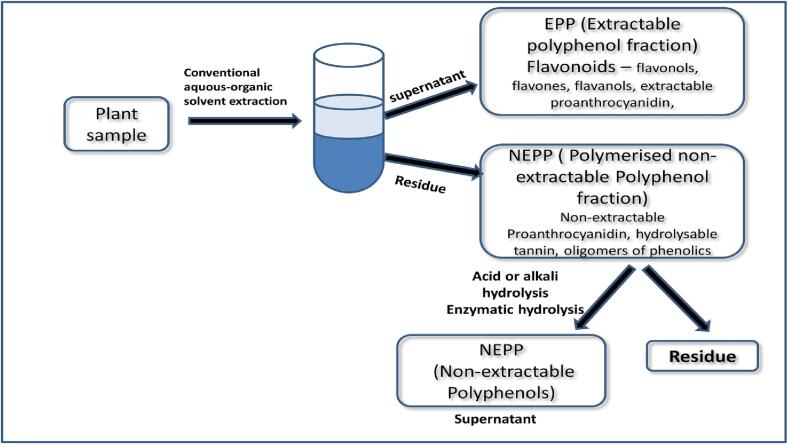
Based on scientific research in the past, the dietary phenolics are conventionally classified in several groups on the basis of their chemical properties and structural diversities (Pérez-Jiménez et al., 2013). But these compounds can also be classified in majorly two classes depending on its association with food matrix components and solubility in conventional aqueous-organic solvents: 1) The soluble phenolics that are present freely in the vacuoles of plant cells together with some low molecular weight soluble phenolics conjugated to the food matrix polysaccharide protein and sometimes fatty acids are mostly referred as extractable polyphenol. Several chemically diverse subgroups of flavonoids (flavanols, anthocyanins, flavones, flavonols), stilbenes, hydroxybenzoic and hydroxycinnamic acids along with some fraction of extractable proanthocyanidins (EPA) are some of the reported EPP found in plants, fruits and vegetables (Carboni Martins et al., 2022, Saura-Calixto, 2012); and 2) The rest of the high molecular weight phenolics, mostly polymerised proanthocyanidin, hydrolysable tannin and to some extent low molecular weight phenolics, which remain bound to the macromolecules in the fibrous residue after aqueous-organic solvent extraction are termed as non-extractable polyphenols (Carboni Martins et al., 2022, Pérez-Jiménez and Torres, 2011).
The non-extractable polyphenols (NEPP) remain associated with cell wall macromolecules mostly protein, dietary fibre, and polysaccharides by covalent, non-covalent, and hydrogen bonds and hydrophobic interactions or confined in the core food matrix (Fig. 1). Therefore, it escapes the conventional aqueous organic extraction process and it is used to get discarded with the corresponding residue (Cheng et al., 2014, Pérez-Jiménez et al., 2013). It encompasses proanthocyanidins commonly known as condensed tannin and hydrolyzable polyphenols and bound phenolic acids (Domínguez-Rodríguez et al., 2017, Domínguez-Rodríguez et al., 2022, Pérez-Jiménez et al., 2013, Pérez-Jiménez and Torres, 2011).
Fig. 1.
Overview of extractable (EPP) and non-extractable polyphenolic (NEPP) compounds present in human diet.
Non-extractable proanthocyanidins (NEPA) or condensed tannins
Proanthocyanidins are the second most predominant plant secondary metabolites after lignin and it constitutes more than twice the content of other flavonoids present in our diet (Carboni Martins et al., 2022, Qi et al., 2022). These are combinations of oligomers and polymers of flavan-3-ol units, commonly known as catechins. The flavan 3-ol units possess the typical C6 C3 C6 flavonoid structural backbone. The fused aromatic ring to the heterocyclic benzopyran ring C is considered as the A ring and the ring with phenyl constituent as the B ring (He et al., 2008, Martínez-Meza et al., 2020). NEPP can be further subdivided in two groups: extractable proanthocyanidins and non-extractable proanthocyanidin (NEPA). The structural divergence of proanthocyanidins rests on the stereochemistry at the chiral carbon and hydroxylation pattern on the B ring of the flavanol monomer extension and end units, the inter-flavan linkage, and the degree of polymerization (Ou & Gu, 2014). Depending on the hydroxylation pattern on both aromatic ring and stereochemistry of the two chiral carbons (C2 and C3) of ring C, flavanol monomers have 4 basic stereochemically different configurations with several monomeric diastereoisomers. The C2 configuration is designated as R whereas the 2S configuration is differentiated by a prefix term enantio (ent.) among which (+) catechins, (-) epicatechin are the two most common monomeric extension units found naturally. Combinations of these monomers give rise to several classes of proanthocyanidins. The most abundant proanthocyanidins are procyanidin, prodelphinidin and propelargonidin made up of (+) catechin or (-) epicatechins with3′,4′-dihydroxyl pattern of extension unit, prodelphinidins with 3′,4′,5′-trihydroxyl pattern ((+)-gallocatechin and/or (-)-epigallocatechin) and propelargonidin with 4′-hydroxyl pattern ((+)-afzelechin and/or (-)-epiafzelechin) units, respectively. Proanthocyanidin monomers are joined through Inter Flavan Linkage (IFL) to form polymers and based on the type of linkage (either α or β) it can be divided into several classes: i) Proanthocyanidins in which flavan-3-ol monomers are joined through C4 - C8 or C4 - C6 linkage are grouped as B-type of proanthocyanidina, and ii) those which are joined by an additional C2 - C7 linkage along with C4 - C8 or C4 - C6 linkage are grouped as type A proanthocyanidins (Ou and Gu, 2014, Rue et al., 2018, He et al., 2008). The most abundant type of proanthocyanidins present in the vegetables is procyanidins which are composed of catechin and epicatechin units, while other less common ones are propelargonidin or prodelphinidin containing (epi)-afzelechin or (epi)-gallocatechin as basic units (Fig. 1). There are several subgroups of procyanidins found in nature from dimers (procyanidin B1-B8, procyanidin A1-A2), trimers (procyanidin C1 and C2) to oligomers, and polymers, based on the number of monomeric units present i.e., the degree of polymerization (Smeriglio et al., 2017). These are found to be associated with the cell wall components and other cellular parts in tegmental tissues and also in fruit peels and stems and barks, seed coats and brans of grains, etc. (Mateos-Martín et al., 2012, Tuohy and Rio, 2014). The mechanism of association of these compounds to the cell wall polysaccharides is discussed below highlighting several reactions taking place. A-type proanthocyanidins are found rarely in nature compared to the B-type. It is detected in fruits (such as avocados, plums, cranberries, and peanuts) and herbs such as curry leaves and cinnamon as well (Xie et al., 2023). Traces of B-type proanthocyanidins can be observed in fruits such as banana, cocoa, grapes, and apricot, cereals and millets for example, sorghum and barley, and nuts such as almonds, etc. (Gu et al., 2003).
Hydrolysable tannin
Hydrolysable tannins are complex polymerized compounds made up of a core sugar moiety that is acylated by gallic acid or ellagic acid subunits and their secondary products. Based on the acylated subunits hydrolysable tannins are mainly of two types; gallotannins (when the monomeric subunits are gallic acid) and ellagitannins (when the monomeric units are ellagic acid) (Domínguez-Rodríguez et al., 2017). Ellagitannins are the transformed version of hexahydroxydiphenic acid (HHDP) produced by hydrolysis (Tuohy & Rio, 2014). Ellagic acid is produced by C—C bonding between the gallolyl units of gallotanins to form hexahydroxydiphenic acid (HHDP), which lactonises spontaneously yielding ellagic acid (Sallam et al., 2021). These secondary metabolites present in a wide variety of fruits, vegetables, nuts, seed coats, cereal grains, and beverages like tea, coffee, cocoa, and red wine (Gu et al., 2004, Smeriglio et al., 2017). Although these compounds can also be present in EPP fraction, a few study reports confirm its presence in residual part after water-organic solvent extraction.
Bound phenolic acids
There is ample evidence to suggest that some phenolic acids remain bound to the food matrix residue even after the extraction of soluble free and esterified phenolic acids. These phenolic acids are mostly derivatives of ferulic acid, such as ferulic acid esterified with a sugar moiety (Rocchetti et al., 2022). Ferulic acid is known to remain bound to cell wall polysaccharides, such as hemicelluloses, via ester linkages. Additionally, the dimer and oligomers of ferulic acid aid in cross-linking polysaccharides to other cell wall insoluble polysaccharides, such as xylanes, lignins, and proteins. To analyze and quantify these phenolics, a step called hydrolysis is required to deconjugate them from the food matrix polysaccharides and proteins (Martínez-Meza et al., 2020, Pérez-Jiménez and Torres, 2011).
Interaction of NEPP with food matrix polysaccharides
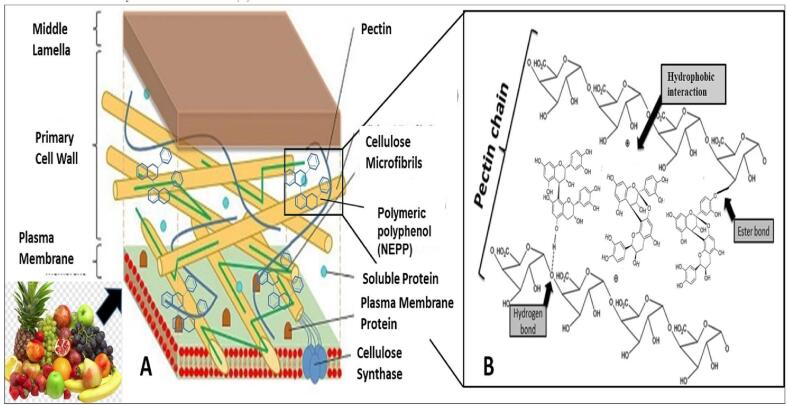
The potential beneficial effects of non-extractable polyphenols depend on their structural and biochemical properties, especially, their unique property to interact with macromolecules of food (Makarewicz et al., 2021). These polymeric polyphenols get attached to the matrix protein, cellulosic polysaccharides and pectin with the help of non-covalent bonds like hydrogen bonds and ester bonds (Wong et al., 2016). The existence of the food matrix, and the chemical components as well, can affect the process of bio-accessibility, bioavailability and the efficiency of biological activity of non-extractable polyphenols and their synergistic interactions with the intestinal microbiota (Iqbal et al., 2022, Saura-Calixto, 2011). The isothermal titration calorimetry (ITC) study of the interaction mechanism between non-extractable polyphenols and food matrix polysaccharides (Le Bourvellec et al., 2009, Watrelot et al., 2013), highlighted the major influencing factors i.e., the chemical and structural composition of NEPP, as well as the degree of polymerization (Carboni Martins et al., 2022), percentage of glycosylation (Chirug et al., 2018), percentage of gallolylation (Bautista-Ortín et al., 2014), stereochemistry of flavan-3-ol subunits, and several physicochemical properties (Bautista-Ortín et al., 2014, Watrelot et al., 2013).The multiple binding site in polymeric procyanidins strongly facilitates its association with pectin molecules. The affinity of interaction is increased by oxidation of proanthocyanidin molecules (Bautista-Ortín et al., 2014).
The polymeric procyanidins possess many ortho phenolic groups and aryl rings which contributes to hydrogen bond formation and hydrophobic interactions. Moreover, the higher the degree of polymerisation, higher the presence of ligands capable of binding the call wall polysaccharides (Liu et al., 2020). Not only the structural diversity of NEPP but the components of food matrix such as presence of cellulose, hemicelluloses and pectins also influences the adsorption and retention of polymeric polyphenols within the matrix polysaccharide network. Fernandes et al. (2020) found that excess branching of arabinan-pectic polysaccharide hampers the interaction between polysaccharide and polyphenolic substances. The plant cell wall is a complex polysaccharide matrix made up of three impregnated but not interlinked networks i.e., a cellulose or xyloglucan network implanted in a matrix of pectin and held immobile by cross-connected glycoproteins (Liu et al., 2020). Ducasse et al. (2010) showed in their research that the use of pectin degrading enzyme during maceration increases proanthocyanidin extraction from grapes as these enzymes degrade the pectin mesh and help release the proanthocyanidin from the bound form. Bindon et al. (2016) found that removal of pectins from grape cell wall reduces the adsorption of proanthocyanidin to the native cell wall components. This study proves the involvement pectins in binding the NEPP through non-covalent interaction. Other than that the solubility of food matrix polysaccharides, molecular size, branching complexity, degree of esterification and overall surface porosity are the major modulatory factor regarding the polysaccharide polyphenol interaction (Liu et al., 2020). The most probable cause of discrepancy in the experimental result of proanthocyanidins binding and extraction efficiency may be due to the oxidation of procyanidin and its absorption in the food polysaccharide matrix or an increase in non-covalent interaction between the components mentioned above (Thilakarathna and Vasantha Rupasinghe, 2022, Ye et al., 2014) (Fig. 2). Ruiz-Garcia et al. (2014) found that hemicellulose fraction has the highest capacity to retain proanthocyanidins. Pectin has the ability to capture the procyanidins in preformed hydrophobic regions by the help of gelling (Le Bourvellec et al., 2005).
Fig. 2.
Structural organization of plant food matrix with non-extractable polyphenol entrapped in it (A) by several chemical interaction mostly noncovalent bonds (B).
Corn and common beans are the sources of phenolic compounds that remain attached to the oligosaccharides. The in vitro digestion process of polyphenols along with oligosaccharides were studied using corn cooked bean chips for snacks. The results showed that the non-digestible polyphenols present in the nondigestible oligosaccharides of corn cooked bean chips reached the colon and effectively showed prebiotic and antioxidative functions (Luzardo-Ocampo et al., 2017). There are a few polysaccharide to be precise, oligosaccharide like fructan and fucoidans (hydrolysed to yield fuco-oligosaccharide) are found to have therapeutic benefits and used for food and nutraceutical and pharmaceutical application (Wang et al., 2023, Wang and Cheong, 2023).
Dietary fibres rich in NEPP were extracted from broccoli stalks in two fractions, total fibre fractions and insoluble dietary fibre fraction. The NEPP content in the total fibre fraction, insoluble fibre fraction and freeze-dried fraction of broccoli stalk was found to be 98%, 97% and 79% of the total polyphenols, respectively. The insoluble dietary fibre fraction was nutritionally important with high content of polyphenols, glucosinolates with high prebiotic score. Environment friendly by-product may be used in food and pharmaceutical industry (Núñez-Gómez et al., 2022).
Several studies have pointed out that the physiological effects of NEPP or dietary fibres when separately given have a less promising effect than when given simultaneously. Insoluble dietary fibres from carob fruit holding NEPP reduced blood pressure and showed hypocholesterolemic effect when given to rats (Macho-González et al., 2017, 2018). Several in vivo studies have shown that dietary fibres along with NEPP synergistically increase the cardiometabolic markers. In a randomised control trial refined wheat grain was compared with whole wheat grain in the diet of unhealthy individuals of overweight and obese category. Whole wheat grain group showed four-fold increase of serum dihydroferulic acid. Refined Wheat grain intake lowered Bifidobacteriales and increased Bacteroidetes while whole wheat grain intake increased Bacteroidetes and Firmicutes but reduced Clostridium with reduction in inflammatory markers (Vitaglione et al., 2015).
Several non-covalent interactions such as hydrophobic interaction, hydrogen bonds helps to form pectin-polyphenol interaction (Hu et al., 2023). The aromatic groups present on the structure of NEPP polymers acts as positive inducer of hydrophobic interaction (Watrelot et al., 2013). Le Bourvellec et al. (2004) observed disruption of hydrogen bonds by applying urea in proanthocyanidin-polysaccharide mixture, therefore proved the involvement of hydrogen bond also. Majority of hydrolysable tannins contents in plants are extracted in conventional water-organic solvent extraction method, leaving behind very less in the food matrix residue (Pérez-Jiménez & Torres, 2011). The proportion of polyphenols to polysaccharides, pH of the extraction medium, ionic strength, reaction time and temperature are also major influencing factors. These factors also influence the extraction rate of bound phenolics from the food matrix (Padayachee et al., 2013, Rocchetti et al., 2022).
The NEPP-polysaccharide interaction exerts a significant effect on the nutritional, biochemical properties and extractability of the NEPP (Le Bourvellec et al., 2009, Renard et al., 2011). It also alters the extractability (Le Bourvellec et al., 2009), susceptibility to enzymes, and fermentability of cell wall biopolymers (Aura et al., 2013). The presence of the food matrix also modifies the bidirectional interactions of NEPP with the intestinal microbiota. NEPP conjugates with insoluble dietary fibre reach the colon before transformation. In a preclinical study wheat bran containing ferulic acid fraction associated with cell wall was found to be more bioavailable than free form of the polyphenols (Rondini et al., 2004). NEPP showed a delayed release of phenolic compounds due to retention in the gel (Mosele et al., 2016).
