Abstract
In Escherichia coli, the repair of lethal DNA damage induced by H2O2 requires exonuclease III, the xthA gene product. Here, we report that both endonuclease IV (the nfo gene product) and exonuclease III can mediate the repair of lesions induced by H2O2 under low-iron conditions. Neither the xthA nor the nfo mutants was sensitive to H2O2 in the presence of iron chelators, while the xthA nfo double mutant was significantly sensitive to this treatment, suggesting that both exonuclease III and endonuclease IV can mediate the repair of DNA lesions formed under such conditions. Sedimentation studies in alkaline sucrose gradients also demonstrated that both xthA and nfo mutants, but not the xthA nfo double mutant, can carry out complete repair of DNA strand breaks and alkali-labile bonds generated by H2O2 under low-iron conditions. We also found indications that the formation of substrates for exonuclease III and endonuclease IV is mediated by the Fpg DNA glycosylase, as suggested by experiments in which the fpg mutation increased the level of cell survival, as well as repair of DNA strand breaks, in an AP endonuclease-null background.
Active oxygen species are a continuous threat to cell integrity, due to their potential to damage DNA, membranes, and proteins. A wide variety of oxidative DNA lesions are formed as a consequence of the attack to both the bases and the sugar-phosphate backbone; the development of repair mechanisms for such lesions aided the success of aerobic life (15).
5′ AP endonucleases are central enzymes in the base excision repair pathway, and besides their participation in the repair of abasic sites, they play an important role in the repair of oxidative DNA lesions by removing 3′ blocks formed upon DNA strand breakage (7).
There are two families of AP endonucleases, each sharing homology with one of the two Escherichia coli prototypes: exonuclease III, encoded by the xthA gene, and endonuclease IV, encoded by the nfo gene. Both families are present in various eukaryotes, and at least in the budding yeast, one member of each is present (7, 8, 17, 25).
In E. coli cells, exonuclease III constitutes approximately 90% of the AP endonuclease activity in crude extracts, whereas endonuclease IV accounts for most of the residual activity (20). Since the two enzymes have similar biochemical activities in DNA repair, the analysis of their biological function has been largely based on the properties of mutant strains lacking one or both genes. xthA mutants are hypersensitive to hydrogen peroxide (14), a phenotype not exhibited by nfo mutants (12). However, the xthA nfo double mutant is even more sensitive to this agent, suggesting that both exonuclease III and endonuclease IV participate in the repair of H2O2-induced lesions, although the former is much more important in the correction of the DNA damage. However, endonuclease IV has been shown to be very important in the repair of certain types of oxidative lesions, such as those induced by bleomycin or NO produced by macrophages (12, 23).
It was demonstrated that iron chelators can protect E. coli cells from the lethal effects of hydrogen peroxide, specially from mode I killing (16). Using alkaline sucrose gradients, Asad and Leitão (6) have shown that although pretreatment of E. coli xthA cultures with iron chelators confers protection against the lethal effects of H2O2, DNA lesions can still be formed under these conditions. In other words, it was demonstrated that DNA damage by H2O2 is not inhibited at all by iron chelators. Instead, a different subset of oxidative DNA lesions predominates under such low-iron conditions, which can be completely repaired in an xthA mutant, as also shown through DNA sedimentation studies (6).
In the present work, we investigated the participation of endonuclease IV in the repair of DNA lesions generated by H2O2 under low-iron conditions. Our findings suggest that either endonuclease IV or exonuclease III can mediate the repair of the DNA lesions caused by this treatment. We also investigated the role of Fpg, the DNA glycosylase that excises damaged purines from DNA (11, 27), and found indications that under low-iron conditions, this enzyme contributes significantly to the generation of substrates for exonuclease III and endonuclease IV in DNA.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains used in this work were derived from E. coli K-12 and are listed in Table 1. The multicopy plasmid pFPG60 (10), expressing the fpg gene, was a kind gift from Serge Boiteux (Département de Radiobiologie et Radiopathologie, CEA Fontenay-aux-Roses, France).
TABLE 1.
