Abstract
Background
S100 calcium-binding protein A9 (S100A9) is a danger-associated molecular pattern molecule that mediates the inflammatory response. Inflammation is essential in aging-related cardiovascular diseases. However, less is known regarding the role of S100A9 in vascular aging.
Methods
S100A9 null mice were used to investigate the role of S100A9 in aging-related pathologies. Artery rings were used to measure the functional characteristics of vascular with a pressurized myograph. Telomere length, Sirtuin activity, oxidative stress, and endothelial nitric oxide synthetase (eNOS) activity were used to elevate vascular senescence. Intraperitoneal glucose tolerance (IPGTT) and insulin sensitivity test (IST) were employed to investigate the effects of S100A9 on insulin resistance. Inflammation response was reflected by the concentration of inflammatory cytokines. The Toll-like receptor 4 (TLR4) and receptor for advanced glycation end products (RAGE) inhibitors were used to identify the downstream molecular mechanisms of S100A9 in aging-induced senescence in endothelial cells.
Results
S100A9 expression in vascular increased with aging in mice and humans. Deficiency of S100A9 alleviated vascular senescence in aged mice, as evidenced by increased telomere length, Sirtuin activity, and eNOS activity. Meanwhile, S100A9 knockout improved endothelium-dependent vasodilatation and endothelial continuity in aged mice. Moreover, the increased insulin resistance, oxidative stress, and inflammation were mitigated by S100A9 deletion in aged mice. In vitro, S100A9 induced senescence in endothelial cells, and that effect was blunted by TLR4 but not RAGE inhibitors.
Conclusion
The present study suggested that S100A9 may contribute to aging-related pathologies and endothelial dysfunction via the TLR4 pathway. Therefore, targeting S100A9/TLR4 signaling pathway may represent a crucial therapeutic strategy to prevent age-related cardiovascular diseases.
Keywords: S100A9, Aging, Vascular senescence, Oxidative stress, Insulin resistance
Highlights
-
•
S100A9 contributed to aging-related pathologies, such as insulin resistance, oxidative stress and inflammation.
-
•
Deficiency of S100A9 alleviated endothelial dysfunction and improved vascular function in aged mice.
-
•
TLR4 pathway was involved in the regulation of S100A9-induced cellular senescence in human aortic endothelial cells.
1. Introduction
Life expectancy is increasing due to improved sanitation, diet, and healthcare. The elderly population (65 years or older) is predicted to rise to 1.5 billion by 2050 [1]. Cardiovascular diseases are the leading cause of death in the elderly population, accounting for 40% of all deaths of the elderly [2]. Aging is an inevitable, independent risk factor for developing cardiovascular disease [3]. However, how aging leads to the occurrence of cardiovascular, especially how aging and cardiovascular disease interact, remains unanswered.
Strong evidence supports that the mechanisms driving aging and cardiovascular disease are interconnected, even overlap. The established mechanisms underlying aging, such as increased inflammation, oxidative stress, metabolic dysfunction, and decreased regeneration, contribute to the pathologic process of cardiovascular together [4,5]. Inflammation is increasingly considered the key factor. Inflammation function as a driver, leading to excessive oxidative stress and metabolic dysfunction [6,7]. Simultaneously, these impairments also fuel inflammation aggravating aging-relegated pathologies [8]. Previous studies revealed that inhibiting inflammation via targeting NOD-like receptor thermal protein domain associated protein 3 (NLRP3) or Toll-like receptor 4 (TLR4) pathway alleviated cardiovascular dysfunction during aging [9,10],indicating the essential role of inflammation in aging-related cardiovascular diseases. Therefore, understanding the mechanisms linking inflammation and cardiovascular dysfunction during aging may help reduce cardiovascular disease occurrence in the elderly and improve lifespan.
Aging-related inflammation occurs without infection, also called sterile inflammation [11]. It is accepted that age-related inflammation is initiated by gradual deterioration and immune system dysfunction during aging [12]. Unlike infection-induced inflammation, aging-related inflammation is characterized by low-grade regulation of cytokines, such as interleukin-1β (IL-1β) and TNF-α [13]. The mechanisms explaining inflammation activation in aging are still unidentified. Many cell processes, such as mitochondrial dysfunction, unbalanced proteolysis, activation of DNA damage response, and metabolic dysfunction, trigger inflammation in aging [14,15]. Additionally, almost all components of an immune system undergo substantial remodeling during aging [16], which may be the key to initiating inflammation in aging.
S100A9 belongs to the S100 protein subfamily and is a calcium- and zinc-binding protein [17]. S100A9 predominantly expresses in neutrophils and monocytes and functions as an alarmin or a danger-associated molecular pattern molecule [18]. S100A9 can activate the MAP-kinase and NF-ĸB signaling pathways via binding to TLR4 and receptors for advanced glycation end products (RAGE) [19,20], thus playing a prominent role in regulating the immune response. Our previous study discovered that S100A9 aggravated lung injury in septic mice [21]. The high expression has also been identified in some cardiovascular diseases [22], such as myocardial infarction and ischemic/repair injury. S100A9 blockage protects the heart from myocardial infarction and ischemic/repair injury [23,24]. Additionally, S100A9 induces senescence in the mesenchymal stromal cell [25]. However, the effects of S100A9 in aging and aging-related cardiovascular dysfunction have not been previously reported.
Various aging-related cardiovascular diseases are linked to impaired vasculature, including hypertension, atherosclerosis, and stroke [3]. The vasculature undergoes continuous remodeling in function and structure during aging [26]. The aged vasculature exhibits an enlarged lumen, increased wall thickening, and stiffness [27]. Endothelial dysfunction plays a prominent role in vasculature aging. Impaired endothelial barriers lead to extracellular matrix accumulation and intimal thickening [28].The endothelial nitric oxide synthetase (eNOS) produces nitric oxide (NO), mediating vasodilatation and inhibiting inflammation and antithrombus [29]. Reduced eNOS activity in the endothelial cell with aging leads to decreased NO production and vascular dysfunction [30]. It has been evidenced that inflammation inhibits eNOS expression and activity [31]. Given the important role of S100A9 in mediating inflammation, we speculated that S100A9 might induce endothelial dysfunction during aging.
In the present study, we aim to investigate the role of S100A9 in aging, focusing on age-related vascular dysfunction. We also investigate the influence of S100A9 on oxidative stress and inflammation pathway to explore the possible underlying mechanism further.
2. Methods
2.1. Animals
S100A9 knockout mice were constructed by Cyagen Biosciences Inc. (Suzhou, China) by CRISPR/Cas-mediated genome engineering. Polymerase chain reaction (PCR) was used to identify the genotyping of mouse offspring. The following primers are used: Primer 1, F1: 5′-CAAAGTCCTAGTGCCCACGGC-3′ R1: 5′-GTGAAAGGAGGCAGAAAGGACATG-3’. Primer 2, F2: 5′-GTATATGTGGAGGGAAGCTGTCTC-3′ R2: 5′-GTGAAAGGAGGCAGAAAGGACATG-3’; Homozygotes mice had one band with 339 bp, wild-type mice had one band with 557 bp, and heterozygotes mice had two bands with 339 bp and 557 bp. Male homozygotes and wild-type mice were used for experiments, and the genotyping results were showed in Fig. S1. All mice were maintained in C57BL/6 N background and reared on a 12 h/12 h light/dark cycle with standard chow and water available ad libitum. 3-month-old mice were defined as the Young group and 24-month-old mice were defined as the Aged group. At the end of the experiments, blood samples were collected and centrifuged at 3000 rpm for 10 min at room temperature to collect the serum as described previously; Then, the serum was stored at −80 °C until analysis. Vascular tissue was quickly isolated, snap-frozen in liquid nitrogen, and stored at −80 °C until analysis. All animal experiments were performed in accordance with the National Institutes of Health Guidelines for the Use of Laboratory Animals. All the study protocols were approved by the Institutional Animal Care and Use Committee of Chongqing Medical University.
2.2. Insulin sensitivity and glucose tolerance test
Insulin (0.5 U/Kg) was injected hypodermically after 8 h of fasting to test the insulin sensitivity (IST). Then, the blood glucose was measured at 0, 30, 60, 90, 120, and 240 min. An intraperitoneal glucose tolerance test (IPGTT) was performed to test the glucose tolerance of mice. After 8 h of fasting, mice were injected intraperitoneally with 2 g/kg glucose. Afterward, blood glucose was measured at 0, 15, 30, 60, 90, and 120 min using tail clippings.