The association of NEPP with cell wall polysaccharides has a significant impact its extractability. The food matrix polysaccharides hinder the bioavailability of these compounds in the upper gastrointestinal tract (Das et al., 2020). This phenomenon helps to carry these compounds to the colon where it gets fermented by the colonic microflora and produces many metabolites such as different phenolic acids (Ozdal et al., 2013) with much more potent biological effects than that of the native compounds. Studies by Aura et al. (2013) put light on the synergistic effect of interconnection between food matrix and non-extractable procyanidin on the metabolic transformation of these compounds in the colon by human colonic microflora. A few studies on the rate of fermentability of NEPP suggested that the presence of dietary fibre amplifies the fermentation of polyphenols (Juśkiewicz et al., 2011). This data was validated by a work done by Saura-Calixto et al. (2010) where the in vitro fermentability of NEPP concentrate was found to be 23%, while NEPP concentrate in presence of dietary fibre showed 53% fermentability. There are several probable hypotheses about this phenomenon but the most convincing one is that the food matrix polysaccharides may act as nutrients for the growth and activity of colonic microflora thus indirectly igniting the fermentation process. The NEPP also modifies the solubility property, reduce viscosity, and also decrease the rigidity of matrix polysaccharide meshwork of plant cell wall through formation of polyphenol-polysaccharide complex (Tudorache et al., 2020) and also provide protection against bacterial, fungal attack and oxidative stress (Liu et al., 2007). Thus, the food matrix components and NEPP both exert a synergistic effect on the colonic fermentation of NEPP providing a significant amount of fermentable NEPP to the gut microbiota for a longer time and keeping the metabolites for a longer time in the human body simultaneously.
Bioavailability and colonic biotransformation of non-extractable polyphenols in gastrointestinal tract
Proanthocyanidin, also known as condensed tannin, is the second most abundant constituent of plant tissue after lignin. The average daily intake of proanthocyanidin is more than twice the total consumption of other extractable flavonoids, making it the major constituent of NEPP fraction (Gu et al., 2003, Qi et al., 2022). Therefore, it can be assumed that the biological effect of NEPP would be of greater magnitude than other flavonoids. However, due to their high molecular weight, the presence of polyhydroxyl groups, and a higher degree of polymerization, the absorption and bio-accessibility of these compounds are quite challenging to achieve in the human GI tract. Studies on the bioavailability of these compounds both in vitro and in vivo are scarce due to inefficient extraction techniques. However, recent advances in its extraction procedure opens the scope of research regarding digestion and bio-accessibility of non-extractable polyphenols. A study by Castello et al. (2018) reported a significant absorption rate as they detected 9.55 μmol (–)-epicatechin and its derivatives in human urine during 48 h following ingestion of 387.58 μmol of (–)-epicatechin measured by UHPLC-ESI-MS/MS in the absence of deconjugating enzymes. In another study using monomeric proanthocyanidins, EGCG showed 0.012% and 0.32% absorption rates in fasted rats and human subjects, respectively within 1 h without the use of deconjugating enzymes (Gan et al., 2018). A recent systematic review by Hu et al. (2018) showed that 704 mg EGCG/day from green tea extract is a safe dose when it is consumed in beverage form.Déprez et al. (2000) revealed that the permeability of proanthocyanidin dimer and trimers through the paracellular route in Caco-2 cell lines is almost similar to the mannitol, a paracellular transport marker. These monomeric, di and trimeric proanthocyanidin is further glucuronudated firstly in the enterocytes and sulfonated and methylated next in the liver (Sallam et al., 2021).
The rate of absorption of monomeric proanthrocyanidin is only 5–10% of extractable polyphenol monomers. On the other hand, proanthocyanidin with a higher degree of polymerization are neither permeable (permeability: 2% of total NEPP) through the cell membrane due to their high polarity as a consequence of hydroxyl groups present on it nor can penetrate through the gap junction probably because of their molecular weight and ability to tighten up the gap junctions by forming complexes with the cellular protein (Sallam et al., 2021). It has been reported that about 95% of non-absorbed proanthocyanidin polymers reach the colon in its intact form to be bio-transformed to produce several metabolites with higher potential benefits (de Freitas et al., 2022, Makarewicz et al., 2021).
To date, several studies have focused on the bioavailability of proanthocyanidin dimers and higher polymers, but a single conclusion cannot be drawn from those due to the contradiction in data. There are a few studies that finally helps to describe this controversial phenomenon. Choy et al. (2013) focused on identifying and characterizing the metabolites of non-extractable polyphenols, and finally observed that about 11% of the ingested grape seed proanthocyanidins were unchanged and the rest of the compounds were identified as polymers having the degree of polymerization from 4 to 6. This observation leads us to conclude that proanthocyanidins consisting of more than 3 subunits are resistant to degradation. Similar results were also observed in an another study conducted by Choy et al. (2014). Thus, these studies further prove that absorption of polymerized proanthocyanidin in the small intestine is negligible. This phenomenon gave a direction for further research on what makes them inaccessible in the small intestine, whether the interaction with polysaccharide has any significant role to play and its fate in the colon, and on the effects of metabolites in the colon, which is the only target organ to assess its biological effects.
The hydrolysable tannins can be hydrolysed to some extents forming sugar moiety and respective ellagic acid, phenolic acid, gallic acid etc by acid, alkali hydrolysis or by esterase enzyme activity at the intestinal mucosa lining. The bioavailability of hydrolysed tannins mostly depends on the absorption rate and further biotransformation of its hydrolysed derivatives in the small intestine and colon, respectively. Wu & Tian (2017) explained the possible mechanism of absorption of hydrolysable tannin in vivo and reported a slow absorption rate of gallic acid than p-coumaric acids. Seeram et al. (2004) found the systemic level of ellagic acid to be significantly low than other phenolics as these compounds get bound irreversibly to protein and minerals. Thus, absorption of hydrolysable tannin is greatly hampered in the small intestine and in turn opens up another chance to be metabolised in the colon with the help of gut microbiota.
Though polyphenols are the most abundant class of phytochemicals ingested through diet, these are recognized as xenobiotics which are mostly found as esters, glycosides, and polymers that hamper their bioavailability. As a result, only 5–10% of the total ingested polyphenols having low molecular weight and less complexity manages to be absorbed in the small intestine; thus, rest of these extractable polyphenols along with the highly polymerized NEPP accumulate in the colon with less than 10% excretion rate through faeces (Cardona et al., 2013). Therefore, it is inevitably clear that these phytochemical compounds are subjected to extensive modification by the colonic microbial enzymes giving rise to several more active low molecular weight metabolites with much more potent biological attributes (Wang et al., 2022).
The colon and GI tract host more than 1,000 different anaerobic microbial entities, including some species that have been found to influence the metabolism of polyphenols, as well as other phytochemicals and xenobiotics (Loo et al., 2020). Depending on the microbial population and the polyphenolic metabolites produced, the general population can be categorized into several subclasses called metabotypes. Understanding these metabotypes is crucial for comprehending the polyphenol-gut microbiota interaction and summarizing their systemic effects (Cani & Everard, 2016). The microbial environment in the gut holds a crucial factor to exert the effectiveness of polyphenolic metabolites because inter-individual variability in microbial communities or specific metabotypes can influence the functionality of polyphenols to a great extent (Fabbrini et al., 2022, Sarkar et al., 2021). Apart from the inter-individual variability, recent scientific investigation focuses on the two-way relationship of polyphenols and gut microbial ecology. Gut microbiota not only influences the biotransformation of polyphenols to produce potent bioactive metabolites but also variation in dietary intake pattern of polyphenols and their metabolites can alter the ecological niche gut microbial population, alter bacteroidetes/firmicutes (B/F) balance by exerting prebiotic as well as antimicrobial effects, therefore, aids beneficial microbial growth by inhibiting pathologenic microorganisms.
Much of the intestinal absorption and metabolism occurs through a common pathway. Natural polyphenols are present in foods as sugar or organic acid conjugates except for the polymeric polyphenols, which remain as a complex polymeric form. Both are hardly absorbed in the small intestine and finally get to the colon unaltered. The following biotransformation by the help of microbial enzymatic catabolism occurs through three major catabolic processes: O-deglycosylations and ester hydrolysis followed by C-ring cleavage; delactonization, demethylation, and dehydroxylation and double bond reduction (Tomás-Barberán et al., 2016). In short, the glycosides of polyphenols are hydrolysed to some extent by the intestinal enzyme β-glucosidase present in the enterocytes or lactase phloridzine hydrolase present in the brush borders of the small intestine followed by absorption and extensive phase I (such as oxidation, reduction, and hydrolysis) and phase II biotransformation (conjugation such as glucuronidation) by the help of 5′ diphosphate glucuronosyltransferase (Sallam et al., 2021) in the enterocytes and hepatocytes, respectively. Later, the conjugated polyphenolic metabolites circulate in the blood in association with protein. The conjugated metabolites can be excreted through biliary circulation in the duodenum and reabsorbed after bacterial enzyme β-glucuronidase in the distal part of the colon and remain in the circulation for a longer time (Manach et al., 2004).
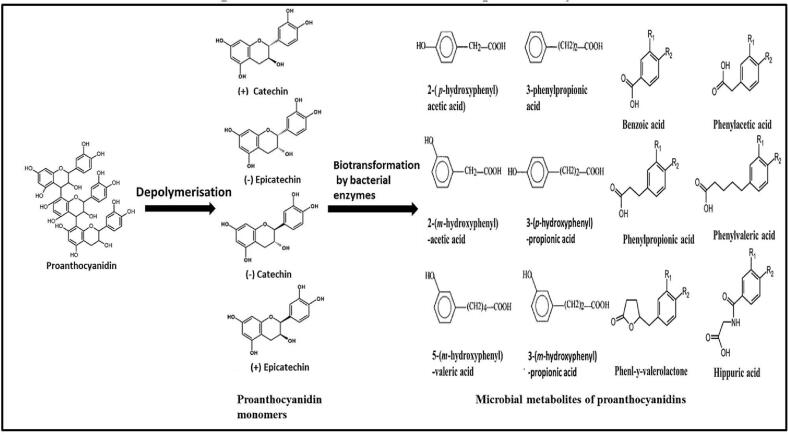
On the contrary, polymeric non-extractable polyphenols along with some extractable glycosides and conjugated metabolites from biliary secretion reach the colon in intact form. Several moieties of the conjugated metabolites are cleaved and the polymeric complex forms are sequentially bio-transformed into monomeric form (Appeldoorn et al., 2009) and then to low molecular weight conjugated metabolites by different microbial enzymes. Studies by several researchers reported the pathway of microbial conversion of monomeric, oligomeric, and polymeric proanthocyanidins, that starts with depolymerization of polymers (in case of oligomeric and polymeric forms), followed by successive production of very first intermediate diphenylpropanol by cleavage of heterocyclic C-ring (C—C bond breakage and removal of methyl ethers by demethylation) and dihydroxyphenylvalerolactone by cleavage of A-ring. These newly formed metabolic intermediates are then catabolized by microbial enzyme-induced delactonisation and decaroxylation (Li et al., 2020, Sánchez-Patán et al., 2012) (Fig. 3). Finally, these are bio-transformed into several phenolic and aromatic acids (such as phenylacetic acid, phenyl propionic acid, phenylvaleric acid, cinnamic acid, benzoic acid, and hippuric acid) with different hydroxylation pattern and aliphatic side chain length (Barroso et al., 2013, Low et al., 2016, Sánchez-Patán et al., 2015) (Table 1). These microbial metabolites can be taken up by the colonocytes and finally delivered to the liver, where these are extorted to phase II metabolism to be evolved as conjugates such as glucuronide, methyl, glycine, and sulfate derivatives before going into the systemic circulation and are absorbed subsequently by different tissues or eliminated through the urine (Margalef et al., 2015).
Fig. 3.
Microbial metabolites of proanthocyanidins.
Table 1.
Metabolites evolved from colonic fermentation of different non-extractable polyphenols (NEPP).