Bacterial strains used in this studya
| Strain | Relevant genotype |
|---|---|
| AB1157 | Wild type |
| BW9091 | xthA |
| BW527 | nfo |
| BW544 | xthA nfo |
| BH110 | xthA nfo fpg |
The AB1157 strain was obtained from our laboratory stock. The BW strains were a gift from Bernard Weiss (University of Michigan Medical School, Ann Arbor). The BH strain was a gift from S. Boiteux (DRR). Both the BW and the BH strains are derivatives of AB1157.
Growth conditions and radioactive labeling.
Bacterial cultures were grown overnight in M9S minimal medium (3) containing 4 g of glucose per liter supplemented with 2.5 mg of Casamino Acids per ml and 10 μg of thiamine per ml at 37°C with shaking. Overnight cultures were diluted 1:40 in fresh M9S medium and cultivated until the mid-exponential phase (2 × 108 cells/ml). Radioactive cultures were grown in M9S medium supplemented with 10 μCi of [methyl-3H]thymidine (6.7 Ci/mmol; New England Nuclear, Boston, Mass.) per ml and 200 μg of 2′-deoxyadenosine per ml until the initial exponential growth phase. Cells were then harvested, resuspended in cold M9S medium, and incubated for 30 min at 37°C to remove the unincorporated [methyl-3H]thymidine from the intracellular pool.
Treatment with metal ion chelators.
Cultures in the mid-exponential phase of growth were incubated for 20 min in M9S medium at 37°C, with shaking, with the iron ion chelator dipyridyl (2,2′-bipyridine; Sigma) at 1 mM. The survival experiments were then conducted as described below. Treatment with the metal ion chelator does not affect cell viability (data not shown).
Survival experiments.
Cultures in the mid-exponential phase of growth, treated or not with 1 mM dipyridyl, were challenged with 5 mM H2O2 (perhydrol [30%]; Merck, Rio de Janeiro, Brazil) in M9S medium at 37°C with shaking. Aliquots were collected after various periods of incubation with H2O2, which was then inactivated by the addition of 5 μg of catalase (EC 1.11.1.6; 9001-05-2; Sigma) per ml. Samples were appropriately diluted in M9 salts solution and spread onto Luria-Bertani (rich) medium (21) solidified with 1.5% agar (Bacto-agar; Difco). CFU were scored after overnight incubation at 37°C. Surviving fractions were expressed as the averages obtained from at least three experiments.
DNA sedimentation studies.
The formation and repair of DNA single-strand breaks were analyzed by sedimentation in alkaline sucrose gradients as described by MacGrath and Williams (22), with slight modifications. Radioactive cultures prepared as described above were treated with H2O2 (5 mM) for 20 min as described for the survival experiments, centrifuged, and resuspended in cold M9S medium. Cells were allowed to recover from DNA damage in nonradioactive M9S medium at 37°C with shaking, and samples were collected after various periods. Undiluted 100-μl aliquots were added on top of 0.2 ml of lysing solution (0.5 M NaOH, 0.01 M EDTA, and 0.05% sodium dodecyl sulfate) layered on the top of a 4.2-ml sucrose gradient of 5 to 20% (wt/vol) in 0.4 M NaCl–0.2 M NaOH–0.01 M EDTA. The tubes were maintained for 30 min at room temperature and then centrifuged in a Beckman SW 50.1 rotor for 120 min at 25,000 rpm and 20°C. After centrifugation, 30 fractions were collected on paper strips (Whatman no. 17) presoaked with 5% trichloroacetic acid, using a peristaltic pump. The paper strips were washed once in ice-cold 5% trichloroacetic acid, once in 95% ethanol, and once in acetone. After drying, the radioactive content of each fraction was determined in a Beckman liquid scintillation counter. The average molecular weights were calculated according to the method described by Ley (19), and the number of DNA strand breaks and alkali-labile bonds per E. coli genome (2.5 × 109 Da) were calculated as described by Ananthaswamy and Eisenstark (2).