2.3. Vascular functional parameters
The vasodilation function of the aorta was measured using a wire myograph as previously [32]. After isolation, 2–3 mm-length arterial rings were mounted into cold oxygenated PBS buffer and slowly heated to 37 °C. The aortic ring was allowed to equilibrate for approximately 75 min, and KCL(80 mM) and norepinephrine (1 μM) were used to contract the ring and assess its vitality. After reaching a plateau, the ring was washed and left quiescent for 45 min before functional measurement. The endotheliun-dependent relaxation was measured by cumulative concentration response to acetylcholine (10−4-10−9 mM) (Sigama, Milano, Italy). Aortic rings were incubated with 100 μM l-NAME (Sigama, Milano, Italy) for 30 min before the dose-response curves to acetylcholine to investigate the role of NO availability in vascular relaxation function. The vasodilation function was described as % change to the maximal vessel diameter.
2.4. Immunohistochemical (IHC) staining
Mice were anesthetized with 5% isoflurane; The aorta was excised and fixed with 4% paraformaldehyde. The human aorta tissues were obtained during the aortic repair surgery in trauma patients from the Department of Cardiothoracic Surgery, Chongqing University Central Hospital, or the Department of Forensic Medicine, College of Basic Medicine, Chongqing Medical University during the medical forensic examinations. Participants or their patients’ family members were informed and they provided consent before the inclusion of these samples in the study. Additionally, the experiments conformed to the principles outlined in the Declaration of Helsinki and the Institutional Ethics Committee of Chongqing University Central Hospital approved all the study protocols. After embedding in paraffin, 7-μm-thick cross-sections were sided and used for IHC analysis. After de-paraffinized and antigen retrieval, the sections were blocked with 10% goat serum and incubated with primary antibodies probing to S100A9 (1: 200) and CD31 (1: 200) at 4 °C overnight, followed by responding secondary antibodies for 60 min at 25 °C. Subsequently, a DAB Substrate Kit (Wuhan Servicebio Biotechnology Co., Ltd., China) was used for the color reaction. Finally, images were captured using a Leica BM4 microscope (Leica Microsystems, Inc., USA) and then quantified using ImageJ software with IHC-Toolbox plug-in according to the mean gray value.
2.5. Western blot
Treated cells or aortic tissues were lysed with RIPA buffer supplemented with protease inhibitor cocktails to access total protein expression or phosphorylation (Beyotime Institute of Biotechnology, China). The aortic tissue nuclei were extracted using a nuclear extraction kit purchased from the Beyotime Institute of Biotechnology to access nuclear protein expression. After centrifugation at 13,000×g at 4 °C for 15 min, the supernatants were collected for subsequent experiments. The protein concentration was measured using BCA assay kits, and an equal amount of proteins were diluted into 5x loading buffer and heated at 100 °C for 10 min. The protein samples were separated using 4–12% gradient gels and MOPS-SDS running buffer provided by Nanjing ACE Biotechnology, then transferred to the PVDF membrane. After blocking with 5% skimmed milk in TBST for 90 min at 20–25 °C, the blots were incubated with primary antibody overnight at 4 °C. After washing with TBST, blots were incubated with corresponding secondary antibodies for 60 min at 20–25 °C. Finally, an ECL reagent was used to detect the protein bands with a gel imaging system (Bio-Rad Laboratories, Inc., USA). Band densities were quantified via Image Lab software (Bio-Rad, USA). The antibodies used in the present experiment are as follows: S100A9 (ab63818), γH2AX phospho S139 (ab22551) (Abcam, UK), (p)-P38 (28796-1-AP), P38 (14064-1-AP), (p)-P65 (82335-1-RR), P65 (80979-1-RR), P21 (28248-1-AP), P53 (60283-2-Ig), NOX1 (17772-1-AP), Histone H3 (68345-1-Ig), GAPDH (60004-1-Ig) (ProteinTech Group, Inc., China), (p)-eNOS (9571), and eNOS (32,027) (Cell Signaling Technology, Inc., USA).
2.6. Enzyme-linked immunosorbent assay (ELISA)
Mice were anesthetized with isoflurane, and blood was obtained via puncture. After centrifugation at 3000 rpm for 10 min at 25 °C, the supernatant was collected as serum and stored at −80 °C until analysis. IL6, IL-1β, and TNFα concentrations were measured using the commercial ELISA Kit (ExCell Bio, Inc., Jiangsu, China) according to the manufacturer's instructions.
2.7. Telomere length measurement
Relative average telomere length was measured using RT-PCR based on the ratio of telomeric repeats and single gene (T/S ratio) as described previously. The primer sequences are as follows: telomere-specific forward, 5′-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3′, telomere-specific reverse, 5′-GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT -3’; acidic ribosomal phosphoprotein PO forward, 5′-ACTGGTCTAGGACCCCGAGAAG-3′, acidic ribosomal phosphoprotein PO reverse, 5′-TCAATGGTGCCTCTGGAGATT-3’.
2.8. Cell culture and treatment
Human aortic endothelial cells (HAECs) were used to assess the direct effects of S100A9 on cellular senescence. HAECs were purchased form rocell Life Science&Technology Co.,Ltd and cultured with ECM containing 5% FBS, 1% endothelial cell growth supplement, and 1% penicillin/streptomycin solution (ScienCell Research Laboratories, Inc.) at 37 °C in a 5% CO2 atmosphere. HAECs were treated with different concentrations of S100A9 (100, 500 and 1000 nM, MedChemExpress, NJ, USA) for 12 h; 50 nM FPS-ZM1 and 5 μM IAXO-102 (MedChemExpress, USA) were used to inhibit the RAGE and TLR4 pathway, respectively, to investigate the role of TLR4 and RAGE on the effects of S100A9. Cell viability was examined by MTT measurement to test the cytotoxic effects of S100A9 and TLR4 or RAGE inhibitors on HAECs. Cell viability was calculated as a percentage of that of control group (Fig. S2). Cell senscence was detected with Senescence associated β-galactosidase (SA-β-Gal) with the Senescence β-Galactosidase Staining Kit (Beyotime Biotechnology, China) according to the manufacturer's instruction. In brief, cells were fixed with a fixative solution for 15 min after washing with PBS. After that, cells were washed with PBS for three times and incubated in staining working solution at 37 °C overnight. The number of SA-β-Gal positive cells were quantified by ImageJ with three different fields, then averaged and presented as percentage.
2.9. Sirtuin and NADPH oxidative activities and NO and MDA content measurement
After extracting the nucleus from HAECs or aortic tissues, the Sirtuin activity was measured using the Epigenase TM Universal SIRT activity assay kit according to the manufacturer's instructions. The NADPH oxidative activity and malondialdehyde (MDA) content in tissue and serum were measured using corresponding assay kits (Nanjing Jiancheng Bioengineering Institute, China). NO content was measured using a total NO assay kit (Beyotime Institute of Biotechnology) as described previously.
2.10. Statistical analysis
The results were presented as mean +SEM. All statistics were performed using GraphPad Prism version 9.0 software (San Diego, CA, USA). The data were analyzed using Student's t-test, one- or two-way ANOVA, followed by Tukey's multiple comparisons tests where appropriate. A P-value <0.05 was considered statistically significant.