| Food Source | Polyphenol | Metabolites | Model | Species | Effect | Reference |
|---|---|---|---|---|---|---|
| Triphala extract(mixture of Terminalia chebula, Terminalia bellerica, and Phyllanthus emblica. | Flavonoids, hydrolysable tannin, condensed tannin |
Analysed (not mentioned specifically) | In vitro fermentation | Human faecal microbiota of obese female adults | No significant changes found in microbial population. Significant changes in metabolite profile and tyrosine, phenyl alanine and tryptophan biosynthesis. |
(Kwandee et al., 2023) |
| Hawthorn (Crataegus pinnatifida) |
Procyanidin | Not analysed | In vivo administration | Mice model | Upragulate growth of Akkermansia, Bacteroides and Adlercreutzia, and decreased Lactobacillus, Bifidobacterium, Blautia, Lachnospiraceae and Subdoligranulum Reduced insulin resistance, oxidative stress |
(Han et al., 2022) |
| peanut skin | Type A procyanidins | Not tested | In vivo administration | Balb/c mice model |
Reverse the ulcerative colitis by improving gut barrier and modu;ating inflammatory cytokines Increase growth of Oscillibacter and Roseburia Decrease growth of Bacteroides, Helicobacter, Parabacteroides, Escherichia-Shigella, and Enterobacter |
(Huang et al., 2022) |
| Grape seed extract | Proanthocyanidin polyphenol extract | Not tested | In vivo administration | Mice model | GSPE normalized the colonic Firmicutes/Bacteroidetes ratios, reversed the relative abundance of Weissella, Faecalibaculum, Bacteroides, Akkermansia and Ruminococcus 1 induced by HFD reduced HFD-induced insulin resistance and increased levels of adiponectin and leptin |
(Du et al., 2021) |
| Pomegranate juice | Hydrolysable tannin | Not mentioned | In vivo fermentation | Insulin resistance mice model | Reduced glucose and lipid metabolic disorder, liver injury and insulin resistance; decreased the Firmicutes/Bacteroides ratio, reduced Coprococcus and Anaerotruncus, and increased Rikenellaceae and liver tumor necrosis factor-alpha and interleukin-1β levels, supressed liver IKKβ and NF-κB phosphorylation; and upragulated liver autophagy-related proteins LC3-II, P62, and Beclin1. |
(Cao et al., 2021) |
| Quebracho wood extract Chestnut wood extract\ Tara pods extract |
profisetinidin condensed tannin hydrolysable ellagitannins hydrolysable gallotannins) |
Not tested | In vitro digestion and fermentation | Human gut microbiata | Increase of genus Akkermansia, Lachnospiraceae and Ruminococcaceae sp. | (Molino et al., 2021) |
| Grape seed | proanthocyanidins | Not analysed | In vivo administration | Mice model | Reduced colitis associated inflammation | (Sheng et al., 2020) |
| Grape seed | Proanthocyanidin extract | Proanthocyanidin polymers | In vivo administration | Rat model | Reduced firmicutes/Bacteroidetes ratio Reduced weight gain, food intake, induced entero hormone secretion. |
(Casanova-Martí et al., 2018b) |
| Grape seed extract | Flavan 3-ol polymers, proanthocyanidins | Not tested | In vivo administration | Overiectomised mice model | Improved Firmicutes:bacteroidetes ratio Prevented menoposal weight gain |
(Jin et al., 2018) |
| – | Pure procyanidins | Not tested | In vivo administration | Mice model | Reduced high fat diet induced obesity Improved Firmicutes to bacteroidetes ratio Increased energy expenditure Improved lipid profile |
(Zheng et al., 2018) |
| Pomegranate extract | Ellagitannin and ellagic acid | Urolithin and ellagic acid | In vitro and in vivo incubation | Human subjects | Increased ellagic acid release from ellaigitannin and increased bioavailability | (González-Sarrías et al., 2015) |
| Cranberry juice | Oligomeric proanthocyanidins | Hydroxyphenyl propionic acid and hydroxyphenyl acetic acid derivatives | In vivo fermentation | Human subject | Increased detectable bioavalability of proanthocyanidin A2 in the plasma | (McKay et al., 2015) |
| Strawberry, fresh berries and processed puree | Ellagitanins and ellagic acid | Urolithins | In vivo fermentation | Human subjects | both samples were found to be efficiently metabolised by human gut microbiota | (Truchado et al., 2012) |
| Grapes | Proanthocyanidin | Valerolactones, phenylvaleric acids, phenylpropionic acids, phenylacetic acids, benzoic acid, cinnamic acids | In vivo fermentation | Rat model | Rich source of henolics in the gut and the NEPA and its metabolites remain bioavailable fr 24 h after ingestion | (Mateos-Martín et al., 2012) |
| Green tea | Flavan 3-ols and proanthocyanidins | Pyrocatechol, pyrogallol, 4-hydroxybenzoic acid, 4-hydroxyphenylacetic acid, 3-methoxy-4-hydroxyphenylacetic acid, hippuric acid, 3-(3-hydroxyphenyl)-3-hydroxypropionic acid, (-)-5-(3′,4′,5′ -trihydroxyphenyl)-γ-valerolactone | In vivo fermentation | Human subject | Reported absorption of flavan 3- ol metabolites in the circulatory system, | (Roowi et al., 2010) |
| Grape seed | Procyanidin dimer | 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-γ-valerolactone., 3-hydroxyphenylacetic acid, 4-hydroxyphenylacetic acid, 3-hydroxyphenylpropionic acid, phenylvaleric acids, monohydroxylatedphenylvalerolactone, and 1-(3′,4′-dihydroxyphenyl)- 3-(2′′,4′′,6′′-trihydroxyphenyl)propan-2-ol | In vitro fermentation | Human faecal microbiota | Procyanidins are metabolised slowly by gut micribiome instead of reapid depolymerisation to flavan 3-ols | (Appeldoorn et al., 2009) |
| Almond skin extract | Oligomeric proanthocyanidin type A | Hydroxyphenylvalerolactones Hydroxyphenylpropionic acids Hydroxyphenylacetic acids Hydroxycinnamic acids Hydroxybenzoic acids Hydroxyhippuric acids |
In vivo fermentation | Human subject | Microbial metabolism increased the bioavilability of proanthocyanidin metabolites | (Urpi-Sarda et al., 2009) |
| Pomegranate extract | Ellagitannin | Urolithins | In vivo fermentation | Human subjects | Urplithins (proanthocyanidin metabolites) remain in circulation much longer and exerts healt benefits | (Seeram et al., 2006) |
| Strawberry,raspberry, walnuts,oak aged red wines | Ellagitannins and ellagic acid | Urolithins | In vivo fermentation | Human subjects | Increased microbial metabolites absorption in the blood confirms colonic microbial catabolism | (Cerdá, Tomás-Barberán, et al., 2005) |
| Walnut extract | Ellagic acid, Ellagitannin | Urolithins | In vitro fermentation | Human faeces | Microbial metabolism of ellaigitannin depends on inter-individual differences of colonic microbial profile | (Cerdá, Periago, et al., 2005) |
| Grape seed extract | Oligomeric proanthocyanidins | 3-Hydroxyphenylpropionic Acid, 3-Hydroxyphenylacetic Acid, 4-Hydroxyphenylacetic Acid, and 4-O-Methylgallic Acid | In vivo fermentation | Human subject | Gradual increase in 3hydroxypropionic acid excretion indicateslong duration resorption of colonic microbial metabolites of proanthocyanidins | (Ward et al., 2004) |
| Chocolate | Oligomeric proanthocyanidins | 3,4-Dihydroxyphenylpropionic acid, m-hydroxyphenylpropionic acid, ferulic acid, 3,4-dihydroxyphenylacetic acid, m-hydroxyphenylacetic acid, phenylacetic acid, vanillic acid, m-hydroxybenzoic acid, p-hydroxybenzoic acid, p-hydroxyhippuric acid, hippuric acid. | In vivo fermentation | Human subject | Increased urinary excretion of proanthocyanidin metabolites authenticates involvement of the same in exerting antioxidant and other biological effects | (Rios et al., 2003) |
| Willow tree shoot (similar to apple and grape seed) | 14C labelled PCA | 2- (p-hydroxyphenyl)acetic acid, 2-(p-hydroxyphenyl)- propionic acid and their m-hydroxy isomers 2-(m-hydroxyphenyl) acetic acid and2- (m-hydroxyphenyl) propionic acid, 5-(m-hydroxyphenyl)valeric acid and phenylpropionic acid |
In vitro incubation | Human faecal microbiota | Confirms release of low molecular weight metabolites of polymeric polyphenols similar to monomeric flavanols | (Déprez et al., 2000) |
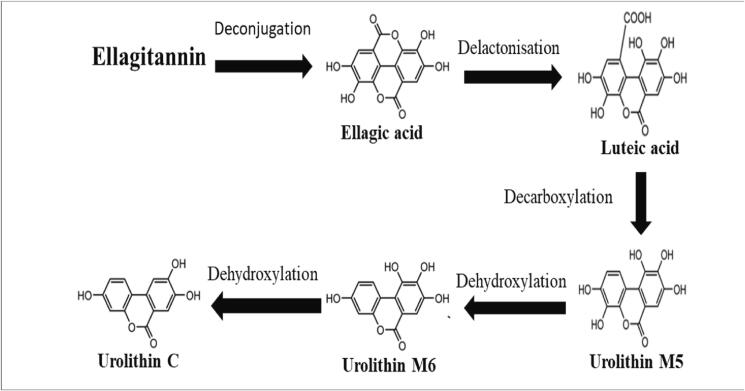
The biotransformation mechanism of hydrolysable tannins by the gut microbial population is somewhat different polymeric NEPA. The gallotannins are hydrolysed to gallic acid and glucose. Hydrolysable tannin can also be degraded by microbial enzymes to produce pyrogallol and phloroglucinol followed by production of acetate and butyrate by further degradation. The microbial tannase cannot degrade ellagitannin due to inability to hydrolyse gallolyl residue of ellagitannins. Ellaagitannins are bio-transformed by a series of action that starts with cleavage of lactone ring, decarboxylation followed by dehydroxylation producing urolithin A and B at the end (Tomás-Barberán et al., 2016) (Fig. 4).
Fig. 4.
Microbial metabolites of ellagitannin.
Therefore, the beneficial health effect of NEPP is accredited more to their metabolites that reach the peripheral circulation than the intact form. The diversity observed in the newly formed phenolic metabolites is due to a varied microbial entity present in the gut, nature, and complexity of the chemical structure of non-extractable polyphenols present in the diet. Although a very few species (Lactobacillus, Clostridium, Eubacterium, and Bacteroides species) are responsible for the phenolic metabolite production (Cerdá et al., 2004, Jin and Hattori, 2012), a few previous human intervention trials have found that not only the inter-individual variation in the daily polyphenol intake but the inter-individual differences in their enterotypes (González-Sarrías et al., 2017), gut microbial community (Manach et al., 2017, Rey et al., 2012) and their metabotypes also can be the influencing factors in the differences observed in the bioavailability and bio-efficacy of polyphenolic compounds and their metabolites (Tomás-Barberán et al., 2016) (Table 1, Table 2).
Table 2.
Major microbial species and microbial enzymes involved in colonic biotransformation of non-extractable polyphenols and its precursors.
| Compounds | Metabolites | Metabolic reactions | Microbial enzymes | Microbes | Reference |
|---|---|---|---|---|---|
| Ellagitannins | Urolithin A and Urolithin B | Dehydroxylation | Tanase | Bifidobacterium pseudocatenulatum INIA P815 | (Gaya et al., 2018) |
| Ellagitannin ellagic acid to 3,8,9,10-tetrahydroxy-urolithin (urolithin M6), 3,8,9-trihydroxy-urolithin (urolithin C) and 3,9-dihydroxy-urolithin (isourolithin A | Isourolithin A | Hydrolysis,lactonisation,delactonisation, decaroxylation and dehydroxylation | Hydrolase, lactonase, delactonase, decarboxylase, o-dehydroxylase | Eggerthellaceae family | (Selma et al., 2017) |
| 1-(3,4,5-Trihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl) propan-2-ol |
1-(3,5-Dihydroxyphenyl)-3- (2,4,6-trihydroxyphenyl)propan-2- ol | Dehydroxylation of the C-ringcleavageproduct | Dehydroxylase | Adlercreutziaequolifaciens | (Takagaki & Nanjo, 2015a) |
| (2S)-1-(3,4-dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (1S) (2R)-1-(3,4-dihydroxyphenyl)-3-(2,4,6trihydroxyphenyl)propan-2-ol (1R) (2S)-1-(3-hydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (2S)and (2R)-1-(3-hydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (2R) |
4-hydroxy-5-hydroxyphenylvaleric acids and 5-hydroxyphenyl-γ-valerolactones |
Degradation of pholoroglucinol moiety | Not known | Flavonifractorplautii | (Takagaki & Nanjo, 2015b) |
| Flavan-3-ols(-) -Epigallocatechin(-) -Gallocatechin(-) -Gallocatechin |
(2S,3S)-Flavan-3,3′,5,5′,7-pentol, (2R,3S)-Flavan-3,3′,5,5′,7-pentol, 1-(3,4,5-Trihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2- ol | Dehydroxylation C-ring cleavage |
Dehydroxylase Reductase |
Adlercreutziaequolifaciens | (Takagaki & Nanjo, 2015b) |
| Proanthocyanidins, flavan-3-ols(-) epicatechin, (-) catechin, and (+) catechin |
(2S)-1-(3,4-dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (1S) (2R)-1-(3,4-dihydroxyphenyl)-3-(2,4,6trihydroxyphenyl)propan-2-ol (1R) |
C-ring cleavage C-ring cleavage |
Reductase Reductase |
Adlercreutziaequolifaciens, Eggerthellalenta | (Takagaki et al., 2014) |
| (2S)-1-(3,4-dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (1S) (2R)-1-(3,4-dihydroxyphenyl)-3-(2,4,6trihydroxyphenyl)propan-2-ol (1R) |
(2S)-1-(3-hydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (2S)and (2R)-1-(3-hydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (2R) |
Dehydroxylation |
Dehydroxylase |
Eggerthellalenta |
(Takagaki et al., 2014) |
| Ellagitannin, ellagic acid | Urolithin C | Delactonisation,decarboxylation,dehydroxylation | Carboxylase,decarboxylase,dehydroxylase | EggerthellaceaeGordonibacterurolithinfaciensGordonibacterpamelaeae | (Selma et al., 2014) |
| (+)-Catechin | (2R)-1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2- ol | C-ring cleavage | Reductase | EggerthellalentaCAT-1 | (J. S. Jin & Hattori, 2012) |
| (-)-Epicatechin |
(2S)-1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | C-ring cleavage | Reductase | EggerthellalentaCAT-1 | (J. S. Jin & Hattori, 2012) |
| (2S)-1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | (2S)-1-(3-Hydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2- ol | Dehydroxylation of the C-ringcleavageproduct | Dehydroxylase | EggerthellalentaCAT-1 | (J. S. Jin & Hattori, 2012) |
| (2R)-1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | (2R)-1-(3-Hydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2- ol | Dehydroxylation of the C-ringcleavageproduct | Dehydroxylase | EggerthellalentaCAT-1 | (J. S. Jin & Hattori, 2012) |
| (+)-Catechin, (-)-epicatechin | 1-(3,4-Dihydroxyphenyl)-3-(2,4,6- trihydroxyphenyl)propan-2- ol | C-ring cleavage | Reductase | LactobacillusplantarumIFPL935 | (Sánchez-Patán et al., 2012) |
| (+)-Catechin, (-)- epicatechin |
1-(3,4-Dihydroxyphenyl)-3- (2,4,6-trihydroxyphenyl)propan-2- ol | C-ring cleavage | Reductase | EggerthellalentarK3 | (Kutschera et al., 2011) |
| 1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | 5-(3,4-Dihydroxyphenyl)-γ-valerolactone, 4-hydroxy-5- (3,4 dihydroxyphenyl)valericacid | Degradation ofthe C-ringcleavageproduct | Dehydroxylase | Flavonifractorplautiistrains DSM6740 and aK2 | (Kutschera et al., 2011) |
| (+)-Catechin, (+)-epicatechin | (2R)-1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2- ol | C-ring cleavage | Reductase | Eggerthella(Eubacterium) spp. | (Wang et al., 2001) |
| (-)-Catechin, (-)-epicatechin | (2S)-1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2- ol | C-ring cleavage | Reductase | Eggerthella(Eubacterium) spp. | (Wang et al., 2001) |
| (-)-Gallocatechin, (-)-epigallocatechin | (2S)-1-(3,4,5-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2- ol | C-ring cleavage | Reductase | Eggerthella(Eubacterium) spp. | (Wang et al., 2001) |
| (2S)-1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | (2S)-1-(3-Hydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2- ol | Dehydroxylation of the C-ringcleavageproduct | Dehydroxylase | Eggerthella(Eubacterium) sp. | (Wang et al., 2001) |
| (2S)-1-(3,4,5-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propa n-2-ol |
(2S)-1-(3,5-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2- ol | Dehydroxylation of the C-ringcleavageproduct | dehydroxylase | Eggerthella(Eubacterium) sp | (Wang et al., 2001) |
A crossover intervention study conducted on healthy volunteers who consumed two pomegranate extract variants containing different amounts of punicalagin and ellagic acid, showed significant inter-individual variability in ellagic acid pharmacokinetics, therefore corroborating the phenomenon observed in the in vitro digestion study (González-Sarrías et al., 2015). Gut microbiota mediated catabolism of polymeric polyphenols plays a crucial role in human health but this tri-factorial cause-effect relationship between microbial ecology, host health and variation in polyphenol consumption has some sort of translucency because several other extrinsic and intrinsic host factors interfere with the diverse microbial community–environment and host interaction (Tomás-Barberán et al., 2016). Therefore, subject stratification on the basis metabotypes has become a necessity in clinical trials regarding gut microbiome induced polyphenol metabolism because metabotype stratification could be used as potential biomarkers of the polyphenol-microbiota interaction.
Biological activities and prophylactic properties of NEPP against intestinal dysbiosis induced local and systemic immune response- Biochemical, molecular, and epigenetic mechanism
It has been widely accepted that the bioavailability of non-extractable complex polyphenols in the gastrointestinal lumen is determined by their structure, orientation of hydroxylation groups, interactions with the intricate food matrix polysaccharide and protein, and the hydrolytic enzymes activity. The modulatory effect of gut microbial niche and the nature of transporters at the gastrointestinal epithelial cells also contributes in the metabolism and utilisation of NEPP (Gonzales et al., 2015, Martínez-Meza et al., 2020). In this context, recent advanced research regarding the mechanism of bioavailability of generally underestimated flavonoids, proanthocyanidin, and hydrolysable tannin including their microbial metabolites have focused on the following possible potential biological activities: 1) Complex polymeric form of non-extractable polyphenols (condensed and hydrolysable tannin) that exert local radical scavenging and anti-adhesive effects in the gastrointestinal cell lining; 2) Immunomodulatory effect of NEPP metabolites after colonic microbial bio-transforation and modulation of hormonal secretion of GI tract affecting systemic metabolic/glucose homeostasis (Williamson & Clifford, 2017); and 3) The systemic effect of bioaccessible monomeric and dimeric flavan-3-olsand their metabolites produced by colonic microbial catabolism (Bladé et al., 2016, Martínez-Meza et al., 2020). There are many speculations about the multidimensional molecular mechanism of NEPP functionality and bioactivity. However, its basic biochemical binding ability to proteins and the ability to bring epigenetic changes in cell functionality by modifying enzyme activities, cell signalling pathways, and gene expression are the most widely investigated field (Bladé et al., 2016). But comprehensive investigation regarding the most prominent immune-modulatory effect of complex polymeric polyphenolics, and their metabolites is still too scarce to draw a definite conclusion. Considering this fact, this section of this review conveys the contemporary acquaintance of the actions of dietary NEPP synchronizing the GI tract makeup, together with their crosstalk with the gut microbiota and intestinal immune system.
Two major mechanisms underlying the biological actions of PACs are widely accepted: the conventional biochemical property, which highlights the ability of polymeric flavan-3-ols to strongly bind to the proteins with its consecutive local and systemic metabolic effects. Secondly, the immuno-molecular and epigenetic mechanisms, which include regulation of intestinal inflammatory responses, (followed by histone modifications, DNA methylation (Xu et al., 2012) and modulation of micro-RNAs (miRNAs). Both these two mechanisms regulate the enzymatic activities, cell signalling pathways, and gene expression therefore, ultimately modulating cell functionality. The presence of multiple hydroxyl groups and aromatic ring structure and a higher degree of polymerization increases its cross-linking potential for the association with protein and other biomolecules (Brás et al., 2010, Martínez-Meza et al., 2020). The interactions between proanthocyanidins and proteins are not only obligatory for the enzyme regulating activity but also for the prevention of receptors-ligand binding and modification of transcription factors of specific DNA receptor binding sites (Bladé et al., 2016, Zhu et al., 2016).
Local antioxidant activity in the gastrointestinal lining
In several in vitro studies, proanthocyanidin rich foods such as grape seed, pomegranate and blueberry extract, and beverages such as wine, tea (Zhang et al. 2020; Ohishi et al., 2016), and cocoa extract (Nabavi et al., 2015) were reported to have direct antioxidant effect. These foods diminished ROS generation and other highly reactive free radicals generated from protein and lipid oxidation through electron donation and resonance stabilization of unstable aroxyl ring. But poor absorption of the proanthocyanidin in its native form denies the relevance of in vivo antioxidant effects (Hollman et al., 2011, Martínez-Meza et al., 2020), while several studies establish a newer concept that its antioxidant effects can be reconsidered only in the case of tissues that comes in contact with a much higher concentration of polymeric forms i.e., cell lining around intestinal lumen (Galleano et al., 2010). Nevertheless, an in vivo experiment demonstrated the capability of grape seed proanthocyanidins to up-regulate the natural antioxidant enzyme activity of superoxide dismutase, glutathione peroxidase, glutathione-S-transferase, and catalase, therefore, simultaneously increasing total radical scavenging activity of hepatic cells (Fernández-Iglesias et al., 2014).