RESULTS
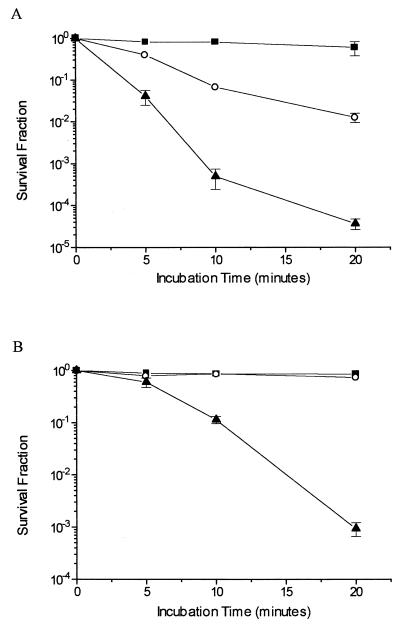
Results of survival experiments with xthA, nfo, and xthA nfo mutant strains are shown in Fig. 1. Experiments in which the cultures were treated with 5 mM H2O2 (Fig. 1A) confirm the data previously described in the literature. It can be observed that the xthA mutant was sensitive to H2O2 while the nfo strain was not and that the xthA nfo double mutant was even more sensitive to this treatment. These results indicate that exonuclease III plays a major role in the repair of DNA lesions generated by H2O2. On the other hand, experiments with cultures pretreated with the iron chelator dipyridyl show a distinct pattern of dependence on AP endonucleases for bacterial survival. Neither the xthA nor the nfo single mutant was significantly inactivated by H2O2 under low-iron conditions. Notwithstanding, the xthA nfo double mutant was highly sensitive to this treatment, indicating that both exonuclease III and endonuclease IV may act in the repair of the oxidative lesions generated under such conditions (Fig. 1B). The wild-type strain was as resistant as the nfo strain to both treatments (data not shown).
FIG. 1.
Survival of E. coli AP endonuclease-deficient strains. Cultures in the mid-exponential phase of growth in M9S medium were treated with 5 mM H2O2 for the indicated times (A) or pretreated with 1 mM dipyridyl for 20 min and then treated with 5 mM H2O2 for the indicated times (B). Values are means ± standard errors. ○, xthA strain; ■, nfo strain; ▴, xthA nfo strain.
In order to analyze the participation of both enzymes in the repair of DNA lesions, we investigated the capacity of the mutant and wild-type strains for repairing strand breaks and alkali labile lesions, using alkaline sucrose gradients. Tables 2 and 3 show the number of DNA strand breaks per genome of the mutant and wild-type strains, immediately after treatment or after different recovery times. The results obtained with cells treated with 5 mM H2O2 indicate that the nfo strain was almost as efficient as the wild-type strain in the repair of DNA strand breaks, while the xthA strain was less efficient. On the other hand, the xthA nfo double mutant was even more deficient in the correction of H2O2-induced lesions (Table 2). This is suggested by both the initial number of DNA strand breaks and the repair kinetics, since repair was already occurring during the 20-min treatment. In contrast, the results obtained with cultures pretreated with dipyridyl (Table 3) reveal that both the xthA and nfo single mutants were proficient in the repair of strand breaks induced by H2O2, unlike the xthA nfo double mutant, which could not carry out complete repair of the DNA lesions formed under these conditions. Despite a very small difference in the initial number of DNA strand breaks, the nfo strain did not differ significantly from the wild-type strain, since both strains were able to repair completely the strand breaks 10 min after the treatment. The xthA strain was also proficient in such repair, although it needed longer periods to remove all the lesions. The small number of DNA strand breaks found in the wild type and xthA and nfo mutants after the 20-min treatment was probably due to repair during the H2O2 exposure period. In contrast, the double mutant strain retained a large number of breaks per genome after treatment. Thus, our results support the idea that both exonuclease III and endonuclease IV act in the repair of DNA damage induced by H2O2 in iron-depleted E. coli. These findings strongly suggest that the lesions formed by H2O2 in the presence of dipyridyl are qualitatively different from those formed in the absence of this iron chelator.
TABLE 2.