3. Results
3.1. S100A9 expression increased in the aged aortas of mice and human
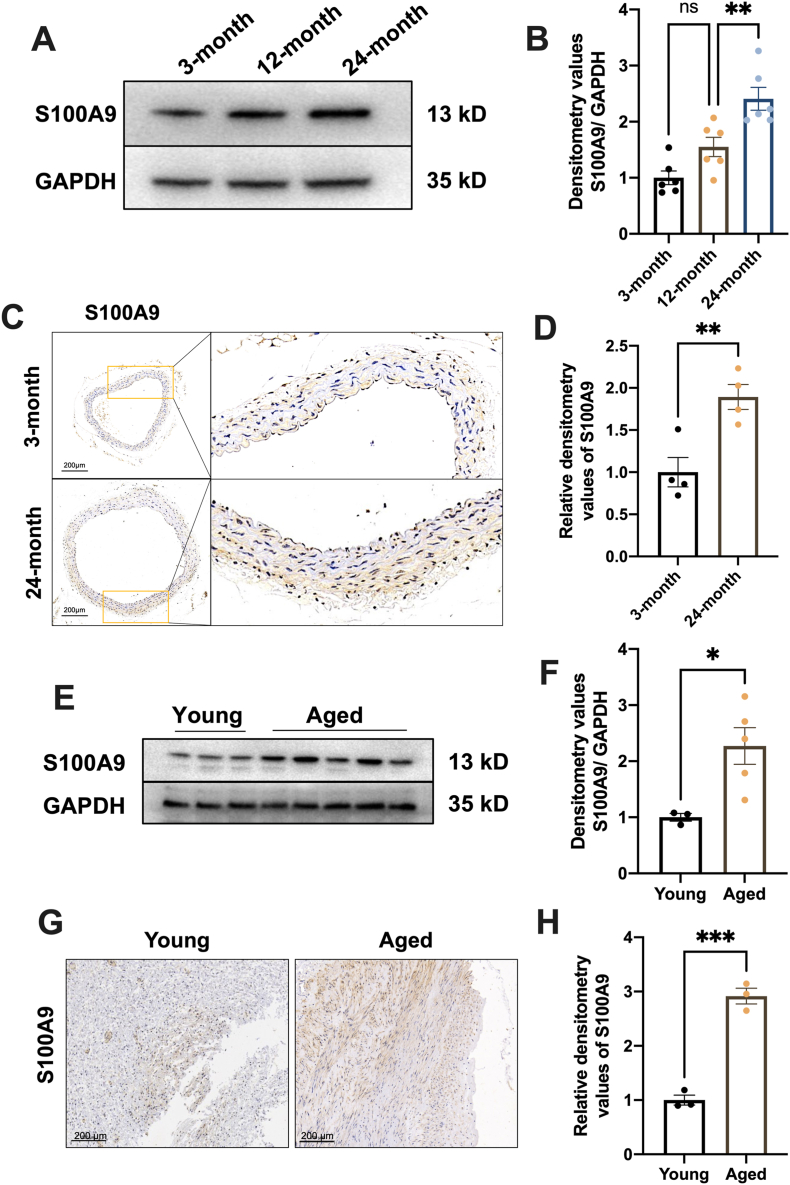
We measured the S100A9 protein expression in mice aorta at different ages to investigate the relation between S100A9 expression and vascular senescence. The results revealed that S100A9 expression increased by 1.5 and 2.4 fold change at 12 and 24 months, respectively, than two months (Fig. 1A and B). The IHC also confirmed the increased S100A9 expression in aged mice aorta (Fig. 1C and D). These data indicated an age-dependent increase of S100A9 expression in the aorta as physiological aging. Furthermore, the S100A9 protein expression was examined in the human vascular from different ages to elevate whether the increased S100A9 expression exists in humans. Consistently, the Western Blots (Fig. 1E and F) and IHC (Fig. 1G and H) results indicated increased S100A9 expression in the aged group (>60 years) than young group (<40 years), suggesting involvement of S100A9 in the aging of human.
Fig. 1.
S100A9 expression increased in the aged aortas of mice and humans. (A) S100A9 expression in the aortas of mice at 3-, 12- and 24-month was measured using Western blots. The statistical results of S100A9 were presented in (B) (n = 6). (C) S100A9 expression in mice aortas was measured using IHC at 3- and 24-month and the related statistical results were displayed in (D) (n = 4). (E) S100A9 expression in young and aged human aortas was measured using Western blots. (F) The statistical results of blots in E (young group, <40 years, n = 3; aged group, >60 years, n = 5). (G) Representative IHC images of S100A9 expression in the aorta of young and aged humans and the related statistical results were depicted in (H) (n = 3). ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
3.2. S100A9 knockout alleviates vascular senescence in aged mice
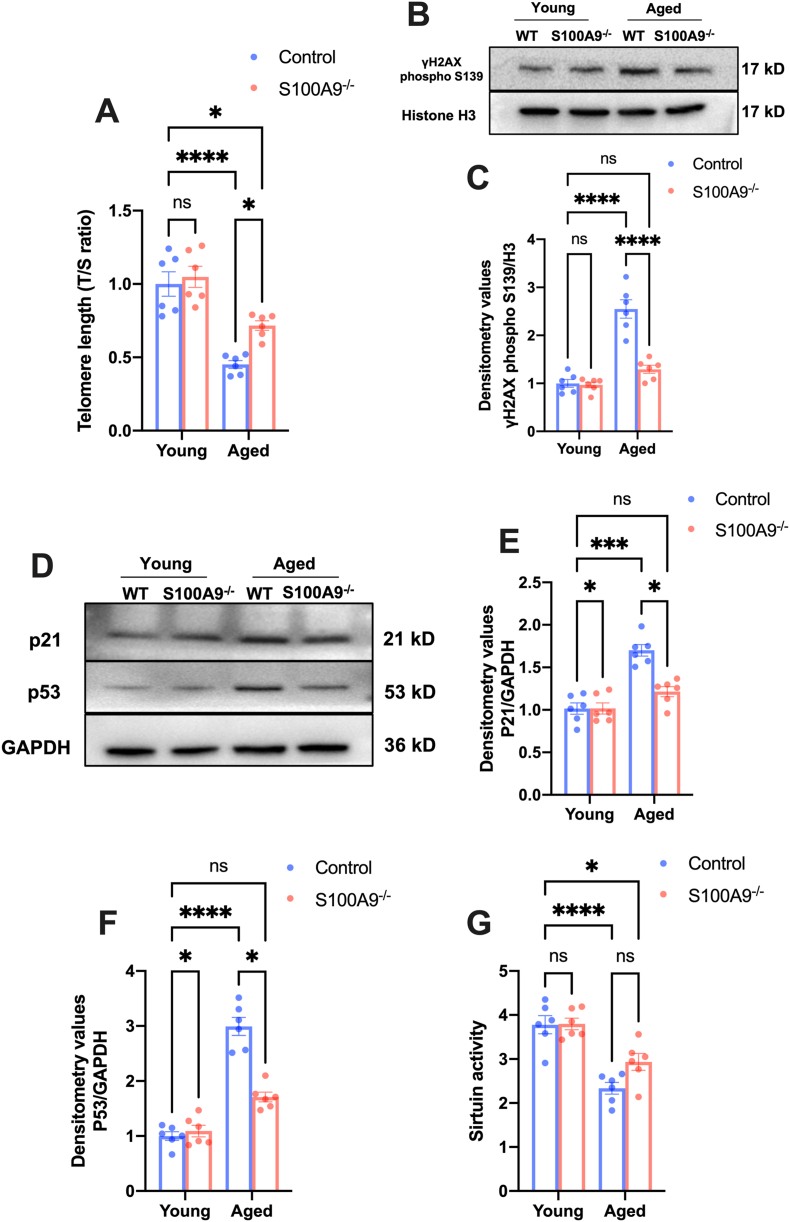
We compared the senescence phenotype in S100A9 null and wildtype mice at 24-months to identify the role of S100A9 in vascular aging. Initially, the telomere length decreased in the vascularity of aged mice and the loss of S100A9 increased the telomere length in aged mice (Fig. 2A). The telomere shortening leads to H2AX phosphorylation at the serin 139 site and DNA damage, followed by p53 and p21 activation, resulting in pathologies during aging [33]. Consistently, loss of S100A9 reduced the γH2AX level (Fig. 2B and C) as well as the p53 and p21 (Fig. 2D, E, and F) expression in the aged vascular. Moreover, the reduced Sirtuin activity is a maker of aging. Loss of S100A9 significantly increased the Sirtuin activity in aged mice aorta (Fig. 2G). All results indicated that increased S100A9 is associated with age-dependent pathologies, and loos of S100A9 alleviate vascular senescence in aged mice.
Fig. 2.
S100A9 knockout alleviates vascular senescence in aged mice. (A) S100A9 knockout increased as evidenced by the increased ratio of telomeric repeats with single-copy gene (T/S ratio) (n = 6). (B) Representative Western blots of Phospho-γH2AX expression in nuclear. The statistical results were illustrated in (C) (n = 6). (D) Representative Western blots of p21 and p53 in mice aortas of different ages. The related statistical results were exhibited in (E) and (F) (n = 6). (G) S100A9 knockout increased the Sirtuin activity in aged mice aorta (n = 6). ns, not significant. *P < 0.05, ***P < 0.001, ****P < 0.0001.