Antibacterial and prebiotic activity of NEPP and its mechanism of action
NEPP is a unique candidate for modification of the enterocytic membrane-microbiota interaction and modification of microbial cell membrane functionality, due to not only its local radical scavenging activity, but also its antibacterial properties resulting from its structural diversity, the presence of an aromatic ring with diverse hydroxylation patterns, and its level of gallolylation. The combination of these factors makes NEPP particularly effective in altering the interaction between enterocytic membranes and microbiota, as well as in modifying microbial cell membrane functionality (Farhadi et al., 2019). Flavan-3-ol monomers catechin can bind to the cell membrane of many Gram-positive as well as Gram-negative pathogenic bacterial species such as Serratia marcescens, Bacillus subtilis, Salmonella choleraesis, Pseudomonas aeruginosa, and Staphylococcus aureus by producing hydrogen peroxide and finally inhibit their growth by altering membrane permeability (Campos et al., 2019, Nawrot-Hadzik et al., 2021). In another study researcher observed that proanthrocyanidins and its monomers showed anti-adhesive property against pathogens to the periodontal tissues especially Porphyromonas gingivalis due to presence of galllyl group in type-B proanthocyanidin and A-linkages in the type-A proanthocyanidins. The structural characteristics also hinders biofilm formation together with collagenase and proteinases activity (Diwani et al., 2020).
Previous reports regarding the antibacterial activity of flavan-3-ol indicated that catechin, which is one of the building blocks of procyanidins could disintegrate the membrane lipid bilayer by reducing its fluidity (Makarewicz et al., 2021). Using isothermal titration calorimetry (Wang et al. (2018) indicated that pectin polysaccharide and procyanidin interact with each other through hydrophobic and hydrogen bonds and synergistically act as growth inhibitory agent against entero-toxicogenic E. coli strain by altering the charge distribution and isoelectric point of the bacterial cell membrane, therefore, can disrupt cell wall stability (Papuc et al., 2017). Disruption of the cell membrane integrity aids the leakage of cellular content such as protein, nucleic acid, and down-regulates the expression of cell surface adhesion protein (Wu et al., 2018).
The other probable mechanisms by which these non-extractable polyphenols mitigate the pathogenic bacterial growth are metal ion chelation developing an iron-deficient gut luminal environment affecting the growth of susceptible microorganisms (Suriyaprom et al., 2022), inhibition of DNA gyrase activity (Bae et al., 2022), inhibition of microbial enzyme activity (Sun et al., 2020), and reduced expression of virulence factors by jeopardizing quorum sensing (Dingeo et al., 2020).
Recently upgraded knowledge about prebiotics reveals that proanthocyanidins and other complex non-absorbable polyphenols have the potency to exert prebiotic effects and play a pivotal role in the modulation of gut microbial composition. Prebiotic effect of non-extractable polymeric polyphenols from grape pomace extracts i.e., rich in polymeric proanthocyanidins showed changes in gut microbial profile with increasing growth of Allobaculum spp. and Roseburia spp. with decease in Desulfovibrio spp.; Lactococcus spp., etc. (Van Hul et al., 2018). In another study increased growth of Akkermansia muciniphila was induced by proanthocyanidin rich grape polyphenol extract treatment (Zhang et al., 2018). In a similar study, supplementation of grape seed proanthocyanidins to mice for short duration (8 days) showed higher growth rate short-term supplementation of grape seed proanthocyanidins by feeding mice with a dose of 500 mg/kg BW/day for 8 days resulted in a lower F/B ratio accompanied with the increased growth of Bacteroides, Phascolarcto-bacterium, Parabacteroides, Sutterella, and Bilophila with lower F/B ratio (Masumoto et al., 2016) and reduced population of Ruminococcacea and Dehalobacteriaceae (Casanova-Martí et al., 2018a). A significant change in gut microbial profile was also observed in mice treated with grape pomace extract for 14 months (Chacar et al., 2018). Similarly, in another study using animal model (pig), cocoa powder rich in flavanol are utilized by probiotics as a substrate and shifted the colonic microbial populations towards Lactobacillus and Bifidobacterium spp. This also improve the gut health by modulating various markers of localized intestinal immunity (Jang et al., 2016).
Red wine polyphenolic extract significantly imparts a positive influence on the growth of Enterococcus, Prevotella, Bifidobacterium, Bacteroides uniformis (Queipo-Ortuño et al., 2012) along with faecal Bifidobacteria and Lactobacillus, which protects intestinal barrier and butyrate producing Faecali bacterium prausnitzii and Roseburia as well (Moreno-Indias et al., 2016). Flavan-3-ols, procyanidin B1, procyanidin B2 exposure significantly enhanced the adhesion of L. acidophilus LA-5 and LA-115 to Caco-2 cell-line and HT-29 cell-lines (Volstatova et al., 2017). Although the adhesion of probiotics to the host does not directly offer any health benefits, it can still have a positive impact by competitively reducing the interaction of pathogens with host cell surface receptor molecules. Furthermore, the prolonged colonization of probiotics in the gut enterocytes can lead to the production of active metabolites such as short chain fatty acids (SCFA) and phenolic acids, as well as the triggering of host cell surface receptor-induced signalling pathways. These effects can contribute to the intended prophylactic and immunomodulatory effects of probiotics (Monteagudo-Mera et al., 2019) (Fig. 5A).
Fig. 5.
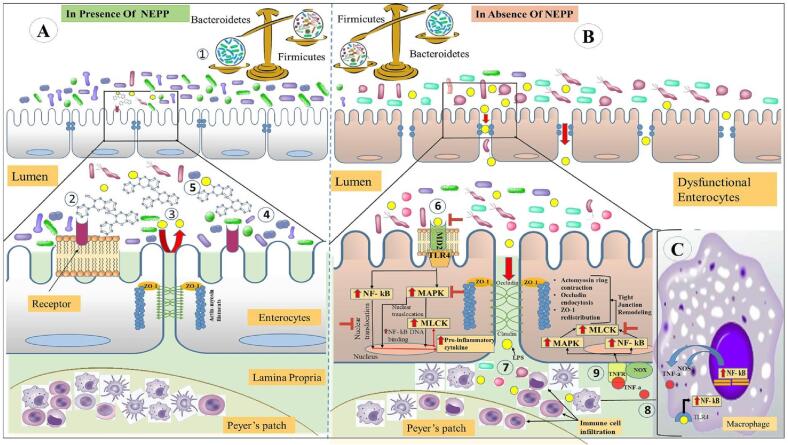
Molecular mechanisms involved in protective effect of non-extractable polyphenols (proanthocyanidin - PAC) against dysbiosis induced mucosal inflammation. [(A) (1) Presence of polymeric polyphenols especially proanthocyanidin maintains the gut microbial balance by aiding the growth of probiotics and diminishing commensal microbial growth. (2) proanthocyanidin binds the epithelium cell membrane by internalizing itself in the lipid bilayers and lipid raft and also occupy the cell surface receptors which in turn reduces endotoxin binding to the receptors and prevent LPS and commensal microbiota entry by halting leakiness of tight junctions (3). (4) probiotics also hinders the unnecessary activation of immune signaling pathways. (5) Proanthocyanidin binds to the endotoxins and maintains the intestinal barrier. (B) (6) Absence of PAC induce dysbiosis and triggers TLR4 mediated NF-kB signaling pathway and activate MAPK and MLCK simultaneously triggering tight junction remodelling and release of pro-inflammatory cytokines. (7) dysfunctional tight junctions increase bacterial toxin influx initiating immune cell infiltration to the lamina propria and initiate TNF-a release from sensitized macrophages (8). (9) TNF-a increases the bacterial influx by activating MAPK and MLCK in the epithelial cells. NOX – NADPH Oxidase; TLR4/MD2 – Toll Like Receptor 4/ Myeloid Differentiation 2 complex; MAPK- Mitogen-Activated Protein Kinase; MLCK- Myosin Light Chain Kinase; NF-kB - Nuclear Factor kappa light chain enhancer of activated B cells; LPS – bacterial Lipopolysaccharide; iNOS – Inducible Nitric Oxide Synthase; TNFR – Tumor Necrosis Factor Receptor; TNF-a - Tumor Necrosis Factor-alpha].
In a randomized, double blind, crossover and controlled study, 22 physically fit individuals were given a flavanol-rich cocoa beverage (494 mg cocoa flavanols/day) for 4 weeks and a significant increase in the growth of Bifidobacterium and Lactobacillus populations was observed along with decreasing trend in Clostridium count (Sorrenti et al., 2020, Tzounis et al., 2011). Another crossover dietary intervention executed on human participants also revealed that administration of polymeric polyphenol-rich blueberry extract for 6 weeks significantly escalated the growth of Bifidobacterium spp. without showing any significant differences in the populations of Bacteroides spp., Prevotella spp., Enterococcus spp., and Clostridium coccoides (Vendrame et al., 2011). A similar outcome was witnessed when 10 human volunteers participated in a study by taking a regular dose of red wine polyphenols for 4 weeks, consecutively. Statistically significant changes were detected in the populations of Enterococcus, Bacteroides, Prevotella, Bacteroides uniformis, Bifidobacterium, Eggerthellalenta, and Blautiacoccoides–Eubacterium rectale species (Queipo-Ortuño et al., 2012). Similar consequences were observed when specific doses of pomegranate extract (25, 100, and 400 mg/mL) given to human subjects gradually increased in probiotic strains such as Bifidobacteria and Lactobacilli (Li et al., 2015).
The large enzymatic diversity helps in the growth of probiotic bacteria in a medium enriched with polyphenols because of their potential to utilize these as an energy source. Various glycosidic enzymes and others enzymes such as aromatic ring-cleavage enzymes, reductases, hydrogenases, decarboxylase, demethylase, isomerase, dehydroxylase that are capable to exhaust/metabolize glycosylated polyphenols as an energy source (Banerjee & Dhar, 2019). Therefore, it is quite evident that absorption and bio-accessibility are not mandatory in the case of these bioactive phenolics to exert a beneficial effect on the host. In summary, although ingestion of high molecular weight polymeric polyphenols is unable to directly influence the cell metabolism and immune functionality due to poor absorption, they can significantly alter the gut microbial composition (Sorrenti et al., 2020). These unavailable NEPP can undergo drastic biodegradation by bacterial enzymes and the produced metabolised can be absorbed into the systemic circulation of human body. Gordonibacter urolithinfaciens and G. pamelaeae of Coriobacteriaceae family and Ellagibacter isourolithinifaciens from Eggerthellaceae family can utilize metabolites of ellagitannins such as urolithins (Selma et al., 2014). Although urolithins have a lower antioxidant capacity than ellagitannin, they can still have many beneficial effects due to their ability to produce estrogen and circulate in the body for a longer period of time. Additionally, a study has shown that highly polymeric procyanidins can decrease the Firmicutes/Bacteroidetes ratio and increase the growth of Akkermansia spp. by a significant amount (Masumoto et al., 2016). Therefore, these polyphenol-rich diets that are not easily bioavailable could play an important role in restoring microbial balance and promoting overall wellbeing in the host.
Preventive effect on metabolic syndrome by regulating entero-endocrine secretion and enzyme inhibition
The distinctive structural properties of proanthocyanidins make them capable of regulating the key molecular mechanisms involved in metabolic syndrome, particularly hyperinsulinemia, altered carbohydrate metabolism, abdominal obesity, hyperlipidemia, and disrupted glucose homeostasis. These factors can ultimately lead to the development of serious health conditions such as cardiovascular disease and type 2 diabetes (Zhang et al., 2023). The prerequisite interactions that can further initiate some other downstream reactions mainly occur in the intestine. Several possible mechanisms involved are inhibition of carbohydrate digesting enzyme and glucose transporters, limiting endotoxemia by increasing gut barrier function, and stimulation of enteroendocrine secretion responsible in regulating hunger and satiety (Corrêa et al., 2019, Strat et al., 2016).
Polyphenols found in the human diet, particularly highly polymerized and non-extractable polyphenols, may have the ability to inhibit the activity of digestive enzymes and their transporters responsible for macronutrient digestion and absorption. This is due to the abundant hydroxyl groups present in polyphenols, which have a strong affinity towards proteins (Eran Nagar et al., 2020, Li et al., 2021). It is well known that the presence of gallolyl group and a high degree of polymerization of procyanidin inhibit the in vitro lipoprotein lipase activity in an in vitro experiment (Li et al., 2021). Gallolylated flavanol monomers such as epigallocatechin gallate and (-)-epigallocatechin 3-O- (3-O-methyl) gallate from oolong tea hinder α-amylase activity (Hanhineva et al., 2014). In another in vivo study executed with both animal and human intervention trial, proanthocyanidins have been found to retard the absorption of triacylglycerol in mice and humans (Zhu & Oteiza, 2022). Flavanol metabolites are also proven to be an effective inhibitory agent against glucose transporters present in peripheral tissues (Martínez-Meza et al., 2020). Several enteroendocrine cells present throughout the lining of the intestinal lumen, secrete different local hormones such as ghrelin, gastrin, somatostatin, and cholecystokinin, and incretins, e.g., glucagon-like peptide-1 (GLP-1), GLP-2, and glucose-dependent insulinotropic polypeptide (GIP), which are recruited for controlling food intake and glucose metabolism (Gribble & Reimann, 2016). Some of the vital functions of GLP1 and GLP2 hormones are up-regulation of insulin, somatostatin secretion, down-regulation of glucagon secretion, predisposition of anorexia and gastroparesis along with upregulation of glycogen synthesis along with maintenance of glucose and lipid metabolism (Lupien-Meilleur et al., 2020). Consumption of proanthocyanidin monomers significantly increases the plasma GLP-1 within a short period (Gowd et al., 2019). A similar result was observed when several researchers provided grape seed procyanidin at 1 g per kg body weight to healthy rats which were on a glucose-loaded feed (González-Abuín, Martínez-Micaelo, Blay, et al., 2014; González-Abuín, Martínez-Micaelo, Margalef, et al., 2014). Increasing the enteroendocrine cell count and its hormone secretion along with the ameliorative effect on proglucagon secretion and convertase activity can be the probable mechanism of action of flavonoids especially the flavanol polymers (Casanova-Martí et al., 2020). Further several subgroups of flavonoids can increase the half-life of GLP 1 and 2 by inhibiting the DPP4 enzymes (Oteiza et al., 2018).
Maintenance of gut intestinal permeability and ameliorative effect against endotoxemia induced local and systemic inflammation
The human intestinal barrier is consistently exposed to a microbial community that thrives in this environment by maintaining a symbiotic relationship with the host. This community plays a critical role in maintaining the host's metabolic and immune response homeostasis. As a result, the intestinal barrier is constantly working to prevent the entry of harmful food components and bacterial endotoxins. When the integrity of the gut barrier is compromised, it can lead to altered immune responses that compromise overall health and wellbeing (Di Tommaso et al., 2021). Sagkan-Ozturk & Arpaci (2022) reviewed a few studies that have revealed that dietary factors such as a high-fat diet, increased stress, and frequent use of antibiotics can induce gut dysbiosis, which ultimately triggers endotoxemia, thus increasing intestinal permeability. Eventually, persistent exposure to such injurious environmental stimuli may aggravate the local and systemic inflammatory response, which further escort barrier dysfunction (Clemente-Postigo et al., 2019). Evidence suggests that endotoxemia is the underlying cause of dysbiosis induced susceptibility of the host to chronic diseases of the alimentary canal such as ulcerative colitis, Crohn’s disease, and irritable bowel syndrome along with systemic metabolic diseases such as cardiovascular disease (Sagkan-Ozturk & Arpaci, 2022), obesity, type 1 and type 2 diabetes (Lu et al., 2018). The intestinal epithelium tissue adapts several strategies such as secretion of thick mucin glycoprotein from goblet cells and recruitment of intercellular tight junction proteins to maintain the barrier integrity against unwanted inflammatory response, bacterial endotoxin and PAMPs (Pathogen Associated Molecular Patterns) such as LPS (lipopolysaccharides) until overwhelming pathogenic bacterial growth over probiotic population (Chistiakov et al., 2014, Ulluwishewa et al., 2011). As a result, the gut-associated lymphoid tissue present in the lamina propria and M cells present in the follicle associated epithelium initiates an abnormal mucosal inflammatory response by activating pattern recognition receptors such as Toll-Like Receptors (TLR4) (Lu et al., 2018). These receptors embedded on the plasma membrane of enterocytes recruit immune cells from the circulatory system and regulate concentrations of proinflammatory mediators in the affected tissues (Chen et al., 2018). Finally, the activated TLR4/myeloid differentiation 2 (MD-2) complex induces phosphorylation of several intracellular protein assemblages, promoting the activation of the nuclear factor kappa-light-chain-enhancer of activated B-cell (NF-κB) signalling pathway which eventually play a significant role in the transcription and translation of inflammatory mediators (Luo et al., 2012) such as cytokines (TNF-α), chemokines, adhesion molecules (CAMs), and different inducible enzymes like nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) (Liu et al., 2017).