DNA strand breaks per genome generated by treatment with 5 mM H2O2 for 20 min and repair kinetics in different E. coli strains
| Recovery time (min) | No. of strand breaks in strain with indicated genotype
|
|||
|---|---|---|---|---|
| Wild type | xthA | nfo | xthA nfo | |
| 0 | 45 | 257 | 63 | 317 |
| 10 | 6 | 104 | 10 | 274 |
| 30 | 0 | 47 | 8 | 193 |
| 60 | 0 | 33 | 1 | 154 |
TABLE 3.
DNA strand breaks per genome generated by treatment with 1 mM dipyridyl for 20 min and then with 5 mM H2O2 for 20 min and repair kinetics in different E. coli strains
| Recovery time (min) | No. of breaks in strain with indicated genotype
|
|||
|---|---|---|---|---|
| Wild type | xthA | nfo | xthA nfo | |
| 0 | 12 | 40 | 3 | 255 |
| 10 | 0 | 9 | 0 | 125 |
| 30 | 0 | 5 | 0 | 60 |
| 60 | 0 | 0 | 0 | 85 |
It is interesting to note that despite the severe repair defect of the xthA nfo strain, significant levels of repair were observed after both treatments. The exact nature of this repair cannot be determined, but it probably reflects the action of recombination and nucleotide excision repair pathways.
Results from our group indicate that copper ions are the mediators of the H2O2 genotoxicity under low-iron conditions (C. E. B. Almeida, R. S. Galhardo, D. L. Felício, J. B. Cabral-Neto, and A. C. Leitão, unpublished data). Fenton-like reactions involving copper ions have been shown to cause extensive base damage, preferentially to guanine residues in DNA (4, 26). It was demonstrated that the Fpg and UvrA proteins are important for the repair of lesions generated by singlet oxygen, a well-known guanine-damaging agent (1, 13), and members of our group demonstrated that these proteins also participate in the repair of the lesions induced by H2O2 in the presence of iron chelators (5). Taken together, these facts suggest that H2O2 can cause extensive guanine oxidative damage under low-iron conditions, which led us to investigate the role of the Fpg protein in the generation of substrates for exonuclease III and endonuclease IV.
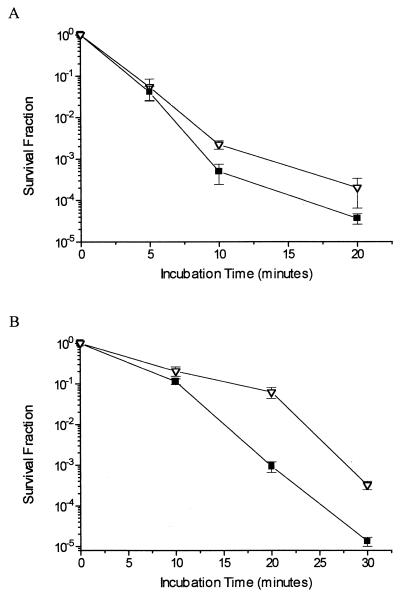
In Fig. 2 are represented the results of survival experiments performed with xthA nfo and xthA nfo fpg mutant strains. In the experiments shown in Fig. 2A, cells were treated with 5 mM H2O2, and in the experiments shown in Fig. 2B, cells were pretreated with 1 mM dipyridyl and then treated with 5 mM H2O2. It should be noted that for Fig. 2B, cells were treated for longer periods (30 min), in order to achieve the same survival level (10−5) for the xthA nfo strain. It is clear that the fpg mutation had only a minor effect, if any, on the viability of AP endonuclease-null cells after H2O2 treatment in the absence of dipyridyl. Nevertheless, this mutation significantly increased resistance of AP endonuclease-null cells to the H2O2 treatment under low-iron conditions, suggesting that under these conditions the Fpg protein generates significant amounts of substrates (i.e., abasic sites) for exonuclease III and endonuclease IV. Longer incubation times with H2O2 were used in cells pretreated with dipyridyl to achieve the same survival level as occurred in experiments with xthA nfo cells without pretreatment with iron chelators.
FIG. 2.
Survival of E. coli xthA nfo and xthA nfo fpg mutant strains. Cultures in the mid-exponential phase of growth in M9S medium were treated with 5 mM H2O2 for the indicated times (A) or pretreated with 1 mM dipyridyl for 20 min and then treated with 5 mM H2O2 for the indicated times (B). Values are means ± standard errors. ■, xthA nfo strain; ▿, xthA nfo fpg strain.