3.3. S100A9 knockout improves vascular function in aged mice
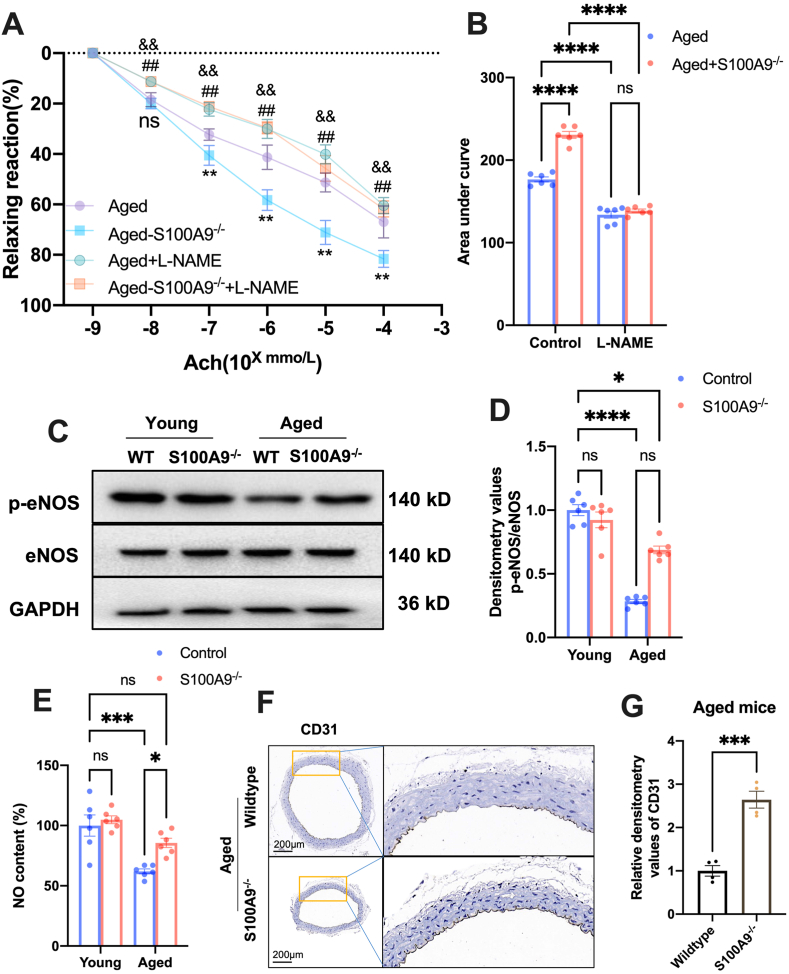
Next, we explored the effects of S100A9 on aged-induced vascular dysfunction. The results demonstrated that the endothelium-dependent vasodilatation induced by Ach was reduced in aged mice, whereas S100A9 knockout significantly improved vascular relaxation (Fig. 3A and B). Moreover, when pretreating with eNOS inhibitor, l-NAME, aged wildtype, and S100A9 null mice did not differ statically (Fig. 3A and B). These data suggested that the effects of S100A9 on vasodilatation were NO-dependent. Consistently, the results revealed that eNOS phosphorylation and NO production were markedly reduced in the aging vascular. However, S100A9 knockout significantly improved eNOS phosphorylation and NO production (Fig. 3C–E). Additionally, we discovered that endothelial cell marker expression, CD31, is reduced and the endothelial cell was discontinuous in aged vascular. Loss of S100A9 elevated the CD31 expression and continuity in the vascularity of aged mice (Fig. 3F and G).
Fig. 3.
S100A9 knockout improves vascular function in aged mice. (A) S100A9 knockout improved endothelium-dependent vasodilatation induced by Ach in aged mice (n = 6). (B) The area under curve of different groups (n = 6). (C) Representative Western blots of Phospho-eNOS in mice aortas of different ages. The related statistical results were shown in (D) (n = 6). (E) S100A9 knockout increased the NO content in the aged mice aorta (n = 6). (F) Representative IHC images of CD31 in the aorta of wild-type and S100A9 null-aged mice and the related statistical results were presented in (G). ns, not significant. *P < 0.05, ***P < 0.001, ****P < 0.0001. ^ ^P < 0.01 Aged vs. Aged-S100A9−/−. ##P < 0.01 Aged + l-NAME vs. Aged. &&P < 0.01 Aged-S100A9−/− + L-NAME vs. Aged-S100A9−/−.
3.4. S100A9 knockout improves insulin sensitivity in aged mice
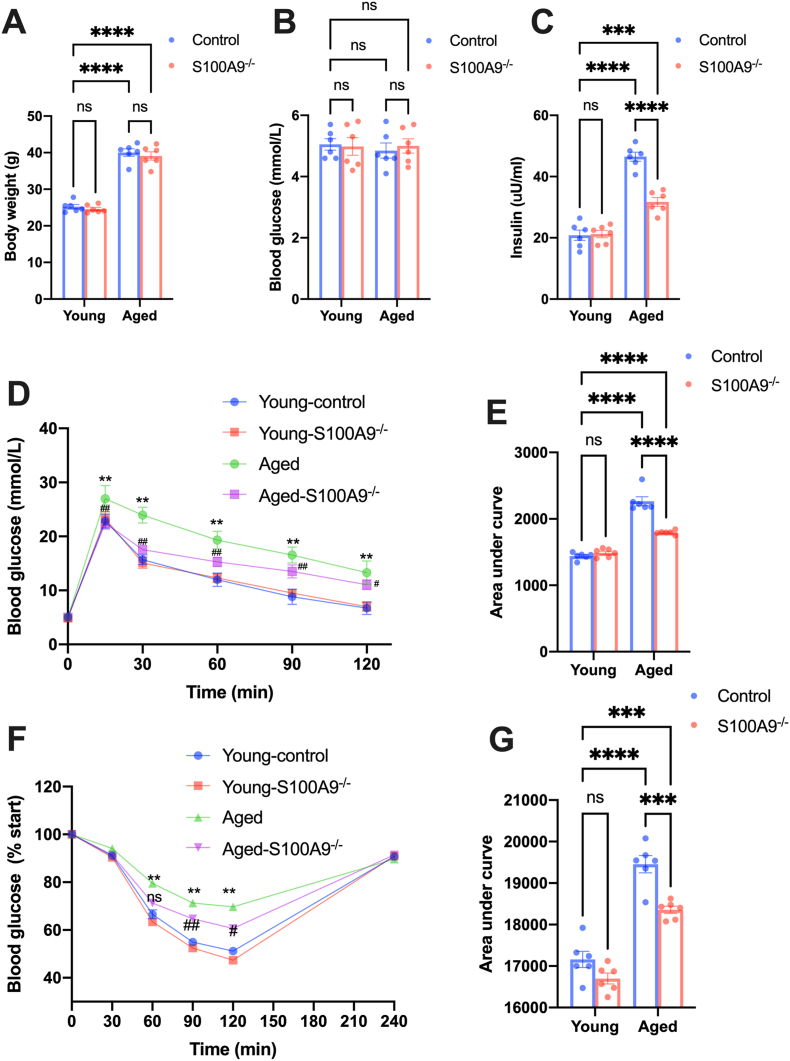
Insulin resistance is responsible for many age-dependent pathologies, including cardiovascular dysfunction [34]. Therefore, we investigated the effects of S100A9 on insulin resistance. Although body weight and fasting glucose was comparable between groups (Fig. 4A and B), the insulin level was significantly increased in aged mice serum, and S100A9 knockout blunted the increased insulin level (Fig. 4C). Moreover, aged mice had higher blood glucose levels after exogenous glucose administration (Fig. 4D and E) and lower blood glucose levels after exogenous insulin stimulation (Fig. 4F and G), indicating impaired insulin sensitivity and increased insulin resistance. S100A9 knockout significantly improved the whole-body insulin sensitivity, as evidenced by IPGTT and IST results (Fig. 4D–G).
Fig. 4.
S100A9 knockout improves insulin sensitivity in aged mice. (A–C) The body weight (A) fasting blood glucose (B) and serum insulin level (C) in mice from different groups (n = 6). (D) The results of the intraperitoneal glucose tolerance test (IPGTT) in mice from different groups. (E) The area under curve of IPGTT results from different groups (n = 6). (F) The results of insulin sensitivity test (IST) in mice from different groups. (G) The area under curve of IST results from different groups (n = 6). ns, not significant. *P < 0.05, ***P < 0.001, ****P < 0.0001. &&P < 0.01 Aged vs. Young-control. #P < 0.05, ##P < 0.01 Aged-S100A9−/− vs. Aged.