Activation of NF-KB signalling pathway activates multiple kinase enzymes such as mitogen-activated protein kinases (MAPKs), phosphoinositide-3-kinases (PI3Ks), and monophosphate-activated protein kinase (AMPK) (Yang et al., 2017), which in turn activates MLCK enzymes that further phosphorylates myosin II regulatory lightchain. As a result, peri-junctional actin-myosin filaments contraction induces tight junction opening thus increasing paracellular permeability (Cunningham & Turner, 2012). Further, this enzyme can also influence the other tight junction proteins such as zona occludin-1and caveolin-1-dependant occluding (Lu et al., 2018). Finally, disruption of local immune homeostasis due to continuous unusual activation of TLRs enhances the permeability favouring the inclusion of bacterial and other potent pro-inflammatory agents in the systemic circulation, commonly known as metabolic endotoxemia (González-Quilen et al., 2019).
The metabolic fate of non-extractable polyphenols like proanthocyanidin and its exceptional ability to bind macromolecules such as polysaccharide and protein due to its structural complexity. This ability not only make it a potent prebiotic agent but also facilitate its preventive effect and also allow it to arrest ligand-receptor binding and the adherence of transcription factors to their respective DNA binding sites (Zhu et al., 2016). Reports are also available to determine the mechanism of action of non-extractable polyphenol like proanthocyanidins in local and systemic inflammation using different in vitro, in vivo, and ex vivo experimental models. Several experiments based on Caco-2 cell line-based models have shown a remarkable curtailment in secretion and gene expression of TNF, IL-6, IL-8 when exposed to several pro-inflammatory agents such as LPS cytokines and other chemical molecules (Denis et al., 2015, Gentile et al., 2015, Wu et al., 2018, Yoshioka et al., 2008) followed by incubation with pure proanthocyanidin extract or botanical extract rich in proanthocyanidin (Bitzer et al., 2015, Gentile et al., 2015, Wu et al., 2018). This phenomenon is often allied to the simultaneous cessation of NF-KB signalling pathways at various levels (Erlejman et al., 2008, Wu et al., 2018). In some in vitro studies, proanthocyanidins have shown a significant decrease in barrier permeability in the context of intestinal dysfunction (Bitzer et al., 2015, Wong et al., 2016). Proanthocyanidin of cocoa having the degree of polymerization 7 (Bitzer et al., 2015) and procyanidin B2 (Bianchi et al., 2019) showed preventive effect against dextran sodium sulfate (DSS)-induced disruption of barrier function in Caco-2 cells and Caco-2/HT29-MTX co-culture model, respectively.
In a few in vivo studies, administration of both nutritional and pharmacological doses of complex non-absorbable polyphenols rich grape seed polyphenolic extract (GSPE) to Wistar rats that were on long-term (17–18 weeks) high fat cafeteria diet, brought about a drastic reduction in TNF- α release (one of the prominent indicators of intestinal permeability) (Gil-Cardoso et al., 2017, Gil-Cardoso et al., 2018, González-Quilen et al., 2019). Therefore, the prophylactic effect of GSPE in maintaining intestinal integrity can be correlated significantly with a reduction in metabolic endotoxemia and systemic inflammation (Gil-Cardoso et al., 2017). Proanthocyanidins are also proven to be effective against the deactivation of pro-inflammatory mediators, e.g., TNF-α and iNOS associated with the up-regulation of intestinal permeability as it helps to deactivate NF-KB signalling pathways, the main effectors for the production of these inflammatory mediators (Contreras et al., 2015). Moreover, proanthocyanidins have also shown a pivotal role in the deactivation of different kinases including MLCK enzyme that is involved in the contraction of peri-junctional actin-myosin filaments that leads to tight junction opening (Yang et al., 2017). In another study by Delehanty et al. (2007), the LPS endocytosis inhibitory effect of proanthocyanidins was examined using HEK 293 cell line (human embryonic kidney cells) that expressed TLR4/MD-2 and CD14 receptors. In this study, proanthocyanidin inhibited the LPS endocytosis by directly binding to the lipid-moiety of LPS molecules, therefore blocking the attachment of LPS molecules to its receptors, the event required for activation of NF-KB signalling pathway (Fig. 5B). Thus, the anti-inflammatory and intestinal barrier-protective attributes of proanthocyanidins prove their eligibility to be used as therapeutic agents for the treatment of intestinal dysfunction related to obesity, IBD, and other metabolic diseases. However, large-scale clinical trials are needed to establish the efficacy of these molecules.
Conclusion
Extensive experimental and epidemiological research established the fact that polymeric polyphenols, being the most predominant phytochemicals in food, exhibit a variety of health-beneficial effects. But polyphenol bio-accessibility and biological assimilability are the primary concern to link polyphenol and human health benefit. Food matrix polysaccharide is the main influencing factor that can modulate the transit of polymeric polyphenols from the small intestine to the colon and therefore indirectly influence the microbiota-induced enzymatic biodegradation. A few studies utilizing spectroscopic, calorimetric, and chromatographic methods, have analyzed the crosstalk between food matrix polysaccharides and high molecular weight polymeric proanthocyanidins and demonstrated that the association occurs as a result of hydrogen bond and hydrophobic interaction and the intensity of such interaction gradually increases with a higher degree of polymerization of proanthocyanidins and higher pectin content present in the food matrix. The physical structure of the food matrix, porosity, particle size also influences the affinity of these polymeric polyphenols to the polysaccharide, therefore passively regulate the extractability of the polymeric polyphenols.
Growing evidence indicates that the health benefits of these phytochemicals might be attributed to the bio-transformed adjoined metabolites (fabricated during the phase I and II catabolism of monomeric flavan-3-ols), especially to metabolites of polymeric NEPP synthesized by microbial catabolism in the colon, rather than to its native conformation found in foods. Association with the food matrix polysaccharide helps to reduce the growth-inhibiting effect of larger polymeric phenolic compound and also aid their further fermentation by the colonic microbiota to produce bioactive metabolites. As a result, the secondary metabolites produced from colonic microbial action also enters the systemic circulation long after the ingestiontherefore, helps to maintain an increased concentration of this bioactive metabolites in biological circulation.
Future perspectives
Till date most of the research solely concentrated on discovering advanced techniques and technologies for the extraction of NEPP. But most of the research ignored the effect of food matrix polysaccharide and other food components such as protein and lipids on the extractability of NEPP. This review summarises the literature available on the interaction between insoluble food matrix polysaccharide. However, there is further scope of further research on the effect of different food processing technologies on the extractability of NEPP. The studies regarding the bioaccessibility of NEPP shows the slow release of bound polyphenols from food matrix enables them to exert therapeutic effect much longer than the extractable polyphenols in the gut and in systemic circulation. Therefore, more elaborate systematic research approach is needed to understand its synergistic activity with the food matrix dietary fibre followed by drawing a more precise conclusion regarding its biological effects.
This review documented a strong inter-connection between the gut microbiota and the therapeutic effect of NEPP in terms of human metabolic homeostasis and modulation of gut microbial ecology or prevention of dysbiosis. The biotransformation of NEPP depends on the metabolites of the species and microbial enzymes involved in catabolism. However, it is quite challenging to identify the wide varieties of NEPP metabolites with their specific therapeutic effect and the mechanism of actions in the human organism. The advanced metabolomic studies i.e., both general and targeted approaches are the new area to be explored along with research regarding applied biology, pharmacokinetics, and molecular level cell biology. However, in-vivo studies should be used to establish the new pathways that are been utilised by the metabolites to exert biological effects. A rigorous clinical trial on human subjects should be taken under consideration to further explore and establish the findings and mechanism of action of these polyphenols and their metabolites revealed in in vitro and in vivo studies.
The use of extra-ordinary advancement in the metabolomic tools and techniques would make it possible to study the complicated metabolomes of gut microorganisms and their influence on metabolites production. Advanced DNA sequencing techniques can be utilised to decode the genomic and metagenomic data of gut microbiota to identify the key genes involved in the metabolism of these underrated polyphenols in the gut. Research in these areas would enable to gather complete knowledge of the therapeutic and nutritional relevance of NEPP, the hidden face of dietary antioxidants.
CRediT authorship contribution statement
Trina Das: Conceptualization, Data curation, Writing – original draft. Niloy Chatterjee: Writing – review & editing. Esra Capanoglu: Resources. Jose M. Lorenzo: Writing – review & editing, Funding acquisition. Arun K. Das: Writing – review & editing. Pubali Dhar: Conceptualization, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to express their deepest gratitude to the University Grants Commission, Government of India, for supporting the research financially.Thanks to GAIN (Axencia Galega de Innovación) for supporting this review (grant number IN607A2019/01).
Contributor Information
Jose M. Lorenzo, Email: jmlorenzo@ceteca.net.
Pubali Dhar, Email: pubalighoshdhar23@gmail.com.
Data availability
No data was used for the research described in the article.
References
- Appeldoorn M.M., Vincken J.P., Aura A.M., Hollman P.C.H., Gruppen H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-γ- valerolactone as the major metabolites. Journal of Agricultural and Food Chemistry. 2009;57(3):1084–1092. doi: 10.1021/jf803059z. [DOI] [PubMed] [Google Scholar]
- Aura A.M., Mattila I., Hyötyläinen T., Gopalacharyulu P., Cheynier V., Souquet J.M., Bes M., Le Bourvellec C., Guyot S., Orešič M. Characterization of microbial metabolism of Syrah grape products in an in vitro colon model using targeted and non-targeted analytical approaches. European Journal of Nutrition. 2013;52(2):833–846. doi: 10.1007/s00394-012-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J.Y., Seo Y.H., Oh S.W. Antibacterial activities of polyphenols against foodborne pathogens and their application as antibacterial agents. Food Science and Biotechnology. 2022;31(8):985–997. doi: 10.1007/s10068-022-01058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Dhar P. Amalgamation of polyphenols and probiotics induce health promotion. Critical Reviews in Food Science and Nutrition. 2019;59(18):2903–2926. doi: 10.1080/10408398.2018.1478795. [DOI] [PubMed] [Google Scholar]
- Barroso E., Sánchez-Patán F., Martín-Alvarez P.J., Bartolomé B., Moreno-Arribas M.V., Peláez C., Requena T., Van De Wiele T., Martínez-Cuesta M.C. Lactobacillus plantarum IFPL935 favors the initial metabolism of red wine polyphenols when added to a colonic microbiota. Journal of Agricultural and Food Chemistry. 2013;61(42):10163–10172. doi: 10.1021/jf402816r. [DOI] [PubMed] [Google Scholar]
- Bautista-Ortín A.B., Cano-Lechuga M., Ruiz-García Y., Gómez-Plaza E. Interactions between grape skin cell wall material and commercial enological tannins. Practical implications. Food Chemistry. 2014;152:558–565. doi: 10.1016/j.foodchem.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Bianchi M.G., Chiu M., Taurino G., Brighenti F., Del Rio D., Mena P., Bussolati O. Catechin and procyanidin B2 modulate the expression of tight junction proteins but do not protect from inflammation-induced changes in permeability in human intestinal cell monolayers. Nutrients. 2019;11(10) doi: 10.3390/nu11102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindon K.A., Li S., Kassara S., Smith P.A. Retention of Proanthocyanidin in Wine-like Solution Is Conferred by a Dynamic Interaction between Soluble and Insoluble Grape Cell Wall Components. Journal of Agricultural and Food Chemistry. 2016;64(44):8406–8419. doi: 10.1021/acs.jafc.6b02900. [DOI] [PubMed] [Google Scholar]
- Bitzer Z.T., Glisan S.L., Dorenkott M.R., Goodrich K.M., Ye L., O’Keefe S.F., Lambert J.D., Neilson A.P. Cocoa procyanidins with different degrees of polymerization possess distinct activities in models of colonic inflammation. Journal of Nutritional Biochemistry. 2015;26(8):827–831. doi: 10.1016/j.jnutbio.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladé C., Aragonès G., Arola-Arnal A., Muguerza B., Bravo F.I., Salvadó M.J., Arola L., Suárez M. Proanthocyanidins in health and disease. BioFactors. 2016;42(1):5–12. doi: 10.1002/biof.1249. [DOI] [PubMed] [Google Scholar]
- Brás N.F., Gonçalves R., Mateus N., Fernandes P.A., Ramos M.J., De Freitas V. Inhibition of pancreatic elastase by polyphenolic compounds. Journal of Agricultural and Food Chemistry. 2010;58(19):10668–10676. doi: 10.1021/jf1017934. [DOI] [PubMed] [Google Scholar]
- Campos E.M., Stehle P., Simon M.C. Microbial metabolites of flavan-3-ols and their biological activity. Nutrients. 2019;11(10) doi: 10.3390/nu11102260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani P.D., Everard A. Talking microbes: When gut bacteria interact with diet and host organs. Molecular Nutrition and Food Research. 2016;60(1):58–66. doi: 10.1002/mnfr.201500406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Ren G., Zhang Y., Qin H., An X., Long Y., Chen J., Yang L. A new way for punicalagin to alleviate insulin resistance: Regulating gut microbiota and autophagy. Food & Nutrition Research. 2021;65 doi: 10.29219/fnr.v65.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni Martins C., Rodrigues R.C., Domeneghini Mercali G., Rodrigues E. New insights into non-extractable phenolic compounds analysis. Food Research International. 2022;157 doi: 10.1016/j.foodres.2022.111487. [DOI] [PubMed] [Google Scholar]
- Cardona F., Andrés-Lacueva C., Tulipani S., Tinahones F.J., Queipo-Ortuño M.I. Benefits of polyphenols on gut microbiota and implications in human health. Journal of Nutritional Biochemistry. 2013;24(8):1415–1422. doi: 10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Casanova-Martí À., González-Abuín N., Serrano J., Blay M.T., Terra X., Frost G., Pinent M., Ardévol A. Long Term Exposure to a Grape Seed Proanthocyanidin Extract Enhances L-Cell Differentiation in Intestinal Organoids. Molecular Nutrition and Food Research. 2020;64(16) doi: 10.1002/mnfr.202000303. [DOI] [PubMed] [Google Scholar]
- Casanova-Martí À., Serrano J., Portune K.J., Sanz Y., Blay M.T., Terra X., Ardévol A., Pinent M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female rats. Food and Function. 2018;9(3):1672–1682. doi: 10.1039/c7fo02028g. [DOI] [PubMed] [Google Scholar]
- Castello F., Costabile G., Bresciani L., Tassotti M., Naviglio D., Luongo D., Ciciola P., Vitale M., Vetrani C., Galaverna G., Brighenti F., Giacco R., Del Rio D., Mena P. Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans. Archives of Biochemistry and Biophysics. 2018;646:1–9. doi: 10.1016/j.abb.2018.03.021. [DOI] [PubMed] [Google Scholar]
- Cerdá B., Espín J.C., Parra S., Martínez P., Tomás-Barberán F.A. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. European Journal of Nutrition. 2004;43(4):205–220. doi: 10.1007/s00394-004-0461-7. [DOI] [PubMed] [Google Scholar]