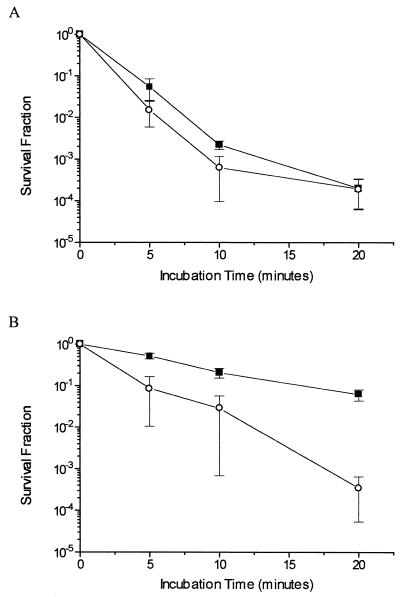
This phenomenon became more evident in survival experiments with the xthA nfo fpg strain carrying the multicopy fpg-expressing plasmid pFPG60. In the experiments shown in Fig. 3A, in which cells were treated with 5 mM H2O2, overproduction of the Fpg protein had no effect upon cell viability after challenge. However, in cultures pretreated with dipyridyl, the presence of the Fpg-overproducing plasmid caused a remarkable increase in the sensitivity to H2O2 (Fig. 3B), confirming that this protein can generate lethal lesions that cannot be repaired in an AP endonuclease-null background.
FIG. 3.
Survival of E. coli BH110, bearing or not bearing the pFPG60 plasmid. Cultures in the mid-exponential phase of growth in M9S medium were treated with 5 mM H2O2 for the indicated times (A) or pretreated with 1 mM dipyridyl for 20 min and then treated with 5 mM H2O2 for the indicated times (B). Values are means ± standard errors. ■, xthA nfo fpg strain; ○, xthA nfo fpg strain transformed with pFPG60.
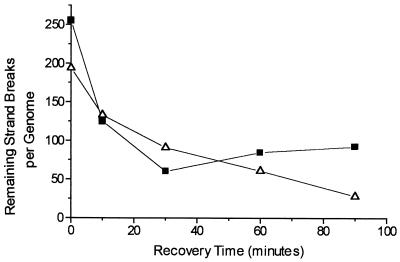
The kinetics of DNA strand break repair in the xthA nfo and xthA nfo fpg strains treated with 1 mM dipyridyl and then with 5 mM H2O2 is shown in Fig. 4. It is noteworthy that an increase in the number of DNA strand breaks occurred after 30 min of recovery in the double mutant strain, suggesting that excision repair of some lesions was initiated but could not be completed. This phenomenon did not occur in the triple mutant strain, strongly suggesting that such excision repair is mediated by the Fpg glycosylase, which removes oxidized purines and leaves abasic sites or DNA strand breaks resulting from AP lyase activity.
FIG. 4.
Kinetics of repair of DNA strand breaks in E. coli BW544 and BH110 pretreated with 1 mM dipyridyl for 20 min and then treated with 5 mM H2O2 for 20 min. Cells were allowed to recover from DNA damage in M9S medium at 37°C with shaking for the indicated times. Values are means ± standard errors. ■, xthA nfo strain; ▵, xthA nfo fpg strain.
DISCUSSION
Our results show that both endonuclease IV and exonuclease III participate in the repair of lesions induced by H2O2 under low-iron conditions, unlike what is observed in the correction of damage induced by H2O2 under physiological iron conditions, where the former plays only a minor role. Such a difference in the repair suggests a qualitative difference in the formation of DNA lesions whether iron is present or not. This hypothesis is further supported by the observation that the participation of endonuclease IV cannot be attributed merely to the induction of nfo gene expression concomitantly with the soxRS regulon, since experiments with strains carrying a soxS′::lacZ fusion failed to detect any induction by the iron chelator (data not shown).