3.5. S100A9 knockout mitigates oxidative stress in the aged mice aorta
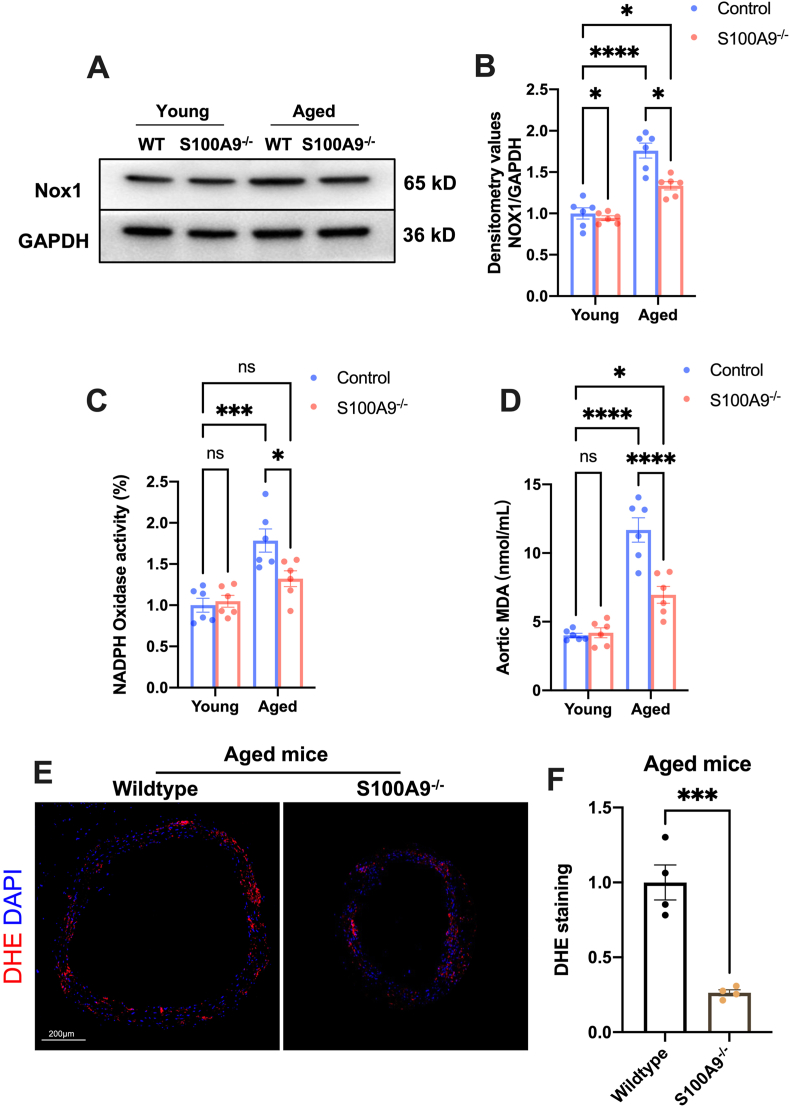
Aging and insulin resistance increase ROS production and lead to oxidative stress injury [35]. The results presented increased Nox1 expression in aged mice aorta (Fig. 5A and B). Similarly, the NADPH oxidase activity and MAD content in the aorta also significantly increased (Fig. 5C and D). However, S100A9 knockout almost blunted the increased NADPH oxidative activity and markedly reduced the NOX1 expression as well as the MDA content in aged mice aorta (Fig. 5A–D). S100A9 also reduced the dihydroethidium (DHE) fluorescence in aorta rings from aged mice (Fig. 5E and F). All these findings suggested that S100A9 knockout inhibited oxidative stress in aged mice aorta.
Fig. 5.
S100A9 knockout mitigates oxidative stress in the aorta of aged mice. (A) Representative Western blots of Nox1 in the aortas from mice in different groups. The statistical results were displayed in (B) (n = 6). (C) The results of NADPH oxidase activity in mice from different groups (n = 6). (D) The MDA content in aortas from different groups (n = 6). (E) Representative images of dihydroethidium (DHE) staining in aortas from different groups and the statistical results were exposed in (F), n = 4. ns, not significant. *P < 0.05, ***P < 0.001, ****P < 0.0001.
3.6. S100A9 knockout attenuates inflammatory response in aged mice
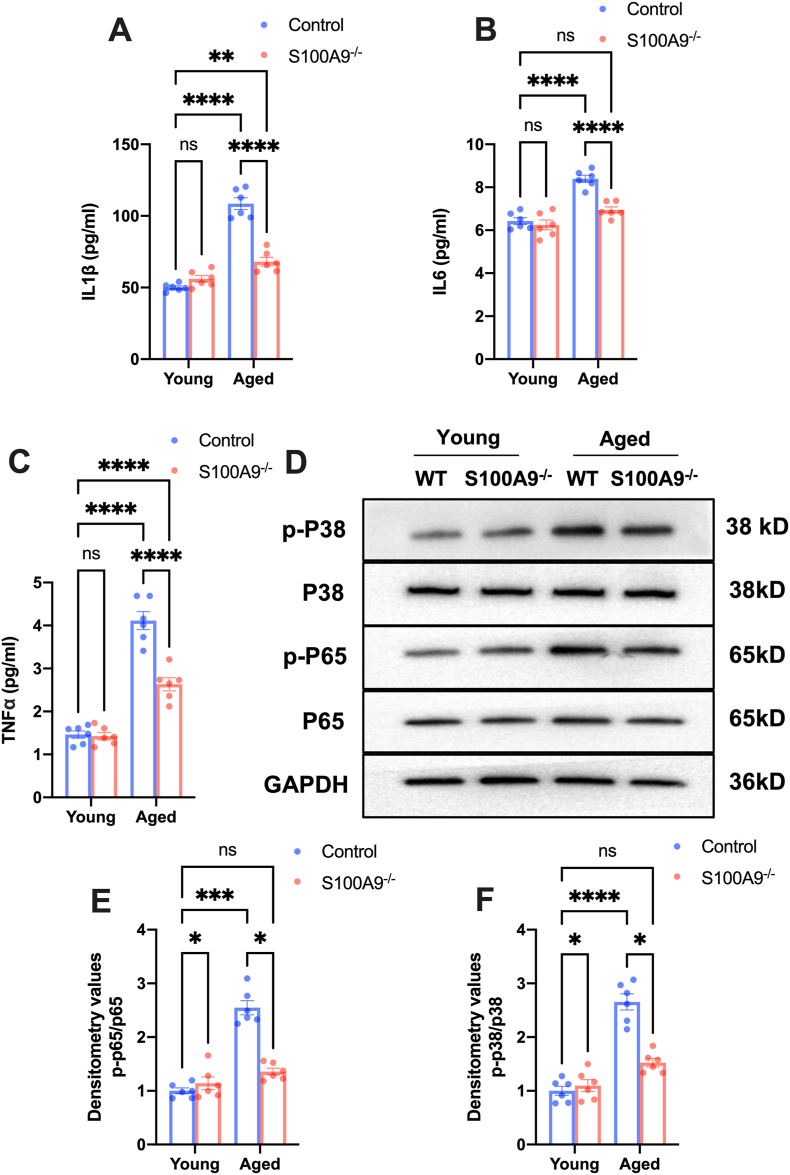
Aging is associated with increased sterile inflammation and S100A9 plays a vital role in triggering immune responses via TLR4 and RAGE pathway. Subsequently, we explored the effects of S100A9 knockout on inflammation in aged mice. The serum levels of interleukin-1β (IL-1β), IL-6, and tumor necrosis factor α (TNF-α) were significantly increased in aged mice compared to young mice, while S100A9 knockout notably attenuated this increase (Fig. 6A–C). Furthermore, S100A9 knockout blunted increased P38 and P65 phosphorylation in aged mice aorta (Fig. 6D–F). These results revealed that S100A9 knockout alleviated inflammation response in aged mice.
Fig. 6.
S100A9 knockout attenuates inflammatory response in aged mice. (A–C) The interleukin 1β (IL-1β), IL-6, and tumor necrosis factor α (TNF-α) levels in the serum of mice in different groups (n = 6). (D) Representative Western blots of Phospho-P38 and P65 in the aortas from mice in different groups. The related statistical results were exhibited in (E) and (F) (n = 6). ns, not significant. *P < 0.05, ***P < 0.001, ****P < 0.0001.