- Chacar S., Itani T., Hajal J., Saliba Y., Louka N., Faivre J.F., Maroun R., Fares N. The Impact of Long-Term Intake of Phenolic Compounds-Rich Grape Pomace on Rat Gut Microbiota. Journal of Food Science. 2018;83(1):246–251. doi: 10.1111/1750-3841.14006. [DOI] [PubMed] [Google Scholar]
- Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A., Yan H., Han C., Chen X., Wang W., Xie C., Qu J., Gong Z., Shi X. Acid and alkaline hydrolysis extraction of non-extractable polyphenols in blueberries: Optimisation by response surface methodology. Czech Journal of Food Sciences. 2014;32(3):218–225. doi: 10.17221/257/2013-cjfs. [DOI] [Google Scholar]
- Chirug L., Okun Z., Ramon O., Shpigelman A. Iron ions as mediators in pectin-flavonols interactions. Food Hydrocolloids. 2018;84:441–449. doi: 10.1016/j.foodhyd.2018.06.039. [DOI] [Google Scholar]
- Chistiakov D.A., Bobryshev Y.V., Kozarov E., Sobenin I.A., Orekhov A.N. Intestinal mucosal tolerance and impact of gut microbiota to mucosal tolerance. Frontiers in Microbiology. 2014;5(DEC) doi: 10.3389/fmicb.2014.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy Y.Y., Jaggers G.K., Oteiza P.I., Waterhouse A.L. Bioavailability of intact proanthocyanidins in the rat colon after ingestion of grape seed extract. Journal of Agricultural and Food Chemistry. 2013;61(1):121–127. doi: 10.1021/jf301939e. [DOI] [PubMed] [Google Scholar]
- Choy Y.Y., Quifer-Rada P., Holstege D.M., Frese S.A., Calvert C.C., Mills D.A., Lamuela-Raventos R.M., Waterhouse A.L. Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food and Function. 2014;5(9):2298–2308. doi: 10.1039/c4fo00325j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente-Postigo M., Oliva-Olivera W., Coin-Aragüez L., Ramos-Molina B., Giraldez-Perez R.M., Lhamyani S., Alcaide-Torres J., Perez-Martinez P., El Bekay R., Cardona F., Tinahone F.J. Metabolic endotoxemia promotes adipose dysfunction and inflammation in human obesity. American Journal of Physiology - Endocrinology and Metabolism. 2019;316(2):E319–E332. doi: 10.1152/ajpendo.00277.2018. [DOI] [PubMed] [Google Scholar]
- Contreras T.C., Ricciardi E., Cremonini E., Oteiza P.I. (-)-Epicatechin in the prevention of tumor necrosis alpha-induced loss of Caco-2 cell barrier integrity. Archives of Biochemistry and Biophysics. 2015;573:84–91. doi: 10.1016/j.abb.2015.01.024. [DOI] [PubMed] [Google Scholar]
- Corrêa T.A.F., Rogero M.M., Hassimotto N.M.A., Lajolo F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Frontiers in Nutrition. 2019;6 doi: 10.3389/fnut.2019.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K.E., Turner J.R. Myosin light chain kinase: Pulling the strings of epithelial tight junction function. Annals of the New York Academy of Sciences. 2012;1258(1):34–42. doi: 10.1111/j.1749-6632.2012.06526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A.K., Nanda P.K., Madane P., Biswas S., Das A., Zhang W., Lorenzo J.M. A comprehensive review on antioxidant dietary fibre enriched meat-based functional foods. Trends in Food Science & Technology. 2020;99:323–336. doi: 10.1016/j.tifs.2020.03.010. [DOI] [Google Scholar]
- de Souza E.L., de Albuquerque T.M.R., dos Santos A.S., Massa N.M.L., de Brito Alves J.L. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities–A review. Critical Reviews in Food Science and Nutrition. 2019;59(10):1645–1659. doi: 10.1080/10408398.2018.1425285. [DOI] [PubMed] [Google Scholar]
- Delehanty J.B., Johnson B.J., Hickey T.E., Pons T., Ligler F.S. Binding and neutralization of lipopolysaccharides by plant proanthocyanidins. Journal of Natural Products. 2007;70(11):1718–1724. doi: 10.1021/np0703601. [DOI] [PubMed] [Google Scholar]
- Denis, M. C., Desjardins, Y., Furtos, A., Marcil, V., Dudonné, S., Montoudis, A., Garofalo, C., Delvin, E., Marette, A., & Levy, E. (2015). Prevention of oxidative stress, inflammation and mitochondrial dysfunction in the intestine by different cranberry phenolic fractions. Clinical Science (London, England : 1979), 128(3), 197–212. https://doi.org/10.1042/CS20140210. [DOI] [PubMed]
- Déprez S., Brezillon C., Rabot S., Philippe C., Mila I., Lapierre C., Scalbert A. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. Journal of Nutrition. 2000;130(11):2733–2738. doi: 10.1093/jn/130.11.2733. [DOI] [PubMed] [Google Scholar]
- Di Tommaso N., Gasbarrini A., Ponziani F.R. Intestinal barrier in human health and disease. International Journal of Environmental Research and Public Health. 2021;18(23) doi: 10.3390/ijerph182312836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Morozova K., Scampicchio M., Ferrentino G. Non-Extractable polyphenols from food by-products: Current knowledge on recovery, characterisation, and potential applications. Processes. 2020;8(8) doi: 10.3390/PR8080925. [DOI] [Google Scholar]
- Dingeo G., Brito A., Samouda H., Iddir M., La Frano M.R., Bohn T. Phytochemicals as modifiers of gut microbial communities. Food and Function. 2020;11(10):8444–8471. doi: 10.1039/d0fo01483d. [DOI] [PubMed] [Google Scholar]
- Diwani N., Fakhfakh J., Athmouni K., Belhaj D., El Feki A., Allouche N., Ayadi H., Bouaziz-Ketata H. Optimization, extraction, structure analysis and antioxidant properties of flavan-3-ol polymers: Proanthocyanidins isolated from Periploca angustifolia using surface response methodology. Industrial Crops and Products. 2020;144 doi: 10.1016/j.indcrop.2019.112040. [DOI] [Google Scholar]
- Domínguez-Rodríguez G., Marina M.L., Plaza M. Strategies for the extraction and analysis of non-extractable polyphenols from plants. Journal of Chromatography A. 2017;1514:1–15. doi: 10.1016/j.chroma.2017.07.066. [DOI] [PubMed] [Google Scholar]
- Domínguez-Rodríguez G., Marina M.L., Plaza M. In vitro assessment of the bioavailability of bioactive non-extractable polyphenols obtained by pressurized liquid extraction combined with enzymatic-assisted extraction from sweet cherry (Prunus avium L.) pomace. Food Chemistry. 2022;385 doi: 10.1016/j.foodchem.2022.132688. [DOI] [PubMed] [Google Scholar]
- Du H., Wang Q., Li T., Ren D., Yang X. Grape seed proanthocyanidins reduced the overweight of C57BL/6J mice through modulating adipose thermogenesis and gut microbiota. Food & Function. 2021;12(18):8467–8477. doi: 10.1039/D1FO01361K. [DOI] [PubMed] [Google Scholar]
- Ducasse M.A., Canal-Llauberes R.M., de Lumley M., Williams P., Souquet J.M., Fulcrand H., Doco T., Cheynier V. Effect of macerating enzyme treatment on the polyphenol and polysaccharide composition of red wines. Food Chemistry. 2010;118(2):369–376. doi: 10.1016/j.foodchem.2009.04.130. [DOI] [Google Scholar]
- Eran Nagar E., Okun Z., Shpigelman A. Digestive fate of polyphenols: Updated view of the influence of chemical structure and the presence of cell wall material. Current Opinion in Food Science. 2020;31:38–46. doi: 10.1016/j.cofs.2019.10.009. [DOI] [Google Scholar]
- Erlejman A.G., Jaggers G., Fraga C.G., Oteiza P.I. TNFα-induced NF-κB activation and cell oxidant production are modulated by hexameric procyanidins in Caco-2 cells. Archives of Biochemistry and Biophysics. 2008;476(2):186–195. doi: 10.1016/j.abb.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Fabbrini M., D’amico F., Barone M., Conti G., Mengoli M., Brigidi P., Turroni S. Polyphenol and Tannin Nutraceuticals and Their Metabolites: How the Human Gut Microbiota Influences Their Properties. Biomolecules. 2022;12(7) doi: 10.3390/biom12070875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadi F., Khameneh B., Iranshahi M., Iranshahy M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytotherapy Research. 2019;33(1):13–40. doi: 10.1002/ptr.6208. [DOI] [PubMed] [Google Scholar]
- Fernandes P.A.R., Le Bourvellec C., Renard C.M.G.C., Wessel D.F., Cardoso S.M., Coimbra M.A. Interactions of arabinan-rich pectic polysaccharides with polyphenols. Carbohydrate Polymers. 2020;230 doi: 10.1016/j.carbpol.2019.115644. [DOI] [PubMed] [Google Scholar]
- Fernández-Iglesias A., Pajuelo D., Quesada H., Díaz S., Bladé C., Arola L., Salvadó M.J., Mulero M. Grape seed proanthocyanidin extract improves the hepatic glutathione metabolism in obese Zucker rats. Molecular Nutrition and Food Research. 2014;58(4):727–737. doi: 10.1002/mnfr.201300455. [DOI] [PubMed] [Google Scholar]
- de Freitas P.L., Miranda J.P.N., França L.M., de Paes A.M., A. Plant-Derived (Poly)phenols and Their Metabolic Outcomes: The Pursuit of a Role for the Gut Microbiota. Nutrients. 2022;14(17) doi: 10.3390/nu14173510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galleano M., Verstraeten S.V., Oteiza P.I., Fraga C.G. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Archives of Biochemistry and Biophysics. 2010;501(1):23–30. doi: 10.1016/j.abb.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Gan R.Y., Li H.B., Sui Z.Q., Corke H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Critical Reviews in Food Science and Nutrition. 2018;58(6):924–941. doi: 10.1080/10408398.2016.1231168. [DOI] [PubMed] [Google Scholar]
- Gaya P., Peirotén Á., Medina M., Álvarez I., Landete J.M. Bifidobacterium pseudocatenulatum INIA P815: The first bacterium able to produce urolithins A and B from ellagic acid. Journal of Functional Foods. 2018;45:95–99. doi: 10.1016/j.jff.2018.03.040. [DOI] [Google Scholar]
- Gentile C., Perrone A., Attanzio A., Tesoriere L., Livrea M.A. Sicilian pistachio (Pistacia vera L.) nut inhibits expression and release of inflammatory mediators and reverts the increase of paracellular permeability in IL-1β-exposed human intestinal epithelial cells. European Journal of Nutrition. 2015;54(5):811–821. doi: 10.1007/s00394-014-0760-6. [DOI] [PubMed] [Google Scholar]
- Gil-Cardoso K., Ginés I., Pinent M., Ardévol A., Arola L., Blay M., Terra X. Chronic supplementation with dietary proanthocyanidins protects from diet-induced intestinal alterations in obese rats. Molecular Nutrition and Food Research. 2017;61(8) doi: 10.1002/mnfr.201601039. [DOI] [PubMed] [Google Scholar]
- Gil-Cardoso K., Ginés I., Pinent M., Ardévol A., Blay M., Terra X. The co-administration of proanthocyanidins and an obesogenic diet prevents the increase in intestinal permeability and metabolic endotoxemia derived to the diet. Journal of Nutritional Biochemistry. 2018;62:35–42. doi: 10.1016/j.jnutbio.2018.07.012. [DOI] [PubMed] [Google Scholar]
- Gonzales G.B., Smagghe G., Grootaert C., Zotti M., Raes K., Camp J.V. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure-activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metabolism Reviews. 2015;47(2):175–190. doi: 10.3109/03602532.2014.1003649. [DOI] [PubMed] [Google Scholar]
- González-Quilen C., Gil-Cardoso K., Ginés I., Beltrán-Debón R., Pinent M., Ardévol A., Terra X., Blay M.T. Grape-seed proanthocyanidins are able to reverse intestinal dysfunction and metabolic endotoxemia induced by a cafeteria diet in wistar rats. Nutrients. 2019;11(5) doi: 10.3390/nu11050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Sarrías A., Espín J.C., Tomás-Barberán F.A. Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects. Trends in Food Science and Technology. 2017;69:281–288. doi: 10.1016/j.tifs.2017.07.010. [DOI] [Google Scholar]
- González-Sarrías A., García-Villalba R., Núñez-Sánchez M.Á., Tomé-Carneiro J., Zafrilla P., Mulero J., Tomás-Barberán F.A., Espín J.C. Identifying the limits for ellagic acid bioavailability: A crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts. Journal of Functional Foods. 2015;19:225–235. doi: 10.1016/j.jff.2015.09.019. [DOI] [Google Scholar]
- Gowd V., Karim N., Shishir M.R.I., Xie L., Chen W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends in Food Science and Technology. 2019;93:81–93. doi: 10.1016/j.tifs.2019.09.005. [DOI] [Google Scholar]
- Gribble F.M., Reimann F. Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annual Review of Physiology. 2016;78:277–299. doi: 10.1146/annurev-physiol-021115-105439. [DOI] [PubMed] [Google Scholar]
- Gu L., Kelm M.A., Hammerstone J.F., Beecher G., Holden J., Haytowitz D., Gebhardt S., Prior R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption. Journal of Nutrition. 2004;134(3):613–617. doi: 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- Gu L., Kelm M.A., Hammerstone J.F., Beecher G., Holden J., Haytowitz D., Prior R.L. Screening of Foods Containing Proanthocyanidins and Their Structural Characterization Using LC-MS/MS and Thiolytic Degradation. Journal of Agricultural and Food Chemistry. 2003;51(25):7513–7521. doi: 10.1021/jf034815d. [DOI] [PubMed] [Google Scholar]
- Han X., Zhao W., Zhou Q., Chen H., Yuan J., Xiaofu Z., Zhang Z. Procyanidins from hawthorn (Crataegus pinnatifida) alleviate lipid metabolism disorder via inhibiting insulin resistance and oxidative stress, normalizing the gut microbiota structure and intestinal barrier, and further suppressing hepatic inflammation. Food & Function. 2022;13(14):7901–7917. doi: 10.1039/D2FO00836J. [DOI] [PubMed] [Google Scholar]
- Hanhineva K., Törrönen R., Bondiapons I., Pekkinen J., Kolehmainen M., Mykkänen H.…Zeng X. Effects of oolong tea polyphenols, EGCG, and EGCG3 ″Me on pancreatic α-amylase activity in vitro. Journal of Agricultural and Food Chemistry. 2014;51(39):9507–9514. doi: 10.1021/jf5032907. [DOI] [PubMed] [Google Scholar]
- He F., Pan Q.H., Shi Y., Duan C.Q. Biosynthesis and genetic regulation of proanthocyanidins in plants. Molecules. 2008;13(10):2674–2703. doi: 10.3390/molecules13102674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollman P.C.H., Cassidy A., Comte B., Heinonen M., Richelle M., Richling E., Serafini M., Scalbert A., Sies H., Vidry S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. Journal of Nutrition. 2011;141(5) doi: 10.3945/jn.110.131490. [DOI] [PubMed] [Google Scholar]
- Hossen M.S., Ali M.Y., Jahurul M.H.A., Abdel-Daim M.M., Gan S.H., Khalil M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharmacological Reports. 2017;69(6):1194–1205. doi: 10.1016/j.pharep.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Hu J., Bi J., Li X., Wu X., Wang W., Yu Q. Understanding the impact of pectin on browning of polyphenol oxidation system in thermal and storage processing. Carbohydrate Polymers. 2023;307 doi: 10.1016/j.carbpol.2023.120641. [DOI] [PubMed] [Google Scholar]
- Hu J., Webster D., Cao J., Shao A. The safety of green tea and green tea extract consumption in adults – Results of a systematic review. Regulatory Toxicology and Pharmacology. 2018;95:412–433. doi: 10.1016/j.yrtph.2018.03.019. [DOI] [PubMed] [Google Scholar]
- Huang M., Han Y., Li L., Rakariyatham K., Wu X., Gao Z., Xiao H. Protective effects of non-extractable phenolics from strawberry against inflammation and colon cancer in vitro. Food Chemistry. 2022;374 doi: 10.1016/j.foodchem.2021.131759. [DOI] [PubMed] [Google Scholar]
- Iqbal A.Z., Javaid N., Hameeda M. Synergic interactions between berry polyphenols and gut microbiota in cardiovascular diseases. Mediterranean Journal of Nutrition and Metabolism. 2022;15(4):555–573. doi: 10.3233/MNM-220071. [DOI] [Google Scholar]
- Jang S., Sun J., Chen P., Lakshman S., Molokin A., Harnly J.M., Vinyard B.T., Urban J.F., Davis C.D., Solano-Aguilar G. Flavanol-enriched cocoa powder alters the intestinal microbiota, tissue and fluid metabolite profiles, and intestinal gene expression in pigs. Journal of Nutrition. 2016;146(4):673–680. doi: 10.3945/jn.115.222968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G., Asou Y., Ishiyama K., Okawa A., Kanno T., Niwano Y. Proanthocyanidin-Rich Grape Seed Extract Modulates Intestinal Microbiota in Ovariectomized Mice. Journal of Food Science. 2018;83(4):1149–1152. doi: 10.1111/1750-3841.14098. [DOI] [PubMed] [Google Scholar]
- Jin J.S., Hattori M. Isolation and characterization of a human intestinal bacterium Eggerthella sp. CAT-1 capable of cleaving the C-Ring of (+)-catechin and (-)-Epicatechin, followed by p-dehydroxylation of the B-ring. Biological and Pharmaceutical Bulletin. 2012;35(12):2252–2256. doi: 10.1248/bpb.b12-00726. [DOI] [PubMed] [Google Scholar]