Our results also point to a significant participation of the Fpg enzyme in the repair of DNA lesions formed by H2O2 under low-iron conditions. This DNA glycosylase clearly mediates the formation of secondary lesions that can be repaired with almost equal efficiency by exonuclease III and endonuclease IV. The determination of the exact nature of these substrates for 5′ AP endonucleases generated by Fpg in vivo, as well as the nature of endonuclease IV-specific damage (18), may contribute substantially to our understanding of the substrate specificity of endonuclease IV and exonuclease III. One candidate is the one-nucleotide gap bordered by 5′ and 3′ phosphoryl groups left by the β-δ-elimination catalyzed by the Fpg protein (24). Another still unresolved matter is the nature of the guanine oxidation products generated by H2O2 under low-iron conditions. The major products formed must at least have a lower potential for lethality than those resulting from the Fpg glycosylase activity, since the absence of this enzyme renders AP endonuclease-null cells more resistant to this treatment.
It is interesting to note that other agents, namely, gamma rays (12) and singlet oxygen (1), generate oxidative DNA lesions that are equally repaired in xthA and nfo mutant strains but not in xthA nfo strains. Furthermore, both agents were reported to induce large amounts of base damage to guanine residues (9). Therefore, it is possible that the repair of oxidative DNA lesions initiated by the Fpg enzyme may require only the xthA or the nfo gene product for its completion.
ACKNOWLEDGMENTS
This work was supported by PRONEX, CNPq, FINEP, CEPG-UFRJ, and FAPERJ.
We thank J. S. Cardoso and A. B. Silva for their expert technical support.
REFERENCES
- 1.Agnez R, Costa de Oliveira R, Di Mascio P, Menck C F M. Involvement of Escherichia coli exonuclease III and endonuclease IV in the repair of singlet oxygen-induced DNA damage. Carcinogenesis. 1996;17:1183–1185. doi: 10.1093/carcin/17.5.1183. [DOI] [PubMed] [Google Scholar]
- 2.Ananthaswamy H N, Eisenstark A. Repair of hydrogen peroxide-induced single-strand breaks in Escherichia coli deoxyribonucleic acid. J Bacteriol. 1977;130:187–191. doi: 10.1128/jb.130.1.187-191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson E H. Growth requirements of virus resistant mutants of Escherichia coli strain B. Proc Natl Acad Sci USA. 1964;32:120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aruoma O I, Halliwell B, Gajewski E, Dizdaroglu M. Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem J. 1991;273:601–604. doi: 10.1042/bj2730601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asad N R, Almeida C E B, Asad L M B O, Felzenswalb I, Leitão A C. Fpg and UvrA proteins participate in the repair of DNA lesions induced by hydrogen peroxide in low iron level in Escherichia coli. Biochimie. 1995;77:262–264. doi: 10.1016/0300-9084(96)88134-x. [DOI] [PubMed] [Google Scholar]
- 6.Asad N R, Leitão A C. Effects of metal ion chelators on DNA strand breaks and inactivation produced by hydrogen peroxide in Escherichia coli: detection of iron-independent lesions. J Bacteriol. 1991;173:2562–2568. doi: 10.1128/jb.173.8.2562-2568.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barzilay G, Hickson I D. Structure and function of apurinic/apyrimidinic endonucleases. Bioessays. 1995;17:713–719. doi: 10.1002/bies.950170808. [DOI] [PubMed] [Google Scholar]
- 8.Bennett R A O. The Saccharomyces cerevisiae ETH1 gene, an inducible homolog of exonuclease III that provides resistance to DNA-damaging agents and limits spontaneous mutagenesis. Mol Cell Biol. 1999;19:1800–1809. doi: 10.1128/mcb.19.3.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boiteux S, Gajewski E, Laval J, Dizdaroglu M. Substrate specificity of the Escherichia coli Fpg protein (formamidopyrimidine-DNA glycosylase): excision of purine lesions in DNA produced by ionizing radiation or photosensitization. Biochemistry. 1992;31:106–110. doi: 10.1021/bi00116a016. [DOI] [PubMed] [Google Scholar]