3.7. S100A9 induces cellular senescence in human aortic endothelial cells via TLR4
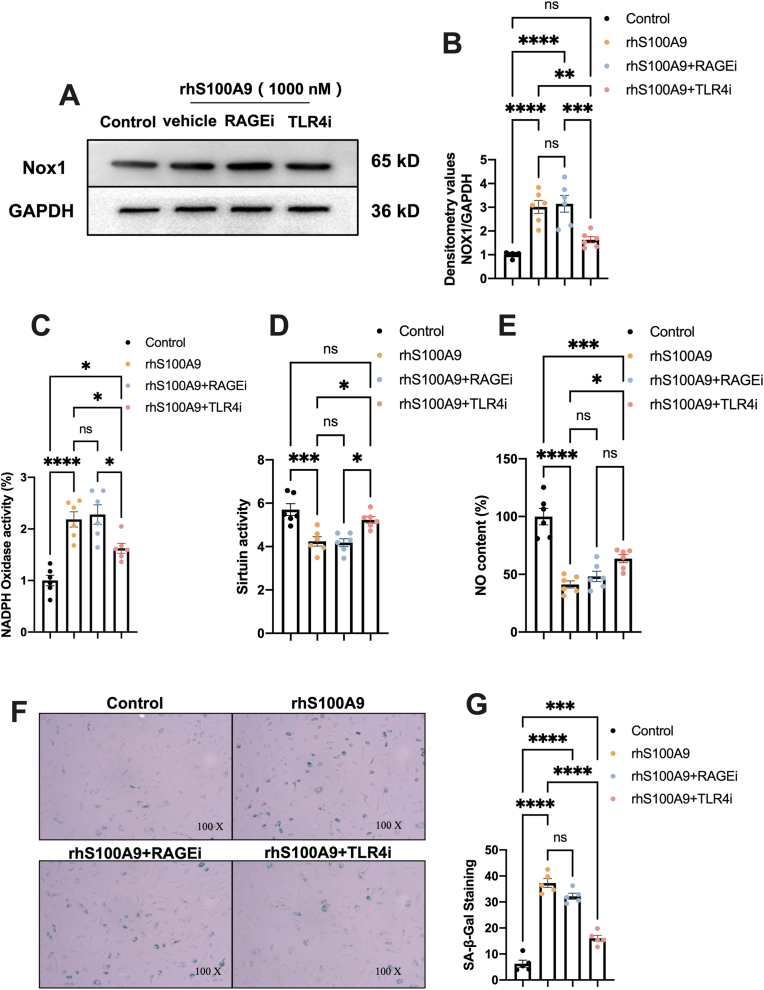
Subsequently, we investigated whether RAGE or TLR4 is responsible for S100A9-induced cellular senescence in aortic endothelial cells. rhS100A9 did not significantly reduced HAECs viability at 12 h, even combination with TLR4 or RAGE inhibitor as shown in Fig. S1 A and B. The rhS100A9 induced Nox1 expression in a concentration-dependent manner, and 1000 nM rhS100A9 increased the Nox1 expression to 3.8 fold-change (Figs. S2C and D). Thus, we chose 1000 nM rhS100A9 for the next experiments. The results revealed that rhS100A9 significantly increased the Nox1 expression (Fig. 7A and B) and NADPH oxidative activity (Fig. 7C) while reducing Sirtuin activity (Fig. 7D) and NO production (Fig. 7E) in endothelial cells. Moreover, rhS100A9 markedly elevated the level of SA-β-Gal activity, an indicator of cardiac senescence (Fig. 7F and G). TLR4 inhibition, but not RAGE, significantly blunted the impaired effects of rhS100A9 on endothelial cells (Fig. 7A–G). These findings indicated that S100A9-induced endothelial injury and senescence are TLR4 dependent.
Fig. 7.
S100A9 induces cellular senescence in human aortic endothelial cells via TLR4. (A) Representative Western blots of Nox1 in HAECs treated with rhS100A9 with TLR4 or RAGE inhibitors. The statistical results were presented in (B) (n = 6). (C–E) The results of NADPH oxidase and Sirtuin activities and NO content in HAECs from different groups (n = 6). (F–G) Representative pictures of SA-β-Gal-stained HAECs and quantification of SA-β-Gal-staining positive cells (n = 5). ns, not significant. *P < 0.05, **P < 0.001, ***P < 0.001, ****P < 0.0001.
4. Discussion
The association between inflammation and aging has been widely accepted. Various stimuli may trigger inflammatory responses, including exogenetic pathogens, endogenous damage-associated molecular patterns, unbalanced metabolism, and gut microbiota, during aging [8]. The excessive baseline inflammation emerges and accelerates the aging as adverse stimulation accumulates. Moreover, aging is accompanied by immune system remodeling, inducing immune dysfunction and leading to age-related diseases [36]. Neutrophils, the immune system's first responder cells, are sensitive to many physiologic changes [37], including aging. The neutrophil functions have been impaired with aging and involve many age-related pathologies [38,39], such as cardiovascular and autoimmune diseases. According to a recent study, the Single-Cell RNA sequencing revealed that the percentage of neutrophils increased with aortic aging [40]. Moreover, S100A9 is an important member of the calcium-binding protein S100 family and constitutes approximately 40% of the cytosolic protein in neutrophils [41]. S100A9 mRNA expression has been increased in aged mice and humans [42], although the specific role of S100A9 in aging remains unidentified. In the present study, we investigated the role of S100A9 in aging using S100A9 null mice for the first time. The results exhibited that S100A9 accelerated the aging-relegated pathologies, including telomere shortening, impaired Sirtuin activity, oxidative stress, and insulin resistance. These findings indicate that S100A9 may be a potential target to delay senescence and prevent age-related diseases.
Cardiovascular disease has caused the most death and disability word widely as lifespan increases [4]. Aging and inflammatory responses promote cardiovascular disease development and progression [5]. Endothelial cells are a constitutive part of the inner layer of the vascular wall and are crucial for normal tone [43]. Besides, endothelial cells are crucial for immune responses by regulating immune cell recruitment and extravasation [29]. Endothelial dysfunction prompts vascular remodeling and impaired vasodilatation, contributing to cardiovascular disease during aging [44,45]. Considering that S100A9 induces inflammation and accelerates senescence in mesenchymal stromal cells [25], we speculated that S100A9 knockout may improve vascular function in aged mice. This study presented that S100A9 deletion improved endothelium-dependent vasodilatation in aged mice. NO, produced primarily by eNOS, is essential for endothelial function [46]. Evidence from clinical and basic research exposed that the eNOS activity and NO production decreased during aging [46]. Consistent with previous findings, our study revealed that the impaired endothelium-dependent vasodilatation was accompanied by reduced eNOS activity and NO production. Our previous studies showed that inflammatory cytokine impaired eNOS activity [47], and we found that S100A9 knockout reduced the content of IL1β, IL6 and TNFα in serum, indicating that S100A9 knockout may improve eNOS function. Indeed, we discovered that S100A9 deficiency significantly increased eNOS phosphorylation at Ser 1177; Thus, increasing NO production in aged mice. These data strongly indicate that S100A9 contributes to vascular dysfunction during aging.
Insulin resistance is one of the hallmarks of aging [48]. The prevalence of impaired glucose tolerance and diabetes mellitus was significantly higher in the aged population [49]. Impaired insulin signal also reduces the NO production and bioactivity aggravates endothelial dysfunction [50]. Although the mechanism of insulin resistance during aging remains unclear, the link between inflammation and insulin resistance has been identified during aging [8]. Inhibiting inflammation may improve insulin sensitivity and prevent age-related cardiovascular dysfunction [51]. The present study did not identify increased fasting blood glucose in aged mice. However, the increased serum insulin levels, impaired IPGTT, and IST evidenced the impaired insulin sensitivity in aged mice. Our study determined that S100A9 deletion improved insulin sensitivity in aged mice. S100A9 knockout also reduced the secretion of inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, which may improve insulin sensitivity and vascular relaxation in aged mice.
Increased reactive oxygen bioavailability and decreased antioxidant protection cause oxidative stress [52]. Oxidative stress has been accepted as an essential adverse factor driving age-related cardiovascular disease [53]. Moreover, eNOS and NO exert anti-oxidative stress effects in endothelial cells via inhibiting ROS generation [54]. Several previous studies reported that S100A9 influence NADPH oxidases activity and Nox1 isoform, and S100A9 knockout reduced myeloperoxidase activity in atherosclerosis mice [55,56]. Therefore, we investigated the effects of S100A9 deletion on oxidative stress in the aged mice aorta. Consistently, we discovered that S100A9 knockout suppressed NADPH oxidases activity, Nox1 expression, and MDA content in aged mice aorta. Our findings and others demonstrated the important role of S100A9 in promoting oxidative stress in cardiovascular diseases.