- Juśkiewicz J., Milala J., Jurgoński A., Król B., Zduńczyk Z. Consumption of polyphenol concentrate with dietary fructo-oligosaccharides enhances cecal metabolism of quercetin glycosides in rats. Nutrition. 2011;27(3):351–357. doi: 10.1016/j.nut.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Kutschera M., Engst W., Blaut M., Braune A. Isolation of catechin-converting human intestinal bacteria. Journal of Applied Microbiology. 2011;111(1):165–175. doi: 10.1111/j.1365-2672.2011.05025.x. [DOI] [PubMed] [Google Scholar]
- Kwandee P., Somnuk S., Wanikorn B., Nakphaichit M., Tunsagool P. Efficacy of Triphala extracts on the changes of obese fecal microbiome and metabolome in the human gut model. Journal of Traditional and Complementary Medicine. 2023;13(2):207–217. doi: 10.1016/j.jtcme.2023.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourvellec C., Bouchet B., Renard C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material. Part III: Study on model polysaccharides. Biochimica et Biophysica Acta - General Subjects. 2005;1725(1):10–18. doi: 10.1016/j.bbagen.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Le Bourvellec C., Guyot S., Renard C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material: Part I. Effect of some environmental parameters. Biochimica et Biophysica Acta - General Subjects. 2004;1672(3):192–202. doi: 10.1016/j.bbagen.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Le Bourvellec C., Guyot S., Renard C.M.G.C. Interactions between apple (Malus x domestica Borkh.) polyphenols and cell walls modulate the extractability of polysaccharides. Carbohydrate Polymers. 2009;75(2):251–261. doi: 10.1016/j.carbpol.2008.07.010. [DOI] [Google Scholar]
- Leri M., Scuto M., Ontario M.L., Calabrese V., Calabrese E.J., Bucciantini M., Stefani M. Healthy effects of plant polyphenols: Molecular mechanisms. International Journal of Molecular Sciences. 2020;21(4) doi: 10.3390/ijms21041250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Christman L.M., Li R., Gu L. Synergic interactions between polyphenols and gut microbiota in mitigating inflammatory bowel diseases. Food and Function. 2020;11(6):4878–4891. doi: 10.1039/d0fo00713g. [DOI] [PubMed] [Google Scholar]
- Li Y., He D., Li B., Lund M.N., Xing Y., Wang Y., Li F., Cao X., Liu Y., Chen X., Yu J., Zhu J., Zhang M., Wang Q., Zhang Y., Li B., Wang J., Xing X., Li L. Engineering polyphenols with biological functions via polyphenol-protein interactions as additives for functional foods. Trends in Food Science and Technology. 2021;110:470–482. doi: 10.1016/j.tifs.2021.02.009. [DOI] [Google Scholar]
- Li Z., Summanen P.H., Komoriya T., Henning S.M., Lee R.-P., Carlson E., Heber D., Finegold S.M. Pomegranate ellagitannins stimulate growth of gut bacteria in vitro: Implications for prebiotic and metabolic effects. Anaerobe. 2015;34:164–168. doi: 10.1016/j.anaerobe.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Liu L.S., Fishman M.L., Hicks K.B. Pectin in controlled drug delivery - A review. Cellulose. 2007;14(1):15–24. doi: 10.1007/s10570-006-9095-7. [DOI] [Google Scholar]
- Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy. 2017;2 doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Le Bourvellec C., Renard C.M.G.C. Interactions between cell wall polysaccharides and polyphenols: Effect of molecular internal structure. Comprehensive Reviews in Food Science and Food Safety. 2020;19(6):3574–3617. doi: 10.1111/1541-4337.12632. [DOI] [PubMed] [Google Scholar]
- Loo Y.T., Howell K., Chan M., Zhang P., Ng K. Modulation of the human gut microbiota by phenolics and phenolic fiber-rich foods. Comprehensive Reviews in Food Science and Food Safety. 2020;19(4):1268–1298. doi: 10.1111/1541-4337.12563. [DOI] [PubMed] [Google Scholar]
- Low D.Y., Hodson M.P., Williams B.A., D’Arcy B.R., Gidley M.J. Microbial biotransformation of polyphenols during in vitro colonic fermentation of masticated mango and banana. Food Chemistry. 2016;207:214–222. doi: 10.1016/j.foodchem.2016.03.108. [DOI] [PubMed] [Google Scholar]
- Lu Y., Li X., Liu S., Zhang Y., Zhang D. Toll-like Receptors and Inflammatory Bowel Disease. Frontiers in Immunology. 2018;9(1):71–80. doi: 10.3389/fimmu.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Guo P., Zhou Q. Role of TLR4/NF-κB in Damage to Intestinal Mucosa Barrier Function and Bacterial Translocation in Rats Exposed to Hypoxia. PLoS ONE. 2012;7(10) doi: 10.1371/journal.pone.0046291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien-Meilleur J., Andrich D.E., Quinn S., Micaelli-Baret C., St-Amand R., Roy D., St-Pierre D.H. Interplay Between Gut Microbiota and Gastrointestinal Peptides: Potential Outcomes on the Regulation of Glucose Control. Canadian Journal of Diabetes. 2020;44(4):359–367. doi: 10.1016/j.jcjd.2019.10.006. [DOI] [PubMed] [Google Scholar]
- Luzardo-Ocampo I., Campos-Vega R., Gaytán-Martínez M., Preciado-Ortiz R., Mendoza S., Loarca-Piña G. Bioaccessibility and antioxidant activity of free phenolic compounds and oligosaccharides from corn (Zea mays L.) and common bean (Phaseolus vulgaris L.) chips during in vitro gastrointestinal digestion and simulated colonic fermentation. Food Research International. 2017;100:304–311. doi: 10.1016/j.foodres.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Macho-González A., Garcimartín A., López-Oliva M.E., Bertocco G., Naes F., Bastida S., Sánchez-Muniz F.J., Benedí J. Fiber purified extracts of carob fruit decrease carbohydrate absorption. Food & Function. 2017;8(6):2258–2265. doi: 10.1039/C7FO00166E. [DOI] [PubMed] [Google Scholar]
- Makarewicz M., Drożdż I., Tarko T., Duda-Chodak A. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants. 2021;10(2):1–70. doi: 10.3390/antiox10020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manach C., Milenkovic D., Van de Wiele T., Rodriguez-Mateos A., de Roos B., Garcia-Conesa M.T., Landberg R., Gibney E.R., Heinonen M., Tomás-Barberán F., Morand C. Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Molecular Nutrition and Food Research. 2017;61(6) doi: 10.1002/mnfr.201600557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: Food sources and bioavailability. American Journal of Clinical Nutrition. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Margalef M., Pons Z., Bravo F.I., Muguerza B., Arola-Arnal A. Plasma kinetics and microbial biotransformation of grape seed flavanols in rats. Journal of Functional Foods. 2015;12:478–488. doi: 10.1016/j.jff.2014.12.007. [DOI] [Google Scholar]
- Martínez-Meza Y., Reynoso-Camacho R., Pérez-Jiménez J. Nonextractable Polyphenols: A Relevant Group with Health Effects. Dietary Polyphenols. 2020;31–83 doi: 10.1002/9781119563754.ch2. [DOI] [Google Scholar]
- Masumoto S., Terao A., Yamamoto Y., Mukai T., Miura T., Shoji T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Scientific Reports. 2016;6 doi: 10.1038/srep31208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos-Martín M.L., Pérez-Jiménez J., Fuguet E., Torres J.L. Non-extractable proanthocyanidins from grapes are a source of bioavailable (epi)catechin and derived metabolites in rats. British Journal of Nutrition. 2012;108(2):290–297. doi: 10.1017/S0007114511005678. [DOI] [PubMed] [Google Scholar]
- McClements D.J., Das A.K., Dhar P., Nanda P.K., Chatterjee N. Nanoemulsion-Based Technologies for Delivering Natural Plant-Based Antimicrobials in Foods. Frontiers in Sustainable Food Systems. 2021;5:208. doi: 10.3389/fsufs.2021.643208. [DOI] [Google Scholar]
- McKay D.L., Chen C.Y.O., Zampariello C.A., Blumberg J.B. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chemistry. 2015;168:233–240. doi: 10.1016/j.foodchem.2014.07.062. [DOI] [PubMed] [Google Scholar]
- Molino S., Lerma-Aguilera A., Jiménez-Hernández N., Gosalbes M.J., Rufián-Henares J.Á., Francino M.P. Enrichment of Food With Tannin Extracts Promotes Healthy Changes in the Human Gut Microbiota. Frontiers in Microbiology. 2021;12 doi: 10.3389/fmicb.2021.625782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteagudo-Mera A., Rastall R.A., Gibson G.R., Charalampopoulos D., Chatzifragkou A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Applied Microbiology and Biotechnology. 2019;103(16):6463–6472. doi: 10.1007/s00253-019-09978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Indias I., Sánchez-Alcoholado L., Pérez-Martínez P., Andrés-Lacueva C., Cardona F., Tinahones F., Queipo-Ortuño M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food and Function. 2016;7(4):1775–1787. doi: 10.1039/c5fo00886g. [DOI] [PubMed] [Google Scholar]
- Mosele J.I., Macià A., Romero M.-P., Motilva M.-J. Stability and metabolism of Arbutus unedo bioactive compounds (phenolics and antioxidants) under in vitro digestion and colonic fermentation. Food Chemistry. 2016;201:120–130. doi: 10.1016/j.foodchem.2016.01.076. [DOI] [PubMed] [Google Scholar]
- Nabavi S., Sureda A., Daglia M., Rezaei P., Nabavi S. Anti-Oxidative Polyphenolic Compounds of Cocoa. Current Pharmaceutical Biotechnology. 2015;16(10):891–901. doi: 10.2174/1389201016666150610160652. [DOI] [PubMed] [Google Scholar]
- Nawrot-Hadzik I., Matkowski A., Hadzik J., Dobrowolska-Czopor B., Olchowy C., Dominiak M., Kubasiewicz-Ross P. Proanthocyanidins and Flavan-3-Ols in the Prevention and Treatment of Periodontitis-Antibacterial Effects. Nutrients. 2021;13(1) doi: 10.3390/nu13010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez-Gómez V., González-Barrio R., Baenas N., Moreno D.A., Periago M.J. Dietary-Fibre-Rich Fractions Isolated from Broccoli Stalks as a Potential Functional Ingredient with Phenolic Compounds and Glucosinolates. International Journal of Molecular Sciences. 2022;23(21):13309. doi: 10.3390/ijms232113309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi T., Goto S., Monira P., Isemura M., Nakamura Y. Anti-inflammatory Action of Green Tea. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry. 2016;15(2):74–90. doi: 10.2174/1871523015666160915154443. [DOI] [PubMed] [Google Scholar]
- Oteiza P.I., Fraga C.G., Mills D.A., Taft D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Molecular Aspects of Medicine. 2018;61:41–49. doi: 10.1016/j.mam.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Ou K., Gu L. Absorption and metabolism of proanthocyanidins. Journal of Functional Foods. 2014;7:43–53. doi: 10.1016/j.jff.2013.08.004. [DOI] [Google Scholar]
- Ozdal T., Capanoglu E., Altay F. A review on protein-phenolic interactions and associated changes. Food Research. 2013 International. [Google Scholar]
- Padayachee A., Netzel G., Netzel M., Day L., Mikkelsen D., Gidley M.J. Lack of release of bound anthocyanins and phenolic acids from carrot plant cell walls and model composites during simulated gastric and small intestinal digestion. Food and Function. 2013;4(6):906–916. doi: 10.1039/c3fo60091b. [DOI] [PubMed] [Google Scholar]
- Papuc C., Goran G.V., Predescu C.N., Nicorescu V., Stefan G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Comprehensive Reviews in Food Science and Food Safety. 2017;16(6):1243–1268. doi: 10.1111/1541-4337.12298. [DOI] [PubMed] [Google Scholar]
- Pérez-Jiménez J., Díaz-Rubio M.E., Saura-Calixto F. Non-extractable polyphenols, a major dietary antioxidant: Occurrence, metabolic fate and health effects. Nutrition Research Reviews. 2013;26(2):118–129. doi: 10.1017/S0954422413000097. [DOI] [PubMed] [Google Scholar]
- Pérez-Jiménez J., Torres J.L. Analysis of nonextractable phenolic compounds in foods: The current state of the art. Journal of Agricultural and Food Chemistry. 2011;59(24):12713–12724. doi: 10.1021/jf203372w. [DOI] [PubMed] [Google Scholar]
- Qi Q., Chu M., Yu X., Xie Y., Li Y., Du Y., Liu X., Zhang Z., Shi J., Yan N. Anthocyanins and Proanthocyanidins: Chemical Structures, Food Sources, Bioactivities, and Product Development. Food Reviews International. 2022 doi: 10.1080/87559129.2022.2029479. [DOI] [Google Scholar]
- Queipo-Ortuño M.I., Boto-Ordóñez M., Murri M., Gomez-Zumaquero J.M., Clemente-Postigo M., Estruch R., Cardona Diaz F., Andrés-Lacueva C., Tinahones F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. The American Journal of Clinical Nutrition. 2012;95(6):1323–1334. doi: 10.3945/ajcn.111.027847. [DOI] [PubMed] [Google Scholar]
- Renard C.M.G.C., Le Quéré J.M., Bauduin R., Symoneaux R., Le Bourvellec C., Baron A. Modulating polyphenolic composition and organoleptic properties of apple juices by manipulating the pressing conditions. Food Chemistry. 2011;124(1):117–125. doi: 10.1016/j.foodchem.2010.05.113. [DOI] [Google Scholar]
- Renard C.M.G.C., Watrelot A.A., Le Bourvellec C. Interactions between polyphenols and polysaccharides: Mechanisms and consequences in food processing and digestion. Trends in Food Science and Technology. 2017;60:43–51. doi: 10.1016/j.tifs.2016.10.022. [DOI] [Google Scholar]
- Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M., Magris M., Hidalgo G., Baldassano R.N., Anokhin A.P., Warner B., Reeder J., Kuczynski J., Caporaso J.G., Lozupone C.A., Lauber C., Clemente J.C., Knights D., Knight R., Gordon J.I. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios L.Y., Gonthier M.P., Rémésy C., Mila I., Lapierre C., Lazarus S.A., Williamson G., Scalbert A. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. The American Journal of Clinical Nutrition. 2003;77(4):912–918. doi: 10.1093/ajcn/77.4.912. [DOI] [PubMed] [Google Scholar]
- Rocchetti G., Gregorio R.P., Lorenzo J.M., Barba F.J., Oliveira P.G., Prieto M.A., Simal-Gandara J., Mosele J.I., Motilva M.J., Tomas M., Patrone V., Capanoglu E., Lucini L. Functional implications of bound phenolic compounds and phenolics–food interaction: A review. Comprehensive Reviews in Food Science and Food Safety. 2022;21(2):811–842. doi: 10.1111/1541-4337.12921. [DOI] [PubMed] [Google Scholar]
- Rondini L., Peyrat-Maillard M.-N., Marsset-Baglieri A., Fromentin G., Durand P., Tomé D., Prost M., Berset C. Bound Ferulic Acid from Bran Is More Bioavailable than the Free Compound in Rat. Journal of Agricultural and Food Chemistry. 2004;52(13):4338–4343. doi: 10.1021/jf0348323. [DOI] [PubMed] [Google Scholar]
- Roowi S., Stalmach A., Mullen W., Lean M.E.J., Edwards And C.A., Crozier A. Green tea flavan-3-ols: Colonic degradation and urinary excretion of catabolites by humans. Journal of Agricultural and Food Chemistry. 2010;58(2):1296–1304. doi: 10.1021/jf9032975. [DOI] [PubMed] [Google Scholar]
- Rue E.A., Rush M.D., van Breemen R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochemistry Reviews. 2018;17(1):1–16. doi: 10.1007/s11101-017-9507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Garcia Y., Smith P.A., Bindon K.A. Selective extraction of polysaccharide affects the adsorption of proanthocyanidin by grape cell walls. Carbohydrate Polymers. 2014;114:102–114. doi: 10.1016/j.carbpol.2014.07.024. [DOI] [PubMed] [Google Scholar]
- Sagkan-Ozturk A., Arpaci A. The comparison of changes in fecal and mucosal microbiome in metabolic endotoxemia induced by a high-fat diet. Anaerobe. 2022;77 doi: 10.1016/j.anaerobe.2022.102615. [DOI] [PubMed] [Google Scholar]
- Sallam I.E., Abdelwareth A., Attia H., Aziz R.K., Homsi M.N., von Bergen M., Farag M.A. Effect of gut microbiota biotransformation on dietary tannins and human health implications. Microorganisms. 2021;9(5) doi: 10.3390/microorganisms9050965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Patán F., Barroso E., Van De Wiele T., Jiménez-Girón A., Martín-Alvarez P.J., Moreno-Arribas M.V., Martínez-Cuesta M.C., Peláez C., Requena T., Bartolomé B. Comparative in vitro fermentations of cranberry and grape seed polyphenols with colonic microbiota. Food Chemistry. 2015;183:273–282. doi: 10.1016/j.foodchem.2015.03.061. [DOI] [PubMed] [Google Scholar]
- Sánchez-Patán F., Tabasco R., Monagas M., Requena T., Peláez C., Moreno-Arribas M.V., Bartolomé B. Capability of lactobacillus plantarum IFPL935 to catabolize flavan-3-ol compounds and complex phenolic extracts. Journal of Agricultural and Food Chemistry. 2012;60(29):7142–7151. doi: 10.1021/jf3006867. [DOI] [PubMed] [Google Scholar]
- Sarkar, P., Abedin, M. M., Singh, S. P., Pandey, A., & Rai, A. K. (2021). Microbial production and transformation of polyphenols. In Current Developments in Biotechnology and Bioengineering: Technologies for Production of Nutraceuticals and Functional Food Products (pp. 189–208). https://doi.org/10.1016/B978-0-12-823506-5.00005-9.