- 10.Boiteux S, O'Connor T R, Laval J. Formamidopyrimidine-DNA-glycosylase of Escherichia coli: cloning and sequencing of the fpg structural gene and overproduction of the protein. EMBO J. 1987;6:3177–3183. doi: 10.1002/j.1460-2075.1987.tb02629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boiteux S, O'Connor T R, Lederer F, Gouyette A, Laval J. Homogeneous Escherichia coli FPG protein. J Biol Chem. 1990;265:3916–3922. [PubMed] [Google Scholar]
- 12.Cunningham R P, Saporito S M, Spitzer S G, Weiss B. Endonuclease IV mutant of Escherichia coli. J Bacteriol. 1986;168:1120–1127. doi: 10.1128/jb.168.3.1120-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czeczot H, Tudek B, Lambert B, Laval J, Boiteux S. Escherichia coli Fpg protein and UvrABC endonuclease repair DNA damage induced by methylene blue plus visible light in vivo and in vitro. J Bacteriol. 1991;173:3419–3424. doi: 10.1128/jb.173.11.3419-3424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demple B, Halbrook J, Linn S. Escherichia coli xth mutants are hypersensitive to hydrogen peroxide. J Bacteriol. 1983;153:1079–1082. doi: 10.1128/jb.153.2.1079-1082.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demple B, Harrison L. Repair of oxidative damage to DNA: enzymology and biology. Annu Rev Biochem. 1994;63:915–948. doi: 10.1146/annurev.bi.63.070194.004411. [DOI] [PubMed] [Google Scholar]
- 16.Imlay J A, Chin S M, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 17.Johnson R E, Torres-Ramos C A, Izumi T, Mitra S, Prakash S, Prakash L. Identification of APN2, the Saccharomyces cerevisiae homolog of the major human AP endonuclease HAP1, and its role in the repair of abasic sites. Genes Dev. 1998;12:3137–3143. doi: 10.1101/gad.12.19.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin J D, Demple B. In vitro detection of endonuclease IV-specific DNA damage formed by bleomycin in vivo. Nucleic Acids Res. 1996;24:885–889. doi: 10.1093/nar/24.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley R D. Postreplication repair in an excision-defective mutant of Escherichia coli: ultraviolet light-induced incorporation of bromodeoxyuridine into parental DNA. Photochem Photobiol. 1973;18:87–95. doi: 10.1111/j.1751-1097.1973.tb06397.x. [DOI] [PubMed] [Google Scholar]
- 20.Ljungquist T, Lindahl T, Flanders P H. Methyl methanesulfonate-sensitive mutant of Escherichia coli deficient in an endonuclease specific for apurinic sites in deoxyribonucleic acid. J Bacteriol. 1976;126:646–653. doi: 10.1128/jb.126.2.646-653.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luria S E, Borrous J W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957;74:461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacGrath R A, Williams R W. Reconstruction “in vivo” of irradiated Escherichia coli deoxyribonucleic acid, the rejoining of broken pieces. Nature. 1966;212:532–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- 23.Nunoshiba T, DeRojas-Walker T, Tannenbaum S R, Demple B. Roles of nitric oxide in inducible resistance of Escherichia coli to activated murine macrophages. Infect Immun. 1995;63:794–798. doi: 10.1128/iai.63.3.794-798.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connor T R, Laval J. Physical association of the 2,6-diamino-4-hydroxy-5-N-formamidopyrimidine DNA glycosylase of E. coli and an activity nicking DNA at apurinic/apyrimidinic sites. Proc Natl Acad Sci USA. 1989;86:5222–5226. doi: 10.1073/pnas.86.14.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popoff S C, Spira A I, Johnson A W, Demple B. Yeast structural gene (APN1) for the major apurinic endonuclease: homology to Escherichia coli endonuclease IV. Proc Natl Acad Sci USA. 1990;87:4193–4197. doi: 10.1073/pnas.87.11.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sagripanti J L, Kraemer K H. Site-specific oxidative DNA damage at polyguanosines produced by copper plus hydrogen peroxide. J Biol Chem. 1989;264:1729–1734. [PubMed] [Google Scholar]
- 27.Tchou J, Kasai H, Shibutani S, Chung M H, Laval J, Grollman A P, Nishimura S. 8-Oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specificity. Proc Natl Acad Sci USA. 1991;88:4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]