S100A9, a calcium- and zinc-binding protein, can activate the immune system via RAGE and TLR4 pathway in the presence of Zn2+ and Ca2+ [20]. We discovered that S100A9 knockout inhibited p38 and p65 signals in aged mice. However, a previous study reported that inhibiting TLR4 prevented the effects of S100A9 on proliferation, whereas inhibiting RAGE promoted them, indicating that TLR4 and RAGE might mediate different effects of S100A9 [57]. Given that the effects of S100A9 on endothelial cell senescence have been uninvestigated before, we inhibited the TLR4 or RAGE pathway in the presence of rhS100A9 in human aortic endothelial cells to investigate the downstream of S100A9. The results revealed that TLR4 inhibition but not RAGE blunted the effects of S100A9 on senescence in endothelial cells, suggesting that S100A9 primarily accelerates senescence via the TLR4 pathway, which is consistent with a previous report that TLR4 is required for S100A9-induced senescence in mesenchymal stromal cells [25]. However, the mechanisms responsible for the different effects of RAGE and TLR4 on S100A9-induced senescence require exploration.
One limitation is that we only tested the effects of RAGE and TLR4 on S100A9-induced senescence in isolated endothelial cells because long-term inhibition of the RAGE and TLR4 pathways in aging is difficult. Therefore, the function of RAGE as a down streaming molecular of S100A9 cannot be excluded during aging. In addition, S100A9 predominantly expresses in neutrophils and monocytes. The Single-Cell RNA Sequencing revealed that the percentage of neutrophils increased with aortic aging and no significant change was observed in monocytes [40].Therefore, we speculated that the it was S100A9 in neutrophils mainly functions during vascular aging. However, we found that S100A9 knockout reduced the inflammatory cytokine levels in serum, indicating the important role of circulating immune cells. So, It needs further study to figure out the increased S100A9 in aorta is mainly resulted from increased neutrophils in aged vascular or circulating immune cells or even endothelial cells. Moreover, we focused on the endothelial dysfunction during aging at the present study. However, the specific effects of S100A9 on cascular smooth muscle cell and endothelial-independent vascular relaxation are also deaerves further study.
5. Conclusions
In conclusion, we demonstrated for the first time that S100A9 plays a vital pathogenic role in vascular dysfunction during aging, likely by aggravating inflammation, insulin resistance, and oxidative stress. Furthermore, TLR4 is essential for S100A9-induced senescence in endothelial cells. S100A9 deletion improved endothelium-dependent vasodilatation in aged mice. S100A9 blockade may provide therapeutic options for aging and cardiovascular diseases with the availability of a small-molecule inhibitor of S100A9.
Funding
This work was supported by the National Natural Science Foundation of China grants (82200422), Science Fund of the First Affiliated Hospital of Chongqing Medical University (PYJJ2021-05), the China Postdoctoral Science Foundation (2022M720601), the Postdoctoral Incubation Project of The First Affiliated Hospital of Chongqing Medical University (CYYY-BSHPYXM-202204), the Natural Science foundation of Chongqing (CSTB2022NSCQ-MSX0913) and Program for Youth Innovation in Future Medicine, Chongqing Medical University (W0168).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102721.
Contributor Information
Yu Zhao, Email: zhaoyu8623@163.com.
Yongzheng Guo, Email: gyz_cardio@hospital.cqmu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Kline K.A., Bowdish D.M. Infection in an aging population. Curr. Opin. Microbiol. 2016;29:63–67. doi: 10.1016/j.mib.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Costantino S., Paneni F., Cosentino F. Ageing, metabolism and cardiovascular disease. J. Physiol. 2016;594:2061–2073. doi: 10.1113/JP270538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moturi S., Ghosh-Choudhary S.K., Finkel T. Cardiovascular disease and the biology of aging. J. Mol. Cell. Cardiol. 2022;167:109–117. doi: 10.1016/j.yjmcc.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 4.North B.J., Sinclair D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liberale L., Badimon L., Montecucco F., Luscher T.F., Libby P., Camici G.G. Inflammation, aging, and cardiovascular disease: JACC review topic of the week. J. Am. Coll. Cardiol. 2022;79:837–847. doi: 10.1016/j.jacc.2021.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papaconstantinou J. The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease. Cells-Basel. 2019;8 doi: 10.3390/cells8111383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 9.Liu H., Chu S., Wu Z. Loss of toll-like receptor 4 ameliorates cardiovascular dysfunction in aged mice. Immun. Ageing. 2021;18:42. doi: 10.1186/s12979-021-00251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marin-Aguilar F., Lechuga-Vieco A.V., Alcocer-Gomez E., Castejon-Vega B., Lucas J., Garrido C., et al. NLRP3 inflammasome suppression improves longevity and prevents cardiac aging in male mice. Aging Cell. 2020;19 doi: 10.1111/acel.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Giosia P., Stamerra C.A., Giorgini P., Jamialahamdi T., Butler A.E., Sahebkar A. The role of nutrition in inflammaging. Ageing Res. Rev. 2022;77 doi: 10.1016/j.arr.2022.101596. [DOI] [PubMed] [Google Scholar]
- 12.Thevaranjan N., Puchta A., Schulz C., Naidoo A., Szamosi J.C., Verschoor C.P., et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21:455–466. doi: 10.1016/j.chom.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharath L.P., Agrawal M., McCambridge G., Nicholas D.A., Hasturk H., Liu J., et al. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metabol. 2020;32:44–55. doi: 10.1016/j.cmet.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y., Simon M., Seluanov A., Gorbunova V. DNA damage and repair in age-related inflammation. Nat. Rev. Immunol. 2023;23:75–89. doi: 10.1038/s41577-022-00751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santoro A., Martucci M., Conte M., Capri M., Franceschi C., Salvioli S. Inflammaging, hormesis and the rationale for anti-aging strategies. Ageing Res. Rev. 2020;64 doi: 10.1016/j.arr.2020.101142. [DOI] [PubMed] [Google Scholar]
- 16.Castelo-Branco C., Soveral I. The immune system and aging: a review. Gynecol. Endocrinol. 2014;30:16–22. doi: 10.3109/09513590.2013.852531. [DOI] [PubMed] [Google Scholar]
- 17.Shabani F., Farasat A., Mahdavi M., Gheibi N. Calprotectin (S100A8/S100A9): a key protein between inflammation and cancer. Inflamm. Res. 2018;67:801–812. doi: 10.1007/s00011-018-1173-4. [DOI] [PubMed] [Google Scholar]
- 18.Bjork P., Bjork A., Vogl T., Stenstrom M., Liberg D., Olsson A., et al. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009;7:e97. doi: 10.1371/journal.pbio.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawakami R., Katsuki S., Travers R., Romero D.C., Becker-Greene D., Passos L., et al. S100A9-RAGE Axis accelerates formation of macrophage-mediated extracellular vesicle microcalcification in diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2020;40:1838–1853. doi: 10.1161/ATVBAHA.118.314087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ursino G., Ramadori G., Hofler A., Odouard S., Teixeira P., Visentin F., et al. Hepatic non-parenchymal S100A9-TLR4-mTORC1 axis normalizes diabetic ketogenesis. Nat. Commun. 2022;13:4107. doi: 10.1038/s41467-022-31803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao B., Lu R., Chen J., Xie M., Zhao X., Kong L. S100A9 blockade prevents lipopolysaccharide-induced lung injury via suppressing the NLRP3 pathway. Respir. Res. 2021;22:45. doi: 10.1186/s12931-021-01641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sreejit G., Nooti S.K., Athmanathan B., Nagareddy P.R. S100A8/A9 in myocardial infarction. Methods Mol. Biol. 2019;1929:739–754. doi: 10.1007/978-1-4939-9030-6_46. [DOI] [PubMed] [Google Scholar]
- 23.Marinkovic G., Koenis D.S., de Camp L., Jablonowski R., Graber N., de Waard V., et al. S100A9 links inflammation and repair in myocardial infarction. Circ. Res. 2020;127:664–676. doi: 10.1161/CIRCRESAHA.120.315865. [DOI] [PubMed] [Google Scholar]