- Saura-Calixto F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. Journal of Agricultural and Food Chemistry. 2011;59(1):43–49. doi: 10.1021/jf1036596. [DOI] [PubMed] [Google Scholar]
- Saura-Calixto F. Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. Journal of Agricultural and Food Chemistry. 2012;60(45):11195–11200. doi: 10.1021/jf303758j. [DOI] [PubMed] [Google Scholar]
- Saura-Calixto F., Pérez-Jiménez J., Touriño S., Serrano J., Fuguet E., Torres J.L., Goñi I. Proanthocyanidin metabolites associated with dietary fibre from in vitro colonic fermentation and proanthocyanidin metabolites in human plasma. Molecular Nutrition and Food Research. 2010;54(7):939–946. doi: 10.1002/mnfr.200900276. [DOI] [PubMed] [Google Scholar]
- Saura-Calixto F., Serrano J., Goñi I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chemistry. 2007;101(2):492–501. doi: 10.1016/j.foodchem.2006.02.006. [DOI] [Google Scholar]
- Seeram N.P., Henning S.M., Zhang Y., Suchard M., Li Z., Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. Journal of Nutrition. 2006;136(10):2481–2485. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- Seeram N.P., Lee R., Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clinica Chimica Acta. 2004;348(1–2):63–68. doi: 10.1016/j.cccn.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Selma M.V., Beltrán D., García-Villalba R., Espín J.C., Tomás-Barberán F.A. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food and Function. 2014;5(8):1779–1784. doi: 10.1039/c4fo00092g. [DOI] [PubMed] [Google Scholar]
- Selma M.V., Beltrán D., Luna M.C., Romo-Vaquero M., García-Villalba R., Mira A., Espín J.C., Tomás-Barberán F.A. Isolation of human intestinal bacteria capable of producing the bioactive metabolite isourolithin a from ellagic acid. Frontiers in Microbiology. 2017;8(AUG) doi: 10.3389/fmicb.2017.01521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng K., Zhang G., Sun M., He S., Kong X., Wang J., Zhu F., Zha X., Wang Y. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food & Function. 2020;11(9):7817–7829. doi: 10.1039/D0FO01418D. [DOI] [PubMed] [Google Scholar]
- Smeriglio A., Barreca D., Bellocco E., Trombetta D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. British Journal of Pharmacology. 2017;174(11):1244–1262. doi: 10.1111/bph.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrenti V., Ali S., Mancin L., Davinelli S., Paoli A., Scapagnini G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients. 2020;12(7):1908. doi: 10.3390/nu12071908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strat K.M., Rowley T.J., Smithson A.T., Tessem J.S., Hulver M.W., Liu D., Davy B.M., Davy K.P., Neilson A.P. Mechanisms by which cocoa flavanols improve metabolic syndrome and related disorders. Journal of Nutritional Biochemistry. 2016;35:1–21. doi: 10.1016/j.jnutbio.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Sun L., Wang Y., Miao M. Inhibition of α-amylase by polyphenolic compounds: Substrate digestion, binding interactions and nutritional intervention. Trends in Food Science and Technology. 2020;104:190–207. doi: 10.1016/j.tifs.2020.08.003. [DOI] [Google Scholar]
- Suriyaprom S., Mosoni P., Leroy S., Kaewkod T., Desvaux M., Tragoolpua Y. Antioxidants of Fruit Extracts as Antimicrobial Agents against Pathogenic Bacteria. Antioxidants. 2022;11(3) doi: 10.3390/antiox11030602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagaki A., Kato Y., Nanjo F. Isolation and characterization of rat intestinal bacteria involved in biotransformation of (-)-epigallocatechin. Archives of Microbiology. 2014;196(10):681–695. doi: 10.1007/s00203-014-1006-y. [DOI] [PubMed] [Google Scholar]
- Takagaki A., Nanjo F. Bioconversion of (-)-epicatechin, (+)-epicatechin, (-)-catechin, and (+)-catechin by (-)-epigallocatechin-metabolizing bacteria. Biological and Pharmaceutical Bulletin. 2015;38(5):789–794. doi: 10.1248/bpb.b14-00813. [DOI] [PubMed] [Google Scholar]
- Takagaki A., Nanjo F. Biotransformation of (-)-epigallocatechin and (-)-gallocatechin by intestinal bacteria involved in isoflavone metabolism. Biological and Pharmaceutical Bulletin. 2015;38(2):325–330. doi: 10.1248/bpb.b14-00646. [DOI] [PubMed] [Google Scholar]
- Thilakarathna W.P.D.W., Vasantha Rupasinghe H.P. Optimization of the Extraction of Proanthocyanidins from Grape Seeds Using Ultrasonication-Assisted Aqueous Ethanol and Evaluation of Anti-Steatosis Activity In Vitro. Molecules. 2022;27(4) doi: 10.3390/molecules27041363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás-Barberán F.A., Espín J.C. Effect of Food Structure and Processing on (Poly)phenol-Gut Microbiota Interactions and the Effects on Human Health. Annual Review of Food Science and Technology. 2019;10:221–238. doi: 10.1146/annurev-food-032818-121615. [DOI] [PubMed] [Google Scholar]
- Tomás-Barberán F.A., Selma M.V., Espín J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Current Opinion in Clinical Nutrition and Metabolic Care. 2016;19(6):471–476. doi: 10.1097/MCO.0000000000000314. [DOI] [PubMed] [Google Scholar]
- Truchado P., Larrosa M., García-Conesa M.T., Cerdá B., Vidal-Guevara M.L., Tomás-Barberán F.A., Espín J.C. Strawberry processing does not affect the production and urinary excretion of urolithins, ellagic acid metabolites, in humans. Journal of Agricultural and Food Chemistry. 2012;60(23):5749–5754. doi: 10.1021/jf203641r. [DOI] [PubMed] [Google Scholar]
- Tudorache M., McDonald J.L., Bordenave N. Gallic acid reduces the viscosity and water binding capacity of soluble dietary fibers. Food and Function. 2020;11(7):5866–5874. doi: 10.1039/d0fo01200a. [DOI] [PubMed] [Google Scholar]
- Tuohy K., Rio D.D. In Diet-Microbe Interactions in the Gut: Effects on Human Health and Disease. 2014. Diet-Microbe Interactions in the Gut: Effects on Human Health and Disease. [DOI] [Google Scholar]
- Tzounis X., Rodriguez-Mateos A., Vulevic J., Gibson G.R., Kwik-Uribe C., Spencer J.P.E. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. American Journal of Clinical Nutrition. 2011;93(1):62–72. doi: 10.3945/ajcn.110.000075. [DOI] [PubMed] [Google Scholar]
- Ulluwishewa D., Anderson R.C., McNabb W.C., Moughan P.J., Wells J.M., Roy N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. Journal of Nutrition. 2011;141(5):769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- Urpi-Sarda M., Monagas M., Khan N., Lamuela-Raventos R.M., Santos-Buelga C., Sacanella E., Castell M., Permanyer J., Andres-Lacueva C. Epicatechin, procyanidins, and phenolic microbial metabolites after cocoa intake in humans and rats. Analytical and Bioanalytical Chemistry. 2009;394(6):1545–1556. doi: 10.1007/s00216-009-2676-1. [DOI] [PubMed] [Google Scholar]
- Van Hul M., Geurts L., Plovier H., Druart C., Everard A., Ståhlman M., Rhimi M., Chira K., Teissedre P.L., Delzenne N.M., Maguin E., Guilbot A., Brochot A., Gérard P., Bäckhed F., Cani P.D. Reduced obesity, diabetes, and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. American Journal of Physiology - Endocrinology and Metabolism. 2018;314(4):E334–E352. doi: 10.1152/ajpendo.00107.2017. [DOI] [PubMed] [Google Scholar]
- Vendrame S., Guglielmetti S., Riso P., Arioli S., Klimis-Zacas D., Porrini M. Six-week consumption of a wild blueberry powder drink increases Bifidobacteria in the human gut. Journal of Agricultural and Food Chemistry. 2011;59(24):12815–12820. doi: 10.1021/jf2028686. [DOI] [PubMed] [Google Scholar]
- Vitaglione P., Mennella I., Ferracane R., Rivellese A.A., Giacco R., Ercolini D., Gibbons S.M., La Storia A., Gilbert J.A., Jonnalagadda S., Thielecke F., Gallo M.A., Scalfi L., Fogliano V. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: Role of polyphenols bound to cereal dietary fiber2–4. The American Journal of Clinical Nutrition. 2015;101(2):251–261. doi: 10.3945/ajcn.114.088120. [DOI] [PubMed] [Google Scholar]
- Volstatova T., Marsik P., Rada V., Geigerova M., Havlik J. Effect of apple extracts and selective polyphenols on the adhesion of potential probiotic strains of Lactobacillus gasseri R and Lactobacillus casei FMP. Journal of Functional Foods. 2017;35:391–397. doi: 10.1016/j.jff.2017.06.005. [DOI] [Google Scholar]
- Wang J., Zhang W., Tang C., Xiao J., Xie B., Sun Z. Synergistic effect of B-type oligomeric procyanidins from lotus seedpod in combination with water-soluble Poria cocos polysaccharides against E. coli and mechanism. Journal of Functional Foods. 2018;48:134–143. doi: 10.1016/j.jff.2018.07.015. [DOI] [Google Scholar]
- Wang L.Q., Meselhy M.R., Li Y., Nakamura N., Min B.S., Qin G.W., Hattori M. The heterocyclic ring fission and dehydroxylation of catechins and related compounds by Eubacterium sp. strain SDG-2, a human intestinal bacterium. Chemical and Pharmaceutical Bulletin. 2001;49(12):1640–1643. doi: 10.1248/cpb.49.1640. [DOI] [PubMed] [Google Scholar]
- Wang M., Cheong K.-L. Preparation, Structural Characterisation, and Bioactivities of Fructans: A Review. Molecules. 2023;28(4):1613. doi: 10.3390/molecules28041613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Li J., Hu T., Zhao H. Metabolic fate of tea polyphenols and their crosstalk with gut microbiota. Food Science and Human Wellness. 2022;11(3):455–466. doi: 10.1016/j.fshw.2021.12.003. [DOI] [Google Scholar]
- Wang M., Veeraperumal S., Zhong S., Cheong K.-L. Fucoidan-Derived Functional Oligosaccharides: Recent Developments, Preparation, and Potential Applications. Foods. 2023;12(4):878. doi: 10.3390/foods12040878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward N.C., Croft K.D., Puddey I.B., Hodgson J.M. Supplementation with grape seed polyphenols results in increased urinary excretion of 3-hydroxyphenylpropionic acid, an important metabolite of proanthocyanidins in humans. Journal of Agricultural and Food Chemistry. 2004;52(17):5545–5549. doi: 10.1021/jf049404r. [DOI] [PubMed] [Google Scholar]
- Watrelot A.A., Le Bourvellec C., Imberty A., Renard C.M.G.C. Interactions between pectic compounds and procyanidins are influenced by methylation degree and chain length. Biomacromolecules. 2013;14(3):709–718. doi: 10.1021/bm301796y. [DOI] [PubMed] [Google Scholar]
- Williamson G., Clifford M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochemical Pharmacology. 2017;139:24–39. doi: 10.1016/j.bcp.2017.03.012. [DOI] [PubMed] [Google Scholar]
- Wong X., Carrasco-Pozo C., Escobar E., Navarrete P., Blachier F., Andriamihaja M., Lan A., Tomé D., Cires M.J., Pastene E., Gotteland M. Deleterious Effect of p-Cresol on Human Colonic Epithelial Cells Prevented by Proanthocyanidin-Containing Polyphenol Extracts from Fruits and Proanthocyanidin Bacterial Metabolites. Journal of Agricultural and Food Chemistry. 2016;64(18):3574–3583. doi: 10.1021/acs.jafc.6b00656. [DOI] [PubMed] [Google Scholar]
- Wu H., Luo T., Li Y.M., Gao Z.P., Zhang K.Q., Song J.Y., Xiao J.S., Cao Y.P. Granny Smith apple procyanidin extract upregulates tight junction protein expression and modulates oxidative stress and inflammation in lipopolysaccharide-induced Caco-2 cells. Food and Function. 2018;9(6):3321–3329. doi: 10.1039/c8fo00525g. [DOI] [PubMed] [Google Scholar]
- Wu S., Tian L. Diverse phytochemicals and bioactivities in the ancient fruit and modern functional food pomegranate (punica granatum) Molecules. 2017;22(10) doi: 10.3390/molecules22101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Wang K., Liu X., Liu G., Hu Z., Zhao L. Characterization and bioactivity of A-type procyanidins from litchi fruitlets at different degrees of development. Food Chemistry. 2023;405 doi: 10.1016/j.foodchem.2022.134855. [DOI] [PubMed] [Google Scholar]
- Xu Z., Du P., Meiser P., Claus J. Proanthocyanidins: Oligomeric structures with unique biochemical properties and great therapeutic promise. Natural Product Communications. 2012;7(3):381–388. doi: 10.1177/1934578x1200700321. [DOI] [PubMed] [Google Scholar]
- Yang H., Huang X., Fang S., He M., Zhao Y., Wu Z., Yang M., Zhang Z., Chen C., Huang L. Unraveling the fecal microbiota and metagenomic functional capacity associated with feed efficiency in pigs. Frontiers in Microbiology. 2017;8(AUG) doi: 10.3389/fmicb.2017.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Yue T., Yuan Y. Evolution of polyphenols and organic acids during the fermentation of apple cider. Journal of the Science of Food and Agriculture. 2014;94(14):2951–2957. doi: 10.1002/jsfa.6639. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y., Akiyama H., Nakano M., Shoji T., Kanda T., Ohtake Y., Takita T., Matsuda R., Maitani T. Orally administered apple procyanidins protect against experimental inflammatory bowel disease in mice. International Immunopharmacology. 2008;8(13–14):1802–1807. doi: 10.1016/j.intimp.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Zhang L., Carmody R.N., Kalariya H.M., Duran R.M., Moskal K., Poulev A., Kuhn P., Tveter K.M., Turnbaugh P.J., Raskin I., Roopchand D.E. Grape proanthocyanidin-induced intestinal bloom of Akkermansia muciniphila is dependent on its baseline abundance and precedes activation of host genes related to metabolic health. Journal of Nutritional Biochemistry. 2018;56:142–151. doi: 10.1016/j.jnutbio.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Song X., Hu X., Chen F., Ma C. Health benefits of proanthocyanidins linking with gastrointestinal modulation: An updated review. Food Chemistry. 2023;404 doi: 10.1016/j.foodchem.2022.134596. [DOI] [PubMed] [Google Scholar]
- Zheng S., Huang K., Zhao C., Xu W., Sheng Y., Luo Y., He X. Procyanidin attenuates weight gain and modifies the gut microbiota in high fat diet induced obese mice. Journal of Functional Foods. 2018;49:362–368. doi: 10.1016/j.jff.2018.09.007. [DOI] [Google Scholar]
- Zhu F. Interactions between cell wall polysaccharides and polyphenols. Critical Reviews in Food Science and Nutrition. 2018;58(11):1808–1831. doi: 10.1080/10408398.2017.1287659. [DOI] [PubMed] [Google Scholar]
- Zhu W., Oteiza P.I. Proanthocyanidins at the gastrointestinal tract: Mechanisms involved in their capacity to mitigate obesity-associated metabolic disorders. Critical Reviews in Food Science and Nutrition. 2022;1–21 doi: 10.1080/10408398.2022.2105802. [DOI] [PubMed] [Google Scholar]
- Zhu W., Xiong L., Peng J., Deng X., Gao J., Li C.M. Molecular Insight into Affinities of Gallated and Nongallated Proanthocyanidins Dimers to Lipid Bilayers. Scientific Reports. 2016;6 doi: 10.1038/srep37680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.