- 24.Li Y., Chen B., Yang X., Zhang C., Jiao Y., Li P., et al. S100a8/a9 signaling causes mitochondrial dysfunction and cardiomyocyte death in response to ischemic/reperfusion injury. Circulation. 2019;140:751–764. doi: 10.1161/CIRCULATIONAHA.118.039262. [DOI] [PubMed] [Google Scholar]
- 25.Shi L., Zhao Y., Fei C., Guo J., Jia Y., Wu D., et al. Cellular senescence induced by S100A9 in mesenchymal stromal cells through NLRP3 inflammasome activation. Aging (Albany NY) 2019;11:9626–9642. doi: 10.18632/aging.102409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ungvari Z., Tarantini S., Donato A.J., Galvan V., Csiszar A. Mechanisms of vascular aging. Circ. Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ungvari Z., Tarantini S., Sorond F., Merkely B., Csiszar A. Mechanisms of vascular aging, A geroscience perspective: JACC focus seminar. J. Am. Coll. Cardiol. 2020;75:931–941. doi: 10.1016/j.jacc.2019.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia G., Aroor A.R., Jia C., Sowers J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta, Mol. Basis Dis. 2019;1865:1802–1809. doi: 10.1016/j.bbadis.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Lee G.H., Hoang T.H., Jung E.S., Jung S.J., Han S.K., Chung M.J., et al. Anthocyanins attenuate endothelial dysfunction through regulation of uncoupling of nitric oxide synthase in aged rats. Aging Cell. 2020;19 doi: 10.1111/acel.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Northcott J.M., Czubryt M.P., Wigle J.T. Vascular senescence and ageing: a role for the MEOX proteins in promoting endothelial dysfunction. Can. J. Physiol. Pharmacol. 2017;95:1067–1077. doi: 10.1139/cjpp-2017-0149. [DOI] [PubMed] [Google Scholar]
- 31.Hu S., Pi Q., Xu X., Yan J., Guo Y., Tan W., et al. Disrupted eNOS activity and expression account for vasodilator dysfunction in different stage of sepsis. Life Sci. 2021;264 doi: 10.1016/j.lfs.2020.118606. [DOI] [PubMed] [Google Scholar]
- 32.Guo Y., Wang X., Jia P., You Y., Cheng Y., Deng H., et al. Ketogenic diet aggravates hypertension via NF-kappaB-mediated endothelial dysfunction in spontaneously hypertensive rats. Life Sci. 2020;258 doi: 10.1016/j.lfs.2020.118124. [DOI] [PubMed] [Google Scholar]
- 33.Cai Y., Liu H., Song E., Wang L., Xu J., He Y., et al. Deficiency of telomere-associated repressor activator protein 1 precipitates cardiac aging in mice via p53/PPARalpha signaling. Theranostics. 2021;11:4710–4727. doi: 10.7150/thno.51739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurauti M.A., Soares G.M., Marmentini C., Bronczek G.A., Branco R., Boschero A.C. Insulin and aging. Vitam. Horm. 2021;115:185–219. doi: 10.1016/bs.vh.2020.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao Y., Fan Y., Li F., Hao Y., Kong Y., Chen C., et al. Phenotypic and functional alterations of monocyte subsets with aging. Immun. Ageing. 2022;19:63. doi: 10.1186/s12979-022-00321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adrover J.M., Nicolas-Avila J.A., Hidalgo A. Aging: a temporal dimension for neutrophils. Trends Immunol. 2016;37:334–345. doi: 10.1016/j.it.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Binet F., Cagnone G., Crespo-Garcia S., Hata M., Neault M., Dejda A., et al. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science. 2020;369 doi: 10.1126/science.aay5356. [DOI] [PubMed] [Google Scholar]
- 39.Lagnado A., Leslie J., Ruchaud-Sparagano M.H., Victorelli S., Hirsova P., Ogrodnik M., et al. Neutrophils induce paracrine telomere dysfunction and senescence in ROS-dependent manner. EMBO J. 2021;40 doi: 10.15252/embj.2020106048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie W., Ke Y., You Q., Li J., Chen L., Li D., et al. Single-cell RNA sequencing and assay for transposase-accessible chromatin using sequencing reveals cellular and molecular dynamics of aortic aging in mice. Arterioscler. Thromb. Vasc. Biol. 2022;42:156–171. doi: 10.1161/ATVBAHA.121.316883. [DOI] [PubMed] [Google Scholar]
- 41.Kozlyuk N., Monteith A.J., Garcia V., Damo S.M., Skaar E.P., Chazin W.J. S100 proteins in the innate immune response to pathogens. Methods Mol. Biol. 2019;1929:275–290. doi: 10.1007/978-1-4939-9030-6_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swindell W.R., Johnston A., Xing X., Little A., Robichaud P., Voorhees J.J., et al. Robust shifts in S100a9 expression with aging: a novel mechanism for chronic inflammation. Sci. Rep. 2013;3:1215. doi: 10.1038/srep01215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casas J.P., Cavalleri G.L., Bautista L.E., Smeeth L., Humphries S.E., Hingorani A.D. Endothelial nitric oxide synthase gene polymorphisms and cardiovascular disease: a HuGE review. Am. J. Epidemiol. 2006;164:921–935. doi: 10.1093/aje/kwj302. [DOI] [PubMed] [Google Scholar]
- 44.Donato A.J., Morgan R.G., Walker A.E., Lesniewski L.A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell. Cardiol. 2015;89:122–135. doi: 10.1016/j.yjmcc.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanabe T., Maeda S., Miyauchi T., Iemitsu M., Takanashi M., Irukayama-Tomobe Y., et al. Exercise training improves ageing-induced decrease in eNOS expression of the aorta. Acta Physiol. Scand. 2003;178:3–10. doi: 10.1046/j.1365-201X.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 46.Kruger-Genge A., Blocki A., Franke R.P., Jung F. Vascular endothelial cell biology: an update. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20184411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu S., Pi Q., Xu X., Yan J., Guo Y., Tan W., et al. Disrupted eNOS activity and expression account for vasodilator dysfunction in different stage of sepsis. Life Sci. 2021;264 doi: 10.1016/j.lfs.2020.118606. [DOI] [PubMed] [Google Scholar]
- 48.Janssen J. Hyperinsulinemia and its pivotal role in aging, obesity, type 2 diabetes, cardiovascular disease and cancer. Int. J. Mol. Sci. 2021:22. doi: 10.3390/ijms22157797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitada M., Koya D. Autophagy in metabolic disease and ageing. Nat. Rev. Endocrinol. 2021;17:647–661. doi: 10.1038/s41574-021-00551-9. [DOI] [PubMed] [Google Scholar]
- 50.Yu Q., Gao F., Ma X.L. Insulin says NO to cardiovascular disease. Cardiovasc. Res. 2011;89:516–524. doi: 10.1093/cvr/cvq349. [DOI] [PubMed] [Google Scholar]
- 51.Kumar P., Liu C., Hsu J.W., Chacko S., Minard C., Jahoor F., et al. Glycine and N-acetylcysteine (GlyNAC) supplementation in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, insulin resistance, endothelial dysfunction, genotoxicity, muscle strength, and cognition: results of a pilot clinical trial. Clin. Transl. Med. 2021;11:e372. doi: 10.1002/ctm2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H., Davies K., Forman H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015;88:314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Izzo C., Vitillo P., Di Pietro P., Visco V., Strianese A., Virtuoso N., et al. The role of oxidative stress in cardiovascular aging and cardiovascular diseases. Life. 2021;11 doi: 10.3390/life11010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflügers Archiv. 2010;459:923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 55.Croce K., Gao H., Wang Y., Mooroka T., Sakuma M., Shi C., et al. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation. 2009;120:427–436. doi: 10.1161/CIRCULATIONAHA.108.814582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benedyk M., Sopalla C., Nacken W., Bode G., Melkonyan H., Banfi B., et al. HaCaT keratinocytes overexpressing the S100 proteins S100A8 and S100A9 show increased NADPH oxidase and NF-kappaB activities. J. Invest. Dermatol. 2007;127:2001–2011. doi: 10.1038/sj.jid.5700820. [DOI] [PubMed] [Google Scholar]
- 57.Kovacic M., Mitrovic-Ajtic O., Beleslin-Cokic B., Djikic D., Suboticki T., Diklic M., et al. TLR4 and RAGE conversely mediate pro-inflammatory S100A8/9-mediated inhibition of proliferation-linked signaling in myeloproliferative neoplasms. Cell. Oncol. 2018;41:541–553. doi: 10.1007/s13402-018-0392-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.